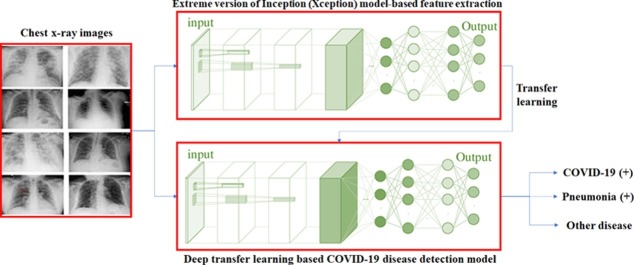
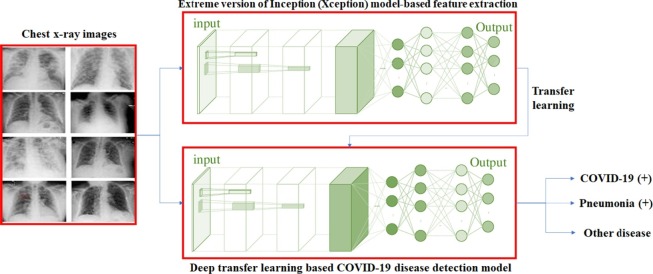
Graphical abstract
Keywords: Deep learning, COVID-19, Chest x-ray, Transfer learning
Abstract
The most widely used novel coronavirus (COVID-19) detection technique is a real-time polymerase chain reaction (RT-PCR). However, RT-PCR kits are costly and take 6-9 hours to confirm infection in the patient. Due to less sensitivity of RT-PCR, it provides high false-negative results. To resolve this problem, radiological imaging techniques such as chest X-rays and computed tomography (CT) are used to detect and diagnose COVID-19. In this paper, chest X-rays is preferred over CT scan. The reason behind this is that X-rays machines are available in most of the hospitals. X-rays machines are cheaper than the CT scan machine. Besides this, X-rays has low ionizing radiations than CT scan. COVID-19 reveals some radiological signatures that can be easily detected through chest X-rays. For this, radiologists are required to analyze these signatures. However, it is a time-consuming and error-prone task. Hence, there is a need to automate the analysis of chest X-rays. The automatic analysis of chest X-rays can be done through deep learning-based approaches, which may accelerate the analysis time. These approaches can train the weights of networks on large datasets as well as fine-tuning the weights of pre-trained networks on small datasets. However, these approaches applied to chest X-rays are very limited. Hence, the main objective of this paper is to develop an automated deep transfer learning-based approach for detection of COVID-19 infection in chest X-rays by using the extreme version of the Inception (Xception) model. Extensive comparative analyses show that the proposed model performs significantly better as compared to the existing models.
1. Introduction
The first case of novel coronavirus (COVID-19) was found in Wuhan, China in December 2019. It was assumed that this virus has been originated from animals that have zoonotic nature. However, the source of this virus has not been identified [1]. The first person has been infected from the Wuhan market in Hubei Province. Thereafter, it has been spread in China and infected persons of any age across the whole World [2]. The disease caused by this novel coronavirus is known as COVID-19. The World Health Organization (WHO) announced COVID-19 a pandemic on 11 February 2020. The total number of infected cases are 7,949,710 with 434,177 causality and 4,087,348 recovered cases on 15 June, 2020 at 11:27 GMT [3]. The second-largest population country, India has 333,008 confirmed cases with 9,520 causality and 169,689 recovered cases.
COVID-19 is a respiratory disease that is instigated by a novel coronavirus. The common symptoms appear in the infected person are fever, cough, sore throat, and difficulty in breathing [4]. Vanishing of taste, tiredness, aches, and nasal blockage can also be observed in some patients [5]. The duration between contamination and the first indication of symptoms may be extended to 14 days [6]. The infection of this virus is transmitted through the droplets of patients such as coughing and sneezing. If the person comes indirectly or indirectly contact with an infected person, then the contacted person gets infected. The vaccines/drugs of this disease are not available until now. Isolation and social distancing are the only solutions to this infection. Therefore, the early detection of infected persons is required to stop the spread of infection.
The most widely used COVID-19 detection technique is real-time polymerase chain reaction (RT-PCR). However, RT-PCR kits are costly and take 6-9 hours to confirm infection in the patient [7]. Due to less sensitivity of RT-PCR, it provides high false-negative results. To resolve this problem, radiological imaging techniques such as chest X-rays and computed tomography (CT) are used to detect and diagnose COVID-19 [8]. In this paper, chest X-rays is preferred over CT scan. The reason behind this is that X-rays machines are available in most of the hospitals. X-rays machines are cheaper than the CT scan machine. Besides this, X-rays have low ionizing radiations than CT scan [9]. COVID-19 reveals some radiological signatures that can be easily detected through chest X-rays. For this, radiologists are required to analyze these signatures. However, it is a time-consuming and error-prone task. Hence, there is a need to automate the analysis of chest X-rays.
The automatic analysis of chest X-rays can be done through deep learning-based approaches, which may accelerate the analysis time. These approaches can train the weights of networks on large datasets as well as fine-tuning the weights of pre-trained networks on small datasets [10]. However, these approaches applied to chest X-rays are very limited to [6]. Hence, the motive of this paper is to develop an automated deep learning-based approach for the detection of infection in chest X-rays.
The main contribution of this paper is:
-
1.
To overcome the less sensitivity of RT-PCR, chest X-rays images are used in this paper to detect and diagnose of COVID-19.
-
2.
In this paper, chest X-rays is preferred over CT scan. The reason behind this is that X-rays machines are available in most of the hospitals. Even, X-rays machine is cheaper than the CT scan machine. Besides this, X-rays has low ionizing radiations than CT scan.
-
3.
COVID-19 reveals some radiological signatures that can be easily detected through chest X-rays. Therefore, automatic analysis of chest X-rays can be done through deep learning-based approaches, which may accelerate the analysis time.
-
4.
Extreme version of inception (Xception) can train the weights of networks on large datasets as well as fine-tuning the weights of pre-trained networks on small datasets.
-
5.
Extensive comparative analyses are also drawn to evaluate the performance of the proposed model by using various performance metrics such as accuracy, f-measure, sensitivity, specificity, and kappa statistics.
The remaining structure of this paper is organized as follows. Section 2 presents related work. The proposed deep learning-based technique is presented in Section 3. Section 4 discusses the experimental results and discussion. The concluding remarks are drawn in Section 5.
2. Related work
Recently, deep learning techniques have been used for the analysis of chest X-rays in a short period. Due to low ionizing radiations and portability of X-rays, it has been preferred over the chest CT scan [11].
Wang et al. [12] developed a deep convolutional neural network (CNN) for the identification of COVID-19 cases from chest X-rays. Their model was trained over 13,975 chest X-ray images. The classification accuracy obtained from the model was 98. 9%. Hemdan et al. [13] developed a COVIDX-Net for automatic detection of coronavirus infected persons using chest X-ray images. COVIDX-Net was trained on 50 normal and 25 confirmed COVID-19 cases. The classification accuracy obtained from COVIDX-Net was 91% for COVID cases. Narin et al. [14] presented three different CNN models such as ResNet-50, Inception-ResNetV2, and InceptionV3 for classification of COVID-19 from the chest X-ray images. ResNet50 provided better classification accuracy of 98% than the other models. Sethy and Behera [15]utilized the pre-trained transfer technique as ResNet-50 for extracting the imaging features from the infected patients. These features were applied to support vector machines (SVM) for classification. The classification accuracy obtained in the developed model was 95. 348%.
Farooq and Hafeez [16] presented a multi-stage fine-tuning scheme for pre-trained ResNet-50 architecture. The developed model named COVIDResNet. The accuracy obtained from COVIDResNet was 96.23%. Asnaoui et al. [17] presented a comparative study of eight transfer learning techniques for the classification of COVID-19 pneumonia. This model was trained on 5856 chest X-ray images. MobileNet-V2 and Inception-V3 provided 96% classification accuracy. Abbas et al. [18] presented a deep CNN named as Decompose, Transfer, and Compose (DeTraC) for distinguishing the symptoms of COVID-19 using chest X-rays. They investigated the irregularities in class boundaries using the decomposition process. DeTraC model attained 95.12% accuracy with a sensitivity of 97.91%. Chowdhury et al. [19] proposed an image argumentation technique with a transfer technique for the detection of coronavirus infection on chest X-ray images. Four well-known pre-trained techniques namely, AlexNet, ResNet-18, DenseNet-201, and SqueezeNet are used for classification. The classification accuracy obtained from SqueezeNet was 98.3% with 99% specificity and 96.7% sensitivity.
Alqudah et al. [20] used machine learning techniques namely, SVM and random forest (RF) for early detection of COVID-19 symptoms in the patients. They utilized the CNN model for feature extraction. After that machine learning techniques are applied to extracted features to classify COVID-19 and non-COVID-19 cases. The accuracies obtained from SVM and RF were 90.5% and 81%, respectively. Ghoshal and Tucker [21] utilized Bayesian CNN (BCNN) to diagnosis the COVID-19 using a chest X-ray. They investigated the significance of dropping weights of BCNN. The correlation between uncertainty and prediction accuracy was investigated. The classification accuracy obtained from BCNN was 90%. Salman et al. [22] utilized a trained CNN for detecting the coronavirus infection on chest X-ray images. The sensitivity and specificity obtained from the model were 100%.
Li and Zhu [23] designed a deep CNN for the extraction of imaging features using chest X-ray images. The developed model is named as COVID-Xpert. DenseNet based transfer learning technique is used to distinguish between COVID-19 and viral pneumonia cases. Karim et al. [24] developed a DeepCOVIDExplainer for automatic detection of COVID-19 symptoms from the patient's chest X-ray. They used an ensemble technique utilizing image processing and transfer learning techniques. The proposed method attained 96.12% classification accuracy for COVID-19 cases. Apostolopoulos and Mpesiana [25] used a transfer learning approach for the detection of coronavirus patterns from the patient's chest X-ray. They utilized 224 confirmed COVID-19, 714 viral pneumonia, and 504 normal images. The accuracy obtained from this model was 98.75% for binary class. Ozturk et al. [26] developed a DarkNet Model for automatic detection of an infected person using chest X-ray images. DarkNet model was used to classify the binary and multi-class problems in COVID-19. It produced the classification accuracies as 98.08% and 87.02% for binary and multi-class problems, respectively.
Medhi et al. [27] implemented deep CNN for detecting and diagnosis of coronavirus infection using chest X-ray. They tested their model on 150 confirmed cases obtained from the Kaggle dataset. The accuracy obtained from their model was 93%. Asif and Wenhui [28] proposed an automatic COVID-19 detection system using chest X-ray images. They used Inception V3 with transfer learning for detecting infection in the patient's chest. Their model was tested on 1341 normal, 1345 viral pneumonia, and 864 COVID-19 images. This model achieved was 96% classification accuracy. Loey et al. [29] implemented a GAN based deep learning technique for COVID-19 detection in chest X-ray. They investigated three models such as AlexNet, GoogleNet, and ResNet-18. GoogleNet model attained 80.6% and 99.9% for four and two class cases. Asnaoui and Chawki [30] presented a comparative study of seven different deep learning architectures for detecting the symptoms of COVID-19 in chest X-ray images. These models were trained over 6087 images. Inception-ResNetV2 provided the classification accuracy of 92.18%.
3. Proposed model
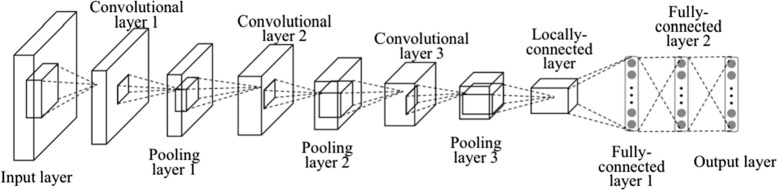
This section discusses the proposed deep transfer learning-based COVID-19 disease detection model. Initially, a deep convolutional network is defined. Thereafter, a deep transfer learning model is defined. Finally, the proposed deep transfer learning-based model is defined. Fig. 1 illustrates the layered structure of the deep convolution neural network.
Fig. 1.
Architecture of deep convolution neural network.
Initially, to extract the features of images, convolutional layer is applied by using different masks. It produces the low-level features. An activate function is then used as [31], [32], [33]:
| (1) |
Here, defines latent demonstration of kth feature of the layer. F represents an activation function, defines lth feature of of the former layers. In case of first layer, lth ⊗ channel of input images with channels. and define the coefficients and biases values, respectively, of kth feature map. Rectified linear unit (ReLU) is utilized as an activation function F. It can be defined as:
| (2) |
If , then, the value of a is 0 and returns a otherwise.
The n-dimensional vector can be decomposed into real numbers by using the Softmax function. It can be computed as:
| (3) |
Here, yj defines the input tensor's parameter.
In this paper, a cross-entropy is used as a loss function. It can be defined as:
| (4) |
Here, defines label of ith image. shows ith parameter of obtained results of softmax function.
To minimize the size of features space, pooling layer is considered. Average and max pooling operations are utilized to obtain average and maximum values, respectively.
Finally, the fully-connected layer is utilized to map the output to flatten and linearly separable space. Softmax is then utilized to test COVID-19 disease in chest x-ray images.
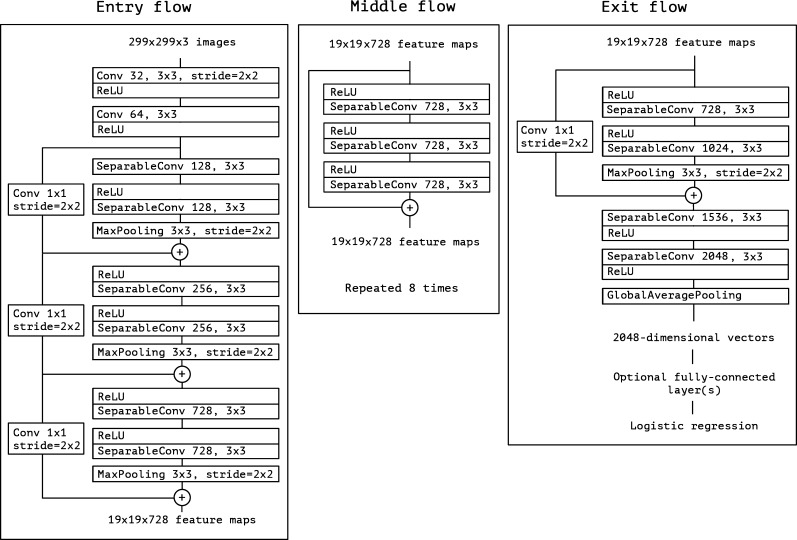
However, a convolutional neural network may suffer from the under-fitting issue, as many potential features may not be extracted. Therefore, in this paper, an extreme version of the Inception (Xception) model is used. Fig. 2 shows the architecture of the xception model (for more details please see [34]).
Fig. 2.
Architecture of extreme version of Inception (Xception) model (adapted from [34]).
Fig. 3 shows the proposed deep transfer learning based COVID-19 disease testing model for chest x-ray images. It utilizes deep convolutional neural networks and Xception model to build the model.
Fig. 3.
Architecture of extreme version of Inception (Xception) model.
4. Experimental results and discussion
4.1. Dataset
We have used three class chest x-ray datasets obtained from [26]. It contains three classes as COVID-19 (+), pneumonia (+) but COVID-19 (-), and other infection except COVID-19 and pneumonia. We have used 70% dataset for training purpose. Remaining dataset is further divided into 10% and 20% fractions for validation and testing purpose, respectively.
4.2. Comparative analyses
Fig. 4 shows the true positive rate and false positive rate analyses among the proposed and some well known competitive models. It is clearly shown that the proposed model achieves a significantly better area under curve (AUC) values as compared to the competitive models.
Fig. 4.
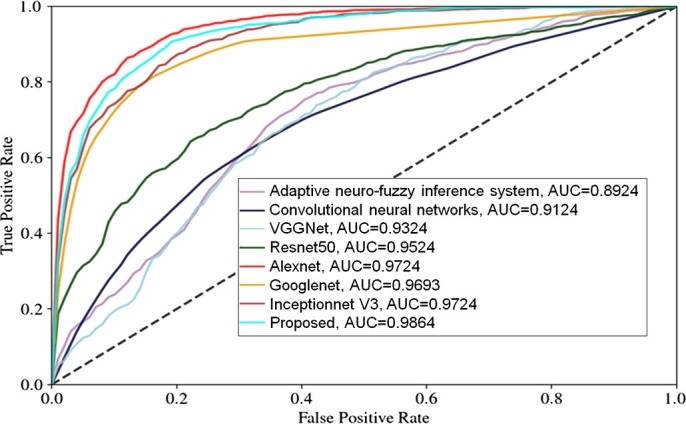
Training and Validation analyses between the proposed and the inceptionnet V3 models.
Table 1 shows training analyses of the proposed and competitive models in terms of accuracy, f-measure, sensitivity, specificity, and kappa statistics. It clearly shows that the proposed model achieves significantly better performance as compared to the competitive models. Even the proposed model shows significantly lesser uncertainty values, which shows the proposed model provides consistent training results.
Table 1.
Training analyses of the proposed deep transfer learning based COVID-19 infection testing model.
| Model | Accuracy | F-measure | Sensitivity | Specificity | Kappa statistics |
|---|---|---|---|---|---|
| Support vector machine | 0.834426 | 0.867797 | 0.867121 | 0.835237 | 0.850837 |
| Random forest | 0.849182 | 0.876271 | 0.876481 | 0.848933 | 0.862536 |
| Back propagation network | 0.854098 | 0.881356 | 0.881557 | 0.853859 | 0.867556 |
| Adaptive neuro-fuzzy inference system | 0.870492 | 0.893222 | 0.893939 | 0.869637 | 0.881667 |
| Convolutional neural networks | 0.886885 | 0.905085 | 0.906198 | 0.885572 | 0.895833 |
| VGGNet | 0.903279 | 0.916949 | 0.918333 | 0.901667 | 0.915436 |
| ResNet50 | 0.919672 | 0.928814 | 0.930348 | 0.917923 | 0.924167 |
| Alexnet | 0.936066 | 0.940678 | 0.942244 | 0.934343 | 0.938333 |
| Googlenet | 0.952459 | 0.952542 | 0.954023 | 0.950931 | 0.952543 |
| Inceptionnet V3 | 0.968852 | 0.964407 | 0.965686 | 0.967687 | 0.966667 |
| Proposed | 0.995246 | 0.986271 | 0.991236 | 0.994615 | 0.980833 |
Table 2 shows verification analyses of the proposed and the competitive models in terms of accuracy, f-measure, sensitivity, specificity, and kappa statistics on the testing dataset. It demonstrates that the proposed model achieves significantly good performance as compared to the competitive models. Even the proposed model shows significantly lesser uncertainty values, which shows the proposed model provides consistent training results.
Table 2.
Testing analyses of the proposed deep transfer learning based COVID-19 infection testing model.
| Model | Accuracy | F-measure | Sensitivity | Specificity | Kappa statistics |
|---|---|---|---|---|---|
| Support vector machine | 0.824959 | 0.861953 | 0.861252 | 0.825806 | 0.843105 |
| Random forest | 0.839546 | 0.870378 | 0.870588 | 0.839286 | 0.854666 |
| Back propagation network | 0.844408 | 0.875421 | 0.875639 | 0.844156 | 0.859626 |
| Adaptive neuro-fuzzy inference system | 0.860616 | 0.887205 | 0.887968 | 0.859706 | 0.873658 |
| Convolutional neural networks | 0.876823 | 0.898998 | 0.900166 | 0.875418 | 0.887696 |
| VGGNet | 0.893031 | 0.910774 | 0.912252 | 0.891269 | 0.901734 |
| ResNet50 | 0.909238 | 0.922559 | 0.924217 | 0.907285 | 0.915772 |
| Alexnet | 0.925446 | 0.934343 | 0.936066 | 0.923461 | 0.929816 |
| Googlenet | 0.941653 | 0.946128 | 0.947798 | 0.939799 | 0.943848 |
| Inceptionnet V3 | 0.957861 | 0.957912 | 0.959416 | 0.956303 | 0.957886 |
| Proposed | 0.974068 | 0.969697 | 0.970921 | 0.972973 | 0.971924 |
5. Conclusion
To overcome the less sensitivity issue with RT-PCR, chest X-rays images were used in this paper to detect and diagnosis of COVID-19. Chest X-rays were preferred over CT scans. As X-rays machines are cheaper than the CT scan machines, therefore, we have preferred chest x-ray images. Besides this, X-rays has low ionizing radiations than CT scan. From the extensive review, it has been reviewed that chest X-rays images of COVID-19 infected patients show some unique patterns and bilateral changes. However, manual COVID-19 testing from chest x-ray images is not an easy task. Therefore, in this paper, an automatic analysis of chest X-rays was achieved using deep learning-based approaches. Deep transfer learning approaches were able to train the weights of networks on large datasets as well as fine-tuning the weights of pre-trained networks on small datasets. Extensive comparative analyses have been performed to evaluate the performance of the proposed model by using various performance metrics such as accuracy, f-measure, sensitivity, specificity, and kappa statistics. Comparative analyses reveal that the proposed model outperforms competitive models. In the near future, the initial parameters of the proposed model can be tuned by using various approaches like parallel Strength Pareto Evolutionary Algorithm-II [35], non-dominated sorting genetic algorithm-III [36], [37], [38], memetic differential evolution, [39], [40], [41], genetic algorithm [42], particle swarm optimization [43], [44], etc. Also, some per-processing techniques can be used to improve the visibility of chest x-ray images such as integrated means filter [45], [46], gain gradient filter [47], [48], etc.
Funding
This work did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
All authors attest that they meet the current International Committee of Medical Journal Editors (ICMJE) criteria for Authorship.
Declaration of Competing Interest
The authors declare that they have no known competing financial or personal relationships that could be viewed as influencing the work reported in this paper.
Acknowledgements
Authors would like to thank Manipal University Jaipur for their kind support.
References
- 1.Boopathi S., Poma A.B., Kolandaivel P. Novel 2019 coronavirus structure, mechanism of action, antiviral drug promises and rule out against its treatment. J Biomol Struct Dyn. 2020:1–10. doi: 10.1080/07391102.2020.1758788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdelli I., Hassani F., Bekkel Brikci S., Ghalem S. In silico study the inhibition of angiotensin converting enzyme 2 receptor of COVID-19 by ammoides verticillata components harvested from western Algeria. J Biomol Struct Dyn. 2020:1–17. doi: 10.1080/07391102.2020.1763199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar S. Will COVID-19 pandemic diminish by summer-monsoon in India? Lesson from the first lockdown. medRxiv, 2020.
- 4.Landry M.D., Geddes L., Moseman A.P., Lefler J.P., Raman S.R., van Wijchen J. Early reflection on the global impact of COVID19, and implications for physiotherapy. Physiotherapy. 2020;107:A1–A3. doi: 10.1016/j.physio.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharifi-Razavi A., Karimi N., Rouhani N. COVID-19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Inf. 2020;35 doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Islam M.M., Hannan T., Sarker L., Ahmed Z. COVID-DenseNet: a deep learning architecture to detect COVID-19 from chest radiology images. 2020. https://doi.org/10.20944/preprints202005.0151.v1
- 7.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020 doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng M.-Y., Lee E.Y., Yang J., Yang F., Li X., Wang H., et al. Imaging profile of the COVID-19 infection: radiologic findings and literature review. Radiology: Cardiothor Imag. 2020;2(1) doi: 10.1148/ryct.2020200034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Latif S., Usman M., Manzoor S., Iqbal W., Qadir J., Tyson G., et al. Leveraging data science to combat COVID-19: a comprehensive review. 2020. https://doi.org/10.36227/techrxiv.12212516.v1 [DOI] [PMC free article] [PubMed]
- 10.Ho T.K.K., Gwak J., Prakash O., Song J.-I., Park C.M. Asian conference on intelligent information and database systems. Springer; 2019. Utilizing pretrained deep learning models for automated pulmonary tuberculosis detection using chest radiography; pp. 395–403. [Google Scholar]
- 11.Singh D., Kumar Vaishali V., Kaur M. Classification of COVID-19 patients from chest CT images using multi-objective differential evolution-based convolutional neural networks. Eur J Clin Microbiol Infect Dis. 2020;39:1–11. doi: 10.1007/s10096-020-03901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L., Wong A. COVID-Net: a tailored deep convolutional neural network design for detection of COVID-19 cases from chest X-ray images. 2020. arXiv:2003.09871 arXiv preprint. [DOI] [PMC free article] [PubMed]
- 13.Hemdan E.E.-D., Shouman M.A., Karar M.E. COVIDX-Net: a framework of deep learning classifiers to diagnose COVID-19 in X-ray images. 2020. arXiv:2003.11055 arXiv preprint.
- 14.Narin A., Kaya C., Pamuk Z. Automatic detection of coronavirus disease (COVID-19) using X-ray images and deep convolutional neural networks. 2020. arXiv:2003.10849 arXiv preprint. [DOI] [PMC free article] [PubMed]
- 15.Sethy P.K., Behera S.K. Detection of coronavirus disease (COVID-19) based on deep features. Preprints. 2020 [Google Scholar]
- 16.Farooq M., Hafeez A. COVID-ResNet: a deep learning framework for screening of COVID19 from radiographs. 2020. arXiv:2003.14395 arXiv preprint.
- 17.Asnaoui K.E., Chawki Y., Idri A. Automated methods for detection and classification pneumonia based on X-ray images using deep learning. 2020. arXiv:2003.14363 arXiv preprint.
- 18.Abbas A., Abdelsamea M.M., Gaber M.M. Classification of COVID-19 in chest X-ray images using detrac deep convolutional neural network. 2020. arXiv:2003.13815 arXiv preprint. [DOI] [PMC free article] [PubMed]
- 19.Chowdhury M.E., Rahman T., Khandakar A., Mazhar R., Kadir M.A., Mahbub Z.B., et al. Can AI help in screening viral and COVID-19 pneumonia? 2020. arXiv:2003.13145 arXiv preprint.
- 20.Alqudah A.M., Qazan S., Alquran H., Qasmieh I.A., Alqudah A. COVID-2019 detection using X-ray images and artificial intelligence hybrid systems. 2020. https://doi.org/10.5455/jjee.204-1585312246
- 21.Ghoshal B., Tucker A. Estimating uncertainty and interpretability in deep learning for coronavirus (COVID-19) detection. 2020. arXiv:2003.10769 arXiv preprint.
- 22.Salman F.M., Abu-Naser S.S., Alajrami E., Abu-Nasser B.S., Alashqar B.A. COVID-19 detection using artificial intelligence. Int J Acad Eng Res. 2020;4(3):18–25. [Google Scholar]
- 23.Li X., Zhu D. COVID-Xpert: an AI powered population screening of COVID-19 cases using chest radiography images. 2020. arXiv:2004.03042 arXiv preprint.
- 24.Karim M., Döhmen T., Rebholz-Schuhmann D., Decker S., Cochez M., Beyan O., et al. Deepcovidexplainer: explainable COVID-19 predictions based on chest X-ray images. 2020. arXiv:2004.04582 arXiv preprint.
- 25.Apostolopoulos I.D., Mpesiana T.A. COVID-19: automatic detection from X-ray images utilizing transfer learning with convolutional neural networks. Phys Eng Sci Med. 2020:1. doi: 10.1007/s13246-020-00865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozturk T., Talo M., Yildirim E.A., Baloglu U.B., Yildirim O., Acharya U.R. Automated detection of COVID-19 cases using deep neural networks with X-ray images. Comput Biol Med. 2020 doi: 10.1016/j.compbiomed.2020.103792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jamil M, Hussain I, et al. Automatic detection of COVID-19 infection from chest X-ray using deep learning. medRxiv, 2020.
- 28.Asif S, Wenhui Y. Automatic detection of COVID-19 using X-ray images with deep convolutional neural networks and machine learning. medRxiv, 2020.
- 29.Loey M., Smarandache F., Khalifa N.E.M. Within the lack of chest COVID-19 X-ray dataset: a novel detection model based on gan and deep transfer learning. Symmetry. 2020;12(4):651. [Google Scholar]
- 30.Elasnaoui K., Chawki Y. Using X-ray images and deep learning for automated detection of coronavirus disease. J Biomol Struct Dyn. 2020:1–22. doi: 10.1080/07391102.2020.1767212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi G., Wang H., Haner M., Weng C., Chen S., Zhu Z. Convolutional neural network based detection and judgement of environmental obstacle in vehicle operation. CAAI Trans Intell Technol. 2019;4(2):80–91. [Google Scholar]
- 32.Tingting Y., Junqian W., Lintai W., Yong X. Three-stage network for age estimation. CAAI Trans Intell Technol. 2019;4(2):122–126. [Google Scholar]
- 33.Basavegowda H.S., Dagnew G. Deep learning approach for microarray cancer data classification. CAAI Trans Intell Technol. 2020;5(1):22–33. [Google Scholar]
- 34.Chollet F. Proceedings of the IEEE conference on computer vision and pattern recognition. 2017. Xception: deep learning with depthwise separable convolutions; pp. 1251–1258. [Google Scholar]
- 35.Singh D., Kumar V. A comprehensive review of computational dehazing techniques. Arch Comput Methods Eng. 2019;26:1395–1413. doi: 10.1007/s11831-018-9294-z. [DOI] [Google Scholar]
- 36.Kaur M., Gianey H.K., Singh D., Sabharwal M. Multi-objective differential evolution based random forest for e-health applications. Mod Phys Lett B. 2019;33(05) [Google Scholar]
- 37.Gupta A., Singh D., Kaur M. An efficient image encryption using non-dominated sorting genetic algorithm-III based 4-D chaotic maps. J Ambient Intell Humaniz Comput. 2020;11(3):1309–1324. [Google Scholar]
- 38.Kaur M., Singh D., Sun K., Rawat U. Color image encryption using non-dominated sorting genetic algorithm with local chaotic search based 5D chaotic map. Future Gener Comput Syst. 2020;107:333–350. [Google Scholar]
- 39.Kaur M., Singh D., Singh Uppal R. Parallel strength Pareto evolutionary algorithm-II based image encryption. IET Image Process. 2020;14(6):1015–1026. [Google Scholar]
- 40.Kaur M., Singh D., Kumar V., Sun K. Color image dehazing using gradient channel prior and guided l0 filter. Inf Sci. 2020;521:326–342. doi: 10.1016/j.ins.2020.02.048. [DOI] [Google Scholar]
- 41.Kaur M., Kumar V., Li L. Color image encryption approach based on memetic differential evolution. Neural Comput Appl. 2019;31(11):7975–7987. [Google Scholar]
- 42.Kaur M., Kumar V. Beta chaotic map based image encryption using genetic algorithm. Int J Bifurc Chaos. 2018;28(11) [Google Scholar]
- 43.Pannu H.S., Singh D., Malhi A.K. Multi-objective particle swarm optimization-based adaptive neuro-fuzzy inference system for benzene monitoring. Neural Comput Appl. 2019;31:2195–2205. [Google Scholar]
- 44.Pannu H.S., Singh D., Malhi A.K. Improved particle swarm optimization based adaptive neuro-fuzzy inference system for benzene detection. CLEAN—Soil Air Water. 2018;46(5) [Google Scholar]
- 45.Singh D., Kumar V., Kaur M. Image dehazing using window-based integrated means filter. Multimed Tools Appl. 2019:1–23. doi: 10.1007/s11042-019-08286-6. [DOI] [Google Scholar]
- 46.Singh D., Kumar V. Dehazing of outdoor images using notch based integral guided filter. Multimed Tools Appl. 2018;77(20):27363–27386. [Google Scholar]
- 47.Singh D., Kumar V. Single image defogging by gain gradient image filter. Sci China Inf Sci. 2019;62(7) [Google Scholar]
- 48.Singh D., Kumar V., Kaur M. Single image dehazing using gradient channel prior. Appl Intell. 2019;49(12):4276–4293. [Google Scholar]