Zinc influx transport proteins function synergistically in rice to maintain zinc homeostasis.
Abstract
The elements Zinc (Zn) and cadmium (Cd) have similar chemical and physical properties, but contrasting physiological effects in higher organisms. In plants, Zn/Cd transport is mediated by various transporter proteins belonging to different families. In this study, we functionally characterized two Zn transporter genes in rice (Oryza sativa), ZINC TRANSPORTER5 (OsZIP5) and ZINC TRANSPORTER9 (OsZIP9), which are tandem duplicates and act synergistically in Zn/Cd uptake. Both genes encode plasma membrane-localized proteins with influx transporter activity. The expression profiles of OsZIP5 and OsZIP9 overlap in the root epidermis and respond to the local Zn status in the root. However, OsZIP9 is also regulated by systemic signals of Zn status from the shoot. OsZIP5 functions redundantly to OsZIP9, but has a relatively weaker effect. Plants with the knockout mutations oszip5, oszip9, or oszip5oszip9 show impaired Zn/Cd uptake. The decreased Zn/Cd levels and growth retardation in the oszip5 mutant are less severe than in the oszip9 mutant. However, the double mutant oszip5oszip9 showed an enhanced Zn deficiency phenotype compared with the single mutants, and few double-knockout plants were able to survive the entire growth cycle without excessive Zn supply. Transgenic plants overexpressing OsZIP9 had markedly enhanced Zn/Cd levels in the aboveground tissues and brown rice. The results of our study fill a gap in current knowledge of Zn uptake and improve our understanding of Zn/Cd accumulation in rice.
Zinc (Zn) and cadmium (Cd) present similar features in terms of chemical and physical properties but have opposite roles in their effects on physiological processes in plants (Sumi et al., 1996; Cai et al., 2019b). Zn is an essential micronutrient that plays an important role in plant growth and development. Zn serves as a cofactor in >300 enzymes and plays key structural roles in many proteins (Vallee and Auld, 1990; Coleman, 1998). Zn deficiency retards plant growth, as characterized by necrosis of the root apex, leaf chlorosis, small leaves, and shortened internodes (Broadley et al., 2012). However, an excess of Zn may cause Zn toxicity, with symptoms such as chlorosis in leaves (Kawachi et al., 2009). Cd is a nonessential toxic heavy metal that is readily taken up by the roots of plants. Cd accumulation in plants can cause disruption in nutrient homeostasis, DNA and membrane damage, and protein dysfunction, which consequently leads to inhibition of plant growth (Sandalio et al., 2001; Hall, 2002). More importantly, the accumulation of Cd in the edible parts of crops can pose risks to human health through the food chain. Since Cd and Zn are chemically and physically similar, they compete with each other for transporters involved in uptake, sequestration, translocation, and remobilization (Sumi et al., 1996; Saifullah et al., 2014; Sarwar et al., 2015; Cai et al., 2019b). Thus, understanding how plants accumulate Zn/Cd will allow for the optimization of Zn and Cd levels for both agriculture and human health.
Rice (Oryza sativa) is a staple crop that provides the majority of dietary nutrients to more than half of the world’s human population (Grusak and DellaPenna, 1999). Unfortunately, compared to other food crops, rice has a relatively lower Zn level but higher Cd content in grains (Chaney et al., 2005). Understanding the control of Zn homeostasis and the Cd response in rice will benefit efforts to genetically improve the rice grain’s nutritional value and food safety (Pinson et al., 2015). A number of transporters have been identified that are involved in Zn and Cd accumulation in rice, including Zn-regulated transporters, iron-regulated transporter-like proteins (ZIPs), and heavy metal ATPases (HMAs; Lee et al., 2010; Olsen and Palmgren, 2014; Bashir et al., 2019; Cai et al., 2019b). Xylem loading of both Zn and Cd in roots relies on ZINC TRANSPORTER7 (OsZIP7) and HEAVY METAL ATPase2 (OsHMA2). Knockout mutants of the OsZIP7 and OsHMA2 genes led to retention of Zn and Cd in the roots and basal regions, which contain basal nodes and serve as the key hubs for mineral nutrient distribution (Yamaji et al., 2013; Sasaki et al., 2015; Tan et al., 2019). Vacuolar sequestration mediated by various transporters is an important strategy for reducing the levels of toxic metals in plants, and OsHMA3 is such a transporter, playing an important role in Zn homeostasis and Cd sequestration in rice (Miyadate et al., 2011; Sasaki et al., 2014; Cai et al., 2019a). The node is a very important structure for distributing mineral elements to developing tissues or panicles through the xylem to the phloem transport pathway (Yamaji and Ma, 2014). OsZIP3, OsZIP7, and OsHMA2 cooperate in the intervascular transfer of Zn/Cd to deliver the ions upward to newly expanding leaves and seeds. It has long been thought that the uptake of Zn in rice roots is mediated by OsZIP1 based on the evidence of induced expression under Zn deficiency and of Zn transport activity in OsZIP1-transformed yeast (Saccharomyces cerevisiae) cells (ZHY3; Ramesh et al., 2003). However, recent evidence indicates that OsZIP1 is a metal-detoxifying transporter that prevents excess Zn and Cd accumulation in rice (Liu et al., 2019). Therefore, the transporter that mediates Zn uptake is yet to be identified.
There are 16 members of the ZIP family in rice (Tiong et al., 2015). Only a small number of ZIP proteins have been studied to determine their biological functions. Transcriptional upregulation of most ZIP genes in response to Zn deficiency and/or Cd stress has been reported in plants (Ramesh et al., 2003; Lee et al., 2010a; Zheng et al., 2018; Tan et al., 2019). Overexpression and/or heterologous expression of these transporter proteins in rice, yeast, and Arabidopsis (Arabidopsis thaliana) was found to disturb Zn homeostasis and alter Cd stress tolerance, confirming their roles in Zn/Cd transport (Ishimaru et al., 2005, 2007; Lee and An, 2009; Lee et al., 2010a; Sasaki et al., 2015; Zheng et al., 2018; Liu et al., 2019; Tan et al., 2019). However, the physiological roles of these proteins in planta (i.e. in Zn/Cd uptake and distribution) have not been defined except for OsZIP3 and OsZIP7. In addition, there is functional redundancy among these transporters. For example, knocking out a single ZIP family gene such as OsZIP1 or OsZIP5 showed no detectable phenotypic changes under normal growth conditions (Lee et al., 2010a; Liu et al., 2019), and single oszip3 or oszip7 gene mutations reduce, but do not abolish, Zn upward delivery. In addition, the overlapping spatiotemporal expression patterns of OsZIP genes also suggest their genetic redundancy. Therefore, the complete picture of ZIP-mediated Zn/Cd accumulation and homeostasis remains to be revealed.
We previously identified numerous transporters, including the OsZIP genes that are responsive to Cd stress, and functionally characterized OsZIP7 (Tang et al., 2014; Tan et al., 2019). In this study, we functionally characterized two rice Zn transporters, OsZIP5 and OsZIP9, and found that they function redundantly in Zn/Cd uptake in roots. Our results will help to close the knowledge gap on Zn/Cd accumulation and pin another point on the genetic control of Zn homeostasis.
RESULTS
Identification of Duplicated ZIP Genes in Rice
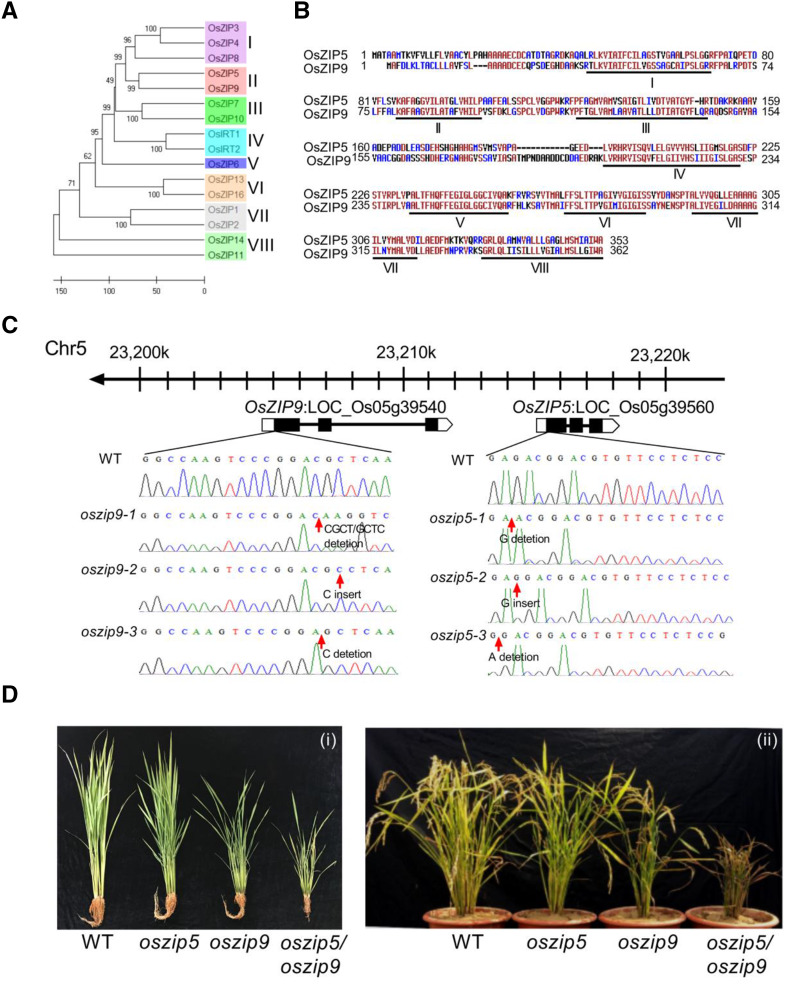
To investigate the evolutionary relationships among the ZIP proteins and identify potential redundancy, the predicted protein sequences of 16 ZIP genes were used for phylogenetic analysis. These proteins were found to group into eight clades, and OsZIP5 (LOC_Os05g39560) and OsZIP9 (LOC_Os05g39540) fell into clade II (Fig. 1A). OsZIP5 and OsZIP9 encode proteins of 353 and 362 amino acids, respectively, and both contain eight putative transmembrane domains (Fig. 1B; Yang et al., 2009). There is a potential metal-binding domain rich in His residues between transmembrane domains III and IV in OsZIP5 and OsZIP9 (Fig. 1B), which is consistent with the results of a previous study (Guerinot, 2000). The two genes are located in a tandem repeat on rice chromosome 5, and both contain three exons and two introns (Fig. 1C). Even though the two genes encode proteins of similar size, OsZIP5 (2,871 bp) is much shorter than OsZIP9 (8,749 bp). Their exons share about 78% sequence identity, but no sequence identity was found for the intron regions, implying that this is an ancient gene duplication.
Figure 1.
Phylogenetic relationship between OsZIP5 and OsZIP9. A, Phylogenetic tree of rice ZIP proteins. The predicted ZIP protein sequences were used to construct the phylogenetic tree using MEGA-X. The scale bar shows the number of amino acid differences per sequence. Clades I to VIII are labeled in different colors. Accession numbers of the predicted rice ZIP proteins are given in Supplemental Table S1. B, Alignment of the predicted amino acid sequences of OsZIP5 and OsZIP9. Identical amino acids are shown in red. Nonconservative amino acids are shown in blue. The membrane-spanning domains are under-lined and labeled I to VIII. C, Schematic diagram of OsZIP5 and OsZIP9 gene structure and the CRISPR/Cas9 guide target sites. Exons and introns are indicated by black rectangles and black lines, respectively. oszip5-1, oszip5-2, oszip5-3, oszip9-1, oszip9-2, and oszip9-3 are single gene mutations; nucleotide deletion and insertion sites are indicated with red arrows. D, Phenotypes of the wild-type (WT) and mutant plants shown at the vegetative growth stage (i) and the grain filling stage (ii).
Functional Redundancy of OsZIP5 and OsZIP9
To understand the physiological roles of OsZIP5 and OsZIP9, we generated three independent knockout mutants of OsZIP5 (oszip5-1, oszip5-2, and oszip5-3) and OsZIP9 (oszip9-1, oszip9-2, and oszip9-3; Fig. 1C), and two independent double-knockout mutation lines (oszip5oszip9-1 and oszip5oszip9-2; Supplemental Fig. S1) using the CRISPR/Cas9 gene editing system. A weak Zn-deficient phenotype was observed for oszip5, but the phenotype was strong for oszip9 and was further enhanced in the double mutants, indicative of the functional redundancy of the two proteins (Fig. 1D).
At the reproductive stage, the Zn-deficient phenotype was barely detectable in the oszip5 mutant, and most of the oszip5 plants showed only slight reductions in stature and yield components (except for tiller number) in comparison to the wild type (Fig. 1D; Supplemental Fig. S2, A–E). A significant decrease in plant height was observed in the oszip9 mutant lines (Fig. 1D; Supplemental Fig. S2A). Knocking out OsZIP9 also resulted in a decrease in grain yield under field conditions. Among the yield components, significant decreases in tiller number, spikelets per panicle, and seed-setting rate were observed in the oszip9 mutant lines, as was 1,000-grain weight, due to Zn deficiency in the mutant (Supplemental Fig. S2, B–E). Also, the oszip5oszip9 double mutants were more severely inhibited with respect to grain yield compared with the wild-type plants, and only a limited number of plants were able to survive the vegetative growth period and produce filled seeds (Fig. 1D; Supplemental Fig. S2, A–E).
When the wild-type and knockout mutant rice lines were grown under hydroponic conditions with a normal physiological Zn concentration (0.4 μm) at the vegetative growth stage, the knockout OsZIP5 plants showed no visible phenotypic changes under normal conditions (Fig. 1D; Supplemental Fig. S2, F and G). However, the mutant oszip9 lines were retarded in growth, and growth defects and necrosis on the developing leaves of oszip9 plants were observed (Fig. 1D). Plant heights were significantly reduced in the oszip9 mutant lines, and the accumulated dry mass in both roots and shoots of oszip9 plants was significantly less than that in wild-type plants (Supplemental Fig. S2, F and G). This reduction was even more severe in the oszip5oszip9 double-mutant plants, which could only survive with excess Zn supplementation (Supplemental Fig. S2, F and G). Taken together, these results suggest that OsZIP5 and OsZIP9 are functionally redundant and that both genes are involved in the control of Zn homeostasis in rice.
Expression Profiles of OsZIP5 and OsZIP9 Overlap in Roots
We examined the expression profiles of OsZIP5 and OsZIP9 using quantitative real-time PCR. Under normal field growth conditions, expression of OsZIP5 was detected in all tissues tested (Supplemental Fig. S3A). But OsZIP9 was only expressed in a limited number of tissues including roots, nodes, and glumes (Supplemental Fig. S3B). Both OsZIP5 and OsZIP9 were expressed at high levels in roots but weakly in shoots (Supplemental Fig. S3, C and D). In hydroponic culture, the expression levels of OsZIP5 and OsZIP9 increased with decreasing Zn concentrations (Supplemental Fig. S3E). OsZIP5 was also induced in the presence of Cd in roots, but OsZIP9 was repressed (Supplemental Fig. S3F). Comparatively, the absolute expression level of OsZIP9 was significantly higher than that of OsZIP5 (Supplemental Fig. S3G). No reliable changes in expression patterns for these two were detected under conditions of Fe, Mn, and Cu deficiency (Supplemental Fig. S3, C and D).
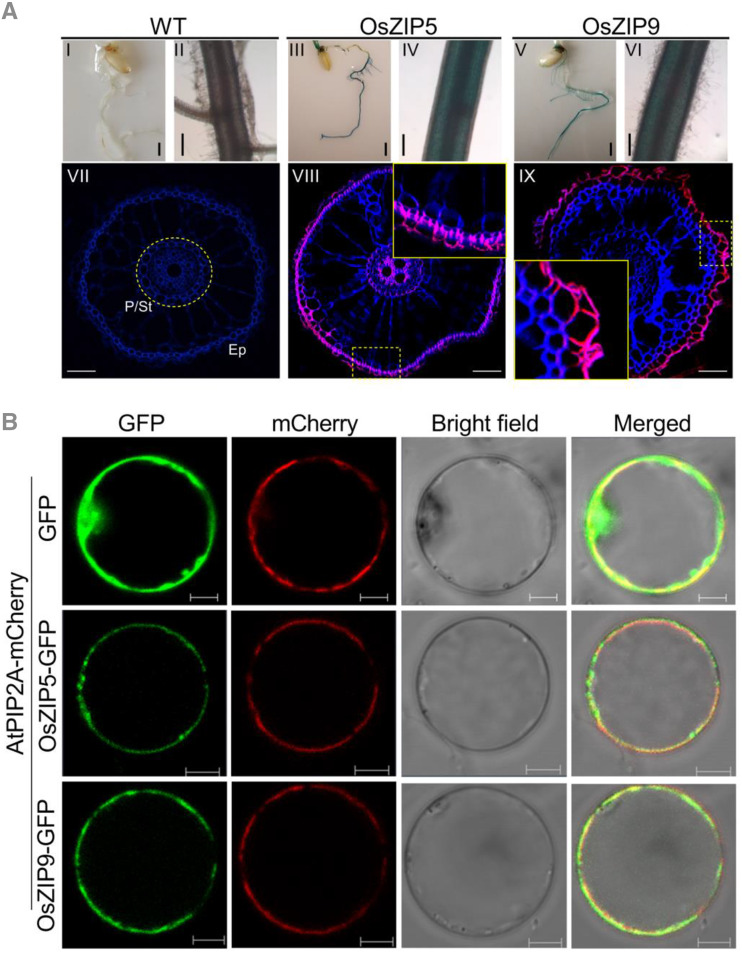
To investigate the tissue-specificity expressions of the two ZIP genes in rice, we developed stable transgenic rice lines expressing the GUS reporter gene driven by the OsZIP5 (−2,422 bp from the start codon) or OsZIP9 (−2,193 bp from the start codon) promoter. GUS staining analysis showed strong expression of both OsZIP5 and OsZIP9 in roots (Fig. 2A). Cross-sectioning and immunostaining using a GUS-specific antibody and counterstaining with 4′,6-diamino-phenylindole (DAPI) for cell wall components revealed that the GUS signals from OsZIP5 and OsZIP9 accumulated mainly in the root epidermis (Fig. 2A, VIII and IX), as well as in the xylem parenchyma cells of enlarged vascular bundles, and in the phloem and xylem of diffuse vascular bundles in the rice node (Supplemental Fig. S4, A, II, III, V, VI, VIII, and IX, and B). OsZIP5 GUS signals also accumulated in the root stele (Fig. 2A, IV and VIII). No signals were observed in the roots, nodes, and leaf sheaths/internodes of wild-type plants (Fig. 2A, I, II, and VII; Supplemental Fig. S4B, I, IV, and VII), demonstrating the specificity of the anti-GUS antibody.
Figure 2.
Tissue specificity and subcellular localization of the OsZIP5 and OsZIP9 proteins. A, Histochemical analysis in roots of wild-type (WT; I and II) and transgenic plants expressing the GUS gene driven by the promoter regions of OsZIP5 (III and IV) and OsZIP9 (V and VI). Immunostaining with an anti-GUS antibody was performed in roots of transgenic rice plants carrying the OsZIP5 or OsZIP9 promoters fused with the GUS gene (VIII and IX) at the vegetative growth stage. Wild-type plants were used as the negative control (VII). The GUS antibody-specific fluorescent signal is shown in red. Blue indicates cell wall autofluorescence after DAPI staining. P, pericycle; St, stele; Ep, epidermis. The insets in VIII and IX represent magnifications of the regions outlined by dotted yellow lines. Scale bars = 1 mm (I–VI) and 50 μm (VII–IX). B, OsZIP5-GFP, OsZIP9-GFP, and AtPIP2A-mCherry (a plasma membrane localization marker) in rice protoplasts. For each localization experiment, ≥20 individual cells were analyzed using a Zeiss LSM880 confocal laser-scanning microscope (Carl Zeiss). Scale bar = 10 μm.
To determine the subcellular localization of the OsZIP5 and OsZIP9 proteins, two vectors expressing the OsZIP5 and OsZIP9 genes fused with the gene for GFP and driven by the cauliflower mosaic virus (CaMV) 35S promoter were constructed (Fig. 2B; Supplemental Fig. S5). When the OsZIP5-GFP or OsZIP9-GFP proteins were transiently expressed in rice protoplasts with AtPIP2-mCherry, a known plasma membrane marker, the GFP signals of both constructs were localized to the plasma membrane (Fig. 2B). Expression of the OsZIP5-GFP and OsZIP9-GFP constructs in Nicotiana benthamiana epidermal cells confirmed this observation (Supplemental Fig. S5).
OsZIP9, But Not OsZIP5, Responds to Long-Distance Signaling of Zn Status
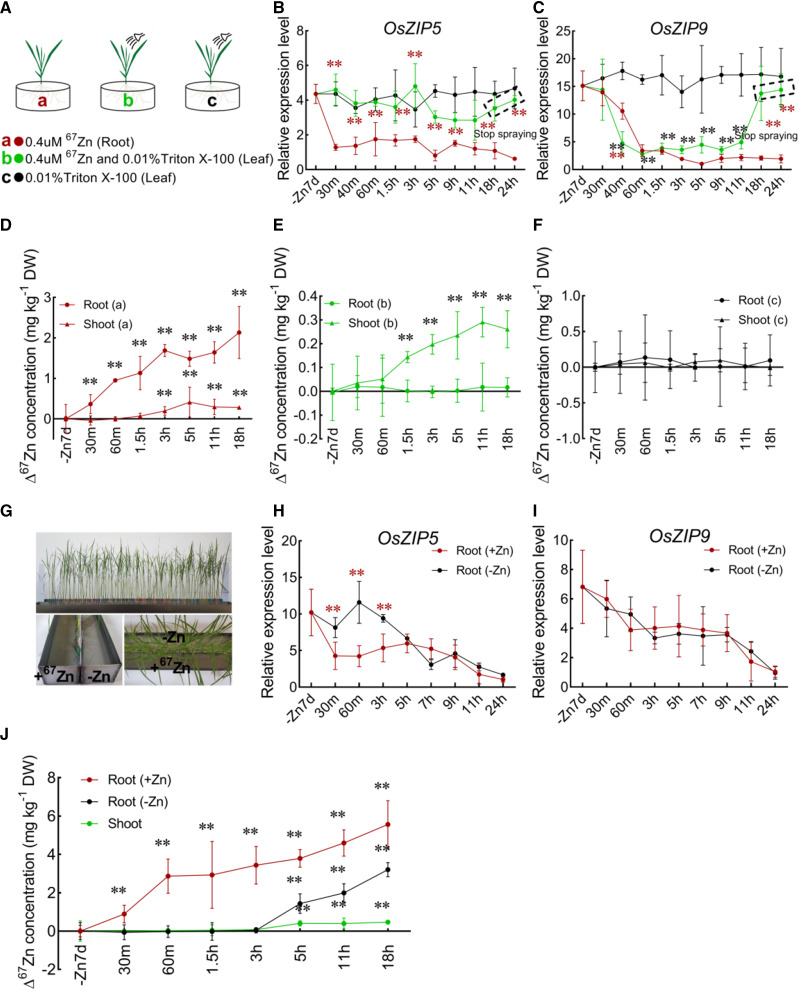
In plants, Zn homeostasis is regulated in a narrow concentration range from deficiency to excess, and both local Zn status and systemic long-distance signals are involved in this process (Sinclair et al., 2018). To investigate whether OsZIP5 and OsZIP9 respond in roots to local signals from roots and to systemic signals from shoots, we performed a Zn foliar-spray experiment using hydroponically grown plants (Fig. 3, A–F). Wild-type plants (4-week-old seedlings cultivated under normal hydroponic conditions) grown in Zn-deficient hydroponic solution for 7 d were supplied with a solution containing 0.4 μm 67ZnSO4 as either a foliar spray or in the hydroculture solution (Fig. 3A). The expression of both OsZIP5 and OsZIP9 in roots decreased rapidly after Zn was supplied in the culture solution (Fig. 3, B and C). However, only OsZIP9 responded to Zn supplied via foliar spray. Expression of OsZIP9 decreased within 40 min to a basal level after application of the foliar Zn spray, but high levels of OsZIP9-specific mRNA were detected at 18 and 24 h after Zn spraying ceased (Fig. 3C). The Δ67Zn concentration in roots and shoots was also examined for −Zn nutrient status after Zn supplementation (Fig. 3, D–F). The Δ67Zn accumulation in roots increased significantly after transfer to the solution containing 0.4 μm 67ZnSO4, while the Δ67Zn accumulation in shoots increased only slightly over the same time period (Fig. 3D). However, for the foliar spray application, Δ67Zn accumulation in the shoots increased, while accumulation in the roots was unchanged (Fig. 3E). This result indicates that the reduction in OsZIP9 expression in the roots treated with Zn foliar spray resulted from Zn-induced systemic signaling, not from an increase in Zn nutrient levels due to translocation of Zn from the leaves to the roots.
Figure 3.
Relative mRNA abundance of OsZIP5- and OsZIP9-specific mRNA in roots responds to shoot Zn status in foliar-spray and split-root experiments. A, Schematic describing treatments a to c; the color scheme is used throughout the figure. B and C, OsZIP5 and OsZIP9 mRNA levels in roots of wild-type plants after foliar spray with Zn solution. D to F, Concentrations of Δ67Zn in roots and shoots for treatments a to c (D–F, respectively). Twenty-eight-day-old seedlings were cultivated in Zn-deficient hydroponic solutions for 7 d, followed by Zn resupply to the leaves as a foliar spray for 11 h. The spray treatments were then stopped for 18 to 24 h. G to I, Real-time PCR analysis of OsZIP5 and OsZIP9 expression in the split-root experiments. Seedlings were grown in a split-compartment culture system (G) in the presence (+67Zn) and absence (−Zn) of Zn. Twenty-eight-day-old plants were transferred to 0.5× KB with no Zn for 7 d to induce Zn deficiency. After 7 d, plants were transferred to split containers in which one-half of the root system was exposed to +67Zn 0.5× KB and the other half was exposed to −67Zn 0.5× KB solution. Roots were collected at different times after transfer to the split containers and relative expression levels of OsZIP5 and OsZIP9 were measured (H and I). J, Concentrations of Δ67Zn in split roots and shoots at different times. Data were compared with those from treatment with 0.4 μm 67ZnSO4 in roots or 0.01% (v/v) Triton X-100 in leaves (B, C, H, and I). All data for Δ67Zn quantification were calculated from the net increase in 67Zn (i.e. the total 67Zn amount minus the natural abundance of 67Zn at different times) and were compared with those for –Zn7d in D to F and J. Values represent means ± sd of biological replicates (n ≥ 3). Statistical comparison was performed by Tukey’s HSD mean-separation test (**P < 0.01). DW, Dry weight.
To further confirm the difference between OsZIP5 and OsZIP9 expression in response to local and long-distance signaling, we performed a hydroponic Zn split-root experiment, in which plants were subjected to a period of Zn starvation and were then moved to the split container (Fig. 3G). Four-week-old plants were cultured in half strength Kimura B (0.5× KB) nutrient solution with no Zn for 7 d and were then transferred to a split container in which one-half of the root system was exposed to +67Zn 0.5× KB solution and the other half to 0.5× KB solution without Zn supplementation (Fig. 3G). It was observed that OsZIP5 can only respond transcriptionally to Zn as a signal that moves from the shoot to the root after initial uptake by the other half of the root, while OsZIP9 can sense sufficiency earlier, and this earlier signal is most likely not Zn but Zn-induced systemic signals (Fig. 3, H–J). The expression of OsZIP5 in the −67Zn side was not suppressed until 3 h after it had been transferred to the split container, and a similar expression level was only detected in both sides 5 h after transfer to the split system, when the Zn absorbed by the roots cultured in the +67Zn side had gradually been transferred to the roots on the −67Zn side (Fig. 3, H and J). In contrast, the expression of OsZIP9 in the −67Zn side decreased quickly, synchronized with that in the +67Zn side (Fig. 3I). Taken together, these results confirm that OsZIP5 is only under the control of local Zn status, and that OsZIP9 is regulated by both local Zn status and systemic long-distance signals.
OsZIP5 and OsZIP9 Function as Influx Transporters for Zn and Cd
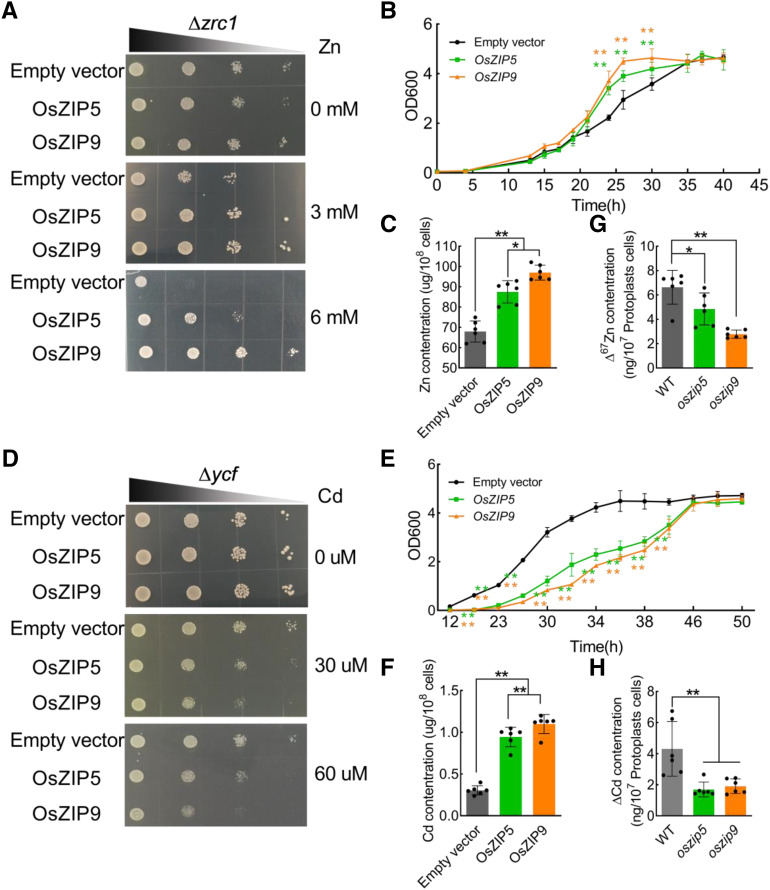
OsZIP5 and OsZIP9 encode putative metal cation transporters (http://octopus.cbr.su.se/). The metal transport ability of these two proteins was first assayed in yeast stains, ∆zrc1 and ∆ycf-135c, which were defective in vacuolar sequestration of Zn and Cd, respectively (Fig. 4). The coding sequences of OsZIP5 and OsZIP9 inserted into the yeast expression vector pYES2-NC were used to transform the two yeast strains, which were grown in nitrogen base medium containing various concentrations of Zn or Cd (Fig. 4, A and D). In the absence of Zn or Cd, the OsZIP5- or OsZIP9-expressing lines grew similarly to the empty vector controls in both the ∆zrc1 and ∆ycf-135c backgrounds (Fig. 4, A and D). However, when the yeast cells were exposed to 3 mm or 6 mm Zn, the lines expressing OsZIP5 and OsZIP9 grew dramatically better than the controls (Fig. 4A). In medium containing 3 mm Zn, quantitative growth analysis clearly showed that the cells expressing OsZIP5 and OsZIP9 grew at a faster rate than cells containing the empty vector, pYES2-NC, and the cells expressing the ZIP transporters accumulated more Zn than did the corresponding controls (Fig. 4, B and C). In medium containing 30 μm and 60 μm Cd, the yeast cells expressing OsZIP5 and OsZIP9 grew slower than the control, and quantitative growth analysis clearly showed that the cells expressing OsZIP5 and OsZIP9 grew at a slower rate than the controls, and yeast cells expressing the ZIP transporters accumulated more Cd than did the controls in the presence of 30 μm Cd (Fig. 4, D–F). As an additional control, expression of OsZIP5 and OsZIP9 did not complement the growth defects of yeast strains Δfet3fet4 and ∆smf1, nor did it increase their concentrations of Fe and Mn (Supplemental Fig. S6).
Figure 4.
Evaluation of the Zn and Cd transport activity of the OsZIP5 and OsZIP9 proteins in yeast mutants and rice protoplasts. A and D, pYES2-OsZIP5, pYES2-OsZIP9, and the empty vector pYES2 were separately introduced into the Zn-sensitive strain Δzrc1 (A) and the Cd-sensitive strain Δycf-135c (D). Yeast strains were cultured on SC-Ura plates containing different concentrations of Zn (A) and Cd (D) at 28°C for 2 to 4 d. B and E, Growth rates of yeast cells. Yeast mutant strains transformed with the empty vector pYES2, pYES2-OsZIP5, or pYES2-OsZIP9 in 3 mm Zn (B) or 30 μm Cd (E) in SC-Ura medium. OD600 values of pYES2-OsZIP5 and pYES2-OsZIP9 strains were significantly different compared with the empty vector strain. C and F, Zn (C) and Cd (F) levels in yeast cells. Yeast mutant strains transformed with the empty vector pYES2, pYES2-OsZIP5, or pYES2-OsZIP9 were treated with 3 mm Zn or 30 μm Cd for 40 to 50 h. G and H, Zn (G) and Cd (H) uptake in rice protoplasts. Rice protoplasts were cultured in W5 buffer with67Zn (G) and Cd (H) for 18 h. Data for ∆67Zn and ∆Cd quantification were calculated from the net increases in 67Zn and Cd (i.e. the total 67Zn amount minus the natural abundance of 67Zn). Values represent means ± sd of biological replicates (n ≥ 3). Statistical comparison was performed by Tukey’s HSD mean-separation test (*P < 0.05 and **P < 0.01). WT, Wild type.
We also used 67Zn and Cd as tracers to directly measure the transport activity of Zn and Cd in rice protoplasts. We found that protoplasts prepared from yong of oszip5 and oszip9 mutant plants had significantly lower Zn and Cd concentrations than did protoplasts from wild-type plants (Fig. 4, G and H). Taken together, these results indicate that OsZIP5 and OsZIP9 function as influx transporters for Zn and Cd.
The OsZIP5 and OsZIP9 Mutants Are Impaired in Zn/Cd Uptake
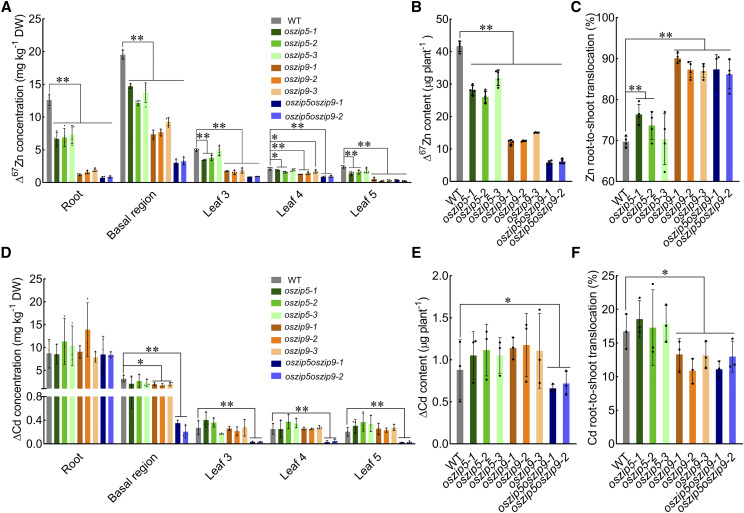
To further functionally characterize the roles OsZIP5 and OsZIP9 in planta, we first performed a short-term (24 h) assay with stable isotope 67Zn to detect their roles in Zn uptake and allocation (Fig. 5). Knocking out OsZIP5 and/or OsZIP9 resulted in a much lower Δ67Zn accumulation in the roots, basal regions, and both the newly expanded leaf (the fifth leaf) and mature leaves (third and fourth leaves), and the reduction was even more severe in the oszip5oszip9 double mutant (Fig. 5A). Cumulatively, the total amount of Δ67Zn accumulated in plants was much lower in the oszip5, oszip9, and oszip5oszip9 plants compared with wild-type plants (Fig. 5B). The root-to-shoot translocation of Δ67Zn was much higher in the oszip5, oszip9, and oszip5oszip9 plants than in the wild type (Fig. 5C). As a control, strontium (Sr) and rubidium (Rb), markers for xylem and phloem transport, respectively (Kuppelwieser and Feller, 1991), did not show any difference in distribution between the knockout lines and the wild-type plants (Supplemental Fig. S7). These results suggested that the uptake of Zn is impaired and the xylem loading of Zn in the roots and distribution in the nodes is not disturbed in these mutants.
Figure 5.
Uptake and allocation of Zn and Cd in different rice organs at the vegetative growth stage. Short-term experiments were conducted with the stable isotopes 67Zn and Cd at the vegetative growth stage. A and D, Distribution of ∆67Zn (A) and Cd (D) in the different organs. B and E, Total content of Δ67Zn (B) and ∆Cd (E) per plant. C and F, 67Zn and Cd root-to-shoot translocation rate. Seedlings of the wild type (WT) and knockout lines were grown in a nutrient solution containing 0.4 μm Zn for 28 d. The seedlings were then transferred to a nutrient solution containing 0.4 μm 67ZnSO4 or 5 μm CdSO4 for 24 h. Roots, basal regions, and individual leaves (three to five older to younger leaves) were harvested separately and subjected to determination of 67Zn and Cd levels by ICP-MS. All data were based on comparisons with wild-type rice plants. Values represent the means ± sd of n ≥ 3 biological replicates. Statistical comparison was performed by Tukey’s HSD mean-separation test (*P < 0.05 and **P < 0.01). DW, Dry weight.
We then tested changes in Zn accumulation in the knockout mutants under hydroponic and field conditions. The OsZIP5 knockout plants showed no significant changes in Zn uptake and accumulation under normal hydroponic conditions (Supplemental Fig. S8A). However, under conditions of Zn deficiency, a decrease in Zn levels was detected in basal regions and shoots of oszip5 plants relative to the wild type (Supplemental Fig. S8B). In contrast, in plants supplied with 0.4 μm Zn, a dramatic decrease in Zn levels was detected in roots, basal regions, and shoots, as well as in the total amount of Zn in oszip9 compared to the wild type (Supplemental Fig. S8, A and C). And the root-to-shoot translocation of Zn was higher in the oszip9 lines than in wild-type plants (Supplemental Fig. S8D). The decrease in Zn accumulation was even more severe in the oszip5oszip9 double mutant (Supplemental Fig. S8C). Consistently, we found that the Zn concentration in the xylem sap was much lower in the oszip9 (but not oszip5) plants compared to the wild type (Supplemental Fig. S8E).
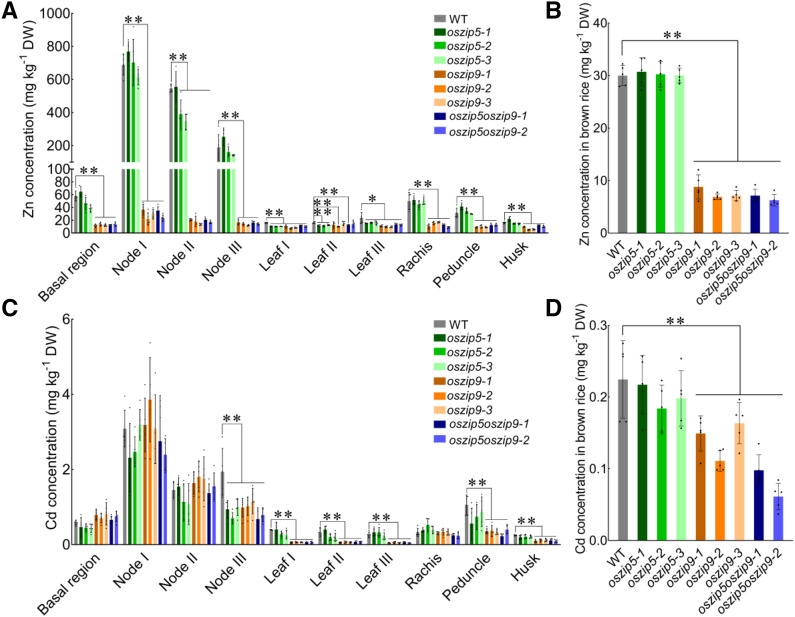
Under field conditions, there were no significant differences in Zn accumulation between OsZIP5 knockout plants and the wild type at harvest (Fig. 6, A and B). However, the concentration of Zn was significantly decreased in the oszip9 lines in all organs, including the basal regions, nodes I to III, leaves I to III, the rachis, peduncle, husks, and brown rice (Fig. 6, A and B). Zn levels in brown rice decreased by ∼60% in the oszip9 plants compared with wild-type rice (Fig. 6B). Also, the oszip5oszip9 double mutant plants were more severely affected in Zn deficiency compared with the oszip9 plants (Fig. 1D). Only a small number of plants were able to survive long enough to produce a few seeds though there was no further decrease of Zn levels in the survived plants (Fig. 6, A and B).
Figure 6.
Accumulation of Zn and Cd in different rice organs at the grain filling stage. Plants of the wild type (WT) and all mutant lines were grown in a paddy field until the grain was ripe. A and C, Concentrations of Zn (A) and Cd (C) in the different tissues. B and D, Zn (B) and Cd (D) concentrations in brown rice. Plants of wild-type and all knockout lines were grown in a paddy field containing 1.80 mg Cd kg−1 and 121.90 mg Zn kg−1 (pH 5.4). All data were based on comparisons with wild-type rice plants. Values represent means ± sd of n = 5 biological replicates. Statistical comparison was performed by Tukey’s HSD mean-separation test (*P < 0.05 and **P < 0.01). DW, Dry weight.
Changes in Cd accumulation were also observed, although the changes were not as dramatic as they were for Zn in short-term or long-term hydroponic culture (Fig. 5, D–F; Supplemental Fig. S9). This is consistent with the finding that NATURAL RESISTANCE-ASSOCIATED MACROPHAGE PROTEIN5 (OsNRAMP5), but not other transporters, is the main entry point for Cd in rice (Sasaki et al., 2012). However, the oszip5oszip9 double mutant plants exhibited lower total Cd levels. Cd levels in the basal regions and xylem sap were much lower in oszip9 and oszip5oszip9 lines than in wild-type plants (Fig. 5D; Supplemental Fig. S9, A and D). The decrease in Cd levels in the above-ground organs is similar to that of Zn under field conditions (Fig. 6, C and D). The oszip9 and oszip5oszip9 plants had lower Cd concentrations in node III, leaves I to III, the peduncle, husks, and brown rice compared with the wild type (Fig. 6, C and D). In brown rice, Cd decreased by 30% to 70% in the oszip9 and oszip5oszip9 mutant plants compared with the wild type (Fig. 6D). No differences were detected in the concentrations of Fe, Mn, and Cu between the wild type and the knockout lines (Supplemental Figs. S10 and S11). Taken together, these results show that OsZIP5 and OsZIP9 play important roles in Zn/Cd uptake in rice.
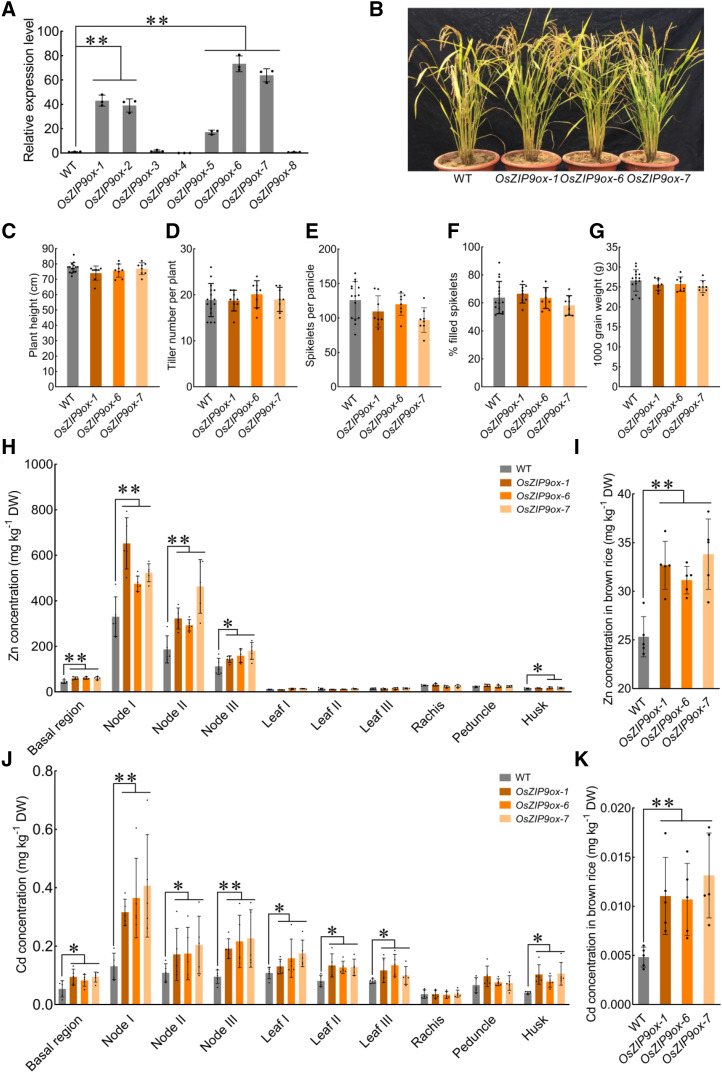
Overexpression of OsZIP9 Enhances Zn/Cd Uptake and Accumulation in Rice
A previous study reported that overexpression of OsZIP5 improved Zn uptake from the soil and altered the Zn distribution pattern so that there was more accumulation in roots, but less in shoots and seeds (Lee et al., 2010a). We generated OsZIP9 overexpression plants and determined the changes in their ionomes. Three OsZIP9ox lines (OsZIP9ox-1, OsZIP9ox-6, and OsZIP9ox-7) were obtained in which the transcript levels were maximally increased by 40- to 70-fold (Fig. 7, A and B). Under field conditions, there were no developmental differences observed between the OsZIP9ox plants and the wild type. Similar plant heights, tiller numbers, spikelet numbers per panicle, seed-setting rates, and 1,000-grain weights were recorded (Fig. 7, C–G). However, unlike the decreased shoot levels of Zn in the OsZIP5ox plants, the OsZIP9ox plants accumulated significantly higher concentrations of Zn in the basal regions, nodes I to III, and the husks compared with wild-type plants (Fig. 7H). In addition, overexpression of OsZIP9 markedly increased the concentration of Zn in brown rice, by 25% to 35%, compared with the wild type (Fig. 7I). The OsZIP9ox plants also accumulated significantly higher concentrations of Cd in nodes I to III, leaves I to III, the husks, and brown rice compared with wild-type plants (Fig. 7, J and K). Hydroponic culture experiments confirmed that overexpression of OsZIP9 increased the Zn/Cd uptake and accumulation (Supplemental Fig. S12).
Figure 7.
Effect of OsZIP9 overexpression on grain yield in rice under field conditions. A, Identification of three transgenic OsZIP9ox lines (OsZIP9ox-1, OsZIP9ox-6, and OsZIP9ox-7) in which the OsZIP9 specific mRNA levels were increased between 40- and 70-fold. B, Phenotypes of wild-type (WT) and OsZIP9ox plants at the grain filling stage. C to G, Plant height (C), tiller number (D), spikelets per panicle (E), percent filled spikelets (F), and 1000-grain weight (G) for the wild type and the OsZIP9ox-1, OsZIP9ox-6, and OsZIP9ox-7 lines. H and J, Concentrations of Zn (H) and Cd (J) in different tissues. I and K, Zn (I) and Cd (K) concentrations in brown rice. Values represent means ± sd of biological replicates (n ≥ 3). Statistical comparison was performed by Tukey’s HSD mean-separation test (*P < 0.05 and **P < 0.01). DW, Dry weight.
DISCUSSION
Ancient gene duplication events and the retention of extant gene pairs have contributed to the evolution of adaption in plants. Duplicate retention might occur via selection for genetic redundancy with divergent expression patterns (Panchy et al., 2016). In this article, we described two duplicated genes, OsZIP5 and OsZIP9, that function redundantly in Zn uptake but have diverged with respect to expression regulation. These two genes are closely linked in terms of physical distance on rice chromosome 5. Amino acid sequence alignment showed a high level of sequence identity between the predicted proteins (Fig. 1, B and C). While Zn deficiency and the consequent growth retardation were only detected in oszip5 plants under Zn-deficient conditions, they were detected in oszip9 and enhanced in the oszip5oszip9 double mutant under normal conditions (Fig. 5; Supplemental Fig. S8). This observation indicated that OsZIP9 plays a major role in the control of Zn homeostasis, and OsZIP5 is redundant to, but weaker than, OsZIP9. Both of the proteins are expressed in root epidermal cells, and the decrease in total Zn accumulation, yeast functional complementation, and rice protoplast assays indicated they function in Zn uptake (Figs. 2, 4, and 5). OsZIP5 signals also accumulated in the root stele; and both OsZIP5 and OsZIP9 are also expressed in parenchyma cells in the node. However, the increase in root-to-shoot translocation of Zn suggested that OsZIP5 and OsZIP9 function redundantly with other transporters such as OsZIP3, OsZIP7, and OsHMA2, and are not indispensable in root Zn xylem loading and intervascular delivery in the node (Fig. 5C; Supplemental Fig. S8D). The increased ratios may result from the induced expression of other transporter genes such as OsZIP3, OsZIP4, and OsZIP7 in response to Zn deficiency caused by mutations in OsZIP5 and OsZIP9 (Ramesh et al., 2003; Cai et al., 2019a; Tan et al., 2019). OsZIP5 and OsZIP9 have diverged in their transcriptional response to Zn deficiency. While both respond quickly to the local Zn status in roots, OsZIP9, but not OsZIP5, can sense the Zn-induced systemic signal in the shoot (Fig. 3). In addition, OsZIP9 is more abundant than OsZIP5 (Supplemental Fig. S3G). Therefore, the following scenario has evolved: OsZIP5 and OsZIP9 function synergistically in Zn uptake and homeostasis control. OsZIP9 monitors the coarse tuning of the Zn status in both roots and shoots, while OsZIP5 acts to fine tune Zn uptake to maintain Zn homeostasis within a narrow concentration range (Supplemental Fig. S13).
It is of great importance to improve both yield and microelemental nutrition in rice. Our results showed that OsZIP5 and OsZIP9 are required to maintain both grain yield and Zn level in brown rice. Knockout of either of the two transporters resulted in significant yield loss and grain-Zn reduction under our field condition (Fig. 6; Supplemental Fig. S2). We noted that Lee et al. (2010a) analyzed two T-DNA insertion alleles of OsZIP5 and found no phenotypic changes. This is consistent with the performance of oszip5 under normal culture conditions in our study (Supplemental Fig. S2, F and G). However, slight phenotypic changes were detected for oszip5 under our field condition (Fig. 1D; Supplemental Fig. S2). This could be explained by our field experiments, which were carried out in Cd-polluted soil, in which Cd may compete with Zn, and which resulted in Zn deficiency. Therefore, functional OsZIP5 and OsZIP9 are fundamental to maintain rice production and Zn nutrition, especially under Zn-deficient condition. Overexpression of OsZIP5 also resulted in yield loss and reduced Zn accumulation in brown rice (Lee et al., 2010a). Such a phenotypic change in OsZIP5 overexpression plants may be due to the disruption of Zn distribution between root and shoot (Lee et al., 2010a). Fortunately, we observed that overexpression of OsZIP9 increased Zn level in brown rice without a yield penalty (Fig. 7), although it is not yet clear whether Zn is increased in polished grain. This indicated that OsZIP9 is a potential transporter gene for Zn biofortication in rice to improve its nutritional value.
Cd accumulation in rice grains is a major concern for food safety because this heavy metal is toxic to humans. Therefore, identifying the genes responsible for Cd transport and partitioning is a prerequisite for breeding Cd-safe rice. We found that OsZIP5 and OsZIP9 play similar (but not the same) roles in Zn versus Cd accumulation. The decrease in Cd levels phenocopies that of Zn in either the OsZIP5 or OsZIP9 mutant compared with wild-type plants under field conditions (Fig. 6). However, the decrease in Cd accumulation is only observed in double mutants, hardly at all in single ones, in hydroponic culture (Fig. 5; Supplemental Fig. S9). This can be explained by the fact that OsNRAMP5, but not other transporters, is the main entry point for Cd in rice (Sasaki et al., 2012). A number of genes involved in Cd accumulation in rice grains have been cloned and characterized, including OsNRAMP1, OsNRAMP5, OsHMA2, OsHMA3, OsLCT1, OsZIP1, OsZIP7, OsIRT1, and OsIRT2 (Nakanishi et al., 2006; Lee and An, 2009; Takahashi et al., 2011, 2012; Uraguchi et al., 2011; Sasaki et al., 2012, 2014; Tang et al., 2017; Liu et al., 2019; Tan et al., 2019). These transporters are facultative Cd transporters that also have affinities for essential microelements. Therefore, Cd hijacks the essential microelement pathway to accumulate in the grain. Mutation of these genes can significantly reduce the Cd content in rice, but it can also reduce the levels of some essential metals, which hinders plant growth. To lower the risk of Cd toxicity, application of microelement fertilizer could decrease Cd levels in rice via molecular competition of the fertilizer with Cd for the OsZIP5 and OsZIP9 transporters, as well as the OsZIP1, OsZIP3, OsZIP7, and OsHMA2 transporter proteins. In addition, certain amino acid residues are important for substrate selection and transport in members of the ZIP protein family. Changing specific residues may increase the substrate specificity of a transporter for a particular element while selectively reducing or eliminating its affinity for other elements (Rogers et al., 2000). Therefore, it is tempting to investigate the structure and function of OsZIP proteins, and to edit the gene sequences to increase their affinity for microelements to improve micronutrition while decreasing Cd levels in rice grains to improve rice quality and safety.
The existence of local and long-distance signaling in plants allows their adaptation to different growth environments (Maas et al., 1988; Vert et al., 2003; Enomoto et al., 2007; Assuncão et al., 2010; García et al., 2013; Kumar et al., 2017; Sinclair et al., 2018). In local regulation, gene expression in roots is important because it allows rapid responses to Zn deficiency in the environment and prevents Zn toxicity in roots under conditions of excess Zn. In systemic regulation, adequate Zn levels in the shoot affects Zn deficiency responses in the roots (Kumar et al., 2017; Sinclair et al., 2018). In Arabidopsis, the dynamics of ZIP4 and ZIP9 gene expression depend on the local root environment, while the MTP2 mRNA abundance is regulated via long-distance signaling (Sinclair et al., 2018). In this study, we have shown that OsZIP5 and OsZIP9 cooperate in the dynamic environment under the control of Zn-related local and systemic long-distance signaling pathways (Fig. 3). In conclusion, our results provide a perspective for further studies on the molecular mechanisms underlying this adaption in rice. It will be fascinating to identify the exact molecular(s) signal and to elucidate the pathway for transducing the Zn signal from shoot to root.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Rice (Oryza sativa ssp. japonica ‘Nipponbare’) was used as the wild type in this study and for the development of all transgenic plant lines. Hydroponic experiments were performed in a standard rice nutrient solution 0.5× KB solution containing 0.4 μm ZnSO4 (pH 5.6; Yamaji et al., 2013), in the glasshouse at 25°C to 30°C. To investigate whether the expression of OsZIP5 and OsZIP9 is affected by metal deficiency, wild-type plants (28 d old) were cultivated in 0.5× KB solution with or without Zn, Fe, Mn, Cu, or Cd. After 7 d, roots, basal regions (0–0.5 cm from the root-shoot junction, containing basal nodes; Sasaki et al., 2015), and shoots were collected and subjected to RNA extraction. For phenotyping and expression of OsZIP5 and OsZIP9 under different Zn or Cd concentrations, seedlings were grown for 7 or 28 d in 0.5× KB solution containing four concentrations of Zn (0, 0.4, 4, or 40 μm ZnSO4) or Cd (0, 5, 20, or 40 μm CdSO4). The nutrient solution was replaced every 2 d. All hydroponic experiments were performed with at least three biological replicates. Field experiments were established in the experimental paddy fields at the Institute of Subtropical Agriculture (ISA) of the Chinese Academy of Sciences in Changsha, China, from 2017 to 2019. Standard agronomical management practices for rice were followed. The soil Cd and Zn concentration were 1.80 mg Cd kg−1 and 121.90 mg Zn kg−1, respectively, and soil pH was 5.4.
Short-Term Experiment with Stable Isotope Zn and Cd
We analyzed the distribution patterns of 67Zn and Cd in 28-d-old seedlings of the knockout lines and wild-type plants, which were grown in 0.5× KB solution containing 0.4 μm 67ZnSO4 (89.60% enrichment; Isoflex), 5 μm CdSO4, and 1 μm each of SrCl2 and RbCl. After 24 h, roots, basal regions and leaves were sampled separately for mineral determination. The roots were washed three times with 5 mm CaCl2 before harvest. ∆67Zn and ∆Cd quantification was calculated as described previously (Yamaji et al., 2013). The translocation rate (%) of Δ67Zn and ∆Cd was calculated by dividing its content in the shoot by its total content in the whole plant, which was the sum of Δ67Zn or ∆Cd contents in all tissues.
Foliar Spray and Split-Root Experiments
In the foliar spray experiment, we administered Zn resupply exclusively to leaves (by spray application). Hydroponically cultivated wild-type plants (4 weeks old) grown in Zn-deficient hydroponic solution for 7 d were sprayed with a solution containing 0.4 μm 67ZnSO4 and 0.01% (v/v) Triton X-100 or a control solution without added Zn. Roots in the solution containing 0.4 μm 67ZnSO4 were evaluated as the control. Total RNA was extracted from roots after foliar spraying at time points from 30 min to 24 h.
In the split-root experiment, 4-week-old plants were transferred to 0.5× KB with no Zn added for 7 d to induce Zn deficiency. After 7 d, plants were transferred to split containers in which one-half of the root system was exposed to (+)67Zn 0.5× KB solution and the other half was exposed to −67Zn 0.5× KB solution. Roots were collected at different times after placement in the split containers.
Expression Pattern of OsZIP5 and OsZIP9
Total RNA was isolated from the different rice tissues sampled at different growth stages from plants grown in a paddy field and under hydroponic conditions. Complementary DNA (cDNA) synthesis and real-time PCR were conducted as described previously (Tan et al., 2019). The absolute quantification of OsZIP5 and OsZIP9 were calculated as described previously (Sui et al., 2018). OsUBC was used as the internal gene expression control using primers 5′-CCGTTTGTAGAGCCATAATTGCA-3′ and 5′ -AGGTTGCCTGAGTCACAGTTAAGTG-3′ (Fang et al., 2015). The following primer pairs were used to amplify OsZIP5 5′-CATGAAGACCAAGGTGCAGAGAAGG-3′ and 5′ - TCACGCCCAGATGGCGATCA-3′, and OsZIP9 5′-ATCTTCTTCTCGCTAACCACAC-3′ and 5′-GCAGCCGCTGCGTCGAGAAT-3′ (Sasaki et al., 2015). Data were collected using an ABI 7900 real-time PCR instrument (Applied Biosystems).
Development of the oszip5 and oszip9 Knockout Lines
The CRISPR/Cas9 system was used to generate knockout lines for the OsZIP5 and OsZIP9 genes (Lu et al., 2017). For the single gene mutations, the pBGK032 vector containing one single-guide RNA (targeting either the OsZIP5 coding sequence (CDS), 5′-GAGAGGAACACGTCCGTCTC-3′, or the OsZIP9 CDS, 5′-GGCCAAGTCCCGGACGCTCA-3′) was introduced into the japonica cv Nipponbare using Agrobacterium tumefaciens strain EHA105. To identify the genotypes of the mutations, genomic DNA was extracted from T2-generation transgenic plants to amplify the target regions by PCR using OsZIP5 primers 5′-CAAGGTGTTCGTCCTGCTCT-3′ and 5′-CGACGGTGTCGACGATGAG-3′, and OsZIP9 primers 5′-GATGGAGGGTTGAGACTGCC-3′ and 5′-GCCTTGAGGGCGAAGAAGAG-3′. For the double gene mutation, the vector containing a double single-guide RNA (targeting the OsZIP5 CDS, 5′-GGTGGCGGCGTGCTACCTGC-3′, and the OsZIP9 CDS, 5′-GGCCAAGTCCCGGACGCTCA-3′) was introduced into cv Nipponbare. The primers used for detection of OsZIP9 were the same as described above. The OsZIP5 primers were 5′-GCAACGGATCGGAGACTGAA-3′ and 5′-TTGACGGAGAGGAACACGTC-3′. No potential off-target cleavage was detected using the web-based tool CRISPR-P (http://cbi.hzau.edu.cn/cgi-bin/CRISPR; Liu et al., 2017). Each gene-editing construct yielded >18 independent transgenic lines. Homozygous mutant lines were selected for further analysis. Mutant lines oszip5-1, oszip5-2, and oszip9-1 were Cas-9 free.
Histochemical Analysis of GUS Expression
For the GUS constructs, a 2,422-bp genomic DNA fragment located upstream of the OsZIP5 initiation codon and a 2,193-bp genomic fragment located upstream of the OsZIP9 initiation codon were amplified from cv Nipponbare genomic DNA using KOD plus DNA polymerase (Toyobo). The open reading frames of the two genes were amplified by PCR using the primer pairs 5′-gacctgcaggcatgcaagcttGCTCCCTCTCGATTATCTCTGTCG-3′ and 5′-ttaccctcagatctaccatggCGCCGATGGCTCACCTGC-3′ for OsZIP5, and 5′-gacctgcaggcatgcaagcttAATATGCAACTCAAAGTCAAATCCA-3′ and 5′-ttaccctcagatctaccatggCTTTTTAGCCTGTGGCCAAAGA-3′ for OsZIP9 (lowercase, underlined letters indicate the additional 21-base 5′ sequences containing the HindIII and NcoI restriction enzyme recognition sites). The amplified promoter fragments were digested with HindIII (5′-AAGCTT-3′) and NcoI (5′-CCATGG-3′) and cloned into the pCAMBIA1301-GUS vector using the ClonExpress II One Step Cloning Kit (www.vazyme.com). The sequences of all constructs were confirmed by DNA sequencing. The constructs were then transformed into cv Nipponbare using A. tumefaciens strain LBA4404 to obtain stable transgenic rice lines.
For GUS staining, all tissues were incubated in a GUS solution kit (www.huayueyang.com.cn) at 37°C overnight. Following staining, the sections were fixed in acetic acid and absolute ethanol (9:1 [v/v]), washed in 95% (v/v) ethanol five times to remove the chlorophyll, and then held in 70% (v/v) ethanol until observation. Sections of various plant tissues were observed using an Olympus BX51 microscope according to the manufacturer’s instructions.
Immunostaining with Anti-GUS Antibody
To detect the GUS protein in transgenic rice tissues, a rabbit anti-GUS polyclonal antibody (BBI) was used as the primary antibody for GUS immunostaining. DAPI was used to stain the cell wall components (Song et al., 2014). Roots and nodes of wild-type and OsZIP promoter-GUS transgenic rice plants were fixed with formaldehyde acetic acid and further studied by paraffin sectioning and immunostaining. Horseradish peroxidase-conjugated goat anti-rabbit IgG (BBI) was used as the secondary antibody at a dilution of 1:200 and the slides were observed via confocal laser-scanning microscopy using a Zeiss LSM700 instrument (Carl Zeiss).
Phylogenetic Analysis
The predicted ZIP protein sequences were retrieved from the Michigan State University Rice Genome Annotation Project Database (http://rice.plantbiology.msu.edu) and the National Center for Biotechnology Information (www.ncbi.nlm.nih.gov/). A phylogenetic tree was constructed with MEGA-X (Kumar et al., 2018) using maximum-likelihood phylogenetic analysis with 1,000 bootstrap replicates. Amino acid sequences of OsZIP5 and OsZIP9 were aligned with ClustalW using the default settings (http://clustalw.ddbj.nig.ac.jp/). Potential transmembrane domains of ZIP protein sequences were identified using TMHMM (Krogh et al., 2001).
Measurement of Metal Ion Concentrations in Xylem Sap
Seedlings of the wild type, three oszip5 lines, and three oszip9 lines were grown in 0.5× KB solution (pH 5.6) containing 0.4 μm Zn or 5 μm Cd for 28 d. Shoots were cut at a postion 4 cm above the roots with a razor. Xylem sap was collected as previously described by Sasaki et al. (2015).
Subcellular Localization of OsZIP5 and OsZIP9
We examined subcellular localization of the OsZIP5 and OsZIP9 proteins in rice protoplasts and Nicotiana benthamiana leaf epidermal cells. The full-length cDNAs of OsZIP5 and OsZIP9 without the stop codon were amplified using primers 5′-tcgagctcaagcttcgaattcATGGCGACGGCGGCGATG-3′ and 5′-gcagcggcagcagccggatccTCCGCCCAGATGGCGATC-3′ for OsZIP5, and 5′-tcgagctcaagcttcgaattcATGGCTTTCGATCTCAAGCTAACC-3′ and 5′-gcagcggcagcagccggatccTCAGCCCAAATACCAAGCAAG-3′ for OsZIP9. Each primer was designed with an additional 21-base sequence on the 5′ end (underlined lowercase letters) that contained restriction enzyme recognition sites. After sequence confirmation and digestion with EcoRI and BamHI, the amplified DNA fragments were cloned into the binary vector pEZR(K)-LN (Zeng et al., 2018), and the constructs were confirmed by Sanger sequencing. The plasmid vectors pEZR(K)-LN-OsZIP5-GFP and pEZR(K)-LN-OsZIP9-GFP containing the GFP gene fusion cassettes were transfected into rice protoplasts. We also performed coexpression experiments with the fluorescently labeled plasma membrane marker pSAT6-mCherry-AtPIP2A. Subcellular localization was further investigated by introducing the pEZR(K)-LN-OsZIP5 and pEZR(K)-LN-OsZIP9 binary vectors along with the HY5 vector (carrying a fluorescently labeled nuclear marker) into N. benthamiana leaves via agroinfiltration. The subcellular localization of the fusion proteins was determined by confocal laser-scanning microscopy using a LSM700 instrument (Zeiss) after incubation at 28°C for 18 to 24 h (Yoo et al., 2007).
Yeast Functional Complementation
The cDNA sequences encoding either the OsZIP5 or OsZIP9 protein were amplified by PCR using primer pairs 5′-ggatccagtgtggtggaattcATGGCGACGGCGGCGATG-3′ and 5′-accttcgaagggccctctagaTCACGCCCAGATGGCGAT-3′ for OsZIP5, and 5′-ggatccagtgtggtggaattcATGGCTTTCGATCTCAAGCTAACC-3′ and 5′-accttcgaagggccctctagaTCAAGCCCAAATACCAAGCAA-3′ for OsZIP9. The 21-base sequences added to the 5′ ends restriction enzyme recognition sites are underlined. The PCR products were cloned into the yeast expression vector pYES2-NC (digestion with EcoRI and XbaI) to generate pYES2-NC-OsZIP5 and pYES2-NC-OsZIP9. These constructs and the empty vector were transformed into the following yeast strains: the Zn-sensitive strain ∆zrc1 (BY4741; MATa his3∆1 leu2∆0 met15∆0 ura3∆0 YMR243c::kanMX4), the Cd-sensitive strain ∆ycf-135c (BY4741; Mata his3∆1 leu2∆0 met15∆0 ura3∆0 YDR135c::kanMX4), the Fe uptake-deficient double mutant ∆fet3fet4 (DEY1453; MATa ade6 can1 his3 leu2 trp1 ura3 fet3-2::HIS3fet4-LEU), and the Mn uptake-deficient mutant ∆smf1 (MATa his2∆0 met15∆0 ura3∆0 YOL122c::KanMX4). The yeast strains were cultured in SC-Ura medium with different metal concentrations as described in a previous report (Miyadate et al., 2011; Yamaji et al., 2013a; Tan et al., 2019; Wang et al., 2019). For measurement of Zn and Cd uptake, yeast transformants were cultured in liquid media supplemented with 3 mm Zn and 30 μm Cd. The OD600 values of the yeast cultures were collected using the NanoDrop One spectrophotometer (Thermo Scientific) at different time points. After 40 to 50 h of incubation with gentle shaking, yeast cells in the media supplemented with 3 mm Zn, 30 μm Cd, 6 μm bathophenanthrolinedisulphonic acid disodium salt hydrate, and 2 mm EGTA were harvested by centrifugation, washed three times with deionized water, and then digested with HNO3. All yeast culture experiments were conducted at least three times independently and gave the same results.
Generation of OsZIP9-Overexpressing Transgenic Plants
To generate the OsZIP9 overexpression lines, the full-length OsZIP9 cDNA sequence was amplified using the primer pairs 5′-tctagaggatccccgggtaccATGGCTTTCGATCTCAAGCTAACC-3′ and 5′-cgatcggggaaattcgagctcTCAAGCCCAAATACCAAGCAA-3′. The 21-base sequences added to the 5′ ends of the primers that contain restriction enzyme recognition sites are underlined. The amplified cDNA fragment was digested with KpnI and SacI and cloned into the pTCK303 vector. After the resulting transgenic seedlings were transplanted to soil, we extracted RNA and prepared cDNA to determine the OsZIP9-specific mRNA levels.
Metal Concentrations in Rice Protoplasts
For protoplast isolation, we used plants grown for 15 d in 0.5× Murashige and Skoog medium. Protoplasts were washed twice with W5 buffer (Wu et al., 2009), transferred to W5 solution containing 0.4 μm 67ZnSO4 and 5 μm CdSO4 and cultured for 18 h before centrifuging at 100g for 10 min to collect the protoplasts. The ∆67Zn and ∆Cd contents in the collected protoplasts were determined by inductively coupled plasma mass spectrometry (ICP-MS).
Elemental Analysis of Plant Tissues
Freshly harvested rice tissues including leaves, basal regions, and roots were immersed for 15 to 20 min in 20 mm disodium EDTA, and then washed three times with 5 mm CaCl2 and twice in ultrapure water. For rice grown in experimental paddy fields, the different organs from wild-type and knockout plants were measured at the grain ripening stage. Tissues were dried at 65°C for 3 to 5 d prior to the determination of metal concentrations. The dried samples were digested with HNO3-HClO4 (6:1 [v/v]) acid mixture as described previously (Yao et al., 2015). The metal concentrations in the digest solutions were determined by ICP-MS using an Agilent 7700 series instrument.
Statistical Analysis
Significant difference tests in this study were performed with Tukey’s honestly significant difference (HSD) mean-separationi test using SAS9.3 software (SAS). Two significance levels were applied, *P < 0.05 and **P < 0.01. Results were shown as the means ± sd of at least three replicated treatments, and each treatment contained 12 to 24 plants.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers Os05g39560 (OsZIP5); Os05g39540 (OsZIP9); and others (listed in Supplemental Table S1).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Double gene mutation of OsZIP5 and OsZIP9 using CRISPR/Cas9 genome editing.
Supplemental Figure S2. Effects of knocking out OsZIP5 and OsZIP9 at the vegetative growth stage and the grain filling stage in rice.
Supplemental Figure S3. Analysis of the expression levels of OsZIP5 and OsZIP9.
Supplemental Figure S4. Cell specificity of OsZIP5 and OsZIP9.
Supplemental Figure S5. Subcellular localization of OsZIP5 and OsZIP9 in N. benthamiana leaves.
Supplemental Figure S6. Evaluation of the Fe and Mn transport activity of OsZIP5 and OsZIP9 in yeast mutants
Supplemental Figure S7. Distribution of strontium and rubidium in a short-term experiment at the vegetative growth stage in rice.
Supplemental Figure S8. Analysis of Zn levels in the oszip5 and oszip9 knockout lines at the vegetative growth stage.
Supplemental Figure S9. Concentrations of Cd in the oszip5 and oszip9 gene knockout lines at the vegetative growth stage.
Supplemental Figure S10. Cu, Fe, and Mn concentrations in five different rice tissues at the vegetative growth stage.
Supplemental Figure S11. Cu, Fe, and Mn concentrations in different tissues of wild-type and all mutant lines at harvest under field conditions.
Supplemental Figure S12. Concentrations of Zn and Cd in the OsZIP9-overexpressing lines during vegetative growth.
Supplemental Figure S13. The working model of OsZIP5 and OsZIP9.
Supplemental Table S1. Gene names, accession numbers, and predicted lengths for the rice ZIP proteins described in this study.
Footnotes
This work was supported by the Chinese Academy of Sciences (grant no. XDA24010404) and the National Natural Science Foundation of China (grant no. 31470443).
References
- Assunção AGL, Herrero E, Lin YF, Huettel B, Talukdar S, Smaczniak C, Immink RGH, van Eldik M, Fiers M, Schat H, et al. (2010) Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proc Natl Acad Sci USA 107: 10296–10301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir K, Seki M, Nishizawa NK(2019) The transport of essential micronutrients in rice. Mol Breed 39: 168 [Google Scholar]
- Broadley M, Brown P, Cakmak I, Rengel Z, Zhao F (2012) Function of Nutrients: Micronutrients. In Marschner P, ed, Marschner’s Mineral Nutrition of Higher Plants, 3rd ed, Chapter 7. Academic Press, San Diego, pp 191–248 [Google Scholar]
- Cai H, Huang S, Che J, Yamaji N, Ma JF(2019a) The tonoplast-localized transporter OsHMA3 plays an important role in maintaining Zn homeostasis in rice. J Exp Bot 70: 2717–2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Xu W, Wang M, Chen W, Li X, Li Y, Cai Y(2019b) Mechanisms and uncertainties of Zn supply on regulating rice Cd uptake. Environ Pollut 253: 959–965 [DOI] [PubMed] [Google Scholar]
- Chaney RL, Angle JS, McIntosh MS, Reeves RD, Li Y-M, Brewer EP, Chen K-Y, Roseberg RJ, Perner H, Synkowski EC, et al. (2005) Using hyperaccumulator plants to phytoextract soil Ni and Cd. Z Naturforsch 60: 190–198 [PubMed] [Google Scholar]
- Coleman JE.(1998) Zinc enzymes. Curr Opin Chem Biol 2: 222–234 [DOI] [PubMed] [Google Scholar]
- Enomoto Y, Hodoshima H, Shimada H, Shoji K, Yoshihara T, Goto F(2007) Long-distance signals positively regulate the expression of iron uptake genes in tobacco roots. Planta 227: 81–89 [DOI] [PubMed] [Google Scholar]
- Fang P, Lu R, Sun F, Lan Y, Shen W, Du L, Zhou Y, Zhou T(2015) Assessment of reference gene stability in Rice stripe virus and Rice black streaked dwarf virus infection rice by quantitative Real-time PCR. Virol J 12: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García MJ, Romera FJ, Stacey MG, Stacey G, Villar E, Alcántara E, Pérez-Vicente R(2013) Shoot to root communication is necessary to control the expression of iron-acquisition genes in Strategy I plants. Planta 237: 65–75 [DOI] [PubMed] [Google Scholar]
- Grusak MA, DellaPenna D(1999) Improving the nutrient composition of plants to enhance human nutrition and health. Annu Rev Plant Physiol Plant Mol Biol 50: 133–161 [DOI] [PubMed] [Google Scholar]
- Guerinot ML.(2000) The ZIP family of metal transporters. Biochim Biophys Acta 1465: 190–198 [DOI] [PubMed] [Google Scholar]
- Hall JL.(2002) Cellular mechanisms for heavy metal detoxification and tolerance. J Exp Bot 53: 1–11 [PubMed] [Google Scholar]
- Ishimaru Y, Masuda H, Suzuki M, Bashir K, Takahashi M, Nakanishi H, Mori S, Nishizawa NK(2007) Overexpression of the OsZIP4 zinc transporter confers disarrangement of zinc distribution in rice plants. J Exp Bot 58: 2909–2915 [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Suzuki M, Kobayashi T, Takahashi M, Nakanishi H, Mori S, Nishizawa NK(2005) OsZIP4, a novel zinc-regulated zinc transporter in rice. J Exp Bot 56: 3207–3214 [DOI] [PubMed] [Google Scholar]
- Kawachi M, Kobae Y, Mori H, Tomioka R, Lee Y, Maeshima M(2009) A mutant strain Arabidopsis thaliana that lacks vacuolar membrane zinc transporter MTP1 revealed the latent tolerance to excessive zinc. Plant Cell Physiol 50: 1156–1170 [DOI] [PubMed] [Google Scholar]
- Krogh A, Larsson B, von Heijne G, Sonnhammer ELL(2001) Predicting transmembrane protein topology with a hidden markov model: Application to complete genomes. J Mol Biol 305: 567–580 [DOI] [PubMed] [Google Scholar]
- Kumar RK, Chu H-H, Abundis C, Vasques K, Rodriguez DC, Chia JC, Huang R, Vatamaniuk OK, Walker EL(2017) Iron-nicotianamine transporters are required for proper long distance iron signaling. Plant Physiol 175: 1254–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K(2018) MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35: 1547–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppelwieser H, Feller U(1991) Transport of Rb and Sr to the ear in mature, excised shoots of wheat: Effects of temperature and stem length on Rb removal from the xylem. Plant Soil 132: 281 [Google Scholar]
- Lee S, An G(2009) Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant Cell Environ 32: 408–416 [DOI] [PubMed] [Google Scholar]
- Lee S, Jeong HJ, Kim SA, Lee J, Guerinot ML, An G(2010a) OsZIP5 is a plasma membrane zinc transporter in rice. Plant Mol Biol 73: 507–517 [DOI] [PubMed] [Google Scholar]
- Lee S, Kim SA, Lee J, Guerinot ML, An G(2010) Zinc deficiency-inducible OsZIP8 encodes a plasma membrane-localized zinc transporter in rice. Mol Cells 29: 551–558 [DOI] [PubMed] [Google Scholar]
- Liu XS, Feng SJ, Zhang BQ, Wang MQ, Cao HW, Rono JK, Chen X, Yang ZM(2019) OsZIP1 functions as a metal efflux transporter limiting excess zinc, copper and cadmium accumulation in rice. BMC Plant Biol 19: 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Ding Y, Zhou Y, Jin W, Xie K, Chen LL(2017) CRISPR-P 2.0: An improved CRISPR-Cas9 tool for genome editing in plants. Mol Plant 10: 530–532 [DOI] [PubMed] [Google Scholar]
- Lu Y, Ye X, Guo R, Huang J, Wang W, Tang J, Tan L, Zhu JK, Chu C, Qian Y(2017) Genome-wide targeted mutagenesis in rice using the CRISPR/Cas9 system. Mol Plant 10: 1242–1245 [DOI] [PubMed] [Google Scholar]
- Maas FM, van de Wetering DAM, van Beusichem ML, Bienfait HF(1988) Characterization of phloem iron and its possible role in the regulation of Fe-efficiency reactions. Plant Physiol 87: 167–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyadate H, Adachi S, Hiraizumi A, Tezuka K, Nakazawa N, Kawamoto T, Katou K, Kodama I, Sakurai K, Takahashi H, et al. (2011) OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol 189: 190–199 [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Ogawa I, Ishimaru Y, Mori S, Nishizawa NK(2006) Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci Plant Nutr 52: 464–469 [Google Scholar]
- Olsen LI, Palmgren MG(2014) Many rivers to cross: The journey of zinc from soil to seed. Front Plant Sci 5: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchy N, Lehti-Shiu M, Shiu SH(2016) Evolution of gene duplication in plants. Plant Physiol 171: 2294–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson SRM, Tarpley L, Yan W, Yeater K, Lahner B, Yakubova E, Huang XY, Zhang M, Guerinot ML, Salt DE(2015) Worldwide genetic diversity for mineral element concentrations in rice grain. Crop Sci 55: 294–311 [Google Scholar]
- Ramesh SA, Shin R, Eide DJ, Schachtman DP(2003) Differential metal selectivity and gene expression of two zinc transporters from rice. Plant Physiol 133: 126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EE, Eide DJ, Guerinot ML(2000) Altered selectivity in an Arabidopsis metal transporter. Proc Natl Acad Sci USA 97: 12356–12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifullah, Sarwar N, Bibi S, Ahmad M, Ok YS(2014) Effectiveness of zinc application to minimize cadmium toxicity and accumulation in wheat (Triticum aestivum L.). Environ Earth Sci 71: 1663–1672 [Google Scholar]
- Sandalio LM, Dalurzo HC, Gómez M, Romero-Puertas MC, del Río LA(2001) Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52: 2115–2126 [DOI] [PubMed] [Google Scholar]
- Sarwar N, Ishaq W, Farid G, Shaheen MR, Imran M, Geng M, Hussain S(2015) Zinc-cadmium interactions: Impact on wheat physiology and mineral acquisition. Ecotoxicol Environ Saf 122: 528–536 [DOI] [PubMed] [Google Scholar]
- Sasaki A, Yamaji N, Ma JF(2014) Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J Exp Bot 65: 6013–6021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Yamaji N, Mitani-Ueno N, Kashino M, Ma JF(2015) A node-localized transporter OsZIP3 is responsible for the preferential distribution of Zn to developing tissues in rice. Plant J 84: 374–384 [DOI] [PubMed] [Google Scholar]
- Sasaki A, Yamaji N, Yokosho K, Ma JF(2012) Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 24: 2155–2167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair SA, Senger T, Talke IN, Cobbett CS, Haydon MJ, Krämer U(2018) Systemic upregulation of MTP2- and HMA2-mediated Zn partitioning to the shoot supplements local Zn deficiency responses. Plant Cell 30: 2463–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WY, Yamaki T, Yamaji N, Ko D, Jung KH, Fujii-Kashino M, An G, Martinoia E, Lee Y, Ma JF(2014) A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc Natl Acad Sci USA 111: 15699–15704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui FQ, Chang JD, Tang Z, Liu WJ, Huang XY, Zhao FJ(2018) Nramp5 expression and functionality likely explain higher cadmium uptake in rice than in wheat and maize. Plant Soil 433: 377–389 [Google Scholar]
- Sumi Y, Itoh MT, Muraki T, Suzuki T(1996) Histochemical staining of cadmium with 2-(8-quinolylazo)-4,5-diphenylimidazole. Histochem Cell Biol 106: 223–227 [DOI] [PubMed] [Google Scholar]
- Takahashi R, Ishimaru Y, Senoura T, Shimo H, Ishikawa S, Arao T, Nakanishi H, Nishizawa NK(2011) The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J Exp Bot 62: 4843–4850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi R, Ishimaru Y, Shimo H, Ogo Y, Senoura T, Nishizawa NK, Nakanishi H(2012) The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ 35: 1948–1957 [DOI] [PubMed] [Google Scholar]
- Tan L, Zhu Y, Fan T, Peng C, Wang J, Sun L, Chen C(2019) OsZIP7 functions in xylem loading in roots and inter-vascular transfer in nodes to deliver Zn/Cd to grain in rice. Biochem Biophys Res Commun 512: 112–118 [DOI] [PubMed] [Google Scholar]
- Tang L, Mao B, Li Y, Lv Q, Zhang L, Chen C, He H, Wang W, Zeng X, Shao Y, et al. (2017) Knockout of OsNramp5 using the CRISPR/Cas9 system produces low Cd-accumulating indica rice without compromising yield. Sci Rep 7: 14438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Mao D, Xu L, Li D, Song S, Chen C(2014) Integrated analysis of miRNA and mRNA expression profiles in response to Cd exposure in rice seedlings. BMC Genomics 15: 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiong J, McDonald G, Genc Y, Shirley N, Langridge P, Huang CY(2015) Increased expression of six ZIP family genes by zinc (Zn) deficiency is associated with enhanced uptake and root-to-shoot translocation of Zn in barley (Hordeum vulgare). New Phytol 207: 1097–1109 [DOI] [PubMed] [Google Scholar]
- Uraguchi S, Kamiya T, Sakamoto T, Kasai K, Sato Y, Nagamura Y, Yoshida A, Kyozuka J, Ishikawa S, Fujiwara T(2011) Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc Natl Acad Sci USA 108: 20959–20964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee BL, Auld DS(1990) Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry 29: 5647–5659 [DOI] [PubMed] [Google Scholar]
- Vert GA, Briat JF, Curie C(2003) Dual regulation of the Arabidopsis high-affinity root iron uptake system by local and long-distance signals. Plant Physiol 132: 796–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liang S, Xiang W, Dai H, Duan Y, Kang F, Chai T(2019) A repeat region from the Brassica juncea HMA4 gene BjHMA4R is specifically involved in Cd2+ binding in the cytosol under low heavy metal concentrations. BMC Plant Biol 19: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu FH, Shen SC, Lee LY, Lee SH, Chan MT, Lin CS(2009) Tape-Arabidopsis Sandwich—a simpler Arabidopsis protoplast isolation method. Plant Methods 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaji N, Ma JF(2014) The node, a hub for mineral nutrient distribution in graminaceous plants. Trends Plant Sci 19: 556–563 [DOI] [PubMed] [Google Scholar]
- Yamaji N, Sasaki A, Xia JX, Yokosho K, Ma JF(2013a) A node-based switch for preferential distribution of manganese in rice. Nat Commun 4: 2442. [DOI] [PubMed] [Google Scholar]
- Yamaji N, Xia J, Mitani-Ueno N, Yokosho K, Feng Ma J(2013) Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant Physiol 162: 927–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Huang J, Jiang Y, Zhang HS(2009) Cloning and functional identification of two members of the ZIP (Zrt, Irt-like protein) gene family in rice (Oryza sativa L.). Mol Biol Rep 36: 281–287 [DOI] [PubMed] [Google Scholar]
- Yao W, Sun L, Zhou H, Yang F, Mao D, Wang J, Chen L, Zhang G, Dai J, Xiao G, et al. (2015) Additive, dominant parental effects control the inheritance of grain cadmium accumulation in hybrid rice. Mol Breed 35: 39 [Google Scholar]
- Yoo SD, Cho YH, Sheen J(2007) Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zeng L, Liu X, Zhou Z, Li D, Zhao X, Zhu L, Luo Y, Hu S(2018) Identification of a G2-like transcription factor, OsPHL3, functions as a negative regulator of flowering in rice by co-expression and reverse genetic analysis. BMC Plant Biol 18: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Chen L, Li X(2018) Arabidopsis and rice showed a distinct pattern in ZIPs genes expression profile in response to Cd stress. Bot Stud 59: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]