A Medicago truncatula cytokinin-signaling transcription factor is required for early symbiotic nodulation and to regulate key Nod factor signaling and endoreduplication genes.
Abstract
Nitrogen-fixing root nodulation in legumes challenged with nitrogen-limiting conditions requires infection of the root hairs by soil symbiotic bacteria, collectively referred to as rhizobia, and the initiation of cell divisions in the root cortex. Cytokinin hormones are critical for early nodulation to coordinate root nodule organogenesis and the progression of bacterial infections. Cytokinin signaling involves regulation of the expression of cytokinin primary response genes by type-B response regulator (RRB) transcription factors. RNA interference or mutation of MtRRB3, the RRB-encoding gene most strongly expressed in Medicago truncatula roots and nodules, significantly decreased the number of nodules formed, indicating a function of this RRB in nodulation initiation. Fewer infection events were also observed in rrb3 mutant roots associated with a reduced Nod factor induction of the Early Nodulin 11 (MtENOD11) infection marker, and of the cytokinin-regulated Nodulation Signaling Pathway 2 (MtNSP2) gene. Rhizobial infections correlate with an expansion of the nuclear area, suggesting the activation of endoreduplication cycles linked to the cytokinin-regulated Cell Cycle Switch 52A (MtCCS52A) gene. Although no significant difference in nucleus size and endoreduplication were detected in rhizobia-infected rrb3 mutant roots, expression of the MtCCS52A endoreduplication marker was reduced. As the MtRRB3 expression pattern overlaps with those of MtNSP2 and MtCCS52A in roots and nodule primordia, chromatin immunoprecipitation-quantitative PCR and protoplast trans-activation assays were used to show that MtRRB3 can interact with and trans-activate MtNSP2 and MtCCS52A promoters. Overall, we highlight that the MtRRB3 cytokinin signaling transcription factor coordinates the expression of key early nodulation genes.
Legume species, such as Medicago truncatula, can form a symbiotic interaction with specific soil nitrogen-fixing bacteria commonly referred to as rhizobia, leading to the formation of new organs on the root system called nodules (Suzaki et al., 2015). Bacteria hosted in nodules find a favorable environment for fixing atmospheric nitrogen into ammonium that can then be assimilated by the host plant. In exchange, bacteria benefit from plant photoassimilates. The symbiotic interaction initiates with a molecular dialogue between the two symbionts: plant roots release specific flavonoids in the rhizosphere, which promote the production of bacterial lipochitooligosaccharidic signals called nodulation factors (NFs; Lerouge et al., 1990; Suzaki et al., 2015). NF perception in root-hair epidermal cells triggers microsymbiont recognition and infection thread (IT) formation. Concomitantly to these epidermal symbiotic events, cortical cells divide several cell layers away from the infection site, leading to formation of a nodule primordium (Kondorosi et al., 2005; Xiao et al., 2014). IT progression across the outer and middle cortex allows bacteria to reach the nodule organ primordium (Fournier et al., 2008; Oldroyd and Downie, 2008; Suzaki et al., 2015). These NF-induced early symbiotic processes rely on a well described signaling cascade, notably involving the activation of a nucleocytoplasmic calcium spiking upstream of the transcriptional regulation of early nodulation genes (Suzaki et al., 2015). Different transcription factors, such as Nodulation Signaling Pathway1 (NSP1) and NSP2, are critical for activation of the early symbiotic infection marker Early Nodulin11 (ENOD11) and nodule formation (Kaló et al., 2005; Smit et al., 2005). Accordingly, the nsp2 loss-of-function mutant does not nodulate. Nodule primordia then differentiate into nitrogen-fixing nodules, which in M. truncatula have an indeterminate growth due to a persistent apical meristem (zone I; Vasse et al., 1990). A rhizobial infection and differentiation zone (zone II) is formed below this apical nodule meristem, where bacteria and plant cells codifferentiate to acquire the ability to fix and assimilate atmospheric nitrogen, respectively, followed by a nitrogen fixation zone (zone III). The differentiation of nodule symbiotic cells is tightly associated with an increase of cell endoreduplication, whose progression is notably controlled by the Anaphase-Promoting Complex (APC) activator Cell Cycle Switch52A (CCS52A) in M. truncatula (Vinardell et al., 2003). MtCCS52A expression is induced during nodule formation prior to nodule differentiation, and reducing the MtCC52A transcript level decreases the endoreduplication rate and inhibits the formation of nitrogen-fixing nodules. In Lotus japonicus, endoreduplication was also suggested to be required for IT progression through the root cortex toward dividing cortical cells (Suzaki et al., 2014). In addition, the two orthologs of MtCCS52A in L. japonicus are expressed in these cortical cells during early nodulation events.
Early nodulation is tightly regulated by plant hormones including cytokinins (CKs; Frugier et al., 2008; Ferguson and Mathesius, 2014; Boivin et al., 2016a; Gamas et al., 2017). This was notably highlighted in M. truncatula and L. japonicus by identification of gain-of-function and loss-of-function mutants affecting orthologs of the Arabidopsis (Arabidopsis thaliana) His Kinase4/Cytokinin Response1 CK receptor (AHK4/CRE1), named CRE1/CHASE (for Cyclases/His kinases Associated Sensory Extracellular) His Kinase 1 in M. truncatula (MtCRE1/MtCHK1), and Lotus His Kinase (LHK1) in L. japonicus (Gonzalez-Rizzo et al., 2006; Murray et al., 2007; Tirichine et al., 2007; Plet et al., 2011; Boivin et al., 2016b). Expression of a constitutively active LHK1 CK receptor is sufficient to induce cell divisions and “spontaneous” nodule organogenesis in the absence of rhizobia (Tirichine et al., 2007), while knockout of MtCRE1 or LHK1 dramatically decreases nodulation in both model legumes (Gonzalez-Rizzo et al., 2006; Murray et al., 2007; Plet et al., 2011). Furthermore, consistent with these early nodulation phenotypes, CK regulates the expression of NF signaling genes, such as, in M. truncatula, Ethylene Response factor required for Nodulation1 (MtERN1), Nodule INception (MtNIN), and MtNSP2, depending on the LHK1/MtCRE1 receptor (Gonzalez-Rizzo et al., 2006; Murray et al., 2007; Tirichine et al., 2007; Heckmann et al., 2011; Plet et al., 2011). CKs have also been shown to negatively regulate rhizobial infections within the root epidermis. Indeed, the mutant affecting LHK1 was named hit1 for hyperinfected 1, as besides its low-nodulation phenotype, it shows a higher number of rhizobial infections (Murray et al., 2007; Held et al., 2014; Liu et al., 2015). In contrast, in M. truncatula, the functionally related low-nodulating cre1 mutant does not show an increased number of infection events but forms ITs that are either blocked in outer layers of MtCRE1 RNA interference (RNAi) roots or tend to have an exaggerated growth and to form sac-like structures in the cre1 mutant (Gonzalez-Rizzo et al., 2006; Plet et al., 2011). More recently, an activation of CK responses in the root epidermis was linked in M. truncatula to a negative regulation of NF signaling (Jardinaud et al., 2016). Indeed, a short-term CK treatment abolishes NF induction of the MtENOD11 infection marker depending on the MtCRE1 receptor, and conversely, depletion of the CK pool specifically in the root epidermis by ectopic expression of a CK oxidase enzyme ensuring CK degradation enhances the NF induction of MtENOD11 expression as well as nodulation. Finally, CK receptors other than LHK1/MtCRE1 have redundant activity in regulating early nodulation and also participate in regulating nitrogen fixation at a later symbiotic stage (Held et al., 2014; Boivin et al., 2016b).
LHK1/MtCRE1 receptors are His kinases whose activation by CK triggers a phosphotransfer signaling pathway similar to bacterial two-component systems (TCSs; Hwang and Sheen, 2001; Heyl and Schmülling, 2003). This pathway involves His phosphotransfer proteins shuttling between the cytosol and the nucleus to activate type-B response regulator (RRB) transcription factors in the nucleus (Imamura et al., 1999; Hwang and Sheen, 2001; Heyl et al., 2013; Kabbara et al., 2018). This ultimately regulates the transcription of CK primary response genes (Sakai et al., 2000), such as type-A response regulators (RRAs) and genes specific to various developmental contexts (Kieber and Schaller, 2018). During M. truncatula early nodulation, the NF signaling gene MtNSP2 notably was proposed to be a direct target of MtCRE1-dependent CK signaling based on the identification in its promoter of MtRRB1 binding sites required for activation of MtNSP2 expression by CK and during early nodulation (Ariel et al., 2012). However, no nodulation phenotype could be identified when silencing or overexpressing MtRRB1. In Arabidopsis roots, the ARR2 RRB transcription factor directly regulates expression of the APC activator subunit-encoding gene AtCCS52A1 (Takahashi et al., 2013). A similar CK regulation of MtCCS52A and endoreduplication may thus occur during early nodulation (Vinardell et al., 2003).
In this study, we aimed to identify a RRB transcription factor in M. truncatula that was relevant for symbiotic nodulation and to characterize potential target genes. From the 31 genes encoding putative RRBs in the genome of M. truncatula, we selected the RRB most closely related to MtRRB1 that shows a structure typical of authentic RRB proteins acting in TCS signaling, namely MtRRB3, which is also the RRB gene most highly expressed in roots and nodules (Gonzalez-Rizzo et al., 2006; Tan et al., 2019). Accordingly, in contrast to MtRRB1, MtRRB3 is able to activate a CK primary target RRA gene in a trans-activation assay. Downregulation of MtRRB3 expression by RNAi, or identification of a mutant, revealed reduced nodulation and rhizobial infection phenotypes, associated with a reduced NF activation of the ENOD11 infection marker. Finally, by a combination of spatial expression and functional assays, we showed that the MtRRB3 transcription factor is able to bind and trans-activate the expression of two genes previously linked to early nodulation, MtNSP2 and MtCCS52A. These results indicate that MtRRB3 coordinates the CK-mediated regulation of key early nodulation genes, consistent with its positive function in regulating rhizobial infections and nodule initiation.
RESULTS
MtRRB3 Positively Regulates Nodulation and Rhizobial Infections in M. truncatula
MtRRB1 binding sites required for regulation of MtNSP2 in response to CK and in early nodulation were previously identified (Ariel et al., 2012), suggesting that this protein could activate transcription of this candidate target gene. However, a closer analysis of the MtRRB1 structure revealed an atypical structure poorly compatible with a role in TCS signaling (Tan et al., 2019). Accordingly, MtRRB1 was unable to trans-activate the promoter of a typical CK primary response gene related to early nodulation (MtRRA11; Supplemental Fig. S1; Op den Camp et al., 2011). A previously published phylogenetic analysis of the whole M. truncatula RRB family revealed that MtRRB3 is the protein with a canonical RRB structure most closely related to MtRRB1 (Tan et al., 2019). In addition, MtRRB3 is the most highly expressed RRB gene and is additionally tightly coexpressed with the MtCRE1 CK receptor in roots and during nodulation. Accordingly, MtRRB3 can efficiently trans-activate the MtRRA11 CK primary response gene (Supplemental Fig. S1). We therefore focused our studies on MtRRB3 with the aim of characterizing a canonical CK-signaling transcription factor involved in nodulation.
To establish the involvement of MtRRB3 in nodulation, we first generated two RNAi constructs specifically targeting MtRRB3 (Fig. 1A, RNAi-1 and RNAi-2). Analysis of steady-state transcript levels of MtRRB3, as well as of MtRRB1 and MtRRB2, its most closely related RRB genes (Tan et al., 2019), revealed that both RNAi constructs efficiently decreased MtRRB3 transcript levels compared to a GUS RNAi control, while MtRRB1 and MtRRB2 expression was not affected. This demonstrates the specificity of the RNAi silencing constructs (Fig. 1B). MtRRB3-silenced roots inoculated with rhizobium had significantly reduced nodule numbers in both RNAi-1 and RNAi-2 roots (Fig. 1C), suggesting a positive role of MtRRB3 during nodule initiation. To independently validate this result, a mutant was identified by targeting-induced local lesions in genomes (TILLING) from an ethyl methane sulfonate (EMS) mutant population using the 3′ region of MtRRB3 corresponding to the trans-activation domain (Fig. 1A; Le Signor et al., 2009). From the allelic series identified, one allele generating a premature stop codon at position 528 (W528*) was selected for further analysis and referred to as rrb3 (Fig. 1A). Analysis of root growth sensitivity to CKs showed that this rrb3 mutant has a reduced sensitivity to benzylaminopurine (BAP) compared to the wild type (Supplemental Fig. S2). In addition, the nodule number was significantly reduced in the rrb3 mutant (Fig. 1D), consistent with the MtRRB3 RNAi nodulation phenotype. This nodulation phenotype was not indirectly due to a root growth defect, since a similar decrease was observed when the nodule number was normalized by the total root-system length (nodule density; Supplemental Fig. S3A). Accordingly, no significant difference between the wild type and the rrb3 mutant was identified regarding either root length, lateral root number, or lateral root density (lateral root number per centimeter of main root; Supplemental Figs. S2 and S4), indicating again that the observed symbiotic phenotype was not indirectly caused by an altered root system architecture of the rrb3 mutant. In addition, the root and shoot dry weights of the rrb3 mutant were not significantly different from those of the wild type under nonsymbiotic conditions (Supplemental Fig. S5A), even though reduced growth of the rrb3 mutant was observed when roots were nodulated, in agreement with its defective nodulation phenotype (Supplemental Fig. S5B). Importantly, the defective nodulation phenotype of rrb3 mutant roots was rescued by an ectopic expression of MtRRB3 (Supplemental Fig. S6). To determine whether this reduced nodulation phenotype was associated with altered rhizobial infection capacity in the mutant, the number of ITs was determined in the rrb3 mutant using a rhizobium strain expressing a LacZ reporter. A decrease in IT number was observed, which remained significant when the IT number was normalized by root length (Fig. 1E, Supplemental Fig. S3B). In agreement, expression of the early symbiotic infection marker MtENOD11 was reduced in rrb3 mutant roots treated for 3 h with NF compared to wild-type roots (Fig. 1F). We further evaluated whether MtRRB3 could also act at a later symbiotic stage to regulate nodule tissue patterning, as well as bacteria differentiation, viability, and/or nitrogen fixation activity. When “live and dead” staining was used on rrb3 nodule sections, no major difference could be identified in the spatial organization of rrb3 mutant nodules, or in the differentiation status or survival of bacteria (Supplemental Fig. S7, A–D). Furthermore, the nitrogen fixation activity of rrb3 mutant nodules was evaluated using an acetylene reduction assay (ARA), which allowed indirect measurement of bacterial nitrogenase activity. Whereas ARA activity per plant was significantly reduced in the rrb3 mutant (Supplemental Fig. S7E), this difference was lost when ARA activity was normalized by nodule number (Supplemental Fig. S7F). This suggests that the decreased ARA activity was caused by reduced nodulation of the rrb3 mutant rather than by a direct effect on nitrogen fixation capacity. Altogether, these results support a positive role for MtRRB3 in nodulation, notably with regard to IT formation and NF induction of the MtENOD11 infection marker.
Figure 1.
Nodulation phenotypes of MtRRB3 RNAi roots and of the rrb3 mutant. A, Schematic representation of the MtRRB3 coding sequence. The mutation site leading to a stop codon in the rrb3 mutant is indicated by a black arrow, and sequences targeted by the two RNAi constructs (RNAi-1 and RNAi-2) are marked by horizontal gray lines. REC, Phosphate receiver domain; MYB, MYB-like DNA binding domain. B, RT-qPCR analysis of MtRRB1, MtRRB2, and MtRRB3 expression in roots expressing MtRBB3 RNAi constructs 1 and 2 or GUS (as control). The GUS RNAi control was set to 1 (dashed line) for each gene to highlight fold changes. Values are the mean of two independent experiments, and error bars represent standard deviations. Student’s t test was performed to assess significant differences (*P < 0.05) relative to the corresponding nontreated control, indicated by asterisks . C, Quantification of nodules formed in vitro on roots expressing MtRRB3 RNAi constructs and GUS (as control) 14 d postinoculation (dpi). Error bars represent the confidence interval (α = 0.05; n > 30 independent transgenic roots per construct). A Kruskal-Wallis test was performed to assess significant differences (α < 0.05), indicated by lowercase letters. D, Quantification of nodules formed 6 and 14 dpi on wild-type (WT) and rrb3 mutant roots. Values are the mean of two independent experiments, and error bars represent the confidence interval (α = 0.05; n > 30 independent plants/genotype for each experiment). A Mann-Whitney test was performed to assess significant differences (α < 0.05), indicated by asterisks. E, Quantification of infection events in wild-type and rrb3 mutant roots 6 dpi using a LacZ rhizobium strain. Values are the mean of two independent experiments, and error bars represent the confidence interval (α = 0.05; n > 5 independent roots/genotype for each experiment). A Mann-Whitney test was performed to assess significant differences (α < 0.05), indicated by asterisks. F, RT-qPCR analysis of MtENOD11 expression in roots treated with NFs (10−8 m NF for 3 h) in wild-type and rrb3 mutant roots. The MtENOD11 expression values in respective untreated roots were set to 1 to highlight fold changes, as indicated by the dashed line. Values are the mean of two independent experiments, and error bars represent standard deviations. Student’s t test was performed to assess significant differences (*P < 0.05) relative to the corresponding nontreated control, indicated by asterisks.
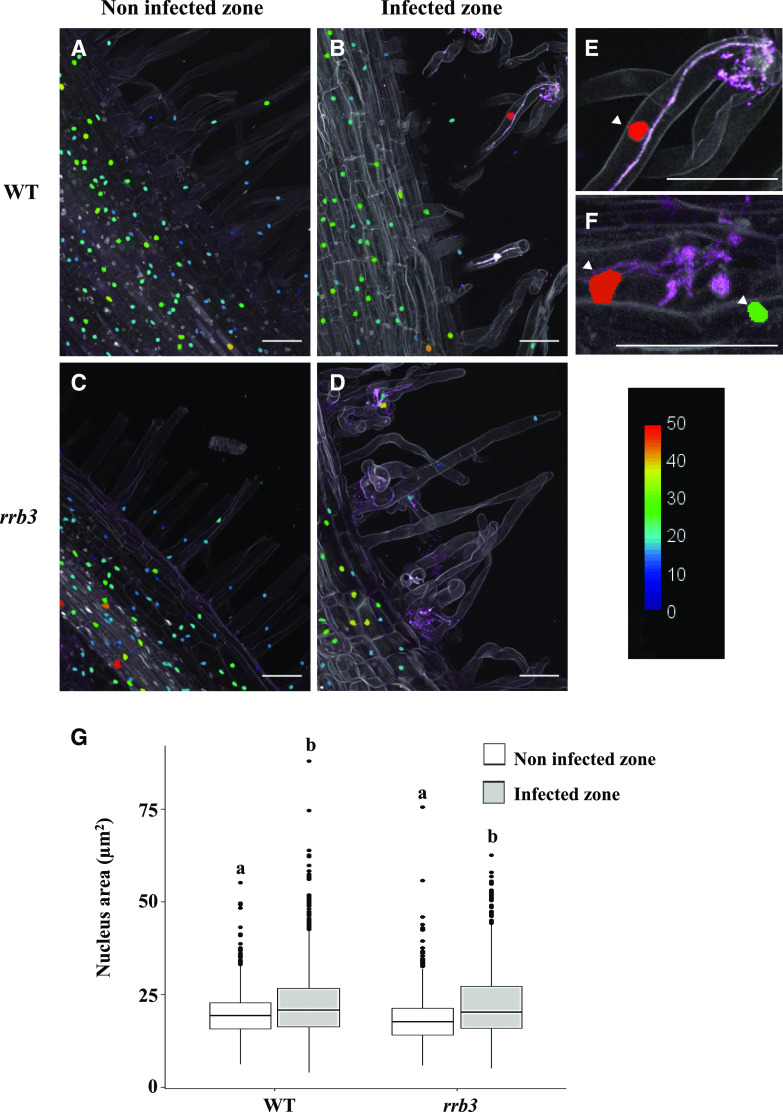
Rhizobial Infections Are Associated with Increased Nuclear Size and the rrb3 Mutant Is Altered in Cytokinin Regulation of the MtCCS52A Endoreduplication Marker
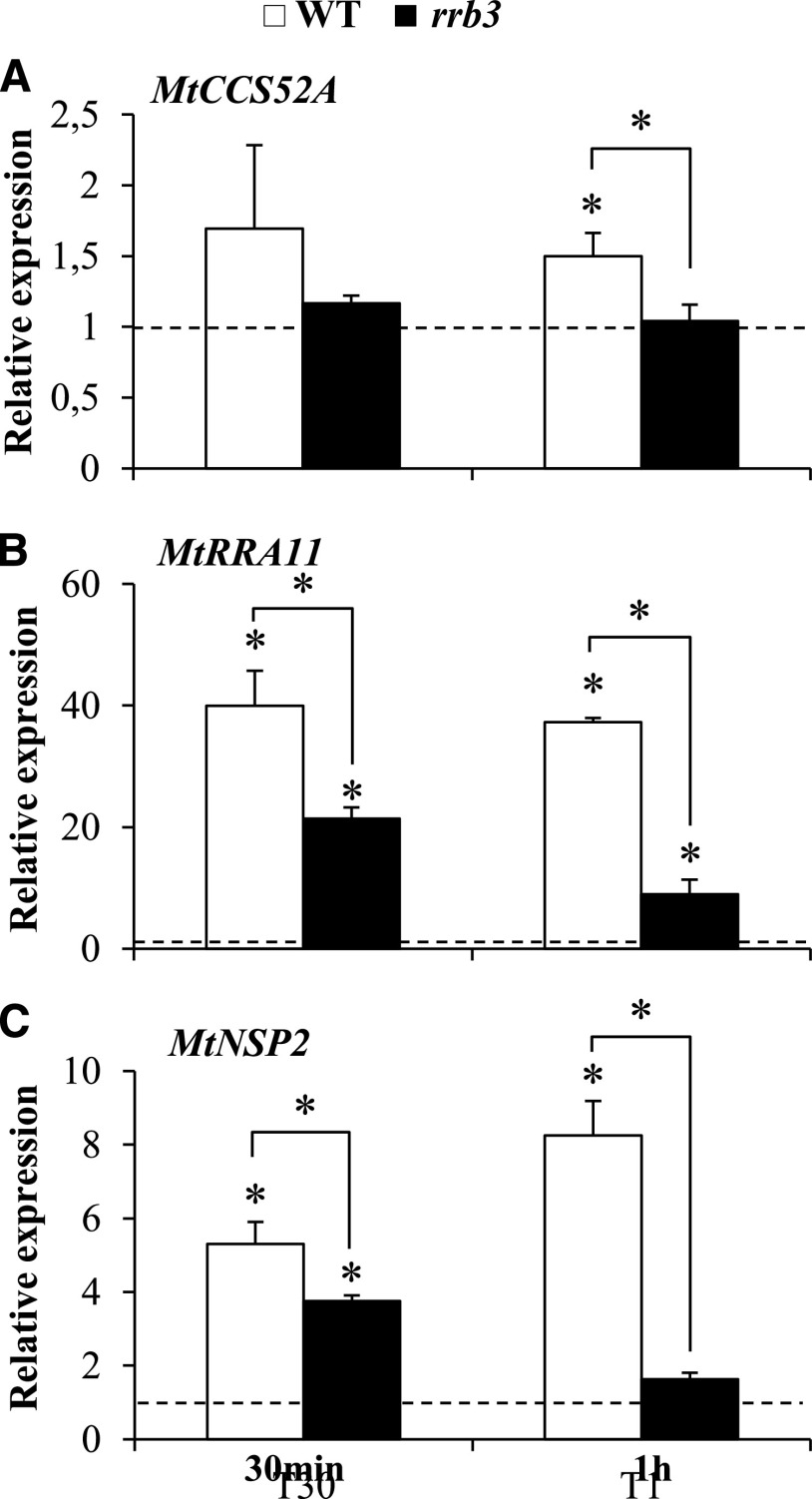
IT progression was previously associated with enlarged nuclei and endoreduplication in L. japonicus, consistent with the expression of LjCCS52A genes during early nodulation (Suzaki et al., 2014), but these features were not described in M. truncatula. We therefore quantified nuclear area in root regions infected or not with rhizobia using confocal microscopy with a strain constitutively expressing a red fluorescent protein (RFP) reporter and 4′,6-diamino-phenylindole (DAPI) staining of nuclei (Fig. 2, A–F). An increase in the nuclear area was indeed observed in infected root regions compared to uninfected root regions in both root hairs (Fig. 2E) and cortical cells (Fig. 2F). However, no significant difference was observed when comparing the nuclear area in the vicinity of developing ITs in wild-type and rrb3 mutant inoculated roots (Fig. 2G). Similarly, flow cytometry analyses revealed no difference in endoreduplication levels either in response to rhizobial inoculation or between wild-type and rrb3 mutant roots (Supplemental Fig. S8). In contrast, we observed that regulation of MtCCS52A expression by a short-term CK treatment (10−7 m BAP for 30 min and 1 h) was decreased in the rrb3 mutant (Fig. 3A). Interestingly, NF regulation of this gene was previously shown to be dependent on the MtCRE1 CK signaling pathway (Supplemental Fig. S9; van Zeijl et al., 2015).
Figure 2.
Increased nuclear area correlates with rhizobial infections. A to D, Representative longitudinal sections of wild-type (WT; A and B) and rrb3 (C and D) DAPI-stained roots 6 dpi with a WSM419-RFP rhizobium strain. Uninfected root regions (A and C) were compared to cells surrounding ITs (B and D). E and F, Magnifications of wild-type ITs in root hairs (E) or root cortex (F). Arrowheads indicate enlarged nuclei associated with infection events. The color scale represents the nuclear area (in micrometers squared). Each picture is representative of the nuclear area pattern observed in n > 15 sections from five independent roots per genotype and two independent experiments. Scale bars = 50 μm. G, Quantification of the nuclear area through a z-stack confocal projection (n > 15 sections from five independent roots per genotype from two independent experiments). A Kruskal-Wallis test was performed to assess significant differences (α < 0.05), indicated by lowercase letters.
Figure 3.
Expression of MtCCS52A, MtRRA11, and MtNSP2 in cytokinin-treated rrb3 mutant roots. A to C, RT-qPCR analysis of MtCCS52A (A), MtRRA11 (B), and MtNSP2 (C) expression in wild-type (WT) and rrb3 mutant roots treated, or not, with 10−7 m BAP for 30 min or 1 h. Values for untreated roots were set to 1 (dashed line) to highlight fold changes. Values are the mean of two independent experiments, and error bars represent standard deviations. Student’s t test was performed to assess significant differences (*P < 0.05) relative to the corresponding nontreated control, indicated by asterisks.
The MtRRB3 Expression Pattern Overlaps with the Expression Domains of the MtNSP2 and MtCSS52A Nodulation Genes
As MtNSP2 was previously shown to be regulated by CKs dependent on MtCRE1, and its promoter being bound by MtRRB1 (Ariel et al., 2012), we also analyzed its regulation in response to a short-term CK treatment (10−7 m BAP for 30 min and 1 h) in wild-type or rrb3 mutant roots (Fig. 3). The expression of MtRRA CK primary response genes previously linked to early nodulation (Gonzalez-Rizzo et al., 2006; Op den Camp et al., 2011; Saur et al., 2011) was reduced in rrb3 mutant roots (Fig. 3B; Supplemental Fig. S10), indicating that CK signaling was indeed impaired in the rrb3 mutant. Similarly, the CK induction of MtNSP2 was also notably reduced after a 1-h BAP treatment (Fig. 3C). These results demonstrate that, as previously shown for MtCCS52A, the expression of MtNSP2 is positively regulated by MtRRB3.
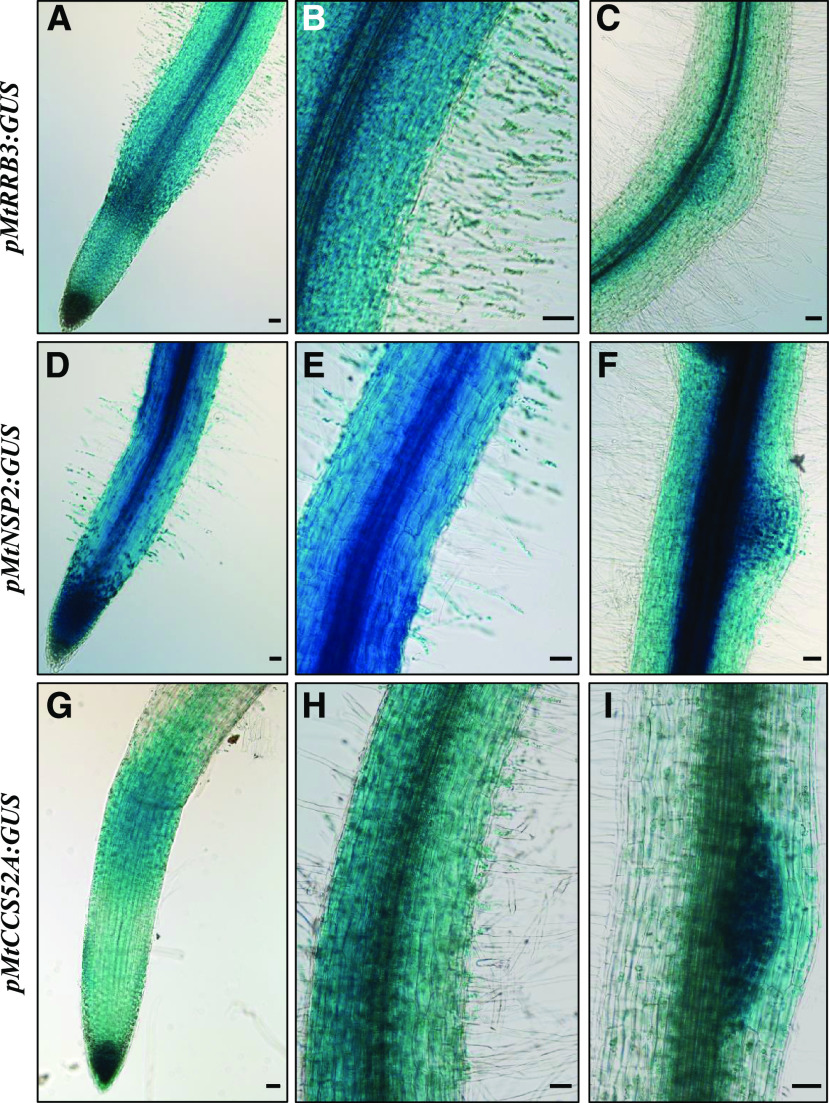
We then analyzed the spatial expression pattern of a MtRRB3 GUS transcriptional fusion in parallel with MtCCS52A GUS and MtNSP2 GUS fusions to determine how these patterns overlapped in rhizobium-infected roots (Fig. 4). Histological analyses revealed that the MtRRB3 GUS transcriptional fusion was detected in the root apex (Fig. 4A), the root hair region susceptible to rhizobia (Fig. 4B), and the nodule primordia (Fig. 4C). Interestingly, both MtNSP2 and MtCCS52A GUS fusions revealed activity colocalizing spatially with MtRRB3 in roots, mostly in the elongation/differentiation zone (Figs. 4, D and G), the differentiated root hair region (Figs. 4, E and H), and the nodule primordia (Figs. 4, F and I).
Figure 4.
MtRRB3, MtNSP2, and MtCCS52A expression patterns in M. truncatula roots. Histochemical analysis of GUS activity of transcriptional fusions for MtRRB3 (A–C), MtNSP2 (D–F), and MtCCS52A (G–I) within the root apex (A, D, and G), in the root region containing rhizobium-susceptible root hairs (B, E, and H) and in 6-dpi nodule primordia (C, F, and I). Each picture is representative of the pattern observed, depending on the construct, in at least 10 independent roots. Scale bars = 100 μm.
Overall, the MtRRB3 protein and the two early-nodulation genes MtNSP2 and MtCCS52A have overlapping spatial expression domains in roots and nodules, which is compatible with a potential direct regulation of these genes by MtRRB3.
MtRRB3 Interacts and Trans-activates the Promoter of MtNSP2 and MtCCS52A
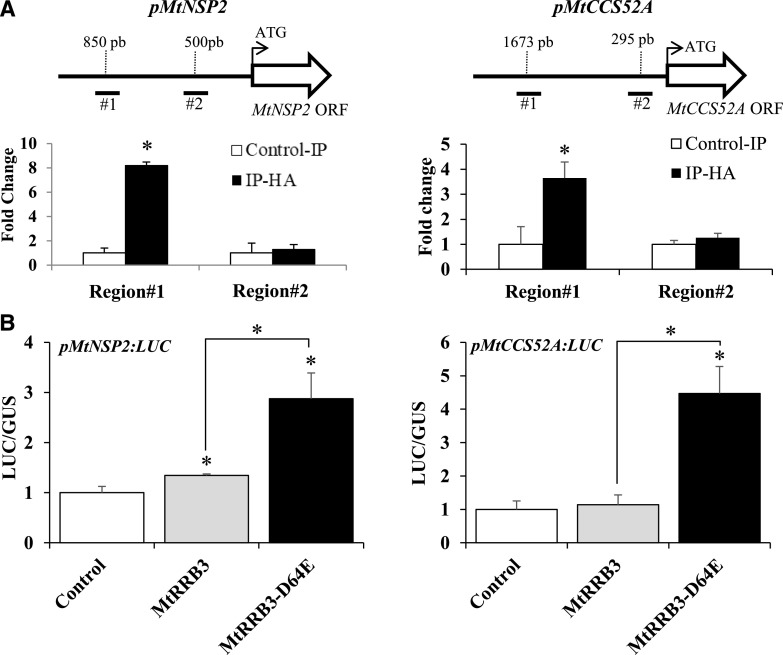
Chromatin immunoprecipitation-quantitative PCR (ChIP-qPCR) assays were then performed in roots transformed with a MtRRB3-human influenza hemagglutinin (HA) construct to test the putative interaction between MtRRB3 and MtNSP2 or MtCCS52A promoters. Immunoprecipitation (IP) with anti-HA antibody revealed enrichment for both MtNSP2 and MtCCS52A promoters compared to IP with anti-IgG antibody (Fig. 5A), indicating that MtRRB3 can bind MtNSP2 and MtCCS52A promoters. In addition, promoter trans-activation assays were carried out in M. truncatula mesophyll protoplasts using the native MtRRB3 protein and a constitutively active form of it (MtRRB3-D64E). In this variant, the conserved Asp involved in the activation of MtRRB3 by phosphotransfer was replaced by a Glu to mimic the phosphorylation effect, leading thus to its constitutive activation even in the absence of CK (Supplemental Fig. S11). Activation of expression of the pMtNSP2::LUC and pMtCCS52A::LUC transcriptional fusions was detected (Fig. 5B), showing the ability of MtRRB3 to transcriptionally activate these two promoters. Taken together, these results indicate that MtRRB3 can positively regulate expression of MtNSP2 and MtCCS52A.
Figure 5.
MtRRB3 binds and transactivates MtNSP2 and MtCCS52A promoters. A, ChIP-qPCR experiments. Roots were transformed with the p35S:MtRRB3-2×HA construct. Chromatin bound by MtRRB3 was collected by IP with an anti-HA antibody. Control IP was performed with an anti-IgG antibody. Specific primers were used to amplify two different regions of the MtNSP2 (left) and MtCCS52A (right) promoters, as shown in the schemes above each graph. IP values were normalized for each promoter as a ratio against the genomic DNA input, expressed as a percentage. Ratios with anti-IgG control were calculated to visualize MtRRB3 binding fold enrichment. Results are the mean of two technical replicates with n > 15 independent transgenic roots per construct, from one representative experiment of three. Error bars represent standard deviations, and Student’s t test was performed to assess significant differences (*P < 0.05) compared to the control IP, indicated by asterisks. B, Trans-activation assays. M. truncatula mesophyll protoplasts were transformed with pMtNSP2:LUC (left) or pMtCCS52A:LUC (right) transcriptional fusions and a vector containing a MtRRB3 native version (MtRRB3), a constitutively active MtRRB3 variant (MtRRB3-D64E), or an empty vector as a negative control. Results are the mean of two technical replicates from one biological experiment of four. Error bars represent standard deviations, and Student’s t test was performed to assess significant differences (*P < 0.05) compared to the control, indicated by asterisks.
DISCUSSION
Initiation of the symbiotic interaction between legumes and rhizobia requires tight coordination between infection and nodule organogenesis, followed by codifferentiation of bacteria and plant cells to allow nitrogen fixation and assimilation (Suzaki et al., 2015). All these processes are finely tuned in time and space by plant hormones that operate at different nodulation stages (Buhian and Bensmihen, 2018). CKs notably play a major role in nodule initiation depending on the MtCRE1/LHK1 receptor, first by positively regulating root cortical cell division and nodule organogenesis, as well as cortical infection, and second by negatively regulating NF signaling and/or rhizobial infections within the root epidermis (Jardinaud et al., 2016; Miri et al., 2016; Gamas et al., 2017). Besides the well described role of CK receptors, little is known about the involvement of downstream TCS elements that are recruited for symbiotic functions. In this study, we show that MtRRB3-defective plants have a decreased ability to form nodules, as previously observed for MtCRE1 (Gonzalez-Rizzo et al., 2006; Plet et al., 2011). In contrast, root length and lateral root number or density were not altered in the rrb3 mutant. The reduced nodule number phenotype in the rrb3 mutant is associated with a reduced number of infection events, whereas no significant difference was previously observed in MtCRE1 RNAi or cre1 mutant roots, even though IT growth in outer layers of the root seemed blocked or altered, respectively. In agreement, short-term NF induction of the MtENOD11 infection marker was not altered in the cre1 mutant (Jardinaud et al., 2016), whereas it was decreased in the rrb3 mutant. As some symbiotic responses are still observed, this suggests that a functional redundancy with other RRBs may exist. In addition, the ectopic expression of MtRRB3 was not sufficient to rescue the cre1 reduced-nodulation phenotype (Supplemental Fig. S12), pointing to a potential functional specialization between RRBs. In addition, our observation that differential symbiotic phenotypes depend on the CK signaling mutant analyzed suggests that MtRRB3 mediates only a subset of the early symbiotic CK function. The greatly increased IT number detected in the L. japonicus lhk1/hit1 (hyperinfected1) mutant (Murray et al., 2007; Held et al., 2014) further indicates that rhizobial infections are differentially regulated between L. japonicus and M. truncatula. Concerning the response to NFs, whereas NF induction of MtENOD11 was potentiated when the CK pool was decreased by ectopically expressing a CK oxidase gene in the root epidermis (Jardinaud et al., 2016), indicating a negative role of CK in the regulation of NF signaling, the response was reduced in the rrb3 mutant. This suggests that identification of antagonistic roles of CK in the regulation of NF signaling is dependent on the strategy used, which different CK metabolism or signaling genes are targeted, and/or the root tissue considered. Overall, these results suggest that MtRRB3 has a positive function in NF signaling and rhizobial infection. Furthermore, differences between infection phenotypes previously identified for CK receptor mutants establish first the diverse ways CK can regulate rhizobial infection, depending on plants and/or root tissues, and second that various RRBs likely contribute to the regulation of early nodulation. More generally, CKs can have variable and sometimes opposite effects on plant-microbe interactions and notably promote either plant defense or susceptibility, depending on pathogens, plant species, and even plant organs (Choi et al., 2011; Boivin et al., 2016a; Shigenaga and Argueso, 2016). It would be interesting, therefore, to determine, on the one hand, whether the positive function of MtRRB3 in rhizobial infection and NF signaling is conserved in other nodulating legumes, such as L. japonicus, where CKs are observed to play a very different role in regulating these processes and, on the other hand, whether MtRRB3 could participate in determining resistance/susceptibility to pathogens, as demonstrated for the Arabidopsis RRB encoding gene ARR2, which promotes resistance against Pseudomonas syringae (Choi et al., 2010).
We explored the hypothesis that some already identified early-nodulation genes may be directly transcriptionally regulated by MtRRB3-dependent CK signaling. We focused on two genes: MtNSP2, previously shown to be regulated by CKs and to have a promoter bound by the atypical MtRRB1 protein (Plet et al., 2011; Ariel et al., 2012), and MtCCS52A, whose closest Arabidopsis homolog was previously shown to be regulated by CK in the root apex through binding of a RRB transcription factor on its promoter (Fülöp et al., 2005; Takahashi et al., 2013). Based on a combination of genetic, reporter fusion, trans-activation, and ChIP analyses, we showed that regulation of both MtNSP2 and MtCCS52A expression relies on interaction with the MtRRB3 transcription factor.
MtNSP2 is a GRAS (gibberellic-acid insensitive [GAI], repressor of gai, and Scarecrow) transcription factor critical for NF signaling and nodule establishment whose expression is regulated by CKs depending on the MtCRE1 receptor (Kaló et al., 2005; Plet et al., 2011; Ariel et al., 2012; van Zeijl et al., 2015). Previous studies showed that the MtNSP2 promoter contains MtRRB1 binding sites (RRBS) required for its induction by CK and for expression in nodule primordia (Ariel et al., 2012). However, MtRRB1 is unable to regulate a MtRRA promoter in a trans-activation assay, consistent with its atypical structure lacking a C-terminal trans-activation domain (Tan et al., 2019). In contrast, MtRRB3 acts as a bona fide TCS RRB, including on the MtNSP2 promoter. This MtRRB3-dependent regulation of the MtNSP2 transcription factor essential for NF signaling and rhizobial infection may explain the reduced NF induction of MtENOD11 expression and the lower number of ITs observed in the rrb3 mutant. In addition, CK-mediated regulation of MtNSP2 expression might be one of the evolutionary innovations associated with the capacity of legume plants to nodulate.
Another gene recruited during nodulation that is a target of MtRRB3 is the APC activator, MtCCS52A, which promotes endoreduplication cycles (Vinardell et al., 2003). In Arabidopsis, there are two CCS52A genes, AtCCS52A1 and AtCCS52A2, which display complementary expression patterns in the root apical meristem (RAM; Vanstraelen et al., 2009). In contrast to the Vinardell et al. (2003) study, we observed that MtCCS52A was expressed not only in nodules but also in roots and at early stages of the interaction with rhizobium, in agreement with data obtained using CCS52A genes in L. japonicus (Suzaki et al., 2014). Interestingly, expression of the M. truncatula CCS52A gene in the RAM recapitulates the combined expression of AtCC52A1 and AtCCS52A2 Arabidopsis genes. As observed in M. truncatula, AtCCS52A1 is regulated by CK depending on the ARR2 transcription factor, to positively control the progression of endoreduplication cycles in the elongation zone of the RAM (Takahashi et al., 2013). In legume roots infected by symbionts, an increase in ploidy levels correlates with an expanded nuclear area and induction of CCS52A genes in response to the progression of either rhizobial ITs in L. japonicus or fungal hyphae from the arbuscular mycorrhizal endosymbiotic fungus Gigaspora margarita in M. truncatula (Suzaki et al., 2014; Carotenuto et al., 2019). We now also show that in M. truncatula, the nuclear area is increased in roots infected by rhizobia, despite there being no identifiable change in endoreduplication levels, which may be due to a lack of sensitivity in detecting discrete endoreduplication events associated with the limited number of rhizobial infections, in contrast to the more extensive mycorrhizal root colonization. Potential ccs52A mutants identified either from the tobacco (Nicotiana tabacum) Tnt1 retrotransposon insertional mutant collection (NF9775 and NF9308; http://bioinfo4.noble.org/mutant/; Tadege et al., 2008) or by TILLING in the same EMS mutant collection used for MtRRB3, did not contain the predicted retrotransposon insertion or did not affect any conserved residue that would be anticipated to generate a nodulation phenotype, respectively. In addition no efficient MtCCS52A RNAi construct could be generated, overall impeding the analysis of any potential IT progression and/or nuclear enlargement phenotype. Despite the fact that no difference in nuclear area could be identified in infected or noninfected rrb3 mutant roots compared to the wild type, the analysis of MtCCS52A expression was sensitive enough to reveal an alteration of its regulation in the rrb3 mutant, indicating that the MtRRB3 transcription factor positively regulates MtCCS52A expression. Overall, the data obtained suggest that MtRRB3-dependent CK regulation of MtCCS52A was recruited at the onset of nodulation, potentially leading to the activation of endoreduplication cycles in root cells associated with IT progression. It would be interesting to determine whether MtRRB3-dependent CK regulation of MtCCS52A expression is also associated with mycorrhizal infections.
CONCLUSION
In this study, we showed that the CK-signaling transcription factor MtRRB3 is required for nodulation, rhizobial infection, and NF signaling, and that these functions correlate with regulation of diverse CK-regulated genes associated with early nodulation, such as MtNSP2 and MtCCS52A. Rhizobial infection and NF signaling response phenotypes are strikingly different in the rrb3 mutant from those of cre1 or lhk1 receptor mutants (Held et al., 2014; Boivin et al., 2016b). This suggests that CK regulates rhizobial infections differently depending on plant species and that other RRBs are functionally redundant with MtRRB3 in regulating nodulation initiation.
MATERIALS AND METHODS
Materials, Plant Growth Conditions, and Treatments
The Medicago truncatula ‘Jemalong A17’ genotype was used in this study. A rrb3 mutant was identified using a TILLING screen on an EMS mutagenized population (Supplemental Table S1; Le Signor et al., 2009; Sánchez et al., 2018), and backcrossed to the wild type. The region used for TILLING was selected, using Codons Optimized to Discover Deleterious Lesions (CODDLE) software (http://wwwuser.cnb.csic.es/∼tiller/sequenceToAnalyse.html), as the most likely to generate stop codons. Homozygous rrb3 mutants introducing a stop codon at position 528 (W528*) were genotyped by PCR and subsequent sequencing of amplicons (primers used are listed in Supplemental Table S1). Seeds were scarified by immersion in pure sulfuric acid for 3 min, rinsed six times with water, and sterilized for 20 min in Chlorofix (8.25 mg L−1; Bayrol). After three washes with sterilized water, seeds were sown on 1.5% (w/v) bacto-agar plates (Gibco) and stratified for 3 d at 4°C in the dark. Germination was triggered by overnight incubation at 24°C in the dark. Germinated seeds were grown either in vitro on 1.5% (w/v) agar plates or in pots containing a mixture of perlite:sand (3:1), and placed in growth chambers with the following conditions: 16 h light at 150 μE intensity, 24°C, and 60% relative air humidity.
For nodulation phenotyping experiments, a Sinorhizobium medicae WSM419 strain (Terpolilli et al., 2008) was used, as were corresponding strains expressing either the lacZ reporter (WSM419 phemA-lacZ; Domonkos et al., 2017) or the pBHR-mRFP construct (WSM419-RFP BRC3915 strain; Smit et al. 2005). Bacteria were grown overnight at 30°C on a yeast extract broth medium (Vervliet et al., 1975) and plants were grown in vitro on a Fahraeus medium without nitrogen (Truchet et al., 1985). Plant roots were then inoculated for 1 h with a bacterial suspension (OD600 = 0.05). Nodules were counted after 6 and 14 d, and root length was measured using Fiji (https://fiji.sc/; Schindelin et al., 2012).
For root architecture phenotyping experiments, germinated seedlings were grown on a low-nitrogen “i” medium (Blondon, 1964). Lateral roots were counted 10 d after germination and primary root lengths were measured using Fiji (https://fiji.sc/; Schindelin et al., 2012).
For CK short-term treatments, germinated seedlings were placed on a grid in a Magenta box containing the low-nitrogen “i” liquid medium (Blondon, 1964) and grown for 5 d under gentle agitation in the same growth chamber described above. Seedlings were treated either with CKs (10−7 m BAP; Sigma) or with NFs (10−8 m) for different incubation times (indicated in figure legends). Roots were collected and immediately frozen in liquid nitrogen for RNA extraction.
For CK sensitivity root growth assays, plants were grown on growth papers (Mega International; https://mega-international.com/) placed on the low-nitrogen “i” medium (Blondon, 1964). After 4 d, plants on growth papers were transferred onto a fresh “i” medium supplemented or not with 10−7 m BAP. The position of root tips was marked at the time of transfer, and root growth from this point was measured after 3 d using Fiji (https://fiji.sc/; Schindelin et al., 2012).
For shoot and root dry weight analyses, germinated seedlings were grown in pots watered with liquid Fahraeus medium (Truchet et al., 1985) supplemented or not with nitrogen (NH4NO3 1 mm). Nitrogen-starved plants were inoculated with rhizobium 7 d after germination, as described above. Roots and shoots were collected 35 d after germination and dried for 7 d in an oven at 60°C before weighing.
Cloning Procedures and Agrobacterium rhizogenes Root Transformation
For the pMtRRB3-GUS transcriptional fusion, a sequence of 2.55 kb upstream of the MtRRB3 start codon (Medtr3g106220; M. truncatula genome v4.0; http://www.medicagogenome.org/; MtrunA17Chr3g0137101; https://medicago.toulouse.inra.fr/MtrunA17r5.0-ANR/; Pecrix et al., 2018) was amplified by PCR from genomic DNA using the Phusion High-Fidelity DNA polymerase (Thermo Scientific) and forward and reverse primers flanked by KpnI and AatII restriction sites, respectively (Supplemental Table S1). The PCR product was then cloned using these enzymes into the pFRN-GUS vector (Boivin et al., 2016b). Transcriptional GUS fusions with MtNSP2 (Medtr3g072710 and MtrunA17_Chr3g0114841 in M. truncatula genome v4.0 and v5.0, respectively) and MtCCS52A (Medtr4g102510 and MtrunA17_Chr4g0057131 in M. truncatula genome v4.0 and v5.0, respectively) promoters (2,542 bp and 2,460 bp, respectively) were previously described in Ariel et al. (2012) and Vinardell et al. (2003), respectively.
For RNAi constructs, specific sequences targeting the MtRRB3 trans-activation domain, which is most divergent at the nucleotide level between M. truncatula RRB genes, were amplified by PCR using the Phusion High-Fidelity DNA polymerase (Thermo Scientific) and the primers indicated in Supplemental Table S1, and subsequently cloned by Gateway recombination into the pDONR207 plasmid (Invitrogen). This sequence was introduced in the pFRN-RNAi destination vector (deriving from pFGC5941; National Center for Biotechnology Information [NCBI] accession no. AY310901; Gonzalez-Rizzo et al., 2006). The GUS-RNAi control vector was previously described (Gonzalez-Rizzo et al., 2006).
For the transcriptional promoter-luciferase (LUC) fusions used in protoplast assays, the ARR6 promoter was removed from the pARR6-LUC plasmid (NCBI accession no. EF090414) using BamHI and NcoI restriction enzymes and replaced by a linker containing BamHI, KpnI, NotI, XhoI, AscI, and NcoI, forming the p0-LUC plasmid. For pMtRRA11-LUC, the MtRRA11 promoter (2,786 bp; Medtr8g038620; M. truncatula genome v4.0; http://www.medicagogenome.org/; MtrunA17Chr8g0352871; https://medicago.toulouse.inra.fr/MtrunA17r5.0-ANR/; Pecrix et al., 2018) was NotI-AscI digested from the pK7GW-pRRA11 plasmid (Op den Camp et al., 2011) and ligated in p0-LUC in NotI and AscI restriction sites. For pMtNSP2-LUC, the MtNSP2 promoter was NotI-AscI digested from the pENTR-pMtNSP2 plasmid (Ariel et al., 2012) and ligated into p0-LUC in the NotI and AscI restriction sites. For the pMtCCS52A-LUC plasmid, pPR97-pMtCCS52A-GUS (Vinardell et al., 2003) and p0-LUC were digested by EcoRI and SmaI and AscI, respectively. Both were then blunted by T4 DNA polymerase and ligated. MtRRB1 (Medtr3g102600; M. truncatula genome v4.0; http://www.medicagogenome.org/; MtrunA17Chr3g0134481; https://medicago.toulouse.inra.fr/MtrunA17r5.0-ANR/; Pecrix et al., 2018) and MtRRB3 complementary DNA (cDNA) were PCR amplified using BamHI- and StuI-containing primers (Supplemental Table S1) and cloned in the pHBT plasmid (NCBI accession number EF090408; Sheen, 1993) downstream of a 35S-CaMV promoter. p35S:MtRRB3-D64E was obtained by site-directed mutagenesis of the p35S:MtRRB3 plasmid using the QuikChange XL Site-Directed Mutagenesis Kit (Agilent) and the appropriate primers (Supplemental Table S1).
For the 35S-CaMV:MtRRB3-HA construct, 35S promoter and MtRRB3 coding sequences were PCR amplified from the pFRN-RNAi vector and M. truncatula root cDNAs, respectively, using the Phusion High-Fidelity DNA polymerase (Thermo Scientific) and forward and reverse primers flanked by KpnI and BamHI restriction sites, respectively, for the promoter, and BamHI and StuI restriction sites, respectively, for the MtRRB3 coding sequence (Supplemental Table S1). These restriction enzymes were then used to clone PCR products into the pFRN-203 vector containing a C-terminal HA tag (Boivin et al., 2016b). The p35S:MtRRB3-D64E-HA construct was prepared by subcloning the MtRRB3-D64E-HA region from the pHBT vector into the pFRN-203 vector using BamHI and StuI restriction enzymes. All plasmids were verified by sequencing.
For in planta experiments, constructs were introduced into the Agrobacterium rhizogenes ‘ARqua1’ strain to transform M. truncatula roots and generate composite plants, as described in Boisson-Dernier et al. (2001). Transformed roots, selected on kanamycin (50 μg mL−1; Sigma), were then transferred onto growth papers (Mega International) on a Fahraeus medium without nitrogen (Truchet et al., 1985). After 1 week, plants were inoculated with rhizobia as described above.
Live and Dead Staining, Nuclear Staining, and Confocal Imaging
For the live and dead staining, nodules from in vitro cultivated plants were harvested at 21 d postinoculation (dpi), embedded in agarose (Eurobio) 4% (w/v), and prepared for “live-dead” staining using the Live/Dead BacLight kit (ref. L7007, Invitrogen), which contains Syto-9 to label living bacteria and propidium iodide (PI) to label dead bacteria and plant cell genomic DNA (Haag et al., 2011). Nodule sections (70 μm) were obtained with a vibratome (VT1200S, Leica) and incubated for 20 min in “live-dead” staining solution supplemented with 0.01% (w/v) calcofluor white M2R (Sigma). Sections were then rinsed in a 50 mm Tris-HCl (pH 7.2) buffer before observation using a LSM880 Zeiss confocal microscope with a 488-nm laser for Syto-9 excitation, a 561-nm laser for PI excitation, and a 405-nm laser for calcofluor excitation.
For nuclear area quantification, roots inoculated with the S. medicae RFP strain were fixed, embedded in 3% (w/v) agarose (Eurobio), sectioned with a vibratome (100 μm thick; VT1200S, Leica), and DAPI stained as described in Carotenuto et al., (2019). Imaging was performed using a LSM880 Zeiss confocal microscope with the 405 nm diode for DAPI excitation and 561 nm diode for RFP excitation. Fluorescence was collected between 425 and 485 nm for DAPI emission and between 595 and 640 nm for RFP emission. Nuclear areas were segmented and measured using Fiji (Schindelin et al., 2012) with a threshold/watershade-based segmentation on maximal z-projection. At least 10 independent sections (from five independent roots) per experimental condition, from two independent experiments, were analyzed.
GUS Staining Assays
Roots of composite plants expressing a reporter construct were stained for GUS activity as described in Pichon et al., (1992). Samples were incubated overnight at 37°C. Tissues were then rinsed with water and cleared in 20% (v/v) sodium hypochlorite for 1 h. Samples were observed with a bright-field light microscope (Olympus BX53) and images were taken with an Olympus DP73 camera. Images included in figures are representative of GUS patterns observed in at least 70% of the transgenic roots (n > 15 roots per replicate; two independent biological experiments).
Flow Cytometry
DNA content of root cell nuclei was assessed as described in Carotenuto et al. (2019). To release nuclei, roots were chopped with a razor blade in buffer containing 45 mm MgCl2, 30 mm sodium citrate, 60 mm MOPS (pH 7.2), and 0.1% (v/v) Triton. Nuclei suspensions were then filtered through 50 μm Celltrics filters (Partec), stained with 50 μg mL−1 PI, and at least 20,000 nuclei per sample were analyzed using a CyFlow cytometer (Partec) with a 488-nm laser for scattering and a 532-nm laser for PI excitation.
ARA
ARAs were performed on individual plants 25 d postrhizobium inoculation following a protocol modified from Koch and Evans (1966). Whole plants were placed in 21-mL glass vials sealed with rubber septa. Acetylene gas (200 μL) was injected into each vial. After a 2-h incubation, ethylene production was measured by injecting 1 mL of vial gas into a gas chromatographer (Agilent Technologies; https://www.agilent.com/) equipped with a GS-Alumina column. Column temperature and gas flow were adjusted at 120°C and 7.5 mL min−1, respectively.
Gene Expression Analysis
For reverse transcription quantitative PCR (RT-qPCR), total RNAs were extracted from frozen roots using the RNeasy plant mini kit (Qiagen). The first-strand cDNA was synthesized from 1.5 μg of total RNA using the Superscript II first-strand synthesis system (Invitrogen). Primer design was performed using OligoPerfect Designer software (https://tools.thermofisher.com/content.cfm?pageid=9716&icid=fr-oligo-6?CID=fl-oligoperfect). Primer combinations showing a minimum amplification efficiency of 90% were retained (Supplemental Table S1), and qPCR reactions were performed according to the manufacturer’s instructions using the Light Cycler Fast Start DNA Master SYBR Green I kit on a Light Cycler 480 apparatus (Roche). Cycling conditions were as follows: 95°C for 10 min, and then 40 cycles at 95°C for 15 s, 60°C for 15 s, and 72°C for 15 s. PCR amplification specificity was verified using a dissociation curve and sequencing of amplicons. MtRBP1 and MtACTIN11 were previously selected as reference genes using Genorm software (http://medgen.ugent.be/∼jvdesomp/genorm/; Gonzalez-Rizzo et al., 2006).
ChIP-qPCR
Roots expressing the 35S-CaMV:MtRRB3-HA construct (∼3 g fresh weight) were used for chromatin purification and immunoprecipitation (IP) as described in Ariel et al. (2012) except that an anti-IgG IP (Abcam172730, dilution 1:1000) was performed as a negative control in parallel to the anti-HA IP (Abcam9110, dilution 1:400). Primers used for the ChIP-qPCR analysis are listed in Supplemental Table S1. qPCR was performed on the input genomic DNA and after IP using the same experimental conditions as for RT-qPCR, but with 45 cycles. For each gene, the input data were used to normalize results obtained after IP.
Protoplast Transactivation Assays
Arabidopsis (Arabidopsis thaliana) protoplasts were prepared and transfected as described by Yoo et al. (2007). For M. truncatula protoplasts, a similar protocol was followed except that 2% (w/v) Cellulase Onozuka R10 (Yakult Pharmaceutical Industry), 1% (w/v) Macerozyme R10 (Yakult Pharmaceutical Industry), and 4% (v/v) Viscozyme (Sigma) enzymes were used to digest cell walls. Arabidopsis and M. truncatula protoplasts (1.6 105) were transfected with 20 µg of a DNA mixture consisting of 9 µg of the LUC reporter construct (pMtRRA11:LUC, pMtNSP2:LUC, or pMtCCS52A:LUC), 10 μg of a plasmid expressing a RRB (p35S:MtRRB1, p35S:MtRRB3, or p35S:MtRRB3-D64E), and 1 µg of the pUBQ10:GUS vector generated in Yoo et al. (2007) and used as an internal control to normalize transfection efficiency. After transfection, protoplasts were incubated overnight at room temperature in darkness. Protoplasts were then harvested by centrifugation (100g for 5 min) and lysed in a protoplast lysis buffer (Yoo et al., 2007); protein extracts were clarified by centrifugation (2,000g for 5 min). For GUS assays, protein extracts were incubated for 30 min at 37°C in a 4-methylumbelliferyl-β-d-glucuronide (Duchefa) substrate mix, and fluorescence (excitation, 365 nm; emission, 455 nm) was measured with a TECAN Infinite200 microplate reader. For LUC assays, protein extracts were mixed with a LUC assay buffer (20 mm tricine [pH 7.8], 5 mm MgCl2, 0.1 mm EDTA, 3.3 mm dithiothreitol, 270 mm CoA, 500 μm luciferin (Duchefa), and 500 μm ATP (Sigma), and luminescence was immediately integrated for 10 s using a TECAN Infinite200 microplate reader.
Statistical Analyses
Statistical analyses were performed with the Xlstat software (https://www.xlstat.com/fr/). We used Student’s t test for RT-qPCR, ChIP-qPCR, and trans-activation assays, and the Mann-Whitney and Kruskal-Wallis tests for phenotyping experiments where n = 2 or n > 2 independent conditions, respectively, are compared.
Accession Numbers
All accession numbers of genes mentioned are listed in Supplemental Table S1.
Supplemental Material
The following supplemental materials are available.
Supplemental Figure S1. Trans-activation of a MtRRA promoter by MtRRB3 but not MtRRB1.
Supplemental Figure S2. rrb3 mutant root growth CK-sensitivity.
Supplemental Figure S3. Nodule and IT densities of the rrb3 mutant.
Supplemental Figure S4. Lateral root phenotype of the rrb3 mutant.
Supplemental Figure S5. Shoot and root dry weight phenotypes of the rrb3 mutant.
Supplemental Figure S6. MtRRB3 complements the rrb3 mutant nodulation phenotype.
Supplemental Figure S7. Late nodulation phenotype of the rrb3 mutant.
Supplemental Figure S8. Ploidy level of rrb3 mutant root cells.
Supplemental Figure S9. MtCCS52A expression in NF-treated cre1 mutant roots.
Supplemental Figure S10. Expression of MtRRA genes previously linked to nodulation in CK-treated rrb3 mutant roots.
Supplemental Figure S11. Trans-activation of a MtRRA promoter by the MtRRB3-D64E construct.
Supplemental Figure S12. MtRRB3 does not complement the cre1 mutant nodulation phenotype.
Supplemental Table S1 List of primers.
Acknowledgments
We thank Fabienne Maillet (Laboratory of Plant-Microbe Interactions), Rene Geurts (Wageningen University and Research), Gabriella Endre (Biological Research Center, Hungarian Academy of Sciences), Leo Serra (Institut Jean-Pierre Bourgin), Peter Mergaert (Institut de Biologie Integrative de la Cellule) and Pascal Ratet (Institut des Sciences des Plantes-Paris, Saclay), for providing NFs, the pKGW-RRGG-243-pRRA11 plasmid, the WSM419-lacZ and WSM419-RFP rhizobium strains, a Fiji macro to quantify nuclear area, and the pPR97 pCCS52A:GUS plasmid, respectively. We also thank students from the Paris-Diderot University for generating additional biological replicates of trans-activation experiments.
Footnotes
This work was supported by the Agence Nationale de la Recherche (ANR), Laboratory of Excellence “Saclay Plant Science” (SPS) and Idex “Plant Phenotyping Pipeline” (3P), and by the Paris-Sud/Paris-Saclay University (PhD fellowship to S.T.).
References
- Ariel F, Brault-Hernandez M, Laffont C, Huault E, Brault M, Plet J, Moison M, Blanchet S, Ichanté JL, Chabaud M, et al. (2012) Two direct targets of cytokinin signaling regulate symbiotic nodulation in Medicago truncatula. Plant Cell 24: 3838–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondon F. (1964) Contribution à l’étude du développement des graminées fourragères ray-grass et dactyle. PhD Thesis. Université de Paris, Paris, pp. 131 [Google Scholar]
- Boisson-Dernier A, Chabaud M, Garcia F, Bécard G, Rosenberg C, Barker DG(2001) Agrobacterium rhizogenes-transformed roots of Medicago truncatula for the study of nitrogen-fixing and endomycorrhizal symbiotic associations. Mol Plant Microbe Interact 14: 695–700 [DOI] [PubMed] [Google Scholar]
- Boivin S, Fonouni-Farde C, Frugier F(2016a) How auxin and cytokinin phytohormones modulate root microbe interactions. Front Plant Sci 7: 1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin S, Kazmierczak T, Brault M, Wen J, Gamas P, Mysore KS, Frugier F(2016b) Different cytokinin histidine kinase receptors regulate nodule initiation as well as later nodule developmental stages in Medicago truncatula. Plant Cell Environ 39: 2198–2209 [DOI] [PubMed] [Google Scholar]
- Buhian WP, Bensmihen S(2018) Mini-review: Nod factor regulation of phytohormone signaling and homeostasis during rhizobia-legume symbiosis. Front Plant Sci 9: 1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carotenuto G, Volpe V, Russo G, Politi M, Sciascia I, de Almeida-Engler J, Genre A(2019) Local endoreduplication as a feature of intracellular fungal accommodation in arbuscular mycorrhizas. New Phytol 223: 430–446 [DOI] [PubMed] [Google Scholar]
- Choi J, Huh SU, Kojima M, Sakakibara H, Paek KH, Hwang I(2010) The cytokinin-activated transcription factor ARR2 promotes plant immunity via TGA3/NPR1-dependent salicylic acid signaling in Arabidopsis. Dev Cell 19: 284–295 [DOI] [PubMed] [Google Scholar]
- Choi J, Choi D, Lee S, Ryu CM, Hwang I(2011) Cytokinins and plant immunity: Old foes or new friends? Trends Plant Sci 16: 388–394 [DOI] [PubMed] [Google Scholar]
- Domonkos Á, Kovács S, Gombár A, Kiss E, Horváth B, Kováts GZ, Farkas A, Tóth MT, Ayaydin F, Bóka K, et al. (2017) NAD1 controls defense-like responses in Medicago truncatula symbiotic nitrogen fixing nodules following rhizobial colonization in a BacA-independent manner. Genes (Basel) 8: 1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson BJ, Mathesius U(2014) Phytohormone regulation of legume-rhizobia interactions. J Chem Ecol 40: 770–790 [DOI] [PubMed] [Google Scholar]
- Fournier J, Timmers ACJ, Sieberer BJ, Jauneau A, Chabaud M, Barker DG(2008) Mechanism of infection thread elongation in root hairs of Medicago truncatula and dynamic interplay with associated rhizobial colonization. Plant Physiol 148: 1985–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frugier F, Kosuta S, Murray JD, Crespi M, Szczyglowski K(2008) Cytokinin: Secret agent of symbiosis. Trends Plant Sci 13: 115–120 [DOI] [PubMed] [Google Scholar]
- Fülöp K, Tarayre S, Kelemen Z, Horváth G, Kevei Z, Nikovics K, Bakó L, Brown S, Kondorosi A, Kondorosi E(2005) Arabidopsis anaphase-promoting complexes: Multiple activators and wide range of substrates might keep APC perpetually busy. Cell Cycle 4: 1084–1092 [PubMed] [Google Scholar]
- Gamas P, Brault M, Jardinaud MF, Frugier F(2017) Cytokinins in symbiotic nodulation: When, where, what for? Trends Plant Sci 22: 792–802 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Rizzo S, Crespi M, Frugier F(2006) The Medicago truncatula CRE1 cytokinin receptor regulates lateral root development and early symbiotic interaction with Sinorhizobium meliloti. Plant Cell 18: 2680–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag AF, Baloban M, Sani M, Kerscher B, Pierre O, Farkas A, Longhi R, Boncompagni E, Hérouart D, Dall’angelo S, et al. (2011) Protection of Sinorhizobium against host cysteine-rich antimicrobial peptides is critical for symbiosis. PLoS Biol 9: e1001169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann AB, Sandal N, Bek AS, Madsen LH, Jurkiewicz A, Nielsen MW, Tirichine L, Stougaard J(2011) Cytokinin induction of root nodule primordia in Lotus japonicus is regulated by a mechanism operating in the root cortex. Mol Plant Microbe Interact 24: 1385–1395 [DOI] [PubMed] [Google Scholar]
- Held M, Hou H, Miri M, Huynh C, Ross L, Hossain MS, Sato S, Tabata S, Perry J, Wang TL, et al. (2014) Lotus japonicus cytokinin receptors work partially redundantly to mediate nodule formation. Plant Cell 26: 678–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyl A, Brault M, Frugier F, Kuderova A, Lindner A-C, Motyka V, Rashotte AM, Schwartzenberg KV, Vankova R, Schaller GE(2013) Nomenclature for members of the two-component signaling pathway of plants. Plant Physiol 161: 1063–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyl A, Schmülling T(2003) Cytokinin signal perception and transduction. Curr Opin Plant Biol 6: 480–488 [DOI] [PubMed] [Google Scholar]
- Hwang I, Sheen J(2001) Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413: 383–389 [DOI] [PubMed] [Google Scholar]
- Imamura A, Hanaki N, Nakamura A, Suzuki T, Taniguchi M, Kiba T, Ueguchi C, Sugiyama T, Mizuno T(1999) Compilation and characterization of Arabidopsis thaliana response regulators implicated in His-Asp phosphorelay signal transduction. Plant Cell Physiol 40: 733–742 [DOI] [PubMed] [Google Scholar]
- Jardinaud M-F, Boivin S, Rodde N, Catrice O, Kisiala A, Lepage A, Moreau S, Roux B, Cottret L, Sallet E, et al. (2016) A laser dissection-RNAseq analysis highlights the activation of cytokinin pathways by Nod factors in the Medicago truncatula root epidermis. Plant Physiol 171: 2256–2276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabbara S, Schmülling T, Papon N(2018) CHASEing cytokinin receptors in plants, bacteria, fungi, and beyond. Trends Plant Sci 23: 179–181 [DOI] [PubMed] [Google Scholar]
- Kaló P, Gleason C, Edwards A, Marsh J, Mitra RM, Hirsch S, Jakab J, Sims S, Long SR, Rogers J, et al. (2005) Nodulation signaling in legumes requires NSP2, a member of the GRAS family of transcriptional regulators. Science 308: 1786–1789 [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Schaller GE(2018) Cytokinin signaling in plant development. Development 145: dev149344. [DOI] [PubMed] [Google Scholar]
- Koch B, Evans HJ(1966) Reduction of acetylene to ethylene by soybean root nodules. Plant Physiol 41: 1748–1750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondorosi E, Redondo-Nieto M, Kondorosi A(2005) Ubiquitin-mediated proteolysis. To be in the right place at the right moment during nodule development. Plant Physiol 137: 1197–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerouge P, Roche P, Faucher C, Maillet F, Truchet G, Promé JC, Dénarié J(1990) Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature 344: 781–784 [DOI] [PubMed] [Google Scholar]
- Le Signor C, Savois V, Aubert G, Verdier J, Nicolas M, Pagny G, Moussy F, Sanchez M, Baker D, Clarke J, et al. (2009) Optimizing TILLING populations for reverse genetics in Medicago truncatula. Plant Biotechnol J 7: 430–441 [DOI] [PubMed] [Google Scholar]
- Liu CW, Breakspear A, Roy S, Murray JD(2015) Cytokinin responses counterpoint auxin signaling during rhizobial infection. Plant Signal Behav 10: e1019982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miri M, Janakirama P, Held M, Ross L, Szczyglowski K(2016) Into the root: How cytokinin controls rhizobial infection. Trends Plant Sci 21: 178–186 [DOI] [PubMed] [Google Scholar]
- Murray JD, Karas BJ, Sato S, Tabata S, Amyot L, Szczyglowski K(2007) A cytokinin perception mutant colonized by Rhizobium in the absence of nodule organogenesis. Science 315: 101–104 [DOI] [PubMed] [Google Scholar]
- Oldroyd GED, Downie JA(2008) Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu Rev Plant Biol 59: 519–546 [DOI] [PubMed] [Google Scholar]
- Op den Camp RHM, De Mita S, Lillo A, Cao Q, Limpens E, Bisseling T, Geurts R(2011) A phylogenetic strategy based on a legume-specific whole genome duplication yields symbiotic cytokinin type-A response regulators. Plant Physiol 157: 2013–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecrix Y, Staton SE, Sallet E, Lelandais-Brière C, Moreau S, Carrère S, Blein T, Jardinaud M-F, Latrasse D, Zouine M, et al. (2018) Whole-genome landscape of Medicago truncatula symbiotic genes. Nat Plants 4: 1017–1025 [DOI] [PubMed] [Google Scholar]
- Pichon M, Journet EP, Dedieu A, de Billy F, Truchet G, Barker DG(1992) Rhizobium meliloti elicits transient expression of the early nodulin gene ENOD12 in the differentiating root epidermis of transgenic alfalfa. Plant Cell 4: 1199–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plet J, Wasson A, Ariel F, Le Signor C, Baker D, Mathesius U, Crespi M, Frugier F(2011) MtCRE1-dependent cytokinin signaling integrates bacterial and plant cues to coordinate symbiotic nodule organogenesis in Medicago truncatula. Plant J 65: 622–633 [DOI] [PubMed] [Google Scholar]
- Sakai H, Aoyama T, Oka A(2000) Arabidopsis ARR1 and ARR2 response regulators operate as transcriptional activators. Plant J 24: 703–711 [DOI] [PubMed] [Google Scholar]
- Sánchez M, Le Signor C, Aubert G, Darchy B, Gallardo K, Thompson RD(2018) Targeting Induced Local Lesions IN Genomes (TILLING) in Medicago truncatula In Cañas LA, and Beltrán JP, eds, Functional Genomics in Medicago Truncatula, Methods in Molecular Biology, 1822. Humana Press, Totowa, NJ, pp 71–82 [DOI] [PubMed] [Google Scholar]
- Saur IML, Oakes M, Djordjevic MA, Imin N(2011) Crosstalk between the nodulation signaling pathway and the autoregulation of nodulation in Medicago truncatula. New Phytol 190: 865–874 [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, et al. (2012) Fiji: An open-source platform for biological-image analysis. Nat Methods 9: 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J.(1993) Protein phosphatase activity is required for light-inducible gene expression in maize. EMBO J 12: 3497–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenaga AM, Argueso CT(2016) No hormone to rule them all: Interactions of plant hormones during the responses of plants to pathogens. Semin Cell Dev Biol 56: 174–189 [DOI] [PubMed] [Google Scholar]
- Smit P, Raedts J, Portyanko V, Debellé F, Gough C, Bisseling T, Geurts R(2005) NSP1 of the GRAS protein family is essential for rhizobial Nod factor-induced transcription. Science 308: 1789–1791 [DOI] [PubMed] [Google Scholar]
- Suzaki T, Ito M, Yoro E, Sato S, Hirakawa H, Takeda N, Kawaguchi M(2014) Endoreduplication-mediated initiation of symbiotic organ development in Lotus japonicus. Development 141: 2441–2445 [DOI] [PubMed] [Google Scholar]
- Suzaki T, Yoro E, Kawaguchi M(2015) Leguminous plants: Inventors of root nodules to accommodate symbiotic bacteria. Int Rev Cell Mol Biol 316: 111–158 [DOI] [PubMed] [Google Scholar]
- Tadege M, Wen J, He J, Tu H, Kwak Y, Eschstruth A, Cayrel A, Endre G, Zhao PX, Chabaud M, et al. (2008) Large-scale insertional mutagenesis using the Tnt1 retrotransposon in the model legume Medicago truncatula. Plant J 54: 335–347 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Kajihara T, Okamura C, Kim Y, Katagiri Y, Okushima Y, Matsunaga S, Hwang I, Umeda M(2013) Cytokinins control endocycle onset by promoting the expression of an APC/C activator in Arabidopsis roots. Curr Biol 23: 1812–1817 [DOI] [PubMed] [Google Scholar]
- Tan S, Debellé F, Gamas P, Frugier F, Brault M(2019) Diversification of cytokinin phosphotransfer signaling genes in Medicago truncatula and other legume genomes. BMC Genomics 20: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpolilli JJ, O’Hara GW, Tiwari RP, Dilworth MJ, Howieson JG(2008) The model legume Medicago truncatula A17 is poorly matched for N2 fixation with the sequenced microsymbiont Sinorhizobium meliloti 1021. New Phytol 179: 62–66 [DOI] [PubMed] [Google Scholar]
- Tirichine L, Sandal N, Madsen LH, Radutoiu S, Albrektsen AS, Sato S, Asamizu E, Tabata S, Stougaard J(2007) A gain-of-function mutation in a cytokinin receptor triggers spontaneous root nodule organogenesis. Science 2680: 104–107 [DOI] [PubMed] [Google Scholar]
- Truchet G, Debellé F, Vasse J, Terzaghi B, Garnerone AM, Rosenberg C, Batut J, Maillet F, Dénarié J(1985) Identification of a Rhizobium meliloti pSym2011 region controlling the host specificity of root hair curling and nodulation. J Bacteriol 164: 1200–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanstraelen M, Baloban M, Da Ines O, Cultrone A, Lammens T, Boudolf V, Brown SC, De Veylder L, Mergaert P, Kondorosi E(2009) APC/C-CCS52A complexes control meristem maintenance in the Arabidopsis root. Proc Natl Acad Sci USA 106: 11806–11811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zeijl A, Op den Camp RHM, Deinum EE, Charnikhova T, Franssen H, Op den Camp HJM, Bouwmeester H, Kohlen W, Bisseling T, Geurts R(2015) Rhizobium lipo-chitooligosaccharide signaling triggers accumulation of cytokinins in Medicago truncatula roots. Mol Plant 8: 1213–1226 [DOI] [PubMed] [Google Scholar]
- Vasse J, de Billy F, Camut S, Truchet G(1990) Correlation between ultrastructural differentiation of bacteroids and nitrogen fixation in alfalfa nodules. J Bacteriol 172: 4295–4306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet G, Holsters M, Teuchy H, Van Montagu M, Schell J(1975) Characterization of different plaque-forming and defective temperate phages in Agrobacterium strains. J Gen Virol 26: 33–48 [DOI] [PubMed] [Google Scholar]
- Vinardell JM, Fedorova E, Cebolla A, Kevei Z, Horvath G, Kelemen Z, Tarayre S, Roudier F, Mergaert P, Kondorosi A, et al. (2003) Endoreduplication mediated by the anaphase-promoting complex activator CCS52A is required for symbiotic cell differentiation in Medicago truncatula nodules. Plant Cell 15: 2093–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao TT, Schilderink S, Moling S, Deinum EE, Kondorosi E, Franssen H, Kulikova O, Niebel A, Bisseling T(2014) Fate map of Medicago truncatula root nodules. Development 141: 3517–3528 [DOI] [PubMed] [Google Scholar]
- Yoo S-D, Cho Y-H, Sheen J(2007) Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat Protoc 2: 1565–1572 [DOI] [PubMed] [Google Scholar]