Abstract
There is limited information regarding clinical characteristics and outcomes of patients with SARS-CoV-2 (COVID-19) disease presenting with ST-segment elevation myocardial infarction (STEMI). In this multicenter retrospective study, we reviewed charts of patients admitted with symptomatic COVID-19 infection and STEMI to a total of 4 hospitals spanning Italy, Lithuania, Spain and Iraq from February 1, 2020 to April 15, 2020. A total of 78 patients were included in this study, 49 (63%) of whom were men, with a median age of 65 [58, 71] years, and high comorbidity burden. During hospitalization, 8 (10%) developed acute respiratory distress syndrome, and 14 (18%) required mechanical ventilation. 19 (24%) patients were treated with primary Percutaneous Coronary Intervention (PCI) and 59 (76%) were treated with fibrinolytic therapy. 13 (17%) patients required cardiac resuscitation, and 9 (11%) died. For the 19 patients treated with primary PCI, 8 (42%) required intubation and 8 (42%) required cardiac resuscitation; stent thrombosis occurred in 4 patients (21%). A total of 5 patients (26%) died during hospitalization. 50 (85%) of the 59 patients initially treated with fibrinolytic therapy had successful fibrinolysis. The median time to reperfusion was 27 minutes [20, 34]. Hemorrhagic stroke occurred in 5 patients (9%). Six patients (10%) required invasive mechanical ventilation; 5 (9%) required cardiac resuscitation, and 4 (7%) died. In conclusion, this is the largest case series to-date of COVID-19 positive patients presenting with STEMI and spans 4 countries. We found a high rate of stent thrombosis, indicating a possible need to adapt STEMI management for COVID-19 patients.
As of May 20, 2020, the total number of confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2; COVID-19) infections worldwide had surpassed 4.5 million cases and the associated reported deaths neared 325,000.1 Systemic viral infections have been associated with acute myocardial infarction and inflammation,2 , 3 and may indeed be a pathophysiologic trigger for plaque rupture and thrombosis. Despite the burgeoning literature regarding the general COVID-19 positive population, little is known about the specific clinical characteristics and outcomes of patients with active COVID-19 infection presenting with ST-segment Elevation myocardial infarction (STEMI). In fact, data thus far have been mostly limited to a small case series of 18 patients in New York City with a reported mortality of 72%,4 and another of 28 patients from northern Italy with a reported mortality of 39%.5
Methods
This retrospective multicenter, medical chart review of consecutive patients hospitalized between February 1, 2020 and April 15, 2020 with COVID-19 and STEMI was conducted at a total of 4 hospitals in Lithuania, Italy, Spain and Iraq. Patients were confirmed as having COVID-19 infection through positive result on polymerase chain reaction testing of a nasopharyngeal sample. COVID negative patients who treated for STEMI in these centers were excluded from this study. Timing of COVID-19 PCR results as it relates to time of Percutaneous Coronary Intervention (PCI) were not available. Data collection was performed by medical doctors at the hospitals where these patients were admitted by reviewing the patients’ electronic medical records as well as their report charts. A 10% random sample from the data set was verified by the same physicians at the collecting sites after data collection was complete to ensure accuracy of the collected information. Data collected included patient demographics, comorbidities, existing medications, laboratory tests, electrocardiogram (ECG) results, echocardiography results, diagnosis during the hospital course, inpatient medication, treatments (fibrinolytic therapy, PCI, vasopressor use, invasive mechanical ventilation), and outcomes (duration of hospitalization, revascularization success, in-hospital reinfarction, and mortality). Home medications were reported based on admission medication reconciliation performed by the admitting physician. Coronary artery disease included previous myocardial infection (MI), coronary artery bypass surgery, previous PCI, angina, and/or abnormal cardiac stress testing.
The choice of initial reperfusion strategy of either fibrinolytic therapy or primary PCI was at the discretion of the treating interventional cardiologist at each site according to institutional guidelines, since international management guidelines do not exist for these patients. Generally, patients presenting with COVID-19 and STEMI in Lithuania, Italy, and Iraq without hemodynamic instability or high-risk features, were treated with fibrinolytic therapy. If successful, patients were discharged home and brought back for invasive revascularization after testing negative for COVID-19 at least 14 days after diagnosis. In Spain, most patients with suspected or confirmed COVID-19 who presented with STEMI were treated through primary PCI.
Successful fibrinolysis was defined as ST-segment resolution by >50% within 60 to 90 min of fibrinolytic administration with absence of chest pain and signs of hemodynamic or electrical instability. Thrombolysis in MI (TIMI) flow grade was assessed angiographically by the performing physician.6 During PCI, door-to-wire crossing time was recorded as a surrogate for reperfusion in accordance with the 2017 European Society of Cardiology guidelines for management of acute MI (AMI) in patients presenting with ST-segment elevation.7 STEMI and stent thrombosis were defined according to the fourth universal definition of MI.8 Post fibrinolysis re-infarction was defined as the presence of any 2 of the following: 1) new ischemic ST segment deviation on ECG; 2) new signs or symptoms suggestive of ongoing ischemia; 3) an increase in cardiac troponin in accordance with the fourth universal definition of MI for post PCI myocardial infarction.8 COVID-19 presentation was classified according the WHO definitions of clinical syndromes associated with COVID-19.9 Chronic kidney disease staging was performed according to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease.10 Dyslipidemia was defined as an LDL > 70 mg/dl, HDL < 50 mg/dl in women and < 40 mg/dl in men.
Continuous variables are presented as median [25th percentile, 75th percentile]. Categorical variables are presented as frequency and percentage. As this analysis utilized de-identified data, it was exempt from Institutional Review Board review.
Results
A total of 78 patients were included in this review, a majority of whom were men, with a median age of 65 [58, 71] years as shown in Table 1 . Patients carried a high comorbidity load; most (43, 55%) had at least 4 comorbidities. The most common comorbidities were dyslipidemia (72, 92%), hypertension (57, 73%), smoking (41, 53%), and diabetes mellitus (21, 27%). During hospitalization, 34 patients (44%) had mild pneumonia, 2 (3%) had severe pneumonia, 8 (10%) developed acute respiratory distress syndrome, 5 (6%) developed septic shock, and a total of 14 (18%) required mechanical ventilation. Median pain to reperfusion time was 12.6 [8, 15] hours. 19 (24%) patients were treated with primary PCI and 59 (76%) were treated with fibrinolytic therapy as the initial reperfusion strategy. A total of 13 patients (17%) required cardiac resuscitation, 7 (9%) re-infarcted during their hospitalization, and 9 (12%) died.
Table 1.
Characteristics of 78 COVID-19 positive patients with ST-Elevation myocardial infarction
| Variables | All patients n (%) median [quartile 1, quartile 3] |
Primary PCI (n = 19) |
Fibrinolytics therapy (n = 59) |
|---|---|---|---|
| Men | 49 (63%) | 9 (47%) | 40 (68%) |
| Age (years) | 65 [58, 71] | 65 [61, 73] | 64 [57, 70] |
| Obesity | 16 (21%) | 2 (11%) | 14 (24%) |
| Country | |||
| Iraq | 44 (56%) | 2 (11%) | 42 (71%) |
| Italy | 22 (28%) | 5 (26%) | 17 (29%) |
| Lithuania | 5 (6%) | 5 (26%) | 0 (0.0%) |
| Spain | 7 (9%) | 7 (37%) | 0 (0.0%) |
| Dyslipidemia | 72 (92%) | 16 (84%) | 56 (95%) |
| Hypertension | 57 (73%) | 15 (79%) | 42 (71%) |
| Current or former smoker | 41 (53%) | 9 (47%) | 32 (54%) |
| Diabetes mellitus | 21 (27%) | 2 (11%) | 19 (32%) |
| Coronary artery disease | 61 (78%) | 14 (74%) | 47 (80%) |
| Previous coronary artery bypass grafting | 9 (11%) | 1 (5%) | 8 (14%) |
| Chronic kidney disease stage | |||
| II | 36 (46%) | 9 (47%) | 27 (46%) |
| III | 31 (40%) | 9 (47%) | 22 (37%) |
| IV | 2 (3%) | 0 (0%) | 2 (3%) |
| Chronic obstructive pulmonary disease | 3 (4%) | 2 (11%) | 1 (2%) |
| Peripheral arterial disease | 3 (4%) | 2 (11%) | 1 (2%) |
| Stroke | 6 (8%) | 6 (32%) | 0 (0%) |
| COVID-19 presentation | |||
| Mild pneumonia | 34 (44%) | 3 (16%) | 31 (53%) |
| Severe pneumonia | 2 (3%) | 0 (0%) | 2 (3%) |
| Acute respiratory distress syndrome | 8 (10%) | 7 (37%) | 1 (2%) |
| Septic shock | 5 (6%) | 2 (11%) | 3 (5%) |
| Medications on admission | |||
| Clopidogrel | 8 (10%) | 1 (5%) | 7 (12%) |
| Aspirin | 17 (22%) | 3 (16%) | 14 (24%) |
| Ticagrelor (1) | 0 (0%) | 0 (0%) | 0 (0%) |
| Warfarin | 3 (4%) | 1 (5%) | 2 (3%) |
| Novel oral anticoagulant | 1 (1%) | 1 (5%) | 0 (0%) |
| Beta blocker | 30 (39%) | 7 (37%) | 23 (39%) |
| Angiotensin II converting enzyme inhibitor | 30 (39%) | 8 (42%) | 22 (37%) |
| Angiotensin receptor blocker | 16 (21%) | 3 (16%) | 13 (22%) |
| Calcium channel blocker | 13 (17%) | 5 (26%) | 8 (14%) |
| Statin | 33 (42%) | 9 (47%) | 24 (41%) |
| Nitrates | 2 (3%) | 0 (0%) | 2 (3%) |
| Hemoglobin (g/dl) | 13 [12, 15] | 14 [12, 15] | 14 [12, 15] |
| Neutrophils (k/cumm) (1) | 8 [6, 10] | 8 [6, 10] | 8 [6, 10] |
| Neutrophil percent (2) | 75 [64, 82] | 80 [64, 84] | 74 [64, 82] |
| Lymphocytes (k/cumm) | 1.8 [1.3, 2.6] | 2 [1.2, 3] | 1.8 [1.3, 2.5] |
| Lymphocyte percent (4) | 17 [12, 28] | 17 [12, 28] | 18 [13, 27] |
| Platelets (k/cumm) | 228 [203, 263] | 240 [165, 263] | 227 [204, 271] |
| Peak troponin I (ng/ml) (5) | 82 [56, 100] | 70 [40, 160] | 83 [58, 98] |
| Ejection fraction after revascularization | 42 [38, 46] | 39 [32, 45] | 43.5 [40, 48] |
| Mechanical ventilation | 14 (18%) | 8 (42%) | 6 (10%) |
| In-hospital hemorrhagic stroke | 5 (6%) | 0 (0%) | 5 (9%) |
| In-hospital ischemic stroke | 3 (4%) | 3 (16%) | 0 (0%) |
| In-hospital resuscitation (1) | 13 (17%) | 8 (42%) | 5 (9%) |
| In-hospital hemodialysis | 6 (8%) | 5 (26%) | 1 (2%) |
| Vasopressor use | 12 (15%) | 6 (32%) | 6 (10%) |
| Length of stay (days) | 2 [2, 4] | 6 [3, 15] | 2 [2, 3] |
| Death | 9 (12%) | 5 (26%) | 4 (7%) |
PCI = percutaneous coronary intervention; STEMI = ST-Elevation Myocardial Infarction; TIMI = thrombolysis in myocardial infarction; Dyslipidemia = LDL > 70 mg/dl, HDL < 50 mg/dl in women and < 40 mg/dl in men; Hypertension = Patients receiving antihypertensive therapy before admission or had to be initiated on antihypertensive therapy during hospitalization due to consistently elevated blood pressure of ≥ 140/90 mmHg; Obesity = Body Mass Index ≥ 30 kg/m2.
Numbers in parentheses after variables indicate missing data.
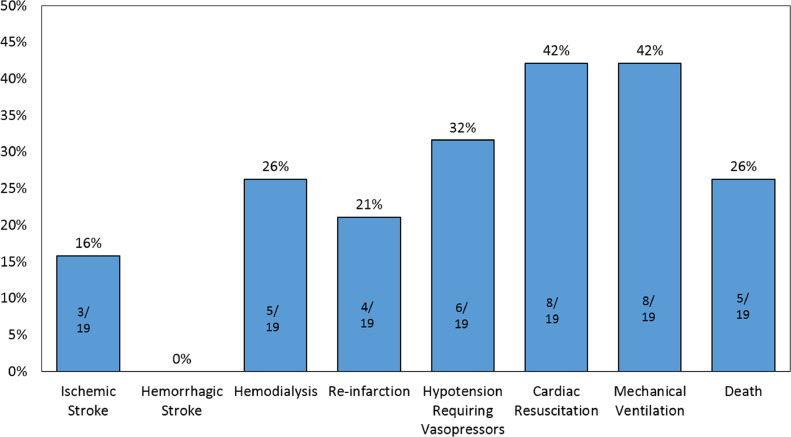
For the 19 patients treated with primary PCI, 1 patient (5%) had no evidence of coronary artery obstruction and was diagnosed with myocarditis. The median door-to-wire time was 128 [90, 210] minutes. The most common culprit artery was the left anterior descending artery (8 patients; 44%). All patients received a drug eluting stent (DES). 8 patients (42%) required invasive mechanical ventilation; additionally, 8 (42%) required cardiac resuscitation during hospitalization. Stent thrombosis occurred in 4 patients (21%). The median peak troponin I was 70 [70, 160] ng/mL, and the median left ventricular ejection fraction after PCI was 39% [32%, 45%]. 5 of these patients (26%) died during hospitalization (Figure 1 ).
Figure 1.
Clinical course and outcomes of COVID-19 patients presenting with STEMI who underwent primary PCI.
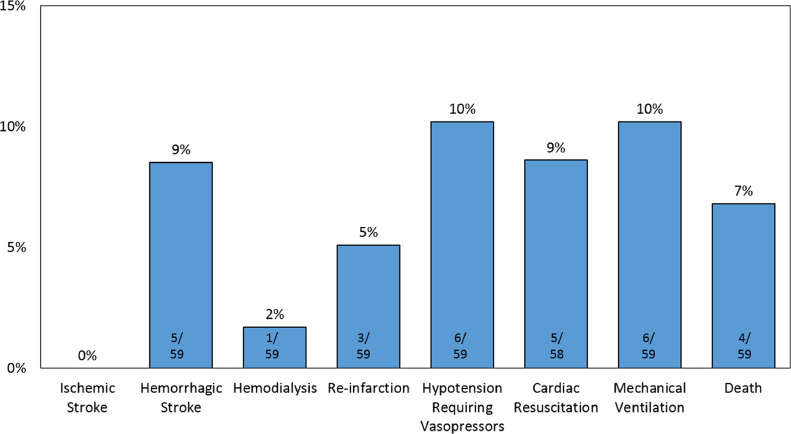
For the 59 patients initially treated with fibrinolytic therapy, 50 patients (85%) had successful fibrinolysis. The median time to reperfusion was 27 [20, 34] minutes. Alteplase was used in 21 (36%) patients, and Tenecteplase was used in 38 (64%). Median peak troponin was 83 [58, 98] ng/ml and median left ventricular ejection fraction after revascularization was 44% [40%, 48%]. Hemorrhagic stroke occurred in 5 patients (9%). 6 patients (10%) required invasive mechanical ventilation; 5 (9%) required cardiac resuscitation, and 4 (7%) died (Figure 2 ). Of the 9 patients who failed fibrinolytic therapy; 1 patient (11%) died before PCI and the remaining 8 (89%) underwent PCI; 2 patients (25%) died following PCI. Of these 9 patients, 7 (78%) had obstructive coronary artery disease, and underwent PCI with a DES; 1 patient (11%) was diagnosed with myocarditis.
Figure 2.
Clinical course and outcomes of COVID-19 patients presenting with STEMI who were treated with fibrinolytic therapy.
Of all 78 STEMI patients positive for COVID-19 infection, 14 (18%) required invasive mechanical ventilation. Their median age was 68 [60, 74] years, and 9 (64%) were men. The most common comorbidities were history of ischemic heart disease (13, 93%) dyslipidemia (13, 93%), hypertension (11, 79%), and smoking (9, 64%). Eight (57%) of the patients who required mechanical ventilation underwent primary PCI, and 6 (43%) were treated with fibrinolytic therapy as the initial reperfusion strategy. Two (14%) patients had a hemorrhagic stroke, 11 (79%) required cardiac resuscitation, and 7 (50%) died. The median length of stay was 14 [9, 15] days.
Discussion
The optimal management strategy for patients with STEMI and concurrent COVID-19 may present a greater challenge than for pre-pandemic STEMI patients. The utilization of fibrinolytic therapy has gained attention recently as a viable option for stable patients presenting with COVID-19 disease and STEMI.11 Experts treating STEMI patients with COVID-19 in China recommend fibrinolytic therapy over primary percutaneous coronary intervention (PCI) in stable patients who present within 12 hours of symptom onset and do not have a contraindication for fibrinolytics.12 However, many questions remain unanswered about this select population. Hence, this study fills a critical knowledge gap by describing the baseline characteristics and comorbidities, presenting laboratory tests, clinical course, revascularization strategies, and outcomes of patients admitted with COVID-19 disease and STEMI.
To our knowledge, this study size of 78 patients represents the largest case series of patients presenting with active COVID-19 and STEMI. The patients in this study were of advanced age and highly comorbid. All patients presented with at least 1 comorbidity, nearly all (75, 96%) had 2 or more comorbidities, and 62 (80%) had 3 or more comorbidities. The overall mortality in this study was 12%, which is lower than what was reported in a smaller case series of 19 patients presenting with COVID-19 and STEMI in New York,4 and another case series of 28 patients in Italy.5 However, this study reported mortality rates for patients with definite outcomes (discharge or death), and longer-term studies may report different mortality rates. Our observed mortality rate in mechanically ventilated patients is within range of previously reported rates in critically ill COVID-19 patients in China and the United States.14 , 15
STEMI is a highly morbid condition, and mortality in this disease is influenced by a plethora of patient characteristics. For example, female gender, old age, anterior MI, and previous coronary artery disease are associated with worse outcomes in these patients.16 Influenza and other viral respiratory infections have been reported to act as triggers for AMI,2 and influenza may modestly increase the risk for STEMI.3 Hence, it is plausible that the COVID-19 infection and systemic inflammatory response may have been a trigger for the STEMI event in many cases.
The standard of care for patients without COVID-19 presenting with STEMI is invasive revascularization within 90 minutes of hospital presentation. Fibrinolytic therapy is recommended if there is an expected delay of more than 90 minutes to invasive revascularization.7 , 17 However, in the ongoing COVID-19 pandemic, multiple expert statements from the United States and China have recommended initiating fibrinolytic therapy as a first line therapy for these stable patients without high-risk features as an effort to mitigate the spread of the disease.11 , 12 , 13 Alarmingly, patients in this study who were treated with PCI had a 21% rate of stent thrombosis, which is much higher than previously reported rates of early stent thrombosis of 1%.18 Further, it has been reported that patients in the intensive care unit for COVID-19 have a remarkably high incidence of thrombotic complications (approximately 31%),19 and that anticoagulation may improve outcomes in such patients who have elevated D-dimer.20 These COVID-19-specific observations could explain why patients in our study had a higher-than-expected rate of stent thrombosis. Therefore, it is important to continue to monitor thrombotic events in this population with larger studies to guide timing and strategies of invasive revascularization.
This study has all the limitations of retrospective studies of prospectively collected data. First, the patient populations, including COVID-19 symptom presentation, were not homogenous across countries. Second, each medical center followed their own institutional guidelines for managing patients presenting with COVID-19 and STEMI. This precluded statistical testing between primary PCI and thrombolysis strategies and may limit the generalizability of our findings. Third, due to small event counts, adjusted analyses were not possible. Fourth, there were missing data points for some procedural characteristics and outcomes. Lastly and most importantly, we did not have a concurrent control group of patients with STEMI at the same centers without concurrent COVID-19 infection.
In conclusion, this is the largest case series to-date of COVID-19 positive patients presenting with STEMI and spans 4 countries. We found a high rate of thrombotic complications and a very high mortality rate. Our results suggest a possible need to adapt STEMI management for patients with concurrent COVID-19 infection.
Author contributions
Anas Hamadeh M.D: Supervision, Conceptualization, Data curation, Methodology, Writing-Original Draft preparation, Writing- Reviewing and Editing. Ali Aldujeli M.D., M.Sc: Supervision, Conceptualization, Investigation, Validation, Writing- Reviewing and Editing. Kasparas Briedis M.D., M.Sc: Conceptualization, Investigation- Data collection, Validation, Writing- Reviewing and Editing. Kristen M. Tecson Ph.D: Formal Analysis, Writing- Reviewing and Editing, Visualization, Funding acquisition. Jorge Sanz Sánchez M.D., Ph.D: Investigation- Data collection, Validation, Writing- Reviewing and Editing. Montazar Al dujeili M.D: Investigation- Data collection, Validation, Writing- Reviewing and Editing. Ammar Al-Obeidi M.D: Investigation- Data collection, Validation, Writing- Reviewing and Editing. Jose Luís M.D., Ph.D: Investigation- Data collection, Validation, Writing- Reviewing and Editing. Remigijus Žaliūnas M.D., M.Sc., Ph.D: Writing- Reviewing and Editing. Robert C. Stoler M.D: Writing- Reviewing and Editing. Peter A. McCullough M.D., M.P.H: Writing- Reviewing and Editing.
Disclosures
The authors have no disclosures.
Footnotes
This work was funded, in part, by the Baylor Health Care System Foundation.
References
- 1.The Center for Systems Science and Engineering (CSSE) at Johns Hopkins University . 2020. Coronavirus COVID-19 global cases. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. Accessed May 20, 2020. [Google Scholar]
- 2.Warren-Gash C, Hayward AC, Hemingway H, Denaxas S, Thomas SL, Timmis AD, Whitaker H, Smeeth L. Influenza infection and risk of acute myocardial infarction in England and Wales: a CALIBER self-controlled case series study. J Infect Dis. 2012;206(11):1652‐1659. doi: 10.1093/infdis/jis597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Claeys MJ, Coenen S, Colpaert C, Bilcke J, Beutels P, Wouters K, Legrand V, Van Damme P, Vrints C. Environmental triggers of acute myocardial infarction: results of a nationwide multiple-factorial population study. Acta Cardiol. 2015;70(6):693‐701. doi: 10.2143/AC.70.6.3120182. [DOI] [PubMed] [Google Scholar]
- 4.Bangalore S., Sharma A., Slotwiner A., Yatskar L., Harari R., Shah B., Ibrahim H., Friedman G.H., Thompson C., Alviar C.L., Chadow H.L., Fishman G.I., Reynolds H.R., Keller N., Hochman J.S. ST-Segment elevation in patients with Covid-19 - a case series [published online ahead of print, 2020 Apr 17] N Engl J Med. 2020 doi: 10.1056/NEJMc2009020. NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stefanini GG, Montorfano M, Trabattoni D, Andreini D, Ferrante G, Ancona M, Metra M, Curello S, Maffeo D, Pero G, Cacucci M, Assanelli E, Bellini B, Russo F, Ielasi A, Tespili M, Danzi GB, Vandoni P, Bollati M, Barbieri L, Oreglia J, Lettieri C, Cremonesi A, Carugo S, Reimers B, Condorelli G, Chieffo A. ST-Elevation Myocardial Infarction in Patients with COVID-19: Clinical and Angiographic Outcomes [published online ahead of print, 2020 Apr 30] Circulation. 2020 doi: 10.1161/CIRCULATIONAHA.120.047525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chesebro JH, Knatterud G, Roberts R, Borer J, Cohen LS, Dalen J, Dodge HT, Francis CK, Hillis D, Ludbrook P. Thrombolysis in Myocardial Infarction (TIMI) Trial, Phase I: A comparison between intravenous tissue plasminogen activator and intravenous streptokinase. Clinical findings through hospital discharge. Circulation. 1987;76(1):142‐154. doi: 10.1161/01.cir.76.1.142. [DOI] [PubMed] [Google Scholar]
- 7.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P, ESC Scientific Document Group 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2018;39(2):119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 8.Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, Executive Group on behalf of the Joint European Society of Cardiology (ESC). American College of Cardiology (ACC) American Heart Association (AHA) World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction Fourth Universal Definition of Myocardial Infarction (2018) J Am Coll Cardiol. 2018;72(18):2231‐2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization; Published March 13, 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected.https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf [Google Scholar]
- 10.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1‐S266. [PubMed] [Google Scholar]
- 11.Szerlip M, Anwaruddin S, Aronow HD, Cohen MG, Daniels MJ, Dehghani P, Drachman DE, Elmariah S, Feldman DN, Garcia S, Giri J, Kaul P, Kapur N, Kumbhani DJ, Meraj PM, Morray B, Nayak KR, Parikh SA, Sakhuja R, Schussler JM, Seto A, Shah B, Swaminathan RV, Zidar DA, Naidu SS. Considerations for cardiac catheterization laboratory procedures during the COVID-19 pandemic perspectives from the Society for Cardiovascular Angiography and Interventions Emerging Leader Mentorship (SCAI ELM) Members and Graduates [published online ahead of print, 2020 Mar 25] Catheter Cardiovasc Interv. 2020 doi: 10.1002/ccd.28887. [DOI] [PubMed] [Google Scholar]
- 12.Zeng J, Huang J, Pan L. How to balance acute myocardial infarction and COVID-19: the protocols from Sichuan Provincial People's Hospital [published online ahead of print, 2020 Mar 11] Intensive Care Med. 2020;46(6):1111‐1113. doi: 10.1007/s00134-020-05993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jing ZC, Zhu HD, Yan XW, Chai WZ, Zhang S. Recommendations from the Peking Union Medical College Hospital for the management of acute myocardial infarction during the COVID-19 outbreak [published online ahead of print, 2020 Mar 31] Eur Heart J. 2020 doi: 10.1093/eurheartj/ehaa258. ehaa258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30] Lancet. 2020;395(10223):497‐506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, Lee M. Characteristics and Outcomes of 21 Critically Ill Patients With COVID-19 in Washington State [published online ahead of print, 2020 Mar 19] JAMA. 2020;323(16):1612‐1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasiljević Z, Stojanović B, Kocev N, Stefanović B, Mrdović I, Ostojić M, Krotin M, Putniković B, Dimković S, Despotović N. [Hospital mortality trend analysis of patients with ST elevation myocardial infarction in the Belgrade area coronary care units] Srp Arh Celok Lek. 2008;136(Suppl 2):84‐96. doi: 10.2298/sarh08s2084v. [DOI] [PubMed] [Google Scholar]
- 17.Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2016 Appropriate use criteria for coronary revascularization in patients with acute coronary syndromes: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2017;69(5):570‐591. doi: 10.1016/j.jacc.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 18.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH, ACCF. AHA. SCAI 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Catheter Cardiovasc Interv. 2012;79(3):453‐495. doi: 10.1002/ccd.23438. [DOI] [PubMed] [Google Scholar]
- 19.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers DAMPJ, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19 [published online ahead of print, 2020 Apr 10] Thromb Res. 2020 doi: 10.1016/j.thromres.2020.04.013. S0049-3848(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin S, Huang M, Li D, Tang N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2 [published online ahead of print, 2020 Apr 3] J Thromb Thrombolysis. 2020:1‐4. doi: 10.1007/s11239-020-02105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]