Abstract
Background
Drug-resistant tuberculosis burdens fragile health systems in sub-Saharan Africa (SSA), complicated by high prevalence of HIV. Several African countries reported large gaps between estimated incidence and diagnosed or treated cases. Our review aimed to identify barriers and facilitators influencing diagnosis and treatment for drug-resistant tuberculosis (DR-TB) in SSA, which is necessary to develop effective strategies to find the missing incident cases and improve quality of care.
Methods
Using an integrative design, we reviewed and narratively synthesised qualitative, quantitative and mixed-methods studies from nine electronic databases: Medline, Global Health, CINAHL, EMBASE, Scopus, Web of Science, International Journal of Tuberculosis and Lung Disease, PubMed and Google Scholar (January 2006 to June 2019).
Results
Of 3181 original studies identified, 55 full texts were screened, and 29 retained. The studies included were from 6 countries, mostly South Africa. Barriers and facilitators to DR-TB care were identified at the health system and patient levels. Predominant health system barriers were laboratory operational issues, provider knowledge and attitudes and information management. Facilitators included GeneXpert MTB/RIF (Xpert) diagnosis and decentralisation of services. At the patient level, predominant barriers were patients being lost to follow-up or dying due to lengthy diagnostic and treatment delays, negative public sector care perceptions, family, work or school commitments and using private sector care. Some patient-level facilitators were HIV positivity and having more symptoms.
Conclusion
Case detection and treatment for DR -TB in SSA currently relies on individual patients presenting voluntarily to the hospital for care. Specific interventions targeting identified barriers may improve rates and timeliness of detection and treatment.
Keywords: tuberculosis, health services research, public health, systematic review
Summary box.
What is already known?
Globally, only 39% and 32% of the estimated drug-resistant tuberculosis (DR-TB) patients are diagnosed and started on appropriate treatment, respectively.
Ten high burden countries in Africa contributed 12% of the estimated global incident cases in 2018, with 54% of these in only Nigeria and South Africa.
For patients who are diagnosed and placed on treatment, delays in access to diagnosis and treatment were up to several months in some sub-Saharan African countries.
What are the new findings?
Laboratory operational challenges as well as inadequate healthcare worker knowledge, attitude and skills were the predominant barriers noted at the health system level.
Predominant patient-level barriers included loss to follow-up and death, as well as inability to pay care-related costs.
Availability of newer diagnostic tools was the predominant health-level facilitator of quicker diagnosis and treatment; however, this did not always translate to significantly higher rates of diagnosis and treatment.
What do the new findings imply?
Implementers and policymakers need to better understand and address various issues that impact DR-TB care at different levels, in order to maximise the impact of new care innovations.
Introduction
Drug-resistant tuberculosis (DR-TB) is a major threat to global health as it undermines gains in TB control, and is especially burdensome to health systems in resource-limited settings.1 Defined as TB resistant to both isoniazid and rifampicin, it is the leading cause of deaths due to antimicrobial resistance and took an estimated 214 000 lives in 2018.2 The 2018 United Nations High-Level resolution to ‘end TB including DR-TB’ by accelerating access to affordable prevention and care, is in line with earlier goals including the Sustainable Development Goals (SDGs) and The End TB Strategy.3–5 To meet these goals, it is essential to synthesise the growing evidence on barriers and facilitators to DR-TB care.
DR-TB is more difficult to diagnose and treat than drug-susceptible TB and is often associated with up to 5.5 times higher treatment costs, longer treatment courses and lower treatment success rates.1 2 6 Globally, only 39% and 32% of the estimated patients diagnosed with DR-TB are started on appropriate treatment, respectively.2 Ten high burden countries (HBCs) in sub-Saharan Africa (SSA) contributed 12% of the 484 000 estimated incident cases in 2018, mostly in Nigeria and South Africa. Nigeria and Mozambique were among 10 countries contributing 75% of the global treatment enrolment gap.2
Gaps in TB care were notedly higher in Africa, where the HIV-associated TB incidence is highest, as HIV further complicates TB care. Of 14 countries classified by WHO as HBCs for TB, DR-TB and HIV, 8 are in Africa.2
For patients with DR-TB who are diagnosed and treated, several studies have reported delays in access running into several months in several SSA countries.7–10 These delays, occurring at patient and health system (provider) levels, contribute to increased morbidity, infection transmission, loss to follow-up and poorer treatment outcomes.11 12 This review examines any synthesised qualitative and quantitative literature, with a view to inform policy and practice in SSA.
Our review question was ‘What are the patient or provider factors associated with delays in tuberculosis diagnosis and treatment in sub-Saharan Africa?’.
Methods
We used a mixed-methods systematic review with an integrative approach13 to analyse data from qualitative, quantitative and mixed-methods literature and assessed quality using the COnsolidated criteria for REporting Qualitative research for qualitative studies (COREQ), Strengthening the Reporting of Observational Studies in Epidemiology -Combined tool (STROBE) and Mixed Methods Appraisal Tool (MMAT) tools, respectively.14–16
We registered the systematic review protocol in the PROSPERO database (https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=106875).
Search strategy
Using a combination of key terms, we searched nine electronic databases: CINAHL, Medline, Embase, Global Health, Scopus, Web of Science, International Journal of Tuberculosis and Lung Disease, PubMed and Google Scholar between January 2006 and June 2017, updating the search in June 2019. The year 2006 was used as this was the date of the first WHO publication guiding the programmatic management of DR-TB.
The Population, Intervention, Comparator and Outcomes (PICO) framework17 and key search terms used are summarised in online supplementary annex 1. The initial search terms were piloted and refined in CINAHL, and replicated in other databases, using appropriate strategies specific to each. The public health librarian at the University of Montreal School of Public Health validated this process.
bmjgh-2019-002280supp001.pdf (168.3KB, pdf)
Study selection and inclusion criteria
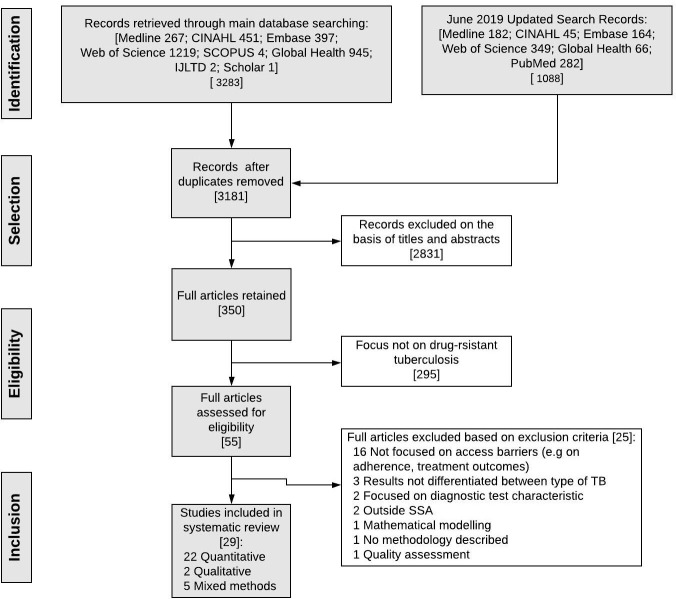
We selected studies (figure 1) based on our inclusion criteria and PICO framework (online supplementary annex 1). Search results were downloaded into EndNote X7.7 and deduplicated. Titles and abstracts were screened, and full texts reviewed to determine studies for inclusion, and reasons for exclusion. All discrepancies or uncertainties were discussed and resolved by consensus during the final review.
Figure 1.
Study selection. SSA, sub-Saharan Africa; TB, tuberculosis.
Data extraction
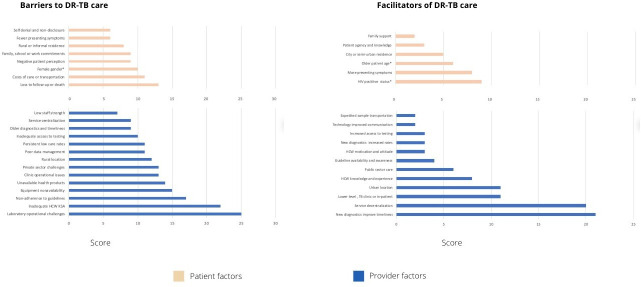
Descriptive and analytical data were extracted (table 1). Study findings and outcomes were grouped quantitatively and qualitatively (table 2). Paired access dimensions and recommendations for some identified barriers, drawn from the studies themselves, were presented in the context of the healthcare access model by Levesque et al (table 3).18 Finally, a summary of access factors based on perceived importance and frequency of appearance19 are presented in figure 2, online supplementary annex 3.
Table 1.
Overview of selected studies
| Study ID number | Study (year), country |
Research design and methods Period |
Populations (number) | Study objectives | Level of care (diagnosis/treatment) | Dimensions of access | Summary of findings | Assessment of study quality (score) |
| Qualitative studies | ||||||||
| 1 | Bieh (2017) Nigeria |
Qualitative FGDs, IDIs and KIIs 2014 |
Patients (11) and health workers (4) | NA | Treatment | Structural and patient dimensions | Treatment delays due to stigma and discrimination, as well as a lack of required hospital tools. | B |
| 2 | Naidoo (2015) South Africa |
Qualitative IDIs (part of a bigger study including a retrospective cohort 2010–2012 |
Patients (26) | NA | Diagnosis and treatment | Structural and patient dimensions | Patients beliefs and knowledge of TB symptoms, wrong perceptions of healthcare and family commitments, compounded by health systems missed opportunities and delays, impact access. | A |
| Quantitative studies | ||||||||
| 3 | Cox (2015) South Africa |
Retrospective trend analysis2009– 2013 | Patients (158) | Time to treatment initiation (TTI) before the decentralisation, during decentralisation and after decentralisation. | Diagnosis and treatment | Structural dimensions | Decentralisation and introducing Xpert were associated with significant reductions in TTI, after initial gains with the LPA. | B |
| 4 | Cox (2017) South Africa |
Retrospective cohort study 2011–2013 |
Patients (2508 in 2011) (2528 in 2013) |
Treatment initiation were assessed among laboratory-diagnosed patients before and after Xpert implementation. | Diagnosis and treatment | Structural and patient dimensions | Patients age and HIV status, as well as diagnostic timeliness delay access. | A |
| 5 | Dlamini-Mvelase (2014) South Africa |
Retrospective cohort study 2011–2012 |
Patients (637) | Availability of confirmatory DST and TTI with Xpert compared with phenotypic and genotypic DST. | Diagnosis | Structural dimensions | Poor adherence to Xpert algorithmwas due to rollout of Xpert preceding training of clinicians | A |
| Quantitative studies | ||||||||
| 6 | Ebonwu (2013) South Africa |
Cross-sectional study 2011 |
Patients (942) | Evaluation of treatment uptake, loss to follow-up and retention of newly diagnosed patients. | Treatment | Structural and patient dimensions | Referrals from hospitals, some health districts, being HIV negative and township place of residence were associated with treatment non-initiation. | A |
| 7 | Evans et al (2018) South Africa |
Retrospective cohort study: First cohort: 2011–2012 (35% Xpert implementation) Second cohort: 20132014 (>90% implementation) |
Patients: First cohort (594) Second cohort 713 |
Compared treatment initiation and TTI forlaboratory-confirmed patients with (first vs second cohort). | Diagnosis and treatment | Structural and patient dimensions | Xpert implementation increased diagnostic capacity and treatment rates. | A |
| 8 | Hanrahan et al (2012) South Afria |
Observational cohort study: 2007– 2008 with MGIT phenotypic DST 2009– 2010 with LPA |
Patients (n=1176 MGIT) and (n=1177 LPA) | Compared data on patients registration before and after an expanded DST algorithm. | Diagnosis and treatment | Structural and patient dimensions | Introducing the faster LPA DST testing cut down time to diagnosis and increased case detection without the expected impact on TTI due to other health system bottlenecks. | A |
| 9 | Hanrahan (2013) South Africa |
Prospective cohort study Jul–Sep 2011 |
Patients (641) | Evaluated diagnostic follow-up and outcomes for a cohort of presumptive patients screened using a single point-of-care Xpert. | Diagnosis and treatment | Structural and patient dimensions | Point-of-care Xpert provided quicker treatment initiation, mostly same day treatment. This was 2 weeks faster than for those started empirically or based on suggestive chest X-ray, and 20 weeks faster than for culture diagnosis. |
A |
| 10 | Iruedo (2017) South Africa |
Retrospective cohort study Jan 2009–Dec 2014 |
Patients (342) | Analysed records of diagnosed patients, comparing diagnostic modalities to assess the Xpert effect on TTI. | Diagnosis and treatment | Structural and patient dimensions | Xpert significantly reduced the time to diagnosis and TTI. This was significantly shorter compared with LPA and culture/phenotypic DST. | A |
| 11 | Jacobson (2012) South Africa |
Retrospective cohort study 2007–2011 |
Patients (197) | Compared records of patients tested using the MTBDRplus and with culture-based DST to determine if TTI from specimen collection was shortened. | Diagnosis and treatment | Structural and patient dimensions | The use of LPA for diagnosis dramatically improved TTI but laboratory and clinical operational delays remained a problem. | A |
| Quantitative studies | ||||||||
| 12 | Jacobson et al (2017) South Africa |
Retrospective cohort in Western Cape: two samples at baseline— for Xpert; and for LPA plus DST 2011– 2013. Prospective cohort in three other provinces: one sample collected at baseline for Xpert; a subsequent one for LPA plus culture-based DST only with detection of RR-TB. 2012– 2013 |
Patients (1332) *Western Cape Province: (835) *Eastern Cape, Free State and Gauteng Province: (497) |
Quantified the time to DST results and proportion of patients potentially placed on suboptimal therapy. | Diagnosis and treatment | Structural and patient dimensions | Incomplete and decreasing adherence to National requirements for DSTimpedes diagnosis rates. Long turnaround time for DST results following RR-TB diagnosis. |
A |
| 13 | Jokwiro et al (2018) Zimbabwe |
Cross-sectional study. 2016– 2017 with two phases: Xpert only for presumptive DR-TB and HIV coinfection: 2016; Xpert recommended for all presumptive patients: 2017. |
Thirteen Xpert assays (13 137 total assays): *2016: (4556) *2017: (8581) |
Compared the use of deploying Xpert only for presumptive DR-TB and HIV coinfection vs Xpert for all presumptive TB patients. | Diagnosis | Structural dimensions | Increased access to Xpert utilisation beyond high-risk groups slightly increased detection of drug susceptible TB, but not DR-TB strains. Persistent HS challenges impeded Xpert utilisation. |
A |
| 14 | Kweza (2018) South Africa |
Cross-sectional survey 2015 |
Patients (1255) | Estimated the proportion of patients missed by PHCs using surveys and testing. | Diagnosis | Structural and patient dimensions | HS missed most patients with TB attending PHCs for TB-related symptoms and for other reasons. | A |
| 15 | McLaren (2017) South Africa |
Healthcare evaluation 2004–2011 |
26 million tests in 429 hospitals | Assessed quality of care in public health facilities by analysing National Health Laboratory Service database for TB tests. | Diagnosis | Structural and patient dimensions | Facilities not adhering to national standards for TB testing. However, DST rates improved steadily over time. Testing rates were transiently affected by policy and guideline changes. |
B |
| Quantitative studies | ||||||||
| 16 | Metcalfe et al (2016) Zimbabwe |
Prospective study: 2011– 2014 |
Patients (352) | Diagnostic accuracy and TTI for Xpert were compared with culture and DST. | Diagnosis and treatment | Structural and patient dimensions | Rapid diagnosis with Xpert was not, in itself, enough to remove health system delays to treatment initiation. | A |
| 17 | Mohr (2017) South Africa |
Retrospective cohort study 2012– 2014 |
Patients (543) | Analysed records of diagnosed patients to assess proportion that could have been diagnosed earlier. | Diagnosis | Structural dimensions | Lack of guideline adherence led to patients not being diagnosed. | A |
| 18 | Moyo et al (2015) South Africa |
Retrospective analysis study: 2008–2013 |
Adolescent patients (71) | Analysed data for adolescents patients to describe frequency of treatment success or failure, loss to follow-up and deaths. | Treatment | Structural and patient dimensions | Treatment refusal and loss to follow-up were the predominant reasons for non-initiation of treatment. | A |
| 19 | Naidoo (2014) South Africa |
Observational analysis of 10 facilities 2008– 2012 |
Patients (541) | Study compared TTI in MDRTBPlus Line Probe Assay vs Xpert-based algorithms. | Diagnosis and treatment | Structural and patient dimensions | Xpert reduced TTI by reducing LTAT. However, patients were being delayed by other steps needed before treatment initiation. | A |
| 20 | Nkosi (2013) South Africa |
Cross-sectional survey 2008 |
Patients (148) | Determined reasons for non-referral of DR-TB patients. | Treatment | Structural and patient dimensions | Poor HCW knowledge of the national DR-TB guidelines, and patients loss to follow-up contributed to non-referrals. | A |
| 21 | Oga-Omenka et al (2019) Nigeria |
Retrospective cohort study. 2015– 2017 |
Patients (996) | Examined treatment rates and TTI using 2015 the TB programme records. | Treatment | Structural and patient dimensions | Geographic location and level of healthcare influenced patient treatment initiation within the time recommended by the National guidelines. | A |
| Quantitative studies | ||||||||
| 22 | Oliwa et al (2018) Kenya |
Cross-sectional study: 2015 |
Patients (82 313) | Analysed National TB programme data for case notification rates, and capacity to perform diagnostic tests. | Diagnosis | Structural and patient dimensions | Despite guideline specifications, Xpert use was suboptimal, negatively affecting diagnosis, especially in children and low risk groups. | A |
| 23 | Timire et al (2019) Zimbabwe |
Cohort study 2017– 2018 |
Patients (133) | Determined the impact of the Hain technology (timeliness and proportion of DST tests). | Diagnosis and treatment | Structural and patient dimensions | While decentralisation and treatment access positively impacted TTI, distance from the NRL hindered timely collection and return of DST. | A |
| 24 | Van Den Handel (2015) South Africa |
Prospective evaluation of different diagnostic approaches 2011– 2013 |
Patients (1449) | Determined the impact of Xpert and decentralisation on patient care in areas with poor access to laboratory services. | Diagnosis | Structural dimensions | Xpert introduction and decentralisation impacted treatment rates and timelines, but did not significantly increase rates of detection. | A |
| Mixed-methods studies | ||||||||
| 25 | Doulla et al (2019) Tanzania |
Qualitative FGDs, IDIs: 2012 Quantitative cross-sectional sample analysis: 2011– 2013 |
Qualitative 45 HCW Quantitative 2759 samples |
Evaluated the effectiveness and stakeholder perception of routine surveillance system for previously treated TB cases. | Diagnosis | Structural dimensions | Delayedspecimen transportation, lack of resources and other laboratory challenges (eg, miscommunication, inconsistent training, etc) delayed diagnosis. | A |
| 26 | Mpagama et al (2019) Tanzania |
Retrospective cohort study and cross-sectional study: 2015 | 28 TB districts 399 patients |
Identified healthcare barriers to implementation of molecular diagnostics and TB collaborative practices in HIV clinics. | Diagnosis and treatment | Structural and patient dimensions | Overall, underdiagnosesoccurred where drug resistance is expected to be prevalent. HCWs lacked the tools, expertise and knowledge to appropriately manage patients with TB. | B |
| Mixed-methods studies | ||||||||
| 27 | Mnyambwa et al (2018) Tanzania |
Retrospective cohort study: 2013– 2016 Qualitative: IDIs |
Chart review: patients (782) Qualitative interviews: TB coordinators (27) |
Assessed the effectiveness of the Xpert GxAlert platformon linkage of patients to care. | Diagnosis and treatment | Structural and patient dimensions | Although the GxAlert platform improved diagnosis, healthcare inconsistencies impaired correct management of patients. | B |
| 28 | Westhuizen et al (2017) South Africa |
Cross-sectional study: 2015 | Medical students (12) | Determined the frequency and impact of occupational TB disease in current medical students and recently graduated doctors. | Diagnosis and treatment | Structural and patient dimensions | Overall, medical students did not have adequate access to the support and services needed for all TB care, including DR-TB. | B |
| 29 | Zimri (2012) South Africa |
Qualitative FGDs and quantitative case control 2011 |
10 FGD with parentsand providers; Case control: 50 patients each arm |
Caregivers of children referred to a specialist paediatric MDR-TB clinic to determine why many child contacts were not brought for assessment. | Diagnosis | Structural and patient dimensions | HCW attitude, coloured ethnicity, the mother being the source case, having a smoker in the house, transport time, cost and number of transitions, and fear of infection were identified as barriers. | A |
DR-TB, drug-resistant TB; DST, drug-sensitivity testing; FGDs, focus group discussions; HCW, healthcare worker; HS, health system; IDI, in-depth interviews; KIIs, key informant interviews; LPA, line probe assay; LTAT, Laboratory turn-around time; MDR, multidrug-resistant TB; MGIT, mycobacteria growth indicator tube; NA, not applicable; NRL, National or Central Reference Laboratory; PHC, Primary Health Clinics; RR-TB, rifampicin-resistant TB; TB, tuberculosis; TTI, time to treatment initiation; Xpert, GeneXpert MTB/RIF Assay.
Table 2.
Quantitative and qualitative findings
| Factor | Quantitative findings 95% CI (study ID) | Qualitative findings (study ID) | ||
| Barrier | Facilitator | Barrier | Facilitator | |
| Healthcare system level barriers (based on the WHO Health Systems Framework) | ||||
| Leadership and governance | ||||
| Guidelines availability and inclusion of low-risk groups |
|
|||
| Service delivery | ||||
| Infrastructure and equipment |
|
|
||
| Decentralisation and integration |
|
|
|
|
| Laboratory operational issues: sputum transportation, turn-around time, misdiagnosis, communication and linkage to care |
|
|
|
|
| Clinic operational issues: patient tracking and follow-up, long waiting times |
|
|||
| Level of care |
|
|||
| Public vs private sector care |
|
|||
| Location and coverage (rural/urban) |
|
|||
| Health workforce | ||||
| Adherence to guidelines |
|
|
||
| Workload and staff numbers | ||||
| HCW knowledge, training, experience and supervision |
|
|
|
|
| HCW motivation and attitude, including stigma and discrimination |
|
|
||
| Health information systems | ||||
| Data management |
|
|
||
| Access to second-line diagnostics, medications and technologies | ||||
| Type of diagnostic test |
|
|
|
|
| Newer diagnostics impact on rates |
|
|||
| Access to testing products |
|
|
|
|
| Health financing | ||||
| TB health financing | ||||
| Patient level (based on the Andersen and Newman health services utilisation model) | ||||
| Predisposing characteristics | ||||
| Sex |
|
|||
| Age |
|
|||
| Pregnancy | ||||
| HIV |
|
|||
| Presenting symptoms and history |
|
|
|
|
| Self-denial and non-disclosure | ||||
| Lifestyle and ethnicity | ||||
| Patient agency, perceptions, and attitudes |
|
|
|
|
| Enabling characteristics | ||||
| Family, school or work support/commitments |
|
|
|
|
| Loss to follow-up or death |
|
|||
| Direct and indirect costs of care | ||||
| Geographic location |
|
|
||
| Need characteristics and health seeking practice | ||||
| Treatment refusal or symptom minimization |
|
|||
| Alternative care |
|
|||
#1—even though a study of patients already on treatment, some issues like discrimination have been shown in other studies to impact patient access to care.
#28—distinguishing between factors related to diagnosis/treatment access from impact of treatment.
aOR, adjusted OR; DR-TB, drug-resistant TB; DST, drug-sensitivity testing; HCW, healthcare worker; LPA, line probe assay; LTAT, Laboratory turnaround time; LTFU, lost to follow-up; NRL, National or Central TB Reference Laboratory; RR-TB, rifampicin-resistant TB; SE, SouthEast; SW, SouthWest; TB, tuberculosis; TTI, time to treatment initiation; Xpert, GeneXpert MTB/RIF Assay.
Table 3.
Paired access dimensions and recommendations
| Structural access dimensions and barriers (study ID #) |
Patients access dimensions and barriers (study ID #) |
Recommendations (study ID #) |
Approachability:
|
Ability to perceive: |
|
| Acceptability: | Ability to seek:
|
|
|
Structural access dimensions and barriers (study ID #) |
Patients access dimensions and barriers (study ID #) |
Recommendations (study ID #) |
Availability: coverage/centralisation of services7 37
|
Ability to reach:
|
|
| Affordability: | Ability to pay:
|
|
HCW, healthcare worker; TB, tuberculosis.
Figure 2.
Summary of barriers and facilitators influencing drug-resistant tuberculosis (DR-TB) diagnosis and treatment in sub-Saharan Africa (SSA), ranked both on frequency of appearance and perceived importance.HCW, healthcare worker; KSA, knowledge, skills and attitude. *Inconclusive results; see table 2.
Quality assessment
We assessed the quality of studies through different critical quality appraisal tools based on study designs. Consensus was reached by discussion. For quantitative studies, we used the STROBE combined tool.14 The COREQ15 and the MMAT was used to appraise mixed-methods studies.16 The quality assessment are provided in online supplementary annex 2.
The overall quality assessments of ‘A’ for high (>70%), ‘B’ (50%–69%) for medium or ‘C’ (<50%) for low were assigned based on independent evaluation by at least two reviewers for each study. The STROBE, COREQ and MMAT tools have been used in several systematic reviews as a basis for excluding low quality studies.20–22
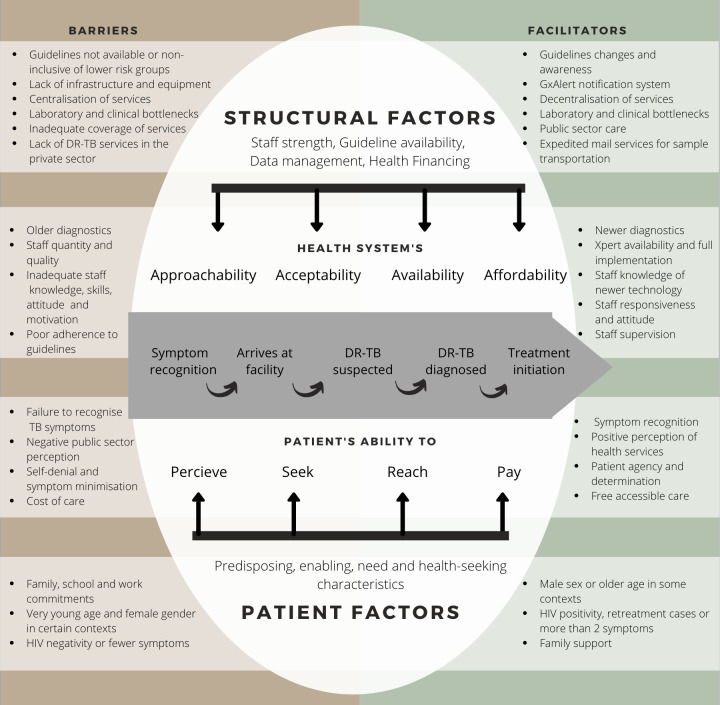
Conceptual framework
We adapted a conceptual framework, mapping the TB care continuum from symptom onset to treatment initiation23 to four corresponding dimensions of access at the provider and patient levels,18 24 and aligned these to identified barriers and facilitators from our review (figure 3). We explained provider factors using the six health systems building blocks described by WHO.25 Patient-level barriers and facilitators were described using the Andersen and Newman individual determinants of healthcare utilisation.26
Figure 3.
An adapted conceptual framework of identified barriers and facilitators to to DR-TB care.
Data analysis
We used an integrative approach13 to develop a narrative analysis of key findings from qualitative, quantitative and mixed-methods studies, due to the high heterogeneity of selected studies. We repeatedly screened, coded and categorised data from each study in four ways: table 1 gives the selected study overview—first author (year) and country, research design, population, intervention (when applicable), summary of barriers and facilitators, the level of care (diagnosis or treatment) in which they occurred and the dimensions of care (provider or patient), the main findings and the quality assessment score.
In table 2, we separated quantitative and qualitative findings for each identified factor. We reported associations that were statistically significant or relevant to our analysis and included representative quotes where available.
In table 3, we used the healthcare access model by Levesque et al18 24 to categorise data into four provider and patient paired dimensions: approachability/ability to perceive; acceptability/ability to seek; availability/ability to reach; affordability/ability to pay and finally appropriateness/ability to engage. The paired dimension of approachability and ability to perceive relates mostly to knowledge of providers and patients about services. Acceptability and the corresponding ability to seek focuses on cultural and social aspects that influence people’s decisions to use health service and the personal autonomy and agency to make these decisions. Availability and the ability to reach refers to the physical existence of health systems and health workers, as well as the physical mobility and work flexibility of patients to reach available health resources. The dimensions of affordability and the corresponding ability to pay reflects the financial implications of health services and the capacity on the side of patients to bear these costs.18
To synthesise the factors identified across the variety of studies, we ranked each barrier and facilitator based on its importance within each study and the number of studies where it appeared (figure 2, online supplementary annex 3). A factor is assigned the maximum score of 3 if it affects >50% of participants or has an OR of <0.65 or >1.5 for quantitative studies; and deemed as being of high importance or repeatedly mentioned across participant types. Factors are assigned a 2 if they affect 25%–50% of participants, or OR 0.65–0.8 or 1.25–1.5 for quantitative studies; or were deemed of moderate importance or by default when mentioned but not rated in qualitative studies. Factors were assigned 1 if affecting few participants and a zero if not mentioned. These scores were added for each study where the factor appeared. A similar method for synthesising mixed-methods reviews has been previously described.19
Patient and public involvement
Patients or members of the public were not involved in this research.
Results
After an initial search yield of 3181 unique studies, 55 full texts met screening criteria, and a final selection of 30 articles were retained (figure 1).
Study characteristics
The majority of the included studies were conducted in South Africa (n=20), with Zimbabwe (n=3), Tanzania (n=3), Nigeria (n=2), Kenya (n=1) and Gabon (n=1) making up the rest. These six countries represent 49.5% of the 77 000 estimated DR-TB incident cases in Africa in 2018.2 There were 3 qualitative, 22 quantitative and 5 mixed-methods studies. Among the quantitative studies, there were 13 retrospective, 3 prospective and 1 mixed cohort studies, and 5 cross-sectional surveys. All of the three qualitative studies employed in-depth interviews, with one study including focus group discussions. Five studies examined access factors related to DR-TB treatment only, 9 on DR-TB diagnosis only and 16 focused on both diagnosis and treatment. Factors impacting access were identified at provider (n=30) and patient (n=24) levels. Sixteen studies explored the influence of diagnostic tools on laboratory turnaround time and on treatment initiation. Table 1 summarises the study characteristics for this review.
Quality appraisal
The results of our quality assessment are shown in online supplementary annex 2. Out of a total of 22 quantitative studies, 20 were classified as A, with 2 scoring B based on the STROBE criteria.14 The two qualitative studies scored A and B using the COREQ tool,15 with one study, graded a C, excluded. Using the MMAT tool,16 four studies were graded A and one as B in mixed methodology.
Provider factors affecting DR-TB diagnosis and treatment
In all 29 retained studies, the most dominant factors affecting DR-TB care were provider-related (table 2, figure 3). Our study highlighted a wide range of specific problems reflecting nearly all aspects of service delivery and health workforce with a few issues related to leadership and governance, and management of health products and information.
Service delivery was, by far, the most predominant provider-related barrier. Laboratory10 27–32 and clinic10 28 31 33 34 operational challenges, as well as centralisation of services7 28 30 32 35–39 and poor linkage between the public and private sector,29 32 hampered care. Inadequate provider knowledge, skill and adherence to national guidelines were also recurring themes.10 27–30 32 34 40–44 These are discussed in more detail in the context of the paired dimensions of access.
Patient-level factors influencing DR-TB diagnosis and treatment
Most patient-level barriers were related to predisposing characteristics including knowledge and perceptions,29 39 40 45 HIV status,7 38 43 44 presenting symptoms,44 46 gender44 46 and age27 43 44 46; and enabling characteristics including geographic location,10 27 28 30 43 47–49 life commitments29 32 40 and the ability to pay for transportation or services.29 32 39 40 A few need and health-seeking characteristics were also identified relating to treatment refusal,36 and choosing private sector care.10 32
Paired access dimensions
We have summarised access factors and recommendations from the reviewed studies into four paired dimensions (table 3).18
Approachability/ability to perceive
We found that provider and patient knowledge about DR-TB services18 were hindered by inadequate leadership and governance, provider training, service delivery, patients’ predisposing and need characteristics.18 25 26 At the systems level, inadequate patient tracking, referrals and follow-up, poor provider knowledge about the service requirements and inadequate guideline availability and non-adherence were identified challenges.10 28 31 32 34 35 40–43 46 50 Patients’ ability to perceive the right services were hampered by health illiteracy, poor perceptions of services, distrust and unmet expectations.31 39 40
At the health systems level, the consequences of poor leadership and governance were reflected in patient non-referral, misdiagnosis and treatment with ineffective regimens.32 42 Guidelines awareness, availability and expansion to include low-risk groups were shown to improve access.32 42 47 51
Provider knowledge, skills and attitude were repeatedly shown to influence access and was a predominant theme (figure 3). Delayed or inadequate training, inexperience and poor supervision of health workers influenced product availability, diagnosis and treatment.10 28 29 32 40 41 Poor adherence to DR-TB testing algorithm, treatment guidelines or referral procedures hampered diagnosis and treatment,27 30 32 34 42–44 with patients often left undiagnosed, untreated, treated with ineffective drugs or only after serious complications.32 42
At the patient level, poor perception of the public sector (overburdened, long waiting times, negative staff attitudes, poor confidentiality, lack of privacy, risk of infection) were some reasons why patients were avoiding the public sector hospitals where DR-TB services could be accessed.29 39 40 45
Wrong disease attribution, symptom minimisation, non-disclosure, treatment refusal and choosing traditional care were also noted as delaying care-seeking.10 32 36 45
Patients seeking care first in the private sector (private hospitals, pharmacies, patent medicine vendors, traditional healers), where the index of suspicion was lower, instead of public sector, where services were available, had lower odds of getting tested.44
Acceptability/ability to seek
Our review found that although provider attitudes and practice were implicated, patients’ predisposing characteristics were predominant in influencing their decisions to use health services.18 25 26 Acceptability challenges were related to poor healthcare worker norms and attitude including confidentiality concerns, stigma and how the care patients received were influenced by their symptoms7 43 44 46 or sociodemographic characteristics.40 45 Patient’s ability to seek were influenced by their sociodemographic characteristics, personal, cultural and social values, disclosure, work and family commitments, use of private sector alternatives and fear of poor infection control.27 29–33 36 39 40 44 50 51
At the provider level, stigma and discrimination towards providers from other hospital workers, and from provider to patients compromised access to and quality of care.10 32 45
At the patient level, living with HIV had conflicting results. Some studies found no association between HIV status and having a DST done, nor with time to treatment.48 52 However, two studies found patients with HIV having overall higher odds of receiving a TB diagnosis.38 44 However, HIV-positive patients had longer times to treatment or were less likely to initiate treatment,7 43 except in one study where treatment initiation rates were higher than in HIV-negative patients.50 In qualitative studies, the fear of an HIV diagnosis delayed health-seeking, and some patients with HIV were seen to have an increased awareness of TB risk.32
Patients presenting with more than any two of TB symptoms (cough, fever, weight loss, night sweats), retreatment cases and undernourished children were more likely to be screened for TB on hospital presentation for other reasons than those presenting with fewer symptoms, new cases and well-nourished children, respectively.44 46 Smear-positive cases and more symptomatic patients were more likely to have a DST done.27 47 Half of the time, previous TB led to faster symptom recognition and care-seeking.32 In one study, being pregnant made accessing DR-TB care more difficult as providers refused to initiate any DR-TB-related care.32
Patient agency and persistence in demanding DR-TB testing where none was offered was noted as a facilitator to DR-TB healthcare, and this was linked to HIV positivity or prior knowledge of the disease, either through an earlier TB infection or knowing another patient with DR-TB.32
Results linking access to patient gender and age were largely inconclusive. In several studies, neither patient gender nor age were found to be associated with diagnosis timeliness or rates,27 30 52 nor with treatment initiation rates or timeliness.36 39 43 48 There were some indications that females44 46 or children whose mothers are the primary TB source,40 or younger age27 43 44 46 were less like to be diagnosed or treated (table 2). One study33 found children to be more likely to initiate treatment than adults.
One study noted other contextual patient factors that were seen to influence DR-TB care. In South Africa, ethnicity and cigarette smoking—with children failing to attend clinic appointments more frequently from coloured ethnicity and homes with cigarette smokers. No particular reason was given for these differences, however, it was acknowledged that these were markers for other socioeconomic and cultural factors needing further research.40
Availability/ability to reach
These were mostly related to service delivery, access to health products and patient tracking on the provider side and geographic access and life commitments on the patient side.18 25 26 Specific health system barriers were related to coverage, bed spaces and centralisation of services; inadequate availability and coverage of health products—equipment and technology, advanced diagnostics and medications; shortages of health personnel; clinic and laboratory errors.7 10 27 28 30–35 37–40 45 47 49 51–53 Patients were prevented from reaching health services when they lived in inaccessible locations or faraway distances, lack of social support and difficulty in transportation, poor sputum specimen, out-migration or death.27 32 33 36 37 40 42 45 49–51
Laboratory operational challenges were the most reoccurring barriers to care (figure 3). Specimen contamination, loss of viability, difficulty in packaging, batching, transportation and delivery of samples10 27 28 delayed diagnosis. Not requesting tests, incomplete records, delayed results were other barriers preventing patients form accessing care.10 28–32 Staff shortages, especially laboratory staff, contributed to diagnostic delays and patient waiting times.10 28 45 There were significant geographical variations, mostly in laboratory operations, which impacted referral, diagnosis and treatment rates.10 27 28 30 33 43 47–49 National programme support to health centres and using expedited mail service for sample transportation were helpful in reducing laboratory delays.28
Poor data management affected patient linkage to care and reporting. Errors including missing patient records in diagnosis or treatment registers, irretrievable request forms, incomplete data entry led to misplaced results, untraceable patients and poor linkage to care.10 28 31 33 34
Inadequate coverage and maintenance of diagnostic equipment, as well as power outages hampered diagnostic capacity10 28 44 45 and staff motivation.45 Where available, using the Xpert notification system improved team communication and facilitated diagnosis.31
Centralisation (in few specialised health centres) of GeneXpert MTB/RIF (Xpert) or other pretreatment requirements like X-rays, and a lack of integration, increased diagnosis time28 39 and resulted in negative patient experiences.45 Service decentralisation (widespread availability of services, and at the different healthcare levels) was, consequently shown to be a major facilitator of access (figure 3), reducing time to diagnosis and treatment and increasing diagnosis rates.7 30 32 35–38 However, patients initiating care at higher facilities had lower odds of getting tested or initiating treatment.36 44 50 Treatment initiation rate was highest among patients diagnosed directly through TB hospitals.43 In one setting, timeliness of treatment was higher among patients initiated as inpatients compared with outpatients.33
The public sector had longer waiting times pushing patients to access care in the private sector, with poor linkages between the two.29 32 Failure or delay in tracking patients, and unavailability of results at appointments prevented access.10 32
Access to newer diagnostics was the principal facilitator of access identified (figure 3). There was an overall consensus that the use of older diagnostic tests (eg, X-rays, drug susceptibility testing (DST) or line probe assay (LPA)), when compared with Xpert, was associated with longer times to diagnosis and treatment.7 28 37–39 41 43 51–53 With the exception of one study,35 Xpert implementation did not result in corresponding increases in diagnosis and treatment rates.7 37 43 54 Also, the average time to DR-TB care remained significantly higher than the national targets in most settings.33 39 41 43 52 53
At the patient level, several studies noted high rates of patients being lost to follow-up or dying before treatment due to non-referrals, data errors, prolonged pretreatment processes and delayed care-seeking.31 42 50
Geographical location of patients was also identified as an access barrier. Patients in an urban/formal settlement accessed care more compared with those in rural, informal settlements or prison. Other variations were likely linked to the healthcare location, as noted above.32 33 50 53 Significant variations in accessing diagnosis and treatment were due to geographical locations of the patients, with urban residence or proximity to care facility increasing likelihood of testing and treatment, as well as reducing time to results and treatment.10 27 28 30 43 47–49
Family, work or school commitments were seen to prevent or interrupt the care process, while the presence of family support enabled care-seeking.29 32 40
Affordability/ability to pay
The financial implications of services were mostly related to the enabling characteristics of patients to pay for care including transportation costs.18 25 26 In our review, we found difficulties in paying for transport to health facilities, and high opportunity costs borne by patients.32 40
Ease of access and cost of health services were some reasons for choice of facility. A lack of money for transport, travel time and numerous bus transfers influenced whether they returned to the facility after initial visit.29 39 40 Seeking care in the private sector was noted as contributing to the high costs of care for patients, as some go the public sector, where care was perceived as poor, only when they could no longer afford private care.32 In another study where high costs of care was noted, majority of the patients sought care in the private sector.29
Discussion
Our review synthesises the diverse knowledge base about obstacles to DR-TB care in SSA to create a consolidated understanding to inform practice. It highlighted several health system and patient barriers.
Our key findings include the role of rapid diagnostics and laboratory operational issues play in facilitating or impeding access. Rapid diagnostic tools, particularly Xpert, play a central role in accessing DR-TB diagnosis and treatment, and their absence would constitute a significant barrier to receiving care.28 38 39 The introduction of these tools has led to a significant reduction in times to care for DR-TB.7 38 39 43 51–53 However, although times were shortened, patients still experienced unnecessary delays in accessing care. The gains of rapid diagnostic technology have, so far, not translated into a commensurate increase in rates of detection and treatment.7 37 39 43 These were likely due to the range of laboratory operational errors identified,10 27–32 and which need to be targeted to improve case finding and treatment rates.
Our review data reveal several missed opportunities for screening and treatment initiation.7 32 34 46 These contribute to the global ‘missing cases’, perpetuate transmission and highlight critical gaps in the care cascade. For example, the inadequate linkage between the private and the public sector occur before access to testing and are beyond the scope of rapid diagnostics. They contribute to the persistence of low diagnostic and treatment rates despite Xpert implementation.
Results for age and sex were found to be divergent, as many studies found both factors not significant in impacting care. In the studies where age was significant, younger age was mostly a barrier,27 43 44 46 except in one study33 where the programme prioritised children and other high-risk groups for Xpert diagnosis and inpatient care. Where most studies found sex not associated with DR-TB care, some found being female or a child or a female patient with DR-TB to be a barrier to care.27 40 44 46 One study found females more likely to have earlier diagnosis, likely due to care-seeking behaviours.52
Several contextual factors like language, religion and culture were not identified by the studies included in this review. Geographic locations of the health centres and of the patients themselves were identified as influencing access to care, and this has been reported by other authors.55 56 Ethnicity and lifestyle were identified in one study to influence access, likely due to socioeconomic and cultural implications.40 The lack of qualitative data on the influence of sex and age on access makes it difficult to draw conclusions about whether the effects seen were due to contextual factors.
To improve patient-level barriers calls for a close examination of social determinants like poverty and geographic access as an addition to biomedical approaches, as recommended by the Commission on Social Determinants of Health (CSDH).57–59 The burdens of infectious diseases like TB are disproportionately borne by patients with certain sociodemographic characteristics. For example, rural patients bear higher treatment costs and report more difficulty with transport to health centres for treatment.60 61 Demographic characteristics such as gender, poverty or ethnicity often interact in complex ways, further increasing vulnerability and disadvantage.62 63 Inadequate knowledge of DR-TB disease and health services was also identified as a major cause of poor health-seeking behaviour among patients. Raising public awareness of symptoms and available resources may contribute to reducing these delays.
The biomedical approach, which focuses more on the use of technology to manage diseases needs to be combined with efforts to tackle root causes and social determinants of DR-TB disease.60 64
Our findings indicate that, in order to overcome prevailing barriers to care, innovative diagnostic tools and treatment require functional, efficient and accessible health systems to reach and track patients who are, themselves, informed and motivated. The high susceptibility of individuals getting harmed from DR-TB, due to the complex interaction between risk factors and available resources, is manifested in their inability to manage risks or recover from the disease effectively.63 This is corroborated by many of the reviewed studies in which diagnosed patients died before they could be initiated on life-saving treatment.31 36 42 50 This highlights the fact that DR-TB continues to be characterised by avoidable morbidity and deaths, especially in SSA, and must be treated with urgency. The raison d’être of rapid diagnostic methods is to improve these outcomes by facilitating quicker diagnosis and treatment. Xpert implementation did not translate to universal increases in diagnosis and treatment rates, presenting a significant setback and missed opportunity in the control of DR-TB.
Gaps in the capacity of the health system to deliver care need to be closed. This would require significant investments at lower levels of care towards more decentralised and ambulatory models of care. In order to fund these efforts, SSA governments need to prioritise and increase health investments and mobilise resources to fund TB control.
Strengths and limitations
The strengths of this review include the adaptation of our conceptual framework to align factors influencing DR-TB care with other well-known frameworks in the field of healthcare access and systems strengthening,18 25 26 using a mixed-methods approach.
This review has a few limitations. First, due to the heterogeneity of study methods and outcome variables, neither summary measures (eg, effect size) nor pooled analysis for specific interventions were determined, as the studies were not sufficiently comparable to each other. Another limitation is related to the location of the studies. Our search showed a dominance of studies from South Africa, with only two from Nigeria, and none from Angola, DR Congo, Ethiopia, Kenya, Mozambique, the other TB/DR-TB/HIV HBCs in the region. This may have affected the generalisability of our findings within the region, as there are likely other barriers in the different other settings that were not identified. However, the relatively higher HIV burden in South Africa, and the country’s quick adoption of newer diagnostics and medications could serve as an example for these other countries as they scale-up services for DR-TB.
Conclusions
The implications of these findings are sobering; they suggest that despite significant progress in cutting down time to diagnosis and treatment by using rapid diagnostics, this is not enough, in itself, to remove all delays to diagnosis, as other barriers persist in the health system.
WHO recognises that DR-TB is a social justice problem and as a threat to global health security, requiring universal access to the tools and services needed for rapid diagnosis, treatment and care.65 Diagnosis and treatment for DR-TB is a complex and multifaceted socioeconomic problem that needs to be addressed using a multisectoral approach.66 Provider-level and patient-level barriers need to be addressed to maximise the impact of advanced diagnostics. Most of the operational problems identified, such as the poor provider knowledge and implementation of DR-TB guidelines or inefficient screening or laboratory processes, are rectifiable, although with a substantial amount of effort and investment. We have identified this review as a call to action for all relevant players.
There is a need for more studies focusing on contextual access dimensions and care cascades from more HBCs in SSA, as this review has highlighted a dominance of studies from South Africa.
Acknowledgments
The authors would like to thank the University of Montreal librarian Sylvie Fontaine for her valuable assistance in conducting the search strategy.
Footnotes
Handling editor: Alberto L Garcia-Basteiro
Twitter: @omenkac, @MMacSeing
Contributors: CO-O, MM-S and CZ conceived the study, DM and CZ supervised the study. CO-O, AT-A and MM-S collected data. CO-O, AT-A, PS and AA analysed the data. All coauthors reviewed and provided feedback on the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Gandhi NR, Nunn P, Dheda K, et al. Multidrug-Resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 2010;375:1830–43. 10.1016/S0140-6736(10)60410-2 [DOI] [PubMed] [Google Scholar]
- 2.WHO Global tuberculosis report 2019, 2019. [Google Scholar]
- 3.UN UN General Assembly High-Level Meeting on the fight against tuberculosis. New York: United Nations, 2018. [Google Scholar]
- 4.Osborn D, Cutter A, Ullah F. Universal sustainable development goals. in Understanding the transformational Challenge for Developed Countries. Report of Study by Stakeholder Forum, 2015. [Google Scholar]
- 5.WHO Gear up to end TB: introducing the end TB strategy. World Health Organization, 2015. [Google Scholar]
- 6.UN UN General Assembly, Seventy-third session, in 18th plenary meeting. New York: United Nations, 2018. [Google Scholar]
- 7.Cox HS, et al. Impact of decentralized care and the Xpert MTB/RIF test on rifampicin-resistant tuberculosis treatment initiation in Khayelitsha, South Africa. Open Forum Infectious Diseases 2015;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heller T, Lessells RJ, Wallrauch CG, et al. Community-Based treatment for multidrug-resistant tuberculosis in rural KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis 2010;14:420–6. [PubMed] [Google Scholar]
- 9.Schaaf HS, Shean K, Donald PR. Culture confirmed multidrug resistant tuberculosis: diagnostic delay, clinical features, and outcome. Arch Dis Child 2003;88:1106–11. 10.1136/adc.88.12.1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mpagama SG, Heysell SK, Ndusilo ND, et al. Diagnosis and interim treatment outcomes from the first cohort of multidrug-resistant tuberculosis patients in Tanzania. PLoS One 2013;8:e62034. 10.1371/journal.pone.0062034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dharmadhikari AS, Mphahlele M, Venter K, et al. Rapid impact of effective treatment on transmission of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2014;18:1019–25. 10.5588/ijtld.13.0834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boyd R, Ford N, Padgen P, et al. Time to treatment for rifampicin-resistant tuberculosis: systematic review and meta-analysis. Int J Tuberc Lung Dis 2017;21:1173–80. 10.5588/ijtld.17.0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whittemore R, Knafl K. The integrative review: updated methodology. J Adv Nurs 2005;52:546–53. 10.1111/j.1365-2648.2005.03621.x [DOI] [PubMed] [Google Scholar]
- 14.STROBE Statement STROBE Statement - Checklist of items that shouldbe included in reports of observational studies, 2007. Available: https://www.strobe-statement.org/fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_combined.pdf [Accessed 15 Sep 2018]. [DOI] [PubMed]
- 15.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349–57. 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- 16.Hong QN, et al. The mixed methods appraisal tool (MMAT) version 2018 for information professionals and researchers. Education for Information 2018:1–7. [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 18.Levesque J-F, Harris MF, Russell G. Patient-Centred access to health care: conceptualising access at the interface of health systems and populations. Int J Equity Health 2013;12:18. 10.1186/1475-9276-12-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clifford BK, Mizrahi D, Sandler CX, et al. Barriers and facilitators of exercise experienced by cancer survivors: a mixed methods systematic review. Supportive Care in Cancer 2018;26:685–700. 10.1007/s00520-017-3964-5 [DOI] [PubMed] [Google Scholar]
- 20.Abegaz TM, Shehab A, Gebreyohannes EA, et al. Nonadherence to antihypertensive drugs: a systematic review and meta-analysis. Medicine 2017;96:e5641. 10.1097/MD.0000000000005641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeve E, To J, Hendrix I, et al. Patient barriers to and enablers of deprescribing: a systematic review. Drugs Aging 2013;30:793–807. 10.1007/s40266-013-0106-8 [DOI] [PubMed] [Google Scholar]
- 22.Pace R, Pluye P, Bartlett G, et al. Testing the reliability and efficiency of the pilot mixed methods appraisal tool (MMAT) for systematic mixed studies review. Int J Nurs Stud 2012;49:47–53. 10.1016/j.ijnurstu.2011.07.002 [DOI] [PubMed] [Google Scholar]
- 23.Yang W-T, Gounder CR, Akande T, et al. Barriers and delays in tuberculosis diagnosis and treatment services: does gender matter? Tuberc Res Treat 2014;2014:1–15. 10.1155/2014/461935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailie J, Schierhout G, Laycock A, et al. Determinants of access to chronic illness care: a mixed-methods evaluation of a national multifaceted chronic disease package for Indigenous Australians. BMJ Open 2015;5:e008103. 10.1136/bmjopen-2015-008103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO Everybody's business-strengthening health systems to improve health outcomes: who's framework for action, 2007. [Google Scholar]
- 26.Andersen R, Newman JF. Societal and individual determinants of medical care utilization in the United States. The Milbank Quarterly 2005;83. [PubMed] [Google Scholar]
- 27.Jacobson KR, Barnard M, Kleinman MB, et al. Implications of failure to routinely diagnose resistance to second-line drugs in patients with rifampicin-resistant tuberculosis on Xpert MTB/RIF: a multisite observational study. Clin Infect Dis 2017;64:1502–8. 10.1093/cid/cix128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doulla BE, Squire SB, MacPherson E, et al. Routine surveillance for the identification of drug resistant tuberculosis in Tanzania: a cross-sectional study of stakeholders' perceptions. PLoS One 2019;14:e0212421. 10.1371/journal.pone.0212421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van der Westhuizen H-M, Dramowski A. When students become patients: TB disease among medical undergraduates in Cape town, South Africa. S Afr Med J 2017;107:475–9. 10.7196/SAMJ.2017.v107i6.12260 [DOI] [PubMed] [Google Scholar]
- 30.Timire C, Sandy C, Kumar AMV, et al. Access to second-line drug susceptibility testing results among patients with Rifampicin resistant tuberculosis after introduction of the Hain® Line Probe Assay in Southern provinces, Zimbabwe. Int J Infect Dis 2019;81:236–43. 10.1016/j.ijid.2019.02.007 [DOI] [PubMed] [Google Scholar]
- 31.Mnyambwa NP, Lekule I, Ngadaya ES, et al. Assessment of GeneXpert GxAlert platform for multi-drug resistant tuberculosis diagnosis and patients' linkage to care in Tanzania. BMC Res Notes 2018;11:121. 10.1186/s13104-018-3235-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naidoo P, van Niekerk M, du Toit E, et al. Pathways to multidrug-resistant tuberculosis diagnosis and treatment initiation: a qualitative comparison of patients' experiences in the era of rapid molecular diagnostic tests. BMC Health Serv Res 2015;15:488. 10.1186/s12913-015-1145-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oga-Omenka C, Zarowsky C, Agbaje A, et al. Rates and timeliness of treatment initiation among drug-resistant tuberculosis patients in Nigeria- a retrospective cohort study. PLoS One 2019;14:e0215542. 10.1371/journal.pone.0215542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohr E, Daniels J, Muller O, et al. Missed opportunities for earlier diagnosis of rifampicin-resistant tuberculosis despite access to Xpert® MTB/RIF. Int J Tuberc Lung Dis 2017;21:1100–5. 10.5588/ijtld.17.0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans D, Sineke T, Schnippel K, et al. Impact of Xpert MTB/RIF and decentralized care on linkage to care and drug-resistant tuberculosis treatment outcomes in Johannesburg, South Africa. BMC Health Serv Res 2018;18:973. 10.1186/s12913-018-3762-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moyo S, J Furin J, Hughes J, et al. Outcomes in adolescents undergoing treatment for drug-resistant tuberculosis in Cape town, South Africa, 2008-2013. Arch Pediatr Infect Dis 2014;2 10.5812/pedinfect.17934 [DOI] [Google Scholar]
- 37.Van Den Handel T, Hampton KH, Sanne I, et al. The impact of Xpert(®) MTB/RIF in sparsely populated rural settings. Int J Tuberc Lung Dis 2015;19:392–8. 10.5588/ijtld.14.0653 [DOI] [PubMed] [Google Scholar]
- 38.Hanrahan CF, Selibas K, Deery CB, et al. Time to treatment and patient outcomes among TB suspects screened by a single point-of-care Xpert MTB/RIF at a primary care clinic in Johannesburg, South Africa. PLoS One 2013;8:e65421 10.1371/journal.pone.0065421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naidoo P, du Toit E, Dunbar R, et al. A comparison of multidrug-resistant tuberculosis treatment commencement times in MDRTBPlus line probe assay and Xpert® MTB/RIF-Based algorithms in a routine operational setting in Cape town. PLoS One 2014;9:e103328 10.1371/journal.pone.0103328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zimri K, Hesseling AC, Godfrey-Faussett P, et al. Why do child contacts of multidrug-resistant tuberculosis not come to the assessment clinic? Public Health Action 2012;2:71–5. 10.5588/pha.12.0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dlamini-Mvelase NR, Werner L, Phili R, et al. Effects of introducing Xpert MTB/RIF test on multi-drug resistant tuberculosis diagnosis in KwaZulu-Natal South Africa. BMC Infect Dis 2014;14:442. 10.1186/1471-2334-14-442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nkosi D, Janssen S, Padanilam X, et al. Factors influencing specialist care referral of multidrug- and extensively drug-resistant tuberculosis patients in Gauteng/South Africa: a descriptive questionnaire-based study. BMC Health Serv Res 2013;13:268. 10.1186/1472-6963-13-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cox H, Dickson-Hall L, Ndjeka N, et al. Delays and loss to follow-up before treatment of drug-resistant tuberculosis following implementation of Xpert MTB/RIF in South Africa: a retrospective cohort study. PLoS Med 2017;14:e1002238–19. 10.1371/journal.pmed.1002238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oliwa JN, Maina J, Ayieko P, et al. Variability in distribution and use of tuberculosis diagnostic tests in Kenya: a cross-sectional survey. BMC Infect Dis 2018;18:328. 10.1186/s12879-018-3237-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bieh KL, Weigel R, Smith H. Hospitalized care for MDR-TB in Port Harcourt, Nigeria: a qualitative study. BMC Infect Dis 2017;17:50. 10.1186/s12879-016-2114-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kweza PF, Van Schalkwyk C, Abraham N, et al. Estimating the magnitude of pulmonary tuberculosis patients missed by primary health care clinics in South Africa. Int J Tuberc Lung Dis 2018;22:264–72. 10.5588/ijtld.17.0491 [DOI] [PubMed] [Google Scholar]
- 47.McLaren ZM, Sharp AR, Zhou J, et al. Assessing healthcare quality using routine data: evaluating the performance of the National tuberculosis programme in South Africa. Trop Med Int Health 2017;22:171–9. 10.1111/tmi.12819 [DOI] [PubMed] [Google Scholar]
- 48.Metcalfe JZ, Makumbirofa S, Makamure B, et al. Xpert(®) MTB/RIF detection of rifampin resistance and time to treatment initiation in Harare, Zimbabwe. Int J Tuberc Lung Dis 2016;20:882–9. 10.5588/ijtld.15.0696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jokwiro A, Timire C, Harries AD, et al. Has the utilisation of Xpert® MTB/RIF in Manicaland Province, Zimbabwe, improved with new guidance on whom to test? Public Health Action 2018;8:124–9. 10.5588/pha.18.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebonwu JI, Tint KS, Ihekweazu C. Low treatment initiation rates among multidrug-resistant tuberculosis patients in Gauteng, South Africa, 2011. Int J Tuberc Lung Dis 2013;17:1043–8. 10.5588/ijtld.13.0071 [DOI] [PubMed] [Google Scholar]
- 51.Hanrahan CF, Dorman SE, Erasmus L, et al. The impact of expanded testing for multidrug resistant tuberculosis using genotype [correction of geontype] MTBDRplus in South Africa: an observational cohort study. PLoS One 2012;7:e49898. 10.1371/journal.pone.0049898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iruedo J, O’Mahony D, Mabunda S, et al. The effect of the Xpert MTB/RIF test on the time to MDR-TB treatment initiation in a rural setting: a cohort study in South Africa’s Eastern Cape Province. BMC Infect Dis 2017;17:1–9. 10.1186/s12879-017-2200-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jacobson KR, Theron D, Kendall EA, et al. Implementation of genotype MTBDRplus reduces time to multidrug-resistant tuberculosis therapy initiation in South Africa. Clin Infect Dis 2013;56:503–8. 10.1093/cid/cis920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naidoo P, du Toit E, Dunbar R, et al. A comparison of multidrug-resistant tuberculosis treatment commencement times in MDRTBPlus line probe assay and Xpert® MTB/RIF-based algorithms in a routine operational setting in Cape town. PLoS One 2014;9:e103328. 10.1371/journal.pone.0103328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sullivan BJ, Esmaili BE, Cunningham CK. Barriers to initiating tuberculosis treatment in sub-Saharan Africa: a systematic review focused on children and youth. Glob Health Action 2017;10:1290317. 10.1080/16549716.2017.1290317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van de Water BJ, Prvu Bettger J, Silva S, et al. Time to drug-resistant tuberculosis treatment in a prospective South African cohort. Glob Pediatr Health 2017;4:2333794X1774414. 10.1177/2333794X17744140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hargreaves JR, Boccia D, Evans CA, et al. The social determinants of tuberculosis: from evidence to action. Am J Public Health 2011;101:654–62. 10.2105/AJPH.2010.199505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rasanathan K, Sivasankara Kurup A, Jaramillo E, et al. The social determinants of health: key to global tuberculosis control. Int J Tuberc Lung Dis 2011;15 Suppl 2:30–6. 10.5588/ijtld.10.0691 [DOI] [PubMed] [Google Scholar]
- 59.CSDH Closing the gap in a generation: health equity through action on the social determinants of health. final report of the Commission on social determinants of health. World Health Organization: Geneva, 2008: 256. [Google Scholar]
- 60.Farmer P. Social inequalities and emerging infectious diseases. Emerg Infect Dis 1996;2:259–69. 10.3201/eid0204.960402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhutta ZA, Salam RA, Das JK, et al. Tackling the existing burden of infectious diseases in the developing world: existing gaps and the way forward. Infect Dis Poverty 2014;3:1 10.1186/2049-9957-3-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marmot M, Allen J, Bell R, et al. Who European review of social determinants of health and the health divide. Lancet 2012;380:1011–29. 10.1016/S0140-6736(12)61228-8 [DOI] [PubMed] [Google Scholar]
- 63.Zarowsky C, Haddad S, Nguyen V-K. Beyond 'vulnerable groups': contexts and dynamics of vulnerability. Glob Health Promot 2013;20:3–9. 10.1177/1757975912470062 [DOI] [PubMed] [Google Scholar]
- 64.WHO Health in all policies: Helsinki statement. framework for country action, 2014. [Google Scholar]
- 65.Organization, W.H Gear up to end TB: introducing the end TB strategy. World Health Organization, 2015. [Google Scholar]
- 66.World Health Organization, Health in all policies: Helsinki statement. framework for country action, 2014. [Google Scholar]
- 67.Mpagama SG, Mbelele PM, Chongolo AM, et al. Gridlock from diagnosis to treatment of multidrug-resistant tuberculosis in Tanzania: low accessibility of molecular diagnostic services and lack of healthcare worker empowerment in 28 districts of 5 high burden TB regions with mixed methods evaluation. BMC Public Health 2019;19:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjgh-2019-002280supp001.pdf (168.3KB, pdf)