Abstract
Endotracheal intubation poses high risk of transmission of severe acute respiratory syndrome coronavirus 2 and other respiratory pathogens. We designed and here describe a protective drape that we believe will greatly reduce this risk. Unlike the intubation box that has been described prior, it is portable, disposable, and does not restrict operator dexterity. We have used it extensively and successfully during the height of the corona virus disease of 2019 outbreak.
Keywords: COVID-19, Novel coronavirus, SARS-CoV-2
Based on data from similar viral outbreaks, endotracheal intubation is considered to be the highest risk procedure for transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 , 2 Various recommendations have been made to decrease transmission during intubation. The use of video laryngoscopy by an experienced provider is currently considered standard of care.3 In addition, the use of a rigid protective intubation box has been proposed.4
At Montefiore Medical Center in the Bronx, New York, we intubated 367 patients with coronavirus disease of 2019 (COVID-19) during the month of April 2020. Our engineering department constructed multiple protective intubation boxes. Unfortunately, in practice at Montefiore, we found intubation boxes to be impractical. They are bulky and heavy, making it difficult to transport them quickly to the various areas throughout the hospital in which emergent intubations occur. In addition, the boxes may limit manual dexterity to such a degree as to make emergent intubations more difficult. Another concern is that the boxes may not protect additional personnel in the room from caudal spread of infectious organisms, which is common during endotracheal intubation.5 Finally, they require meticulous disinfection after each use, a process which itself carries some risk of disease transmission. Because of these limitations, the use of intubation boxes was abandoned shortly after deployment in our hospitals.
In order to overcome these limitations, we designed and have put into regular practice a disposable protective intubation drape for emergent intubation of patients with known or suspected SARS-Co-V-2 infection. The potential utility of plastic sheeting to limit the spread of infectious organisms during extubation has recently been demonstrated.6 The use of such sheeting has not been described for use in endotracheal intubation.
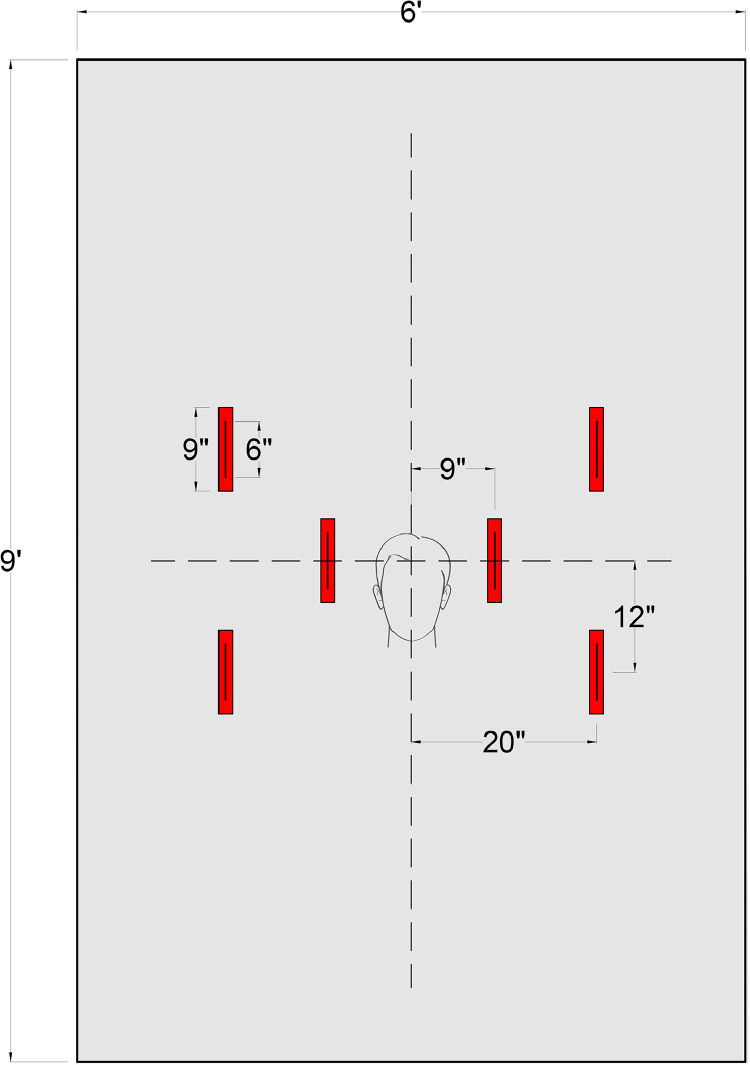
To our knowledge, ours is the first reported use of a clear plastic drape that has been specifically designed to prevent the spread of infectious organisms during endotracheal intubation. The drape consists of a 6-foot wide by 9-foot long thin (2 mil.) clear plastic sheet. As per the template in Figure 1 , 6 parallel 9-inch long by 2-inch wide pieces of tape were placed along the drape at regular intervals. Openings were then cut lengthwise through the center of the tape to create the hand insertion ports. When 2 personnel are used, the time to cut and assemble a single intubation drape is under 5 minutes (see Video 1). The drape should be disposed after each use. In order to limit spread of infectious organisms during disposal, we recommend rolling the sheet under in a fashion that the surface remains in contact with the patient and not the providers. After rolling under, the sheet should be folded and disposed of in a medical waste container.
Fig 1.
Drape template
This drape is easily portable to any location. In addition, we have found that that the drape allows for good manual dexterity. We have used it extensively at Montefiore Medical Center during the COVID 19 crisis with various forms of video laryngoscopy (see Video 2). The flexible property of the drape allows for the intubation of patients without limiting manual dexterity. The drape is potentially protective not only of the intubator but also of other providers in the room who are usually located caudal to the patient's head.
The use of the drape may be limited by reduction in operator visibility. In addition, use in emergent situations can be somewhat confusing to those who are not familiar with the drape. For this reason, we designed the openings in the drape to be symmetric so that there can be no confusion over the proper orientation of the drape in cranial-caudad orientation.
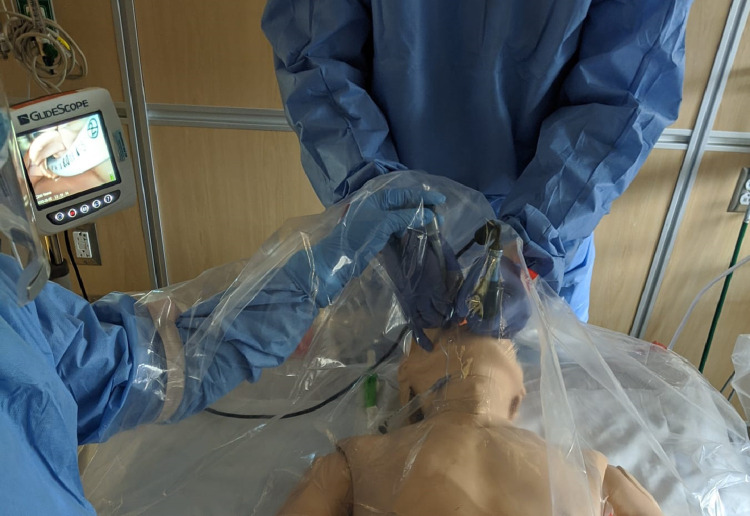
In terms of sheet thickness, we found that 2 mil provides a reasonable balance of visibility and durability, with thinner sheeting being less durable and thicker sheeting creating a greater loss in visibility. In addition, the 2 mil sheeting does not adhere to the patient's skin and provides a degree of “tenting” that permits adequate separation between patient and provider so that instruments may be manipulated under the sheet with ease (Fig 2 ).
Fig 2.
Intubator and assistant are shown utilizing the drape
Due to the overwhelming clinical burden imposed by COVID-19 in at our hospitals, we were unfortunately unable to collect data on comparative intubation success rate with and without the drape. In addition, we were not able to study whether the use of the drape actually reduced the rate of transmission from patient to provider during intubation. However, after initial experience with the drape the directors of all 3 emergency departments on the main Montefiore Medical Center campuses as well as the Division of Critical Care Medicine have requested distribution of the drape to all of their patient care areas. So far 900 drapes have been produced and distributed to these areas, with multiple requests to restock these drapes fulfilled.
We would like to share the design of this drape with all and hope to decrease the rate of patient to provider transmission of the SARS-Co-V-2 virus. We hope that future studies will be able to investigate both the effects of the use of the protective drape on first-pass intubation success as well as the degree to which the drape may be effective at preventing disease transmission in clinical practice.
Footnotes
Conflicts of interest: None to report.
All authors have read and approved the submission of the manuscript to ICHE.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2020.06.212.
Appendix. SUPPLEMENTARY MATERIALS
References
- 1.Fowler A., Guest CB, Lapinsky SE, et al. Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. AJRCCM. 2004;169:1198–1202. doi: 10.1164/rccm.200305-715OC. [DOI] [PubMed] [Google Scholar]
- 2.Raboud J, Shigayeva A, McGeer A, et al. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLOS ONE. 2010;5:e10717. doi: 10.1371/journal.pone.0010717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alhazzani W., Arabi Y.M., Loeb M., et al. Surviving sepsis campaign. Crit Care Med. 2020;48:e440–e469. doi: 10.1097/CCM.0000000000004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canelli R., Connor C.W., Gonzalez M., et al. Barrier enclosure during endotracheal intubation. NEJM. 2020;382:1957–1958. doi: 10.1056/NEJMc2007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan M.T.V., Chow B.K., Lo T., et al. Exhaled air dispersion during bag-mask ventilation and sputum suctioning-Implications for infection control. Sci Rep. 2018;8:1–98. doi: 10.1038/s41598-017-18614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matava C., Yu J., Denning S., et al. Clear plastic drapes may be effective at limiting aerosolization and droplet spray during extubation: implications for COVID-19. Can J Anaesth. 2020;67:902–904. doi: 10.1007/s12630-020-01649-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.