Abstract
Checkpoint inhibitor immunotherapy has revolutionised cancer treatment since its inception. During an inflammatory response, activated cytotoxic T cells expressing programmed cell death protein 1 (PD-1) interact with programmed cell death-ligand 1 (PD-L1) on peripheral tissues to thwart an autoimmune reaction. Cancer cells upregulate PD-L1 expression to evade the immune system and are vulnerable to attack in the presence of PD-1 or PD-L1 checkpoint inhibitors. However, blockade of this pathway also contributes to the unintended side effect of autoimmune endocrinopathies. Atezolizumab, a checkpoint inhibitor against PD-L1, is associated with the rare complication of type 1 diabetes. We present a case of glutamic acid decarboxylase antibody-positive type 1 diabetes developing in a patient with a long-standing history of well-controlled type 2 diabetes following treatment with atezolizumab for metastatic renal cell carcinoma.
Keywords: diabetes, chemotherapy
Background
Immunotherapy has revolutionised cancer treatment and led to clinical improvements in outcomes for metastatic malignancies previously deemed incurable. Currently available drugs target molecules that regulate immune checkpoints such as cytotoxic T-lymphocyte-associated protein 4 and programmed cell death protein 1 or ligand 1 (PD-1 or PD-L1).1 2 Checkpoint immune inhibitors operate by manipulating the body’s own immune T cells to recognise cancer cells as foreign, thereby making the cancer cells vulnerable to immune attack.1–3 Checkpoint immune inhibitors lead to a bolstering of T-cell activity, which is responsible for both the desired antitumour response and also the unintended autoimmune side effects.4–6 Atezolizumab, an engineered humanised IgG1 isotype checkpoint inhibitor against PD-L1, is approved by the USA Food and Drug Administration for use in advanced urothelial,7 lung8 and breast9 cancers and is under investigation for other cancers including renal cell carcinoma.10 Associated autoimmune endocrine side effects are uncommon and include hypothyroidism (5%), hyperthyroidism (2%), adrenal insufficiency (<1%) and type 1 diabetes mellitus (T1DM) with diabetic ketoacidosis (DKA) (<1%).4 6 11–20 As a result of the success that this group of cancer chemotherapeutic agents, the number of reports of immune checkpoint inhibitor endocrinopathies is expected to grow. We present a case of glutamic acid decarboxylase (GAD) antibody-positive T1DM developing in a patient with a history of long-standing well-controlled type 2 diabetes mellitus (T2DM) following treatment with atezolizumab for metastatic renal cell carcinoma.
Case presentation
A 64-year-old man with a history of presumed T2DM, renal cell carcinoma with metastasis to liver, congestive heart failure and postablative hypothyroidism was admitted to the hospital after presenting to an outpatient oncology appointment with polyuria, polydipsia, blurred vision and hyperglycaemia (glucose 609 mg/dL). High-dose glucocorticoids (prednisone 60 mg × three doses over the 12 hours prior to imaging) taken for an intravenous contrast allergy in preparation for a CT scan were thought to contribute to the hyperglycaemia. Inpatient evaluation showed serum bicarbonate 16 mEq/dL and +2 urine ketones which were concerning for DKA (table 1).
Table 1.
Admission laboratory evaluation
| Serum values (range) | Results |
| Plasma glucose (70–99 mg/dL) | 609 mg/dL |
| Acetone, blood (ref: negative) | Mildly elevated |
| Anion gap (4–16) | 22 |
| Serum bicarbonate (22–26 mmol/L) | 16 mmol/L |
| Urine ketones (negative) | 2+ |
| Haemoglobin A1c (≤5.6%) | 8.3 % |
| Venous pH (7.32–7.44) | 7.29 |
| Glutamic acid decarboxylase antibody-65 (0–5 IU/mL) | >250 IU/mL |
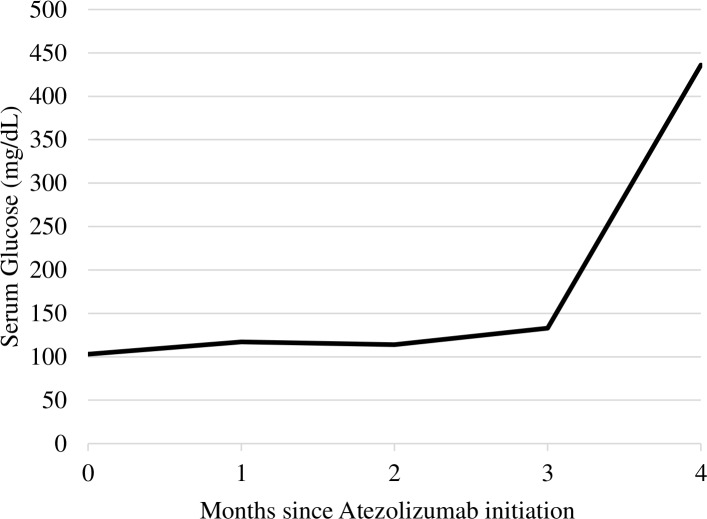
At the time of presentation, he had received eight cycles of combination immunotherapy with atezolizumab (PD-L1 inhibitor), interleukin-2 (IL-2) and bevacizumab (vascular endothelial growth factor-A inhibitor) over the course of the previous 3 months for metastatic renal cancer. Since developing capillary leak syndrome, a complication of IL-2 treatment, 2 months prior to admission, he was also treated with high-dose glucocorticoids (hydrocortisone 200 mg) every 2 weeks before each chemotherapy cycle. The patient was originally diagnosed with T2DM by his primary care provider 5 years prior to presentation at our institution. He was presumed to have T2DM based on his initial clinical presentation which included obesity (body mass index 35.8 kg/m2) and other features of metabolic syndrome including hypertension and hyperlipidaemia, absence of DKA, good glucose control on oral medications and strong family history of T2DM. Glucose levels had been previously well controlled with haemoglobin A1c of 5.5% and he did not have evidence of microvascular or macrovascular complications. Three months prior to presentation, linagliptin and metformin were discontinued due to nausea with chemotherapy. Off of oral hypoglycaemic medications, glucose levels were initially 80–90 mg/dL on diet alone; however, glucose levels rose to 300–400 mg/dL over the month prior to admission (figure 1) despite no changes in diet or activity and included times when the patient was not receiving glucocorticoid therapy.
Figure 1.
Serum glucose trend since initiation of atezolizumab.
Investigations
Because of the presentation with recent decompensation in glucose control, DKA and the prior use of atezolizumab, a PD-L1 inhibitor known to trigger the onset of autoimmune disorders including autoimmune diabetes, GAD-65 antibodies were checked and found to be elevated at >250 IU/mL (<0.5 U/mL) (table 1). These results are consistent with autoimmune or T1DM.
Treatment
On hospital admission, the patient was treated with intravenous fluids and a continuous intravenous insulin infusion. Once DKA is resolved, he was transitioned to a subcutaneous basal-bolus insulin regimen with glargine and aspart insulins.
Outcome and follow-up
Since discharge from the hospital, glucose levels have been well controlled on a regimen of glargine insulin 40 units per day and aspart insulin 10 units before meals. He has not experienced any further episodes of DKA. Despite the development of T1DM, he continues enrollment in the atezolizumab clinical trial as the tumour has responded to treatment based on surveillance scans displaying decreased size of hepatic metastases.
Discussion
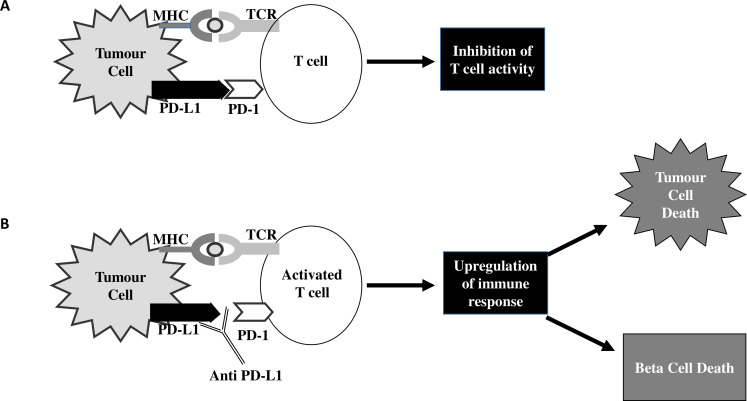
Immune checkpoint inhibitors block the ability of cancer cells to evade the immune system through a complex process.1 3 21 During an inflammatory response, T cell activation induces PD-1 receptor expression, which interacts with either PD-L1 or PD-L2 in peripheral tissue (figure 2). This interaction of PD-1 receptor protein with PD-L generates an inhibitory signal which downregulates T cell activity, suppressing autoimmunity.21 22 Human cancer cells have been shown to take advantage of this inhibitory pathway by expressing high levels of PD-L1.1 3 21 In addition to expression in malignancy, PD-1 and PD-L1 are also present on B cells, dendritic cells, macrophages, T cells, vascular endothelial cells and pancreatic beta cells.22–25
Figure 2.
Programmed cell death-ligand 1 (PD-L-1) pathway. (A) Upregulation of programmed cell death protein 1 (PD-1) pathway leads to increased inhibition of T cell/ immune response. (B) Inhibition of the PD-1 pathway via anti-PD-L1 leads to upregulation of immune response. MHC, major histocompatibility complex; TCR, T cell receptor.
Adverse endocrine events related to checkpoint inhibitor therapy include autoimmune endocrinopathies such as thyroiditis, adrenal insufficiency, hypophysitis and T1DM.4 6 13 26–34 T1DM is a rare consequence of PD-L1 inhibitor therapy (atezolizumab, avelumab, durvalumab).12 14 15 17–20 Human pancreatic beta cells are known to respond to increased inflammation with upregulation of PD-L1 expression to limit autoimmunity.22 Decreased expression of PD-1 or PD-L1 in human islet cells is associated with a higher risk of developing T1DM.22 26 35 Similarly, individuals with lower serum PD-L1 levels have a higher likelihood of developing T1DM.36 A proposed mechanism underlying the development of T1DM with PD-L1 inhibitor therapy is that PD-1–PD-L1 pathway blockade leads to activation of autoreactive T cells that target pancreatic islet beta cells. An immune-mediated destruction of these cells ensues with manifestations of diabetes developing when 80%–90% of the pancreatic beta cells have been destroyed.26 37 Thus, decreased PD-1–PD-L1 interaction, an intended effect of PD-L1 inhibitors, augments the risk of T1DM.4 6 20 37 Conversely, in some studies, patients with cancer with checkpoint inhibitor-mediated immune-related adverse events such as T1DM are more likely to respond to immunotherapy.5 38
Only 12 cases of PD-L1 inhibitor-associated T1DM have been published—seven associated with atezolizumab,11–13 15 18–20 two with avelumab,17 39 two with durvalumab14 20 and one with anti-PD-L1 therapy not specified by name40 (table 2). Three cases of atezolizumab-induced T1DM arose in patients with high-grade metastatic urothelial papillary cancer,11 12 20 three arose in patients with metastatic lung adenocarcinoma,13 15 18 and one arose in renal cell carcinoma.19 The shortest time to onset of T1DM occurred in a 63-year-old woman with renal cell carcinoma 3 weeks after the initiation of atezolizumab treatment.19 DKA was the initial presentation in 11 of the PD-L1 inhibitor cases of T1DM and none had a previous history of diabetes.11–15 17 18 20 40 Five patients had positive GAD-65 antibodies12 18 20 27 39 whereas the other seven were presumed to have T1DM based on their clinical presentation.11 13–15 17 19 40 In contrast to these 12 cases, our patient had an existing diagnosis of T2DM prior to treatment with atezolizumab.
Table 2.
Case reports of PD-L1 inhibitor-induced type 1 diabetes
| Case report | Age | Sex | PD-L1 therapy | Type of cancer | Pre-existing DM |
Time from starting PDL1 inhibitor | Presentation | BG (mg/ dL) |
C-peptide (ng/mL) | GAD-65 antibody |
| Hickmott et al11 | 57 | M | Atezolizumab | Metastatic urothelial cancer | No | 15 weeks | DKA | 432 | 0.65 (1.0–7.1) |
Negative |
| Kapke et al12 | 63 | F | Atezolizumab | High-grade urothelial cancer | No | 6 weeks | DKA | 801 | 0.02 (1.1–4.4) |
Positive >250 IU/mL |
| Way et al20 | NR | M | Atezolizumab | Metastatic papillary urothelial cancer | No | 9 weeks | DKA | 336 | 0.6 (1.1–4.3) |
Positive 28.4 IU/mL |
| Sothornwit et al18 | 52 | F | Atezolizumab | Advanced non-small cell lung cancer | No | 24 weeks | DKA | 332 | <0.012 | Positive 7.2 IU/mL |
| Patti et al15 | 70 | F | Atezolizumab | Stage 4 non-small cell lung adenocarcinoma | No | 9 weeks | DKA | 1015 | Reported normal | Negative |
| Lanzolla et al13 | 60 | M | Atezolizumab | Metastatic lung adenocarcinoma | No | 12 weeks | DKA | 549 | 0.7 (>1) |
Negative |
| Stamatouli et al19 | 63 | F | Atezolizumab | Renal cell carcinoma | No | 3 weeks | NR | NR | NR | NR |
| Shibayama et al17 | 81 | F | Avelumab | Metastatic Merkel cell carcinoma | No | 20 weeks | DKA | 483 | Undetectable | Negative |
| Atkins et al10 | 50 | M | Avelumab | Metastatic tonsillar squamous cell cancer | No | 4 weeks | DKA | 340 | 28.7 | Positive 128.1 IU/mL |
| Way et al20 | 84 | F | Durvalumab | Metastatic squamous cell carcinoma of nasopharynx | No | 16 weeks | DKA | 488 | 0.4 (1.1–4.3) |
Positive 13 IU/mL |
| Marchand et al14 | 69 | M | Durvalumab | Pulmonary adenocarcinoma | No | 4 weeks | DKA | 558 | <0.01 | Negative |
| Mellati et al40 | 70 | M | PD-L1 unspecified | Advanced adenocarcinoma of lung | No | 15 weeks | DKA | 410 | 0.3 (1–7.1) |
Negative |
BG, blood glucose; DKA, diabetic ketoacidosis; DM, diabetes mellitus; F, female; GAD, glutamic acid decarboxylase; M, male; NR, not reported; PD-L1, programmed cell death ligand 1.
Our case and the previously reported cases point to the importance of having a high level of suspicion and subsequent screening for T1DM in patients receiving PD-L1 inhibitors presenting new onset with hyperglycaemia. Based on our experience of the development of T1DM in a patient with presumed T2DM, a high level of suspicion for conversion to T1DM/autoimmune diabetes is also warranted for patients with T2DM when an unexplained decompensation in glucose control develops and/or the development of DKA. While the incidence of type 1 diabetes in patients receiving check point inhibitors is still low, the increasing indications for their use of check point inhibitors in cancer treatment portend a greater number of cases in the future. Patients receiving check point inhibitors typically undergo frequent laboratory studies that include glucose levels. Those with an unexplained upward trend in glucose levels should be considered for more frequent glucose monitoring (eg, home glucose monitoring with a glucometer) and testing for GAD-65 antibody. The additional monitoring would allow for earlier identification of at-risk patients potentially preventing hyperglycaemic decompensation and hospitalisation. While these patients would likely be treated initially with insulin because of the severe hyperglycaemia, insulin should also be a first-line therapy for decompensated hyperglycaemia in patients receiving PD-L1 inhibitors due to the possibility of type 1 diabetes.
Learning points.
Through blockade of the programmed cell death protein 1 and programmed cell death ligand 1 (PD-L1) pathway, cancer cells are less able to evade the immune response and become vulnerable to destruction by cytotoxic T cells. However, this blockade can also lead to an unintended increase in autoimmune destruction of cells including pancreatic insulin producing beta cells.
This case is the first to describe type 1 diabetes mellitus (T1DM) secondary to atezolizumab therapy developing in a patient previously diagnosed with type 2 diabetes mellitus (T2DM).
Screening for T1DM in patients receiving PD-L1 inhibitors is critical when patients present with new onset hyperglycaemia and in when patients with T2DM present with a decompensation in glucose control and/or diabetic ketoacidosis.
Footnotes
Twitter: @diabetes
Contributors: KDS and WR participated in the care of the reported patient. KDS, AC and WR contributed to the development and writing of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol 2017;8:561. 10.3389/fphar.2017.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diesendruck Y, Benhar I. Novel immune check point inhibiting antibodies in cancer therapy-Opportunities and challenges. Drug Resist Updat 2017;30:39–47. 10.1016/j.drup.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 3.Velcheti V, Schalper K. Basic overview of current immunotherapy approaches in cancer. Am Soc Clin Oncol Educ Book 2016;35:298–308. 10.1200/EDBK_156572 [DOI] [PubMed] [Google Scholar]
- 4.Ferrari SM, Fallahi P, Elia G, et al. Autoimmune endocrine dysfunctions associated with cancer immunotherapies. Int J Mol Sci 2019;20. 10.3390/ijms20102560. [Epub ahead of print: 24 May 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Judd J, Zibelman M, Handorf E, et al. Immune-related adverse events as a biomarker in non-melanoma patients treated with programmed cell death 1 inhibitors. Oncologist 2017;22:1232–7. 10.1634/theoncologist.2017-0133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sznol M, Postow MA, Davies MJ, et al. Endocrine-Related adverse events associated with immune checkpoint blockade and expert insights on their management. Cancer Treat Rev 2017;58:70–6. 10.1016/j.ctrv.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909–20. 10.1016/S0140-6736(16)00561-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vansteenkiste J, Wauters E, Park K, et al. Prospects and progress of atezolizumab in non-small cell lung cancer. Expert Opin Biol Ther 2017;17:781–9. 10.1080/14712598.2017.1309389 [DOI] [PubMed] [Google Scholar]
- 9.Basile D, Pelizzari G, Vitale MG, et al. Atezolizumab for the treatment of breast cancer. Expert Opin Biol Ther 2018;18:595–603. 10.1080/14712598.2018.1469619 [DOI] [PubMed] [Google Scholar]
- 10.Atkins MB, Tannir NM. Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat Rev 2018;70:127–37. 10.1016/j.ctrv.2018.07.009 [DOI] [PubMed] [Google Scholar]
- 11.Hickmott L, De La Peña H, Turner H, et al. Anti-PD-L1 atezolizumab-Induced autoimmune diabetes: a case report and review of the literature. Target Oncol 2017;12:235–41. 10.1007/s11523-017-0480-y [DOI] [PubMed] [Google Scholar]
- 12.Kapke J, Shaheen Z, Kilari D, et al. Immune checkpoint inhibitor-associated type 1 diabetes mellitus: case series, review of the literature, and optimal management. Case Rep Oncol 2017;10:897–909. 10.1159/000480634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lanzolla G, Coppelli A, Cosottini M, et al. Immune checkpoint blockade anti-PD-L1 as a trigger for autoimmune polyendocrine syndrome. J Endocr Soc 2019;3:496–503. 10.1210/js.2018-00366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchand L, Thivolet A, Dalle S, et al. Diabetes mellitus induced by PD-1 and PD-L1 inhibitors: description of pancreatic endocrine and exocrine phenotype. Acta Diabetol 2019;56:441–8. 10.1007/s00592-018-1234-8 [DOI] [PubMed] [Google Scholar]
- 15.Patti R, Malhotra S, Sinha A, et al. Atezolizumab-Induced new onset diabetes mellitus with ketoacidosis. Am J Ther 2018;25:e565–8. 10.1097/MJT.0000000000000644 [DOI] [PubMed] [Google Scholar]
- 16.Perdigoto AL, Quandt Z, Anderson M, et al. Checkpoint inhibitor-induced insulin-dependent diabetes: an emerging syndrome. Lancet Diabetes Endocrinol 2019;7:421–3. 10.1016/S2213-8587(19)30072-5 [DOI] [PubMed] [Google Scholar]
- 17.Shibayama Y, Kameda H, Ota S, et al. Case of fulminant type 1 diabetes induced by the anti-programmed death-ligand 1 antibody, avelumab. J Diabetes Investig 2019;10:1385–7. 10.1111/jdi.13022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sothornwit J, Phunmanee A, Pongchaiyakul C. Atezolizumab-induced autoimmune diabetes in a patient with metastatic lung cancer. Front Endocrinol 2019;10:352. 10.3389/fendo.2019.00352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stamatouli AM, Quandt Z, Perdigoto AL, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes 2018;67:1471–80. 10.2337/dbi18-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Way J, Drakaki A, Drexler A, et al. Anti-PD-L1 therapy and the onset of diabetes mellitus with positive pancreatic autoantibodies. BMJ Case Rep 2017;2017. 10.1136/bcr-2017-220415. [Epub ahead of print: 04 Oct 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang Y, Chen M, Nie H, et al. PD-1 and PD-L1 in cancer immunotherapy: clinical implications and future considerations. Hum Vaccin Immunother 2019;15:1111–22. 10.1080/21645515.2019.1571892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osum KC, Burrack AL, Martinov T, et al. Interferon-Gamma drives programmed death-ligand 1 expression on islet β cells to limit T cell function during autoimmune diabetes. Sci Rep 2018;8:8295. 10.1038/s41598-018-26471-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang SC, Latchman YE, Buhlmann JE, et al. Regulation of PD-1, PD-L1, and PD-L2 expression during normal and autoimmune responses. Eur J Immunol 2003;33:2706–16. 10.1002/eji.200324228 [DOI] [PubMed] [Google Scholar]
- 24.Wang G, Zhang S, Wang F, et al. Expression and biological function of programmed death ligands in human placenta mesenchymal stem cells. Cell Biol Int 2013;37:137–48. 10.1002/cbin.10024 [DOI] [PubMed] [Google Scholar]
- 25.Yamazaki T, Akiba H, Iwai H, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol 2002;169:5538–45. 10.4049/jimmunol.169.10.5538 [DOI] [PubMed] [Google Scholar]
- 26.Ansari MJI, Salama AD, Chitnis T, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med 2003;198:63–9. 10.1084/jem.20022125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aziz K, Shahbaz A, Umair M, et al. Avelumab inducing hypothyroidism and hypoadrenalism: a case report and review of literature. Excli J 2018;17:526–30. 10.17179/excli2018-1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 2018;4:173–82. 10.1001/jamaoncol.2017.3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girotra M, Hansen A, Farooki A, et al. The current understanding of the endocrine effects from immune checkpoint inhibitors and recommendations for management. JNCI Cancer Spectr 2018;2:pky021. 10.1093/jncics/pky021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guerrero E, Johnson DB, Bachelot A, et al. Immune checkpoint inhibitor-associated hypophysitis-World health organisation VigiBase report analysis. Eur J Cancer 2019;113:10–13. 10.1016/j.ejca.2019.03.002 [DOI] [PubMed] [Google Scholar]
- 31.Imblum BA, Baloch ZW, Fraker D, et al. Pembrolizumab-induced thyroiditis. Endocr Pathol 2019;30:163–7. 10.1007/s12022-019-9579-2 [DOI] [PubMed] [Google Scholar]
- 32.Kobayashi T, Iwama S, Yasuda Y, et al. Patients with antithyroid antibodies are prone to develop destructive thyroiditis by nivolumab: a prospective study. J Endocr Soc 2018;2:241–51. 10.1210/js.2017-00432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, Masood A, Bari S, et al. Autoimmune diabetes and thyroiditis complicating treatment with nivolumab. Case Rep Oncol 2017;10:230–4. 10.1159/000456540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohara N, Kobayashi M, Ohashi K, et al. Isolated adrenocorticotropic hormone deficiency and thyroiditis associated with nivolumab therapy in a patient with advanced lung adenocarcinoma: a case report and review of the literature. J Med Case Rep 2019;13:88. 10.1186/s13256-019-2002-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujisawa R, Haseda F, Tsutsumi C, et al. Low programmed cell death-1 (PD-1) expression in peripheral CD4(+) T cells in Japanese patients with autoimmune type 1 diabetes. Clin Exp Immunol 2015;180:452–7. 10.1111/cei.12603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pizarro C, García-Díaz DF, Codner E, et al. PD-L1 gene polymorphisms and low serum level of PD-L1 protein are associated to type 1 diabetes in Chile. Diabetes Metab Res Rev 2014;30:761–6. 10.1002/dmrr.2552 [DOI] [PubMed] [Google Scholar]
- 37.Yadav D, Hill N, Yagita H, et al. Altered availability of PD-1/PD ligands is associated with the failure to control autoimmunity in NOD mice. Cell Immunol 2009;258:161–71. 10.1016/j.cellimm.2009.04.006 [DOI] [PubMed] [Google Scholar]
- 38.Indini A, Di Guardo L, Cimminiello C, et al. Immune-related adverse events correlate with improved survival in patients undergoing anti-PD1 immunotherapy for metastatic melanoma. J Cancer Res Clin Oncol 2019;145:511–21. 10.1007/s00432-018-2819-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atkins PW, Thompson DM. Combination avelumab and utomilumab immunotherapy can induce diabetic ketoacidosis. Diabetes Metab 2018;44:514–5. 10.1016/j.diabet.2017.05.005 [DOI] [PubMed] [Google Scholar]
- 40.Mellati M, Eaton KD, Brooks-Worrell BM, et al. Anti-PD-1 and Anti-PDL-1 monoclonal antibodies causing type 1 diabetes. Diabetes Care 2015;38:e137–8. 10.2337/dc15-0889 [DOI] [PubMed] [Google Scholar]