Abstract
The mechanisms regulating viral pathogenesis of human papillomavirus (HPV) associated oropharyngeal squamous cell cancers (OPSCC) are not well understood. In the cervix, activation of DNA damage repair pathways is critical for viral replication but little is known about their role in OPSCC. APOBEC factors have been shown to be increased in OPSCC but the significance of this is unclear. We therefore examined activation of DNA damage and APOBEC factors in HPV-induced OPSCC. Our studies show significantly increased levels of pCHK1, FANCD2, BRCA1, RAD51, pSMC1 and γH2AX foci in HPV-positive samples as compared to HPV-negative while the ATM effector kinase, pCHK2, was not increased. Similar differences were observed when the levels of proteins were examined in OPSCC cell lines. In contrast, the levels of APOBEC3B and 3A were found to be similar in both HPV-positive and -negative OPSCC. Our studies suggest members of ATR pathway and FANCD2 may be important in HPV-induced OPSCC.
Keywords: Head and neck cancer, HPV, DNA damage, ATR, FANCD2, APOBEC3A, APOBEC3B
Introduction
Over the last several decades there has been a rapid increase in the incidence of human papillomavirus (HPV) associated oropharyngeal squamous cell carcinomas (OPSCC) in Western countries, and the number of cases will likely soon surpass those of cervical cancer [1]. Although greater than 400 types of HPV have been identified, over 90% of HPV related OPSCC is caused by HPV16 [2–4]. While the progression to cervical cancer via dysplastic precancerous lesions has been well characterized, the pathogenesis of HPV induced OPSCC remains an enigma as dysplastic lesions have rarely been found.
In HPV positive cervical cancers as well as precursor lesions, the host DNA damage response (DDR), including the ataxia telangiectasia mutated-dependent (ATM) pathway and the ataxia telangiectasia mutated-dependent DNA-related (ATR) pathway, have been shown to be constitutively activated [5]. The ATM pathway is activated in response to DNA double-strand breaks resulting in phosphorylation of several downstream effector proteins, including the kinase CHK2, the modified histone H2AX, as well as members of the homologous recombination repair arm such as BRCA1 and RAD51 [6]. The ATR pathway responds to replication stress and the presence of single-stranded DNA at stalled replication forks. It also signals by phosphorylating specific effector proteins, such as the kinase CHK1, H2AX and FANCD2, a key regulator of the Fanconi anemia (FA) pathway along with BRCA1 and RAD51 [7,8]. Previous studies have shown that activation of DDR proteins in HPV positive cells is required for completion of the viral life cycle in the cervical epithelium [5]. These DDR factors are preferentially recruited to HPV genomes and are necessary for differentiation-dependent amplification [5,9]. Importantly examination of biopsy materials revealed that the distribution and levels of DDR factors increase with the grade of cervical lesion. This suggests that activation of DDR in HPV positive genital epithelia is important for viral replication as well as transformation [10]. It is, however, not clear if similar activation of the DDR occurs in HPV positive lesions of the head and neck and whether this is a characteristic of most cancers of this region or specific to those caused by viral infection.
Several recent studies have also demonstrated the upregulation of apolipoprotein B mRNA editing enzyme catalytic (APOBEC) family of proteins in head and neck cancer [11,12]. APOBEC is a cytidine deaminase enzyme that functions to convert cytosine to uracil. The human APOBEC family is composed of at least 11 deaminases [13,14]. These were initially considered as viral restriction factors against several viruses including HPV [15–20]. Recent studies have identified additional important roles for these family members in diverse cellular process including promoting host genome mutations that contribute to cellular transformation [21–24]. Furthermore, sequence data from TCGA indicate that head and neck as well as cervical, bladder, lung, ovarian and breast tumors, contain genome-wide cytosine mutations consistent with APOBEC3 deamination of cellular DNA [23, 25–28]. It has also been reported that APOBEC3s may play a role in activation of the replication stress response [29,30], however it is unclear what role these factors play in HPV induced OPSCC and whether APOBEC3 proteins are linked to effects of DDR factors in virally induced cancers. Finally, APOBEC3 enzymes have also been linked to alphavirus diversity and evolution.
In this study, we investigated the expression levels and distributions of DNA damage repair factors in head and neck cancers through a comparative analysis of both HPV-positive and -negative OPSCC biopsy specimens as well as head and neck cancer cell lines. Examination of APOBEC3 proteins indicated elevated but similar levels in HPV positive and negative OPSCC with no strong correlation to DDR factors. Our studies demonstrate the increased activation of DDR factors, particularly members of the ATR pathway and FANCD2, in HPV-positive as compared to HPV-negative head and neck cancers as well as normal tissues.
Materials and Methods
Tissue samples and HPV testing
Archived specimens for retrospective analysis were collected from patients diagnosed with OPSCC at Northwestern Memorial Hospital, Department of Pathology under a Northwestern University IRB-approved protocol. All patients provided written informed consent. The formalin-fixed paraffin-embedded (FFPE) tissues selected for this study were used for construction of tissue microarrays (TMAs) as three 1.5 mm cores per case, comprised of two tumor cores and one core of adjacent histologically normal squamous mucosa, verified by a pathologist (K.P.). Tonsil cores from healthy donors were included in selection for the TMA as staining controls. Five μm sections were cut from tissue and TMA blocks for staining with hematoxylin and eosin (H&E) and for IHC. All cases were tested for HPV positivity by p16 immunohistochemistry (IHC) and interpreted as positive when >70% strong tumor staining was present. We also performed high-risk (HR) HPV testing on all cases using DNA and mRNA in situ hybridization (ISH). DNA-ISH: ZytoFast® HPV High-Risk (HR) Types Probe specific for oncogenic E6/E7 from 15 HPV types, combined with the Zytofast PLUS Implementation Kit. mRNA-ISH: RNAscope 2.0 HD Detection Kit (Brown, ACD). RNAscope testing used a probe against 18 high-risk (HR) HPV genotypes E6/E7 mRNA (RNAscope Probe HPV-HR18, ACD). Slides were processed according to the manufacturer’s instructions; the bacterial gene DapB was used as a negative control and the housekeeping gene POLR2A served as a positive control.
Cell lines
The UPCI:SCC154 and SCC25 cell lines were purchased from ATCC. The SCC9 and SCC68 lines were a generous gift from Dr. Kathleen Green, Northwestern University. The UPCI:SCC090 cell line was kindly obtained from Dr. Robert Ferris in University of Pittsburgh Hilman Cancer Center. The UPCI cell lines were cultured and maintained in DMEM supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin, non-essential amino acids and 2mM L-glutamine. The SCC cell lines were cultured using DMEM/F12 supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin and hydrocortisone. All cell lines were incubated on 37°C and passaged via trypsinization.
Immunofluorescence/ Immunocytochemistry
Tissue sample sections were de-paraffinized by a series of washes in xylene, ethanol, and permeabilized with triton x-100. Antigen retrieval was performed in citrate buffer. Sections were blocked with normal goat serum (NGS) and probed with primary antibodies in NGS at 4°C overnight. After three washes in PBS with triton x-100, Alexaflour secondary antibodies in NGS were added followed by DAPI incubation, and coverslips mounted. For cell lines, cells were grown on treated coverslips under media. Prior to analysis, the cells were fixed with 4% paraformaldehyde, permeabilized with Triton-X100, blocked with NGS, and probed with primary antibodies in NGS at 4°C overnight. Afterwards the identical wash protocol described above was performed, slides were incubated with secondary antibodies followed by DAPI incubation, and coverslips mounted. The APOBEC3B antibodies from Novus and mAb 5210-87-13 were used in IF studies and demonstrated similar focal staining in nuclei.
Microscopy and ImageJ analysis
Images were taken on a Zeiss Axioscope and imported into ImageJ for analysis. The percentage of positive-staining cells was evaluated by comparing the number of cells with nuclear focal staining to the total number of cells as determined by DAPI staining. The counting of foci number was performed using ImageJ macro and greater than 20 cells were examined in each group. The specific staining images was converted to 8 bits, and threshold was adjusted to 185 to 255 for a specific staining mask. The image was processed as binary and a watershed was applied. Next the foci number was determined using the particles analysis tool. Total signal area was calculated as the mean IF intensity over the region minus mean background intensity, and multiplied by the regions total area. Gretaer than 10 cells were examined in each group.
Western blot analysis
Whole-cell lysates were extracted using RIPA buffer with added protease inhibitor cocktail. Protein concentrations were quantified using a Bradford assay and 20 μg of protein lysates were loaded per well, followed by separation by electrophoresis on SDS-polyacrylamide gels. Proteins were then transferred to an Immoblion-P, PVDF membrane and probed with primary and secondary antibodies. Blots were developed using ECL, chemiluminescence visualized on Licor Odyssey FL imager.
Antibodies
p16 (#550834; BDPharmingen), phosphorylated CHK1 (pCHK1) (#12302; CST), phosphorylated CHK2 (pCHK2) (#2661; CST), FANCD2 (#100182; Novus), phosphorylated H2Ax (γH2AX) (#05636 Millipore), phosphorylated SMC1 (pSMC1) (#4805S; CST), BRCA1 (#OP92; Millipore), RAD51 (#NB100148; Novus), APOBEC3A (#HPA043237; Sigma), APOBEC3B (#NBP256411; Novus), APOBEC3B (mAb 5210-87-13) (Gift from Reuben Harris lab)) [31]
Statistical analysis
IBM SPSS Statics24 software was used for statistical analysis and the generation of graphs. Comparison between two groups was performed with unpaired Student’s t test. Multigroup analysis was performed with Kruskall-Wallace tests, and P values were adjusted using the Holm-Sidak methods. P values of <0.05 were considered to be statistically significant.
Results
Biopsy tissue sample selection and HPV status evaluation
To investigate the correlation between HPV status and activation of DDR factors we conducted a retrospective review of head and neck cancer specimens involving either the palatine tonsils or the base of tongue. A total of 41 cases were selected based on adequate tumor tissue present on the slides. These cases underwent both p16 IHC and HPV DNA ISH testing (Fig.1A) to determine viral status. Twenty-eight cases were identified that were positive for both along with 9 additional cases that were negative for both (Fig.1B). Of the HPV positive samples, 7 samples included a normal margin region adjacent to the cancer and these regions were used as negative controls (Fig.1C).
Fig. 1. Tissue samples or oropharyngeal cancers and marginal regions.

(A). Left panels: Representative immunohistochemical analysis for p16 in OPSCC tissue samples. Right panels: Representative example of in situ hybridization of HPV 16 DNA. (B) Tabulation of p16 positivity and HPV 16 status in tissue samples. (C) Hematoxylin and eosin (HE) staining of tumor samples. Seven samples included a normal margin region adjacent to the cancer. Epithelial layers in normal regions were divided into 4 categories for assessment: basal, parabasal, intermediate, and superficial.
Expression of DNA Damage Repair Factors expression is elevated in HPV positive oropharyngeal cancers
We next sought to determine if there was a correlation with activation of DDR factors and HPV status in OPSCC. For this analysis, the expression of ATM, ATR and FA pathway related proteins including pCHK1, pCHK2, FANCD2, BRCA1, RAD51, γH2AX and pSMC1 was examined by IF using biopsy samples. These factors were examined previously in HPV positive cervical cancers and were chosen so comparisons could be made [10]. In HPV positive and negative samples, all of these factors exhibited discrete focal staining in the nuclei of cells, which is a marker of pathway activation (Fig.2A). To compare levels of activation we used two measures of activity. First we determined the percentages of cells that exhibited any focal localization of these factors. Second we counted the average number of foci in positive cells and used that as well to make comparisons. These assays were used as the average nuclear signal intensity between samples was judged to be too inconsistent to utilize reliably.
Fig. 2. Expression patterns of DNA damage factors in tumor specimens.
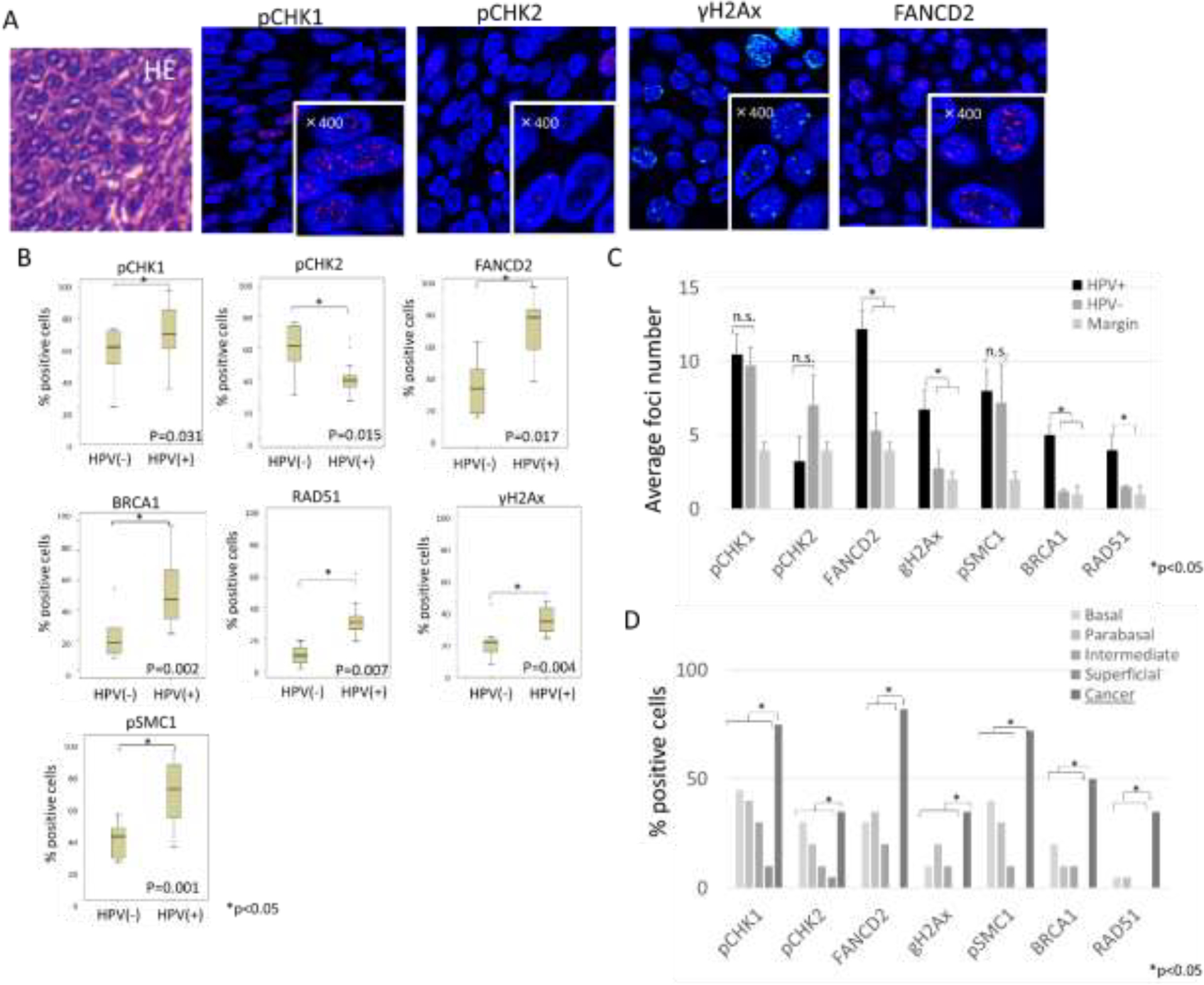
(A) Representative example of HE staining and Immunofluorescence staining of DNA damage factors in HPV positive cancer region. High magnification (x400) is shown in lower right corner and indicates the presence of positive foci. (B) Comparative analysis of the percentages of cells that exhibited any focal localization of DDR factors between HPV positive and negative OPSCC samples. Error bars represent the SD between samples. *P <0.05. (C) Quantitation of average nuclear foci number per positive cell. (D) Comparative analysis of the percentages of cells that exhibited any focal localization of DDR factors between HPV positive OPSCC and normal regions of epithelia in margin (20 or more cells per group were examined).
Members of the ATR pathway were examined first to determine if there were differences between HPV positive and negative OPSCC as well as in normal cells found in margin regions. In HPV positive samples, the number of cells exhibiting pCHK1 foci was significantly higher than in HPV negative OPSCC samples (Figure 2B). In HPV positive OPSCC the average number of pCHK1 positive foci per cell was greater than 10. While the number of positive cells in HPV negative lesions was reduced, the average number of foci per positive cell was similar (Fig.2C). In normal epithelia present in the margin regions, pCHK1 positive foci were detected in basal cells and decreased upon differentiation but at substantially reduced numbers. When comparing cancer and margin region, statistically higher levels of pCHK1 positive nuclei were observed in cancer cells (Fig.2D). Significant differences were also observed with cells staining positive for FANCD2. The percentage of HPV positive cells with FANCD2 positive foci was significantly higher compared to HPV negative cancers. In addition, the average number of foci found in HPV positive cancers was also substantially increased (Fig.2B, C). In normal epithelia located at margins, cells with FANCD2 positive foci were primarily detected in basal layers, and this was significantly lower than in HPV positive samples (Fig.2D). We next examined the distribution of the DNA damage associated phosphorylated histone γH2AX. In HPV positive samples the average number of γH2AX positive foci along with BRCA1 and RAD51 was greater than that seen in HPV negative cancers (Fig.2B). The percentage of γH2AX, BRCA1 and RAD51 positive cells was also substantially increased in HPV positive samples than HPV negative as well as normal cells located in the margin area. With respect to the cohesin, SMC1, which can be phosphorylated by both ATM and ATR DNA damage kinases, the number of cells with positive foci was also substantially higher in HPV positive cancers than in negative lesions. We conclude that members of the ATR pathway including CHK1, RAD51, FANCD2, BRCA1 and SMC1 are present at increased levels in HPV positive OPSCC.
We next examined tissue samples for the presence of pCHK2, a member of the ATM pathway, and found that the average numbers of pCHK2 nuclei foci in HPV positive cancers was reduced compared with other DDR proteins (Fig.2B). Although the number of pCHK2 positive cells in HPV positive cancer was higher than non-cancerous cells in the margin, there was no differences between HPV positive and negative cancers (Fig.2C, D). Overall our data indicate that the expression of ATR target proteins is substantially increased in HPV positive OPSCC specimens with over 70% of cells containing positive foci (Fig.2D) while the number of cells with foci containing the ATM factor pCHK2 are not substantially increased.
APOBEC3A and 3B expression is increased in HPV positive oropharyngeal cancer specimens
A family of proteins that has been demonstrated to cause replication stress leading to DDR activation are APOBECs. We next investigated the state of APOBEC3A and 3B in HPV positive OPSCC. IF analysis of HPV positive and negative lesions using an antibody against APOBEC3A showed localization to both nuclear and cytoplasmic foci. Staining with an antibody to APOBEC3B, which may also cross-react with other APOBEC3 proteins, showed localization to foci in the nuclei of HPV positive OPSCC tissue (Fig.3A) with a slight statistical significance with for HPV positive versus negative lesions (Fig.3B). In the margin region, the number of APOBEC3A-positive cells was highest in the intermediate layer whereas APOBEC3B was found primarily in basal and parabasal layers (Fig.3C). When we compared the number of positive cells in the cancer region versus margin epithelium, APOBEC3B showed higher levels as compared to normal cells while APOBEC3A did not show statistical differences between the two regions (Fig.3D).
Fig. 3. Expression patterns of APOBEC3A and 3B in tumor specimens.
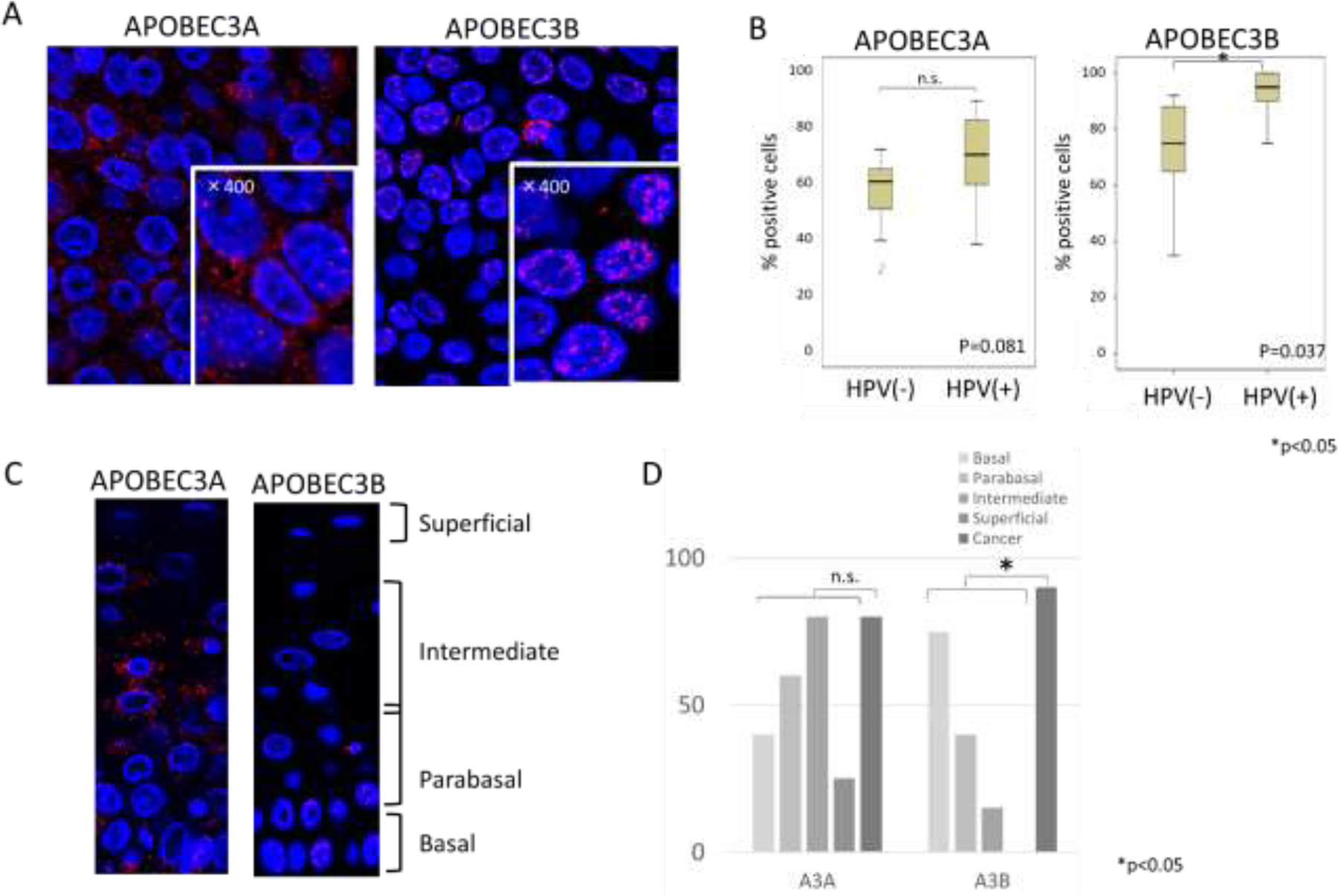
(A) Representative immunofluorescence staining of APOBEC3A and 3B in HPV positive tumor samples. High magnification (x400) are shown in lower right corner. APOBEC3A antibody used in this analysis was from Sigma while APOBEC3B antibody was from Novus.
(B) Comparative analysis of the percentages of cells that exhibited any focal localization of APOBEC3A and 3B between HPV positive and negative OPSCC samples.
(C) Immunofluorescence staining of APOBEC3A and 3B in normal epithelial layers
(D) Comparative analysis of the percentages of cells that exhibited any focal localization of APOBEC3A and 3B between HPV positive OPSCC and normal epithelia (20 or more cells per group were examined). (P value; 0.17 (APOBEC3A), 0.01(APOBEC3B))
Levels of DNA Damage Repair Factors except for pCHK2 are increased in HPV positive oropharyngeal cancer cell lines
In the above analysis we evaluated the expression of DDR and APOBEC factors by IF screening but were unable to compare total protein levels. For this reason we complemented the tumor sample analysis with an examination of established HPV-positive and -negative OPSCC cell lines. The presence of HPV16 in each lines was first confirmed by RT-PCR for HPV16-E6.
Western analysis demonstrated that the total levels of FANCD2, γH2AX, BRCA1, pSMC1 and RAD51 proteins are increased in HPV positive lines. In contrast, the levels of pCHK1 and pCHK2 exhibited only modest differences between the HPV positive and negative lines (Fig.4A). We next examined cells by IF and similar to the biopsy studies we observed large numbers of DDR-positive foci in both HPV positive lines, however most cells in both sets of cell lines appeared positive and the numbers of foci were too variable to compare (Figure 4B). We did however notice that the foci in HPV positive cells appeared larger than those in HPV negative lines. We therefore quantified total area of foci in the nuclei of 10 or more cells using automated particle analysis in ImageJ software as with tumor samples and tabulated the data. Our data indicate that DDR foci are larger in HPV positive samples. We conclude that HPV positive lines had statistically higher levels of the DDR proteins FANCD2, γH2Ax than the HPV negative lines except for pCHK2 which exhibited similar values (Fig.4C).
Fig. 4. Analysis of DNA Damage Repair proteins in established HPV positive and negative OPC cell lines.
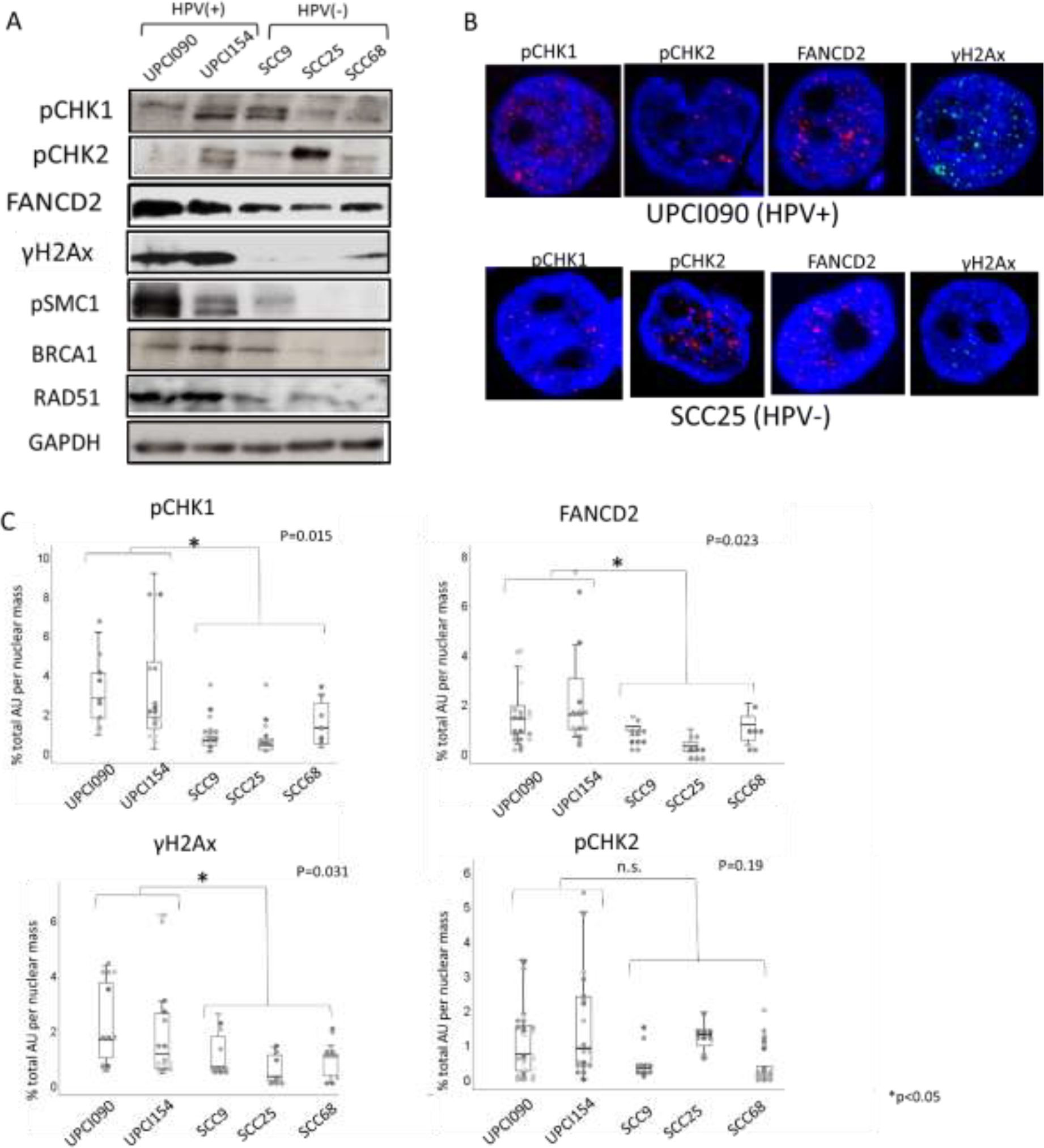
(A) Western blot analysis of DDR factors in HPV positive cell lines (UPCI090 and 154) and negative lines (SCC9, SCC25 and SCC68). GAPDH was evaluated as a loading control.
(B) Immunofluorescence of DDR factors in HPV positive cell line (UPCI090) and HPV negative line (SCC25).
(C) Quantitation of area of foci as percent of total nuclear size using automated particle analysis in ImageJ software (10 cells per group were examined)
APOBEC3A and 3B expression are increased in HPV positive head and neck cancer cell lines
We next examined the levels of APOBEC3B proteins in the OPSCC cell lines using an antibody that has been shown to accurately detect the 37kd form this protein in Western analysis. Our studies show variable levels of expression of APOBEC3B in both HPV positive and negative cell lines. While both express higher levels than those seen in normal HFKs, we are unable to conclude that one is consistently higher than the other (Fig.5A). Using an antibody to APOBEC3A, we observed slightly elevated levels in HPV positive versus negative cell lines (Fig.5A). Furthermore, IF analyses indicated APOBEC3A was found localized in both nuclei and cytoplasm whereas APOBEC3B was mainly present as nuclear foci (Fig.5B) similar to observations in tissue samples. This APOBEC3B antibody may also cross-react with other APOBEC3 proteins. We found no statistically significant differences in the average number of foci between HPV positive and HPV negative cell lines (Fig.5C; greater than 20 cells per group were examined). Multicolor immunofluorescence revealed that foci of γH2AX, RAD51 and FANCD2 were present in cells that were also enriched for APOBEC3B/3G but we detected no co-localization of the two sets of factors (Fig.5D).
Fig. 5. Analysis of APOBEC3A and 3B levels and localization in HPV positive and negative OPC cell lines.
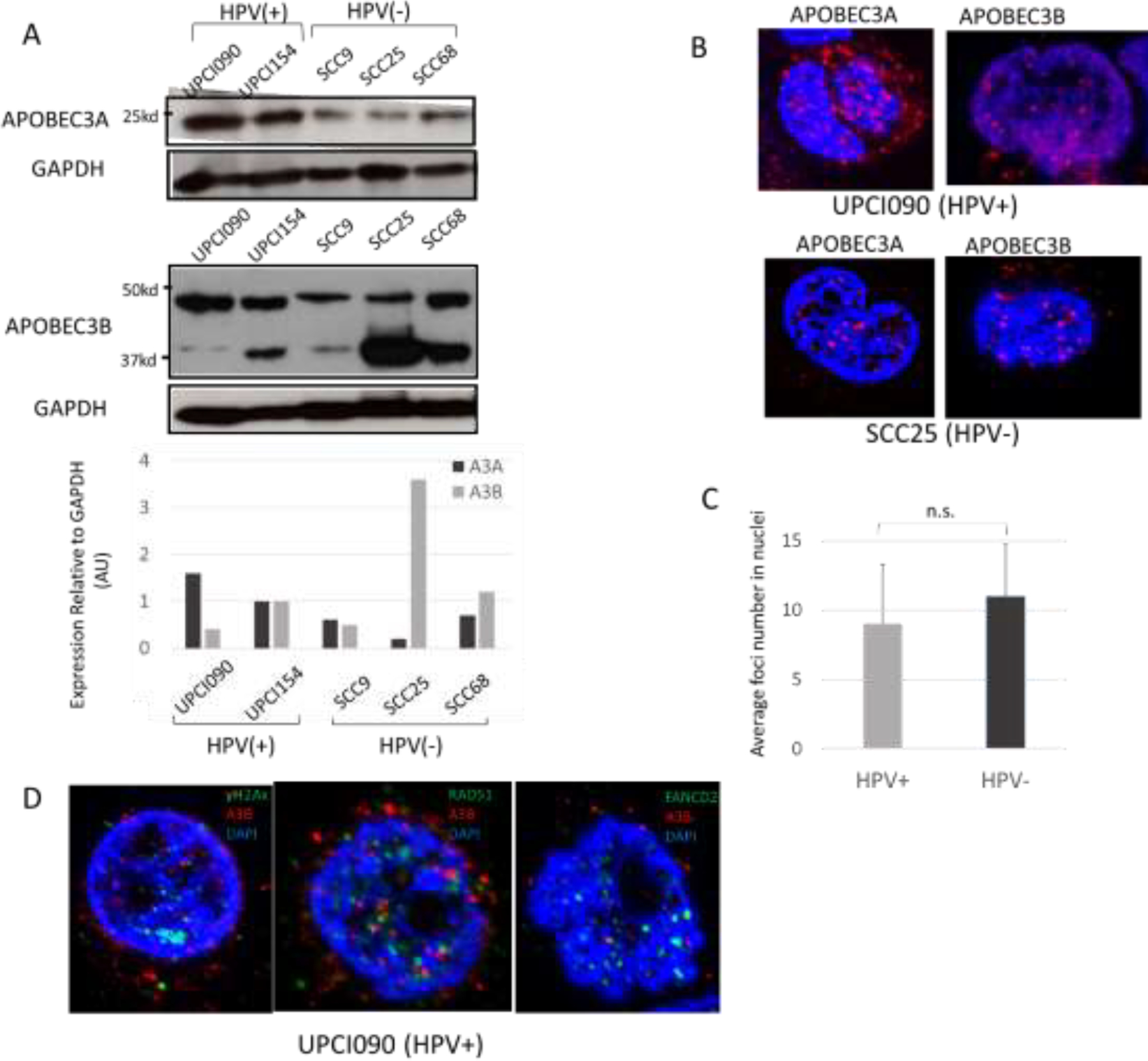
(A) Western blot analysis of APOBEC3A and 3B in HPV positive and negative cell lines. GAPDH was evaluated as a loading control. Quantitation of band intensity was analyzed by ImageJ software. For Western analysis, APOBEC3A antibody from Sigma and APOBEC3B antibody mAb 5210-87-13 (a gift from Harris lab) were used.
(B) Representative Immunofluorescence of APOBEC3A and 3B in HPV positive cell line (UPCI090) and HPV negative line (SCC25). For IF analysis, APOBEC3A antibody from Sigma and APOBEC3B antibody mAb 5210-87-13 (a gift from Harris lab) were used.
(C) Quantitation of average nuclear foci number in both HPV positive and negative cell lines (10 cells were examined in each group). P=0.24.
(D) Multicolor immunofluorescence of APOBEC3B and γH2AX, RAD51, FANCD2 in HPV positive cell line (UPCI090). Merged picture is shown.
Discussion
HPV positive OPSCC are traditionally treated with either a combination of radiation and chemotherapy, or surgery followed by radiation. However, the side effects and long-term morbidity associated with these therapies can be severe as head and neck cancers are highly associated with impairment of important functions such as swallowing, speaking and breathing. It is therefore important to identify new prognostic markers as well as therapeutics. Critical to this is the identification of the molecular mechanisms regulating viral pathogenesis in head and neck cancers. While extensive research has helped to unveil the molecular mechanisms of pathogenesis in HPV related cervical cancers, the same cannot be said for HPV related OPSCC. Furthermore, little is known about how the virus replicates and persists in oropharyngeal tissues and there are no currently defined clinical biomarkers or targetable genetic alterations for either HPV- and non-HPV related OPSCC [35].
In cervical cancers both ATM and ATR pathways are constitutively activated by the HPV proteins E6 and E7. Activation of both pathways is critical for viral replication particularly for differentiation-dependent viral genome amplification which precedes virion production[5,33–36]. Our analysis indicates that pCHK1, a member of the ATR pathway, and FANCD2 as well as BRCA1, RAD51 and γH2AX are all increased in HPV positive OPSCC. FANCD2 is one of the key components of the FA pathway which cross talks with both the ATM and ATR pathways to regulate cell cycle progression and to repair interstrand cross-linked DNAs [34]. ATR directly phosphorylates several proteins in FA pathway which are required for optimal FANCD2 monoubiquitination and thus activation [38–40]. Similarly, ATR regulates the formation of nuclear foci containing homologous recombination factors such as γH2AX, BRCA1, BRCA2 as well as recruiting RAD51 to sites of damage. ATM can also phosphorylate FANCD2, but this leads to an S-phase arrest and is not involved in DNA damage repair [41]. Interestingly FA patients have an increased incidence of head and neck cancer and deficiencies in the FA pathway increased progression to HPV-positive OPSCC in E7 transgenic mice, suggesting that an intact FA pathway may regulate viral transformation in these tissues[42,43]. We observed similarities in our analysis of biopsy samples and cell lines derived from HPV positive and negative OPSCC. Examination of established cell lines allowed for the determination of total levels of DDR proteins and this correlated with our observations of foci in biopsy samples. In culture, the most highly proliferative cells are selected for and this may suggest why both sets of lines exhibited similarly high levels of foci. However, our studies indicate that the size of the foci in HPV positive cells were significantly larger than in negative lines. Overall, our findings suggest higher levels of activation of ATR pathway and FANCD2 in HPV positive cancers indicating these factors may play critical roles in head and neck region. The role of the ATR pathway and FANCD2 factors in particular in OPSCC is a critical area for future study.
In contrast to the activation observed with members of the ATR family, pCHK2, the central kinase of ATM pathway was not preferentially activated in HPV positive lesions and even appeared to be more activated in HPV negative OPSCC. This suggests there may be important differences in ATM and ATR activation by HPV proteins in the oropharynx in comparison to the cervix. In the cervix, activation of ATM pathway is critical for amplification [5,44,45] and it is possible that low levels of activation seen in normal oropharyngeal tissues is sufficient for viral replication and no further increases are needed. Alternatively, activation of the ATR pathway by itself may be sufficient in oropharyngeal tissues for viral replication but since no cells harboring HPV episomes or amplifying viral DNAs in the oropharynx have yet been identified, it is difficult to test this hypothesis. Finally, it is also possible that oropharyngeal cells that maintain HPV episomes initially had high levels of ATM activation but that reductions occurred during progression that resulted in the integrated state of HPV genomes seen in most OPSCC lesions. Testing these models is an important area for study but will have to await isolation of HPV positive oropharyngeal lesions that maintain viral genomes as episomes.
Data from the TCGA indicate high levels of APOBEC3B are present in HPV positive OPSCC [9] and our studies are consistent with this observation. High levels of the 37kd APOBEC3B were observed by Western in HPV-positive cell lines and correlated with high numbers of APOBEC3B-positive foci in biopsy samples. No significant difference was however observed between HPV positive and negative OPSCC though both were increased relative to normal cells. Our studies also indicated increased levels of APOBEC3A and other studies have reported increased levels of APOBEC3A in HPV-positive OPSCC [46] but there is disagreement as to whether this is due to post-translational stabilization or increased transcription. Our immunofluorescence analysis of AOBEC3B indicated a focal nuclear localization. Previous studies from the Harris lab using transiently overexpressed APOBEC3B showed a pan nuclear localization [31] while other studies have reported the presence of foci [47]. This difference could be due to the cell lines examined or that the antibodies used can cross react with other APOBEC3 family members including the 50kd APOBEC3G which is a cytoplasmic protein. A more detailed analysis in the future examining cells in which each of the individual family members has been knocked down could clarify these issues. Our Western analysis readily identified the 37kd APOBEC3B band and showed variable levels between HPV positive and negative OPSCC so no conclusion about differences could be made.
An association of APOBEC proteins with DNA damage has previously been suggested Studies have indicated that APOBEC3B can be activated in response to DNA damage, specifically through the ATR kinase, and this further contributes to DNA breaks in a deaminase-dependent manner(29). High levels of APOBEC3A can also lead to induction of DNA breaks by the base excision repair pathway initiated by cellular uracil-DNA glycosylases and leading to activation of DDR(30). A mechanistic linkage between APOBECs and DNA damage, particularly ATR and FA pathway, may also help to explain HPV induced carcinogenesis in the oropharynx. Further analyses to investigate the association between DNA damage and APOBECs may be useful in the development of therapeutics against HPV infection or for an effective screening strategy.
Conclusion
HPV positive OPSCC exhibit increased activation of the ATR pathway as compared to HPV negative lesions or normal epithelia in marginal regions. Using IF in biopsy samples or proteins levels in OPSCC cells lines as indicators, the levels of FANCD2, pCHK1, BRCA1, RAD51 along with γH2AX were increased in HPV positive OPSCC. The ATM effector kinase, pCHK2, was not increased. Whether cells with increased levels of APOBEC3B exhibit elevated activation of ATR pathway members and FANCD2 requires a more detailed analysis in the future. Overall our studies suggest that members of the ATR pathway and FANCD2 may be important in HPV induced disease in the oropharynx.
Acknowledgement:
We thank the Reuben Harris lab for the generous gifts of the 5210-87-13 monoclonal antibody, additional reagents and helpful discussions on APOBEC3B/3G localization.
Funding Source: LAL was supported by grants from the National Cancer Institute (RO1CA 059655 and RO1CA142861). The research was partially supported by grants from Walter Newman family and IDP Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflict of interest.
References
- [1].Castellsague X, Alemany L, Quer M, Halec G, Quiros B, Tous S, et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J Natl Cancer Inst. 2016;108(6):djv403. [DOI] [PubMed] [Google Scholar]
- [2].Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–75. [DOI] [PubMed] [Google Scholar]
- [3].Pastrana DV, Peretti A, Welch NL, Borgogna C, Olivero C, Buck CB, et al. Metagenomic Discovery of 83 New Human Papillomavirus Types in Patients with Immunodeficiency. mSphere. 2018; 3(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tirosh O, Conlan S, Deming C, Lee-Lin SQ, Huang X, Kong HH, et al. Expanded skin virome in DOCK8-deficient patients. Nat Med. 2018;24(12):1815–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Moody CA, Laimins LA. Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation. PLoS Pathog. 2009;5(10):e1000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Eyfjord JE, Bodvarsdottir SK. Genomic instability and cancer: networks involved in response to DNA damage. Mutat Res. 2005;592(1–2):18–28. [DOI] [PubMed] [Google Scholar]
- [7].Sulli G, Di Micco R, d’Adda di Fagagna F. Crosstalk between chromatin state and DNA damage response in cellular senescence and cancer. Nat Rev Cancer. 2012;12(10):709–20. [DOI] [PubMed] [Google Scholar]
- [8].Kee Y, D’Andrea AD. Expanded roles of the Fanconi anemia pathway in preserving genomic stability. Genes Dev. 2010;24(16):1680–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mehta K, Laimins L. Human Papillomaviruses Preferentially Recruit DNA Repair Factors to Viral Genomes for Rapid Repair and Amplification. MBio. 2018;9(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Spriggs CC, Blanco LZ, Maniar KP, Laimins LA. Expression of HPV-induced DNA Damage Repair Factors Correlates With CIN Progression. Int J Gynecol Pathol. 2019;38(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Warren CJ, Westrich JA, Doorslaer KV, Pyeon D. Roles of APOBEC3A and APOBEC3B in Human Papillomavirus Infection and Disease Progression. Viruses. 2017;9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, et al. Signatures of mutational processes in human cancer. Nature. 2013;500(7463):415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Goila-Gaur R, Strebel K. HIV-1 Vif, APOBEC, and intrinsic immunity. Retrovirology. 2008;5:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harris RS, Liddament MT. Retroviral restriction by APOBEC proteins. Nat Rev Immunol. 2004;4(11):868–77. [DOI] [PubMed] [Google Scholar]
- [15].Malim MH. APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos Trans R Soc Lond B Biol Sci. 2009;364(1517):675–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Okeoma CM, Lovsin N, Peterlin BM, Ross SR. APOBEC3 inhibits mouse mammary tumour virus replication in vivo. Nature. 2007;445(7130):927–30. [DOI] [PubMed] [Google Scholar]
- [17].Nguyen DH, Hu J. Reverse transcriptase- and RNA packaging signal-dependent incorporation of APOBEC3G into hepatitis B virus nucleocapsids. J Virol. 2008;82(14):6852–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gee P, Ando Y, Kitayama H, Yamamoto SP, Kanemura Y, Ebina H, et al. APOBEC1-mediated editing and attenuation of herpes simplex virus 1 DNA indicate that neurons have an antiviral role during herpes simplex encephalitis. J Virol. 2011;85(19):9726–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Warren CJ, Xu T, Guo K, Griffin LM, Westrich JA, Lee D, et al. APOBEC3A functions as a restriction factor of human papillomavirus. J Virol. 2015;89(1):688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Cheng AZ, Yockteng-Melgar J, Jarvis MC, Malik-Soni N, Borozan I, Carpenter MA, et al. Epstein-Barr virus BORF2 inhibits cellular APOBEC3B to preserve viral genome integrity. Nat Microbiol. 2019;4(1):78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nowarski R, Kotler M. APOBEC3 cytidine deaminases in double-strand DNA break repair and cancer promotion. Cancer Res. 2013;73(12):3494–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Green AM, Landry S, Budagyan K, Avgousti DC, Shalhout S, Bhagwat AS, et al. APOBEC3A damages the cellular genome during DNA replication. Cell Cycle. 2016;15(7):998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Burns MB, Temiz NA, Harris RS. Evidence for APOBEC3B mutagenesis in multiple human cancers. Nat Genet. 2013;45(9):977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chan K, Roberts SA, Klimczak LJ, Sterling JF, Saini N, Malc EP, et al. An APOBEC3A hypermutation signature is distinguishable from the signature of background mutagenesis by APOBEC3B in human cancers. Nat Genet. 2015;47(9):1067–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kuong KJ, Loeb LA. APOBEC3B mutagenesis in cancer. Nat Genet. 2013;45(9):964–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Leonard B, Hart SN, Burns MB, Carpenter MA, Temiz NA, Rathore A, et al. APOBEC3B upregulation and genomic mutation patterns in serous ovarian carcinoma. Cancer Res. 2013;73(24):7222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Middlebrooks CD, Banday AR, Matsuda K, Udquim KI, Onabajo OO, Paquin A, et al. Association of germline variants in the APOBEC3 region with cancer risk and enrichment with APOBEC-signature mutations in tumors. Nat Genet. 2016;48(11):1330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vieira VC, Leonard B, White EA, Starrett GJ, Temiz NA, Harris RS, et al. Human papillomavirus E6 triggers upregulation of the antiviral and cancer genomic DNA deaminase APOBEC3B. mBio. 2014; 5(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Shimizu A, Fujimori H, Minakawa Y, Matsuno Y, Hyodo M, Murakami Y, et al. Onset of deaminase APOBEC3B induction in response to DNA double-strand breaks. Biochem Biophys Rep. 2018;16:115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Landry S, Narvaiza I, Linfesty DC, Weitzman MD. APOBEC3A can activate the DNA damage response and cause cell-cycle arrest. EMBO Rep. 2011;12(5):444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Brown WL, Law EK, Argyris PP, Carpenter MA, Harris RS, et al. A Rabbit Monoclonal Antibody against the Antiviral and Cancer Genomic DNA Mutating Enzyme APOBEC3B. Antibodies 2019; 8(3):E47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kang H, Kiess A, Chung CH. Emerging biomarkers in head and neck cancer in the era of genomics. Nat Rev Clin Oncol. 2015;12(1):11–26. [DOI] [PubMed] [Google Scholar]
- [33].Gillespie KA, Mehta KP, Laimins LA, Moody CA. Human papillomaviruses recruit cellular DNA repair and homologous recombination factors to viral replication centers. J Virol. 2012;86(17):9520–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].McKinney CC, Hussmann KL, McBride AA. The Role of the DNA Damage Response throughout the Papillomavirus Life Cycle. Viruses. 2015;7(5):2450–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hong S, Cheng S, Iovane A, Laimins LA. STAT-5 Regulates Transcription of the Topoisomerase IIbeta-Binding Protein 1 (TopBP1) Gene To Activate the ATR Pathway and Promote Human Papillomavirus Replication. MBio. 2015;6(6):e02006–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Edwards TG, Helmus MJ, Koeller K, Bashkin JK, Fisher C. Human papillomavirus episome stability is reduced by aphidicolin and controlled by DNA damage response pathways. J Virol. 2013;87(7):3979–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Grompe M FANCD2: a branch-point in DNA damage response? Nat Med. 2002;8(6):555–6. [DOI] [PubMed] [Google Scholar]
- [38].Andreassen PR, D’Andrea AD, Taniguchi T. ATR couples FANCD2 monoubiquitination to the DNA-damage response. Genes Dev. 2004;18(16):1958–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Collins NB, Wilson JB, Bush T, Thomashevski A, Roberts KJ, Jones NJ, et al. ATR-dependent phosphorylation of FANCA on serine 1449 after DNA damage is important for FA pathway function. Blood. 2009;113(10):2181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ishiai M, Kitao H, Smogorzewska A, Tomida J, Kinomura A, Uchida E, et al. FANCI phosphorylation functions as a molecular switch to turn on the Fanconi anemia pathway. Nat Struct Mol Biol. 2008;15(11):1138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Taniguchi T, Garcia-Higuera I, Xu B, Andreassen PR, Gregory RC, Kim ST, et al. Convergence of the fanconi anemia and ataxia telangiectasia signaling pathways. Cell. 2002;109(4):459–72. [DOI] [PubMed] [Google Scholar]
- [42].Hoskins EE, Morreale RJ, Werner SP, Higginbotham JM, Laimins LA, Lambert PF, et al. The fanconi anemia pathway limits human papillomavirus replication. J Virol. 2012;86(15):8131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Park JW, Pitot HC, Strati K, Spardy N, Duensing S, Grompe M, et al. Deficiencies in the Fanconi anemia DNA damage response pathway increase sensitivity to HPV-associated head and neck cancer. Cancer Res. 2010;70(23):9959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hong S, Dutta A, Laimins LA. The acetyltransferase Tip60 is a critical regulator of the differentiation-dependent amplification of human papillomaviruses. J Virol. 2015;89(8):4668–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Anacker DC, Gautam D, Gillespie KA, Chappell WH, Moody CA. Productive replication of human papillomavirus 31 requires DNA repair factor Nbs1. J Virol. 2014;88(15):8528–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kondo S, Wakae K, Wakisaka N, Nakanishi Y, Ishikawa K, Komori T, et al. APOBEC3A associates with human papillomavirus genome integration in oropharyngeal cancers. Oncogene. 2017;36(12):1687–97. [DOI] [PubMed] [Google Scholar]
- [47].Siriwardena SU, Perena MLW, Senevirathne V, Stewart J, Bhagwat AS. A Tumor-Promoting Phorbol Ester Causes a Large Increase in APOBEC3A Expression and a Moderate Increase in APOBEC3B Expression in a Normal Human Keratinocyte Cell Line without Increasing Genomic Uracils. Mol Cell Biol. 2018;39(1):e00238–18. Doi:10.1128 [DOI] [PMC free article] [PubMed] [Google Scholar]


