Abstract
Chloroquine (CQ) and its analogue hydroxychloroquine (HCQ) have been thrust into our everyday vernacular because some believe, based on very limited basic and clinical data, that they might be helpful in preventing and/or lessening the severity of the pandemic coronavirus disease 2019 (COVID-19). However, lacking is a temperance in enthusiasm for their possible use as well as sufficient perspective on their effects and side-effects. CQ and HCQ have well-known properties of being diprotic weak bases that preferentially accumulate in acidic organelles (endolysosomes and Golgi apparatus) and neutralize luminal pH of acidic organelles. These primary actions of CQ and HCQ are responsible for their anti-malarial effects; malaria parasites rely on acidic digestive vacuoles for survival. Similarly, de-acidification of endolysosomes and Golgi by CQ and HCQ may block severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) integration into host cells because SARS-CoV-2 may require an acidic environment for its entry and for its ability to bud and infect bystander cells. Further, de-acidification of endolysosomes and Golgi may underly the immunosuppressive effects of these two drugs. However, modern cell biology studies have shown clearly that de-acidification results in profound changes in the structure, function and cellular positioning of endolysosomes and Golgi, in signaling between these organelles and other subcellular organelles, and in fundamental cellular functions. Thus, studying the possible therapeutic effects of CQ and HCQ against COVID-19 must occur concurrent with studies of the extent to which these drugs affect organellar and cell biology. When comprehensively examined, a better understanding of the Janus sword actions of these and other drugs might yield better decisions and better outcomes.
Keywords: Chloroquine, Hydroxychloroquine, COVID-19, Endolysosome, Golgi, de-acidification
Highlights
-
•
Chloroquine (CQ) and hydroxychloroquine (HCQ) de-acidify acidic organelles and alter cell biology.
-
•
De-acidifying effects of CQ and HCQ disturb SARS-CoV2 viral biology.
-
•
De-acidifying effects of CQ and HCQ affect immune responses.
1. Introduction
Chloroquine (CQ) and its analogue hydroxychloroquine (HCQ) have long been used for prophylactic treatment against and treatment of malaria; a condition caused by infection of red blood cells with the parasite plasmodium [1,2]. CQ and HCQ are very similar drugs; HCQ has an extra hydroxyl group at the end of the side chain, and both have similar profiles for drug absorption, distribution, metabolism, and excretion. While HCQ is as active as is CQ against Plasmodium falciparum malaria, it is much less active against CQ-resistant malaria [3]. Furthermore, the wide-spread emergence of CQ-resistant strains of malaria parasites has diminished the effectiveness and use of these drugs in malaria management [4,5].
Beside their anti-malaria effects, CQ and HCQ both exert immunomodulatory and immunosuppressive effects; they are useful in the management of rheumatic diseases, lupus erythematosus, and dermatological disorders [6]. Limited preliminary findings have suggested that CQ and HCQ might exhibit antiviral effects against the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [7,8]; the viral cause of the global pandemic coronavirus disease 2019 (COVID-19) [[9], [10], [11]]. The USA Food and Drug Administration (FDA) issued an Emergency Use Authorization (EUA) for the use of CQ and HCQ in COVID-19 and there are now over one dozen clinical trials and one state-wide clinical trial (South Dakota) on-going to determine their therapeutic effectiveness in patients living with COVID-19. However, and contrary to public pronouncements, these are not safe drugs; CQ more than HCQ have associated with them side-effects and toxicity profiles including cardiotoxicity, ocular toxicity, and neuromyotoxicity [[12], [13], [14], [15]]. When these drugs were being approved for marketing, cell biology was in its infancy and little was known about de-acidification-induced organellar changes. To enhance the discussion of possible use of CQ and HCQ against COVID-19, it is important for the public, for researchers and for clinicians to appreciate better the effects of CQ and HCQ from a modern cell biology perspective.
2. De-acidifying effects of CQ and HCQ on acidic organelles
2.1. CQ and HCQ de-acidify digestive acidic vacuoles of malaria parasites
CQ (pKa1 = 8.1, pKa2 = 10.2) and HCQ (pKa1 = 8.3, pKa2 = 9.7) are diprotic weak base drugs that are present in protonated or unprotonated forms. Unprotonated forms of CQ and HCQ can diffuse freely across membranes into endolysosomes and Golgi; once protonated, CQ and HCQ are trapped within these acidic organelles [16]. The driving force for intra-vesicular accumulation of CQ and HCQ is proportional to the square of the hydrogen ion gradient; the accumulation is much larger than that of a monoprotic weak base like ammonia chloride, which is proportional to the hydrogen ion gradient [17,18]. Therefore, CQ and HCQ are preferentially concentrated in and raises the pH of acidic digestive vacuoles (pH of 5.2) of plasmodium parasites that cause malaria [[19], [20], [21]]; the anti-parasitic actions are due to preventing the polymerization of heme into hemozoin [22].
2.2. CQ and HCQ de-acidify acidic organelles in mammalian cells
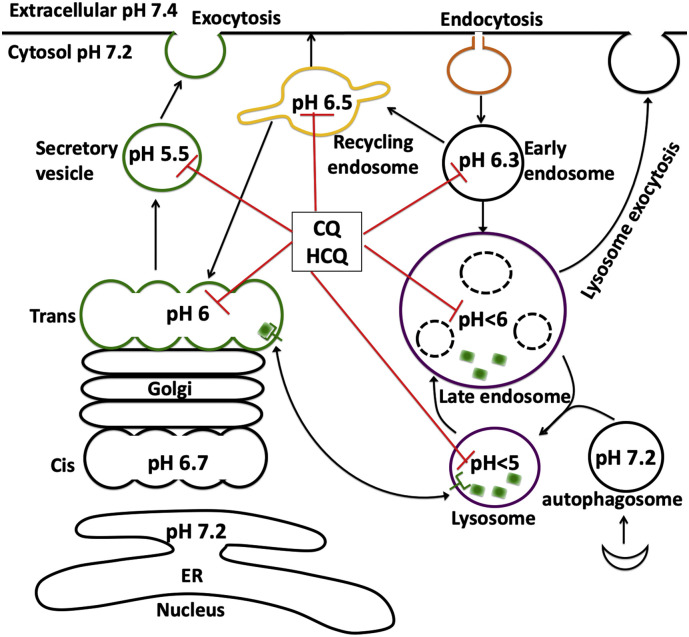
Modern cell biology is populated by extensive findings about the structure and function of membrane bound organelles that contain a plethora of channels, receptors and transporters regulating organellar function and the biology of the cell. The selective permeability of the organellar membranes creates a unique luminal microenvironment; one that is distinct from the environment of the surrounding cytosol. For acidic organelles, a key requirement for optimal function is maintenance of an acidic lumen. This luminal pH is regulated and maintained by a balance between proton pumping by vacuolar-ATPase (v-ATPase), counterion conductance, and intrinsic proton leaks [[23], [24], [25], [26], [27], [28]]. Membrane bound vesicles in the endocytic pathway (early endosome, recycling endosome, late endosome, and lysosomes) and the biosynthetic secretory pathway (Golgi apparatus and secretory vesicles) all display varying degrees of acidity (Fig. 1 ). The acidic pH enables efficient sorting and trafficking of newly synthesized and internalized molecules and/or membranes to their intended destinations for proper processing and function [23,29,30].
Fig. 1.
CQ and HCQ de-acidify acidic organelles.
Membrane bound vesicles in the endocytic pathway (early endosome, recycling endosome, late endosome, and lysosomes) and the biosynthetic secretory pathway (Golgi apparatus and secretory vesicles) all display varying degrees of acidity, and these vesicles rapidly acidify as they progress along the endocytic or secretory pathway. As diprotic weak bases, CQ and HCQ are taken up by cells and trapped in these acidic organelles, where they neutralize pH and alter their structure, function, and trafficking.
The endolysosome system is a dynamic interconnected network with morphological and functional heterogeneity, and this system exhibits complex interactions with other organelles. Extracellular macromolecules and membrane components are up-taken into endosomes by a variety of endocytic pathways and can either be trafficked through early endosomes to recycling endosomes, which mediates receptor recycling to plasma membrane or Golgi apparatus, or can transition to late endosomes and fusion with lysosomes [26,[31], [32], [33]]. As mentioned above, a hallmark feature of the endolysosome system is their acidic luminal pH [[25], [26], [27], [28]] that is critical for the activity of up to 60 different pH sensitive hydrolytic enzymes including proteases, lipases and nucleases [33].
Early endosomes act as a major sorting station and their acidic nature enables internalized ligands to be dissociated from the endocytosed cell surface membrane receptors to which they were bound. The receptors unbound with ligand can then be recycled back to the cell surface or can traffic to Golgi apparatus via recycling endosomes; dissociated ligands are transported through late endosomes to lysosomes for degradation. The maturation of early endosomes to late endosomes is characterized by an increased number of intralumenal vesicles and the formation of multivesicular bodies; a process requiring an acidic intraluminal pH [34]. The acidic pH and acidic hydrolases are critical for degradation of cytosolic proteins and obsolete organelles via the formation of autophagosomes followed by fusion with late endosomes/lysosomes; the so-called “self-eating” or autophagy that is essential for cell homeostasis and development [35,36]. In addition to the above, endolysosomes control a range of physiological functions including antigen processing, membrane repair, nutrient sensing, and ion homeostasis [[37], [38], [39], [40]].
Endolysosome pH can be disturbed by many factors. Classical lysosomotropic weak bases such as NH4Cl, CQ, and methylamine [41] as well as many FDA approved drugs (possibly weak bases) including antiretroviral drugs [42] and anti-cancer drugs [43] tend to accumulate in acidic endolysosomes and neutralize their pH. Carboxylic ionophores, such as monensin and nigericin, can cause electroneutral exchange of protons across the membrane against other monovalent cations [44]. Inhibitors of v-ATPase (bafilomycin and concanamycin) rapidly elevate the pH in acidic organelles [45]. Abnormal accumulation in endolysosomes of low-density lipoprotein (LDL) cholesterol [46], HIV-1 viral proteins such as Tat and gp120 [47,48], and intracellular pathogens including Mycobacterium tuberculosis [49,50], uropathogenic E. coli [51], and coxiella burnetii [52] also cause endolysosome de-acidification. Further, endolysosome pH is affected by alterations in ion permeability (K+, Cl−, Ca2+, Fe2+, Zn2+) across endolysosome membranes, changes in endolysosome membrane potential, cellular nutritional status, and cellular signaling [[53], [54], [55], [56], [57], [58]]. Significantly, defective endolysosome pH regulation has been implicated in a growing number of human diseases including osteopetrosis, renal tubular acidosis, cutis laxa [59], Dent's disease [60], Christianson syndrome, autism and attention deficit hyperactivity disorder [61]. Furthermore, it is being increasingly linked to cellular aging, synaptic dysfunction, cancer, and neurodegenerative disorders including Alzheimer's and Parkinson's disease [53] and HIV-associated neurocognitive disorders [47,[62], [63], [64], [65]].
CQ and its analogues HCQ (Fig. 1) are concentrated in acidic endolysosomes [[66], [67], [68]] where they neutralize endolysosome pH [20,55], induce markedly enlargement of endolysosomes [69,70], change the positioning of endolysosomes from perinuclear to the periphery of cells [48,71], and lead to lysosome membrane permeabilization [54,72,73]. CQ-induced endolysosome de-acidification results in the accumulation and aggregation of undegraded substrates and atypical cleavages that lead to generation of toxic intermediates [42,74]. CQ-induced lysosomal membrane permeabilization leads to the translocation of lysosomal contents (eg. cathepsins) to the cytoplasm and to the induction of mitochondria damage and cell death [75,76]. CQ-induced endolysosome de-acidification also impairs vesicular fusion and inhibits autophagic flux by decreasing autophagosome-lysosome fusion [70,77]. In addition, CQ enhances lysosome exocytosis [72,78] and the release of exosomes [79]. However, CQ does not affect endocytosis [70].
Golgi apparatus helps process, sort and traffic proteins and lipids destined for secretion, membranes, and organelles. Sub-compartments of Golgi are mildly acidic; pH values range from 6.7 at cis-Golgi to 6.0 at trans-Golgi [29]. Secretory vesicles (constitutive or regulated) are more acidic; luminal pH ranges from 5.2 to 5.7 [23,24]. Consistent with the view that an acidic environment is critical for the processing of proteins and lipids, deacidification of Golgi results in defects in posttranslational modifications and processing of secreted proteins. For example, glycosylation is pH-sensitive [80] and an increase of 0.2 pH units results in decreased glycosylation [81]. In terms of sorting and trafficking of proteins and lipids, deacidification impaired anterograde transport from Golgi to secretary vesicles [82], retrograde transport from Golgi back to the endoplasmic reticulum (ER) [83], the delivery of lysosomal hydrolases to lysosomes via mannose-6-phosphate receptor (M6PR) [78,84], the integrity of Golgi itself [85,86], and the sorting and proteolytic maturation of prohormones in secretory granules [87]. Thus, it is not surprising that defective Golgi pH regulation has been implicated in a number of human diseases including autosomal recessive Cutis Laxa type II [88] and multigenerational non-syndromic intellectual disability [89].
Similar to endolysosomes, CQ and HCQ (Fig. 1) are concentrated in and neutralize the pH of acidic Golgi [29], and induce marked dilatation of the Golgi cisternae [90]. Functionally, CQ-induced de-acidification results in glycosylation deficits [81], deficits in the formation of functional transport vesicles, and the inability of budding vesicles to pinch off and form functional transport vesicles [91,92]. CQ also changed distribution patterns of mannose-6-phosphate receptors and decreased the delivery of lysosomal enzymes into lysosomes via mannose-6-phosphate receptors [93]; the latter process might be responsible for CQ-induced changes in lysosome exocytosis [72,78]. Furthermore, CQ-induced de-acidification also leads to deficits in sorting and proteolytic maturation of the prohormones pro-somatostatin [94], adrenocorticotropic hormone (ACTH) [95], and pro-insulin [96].
2.3. Summary of cell biology concerns
As diprotic weak bases, CQ and HCQ are taken up by cells and trapped in acidic organelles; they are only extruded by exocytosis and/or through the multidrug resistance protein p-glycoprotein [[97], [98], [99]].Through their ability to deacidify acidic organelles along endocytic and biosynthetic secretory pathways, CQ and HCQ disturb many key aspects of cell biology including organellar biology and inter-organellar signaling, many of which are linked to their antiviral and immunosuppressive effects. Decades of clinical usage have shown that CQ and HCQ are relatively safe drugs. They are even commonly used during pregnancy in patients with autoimmune disorders. Recent systematic reviews and meta-analyses suggest that maternal HCQ use during pregnancy does not increase risk of major congenital malformations [100,101]. The reported tissue side effects of CQ and HCQ such as retinopathy [102,103], cardiomyopathy [104], neuromyotoxicity [105] are likely due to abnormal accumulation of these drugs in tissues with chronic use [12,106]. The unexpected high rate of side effects including cardiac arrhythmias during the COVID-19 pandemic may be related to higher doses of HCQ used, the older ages of the patients, and to drug-drug interactions [107]. Because of their de-acidifying effects, CQ and HCQ could alter the pharmacokinetics and/or pharmacodynamics of other concurrently used drugs. One such example is the use of CQ and HCQ in combination with chemotherapeutic drugs to enhance the efficacy of tumor cell killing [108]. Thus, from a modern cell biology perspective, a blueprint exists that should guide future studies of possible effects and side effects of acute and chronic CQ and HCQ.
3. Coronaviruses and organellar pH
Coronaviruses are single-stranded RNA virus that are enveloped with crown-like spikes on the surface. Various types of human coronaviruses cause acute lung injury and acute respiratory distress syndrome that results in pulmonary failure; these include severe acute respiratory syndrome coronavirus (SARS-CoV), H5N1 influenza A (H1N1), Middle East respiratory syndrome coronavirus (MERS-CoV), and most recently SARS-CoV-2 the root cause of the current COVID-19 pandemic [109]. Unlike the malaria-causing parasite that has its own acidic digestive vacuole, viruses use host cell mechanisms for entry and replication. Many viruses are endocytosed into endolysosomes following interactions with cell surface proteins, lipids and sugar moieties [110].
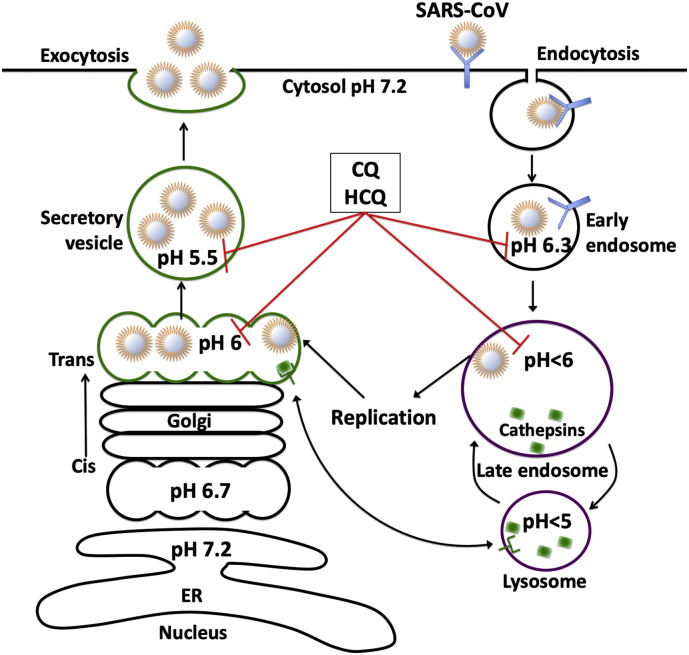
Like other enveloped viruses, SARS-CoV-2 enters host cells and utilizes host cell machinery for replication. The spiked glycoprotein on the outer surfaces of coronaviruses are responsible for the attachment and entry of the virus to host cells via receptor-mediated endocytosis with the assistance of angiotensin-converting enzyme 2 (ACE2) [7,8,111] and possibly other co-receptors [112]. Once inside endosomes, SARS-CoV-2 is either metabolized by pH-sensitive hydrolytic enzymes or it escapes from these organelles. For SARS-CoV [[113], [114], [115], [116], [117], [118], [119]], pH-dependent, furin or cathepsin L-mediated cleavage of spike envelope protein appears to facilitate viral envelope fusion with endosomes and the virus with its genomic contents are released into the cytoplasm of the host cell. Similar to SARS-CoV [120], SARS-CoV-2 replication occurs in the cytoplasm and it may assemble and mature in Golgi from which it is released via secretary vesicles (Fig. 2 ).
Fig. 2.
Coronaviruses and organellar pH.
SARS-CoV enters host cells via endocytosis and utilizes host cell machinery for replication. Once inside endosomes, SARS-CoV escapes from these organelles via pH- and cathepsin L-dependent mechanism. Following replication in the cytoplasm SARS-CoV may assemble and mature in trans-Golgi, from which it is released via secretary vesicles. By de-acidifying these acidic organelles, CQ and HCQ may block virus entry and affect post-translational modifications including the proteolysis and glycosylation of SARS-CoV.
Other enveloped viruses like influenza A and Ebola, also use the acidic environment of endosomes or endolysosome hydrolases to drive the fusion of viral membranes with endosome membranes and the release of viral genomic content into the cytoplasm [121]. As such, endolysosome de-acidification with a v-ATPase inhibitor [122] or CQ [123,124] has been used frequently to inhibit cellular entry of enveloped virus in vitro. Once replicated in the cytoplasm, some viruses are packaged in trans-Golgi network where low pH facilitates the maturation of the virus. CQ, by de-acidifying Golgi, impairs the maturation of viruses and decreases viral infection, in part, by increasing the accumulation of, for example, non-infectious herpes simplex virus 1 particles [125], HIV-1 [126] and flavivirus [[126], [127], [128], [129]]. Currently it is not clear whether CQ affects membrane invagination and viral packing into the trans-Golgi network or the extracellular release of mature virus.
Given the above findings, it is not surprising that CQ and HCQ are being tested for their possible effectiveness against SARS-CoV-2. Preliminary in vitro studies have shown that both CQ [9] and HCQ [10,11] exhibit antiviral effects against SARS-CoV-2. Although the underlying mechanisms are not fully understood, it is possible that endolysosome de-acidification by CQ (Fig. 2) may block pH-dependent, furin - or cathepsin L-mediated cleavage of the spike envelope protein that facilitates viral envelope fusion with endosome membranes [115,116,118,119]. Further, CQ-induced Golgi de-acidification may result in decreased expression levels of ACE2. Such mechanisms have been implicated previously with SARS-CoV; CQ decreased the binding of SARS-CoV spike protein with ACE2 [8] and CQ-induced Golgi de-acidification (Fig. 2) affected post-translational modifications including the proteolysis and glycosylation of SARS-CoV virions [8].
In the context of COVID-19, CQ and HCQ are often used in combination with antivirals and other drugs such as azithromycin and zinc [130,131]. By de-acidifying acidic organelles, CQ and CQ could alter the cellular distribution of these drugs. Azithromycin, a macrolide antibiotic, has been used against Zika [132] and Ebola viruses [133]. In vitro evidence indicates that HCQ and azithromycin show synergistic effects on inhibiting SARS-CoV-2 [130]. As a weak base, azithromycin (pKa = 8.5) also tends to accumulate in acidic environments [134]. By de-acidifying acidic organelles, CQ and HCQ could prevent the accumulation of azithromycin in acidic organelles and increase its concentrations in cytosol where azithromycin exerts inhibitory effects on viral replication [130]. On the other hand, such CQ- and HCQ-induced cellular redistributions of azithromycin may exaggerate its QT prolongation effects [135] as observed in the context of COVID-19 [136]. In vitro evidence indicates that zinc inhibits SARS-CoV [137]. In a preprint paper [131], the authors reason that CQ, as a zinc ionophore [138], could increase cytosolic concentrations of zinc to inhibit RNA-dependent RNA polymerase. This is contrary to the original observation that CQ is a zinc ionophore [138], in which chloroquine was observed as a zinc ionophore that targets zinc to lysosomes. Nonetheless, zinc has been shown to inhibit the activity of furin [139] and cathepsins [140]. Interestingly, the activity of furin is also pH-dependent; furin's optimum pH is 6.0 [141]. Thus, in addition to its de-acidifying effects, CQ-induced accumulation of zinc in endolysosomes could further inhibit activities of furin and cathepsins that are responsible for cleavage of SARS-CoV spike proteins and viral entry.
De-acidifying acidic organelles may not be the only mechanism whereby CQ and HCQ interfere with the biology of SARS-CoV2. In human epithelial lung cells, CQ inhibits the phosphorylation of p38 mitogen-activated protein kinase (MAPK) and inhibits the release of human coronavirus from infected cells [142]. Thus, CQ and HCQ may exert its effects on SARS-CoV-2 via altering protein kinase activities.
3.1. Concerns about the possible antiviral effects of CQ and HCQ
Currently, there exist very limited evidence supporting the use of CQ and HCQ against SARS-CoV-2 and COVID-19. First, almost all in vitro studies reported to date were conducted in epithelioid cells derived from the kidney of African green monkeys (Vero E6 cells) or human hepatomas (Huh7 cells) [129,143,144]. Vero E6 cells are extremely permissiveness for viral replication including coronaviruses, which is due in part to genetic defects in interferon production [145,146] and defects in innate antiviral responses [147]. Huh7 cells are also highly permissive for virus replication due to defective retinoic acid-inducible gene I signaling, impaired interferon signaling, and defects in innate antiviral responses [148,149]. Thus, it is not clear how finding from these cells are translatable to human clinical trials. Second, CQ and HCQ change the pH of endolysosomes and Golgi and cause multiple morphological and functional changes to these organelles including swelling and altered exocytotic release of virus. These changes may result in the formation of intracellular SARS-CoV-2 reservoirs capable of being re-activated when pH normalizes. Most recently (June 23, 2020), several clinical studies have reported on the favorable use of CQ and HCQ for the treatment of COVID-19 [[150], [151], [152], [153], [154], [155], [156], [157], [158], [159]]. However, other studies have reported no significant beneficial effects and some detrimental effects [[160], [161], [162]]. Multiple clinical trials testing the possible effectiveness of CQ and HCQ against COVID-19 are ongoing [163], the results of these studies will be important as more people require treatments for COVID-19.
4. Immunomodulatory properties of CQ and HCQ
The immunomodulatory properties of CQ and HCQ have long been recognized and these drugs continue to be used clinically for the treatment of rheumatoid arthritis, systemic lupus erythematosus and other inflammatory rheumatic diseases [164]. Although their mechanisms of action remain under investigation, the immunomodulatory effects are likely due to their accumulation in and de-acidification of acidic compartments; endolysosomes and Golgi apparatus of immune cells. The importance of CQ- and HCQ-induced de-acidification as an important mediator in immune modulation may be due to impaired maturation of lysosomes and blockade of fusions between autophagosomes and lysosomes. Indeed, lysosomal degradation of endocytosed or autophagocytosed proteins affects antigen processing and MHC class II presentation [165,166]. This is consistent with findings that CQ and HCQ inhibit MHC class II expression, antigen presentation and immune activation [167]. Further, RNA and DNA binding to toll-like receptor 7 (TLR7) and TLR9 in endosomes results in TLR signaling activation and production of pro-inflammatory cytokines [168]. Thus, CQ- and HCQ-induced endolysosome de-acidification could interfere with ligand binding with TLRs and inhibition of TLR signaling and the production of pro-inflammatory cytokines [164,169].
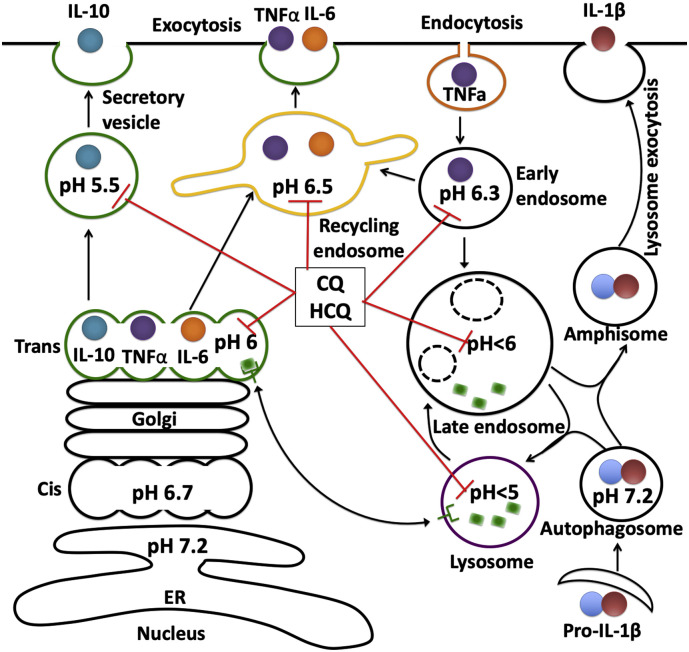
CQ and HCQ also affect immunoregulation by inhibiting the release of various pro-inflammatory cytokines such as IL-1, IFNα and TNFα [98,170]. Most cytokines require conversion to soluble more active mature forms and CQ and HCQ by deacidifying Golgi block such conversion of, for example, pro-TNFα to its soluble mature form [170,171]. Two pathways for cytokine secretion also require acidic vesicles. The classical pathway for cytokine secretion (Fig. 3 ) involves direct transport along trans-Golgi secretory pathways to the extracellular space (eg. IL-10) or indirectly though recycling endosomes to the extracellular space (egs. TNFα, IL-6, and IL-10) [172]. The non-classical pathway for cytokine secretion (Fig. 3) involves transport of cytokines (eg. IL-1β) into autophagosomes and fusion with endosomes to form amphisomes before being released into the extracellular space [173,174]. By deacidifying endolysosomes and Golgi, CQ and HCQ could reduce the secretion of, for example, the proinflammatory cytokines TNF-α, IL-1β and IL-6 [98,170]. Because elevated systemic IL-6 levels in patients with COVID-19 have been implicated as a relevant parameter in predicting severity of disease and the need for intensive care [175], CQ and HCQ may alter the course of COVID-19 by controlling IL-6 and delaying or preventing the development of critical stages of the disease. In addition, CQ could decrease the surface expression of TNFα receptors and thereby inhibit receptor-mediated TNFα signaling [176].
Fig. 3.
CQ and HCQ affect cytokine release.
The classical pathway for cytokine secretion involves direct transport along trans Golgi secretory pathways to the extracellular space (eg. IL-10) or indirectly though recycling endosomes to the extracellular space (egs. TNFα, IL-6, and IL-10). The non-classical pathway for cytokine secretion involves transport of cytokines (eg. IL-1β) into autophagosomes and fusion with endosome and form amiphisome before being released into the extracellular space. By deacidifying endolysosomes and Golgi, CQ and HCQ could reduce the secretion of, for example, the proinflammatory cytokine TNF-α, IL-1β and IL-6.
5. Summary
The FDA issued an Emergency Use Authorization for the use of CQ and HCQ against COVID-19. The clinical effectiveness of CQ and HCQ against COVID-19 is being studied in over a dozen controlled clinical trials. However, the safety of using these drugs may be questioned because limited attention has been paid to the underlying cell biology mechanisms of action. Furthermore, clinical outcomes including inflammation and immunomodulation must be considered especially if they are to be used chronically and/or prophylactically.
Declaration of Competing Interest
The authors declare that this manuscript was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Research reported in this publication was supported by the National Institute of Mental Health [R01MH100972, 2014], National Institute of Mental Health [R01MH105329, 2015], and National Institute of Mental Health [R01MH119000, 2019].
References
- 1.Winzeler E.A. Malaria research in the post-genomic era. Nature. 2008;455(7214):751–756. doi: 10.1038/nature07361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White N.J. Malaria: a molecular marker of artemisinin resistance. Lancet. 2014;383(9927):1439–1440. doi: 10.1016/S0140-6736(14)60656-5. [DOI] [PubMed] [Google Scholar]
- 3.Warhurst D.C., Steele J.C., Adagu I.S., Craig J.C., Cullander C. Hydroxychloroquine is much less active than chloroquine against chloroquine-resistant Plasmodium falciparum, in agreement with its physicochemical properties. J. Antimicrob. Chemother. 2003;52(2):188–193. doi: 10.1093/jac/dkg319. [DOI] [PubMed] [Google Scholar]
- 4.Wellems T.E., Plowe C.V. Chloroquine-resistant malaria. J. Infect. Dis. 2001;184(6):770–776. doi: 10.1086/322858. [DOI] [PubMed] [Google Scholar]
- 5.Price R.N., von Seidlein L., Valecha N., Nosten F., Baird J.K., White N.J. Global extent of chloroquine-resistant Plasmodium vivax: a systematic review and meta-analysis. Lancet Infect. Dis. 2014;14(10):982–991. doi: 10.1016/S1473-3099(14)70855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Bari M.A. Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J. Antimicrob. Chemother. 2015;70(6):1608–1621. doi: 10.1093/jac/dkv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., Somasundaran M., Sullivan J.L., Luzuriaga K., Greenough T.C., Choe H., Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In vitro antiviral activity and projection of optimized dosing Design of Hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McChesney E.W. Animal toxicity and pharmacokinetics of hydroxychloroquine sulfate. Am. J. Med. 1983;75(1A):11–18. doi: 10.1016/0002-9343(83)91265-2. [DOI] [PubMed] [Google Scholar]
- 13.Farrell D.F. Retinal toxicity to antimalarial drugs: chloroquine and hydroxychloroquine: a neurophysiologic study. Clin. Ophthalmol. 2012;6:377–383. doi: 10.2147/OPTH.S27731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding H.J., Denniston A.K., Rao V.K., Gordon C. Hydroxychloroquine-related retinal toxicity. Rheumatology (Oxford) 2016;55(6):957–967. doi: 10.1093/rheumatology/kev357. [DOI] [PubMed] [Google Scholar]
- 15.Raines M.F., Bhargava S.K., Rosen E.S. The blood-retinal barrier in chloroquine retinopathy. Invest. Ophthalmol. Vis. Sci. 1989;30(8):1726–1731. [PubMed] [Google Scholar]
- 16.Martin R.E., Marchetti R.V., Cowan A.I., Howitt S.M., Broer S., Kirk K. Chloroquine transport via the malaria parasite’s chloroquine resistance transporter. Science. 2009;325(5948):1680–1682. doi: 10.1126/science.1175667. [DOI] [PubMed] [Google Scholar]
- 17.Roos A., Boron W.F. Intracellular pH. Physiol. Rev. 1981;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- 18.de Duve C., de Barsy T., Poole B., Trouet A., Tulkens P., Van Hoof F. Commentary. Lysosomotropic agents. Biochem. Pharmacol. 1974;23(18):2495–2531. doi: 10.1016/0006-2952(74)90174-9. [DOI] [PubMed] [Google Scholar]
- 19.Skinner-Adams T.S., Stack C.M., Trenholme K.R., Brown C.L., Grembecka J., Lowther J., Mucha A., Drag M., Kafarski P., McGowan S., Whisstock J.C., Gardiner D.L., Dalton J.P. Plasmodium falciparum neutral aminopeptidases: new targets for anti-malarials. Trends Biochem. Sci. 2010;35(1):53–61. doi: 10.1016/j.tibs.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Schlesinger P.H., Krogstad D.J., Herwaldt B.L. Antimalarial agents: mechanisms of action. Antimicrob. Agents Chemother. 1988;32(6):793–798. doi: 10.1128/aac.32.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuhn Y., Rohrbach P., Lanzer M. Quantitative pH measurements in Plasmodium falciparum-infected erythrocytes using pHluorin. Cell. Microbiol. 2007;9(4):1004–1013. doi: 10.1111/j.1462-5822.2006.00847.x. [DOI] [PubMed] [Google Scholar]
- 22.Loria P., Miller S., Foley M., Tilley L. Inhibition of the peroxidative degradation of haem as the basis of action of chloroquine and other quinoline antimalarials. Biochem. J. 1999;339(Pt 2):363–370. [PMC free article] [PubMed] [Google Scholar]
- 23.Paroutis P., Touret N., Grinstein S. The pH of the secretory pathway: measurement, determinants, and regulation. Physiology (Bethesda) 2004;19:207–215. doi: 10.1152/physiol.00005.2004. [DOI] [PubMed] [Google Scholar]
- 24.Demaurex N. pH homeostasis of cellular organelles. News Physiol. Sci. 2002;17:1–5. doi: 10.1152/physiologyonline.2002.17.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Mindell J.A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 2012;74:69–86. doi: 10.1146/annurev-physiol-012110-142317. [DOI] [PubMed] [Google Scholar]
- 26.Huotari J., Helenius A. Endosome maturation. EMBO J. 2011;30(17):3481–3500. doi: 10.1038/emboj.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGuire C., Stransky L., Cotter K., Forgac M. Regulation of V-ATPase activity. Front. Biosci. (Landmark Ed) 2017;22:609–622. doi: 10.2741/4506. [DOI] [PubMed] [Google Scholar]
- 28.Prasad H., Rao R. Histone deacetylase-mediated regulation of endolysosomal pH. J. Biol. Chem. 2018;293(18):6721–6735. doi: 10.1074/jbc.RA118.002025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellokumpu S. Golgi pH, ion and redox homeostasis: how much do they really matter? Front. Cell Dev. Biol. 2019;7:93. doi: 10.3389/fcell.2019.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weisz O.A. Organelle acidification and disease. Traffic. 2003;4(2):57–64. doi: 10.1034/j.1600-0854.2003.40201.x. [DOI] [PubMed] [Google Scholar]
- 31.Bright N.A., Gratian M.J., Luzio J.P. Endocytic delivery to lysosomes mediated by concurrent fusion and kissing events in living cells. Curr. Biol. 2005;15(4):360–365. doi: 10.1016/j.cub.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 32.Luzio J.P., Pryor P.R., Bright N.A. Lysosomes: fusion and function. Nat. Rev. Mol. Cell. Biol. 2007;8(8):622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- 33.de Duve C. The lysosome turns fifty. Nat. Cell Biol. 2005;7(9):847–849. doi: 10.1038/ncb0905-847. [DOI] [PubMed] [Google Scholar]
- 34.Matsuo H., Chevallier J., Mayran N., Le Blanc I., Ferguson C., Faure J., Blanc N.S., Matile S., Dubochet J., Sadoul R., Parton R.G., Vilbois F., Gruenberg J. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 2004;303(5657):531–534. doi: 10.1126/science.1092425. [DOI] [PubMed] [Google Scholar]
- 35.Spiering M.J. Tor comes to the fore in autophagy. J. Biol. Chem. 2019;294(48):18519–18520. doi: 10.1074/jbc.CL119.011710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24(1):9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pu J., Guardia C.M., Keren-Kaplan T., Bonifacino J.S. Mechanisms and functions of lysosome positioning. J. Cell Sci. 2016;129(23):4329–4339. doi: 10.1242/jcs.196287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casey J.R., Grinstein S., Orlowski J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell. Biol. 2010;11(1):50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 39.Christensen K.A., Myers J.T., Swanson J.A. pH-dependent regulation of lysosomal calcium in macrophages. J. Cell Sci. 2002;115(Pt 3):599–607. doi: 10.1242/jcs.115.3.599. [DOI] [PubMed] [Google Scholar]
- 40.Xu H., Ren D. Lysosomal physiology. Annu. Rev. Physiol. 2015;77:57–80. doi: 10.1146/annurev-physiol-021014-071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ohkuma S., Poole B. Cytoplasmic vacuolation of mouse peritoneal macrophages and the uptake into lysosomes of weakly basic substances. J. Cell Biol. 1981;90(3):656–664. doi: 10.1083/jcb.90.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hui L., Ye Y., Soliman M.L., Lakpa K.L., Miller N.M., Afghah Z., Geiger J.D., Chen X. Antiretroviral drugs promote amyloidogenesis by de-acidifying endolysosomes. J. NeuroImmune Pharmacol. 2019 doi: 10.1007/s11481-019-09862-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhitomirsky B., Assaraf Y.G. Lysosomal accumulation of anticancer drugs triggers lysosomal exocytosis. Oncotarget. 2017;8(28):45117–45132. doi: 10.18632/oncotarget.15155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reijngoud D.J., Oud P.S., Tager J.M. Effect of ionophores on intralysosomal pH. Biochim. Biophys. Acta. 1976;448(2):303–313. doi: 10.1016/0005-2736(76)90244-3. [DOI] [PubMed] [Google Scholar]
- 45.Huss M., Wieczorek H. Inhibitors of V-ATPases: old and new players. J. Exp. Biol. 2009;212(Pt 3):341–346. doi: 10.1242/jeb.024067. [DOI] [PubMed] [Google Scholar]
- 46.Hui L., Soliman M.L., Geiger N.H., Miller N.M., Afghah Z., Lakpa K.L., Chen X., Geiger J.D. Acidifying endolysosomes prevented low-density lipoprotein-induced amyloidogenesis. J. Alzheimers Dis. 2019;67(1):393–410. doi: 10.3233/JAD-180941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hui L., Chen X., Haughey N.J., Geiger J.D. Role of endolysosomes in HIV-1 Tat-induced neurotoxicity. ASN Neuro. 2012;4(4):243–252. doi: 10.1042/AN20120017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Datta G., Miller N.M., Afghah Z., Geiger J.D., Chen X. HIV-1 gp120 promotes lysosomal exocytosis in human Schwann cells. Front. Cell. Neurosci. 2019;13:329. doi: 10.3389/fncel.2019.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chicurel M., Garcia E., Goodsaid F. Modulation of macrophage lysosomal pH by Mycobacterium tuberculosis-derived proteins. Infect. Immun. 1988;56(2):479–483. doi: 10.1128/iai.56.2.479-483.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huynh K.K., Grinstein S. Regulation of vacuolar pH and its modulation by some microbial species. Microbiol. Mol. Biol. Rev. 2007;71(3):452–462. doi: 10.1128/MMBR.00003-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miao Y., Li G., Zhang X., Xu H., Abraham S.N. A TRP Channel senses lysosome neutralization by pathogens to trigger their expulsion. Cell. 2015;161(6):1306–1319. doi: 10.1016/j.cell.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samanta D., Clemente T.M., Schuler B.E., Gilk S.D. Coxiella burnetii type 4B secretion system-dependent manipulation of endolysosomal maturation is required for bacterial growth. PLoS Pathog. 2019;15(12) doi: 10.1371/journal.ppat.1007855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colacurcio D.J., Nixon R.A. Disorders of lysosomal acidification-the emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res. Rev. 2016;32:75–88. doi: 10.1016/j.arr.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khan N., Halcrow P.W., Lakpa K.L., Afghah Z., Miller N.M., Dowdy S.F., Geiger J.D., Chen X. Two-pore channels regulate Tat endolysosome escape and Tat-mediated HIV-1 LTR transactivation. FASEB J. 2020;34(3):4147–4162. doi: 10.1096/fj.201902534R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khan N., Lakpa K.L., Halcrow P.W., Afghah Z., Miller N.M., Geiger J.D., Chen X. BK channels regulate extracellular Tat-mediated HIV-1 LTR transactivation. Sci. Rep. 2019;9(1):12285. doi: 10.1038/s41598-019-48777-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Korolchuk V.I., Saiki S., Lichtenberg M., Siddiqi F.H., Roberts E.A., Imarisio S., Jahreiss L., Sarkar S., Futter M., Menzies F.M., O’Kane C.J., Deretic V., Rubinsztein D.C. Lysosomal positioning coordinates cellular nutrient responses. Nat. Cell Biol. 2011;13(4):453–460. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lawrence R.E., Zoncu R. The lysosome as a cellular centre for signalling, metabolism and quality control. Nat. Cell Biol. 2019;21(2):133–142. doi: 10.1038/s41556-018-0244-7. [DOI] [PubMed] [Google Scholar]
- 58.Rahman N., Ramos-Espiritu L., Milner T.A., Buck J., Levin L.R. Soluble adenylyl cyclase is essential for proper lysosomal acidification. J. Gen. Physiol. 2016;148(4):325–339. doi: 10.1085/jgp.201611606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esmail S., Kartner N., Yao Y., Kim J.W., Reithmeier R.A.F., Manolson M.F. Molecular mechanisms of cutis laxa- and distal renal tubular acidosis-causing mutations in V-ATPase a subunits, ATP6V0A2 and ATP6V0A4. J. Biol. Chem. 2018;293(8):2787–2800. doi: 10.1074/jbc.M117.818872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jentsch T.J. Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J. Physiol. 2007;578(Pt 3):633–640. doi: 10.1113/jphysiol.2006.124719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kondapalli K.C., Prasad H., Rao R. An inside job: how endosomal Na(+)/H(+) exchangers link to autism and neurological disease. Front. Cell. Neurosci. 2014;8:172. doi: 10.3389/fncel.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gelman B.B., Soukup V.M., Holzer C.E., 3rd, Fabian R.H., Schuenke K.W., Keherly M.J., Richey F.J., Lahart C.J. Potential role for white matter lysosome expansion in HIV-associated dementia. J. Acquir. Immune Defic. Syndr. 2005;39(4):422–425. doi: 10.1097/01.qai.0000164250.41475.f2. [DOI] [PubMed] [Google Scholar]
- 63.Spector S.A., Zhou D. Autophagy: an overlooked mechanism of HIV-1 pathogenesis and neuroAIDS? Autophagy. 2008;4(5):704–706. doi: 10.4161/auto.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou D., Spector S.A. Human immunodeficiency virus type-1 infection inhibits autophagy. Aids. 2008;22(6):695–699. doi: 10.1097/QAD.0b013e3282f4a836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cysique L.A., Hewitt T., Croitoru-Lamoury J., Taddei K., Martins R.N., Chew C.S., Davies N.N., Price P., Brew B.J. APOE epsilon4 moderates abnormal CSF-abeta-42 levels, while neurocognitive impairment is associated with abnormal CSF tau levels in HIV+ individuals - a cross-sectional observational study. BMC Neurol. 2015;15:51. doi: 10.1186/s12883-015-0298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Solomon V.R., Lee H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur. J. Pharmacol. 2009;625(1–3):220–233. doi: 10.1016/j.ejphar.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 67.Morissette G., Moreau E., Marceau C.G.R.F. Massive cell vacuolization induced by organic amines such as procainamide. J Pharmacol Exp Ther. 2004;310(1):395–406. doi: 10.1124/jpet.104.066084. [DOI] [PubMed] [Google Scholar]
- 68.Marceau F., Bawolak M.T., Lodge R., Bouthillier J., Gagne-Henley A., Gaudreault R.C., Morissette G. Cation trapping by cellular acidic compartments: beyond the concept of lysosomotropic drugs. Toxicol. Appl. Pharmacol. 2012;259(1):1–12. doi: 10.1016/j.taap.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Yoon Y.H., Cho K.S., Hwang J.J., Lee S.J., Choi J.A., Koh J.Y. Induction of lysosomal dilatation, arrested autophagy, and cell death by chloroquine in cultured ARPE-19 cells. Invest. Ophthalmol. Vis. Sci. 2010;51(11):6030–6037. doi: 10.1167/iovs.10-5278. [DOI] [PubMed] [Google Scholar]
- 70.Mauthe M., Orhon I., Rocchi C., Zhou X., Luhr M., Hijlkema K.J., Coppes R.P., Engedal N., Mari M., Reggiori F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy. 2018;14(8):1435–1455. doi: 10.1080/15548627.2018.1474314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson D.E., Ostrowski P., Jaumouille V., Grinstein S. The position of lysosomes within the cell determines their luminal pH. J. Cell Biol. 2016;212(6):677–692. doi: 10.1083/jcb.201507112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Circu M., Cardelli J., Barr M.P., O'Byrne K., Mills G., El-Osta H. Modulating lysosomal function through lysosome membrane permeabilization or autophagy suppression restores sensitivity to cisplatin in refractory non-small-cell lung cancer cells. PLoS One. 2017;12(9) doi: 10.1371/journal.pone.0184922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sironi J., Aranda E., Nordstrom L.U., Schwartz E.L. Lysosome membrane permeabilization and disruption of the molecular target of rapamycin (mTOR)-lysosome interaction are associated with the inhibition of lung cancer cell proliferation by a Cchloroquinoline analog. Mol. Pharmacol. 2019;95(1):127–138. doi: 10.1124/mol.118.113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caporaso G.L., Gandy S.E., Buxbaum J.D., Greengard P. Chloroquine inhibits intracellular degradation but not secretion of Alzheimer beta/A4 amyloid precursor protein. Proc. Natl. Acad. Sci. U. S. A. 1992;89(6):2252–2256. doi: 10.1073/pnas.89.6.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boya P. Lysosomal function and dysfunction: mechanism and disease. Antioxid. Redox Signal. 2012;17(5):766–774. doi: 10.1089/ars.2011.4405. [DOI] [PubMed] [Google Scholar]
- 76.Wang F., Gomez-Sintes R., Boya P. Lysosomal membrane permeabilization and cell death. Traffic. 2018;19(12):918–931. doi: 10.1111/tra.12613. [DOI] [PubMed] [Google Scholar]
- 77.Boya P., Gonzalez-Polo R.A., Poncet D., Andreau K., Vieira H.L., Roumier T., Perfettini J.L., Kroemer G. Mitochondrial membrane permeabilization is a critical step of lysosome-initiated apoptosis induced by hydroxychloroquine. Oncogene. 2003;22(25):3927–3936. doi: 10.1038/sj.onc.1206622. [DOI] [PubMed] [Google Scholar]
- 78.Kokkonen N., Rivinoja A., Kauppila A., Suokas M., Kellokumpu I., Kellokumpu S. Defective acidification of intracellular organelles results in aberrant secretion of cathepsin D in cancer cells. J. Biol. Chem. 2004;279(38):39982–39988. doi: 10.1074/jbc.M406698200. [DOI] [PubMed] [Google Scholar]
- 79.Ortega F.G., Roefs M.T., de Miguel Perez D., Kooijmans S.A., de Jong O.G., Sluijter J.P., Schiffelers R.M., Vader P. Interfering with endolysosomal trafficking enhances release of bioactive exosomes. Nanomedicine. 2019;20:102014. doi: 10.1016/j.nano.2019.102014. [DOI] [PubMed] [Google Scholar]
- 80.Axelsson M.A., Karlsson N.G., Steel D.M., Ouwendijk J., Nilsson T., Hansson G.C. Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O-glycosylation of mucins. Glycobiology. 2001;11(8):633–644. doi: 10.1093/glycob/11.8.633. [DOI] [PubMed] [Google Scholar]
- 81.Rivinoja A., Kokkonen N., Kellokumpu I., Kellokumpu S. Elevated Golgi pH in breast and colorectal cancer cells correlates with the expression of oncofetal carbohydrate T-antigen. J. Cell. Physiol. 2006;208(1):167–174. doi: 10.1002/jcp.20653. [DOI] [PubMed] [Google Scholar]
- 82.Chanat E., Huttner W.B. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J. Cell Biol. 1991;115(6):1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palokangas H., Ying M., Vaananen K., Saraste J. Retrograde transport from the pre-Golgi intermediate compartment and the Golgi complex is affected by the vacuolar H+-ATPase inhibitor bafilomycin A1. Mol. Biol. Cell. 1998;9(12):3561–3578. doi: 10.1091/mbc.9.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ghosh P., Dahms N.M., Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat. Rev. Mol. Cell. Biol. 2003;4(3):202–212. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- 85.Linstedt A.D., Mehta A., Suhan J., Reggio H., Hauri H.P. Sequence and overexpression of GPP130/GIMPc: evidence for saturable pH-sensitive targeting of a type II early Golgi membrane protein. Mol. Biol. Cell. 1997;8(6):1073–1087. doi: 10.1091/mbc.8.6.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Puri S., Bachert C., Fimmel C.J., Linstedt A.D. Cycling of early Golgi proteins via the cell surface and endosomes upon lumenal pH disruption. Traffic. 2002;3(9):641–653. doi: 10.1034/j.1600-0854.2002.30906.x. [DOI] [PubMed] [Google Scholar]
- 87.Schmidt W.K., Moore H.P. Ionic milieu controls the compartment-specific activation of pro-opiomelanocortin processing in AtT-20 cells. Mol. Biol. Cell. 1995;6(10):1271–1285. doi: 10.1091/mbc.6.10.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kornak U., Reynders E., Dimopoulou A., van Reeuwijk J., Fischer B., Rajab A., Budde B., Nurnberg P., Foulquier F., A.D.-T.S. Group, Lefeber D., Urban Z., Gruenewald S., Annaert W., Brunner H.G., van Bokhoven H., Wevers R., Morava E., Matthijs G., Van Maldergem L., Mundlos S. Impaired glycosylation and cutis laxa caused by mutations in the vesicular H+-ATPase subunit ATP6V0A2. Nat. Genet. 2008;40(1):32–34. doi: 10.1038/ng.2007.45. [DOI] [PubMed] [Google Scholar]
- 89.Khayat W., Hackett A., Shaw M., Ilie A., Dudding-Byth T., Kalscheuer V.M., Christie L., Corbett M.A., Juusola J., Friend K.L., Kirmse B.M., Gecz J., Field M., Orlowski J. A recurrent missense variant in SLC9A7 causes nonsyndromic X-linked intellectual disability with alteration of Golgi acidification and aberrant glycosylation. Hum. Mol. Genet. 2019;28(4):598–614. doi: 10.1093/hmg/ddy371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thorens B., Vassalli P. Chloroquine and ammonium chloride prevent terminal glycosylation of immunoglobulins in plasma cells without affecting secretion. Nature. 1986;321(6070):618–620. doi: 10.1038/321618a0. [DOI] [PubMed] [Google Scholar]
- 91.Hiebsch R.R., Raub T.J., Wattenberg B.W. Primaquine blocks transport by inhibiting the formation of functional transport vesicles. Studies in a cell-free assay of protein transport through the Golgi apparatus. J. Biol. Chem. 1991;266(30):20323–20328. [PubMed] [Google Scholar]
- 92.Oda K., Ikehara Y. Weakly basic amines inhibit the proteolytic conversion of proalbumin to serum albumin in cultured rat hepatocytes. Eur. J. Biochem. 1985;152(3):605–609. doi: 10.1111/j.1432-1033.1985.tb09238.x. [DOI] [PubMed] [Google Scholar]
- 93.Brown W.J., Constantinescu E., Farquhar M.G. Redistribution of mannose-6-phosphate receptors induced by tunicamycin and chloroquine. J. Cell Biol. 1984;99(1 Pt 1):320–326. doi: 10.1083/jcb.99.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu H., Shields D. Prohormone processing in the trans-Golgi network: endoproteolytic cleavage of prosomatostatin and formation of nascent secretory vesicles in permeabilized cells. J. Cell Biol. 1993;122(6):1169–1184. doi: 10.1083/jcb.122.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moore H.P., Gumbiner B., Kelly R.B. Chloroquine diverts ACTH from a regulated to a constitutive secretory pathway in AtT-20 cells. Nature. 1983;302(5907):434–436. doi: 10.1038/302434a0. [DOI] [PubMed] [Google Scholar]
- 96.Orci L., Ravazzola M., Amherdt M., Madsen O., Perrelet A., Vassalli J.D., Anderson R.G. Conversion of proinsulin to insulin occurs coordinately with acidification of maturing secretory vesicles. J. Cell Biol. 1986;103(6 Pt 1):2273–2281. doi: 10.1083/jcb.103.6.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vezmar M., Georges E. Direct binding of chloroquine to the multidrug resistance protein (MRP): possible role for MRP in chloroquine drug transport and resistance in tumor cells. Biochem. Pharmacol. 1998;56(6):733–742. doi: 10.1016/s0006-2952(98)00217-2. [DOI] [PubMed] [Google Scholar]
- 98.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect. Dis. 2003;3(11):722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhitomirsky B., Yunaev A., Kreiserman R., Kaplan A., Stark M., Assaraf Y.G. Lysosomotropic drugs activate TFEB via lysosomal membrane fluidization and consequent inhibition of mTORC1 activity. Cell Death Dis. 2018;9(12):1191. doi: 10.1038/s41419-018-1227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sperber K., Hom C., Chao C.P., Shapiro D., Ash J. Systematic review of hydroxychloroquine use in pregnant patients with autoimmune diseases. Pediatr. Rheumatol. Online J. 2009;7:9. doi: 10.1186/1546-0096-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaplan Y.C., Ozsarfati J., Nickel C., Koren G. Reproductive outcomes following hydroxychloroquine use for autoimmune diseases: a systematic review and meta-analysis. Br. J. Clin. Pharmacol. 2016;81(5):835–848. doi: 10.1111/bcp.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bernstein H., Zvaifler N., Rubin M., Mansour A.M. The ocular deposition of chloroquine. Investig. Ophthalmol. 1963;2:384–392. [PubMed] [Google Scholar]
- 103.Schroeder R.L., Gerber J.P. Chloroquine and hydroxychloroquine binding to melanin: some possible consequences for pathologies. Toxicol. Rep. 2014;1:963–968. doi: 10.1016/j.toxrep.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ratliff N.B., Estes M.L., Myles J.L., Shirey E.K., McMahon J.T. Diagnosis of chloroquine cardiomyopathy by endomyocardial biopsy. N. Engl. J. Med. 1987;316(4):191–193. doi: 10.1056/NEJM198701223160405. [DOI] [PubMed] [Google Scholar]
- 105.Estes M.L., Ewing-Wilson D., Chou S.M., Mitsumoto H., Hanson M., Shirey E., Ratliff N.B. Chloroquine neuromyotoxicity. Clinical and pathologic perspective. Am. J. Med. 1987;82(3):447–455. doi: 10.1016/0002-9343(87)90444-x. [DOI] [PubMed] [Google Scholar]
- 106.McChesney E.W., Banks W.F., Jr., Fabian R.J. Tissue distribution of chloroquine, hydroxychloroquine, and desethylchloroquine in the rat. Toxicol. Appl. Pharmacol. 1967;10(3):501–513. doi: 10.1016/0041-008x(67)90089-0. [DOI] [PubMed] [Google Scholar]
- 107.Juurlink D.N. 2020. Safety Considerations with Chloroquine, Hydroxychloroquine and Azithromycin in the Management of SARS-CoV-2 Infection, CMAJ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maycotte P., Aryal S., Cummings C.T., Thorburn J., Morgan M.J., Thorburn A. Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy. 2012;8(2):200–212. doi: 10.4161/auto.8.2.18554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Staring J., Raaben M., Brummelkamp T.R. Viral escape from endosomes and host detection at a glance. J. Cell Sci. 2018;131(15) doi: 10.1242/jcs.216259. [DOI] [PubMed] [Google Scholar]
- 111.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020;94(7) doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li R., Pei S., Chen B., Song Y., Zhang T., Yang W., Shaman J. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2) Science. 2020;368(6490):489–493. doi: 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Glowacka I., Bertram S., Muller M.A., Allen P., Soilleux E., Pfefferle S., Steffen I., Tsegaye T.S., He Y., Gnirss K., Niemeyer D., Schneider H., Drosten C., Pohlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011;85(9):4122–4134. doi: 10.1128/JVI.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc. Natl. Acad. Sci. U. S. A. 2005;102(33):11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Simmons G., Reeves J.D., Rennekamp A.J., Amberg S.M., Piefer A.J., Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc. Natl. Acad. Sci. U. S. A. 2004;101(12):4240–4245. doi: 10.1073/pnas.0306446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang Z.Y., Huang Y., Ganesh L., Leung K., Kong W.P., Schwartz O., Subbarao K., Nabel G.J. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 2004;78(11):5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G., Jiang C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008;18(2):290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bosch B.J., Bartelink W., Rottier P.J. Cathepsin L functionally cleaves the severe acute respiratory syndrome coronavirus class I fusion protein upstream of rather than adjacent to the fusion peptide. J. Virol. 2008;82(17):8887–8890. doi: 10.1128/JVI.00415-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoffmann M., Kleine-Weber H., Pohlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol. Cell. 2020;78(4):779–784. doi: 10.1016/j.molcel.2020.04.022. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ng M.L., Tan S.H., See E.E., Ooi E.E., Ling A.E. Proliferative growth of SARS coronavirus in Vero E6 cells. J. Gen. Virol. 2003;84(Pt 12):3291–3303. doi: 10.1099/vir.0.19505-0. [DOI] [PubMed] [Google Scholar]
- 121.White J.M., Whittaker G.R. Fusion of enveloped viruses in endosomes. Traffic. 2016;17(6):593–614. doi: 10.1111/tra.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ochiai H., Sakai S., Hirabayashi T., Shimizu Y., Terasawa K. Inhibitory effect of bafilomycin A1, a specific inhibitor of vacuolar-type proton pump, on the growth of influenza A and B viruses in MDCK cells. Antivir. Res. 1995;27(4):425–430. doi: 10.1016/0166-3542(95)00040-s. [DOI] [PubMed] [Google Scholar]
- 123.Ooi E.E., Chew J.S., Loh J.P., Chua R.C. In vitro inhibition of human influenza A virus replication by chloroquine. Virol. J. 2006;3:39. doi: 10.1186/1743-422X-3-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Di Trani L., Savarino A., Campitelli L., Norelli S., Puzelli S., D’Ostilio D., Vignolo E., Donatelli I., Cassone A. Different pH requirements are associated with divergent inhibitory effects of chloroquine on human and avian influenza A viruses. Virol. J. 2007;4:39. doi: 10.1186/1743-422X-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Harley C.A., Dasgupta A., Wilson D.W. Characterization of herpes simplex virus-containing organelles by subcellular fractionation: role for organelle acidification in assembly of infectious particles. J. Virol. 2001;75(3):1236–1251. doi: 10.1128/JVI.75.3.1236-1251.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Savarino A., Lucia M.B., Rastrelli E., Rutella S., Golotta C., Morra E., Tamburrini E., Perno C.F., Boelaert J.R., Sperber K., Cauda R. Anti-HIV effects of chloroquine: inhibition of viral particle glycosylation and synergism with protease inhibitors. J. Acquir. Immune Defic. Syndr. 2004;35(3):223–232. doi: 10.1097/00126334-200403010-00002. [DOI] [PubMed] [Google Scholar]
- 127.Randolph V.B., Winkler G., Stollar V. Acidotropic amines inhibit proteolytic processing of flavivirus prM protein. Virology. 1990;174(2):450–458. doi: 10.1016/0042-6822(90)90099-d. [DOI] [PubMed] [Google Scholar]
- 128.D’Alessandro S., Scaccabarozzi D., Signorini L., Perego F., Ilboudo D.P., Ferrante P., Delbue S. The use of antimalarial drugs against viral infection. Microorganisms. 2020;8(1) doi: 10.3390/microorganisms8010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;105938 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Andreani J., Le Bideau M., Duflot I., Jardot P., Rolland C., Boxberger M., Wurtz N., Rolain J.M., Colson P., La Scola B., Raoult D. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb. Pathog. 2020;145:104228. doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.P. Carlucci, T. Ahuja, C.M. Petrilli, H. Rajagopalan, S. Jones, J. Rahimian, Hydroxychloroquine and azithromycin plus zinc vs hydroxychloroquine and azithromycin alone: outcomes in hospitalized COVID-19 patients, medRxiv (2020) 2020.05.02.20080036.
- 132.Retallack H., Di Lullo E., Arias C., Knopp K.A., Laurie M.T., Sandoval-Espinosa C., Mancia Leon W.R., Krencik R., Ullian E.M., Spatazza J., Pollen A.A., Mandel-Brehm C., Nowakowski T.J., Kriegstein A.R., DeRisi J.L. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc. Natl. Acad. Sci. U. S. A. 2016;113(50):14408–14413. doi: 10.1073/pnas.1618029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Madrid P.B., Panchal R.G., Warren T.K., Shurtleff A.C., Endsley A.N., Green C.E., Kolokoltsov A., Davey R., Manger I.D., Gilfillan L., Bavari S., Tanga M.J. Evaluation of Ebola virus inhibitors for drug repurposing. ACS Infect. Dis. 2015;1(7):317–326. doi: 10.1021/acsinfecdis.5b00030. [DOI] [PubMed] [Google Scholar]
- 134.Kong F.Y., Rupasinghe T.W., Simpson J.A., Vodstrcil L.A., Fairley C.K., McConville M.J., Hocking J.S. Pharmacokinetics of a single 1g dose of azithromycin in rectal tissue in men. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0174372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Choi Y., Lim H.S., Chung D., Choi J.G., Yoon D. Risk evaluation of azithromycin-induced QT prolongation in real-world practice. Biomed. Res. Int. 2018;2018:1574806. doi: 10.1155/2018/1574806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chorin E., Dai M., Shulman E., Wadhwani L., Bar-Cohen R., Barbhaiya C., Aizer A., Holmes D., Bernstein S., Spinelli M., Park D.S., Chinitz L.A., Jankelson L. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat. Med. 2020;26(6):808–809. doi: 10.1038/s41591-020-0888-2. [DOI] [PubMed] [Google Scholar]
- 137.te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11) doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xue J., Moyer A., Peng B., Wu J., Hannafon B.N., Ding W.Q. Chloroquine is a zinc ionophore. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0109180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Podsiadlo P., Komiyama T., Fuller R.S., Blum O. Furin inhibition by compounds of copper and zinc. J. Biol. Chem. 2004;279(35):36219–36227. doi: 10.1074/jbc.M400338200. [DOI] [PubMed] [Google Scholar]
- 140.Lockwood T.D. Lysosomal metal, redox and proton cycles influencing the CysHis cathepsin reaction. Metallomics. 2013;5(2):110–124. doi: 10.1039/c2mt20156a. [DOI] [PubMed] [Google Scholar]
- 141.Anderson E.D., VanSlyke J.K., Thulin C.D., Jean F., Thomas G. Activation of the furin endoprotease is a multiple-step process: requirements for acidification and internal propeptide cleavage. EMBO J. 1997;16(7):1508–1518. doi: 10.1093/emboj/16.7.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kono M., Tatsumi K., Imai A.M., Saito K., Kuriyama T., Shirasawa H. Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: involvement of p38 MAPK and ERK. Antivir. Res. 2008;77(2):150–152. doi: 10.1016/j.antiviral.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Colson P., Rolain J.M., Lagier J.C., Brouqui P., Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents. 2020;105932 doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Colson P., Rolain J.M., Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int. J. Antimicrob. Agents. 2020;55(3):105923. doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Emeny J.M., Morgan M.J. Regulation of the interferon system: evidence that Vero cells have a genetic defect in interferon production. J. Gen. Virol. 1979;43(1):247–252. doi: 10.1099/0022-1317-43-1-247. [DOI] [PubMed] [Google Scholar]
- 146.Mosca J.D., Pitha P.M. Transcriptional and posttranscriptional regulation of exogenous human beta interferon gene in simian cells defective in interferon synthesis. Mol. Cell. Biol. 1986;6(6):2279–2283. doi: 10.1128/mcb.6.6.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Chew T., Noyce R., Collins S.E., Hancock M.H., Mossman K.L. Characterization of the interferon regulatory factor 3-mediated antiviral response in a cell line deficient for IFN production. Mol. Immunol. 2009;46(3):393–399. doi: 10.1016/j.molimm.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 148.Sumpter R., Jr., Loo Y.M., Foy E., Li K., Yoneyama M., Fujita T., Lemon S.M., Gale M., Jr. Regulating intracellular antiviral defense and permissiveness to hepatitis C virus RNA replication through a cellular RNA helicase, RIG-I. J. Virol. 2005;79(5):2689–2699. doi: 10.1128/JVI.79.5.2689-2699.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Devhare P.B., Desai S., Lole K.S. Innate immune responses in human hepatocyte-derived cell lines alter genotype 1 hepatitis E virus replication efficiencies. Sci. Rep. 2016;6:26827. doi: 10.1038/srep26827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T., Honore S., Colson P., Chabriere E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. 105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen J., Liu D., Liu L., Liu P., Xu Q., Xia L., Ling Y., Huang D., Song S., Zhang D., Qian Z., Li T., Shen Y., Lu H. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49(2):215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Guérin V.L., Thomas J., Lardenois T., Lacrosse P., Sarrazin E., Regensberg de Andreis N., Wonner M. 2020. Azithromycin and Hydroxychloroquine Accelerate Recovery of Outpatients with Mild/Moderate COVID-19. Preprints 2020050486. [Google Scholar]
- 153.Barbosa S.D.S.R., Esper R., Teiichi Costa Oikawa F., Machado Castro M., Razuk-Filho A., Benedito Batista Junior P., Lotze S.W., Nunes da Rocha C., de Sá Cunha Filho R., Barbosa de Oliveira S.E., Ribeiro P.L., Vigar Martins V.C., Silva Braga Bueno F., Ligeiro Gonçalves Esper P., Fagundes Parrillo E. Empirical treatment with hydroxychloroquine and azithromycin for suspected cases of COVID-19 followed-up by telemedicine. Dropbox. 2020 [Google Scholar]
- 154.B. Davido, T. Lansaman, C. Lawrence, J.-C. Alvarez, F. Bouchand, P. Moine, V. Perronne, A. Le Gal, D. Annane, C. Perronne, P. De Truchis, Hydroxychloroquine plus azithromycin: a potential interest in reducing in-hospital morbidity due to COVID-19 pneumonia (HI-ZY-COVID)?, medRxiv (2020) 2020.05.05.20088757.
- 155.M.S. Kim, S.-W. Jang, Y.-K. Park, B.-o. Kim, T.-H. Hwang, S.H. Kang, W.J. Kim, H.-W. Park, W. Yang, J. Jang, M.H. An, Treatment Response to Hydroxychloroquine, Lopinavir/Ritonavir, and Antibiotics for Moderate COVID 19: A First Report on the Pharmacological Outcomes from South Korea, medRxiv (2020) 2020.05.13.20094193.
- 156.M.H. Shabrawishi, A.Y. Naser, H. Alwafi, A.M. Aldobyany, A.A. Touman, Negative nasopharyngeal SARS-CoV-2 PCR conversion in Response to different therapeutic interventions, medRxiv (2020) 2020.05.08.20095679. [DOI] [PMC free article] [PubMed]
- 157.Z. Chen, J. Hu, Z. Zhang, S. Jiang, S. Han, D. Yan, R. Zhuang, B. Hu, Z. Zhang, Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial, medRxiv (2020) 2020.03.22.20040758.
- 158.Huang M., Tang T., Pang P., Li M., Ma R., Lu J., Shu J., You Y., Chen B., Liang J., Hong Z., Chen H., Kong L., Qin D., Pei D., Xia J., Jiang S., Shan H. Treating COVID-19 with chloroquine. J. Mol. Cell Biol. 2020;12(4):322–325. doi: 10.1093/jmcb/mjaa014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.W. Tang, Z. Cao, M. Han, Z. Wang, J. Chen, W. Sun, Y. Wu, W. Xiao, S. Liu, E. Chen, W. Chen, X. Wang, J. Yang, J. Lin, Q. Zhao, Y. Yan, Z. Xie, D. Li, Y. Yang, L. Liu, J. Qu, G. Ning, G. Shi, Q. Xie, Hydroxychloroquine in patients mainly with mild to moderate COVID-19: an open-label, randomized, controlled trial, medRxiv (2020) 2020.04.10.20060558.
- 160.J. Magagnoli, S. Narendran, F. Pereira, T. Cummings, J.W. Hardin, S.S. Sutton, J. Ambati, Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid-19, medRxiv (2020) 2020.04.16.20065920. [DOI] [PMC free article] [PubMed]
- 161.S. Singh, A. Khan, M. Chowdhry, A. Chatterjee, Outcomes of Hydroxychloroquine Treatment Among Hospitalized COVID-19 Patients in the United States- Real-World Evidence From a Federated Electronic Medical Record Network, medRxiv (2020) 2020.05.12.20099028.
- 162.Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J., Weinberg P., Kirkwood J., Muse A., DeHovitz J., Blog D.S., Hutton B., Holtgrave D.R., Zucker H.A. Association of Treatment with Hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York State. JAMA. 2020;323(24):2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schrezenmeier E., Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat. Rev. Rheumatol. 2020;16(3):155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 165.Lotteau V., Teyton L., Peleraux A., Nilsson T., Karlsson L., Schmid S.L., Quaranta V., Peterson P.A. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990;348(6302):600–605. doi: 10.1038/348600a0. [DOI] [PubMed] [Google Scholar]
- 166.Munz C. Autophagy beyond intracellular MHC class II antigen presentation. Trends Immunol. 2016;37(11):755–763. doi: 10.1016/j.it.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 167.Belizaire R., Unanue E.R. Targeting proteins to distinct subcellular compartments reveals unique requirements for MHC class I and II presentation. Proc. Natl. Acad. Sci. U. S. A. 2009;106(41):17463–17468. doi: 10.1073/pnas.0908583106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ewald S.E., Lee B.L., Lau L., Wickliffe K.E., Shi G.P., Chapman H.A., Barton G.M. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456(7222):658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kuznik A., Bencina M., Svajger U., Jeras M., Rozman B., Jerala R. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J. Immunol. 2011;186(8):4794–4804. doi: 10.4049/jimmunol.1000702. [DOI] [PubMed] [Google Scholar]
- 170.Jang C.H., Choi J.H., Byun M.S., Jue D.M. Chloroquine inhibits production of TNF-alpha, IL-1beta and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes. Rheumatology (Oxford) 2006;45(6):703–710. doi: 10.1093/rheumatology/kei282. [DOI] [PubMed] [Google Scholar]
- 171.Jeong J.Y., Jue D.M. Chloroquine inhibits processing of tumor necrosis factor in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Immunol. 1997;158(10):4901–4907. [PubMed] [Google Scholar]
- 172.Murray R.Z., Stow J.L. Cytokine secretion in macrophages: SNAREs, Rabs, and membrane trafficking. Front. Immunol. 2014;5:538. doi: 10.3389/fimmu.2014.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhang M., Kenny S.J., Ge L., Xu K., Schekman R. Translocation of interleukin-1beta into a vesicle intermediate in autophagy-mediated secretion. Elife. 2015;4 doi: 10.7554/eLife.11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Carta S., Lavieri R., Rubartelli A. Different Members of the IL-1 Family Come Out in Different Ways: DAMPs vs. Cytokines? Front. Immunol. 2013;4:123. doi: 10.3389/fimmu.2013.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Herold T., Jurinovic V., Arnreich C., Lipworth B.J., Hellmuth J.C., Bergwelt-Baildon M.V., Klein M., Weinberger T. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J. Allergy Clin. Immunol. 2020;146(2020):128–136.e4. doi: 10.1016/j.jaci.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Jeong J.Y., Choi J.W., Jeon K.I., Jue D.M. Chloroquine decreases cell-surface expression of tumour necrosis factor receptors in human histiocytic U-937 cells. Immunology. 2002;105(1):83–91. doi: 10.1046/j.0019-2805.2001.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]