Abstract
Introduction:
Parathyroid carcinoma (PC) is an uncommon clinical entity. Identification and appropriate treatment of PC remains a challenge. In this study, we explore clinico-radiological features suggestive of PC, the utility of Castleman's histopathological criteria for the diagnosis of PC and discuss the MD-Anderson prognostic stratification system for PC.
Methods:
Retrospective analysis (case series) of patients who were treated at our tertiary oncology institution between January 2009 and December 2018 with an eventual diagnosis of PC.
Results:
The study group comprised 15 patients. The most common presentation was related to the musculoskeletal system (n = 9, 60%). In one of the cases, ultrasound features were recorded to be suspicious of PC. The highest histopathological correlation with PC was found with capsular and vascular invasion (n = 12, 80%). The primary tumor was found in inferior parathyroid glands in most cases (n = 12, 80%). The average tumor size was 2.47 cm. Six patients (40%) received adjuvant radiotherapy (RT). Three patients (20%) developed recurrence, all having distant metastasis. Overall survival (OS) at 3 years was 92.31% and disease-free survival (DFS) of 76.61%.
Conclusion:
The presence of certain features on ultrasonography might suggest PC preoperatively. Among Castleman's criteria, capsular invasion and vascular invasion had a maximum association with PC in our series. At present, there is no evidence to routinely give adjuvant RT to all patients with PC.
Keywords: Brown tumors, Castleman's criteria, parathormone, parathyroid carcinoma, pimary hyperparathyroidism
INTRODUCTION
The incidence of primary hyperparathyroidism (PHPT) as reported in western literature is around 1% and its incidence rises to 2% beyond 55 years.[1] The most common cause for PHPT is parathyroid adenoma (85%) followed by parathyroid hyperplasia (14%) and in 1% of the patients, it could be due to parathyroid carcinoma (PC).[2,3] Identifying patients with PHPT due to PC is difficult and challenging. The incidence of hyperparathyroidism in western literature varies from 34 to 120 per 100,000 persons[4,5]; similar data from the Indian subcontinent is not available. Most patients in the available literature from the western series lack overt clinical symptoms and are diagnosed incidentally.[6] However, in India patients with PHPT and PC present with significant skeletal and renal manifestations, which makes it challenging to differentiate between the two clinical entities.[6,7]
It was only after the landmark article by Shantz and Castleman in 1973,[8] in which they published histopathologic criteria to diagnose PC [Table 1], that various case series appeared in the literature giving their experience of this rare disease. Another article published by MD Anderson Cancer Center in 2017 tried to address a completely different aspect of this multispectral disease.[9] They addressed issues pertaining to the chances of recurrence and possible stratification to identify risk groups. Due to the rarity of disease; large trials are not feasible to provide insights into management strategies for PC.[10,11,12] Our study is an attempt to not only bring out our experience with cases of PC over a period of 10 years but also explore clinico-radiological features suggestive of PC, the utility of Castleman's criteria in making a diagnosis as well as discuss the MD Anderson prognostic stratification system in relation to PC in our cases.
Table 1.
Shantz and Castleman histopathologic criteria to diagnose parathyroid carcinoma
| Capsular invasion |
|---|
| Vascular invasion |
| Mitotic figures |
| Fibrous bands |
| Trabecular architecture |
METHODS AND RESULTS
This is a retrospective analysis of the data of patients who were treated at our institution over a period of 10 years between January 2009 and December 2018 and had an eventual diagnosis of PC in the final histopathology diagnosis following surgery. A total of 15 cases of PC that were operated at our center were identified. All reports and data of the patients were retrieved from hospital medical records. Senior head and neck pathologists at our institute reviewed all blocks and slides of the patients included in this series. The patients have been under follow up for a period varying from 1 to 4 years with an average of 1.6 years. Demographic, etiologic, pathologic, and clinico-radiological presentation data were recorded and analyzed by IBM SPSS version 20.0.
Demographic details
The mean age at presentation was 48.60 years (SD 14.5 years) with a range between 22 and 73 years. There was no significant male to female predilection, with 53.3% of patients being male.
Clinical presentation and radiological features
The most common presentation was related to the musculoskeletal system in 60% of patients [Table 2]. These included multiple osteolytic lesions, brown tumors, fractures, backaches, and joint pains. Patients presented with neck swelling in 13% cases and abdominal symptoms in 6.7%. 20% of the patients were asymptomatic, with incidental detection during evaluation for other unrelated pathologies. Also, in one of these cases, preoperative ultrasound features of the parathyroid were suspicious of PC with findings of exophytic component, ill-defined margins with the thyroid gland, central and peripheral vascularity, and micro-calcifications. There are similar studies in the literature which mention the possibility of differentiating PC from adenoma based on preoperative ultrasonography.[13]
Table 2.
Clinical and biochemical findings along with surgery and recurrence details
| Age | Sex | Presenting complaints | Pre Op PTH | Pre Op Ca | Imaging localization | Pre-op suspicion | Surgery | Recurrence (if yes, time since surgery) |
|---|---|---|---|---|---|---|---|---|
| 40 | F | Neck swelling | 82.00 | 8.50 | Yes (USG, MIBI, PET CT) |
Parathyroid adenoma | Parathyroid excision + hemithyroidectomy | Yes (4.5 months) |
| 32 | M | Multiple osteolytic lesions, brown tumor, fractures | 1861.90 | 17.80 | Yes (USG, CT) |
Parathyroid carcinoma | Parathyroid excision + hemithyroidectomy | No |
| 73 | F | Incidental finding (after screening for osteoporosis) | 263.00 | 11.70 | Yes (MIBI, CT) |
Parathyroid adenoma | Parathyroid excision | No |
| 22 | F | Multiple osteolytic lesions, brown tumor | 910.00 | 13.33 | Yes (USG) |
Parathyroid adenoma | Parathyroid excision + hemithyroidectomy | No |
| 49 | M | Osteolytic lesions, pancreatic necrosis, nephrolithiasis | 1389.50 | 10.99 | Yes (MIBI) |
Parathyroid carcinoma | Parathyroid excision + hemithyroidectomy | Yes (6 months) |
| 46 | F | Osteolytic lesion | 1098.00 | 15.63 | Yes (CT, USG, MIBI) |
Parathyroid adenoma | Parathyroid excision | No |
| 25 | M | Abdominal pain/pancreatitis | 1072.00 | 17.80 | Yes (MIBI) |
Parathyroid adenoma | Parathyroid excision + hemithyroidectomy | No |
| 62 | M | Fracture, brown tumor | 826.00 | 15.90 | Yes (MIBI, PET CT) |
Parathyroid carcinoma | Parathyroid excision + hemithyroidectomy | No |
| 67 | M | Weakness, backache, anorexia | 850.00 | 12.40 | Yes (USG, MIBI, PET CT) |
Parathyroid adenoma | Parathyroid excision + hemithyroidectomy | No |
| 49 | M | Multiple osteolytic lesions, mandibular fracture, renal cysts | 839.00 | 12.10 | Yes (USG-ill-defined margins, microcalcifications), CT, MIBI |
Parathyroid carcinoma | Parathyroid excision + hemithyroidectomy | No |
| 60 | F | Joint pains, lethargy, nephrolithiasis | 365.00 | 12.20 | Yes (USG, MIBI) |
Parathyroid adenoma | Parathyroid excision + hemithyroidectomy | No |
| 45 | M | Neck swelling, backache | NA | 13.44 | Yes (MIBI) |
Parathyroid adenoma | Parathyroid excision + hemithyroidectomy | No |
| 55 | F | Fracture, Scalp swelling | NA | 9.20 | NA | Parathyroid Adenoma | Parathyroid excision + hemithyroidectomy | Yes (9 months) |
| 51 | F | Incidental finding (hypothyroid for 20 years, USG neck done) | 171.00 | NA | Yes (USG, MIBI) |
Parathyroid adenoma | Parathyroid excision + hemithyroidectomy | No |
| 53 | M | Incidental finding (regular health check-up-raised calcium) | 363.00 | 13.90 | Yes (MRI, MIBI) |
Parathyroid adenoma | Parathyroid excision | No |
M: Male, F: Female, USG: Ultrasonography, CT: Computed tomography, PET CT: Positron emission tomography with CT, MRI: Magnetic Resonance Imaging, MIBI: Technetium - 99m-Sestamibi scan
The mean preoperative serum calcium in our series was 13.51 mg/dL (SD 2.69). Our institutional normal range for serum calcium is between 8.6 and 10 mg/dL. Two of the patients (13.3%) had serum Ca more than 14 mg/dL. The mean preoperative PTH was 826.61 pg/mL (SD 507.24) in our series.
Histopathology
At our institute, the histopathological diagnosis of PC is based on Castleman criteria, which includes capsular invasion, vascular invasion, mitotic figures, fibrous bands, and trabecular architecture. Both capsular invasion and vascular invasion were detected in nine cases [Table 3]. In the rest of six cases, at least capsular or vascular invasion was present (three cases with capsular invasion alone and three cases with vascular invasion alone). In cases having only one of these two criteria, other histopathological features, as well as clinico-radiological findings, were also taken into consideration for diagnosis of PC. Thick fibrous bands were detected in only six cases, while increased mitosis was seen in only one case. Necrosis was noticed in two cases only and macro nucleoli in five cases. Extra parenchymal extension or extension into surrounding tissues was identified histologically in eight cases.
Table 3.
Histopathology findings of cases of parathyroid carcinoma in the present series
| Histology | No. of patients | Percentage |
|---|---|---|
| Capsular invasion | 12 | 80.00 |
| Vascular invasion | 12 | 80.00 |
| Lymphatic invasion | 7 | 46.66 |
| Trabecular architecture | 0 | 0.00 |
| Increased mitosis | 1 | 6.66 |
| Thick fibrous bands | 6 | 40.00 |
| Necrosis | 2 | 13.33 |
| Macronucleoli | 5 | 33.33 |
The primary tumor was found in the right inferior parathyroid glands in six patients (40%) and in the left inferior parathyroid gland in another six patients (40%). And therefore 80% (n = 12) of the cases had the tumor in the inferior parathyroid glands. Only two tumors were found in the right superior and none in the left superior parathyroid gland. The exact site of the primary tumor was not clear in one patient. The tumor size was available for 11 patients. The average tumor size was 2.47 cm (SD 0.88).
Overall survival and disease-free survival
In our series, the overall survival at 3 years was 92.31% (SE 7.3%, 95% CI 56.64–98.88%) [Figure 1]. The DFS at 3 years was 76.61% (SE 11.91%, 95% CI 43.33–91.86%) [Figure 2]. There were recurrences in 3 of the patients (20%) at time intervals of 4.5 months (distant metastasis to lungs, liver, and bones), 6 months (distant metastasis to liver and bones), and 9 months (distant bony metastasis) from the date of surgery. There was no case of locoregional recurrence. One of the patients, who had developed distant metastasis at 4.5 months, died during the study period at a time interval of 5.5 months from the date of surgery.
Figure 1.
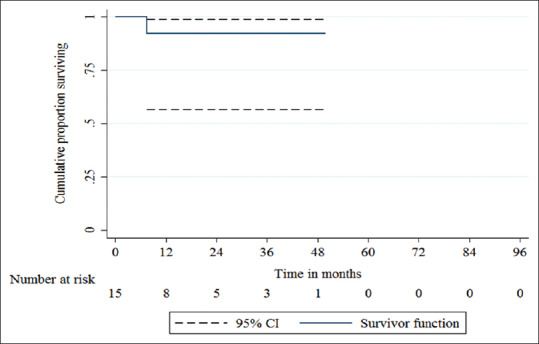
The overall survival (OS) at 3 years was 92.31% (SE 7.3%, 95% CI 56.64–98.88%)
Figure 2.
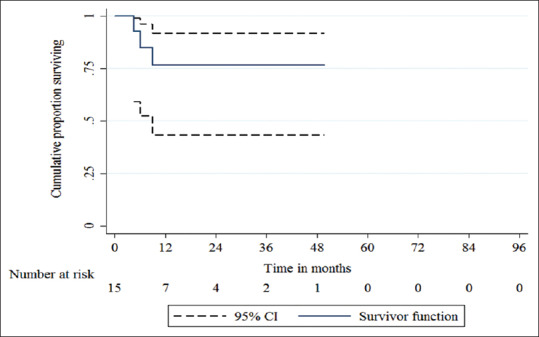
The disease-free survival (DFS) at 3 years was 76.61% (SE 11.91%, 95% CI 43.33–91.86%)
Radiotherapy
At our institute, we offer adjuvant RT to patients with vascular invasion or extra-parenchymal extension of disease. Six patients (40%) in our study received RT [Table 4]. One of the patients was advised for RT but the patient refused it. Out of these six patients, one developed recurrence in the form of distant metastasis. Among the nine patients who did not receive RT, two developed recurrences (distant metastasis in both patients).
Table 4.
Radiotherapy (RT)-related data
| RT status | Percentage of patients and outcomes | |
|---|---|---|
| Advised RT (n=7) | 46.7% | |
| Received RT (n=6) | 40% (one patient refused RT) | |
| Recurrence with RT (n=1)* | 16.7% (of patients receiving RT) | |
| Not received RT (n=9) | 60% | |
| Recurrence without RT (n=2)* | 22.2% (of non RT patients) | |
*Type of recurrence in all three patients was “distant metastasis”
Prognostic scores for recurrence based on MD Anderson Scoring system
MD Anderson prognostic scoring system for PC recurrence is based on three factors viz. age >65 years at presentation, preoperative hypercalcemia >15 mg/dL, and postoperative histopathological report suggestive of vascular invasion [Table 5]. The presence of zero, one, or more than one of these factors, stratifies the patient into low, intermediate, or high-risk groups for recurrence, respectively.[9] Based on the MD Anderson Prognostic scoring system for PC recurrence [Table 6], two patients were in the low-risk group for recurrence, nine in the moderate risk group, and four in the high-risk group. All patients in the high-risk group had 2 factors positive. There was no patient with all the three risk factors positive. There were a total of three cases of recurrence. All were stratified as the moderate-risk group. Of the four patients stratified as the high-risk group for recurrence by the scoring system, none developed recurrence during the study period.
Table 5.
MD Anderson prognostic criteria for parathyroid carcinoma recurrence
| Risk Group | Classification | No. of adverse factors present |
|---|---|---|
| 0 | Low risk | 0 |
| I | Moderate Risk | 1 |
| II | High Risk | 2 |
| III | High Risk | 3 |
Note - The adverse factors include vascular invasion, Age at presentation >65 years and serum calcium level >15 mg/dL
Table 6.
Findings based on MD Anderson Scoring system
| Age at presentation | Vascular invasion | Serum calcium (mg/dL) | Number of factors present | Risk stratification for recurrence | Received postoperative RT | Recurrence/type of recurrence (if present) |
|---|---|---|---|---|---|---|
| 45 | No | 13.44 | 0 | Low | No | No |
| 51 | Yes | NA | 1 | Moderate | No | No |
| 49 | Yes | 10.99 | 1 | Moderate | No | Yes (distant metastasis) |
| 46 | No | 15.63 | 1 | Moderate | No | No |
| 22 | Yes | 13.33 | 1 | Moderate | No | No |
| 73 | Yes | 11.70 | 2 | High | No | No |
| 32 | Yes | 17.80 | 2 | High | No | No |
| 49 | No | 12.10 | 0 | Low | No | No |
| 53 | Yes | 13.90 | 1 | Moderate | Yes | No |
| 55 | Yes | 9.20 | 1 | Moderate | No | Yes (distant metastasis) |
| 25 | Yes | 17.80 | 2 | High | Yes | No |
| 40 | Yes | 8.50 | 1 | Moderate | Yes | Yes (distant metastasis) |
| 67 | Yes | 12.40 | 2 | High | Yes | No |
| 62 | Yes | 15.90 | 2 | High | Yes | No |
| 60 | Yes | 12.20 | 1 | Moderate | Yes | No |
NA: Not available in records
DISCUSSION
PC is a rare endocrine malignancy requiring a high index of suspicion for making a diagnosis. This series represents our institution's experience with PC during the past 10 years.
Demographic details and presentation
The average age at the time of diagnosis in our case series was 48.6 years, which is in concordance with other case series [Table 7], where the most common age of diagnosis was the fifth decade.[2,14,15,16,17,18] There was no significant male to female predilection in the present series.
Table 7.
Comparison of a few published single institution experiences of parathyroid carcinoma
| Mayo Clinic 1992[16] | MD Anderson Cancer Center 2003[14] | Tata Memorial Hospital 2019 | |
|---|---|---|---|
| Period of review | 1920-1990 | 1980-2002 | 2009-2018 |
| No. of patients | 43 | 27 | 15 |
| Male/female | 21/22 | 16/11 | 8/7 |
| Mean age (years) | 54 | 46.7 | 48.6 |
| Mean Calcium | 14.6 | 13.4 | 13.51 |
| Mean PTH | 10.2 times the upper limit of normal | N/A | 826.61 pg/mL |
| Commonest presenting complaints | Musculoskeletal-91% | Constitutional symptoms (fatigue, weight loss, anorexia, memory deficit, paresthesias)-70% | Musculoskeletal-60% |
| Neck masses | 14 patients (45%) | 4 patients (15%) | 2 patients (13%) |
| Commonest histopathology finding | Capsular invasion (100%) | Fibrous bands (44%) | Capsular invasion (80%) vascular invasion (80%) |
| Death | 17/39 (44%) parathyroid carcinoma related 4/39 (9%) other causes |
5/27 (19%) parathyroid carcinoma related 3/27 (11%) other causes |
1/15 (6.67%) at 3 years parathyroid carcinoma related |
| Disease-free survival | 36% at 3 years | 68% at 5 years | 76.61% at 3 years |
| Overall survival | 69% at 5 years | 85% at 5 years | 92.31% at 3 years |
| Recurrence rates | 67% | 42% | 20% |
| Distant metastatic sites | Lung, mediastinum | Lung, bones, brain 4 patients (15%) |
Lung., liver, bones 3 patients (20%) |
| Radiotherapy | 6 patients for recurrent or metastatic disease 4 no response 1 no data 1 disease-free |
6 adjuvant, 5 disease-free 2 for recurrent disease, 1 no response, 1 prolonged response |
6 adjuvant 5 disease-free at 3 years 1 recurrence (distant) |
In India, PHPT is known to present with overt skeletal and renal manifestations.[6] In the western world, where asymptomatic hyperparathyroidism is more common, features such as skeletal and renal manifestation are more suggestive of PC.[7] Therefore, it becomes more difficult in our group of PHPT patients to suspect PC preoperatively. Our study has tried to focus on the preoperative clinico-radiological factors which suggested the possibility of PC in these cases.
Radiological findings
In addition to the clinical findings, certain features on imaging (USG— Figure 3), as seen in one of our cases, such as vascularity and micro-calcifications in a nodule outside thyroid near the lower pole could hint at a diagnosis of PC. Nam et al., in an earlier study, had also mentioned similar findings that certain features (namely heterogeneous echotexture, irregular shape, non-circumscribed margin, intra-nodular calcifications, and local invasion) on preoperative ultrasonography of the parathyroid, might be helpful in differentiation of PC from adenoma.[13] Further research in this aspect could possibly lead to the development of a reporting system similar to the thyroid imaging reporting and data system (TIRADS) for parathyroid glands in the future.
Figure 3.
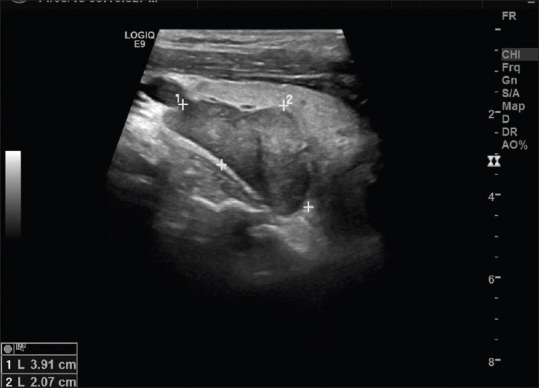
Ultrasonography (USG) image of preoperative suspicious parathyroid carcinoma showing a hypoechoic parathyroid image with ill-defined margins and micro-calcifications
Histopathology
Among the classical histopathological features of PC described by Schantz and Castleman,[8] we found the highest association with capsular and vascular invasion in up to 80% for both [Table 3]. Some other researchers have suggested the use of features such as necrosis and macronuclei as well for histopathologic diagnosis of PC.[19] Clayman et al. also mentioned a similar observation that classic pathologic features were not always present in all PCs.[20] In their series of PC, they noticed fibrous bands in 44% cases, mitoses in 40% cases, and vascular invasion in 37% cases. The capsular invasion was observed in 26% of patients, and trabecular architecture and lymphatic invasion were observed in only 11% of patients.
The predilection of PC for inferior parathyroid glands has been reported in many case series.[3,17,21] In our series, 80% (n = 12) of PC was found in inferior parathyroid glands. The average tumor size was 2.47 ± 0.88 cm. The MD Anderson case series reported an average tumor size of 2.5 cm while other series have reported an average tumor size up to 3.3 cm.[14,22,23] There was no correlation between tumor size and recurrence in our series as well as in other series reported in the literature.[24]
Radiotherapy
In our series, six patients were given adjuvant RT on the basis of the presence of extra glandular extension and vascular invasion. However, there was no significant statistical difference between a patient receiving and not receiving RT in terms of developing a recurrence. Literature is divided by the role of RT in the PC. Munson et al. reviewed a case series from the Mayo clinic, in which four patients received postoperative RT in PC.[25] At the end of the study, all four cases were disease-free. Another study from Princess Margaret hospital mentions six patients of PC who received RT and with a mean follow-up of 62.3 months were disease-free.[18] In the MD Anderson case series,[24] one out of six patients who received adjuvant RT developed recurrence similar to the recurrence seen in our series. At present, in our opinion, based on data from a very small number of cases available in the literature, it is not possible to suggest adjuvant RT as a routine in cases of PC.
OS and DFS
The DFS at 3 years was 76.61%, similar in comparison with the MD Anderson case series.[14] In our series, recurrence was seen in three patients. Among these, one patient died during the study period. The 5-year OS for MD Anderson case series was 85% while for the Mayo clinic case series, it was 76.9%. Median survival for our case series for the study period was 13.4 months and the range was 0.84–49.74 months.
MD Anderson Scoring system
Angelica et al., based on case series from MD Anderson, mention a prognostic scoring system using vascular invasion, age, and serum calcium level at initial parathyroidectomy to predict recurrence. As has been pointed out earlier, there is no relation of prognosis with the size of the tumor in PC, so tumor, node, and metastasis (TNM) type of staging system may not be used. There are no clear-cut criteria regarding the indications for adjuvant RT in PC. If a reliable prognostic scoring system to identify the chances of recurrence can be designed, it can help in making treatment decisions regarding which patients should be offered adjuvant RT and which patient requires close follow-up. MD Anderson prognostic scoring system attempts to do the same. We evaluated our case series based on this prognostic scoring system [Tables 5 and 6]. None of the patients in the low or the high-risk group developed recurrence after treatment completion. All the three patients who developed recurrence in our series were in the moderate-risk group. All three developed distant metastasis. Hence, suggesting that the prognostic scoring may not be applicable universally, or that it needs to be validated across different series.
Limitations
Despite a 10-year long review period, due to the rarity of PC, there were only 15 patients in this series. This brings about limitations in making any strong observations or conclusions based on the findings. Another limitation we find in our study is a shorter average follow-up period of 1.6 years. This has likely resulted due to the spread of the patients in this series over a large geographical area in the country and the distances they had to travel for follow-up in the hospital.
CONCLUSION
The presence of certain features on ultrasonography might suggest PC preoperatively. Among Castleman's criteria, capsular invasion and vascular invasion had a maximum association with PC in our series. At present, there is no evidence to routinely give adjuvant RT to all patients with PC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.AACE/AAES-position-statement, in Endocrine Practice. 2005:50–4. [Google Scholar]
- 2.Shane E, Bilezikian JP. Parathyroid carcinoma: A review of 62 patients. Endocr Rev. 1982;3:218–26. doi: 10.1210/edrv-3-2-218. [DOI] [PubMed] [Google Scholar]
- 3.Cohn K, Silverman M, Corrado J, Sedgewick C. Parathyroid carcinoma: The Lahey Clinic experience. Surgery. 1985;98:1095–100. [PubMed] [Google Scholar]
- 4.Heath H, 3rd, Hodgson SF, Kennedy MA. Primary hyperparathyroidism: Incidence, morbidity, and potential economic impact in a community. N Engl J Med. 1980;302:189–93. doi: 10.1056/NEJM198001243020402. [DOI] [PubMed] [Google Scholar]
- 5.Yeh MW, Ituarte PH, Zhou HC, Nishimoto S, Liu IL, Harari A, et al. Incidence and prevalence of primary hyperparathyroidism in a racially mixed population. J Clin Endocrinol Metab. 2013;98:1122–9. doi: 10.1210/jc.2012-4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishra SK, Agarwal G, Kar DK, Gupta SK, Mithal A, Rastad J. Unique clinical characteristics of primary hyperparathyroidism in India. Br J Surg. 2001;88:708–14. doi: 10.1046/j.0007-1323.2001.01775.x. [DOI] [PubMed] [Google Scholar]
- 7.Shane E, Bilezikian J. Parathyroid carcinoma. In: Williams CJ, Green MR, Raghaven D, editors. Textbook of Uncommon Cancer. New York: Wiley & Sons; 1987. [Google Scholar]
- 8.Schantz A, Castleman B. Parathyroid carcinoma. A study of 70 cases. Cancer. 1973;31:600–5. doi: 10.1002/1097-0142(197303)31:3<600::aid-cncr2820310316>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Silva-Figueroa AM, Hess KR, Williams MD, Clarke CN, Christakis I, Graham PH, et al. Prognostic scoring system to risk stratify parathyroid carcinoma. J Am Coll Surg. 2017 doi: 10.1016/j.jamcollsurg.2017.01.060. doi: 101016/jjamcollsurg 201701060. [DOI] [PubMed] [Google Scholar]
- 10.Lee PK, Jarosek SL, Virnig BA, Evasovich M, Tuttle TM. Trends in the incidence and treatment of parathyroid cancer in the United States. Cancer. 2007;109:1736–41. doi: 10.1002/cncr.22599. [DOI] [PubMed] [Google Scholar]
- 11.Schaapveld M, Jorna FH, Aben KK, Haak HR, Plukker JT, Links TP. Incidence and prognosis of parathyroid gland carcinoma: A population-based study in The Netherlands estimating the preoperative diagnosis. Am J Surg. 2011;202:590–7. doi: 10.1016/j.amjsurg.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 12.Brown S, O'Neill C, Suliburk J, Sidhu S, Sywak M, Gill A, et al. Parathyroid carcinoma: Increasing incidence and changing presentation. ANZ J Surg. 2011;81:528–32. doi: 10.1111/j.1445-2197.2010.05594.x. [DOI] [PubMed] [Google Scholar]
- 13.Nam M, Jeong HS, Shin JH. Differentiation of parathyroid carcinoma and adenoma by preoperative ultrasonography. Acta Radiol. 2017;58:670–5. doi: 10.1177/0284185116666418. [DOI] [PubMed] [Google Scholar]
- 14.Busaidy NL, Jimenez C, Habra MA, Schultz PN, El-Naggar AK, Clayman GL, et al. Parathyroid carcinoma: A 22-year experience. Head Neck. 2004;26:716–26. doi: 10.1002/hed.20049. [DOI] [PubMed] [Google Scholar]
- 15.Wang O, Xing X, Meng X. Comparison of clinical characteristics in primary hyperparathyroidism among different pathologic types. Chinese J Practical Intern Med. 2006 [Google Scholar]
- 16.Wynne AG, van Heerden J, Carney JA, Fitzpatrick LA. Fitzpatrick, Parathyroid carcinoma: Clinical and pathologic features in 43 patients. Medicine (Baltimore) 1992;71:197–205. [PubMed] [Google Scholar]
- 17.Holmes EC, Morton DL, Ketcham AS. Parathyroid carcinoma: A collective review. Ann Surg. 1969;169:631–40. doi: 10.1097/00000658-196904000-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow E, Tsang RW, Brierley JD, Filice S. Parathyroid carcinoma—The princess margaret hospital experience. Int J Radiat Oncol Biol Phys. 1998;41:569–72. doi: 10.1016/s0360-3016(98)00098-4. [DOI] [PubMed] [Google Scholar]
- 19.Kameyama K, DeLellis RA, Lloyd RV, Kakudo K, Takami HE. Parathyroid carcinomas: Can clinical outcomes for parathyroid carcinomas be determined by histologic evaluation alone? Endocr Pathol. 2002;13:135–9. doi: 10.1385/ep:13:2:135. [DOI] [PubMed] [Google Scholar]
- 20.Clayman GL, Gonzalez HE, El-Naggar A, Vassilopoulou-Sellin R. Parathyroid carcinoma: Evaluation and interdisciplinary management. Cancer. 2004;100:900–5. doi: 10.1002/cncr.20089. [DOI] [PubMed] [Google Scholar]
- 21.Flye MW, Brennan MF. Surgical resection of metastatic parathyroid carcinoma. Ann Surg. 1981;193:425–35. doi: 10.1097/00000658-198104000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. Two hundred eighty-six cases of parathyroid carcinoma treated in the US between 1985–1995: A National Cancer Data Base Report. Cancer. 1999;86:538–44. doi: 10.1002/(sici)1097-0142(19990801)86:3<538::aid-cncr25>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 23.Kleinpeter KP, Lovato JF, Clark PB, Wooldridge T, Norman ES, Bergman S, et al. Is parathyroid carcinoma indeed a lethal disease? Ann Surg Oncol. 2005;12:260–6. doi: 10.1245/ASO.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 24.Owen RP, Silver CE, Pellitteri PK, Shaha AR, Devaney KO, Werner JA, et al. Parathyroid carcinoma: A review. Head Neck. 2011;33:429–36. doi: 10.1002/hed.21376. [DOI] [PubMed] [Google Scholar]
- 25.Munson ND, Foote RL, Northcutt RC, Tiegs RD, Fitzpatrick LA, Grant CS, et al. Parathyroid carcinoma: Is there a role for adjuvant radiation therapy? Cancer. 2003;98:2378–84. doi: 10.1002/cncr.11819. [DOI] [PubMed] [Google Scholar]


