A single transcription factor regulates the accumulation of several color compounds in tomato fruit.
Abstract
Tomato (Solanum lycopersicum) fruit ripening is accompanied by the degradation of chlorophylls and the accumulation of carotenoids and flavonoids. Tomato SlMYB72 belongs to the R2R3 MYB subfamily, is located in the nucleus, and possesses transcriptional activator activity. Down-regulation of the SlMYB72 gene produced uneven-colored fruits; that is, dark green spots appeared on immature and mature green fruits, whereas yellow spots appeared on red fruits. Down-regulation of SlMYB72 increased chlorophyll accumulation, chloroplast biogenesis and development, and photosynthesis rate in fruits. This down-regulation decreased lycopene content, promoted β-carotene production and chromoplast development, and increased flavonoid accumulation in fruits. RNA sequencing analysis revealed that down-regulation of SlMYB72 altered the expression levels of genes involved in the biosynthesis of chlorophylls, carotenoids, and flavonoids. SlMYB72 protein interacted with the auxin response factor SlARF4. SlMYB72 directly targeted protochlorophyllide reductase, Mg-chelatase H subunit, and knotted1-like homeobox2 genes and regulated chlorophyll biosynthesis and chloroplast development. SlMYB72 directly bound to phytoene synthase, ζ-carotene isomerase, and lycopene β-cyclase genes and regulated carotenoid biosynthesis. SlMYB72 directly targeted 4-coumarate-coenzyme A ligase and chalcone synthase genes and regulated the biosynthesis of flavonoids and phenolic acid. The uneven color phenotype in RNA interference-SlMYB72 fruits was due to uneven silencing of SlMYB72 and uneven expression of chlorophyll, carotenoid, and flavonoid biosynthesis genes. In summary, this study identified important roles for SlMYB72 in the regulation of chlorophyll, carotenoid, and flavonoid metabolism and provided a potential target to improve fruit nutrition in horticultural crops.
Tomato (Solanum lycopersicum) is the second largest vegetable crop in the world and has been a classical model system for understanding fruit development and ripening (Klee and Giovannoni, 2011). Tomato fruit development and ripening include starch accumulation and degradation and the accumulation of soluble sugars, carotenoids, and volatile organic compounds (Klee and Giovannoni, 2011). Chlorophyll content and photosynthesis activity in green fruits affect the starch level and nutritional components of tomato fruits (Nadakuduti et al., 2014). Chlorophyll biosynthesis can be divided into three parts, namely, formation of 5-aminolevulinic acid, biosynthesis of protoporphyrin IX from eight molecules of 5-aminolevulinic acid, and biosynthesis of chlorophyll a and b in the magnesium branch. Chlorophyll accumulation and chloroplast development are influenced by several genes. For example, the Deetiolated1/High Pigment2 and UV-damaged DNA-Binding Protein1/High Pigment1 negatively regulate chlorophyll biosynthesis and chloroplast formation in tomato fruits (Kolotilin et al., 2007; Rohrmann et al., 2011). Other examples are the two Golden2-like transcription factor (TF) genes, SlGLK1 and SlGLK2, which are required for chlorophyll accumulation and chloroplast formation (Waters et al., 2009). The expressional latitudinal gradient of SlGLK2 regulates the uneven accumulation of chlorophylls in tomato fruits (Nguyen et al., 2014). The APRR2-like gene, a homolog of SlGLK2, regulates chlorophyll accumulation and chloroplast development in tomato fruits (Pan et al., 2013). In addition, two Knotted1-like Homeobox (KNOX) proteins, TKN2 and TKN4, activate the expression of SlGLK2 and APRR2-like genes and increase chloroplast development in tomato fruits (Nadakuduti et al., 2014). According to Meng et al. (2018), the BEL1-like Homeodomain11 gene SlBEL11 is involved in chloroplast development and chlorophyll accumulation in tomato fruits. Sagar et al. (2013) and Yuan et al. (2018, 2019) reported that auxin response genes, SlARF4, SlARF6, and SlARF10, regulate chlorophyll accumulation and chloroplast development in tomato fruits.
The visible evidence of tomato fruit ripening is the change in color from green to red, which is accompanied by the transition of chloroplasts into chromoplasts and the biosynthesis and accumulation of carotenoids in the chromoplasts of fruit cells (Klee and Giovannoni, 2011). In carotenoid biosynthesis, geranylgeranyl pyrophosphate is converted to phytoene and lycopene, and cyclization of lycopene produces α- and β-carotene (Arango et al., 2014). Carotenoid accumulation in tomato can be regulated by several TFs, such as MADS-box TFs, RIN, TAGL1, and TDR4; the SQUAMOSA promoter binding protein-like LeSPL-CNR; the NAC domain proteins SlNAC4 and NOR; the HD-Zip homeobox protein LeHB-1; and the AP2/ERF proteins SlERF6 and SlAP2a (Vrebalov et al., 2002, 2009; Manning et al., 2006; Lin et al., 2008; Itkin et al., 2009; Chung et al., 2010; Martel et al., 2011; Bemer et al., 2012; Lee et al., 2012; Zhu et al., 2014). These TFs also affect fruit softening, flavor biosynthesis, and ethylene production, which may possibly induce carotenoid biosynthesis in tomato fruit (Su et al., 2015); therefore, TFs may regulate carotenoid biosynthesis indirectly via the ethylene pathway.
Chromoplasts are derived from preexisting plastids, such as chloroplasts, and the transition from chloroplast to chromoplast is synchronous for all plastids in one cell (Egea et al., 2011). The differentiation process includes breakdown of chlorophylls, disruption of the thylakoid membrane, formation of the inner membrane envelope of chromoplast, and the appearance of carotenoid-containing crystalloids (Egea et al., 2011). The tomato ripening inhibitor (rin) mutant has an increased number of chromoplasts per cell in breaker fruit compared with wild-type fruit (Vrebalov et al., 2002); hence, the RIN gene is involved in chromoplast biogenesis. The ORANGE (OR) gene encodes a plastidial DNA J Cys-rich domain-containing protein and is an important regulator for carotenoid biosynthesis (Lu et al., 2006; Zhou et al., 2015; Yazdani et al., 2019). Chayut et al. (2017) reported a melon (Cucumis melo) OR gene (CmOR) that is involved in the transition from chloroplast to chromoplast.
Flavonoids have excellent antioxidant and antiinflammatory capacities (Havsteen, 2002; Hannum, 2004). Tomato fruit ripening is associated with an increase in flavonoid accumulation in the epidermis (España et al., 2014). Flavonoids comprise a large family of aromatic molecules that are synthesized from the stepwise condensation of 4-coumaroyl-CoA, a product of the phenylpropanoid metabolism pathway (Winkel-Shirley, 2001). R2R3 MYBs have been reported to play an important role in flavonoid biosynthesis (Xu et al., 2015). Arabidopsis (Arabidopsis thaliana) AtMYB12 functions as an activator of flavonol biosynthesis by positively regulating the expression of flavonol biosynthetic genes (Mehrtens et al., 2005). The overexpression of two MYB TFs (CsMYB5-1 and CsMYB5-2) dramatically increases the flavonoid content of alfalfa (Medicago sativa; Zheng et al., 2019). MYB TFs interact with MYB-bHLH-WDR complexes that regulate flavonoid biosynthesis (Baudry et al., 2004; Li, 2014; Xu et al., 2015).
Recently, several R2R3 MYBs have been reported to be involved in chlorophyll and carotenoid accumulation. CrMYB68, an R2R3-MYB TF, negatively regulates the expression of CrBCH2 and CrNCED5 genes and the accumulation of α- and β-carotenoids in the flavedo of Citrus reticulata (Zhu et al., 2017). In kiwifruit (Actinidia deliciosa), MYB7, another R2R3-MYB TF, regulates carotenoid accumulation via the activation of the LYCOPENE β-CYCLASE (LCYB) gene and modulates chlorophyll biosynthesis (Ampomah-Dwamena et al., 2019). In this study, we found that an R2R3-MYB TF, SlMYB72, simultaneously regulates the metabolism of chlorophylls, carotenoids, and flavonoids in tomato fruits.
RESULTS
SlMYB72 Gene Sequence, Expression Analysis, Protein Subcellular Localization, and Transcriptional Activity
The SlMYB72 gene has an open reading frame (ORF) of 726 bp that encodes a protein containing 242 amino acid residues. Sequence analysis revealed that the SlMYB72 protein contains an R2R3 domain in the N terminus (Supplemental Fig. S1A) and highly conserved triplet Trp residues in each repeat, and the characteristic Trp residues are located at positions 17, 37, and 57 of the R2 repeat (Supplemental Fig. S1A). Phylogenetic analysis of tomato MYB family proteins revealed that SlMYB72, SlMYB83, SlMYB81, SlMYB85, SlMYB68, and SlMYB66 were clustered into the tomato S7 subfamily, which was close to the Arabidopsis S7 subfamily (Supplemental Fig. S1B). The AtMYB11, AtMYB12, and AtMYB111 genes from the S7 subfamily are involved in flavonoid biosynthesis in tomato (Stracke et al., 2010). The expression profiles of the SlMYB72 gene in tomato plants were analyzed by reverse transcription quantitative PCR (RT-qPCR). The SlMYB72 gene was highly expressed in roots, flowers, and fruits at different stages (Fig. 1A). The spatial expression pattern of the SlMYB72 gene was analyzed through a transgenic tomato in which the GUS gene was driven by the promoter sequence of the SlMYB72 gene. GUS staining was strong in the fruit and the stamen of the flower (Fig. 1B).
Figure 1.
Expression patterns of SlMYB72 gene and subcellular localization analysis of SlMYB72 protein in tomato. A, RT-qPCR analysis of SlMYB72 expression level. Bars from left to right are as follows: Rt, root; St, stem; Lf, leaf; Fl, flower; Img, early immature green fruit; Img2, late immature green fruit; Mg, mature green fruit; Bf, breaker fruit; Of, orange fruit; Rf, red fruit. The data represent means ± sd of four biological replicates. B, Expression pattern of SlMYB72 revealed by the expression of the GUS reporter gene driven by the SlMYB72 promoter. Bars = 1 mm. C, Subcellular localization analysis of SlMYB72 protein. The SlMYB72-GFP fusion protein was transiently expressed in Nicotiana benthamiana leaves. Nuclei were identified by 4′,6-diamidino-2-phenylindole (DAPI) staining. Bars = 50 μm. D, Transcriptional activation activity of SlMYB72 protein. pGBKT7-SlMYB72 fusion vector, negative control, and positive control were transformed into Y2HGold yeast cells. The yeast cells were cultivated on SD-Trp/His/Ade.
The subcellular location of the SlMYB72 protein was determined by a fusion protein with GFP. The green fluorescence of the SlMYB72-GFP fusion protein was distributed in the nucleus (Fig. 1C), indicating nuclear localization of SlMYB72. The transcriptional activity of the SlMYB72 protein was analyzed by using a GAL4-responsive reporter system in yeast. SlMYB72 was fused to the GAL4 DNA-binding domain to form a pGBKT7-SlMYB72 fusion plasmid. Yeast cells with the pGBKT7-SlMYB72 plasmid grew well on synthetic dextrose (SD) medium lacking Trp, His, and Ade, whereas yeast with negative control pGBKT7 did not grow (Fig. 1D). Hence, SlMYB72 has transcriptional activation activity.
SlMYB72 Negatively Affects Chlorophyll Accumulation, Chloroplast Biogenesis, and Photosynthesis in Tomato Fruits
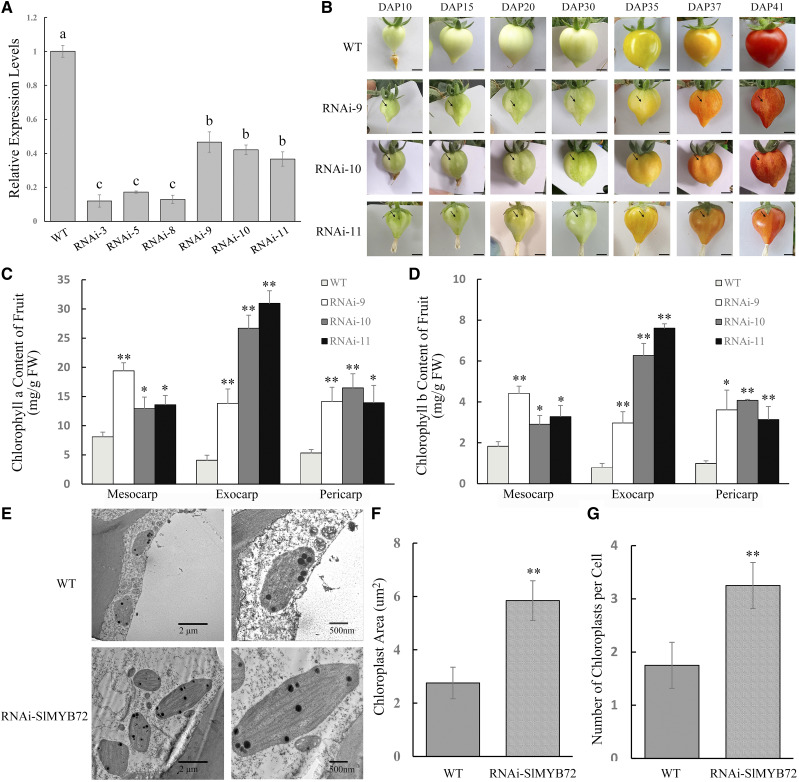
Six down-regulated transgenic lines (RNA interference [RNAi]-SlMYB72) and 10 up-regulated transgenic lines (over expression [OE]-SlMYB72) corresponding to independent transformation events were produced to investigate the functions of the SlMYB72 gene in tomato. Analysis of the expression levels of the transgenic lines by RT-qPCR showed that SlMYB72 expression levels decreased in the six RNAi-SlMYB72 lines (Fig. 2A) and increased in the 10 OE-SlMYB72 lines (Supplemental Fig. S2). The SlMYB72 overexpression lines had no obvious change in fruit phenotype. By contrast, the RNAi-SlMYB72 lines exhibited numerous dark green spots of variable size on immature green and mature green fruits, and these dark green spots changed to yellow spots at the orange and red fruit stages (Fig. 2B). Lines 3, 5, and 8 with the most highly reduced expression levels of SlMYB72 did not produce seeds. Therefore, lines 9, 10, and 11 were used for the generation of T3 homozygous lines and used for further analysis (Fig. 2A).
Figure 2.
Generation of RNAi-SlMYB72 plants and analysis of chlorophyll accumulation and chloroplast development in RNAi-SlMYB72 plants. A, RT-qPCR analysis of the expression of SlMYB72 in transgenic lines. The data represent means ± sd of four biological replicates. The relative expression levels of genes were compared between the RNAi-SlMYB72 (RNAi) and wild-type (WT) plants. Different lowercase letters indicate significant differences. B, Fruit phenotypes. Bars = 0.5 cm. C, Chlorophyll a contents in mesocarps, exocarps, and pericarps of wild-type and RNAi-SlMYB72 fruits. D, Chlorophyll b contents in mesocarps, exocarps, and pericarps of wild-type and RNAi-SlMYB72 fruits. FW, Fresh weight. E, TEM observation of chloroplasts in mesocarps of wild-type and RNAi-SlMYB72 fruits. Bars = 2 μm (left) and 500 nm ( right). F, Analysis of chloroplast size. G, Number of chloroplasts per cell. The data represent means ± sd of three biological replicates in C, D, F, and G. Asterisks indicate significant differences between the transgenic and wild-type plants (*P < 0.05 and **P < 0.01), as determined by Student’s t test.
Tomato fruit pericarp consists of exocarp, mesocarp, and endocarp. The chlorophyll contents in total pericarps, exocarps, and mesocarps of mature green fruits of RNAi-SlMYB72 and OE-SlMYB72 lines were analyzed by HPLC. OE-SlMYB72 lines showed no change in the contents of chlorophylls a and b compared with wild-type plants (Supplemental Fig. S3). But RNAi-SlMYB72 lines showed higher contents of chlorophylls a and b in dark green regions of fruits than wild-type fruits at the mature green stage (Fig. 2, C and D). Transmission electron microscopy (TEM) analysis of the mesocarp of the dark green regions of fruits revealed that the chloroplast size and the number of chloroplasts increased in RNAi-SlMYB72 lines compared with those in wild-type plants (Fig. 2, E–G). But there was no obvious difference in grana structure between RNAi-SlMYB72 and wild-type plants. These results indicated that SlMYB72 is involved in chlorophyll accumulation and chloroplast biogenesis and development.
The increased chlorophyll accumulation in fruits may affect photosynthesis in the transgenic lines. The photosynthetic performance of the fruits of RNAi-SlMYB72 was measured. The photochemical potential was elevated in the transgenic lines compared with that in the wild type (Supplemental Fig. S4A). The values of RNAi-SlMYB72 lines for the effective photochemical quantum yield of PSII were higher than those of the fruits of wild-type plants (Supplemental Fig. S4B). Thus, SlMYB72 negatively affects photosynthesis in fruits of tomato plants.
SlMYB72 Affects Carotenoid Accumulation and Chromoplast Biogenesis in Tomato Fruits
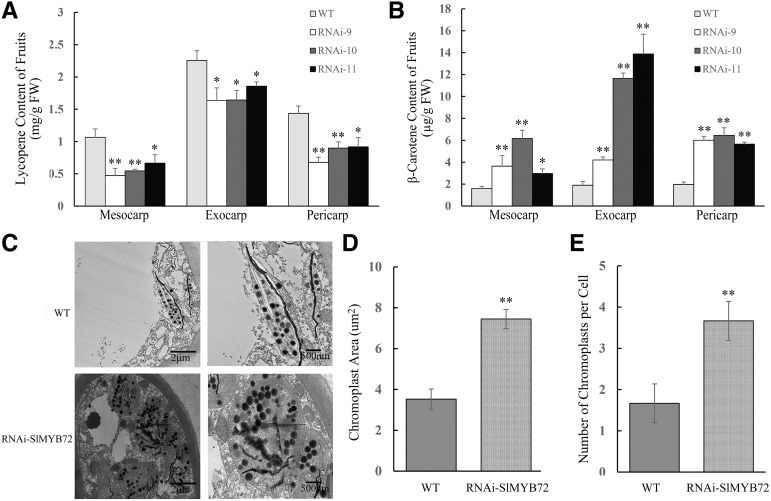
Carotenoid contents in the red fruits of OE-SlMYB72 and RNAi-SlMYB72 lines were determined by HPLC. The OE-SlMYB72 lines showed no change in the contents of lycopene and β-carotene in pericarps of red fruits compared with wild-type plants (Supplemental Fig. S3). The lycopene contents obviously decreased (Fig. 3A), whereas the β-carotene contents increased in the yellow regions of the mesocarps, exocarps, and pericarps of RNAi-SlMYB72 fruits compared with those in wild-type fruits (Fig. 3B). Furthermore, chromoplasts in the yellow regions of RNAi-SlMYB72 fruits were analyzed by TEM (Fig. 3C). The chromoplast size and number of chromoplasts per cell of the RNAi-SlMYB72 lines increased compared with those of wild-type plants (Fig. 3, D and E). The results demonstrated that SlMYB72 affects carotenoid biosynthesis as well as chromoplast biogenesis and development in tomato fruits.
Figure 3.
Analysis of carotenoid accumulation and chromoplast development in RNAi-SlMYB72 plants. A, Lycopene contents in mesocarps, exocarps, and pericarps of wild-type (WT) and RNAi-SlMYB72 (RNAi) plants. B, β-Carotene contents in mesocarps, exocarps, and pericarps of wild-type and RNAi-SlMYB72 plants. FW, Fresh weight. C, TEM observation of chromoplasts in mesocarps of wild-type and RNAi-SlMYB72 fruits. Bars = 2 μm (left) and 500 nm (right). D, Analysis of chromoplast size. E, Number of chromoplasts per cell. The data represent means ± sd of three biological replicates. Asterisks indicate significant differences between transgenic and wild-type plants (*P < 0.05 and **P < 0.01), as determined by Student’s t test.
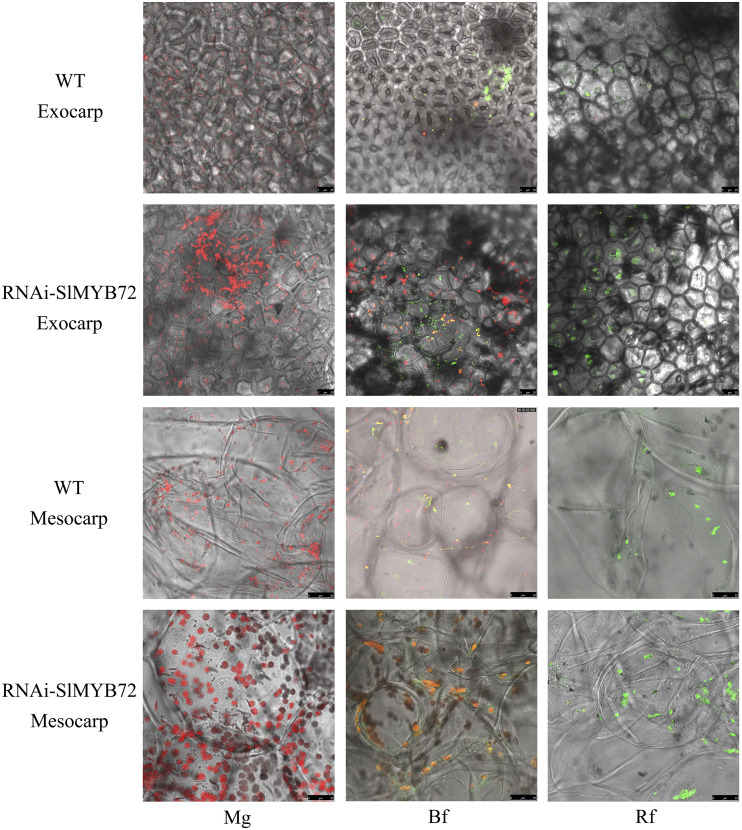
The autofluorescence of chloroplasts and chromoplasts in green, breaker, and red fruits was further detected using confocal laser scanning microscopy. Chloroplast autofluorescence was considerably stronger in the exocarps and mesocarps of RNAi-SlMYB72 green fruits and chromoplast autofluorescence was stronger in RNAi-SlMYB72 red fruits than in the wild type (Fig. 4). At the breaker stage, the exocarps and mesocarps of the RNAi-SlMYB72 line exhibited higher autofluorescence of intermediated plastids than wild-type plants (Fig. 4). These results further demonstrated that the SlMYB72 gene is involved in the biogenesis of chloroplasts and chromoplasts in tomato fruits.
Figure 4.
Fluorescence observation of plastids at three stages of RNAi-SlMYB72 fruits. Autofluorescence of chlorophylls and carotenoids was observed via confocal laser scanning microscopy in the mesocarp and exocarp of tomato fruits at the following stages from left to right: Mg, mature green; Bf, breaker; Rf, red. WT, Wild-type plants. Bars = 25 μm.
SlMYB72 Negatively Affects the Accumulation of Flavonoids and Phenolic Acids
The RNAi-SlMYB72 fruits, which exhibited yellow spots at the red fruit stage (Fig. 2B), have a similar phenotype to tomato fruits containing high flavonoid contents, as reported by Luo et al. (2008). The flavonoids in pericarps of the red fruits of OE-SlMYB72 and RNAi-SlMYB72 lines were further analyzed by HPLC. The total flavonoid content in OE-SlMYB72 did not change compared with wild-type plants (Supplemental Fig. S3). However, the total flavonoid, rutin, quercetin, total phenolic acid, gallic acid, and chlorogenic acid contents in RNAi-SlMYB72 red fruits obviously increased compared with those in wild-type plants (Fig. 5, A–F).
Figure 5.
Flavonoid accumulation in the pericarps of RNAi-SlMYB72 plants. A, Total flavonoid content. B, Rutin content. C, Quercetin content. D, Total phenolic content. E, Gallic acid content. F, Chlorogenic acid content. FW, Fresh weight; RNAi, RNAi-SlMYB72 lines; WT, wild-type plants. The data represent means ± sd of three biological replicates. Asterisks indicate significant differences between transgenic and wild-type plants (**P < 0.01), as determined by Student’s t test. G, Autofluorescence observation of flavonoid accumulation in mesocarps of RNAi-SlMYB72 fruits. The greenish color at the mesocarp cell indicates the accumulation of kaempferol derivatives, whereas orange indicates quercetin derivatives in plants. Bars = 200 μm.
The flavonoids in RNAi-SlMYB72 fruits were stained with diphenylboric acid-2-amino ethyl ester and imaged by confocal laser scanning microscopy. The signals of orange and green fluorescence in fruits of RNAi-SlMYB72 lines were stronger than those in wild-type plants; hence, the kaempferol and quercetin contents in RNAi-SlMYB72 fruits were higher compared with those in wild-type fruits (Fig. 5G).
Down-Regulation of SlMYB72 Expression Alters the Expression of Genes Involved in Chlorophyll Metabolism, Photosynthesis, and Carotenoid Metabolism
RNA sequencing (RNA-seq) transcriptome analysis was performed to determine the underlying molecular basis of the RNAi-SlMYB72 phenotype. Gene expression analysis under the criterion of a false discovery rate < 0.05 revealed that the RNAi-SlMYB72 fruit contains 5,260 differentially expressed genes (DEGs), including 784 down-regulated and 4,476 up-regulated DEGs (Supplemental Table S1).
Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses showed that the down-regulation of SlMYB72 affected multiple metabolic pathways, including photosynthesis, photosynthesis-antenna proteins, carbon fixation in photosynthetic organisms, starch and Suc metabolism, carotenoid biosynthesis, flavonoid biosynthesis, fatty acid biosynthesis, elongation, and degradation, and phenylpropanoid biosynthesis (Supplemental Fig. S5A; Supplemental Table S2). MapMan analysis further identified altered metabolic pathways in RNAi-SlMYB72 lines, such as light reactions, photorespiration, cell wall, lipids, terpenes, and flavonoids (Supplemental Fig. S5B; Supplemental Table S3).
SlMYB72 Interacts with SlARF4 and Targets PROTOCHLOROPHYLLIDE REDUCTASE, Mg-CHELATASE H SUBUNIT, and TKN2 Genes
SlARF4 negatively regulates chlorophyll accumulation, which is consistent with the phenotypes of SlMYB72 (Sagar et al., 2013). We performed yeast two-hybrid (Y2H) analysis to determine the interactions between SlMYB72 and SlARF4. SlMYB72 exhibited strong interaction ability with SlARF4 (Fig. 6A). The interaction between SlMYB72 and SlARF4 was subsequently confirmed using the bimolecular fluorescence complementation (BiFC) method, and the results showed that SlMYB72 and SlARF4 proteins interacted in the nucleus (Fig. 6B). An in vitro pull-down assay was performed to confirm the protein interaction. Purified recombinant SlARF4-His was incubated with SlMYB72-GST, and the interacting proteins were pulled down with glutathione resin. We found that SlARF4-His was recovered only with SlMYB72 as bait (Fig. 6C). These results indicated that SlMYB72 can interact with SlARF4.
Figure 6.
SlMYB72 interacts with SlARF4 and targets the genes involved in chlorophyll accumulation and chloroplast development. A, Y2H analysis of protein interactions between SlMYB72 and SlARF4. AD-53 and BD-53 were used as positive controls; AD+BD, AD-SlMYB72+BD, and AD+BD-SlARF4 were used as negative controls. SD-LW, Selective medium lacking Leu and Trp; SD-LWAH, selective medium lacking Leu, Trp, Ade, and His. B, BiFC assays validating the interactions between SlARF4 and SlMYB72 in nuclei. nYFP and cYFP represent the N- and C-terminal parts of YFP, respectively. The SlMYB72-cYFP and SlARF4-nYFP constructs were cotransformed into N. benthamiana leaves via Agrobacterium tumefaciens-mediated infiltration. cYFP+nYFP, SlMYB72-cYFP+nYFP, and cYFP+SlARF4-nYFP were used as negative controls. The fluorescence emitted by YFP was examined through confocal microscopy. Nuclei were identified by 4′,6-diamidino-2-phenylindole (DAPI) staining. Bars = 50 μm. C, The interaction of SlMYB72 with SlARF4 was detected by in vitro pull-down assays. The GST-SlMYB72 and His-SlARF4 fusion proteins were purified from Escherichia coli. Anti-His antibody was used to detect the His-SlARF4 protein, and anti-GST antibody was used to detect the amount of GST-SlMYB72 protein. D, Expression levels of CHLH, POR, and TKN2 in pericarps of mature green fruits. RNAi, RNAi-SlMYB72 lines; WT, wild-type plants. The data represent means ± sd of four biological replicates. The relative expression levels of genes were compared between the RNAi-SlMYB72 and wild-type plants. E to G, EMSA showing the binding of SlMYB72 to the promoters of CHLH, POR, and TKN2, respectively. Biotin-labeled DNA probes from native promoter or mutants were incubated with GST-SlMYB72 protein, and the DNA-protein complexes were separated on 6% (w/v) native polyacrylamide gels. H, ChIP-qPCR assay for the direct binding of SlMYB72 to CHLH, POR, and TKN2 genes. Values are percentages of DNA fragments that coimmunoprecipitated with anti-FLAG antibodies or nonspecific antibodies (anti-IgG) relative to the input DNA. The data represent means ± sd of four biological replicates. Asterisks indicate significant differences between transgenic and wild-type plants (**P < 0.01), as determined by Student’s t test.
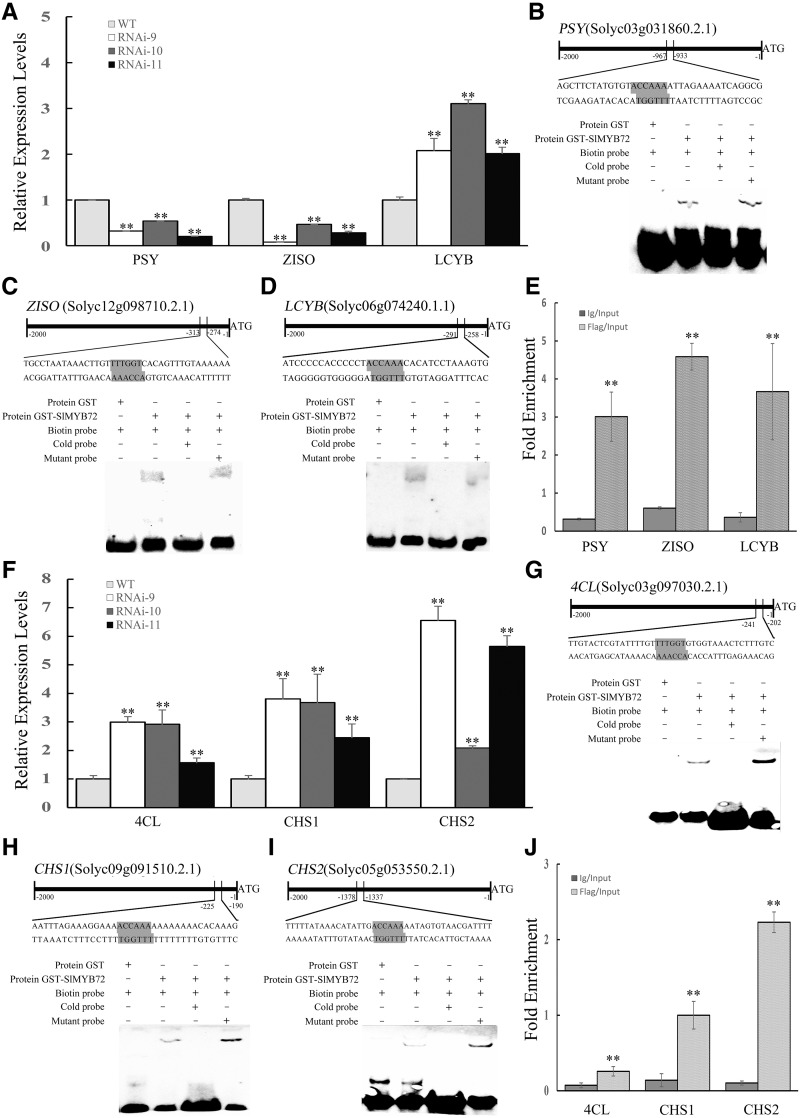
Analysis of the promoters of the six DEGs involved in chlorophyll accumulation showed that the promoters of PROTOCHLOROPHYLLIDE REDUCTASE (POR), Mg-CHELATASE H SUBUNIT (CHLH), and a class I KNOX gene (TKN2) contain an ACCAAC/ACCAAA element, which is an R2R3-MYB-binding motif. The expression of POR, CHLH, and TKN2 genes increased in RNAi-SlMYB72 plants (Fig. 6D). The direct binding of the SlMYB72 protein to the promoters of POR, CHLH, and TKN2 genes was analyzed by electrophoretic mobility shift assay (EMSA). The GST and SlMYB72 fusion protein (GST-tSlMYB72) was successfully purified (Supplemental Fig. S6). The GST-tSlMYB72 fusion protein bound biotin-labeled probes containing the ACCAAC/ACCAAA motif derived from the promoters of POR, CHLH, and TKN2 genes, resulting in mobility shifts. The unlabeled fragments of POR, CHLH, and TKN2 genes as competitors effectively abolished the mobility shift in a dose-dependent manner. The biotin-labeled probes incubated with only GST did not produce a mobility shift (Fig. 6, E–G). The data indicated that the specific target of SlMYB72 is the promoters of POR, CHLH, and TKN2 genes. The interaction between SlMYB72 and the promoters of POR, CHLH, and TKN2 genes was further confirmed by chromatin immunoprecipitation (ChIP) coupled with qPCR in vivo. The promoter regions of POR, CHLH, and TKN2 were specifically enriched using FLAG antibodies and not by nonspecific antibodies (IgG; Fig. 6H). Thus, SlMYB72 directly targets the promoters of POR, CHLH, and TKN2 genes.
SlMYB72 Directly Targets PHYTOENE SYNTHASE, ζ-CAROTENE ISOMERASE, and LCYB Genes
Ten DEGs have been found to be related to carotenoid metabolism. Sequence analysis showed that the promoters of PHYTOENE SYNTHASE (PSY), ζ-CAROTENE ISOMERASE (ZISO), and LCYB genes contain the ACCAAC/ACCAAA element. RT-qPCR analysis revealed that PSY and ZISO genes decreased and the LCYB gene increased in RNAi-MYB72 plants (Fig. 7A). EMSA showed that the GST-tSlMYB72 fusion protein targeted biotin-labeled probes containing the ACCAAC/ACCAAA motif derived from the promoters of PSY, ZISO, and LCYB genes, resulting in mobility shifts (Fig. 7, B–D). Thus, the specific targets of SlMYB72 are the promoters of PSY, ZISO, and LCYB genes. ChIP-qPCR was performed to confirm the interaction between SlMYB72 and the promoters of PSY, ZISO, and LCYB genes in vivo. The promoter regions of PSY, ZISO, and LCYB were specifically enriched using FLAG antibodies (Fig. 7E) compared with the negative control (IgG). Therefore, our data indicated that SlMYB72 directly targets the promoters of PSY, ZISO, and LCYB genes.
Figure 7.
SlMYB72 targets the genes involved in the biosynthesis of carotenoids and flavonoids. A, Expression levels of PSY, ZISO, and LCYB genes in pericarps of red fruits. RNAi, RNAi-SlMYB72 lines; WT, wild-type plants. The data represent means ± sd of four biological replicates. The relative expression levels of genes were compared between the RNAi-SlMYB72 and wild-type plants. B to D, EMSA showing the binding of SlMYB72 to the promoters of PSY, ZISO, and LCYB genes, respectively. Biotin-labeled DNA probes from native promoter or mutants were incubated with GST-SlMYB72 protein, and the DNA-protein complexes were separated on 6% native polyacrylamide gels. E, ChIP-qPCR assay for the direct binding of SlMYB72 to PSY, ZISO, and LCYB genes. Values are percentages of DNA fragments that coimmunoprecipitated with anti-FLAG antibodies or nonspecific antibodies (anti-IgG) relative to the input DNA. F, Expression levels of 4CL, CHS1, and CHS2 genes in pericarps of red fruit. G to I, EMSA showing the binding of SlMYB72 to the promoters of 4CL, CHS1, and CHS2 genes, respectively. J, ChIP-qPCR assay for the direct binding of SlMYB72 to the 4CL, CHS1, and CHS2 genes. The data represent means ± sd of four biological replicates in A, E, F, and J. Asterisks indicate significant differences between transgenic and wild-type plants (**P < 0.01), as determined by Student’s t test.
SlMYB72 Directly Targets 4-COUMARATE-COENZYME A LIGASE, CHALCONE SYNTHASE1, and CHALCONE SYNTHASE2 Genes
DEGs related to flavonoid biosynthesis exist in RNAi-SlMYB72 fruits. Sequence analysis revealed that the promoters of 4-COUMARATE-COENZYME A LIGASE (4CL), CHALCONE SYNTHASE1 (CHS1) and CHS2 genes contain the ACCAAC/ACCAAA motif. The expression of 4CL, CHS1, and CHS2 genes increased in RNAi-SlMYB72 plants (Fig. 7F). The interactions between SlMYB72 protein and the promoters were analyzed via the EMSA method. The GST-tSlMYB72 fusion protein targeted biotin-labeled probes containing the ACCAAC/ACCAAA motif of the promoters of 4CL, CHS1, and CHS2 genes (Fig. 7, G–I). ChIP-qPCR further confirmed the interaction between SlMYB72 and the promoters of 4CL, CHS1, and CHS2 genes in vivo (Fig. 7J).
The Uneven Color Phenotype Is the Result of Uneven Silencing of the SlMYB72 Gene in Tomato Fruit
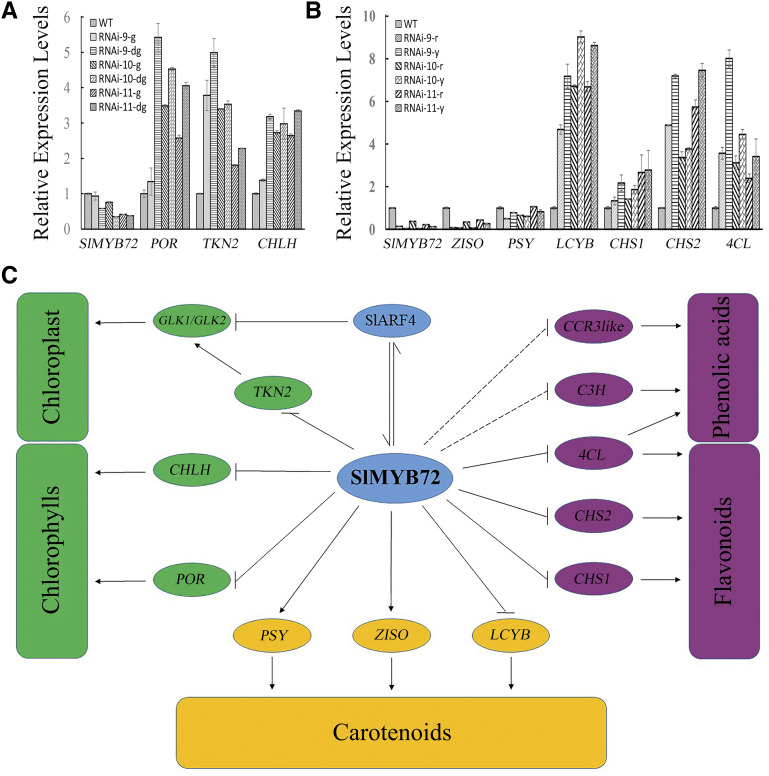
RT-qPCR was performed to analyze the differential expression levels of SlMYB72 between the different color regions in the pericarps of the RNAi-SlMYB72 mature green and red fruits to better understand the uneven color phenotype in RNAi-SlMYB72 fruits. The dark green regions showed a lower expression level of SlMYB72 than that in the normal green regions of the pericarps of RNAi-SlMYB72 mature green fruits. Moreover, wild-type fruits showed the highest expression level of SlMYB72 (Fig. 8A). POR, CHLH, and TKN2 genes had opposite expression patterns from SlMYB72, and the dark green regions had the highest expression levels of the three genes in the mature green fruits of RNAi-SlMYB72 and wild-type plants (Fig. 8A). In the RNAi-SlMYB72 red fruits, the yellow pericarp regions also showed a lower SlMYB72 expression level than the normal red regions (Fig. 8B). The expression patterns of PSY and ZISO genes were consistent with those of SlMYB72, whereas LCYB, 4CL, CHS1, and CHS2 genes had opposite expression patterns from those of SlMYB72 in the red fruits of RNAi-SlMYB72 and wild-type plants (Fig. 8B). Our results indicated that the uneven silencing of SlMYB72 led to the uneven color phenotype in RNAi-SlMYB72 fruits.
Figure 8.
A, RT-qPCR analysis of the expression levels of SlMYB72, CHLH, POR, and TKN2 genes. B, RT-qPCR analysis of the expression levels of SlMYB72, PSY, ZISO, LCYB, 4CL, CHS1, and CHS2 genes. RNAi, RNAi-SlMYB72 lines; WT, wild-type plants. Fruit regions are indicated as follows: dg, dark green region of mature green fruit; g, normal green region of mature green fruit; r, normal red region of red fruit; y, yellow region of red fruit. The data represent means ± sd of four biological replicates in A and B. The relative expression levels of genes were compared between the RNAi and wild-type plants. C, Proposed model to illustrate how SlMYB72 regulates the metabolisms of chlorophylls, carotenoids, and flavonoids in tomato fruit. SlMYB72 negatively regulates chlorophyll biosynthesis and chloroplast development by its interaction with SlARF4 and the direct inhibition of the expression of POR, CHLH, and TKN2 genes. SlMYB72 modulates carotenoid biosynthesis by the direct promotion of the expression of PSY and ZISO genes and the inhibition of LCYB gene. SlMYB72 negatively regulates flavonoid biosynthesis by the direct inhibition of the expression of 4CL and CHS genes. The greenish color represents chloroplast, chlorophylls, and related genes, yellow color represents carotenoids and related genes, whereas purple color represents the flavonoids, phenolic acids, and related genes.
DISCUSSION
The ripening process of tomato includes the conversion of chloroplasts into chromoplasts, the breakdown of chlorophylls, and carotenoid accumulation (Egea et al., 2011). The chlorophyll content of fruit contributes to the color and nutritional quality of tomato. In this study, we demonstrated that the R2R3-MYB TF gene SlMYB72 simultaneously regulates the metabolism of chlorophylls, carotenoids, and flavonoids in tomato fruits.
SlMYB72 Regulates Chlorophyll Accumulation and Chloroplast Development
MYB genes reportedly regulate many aspects of secondary metabolism, such as that of carotenoids and flavonoids, and infrequently reported functions in chlorophyll metabolism. Ampomah-Dwamena et al. (2019) reported that the overexpression of the kiwifruit MYB7 gene in N. benthamiana alters chlorophyll accumulation and increases the expression of the chlorophyll biosynthesis genes NbGGR and NbSGR1. However, evidence showing that MYB7 directly targets chlorophyll-related genes is lacking. POR is the key enzyme in the chlorophyll biosynthetic pathway and the catalytic conversion from chlorophyllide a to chlorophyll a and chlorophyllide b (Meng et al., 2018). The miR171-targeted scarecrow-like protein genes negatively regulate chlorophyll accumulation via the inhibition of POR expression (Ma et al., 2014). CHLH encodes the H subunit of Mg-chelatase, which is involved in the conversion of protoporphyrin IX to Mg-protoporphyrin IX during chlorophyll biosynthesis (Meng et al., 2018). In this study, the expression of POR and CHLH genes and the chlorophyll accumulation were increased in RNAi-SlMYB72 lines, and SlMYB72 directly targeted the POR and CHLH genes (Figs. 2 and 6). Our results indicated that SlMYB72 regulates chlorophyll accumulation by directly binding to POR and CHLH genes.
TKN2 regulates chloroplast development by positively modulating the expression of SlGLK2 and SlAPRR2-like genes (Nadakuduti et al., 2014). Pan et al. (2013) reported that overexpression of the SlAPRR2-like gene increases plastid number and area in tomato. According to our data, the expression of SlAPRR2-like and TKN2 genes and chloroplast development obviously increased in the fruits of RNAi-SlMYB72 lines (Figs. 2 and 6). SlMYB72 directly targeted the promoter of TKN2; therefore, SlMYB72 regulates chloroplast development by directly binding to the TKN2 gene.
Our results showed that SlMYB72 can interact with the SlARF4 protein (Fig. 6). In ‘Micro-Tom’ tomato, SlARF4-silenced lines enhance chlorophyll accumulation and chloroplast development, and SlARF4 may transcriptionally inhibit SlGLK1 (Sagar et al., 2013). SlARF4 also negatively regulates the expression of SlGLK2 and SlAPRR2-like genes (Nadakuduti et al., 2014). Nguyen et al. (2014) reported that SlGLK1 and SlGLK2 had similar functions; however, SlGLK1 acts largely in leaves, whereas SlGLK2 acts in the fruits of tomato. The ‘Micro-Tom’ tomato has two null alleles of SlGLK2 (Powell et al., 2012). Nguyen et al. (2014) reported that the overexpression of SlGLK1 produces dark-green fruits and increases chlorophyll accumulation and chloroplast development in uniformly ripening tomato ‘M82’. The data showed that SlGLK1 might function in the leaves and fruits of ‘Micro-Tom’ tomato. We presumed that SlMYB72 and SlARF4 regulate chlorophyll accumulation and chloroplast development via the transcriptional inhibition of the SlGLK1 gene and that TKN2 regulates chloroplast development via the transcriptional activation of SlGLK1 in ‘Micro-Tom’ tomato fruits. In the future, the function of SlGLK1 in tomato fruit will be further studied using the CRISPR/Cas9 method.
MYB72 Regulates Carotenoid Accumulation
PSY catalyzes the production of phytoene from two molecules of geranylgeranyl pyrophosphate in the carotenoid biosynthesis pathway. Three PSY genes have been identified in the tomato genome, and only PSY1 has been expressed in the breaker and red stages of the fruits (Kang et al., 2014). The loss-of-function mutation of PSY1 results in the yellow flesh phenotype of the tomato fruit (Kang et al., 2014). Fraser et al. (2007) reported that the overexpression of PSY1 increased the carotenoid content in tomato fruit. The ZISO gene is involved in the biosynthesis of lycopene from phytoene (Ampomah-Dwamena et al., 2019). In our study, lycopene content obviously decreased in red fruits, and the expression of PSY1 and ZISO genes also decreased in RNAi-SlMYB72 plants. SlMYB72 directly targeted the promoters of PSY1 and ZSIO genes (Figs. 3 and 7). The decreased lycopene content in RNAi-SlMYB72 plants can be explained by the decreased expression of PSY1 and ZISO genes regulated by the SlMYB72 protein.
The LCYB gene encodes the enzyme that catalyzes the conversion of lycopene into β-carotene (Sagawa et al., 2016). For example, the overexpression of LCYB2 in sweet potato (Ipomoea batatas) considerably increases the contents of β-carotene (Kang et al., 2018). In addition, overexpression of the kiwifruit MYB7 gene in N. benthamiana increases carotenoid content, and MYB7 directly regulates the expression of the AdLCYB gene (Ampomah-Dwamena et al., 2019). Our data showed that β-carotene content and the expression of the two LCYB genes increased in the RNAi-SlMYB72 lines and SlMYB72 directly targeted the LCYB genes (Figs. 3 and 7). Lycopene content decreased in RNAi-SlMYB72 fruits, and lycopene is needed to produce β-carotene; however, the amount of lycopene was about 100 times higher than that of β-carotene in RNAi-SlMYB72 fruits (Fig. 3). Therefore, decreased lycopene content does not limit the β-carotene content in fruits. The increased β-carotene contents in RNAi-SlMYB72 lines can be explained by the increased expression of LCYB genes regulated by SlMYB72. Our results indicated that SlMYB72 regulates the biosynthesis of lycopene and β-carotene by directly targeting carotenoid biosynthesis genes, including PSY1, ZISO, and LCYB genes.
MYB72 Regulates Flavonoid Accumulation
4CL catalyzes the conversion of l-Phe to 4-coumaroyl-CoA, which is the precursor of flavonoids. 4CL is a key enzyme in flavonoid biosynthesis and affects the flux through different phenylpropanoid pathways (Lavhale et al., 2018). Kim et al. (2014) reported that the overexpression of SbC4H and Sb4CL in Scutellaria baicalensis roots increases flavone contents; hence, C4H and 4CL have important roles in flavonoid biosynthesis. CHS is involved in the sequential condensation of three malonyl-CoA molecules and one p-coumaroyl to form naringenin chalcone, which is the first committed step in flavonoid biosynthesis. Down-regulation of the CHS gene reduces flavonoid content and pink fruits in tomato (Schijlen et al., 2007). In Arabidopsis, MYB12 functions as an activator of flavonoid biosynthesis by the transcriptional activation of CHS (Mehrtens et al., 2005). SlMYB12 plays a positive role in the flavonoid biosynthesis pathway in tomato (Ballester et al., 2010; Wang et al., 2018). In this study, RNAi-SlMYB72 lines exhibited higher contents of total flavonoids, rutin, and quercetin than the wild type, and the expression of 4CL and CHSs in the transgenic lines obviously increased compared with those in the wild type (Figs. 5 and 7). SlMYB72 regulated the expression of 4CL and the two CHS genes by directly binding to the promoters of these genes. Our results indicated that SlMYB72 regulates flavonoid biosynthesis by directly targeting 4CL and CHS genes and confirmed the important roles of these genes in the flavonoid biosynthesis pathway. In addition, RNAi-SlMYB72 lines showed higher levels of chlorogenic acid and gallic acid than the wild type. The expression of P-COUMAROYL-COENZYME A 3-HYDROXYLASE (C3H) and CCR-like4 genes also increased in the RNAi-SlMYB72 lines (Figs. 5 and 7). Our data showed that SlMYB72 is involved in phenolic acid biosynthesis by regulating the expression of C3H and CCR-like4 genes.
Uneven Silencing of SlMYB72 Leads to the Uneven Color Phenotype in Fruit
In grasses, basipetal leaf differentiation, chloroplast development, and photosynthetic competency are accompanied by a gradient of differential gene expression (Li et al., 2010). In tomato, a latitudinal gradient of SlGLK2 expression results in a typically uneven coloration of green and ripe fruit. In addition, numerous genes, including KNOX, WRKY, and RAVB3/NGATHA gene families, exhibit expression gradients through the fruit (Nguyen et al., 2014). Two KNOX genes, TKN2 and TKN4, act upstream of SlGLK2 to establish their latitudinal gradient of expression across a developing fruit, resulting in a gradient of chloroplast development (Nadakuduti et al., 2014). In this study, RNAi-SlMYB72 plants exhibited uneven-colored fruits, and the SlMYB72 gene showed lower expression levels in the dark green regions than in the normal green regions of RNAi-SlMYB72 green fruits. POR, CHLH, and TKN2 genes also had different expression levels between the dark green and normal green regions (Fig. 8). SlMYB72 showed a lower expression level in the yellow regions than in the normal red region of RNAi-SlMYB72 red fruits, and the expression levels of LCYB, PSY, ZISO, 4CL, CHS1, and CHS2 genes were also different between the yellow and normal red regions of RNAi-SlMYB72 fruits (Fig. 8). Our results demonstrated that the different expression of SlMYB72 across RNAi-SlMYB72 fruits resulted in uneven coloration and further confirmed the important roles of SlMYB72 in the coloration of developing tomato fruit. Our results also proved that SlMYB72 affects the coloration of fruits via the transcriptional regulation of POR, CHLH, TKN2, LCYB, PSY, ZISO, 4CL, CHS1, and CHS2 genes.
In tomato, fruits with greater numbers of plastids have higher levels of chlorophyll, carotenoids, and flavonoids (Cocaliadis et al., 2014), which suggests a close relationship among the metabolism of chlorophylls, carotenoids, and flavonoids. Our study demonstrated that the close relationship was modulated by the SlMYB72 gene in tomato fruit. Moreover, overexpression of SlMYB72 did not affect the accumulation of chlorophyll, carotenoid, and flavonoid in tomato fruits. The possible reason is that the expression of SlMYB72 may be strictly controlled by microRNA. Another reason is that the function of SlMYB72 may be required for SlARF4. In summary, SlMYB72 is involved in the metabolism of chlorophyll, carotenoid, and flavonoid in tomato fruits. SlMYB72 regulates chlorophyll accumulation by directly interacting with SlARF4 and binding to POR, CHLH, and TKN2 genes. SlMYB72 regulates the biosynthesis of lycopene and β-carotene by directly targeting carotenoid biosynthesis genes, including PSY, ZISO, and LCYB, and regulates flavonoid biosynthesis by directly targeting 4CL and CHS genes (Fig. 8C). Our study demonstrates the important roles of an R2R3 MYB gene in the regulation of secondary metabolism in tomato fruits and provides a candidate gene for the improvement of fruit nutrition by genetic engineering.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Tomato (Solanum lycopersicum) ‘Micro-Tom’ was maintained in our laboratory. Plants were cultivated under standard greenhouse conditions with a 16-h-day/8-h-night cycle, 25°C/20°C day/night temperature, 60% humidity, and 250 mol m−2 s−1 intense light.
Subcellular Localization and Transcriptional Activity Assay
The ORF sequence of the SlMYB72 gene was cloned into PCX-DG vector to generate a SlMYB72-GFP fusion expression vector under the control of the cauliflower mosaic virus (CaMV) 35S promoter. Nicotiana benthamiana leaves were transiently transformed with SlMYB72-GFP vector using Agrobacterium tumefaciens strain GV3101. GFP fluorescence was observed through confocal laser scanning microscopy. All transient expression assays were repeated at least three times. Transcriptional activity assay was performed according to Yuan et al. (2019). The ORF sequence of SlMYB72 was amplified and ligated into pGBKT7 containing a GAL4 DNA-binding domain to obtain the pGBKT7-SlMYB72 vector. The pGBKT7-SlMYB72, negative control (pGBKT7), and positive control (pGBKT7-PtrGRA) vectors were transformed into Y2HGold yeast cells. The yeast cells were cultivated on SD-Trp/His/Ade and cultured in medium without Trp (SD-Trp) or without Trp, His, and Ade (SD-Trp/His/Ade). Transcriptional activity was assayed by the growth status as well as α-galactosidase activity. The primers used are listed in Supplemental Table S4.
Plasmid Construction and Plant Transformation
The SlMYB72 promoter sequence was ligated into the pLP100 vector containing the GUS gene for the construction of the GUS staining vector. For construction of the RNAi-SlMYB72 vector, a 200-bp target fragment of the SlMYB72 gene was inserted around a GUS gene spacer in pCAMBIA2301 under the control of a CaMV 35S promoter and a nopaline synthase terminator. For construction of the SlMYB72-FLAG overexpression vector, a pair of primers with FLAG sequences was used to amplify the ORF sequence of the SlMYB72 gene. Then, the ORF sequence was cloned into plant binary vector 35S pLP100 with CaMV 35S as its promoter. The cv Micro-Tom plants were transformed using the A. tumefaciens-mediated method (Deng et al., 2012). Transgenic seeds of T2 and T3 generations were screened by one-half-strength Murashige and Skoog medium with 100 mg L−1 kanamycin. The primer sequence used is listed in Supplemental Table S4.
RT-qPCR and GUS Staining
RT-qPCR was performed with four biological replicates. The four replicate samples were collected from four different plants. For each replicate, 1 g of sample was harvested for total RNA isolation using the RNeasy Plant Mini Kit (Qiagen). RNA was reverse transcribed into cDNA by the HiScript II Q Select RT SuperMix (Vazyme Biotech). RT-qPCR was performed using the CFX96 real-time PCR detection system (Bio-Rad) following the method described by Zhang et al. (2015). The relative expression levels of genes were calculated from the ΔΔCt values and normalized with actin and ubiquitin genes. The primer sequences used are listed in Supplemental Table S4.
For GUS staining, tissues were collected and infiltrated for 15 min with GUS staining solution (0.1 m sodium phosphate buffer, pH 7.2, and 10 mm EDTA). The samples were incubated in the GUS staining solution at 37°C for 24 h. Then, the samples were washed via a series of graded ethanol and observed with a light microscope.
Measurement of Chlorophylls, Carotenoids, and Flavonoids
Chlorophyll and carotenoid contents were measured using HPLC according to the methods described by Saladié et al. (2014). All samples were protected from light and heat during all steps of the extraction procedure. Lyophilized tissue samples (20 mg) were used for extraction. HPLC analysis was performed on an Agilent 1260 Series liquid chromatograph system (Agilent Technologies). A YMC C30 column (4.6 × 250 mm) was employed, and the column temperature was maintained at 30°C. The injection volume for all samples was 10 μL, and detections were made at 450 nm. Flavonoid content was determined according to the method described by Jian et al. (2019). All the measurements were performed with three biological replicates. The three replicate samples were collected from three different plants, and each sample contained three fruits from the same plant.
Measurement of Photosynthesis Rates and Observation of Autofluorescence in Fruits
Photosynthesis rates were measured using the PAM-2500 pulse-amplitude modulation fluorometer (Walz) according to the method described by Maury et al. (1996). The autofluorescence of chloroplasts and chromoplasts was observed using confocal laser scanning microscopy. The emitted fluorescence was collected at 650 to 750 nm for chlorophylls and at 600 to 700 nm for carotenoids. Plastids in fruit mesocarp were examined via an FEI Tecnai T12 twin transmission electron microscope based on the method described by Nguyen et al. (2014). The tissues were saturated for 1.5 h in a freshly prepared solution of 0.25% (w/v) diphenylboric acid-2-amino ethyl ester and 0.00375% (v/v) Triton X-100 and imaged by confocal laser scanning microscopy for the observation of flavonoid autofluorescence (Stracke et al., 2007).
RNA-Seq Analysis
The red fruits (45 DPA) of wild-type and RNAi-SlMYB72 plants were collected for RNA-seq analysis. Total RNA was extracted using a DNeasy Plant Mini Kit (Qiagen), and RNA-seq was performed at Shanghai Majorbio Biopharm Technology as described by Zhang et al. (2015). KEGG pathway analysis was carried out using KOBAS (http://kobas.cbi.pku.edu.cn/kobas3). Pathway enrichment was analyzed using the Benjamini and Hochberg correction method with false discovery rate < 0.05. Functional analysis of DEGs was performed using MapMan software (Thimm et al., 2004).
Y2H Assays
Y2H assays were conducted following the manufacturer’s instructions (Clontech). The ORF sequence of SlMYB72 was cloned into the pGADT7 vector. SlARF4 possessed transcriptional activator activity; therefore, a truncated SlARF4 sequence (1–2,331) was cloned, in which the truncated SlARF4 protein did not show activator activity. The truncated SlARF4 sequence (1–2,331) was cloned into the pGBDT7 vector. The AD and BD fusion constructs were cotransformed into Y2HGold yeast cells and screened on SD medium lacking Trp and Leu and SD medium lacking Trp, Leu, His, and Ade. Protein interactions were analyzed on the basis of growth status and α-galactosidase activity. The primers used are listed in Supplemental Table S4.
BiFC and Pull-Down Assay
Vectors pXY106 and pXY104, which respectively carry the N- and C-terminal halves of yellow fluorescent protein, were used in the BiFC assay. The ORF sequences of SlMYB72 and SlARF4 were cloned into pXY104 and pXY106 vectors, respectively. The vectors were cotransformed into N. benthamiana leaves and observed by confocal laser scanning microscopy. For pull-down assays, 5 μg of GST tag-fused SlMYB72 protein was incubated with GST bind resin (GenScript, L0026) at 4°C for 8 h, washed with phosphate-buffered saline, and mixed with 2 μg of purified His tag-fused SlARF4 protein. The incubation continued for another 6 h, after which the resin was washed with binding buffer three times, and 100 μL of elution buffer was added for 1 h at 4°C. The results were analyzed by western blotting using an anti-His antibody (Proteintech).
EMSA and ChIP-qPCR Analysis
The ORF sequence of SlMYB72 was cloned and fused with the GST tag in pGEX-4T-1 vectors (GE Healthcare Life Science) and expressed in Escherichia coli strain BM Rosetta (DE3) by induction with 0.5 mm isopropyl-β-d-thiogalactopyranoside for 6 h at 20°C. The recombinant proteins were purified with a GST-tagged protein purification kit (Clontech). The probes that contain the ACCAAC/ACCAAA motif sequence from the promoters of POR, CHLH, TKN2, PSY, ZISO, LCYBs, 4CL, CHS1, and CHS2 were biotin labeled with a light shift chemiluminescent EMSA kit (Thermo Fisher Scientific). The unlabeled sequence was used as the competitor, and an AAAAAA fragment was used as the mutant probe in the assay. ChIP-qPCR assay was performed following the method described by Qin et al. (2012). Fruit tissues of SlMYB72-FLAG overexpression plants were used for formaldehyde cross-linking. All primer sequences used are listed in Supplemental Table S4.
Statistical Analyses
Duncan’s multiple range test or Student’s t test was used for statistical analysis.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers Solyc07g055000 (SlMYB72), Solyc11g069190 (SlARF4), Solyc04g015750 (CHLH), Solyc12g013710 (POR), Solyc02g081220 (TKN2), Solyc03g031860 (PSY), Solyc12g098710 (ZISO), Solyc04g040200 (LCYB), Solyc03g097030 (4CL), Solyc09g091510 (CHS1), and Solyc05g053550 (CHS2).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Sequences and phylogenetic analysis of SlMYB72 protein.
Supplemental Figure S2. RT-qPCR analysis of the expression of SlMYB72 in overexpression lines.
Supplemental Figure S3. Analysis of accumulations of chlorophylls, carotenoids, and flavonoids in pericarps of OE-SlMYB72 fruits.
Supplemental Figure S4. Photosynthetic performance in fruits of RNAi-SlMYB72 plants.
Supplemental Figure S5. RNA-seq analysis of RNAi-SlMYB72 plants.
Supplemental Figure S6. SDS-PAGE for protein purification and pull-down assay for SlMYB72 interaction with SlARF4.
Supplemental Table S1. DEGs in RNAi-SlMYB72 versus wild-type plants.
Supplemental Table S2. KEGG pathway enrichment analyses.
Supplemental Table S3. MapMan analysis of DEGs.
Supplemental Table S4. Primers used in this study.
Acknowledgments
We thank Jianye Chen and Jianfei Kuang for providing experimental instruction and instruments.
Footnotes
This work was supported by the National Key Research and Development Program (grant no. 2016YFD0400100), the National Natural Science Foundation of China (grant no. 31960618), the Graduate Research and Innovation Foundation of Chongqing, China (grant no. CYB19071), and the Project on Promoting Scientific Research Cooperation and Talents Training with America and Oceania (grant no. 0903005109137).
References
- Ampomah-Dwamena C, Thrimawithana AH, Dejnoprat S, Lewis D, Espley RV, Allan AC(2019) A kiwifruit (Actinidia deliciosa) R2R3-MYB transcription factor modulates chlorophyll and carotenoid accumulation. New Phytol 221: 309–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arango J, Jourdan M, Geoffriau E, Beyer P, Welsch R(2014) Carotene hydroxylase activity determines the levels of both α-carotene and total carotenoids in orange carrots. Plant Cell 26: 2223–2233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester AR, Molthoff J, de Vos R, Hekkert B, Orzaez D, Fernández-Moreno JP, Tripodi P, Grandillo S, Martin C, Heldens J, et al. (2010) Biochemical and molecular analysis of pink tomatoes: Deregulated expression of the gene encoding transcription factor SlMYB12 leads to pink tomato fruit color. Plant Physiol 152: 71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry A, Heim MA, Dubreucq B, Caboche M, Weisshaar B, Lepiniec L(2004) TT2, TT8, and TTG1 synergistically specify the expression of BANYULS and proanthocyanidin biosynthesis in Arabidopsis thaliana. Plant J 39: 366–380 [DOI] [PubMed] [Google Scholar]
- Bemer M, Karlova R, Ballester AR, Tikunov YM, Bovy AG, Wolters-Arts M, Rossetto P, Angenent GC, de Maagd RA(2012) The tomato FRUITFULL homologs TDR4/FUL1 and MBP7/FUL2 regulate ethylene-independent aspects of fruit ripening. Plant Cell 24: 4437–4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chayut N, Yuan H, Ohali S, Meir A, Sa’ar U, Tzuri G, Zheng Y, Mazourek M, Gepstein S, Zhou X, et al. (2017) Distinct mechanisms of the ORANGE protein in controlling carotenoid flux. Plant Physiol 173: 376–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MY, Vrebalov J, Alba R, Lee J, McQuinn R, Chung JD, Klein P, Giovannoni J(2010) A tomato (Solanum lycopersicum) APETALA2/ERF gene, SlAP2a, is a negative regulator of fruit ripening. Plant J 64: 936–947 [DOI] [PubMed] [Google Scholar]
- Cocaliadis MF, Fernández-Muñoz R, Pons C, Orzaez D, Granell A(2014) Increasing tomato fruit quality by enhancing fruit chloroplast function: A double-edged sword? J Exp Bot 65: 4589–4598 [DOI] [PubMed] [Google Scholar]
- Deng W, Yang Y, Ren Z, Audran-Delalande C, Mila I, Wang X, Song H, Hu Y, Bouzayen M, Li Z(2012) The tomato SlIAA15 is involved in trichome formation and axillary shoot development. New Phytol 194: 379–390 [DOI] [PubMed] [Google Scholar]
- Egea I, Bian W, Barsan C, Jauneau A, Pech JC, Latché A, Li Z, Chervin C(2011) Chloroplast to chromoplast transition in tomato fruit: Spectral confocal microscopy analyses of carotenoids and chlorophylls in isolated plastids and time-lapse recording on intact live tissue. Ann Bot 108: 291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- España L, Heredia-Guerrero JA, Reina-Pinto JJ, Fernández-Muñoz R, Heredia A, Domínguez E(2014) Transient silencing of CHALCONE SYNTHASE during fruit ripening modifies tomato epidermal cells and cuticle properties. Plant Physiol 166: 1371–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser PD, Enfissi EM, Halket JM, Truesdale MR, Yu D, Gerrish C, Bramley PM(2007) Manipulation of phytoene levels in tomato fruit: Effects on isoprenoids, plastids, and intermediary metabolism. Plant Cell 19: 3194–3211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum SM.(2004) Potential impact of strawberries on human health: A review of the science. Crit Rev Food Sci Nutr 44: 1–17 [DOI] [PubMed] [Google Scholar]
- Havsteen BH.(2002) The biochemistry and medical significance of the flavonoids. Pharmacol Ther 96: 67–202 [DOI] [PubMed] [Google Scholar]
- Itkin M, Seybold H, Breitel D, Rogachev I, Meir S, Aharoni A(2009) TOMATO AGAMOUS-LIKE 1 is a component of the fruit ripening regulatory network. Plant J 60: 1081–1095 [DOI] [PubMed] [Google Scholar]
- Jian W, Cao H, Yuan S, Liu Y, Lu J, Lu W, Li N, Wang J, Zou J, Tang N, et al. (2019) SlMYB75, an MYB-type transcription factor, promotes anthocyanin accumulation and enhances volatile aroma production in tomato fruits. Hortic Res 6: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B, Gu Q, Tian P, Xiao L, Cao H, Yang W(2014) A chimeric transcript containing Psy1 and a potential mRNA is associated with yellow flesh color in tomato accession PI 114490. Planta 240: 1011–1021 [DOI] [PubMed] [Google Scholar]
- Kang C, Zhai H, Xue L, Zhao N, He S, Liu Q(2018) A lycopene β-cyclase gene, IbLCYB2, enhances carotenoid contents and abiotic stress tolerance in transgenic sweetpotato. Plant Sci 272: 243–254 [DOI] [PubMed] [Google Scholar]
- Kim YS, Kim YB, Kim Y, Lee MY, Park SU(2014) Overexpression of cinnamate 4-hydroxylase and 4-coumaroyl CoA ligase prompted flavone accumulation in Scutellaria baicalensis hairy roots. Nat Prod Commun 9: 803–807 [PubMed] [Google Scholar]
- Klee HJ, Giovannoni JJ(2011) Genetics and control of tomato fruit ripening and quality attributes. Annu Rev Genet 45: 41–59 [DOI] [PubMed] [Google Scholar]
- Kolotilin I, Koltai H, Tadmor Y, Bar-Or C, Reuveni M, Meir A, Nahon S, Shlomo H, Chen L, Levin I(2007) Transcriptional profiling of high pigment-2dg tomato mutant links early fruit plastid biogenesis with its overproduction of phytonutrients. Plant Physiol 145: 389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavhale SG, Kalunke RM, Giri AP(2018) Structural, functional and evolutionary diversity of 4-coumarate-CoA ligase in plants. Planta 248: 1063–1078 [DOI] [PubMed] [Google Scholar]
- Lee JM, Joung JG, McQuinn R, Chung MY, Fei Z, Tieman D, Klee H, Giovannoni J(2012) Combined transcriptome, genetic diversity and metabolite profiling in tomato fruit reveals that the ethylene response factor SlERF6 plays an important role in ripening and carotenoid accumulation. Plant J 70: 191–204 [DOI] [PubMed] [Google Scholar]
- Li P, Ponnala L, Gandotra N, Wang L, Si Y, Tausta SL, Kebrom TH, Provart N, Patel R, Myers CR, et al. (2010) The developmental dynamics of the maize leaf transcriptome. Nat Genet 42: 1060–1067 [DOI] [PubMed] [Google Scholar]
- Li S.(2014) Transcriptional control of flavonoid biosynthesis: Fine-tuning of the MYB-bHLH-WD40 (MBW) complex. Plant Signal Behav 9: e27522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, Hong Y, Yin M, Li C, Zhang K, Grierson D(2008) A tomato HD-Zip homeobox protein, LeHB-1, plays an important role in floral organogenesis and ripening. Plant J 55: 301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Van Eck J, Zhou X, Lopez AB, O’Halloran DM, Cosman KM, Conlin BJ, Paolillo DJ, Garvin DF, Vrebalov J, et al. (2006) The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high levels of β-carotene accumulation. Plant Cell 18: 3594–3605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Butelli E, Hill L, Parr A, Niggeweg R, Bailey P, Weisshaar B, Martin C(2008) AtMYB12 regulates caffeoyl quinic acid and flavonol synthesis in tomato: Expression in fruit results in very high levels of both types of polyphenol. Plant J 56: 316–326 [DOI] [PubMed] [Google Scholar]
- Ma Z, Hu X, Cai W, Huang W, Zhou X, Luo Q, Yang H, Wang J, Huang J(2014) Arabidopsis miR171-targeted scarecrow-like proteins bind to GT cis-elements and mediate gibberellin-regulated chlorophyll biosynthesis under light conditions. PLoS Genet 10: e1004519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning K, Tör M, Poole M, Hong Y, Thompson AJ, King GJ, Giovannoni JJ, Seymour GB(2006) A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat Genet 38: 948–952 [DOI] [PubMed] [Google Scholar]
- Martel C, Vrebalov J, Tafelmeyer P, Giovannoni JJ(2011) The tomato MADS-box transcription factor RIPENING INHIBITOR interacts with promoters involved in numerous ripening processes in a COLORLESS NONRIPENING-dependent manner. Plant Physiol 157: 1568–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury P, Mojayad F, Berger M, Planchon C(1996) Photochemical response to drought acclimation in two sunflower genotypes. Physiol Plant 98: 57–66 [Google Scholar]
- Mehrtens F, Kranz H, Bednarek P, Weisshaar B(2005) The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis. Plant Physiol 138: 1083–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Fan Z, Zhang Q, Wang C, Gao Y, Deng Y, Zhu B, Zhu H, Chen J, Shan W, et al. (2018) BEL1-LIKE HOMEODOMAIN 11 regulates chloroplast development and chlorophyll synthesis in tomato fruit. Plant J 94: 1126–1140 [DOI] [PubMed] [Google Scholar]
- Nadakuduti SS, Holdsworth WL, Klein CL, Barry CS(2014) KNOX genes influence a gradient of fruit chloroplast development through regulation of GOLDEN2-LIKE expression in tomato. Plant J 78: 1022–1033 [DOI] [PubMed] [Google Scholar]
- Nguyen CV, Vrebalov JT, Gapper NE, Zheng Y, Zhong S, Fei Z, Giovannoni JJ(2014) Tomato GOLDEN2-LIKE transcription factors reveal molecular gradients that function during fruit development and ripening. Plant Cell 26: 585–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Bradley G, Pyke K, Ball G, Lu C, Fray R, Marshall A, Jayasuta S, Baxter C, van Wijk R, et al. (2013) Network inference analysis identifies an APRR2-like gene linked to pigment accumulation in tomato and pepper fruits. Plant Physiol 161: 1476–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ALT, Nguyen CV, Hill T, Cheng KL, Figueroa-Balderas R, Aktas H, Ashrafi H, Pons C, Fernández-Muñoz R, Vicente A, et al. (2012) Uniform ripening encodes a Golden 2-like transcription factor regulating tomato fruit chloroplast development. Science 336: 1711–1715 [DOI] [PubMed] [Google Scholar]
- Qin G, Wang Y, Cao B, Wang W, Tian S(2012) Unraveling the regulatory network of the MADS box transcription factor RIN in fruit ripening. Plant J 70: 243–255 [DOI] [PubMed] [Google Scholar]
- Rohrmann J, Tohge T, Alba R, Osorio S, Caldana C, McQuinn R, Arvidsson S, van der Merwe MJ, Riaño-Pachón DM, Mueller-Roeber B, et al. (2011) Combined transcription factor profiling, microarray analysis and metabolite profiling reveals the transcriptional control of metabolic shifts occurring during tomato fruit development. Plant J 68: 999–1013 [DOI] [PubMed] [Google Scholar]
- Sagar M, Chervin C, Mila I, Hao Y, Roustan JP, Benichou M, Gibon Y, Biais B, Maury P, Latché A, et al. (2013) SlARF4, an auxin response factor involved in the control of sugar metabolism during tomato fruit development. Plant Physiol 161: 1362–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagawa JM, Stanley LE, LaFountain AM, Frank HA, Liu C, Yuan YW(2016) An R2R3-MYB transcription factor regulates carotenoid pigmentation in Mimulus lewisii flowers. New Phytol 209: 1049–1057 [DOI] [PubMed] [Google Scholar]
- Saladié M, Wright LP, Garcia-Mas J, Rodriguez-Concepcion M, Phillips MA(2014) The 2-C-methylerythritol 4-phosphate pathway in melon is regulated by specialized isoforms for the first and last steps. J Exp Bot 65: 5077–5092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schijlen EG, de Vos CH, Martens S, Jonker HH, Rosin FM, Molthoff JW, Tikunov YM, Angenent GC, van Tunen AJ, Bovy AG(2007) RNA interference silencing of chalcone synthase, the first step in the flavonoid biosynthesis pathway, leads to parthenocarpic tomato fruits. Plant Physiol 144: 1520–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Ishihara H, Huep G, Barsch A, Mehrtens F, Niehaus K, Weisshaar B(2007) Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J 50: 660–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracke R, Jahns O, Keck M, Tohge T, Niehaus K, Fernie AR, Weisshaar B(2010) Analysis of PRODUCTION OF FLAVONOL GLYCOSIDES-dependent flavonol glycoside accumulation in Arabidopsis thaliana plants reveals MYB11-, MYB12- and MYB111-independent flavonol glycoside accumulation. New Phytol 188: 985–1000 [DOI] [PubMed] [Google Scholar]
- Su L, Diretto G, Purgatto E, Danoun S, Zouine M, Li Z, Roustan JP, Bouzayen M, Giuliano G, Chervin C(2015) Carotenoid accumulation during tomato fruit ripening is modulated by the auxin-ethylene balance. BMC Plant Biol 15: 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimm O, Bläsing O, Gibon Y, Nagel A, Meyer S, Krüger P, Selbig J, Müller LA, Rhee SY, Stitt M(2004) MAPMAN: A user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes. Plant J 37: 914–939 [DOI] [PubMed] [Google Scholar]
- Vrebalov J, Pan IL, Arroyo AJM, McQuinn R, Chung M, Poole M, Rose J, Seymour G, Grandillo S, Giovannoni J, et al. (2009) Fleshy fruit expansion and ripening are regulated by the tomato SHATTERPROOF gene TAGL1. Plant Cell 21: 3041–3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J(2002) A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science 296: 343–346 [DOI] [PubMed] [Google Scholar]
- Wang S, Chu Z, Jia R, Dan F, Shen X, Li Y, Ding X(2018) SlMYB12 regulates flavonol synthesis in three different cherry tomato varieties. Sci Rep 8: 1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters MT, Wang P, Korkaric M, Capper RG, Saunders NJ, Langdale JA(2009) GLK transcription factors coordinate expression of the photosynthetic apparatus in Arabidopsis. Plant Cell 21: 1109–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel-Shirley B.(2001) Flavonoid biosynthesis: A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126: 485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Dubos C, Lepiniec L(2015) Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci 20: 176–185 [DOI] [PubMed] [Google Scholar]
- Yazdani M, Sun Z, Yuan H, Zeng S, Thannhauser TW, Vrebalov J, Ma Q, Xu Y, Fei Z, Van Eck J, et al. (2019) Ectopic expression of ORANGE promotes carotenoid accumulation and fruit development in tomato. Plant Biotechnol J 17: 33–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Mei L, Wu M, Wei W, Shan W, Gong Z, Zhang Q, Yang F, Yan F, Zhang Q, et al. (2018) SlARF10, an auxin response factor, is involved in chlorophyll and sugar accumulation during tomato fruit development. J Exp Bot 69: 5507–5518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Xu X, Gong Z, Tang Y, Wu M, Yan F, Zhang X, Zhang Q, Yang F, Hu X, et al. (2019) Auxin response factor 6A regulates photosynthesis, sugar accumulation, and fruit development in tomato. Hortic Res 6: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yan F, Tang Y, Yuan Y, Deng W, Li Z(2015) Auxin response gene SlARF3 plays multiple roles in tomato development and is involved in the formation of epidermal cells and trichomes. Plant Cell Physiol 56: 2110–2124 [DOI] [PubMed] [Google Scholar]
- Zheng G, Fan C, Di S, Wang X, Gao L, Dzyubenko N, Chapurin V, Pang Y(2019) Ectopic expression of tea MYB genes alter spatial flavonoid accumulation in alfalfa (Medicago sativa). PLoS ONE 14: e0218336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Welsch R, Yang Y, Álvarez D, Riediger M, Yuan H, Fish T, Liu J, Thannhauser TW, Li L(2015) Arabidopsis OR proteins are the major posttranscriptional regulators of phytoene synthase in controlling carotenoid biosynthesis. Proc Natl Acad Sci USA 112: 3558–3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F, Luo T, Liu C, Wang Y, Yang H, Yang W, Zheng L, Xiao X, Zhang M, Xu R, et al. (2017) An R2R3-MYB transcription factor represses the transformation of α- and β-branch carotenoids by negatively regulating expression of CrBCH2 and CrNCED5 in flavedo of Citrus reticulate. New Phytol 216: 178–192 [DOI] [PubMed] [Google Scholar]
- Zhu M, Chen G, Zhang J, Zhang Y, Xie Q, Zhao Z, Pan Y, Hu Z(2014) The abiotic stress-responsive NAC-type transcription factor SlNAC4 regulates salt and drought tolerance and stress-related genes in tomato (Solanum lycopersicum). Plant Cell Rep 33: 1851–1863 [DOI] [PubMed] [Google Scholar]