Integrated root phenotypes that optimize water use efficiency and overcome mechanical impedance from dry, hard soils are beneficial for drought tolerance in maize.
Abstract
To test the hypothesis that multiple integrated root phenotypes would co-optimize drought tolerance, we phenotyped the root anatomy and architecture of 400 mature maize (Zea mays) genotypes under well-watered and water-stressed conditions in the field. We found substantial variation in all 23 root phenes measured. A phenotypic bulked segregant analysis revealed that bulks representing the best and worst performers in the field displayed distinct root phenotypes. In contrast to the worst bulk, the root phenotype of the best bulk under drought consisted of greater cortical aerenchyma formation, more numerous and narrower metaxylem vessels, and thicker nodal roots. Partition-against-medians clustering revealed several clusters of unique root phenotypes related to plant performance under water stress. Clusters associated with improved drought tolerance consisted of phene states that likely enable greater soil exploration by reallocating internal resources to greater root construction (increased aerenchyma content, larger cortical cells, fewer cortical cell files), restrict uptake of water to conserve soil moisture (reduced hydraulic conductance, narrow metaxylem vessels), and improve penetrability of hard, dry soils (thick roots with a larger proportion of stele, and smaller distal cortical cells). We propose that the most drought-tolerant–integrated phenotypes merit consideration as breeding ideotypes.
Drought is the most substantial threat to crop yields worldwide (Boyer, 1982) and is expected to offset yield increases attained from technological advances and the elevated carbon dioxide fertilization effect (Lobell et al., 2011). Maize (Zea mays) is one of the most economically and nutritionally important crops worldwide, and its production is highly vulnerable to drought in the face of climate change (Dai, 2013)—particularly in the low input agricultural ecosystems of the Global South, where almost two-thirds of the world’s maize production area is found (Dowswell et al., 1996). Roots are a natural and often underexploited target for crop improvement under drought stress as the organ most responsible for the uptake of water (Vadez, 2014; Bishopp and Lynch, 2015).
Root system architecture largely determines the spatiotemporal capture of water within the soil profile (Lynch, 1995). Several phenes (“phene” is to phenotype as “gene” is to genotype; Lynch, 2011; Pieruschka and Poorter, 2012; York et al., 2013) contribute to overall root system architecture and have important roles in resource capture, particularly in resource-limiting environments. Root phenotypes can substantially improve the capture of both mobile resources such as water or nitrogen (N), and immobile nutrients such as phosphorus (P; Lynch and Brown, 2001, 2012; Ho et al., 2005; Yu et al., 2014). Root-growth angle is a key phene for accessing nutrients in the soil, especially in poor soils (Lynch, 1995, 2013, 2019). Roots with steep angles are ideal for accessing mobile nutrients that quickly move through the soil profile and become more concentrated at greater depth, such as water and N (Trachsel et al., 2013; Uga et al., 2013; Lynch and Wojciechowski, 2015). Shallow-angled roots are better able to access nutrients more concentrated in the shallow soil layers, such as P (Lynch and Brown, 2001; Liao et al., 2004; Ho et al., 2005; Zhu et al., 2005). The length and density of lateral roots is also important for plant performance. Maize plants with fewer, longer lateral roots are more ideal for capturing mobile nutrients by reducing the inter- and intraplant competition (Wasson et al., 2012; Zhan et al., 2015; Zhan and Lynch, 2015) while plants with denser, shorter laterals are more suited for foraging P in the topsoil (Postma et al., 2014; Jia et al., 2018). The distance to the first lateral root may also be important—a longer distance to the first lateral would decrease intraplant competition for mobile resources (York and Lynch, 2015).
Multiple root anatomical phenes are associated with the hydraulic safety and conductive ability of the plant. Variation in root metaxylem size is related to xylem function under drought stress (Hacke and Sperry, 2001). Narrower vessels are correlated with enhanced drought tolerance and are less susceptible to cavitation (Richards and Passioura, 1989; Comas et al., 2013). Richards and Passioura (1989) found that selecting for narrower metaxylem vessels in wheat (Triticum aestivum) seminal roots led to greater yields under drought with no penalty under well-watered conditions. Restricted hydraulic conductance may be beneficial for mitigating drought effects by reducing transpiration via induced stomatal closure (Vadez et al., 2014), increasing water-use efficiency by reducing the overall shoot size, and retaining moisture at the root tips to enable further growth through the soil profile (Lynch, 2019). Restricted hydraulic conductance may also contribute to “water banking,” a phenomenon where soil moisture is depleted at slower rates to sustain the plant until later in the growth season during the critical reproductive stages (Feng et al., 2016). In chickpea (Cicer arietinum), conservative water usage was found to be more beneficial than deep or profuse rooting (Zaman-Allah et al., 2011).
Several root anatomical phenes are important for reducing the metabolic costs of root maintenance. Root cortical aerenchyma are air-filled lacunae that form in the root cortex from programmed cell death (Drew et al., 2000). Aerenchyma replace the respiring cortical tissue, thereby reducing the overall metabolic burden to maintain the root tissue (Saengwilai et al., 2014a; Chimungu et al., 2015b). A root with high aerenchyma content demands less carbon, N, and P for metabolic maintenance, and these remobilized nutrients are reallocated to greater root growth, facilitating greater soil exploration and subsequent soil resource acquisition (Postma et al., 2014; Galindo-Castañeda et al., 2018). Greater aerenchyma formation in maize is associated with greater biomass (Zhu et al., 2010; Jaramillo et al., 2013; Chimungu et al., 2015b; Díaz et al., 2018) and greater yields (Chimungu et al., 2015b) under water deficit. Similarly, a cortex comprised of larger cortical cells arranged in fewer cortical cell files may reduce the metabolic burden of the entire cortex (Colombi et al., 2019), and is associated with improved yields in water-limiting environments (Chimungu et al., 2014a, 2014b).
Phenotypic plasticity of root phenes is a widespread and important phenomenon for the enhanced capture of edaphic resources. Several abiotic and biotic factors limit crop productivity, and phenotypic plasticity may enable plants to adapt to spatial and temporal changes in their environment. Several root anatomical and architectural phenes display phenotypic plasticity. In water-stress conditions, metaxylem vessel number increased in soybean (Glycine max; Prince et al., 2017) and xylem vessel diameter and number and stele diameter were plastic in wheat and rice (Oryza sativa; Kadam et al., 2017). In water-stress conditions, plasticity in lateral root length and density correlated with greater shoot biomass and water uptake (Kano et al., 2011; Kano-Nakata et al., 2013). The number of nodal roots in rice (Suralta et al., 2010) and maize (Gao and Lynch, 2016) display plastic responses to water deficit. In low P environments, plasticity in secondary root growth was associated with greater root depth and shoot growth in common bean (Phaseolus vulgaris; Strock et al., 2018), and plasticity in root hair length was associated with increased plant performance (Zhu et al., 2010). Root anatomical and architectural phenes express a wide range of plastic responses in a wide range of environments and edaphic stresses. However, it remains unclear which plastic responses are adaptive responses to edaphic stress.
Desiccation increases the strength of many agricultural soils, and several root phenes regulate the penetration of hard soil (Bengough et al., 2011; Potocka and Szymanowska-Pułka, 2018; Vanhees et al., 2020). As a result of high mechanical impedance, root diameter increases (Eavis, 1972; Rich and Watt, 2013; Popova et al., 2016) or decreases (Potocka and Szymanowska-Pułka, 2018), and the number and length of lateral roots decreases (Grzesiak et al., 2002; Colombi and Walter, 2016). There is some debate over the role root size plays for penetrative ability. Thick roots are thought to increase the mechanical strength of the root to prevent deflections or buckling (Clark et al., 2003), while thin roots are thought to improve growth into pores in hardened soil (Potocka and Szymanowska-Pułka, 2018; Vanhees et al., 2020). Root tensile strength and penetrative ability increases with increasing stele size in maize (Chimungu et al., 2015a). An increase in root diameter most often results from an increase in radial cortical cell size (Veen, 1982; Colombi et al., 2019). Large cortical cells decrease the energy costs associated with tissue maintenance, thereby facilitating greater root growth, particularly in penetration-resistant soils (Colombi et al., 2019). However, small cells in the distal cortex improved root stabilization preventing buckling or ovalization (Chimungu et al., 2015a). Having a larger number of thick roots is associated with high penetrative ability (Materechera et al., 1992). Root angle may also play a role in overcoming mechanical impedance. As a result of topsoil penetration resistance, maize root systems become shallower and stay shallow as water is depleted and soil hardens further (Colombi et al., 2018), but steep-angled roots may be more capable of applying a greater proportion of force in the vertical direction, thereby increasing the likelihood to penetrate (Correa et al., 2019).
Ideotype breeding is a promising method for generating high-yielding material in a particular environment (Donald, 1968; Lynch, 2019). An ideotype may consist of any number of phenotypic characteristics to optimize performance in a given environment (Peng et al., 2008). The “steep, cheap, and deep” (SCD) root ideotype hypothesized a combination of architectural, anatomical, and physiological phene states contributing to superior water and N acquisition in a resource-limited environment in maize (Lynch, 2013). By being “steeper” (i.e. steeper root architecture) and “cheaper” (i.e. reduced metabolic maintenance costs to allow greater root construction), the SCD ideotype facilitates deeper rooting to access deep water stores. However, the use of that deep water must be optimized. Conservative, rather than profligate, water use was key for terminal drought tolerance in chickpea (Zaman-Allah et al., 2011) and pearl millet (Pennisetum glaucum; Vadez et al., 2013). Subsequently, an ideotype that maximizes root water uptake may not be one that maximizes yield production.
There is no single root phenotype that is ideal across all environments (Dathe et al., 2016; Rangarajan et al., 2018; Strock et al., 2019a). Access to resources may be influenced by some other aspect of the environment, like soil texture (Dathe et al., 2016) or rainfall (Dunbabin et al., 2003). Ultimately, the water use strategy (i.e. water spending or water saving) is likely dependent on the distribution and size of water stores in the soil. Access to resources may also be a result of the phenotype itself. Some of these integrated phenotypes may be synergistic, like the multiplicative increase in P acquisition by longer root hairs coupled with shallow basal roots in common bean (Miguel et al., 2015). A root phenotype that demonstrates utility for improved drought tolerance may inadvertently impair acquisition of other necessary nutrients. For instance, developing fewer lateral branches is advantageous for acquiring mobile resources, like water or N, but maladaptive for capturing immobile resources concentrated in the shallow layers, like P (Postma et al., 2014; Zhan et al., 2015; Zhan and Lynch, 2015; Jia et al., 2018). A phene state’s synergistic or antagonistic effects are largely dependent on the other components of its phenotype (Rangarajan et al., 2018). For example, root tissue containing numerous small cortical cells is maladaptive for drought tolerance, but a high degree of aerenchyma formation compensates to increase metabolic parsimony. Thus, it is important to consider how fitness optima are related to individual phene aggregates, or the integrated phenotype.
Furthermore, there may actually be several integrated phenotypes resulting in optimal fitness outcomes instead of only one perfect phenotype posited by a single ideotype. In common bean, (Rangarajan et al., 2018) several in silico-integrated phenotypes have been reported, including different combinations of basal root whorl number, basal root growth angle, adventitious root number, and lateral root branching density, which resulted in comparable biomass under low P. When all architectural and anatomical phenes are considered, the number of optimal integrated phenotypes will be exponentially greater. Exploiting highly diverse genetic and phenotypic material would enable identification of multiple integrated phenotypes correlated with improved fitness in crops exposed to stressful conditions in the field (Donald, 1968; Chitwood and Topp, 2015).
The utility of individual components of the SCD ideotype has been validated in isophenic studies under limited N (Saengwilai et al., 2014a, 2014b; Zhan and Lynch, 2015; Dathe et al., 2016) and water (Zhu et al., 2010; Chimungu et al., 2014a, 2014b, 2015b; Gao and Lynch, 2016). Integrated architectural phenotypes optimizing resource acquisition have been evaluated in silico for maize with suboptimal N availability (Dathe et al., 2016) and for common bean under limited N and P (Rangarajan et al., 2018). While in silico research is useful in this context because it permits the analysis of many phenotypic combinations in many environments, these combinations need to be validated in the field. Several population-specific integrated root phenotypes in common bean were reported (Strock et al., 2019a) to have variable fitness under conditions of non-stress, drought, and low-fertility soils. Likewise, York and Lynch (2015) reported the additive effect of multiple architectural root phenes on shoot growth under deficient N, where a substantial portion of the variance related to shoot growth under low N was related to six specific architectural phenes. However, both studies were limited to integrated root architectural phenes, and did not account for anatomical phene interactions.
To test the hypothesis that multiple integrated phenotypes are related to plant fitness under drought, we phenotyped the root systems of 400 maize genotypes under well-watered and water-deficit stress conditions in the field to identify unique clusters of root phenotypes related to plant performance. Our aim was to (1) quantify variation in anatomical and architectural phenes in well-watered and water-stressed conditions in the field; and (2) identify combinations of root phenes associated with drought tolerance.
RESULTS
Wide Natural Diversity in Root Phenotypes under Stress and Non-Stress Conditions
Water deficit on average reduced vegetative biomass by 21.4% and dry cob weight by 39.1% (Supplemental Fig. S1). There was wide natural variation for all root phenes measured under both well-watered and water-deficit conditions (Table 1; Supplemental Fig. S2). Extensive phenotypic diversity was accompanied by wide variation in flowering time (Supplemental Fig. S3).
Table 1. Means and 95% confidence intervals of all root phenes under well-watered and water-stressed conditions.
The significance of treatment effects on each root was evaluated using a Mann–Whitney test.
| Phene No. | Phene | Units | Well-Watered | Water Stress | P |
|---|---|---|---|---|---|
| 1 | Root angle | ° from soil surface | 55.307 ± 0.832 | 52.766 ± 0.809 | <0.0001 |
| 2 | Lateral root branching frequency | no. lateral roots/cm axial root | 10.34 ± 1.698 | 10.796 ± 1.724 | 0.3042 |
| 3 | Distance to first lateral root | mm | 50.239 ± 1.644 | 48.859 ± 1.623 | 0.3186 |
| 4 | Lateral root length | mm | 161.466 ± 3.445 | 159.11 ± 3.393 | 0.4051 |
| 5 | Root cross-sectional area | mm2 | 2.61 ± 0.042 | 2.401 ± 0.042 | <0.0001 |
| 6 | Total cortex area | mm2 | 1.89 ± 0.03 | 1.742 ± 0.03 | <0.0001 |
| 7 | Total stele area | mm2 | 0.719 ± 0.015 | 0.659 ± 0.014 | <0.0001 |
| 8 | Cortex: stele | N/A | 2.795 ± 0.045 | 2.79 ± 0.039 | 0.5884 |
| 9 | Aerenchyma area | mm2 | 0.371 ± 0.02 | 0.257 ± 0.016 | <0.0001 |
| 10 | Aerenchyma percent of cortex | % | 19.055 ± 0.941 | 14.667 ± 0.776 | <0.0001 |
| 11 | Cortical cell file number | no. | 13.294 ± 0.182 | 12.166 ± 0.131 | <0.0001 |
| 12 | Cortical cell size (Z0) | μm2 | 275.013 ± 5.832 | 307.639 ± 6.331 | <0.0001 |
| 13 | Cortical cell size (Z1) | μm2 | 332.406 ± 5.739 | 322.941 ± 6.915 | 0.0086 |
| 14 | Cortical cell size (Z2) | μm2 | 188.167 ± 3.556 | 185.453 ± 3.477 | 0.1656 |
| 15 | Median metaxylem area | mm2 | 6.56e−03 ± 1.1e−04 | 6.14e−03 ± 1.10e−04 | <0.0001 |
| 16 | Minimum metaxylem area | mm2 | 1.65e−03 ± 7e−05 | 1.74e−03 ± 8e−05 | 0.3412 |
| 17 | Maximum metaxylem area | mm2 | 1.02e−02 ± 1.6e−04 | 9.36e−03 ± 1.6e−04 | <0.0001 |
| 18 | Range metaxylem area | mm2 | 8.58e−03 ± 1.6e−04 | 7.62e−03 ± 1.5e−04 | <0.0001 |
| 19 | Metaxylem number | no. | 16.777 ± 0.283 | 15.861 ± 0.281 | <0.0001 |
| 20 | Total metaxylem area | mm2 | 0.105 ± 0.002 | 0.092 ± 0.002 | <0.0001 |
| 21 | Hydraulic conductance | mm3 s−1 | 5.86e−05 ± 2.8e−06 | 5.92e−05 ± 2.6e−06 | 0.1159 |
Phenotypic expression was not significantly affected by drought for only seven phenes (lateral root branching frequency, cortical cell size [Z2], distance to the first lateral, lateral length, minimum metaxylem vessel area, cortex to stele ratio, and hydraulic conductance), but watering regime had a significant effect on distributions of all other root phenes (Table 1; Supplemental Fig. S2). Root cross-sectional, cortical, and stele areas were reduced 8% under water deficit stress. Nearly all metaxylem-related phenes were significantly affected by the watering treatment except for minimum metaxylem vessel area. Under water stress, maximum metaxylem vessel area, median metaxylem vessel area, and number of metaxylem were reduced 5.7%, 6.4%, and 5.5%, respectively. Likewise, the range of metaxylem vessel area size and total metaxylem vessel area were reduced 11.2% and 12.5% as a result of water stress, respectively. The cortical phenes exhibited varying responses to water stress. Aerenchyma content as a measure of area and percentage of the cortex was reduced 30.8% and 23%, respectively, under water stress. Cortical cell file number decreased 8.4% under water stress. Cortical cell size increased 11.9% in the outer region of the cortex while cortical cell size in the midcortex decreased 2.9% as a result of water stress. For architecture, only root angle was significantly affected by water deficit, which was reduced by 4.5% under water deficit stress.
Distinct Root Phenotypes Associated with the Strongest and Poorest Performers in the Field
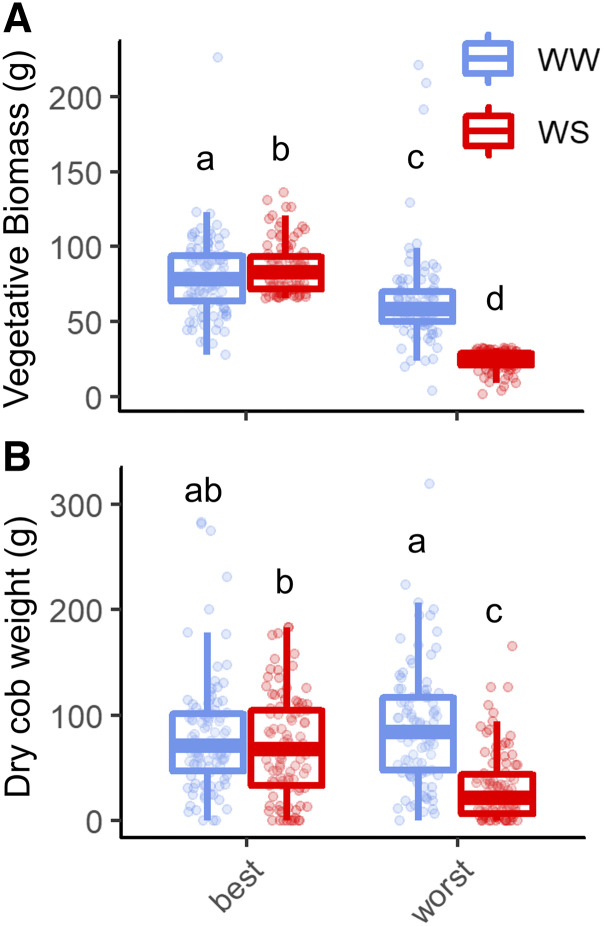
The phenotypic bulked segregant analysis selected 200 individual plants to comprise two pools, or bulks, related to plant performance under water stress. These bulks displayed similar vigor under well-watered conditions—similar yield in both bulks although shoot vegetative biomass was larger in the best bulk—but differing responses to water stress. Shoot biomass increased by 7.5% and cob weight was unchanged under water stress in the bulk of best performers (hereafter “best bulk”; Fig. 1). In contrast, the bulk of poor performers (hereafter “worst bulk”) exhibited a 62% reduction in shoot dry biomass and a 64% reduction in dry cob weight. Additionally, the best bulk reached pollen shed and silking earlier compared to the worst bulk (Supplemental Fig. S4).
Figure 1.
Physiological measures for the best and worst bulks. Boxplots displaying vegetative shoot biomass (A) and dry cob weight (B) under well-watered (WW) and water-stressed (WS) conditions. Letters above each boxplot denote statistically significant differences per Kruskal–Wallis comparison tests with a Benjamini–Hochberg adjustment (α ≤ 0.05).
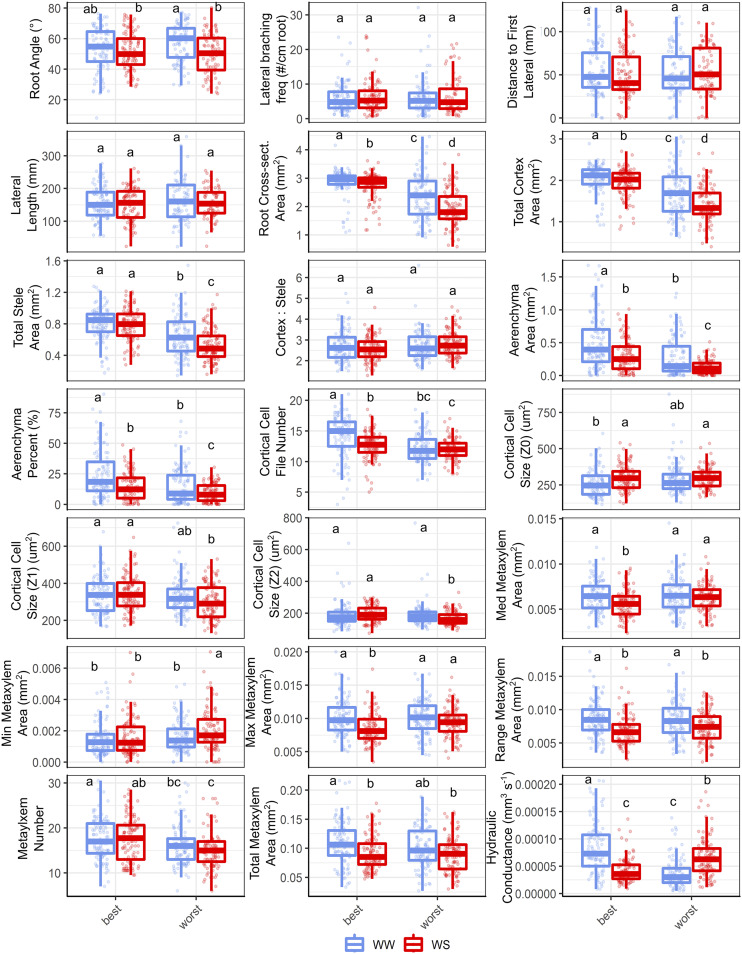
Despite similar fitness in non-stress conditions, the average root phenotypes corresponding to each bulk under well-watered conditions differed in some respects (Fig. 2; Supplemental Table S1). In the best bulk, aerenchyma content (area and percentage of the cortex), root size (root cross-sectional area, cortex area and stele area), and cortical cell file number were greater under non-stress conditions. Metaxylem size-related phenes did not differ between bulks under non-stress conditions, but metaxylem number and hydraulic conductance were greater in the best bulk.
Figure 2.
Phenotypic bulked segregant analysis showing distinct root phenotypic differences between two bulks representing the best and worst performers in the field under well-watered (WW) and water-stressed (WS) conditions. Boxplot displays the median of each group bounded by the first and third quartile with individuals marked as points. Letters above each boxplot denote similarity among groups per Kruskal–Wallis comparison tests with a Benjamini–Hochberg adjustment (α ≤ 0.05).
Root phenotypic differences between bulks were more exaggerated under water stress (Fig. 2; Supplemental Table S2). Compared to the worst bulk, aerenchyma content by area and by percentage of the cortex was 136% and 8% greater, respectively, in the best bulk under water stress. Cortical cell size in the distal cortex was no different between bulks under water stress, but cell size in the midcortex and interior cortex were both 15% larger. Cortical cell file number was 4.5% greater in the best bulk. Despite both bulks displaying an equal range of metaxylem vessel areas and total metaxylem area under water stress, the best bulk contained 17% more metaxylem vessels that were 10% to 15% narrower (minimum, median, and maximum area) than those of the worst bulk. The reduction in metaxylem vessel size coincided with a significant decrease in theoretical hydraulic conductance in the best bulk under water stress, which was 35% lower than that of the worst bulk. In the best bulk under water stress, the root cross-sectional area, the total cortical area, and the total stele area were 40% greater than the worst bulk, and there were no differences in the proportion of cortex to stele.
The degree of plasticity of each root phene was assessed in each bulk as the relative change in each phene’s state as a result of water stress (Fig. 3; Supplemental Table S2). A negative plasticity value indicates the phene’s dimensions lessened whereas a positive plasticity value indicates enlarging as a result of water stress. Plasticity in several root phenes—lateral root branching frequency, distance to the first lateral, lateral length, aerenchyma area and percent, and cortical cell size (Z0)—were consistently positive in both bulks, indicating that the dimensions of these phenes increased under water stress. All other plastic responses varied between bulks. For root angle, the plasticity values were positive in the best bulk but negative in the worst bulk. Plasticity in all cortical cell file number and root size phenes—root cross-sectional area, total cortex area, stele area, cortex:stele—was very low, but plasticity in the best bulk was more negative than that of the worst bulk. Cortical cell size in the mid- and interior regions of the cortex displayed low negative plasticity in the worst bulk, but positive plasticity in the best bulk. Plasticity in median and maximum metaxylem vessel number was negative in the best bulk, in contrast to the worst bulk where these values were nearly zero. Plasticity of minimum metaxylem area was positive in both bulks, but the magnitude of plasticity was greater in the worst bulk. Plasticity in hydraulic conductance was negative in the best bulk, contrasting directly with the high positive plasticity value displayed by the worst bulk.
Figure 3.
Plasticity of all root phenes shown as the mean relative difference in phenotypes between water treatments for individuals in the best (purple) and worst (gold) bulks. Phenes in bold indicate significant differences in plastic responses to drought between bulks according to Mann–Whitney tests (α ≤ 0.05).
Unique Clusters of Root Phenotypes Related To Flowering Time and Stress Tolerance
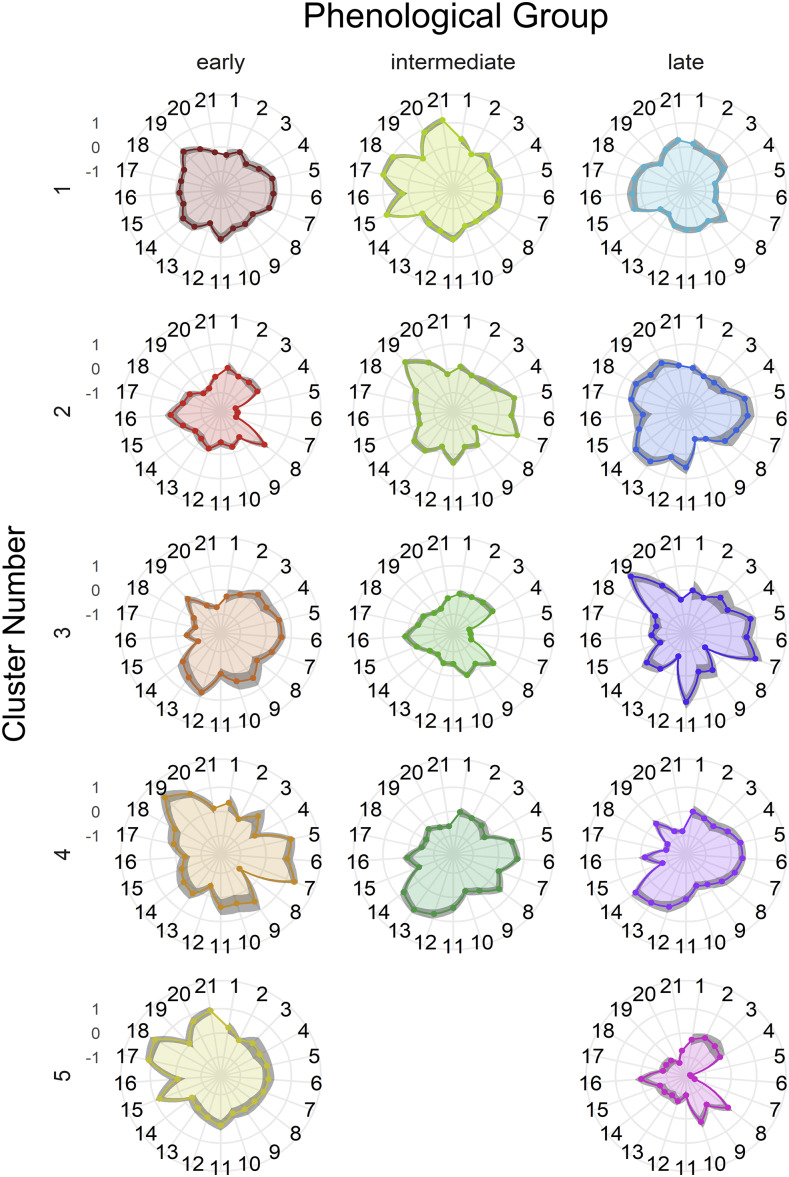
All individual plants grown under the water stress treatment were segregated into three phenological groups measured by growing degree days to pollen shed (GDD_pollen; Supplemental Fig. S3): early flowering (<1,275 GDD_pollen), intermediate flowering (>1,275, <1,425 GDD_pollen), and late flowering (>1,425 GDD_pollen). There were 214, 394, and 192 individual plants in the early, intermediate, and late phenological groups, respectively. Each phenological group was applied to a partition around the medians (PAM) clustering algorithm. PAM clustering identified five, four, and five unique clusters of root phenotypes associated with contrasting performance under water stress in the early, intermediate, and late phenological groups, respectively. The membership size of each cluster varied.
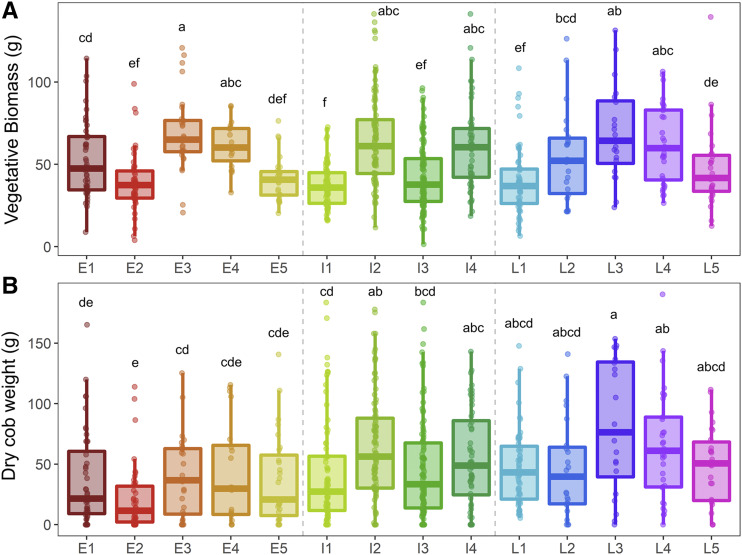
There were five clusters of unique root phenotypes identified in the early flowering group, and each cluster contained 46, 47, 30, 20, and 29 individuals (Supplemental Figs. S5 and S6). All clusters had equal vigor for vegetative biomass under non-stress conditions, except for cluster E3, which was slightly larger. Clusters E3 and E4 accumulated the most vegetative biomass under water stress, while clusters E2 and E5 accumulated the least (Fig. 4). Yield was similar among clusters under both non-stress conditions and water deficit stress (Fig. 4; Supplemental Fig. S7).
Figure 4.
Plant performance of each cluster under water stress. Boxplots of shoot vegetative biomass (A) and dry cob weight (B) of all clusters under water stress. Boxplot displays the median bounded by the first and third quartile with individual members marked with points. Gray dotted lines separate boxplots into the early (E), intermediate (I), and late (L) phenological groups. Letters above boxplots denote statistical similarity between groups per Kruskal–Wallis comparison tests with a Benjamini–Hochberg adjustment (α ≤ 0.05). Corresponding well-watered data are shown in Supplemental Figure S7.
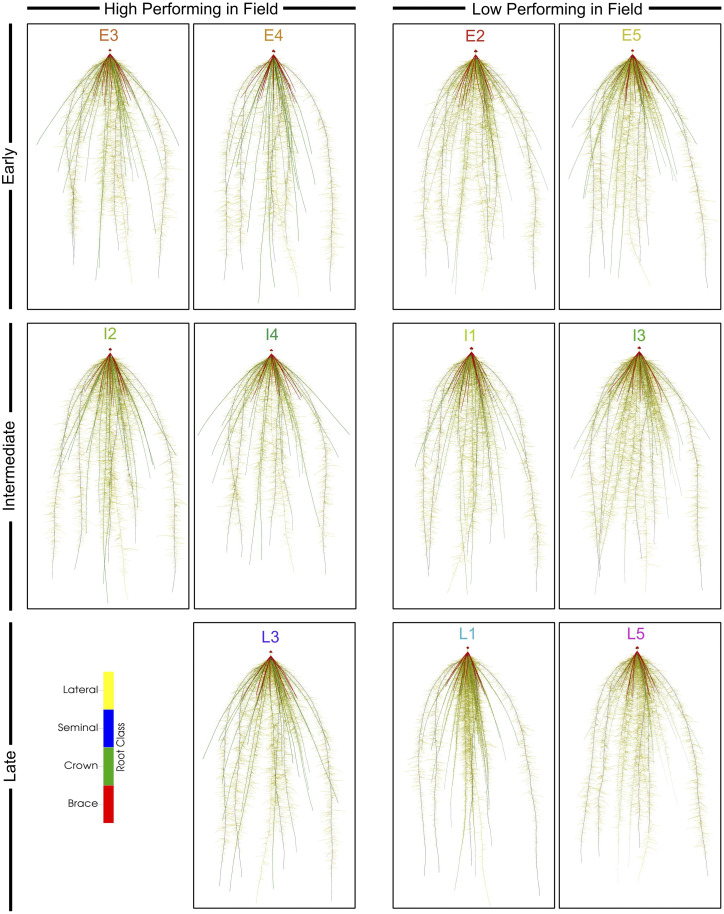
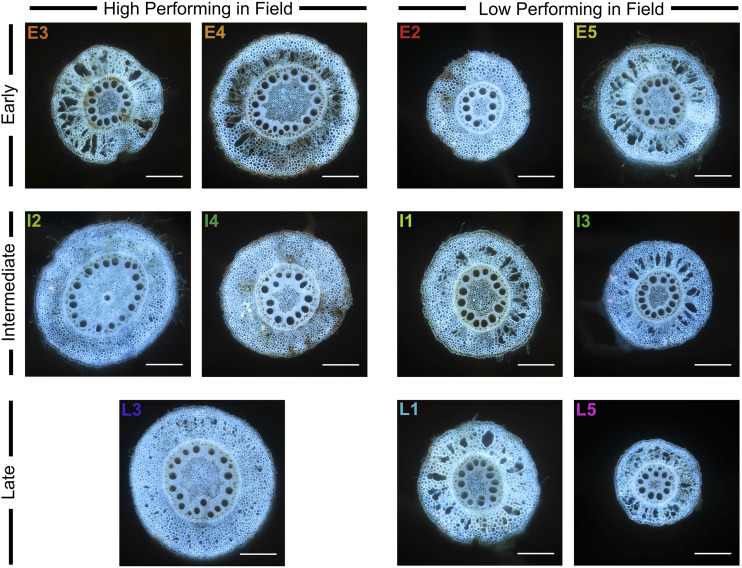
Despite showing high fitness under water stress, clusters E3 and E4 from the early phenological group presented root phenotypes with varied phene states (Figs. 5, 6, and 7). In relation to the entire pool of individuals, clusters E3 and E4 shared phene states of greater aerenchyma area, large cortical cell file number, large cortical cells in the midcortex and interior cortex, longer distance to the first lateral, and a large cortex area. Cortical cell size in the distal cortex was larger than average in cluster E3, and smaller than average in cluster E4. Root cross-sectional area was larger in cluster E4 than E3, and a larger proportion of the root cross section was stele. Metaxylem phenotypes contrasted substantially. Cluster E3 contained an average number of narrow vessels, which led to a smaller total metaxylem vessel area and severely restricted hydraulic conductance. Cluster E4 contained a greater number of average-sized metaxylem vessels, resulting in a larger total metaxylem vessel area and an average hydraulic conductance.
Figure 5.
Phenotypic means among clusters within early, intermediate, and late flowering groups under water stress as determined with PAM clustering surrounded by a 95% confidence interval in the shaded region. Phenotype data displayed has been centered and scaled. Numbers around the circumference designate the phene as assigned in Table 1. Exact values of means and 95% confidence intervals are disclosed in Supplemental Table S3.
Figure 6.
Graphical rendering showing the medoid architectural phenotype for the five best and six worst performing clusters in each phenological group under water stress. These images were generated in the program OpenSimRoot. Roots are colored according to their class.
Figure 7.
Root anatomy cross-section images of the medoid phenotype for the five best and six worst performing clusters in each phenological group (early, intermediate, and late) under water stress. Images were captured using LAT. Scale bars = 0.5 mm.
There were four clusters of unique root phenotypes identified in the intermediate phenological group, containing 75, 90, 107, and 52 individuals in each cluster (Supplemental Figs. S5 and S8). Individuals in clusters I2 and I4 accumulated greater shoot vegetative biomass than those in clusters I1 and I3, despite equal vigor under non-stress conditions (Fig. 4, Supplemental Fig. S7). Individuals in cluster I2 also amassed greater dry cob weight under water stress compared to I1 (Fig. 4).
Many components of the integrated phenotypes for clusters I2 and I4 of the intermediate phenological group were similar, while others differed (Figs. 5, 6, and 7). Relative to other individuals in the entire pool, individuals in clusters I2 and I4 displayed phenotypes consisting of an average cortical cell file number, large cortical cells in the interior cortex, and thick roots (root cross-sectional and cortex areas). Clusters I2 and I4 mutually presented narrow metaxylem vessels, but contrasted in other metaxylem-related phenes. Cluster I2 was comprised of many, narrow metaxylem within an average range of vessel areas, resulting in an average total metaxylem vessel area but reduced hydraulic conductance. On the other hand, the average root phenotype of cluster I4 consisted of few, narrow metaxylem vessels within a narrow range of vessel sizes associated with a reduced total metaxylem vessel area and severely reduced hydraulic conductance. Root phenotypes in cluster I2 exhibited average-sized cortical cells in the distal and midcortex, long lateral roots, and a larger proportion of the root comprising stele area. Contrary to cluster I2, cluster I4 displayed an average root phenotype composed of large cortical cells in the distal and midcortex regions and short lateral roots.
There were five clusters of unique root phenotypes identified in the late phenological group that contained 57, 24, 20, 33, and 28 individuals in each cluster (Supplemental Figs. S5 and S9). These clusters displayed similar shoot vegetative biomass accumulation under non-stress conditions except for L1, which was smaller (Supplemental Fig. S7). Cluster L3 accumulated the most vegetative biomass and yield under water stress (Fig. 4). Compared to those of other clusters in the late phenological group, the integrated phenotype representing cluster L3 was distinct even from high-performing clusters of the other phenological groups (Figs. 5, 6, and 7). The integrated phenotype of cluster L3 appeared as one with greater aerenchyma area, larger cortical cell file number, small cortical cells in the distal cortex, average-sized cells in mid- and interior cortex, more numerous and narrower metaxylem vessels, reduced hydraulic conductance, and thick roots with a proportionally larger stele.
The five best performing clusters overall (E3, E4, I2, I4, and L3) compared similarly under both treatments, although shoot vegetative biomass of E4 was smaller than the other clusters under non-stress (Fig. 4; Supplemental Fig. S7). These clusters varied in their overall phenotype (Figs. 5, 6, and 7), but there were many shared phene states under water stress between multiple clusters (Supplemental Table S3). Root cross-sectional area and total cortical area were consistently of average size for all clusters except for E4, which was slightly larger. Total stele area was similarly large in clusters E4, I2, and L3 while stele area in clusters E3 and I4 was slightly smaller. Aerenchyma content (area and percent of the cortex) was high in all clusters, although aerenchyma area was slightly reduced in I2. Cell size of each region of the cortex varied significantly between clusters, but cortical cell size in the midregion was most commonly of average size. Median metaxylem vessel area was of average size in E4, I2, and I4, but was reduced in clusters E3 and L3. Clusters E4, I2, and L3 contained significantly more metaxylem vessels than E3 and I4. The variation in metaxylem phenotypes resulted in a wide range of hydraulic conductance measurements, although E3, I4, and L3 maintained similar volumetric water uptake rates under water stress.
DISCUSSION
We observed wide variation in root anatomical and architectural phenes coinciding with varying sensitivity to drought (Supplemental Fig. S2). Most phenes showed positive or negative shifts in their mean values and phenotypic distributions as a result of water stress, suggesting that most root phenes are plastic and highly responsive to drought. The means and phenotypic distributions of only lateral root branching frequency, cortical cell size in the inner cortex, distance to the first lateral, lateral root length, minimum metaxylem vessel area, and the ratio of total cortex area to total stele area were unaffected by water stress (Table 1; Supplemental Fig. S2). These phenes are still likely to be plastic but may be limited to a specific phenotypic range not exceeding that observed in the well-watered environment. Also, the drought stress experienced in our fields was relatively mild.
Phenotypic bulked segregant analysis identified groups of drought-tolerant (best bulk) and drought-sensitive (worst bulk) individuals. The average root phenotype of the top bulk was distinctly different from that of the bottom bulk (Fig. 2). Growing degree days to pollen shed and silking in the best bulk were lower than the worst bulk (Supplemental Fig. S4), suggesting that individuals in the best bulk were more likely to escape drought effects compared to those in the later flowering worst bulk. Therefore, phenology must also be considered in relation to drought-tolerance strategies.
Varying degrees of plasticity were observed in the bulked segregant analysis (Fig. 3). Plasticity appeared to be conserved across several phenes, whose dimensions increased as a result of water stress in both bulks. Aerenchyma content similarly increased in both bulks, which has been demonstrated to be a valuable phenotype under drought (Zhu et al., 2010; Jaramillo et al., 2013). For the root architecture, increases in lateral root length and distance to the first lateral may facilitate more efficient capture of mobile resources (Lynch, 2013; York and Lynch, 2015), but an increase in lateral root branching frequency may inhibit deeper root growth by limiting the amount of resources available for greater axial root construction (Zhan et al., 2015). Roots of the best performers were more likely to grow at a steeper angle as a result of water stress compared to the worst performers whose root systems were more likely to become shallower. Steep root angles have been associated with improved drought tolerance (Uga et al., 2013). Cortical cells in the mid- and interior cortex enlarged and cortical cell file number decreased in the best bulk, implying that individuals of the best bulk developed a more economical cortex under water stress (Jaramillo et al., 2013). Plasticity in metaxylem phenes appeared to have direct consequences on water use. In the best bulk, median and maximum metaxylem area decreased under water stress, which coincided with a reduction in hydraulic conductance. In contrast, metaxylem vessel area in the worst bulk increased as a result of drought, most dramatically in the minimum vessel area, resulting in a dramatic increase in hydraulic conductance. This suggests that maize lines that effectively decrease their water uptake under drought are more likely to perform well than those that spend their water quickly (Vadez et al., 2014).
PAM clustering identified several clusters of unique root phenotypes and these clusters were related to phenology and drought tolerance (Figs. 5, 6, and 7; Supplemental Fig. S5). There were five, four, and five clusters identified in the early, intermediate, and late phenological groups, respectively. Fitness under well-watered conditions varied little (Supplemental Fig. S7), but these clusters contrasted significantly in fitness under water stress (Fig. 4). Individuals in clusters E3, E4, I2, I4, and L3 appeared to be most tolerant, and clusters E2, E5, I1, I3, L1, and L5 were most sensitive to drought (Fig. 4). Several integrated phenotypes related to enhanced fitness under drought stress were detected in this maize diversity panel. Previous studies evaluated the fitness of integrated phenotypes in silico under limited P and N, and multiple phenotypes were beneficial under stressful conditions (Dunbabin et al., 2003; Dathe et al., 2016; Rangarajan et al., 2018). This study empirically demonstrates the utility of integrated phenotypes in the field under drought stress. Certain phene states were common among several integrated phenotypes. However, these integrated phenotypes largely differed from one another, and co-optimized three main strategies to best tolerate drought stress.
First, several integrated phenotypes may optimize parsimonious maintenance costs to facilitate greater root construction and soil exploration. Phene states such as increased aerenchyma formation (Jaramillo et al., 2013; Chimungu et al., 2015b), reduced cortical cell files (Chimungu et al., 2014a), and larger cortical cells (Chimungu et al., 2014b) have been shown to relate to improved fitness under drought. The phenotypic bulked segregant analysis showed that individuals in the best bulk likely contained greater aerenchyma content and larger cortical cells in the mid- and interior cortex under water stress (Fig. 2). Likewise, PAM clustering identified three clusters exhibiting high fitness under water stress with greater aerenchyma content (E3, E4, and L3) and/or larger cortical cells in the distal (E3 and I4), middle (E3 and I4), and inner cortex (E3; Fig. 5; Supplemental Table S3). Increased aerenchyma content and larger cortical cells were previously shown to enhance drought tolerance and overcome mechanical impedance by reducing the metabolic cost associated with root tissue maintenance and supporting further soil exploration with greater root construction (Chimungu et al., 2014b, 2015b; Colombi et al., 2019). In the phenotypic bulked segregant analysis, cortical cell size in the distal cortex did not differ among bulks under water stress but was smaller than the middle and interior cortical regions (Fig. 2). A similar trend was seen for clusters E4, I2, and L3 (Figs. 5 and 7), and may have implications for root mechanical strength.
Second, many integrated phenotypes could optimize penetrability of drying and hardening soils. Thick roots with larger steles were reported as advantageous phene states to best surmount mechanical impedance by maximizing penetrative ability (Clark et al., 2003; Colombi and Walter, 2016). Thicker roots with a larger proportion of stele were identified as beneficial phene states under water stress in the bulked segregant analysis (Fig. 2) and PAM clustering (E4, I2, L3; Figs. 5, 6, and 7). Root thickening under mechanical impedance often corresponds to radial enlarging of cortical cells (Veen, 1982; Colombi et al., 2019), but retaining small distal cortical cells is beneficial for increasing root penetration (Chimungu et al., 2015a). Small distal cortical cells coupled with larger cells in the remaining cortex were shown to be related to enhanced drought tolerance in the bulked segregant analysis and in PAM clustering (clusters E4, I2, and L3), perhaps to better penetrate hardening soils. Additionally, the presence of aerenchyma content has no effect on root mechanical strength (Striker et al., 2006), which suggests a positive interaction among small distal cortical cells, large cortical cells in the middle and interior cortex, and increased aerenchyma content, which optimizes tissue maintenance costs without compromising mechanical integrity.
Third, several integrated root phenotypes likely optimized slower water extraction from the soil to conserve soil moisture. Metaxylem phene states promoting restricted hydraulic conductance, such as uniformly narrow vessels, would be advantageous under drought conditions. Narrower metaxylem vessels are advantageous for reducing root hydraulic conductance to encourage water-banking (Richards and Passioura, 1989; Feng et al., 2016) and inhibit cavitation (Hacke and Sperry, 2001). The phenotypic bulked segregant analysis indicated that root phenotypes containing more numerous, narrower metaxylem vessels and the corresponding reduction in hydraulic conductance were more advantageous under water stress compared to fewer, wider metaxylem (Fig. 2). Likewise, PAM clustering revealed clusters of root phenotypes containing more numerous, narrower metaxylem vessels and subsequent restricted hydraulic conductance and improved drought tolerance (E3, I4, and L3). Cluster E4 demonstrated high fitness under water stress, and displayed an increased number of average-sized vessels that resulted in a large total metaxylem vessel area but average root hydraulic conductance relative to the rest of the maize diversity panel. Perhaps, in certain cases, constrained soil water use is not favored over higher rates of extraction.
There has been much debate on whether water-spending or water-saving strategies are more advantageous under water deficit (Zaman-Allah et al., 2011; Carvalho et al., 2015). The phenotypic bulked segregant analysis and PAM clustering indicated that water-saving is the most universally valuable water-use strategy, as most of the best performing clusters displayed restricted hydraulic conductance via narrower metaxylem vessels. Depleting soil water reserves slowly (i.e. water banking) avoids drought effects during the crucial anthesis-silking interval and keeps root tips hydrated for continued growth (Feng et al., 2016; Lynch, 2019). Furthermore, maize has a relatively shallow-angled root system in comparison to other crops (Zhang et al., 2014). If maize retains its shallow distribution, water extraction must be constrained to preserve soil moisture in the already dry shallow soil layers. However, cluster E4 exhibited the greatest hydraulic conductance measures of all high-performing clusters, though still average overall. This suggests that the water-use strategy is not as important for the early phenological group, because these individuals are more likely to successfully reproduce by escaping the most severe drought period. Narrower metaxylem vessels are also better in preventing cavitation (Hacke and Sperry, 2001). Cavitation is likely to be prevalent throughout the maize hydraulic system, but its lasting impact on survival is poorly understood. Nevertheless, a root phenotype containing large metaxylem vessels was never beneficial for drought tolerance in any phenological group in this study.
The poor performing clusters often contained several similar phene elements as the best performers in each phenological group, but we presume that the combinatorial effects with the remaining phenes must have had antagonistic effects. For instance, cortical cell size in clusters E2 and E5 (poor fitness under water stress), like E4 (high fitness under water stress), was small. However, E2 and E5 also contained little aerenchyma content, thin roots, and few metaxylem vessels. High aerenchyma content does not impair overall mechanical strength (Striker et al., 2006), but does reallocate resources otherwise, supporting maintenance costs to facilitate greater root growth and soil exploration (Zhu et al., 2010; Jaramillo et al., 2013; Lynch, 2014). It is not known if thin roots are disadvantageous because they are not as mechanically strong to penetrate hard soils (Clark et al., 2003), but they may be better able to access small soil pores to continue growing (Potocka and Szymanowska-Pułka, 2018; Vanhees et al., 2020). This suggests that some phene states, like small cortical cells, can be beneficial or antagonistic depending on the other components of the overall root phenotype and other environmental factors, such as the soil structure.
None of the clusters we identified were precisely analogous to the SCD ideotype, although some were partially congruent with it. The SCD ideotype was developed for environments in which water availability is greater at depth, but may not be optimal in dry environments relying on high in-season rainfall (Leitner et al., 2014). We observed little evidence of root angle playing a central role for drought-tolerance mechanisms, as posited by the SCD ideotype. This may be an artifact of the pivot irrigation system used—which, by periodically providing water in the more easily accessible shallow soil layers, placed little selection pressure on roots to grow deep. Many of the phenes associated with reducing the metabolic burden for root tissue, like increased aerenchyma production and larger cortical cells organized in fewer cell files, were commonly identified in clusters that performed well under drought conditions. However, there may be biomechanical tradeoffs associated with these phenotypes making them maladapted to drying, hardening soils. While aerenchyma content has no relationship with root strength (Striker et al., 2006), smaller cortical cells, particularly in the distal cortex, are beneficial for root penetration (Chimungu et al., 2015a), despite making that region more metabolically costly.
The integrated phenotypes discussed here optimize fitness outcomes in this specific drought-stressed environment, but an average phenotype may best co-optimize adaptation to a variety of environments and stresses (Dathe et al., 2016; Rangarajan et al., 2018; Strock et al., 2019a). In common bean, Strock et al. (2019a) identified a root architectural integrated phenotype of the most average dimensions that performed reliably under a variety of stresses in multiple environments. although they were rarely the best performers at the field location, these bean varieties consistently yielded well across all environments and stresses as opposed to those specifically adapted for a particular environment. Therefore, we must weigh the benefits of developing maize lines with exceptional performance applied in a narrow range against lines that perform consistently well enough across a wide range of environments.
This study examined the interactions among four architectural and 18 anatomical phenes, but there are additional phenes that may improve drought tolerance in cooperation with other phenes. Maize genotypes with fewer nodal roots allocate more carbon toward deeper axial and greater lateral root growth and minimize inter- and intraplant competition for mobile resources, like water or N (Lynch, 2013; Gao and Lynch, 2016; Guo and York, 2019). A greater number of crown roots increases the likelihood of penetrating dry topsoil to access deep soil water stores (Materechera et al., 1992). If true, an increase in aerenchyma formation may compensate for the increased costs to construct and maintain a greater number of crown roots (York et al., 2013). Variation in root-hair morphology may influence the ability to acquire water from the soil (Carminati et al., 2017) and the anchoring of the root tip for improved penetrative ability (Bengough et al., 2011). At the molecular level, aquaporins likely play a crucial role in regulating the radial conductivity and uptake of water from the soil (Chaumont and Tyerman, 2014). Finally, although not evaluated here, some shoot traits may play a key role in drought tolerance mechanisms. Wide variation in shoot traits exists within the WI Diversity (WiDiv) panel (Mazaheri et al., 2019), but their relationships with integrated root phenotypes and drought tolerance is largely unknown. Future studies should integrate these other phenes to elucidate their interactions with the phenes investigated here. Architecture and anatomy also vary by node (York and Lynch, 2015; Yang et al., 2019), so it may be necessary to expand future phenotyping efforts to accommodate multiphenic and multinodal aggregates.
Selection for integrated phenotypes instead of single phene states is necessary in breeding programs moving forward, but is difficult to accomplish. First, many of these phenes exhibit a high degree of plasticity. Plasticity may promote adaptation to the changing local environment, but plasticity may be maladaptive in the long term (Lynch, 2013, 2018). Thus, the utility of plasticity is poorly understood. Second, genetic control of many of these phenes is highly quantitative with small effects. Selection for traits with small effects makes breeding efforts difficult. Rather than seeking individual genes influencing single phenes, adopting alternative genetic methods like multitrait genome-wide association studies or genomic selection may be more appropriate to incorporate with integrated phenotypic analysis.
In this study, integrated root architectural and anatomical phenotypes of maize were quantified under non-stress and drought stress conditions in the field. Substantial phenotypic variation was observed for all phenes in both environments, and specific phene aggregates, or integrated phenotypes, were associated with drought tolerance. Identification of several clusters of unique root phenotypes within the WiDiv under water stress revealed that these phenotypes are related to water acquisition differences, phenology-dependent water use, penetration of hardened soils, and economical tissue maintenance costs. Addressing integrated phenotypes, as opposed to a single phene, will be an invaluable tool for breeders to develop crop varieties suited to a given agroecosystem.
MATERIALS AND METHODS
Plant Materials, Field Conditions, and Experimental Design
Subsets of 400 maize (Zea mays) genotypes derived from the WiDiv panel (Hansey et al., 2011) were evaluated for root phenotypes under well-watered and water-stress conditions in the field in 2016. The WiDiv panel is a large collection of inbred maize lines that represent the genetic diversity of maize grown in the upper Midwest region of the United States.
All experiments were performed in the field at the Apache Root Biology Center located in Willcox, Arizona (32° 29 099 N, 109° 419 3099 W). Trials were carried out in a Grab loam soil (coarse-loamy, mixed, thermic Typic Torrifluvent) from May through October in 2016. Each genotype was planted in a single-row plot containing 20 individuals per plot. Rows were spaced 75-cm apart while plants were spaced 23-cm apart. Two replications of the WiDiv panel were grown in both the well-watered and water-deficit treatments in a randomized complete block design. Fertilizer and lime application were adjusted based on soil test results to meet the nutritional requirements for maize production. Pest and pathogen control were conducted as needed. Experiments were irrigated using a center pivot irrigation system. Well-watered and water-stress treatment were induced in separate blocks. Water deficit was induced four weeks after planting by withholding 50% of the water needed to sustain adequate growth in the well-watered blocks. The water-stress treatment was monitored over the course of the season using PR2 multidepth soil moisture probes (Dynamax). As a result, a 21% reduction in biomass and a 39% reduction in yield was achieved under water deficit (Supplemental Fig. S1).
Phenotypic Analysis
Root architecture and root anatomy was phenotyped. Maize root phenotypes were evaluated using the shovelomics method (Trachsel et al., 2010) coupled with Digital Imaging of Root Traits (Das et al., 2015) and laser ablation tomography (LAT; Hall et al., 2019; Strock et al., 2019b) for architectural and anatomical analysis, respectively. In short, three representative plants were excavated in a soil monolith using a standard spade one-shovel-head–deep and wide. Root crowns were soaked in water for 15 min to loosen soil and then washed with low pressure to remove soil completely. All three representative root crowns were included for architectural imaging and one representative crown was selected for anatomical analysis. Four architectural phenes were evaluated from images of clean root crowns using the tool Digital Imaging of Root Traits (Das et al., 2015): root growth angle, lateral root branching frequency, distance to the first lateral root, and lateral root length (Table 1). Architecture across both seasons were averaged. Samples for root anatomy were collected by excising a fourth whorl root sample 5 to 8 cm from the base of the root. The root sample was preserved in 75% (v/v) ethanol and ablated using LAT (Strock et al., 2019b). During LAT, a root sample is moved into a 355-nm Avia 7000 pulsed laser and vaporized in the camera focal plane ahead of an imaging stage. Simultaneously, a Canon T3i camera with a 53 micro lens (MP-E 65 mm) was used to capture images of the root cross section. Root cross-sectional images were analyzed and quantified for 18 anatomical phenes using the program RootScan 2.0 (https://plantscience.psu.edu/research/labs/roots/methods/computer/rootscan) and the software MIPAR (Table 1; Sosa et al., 2014). Two images were collected and averaged for each fourth whorl crown-root sample. The theoretical hydraulic conductance was calculated as the volumetric flow rate (cubic millimeters per second) based on the dimensions of the metaxylem vessels using the Exact equations (Berker, 1963; Lewis and Boose, 1995), which estimate the movement of water through noncircular conduits.
Phenological data were collected on the WiDiv panel under non-stress conditions in Arlington, Wisconsin in 2015.
Data Analysis
All data analyses were performed in R v. 3.6.2 (R Core Team, 2019) with graphical illustration using “ggplot2” (Wickham, 2016). Phenotypic bulked segregant analysis was performed to evaluate two pools, or bulks, of phenotypes representing the best and worst performers under water stress. Each bulk consisted of the best- and worst-performing quartile of vegetative biomass accumulators under water stress but had similar vigor under well-watered conditions. The root phenotype of each bulk and each treatment was compared for significant differences using a Kruskal–Wallis multiple comparison test with a Benjamini–Hochberg P-value correction (α < 0.05). The plastic response to drought of each phene in each bulk was calculated as the relative change in the phene’s dimensions as a result of the water-stress treatment expressed as a percent. The mean plasticity response for each phene was compared between bulks using a Mann–Whitney test (α < 0.05).
To identify groups of similar root phenotypes, all individuals grown under drought were analyzed using PAM clustering, a type of k-medoids clustering, to partition the data into unique clusters of phene combinations associated with performance under stress using the packages “cluster” (Maechler et al., 2019) and “fpc” (Hennig, 2019). K-medoids is a robust clustering algorithm that is less sensitive to noise and outliers compared to more common clustering methods. Before clustering, the data for every individual was centered and scaled and missing data were imputed using the K-Nearest-Neighbors algorithm as part of the “caret” (Kuhn, 2019) and “RANN” (Arya et al., 2019) packages. The number of clusters was determined by the within-sums-of-squares values. As a result, 14 unique clusters of root phenotypes related to shoot vegetative biomass accumulation were selected: five in the early groups, four in the intermediate groups, and five in the late phenological groups. The robustness of the PAM clustering method was validated using the average predictive accuracy of 1,000 iterations of a linear discriminant analysis. Each model generated for the early, intermediate, and late phenology groups achieved cluster classification accuracies of 79%, 84%, and 87%. Kruskal–Wallis comparison tests with a Benjamini–Hochberg P-value adjustment were used to identify significant phenotypic and performance differences between clusters (α < 0.05). All WiDiv root phenotype data and the R script to conduct this analysis is available at https://doi.org/10.5281/zenodo.3728116.
Architectural models of each cluster were constructed using the functional–structural plant model OpenSimRoot (Postma et al., 2017). Existing maize parameters were modified to incorporate the aerenchyma percentage, root growth angle, lateral root branching frequency, and diameter of nodal roots from each cluster medoid. Measurements were collected from fourth whorl roots, so angle and diameter parameters were adjusted independently for each whorl using the difference from the medoid value and the overall mean, while aerenchyma percentage and lateral root branching frequency measurements were applied to all whorls of crown roots. Low N stress was imposed as a proxy for drought stress because water and nitrate are likely to have similar distributions by depth over the course of the growing season under those circumstances (Asbjornsen et al., 2008; Dathe et al., 2016). Soil nitrate and water movement were modeled in OpenSimRoot using SWMS3D (Simbnek et al., 1995).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Plant performance of the WiDiv panel under well-watered (WW, blue) and water-stressed (WS, red) conditions.
Supplemental Figure S2. Distributions of individual phenes under well-watered (WW, blue) and water-stressed (WS, red) conditions.
Supplemental Figure S3. Variation in phenology of the WiDiv panel.
Supplemental Figure S4. Phenology of the best and worst bulk.
Supplemental Figure S5. PAM clustering of root phenotypes under water stress.
Supplemental Figure S6. Animated GIF of 3D pseudo-principal component analysis (PCA) plot of the first three principal components for the early phenology group.
Supplemental Figure S7. Plant performance for all clusters under well-watered conditions.
Supplemental Figure S8. Animated GIF of 3D pseudo-principal component analysis (PCA) plot of the first three principal components for the intermediate phenology group.
Supplemental Figure S9. Animated GIF of 3D pseudo-principal component analysis (PCA) plot of the first three principal components for the late phenology group.
Supplemental Table S1. Root phenotypic means of each bulk under well-watered (WW) and water-stressed (WS) conditions.
Supplemental Table S2. Mean plasticity values calculated for each phene in the best and worst bulks.
Supplemental Table S3. Summary statistics for each cluster including the means and 95% confidence intervals for each phene under water stress.
Acknowledgments
We thank Robert Snyder, Patricio Cid, and Bishow Poudel for technical support. We thank Shawn Kaeppler and Kathryn Michel for supplying WiDiv maize seed and phenology data. We also thank Christopher Strock for helpful discussions.
Footnotes
This work was supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture (grant nos. 2014–67013–21572 and 2017–67013–26192) and the Howard G. Buffet Foundation.
Articles can be viewed without a subscription.
References
- Arya S, Mount D, Kemp SE, Jefferis G (2019) RANN: Fast Nearest Neighbour Search (Wraps ANN Library) Using L2 Metric. https://cran.r-project.org/web/packages/RANN/RANN.pdf (April 10, 2020)
- Asbjornsen H, Shepherd G, Helmers M, Mora G(2008) Seasonal patterns in depth of water uptake under contrasting annual and perennial systems in the Corn Belt Region of the Midwestern US. Plant Soil 308: 69–92 [Google Scholar]
- Bengough AG, McKenzie BM, Hallett PD, Valentine TA(2011) Root elongation, water stress, and mechanical impedance: A review of limiting stresses and beneficial root tip traits. J Exp Bot 62: 59–68 [DOI] [PubMed] [Google Scholar]
- Berker R. (1963) Integration of the equations of motion of an incompressible viscous fluid [in French] In Flugge S, ed., Encyclopedia of Physics, Vol. 2, Springer, Berlin, Germany, pp 1–384 [Google Scholar]
- Bishopp A, Lynch JP(2015) The hidden half of crop yields. Nat Plants 1: 15117. [DOI] [PubMed] [Google Scholar]
- Boyer JS.(1982) Plant productivity and environment. Science 218: 443–448 [DOI] [PubMed] [Google Scholar]
- Carminati A, Passioura JB, Zarebanadkouki M, Ahmed MA, Ryan PR, Watt M, Delhaize E(2017) Root hairs enable high transpiration rates in drying soils. New Phytol 216: 771–781 [DOI] [PubMed] [Google Scholar]
- Carvalho ECD, Martins FR, Soares AA, Oliveira RS, Muniz CR, Araújo FS(2015) Hydraulic architecture of lianas in a semiarid climate: Efficiency or safety? Acta Bot Bras 29: 198–206 [Google Scholar]
- Chaumont F, Tyerman SD(2014) Aquaporins: Highly regulated channels controlling plant water relations. Plant Physiol 164: 1600–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimungu JG, Brown KM, Lynch JP(2014a) Reduced root cortical cell file number improves drought tolerance in maize. Plant Physiol 166: 1943–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimungu JG, Brown KM, Lynch JP(2014b) Large root cortical cell size improves drought tolerance in maize. Plant Physiol 166: 2166–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimungu JG, Loades KW, Lynch JP(2015a) Root anatomical phenes predict root penetration ability and biomechanical properties in maize (Zea mays). J Exp Bot 66: 3151–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chimungu JG, Maliro MFA, Nalivata PC, Kanyama-Phiri G, Brown KM, Lynch JP(2015b) Utility of root cortical aerenchyma under water limited conditions in tropical maize (Zea mays L.). Field Crops Res 171: 86–98 [Google Scholar]
- Chitwood DH, Topp CN(2015) Revealing plant cryptotypes: Defining meaningful phenotypes among infinite traits. Curr Opin Plant Biol 24: 54–60 [DOI] [PubMed] [Google Scholar]
- Clark LJ, Whalley WR, Barraclough PB(2003) How do roots penetrate strong soil? Plant Soil 255: 93–104 [Google Scholar]
- Colombi T, Herrmann AM, Vallenback P, Keller T(2019) Cortical cell diameter is key to energy costs of root growth in wheat. Plant Physiol 180: 2049–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombi T, Torres LC, Walter A, Keller T(2018) Feedbacks between soil penetration resistance, root architecture and water uptake limit water accessibility and crop growth—a vicious circle. Sci Total Environ 626: 1026–1035 [DOI] [PubMed] [Google Scholar]
- Colombi T, Walter A(2016) Root responses of triticale and soybean to soil compaction in the field are reproducible under controlled conditions. Funct Plant Biol 43: 114–128 [DOI] [PubMed] [Google Scholar]
- Comas LH, Becker SR, Cruz VMV, Byrne PF, Dierig DA(2013) Root traits contributing to plant productivity under drought. Front Plant Sci 4: 442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa J, Postma JA, Watt M, Wojciechowski T(2019) Root system architectural plasticity and soil compaction: A review. J Exp Bot 70: 6019–6034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai A.(2013) Increasing drought under global warming in observations and models. Nat Clim Chang 3: 52–58 [Google Scholar]
- Das A, Schneider H, Burridge J, Ascanio AKM, Wojciechowski T, Topp CN, Lynch JP, Weitz JS, Bucksch A(2015) Digital imaging of root traits (DIRT): A high-throughput computing and collaboration platform for field-based root phenomics. Plant Methods 11: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dathe A, Postma JA, Postma-Blaauw MB, Lynch JP(2016) Impact of axial root growth angles on nitrogen acquisition in maize depends on environmental conditions. Ann Bot 118: 401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz AS, Aguiar GM, Pereira MP, de Castro EM, Magalhães PC, Pereira FJ(2018) Aerenchyma development in different root zones of maize genotypes under water limitation and different phosphorus nutrition. Biol Plant 62: 561–568 [Google Scholar]
- Donald CM.(1968) The breeding of crop ideotypes. Euphytica 17: 385–403 [Google Scholar]
- Dowswell CR, Paliwal RL, Cantrell RP(1996) Maize in the Third World. Westview Press, Boulder, CO [Google Scholar]
- Drew MC, He CJ, Morgan PW(2000) Programmed cell death and aerenchyma formation in roots. Trends Plant Sci 5: 123–127 [DOI] [PubMed] [Google Scholar]
- Dunbabin V, Diggle A, Rengel Z(2003) Is there an optimal root architecture for nitrate capture in leaching environments? Plant Cell Environ 26: 835–844 [DOI] [PubMed] [Google Scholar]
- Eavis BW.(1972) Soil physical conditions affecting seedling root growth. Plant Soil 36: 613–622 [Google Scholar]
- Feng W, Lindner H, Robbins NE II, Dinneny JR(2016) Growing out of stress: The role of cell- and organ-scale growth control in plant water-stress responses. Plant Cell 28: 1769–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-Castañeda T, Brown KM, Lynch JP(2018) Reduced root cortical burden improves growth and grain yield under low phosphorus availability in maize. Plant Cell Environ 41: 1579–1592 [DOI] [PubMed] [Google Scholar]
- Gao Y, Lynch JP(2016) Reduced crown root number improves water acquisition under water deficit stress in maize (Zea mays L.). J Exp Bot 67: 4545–4557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesiak S, Grzesiak MT, Filek W, Hura T, Stabryła J(2002) The impact of different soil moisture and soil compaction on the growth of triticale root system. Acta Physiol Plant 24: 331–342 [Google Scholar]
- Guo H, York LM(2019) Maize with fewer nodal roots allocates mass to more lateral and deep roots that improve nitrogen uptake and shoot growth. J Exp Bot 70: 5299–5309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacke UG, Sperry JS(2001) Functional and ecological xylem anatomy. Perspect Plant Ecol Evol Syst 4: 97–115 [Google Scholar]
- Hall B, Lanba A, Lynch J(2019) Three-dimensional analysis of biological systems via a novel laser ablation technique. J Laser Appl 31: 22602 [Google Scholar]
- Hansey CH, Johnson JM, Sekhon RS, Kaeppler SM, de Leon N(2011) Genetic diversity of a maize association population with restricted phenology. Crop Sci 51: 704–715 [Google Scholar]
- Hennig C. (2019) fpc: Flexible procedures for clustering. https://cran.r-project.org/web/packages/fpc/fpc.pdf (April 10, 2020)
- Ho MD, Rosas JC, Brown KM, Lynch JP(2005) Root architectural tradeoffs for water and phosphorus acquisition. Funct Plant Biol 32: 737–748 [DOI] [PubMed] [Google Scholar]
- Jaramillo RE, Nord EA, Chimungu JG, Brown KM, Lynch JP(2013) Root cortical burden influences drought tolerance in maize. Ann Bot 112: 429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Liu P, Lynch JP(2018) Greater lateral root branching density in maize improves phosphorus acquisition from low phosphorus soil. J Exp Bot 69: 4961–4970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadam NN, Tamilselvan A, Lawas LMF, Quinones C, Bahuguna RN, Thomson MJ, Dingkuhn M, Muthurajan R, Struik PC, Yin X, et al. (2017) Genetic control of plasticity in root morphology and anatomy of rice in response to water-deficit. Plant Physiol 174: 2302–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Inukai Y, Kitano H, Yamauchi A(2011) Root plasticity as the key root trait for adaptation to various intensities of drought stress in rice. Plant and Soil 342: 117–128 [Google Scholar]
- Kano-Nakata M, Gowda VRP, Henry A, Serraj R, Inukai Y, Fujita D, Kobayashi N, Suralta RR, Yamauchi A(2013) Functional roles of the plasticity of root system development in biomass production and water uptake under rainfed lowland conditions. F Crop Res 144: 288–296 [Google Scholar]
- Kuhn M. (2019) caret: Classification and Regression Training. https://cran.r-project.org/web/packages/caret/caret.pdf. Accessed April 10, 2020.
- Leitner D, Meunier F, Bodner G, Javaux M, Schnepf A(2014) Impact of contrasted maize root traits at flowering on water stress tolerance—A simulation study. Field Crops Res 165: 125–137 [Google Scholar]
- Lewis AM, Boose ER(1995) Estimating volume flow rates through xylem conduits. Am J Bot 82: 1112–1116 [Google Scholar]
- Liao H, Yan X, Rubio G, Beebe SE, Blair MW, Lynch JP(2004) Genetic mapping of basal root gravitropism and phosphorus acquisition efficiency in common bean. Funct Plant Biol 31: 959–970 [DOI] [PubMed] [Google Scholar]
- Lobell DB, Schlenker W, Costa-Roberts J(2011) Climate trends and global crop production since 1980. Science 333: 616–620 [DOI] [PubMed] [Google Scholar]
- Lynch J.(1995) Root architecture and plant productivity. Plant Physiol 109: 7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP.(2011) Root phenes for enhanced soil exploration and phosphorus acquisition: Tools for future crops. Plant Physiol 156: 1041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP.(2013) Steep, cheap and deep: An ideotype to optimize water and N acquisition by maize root systems. Ann Bot 112: 347–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP.(2014) Root phenes that reduce the metabolic costs of soil exploration: Opportunities for 21st century agriculture. Plant Cell Environ 38: 1775–1784 [DOI] [PubMed] [Google Scholar]
- Lynch JP.(2018) Rightsizing root phenotypes for drought resistance. J Exp Bot 69: 3279–3292 [DOI] [PubMed] [Google Scholar]
- Lynch JP.(2019) Root phenotypes for improved nutrient capture: An underexploited opportunity for global agriculture. New Phytol 223: 548–564 [DOI] [PubMed] [Google Scholar]
- Lynch JP, Brown KM(2001) Topsoil foraging—an architectural adaptation of plants to low phosphorus availability. Plant Soil 237: 225–237 [Google Scholar]
- Lynch JP, Brown KM(2012) New roots for agriculture: Exploiting the root phenome. Philos Trans R Soc Lond B Biol Sci 367: 1598–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP, Wojciechowski T(2015) Opportunities and challenges in the subsoil: Pathways to deeper rooted crops. J Exp Bot 66: 2199–2210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maechler M, Rousseeuw P, Struyf A, Hubert M, Hornik K (2019) Cluster: Cluster Analysis Basics and Extensions. R packages. 2.1.0 https://cran.r-project.org/web/packages/cluster/citation.html (April 10, 2020)
- Materechera SA, Alston AM, Kirby JM, Dexter AR(1992) Influence of root diameter on the penetration of seminal roots into a compacted subsoil. Plant Soil 144: 297–303 [Google Scholar]
- Mazaheri M, Heckwolf M, Vaillancourt B, Gage JL, Burdo B, Heckwolf S, Barry K, Lipzen A, Ribeiro CB, Kono TJY, et al. (2019) Genome-wide association analysis of stalk biomass and anatomical traits in maize. BMC Plant Biol 19: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel MA, Postma JA, Lynch JP(2015) Phene synergism between root hair length and basal root growth angle for phosphorus acquisition. Plant Physiol 167: 1430–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Khush GS, Virk P, Tang Q, Zou Y(2008) Progress in ideotype breeding to increase rice yield potential. Field Crops Res 108: 32–38 [Google Scholar]
- Pieruschka R, Poorter H(2012) Phenotyping plants: Genes, phenes and machines. Funct Plant Biol 39: 813–820 [DOI] [PubMed] [Google Scholar]
- Popova L, van Dusschoten D, Nagel KA, Fiorani F, Mazzolai B(2016) Plant root tortuosity: An indicator of root path formation in soil with different composition and density. Ann Bot 118: 685–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma JA, Dathe A, Lynch JP(2014) The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiol 166: 590–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma JA, Kuppe C, Owen MR, Mellor N, Griffiths M, Bennett MJ, Lynch JP, Watt M(2017) OpenSimRoot: Widening the scope and application of root architectural models. New Phytol 215: 1274–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocka I, Szymanowska-Pułka J(2018) Morphological responses of plant roots to mechanical stress. Ann Bot 122: 711–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince SJ, Murphy M, Mutava RN, Durnell LA, Valliyodan B, Shannon JG, Nguyen HT(2017) Root xylem plasticity to improve water use and yield in water-stressed soybean. J Exp Bot 68: 2027–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangarajan H, Postma JA, Lynch JP(2018) Co-optimization of axial root phenotypes for nitrogen and phosphorus acquisition in common bean. Ann Bot 122: 485–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2019) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- Rich SM, Watt M(2013) Soil conditions and cereal root system architecture: Review and considerations for linking Darwin and Weaver. J Exp Bot 64: 1193–1208 [DOI] [PubMed] [Google Scholar]
- Richards RA, Passioura JB(1989) A breeding program to reduce the diameter of the major xylem vessel in the seminal roots of wheat and its effect on grain-yield in rain-fed environments. Aust J Agric Res 40: 943–950 [Google Scholar]
- Saengwilai P, Nord EA, Chimungu JG, Brown KM, Lynch JP(2014a) Root cortical aerenchyma enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiol 166: 726–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengwilai P, Tian X, Lynch JP(2014b) Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiol 166: 581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simbnek J, Huang K, van Genuchten MT (1995) The SWMS 3D Code for Simulating Water Flow and Solute Transport in Three-Dimensional Variably-Saturated Media. U.S. Salinity Laboratory Agricultural Research Service. https://www.pc-progress.com/documents/programs/SWMS_3D.pdf (December 17, 2019)
- Sosa JM, Huber DE, Welk B, Fraser HL(2014) Development and application of MIPAR: A novel software package for two- and three-dimensional microstructural characterization. Integr Mater Manuf Innov 3: 123–140 [Google Scholar]
- Striker GG, Insausti P, Grimoldi AA, Leon RJC(2006) Root strength and trampling tolerance in the grass Paspalum dilatatum and the dicot Lotus glaber in flooded soil. Funct Ecol 20: 4–10 [Google Scholar]
- Strock CF, Burridge J, Massas ASF, Beaver J, Beebe S, Camilo SA, Fourie D, Jochua C, Miguel M, Miklas PN, et al. (2019a) Seedling root architecture and its relationship with seed yield across diverse environments in Phaseolus vulgaris. Field Crops Res 237: 53–64 [Google Scholar]
- Strock CF, Morrow de la Riva L, Lynch JP(2018) Reduction in root secondary growth as a strategy for phosphorus acquisition. Plant Physiol 176: 691–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strock CF, Schneider HM, Galindo-Castañeda T, Hall BT, Van Gansbeke B, Mather DE, Roth MG, Chilvers MI, Guo X, Brown K, et al. (2019b) Laser ablation tomography for visualization of root colonization by edaphic organisms. J Exp Bot 70: 5327–5342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suralta RR, Inukai Y, Yamauchi A(2010) Dry matter production in relation to root plastic development, oxygen transport, and water uptake of rice under transient soil moisture stresses. Plant Soil 332: 87–104 [Google Scholar]
- Trachsel S, Kaeppler SM, Brown KM, Lynch JP(2010) Shovelomics: High throughput phenotyping of maize (Zea mays L.) root architecture in the field. Plant Soil 341: 75–87 [Google Scholar]
- Trachsel S, Kaeppler SM, Brown KM, Lynch JP(2013) Maize root growth angles become steeper under low N conditions. Field Crops Res 140: 18–31 [Google Scholar]
- Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, et al. (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 45: 1097–1102 [DOI] [PubMed] [Google Scholar]
- Vadez V.(2014) Root hydraulics: The forgotten side of roots in drought adaptation. Field Crops Res 165: 15–24 [Google Scholar]
- Vadez V, Kholova J, Medina S, Kakkera A, Anderberg H(2014) Transpiration efficiency: New insights into an old story. J Exp Bot 65: 6141–6153 [DOI] [PubMed] [Google Scholar]
- Vadez V, Kholová J, Yadav RS, Hash CT(2013) Small temporal differences in water uptake among varieties of pearl millet (Pennisetum glaucum (L.) R. Br.) are critical for grain yield under terminal drought. Plant Soil 371: 447–462 [Google Scholar]
- Vanhees DJ, Loades KW, Bengough GA, Mooney SJ, Lynch JP(2020) Root anatomical traits contribute to deeper rooting of maize (Zea mays L.) under compacted field conditions. J Exp Bot 10.1093/jxb/eraa165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veen BW.(1982) The influence of mechanical impedance on the growth of maize roots. Plant Soil 66: 101–109 [Google Scholar]
- Wasson AP, Richards RA, Chatrath R, Misra SC, Prasad SVS, Rebetzke GJ, Kirkegaard JA, Christopher J, Watt M(2012) Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J Exp Bot 63: 3485–3498 [DOI] [PubMed] [Google Scholar]
- Wickham H.(2016) ggplot2: Elegant Graphics for Data Analysis. Springer, New York [Google Scholar]
- Yang JT, Schneider HM, Brown KM, Lynch JP(2019) Genotypic variation and nitrogen stress effects on root anatomy in maize are node specific. J Exp Bot 70: 5311–5325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- York LM, Lynch JP(2015) Intensive field phenotyping of maize (Zea mays L.) root crowns identifies phenes and phene integration associated with plant growth and nitrogen acquisition. J Exp Bot 66: 5493–5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- York LM, Nord EA, Lynch JP(2013) Integration of root phenes for soil resource acquisition. Front Plant Sci 4: 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, White PJ, Hochholdinger F, Li C(2014) Phenotypic plasticity of the maize root system in response to heterogeneous nitrogen availability. Planta 240: 667–678 [DOI] [PubMed] [Google Scholar]
- Zaman-Allah M, Jenkinson DM, Vadez V(2011) A conservative pattern of water use, rather than deep or profuse rooting, is critical for the terminal drought tolerance of chickpea. J Exp Bot 62: 4239–4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan A, Lynch JP(2015) Reduced frequency of lateral root branching improves N capture from low-N soils in maize. J Exp Bot 66: 2055–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan A, Schneider H, Lynch JP(2015) Reduced lateral root branching density improves drought tolerance in maize. Plant Physiol 168: 1603–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Postma JA, York LM, Lynch JP(2014) Root foraging elicits niche complementarity-dependent yield advantage in the ancient ‘three sisters’ (maize/bean/squash) polyculture. Ann Bot 114: 1719–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Brown KM, Lynch JP(2010) Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant Cell Environ 33: 740–749 [DOI] [PubMed] [Google Scholar]
- Zhu J, Kaeppler SM, Lynch JP(2005) Topsoil foraging and phosphorus acquisition efficiency in maize (Zea mays). Funct Plant Biol 32: 749. [DOI] [PubMed] [Google Scholar]