Abstract
With the increasing demand to provide more detailed quality attributes, more sophisticated glycan analysis tools are highly desirable for biopharmaceutical manufacturing. Here, we performed an intact glycopeptide analysis method to simultaneously analyze the site-specific N- and O-glycan profiles of the recombinant erythropoietin Fc (EPO-Fc) protein secreted from a Chinese hamster ovary glutamine synthetase stable cell line and compared the effects of two commercial culture media, EX-CELL (EX) and immediate advantage (IA) media, on the glycosylation profile of the target protein. EPO-Fc, containing the Fc region of immunoglobulin G1 (IgG1) fused to EPO, was harvested at Day 5 and 8 of a batch cell culture process followed by purification and N- and O-glycopeptide profiling. A mixed anion exchange chromatographic column was implemented to capture and enrich N-linked glycopeptides. Using intact glycopeptide characterization, the EPO-Fc was observed to maintain their individual EPO and Fc N-glycan characteristics in which the EPO region presented bi-, tri-, and tetra-branched N-glycan structures, while the Fc N-glycan displayed mostly biantennary glycans. EPO-Fc protein generated in EX medium produced more complex tetra-antennary N-glycans at each of the three EPO N-sites while IA medium resulted in a greater fraction of bi- and tri-antennary N-glycans at these same sites. Interestingly, the sialylation content decreased from sites 1–4 in both media while the fucosylation progressively increased with a maximum at the final IgG Fc site. Moreover, we observed that low amounts of Neu5Gc were detected and the content increased at the later sampling time in both EX and IA media. For O-glycopeptides, both media produced predominantly three structures, N1F1F0SOG0, N1H1F0S1G0, and N1H1F0S2G0, with lesser amounts of other structures. This intact glycopeptide method can decipher site-specific glycosylation profile and provide a more detailed characterization of N- and O-glycans present for enhanced understanding of the key product quality attributes such as media on recombinant proteins of biotechnology interest.
Keywords: CHO-GS cells, culture media, EPO-Fc protein, intact glycopeptide analysis, site-specific glycosylation
1 |. INTRODUCTION
The glycan profile of therapeutic proteins expressed in Chinese hamster ovary (CHO) cells is a critical quality attribute in biopharmaceutical manufacturing because more than half of the biotherapeutic drugs in the market are glycoproteins and the majority are secreted from CHO cells (Lalonde & Durocher, 2017). As a result, CHO cells are currently the most prevalent platform for biotherapeutic production and monitoring and control of their glycan patterns is an important objective of the biotechnology industry since the glycosylation patterns on therapeutic proteins can sometimes affect their bioactivity, immunogenicity, drug efficiency, and clinical safety (Hossler, Khattak, & Li, 2009). For example, the removal of core fucosylation can significantly improve the antibody-dependent cell-mediated cytotoxicity and increase antibody binding efficiency to the FcγRIIIa receptor (Yamane-Ohnuki & Satoh, 2009) and its binding to natural killer cells (Niwa et al., 2005). Elimination of terminal galactose on immunoglobulin G1 (IgG1) reduces its binding to C1q complex and impairs complement-dependent cytotoxicity activity (Hodoniczky, Zheng, & James, 2005; Reusch & Tejada, 2015; Thomann, Reckermann, Reusch, Prasser, & Tejada, 2016). Terminal sialylation on glycoproteins such as erythropoietin (EPO) is important for circulatory half-life in blood (Heffner, Wang, Hizal, Can, & Betenbaugh, 2018; Son, Jeong, Park, & Kim, 2011). The existence of N-glycolylneuraminic acid (Neu5Gc) in the final drug product represents a potential immunogenic chemical because human cells do not typically generate Neu5Gc due to a genetic mutation of the CMP-Neu5Ac hydroxylase (CMAH) gene (Irie, Koyama, Kozutsumi, Kawasaki, & Suzuki, 1998; Peri, Kulkarni, Feyertag, Berninsone, & Alvarez-Ponce, 2018). Therefore, the detailed characterization and control of the glycosylation profile of therapeutic proteins are pivotal to ensure the efficacy and safety of final drug products.
According to recent regulatory documents (Fournier, 2015), not only the glycan composition, including mannosylation, galactosylation, fucosylation, and sialylation, but also the glycan branch distribution and even the glycosylation site information can be useful and should be well characterized if possible. Indeed, the control and monitoring of the glycan profile of therapeutic drugs to ensure lot-to-lot consistency and accuracy is a continual challenge for manufacturers. Given the nontemplate driven nature of posttranslational glycosylation processes, various parameters intracellularly and extracellularly can affect the final glycoforms on glycoproteins in mammalian cells. The glycosylation process initiates in the lumen of the endoplasmic reticulum followed by elongation and completion in the Golgi apparatus (Spiro, 2002; Varki & Schauer, 2009). As an enzyme-directed, site-specific process, the activity and availability of the relevant glycosyltransferases, and the metabolite fluxes through nucleotide sugar synthesis and transportation pathways are critical to the final glycan profile of recombinant proteins (Chung, Majewska, Wang, Paul, & Betenbaugh, 2017). Numerous process effects, such as the composition and nutrients in the culture media, harvest time, culture temperature, pH, CO2, and dissolved oxygen level can also affect the final glycoforms of therapeutic proteins (Hossler et al., 2009). Among all the variations, the culture media composition dictates the nutrients incorporated, which can directly affect the final glycan profile of recombinant proteins. For example, the feeding of uridine, manganese chloride, and galactose in the culture media can improve antibody galactosylation better than individual additions, and the extent of antibody galactosylation can be adjusted by changing their concentrations in the culture media (Gramer et al., 2011; Wang Chung, Chough, & Betenbaugh, 2018). Supplementation with 20–40 mM N-acetylmannosamine (ManNAc), an intermediate precursor in the sialyation pathway, can also increase the protein sialylation about 10–20% (Gu & Wang, 1998). Thus, the variety of glycosyltransferase types and contents, nutrient feedings, and possess parameters can lead to considerable diversity in the glycosylation of recombinant IgG and other therapeutic glycoprotein products. The glycans generated can further pose a daunting challenge for analytics processing (Zhang, Luo, & Zhang, 2016).
Advanced analytical techniques based on mass spectrometry have revolutionized the identification and characterization of glycans (Han & Costello, 2013; Yang, Chatterjee, & Cipollo, 2018). Current approaches for glycan analysis typically include the release of carbohydrates from glycoproteins (deglycosylation), either by enzymatic or chemical reactions (Han & Costello, 2013). For N-glycans, peptide:N-glycosidase F, which reacts between the innermost N-acetylglucosamine and asparagine residues, is currently the most effective enzyme to cleave most N-glycans. For O-glycans, so far no single enzyme was found to be able to remove all the types of O-glycans, but O-glycosidase can specifically remove core 1 and 3 O-glycans (Koutsioulis, Landry, & Guthrie, 2008). Chemical reaction, β elimination, is still the prevalent method for O-glycan release, which may pose low efficiency and high detection noise drawbacks (Mulagapati, Koppolu, & Raju, 2017). Derivatization with a fluorescent tag, such as 2-aminobenzamide and 2-aminobenzoic acid through reductive amination or glycan permethylation are effective approaches to increase the detection sensitivity and gain glycan structural information (Han & Costello, 2013; Zhang et al., 2016). However, a major challenge for released glycan analysis is that it cannot provide site-specific glycan information, and often requires multiple sample preparation steps, which may be time-consuming.
A complementary approach for glycan analysis is intact glycopeptide characterization. With glycans remaining attached to the protein sites, this strategy can elucidate the glycopeptide amino acid sequence, the glycosylation site information, and the glycan composition simultaneously (Mayampurath et al., 2014; Stadlmann, Pabst, Kolarich, Kunert, & Altmann, 2008). Due to the inherent complexities of intact glycopeptides, this analytical method faces several complications: (a) the glycopeptides can be suppressed by nonglycopeptides in the mass spectrometry because of their low ionization efficiency (Cao et al., 2016). Therefore, a glycopeptide enrichment step may help to enhance the mass spectrometry performance. (b) The commonly used collision-induced dissociation fragmentation method was reported to have difficulty in generating a diverse collection of peptide backbone fragments, which can make peptide sequence identification problematic (Cao et al., 2016). As an alternative approach, higher-energy C-trap dissociation (HCD) fragmentation, often equipped with orbitrap detection, represents a potentially powerful tool for generating high resolution tandem mass spectrometry (MS/MS) spectra. HCD can provide superior performance in representing low m/z glycan oxonium ions, which normally contain signal ions for glycopeptides (Cao et al., 2016). By supporting multiple cleavage events, HCD fragmentation can provide a wealth of peptide identifications (Cao et al., 2016; Frese et al., 2011; Jedrychowski et al., 2011).
Therefore, in this study, we applied an intact glycopeptide method to determine the site-specific glycosylation profiles for both the N- and O-glycans of a recombinant fusion protein EPO-Fc produced by CHO cells. Containing the well-studied Fc region of IgG1 fused to EPO, this fusion protein was stably expressed in a commercial CHO-glutamine synthetase (CHO-GS) cell line to test the intact glycopeptide analysis method. Furthermore, the impact of adding an anion-exchange chromatographic column to capture N-glycopeptides and the enhancement of the N-glycopeptide abundance was compared with performance without the step. To examine the role that culture media plays on glycopeptides and glycan structures generated, we evaluated two different media: EX-CELL (EX) medium and immediate advantage (IA) medium from Millipore Sigma-Aldrich and compared the effects of media difference on the EPO-Fc N- and O-glycan profiles using this intact glycopeptide method as illustrated in Figure 1 and the workflow in Figure S1. In particular, we were able to distinguish the differences in the glycan structures at each site as well as the levels of sialylation and fucosylation present at each site, including the three EPO and one Fc N-glycan sites. The glycopeptide analytical method was also used to identify the predominant O-glycans in the different media as well. These findings demonstrate the potential of intact glycopeptide analysis to provide a robust and detailed profiling of both N- and O-glycans at specific sites, which will allow biotechnologists to better understand and control the glycan compositions emerging from CHO cells and other production hosts in the coming decades.
FIGURE 1.
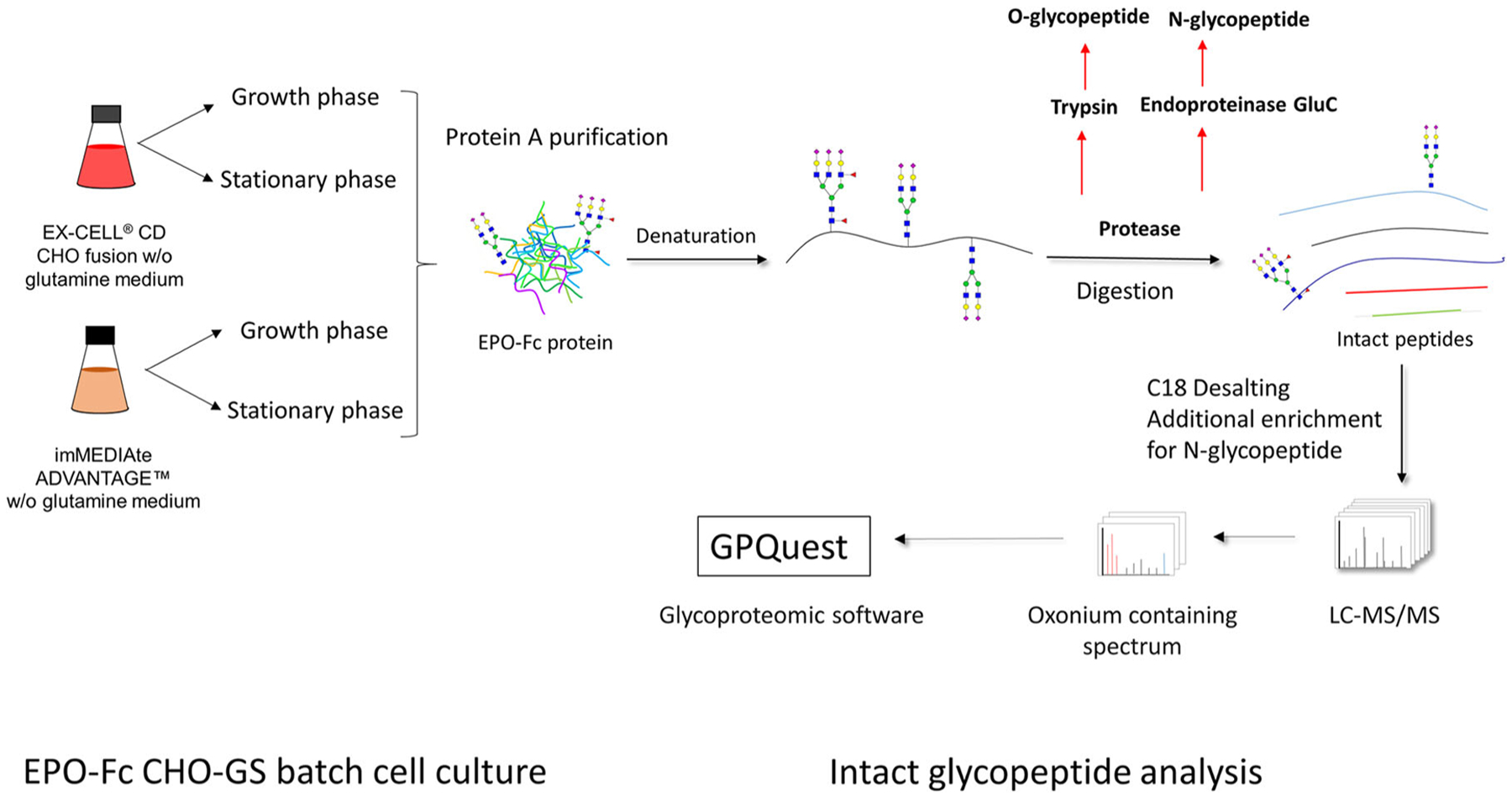
Illustration of intact glycopeptide characterization method applied in this study. CHO, Chinese hamster ovary; EPO, erythropoietin; GS, glutamine synthetase; LC-MS/MS, liquid chromatography with tandem mass spectrometry
2 |. MATERIALS AND METHODS
2.1 |. Cell culture
The recombinant EPO-Fc expressing CHO-GS cell line was graciously provided by Millipore-Sigma-Aldrich (Rockville, MD) as part of the AMBIC intact glycopeptide project. This cell line was cultured in both EX-CELL® CD CHO Fusion Medium (Catalog No. 14365C; Millipore-Sigma-Aldrich), and imMEDIAte Advantage Medium (Catalog No. 87093C; Millipore-Sigma-Aldrich) individually. These two media were abbreviated as EX and IA media respectively in this study. EX and IA media are commercial proprietary media graciously provided by Millipore Sigma-Aldrich. EX is a first-generation chemically defined cell culture medium. IA is a second-generation chemically defined medium with a more restricted raw material content focusing on cell growth and productivity. IA contains a streamlined composition so that customers can tailor the formulation later with various raw materials for increasing glycosylation complexity as desired. Cell culture was performed in a humidified 37°C incubator with 5% CO2 and 130 rpm shaking speed. Cells were subcultured every 3–4 days at a seeding density of 0.5 × 106 cells/ml media in 125 ml shake flasks with 30 ml media.
To monitor cell growth rates, recombinant EPO-Fc cells were seeded at 0.3 × 106 cells/ml in 30 ml culture EX and IA media separately. Three biological repeats were performed. Cell density was measured every 24 hr using a hemocytometer, and viability was determined by distinguishing live cells from dead cells using trypan blue staining (Thermo Fisher Scientific, Bremen, Germany). During the cell growth study, cell supernatants were collected every 24 hr and subject to metabolite analysis and EPO-Fc characterization using immunoblotting and Coomassie blue staining as described below. Metabolites (glucose and lactate) were measured by using an YSI 2700 D dual channel select biochemistry analyzer (YSI Life Sciences, Yellow Springs, OH).
2.2 |. Immunoblots and Coomassie blue staining
Equal volumes (40 μl) of daily supernatants from EX medium-cultured and IA medium-cultured EPO-Fc cells in the growth study were denatured and reduced with 4× sample buffer by boiling at 95°C for 5 min. The 4× sample buffer was made of 90% 4× Laemmli Sample Buffer (Bio-Rad, Hercules, CA) and 10% 2-mercaptoethanol (Millipore Sigma-Aldrich). After cooling, supernatant samples were fractionated on 10% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE), followed by transfer to a polyvinylidene fluoride membrane (Bio-Rad). Rabbit anti-human EPO (Abcam, Cambridge, MA) was used as the primary antibody in immunoblots. Horseradish peroxidase (HRP)-linked anti-rabbit IgG was used as secondary antibody for EPO detection (Cell Signaling Technology, Danvers, MA), as previously described (Yin et al., 2018). HRP-linked anti-human IgG from Abcam was used to detect Fc protein expression. The HRP signal was detected using Immun-Star Western Chemiluminescent Kit (Bio-Rad) with imaging performed on a Molecular Imager® ChemiDoc™ XRS (Bio-Rad) with Quantity One Software (Bio-Rad). For Coomassie blue staining, the purified EPO-Fc protein was subjected to SDS-PAGE and followed by staining in Coomassie blue solution (0.1% Coomassie blue R250 (Thermo Fisher Scientific), 10% acetic acid, 50% methanol, and 40% H2O) overnight and destaining in destaining solution (10% acetic acid, 50% methanol and 40% H2O) for 6 hr, replacing the solution every 2 hours.
2.3 |. EPO-Fc protein purification
The culture supernatant containing recombinant EPO-Fc protein was first filtered through a 0.22-μm-pore-size membrane, and then mixed with protein A agarose bead slurry (Vector Lab, Burlingame, CA) and incubated at room temperature for 3 hr on a rotator. Then the supernatant was loaded onto a column to retain the agarose beads. After three washes with phosphate buffered saline (PBS) buffer, the bound EPO-Fc was eluted with 0.1 M glycine, pH 2.7. The pH of eluate was neutralized by adding 1 M Tris-HCl, pH 9.0. The EPO-Fc eluate was subsequently dialyzed against PBS buffer using Amicon ultra-flow filter with molecular weight cut-off 10 kDa (Millipore). The final protein concentration was measured by bicinchoninic acid assay (Thermo Fisher Scientific). The purified EPO-Fc protein purity was assessed by 10% SDS-PAGE followed by Coomassie blue staining.
2.4 |. Enzymatic digestion of purified EPO-Fc protein
Two milligrams of purified EPO-Fc protein from EX medium and IA medium respectively was denatured in 8.0 M urea/1.0 M ammonium bicarbonate buffer, and reduced in 10 mM 1,4-dithiothreitol at 37°C for 1.0 hr. Proteins were alkylated in 10 mM iodoacetamide at room temperature for 30 min in the dark. Samples were diluted fivefold with nuclease-free water to decrease the concentration of urea. Endoproteinase GluC (New England BioLabs, Ipswich, MA) for N-glycopeptide or trypsin (New England BioLabs) for O-glycopetide (protein:enzyme = 50:1, w/w) were separately added to each sample to digest proteins overnight with shaking at 37°C. We chose Endoproteinase GluC and trypsin for N- and O-glycopeptide analysis, respectively, because (a) GluC can divide EPO-Fc protein into individual glycopeptides with only one glycan site remaining on each glycopeptide, while trypsin cannot separate the first two N-glycosites of EPO-Fc; (b) the O-glycopeptide after GluC digestion was too large to be ionized for routine MS detection while trypsin digestion can generate O-glycopeptide of EPO-Fc with appropriate length for MS detection. After digestion, samples were centrifuged at 13,000g for 15 min to remove precipitates, followed by desalting on a C18 cartridge (Waters Company, Milford, MA) according to the manufacturer’s instructions. The peptide samples were dried in a Speed-Vac (ThermoFisher Scientific, Pittsburgh, PA) and stored at −80°C before LC–MS/MS analysis.
2.5 |. Mixed anion exchange (MAX) column for N-glycopeptide enrichment
Oasis MAX is an anion-exchange and reversed-phase polymer column (Waters Company). The protocol for using MAX column for N-glycopeptide enrichment was developed previously by Yang et al. (2017). Briefly, after C18 desalting, peptides were eluted in 60% acetonitrile (ACN), 0.1% trifluoroacetic acid (TFA). Then the binding solution was adjusted to 95% ACN, 1% TFA for N-glycopeptide enrichment using Oasis MAX 1 cc vacuum cartridge (Waters Company). Before loading samples, MAX columns were first equilibrated in 1 ml of ACN for three times, then 1 ml 100 mM triethylammonium acetate for three times, 1 ml water for three times, and finally 1 ml 95% ACN, 1% TFA for three times. Next, the peptide samples in 95% ACN, 1% TFA solution were loaded and washed by 1 ml of 95% ACN, 1% TFA for three times. Finally, bound glycopeptides were eluted in 400 μl of 50% ACN, 0.1% TFA. Concentration of the eluted peptides were determined using Nanodrop spectrophotometer (ThermoFisher Scientific) to measure 280 nm absorbance for tryptophan and tyrosine. The peptide samples were dried in Speed-Vac and stored at −80°C before LC–MS/MS analysis. The component percentage shown in this study is based on the volume ratio (v/v) unless otherwise specified.
2.6 |. Nano LC–MS/MS analysis
The dried peptide sample was resuspended in 3% ACN and 0.1% formic acid (FA). The samples were analyzed on a Q-Extractive mass spectrometer (Thermo Fisher Scientific). The Nano LC-MS/MS parameters were described previously in Yang et al. (2018). First, samples were separated by a Dionex Ultimate 3000 RSLC nano system (Thermo Fisher Scientific) with a PepMap RSLC C18 column (75 μm × 50 cm, 2 μm; Thermo Fisher Scientific) protected by an Acclaim PepMapC18 column (100 μm × 2 cm, 5 μm; Thermo Fisher Scientific). For intact glycopeptides, the mobile phase consisted of 0.1% FA and 3% ACN in water (A) and 0.1% FA, 90% ACN in water (B) using a gradient elution of 0–2% B, 1 min; 2–8% B, 9 min; 8–31% B, 80 min, 31–38% B, 20 min; 38–95% B, 5 min; 95% B, 10 min; 95–2% B, 4 min (Yang et al., 2018). The flow rate was kept at 0.3 μl/min. Data-dependent HCD MS/MS fragmentation was performed on the 12 most abundant ions. The spray voltage was set to 1.5 kV. Spectra MS1 (automatic gain control [AGC]) target 3 × 106 and maximum injection time 60 ms) were collected from 400 to 2,000 m/z at a resolution of 70,000 followed by data-dependent HCD MS/MS (at a resolution of 35,000, normalized collision energy 32%, intensity threshold of 4.2 × 104, AGC target 2 × 105 and maximum injection time 120 ms) of the 12 most abundant ions using an isolation window of 1.4 m/z (Yang et al., 2018). Charge state screening was enabled to reject singly charged ions and ions with more than eight charges. A dynamic exclusion time of 30 s was used to discriminate against previously selected ions. For tryptic peptides, 110 m/z was set as the fixed first mass in MS/MS fragmentation to include all oxonium ions of glycopeptides (Yang et al., 2018).
2.7 |. Data analysis
For intact glycopeptide identification, the MS data were searched using an in-house glycopeptide analysis software, GPQuest 3.0, based on GPQuest (Toghi Eshghi et al., 2015). Acquired MS/MS spectra were searched against the RefSeq Cricetulus griseus protein database downloaded from the NCBI website with last update June 01, 2016, which contained 46,402 proteins. The human erythropoietin fasta protein sequence and human IgG1 Fc region sequence were also added to the protein database. Database search parameters were set as follows: a maximum of two missed cleavage sites permitted from GluC or trypsin digestion, 10 ppm precursor mass tolerance, 0.06 Da fragment mass tolerance, carbamidomethylation (C, +57.0215 Da) as a fixed modification, and oxidization (M, +15.9949 Da) and deamidation (N, +0.98 Da) as dynamic modifications. Spectral counting was used to quantify the peptides identified from LC-MS/MS data. The parameters for mass tolerance of MS1 and MS2 were 10 and 20 ppm, respectively. The spectra containing an oxonium ion m/z 204.09 were chosen for further searching. Results were filtered based on the following criteria: (a) false discovery rate <1%, (b) ≥3 glycopeptide spectra matches (PSMs) for each peptide were required, (c) all the PSMs should be annotated by at least one N-linked glycans. All the annotated intact glycopeptides were presented in Figures S2.
3 |. RESULTS
3.1 |. Comparison of EX-CELL and IA media on EPO-Fc CHO cell growth and protein production
First, EPO-Fc expressing cell growth profiles cultured in EX-CELL (EX) medium and IA medium were obtained for three biological repeats. As shown in Figure 2a, EPO-Fc cells in IA medium exhibited an extended exponential growth phase (Days 2–6) compared with that in EX medium (Days 2–5) and as a result reached a higher cell density than those in EX-CELL medium. On Day 6, EPO-Fc cells in IA medium reached a maximum cell density around 13 million/ml on Day 7 while EX medium reached a maximum cell density of about 8.4 million/ml at Day 6. Furthermore, the viability in EX medium started to drop and declined gradually from Day 6 while the viability in IA medium started to drop on Day 8 but quickly declined to 0 by Day 9. In addition, as a complement to the growth and viability studies, we conducted metabolite analysis of lactate accumulation and glucose consumption (Figure 2c,d) to gain further insight into the relative impact of EX and IA media on CHO cell metabolism. As shown in Figure 2d, IA medium had higher glucose level (5,800 mg/L) than that (5,400 mg/L) in EX medium at Day 0. Cells in EX medium also exhibited a relatively slower glucose consumption and lactate accumulation rates compared with those in IA medium. By Day 8, EPO-Fc cells in IA medium had completely consumed all the glucose and taken up most of the lactate, which correlated with the drop of cell number and viability observed at Day 9 in IA medium. At Day 9, small amounts of glucose and lactate remained in EX medium, which is also consistent with the more gradual decline in cell density and viability.
FIGURE 2.
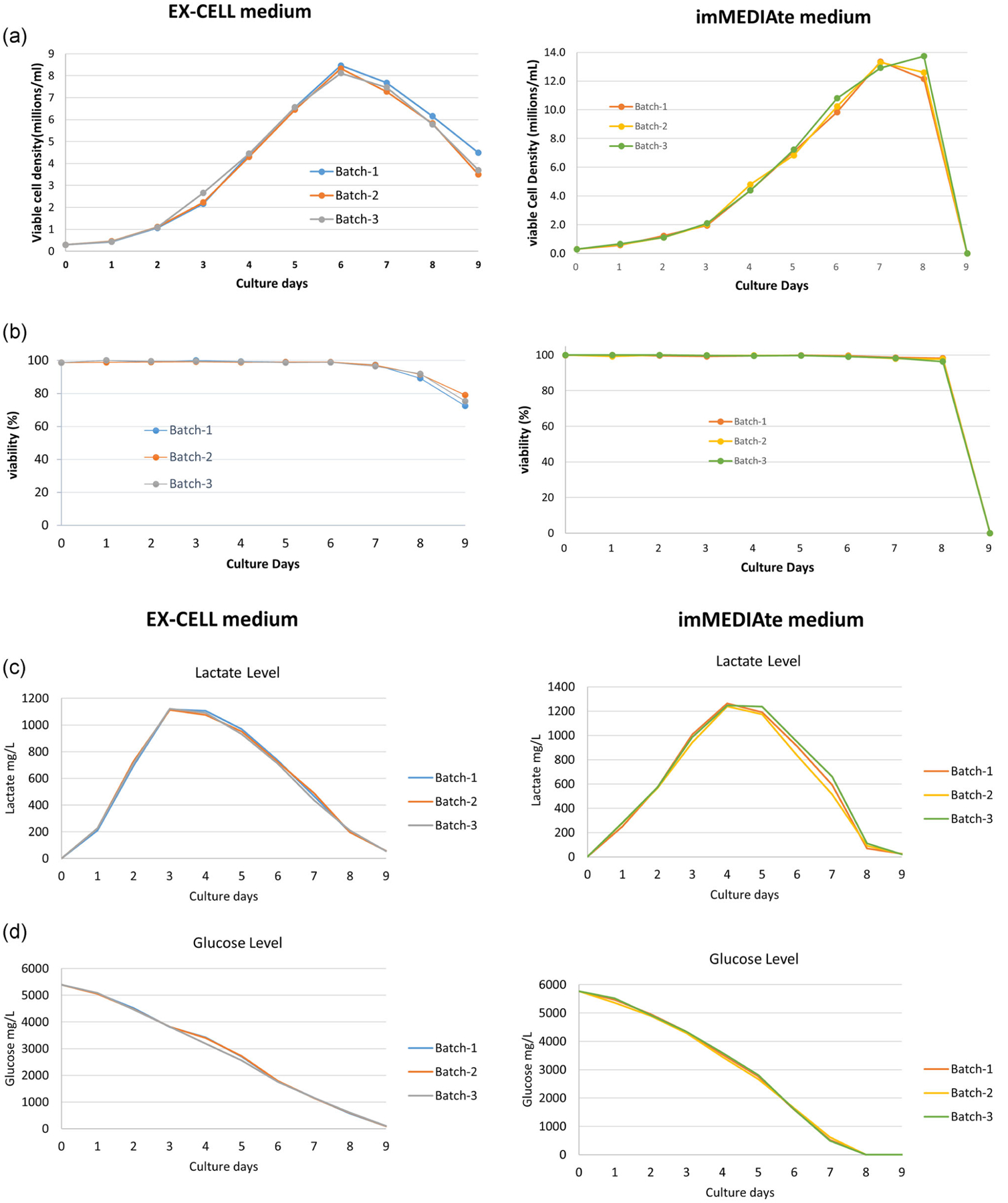
Growth study of EPO-Fc CHO-GS cells in EX-CELL (EX) and imMEDIAte ADVANTAGE media (left and right panels, respectively). (a) The viable cell density of EPO-Fc cells. (b) The viability of EPO-Fc cells. (c) The lactate level in the culture media. (d) The glucose level in the culture media. Please note that the EPO-Fc cell density declined on Day 8 in EX-CELL medium cell culture but the viability remained more than 90%, so we still recognized Day 8 as the stationary phase in the cell growth cycle. EPO, erythropoietin; GS, glutamine synthetase
Next, the abundance and quality of EPO-Fc protein secreted into EX and IA culture media were evaluated. Briefly, equal amounts of supernatant were harvested every day of the growth study and subjected to SDS-PAGE followed by Coomassie blue staining. Purified EPO-Fc protein was used as a positive control. As shown in Figure S3A, EPO-Fc protein was the most abundant protein in the supernatant from both EX and IA media cell culture during the growth cycle. In addition, anti-EPO and anti-IgG immunoblots were applied on the daily supernatant samples from the same EX and IA growth study, respectively. In Figure S3B, the intensity of EPO and IgG signal in their respective immunoblots increased gradually with increasing time in culture in both EX and IA media. However, we observed some degradation of the protein for the anti-IgG immunoblot from Days 6 to 8 in the IA medium cell culture but not in EX medium and no obvious degradation of EPO-Fc protein for the anti-EPO immunoblot results in both EX and IA media (Figure S3B). Therefore, some degradation of recombinant EPO-Fc protein during the later days for batch cell growth in IA basal medium may be attributed to the changed concentration of secreted proteases and reductases in IA medium or an increase of truncated or missed glycosylation on EPO-Fc protein. Alternatively, recombinant EPO-Fc protein maintained its integrity in the EX medium. This difference may have been due, in part, to the composition differences of the two media which can affect the cell state and secretory processes. In particular, the IA medium was designed with a streamlined composition which may have affected secretion of certain components at later days for this simple batch cell culture process.
3.2 |. N-glycopeptide enrichment for improving intact glycopeptide MS identification
Before the study of media influence on the glycan profile of EPO-Fc protein using intact glycopeptide analysis, to improve the EPO-Fc glycopeptide abundance for better MS capture and characterization, chromatographic columns can be applied to concentrate the glycopeptide quantities from digested EPO-Fc peptides. Due to the hydrophilic features of glycans or glycopeptides, strong anion-exchange columns such as the MAX column can be used for highly sensitive and selective extraction of acidic compounds, such as N-glycopeptides (Hagglund, Bunkenborg, Elortza, Jensen, & Roepstorff, 2004). In this study, we adopted the MAX column for EPO-Fc N-glycopeptide enrichment and conducted a comparison on the N-glycopeptide analysis for IA Day 6 culture supernatant with and without MAX column enrichment as shown in Figure 3a workflow. Briefly, conditioned media from Day 6 EPO-Fc cell culture in IA medium was harvested and subjected to protein A purification to obtain EPO-Fc protein. Then purified EPO-Fc protein from three biological repeats was digested using GluC endoproteinase individually followed by C18 column desalting of the digested peptides. Subsequently, some desalted peptides were subjected to the MAX column for N-glycopeptide enrichment. Finally, equal amounts(0.4 μg) of peptides from before and after MAX enrichment were subjected to LC-MS/MS analysis.
FIGURE 3.
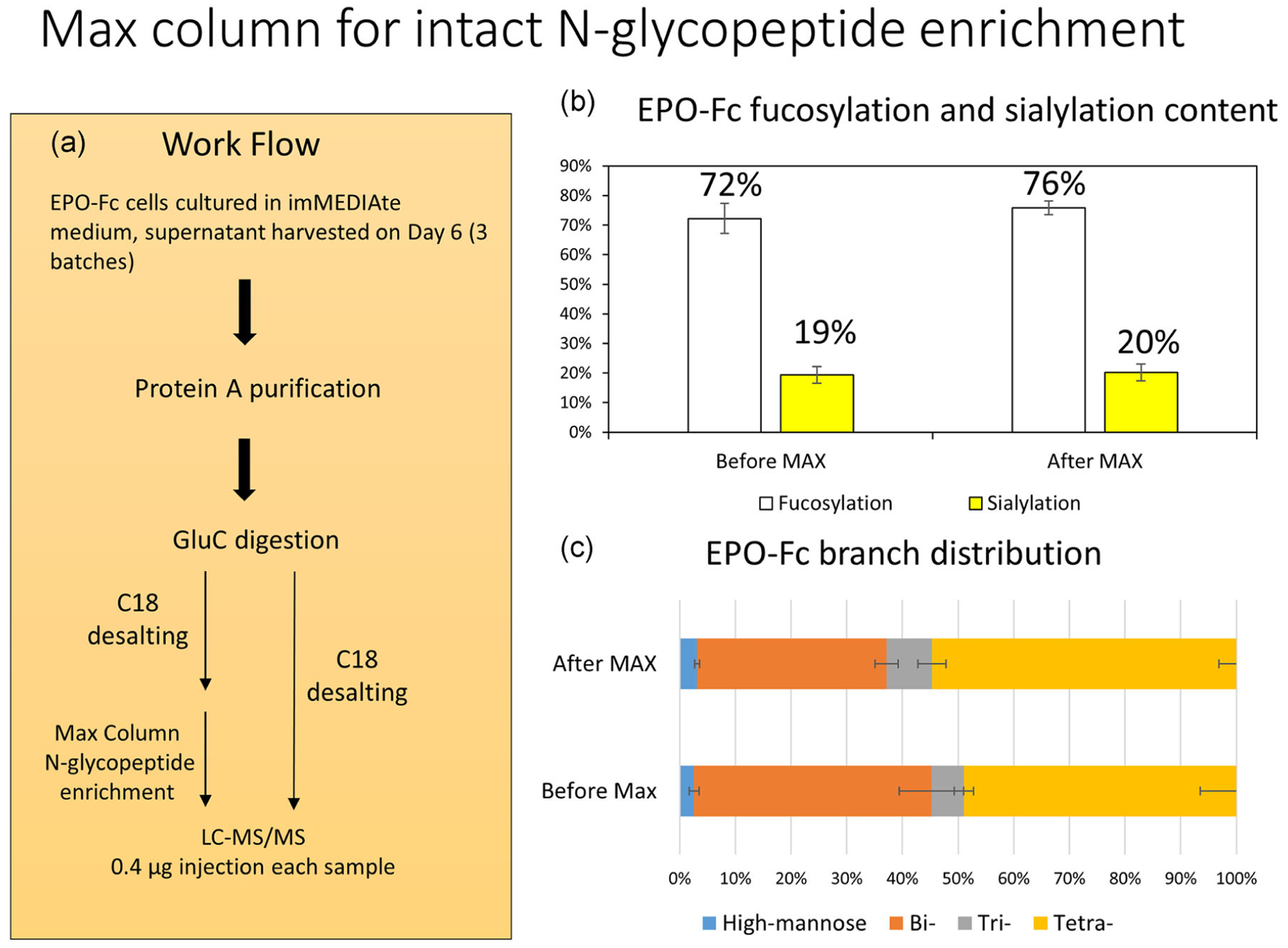
The application of MAX column for N-glycopeptide enrichment. (a) The workflow of MAX column comparison study. (b) The EPO-Fc fucosylation and sialylation content before and after MAX column enrichment. (c) The EPO-Fc N-glycan branch distribution before and after MAX enrichment. The p-values for high-mannose, bi-, tri-, and tetra-antennary glycan types before and after MAX column enrichment is 0.5, 0.12, 0.35, and 0.32, respectively. EPO, erythropoietin; MAX, mixed anion exchange
The characterization of N-glycopeptide mass spectrometric performance is tabulated in Table 1. On average, more glycopeptides and glycan types were detected using MAX column enrichment (51.67 ± 1.25 PSM and 24 ± 0.81 glycan types detected before MAX enrichment; 85.33 ± 4.78 PSM and 31 ± 0.81 glycan types detected after MAX enrichment). Importantly, the representative oxonium ion (m/z = 204.09) signal intensity was increased nearly 10-fold using MAX column to enrich the N-glycopeptides. Next, a fucosylation and sialylation comparison were performed as shown in Figure 3b. While the fucosylation content of EPO-FC protein (76%) was slightly higher than the sample without MAX column enrichment (72%), there was no significant fucosylation level difference between MAX column sample and the control sample (Figure 3b). Moreover, the sialylation content of the EPO-Fc protein also displayed similar content with or without MAX enrichment (Figure 3b). We also explored the N-glycan branch distribution of EPO-Fc protein with and without MAX enrichment. The MAX column enrichment increased the tri- and tetra-antennary content of EPO-Fc protein slightly while decreasing the bi- antennary content, but this result is not significantly different for the three biological repeats. The high-mannose type glycans present similar abundance with or without MAX column enrichment. Furthermore, with more N-glycopeptides and N-glycan types detected after MAX column enrichment, we acquired more consistent results among three biologic repeats compared with the control unenriched counterparts, which identified fewer N-glycopeptides and glycan types. Collectively, these results indicated that MAX column can efficiently enrich N-glycopeptides leading to a more in-depth profiling analysis of the glycans presenting on EPO-Fc glycoprotein.
TABLE 1.
The comparison of MS characterization of EPO-Fc protein with and without MAX column enrichment
| Batch-1 | Batch-2 | Batch-3 | Average | Standard deviation | |
|---|---|---|---|---|---|
| Control | |||||
| Total glycopeptide (PSM) | 50 | 53 | 52 | 51.67 | 1.247219 |
| Glycan types | 23 | 25 | 24 | 24 | 0.816497 |
| Oxonium ion (m/z = 204.09) intensity (glycan signal ion) | 3.99E6 | 3.42E6 | 3.63E6 | ||
| Max column | |||||
| Total glycopeptide (PSM) | 92 | 83 | 81 | 85.33 | 4.784233 |
| Glycan types | 32 | 31 | 30 | 31 | 0.816497 |
| Oxonium ion (m/z = 204.09) intensity (glycan signal ion) | 2.48E7 | 1.31E7 | 1.17E7 | ||
Abbreviations: EPO, erythropoietin; MS, mass spectrometry; MAX, mixed anion exchange; PSM, peptide-spectrum match.
3.3 |. Comparison of media influence on EPO-Fc N-glycans using intact N-glycopeptide characterization
After evaluating the performance of MAX column for N-glycopeptide enrichment, we next studied the EPO-Fc glycan profile in EX and IA media using the intact glycopeptide characterization method. The workflow is displayed in Figure S1. Briefly, conditioned media from Day 5 (see Figure 2 growth phase) and Day 8 (see Figure 2 stationary phase) in both EX and IA media cell culture were collected and recombinant EPO-Fc protein was obtained and purified from the culture broth. Next, equal amounts of EPO-Fc protein were subjected to GluC endoproteinase digestion followed by C18 column desalting and MAX column enrichment for N-glycopeptide preparation. Finally equal amounts (0.8 μg) of MAX-enriched peptides were subjected to nano LC-MS/MS analysis.
First, we assessed the size difference of purified EPO-Fc protein from EX and IA media culture. Equal amounts of the purified EPO-Fc protein from both Day 5 and 8 EX and IA media cell cultures were loaded onto an SDS-PAGE gel followed by Coomassie blue staining. The result shown in Figure 4a indicates that EX medium generated a more homogenous and slightly larger EPO molecule during both the growth and stationary phases compared with their corresponding counterparts from IA medium cell culture. To further explore the effect of media on EPO-Fc protein glycan characteristics, for EPO-Fc N-glycopeptide analysis, the distribution of glycan antennary was studied and is displayed at each of the four N-glycan sites in Figure 4b. There were more combined bi-/tri-antennary glycans present in IA medium than EX medium both in the growth phase and stationary phase at the first three N-glycan sites. In contrast, the EX medium tended to include a larger fraction of tetra-antennary structures at both Days 5 and 8. As expected, at the fourth N-glycan site, both Days 5 and 8 samples in both EX and IA media present solely bi-antennary structures, consistent with previous studies noting that the N-glycans on the antibody Fc region is predominantly a bi-antennary structure (Jefferis, 2009). Alternatively, the prevalent N-glycan types on recombinant EPO protein expressed in CHO cells are often tri- and tetra-branched structures (Son et al., 2011; Wang et al., 2019; Yin et al., 2015). Therefore, as a fusion protein, EPO-Fc maintained their individual EPO and Fc N-glycan characteristics, which may be attributed to their structural inherence. The N-glycopeptide analysis was in keeping with Coomassie blue gel result with the shown in Figure 4a. The EX media generated larger molecules including a large fraction of tetra-antennary glycans in Figure 4b, consistent with the more uniform and slightly larger protein band observed for the Coomassie gel result. Alternatively, the IA medium generated a more diverse collection of bi- and tri-antennary branched glycans which is in accordance with broader EPO-Fc band observed for the IA-derived samples on the Coomassie blue gel.
FIGURE 4.
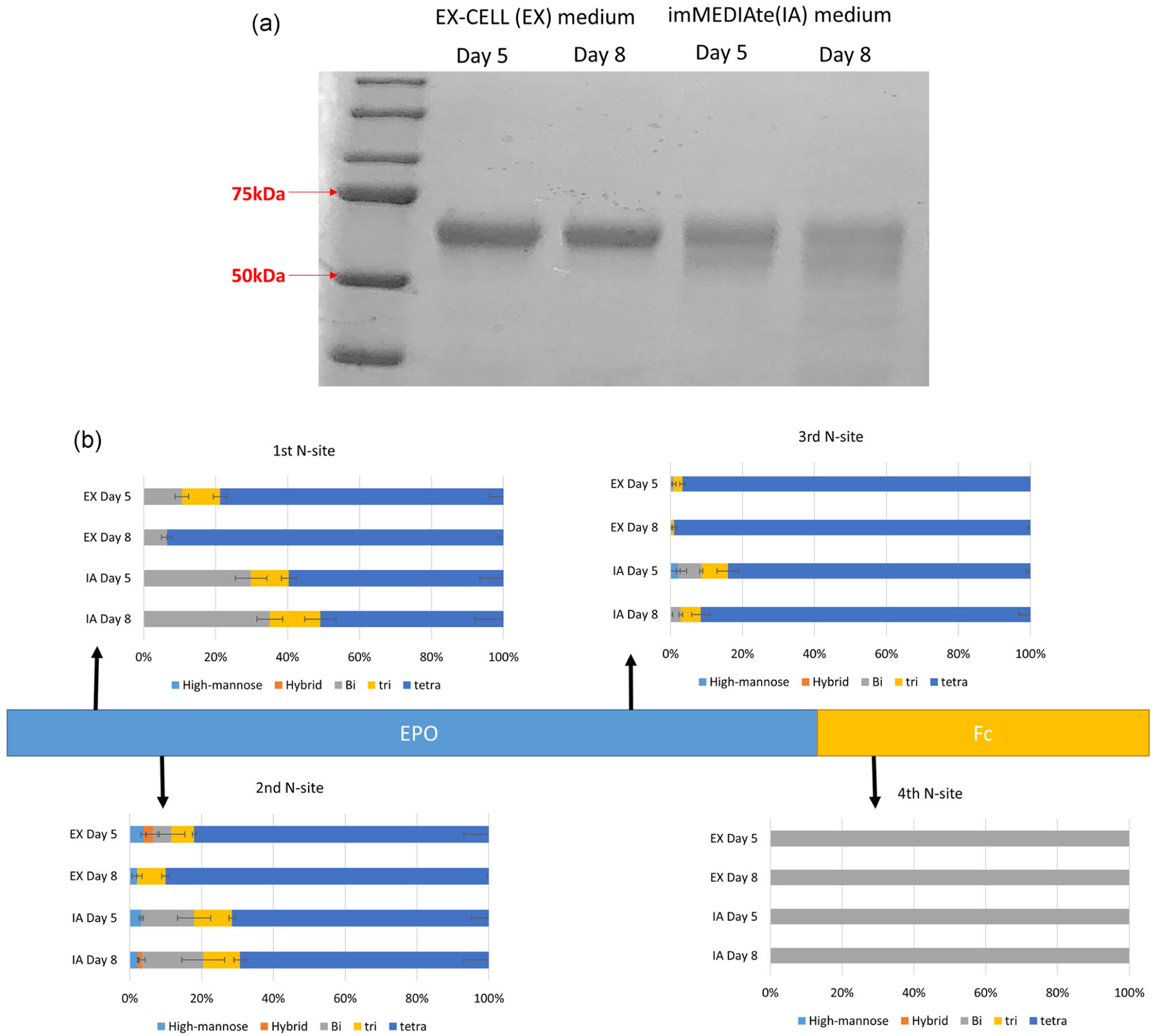
(a) Coomassie blue staining result of purified EPO-Fc protein from both exponential and stationary phases in EX and IA media culture, respectively. Equal amount of purified EPO-Fc protein was loaded. (b) The site-specific N-glycan antennary analysis of EPO-Fc protein. CHO-GS EPO-Fc expressing cells were cultured in EX and IA media individually and harvested at both exponential and stationary phases. CHO-GS, Chinese hamster ovary glutamine synthetase; EPO, erythropoietin; EX, EX-CELL; IA, immediate advantage
We further evaluated the sialylation content (N-acetylneuraminic acid [Neu5Ac] and Neu5Gc) of N-glycans on the EPO-Fc protein. The total Neu5Ac sialylation site occupancy of Days 5 and 8 samples in both EX and IA media cell culture is shown in Figure 5a. In all tested samples, the Neu5Ac content in growth phase was similar to that in stationary phase for EX medium, while the Neu5Ac level decreased with cell culture time in IA medium. Site-specific analysis of Neu5Ac content on the four N-glycan sites (first three of EPO N-glycan sites, the fourth of Fc N-glycan site) is shown in Figure 5b. The Neu5Ac sialylation content decreased gradually from the first to the fourth N-glycan sites, which is consistent with our analysis of wild-type EPO protein glycans from CHO cell cultures (Wang et al., 2019). In the current study, the fourth N-glycan site was fully unsialylated (Figure 5b). Similar to the total Neu5Ac sialylation trend in Figure 5a, the Neu5Ac sialylation level in the growth phase was consistent with that in stationary phase at the first three N-sites for EX culture, while the Neu5Ac sialylation level decreased along with cell culture time in IA medium for the first three N-sites. Further details about the sialic acid content at the specific sites is summarized in Figure S4. This analysis revealed that the most widely observed N-glycans include only one sialic acid (S1) for all tested samples at the first three N-glycan sites. N-glycans with two or three sialic acids (S2 and S3) represented a smaller fraction of the N-glycans at each site while N-glycans with four sialic acids (S4) were rare and only traceably detected at the first N-site on the Day 5 EX cultured and the second N-site on the Day 8 IA cultured EPO-Fc samples. Therefore, EX medium can maintain the EPO-Fc protein Neu5Ac sialylation content along with cell culture time while sialylation content decreased slightly with culture duration for the IA medium. On the other hand, the total and site-specific Neu5Gc sialylation analysis is presented in Figure 5c,d. The Neu5Gc sialylation analysis, shown in Figure 5c, revealed that low but detectable amounts of Neu5Gc were detected in all tested samples with Day 8 cultured EPO-Fc protein exhibiting more Neu5Gc content than Day 5 culture samples in both EX and IA media. For site-specific Neu5Gc analysis (Figure 5d), Neu5Gc content was highest at the first N-glycan site during stationary phase in both media. At second N-site, Neu5Gc was only detected at the stationary phase in IA medium. The third N-glycan site also contained detectable Neu5Gc content with very low levels as the second site and none detected at the fourth site.
FIGURE 5.
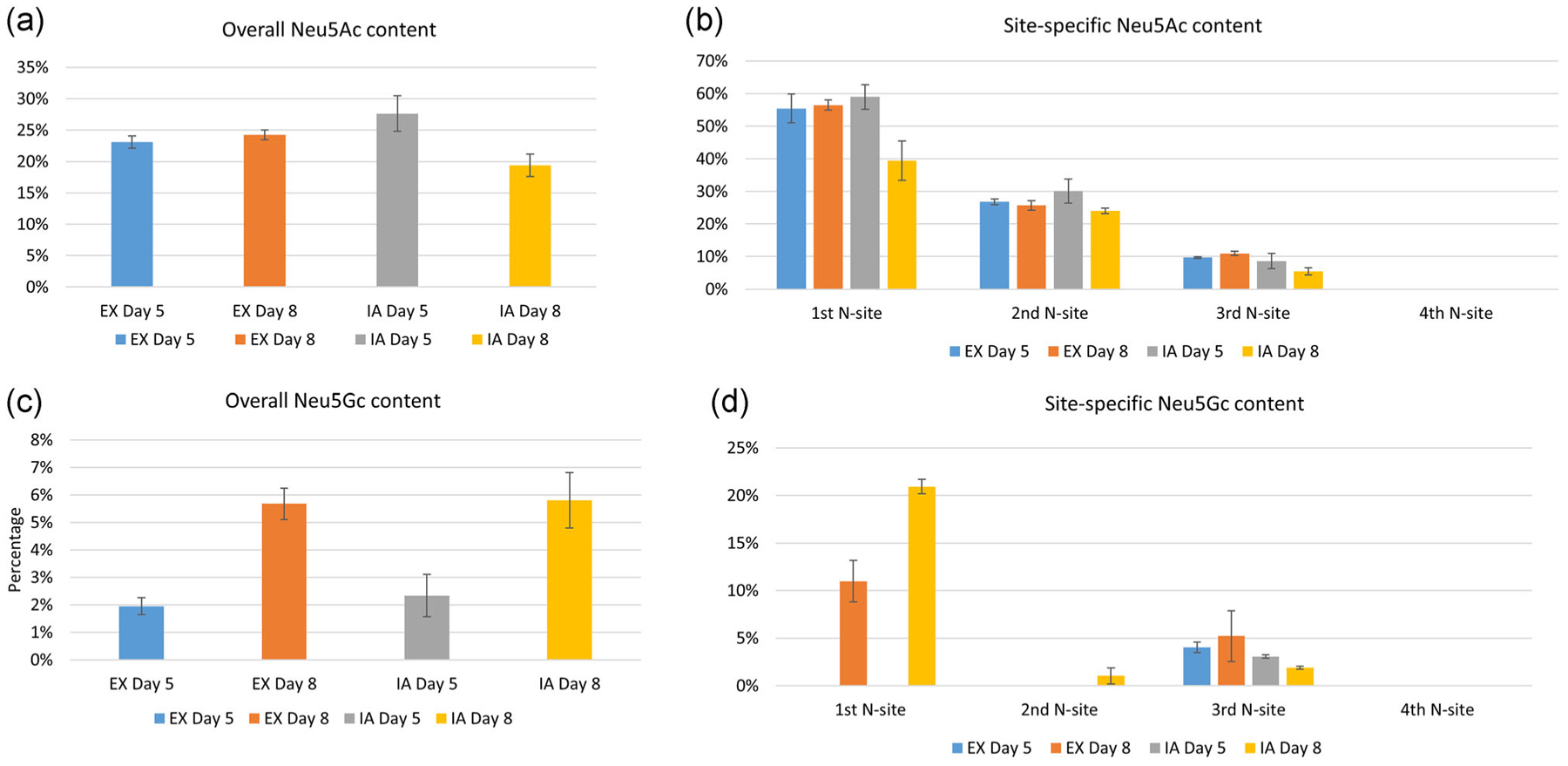
The N-glycan sialylation analysis of EPO-Fc protein. (a,c) The overall Neu5Ac and Neu5Gc content with four N-sites summed up together. (b,d) The site-specific Neu5Gc and Neu5Ac content. Neu5Gc, N-glycolylneuraminic acid; Neu5Ac, N-acetylneuraminic acid
The total and site-specific fucosylation analysis is shown in Figure 6a,b respectively. EPO-Fc from the IA cultured medium had a slightly higher fucosylation content than EX cultured samples during both growth and stationary phases (Figure 6a). For the site-specific analysis (Figure 6b), interestingly, in contrast to the sialylation analysis, the first N-glycan site had the lowest fucosylation occupancy compared with the other N-glycan sites. Furthermore, the fourth N-glycan site was fully fucosylated in all tested samples, and the fucosylation content also remained relatively similar over time at the second, third, and fourth N-glycan site for both EX and IA media. The culture media difference in fucosylation content mainly affected the first N-glycan site, in which the IA medium exhibited a higher fucosylation level compared with its counterparts in EX medium at same time points.
FIGURE 6.
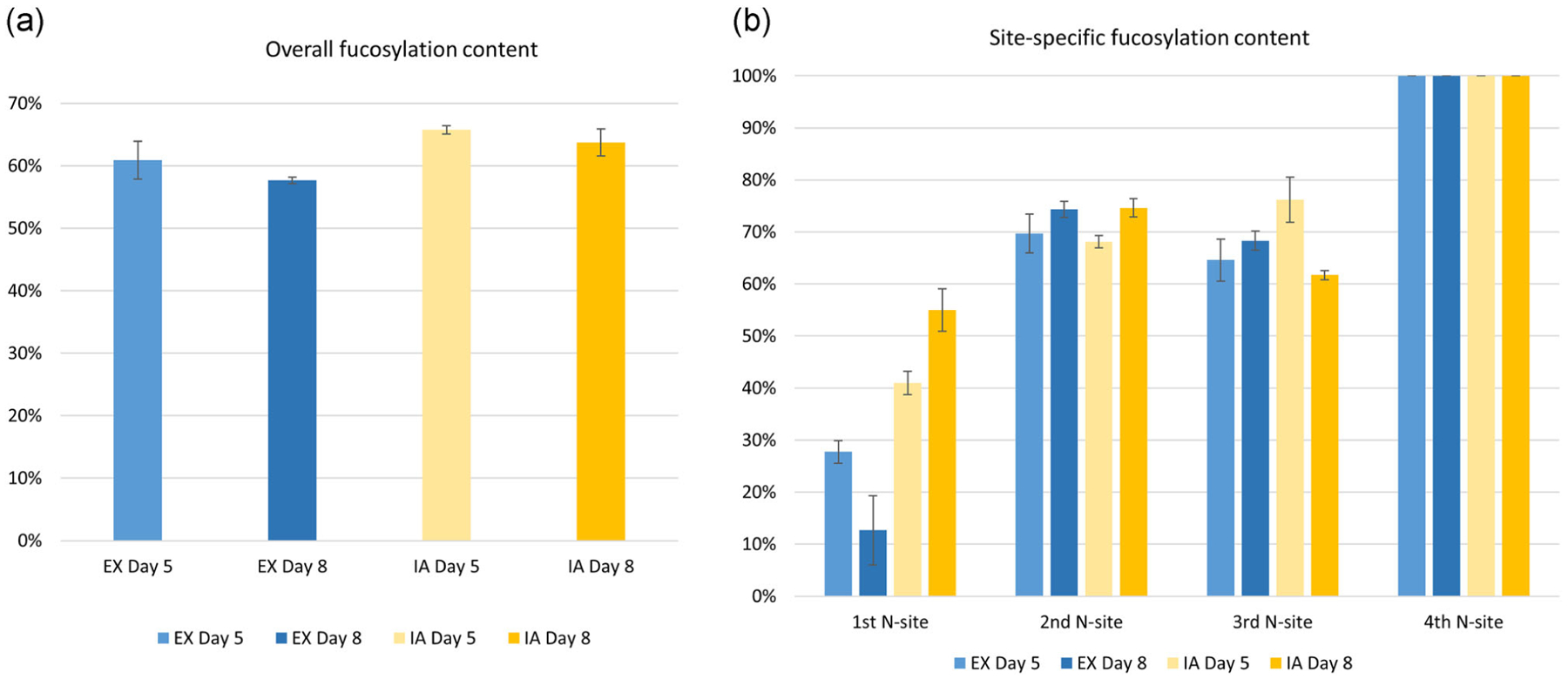
The N-glycan fucosylation analysis of EPO-Fc protein. (a) The overall fucosylation content of EPO-Fc protein with four N-sites summed up together. (b) The site-specific fucosylation content of EPO-Fc protein. EPO, erythropoietin
3.4 |. Comparison of different media impact on EPO-Fc O-glycan profile using intact O-glycopeptide characterization
Finally, we studied the O-glycan profile of EPO-Fc protein cultured in both EX and IA media. The workflow of O-glycopeptide analysis is also illustrated in Figure S1. After EPO-Fc protein purification, trypsin digestion, and C18 desalting, equal amounts (0.8 μg) of two biological O-glycopeptide repeats were subjected to nano LC-MS/MS analysis. The identified O-glycan are summarized in Table 2. In all tested samples, GalNAc-Gal, GalNAc-Gal-SA, and GalNAc-Gal-SA2 (N1F1F0SOG0, N1H1F0S1G0, and N1H1F0S2G0) represented the three major types of O-glycans identified on EPO-Fc protein. Moreover, more O-glycan types were identified in the EX medium in both the growth (seven types) and stationary phases (six types) compared with the types observed at their corresponding time points in IA medium (five types and four types for growth and stationary phases, respectively), indicating that EX medium results in a more diverse O-glycan profile on EPO-Fc protein than IA medium.
TABLE 2.
The O-glycan analysis of erythropoietin-Fc protein using O-glycopeptide characterization method
| O-Glycan types | EX-Day 5 (%) | EX-Day 8 (%) | IA-Day 5 (%) | IA-Day 8 (%) |
|---|---|---|---|---|
| N1H0F0S0G0 | 0 | 7 | 0 | 0 |
| N1H1F0S0G0 | 20 | 25 | 21 | 24 |
| N1H1F0S1G0 | 46 | 50 | 57 | 48 |
| N1H1F0S2G0 | 26 | 10 | 15 | 26 |
| N1H2F1S0G0 | 2 | 0 | 2 | 0 |
| N2H2F0S2G0 | 2 | 0 | 0 | 0 |
| N2H2F1S0G0 | 2 | 5 | 4 | 2 |
| N3H1F1S0G0 | 2 | 2 | 0 | 0 |
4 |. DISCUSSION
Intact glycopeptide profiling characterizing the site-specific glycans has emerged as an alternative and complementary glycan analysis tool in recent years. Its application includes biomarker discovery, disease diagnosis and biopharmaceutical product quality control (Bora de Oliveira, Spencer, Barton, & Agarwal, 2017; Kolarich, Jensen, Altmann, & Packer, 2012; Moh, Lin, Thaysen-Andersen, & Packer, 2016; Narimatsu et al., 2018). Here we adopted this glycoproteomic method for evaluating the effects of different media compositions on the glycan profile of a recombinant protein, EPO-Fc, secreted from CHO cells. Compared with the traditional released glycan analysis method, this approach can elucidate the glycopeptide sequence, glycan site information, and the glycan structural composition simultaneously (Mayampurath et al., 2014). Moreover, this approach requires a less extensive sample preparation process with a turnover time for results in <2 days. In this study, we applied two different media, EX-CELL (EX) medium and IA medium from Millipore-Sigma-Aldrich, and compared the effects of the media on the EPO-Fc N- and O-glycan profiles using this intact glycopeptide method. For N-glycosylation, the sialylation content decreased for the first through fourth N-glycan sites. Even as a fusion protein, EPO-Fc maintained the N-glycan characteristics that would be expected for the individual EPO (sites 1–3) and Fc (fourth site) elements. EPO N-glycans presented bi-, tri-, and tetra-branched glycan structures with various sialylation and fucosylation levels at each site, while the Fc N-glycan displayed mostly fully fucosylated, unsialylated bi-antennary N-glycans. For the O-glycans, there was a prominence of three main glycotypes (N1F1F0SOG0, N1H1F0S1G0, and N1H1F0S2G0) for both media compositions.
For glycopeptide sample preparation, an enrichment step can serve to increase the abundance of glycopeptides among all the digested peptides. For N-glycans, we evaluated the MAX column for N-glycopeptide enrichment and the result shows that more N-glycopeptides and more consistent N-glycan types were detected after MAX column enrichment. For O-glycans, a HyperSep retain AX (RAX) (Thermo Fisher Scientific) column enrichment method was tested (Yang et al., 2017) but not implemented due to low capture efficiency. There are two possible reasons for this phenomenon: (a) the O-glycan on EPO-Fc protein has generally low occupancy and less diversity (Tsuda, Kawanishi, Ueda, Masuda, & Sasaki, 1990). Indeed, using β-elimination method to release O-glycan, we only obtained three types (N1H0F0S0G0, F0N1H1S0G0, and N1H1F0S1G0) of O-glycan on EPO-Fc protein (data not shown), which is consistent with previous studies (Lauber, Koza, & Chambers, 2015) and (b) RAX affinity may have binding bias, which is specific to a certain type of glycan structures and may be inefficient for enriching O-glycan-rare samples (Cao et al., 2016).
We tested two different commercial culture media, EX and IA media from Millipore Sigma-Aldrich, and their influence on the complexity of glycan profiles of recombinant EPO-Fc protein secreted from a CHO-GS cell line. Coomassie blue profiling (Figure 4a) indicated that the N-glycans produced by cells cultured in the EX medium varied more in size and produced a broader distribution of EPO-Fc protein than that in the IA medium. Furthermore, the detailed glycopeptide antennary analysis (Figure 4b) based on glycopeptide spectra match (PSM) revealed a similar wider distribution of glycan types in the IA medium. While the EX medium tended to produce predominantly tetra-antennary N-glycans on the first, second, and third sites, CHO cells in IA cell culture produced more bi- and tri-antennary structures in addition to the tetra-antennary features at those same three sites. We also summarized the total N-glycan types identified on the four N-sites of EPO-Fc protein, which also revealed that more tetra-branched and fewer tri- and bi-branched glycan types were detected at both Days 5 and 8 in EX culture medium than those obtained in IA culture medium (Figure S5). Interestingly, O-glycopeptide analysis revealed that both media produced the highest relative amount of structures containing GalNAc-Gal, GalNAc-Gal-SA, and GalNAc-Gal-SA2 (N1H1F0S0G0, N1H1F0S1G0, and N1H1F0S2G0) but the other structures varied somewhat in the two media compositions. Collectively, our results indicate that IA medium produced more diverse N-glycopeptide profile while the EPO-Fc from EX medium tended to contain more tetra-antennary N-glycans. Previous studies by commercial vendors have indicated that the two media will produce different growth and glycan profiles for recombinant proteins generated from CHO cells, and CHO cells grown in the IA medium may produce a less complex glycosylation product than that in EX medium (personal conversation with Dr. Frank Swartzwelder from Millipore Sigma-Aldrich). Moreover, it is also worthwhile to note that the cell growth is enhanced in IA medium culture with higher cell densities, which may lead to less complete glycan modifications perhaps due to the differing allocation of resources or protein transport rates through the Golgi apparatus.
While Neu5Ac is the predominant sialylation type for recombinant glycoproteins in CHO cells, we still detected low amounts of Neu5Gc in all tested samples (Figure 5). Neu5Gc, as a side-product in CHO cell culture, should be limited in the final drug product if possible. Interestingly, from our intact glycopeptide analysis, the Neu5Gc amount increased significantly at later times (Day 8) compared with that in the earlier growth phase (Day 5) in both EX and IA media for EPO-Fc cell culture (Figure 5c). After CMP-Neu5Ac is synthesized and released from the nucleus into the cytosol, CMP-Neu5Ac can be converted into CMP-Neu5Gc with the assistance of CMAH enzyme (Wickramasinghe & Medrano, 2011). Then both CMP-Neu5Ac and CMP-Neu5Gc in the cytosol will be transported to the Golgi by the same transporter SLC35A1 and involved in the protein glycosylation process (Wickramasinghe & Medrano, 2011). The activity and availability of CMAH enzyme directly affect the incorporation of Neu5Gc into glycoproteins (Malykh, Shaw, & Schauer, 1998). We propose that the prolonged cell culture time may serve to change CMAH and NADH (a cofactor for CMAH) levels in CHO cells and as a result, the distribution of Neu5Ac and Neu5Gc metabolites intracellularly, which further impacts the Neu5Gc content on glycoproteins.
In summary, we have demonstrated a rapid glycopeptide analysis method can reveal site-specific glycan information on recombinant EPO-Fc protein secreted from CHO cells in different media compositions. Retaining glycans on the peptides enables users to simultaneously elucidate the glycan structure on the specific glycosylation sites and provide insights about how proteins are modified at different points along the polypeptide chain under different culture conditions. Using this intact glycopeptide characterization method, EX medium was revealed to produce a more complex tetra-antennary N-glycan profile than IA medium for EPO-Fc cell cultures. For N-glycosylation analysis, the sialylation content decreased with increasing N-glycan sites of EPO-Fc, which is opposite to the trend of fucosylation content at these same four N-glycan sites and consistent with the known profiles of the individual structural regions of the composite protein. EPO N-glycans from EPO-Fc protein presented bi-, tri-, and tetra-branched glycans structures with various sialylation and fucosylation levels at each site, while Fc N-glycan displayed mostly fully fucosylated, unsialylated bi-antennary glycans. O-glycan profiles indicated a predominance of the three major structures in both the EX and IA media and lesser amounts of other glycans. In total, intact glycopeptide characterization may become increasingly important as an analytical tool for biopharmaceutical manufacturing by producing protein therapeutics with well-defined physical properties.
Supplementary Material
ACKNOWLEDGMENT
This study was supported by Advanced Mammalian Biomanufacturing Innovation Center (AMBIC), which is an Industry-University Cooperative Research Center Program under US National Science Foundation (grant no. 1624684) and the National Science Foundation (grant no. CBET-1512265). The authors would like to acknowledge the support and contributions of Dr. Frank Swartzwelder and other AMBIC mentors as part of this effort. One of the industrial members of AMBIC is Millipore-Sigma, which provided media that was used in this study.
Footnotes
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interests.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- Bora de Oliveira K, Spencer D, Barton C, & Agarwal N (2017). Site-specific monitoring of N-Glycosylation profiles of a CTLA4-Fc-fusion protein from the secretory pathway to the extracellular environment. Biotechnology and Bioengineering, 114(7), 1550–1560. 10.1002/bit.26266 [DOI] [PubMed] [Google Scholar]
- Cao L, Qu Y, Zhang Z, Wang Z, Prytkova I, & Wu S (2016). Intact glycopeptide characterization using mass spectrometry. Expert Review of Proteomics, 13(5), 513–522. 10.1586/14789450.2016.1172965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung C-Y, Majewska NI, Wang Q, Paul JT, & Betenbaugh MJ (2017). SnapShot: N-glycosylation processing pathways across Kingdoms. Cell, 171(1), 258–258. 10.1016/j.cell.2017.09.014 [DOI] [PubMed] [Google Scholar]
- Fournier J (2015). A review of glycan analysis requirements. BioPharm International, 28(10), 32–37. [Google Scholar]
- Frese CK, Altelaar AF, Hennrich ML, Nolting D, Zeller M, Griep-Raming J, & Mohammed S (2011). Improved peptide identification by targeted fragmentation using CID, HCD and ETD on an LTQ-Orbitrap Velos. Journal of Proteome Research, 10(5), 2377–2388. 10.1021/pr1011729 [DOI] [PubMed] [Google Scholar]
- Gramer MJ, Eckblad JJ, Donahue R, Brown J, Shultz C, Vickerman K, & van Berkel PH (2011). Modulation of antibody galactosylation through feeding of uridine, manganese chloride, and galactose. Biotechnology and Bioengineering, 108(7), 1591–1602. 10.1002/bit.23075 [DOI] [PubMed] [Google Scholar]
- Gu X, & Wang DI (1998). Improvement of interferon-gamma sialylation in Chinese hamster ovary cell culture by feeding of N-acetylmannosamine. Biotechnology and Bioengineering, 58(6), 642–648. [PubMed] [Google Scholar]
- Hagglund P, Bunkenborg J, Elortza F, Jensen ON, & Roepstorff P (2004). A new strategy for identification of N-glycosylated proteins and unambiguous assignment of their glycosylation sites using HILIC enrichment and partial deglycosylation. Journal of Proteome Research, 3(3), 556–566. [DOI] [PubMed] [Google Scholar]
- Han L, & Costello CE (2013). Mass spectrometry of glycans. Biochemistry, 78(7), 710–720. 10.1134/S0006297913070031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner KM, Wang Q, Hizal DB, Can Ö, & Betenbaugh MJ (2018). Glycoengineering of mammalian expression systems on a cellular level, Advances in Biochemical Engineering/Biotechnology. Berlin, Heidelberg: Springer. [DOI] [PubMed] [Google Scholar]
- Hodoniczky J, Zheng YZ, & James DC (2005). Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnology Progress, 21(6), 1644–1652. 10.1021/bp050228w [DOI] [PubMed] [Google Scholar]
- Hossler P, Khattak SF, & Li ZJ (2009). Optimal and consistent protein glycosylation in mammalian cell culture. Glycobiology, 19(9), 936–949. 10.1093/glycob/cwp079 [DOI] [PubMed] [Google Scholar]
- Irie A, Koyama S, Kozutsumi Y, Kawasaki T, & Suzuki A (1998). The molecular basis for the absence of N-glycolylneuraminic acid in humans. Journal of Biological Chemistry, 273(25), 15866–15871. 10.1074/jbc.273.25.15866 [DOI] [PubMed] [Google Scholar]
- Jedrychowski MP, Huttlin EL, Haas W, Sowa ME, Rad R, & Gygi SP (2011). Evaluation of HCD- and CID-type fragmentation within their respective detection platforms for murine phosphoproteomics. Molecular & Cellular Proteomics, 10(12), M111 10.1074/mcp.M111.009910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis R (2009). Glycosylation as a strategy to improve antibody-based therapeutics. Nature Reviews. Drug Discovery, 8(3), 226–234. [DOI] [PubMed] [Google Scholar]
- Kolarich D, Jensen PH, Altmann F, & Packer NH (2012). Determination of site-specific glycan heterogeneity on glycoproteins. Nature Protocols, 7(7), 1285–1298. 10.1038/nprot.2012.062 [DOI] [PubMed] [Google Scholar]
- Koutsioulis D, Landry D, & Guthrie EP (2008). Novel endo-alpha-N-acetylgalactosaminidases with broader substrate specificity. Glycobiology, 18(10), 799–805. 10.1093/glycob/cwn069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde M-E, & Durocher Y (2017). Therapeutic glycoprotein production in mammalian cells. Journal of Biotechnology, 251, 128–140. 10.1016/j.jbiotec.2017.04.028 [DOI] [PubMed] [Google Scholar]
- Lauber MA, Koza SM, & Chambers EE (2015, August). Comprehensive Characterization of the N and O-Linked Glycosylation of a Recombinant Human EPO [Application Notes]. Retrieved from http://www.waters.com/waters/library.htm?locale=en_US&lid=134855745
- Malykh YN, Shaw L, & Schauer R (1998). The role of CMP-N-acetylneuraminic acid hydroxylase in determining the level of N-glycolylneuraminic acid in porcine tissues. Glycoconjugate Journal, 15(9), 885–893. [DOI] [PubMed] [Google Scholar]
- Mayampurath A, Yu C-Y, Song E, Balan J, Mechref Y, & Tang H (2014). Computational framework for identification of intact glycopeptides in complex samples. Analytical Chemistry, 86(1), 453–463. 10.1021/ac402338u [DOI] [PubMed] [Google Scholar]
- Moh ESX, Lin C-H, Thaysen-Andersen M, & Packer NH (2016). Site-specific N-glycosylation of recombinant pentameric and hexameric human IgM. Journal of the American Society for Mass Spectrometry, 27(7), 1143–1155. 10.1007/s13361-016-1378-0 [DOI] [PubMed] [Google Scholar]
- Mulagapati S, Koppolu V, & Raju TS (2017). Decoding of O-linked glycosylation by mass spectrometry. Biochemistry, 56(9), 1218–1226. 10.1021/acs.biochem.6b01244 [DOI] [PubMed] [Google Scholar]
- Narimatsu H, Kaji H, Vakhrushev SY, Clausen H, Zhang H, Noro E, & Sun Y (2018). Current technologies for complex glycoproteomics and their applications to biology/disease-driven glycoproteomics. Journal of Proteome Research, 17, 4097–4112. 10.1021/acs.jproteome.8b00515 [DOI] [PubMed] [Google Scholar]
- Niwa R, Sakurada M, Kobayashi Y, Uehara A, Matsushima K, Ueda R, & Shitara K (2005). Enhanced natural killer cell binding and activation by low-fucose IgG1 antibody results in potent antibody-dependent cellular cytotoxicity induction at lower antigen density. Clinical Cancer Research, 11(6), 2327–2336. 10.1158/1078-0432.ccr-04-2263 [DOI] [PubMed] [Google Scholar]
- Peri S, Kulkarni A, Feyertag F, Berninsone PM, & Alvarez-Ponce D (2018). Phylogenetic distribution of CMP-Neu5Ac hydroxylase (CMAH), the enzyme synthetizing the proinflammatory human xenoantigen Neu5Gc. Genome Biology and Evolution, 10(1), 207–219. 10.1093/gbe/evx251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusch D, & Tejada ML (2015). Fc glycans of therapeutic antibodies as critical quality attributes. Glycobiology, 25(12), 1325–1334. 10.1093/glycob/cwv065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YD, Jeong YT, Park SY, & Kim JH (2011). Enhanced sialylation of recombinant human erythropoietin in Chinese hamster ovary cells by combinatorial engineering of selected genes. Glycobiology, 21(8), 1019–1028. 10.1093/glycob/cwr034 [DOI] [PubMed] [Google Scholar]
- Spiro RG (2002). Protein glycosylation: Nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology, 12(4), 43R–56R. 10.1093/glycob/12.4.43R [DOI] [PubMed] [Google Scholar]
- Stadlmann J, Pabst M, Kolarich D, Kunert R, & Altmann F (2008). Analysis of immunoglobulin glycosylation by LC-ESI-MS of glycopeptides and oligosaccharides. Proteomics, 8(14), 2858–2871. 10.1002/pmic.200700968 [DOI] [PubMed] [Google Scholar]
- Thomann M, Reckermann K, Reusch D, Prasser J, & Tejada ML (2016). Fc-galactosylation modulates antibody-dependent cellular cytotoxicity of therapeutic antibodies. Molecular Immunology, 73, 69–75. 10.1016/j.molimm.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Tsuda E, Kawanishi G, Ueda M, Masuda S, & Sasaki R (1990). The role of carbohydrate in recombinant human erythropoietin. European Journal of Biochemistry, 188(2), 405–411. http://www.ncbi.nlm.nih.gov/pubmed/2156701 [DOI] [PubMed] [Google Scholar]
- Toghi Eshghi S, Shah P, Yang W, Li X, & Zhang H (2015). GPQuest: a spectral library matching algorithm for site-specific assignment of tandem mass spectra to intact N-glycopeptides. Analytical chemistry, 87(10), 5181–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varki A, & Schauer R (2009). Essentials of Glycobiology In Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, & Etzler ME (Eds.), Sialic Acids (2nd ed). New York: Cold Spring Harbor. [PubMed] [Google Scholar]
- Wang Q, Chung C, Chough S, & Betenbaugh MJ (2018). Antibody glycoengineering strategies in mammalian cells. Biotechnology and Bioengineering, 115, 1378–1393. 10.1002/bit.26567 [DOI] [PubMed] [Google Scholar]
- Wang Q, Chung C-Y, Yang W, Yang G, Chough S, Chen Y, & Zhang H (2019). Combining butyrated ManNAc with glycoengineered CHO cells improves EPO glycan quality and production. Biotechnology Journal, 14(4), 1800186 10.1002/biot.201800186 [DOI] [PubMed] [Google Scholar]
- Wickramasinghe S, & Medrano JF (2011). Primer on genes encoding enzymes in sialic acid metabolism in mammals. Biochimie, 93(10), 1641–1646. 10.1016/j.biochi.2011.06.002 [DOI] [PubMed] [Google Scholar]
- Yamane-Ohnuki N, & Satoh M (2009). Production of therapeutic antibodies with controlled fucosylation. mAbs, 1(3), 230–236. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2726589/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Hu Y, Sun S, Ouyang C, Yang W, Wang Q, & Zhang H (2018). Comprehensive glycoproteomic analysis of Chinese hamster ovary cells. Analytical Chemistry, 90(24), 14294–14302. 10.1021/acs.analchem.8b03520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Chatterjee S, & Cipollo J (2018). The glycoproteomics-MS for studying glycosylation in cardiac hypertrophy and heart failure. Proteomics: Clinical Applications, 12(5), e1700075 10.1002/prca.201700075 [DOI] [PubMed] [Google Scholar]
- Yang W, Shah P, Hu Y, Toghi Eshghi S, Sun S, Liu Y, & Zhang H (2017). Comparison of enrichment methods for intact N- and O-linked glycopeptides using strong anion exchange and hydrophilic interaction liquid chromatography. Analytical Chemistry, 89(21), 11193–11197. 10.1021/acs.analchem.7b03641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin B, Gao Y, Chung CY, Yang S, Blake E, Stuczynski MC, & Betenbaugh MJ (2015). Glycoengineering of Chinese hamster ovary cells for enhanced erythropoietin N-glycan branching and sialylation. Biotechnology and Bioengineering, 112(11), 2343–2351. [DOI] [PubMed] [Google Scholar]
- Yin B, Wang Q, Chung C-Y, Ren X, Bhattacharya R, Yarema KJ, & Betenbaugh MJ (2018). Butyrated ManNAc analog improves protein expression in Chinese hamster ovary cells. Biotechnology and Bioengineering, 115(6), 1531–1541. 10.1002/bit.26560 [DOI] [PubMed] [Google Scholar]
- Zhang L, Luo S, & Zhang B (2016). Glycan analysis of therapeutic glycoproteins. mAbs, 8(2), 205–215. 10.1080/19420862.2015.1117719 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


