A high-resolution crystal structure of the sorghum cinnamate 4-hydroxylase (SbC4H) has implications for the functional properties of this protein in phenylpropanoid metabolism.
Abstract
Cinnamate 4-hydroxylase (C4H; CYP73A) is a cytochrome P450 monooxygenase associated externally with the endoplasmic reticulum of plant cells. The enzyme uses NADPH-cytochrome P450 reductase as a donor of electrons and hydroxylates cinnamic acid to form 4-coumaric acid in phenylpropanoid metabolism. In order to better understand the structure and function of this unique class of plant P450 enzymes, we have characterized the enzyme C4H1 from lignifying tissues of sorghum (Sorghum bicolor), encoded by Sobic.002G126600. Here we report the 1.7 Å resolution crystal structure of CYP73A33. The obtained structural information, along with the results of the steady-state kinetic analysis and the absorption spectroscopy titration, displays a high degree of similarity of the structural and functional features of C4H to those of other P450 proteins. Our data also suggest the presence of a putative allosteric substrate-binding site in a hydrophobic pocket on the enzyme surface. In addition, comparing the newly resolved structure with those of well-investigated cytochromes P450 from mammals and bacteria enabled us to identify those residues of critical functional importance and revealed a unique sequence signature that is potentially responsible for substrate specificity and catalytic selectivity of C4H.
The superfamily of cytochrome P450 enzymes includes a large group of heme-thiolate monooxygenase enzymes that catalyze the transfer of a single oxygen atom from O2 to a broad range of substrates in organisms from all kingdoms of life, including eubacteria, archaea, protists, fungi, plants, and animals (Dawson and Sono, 1987; Nelson et al., 1993). In plants, the P450 proteins constitute one of the biggest superfamilies; P450-encoding sequences comprise up to 1% of the genome in some plant species (Nelson et al., 2008; Mizutani and Sato, 2011). Plant P450 enzymes participate in a variety of metabolic pathways to produce both primary and secondary metabolites, including phenylpropanoids (Chapple, 1998; Mizutani and Ohta, 2010; Rustgi et al., 2019). Most of the plant P450s have been shown to prefer a single substrate, in contrast to the broad substrate preference characteristic of the majority of animal P450 enzymes (Guengerich, 1991; Renault et al., 2014).
The first step of phenylpropanoid metabolism in plants is the deamination of l-Phe, which is catalyzed by Phe ammonia lyase (PAL). This reaction results in the formation of cinnamic acid that is subsequently hydroxylated at the 4-position giving rise to p-coumaric acid (Fig. 1). This hydroxylation is catalyzed by cinnamate 4-hydroxylase (C4H; E.C. 1.14.13.11), an enzyme belonging to the CYP73A family of P450 enzymes. C4H can also be termed a class II monooxygenase, where the electrons necessary for catalysis are provided by NADPH cytochrome P450 reductase (CPR), a flavoprotein colocalized with C4H on the exterior surface of the membrane of the endoplasmic reticulum (Werck-Reichhart and Feyereisen, 2000). p-Coumaric acid serves as a precursor of myriad organic compounds that are essential for plant metabolism, structure, development, and defense, including ubiquinone, coumarins, stilbenes, chalcones, flavonoids, hydroxycinnamates, hydroxycinnamyl alcohols, lignans, and lignin (Treutter, 2006; Vanholme et al., 2010; Schreiner et al., 2012; Cheynier et al., 2013; Block et al., 2014; Shahidi and Ambigaipalan, 2015). Additional hydroxylation reactions in the monolignol biosynthetic pathway occur at the 3- and 5-positions of the benzene ring by p-coumarate-3-hydroxylase, p-coumaroyl shikimate 3′-hydroxylase, and ferulate-5-hydroxylase, respectively (Meyer et al., 1996; Schoch et al., 2001; Franke et al., 2002a, 2002b; Barros et al., 2019).
Figure 1.

Hydroxylation reaction of C4H enzyme converting trans-cinnamic acid to p-coumaric acid.
Downregulation of C4H in tobacco (Nicotiana tabacum), alfalfa (Medicago sativa), and Arabidopsis (Arabidopsis thaliana) results in a proportional decrease in lignin content (Sewalt et al., 1997; Blount et al., 2000; Van Acker et al., 2013). Reduction of C4H activity in tobacco through an antisense repression results in both reduced content and altered subunit composition of lignin (Sewalt et al., 1997). In addition, C4H of tobacco has been proposed to create a metabolic channel on the ER through which cinnamic acid is transferred between PAL and C4H without diffusion into the cytosol (Achnine et al., 2004). This means that modifications to C4H may have a greater impact on metabolic flux than would be expected based solely on its metabolic role.
Like other cytochrome P450 enzymes, C4H activates molecular oxygen and eventually inserts one oxygen atom into a C-H bond while reducing the other oxygen atom to water. Its instability, low abundance, and membrane localization impede the use of conventional purification methods for this protein (Chapple, 1998). Consequently, despite diverse and important roles of C4H enzymes in plants, no crystal structure of any of these proteins has been resolved so far. We carried out the determination of crystal structure of CYP73A33 protein and systematic enzyme kinetic and thermodynamic assays to fully understand the basis of substrate specificity of C4H enzymes.
The C4 grass sorghum (Sorghum bicolor) is receiving considerable attention as a climate-resilient lignocellulosic feedstock for the production of renewable fuels and chemicals, in part because of its ability to tolerate both heat and drought (Farre and Faci, 2006; Wang et al., 2014). Manipulation of lignin concentration and lignin subunit composition in sorghum through the incorporation of certain brown midrib (bmr) mutations has been shown to result in improved rumen digestibility (Porter et al., 1978) and greater efficiency of enzymatic saccharification of sorghum biomass (Saballos et al., 2008; Dien et al., 2009). In order to maximize the toolkit for manipulating the cell wall composition and redirecting the metabolic flux to phenylpropanoids with health-promoting properties for humans and farm animals, it is important to have a detailed understanding of substrate specificity and catalytic mechanisms of the enzymes involved in the biosynthesis of monolignols. We have reported detailed structural and catalytic analyses of participating enzymes in sorghum and switchgrass (Panicum virgatum), specifically hydroxycinnamoyl transferases (Walker et al., 2013), caffeic acid O-methyltransferase (Walker et al., 2016), and cinnamoyl-CoA O-methyl transferase (Green et al., 2014), cinnamyl alcohol dehydrogenases (Jun et al., 2017), cinnamoyl-CoA reductases (Sattler et al., 2017), PAL (Jun et al., 2018), and peroxidases (Moural et al., 2017). Here we present the crystal structure of sorghum C4H (SbC4H1), which is encoded by the gene Sobic.002G126600 and, according to the accepted cytochrome P450 nomenclature (Nelson, 2006), is classified as CYP73A33 protein. We also reconstituted the membrane-bound monooxygenase system consisting of SbC4H1 and CPR from sorghum (SbCPR) and demonstrated its activity in 4-hydroxylation of trans-cinnamic acid. The high-resolution structure of SbC4H1 provides critical insight into how the substrate-binding pocket evolved to be highly selective for trans-cinnamic acid. Considering the high level of amino acid sequence similarity, the enzymes encoded by sorghum genes SbC4H2 (Sobic.03G337400.1) and SbC4H3 (Sobic.04G141200.1) are predicted to also have C4H activity.
RESULTS
Interactions of SbC4H1 with Substrates Studied by Absorbance Spectroscopy
The absorbance spectrum of purified recombinant C4H from sorghum (SbC4H1) possesses all spectral features typical to cytochrome P450 enzymes and exhibits the Soret band of the ferric protein centered at 420 nm, which suggests a predominance of the low-spin state of the heme iron in the substrate-free heme protein. The absorbance spectrum of the carbonyl complex of the dithionite-reduced (ferrous) enzyme exhibits a maximum of the Soret band at 449 nm consistent with the cytochrome P450 nature of the protein (Supplemental Fig. S1).
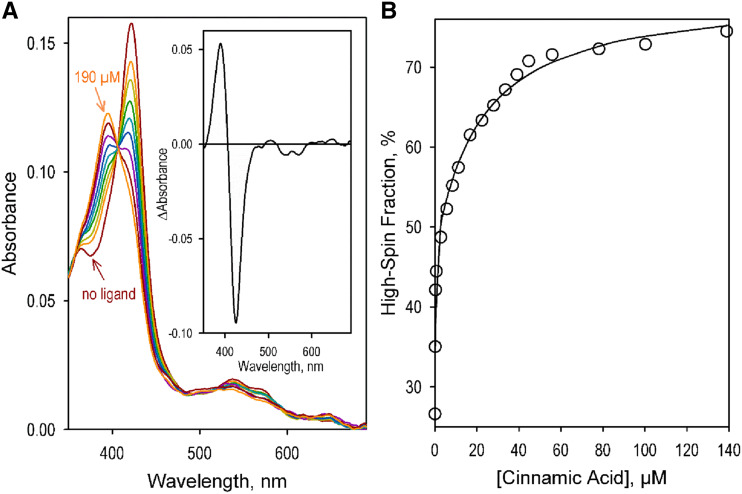
The interactions of the SbC4H1 enzyme with its natural substrates, trans-cinnamic acid and 2-naphthoic acid, a fluorogenic substrate that showed high affinity and rapid turnover with C4H from Helianthus tuberosus (CYP73A1; Schalk et al., 1997), were studied with advanced UV-visable absorbance spectroscopy. In these studies, we applied the technique of principal component analysis (PCA; Halaka et al., 1985; Davydov et al., 1995) to analyze the substrate-induced changes in protein absorbance. Combination of PCA with approximation of the spectra of principal components with a combination of prototypical spectra of absorbance of SbC4H1 in different states of ligation of the heme iron allowed us to interpret in terms of ligand-induced changes in the partitioning between the high- and low-spin states of the heme protein (Fig. 2).
Figure 2.
Interactions of SbC4H1 with trans-cinnamic acid. A, A series of absorbance spectra obtained in a titration of 1 μm SbC4H1 with trans-cinnamic acid. The spectra shown in the graph correspond to 0, 0.14, 0.28, 1.4, 5.6, 11, 22, 39, and 190 μm of the substrate added. Spectra for 0 and 190 μm are indicated by arrows; the spectra between these two concentrations displayed effects proportional to the concentrations added. The inset shows the spectrum of the first principal component obtained with PCA. B, Changes in the content of the high-spin fraction of the heme protein caused by its interaction with trans-cinnamic acid. The solid line shows the approximation of the data set with the equation deduced for the parallel mode of ligand interactions with two distinct binding sites (Fernando et al., 2006).
The substrate-induced changes in the C4H1 absorbance were typical for the interactions of cytochromes P450 with Type-I ligands and suggested a displacement of the spin equilibrium toward the high-spin state of the heme protein (Fig. 2A, inset). The shape of the dependencies of the high-spin fraction on the concentrations of substrates suggests the presence of two substrate-binding sites with different affinities. These titration curves closely obey the equation deduced for a parallel mode of interactions at two distinct ligand binding sites (Fig. 2B; Fernando et al., 2006). As is apparent from the parameters in Table 1, which were obtained from the fitting of the experimental data to this equation, the high-affinity binding event resulted in an 8% to 15% increase in the content of the high-spin form and was characterized by the dissociation constant (KD1) of 0.02 and 0.15 μm for trans-cinnamic acid and 2-naphthoic acid, respectively. Low amplitude of the substrate-induced spin shift indicates that the substrate-binding does not result in any significant displacement of the water molecule that serves as a sixth ligand of the heme iron in the low-spin state of the heme protein. Importantly, the amplitude of the spin shift considerably increases after the binding of the second substrate molecule and reaches 46% to 47% (Table 1).
Table 1. Parameters of interactions of SbC4H1 with the substrates and products of their oxidation determined by absorbance spectroscopy.
The estimates given in the table represent the averages of 5 individual measurements. The “±” values are the confidence intervals calculated for P = 0.05.
| Ligand | Ligand-Induced Spin Shift | |||||||
|---|---|---|---|---|---|---|---|---|
| KD1 | KD2 | High-affinity phase | Total | |||||
| μm | μm | % | ||||||
| Trans-cinnamic acid | 0.025 | ±0.021 | 28 | ± 8 | 16 | ± 7 | 47 | ±6 |
| 2-Naphthoic acid | 0.076 | ±0.046 | 32 | ±12 | 7.8 | ±2.4 | 46 | ±7 |
Enzyme Kinetic Assays
C4H catalyzes the 4-hydroxylation of trans-cinnamic acid using two electrons from its redox partner, CPR. In our studies of the enzymatic activity of C4H, we used a reconstituted membranous system where the P450 enzyme interacts with preformed proteoliposomes containing a full-length CPR enzyme. Besides the use of SbCPR, the native electron donor partner of SbC4H, we also probed the activity of the enzyme with recombinant CPR from rat (Rattus norvegicus ssp. domestica; rCPR) as a surrogate redox partner. In our experiments with both redox partners, we maintained their concentration at two times higher than the concentration of SbC4H1 in order to achieve the optimal activity of the system.
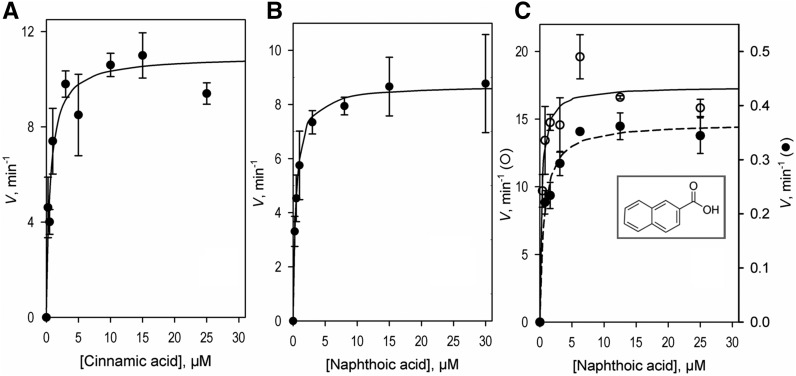
The steady-state kinetics experiments with the use of trans-cinnamic acid as a substrate were performed with monitoring of the product formation by liquid chromatography mass spectrometry (LC-MS; Fig. 3). As an alternative approach we employed fluorimetric detection of the activity of SbC4H1 using the fluorogenic substrate 2-naphthoic acid.
Figure 3.
Activity assays with SbC4H1. A and B, Dependence of the rate of oxidation of trans-cinnamic (A) and 2-naphthoic (B) acids on the concentration of the indicated substrates were obtained by determining the amounts of the formed products (p-coumaric acid and 6-HNA, respectively) through LC-MS assays. The initial rates of reaction were determined from the amount of product formed after 3 min of incubation. In each case, the concentration of NADPH and SbC4H1 (reconstituted with SbCPR) were held constant at 120 μm and 4 nm, respectively. C, Rate of oxidation of 2-naphthoic acid determined by fluorimetric assay. Dependence of the reaction rate on the concentration of substrate was obtained with SbC4H1 reconstituted with SbCPR (open circles) and rat CPR (solid circles). Molar concentrations of the formed product were determined from the intensity of fluorescence based on a calibration curve obtained by measuring the intensity of fluorescence of the product (6-HNA) added to the incubation mixture at increasing concentrations from 0.04 to 0.4 μm. Solid lines show the results of fitting of the experimental datasets to the Michaelis-Menten equation. Each experiment was repeated two to five times to acquire the sd (indicated as error bars).
Kinetic parameters obtained with both substrates with two different electron donors (SbCPR and rCPR) are shown in Table 2. It is apparent that the two substrates are quite similar in terms of the rates of turnover and values of KM exhibited with SbC4H1. However, our studies revealed a large contrast between SbCPR and rCPR in terms of the efficiency of the electron transfer to SbC4H1. As is evident from Table 2, the rates of turnover exhibited in a pair with SbCPR were ∼40 times higher than those observed in a system reconstituted with rCPR. By contrast, there were no substantial differences between the two reconstituted systems in the values of KM. This result indicates that the CPRs, as the electron donors to SbC4H1, do not affect the binding affinity of the substrate, but substitution of the native electron-donor partner (SbCPR) with its alternative (rCPR) significantly reduces the electron transfer efficiency and, consequently, substantially decreases the rate of turnover.
Table 2. Kinetic parameters of SbC4H1 for 2-naphthoic and trans-cinnamic acids by LC-MS and fluorometric assays.
| Substrate | KM | Vmax |
|---|---|---|
| μm | min−1 | |
| LC-MS Assay | ||
| Trans-cinnamic acid | 0.61 ± 0.15 | 12.2 ± 0.6 |
| 2-Naphthoic acid | 0.76 ± 0.22 | 7.7 ± 0.2 |
| Fluorometric Assay | ||
| 2-Naphthoic acid (SbCPR) | 0.34 ± 0.11 | 17.5 ± 1.0 |
| 2-Naphthoic acid (rCPR) | 0.68 ± 0.16 | 0.36 ± 0.02 |
Interactions of SbC4H1 with HEPES
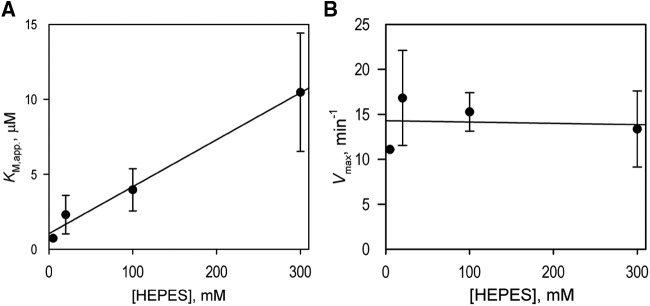
When setting up the conditions for our kinetic assays, we noticed that the use of HEPES buffer in place of the potassium phosphate buffer resulted in a considerable increase in the KM values exhibited by the enzyme. Therefore, we studied this effect in more detail by performing a series of kinetic assays with 2-naphthoic acid as the substrate at increasing concentrations of HEPES. The assays conducted in 5, 20, 100, and 300 mm of HEPES yielded KM values of 0.73 ± 0.05, 2.31 ± 1.28, 3.96 ± 1.41, and 10.47 ± 3.94 μm, respectively. As shown in Figure 4, the value of KM displays a linear dependence on the concentration of HEPES, whereas no considerable changes in Vmax were observed, indicating a competitive mode of inhibition by HEPES.
Figure 4.
Effect of HEPES on the kinetic parameters of hydroxylation of 2-naphthoic acid by C4H1. A and B, Effect of the concentration of HEPES buffer on the values of apparent KM (KM,app) and the maximal velocity (Vmax), respectively. The ionic strength of the buffer was kept constant (0.21 m) by addition of the appropriate amounts of KCl. The solid lines show the linear approximations of the datasets. The assays were conducted in 5, 20, 100, and 300 mm HEPES, and the value of KM,app displays a linear dependence on the concentrations of HEPES, whereas no statistically significant changes in the value of Vmax were detected. Each experiment was repeated two to five times to acquire the sd (indicated as error bars).
The value of the inhibition constant (Ki) was determined from the dependence of the apparent KM value (KM,app.) on the concentration of inhibitor using the canonical relationship for competitive inhibition (equation 4.1 of Cornish-Bowden [1976])
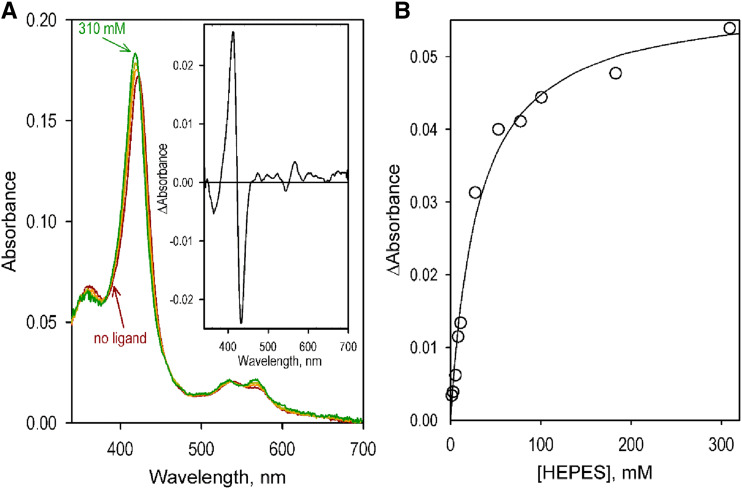
Application of this equation to the dataset shown in Figure 3 results in the Ki value of 33 ± 23 mm. The apparent competitive relationship between HEPES and the substrate (2-naphthoic acid in the above experiments) revealed in our kinetic assays prompted us to study the interactions of SbC4H1 with HEPES with advanced UV-visible absorbance spectroscopy. The series of spectra obtained in the titration experiments were subjected to PCA to determine the dependency of their amplitude on the concentration of the bound ligand. As shown in Figure 5, addition of increased concentrations of HEPES resulted in readily detectable spectral changes, namely a blue shift and broadening of the Soret band of the low-spin heme protein. These changes may be interpreted as resulting from a displacement of the water molecule that serves as the sixth ligand of the heme iron with the hydroxyl group of the bound HEPES molecule. Fitting of the titration curves with a hyperbolic (Michaelis-Menten) equation yields an estimated dissociation constant (KD) of 29 ± 6 mm. A good correspondence of this value to the value of Ki derived from our kinetic assays (33 ± 23 mm) corroborates the above conclusion regarding a competitive relationship between HEPES and the substrates of SbC4H1 in their interactions with the enzyme. As will be discussed later, this quite unexpected finding may find its explanations in a significant resemblance between the structure of the HEPES molecule and the structure of p-coumaric acid, the product of catalytic turnover.
Figure 5.
Interactions of SbC4H1 with HEPES. A, A series of absorbance spectra obtained by titration of 1.6 μm SbC4H1 with HEPES. The spectra shown in the graph correspond to 0, 25, 53, and 310 mm of HEPES added. The inset shows the spectrum of the first principal component (98.8% of the total changes in absorbance) obtained with PCA. B, Changes in the difference between absorbance at 412 and at 432 nm caused by SbC4H1 interactions with HEPES. The solid line shows the approximation of the data set with the hyperbolic (Michaelis-Menten) equation with KD = 28.8 μm.
Overall Structure of SbC4H1
Detergent-solubilized SbC4H1 crystallizes in the P3221 space group with diffraction up to 1.7 Å resolution. The final Rwork was 19.85%, Rfree was 21.37%, and root mean-square deviation from ideal geometry of the model was 0.005 Å for bonds and 0.73° for angles. Diffraction data and refinement statistics are listed in Table 3. The lattice packing of SbC4H1 crystal was through one molecule in the asymmetric unit (Fig. 6).
Table 3. X-ray diffraction data and refinement statistics for SbC4H1.
| Data Collection | SbC4H |
|---|---|
| Space group | P3221 |
| Cell Dimensions | |
| a, b, c (Å) | 132.278, 132.278, 79.431 |
| α, β, γ (°) | 90.00, 90.00, 120.00 |
| Resolution (Å) | 65.27–1.7 (1.761–1.7) |
| Rmerge | 0.3031 (2.319) |
| Wavelength (Å) | 1 |
| Unique reflections | 87984 (8712) |
| Completeness (%) | 99.92 (99.92) |
| <I>/σI | 4.75 (1.12) |
| CC1/2 | 0.995 (0.462) |
| Redundancy | 18.6 (17.1) |
| Refinement | |
| Rwork/Rfree | 0.1985/0.2137 (0.3093/0.3279) |
| Number of atoms | 4,686 |
| Proteins and ligands | 3,842 |
| Water | 736 |
| B-factors (Å2) | |
| All atoms | 23.73 |
| Solvent | 35.53 |
| Root-Mean-Square Deviations | |
| Bonds (Å) | 0.005 |
| Angles (°) | 0.73 |
| Ramachandrans | |
| % Favored | 97.44 |
| % Outliers | 0.21 |
| Clashscore | 4.71 |
| TLS groups | 3 |
Figure 6.
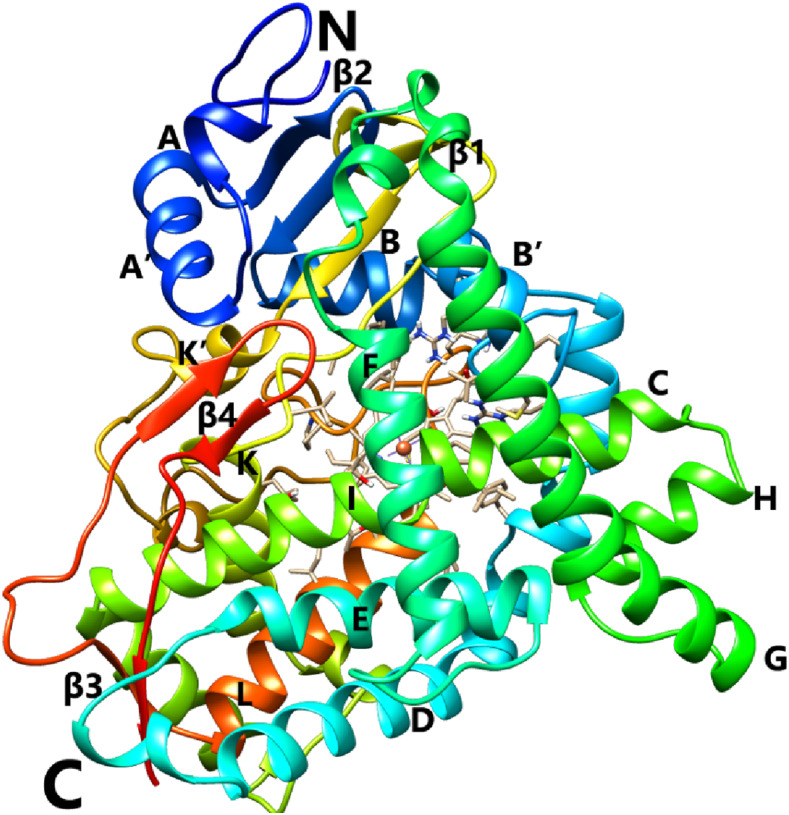
Ribbon diagram representing the crystal structure of SbC4H1. Secondary structure elements have been numbered sequentially as A to L and β1 to β4. “N” and “C” refer to the N- and C-terminal regions, respectively. Color compositions indicate different secondary structural elements in SbC4H1. The heme is represented by a stick model. Molecular graphics images were produced using the Chimera package (UCSF).
The overall fold of SbC4H1 displayed a typical cytochrome P450 folding, a helix-rich triangular prism-like shape (Fig. 6). It consists of 16 α-helices (A, A′, B, B′, C, D, E, F, F′, G, H, I, J, K, K′, and L) and four β-strands (β1–β4). These helices are similar to those observed among other P450 structures and thus were labeled according to the previously established scheme (Poulos et al., 1987). Despite certain specifics of SbC4H1 as to the length and orientation of individual elements of the secondary structure (and especially those located at the periphery of the molecule), the core elements at both proximal and distal sides of the heme, such as the loop preceding helix L and the oxygen activation region in the middle of helix I, exhibit three-dimensional structures typical of all cytochrome P450 proteins. Both the F and G helices in SbC4H1 have length commensurate with that observed in mammalian enzymes, being therefore much longer than the corresponding elements in bacterial P450s. However, the F-G loop is shorter than that typical of the mammalian P450 structures. The electron density that corresponds to the first nine amino acids at the N terminus of SbC4H1 is not visible, indicating a disordered structure of this region. These disordered residues correspond to the “MAKKTSSKG” N-terminal peptide, which replaces the native sequence 1MDLVLLEKALLGLFAAAVLAVAVAKLTGKRY31 in our construct. This replacement was made to increase protein solubility and facilitate crystallization. For ease of reading and comparing with other P450 structures, the original (full-sequence) numbering of residues was used throughout the article.
Heme Environment, Active Site, and Substrate Binding
The heme-iron center located in the central part of the SbC4H1 molecule is deeply buried inside the enzyme core. The heme iron is tethered to the Cys-443 residue, which serves as a fifth ligand at the proximal side of the heme. The adjacent Pro residue, Pro-444, which is rarely present in mammalian P450 sequences but is often observed in plant and bacterial P450 proteins, is in the trans conformation. Similar to what has been observed in other P450 structures, most residues surrounding the heme of SbC4H1, such as those establishing the B′/C turn, the central region of helix I, the N-terminal half of the L helix, and its N-terminal side loop, are hydrophobic in nature. Of special interest is Phe-436, positioned most closely to the sulfur-iron bond at the proximal side. The two propionates of the porphyrin ring are coordinated to two positively charged (Arg-101 and Arg-441) and two hydrogen-bond-forming (Trp-126 and His-373) residues.
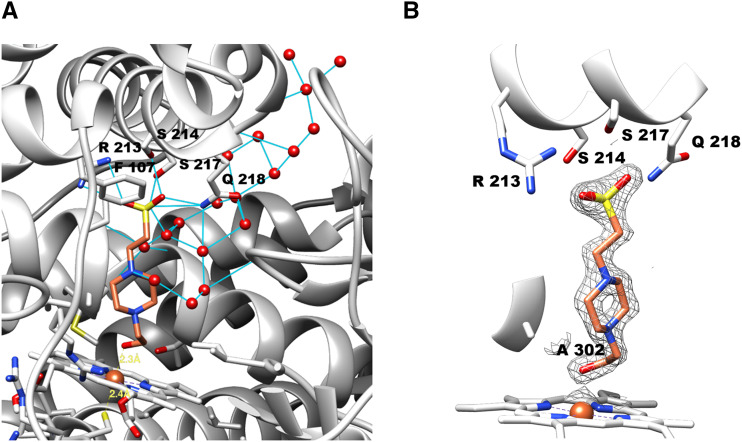
At the early stage of refinement, a significant electron density was identified at the distal side cavity formed by the helix-I, -F, -B′, and the β-turn between β4-1 and β4-2 (Fig. 7). Considering the shape of electron density, it was identified as belonging to a bound molecule of HEPES, which was present at 100 mm in the crystallization buffer. Our efforts to lower the concentration of HEPES gradually down to 20 mm, or to replace HEPES with a buffer like Tris, Bis-tris, and potassium phosphate, yielded no crystals. In addition, soaking the SbC4H1 crystals in solutions of cinnamic acid, 2-naphthoic acid, p-coumaric acid, 6-hydroxy-2-naphthoic acid (6-HNA), or His, a known axial ligand of the P450 heme iron, did not result in replacement of the bound HEPES molecule by those ligands. The discovery of a HEPES molecule bound to the heme pocket is consistent with the inhibitory effect of HEPES on SbC4H1 and demonstrates binding of HEPES to the heme pocket of SbC4H1, where it apparently replaces a water molecule as the sixth ligand of the heme iron.
Figure 7.
Molecular environment around a HEPES molecule bound at the active site. A, Active site of SbC4H1. B, Electron density (2 σ) for the HEPES molecule. HEPES and heme are represented as stick models. The backbone of SbC4H1 is represented as a ribbon diagram, and thin blue lines represent hydrogen bonds or ionic interactions. All residues that contribute to HEPES binding are labeled according to their single-letter abbreviations and numbered according to sequence. Water molecules are depicted by red balls. Molecular graphics images were produced using the Chimera package (UCSF, under grant no. NIH P41 RR–01081).
Despite the low binding affinity of HEPES to SbC4H1, which is explainable by the highly polar nature of the HEPES molecule, the high concentration of HEPES in the crystallization buffer allowed it to be bound as an axial ligand to the heme iron. The bound HEPES is surrounded by hydrophobic residues such as Phe-107, Val-118, Phe-119, Val-301, Ala-302, Ile-367, Val-371, and Phe-484. Its sulfonate group is positioned in proximity to the side chains of Arg-213, Ser-214, Ser-217, and Gln-218 located in the F helix for both electrostatic interaction and hydrogen bond formation. The hydroxyl group of the HEPES molecule appears to be coordinated to the heme iron as the sixth ligand and forms a hydrogen bond with the backbone carbonyl oxygen of Ala-302. The same backbone oxygen of Ala-302 also forms a hydrogen bond with the side chain of Thr-306. In addition, the center of helix I, 300NVAAIETT307, which contains both Ala-302 and Thr-306, has one water molecule tightly captured by the backbone carbonyl oxygen of Asn-300 and Val-301 and the backbone nitrogen of Ile-304 and Glu-305. The side chain of Thr-307 is in hydrogen bond interaction with the backbone carbonyl oxygen of Ala-303. Consequently, the central region of helix I is deformed due to the absence of the regular α-helical hydrogen bonds.
Channels
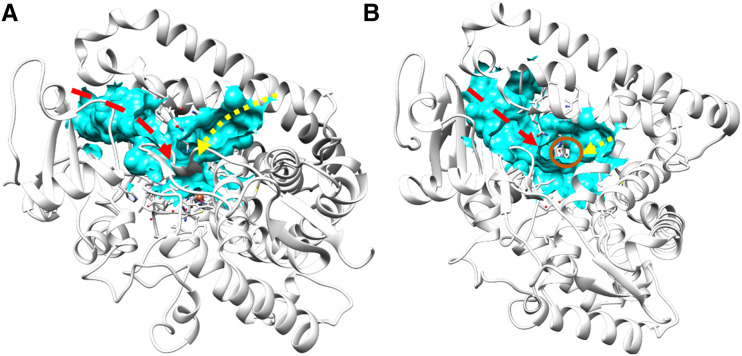
The active site of SbC4H1 is channeled through the enzyme and is connected to the surface of the enzyme and thus to the bulk solvent. Based on calculations performed with the CASTp 3.0 server, contiguous pockets had a solvent-accessible surface area of 1,352 Å2 and a volume of 701 Å3. There are four main parts of this large cavity: the heme pocket itself, a central stem connected to the distal side of the heme, and two lobes radiating from this stem in a Y-shaped manner (Fig. 8). The larger lobe in the left-side of the Y (Fig. 8) represents a funnel-shaped channel lined by the F and G helices that may accommodate substrate access and product egress. In the crystal structure, the smaller, right-side lobe of the Y (Fig. 8) is filled with water molecules and has an opening between helices F, E, and I. This channel is likely to enable water and proton access for the heme. However, the presence of other smaller water-access channels cannot be ruled out, as demonstrated in a previous study using molecular dynamic simulation with other P450 enzymes, where new access channels were created after rotation of the side chains initially blocking them (Dubey and Shaik, 2019). The observed HEPES ligand was found at the stem on the distal side of the heme. Although the gate over the larger lobe, which is established between the F/G loop and helix B′, is open, the channel appears fairly closed off upon ligand binding, as noted in other P450 enzymes (Liou et al., 2016; Dubey and Shaik, 2019). The larger lobe is connected to the surface of the enzyme through a channel ∼6 Å wide and is formed primarily by helices F, F′, and G, the B/B′ loop, and helix B′ on one side and by the B′/C and A/A′ loops, the β-turn between β1-1 and β1-2, β1-2, and the β-turn between β4-1 and β4-2 on the opposing side. Compared to ferruginol synthase from Salvia miltiorrhiza (Protein Data Bank [PDB] ID 5YLW; Gu et al., 2019), the open cleft of SbC4H1 is slightly wider, probably due to the more extended F and G helices. The F and G helices of SbC4H1 are significantly longer than bacterial P450s such as P450cam from Pseudomonas putida (PDB IDs 7CPP and 5IK1, respectively). There are clustered positively charged residues, such as Arg-76, Arg-103, and Arg-232 in β1-2, the B/B′ loop, and helix G, respectively, at the entrance port of the larger lobe.
Figure 8.
Channels in the crystal structure of SbC4H1. A, Side view perpendicular to the heme plane. B, Top view parallel to the heme plane. Channels are represented in cyan. Red and yellow arrows indicate the hypothetical channels for substrate and water, respectively. The heme iron is indicated by a red circle. Molecular graphics images were produced using the Chimera package (UCSF, under grant no. NIH P41 RR–01081).
Molecular Docking
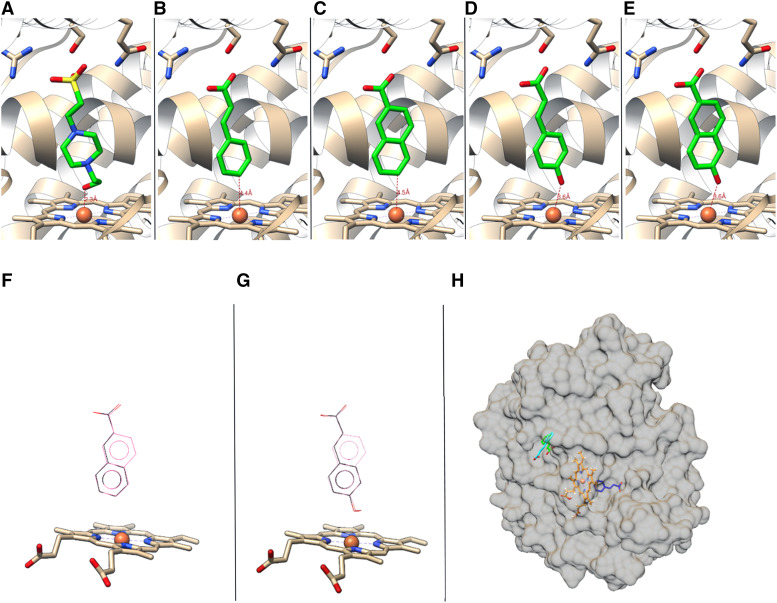
To model the binding of the substrate, trans-cinnamic acid, and the product, p-coumaric acid, the ligands were docked into the crystal structure of SbC4H1 using AutoDock Vina (Trott and Olson, 2010). As a control, HEPES was also docked into the same structure (Fig. 9). The docked positions of the lowest ΔG showed both trans-cinnamic and p-coumaric acid bound at the same position as the crystallographic HEPES molecule (Fig. 9, B and D), where their propionyl groups interacted with the guanidinium side chain of Arg-213, while also being within hydrogen-bonding distance from Ser-214 and Gln-218. Reliability of the docking is supported by the fact that the position and orientation of both docked molecules, trans-cinnamic and p-coumaric acid, as well as the docked HEPES molecule, coincide well with the position and orientation of the HEPES molecule present in the crystallographic structure (Fig. 9, A, C, and E). The docking energies (ΔGb) for trans-cinnamic acid, 2-naphthoic acid, p-coumaric acid, and 6-hydroxy-2-naphthoate were −7.6, −8.2, −7.9, and −8.7 kcal/mol, respectively. Overall, molecular docking showed trans-cinnamic and 2-naphthoic acid docked in almost the same location and orientation (Fig. 9F). The same was true for the p-coumaric acid and 6-hydroxy-2-naphthoate pair (Fig. 9G). In addition, a second binding site for trans-cinnamic acid identified on the surface of SbC4H1 had a slightly different orientation and reduced ΔGb value of either −6.6 or −6.1 kcal/mol (Fig. 9H). The pocket was located at the concave area between helices K and L, which is surrounded by Leu-347, Gln-354, and Leu-450 (Fig. 9H).
Figure 9.
Positions for the ligand, substrate and product molecules. A to E, Crystallographic position of HEPES (A) and molecular docking positions of trans-cinnamic acid (B), 2-naphthoic acid (C), p-coumaric acid (D), and 6-HNA (E) to SbC4H1. F and G, Two substrates of SbC4H1, cinnamic acid (black) and 2-naphthoic acid (pink), docked in the same location and orientation (F), and the same was true for two corresponding products, p-coumaric acid (black) and 6-HNA (pink; G). H, Docked position of trans-cinnamic acid at the surface with lower affinity, ΔGb, of either −6.6 (green) or −6.1 (cyan) kcal mol−1. Trans-cinnamic acid at the active site is indicated in blue, and the heme in orange. Figures were generated by Molecular graphics images, produced using the Chimera package (UCSF, under grant no. NIH P41 RR–01081).
Structural Alignment of SbC4H1 with other P450s
To identify structural homologs and correlate their sequences with SbC4H1, the amino acid sequence of SbC4H1 was used to perform a similarity search among the crystal structures of all P450s deposited in the PDB using BLAST (Altschul et al., 1997). In addition, a chain fold search with DALI (Holm and Sander, 1993; Holm and Rosenström, 2010) was performed using the atomic coordinates of SbC4H1 to identify closely related structural homologs in the PDB.
The top 10 hits by BLAST on structures deposited in the PDB (identification numbers in parentheses) were CYP76AH1 (ferruginol synthase) from S. miltiorrhiza at 31% amino acid identity (5YLW), CYP17A1 from Danio rerio at 28.9% (6B82), human (Homo sapiens) CYP17SA1 at 28.54% (4NKV and 3RUK), CYP2B4 from rabbit (Oryctolagus cuniculus; 6BWW and others), human CYP1A1 at 27.69% (4I8V), human CYP2C9 at 28.76% (5X24 and others), human CYP1A2 at 27.41% (2HI4), bovine (Bos Taurus) CYPC21 at 27.36% (3QZ1), human CYP1B1 at 27.78% (3PM0), and human CYP21A2 at 27.32% (5VBU and 4Y8W). P450cam from Pseudomonas putida (5IK1) shows only 17.6% identity. On the other hand, the top 10 hits in the DALI search were human CYP1A2 (2HI4), CYP19A1 (3EQM), and CYP3A4 (1TQN), with Z-scores of 38.1, 37.8, and 37.6, respectively; P450 BM3 from Bacillus megaterium (3HF2), with a Z-score of 37.3; CYP120A1 from Synechocystis sp. (2VE3), with a Z-score of 36.8; human CYP46A1 (4J14), with a Z-score of 36.7; CYP170A1 from Streptomyces coelicolor (3DBG), with a Z-score of 35.3; human CYP11A1 from H. sapiens (3NA1), with a Z-score of 34.8; CYP134A1 from Bacillus subtilis (3NC7), with a Z-score of 32.5; and CYP245A1 from Streptomyces sp. TP-A0274 (2Z3U), with a Z-score of 30.8.
Oddly, despite the high amino acid sequence identity between SbC4H1 and ferruginol synthase (CYP76AH1, 5YLW) from S. miltiorrhiza, ferruginol synthase did not appear in any DALI results. However, pairwise structural comparison between SbC4H1 and ferruginol synthase by the DALI server resulted in a Z-score of 44.3, which confers relatively superimposable structures. Thus, a detailed sequence comparison of SbC4H1 was conducted with the top three P450s, CYP76AH1 from S. miltiorrhiza, CYP17A1 from D. rerio (4R1Z), and CYP2B4 from rabbit (1PO5). Despite relatively low levels of sequence identity and differences in length of the individual secondary structures, the locations of individual secondary structural elements are conserved (Fig. 10). In particular, the spatial position and relative orientation of the heme and helices I and L are conserved among all P450 enzymes compared. Similar to other P450s, most residues around the heme of SbC4H1 are conservatively hydrophobic, whereas the residues constituting helices F, F′, and G are relatively poorly conserved. According to analysis with the University of California, San Francisco (UCSF) Chimera package (Pettersen et al., 2004), 11 of 31 residues located around the heme (within 3.6 Å of the heme iron) are conserved among P450 structures under comparison. The list of conserved residues includes Leu-91, Arg-101, Trp-126, Arg-130, Thr-306, Pro-435, Phe-436, Arg-441, Cys-443, Gly-445, and Ala-449 (Fig. 10). Although most of these residues are in helices C, I, and L, some belong to helices B and K. Those include completely conserved Arg-101, Trp-126, Arg-130, and Arg-441, which establish hydrogen bonds with the heme porphyrin ring in all compared structures. The sequence signature of the heme-binding loop of cytochromes P450, which according to PROSITE (Falquet et al., 2002) is [FW]-[SGNH]-x-[GD]-|F|-[RKHPT]-|P|-C-[LIVMFAP]-[GAD], is conserved in SbC4H1 as 436FGVGRRSCPG445. Another highly conserved region in helix I with a general signature motif of [AG]-[AG]-x-[ED]-T (present in SbC4H1 as 302AAIET306) is known to be important for proton transfer and oxygen activation (Sen and Thiel, 2014).
Figure 10.
Multiple-sequence alignment of the amino acid sequence and secondary structure of C4Hs. A, Secondary structure elements are highlighted in green for α-helices and red for β-strands. Sixteen α-helices and four β-strands are labeled in bold above sequences in blue boxes. The 11 conserved residues around the heme are indicated in bold with an asterisk above. Signature residues that are conserved in all compared C4Hs, 290DNVLYIVENINVAAIETTLWSIEWGIAELVNHP322, 435PFGVGRRSCPGIILALPI452, 218QSFEYNYGDFIP229, and 101RTRNVVFDIFTG112, are in orange-dotted boxes. B, A phylogenetic tree was generated with Phylogeny.fr (www.phylogeny.fr/; Dereeper, A et al., 2008) for SbC4H1 (S. bicolor), GmC4H (G. max, NP_001237317.1), AtC4H (Arabidopsis, NP_180607.1), ZmC4H (Z. mays, PWZ11997.1), ObC4H (O. brachyantha, XP_006654260.1), CYP76AH1 (S. miltiorrhiza, PDB 5YLW), Cyp17A1 (D. rerio, PDB 4R1Z), and Cyp2B4 (O. cuniculus, PDB 1PO5). Branch support values (%) are shown in red (Scale bar = 0.6 amino acid substitutions per site). Based on sequence similarity, SbC4H1 is closely related to C4H from Z. mays and O. brachyantha.
DISCUSSION
Structural Implications of the Catalytic Mechanism
Based on the fact that C4H enzymes display a strong preference for cinnamic acid as a single substrate, the substrate-binding pocket was examined in order to pinpoint specific binding interactions between SbC4H1 and the docked cinnamate.
Given that the crystal structure of SbC4H1 contained a HEPES molecule at the substrate-binding site, that docked HEPES, trans-cinnamic acid, and p-coumaric acid were at nearly equivalent positions with the crystallographic HEPES molecule, and that all have remarkably similar molecular geometry, we deemed the crystallographic HEPES to be an appropriate model for analyzing the molecular contacts of SbC4H1 with the bound substrate. This analysis indicates that SbC4H1 uses the hydrophobic side chains of Phe-107, Val-118, Phe-119, Val-301, Ala-302, Ile-367, Val-371, and Phe-484 for binding phenylpropene, the hydrophobic portion of the trans-cinnamic acid. The sulfonate group of the HEPES is at a position in space nearly equivalent to that of the carboxyl group of trans-cinnamic acid or p-coumaric acid, which suggests that Arg-213, Ser-214, and Gln-218, located in the F helix and conserved among C4H enzymes, establish electrostatic interactions and a hydrogen bond with trans-cinnamic acid. The hydroxyl group of HEPES is bound as the sixth ligand to the heme iron, and simultaneously establishes a hydrogen bond with the backbone carbonyl oxygen of Ala-302 (Fig. 7B). Given the close proximity of the piperazinyl ring to the docked phenyl or phenol ring of trans-cinnamic/p-coumaric acid, this positional configuration is consistent with the ferrous iron ligated to molecular oxygen or to the para-hydroxyl group in p-coumaric acid. The same atom of Ala-302 also forms a hydrogen bond with the side chain of Thr-306. Both residues are located in the kinked region in the middle of helix I, 300NVAAIETT307, and hold a water molecule through hydrogen bonds with the backbone atoms of Asn-300, Val 301, Ile-304, and Glu-305. The carbonyl oxygen of Ala-303 is also in a hydrogen bond interaction with the side chain of Thr-307. Similar to what is observed with Thr-252 in P450cam (Gerber and Sligar, 1992; Vidakovic et al., 1998; Schlichting et al., 2000), Thr-306 in SbC4H1 appears to provide key hydrogen bonds establishing the kink in α-helix I, while Glu-305 interacts with the side chains of Arg-175 and Gln-179 at the highly solvated, concave-shaped enzyme surface. All residues participating in this hydrogen bond network are strongly conserved among C4H enzymes, indicating their important functional role and structural conservation.
The system of hydrogen bonds described above closely resembles what is observed in many other P450 enzymes (Gerber and Sligar, 1992; Hamdane et al., 2008; Pochapsky et al., 2010) and what was first demonstrated in P450cam (Martinis et al., 1989; Gerber and Sligar, 1992; Vidakovic et al., 1998). The water molecule sequestered at the kink of α-helix I and coordinated to Thr-306 and Glu-305 in SbC4H1 is analogous to WAT901 in P450cam (Schlichting et al., 2000), where it is hydrogen-bonded to the Asp-251 and Thr-252 pair located at similar positions at the “groove” of α-helix I (Gerber and Sligar, 1992; Vidakovic et al., 1998; Schlichting et al., 2000). The system of hydrogen bonds formed by this water molecule and the side chains and backbones of the adjacent residues forms a proton relay network connected to bulk water at the SbC4H1 enzyme surface. By analogy to what is established for P450cam, we can imply, therefore, that the acid-alcohol pair of Glu-305 and Thr-306 may play a key role in ensuring timely delivery of two protons necessary for the catalytic turnover. Coordination of proton delivery with catalytic turnover is necessary to prevent uncoupling of electron transfer from substrate monooxygenation, which would result in production of hydrogen peroxide and other reactive oxygen species by futile cycling of the enzyme (Loida and Sligar, 1993; Makris et al., 2002; Zangar et al., 2004).
Catalytic Inefficiency of SbC4H1 in Combination with a Heterologous Redox Partner
Another important prerequisite for a high rate of catalysis and the tight coupling of catalytic turnover to NADPH consumption is efficient synchronization of electron flow from CPR to P450 with substrate binding and monooxygenation. This involves fine tuning of interactions in the CPR-P450 pair, their modulation by substrate, and the coordination of CPR conformational dynamics with catalytic cycling (Hlavica, 2007; Iyanagi, 2019). Studies of electron transfer between heterologous redox partners are instrumental in exploring the mechanisms of formation of electron transfer complexes and related conformational rearrangements in cytochrome P450s and their reductases (Davydov et al., 2000; Anandatheerthavarada et al., 2001; Fairhead et al., 2005; Davydov et al., 2010; Hlavica, 2015; Zhang et al., 2018). In order to probe the conservation of these mechanisms between the mammalian microsomal P450 system and the pairing of SbC4H with its native reductase, we compared the kinetic parameters of monooxygenation reactions catalyzed by the SbC4H1-SbCPR pair to those exhibited by the SbC4H1-rat CPR (rCPR) pair. Another aim of our studies with rCPR was to probe the use of closely characterized and readily available mammalian enzyme as a surrogate electron donor partner in further studies with SbC4H1. According to our results, the rates of turnover exhibited by the SbC4H1-SbCPR pair are ∼40 times higher than those observed in the SbC4H1 reconstituted with rCPR. However, no substantial differences in the values of KM were detected. These results suggest that there are significant differences between plant and mammalian systems in the mechanisms of P450-CPR interactions and related conformational dynamics of the proteins. Consequently, low activity of SbC4H1 combined with rCPR should preclude the use of the latter protein in further studies with SbC4H1 and other SbC4Hs.
A Putative Allosteric Binding Site
Both the titration experiment (Fig. 2) and the molecular docking studies (Fig. 9H) strongly suggest the presence of a second substrate-binding site located at the surface of SbC4H1. The second trans-cinnamic acid was docked at the hydrophobic pocket located between helices K and L in proximity to the heme pocket. One of the constituting hydrophobic residues, Leu-450 in helix L, is located right next to the Cys loop (heme-binding loop) and helix K (L-347/Q-354), which flanks the proximal side of the heme pocket. Thus, binding of a trans-cinnamic acid molecule at this site could alter the heme pocket geometry. These changes may initiate a more efficient displacement of the water molecule from the vicinity of the heme iron and, consequently, be responsible for a more pronounced low-to-high spin shift observed in the low-affinity binding phase of the titration experiments (Fig. 2). Furthermore, the location of the second binding site is close to the interface between the FMN-binding domain and the heme-binding domain in the structure of P450BM3 (Sevrioukova et al., 1999). Therefore, the association at this site may also alter the affinity of the SbC4H-SbCPR interaction and/or affect the rate of electron transfer.
Based on the above analysis, and taking into account that (1) the first substrate-binding event produces only a low-amplitude (∼8%–15%) spin shift in the heme protein; (2) the binding of the second substrate molecule results in a considerably more prominent (∼45%) increase in the high-spin content; and (3) the high-spin state of the enzyme-substrate complex cytochromes P450 is commonly recognized as more favorable for their catalytic turnover (Blanck et al., 1983; Fisher and Sligar, 1985; Denisov et al., 2007), it is tempting to propose a regulatory role for the second substrate-binding site. Allosteric interactions at this site may help to fine tune the functional properties of SbC4H1 and adjust them depending on the cellular concentration of the trans-cinnamic acid.
C4H Signature Residues
Superimposing the crystal structure of SbC4H1 on those of widely investigated P450s such as P450cam, P450BM3, and CYP1A reveals that the key secondary structural elements align reasonably well, despite low shared sequence identity among these proteins (Fig. 10A). Among the deposited coordinates of P450s in the PDB, ferruginol synthase from S. miltiorrhiza (5YLW) provides the structure most similar to that of SbC4H1 in terms of both amino acid sequence (31% similarity) and 3D structure (Z-score of 44.3) according to our BLAST and DALI searches (Fig. 10). Although there are two atypical plant P450 structures in the PDB, allene oxide synthases from Parthenium argentatum (Li et al., 2008) and Arabidopsis (Lee et al., 2008), ferruginol synthase is the only class II plant P450 for which a crystal structure is available in the PDB. This enzyme catalyzes monooxygenation that converts abieta-8,11,13-triene into ferruginol, an intermediate in tanshinone biosynthesis (Guo et al., 2013). However, the sequence of this protein is somewhat distant from those of other C4H enzymes (Fig. 10B). On the other hand, a BLAST search of the nonredundant protein sequence database of the National Center for Biotechnology Information (https://blast.ncbi.nlm.nih.gov) using the SbC4H1 sequence yields several monocot C4H enzymes, including enzymes from Panicum miliaceum, Panicum hallii, Zea mays, Miscanthus × giganteus, and Oryza brachyantha, for which sequence identity was as high as 96.41%, 96.21%, 96.21%, 96.0%, and 90.42%, respectively. In the same search, the eudicot C4H enzymes from Glycine max and Arabidopsis showed identity of only 77.14% and 76.29%, respectively.
The crystal structure of SbC4H1 and its sequence alignment with many C4Hs from monocots and dicots revealed several unique signature sequences. The sequence 290DNVLYIVENINVAAIETTLWSIEWGIAELVNHP322, which spans the entire helix I at the proximal side of the heme, is conserved among most C4H isozymes and the five C4H enzymes compared (Fig. 10A). A BLAST search with this sequence reveals many unnamed hypothetical proteins in the nonredundant protein sequence database of the National Center for Biotechnology Information that are likely to be C4H enzymes.
Despite the unique amino acid sequence that is highly conserved among SbC4H1 and other CYP73A superfamily enzymes, the center of helix I in SbC4H, 300NVAAIETT307, displays distorted helix geometry with disrupted normal backbone hydrogen bonds and captures a water molecule through multiple hydrogen bonds in the cavity, as in other P450 structures. As described in the “Results” section, the observed intricate hydrogen bond network and salt bridge in the distorted 300NVAAIETT307 and captured water molecule that are connected to the bulk solvent through a small water-filled lobe could activate iron-bound reduced oxygen by shuttling protons from the bulk solvent through the water-filled channel.
Importantly, the pair of two adjoined Ala residues (Ala-302 and Ala-303 in SbC4H1), which is conserved among C4H enzymes, is rarely found in other P450 proteins, where the respective positions are occupied by either Ala-Gly or Gly-Gly pairs (Schalk et al., 1999). Ala-302 is the residue positioned most closely to the heme, and its carbonyl oxygen establishes the hydrogen bond with the heme-coordinating hydroxyl group of bound HEPES in the crystal structure of SbC4H1. In addition, Ala-302 together with Val-301 forms a part of the Van der Waals surface of the wall adjacent to the bound substrate molecule. Substitution of Ala-302 and/or Ala-303 in CYP73A1 with a Gly residue(s), which is more common at those positions, results in a considerable decrease in the enzyme affinity to substrate, and also decreases catalytic efficiency and stability (Schalk et al., 1999). These observations indicate a critical role of this Ala duo in the structural stability of the enzyme and in positioning the substrate in the active site.
Another region of SbC4H1, 435PFGVGRRSCPGIILALPI452, which contains an invariant Cys, spans the N-terminal half of the L helix and its N-terminal loop on the proximal side of the heme and is highly conserved among the above listed plants and many other monocots and dicots. A fragment of this conserved sequence, 436FGVGRRSCPT445, called a Cys-pocket or β-bulge, encloses the Fe-S in a hydrophobic environment. The presence of residue Pro-444 adjacent to Cys-443, which is rare in mammalian P450 proteins but common in many bacterial and plant P450s, is somewhat unexpected considering the geometric constraints from Pro (Schalk et al., 1999). Mutation of the corresponding Pro in CYP73A1 results in disruption of the heme-protein interaction and loss of catalytic activity, probably due to impaired O2 binding and reduction (Schalk et al., 1999). Pro was also proposed to be essential for the correct insertion of the heme into the apoprotein due to its cis-trans isomerization (Schalk et al., 1999). In the crystal structure of SbC4H1, Pro-444 is in trans conformation and the overall Cα tract is superimposable on that in the non-Pro-containing P450s, such as Cyp17A1 (4R1Z), Cyp2B4 (1PO5), and P450BM3 (1SMJ). The guanidinium side chain of the highly conserved Arg-441 in the same sequence motif of SbC4H1 establishes a salt bridge with the heme propionate with 3.00 Å. Based on the results of molecular dynamics simulation, the corresponding interaction was proposed to be an aqueduct gating mechanism in human CYP3A4 (Fishelovitch et al., 2010). When this Arg residue rotates and breaks the salt bridge, a connection is established between a cluster of active-site water molecules and the bulk solvent, shuttling the protons to the active site through ordered water molecules (Fishelovitch et al., 2010).
Residues at the potential substrate entry port, the F/G loop, and the B′-helix are well conserved as 218QSFEYNYGDFIP229 and 101RTRNVVFDIFTG112, respectively, among the C4H enzymes compared. In addition, Arg-76, Arg-103, and Arg-232, which are at or near the entrance port, are connected with neighboring residues through electrostatic interaction. Importance of the Arg at the equivalent position has been proposed before in P450BM3 (Dubey et al., 2016; Li and Poulos, 1999). In addition, most of the observed channel-forming residues are conserved among C4Hs. In particular, the amino acid sequences of the F and G helices are well conserved among the C4Hs compared, although the corresponding regions are known to have a low level of similarity, offering unique substrate specificity for various substrates for each P450 enzyme.
The hydrophobic residues establishing the wall of the substrate-binding pocket of SbC4H—Phe-107, Val-118, Phe-119, Val-301, Ala-302, Ile-367, Val-371, and Phe-484—together with those interacting with the sulfate group of HEPES—Arg-213, Ser-214, and Gln-218—were completely conserved.
Specific coordination of propionate groups of heme is required by the polar and/or charged residues to accommodate their polarity and negative charge in the hydrophobic interior of the P450. The participating residues were conserved among all compared P450s, except for His-373, which is replaced by Arg in ferruginol synthase (Fig. 10).
CONCLUSION
Lignin is derived from the polymerization of monolignols and other aromatic compounds, and the first two steps that give rise to monolignol precursors are catalyzed by PAL and C4H. C4H has been proposed to be physically associated with PAL to create a metabolic channel, which is responsible for ensuring efficiency of phenylpropanoid metabolism. In this article, the membrane-bound monooxygenase system consisting of SbC4H1 and SbCPR was reconstituted, and its activity in 4-hydroxylation of trans-cinnamic acid was demonstrated. The comprehensive characterization of SbC4H1, including the first crystal structure of C4H, indicates multiple highly conserved residues at the substrate-binding sites and a potential regulatory role of the secondary binding pocket for the substrate. Overall, our results contribute to the fundamental knowledge necessary to tune the chemical composition in the secondary cell walls of plants.
MATERIALS AND METHODS
Chemicals
Analytical-grade chemicals were obtained from Sigma-Aldrich, Thermo Fisher, and AlfaAesar. In addition, bovine liver l-α-phosphatidylethanolamine and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphate (phosphatidic acid; Avanti Polar Lipids), octyl-β-d-glucopyranoside (Fluka Honeywell Specialty Chemicals) were used. Crystal screening solutions were obtained from Hampton Research.
Expression and Purification of Recombinant SbC4H
The SbC4H1 complementary DNA corresponding to the sorghum (Sorghum bicolor) gene Sobic.002G126600 was modified: with the sequence encoding the N-terminal transmembrane anchor truncated, it was cloned into the pET30a vector for overexpression. The vector was introduced into Escherichia coli BL21(DE3) cells with GroEL (Nishihara et al., 2000). Ten milliliters of lysogeny broth was inoculated with cells from a glycerol stock and grown overnight at 37°C in an orbital shaker incubator before being transferred into 800 mL Terrific Broth medium complemented with 50 μg mL−1 spectinomycin and 50 μg mL−1 kanamycin. The cells were grown at 37°C until the culture OD600 was >1.0, and then the temperature was reduced to 28°C before adding 80 mg L−1 5-aminolevulinic acid and 1 mm isopropylthio-β-galactoside. The cells were induced for 48 to 72 h and then harvested by centrifugation at 8,000g for 15 min at 4°C. Protease inhibitor cocktail (Sigma-Aldrich) was added to buffer A (100 mm HEPES [pH 7.4] and 10% [v/v] glycerol) with 0.5 m KCl before resuspending. After sonicating on ice for 30 min (model 450 Sonifier; Branson Ultrasonics), 10% (w/v) CHAPS stock solution was added to a final concentration of 0.5% (w/v). The suspension was stirred overnight at 4°C before removing cell debris by centrifugation at 31,000g for 1 h. The clarified lysate was loaded onto a nickel-NTA column at 4°C (Qiagen) and washed with buffer A with 20 mm imidazole, 0.5 m KCl, 0.5% (w/v) CHAPS, and 2 mm 2-mercaptoethanol. The column was washed with buffer A with 150 mm KCl, 0.5% (w/v) CHAPS, and 2 mm 2-mercaptoethanol to decrease the salt concentration, then eluted with buffer A with 100 mm His, 0.5% (w/v) CHAPS, and 2 mm 2-mercaptoethanol. The eluted protein was buffer-exchanged through an Amicon 8050 ultrafiltration cell (100-kD cutoff membrane; Millipore) against buffer B (20 mm HEPES and 10% [v/v] glycerol) with an additional 100 mm KCl. The protein solution was then applied to 2 mL carboxymethyl cellulose resin (Bio-Rad) equilibrated with the same buffer and eluted with buffer B with 150 mm KCl. The purity of the eluted fraction was analyzed by SDS-PAGE, followed by staining with Coomassie brilliant blue, and the concentration was determined by CO spectrum. Purified SbC4H1 was mixed with the CO-saturated membrane solubilization buffer (100 mm potassium phosphate [pH 7.3], 10% [v/v] glycerol, 0.5% [w/v] sodium chloride, 0.4% [v/v] Igepal CO-630, and 1 mm EDTA) at a 1:50 ratio, and the spectrum was recorded. Approximately 0.1 μg of sodium dithionite was then added to the solution to reduce SbC4H1. The spectrum was recorded again after 1 min and the concentration was determined by the difference between the two spectra. The extinction coefficient was 0.091 μm cm−1 for the A450. The protein was concentrated with Vivaspin 20 (Sartorius) to 15 mg mL−1.
Crystallization and Structure Determination
C4H crystals were grown by the hanging drop method at 277 K. Crystals were obtained by mixing the pure protein (15 mg mL−1 in 120 mm potassium phosphate [pH 7.4], 0.5 m Suc, and 1 mm EDTA) with an equal volume of reservoir solution that consisted of 0.1 m HEPES (pH 7.5), 12% (v/v) isopropanol, and 14% (w/v) PEG 2000. Crystals started to appear after 10 d. SbC4H1 crystallized in the P3221 space group, diffracting to 1.70 Å resolution. Data were collected at the Advanced Light Source beamline 5.0.2 with a wavelength of 1.0 Å. The software package HKL2000 was used for diffraction data processing (Otwinowski and Minor, 1997). Initial phasing of diffraction data was performed by molecular replacement with the PHENIX Phaser (Adams et al., 2010) using the coordinates of CYP17A1 from Danio rerio (PDB ID 4R1Z) as a search model. Refinement and model building were done using PHENIX and Coot (Emsley et al., 2010). Diffraction data statistics are listed in Table 3. Crystallographic data and coordinates were deposited in the PDB.
Expression and Purification of Recombinant CPR Enzymes
Recombinant NADPH CPR from rat (Rattus norvegicus domestica) liver (rCPR) was expressed in E. coli Topp3 cells and purified as described (Harlow and Halpert, 1998). Recombinant full-length CPR complementary DNA from sorghum (SbCPR) was cloned into in pET30a and expressed in E. coli Rosetta cells (MilliporeSigma). For large-scale culture, 3 L of Luria-Bertani broth supplemented with 25 μg mL−1 chloramphenicol and 50 μg mL−1 kanamycin was shaken at 37°C, 220 rpm until the OD600 reached 0.6. The temperature was reduced to 25°C before adding 0.5 mm isopropylthio-β-galactoside. The cells were induced overnight and then harvested by centrifugation at 8,000g for 15 min at 4°C. Protease inhibitor cocktail (Sigma-Aldrich) was added to buffer A (100 mm HEPES [pH 7.4] and 10% [v/v] glycerol) with 0.5 m KCl before resuspending. After sonicating on ice for 30 min (model 450 Sonifier; Branson Ultrasonics), 0.5% (v/v) Igepal was added to the cells. The suspension was stirred overnight at 4°C before removing cell debris by centrifugation at 31,000g for 1 h. The clarified lysate was loaded onto a nickel-NTA column (Qiagen) equilibrated with the same buffer used to prepare the lysate. The column was washed with this buffer containing an additional 20 mm imidazole and then eluted with the same buffer with 250 mm imidazole. The detergent was removed by adding Bio-beads SM-2 (Bio-Rad) into the solution, which was stirred for 30 min before being concentrated. The buffer was then exchanged by an Amicon 8050 ultrafiltration cell with a 30 kD cutoff membrane (Millipore) to buffer C (20 mm Tris, 10% [v/v] glycerol, and 0.5% [v/v] Igepal) with 100 mm KCl. A DEAE column (8 mL) was equilibrated with the same buffer before loading the protein fraction, and then the column was washed with equilibration buffer. The full-length CPR was eluted with buffer C containing 200 mm KCl and its purity was confirmed by SDS-PAGE.
Preparation of CPR-Containing Proteoliposomes and Reconstitution of SbC4H1-containing Monooxygenase System
Giant liposomes used in this study were prepared by the octyl glucoside dialysis/sorption technique (Rodgers et al., 2018). To this end, we prepared 7.5 mg of a mixture of phosphatidylcholine, phosphatidyl diethanolamine, and phosphatidic acid prepared as solutions in chloroform in a 2:1:0.6 ratio (by weight). The chloroform was removed by evaporation under a stream of argon gas and subsequent drying under vacuum for 2 h. The phospholipids were suspended in 1.25 mL of argon-saturated buffer D (100 mm HEPES [pH 7.4], 150 mm KCl, 0.5 mm EDTA, 1 mm dithiothreitol, 20% [v/v] glycerol) containing 1.54% (v/v) octylglycoside by vigorous stirring with a vortex mixer under argon atmosphere. After 2 h of incubation with continuous stirring at room temperature under argon atmosphere, the suspension was diluted to a final volume of 4.5 mL with argon-saturated buffer D without octyl glucoside. The suspension was supplemented with 15 nmol of rCPR or SbCPR, incubated at room temperature for 45 min, placed in a bag of Spectra/Por 6 dialysis membrane with a molecular weight cutoff of 25 kD (Spectrum Chemical), and dialyzed at 4°C under argon atmosphere three times (each lasting for 12–24 h) against 0.5 L buffer D containing 3.5 mL of Bio-Beads SM-2 Adsorbent (Bio-Rad) to remove the detergent. The duration of dialysis between each change of buffer was 24 h. The suspension was stored at −80°C until use. In order to reconstitute the SbC4H1-containing monooxygenase system, the CPR-containing proteoliposomes were supplemented with purified SbC4H1 protein at a molar ratio of 0.5:1 to proteoliposomal CPR. The mixture was incubated at stirring under argon atmosphere at 4°C for 12 to 16 h prior to its use in kinetic assays.
Kinetic Assay for trans-Cinnamic Acid by LC-MS
The activity of SbC4H1 was monitored with LC-MS based on the rate of production of p-coumaric acid. Samples were analyzed using an LC-20AD series HPLC system (Shimadzu) fitted with an HTC PAL autosampler (LEAP Technologies). Chromatography was performed using two columns, a Luna C18 column (50 × 2.0 mm, 5 μm; Phenomenex) and a Luna C18 column (100 × 2.0 mm, 5 μm; Phenomenex). Mobile phase A consisted of 0.05% (v/v) formic acid and 0.2% (v/v) acetic acid (MilliporeSigma) in water, and mobile phase B comprised 90% (v/v) acetonitrile (MilliporeSigma), 9.9% (v/v) water, and 0.1% (v/v) formic acid (Fischer Scientific). Quantitation was conducted on an API 4000 Q-Trap MS system (Applied Biosystems/MDS Sciex) with turbospray electrospray ionization operating in positive ion mode. For p-coumaric acid, the initial condition was 10% mobile phase B and 90% mobile phase A for 0.3 min. The concentration of mobile phase B was ramped up to 95% at 2.2 min and held constant for 0.1 min, followed by a linear gradient back to 10% B for 0.9 min. For 6-HNA, the initial condition was 10% mobile phase B and 90% mobile phase A for 0.3 min. The concentration of mobile phase B was ramped up to 75% at 2.2 min and held constant for 0.1 min, then ramped down to 10% again at 3.1 min and held constant for 0.9 min. The MS tuning parameters used for all the compounds were as follows: collision gas, medium; curtain gas, 20; ion spray voltage, 4,900; ion source gas 1, 35; ion source gas 2, 55; desolvation temperature, 600°C; declustering potential, 70 V; entrance potential, 10 V; cell exit potential, 15 V; and collision energy, 25 eV for both p-coumaric acid and 6-HNA.
The reaction was conducted in 500 μL 120 mm KPi and 20% (v/v) glycerol buffer. SbC4H1 reconstituted with CPR in proteoliposomal system as described above was added to a final concentration of 4 nm. Reaction was initiated by addition of 20 mm stock solution of NADPH to a final concentration of 120 μm NADPH. The reaction system was incubated in 30°C for 3 min and formic acid was added to a final concentration of 20% (v/v) to quench the reaction.
Fluorometric Assay of C4H-Dependent Hydroxylation of 2-Naphthoic Acid
Hydroxylation of 2-NA catalyzed by C4H results in the formation a fluorescent product, 6-HNA. The reaction was monitored with a real-time continuous fluorometric assay. A suspension of SbC4H1-supplemented proteoliposomes was added to 250 μL of 0.125 m potassium phosphate buffer (pH 7.4) containing 1 mm Glc-6-phosphate and 2 U/mL of Glc-6-phosphate dehydrogenase. The final concentration of SbC4H1 was equal to 7.9 and 29 nm for the assays with SbCPR and rCPR, respectively. The mixture was placed into a 5 × 5-mm2 quartz cell with continuous stirring at a constant temperature of 30°C. An aliquot of a 20 mm stock solution of 2-NA in acetone was added to attain the desired substrate concentration in the range 0.4 to 25 μm. The reaction was initiated by addition of 20 mm NADPH to a final concentration of 50 μm. The assay for HEPES inhibition was conducted in the same way using SbCPR, except that the reaction buffer contained HEPES at 5, 20, 100, and 300 mm. The ionic strength of the buffer was kept constant equal to 0.125 m potassium phosphate (I = 0.21 m) by adding appropriate amounts of KCl. Increase in the concentration of the product, 6-HNA, was monitored with a Cary Eclipse fluorometer (Agilent Technologies). Excitation and emission wavelengths were set at 300 and 450 nm, respectively. The rate of reaction was determined from the slope of the initial 1-min part of the kinetic curve (See Supplemental Fig. S2). Turnover rate was calculated using the calibration coefficient determined from measurements of the fluorescence intensity of 6-HNA added to the incubation mixture at concentrations increasing from 0.04 to 0.4 μm.
Molecular Docking
HEPES, cinnamic acid, p-coumaric 2-naphthoic acid, and 6-hydroxy-napthoate were docked into the active site of SbC4H1 by AutoDock Vina (Trott and Olson, 2010); ligands and grids were prepared for docking using AutoDock Tools (Morris et al., 2009). The lowest energy binding positions were found by docking into a 20 Å × 20 Å × 20 Å grid centered at the coordinates of the crystallographic HEPES molecule (61.065, 35.739, and 67.698 Å).
Studies of Interactions of SbC4H1 with Substrates and HEPES with Absorption Spectroscopy
The absorbance spectra were recorded with a MC2000-2 multichannel CCD rapid scanning spectrometer (Ocean Optics) using a PX2 pulsing xenon lamp as a light source. Titration experiments were performed in 125 mm potassium phosphate buffer under continuous stirring at 25°C at 1- to 2-μm concentration of SbC4H1. The substrates were added as 20 mm stock solutions in acetone. Concentration of the stock solution of HEPES was 1 m and its pH was adjusted to 7.4 with addition of NaOH.
The series of absorbance spectra obtained in titration experiments were analyzed using the PCA procedure described previously (Davydov et al., 1995, 1999; Renault et al., 2014). To interpret the changes in the spectra of absorbance in terms of the concentration of P450 in different states of ligation of the heme iron we used a least-squares fitting of the spectra of the first and second principal components to the set of spectral standards of the pure high spin, substrate-free ferric low-spin, and nitrogen-coordinated (Type II-substrate-bound) ferric low-spin SbC4H1. The set of spectral standards used in this study was obtained from series of spectra reflecting the temperature-, pressure-, and ligand-induced spectral changes in SbC4H1, as described earlier for P450BM3, P450eryF, and CYP3A4 (Davydov et al., 1999, 2002; Fernando et al., 2006). In this process, the set of spectral standards obtained for CYP3A4 was used as a prototype. The dependence of the fraction of the high-spin heme protein or the loading factor of the first principal component on substrate concentration was used to determine the parameters of the interactions by fitting these curves to an appropriate equation using a combination of Marquardt and Nelder-Mead nonlinear regression algorithms (Davydov et al., 1995). All data treatment procedures and curve fitting were performed using SpectraLab software (Davydov et al., 1995, 2016). The software package is available for download at http://cyp3a4.chem.wsu.edu/spectralab.html. It can also be obtained from the author D.R.D. upon request.
Accession Numbers
Sequence data from this article can be found in the EMBL/GenBank data libraries under accession numbers XP_002461939.1 (SbC4H1) and XP_002444097.1 (SbCPR).
The structure discussed in this manuscript can be found at www.rcsb.org deposited under PDB code 6VBY.
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. C4H-CO spectrum.
Supplemental Figure S2. Kinetics of oxidation of p-naphthoic acid by SbC4H1 in reconstituted systems with SbCPR and rat CPR.
Acknowledgments
We thank Tammy Gries for technical assistance with the expression of recombinant proteins in E. coli and John T. Rodgers for assistance with LC-MS. We thank Sebastien Santini for releasing the Phylogeny.fr for phylogenetic tree building.
Footnotes
This work was supported by the National Science Foundation (grant no. CHE 1804699), the Murdock Charitable Trust (to C.H.K.), the U.S. Department of Energy Offices of Science, providing Advanced Light Source (beamline 5.0.2) User Facility resources (contract no. DE–AC02–05CH11231), and Energy Efficiency and Renewable Energy, Bioenergy Technologies Office, sponsored by the department’s International Affairs (grant no. DE–PI0000031 to W.V.), and the U.S. Department of Agriculture/National Institute of Food and Agriculture Biomass Research and Development Initiative (grant no. 2011–1006–30358 to W.V.).
References
- Achnine L, Blancaflor EB, Rasmussen S, Dixon RA(2004) Colocalization of L-phenylalanine ammonia-lyase and cinnamate 4-hydroxylase for metabolic channeling in phenylpropanoid biosynthesis. Plant Cell 16: 3098–3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PD, Afonine PV, Bunkóczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, et al. (2010) PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66: 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ(1997) Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandatheerthavarada HK, Amuthan G, Biswas G, Robin MA, Murali R, Waterman MR, Avadhani NG(2001) Evolutionarily divergent electron donor proteins interact with P450MT2 through the same helical domain but different contact points. EMBO J 20: 2394–2403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros J, Escamilla-Trevino L, Song L, Rao X, Serrani-Yarce JC, Palacios MD, Engle N, Choudhury FK, Tschaplinski TJ, Venables BJ, et al. (2019) 4-Coumarate 3-hydroxylase in the lignin biosynthesis pathway is a cytosolic ascorbate peroxidase. Nat Commun 10: 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanck J, Rein H, Sommer M, Ristau O, Smettan G, Ruckpaul K(1983) Correlations between spin equilibrium shift, reduction rate, and N-demethylation activity in liver microsomal cytochrome P-450 and a series of benzphetamine analogues as substrates. Biochem Pharmacol 32: 1683–1688 [DOI] [PubMed] [Google Scholar]
- Block A, Widhalm JR, Fatihi A, Cahoon RE, Wamboldt Y, Elowsky C, Mackenzie SA, Cahoon EB, Chapple C, Dudareva N, et al. (2014) The origin and biosynthesis of the benzenoid moiety of ubiquinone (coenzyme Q) in Arabidopsis. Plant Cell 26: 1938–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blount JW, Korth KL, Masoud SA, Rasmussen S, Lamb C, Dixon RA(2000) Altering expression of cinnamic acid 4-hydroxylase in transgenic plants provides evidence for a feedback loop at the entry point into the phenylpropanoid pathway. Plant Physiol 122: 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple C.(1998) Molecular-genetic analysis of plant cytochrome P450-dependent monooxygenases. Annu Rev Plant Physiol Plant Mol Biol 49: 311–343 [DOI] [PubMed] [Google Scholar]
- Cheynier V, Comte G, Davies KM, Lattanzio V, Martens S(2013) Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol Biochem 72: 1–20 [DOI] [PubMed] [Google Scholar]
- Cornish-Bowden A.(1976) Principles of Enzyme Kinetics. Butterworth-Heinemann, Oxford, United Kingdom [Google Scholar]
- Davydov DR, Deprez E, Hoa GHB, Knyushko TV, Kuznetsova GP, Koen YM, Archakov AI(1995) High-pressure-induced transitions in microsomal cytochrome P450 2B4 in solution: Evidence for conformational inhomogeneity in the oligomers. Arch Biochem Biophys 320: 330–344 [DOI] [PubMed] [Google Scholar]
- Davydov DR, Hui Bon Hoa G, Peterson JA(1999) Dynamics of protein-bound water in the heme domain of P450BM3 studied by high-pressure spectroscopy: Comparison with P450cam and P450 2B4. Biochemistry 38: 751–761 [DOI] [PubMed] [Google Scholar]
- Davydov DR, Kariakin AA, Petushkova NA, Peterson JA(2000) Association of cytochromes P450 with their reductases: Opposite sign of the electrostatic interactions in P450BM-3 as compared with the microsomal 2B4 system. Biochemistry 39: 6489–6497 [DOI] [PubMed] [Google Scholar]
- Davydov DR, Kumar S, Halpert JR(2002) Allosteric mechanisms in P450eryF probed with 1-pyrenebutanol, a novel fluorescent substrate. Biochem Biophys Res Commun 294: 806–812 [DOI] [PubMed] [Google Scholar]
- Davydov DR, Sineva EV, Sistla S, Davydova NY, Frank DJ, Sligar SG, Halpert JR(2010) Electron transfer in the complex of membrane-bound human cytochrome P450 3A4 with the flavin domain of P450BM-3: The effect of oligomerization of the heme protein and intermittent modulation of the spin equilibrium. Biochim Biophys Acta 1797: 378–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davydov DR, Yang Z, Davydova N, Halpert JR, Hubbell WL(2016) Conformational mobility in cytochrome P450 3A4 explored by pressure-perturbation EPR spectroscopy. Biophys J 110: 1485–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson JH, Sono M(1987) Cytochrome P-450 and chloroperoxidase: Thiolate-ligated heme enzymes. Spectroscopic determination of their active-site structures and mechanistic implications of thiolate ligation. Chem Rev 87: 1255–1276 [Google Scholar]
- Denisov IG, Baas BJ, Grinkova YV, Sligar SG(2007) Cooperativity in cytochrome P450 3A4: Linkages in substrate binding, spin state, uncoupling, and product formation. J Biol Chem 282: 7066–7076 [DOI] [PubMed] [Google Scholar]
- Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard J, Guindon S, Lefort V, Lescot M(2008) Phylogeny. fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36: W465–W469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien BS, Sarath G, Pedersen JF, Sattler SE, Chen H, Funnell-Harris DL, Nichols NN, Cotta MA(2009) Improved sugar conversion and ethanol yield for forage sorghum (Sorghum bicolor L. Moench) lines with reduced lignin contents. BioEnergy Res 2: 153–164 [Google Scholar]
- Dubey KD, Shaik S(2019) Cytochrome P450—the wonderful nanomachine revealed through dynamic simulations of the catalytic cycle. Acc Chem Res 52: 389–399 [DOI] [PubMed] [Google Scholar]
- Dubey KD, Wang B, Shaik S(2016) Molecular dynamics and QM/MM calculations predict the substrate-induced gating of cytochrome P450 BM3 and the regio- and stereoselectivity of fatty acid hydroxylation. J Am Chem Soc 138: 837–845 [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K(2010) Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66: 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhead M, Giannini S, Gillam EMJ, Gilardi G(2005) Functional characterisation of an engineered multidomain human P450 2E1 by molecular Lego. J Biol Inorg Chem 10: 842–853 [DOI] [PubMed] [Google Scholar]
- Falquet L, Pagni M, Bucher P, Hulo N, Sigrist CJ, Hofmann K, Bairoch A(2002) The PROSITE database, its status in 2002. Nucleic Acids Res 30: 235–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farre I, Faci JM(2006) Comparative response of maize (Zea mays L.) and sorghum (Sorghum bicolor L. Moench) to deficit irrigation in a Mediterranean environment. Agric Water Manage 83: 135–143 [Google Scholar]
- Fernando H, Halpert JR, Davydov DR(2006) Resolution of multiple substrate binding sites in cytochrome P450 3A4: The stoichiometry of the enzyme-substrate complexes probed by FRET and Job’s titration. Biochemistry 45: 4199–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishelovitch D, Shaik S, Wolfson HJ, Nussinov R(2010) How does the reductase help to regulate the catalytic cycle of cytochrome P450 3A4 using the conserved water channel? J Phys Chem B 114: 5964–5970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MT, Sligar SG(1985) Control of heme protein redox potential and reduction rate: Linear free energy relation between potential and ferric spin state equilibrium. J Am Chem Soc 107: 5018–5019 [Google Scholar]
- Franke R, Hemm MR, Denault JW, Ruegger MO, Humphreys JM, Chapple C(2002a) Changes in secondary metabolism and deposition of an unusual lignin in the ref8 mutant of Arabidopsis. Plant J 30: 47–59 [DOI] [PubMed] [Google Scholar]
- Franke R, Humphreys JM, Hemm MR, Denault JW, Ruegger MO, Cusumano JC, Chapple C(2002b) The Arabidopsis REF8 gene encodes the 3-hydroxylase of phenylpropanoid metabolism. Plant J 30: 33–45 [DOI] [PubMed] [Google Scholar]
- Gerber NC, Sligar SG(1992) Catalytic mechanism of Cytochrome P450—evidence for a distal charge relay. J Am Chem Soc 114: 8742–8743 [Google Scholar]
- Green AR, Lewis KM, Barr JT, Jones JP, Lu F, Ralph J, Vermerris W, Sattler SE, Kang C(2014) Determination of the structure and catalytic mechanism of Sorghum bicolor caffeic acid O-methyltransferase and the structural impact of three brown midrib12 mutations. Plant Physiol 165: 1440–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M, Wang M, Guo J, Shi C, Deng J, Huang L, Huang L, Chang Z(2019) Crystal structure of CYP76AH1 in 4-PI-bound state from Salvia miltiorrhiza. Biochem Biophys Res Commun 511: 813–819 [DOI] [PubMed] [Google Scholar]
- Guengerich FP.(1991) Reactions and significance of cytochrome P-450 enzymes. J Biol Chem 266: 10019–10022 [PubMed] [Google Scholar]
- Guo J, Zhou YJ, Hillwig ML, Shen Y, Yang L, Wang Y, Zhang X, Liu W, Peters RJ, Chen X, Zhao ZK, Huang L(2013) CYP76AH1 catalyzes turnover of miltiradiene in tanshinones biosynthesis and enables heterologous production of ferruginol in yeasts. Proc Natl Acad Sci USA 110: 12108–12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaka FG, Babcock GT, Dye JL(1985) The use of principal component analysis to resolve the spectra and kinetics of cytochrome c oxidase reduction by 5,10-dihydro-5-methyl phenazine. Biophys J 48: 209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdane D, Zhang H, Hollenberg P(2008) Oxygen activation by cytochrome P450 monooxygenase. Photosynth Res 98: 657–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow GR, Halpert JR(1998) Analysis of human cytochrome P450 3A4 cooperativity: Construction and characterization of a site-directed mutant that displays hyperbolic steroid hydroxylation kinetics. Proc Natl Acad Sci USA 95: 6636–6641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hlavica P.(2007) Control by substrate of the cytochrome p450-dependent redox machinery: Mechanistic insights. Curr Drug Metab 8: 594–611 [DOI] [PubMed] [Google Scholar]
- Hlavica P. (2015) Mechanistic basis of electron transfer to cytochromes P450 by natural redox partners and artificial donor constructs. In Hrycay EG, Bandiera SM, eds, Monooxygenase, Peroxidase and Peroxygenase Properties and Mechanisms of Cytochrome P450, Advances in Experimental Medicine and Biology, Vol. 851. Springer International, Cham, Switzerland, pp 247–297 [DOI] [PubMed] [Google Scholar]
- Holm L, Rosenström P(2010) Dali server: Conservation mapping in 3D. Nucleic Acids Res 38: W545–W549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm L, Sander C(1993) Protein structure comparison by alignment of distance matrices. J Mol Biol 233: 123–138 [DOI] [PubMed] [Google Scholar]
- Iyanagi T.(2019) Molecular mechanism of metabolic NAD(P)H-dependent electron-transfer systems: The role of redox cofactors. Biochim Biophys Acta Bioenerg 1860: 233–258 [DOI] [PubMed] [Google Scholar]
- Jun S-Y, Sattler SA, Cortez GS, Vermerris W, Sattler SE, Kang C(2018) Biochemical and structural analysis of substrate specificity of a phenylalanine ammonia-lyase. Plant Physiol 176: 1452–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun S-Y, Walker AM, Kim H, Ralph J, Vermerris W, Sattler SE, Kang C(2017) The enzyme activity and substrate specificity of two major cinnamyl alcohol dehydrogenases in sorghum (Sorghum bicolor), SbCAD2 and SbCAD4. Plant Physiol 174: 2128–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D-S, Nioche P, Hamberg M, Raman CS(2008) Structural insights into the evolutionary paths of oxylipin biosynthetic enzymes. Nature 455: 363–368 [DOI] [PubMed] [Google Scholar]
- Li H, Poulos TL(1999) Fatty acid metabolism, conformational change, and electron transfer in cytochrome P-450(BM-3). Biochim Biophys Acta 1441: 141–149 [DOI] [PubMed] [Google Scholar]
- Li L, Chang Z, Pan Z, Fu Z-Q, Wang X(2008) Modes of heme binding and substrate access for cytochrome P450 CYP74A revealed by crystal structures of allene oxide synthase. Proc Natl Acad Sci USA 105: 13883–13888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou S-H, Mahomed M, Lee Y-T, Goodin DB(2016) Effector roles of putidaredoxin on cytochrome P450cam conformational states. J Am Chem Soc 138: 10163–10172 [DOI] [PubMed] [Google Scholar]
- Loida PJ, Sligar SG(1993) Molecular recognition in cytochrome P-450: Mechanism for the control of uncoupling reactions. Biochemistry 32: 11530–11538 [DOI] [PubMed] [Google Scholar]
- Makris TM, Davydov R, Denisov IG, Hoffman BM, Sligar SG(2002) Mechanistic enzymology of oxygen activation by the cytochromes P450. Drug Metab Rev 34: 691–708 [DOI] [PubMed] [Google Scholar]
- Martinis SA, Atkins WM, Stayton PS, Sligar SG(1989) A conserved residue of cytochrome P-450 is involved in heme-oxygen stability and activation. J Am Chem Soc 111: 9252–9253 [Google Scholar]
- Meyer K, Cusumano JC, Somerville C, Chapple CC(1996) Ferulate-5-hydroxylase from Arabidopsis thaliana defines a new family of cytochrome P450-dependent monooxygenases. Proc Natl Acad Sci USA 93: 6869–6874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M, Ohta D(2010) Diversification of P450 genes during land plant evolution. Annu Rev Plant Biol 61: 291–315 [DOI] [PubMed] [Google Scholar]
- Mizutani M, Sato F(2011) Unusual P450 reactions in plant secondary metabolism. Arch Biochem Biophys 507: 194–203 [DOI] [PubMed] [Google Scholar]
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ(2009) AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem 30: 2785–2791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moural TW, Lewis KM, Barnaba C, Zhu F, Palmer NA, Sarath G, Scully ED, Jones JP, Sattler SE, Kang C(2017) Characterization of class III peroxidases from switchgrass. Plant Physiol 173: 417–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DR.(2006) Cytochrome P450 nomenclature, 2004 In Phillips IR, and Shephard EA, eds, Cytochrome P450 Protocols. Humana Press, Totowa, NJ, pp 1–10 [Google Scholar]
- Nelson DR, Kamataki T, Waxman DJ, Guengerich FP, Estabrook RW, Feyereisen R, Gonzalez FJ, Coon MJ, Gunsalus IC, Gotoh O, et al. (1993) The P450 superfamily: Update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol 12: 1–51 [DOI] [PubMed] [Google Scholar]
- Nelson DR, Ming R, Alam M, Schuler MA(2008) Comparison of cytochrome P450 genes from six plant genomes. Trop Plant Biol 1: 216–235 [Google Scholar]
- Nishihara K, Kanemori M, Yanagi H, Yura T(2000) Overexpression of trigger factor prevents aggregation of recombinant proteins in Escherichia coli. Appl Environ Microbiol 66: 884–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W (1997) Processing of X-ray diffraction data collected in oscillation mode. In Carter CW, Jr, ed, Macromolecular Crystallography Part A. Methods in Enzymology, Vol. 276. Elsevier, Amsterdam, pp 307–326 [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE(2004) UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem 25: 1605–1612 [DOI] [PubMed] [Google Scholar]
- Pochapsky TC, Kazanis S, Dang M(2010) Conformational plasticity and structure/function relationships in cytochromes P450. Antioxid Redox Signal 13: 1273–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K, Axtell J, Lechtenberg V, Colenbrander V(1978) Phenotype, fiber composition, and in vitro dry matter disappearance of chemically induced brown midrib (bmr) mutants of sorghum. Crop Sci 18: 205–208 [Google Scholar]
- Poulos TL, Finzel BC, Howard AJ(1987) High-resolution crystal structure of cytochrome P450cam. J Mol Biol 195: 687–700 [DOI] [PubMed] [Google Scholar]
- Renault H, Bassard J-E, Hamberger B, Werck-Reichhart D(2014) Cytochrome P450-mediated metabolic engineering: Current progress and future challenges. Curr Opin Plant Biol 19: 27–34 [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Davydova NY, Paragas EM, Jones JP, Davydov DR(2018) Kinetic mechanism of time-dependent inhibition of CYP2D6 by 3,4-methylenedioxymethamphetamine (MDMA): Functional heterogeneity of the enzyme and the reversibility of its inactivation. Biochem Pharmacol 156: 86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustgi S, Springer A, Kang C, von Wettstein D, Reinbothe C, Reinbothe S, Pollmann S(2019) Allene oxide synthase and hydroperoxide lyase, two non-canonical cytochrome P450s in Arabidopsis thaliana and their different roles in plant defense. Int J Mol Sci 20: 3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saballos A, Vermerris W, Rivera L, Ejeta G(2008) Allelic association, chemical characterization and saccharification properties of brown midrib mutants of sorghum (Sorghum bicolor L. Moench). BioEnergy Res 1: 193–204 [Google Scholar]
- Sattler SA, Walker AM, Vermerris W, Sattler SE, Kang C(2017) Structural and biochemical characterization of cinnamoyl-CoA reductases. Plant Physiol 173: 1031–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalk M, Batard Y, Seyer A, Nedelkina S, Durst F, Werck-Reichhart D(1997) Design of fluorescent substrates and potent inhibitors of CYP73As, P450s that catalyze 4-hydroxylation of cinnamic acid in higher plants. Biochemistry 36: 15253–15261 [DOI] [PubMed] [Google Scholar]
- Schalk M, Nedelkina S, Schoch G, Batard Y, Werck-Reichhart D(1999) Role of unusual amino acid residues in the proximal and distal heme regions of a plant P450, CYP73A1. Biochemistry 38: 6093–6103 [DOI] [PubMed] [Google Scholar]
- Schlichting I, Berendzen J, Chu K, Stock AM, Maves SA, Benson DE, Sweet RM, Ringe D, Petsko GA, Sligar SG(2000) The catalytic pathway of cytochrome p450cam at atomic resolution. Science 287: 1615–1622 [DOI] [PubMed] [Google Scholar]
- Schoch G, Goepfert S, Morant M, Hehn A, Meyer D, Ullmann P, Werck-Reichhart D(2001) CYP98A3 from Arabidopsis thaliana is a 3′-hydroxylase of phenolic esters, a missing link in the phenylpropanoid pathway. J Biol Chem 276: 36566–36574 [DOI] [PubMed] [Google Scholar]
- Schreiner M, Mewis I, Huyskens-Keil S, Jansen M, Zrenner R, Winkler J, O’Brien N, Krumbein A(2012) UV-B-induced secondary plant metabolites-potential benefits for plant and human health. Crit Rev Plant Sci 31: 229–240 [Google Scholar]
- Sen K, Thiel W(2014) Role of two alternate water networks in Compound I formation in P450eryF. J Phys Chem B 118: 2810–2820 [DOI] [PubMed] [Google Scholar]
- Sevrioukova IF, Li H, Zhang H, Peterson JA, Poulos TL(1999) Structure of a cytochrome P450-redox partner electron-transfer complex. Proc Natl Acad Sci USA 96: 1863–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewalt V, Ni W, Blount JW, Jung HG, Masoud SA, Howles PA, Lamb C, Dixon RA(1997) Reduced lignin content and altered lignin composition in transgenic tobacco down-regulated in expression of L-phenylalanine ammonia-lyase or cinnamate 4-hydroxylase. Plant Physiol 115: 41–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahidi F, Ambigaipalan P(2015) Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects–A review. J Funct Foods 18: 820–897 [Google Scholar]
- Treutter D.(2006) Significance of flavonoids in plant resistance: A review. Environ Chem Lett 4: 147 [Google Scholar]
- Trott O, Olson AJ(2010) AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31: 455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Acker R, Vanholme R, Storme V, Mortimer JC, Dupree P, Boerjan W(2013) Lignin biosynthesis perturbations affect secondary cell wall composition and saccharification yield in Arabidopsis thaliana. Biotechnol Biofuels 6: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanholme R, Demedts B, Morreel K, Ralph J, Boerjan W(2010) Lignin biosynthesis and structure. Plant Physiol 153: 895–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidakovic M, Sligar SG, Li H, Poulos TL(1998) Understanding the role of the essential Asp251 in cytochrome p450cam using site-directed mutagenesis, crystallography, and kinetic solvent isotope effect. Biochemistry 37: 9211–9219 [DOI] [PubMed] [Google Scholar]
- Walker AM, Hayes RP, Youn B, Vermerris W, Sattler SE, Kang C(2013) Elucidation of the structure and reaction mechanism of sorghum hydroxycinnamoyltransferase and its structural relationship to other coenzyme a-dependent transferases and synthases. Plant Physiol 162: 640–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker AM, Sattler SA, Regner M, Jones JP, Ralph J, Vermerris W, Sattler SE, Kang C(2016) The structure and catalytic mechanism of Sorghum bicolor caffeoyl-CoA O-methyltransferase. Plant Physiol 172: 78–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Rezenom YH, Cho K-C, Tran JL, Lee DG, Russell DH, Gill JJ, Young R, Chu K-H(2014) Cultivation of lipid-producing bacteria with lignocellulosic biomass: Effects of inhibitory compounds of lignocellulosic hydrolysates. Bioresour Technol 161: 162–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werck-Reichhart D, Feyereisen R(2000) Cytochromes P450: A success story. Genome Biol 1: reviews3003.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangar RC, Davydov DR, Verma S(2004) Mechanisms that regulate production of reactive oxygen species by cytochrome P450. Toxicol Appl Pharmacol 199: 316–331 [DOI] [PubMed] [Google Scholar]
- Zhang W, Du L, Li FW, Zhang XW, Qu ZP, Hang L, Li Z, Sun JR, Qi FX, Yao QP, et al. (2018) Mechanistic insights into interactions between bacterial class I P450 enzymes and redox partners. ACS Catal 8: 9992–10003 [Google Scholar]