Abstract
Background:
Mesenchymal stem cell (MSC)-based cell transplantation is an effective means of treating chronic liver injury, fibrosis and end-stage liver disease. However, extensive studies have found that only a small number of transplanted cells migrate to the site of injury or lesion, and repair efficacy is very limited.
Methods:
Bone marrow-derived MSCs (BM-MSCs) were generated that overexpressed the erythropoietin (EPO) gene using a lentivirus. Cell Counting Kit-8 was used to detect the viability of BM-MSCs after overexpressing EPO. Cell migration and apoptosis were verified using Boyden chamber and flow cytometry, respectively. Finally, the anti-fibrosis efficacy of EPO-MSCs was evaluated in vivo using immunohistochemical analysis.
Results:
EPO overexpression promoted cell viability and migration of BM-MSCs without inducing apoptosis, and EPO-MSC treatment significantly alleviated liver fibrosis in a carbon tetrachloride (CCl4) induced mouse liver fibrosis model.
Conclusion:
EPO-MSCs enhance anti-fibrotic efficacy, with higher cell viability and stronger migration ability compared with treatment with BM-MSCs only. These findings support improving the efficiency of MSCs transplantation as a potential therapeutic strategy for liver fibrosis.
Keywords: BM-MSCs, Erythropoietin, Migration, Anti-fibrosis efficacy, Liver fibrosis
Introduction
Viruses, ethanol, drugs and other sources can cause chronic liver injury. Long-term or repeated stimulation may also lead to liver cirrhosis and even liver failure [1]. Studies show that the common pathological basis of many chronic liver diseases is liver fibrosis [2]. Liver fibrosis is an intermediate process of transformation from a variety of chronic liver diseases to liver cirrhosis, which is mainly characterized by the activation of hepatic stellate cells (HSCs) and excessive deposition of extracellular matrix (ECM) [3, 4]. However, ideal drugs for the treatment of liver fibrosis are lacking.
Mesenchymal stem cells (MSCs) are nonhematopoietic cells that can be isolated from a spectrum of tissues such as bone marrow, adipose tissue, dental pulp, placenta, amniotic membrane and umbilical cord [5]. Increasing evidence from preclinical and clinical studies indicates that MSCs from different sources can repair damaged liver tissue, improve liver function and reduce liver fibrosis [6, 7]. Antifibrosis efficacy may be closely related to the migration and homing efficiency of MSCs. The local microenvironment also affects MSC quality. Numerous in vivo studies have found that only a small number of transplanted cells migrate to the injury or lesion site, and antifibrosis efficacy is modest [8]. Consequently, for chronic injury, especially liver fibrosis, understanding how to improve the directional migration ability of MSCs and increase the number of transplanted cells is crucial for improving antifibrosis efficacy.
EPO, a glycoprotein hormone that is mainly produced by the kidneys, promotes proliferation and differentiation of erythrocyte progenitor cells. EPO functions to protect kidneys. However, emerging evidence indicates that EPO also functions in neuroprotection [9], anti-inflammation [10], anti-oxidation [11] and apoptosis [12], as the erythropoietin receptor (EPOR) is distributed not only in kidneys, but also in other systems. Because of the distribution of EPOR on MSCs, EPO pretreatment improves MSC quality, which is beneficial for using MSCs to treat ulcers [13]. However, EPO has a short half-life, which makes achieve ideal MSCs quality after a single treatment difficult. In this study, we established MSCs that stably expressed the EPO gene using lentivirus infection. We investigated cell viability and migration of MSCs upon EPO challenge and evaluated the anti-fibrosis efficacy of EPO-MSCs. Our data provide a novel perspective for improving the efficiency of MSC-based cell therapy for liver fibrosis.
Materials and methods
Animal
All animal experiments were approved by the Ethics Committee of Guizhou Medical University. We used 27 6-week-old adult female C57BL/6 mice (18–20 g) from the Experimental Animal Center of Guizhou Medical University of China. Mice were housed in a temperature-controlled environment (22 ± 2 °C) under standard 12 h light/dark conditions and received food and water ad libitum.
Isolation of MSCs from bone marrow
Three 6-week-old adult female C57BL/6 mice were euthanized for MSC preparation. Bone marrow-derived MSCs (BM-MSCs) were isolated and expanded in vitro as previously described [14]. Cells were cultured in low Dulbecco’s modified Eagle’s medium (L-DMEM, Gibco, Gaithersburg, MD, USA) supplemented with 10% FBS (Gibco) and penicillin–streptomycin (100 U/ml; Gibco). BM-MSCs were dissociated with a 0.25% trypsin–EDTA solution (Sigma, St. Louis, MO, USA) when approximately 80–90% of cells were confluent and subcultured at a ratio of 1:2. BM-MSCs at passages 3–10 were used for experiments.
Flow cytometry analysis of BM-MSC marker expression
BM-MSCs were identified by analyzing expression of cell-surface markers using a BECKMAN FC500 MCL flow cytometer (BECKMAN COULTER, Brea, CA, USA), including cluster of differentiation (CD) CD34, CD45, CD90 and CD105 (all from Lincolin Park, NJ, USA). Assays were as described previously [15], and appropriate isotype antibodies were used as negative controls.
Establishment of BM-MSCs Stably overexpressing EPO gene
The protocol for establishing an overexpressing EPO lentivirus vector was reported previously [16]. After the lentivirus vector was constructed, viral packaging was carried out in HEK 293T (ATCC, ACS-4500) cells. Titers were measured using 50% tissue culture infective dose (TCID50) assays on HEK 293T cells as described previously [17].
Real-time PCR
Total RNA was extracted with TRIzol (Invitrogen, Carlsbad, CA, USA) and treated with gDNA Eraser (Dalian, China). CDNA was generated with PrimeScript RT Enzyme (Takara) using Oligo(dT) primer. Realtime PCR was by a Bio-Rad CFX96 system using TB Green Premix Ex Taq II (Takara). Specific primers for EPO were 5′-AGCCACCAGAGACCCTTCAG-3′ (forward) and 5′-GAGTGTTCGGAGTGGAGCAG-3′ (reverse), for EPOR were 5′-GGGCTCCGAAGAACTTCTGTG-3′ (forward) and 5′-TGACTTTCGTGACTCACCCTC-3′ (reverse), for TGF-β were 5′-CTTCAATACGTCAGACATTCGGG-3′ (forward) and 5′-GTAACGCCAGGAATTGTTGCTA-3′ (reverse), for IL-6 were 5′- CTGCAAGAGACTTCCATCCAG-3′ (forward) and 5′-AGTGGTATAGACAGGTCTGTTGG-3′ (reverse), for MMP-9 were 5′-GGACCCGAAGCGGACATTG-3′ (forward) and 5′-CGTCGTCGAAATGGGCATCT-3′ (reverse) and for β-actin were 5′-GGCTGTATTCCCCTCCATCG-3′ (forward) and 5′-CCAGTTGGTAACAATGCCATGT-3′ (reverse). Data were calculated with Bio-Rad CFX manager software v.2.1 by the ΔΔCT method [18] and expressed as relative quantities after β-actin normalization.
ELISA
BM-MSCs were resuspended at a density of 5 × 104 cells/mL and seeded in 6-well culture plates with 1.5 mL cell growth medium. The next day, cells were infected with the lentivirus for overexpressing EPO at multiplicity of infection (MOI) 10 for 2 h. Cells were washed with PBS (Gibco) and cultured in cell growth medium. At 48 h after infection, 200 μL culture medium supernatant was collected for ELISA assays according to the manufacturer’s instructions (Abcam). EPO concentrations were calculated according to a standard curve.
Cell viability
Cell viability was determined using Cell Counting Kit-8 (CCK8, Dojindo, Tokyo, Japan) assays. BM-MSCs at passage 3 were plated in 96-well plates (Corning, Corning, NY, USA) at 103 cells/well. After infecting with lentivirus for 24, 48 or 72 h, cell proliferation was measured with absorbance at 450 nm with CCK-8 according to the manufacturer’s instructions. Measurements were in triplicate for each experiment, and all experiments were repeated 3 times.
Cell migration
After 48 h infection with lentivirus or control, MSCs were starved with serum-free medium for 30 min. Cells were digested with 0.25% trypsin–EDTA and resuspended with F12/DMEM and density adjusted to 8 × 105 cells/ml. Transfilter migration of MSCs toward hepatocyte growth factor (HGF, Sigma) was studied using a 48-well modified Boyden chamber (Neuro Probe, Gaithersburg, MD, USA) as reported previously [19]. Experiments were performed in triplicate and repeated independently three times.
Apoptosis assay
To analyze the effect of EPO on apoptosis of BM-MSCs, 48 h after lentivirus infection, cells were collected and made into single-cell suspensions. Apoptosis staining was with Annexin V/PI kits (BD Biosciences) according to the manufacturer’s instructions, and analyzed by a BECKMAN FC500 MCL flow cytometer (BECKMAN COULTER).
Animal experiment
A total of 24 6-week-old adult female C57BL/6 mice were used for antifibrosis efficacy evaluation and randomly divided into four groups (sham, CCl4, CCl4 + NC-MSCs and CCl4 + EPO-MSCs). Among the liver fibrosis models, 18 mice were induced to have liver fibrosis by intraperitoneal (i.p.) administration of CCl4 (diluted at 1:4 in olive oil) (5 ml/kg body weight), twice a week for 5 weeks. Six mice were ip injected equally with olive oil alone as sham group. The treatment groups were ip injected with 1 × 106 lentivirus-infected BM-MSCs at 2 weeks after first CCl4 administration. Mice were euthanized for serum biochemistry and immunohistochemistry assays at 6 weeks after first CCl4 administration.
Western blot
Western blot analysis was performed as previously described (19). Antigen–antibody complexes were visualized by enhanced chemiluminescence (Biological Industries, Beit Haemek, Israel). Proteins of quite distinct molecular weight, Akt, ERK1/2 and Actin were separately probed by cutting the nitrocellulose membrane according to the protein molecular weight marker.
Serum biochemistry
Blood was collected from mouse ophthalmic arteries with a nonanticoagulant tube. Serum was obtained by centrifugation after sedimentation. Serum levels of ALT and AST were measured using standard enzymatic procedures according to the manufacturers’ instructions (Abcam, Cambridge, UK).
Immunocytochemistry
Liver specimens were fixed in 4% paraformaldehyde and embedded in paraffin blocks. After separation and rehydration, liver sections (5 μm) were incubated with anti-a-SMA, anti-collagen-I and anti-fibronectin (all diluted 1:200, from Servicebio, Wuhan, China). All samples were observed using an inverted microscope with a ×20 objective (Olympus, Tokyo, Japan) and areas of positive staining were quantified using NIH Image J software.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics 19 software. Differences between groups were analyzed using one-way analysis of variance (ANOVA). The Pearson correlation analysis was used to assess correlations. Two-sided p values < 0.05 were considered statistically significant.
Results
EPO overexpressing increases EPO secretion
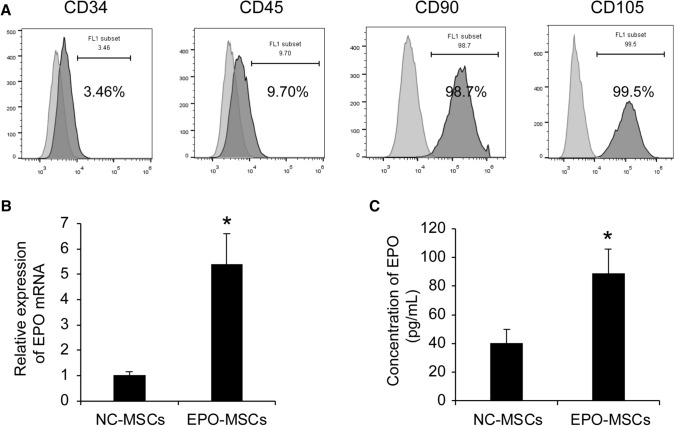
BM-MSCs were prepared from adult mice bone marrow and characteristics are in Fig. 1A. Consistent with other reports [20], CD90 and CD105, two putative markers for MSCs, were detected on cell surfaces, with no expression of hematopoietic markers CD34 and CD45. Infected BM-MSCs showed higher levels of EPO in transcription and secretion than the control group (Fig. 1B, C, p < 0.05). These data indicated that BM-MSCs infected by lentivirus had significantly increased EPO expression.
Fig. 1.
Detection of EPO expression. A Flow cytometry analysis of surface marker expression on BM-MSCs. B Quantitative PCR was performed to quantify EPO expression. Values are normalized with β-actin mRNA and expressed as a percentage of the control value, as the normalized expression of BM-MSCs infected with negative control (NC). Data represent mean ± SEM from three independent experiments. *p < 0.05. C ELISA assays for secretion of EPO in supernatants. Data are mean ± SEM from three independent experiments. *p < 0.05
EPO overexpression promotes BM-MSC viability and migration
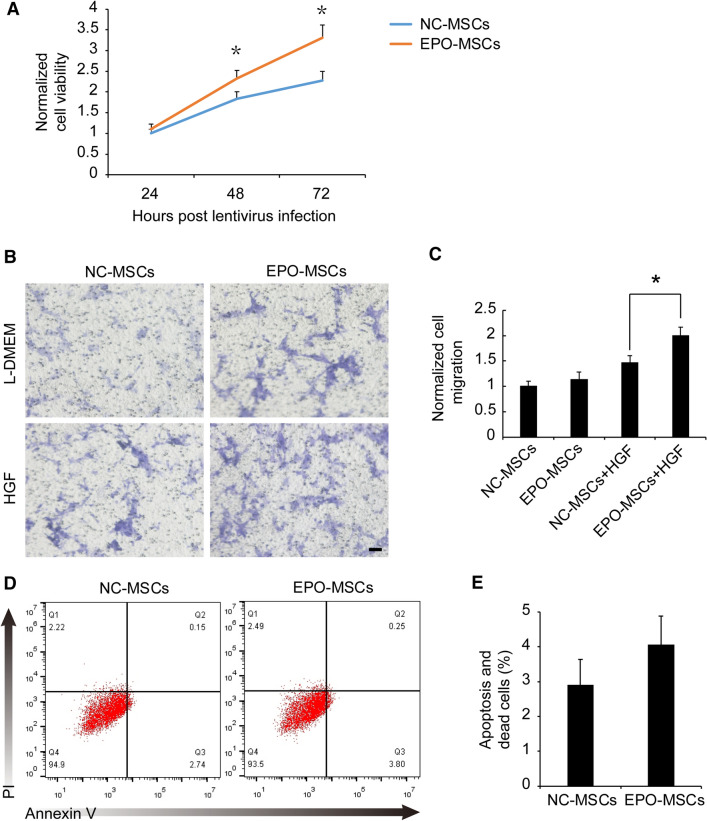
To confirm the effects of EPO overexpression on BM-MSCs, cell viability was evaluated using CCK8 assays. EPO significantly increased BM-MSC cell viability at 48 and 72 h after lentivirus infection, ranging from 1.8 ± 0.17 to 2.3 ± 0.20 at 48 h and 2.3 ± 0.22 to 3.3 ± 0.31 at 72 h, with no significant effect on cell viability at 24 h (Fig. 2A, p < 0.05). We examined the migration ability of BM-MSCs toward HGF using a Boyden chamber device. EPO overexpression had no effect on the migration of BM-MSCs without HGF in the lower chamber. EPO overexpression significantly promoted the migration of MSCs in the presence of 50 ng/mL HGF in the lower chamber, ranging from 1.46 ± 0.15-fold to 1.99 ± 0.17-fold (Fig. 2B, C, p < 0.05). EPO overexpression did not affect apoptosis (Fig. 2D, E). These data demonstrated that EPO overexpression promotes cell viability and migration of BM-MSCs.
Fig. 2.
Effects of EPO on cell viability migration and apoptosis. A Cell viability was analyzed using CCK8 assays after 24, 48 and 72 h of lentivirus infection. Data are mean ± SEM from three independent experiments. *p < 0.05. B Transfilter migration of BM-MSCs after 48 h infection. Images are representative of migratory cells. Bar = 100 μm. C Values were normalized to NC-MSCs without HGF. Data are mean ± SEM from three independent experiments. *p < 0.05. D BM-MSCs infected with lentivirus were processed for flow cytometry for apoptotic and dead cells. E Graphs show percentage of apoptotic and dead cells in control and EPO-overexpressing BM-MSCs. Data are mean ± SEM from three independent experiments
EPO overexpression increases the phosphorylation of Akt and ERK1/2
To explain how EPO secreted from EPO-MSCs promotes cell viability and migration without apoptosis, the intracellular signaling cascades induced by the interaction of EPO with EPOR in MSCs were detected. Real-time PCR showed that EPOR mRNA expression in MSCs after EPO overexpression was upregulated compared with NC-MSCs group (Fig. 3A, p < 0.05). However, it is reported that the enhanced viability and migration are related to the activation of PI3K/Akt and ERK. Following EPO overexpression, phosphorylation levels of PI3K/Akt and ERK1/2 signaling pathways were elevated (Fig. 3B). These data demonstrated that EPO overexpression promotes viability and migration of BM-MSCs by activating PI3K/Akt and ERK1/2 signaling pathways.
Fig. 3.
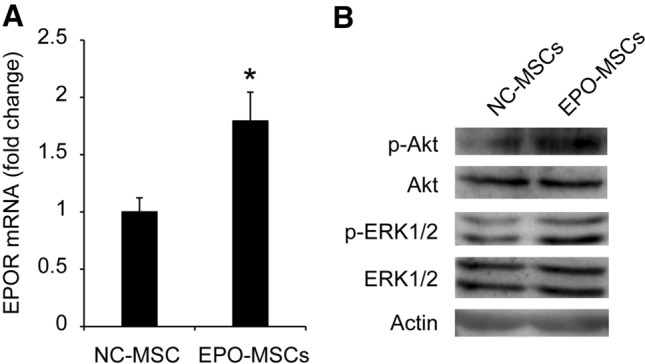
The intracellular signaling cascades induced by EPO overexpression in MSCs. A Quantitative PCR was performed to quantify EPOR mRNA expression. Values are normalized with β-actin mRNA and expressed as a percentage of the control value, as the normalized expression of BM-MSCs infected with negative control (NC). Data represent mean ± SEM from three independent experiments. *p < 0.05. B EPO overexpression on phosphorylation of Akt and ERK1/2 detected by western blot
EPO enhance BM-MSCs migration to the site of liver injury
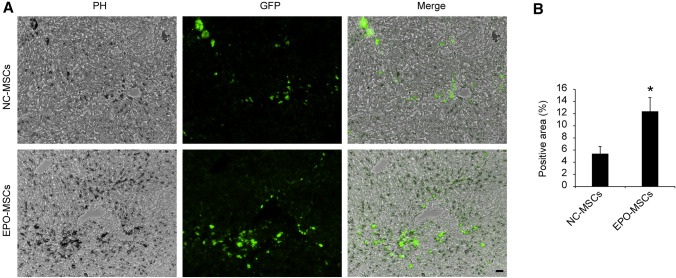
We collected liver specimens and made 5-μm frozen sections to observe the number of GFP-positive cells in liver tissue. Fluorescence microscopy showed that most MSCs were localized in the tissue around the hepatic conduit (Fig. 4A), with more GFP-positive cells in the EPO-MSC groups than the NC-EPO groups (Fig. 4B, p < 0.05). These data illustrated that EPO enhance BM-MSCs migration to the site of liver injury.
Fig. 4.
EPO-MSC migrates to the site of liver fibrosis and promotes antifibrosis therapy. A Fluorescence images of GFP-positive MSCs in mouse livers. Bar = 100 μm. B GFP-positive areas quantified using Image J. Data are mean ± SEM. *p < 0.05
EPO-MSCs improve the anti-fibrosis efficacy of liver fibrosis
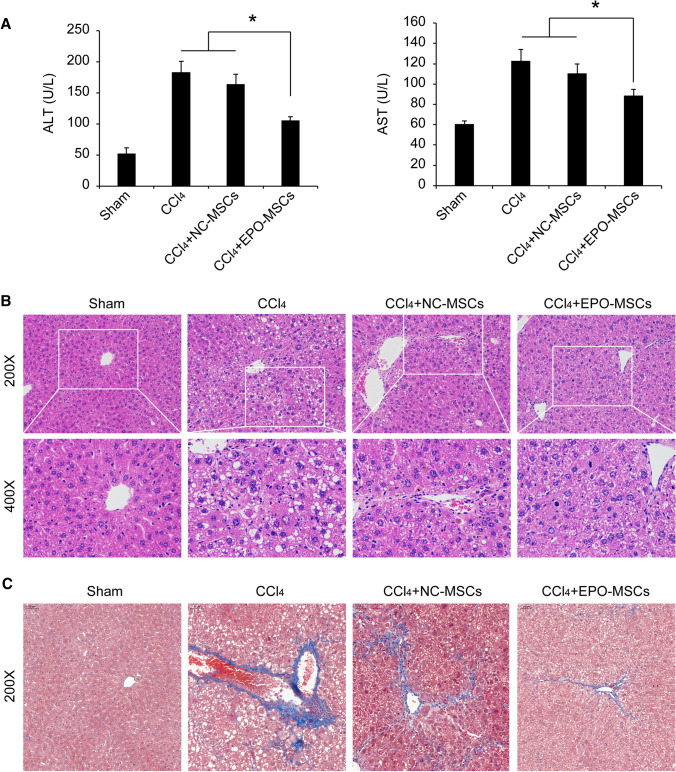
To evaluate the antifibrosis efficacy of EPO-MSCs in vivo, we established a liver fibrosis model. Representative images (Fig. 5) confirmed characteristics of liver fibrosis. ALT and AST levels were significantly elevated in serum after continuous intraperitoneal injection of CCl4 for 6 weeks. Pathological changes showed that nuclei were different in size and had irregular shapes, hepatic steatosis was accompanied by balloon degeneration, lymphocytes were surrounded by lymphocytes, necrosis was seen in hepatocytes in the central vein, and portal area fibrous hyperplasia was accompanied by obvious bridging. Also, α-SMA, collagen fiber and fibronectin were highly expressed compared to an olive oil group (Figs. 5 and 6). EPO-MSCs significantly improved hepatic fibrosis symptoms, mainly manifested as liver function improvement, pathological symptom alleviation, and decreases in α-SMA and fibronectin expression. No efficacy against liver fibrosis was seen for BM-MSCs alone. These data illustrated that EPO-MSCs improved antifibrosis efficacy against liver fibrosis.
Fig. 5.
EPO-MSCs alleviate pathological symptoms of liver fibrosis. A Serum levels of ALT and AST. Data represent the mean ± SEM, n = 6. *p < 0.05. B and C Representative histology of H&E and Masson staining
Fig. 6.
EPO-MSCs improve antifibrosis efficacy against liver fibrosis. A Representative immunohistochemical staining of a-SMA and fibronectin. Bar = 200 μm. B and C Quantification of positive staining areas by Image J software. Data are mean ± SEM, n = 6. *p < 0.05
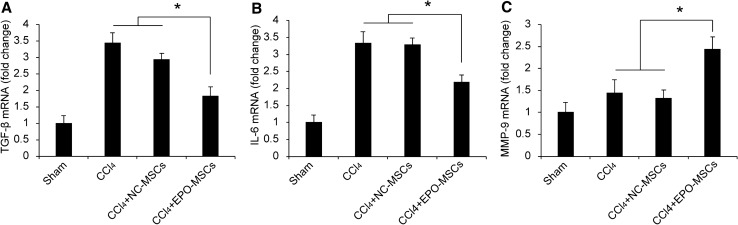
EPO-MSCs promote the expression of cytokines against liver injury
We analyzed TGF-β, IL-6 and MMP-9 hepatic gene expression after EPO-MSCs treatment using real-time PCR (Fig. 7). After EPO-MSC treatment, TGF-β and IL-6 mRNA expression in liver was downregulated compared to a model group (CCl4) or model mice treated with NC-MSCs. MMP-9 was upregulated in the EPO-MSC treatment group compared with control groups.
Fig. 7.
A-C TGF-β, IL-6 and MMP-9 expression. Sham-treated mice were used as controls and used as 1 in normalization, n = 6. Data are mean ± SEM from three independent experiments. *p < 0.05
Discussion
In the past decades, MSC-based cell therapeutics have been pursued aggressively. To date, more than 800 clinical trials have been conducted with MSCs, including umbilical cord-derived MSCs for the treatment of coronavirus disease 2019 (COVID-19) (Clinicaltrials.gov, NCT Number: NCT04269525, NCT04288102, NCT04313322 and NCT04273646). Among these trials, more than 50 are for liver diseases. We and other investigators found that the number of MSCs arriving at injured tissue through systemic administration is limited [8, 19]. Therefore, we should investigate effective strategies to improve the quality and migration ability of MSCs toward injured tissue.
MSCs could be an ideal seed cell for cell therapy for liver fibrosis because of the following characteristics. First, MSCs have low immunogenicity and immunosuppression, and has extensive immunomodulatory functions that affect both innate immunity and adaptive immunity [5] and benefit autologous and allogeneic transplantation. Second, both endogenous and exogenous MSCs have strong inherent tropism toward injured and inflammatory tissue [21]. Increasing evidence from preclinical and clinical studies indicates that HGF, TGF-β and VEGF induce migration of MSCs toward inflammatory sites [22, 23], Third, MSCs various soluble factors and microvesicles that reduce inflammation, improve the microenvironment of the injured site and promote tissue repair and regeneration [24]. Furthermore, MSCs have the potential to differentiate into hepatocytes to replace damaged cells or tissues [25], although this finding is controversial. Based on the migratory capacity and antifibrotic effects of MSCs, we hypothesized that antifibrotic efficacy would likely improve by increasing the cell viability and chemotactic migration ability of MSCs.
A recent study demonstrated that EPO mobilizes MSCs grafted through caudal veins to areas of bone defect to participate in the bone regeneration [26]. However, our study indicated that overexpression of EPO gene significantly increased the cell viability and chemotactic migration ability of MSCs by activating PI3K/Akt and ERK1/2 signaling pathways. This may be the mechanism by which EPO-MSCs ameliorated chronic liver injury in C57BL/6 mice receiving ip injection of CCl4 for 6 weeks.
Hepatocyte damage leads to increased serum ALT and AST [27]. Consistently, in our study, 6 weeks after intraperitoneal injection of CCl4, levels of serum ALT and AST increased significantly compared to the sham group. EPO-MSCs significantly decreased the levels of serum ALT and AST, suggesting that EPO-MSCs had hepatoprotective effects (Fig. 5A). The typical characteristics of hepatic fibrosis are the activation of HSC and excessive deposition of ECM3. As a unique marker of HSC activation, α-SMA is positively correlated with proliferation of HSCs and degree of liver fibrosis [28]. Accordingly, we detected expression of α-SMA by immunohistochemistry. The results showed that EPO-MSCs downregulated expression of α-SMA, consistent with the evaluation of liver fibrosis. Fibronectin is a macromolecular glycoprotein with widely distributed collagen fibers that are critical for fibrogenesis [29, 30]. Increased pathological changes and collagen fibers were observed in liver fibrosis progression using immunohistochemistry. Our study found that hepatic fibrosis symptoms were significantly improved by EPO-MSC treatment, mainly manifested as pathological damage alleviation and decreased fibronectin expression (Figs. 5 and 6). The results were consistent with Masson staining (Fig. 5C), a common procedure for collagen deposition, suggesting that EPO-MSCs had stronger suppression effect on hepatic fibrosis compared with MSCs alone.
Immune cells and cytokines in the liver are involved in the progression of liver fibrosis [31]. Kupffer cells (KCs) are inherent macrophages in the liver that produce cytokines such as TGF-β and IL-6 that promote the activation, proliferation, migration and survival of hepatic stellate cells [32]. We detected expression of TGF-β and IL-6 by real-time PCR. Levels of these cytokines were downregulated by EPO-MSC treatment. KC-derived MMP9 is a key regulatory factor of ECM degradation and remodeling in liver fibrosis regression [33] and are critical in liver fibrosis resolution. The level of MMP-9 was downregulated by EPO-MSC treatment, corresponding with the evaluation of liver fibrosis. Although we observed that EPO-MSCs enhance anti-fibrosis efficacy, their molecular mechanism is not clear and will be the direction of our further research.
Collectively, this study demonstrated that MSCs stably expressing EPO showed better antifibrotic efficacy, higher cell viability and stronger migration ability. These findings provide a novel perspective for the development of MSC-based cell therapy for liver fibrosis.
Acknowledgements
We thank Hanbio Biotechnology Co., Ltd. (Shanghai, China) for lentivirus assistance; International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript. This work was supported by Grants from Foundation of Guizhou Provincial Department of Science and Technology (Nos. [2017] 2873 and [2017] 5718), The Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (No. 2018PT31048), Science and Technology project of Guiyang City (No. ZKHT [2017]-5-10), Guizhou Province Science and Technology supporting Plan (No. [2018] 2757).
Compliance with ethical standards
Ethical statement
The animal studies were performed after receiving approval of the Ethics Committee of Guizhou Medical University (approval No. 2000799).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yanhua Zhou, Email: 36088035@qq.com.
Jianwei Xu, Email: 363912577@qq.com.
References
- 1.Elpek GÖ. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: an update. World J Gastroenterol. 2014;20:7260–7276. doi: 10.3748/wjg.v20.i23.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khatun M, Ray RB. Mechanisms underlying hepatitis C virus-associated hepatic fibrosis. Cells. 2019;8:1249. doi: 10.3390/cells8101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karsdal MA, Daniels SJ, Holm Nielsen S, Bager C, Rasmussen DGK, Loomba R, et al. Collagen biology and non-invasive biomarkers of liver fibrosis. Liver Int. 2020;40:736–750. doi: 10.1111/liv.14390. [DOI] [PubMed] [Google Scholar]
- 4.Dolin CE, Arteel GE. The matrisome, inflammation, and liver disease. Semin Liver Dis. 2020;40:180–188. doi: 10.1055/s-0039-3402516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naji A, Eitoku M, Favier B, Deschaseaux F, Rouas-Freiss N, Suganuma N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76:3323–3348. doi: 10.1007/s00018-019-03125-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi JS, Ryu HA, Cheon SH, Kim SW. Human adipose derived stem cells exhibit enhanced liver regeneration in acute liver injury by controlled releasing hepatocyte growth factor. Cell Physiol Biochem. 2019;52:935–950. doi: 10.33594/000000065. [DOI] [PubMed] [Google Scholar]
- 7.Hu C, Wu Z, Li L. Mesenchymal stromal cells promote liver regeneration through regulation of immune cells. Int J Biol Sci. 2020;16:893–903. doi: 10.7150/ijbs.39725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu C, Zhao L, Duan J, Li L. Strategies to improve the efficiency of mesenchymal stem cell transplantation for reversal of liver fibrosis. J Cell Mol Med. 2019;23:1657–1670. doi: 10.1111/jcmm.14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Auzmendi J, Puchulu MB, Rodríguez JCG, Balaszczuk AM, Lazarowski A, Merelli A. EPO And EPO-receptor system as potential actionable mechanism to protection of brain and heart in refractory epilepsy and SUDEP. Curr Pharm Des. 2020;26:1356–1364. doi: 10.2174/1381612826666200219095548. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura S, Sho M, Koyama F, Ueda T, Nishigori N, Inoue T, et al. Erythropoietin attenuates intestinal inflammation and promotes tissue regeneration. Scand J Gastroenterol. 2015;50:1094–1102. doi: 10.3109/00365521.2015.1020861. [DOI] [PubMed] [Google Scholar]
- 11.Chen LN, Sun Q, Liu SQ, Hu H, Lv J, Ji WJ, et al. Erythropoietin improves glucose metabolism and pancreatic β-cell damage in experimental diabetic rats. Mol Med Rep. 2015;12:5391–5398. doi: 10.3892/mmr.2015.4006. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Tao T, Xu J, Liu Z, Zou Z, Jin M. HIF1alpha attenuates neuronal apoptosis by upregulating EPO expression following cerebral ischemiareperfusion injury in a rat MCAO model. Int J Mol Med. 2020;45:1027–1036. doi: 10.3892/ijmm.2020.4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu H, Wu X, Wang Z, Li L, Chen W, Yang M, et al. Erythropoietin-activated mesenchymal stem cells promote healing ulcers by improving microenvironment. J Surg Res. 2016;205:464–473. doi: 10.1016/j.jss.2016.06.086. [DOI] [PubMed] [Google Scholar]
- 14.Parekkadan B, Upadhyay R, Dunham J, Iwamoto Y, Mizoguchi E, Mizoguchi A, et al. Bone marrow stromal cell transplants prevent experimental enterocolitis and require host CD11b + splenocytes. Gastroenterology. 2011;140:966–975. doi: 10.1053/j.gastro.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Han Z, Han Z, He Z. Mesenchymal stem cell-conditioned media suppresses inflammation-associated overproliferation of pulmonary artery smooth muscle cells in a rat model of pulmonary hypertension. Exp Ther Med. 2016;11:467–475. doi: 10.3892/etm.2015.2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han XP, Zhang FQ, Tan XS, Liu L, Ma WX, Ou-Yang HF, et al. EPO modified MSCs can inhibit asthmatic airway remodeling in an animal model. J Cell Biochem. 2018;119:1008–1016. doi: 10.1002/jcb.26268. [DOI] [PubMed] [Google Scholar]
- 17.Zeng J, Bao J, Wang H, Zhang H, Zhang J, Wang W, et al. Construction and identification of mouse BTLA lentiviral expression vector. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2013;29:261–264. [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.He L, Wang X, Kang N, Xu J, Dai N, Xu X, et al. MiR-375 inhibits the hepatocyte growth factor-elicited migration of mesenchymal stem cells by downregulating Akt signaling. Cell Tissue Res. 2018;372:99–114. doi: 10.1007/s00441-017-2765-y. [DOI] [PubMed] [Google Scholar]
- 20.Lin H, Luo X, Jin B, Shi H, Gong H. The effect of EPO gene overexpression on proliferation and migration of mouse bone marrow-derived mesenchymal stem cells. Cell Biochem Biophys. 2015;71:1365–1372. doi: 10.1007/s12013-014-0358-x. [DOI] [PubMed] [Google Scholar]
- 21.Encabo-Berzosa MDM, Sancho-Albero M, Crespo A, Andreu V, Sebastian V, Irusta S, et al. The effect of PEGylated hollow gold nanoparticles on stem cell migration: potential application in tissue regeneration. Nanoscale. 2017;9:9848–9858. doi: 10.1039/C7NR01853C. [DOI] [PubMed] [Google Scholar]
- 22.Tang Y, Liu L, Wang P, Chen D, Wu Z, Tang C. Periostin promotes migration and osteogenic differentiation of human periodontal ligament mesenchymal stem cells via the Jun amino-terminal kinases (JNK) pathway under inflammatory conditions. Cell Prolif. 2017;50:e12369. doi: 10.1111/cpr.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wei B, Bai X, Chen K, Zhang X. SP600125 enhances the anti-apoptotic capacity and migration of bone marrow mesenchymal stem cells treated with tumor necrosis factor-alpha. Biochem Biophys Res Commun. 2016;475:301–307. doi: 10.1016/j.bbrc.2016.05.107. [DOI] [PubMed] [Google Scholar]
- 24.Sherman LS, Condé-Green A, Sandiford OA, Rameshwar P. A discussion on adult mesenchymal stem cells for drug delivery: pros and cons. Ther Deliv. 2015;6:1335–1346. doi: 10.4155/tde.15.80. [DOI] [PubMed] [Google Scholar]
- 25.Kamel MM, Baz HGE, Demerdash Z, Hassan S, Salah F, Mansour WA, et al. Cord blood-derived mesenchymal stem cells with hepatogenic differentiation potential ameliorate chronic liver affection in experimental models. Adv Clin Exp Med. 2018;27:1329–1339. doi: 10.17219/acem/70430. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Huang Z, Li B, Zhang Z, Liu L. Mobilization of transplanted bone marrow mesenchymal stem cells by erythropoietin facilitates the reconstruction of segmental bone defect. Stem Cells Int. 2019;2019:5750967. doi: 10.1155/2019/5750967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sha M, Gao Y, Deng C, Wan Y, Zhuang Y, Hu X, et al. Therapeutic effects of AdipoRon on liver inflammation and fibrosis induced by CCl4 in mice. Int Immunopharmacol. 2020;79:106157. doi: 10.1016/j.intimp.2019.106157. [DOI] [PubMed] [Google Scholar]
- 28.Zou GL, Zuo S, Lu S, Hu RH, Lu YY, Yang J, et al. Bone morphogenetic protein-7 represses hepatic stellate cell activation and liver fibrosis via regulation of TGF-β/Smad signaling pathway. World J Gastroenterol. 2019;25:4222–4234. doi: 10.3748/wjg.v25.i30.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu XY, Liu RX, Hou F, Cui LJ, Li CY, Chi C, et al. Fibronectin expression is critical for liver fibrogenesis in vivo and in vitro. Mol Med Rep. 2016;14:3669–3675. doi: 10.3892/mmr.2016.5673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Attallah AM, Abdallah SO, Attallah AA, Omran MM, Farid K, Nasif WA, et al. Diagnostic value of fibronectin discriminant score for predicting liver fibrosis stages in chronic hepatitis C virus patients. Ann Hepatol. 2013;12:44–53. doi: 10.1016/S1665-2681(19)31384-5. [DOI] [PubMed] [Google Scholar]
- 31.Haghgoo SM, Sharafi H, Alavian SM. Serum cytokines, adipokines and ferritin for non-invasive assessment of liver fibrosis in chronic liver disease: a systematic review. Clin Chem Lab Med. 2019;57:577–610. doi: 10.1515/cclm-2018-0357. [DOI] [PubMed] [Google Scholar]
- 32.Wu S, Liu L, Yang S, Kuang G, Yin X, Wang Y, et al. Paeonol alleviates CCl4-induced liver fibrosis through suppression of hepatic stellate cells activation via inhibiting the TGF-β/Smad3 signaling. Immunopharmacol Immunotoxicol. 2019;41:438–445. doi: 10.1080/08923973.2019.1613427. [DOI] [PubMed] [Google Scholar]
- 33.Feng M, Ding J, Wang M, Zhang J, Zhu X, Guan W. Kupffer-derived matrix metalloproteinase-9 contributes to liver fibrosis resolution. Int J Biol Sci. 2018;14:1033–1040. doi: 10.7150/ijbs.25589. [DOI] [PMC free article] [PubMed] [Google Scholar]