Abstract
Background
Locally advanced pancreatic cancer (LAPC) remains a challenge for current treatments. Local destructive therapies, such as irreversible electroporation (IRE) and radiofrequency ablation (RFA), were used more and more frequently in the treatment of LAPC.
Objective
This study aimed to compare the efficacy of IRE with RFA in patients with LAPC.
Methods
From August 2015 to August 2017, 58 LAPC patients after IRE or RFA therapy, which was performed through open approach, were retrospectively reviewed. The survival outcomes after IRE (36 patients) and RFA (18 patients) were compared after propensity score matching (PSM) analysis.
Results
Before PSM analysis, IRE after the induction chemotherapy resulted in significant higher overall survival (OS) rates and progression‐free survival (PFS) rates to RFA (2‐year OS, 53.5% vs 30.8%, P = .013; 2‐year PFS, 28.4% vs 12.1%, P = .043). After PSM analysis, compared with RFA, the survival benefit of IRE was even more obvious, (2‐year OS, 53.5% vs 27.0%, P = .010; 2‐year PFS, 28.4% vs 6.4%, P = .018). For patients with tumor larger than 4 cm, IRE resulted in comparable OS and PFS between RFA and IRE while IRE also achieved better long‐term OS to RFA for those with tumor smaller than 4 cm. Multivariate analysis illustrated that IRE was a favorable prognostic factor in terms of OS and PFS in patients with LAPC.
Conclusions
IRE after induction chemotherapy is superior to RFA after induction chemotherapy for treating LAPC patients while these two therapies have comparable efficacy for tumors which were larger than 4 cm.
Keywords: chemotherapy, irreversible electroporation, locally advanced pancreatic cancer, prognosis, radiofrequency ablation
Locally advanced pancreatic cancer (LAPC) is a devastating disease while the standard chemotherapy remains an unmet need in the management of LAPC. Only a multidisciplinary approach can be effective in obtaining both a local tumor reduction and a systemic control of disease. Local destructive therapies were important components of the multidisciplinary treatment. Radiofrequency ablation (RFA) and irreversible electroporation (IRE) have been proposed as new treatment options in the multimodal treatment of the LAPC. Until recently, there is a lack of studies comparing treatment effects between IRE and RFA. Therefore, we aimed to investigate the effect of IRE versus RFA after the induction chemotherapy on long‐term OS and progression‐free survival (PFS) in patents with LAPC. It was the first time to show that IRE resulted in better OS and PFS than RFA after the induction chemotherapy in patients with LAPC and should be considered as the first‐line ablation modality. A new ablative method, RFA ablation followed by tumor margin accentuation by IRE, is also considerable.
![]()
1. INTRODUCTION
Pancreatic cancer (PC) is associated with poor survival with a dismal 5‐year survival rate of only 7%. 1 There was little significant progress in the treatment of PC during the past two decades. 2 , 3 Although surgery provides the best chance to obtain better survival, only 15% of patients were eligible candidates for surgery. More than half (55%) of patients have metastatic PC. Another 40% of patients were classified as locally advanced PC (LAPC), which were characterized with vascular involvement prohibiting upfront resection. 4 , 5 , 6 There was no consensus on the most suitable treatment for patients with LAPC. The most frequently recommended treatment was chemotherapy and chemoradiotherapy, which only achieved modest survival benefit for patients with LAPC. 7 The median overall survival (OS) was only 9‐12 months for LAPC patients treated with chemotherapy or chemoradiotherapy. 8 , 9 , 10 In addition, it was shown that locally destructive disease was responsible for half of mortalities in patients with LAPC, although distant metastasis was found to be the most common form of disease progression, 11 indicating the importance of local destructive therapies. Considering the limited success of current therapy for the local control of disease and prolonging survival of patients with LAPC, novel local destructive therapies have been tried and viewed as more and more important treatments. 12
Nowadays, new insights have been focused on some novel local therapies as new treatment options for LAPC, including radiofrequency ablation (RFA) and irreversible electroporation (IRE). 13 Radiofrequency ablation has been applied in solid organ malignancies, such as renal carcinoma, 14 hepatocellular carcinoma, 15 and LAPC. 16 , 17 , 18 Also, as a subsequent treatment after induction chemotherapy in LAPC, there were many studies illustrating the survival benefit of IRE. 19 , 20 , 21 As a nonthermal method, IRE creates defects in cell membrane through the transmission of high‐voltage currents through the tumor, inducing loss of homeostasis and apoptotic death of tumor cells. 21 However, there is only limited evidence of which ablation method is survival beneficial to the LAPC patients. 22 Therefore, the primary aim of this study was the OS comparison and the secondary aim was the progression‐free survival (PFS) comparison in LAPC patients who received IRE and RFA after the induction chemotherapy.
2. METHODS
2.1. Patients
Patients who were diagnosed with LAPC and had received IRE or RFA combined with induction chemotherapy from August 2015 to August 2017 at Sun Yat‐sen University Cancer Center were retrospectively reviewed. The diagnosis of LAPC and the final therapy were confirmed by a multidisciplinary team, which included specialized pancreatic surgeons, oncologists, pathologists, and radiologists. Patients who were pathologically confirmed pancreatic adenocarcinoma and radiologically confirmed LAPC were included into this study. Locally advanced pancreatic cancer was defined as the description of AJCC staging system for pancreatic cancer. 23 , 24 All LAPC patients had received four months of induction chemotherapy (FOLFIRINOX or Gem‐based chemotherapy) 25 and those who were also judged as unresectable ones after induction chemotherapy were included in this study. A total of 378 patients were included into this study and 303 patients were excluded based on the following exclusion criteria: (a) second primary cancer; (b) distant metastases; (c) other treatments, such as surgical resection and radiotherapy; (d) a history of heart arrhythmia; and (e) missing or incomplete information. Finally, 75 patients were enrolled into this study. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and obtained approval from the Ethics Committee of Sun Yat‐sen University Cancer Center. Written informed consent was obtained from all patients.
2.2. Data and treatment procedure
The associated clinical data were retrieved and analyzed. Carbohydrate antigen 19‐9 (CA19‐9) and carcinoembryonic antigen (CEA) were taken after chemotherapy and prior to ablation. Before induction chemotherapy, which was conducted as the procedures described in our previous study, 25 biopsy was finished for all patients and tumor grade was determined. After induction chemotherapy, as long as no metastases were detected, IRE or RFA was performed and the same line of chemotherapy was followed after IRE or RFA therapy. A diagnostic laparoscopy is adopted to confirm that no metastasis is present. If none is found, IRE or RFA is performed. As shown in our previous study, 25 specialized pancreatic surgeons performed all IRE and RFA, which were performed using an open technique and guided by intraoperative ultrasound. The general anesthesia with deep neuromuscular block was adopted. In the procedure of IRE, under the guidance of ultrasound during surgery, 2 to 6 probes were adopted to create an electric field around the tumor, which caused nanoscale pore formation in the cell membrane. Also, the electrode of RFA was placed at the center of tumor. The ablation of IRE and RFA was monitored with ultrasound during surgery. The same line of chemotherapy was performed 7‐14 days after IRE treatment. According to the guidelines from National Comprehensive Cancer Network (NCCN), 5 4 cm was adopted as the cutoff value of tumor size in this study. Tumors which were larger than 4 cm were classified as large ones for pancreatic cancer. To further compare the efficacy of IRE and RFA in LAPC patients with large tumors, the survival comparisons of IRE and RFA were conducted in patients with tumors that are larger than 4 cm.
2.3. Follow‐up
The follow‐up procedures, including hematological examination, such as CA19‐9 and CEA analysis, and radiological examination (abdominal CT or MRI) were regularly performed for patients, who had the first one at approximately 1 month after IRE or RFA and the following ones every 2‐3 months thereafter. OS and PFS were defined as the duration from the date of induction chemotherapy until death or disease progression. If no endpoint event was observed, the date of last follow‐up was also used to calculate OS or PFS. The last follow‐up was completed on September 30, 2018.
2.4. Propensity score matching (PSM) analysis
To minimize selection bias, PSM analysis was utilized based on the following factors: age, gender, tumor site, tumor size, tumor grade, TNM stage, CA19‐9, and CEA. A two‐to‐one nearest‐neighbor matching algorithm 26 and “MatchIt” package in R software were adopted to perform PSM analysis.
2.5. Statistical analysis
The independent sample t test, Mann‐Whitney U test, and chi‐square test were used to compare the continuous and categorical variables, respectively. The survival differences in terms of OS and PFS were compared by the log‐rank test and survival curves were analyzed using the Kaplan‐Meier method. Prognostic factors of survival and the associated corresponding 95% confidence intervals (CIs) were determined by multivariate analyses using the Cox regression model. Statistical significance was considered when two‐tailed P value < .05 was obtained. All statistical analyses were performed using R software version 3.4.2 software (R Foundation for Statistical Computing, Vienna, Austria).
3. RESULTS
3.1. Patient
A total of 75 consecutive LAPC patients were identified to have IRE or RFA after the induction chemotherapy. Among this cohort, 8 patients were excluded due to other treatments other than IRE or RFA. Additional patients were excluded due to metastatic diseases developed after the induction chemotherapy (n = 5), a history of second primary malignant tumors (n = 3), or a history of heart arrhythmia (n = 1). After the exclusion process, there were 58 patients available for analysis (IRE: 36 and RFA: 22). The baseline characteristics of patients allocated to IRE or RFA were described in Table 1. Patients in the IRE group were likely to have tumors located in the head of pancreas while tumors with tumor‐node‐metastasis (TNM) stage III was a little more frequently observed in patients in this group. After PSM analyses, 36 patients in the IRE group and 18 patients in the RFA group were matched and compared. FOLFIRINOX‐ and Gem‐based chemotherapy were applied to 21 (58.3%) and 15 (41.7%) patients in the IRE group, which was similar with that of the RFA group. All other factors were balanced between two groups after PSM analysis.
Table 1.
Comparisons of clinical and imaging characteristics of patients
| Characteristic | Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
Chemotherapy + IRE n (%) |
Chemotherapy + RFA n (%) | Total number | P value | Chemotherapy + IRE n (%) | Chemotherapy + RFA n (%) | Total number | P value | ||
| Total number | 36 (62.1) | 22 (37.9) | 58 | 36 (66.7) | 18 (33.3) | 54 | |||
| Age (y) a | ≤60 | 20 (69.0) | 9 (31.0) | 29 | .417 | 20 (74.1) | 7 (25.9) | 27 | .387 |
| >60 | 16 (55.2) | 13 (44.8) | 29 | 16 (59.3) | 11 (40.7) | 27 | |||
| Gender a | Female | 19 (67.9) | 9 (32.1) | 28 | .427 | 19 (67.9) | 9 (32.1) | 28 | .847 |
| Male | 17 (56.7) | 13 (43.3) | 30 | 17 (65.4) | 9 (34.6) | 26 | |||
| Tumor size a (cm) | ≤2 | 1 (100.0) | 0 (0.0) | 1 | .505 | 1 (100.0) | 0 (0.0) | 1 | .685 |
| 2 ~ 4 | 20 (66.7) | 10 (33.3) | 30 | 20 (69.0) | 9 (31.0) | 29 | |||
| >4 | 15 (55.6) | 12 (44.4) | 27 | 15 (62.5) | 9 (37.5) | 24 | |||
| Tumor grade a | Well | 3 (60.0) | 2 (40.0) | 5 | .994 | 3 (60.0) | 2 (40.0) | 5 | .908 |
| Moderate | 20 (62.5) | 12 (37.5) | 32 | 20 (69.0) | 9 (31.0) | 29 | |||
| Poor | 13 (61.9) | 8 (38.1) | 21 | 13 (65.0) | 7 (35.0) | 20 | |||
| LN metastasis | Absent | 9 (52.9) | 8 (47.1) | 17 | .387 | 9 (60.0) | 6 (40.0) | 15 | .536 |
| Present | 27 (65.9) | 14 (34.1) | 41 | 27 (69.2) | 12 (30.8) | 39 | |||
| Tumor site a | Head | 18 (64.3) | 10 (35.7) | 28 | .018 | 18 (64.3) | 10 (35.7) | 28 | .207 |
| Body | 15 (78.9) | 4 (21.1) | 19 | 15 (78.9) | 4 (21.1) | 19 | |||
| Tail | 3 (27.3) | 8 (72.7) | 11 | 3 (42.9) | 4 (57.1) | 7 | |||
| TNM stage a | IIB | 4 (30.8) | 9 (69.2) | 13 | .020 | 4 (40.0) | 6 (60.0) | 10 | .067 |
| III | 32 (71.1) | 13 (28.9) | 45 | 32 (72.7) | 12 (27.3) | 44 | |||
| WBC (*109) | ≤10 | 32 (61.5) | 20 (38.5) | 52 | .589 | 32 (66.7) | 16 (33.3) | 48 | .687 |
| >10 | 4 (66.7) | 2 (33.3) | 6 | 4 (66.7) | 2 (33.3) | 6 | |||
| HGB (g/L) | ≤120 | 1 (50.0) | 1 (50.0) | 2 | .619 | 1 (50.0) | 1 (50.0) | 2 | .560 |
| >120 | 35 (62.5) | 21 (37.5) | 56 | 35 (67.3) | 17 (32.7) | 52 | |||
| PLT (*109) | ≤300 | 31 (60.8) | 20 (39.2) | 51 | .698 | 31 (64.6) | 17 (35.4) | 48 | .651 |
| >300 | 5 (71.4) | 2 (28.6) | 7 | 5 (83.3) | 1 (16.7) | 6 | |||
| ALT (U/L) | ≤40 | 26 (66.7) | 13 (33.3) | 39 | .390 | 26 (70.3) | 11 (29.7) | 37 | .536 |
| > 40 | 10 (52.6) | 9 (47.4) | 19 | 10 (58.8) | 7 (41.2) | 17 | |||
| AST (U/L) | ≤ 40 | 29 (63.0) | 17 (37.0) | 46 | .752 | 29 (67.4) | 14 (32.6) | 43 | .537 |
| >40 | 7 (58.3) | 5 (41.7) | 12 | 7 (63.6) | 4 (36.4) | 11 | |||
| ALP (U/L) | ≤100 | 19 (61.3) | 12 (38.7) | 31 | .556 | 19 (65.5) | 10 (34.5) | 29 | .539 |
| >100 | 17 (63.0) | 10 (37.0) | 27 | 17 (68.0) | 18 (72.0) | 25 | |||
| GGT (U/L) | ≤45 | 19 (70.4) | 8 (29.6) | 27 | .283 | 19 (73.1) | 7 (26.9) | 26 | .395 |
| >45 | 17 (54.8) | 14 (45.2) | 31 | 17 (60.7) | 11 (39.3) | 28 | |||
| ALB (g/L) | ≤ 40 | 4 (44.4) | 5 (55.6) | 9 | .278 | 4 (50.0) | 4 (50.0) | 8 | .418 |
| >40 | 32 (65.3) | 17 (34.7) | 49 | 32 (69.6) | 14 (30.4) | 46 | |||
| TBIL (µmol/L) | ≤20.5 | 27 (61.4) | 17 (38.6) | 44 | .553 | 27 (67.5) | 13 (32.5) | 40 | .536 |
| >20.5 | 9 (64.3) | 5 (35.7) | 14 | 9 (64.3) | 5 (35.7) | 14 | |||
| IBIL (µmol/L) | ≤15 | 32 (62.7) | 19 (37.3) | 51 | .540 | 32 (68.1) | 15 (31.9) | 47 | .674 |
| >15 | 4 (57.1) | 3 (42.9) | 7 | 4 (57.1) | 3 (42.9) | 7 | |||
| CRP (ng/L) | ≤3 | 24 (64.9) | 13 (35.1) | 37 | .585 | 24 (64.9) | 13 (35.1) | 37 | .763 |
| >3 | 12 (57.1) | 9 (42.9) | 21 | 12 (70.6) | 5 (29.4) | 17 | |||
| CEA (ng/mL) a | ≤5 | 21 (70.0) | 9 (30.0) | 30 | .250 | 21 (72.4) | 8 (27.6) | 29 | .378 |
| >5 | 15 (53.6) | 13 (46.4) | 28 | 15 (60.0) | 10 (40.0) | 25 | |||
| CA19‐9 (U/mL) a | ≤35 | 9 (60.0) | 6 (40.0) | 15 | .501 | 9 (64.3) | 5 (35.7) | 14 | .536 |
| >35 | 27 (62.8) | 16 (37.2) | 43 | 27 (67.5) | 13 (32.5) | 40 | |||
| HBsAg | Negative | 33 (62.3) | 20 (37.7) | 53 | .635 | 33 (66.0) | 17 (34.0) | 50 | .593 |
| Positive | 3 (60.0) | 2 (40.0) | 5 | 3 (75.0) | 1 (25.0) | 4 | |||
| Chemotherapy | FOLFIRINOX | 21 (67.7) | 10 (32.3) | 31 | .420 | 21 (72.4) | 8 (27.6) | 29 | .394 |
| Gem | 15 (55.6) | 12 (44.4) | 27 | 15 (60.0) | 10 (40.0) | 25 | |||
Abbreviations: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase; CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; CRP, C‐reactive protein; GGT, glutamyl transpeptidase; HBsAg, hepatitis B surface antigen; HBsAg, hepatitis B surface antigen; IBIL, indirect bilirubin; LN, lymph node metastasis; PLT, platelet count; TBIL, total bilirubin; TNM, tumor‐node‐metastasis stage; WBC, white blood cell count.
Variables that were used for propensity score matching analysis.
3.2. Survival and tumor progression analysis
The whole study cohort was regularly followed up at a median time of 10.0 months (range 1.2‐75.0 months). The 1‐ and 2‐year OS rates were 60.7% and 42.5%, respectively. A total of 7 (19.4%) deaths and 11 (61.1%) deaths were observed in the IRE and RFA groups, respectively (P = .005). Before PSM analysis, the 1‐ and 2‐year OS rates for patients in the IRE and RFA groups were 71.4%, 53.5% and 41.3%, 30.8%, respectively (P = .013, Figure 1A). After PSM analysis, patients in the IRE group still had significant higher OS rates than those in the RFA group (1‐year OS, 71.4% vs 40.5%; 2‐year OS, 53.5% vs 27.0%; P = .010, Figure 1B).
Figure 1.
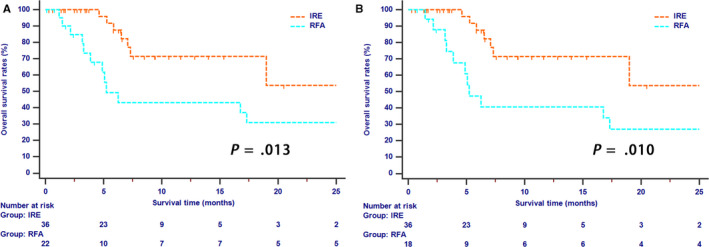
The Kaplan‐Meier survival curves of overall survival stratified by treatment strategies for patients with LAPC before (A) and after (B) propensity score matching
Tumor progression was recorded in 17 (47.2%) and 15 (83.3%) patients in the IRE and RFA groups, respectively (P = .018). Local recurrences were observed in 6 (16.6%) patients in the IRE group and 5 (27.7%) patients in the RFA group. In terms of distant metastases, patients in the RFA group have more cases (n = 10, 55.5%) than those in IRE group (n = 11, 30.5%). The 1‐ and 2‐year PFS rates for patients in the IRE and RFA groups were 28.4% and 28.4%, and 30.3% and 12.1%, respectively (P = .043, Figure 2A) before PSM analysis while after PSM analysis, 1‐ and 2‐year PFS rates for patients in the IRE and RFA groups were 28.4% and 28.4%, and 25.7% and 6.4%, respectively (P = .018, Figure 2B).
Figure 2.

The Kaplan‐Meier survival curves of progression‐free survival stratified by treatment strategies for patients with LAPC before (A) and after (B) propensity score matching
Tumor size was an important factor which may have an effect on the efficacy of ablation therapy. Four centimeter was adopted as the cutoff value of tumor size in this study. Fifteen (41.7%) patients and 9 (50%) patients had tumors which were larger than 4 cm in the IRE and RFA groups, respectively. For cases with LAPC larger than 4 cm, long‐term OS (P = .675, Figure 3A) and PFS (P = .098, Figure 3B) rates were similar between two groups. However, patients whose tumor sizes were smaller than 4 cm had significantly higher OS rates in the IRE group than those in the RFA group (P < .001, Figure 4A). In addition, the survival benefit for PFS were also different between two groups, although the difference was not significant (P = .080, Figure 4B).
Figure 3.
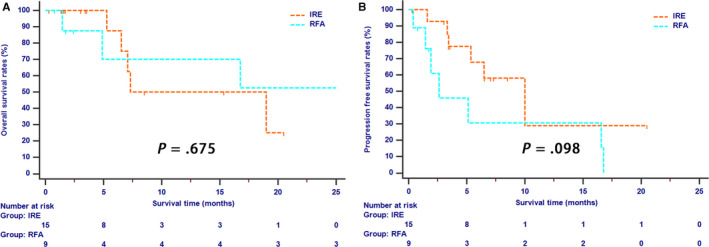
The Kaplan‐Meier survival curves of overall survival (A) and progression‐free survival (B) stratified by treatment strategies for LAPC patients whose tumor was larger than 4 cm
Figure 4.
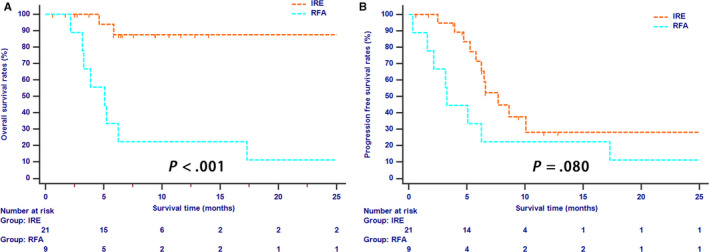
The Kaplan‐Meier survival curves of overall survival (A) and progression‐free survival (B) stratified by treatment strategies for LAPC patients whose tumor was smaller than 4 cm
3.3. Prognostic factors associated with OS and PFS
As shown in Table 2, univariate analysis revealed that IRE treatment, tumor grade, ALB, and CRP were associated with OS. Moreover, multivariate analysis identified several independent prognostic factors, including chemotherapy followed by IRE treatment (HR = 4.120; 95% CI, 1.493‐11.371; P = .006) and ALB level (HR = 0.240, 95% CI, 0.074‐0.780, P = .018). For PFS analysis, the only factor identified by univariate and multivariate analyses was IRE treatment (IRE vs RFA, HR = 2.330; 95% CI, 1.138‐4.768; P = .021) (Table 3).
Table 2.
Univariate and multivariate analyses of OS in patients
| Characteristic | Before PSM | After PSM | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | HR | 95% CI | P | ||
| Age (y) | ≤60/ >60 | 1.408 | 0.565‐3.508 | .462 | NI | 1.155 | 0.455‐2.933 | .761 | NI | ||||
| Gender | Female/ Male | 1.515 | 0.596‐3.851 | .383 | NI | 1.529 | 0.592‐3.951 | .380 | NI | ||||
| Tumor size (cm) | ≤2/2 ~ 4/>4 | 0.927 | 0.394‐2.180 | .861 | NI | 1.109 | 0.458‐2.687 | .818 | NI | ||||
| Tumor grade | Well/ Moderate/ Poor | 2.921 | 1.259‐6.774 | .013 | 2.715 | 1.121‐6.575 | .027 | 2.574 | 1.114‐5.949 | .027 | 2.162 | 0.894‐5.227 | .087 |
| LN metastasis | Absent/ Present | 2.551 | 0.741‐8.787 | .138 | NI | 2.563 | 0.737‐8.916 | .139 | NI | ||||
| Tumor site | Head/ Body/ Tail | 1.151 | 0.629‐2.103 | .649 | NI | 1.170 | 0.608‐2.249 | .638 | NI | ||||
| WBC (*109) | ≤10/ >10 | 1.642 | 0.477‐5.651 | .432 | NI | 1.662 | 0.480‐5.761 | .423 | NI | ||||
| HGB (g/L) | ≤120/ >120 | 0.556 | 0.073‐4.221 | .570 | NI | 0.540 | 0.071‐4.119 | .552 | NI | ||||
| PLT (*109) | ≤300/ >300 | 0.898 | 0.260‐3.105 | .865 | NI | 1.269 | 0.357‐4.512 | .712 | NI | ||||
| ALT (U/L) | ≤40/ >40 | 0.760 | 0.287‐2.016 | .581 | NI | 0.697 | 0.247‐1.966 | .495 | NI | ||||
| AST (U/L) | ≤40/ >40 | 1.466 | 0.484‐4.440 | .499 | NI | 1.059 | 0.305‐3.671 | .929 | NI | ||||
| ALP (U/L) | ≤100/ >100 | 0.900 | 0.361‐2.244 | .822 | NI | 0.864 | 0.334‐2.233 | .763 | NI | ||||
| GGT (U/L) | ≤45/ >45 | 1.205 | 0.488‐2.973 | .686 | NI | 1.218 | 0.482‐3.075 | .676 | NI | ||||
| ALB (g/L) | ≤40/ >40 | 0.167 | 0.060‐0.467 | .001 | 0.189 | 0.062‐0.581 | .004 | 0.200 | 0.068‐0.593 | .004 | 0.240 | 0.074‐0.780 | .018 |
| TBIL (µmol/L) | ≤20.5/ >20.5 | 0.661 | 0.192‐2.270 | .511 | NI | 0.656 | 0.190‐2.268 | .505 | NI | ||||
| IBIL (µmol/L) | ≤15/ >15 | 1.270 | 0.288‐5.598 | .752 | NI | 1.318 | 0.297‐5.853 | .716 | NI | ||||
| CRP (ng/L) | ≤3/ >3 | 2.613 | 1.047‐6.519 | .039 | 1.384 | 0.511‐3.746 | .523 | 3.127 | 1.185‐8.250 | .021 | 1.848 | 0.603‐5.662 | .282 |
| CEA (ng/mL) | ≤5/ >5 | 1.488 | 0.556‐3.979 | .429 | NI | 1.674 | 0.626‐4.476 | .304 | NI | ||||
| CA19‐9 (U/ml) | ≤35/ >35 | 1.967 | 0.643‐6.020 | .236 | NI | 2.103 | 0.684‐6.468 | .195 | NI | ||||
| HBsAg | Negative/ Positive | 0.882 | 0.116‐6.686 | .903 | NI | 0.908 | 0.119‐6.918 | .926 | NI | ||||
| Group | IRE/ RFA | 3.151 | 1.220‐8.140 | .018 | 3.870 | 1.426‐10.503 | .008 | 3.320 | 1.264‐8.718 | .015 | 4.120 | 1.493‐11.371 | .006 |
| Chemotherapy type | FOLFIRINOX/ Gem | 0.812 | 0.508‐1.299 | .385 | NI | 0.729 | 0.445‐1.192 | .208 | NI | ||||
Abbreviations: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase; CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; CRP, C‐reactive protein; GGT, glutamyl transpeptidase; HBsAg, hepatitis B surface antigen; HBsAg, hepatitis B surface antigen; IBIL, indirect bilirubin; LN, lymph node metastasis; PLT, platelet count; TBIL, total bilirubin; TNM, tumor‐node‐metastasis stage; WBC, white blood cell count.
Table 3.
Univariate and multivariate analyses of PFS in patients
| Characteristic | Before PSM | After PSM | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | ||||||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95% CI | P | HR | 95% CI | P | ||
| Age (years) | ≤60/ >60 | 1.142 | 0.572‐2.279 | .707 | NI | 0.927 | 0.457‐1.884 | .835 | NI | ||||
| Gender | Female/ Male | 1.448 | 0.722‐2.902 | .297 | NI | 1.650 | 0.803‐3.390 | .173 | NI | ||||
| Tumor size (cm) | ≤2/ 2 ~ 4/ >4 | 0.950 | 0.497‐1.815 | .876 | NI | 1.141 | 0.587‐2.215 | .697 | NI | ||||
| Tumor grade | Well/ Moderate/ Poor | 1.232 | 0.691‐2.199 | .480 | NI | 1.098 | 0.619‐1.950 | .749 | NI | ||||
| LN metastasis | Absent/ Present | 1.434 | 0.645‐3.189 | .376 | NI | 1.444 | 0.646‐3.226 | .370 | NI | ||||
| Tumor site | Head/ Body/ Tail | 0.973 | 0.613‐1.545 | .909 | NI | 1.077 | 0.653‐1.776 | .771 | NI | ||||
| WBC (*109) | ≤10/ >10 | 1.560 | 0.592‐4.109 | .369 | NI | 1.542 | 0.583‐4.078 | .383 | NI | ||||
| HGB (g/L) | ≤120/ >120 | 0.515 | 0.122‐2.180 | .367 | NI | 0.520 | 0.122‐2.205 | 0.375 | NI | ||||
| PLT (*109) | ≤300/ >300 | 0.434 | 0.132‐1.427 | .169 | NI | 0.604 | 0.181‐2.011 | .411 | NI | ||||
| ALT (U/L) | ≤40/ >40 | 0.800 | 0.380‐1.683 | .557 | NI | 0.832 | 0.384‐1.803 | .642 | NI | ||||
| AST (U/L) | ≤40/ >40 | 1.275 | 0.553‐2.943 | .569 | NI | 1.025 | 0.421‐2.495 | .956 | NI | ||||
| ALP (U/L) | ≤100/ >100 | 0.578 | 0.284‐1.175 | .130 | NI | 0.578 | 0.279‐1.200 | .142 | NI | ||||
| GGT (U/L) | ≤45/ >45 | 0.606 | 0.300‐1.225 | .163 | NI | 0.635 | 0.309‐1.303 | .216 | NI | ||||
| ALB (g/L) | ≤40/ >40 | 0.392 | 0.165‐0.930 | .034 | 0.372 | 0.156‐0.888 | 0.026 | 0.471 | 0.189‐1.175 | .107 | NI | ||
| TBIL (µmol/L) | ≤20.5/ >20.5 | 0.594 | 0.243‐1.453 | .254 | NI | 0.544 | 0.220‐1.345 | .187 | NI | ||||
| IBIL (µmol/L) | ≤ 5/ >15 | 1.580 | 0.603‐4.137 | .352 | NI | 1.556 | 0.592‐4.088 | .370 | NI | ||||
| CRP (ng/L) | ≤3/ >3 | 0.975 | 0.461‐2.062 | .946 | NI | 1.120 | 0.512‐2.450 | .777 | NI | ||||
| CEA (ng/mL) | ≤5/ >5 | 1.312 | 0.638‐2.699 | .460 | NI | 1.487 | 0.724‐3.053 | .280 | NI | ||||
| CA19‐9 (U/ml) | ≤35/ >35 | 1.735 | 0.749‐4.021 | .199 | NI | 1.846 | 0.794‐4.291 | .155 | NI | ||||
| HBsAg | Negative/ Positive | 1.258 | 0.380‐4.164 | .707 | NI | 1.230 | 0.371‐4.082 | .735 | NI | ||||
| Group | IRE/ RFA | 2.041 | 1.009‐4.129 | .047 | 2.125 | 1.043‐4.330 | 0.038 | 2.330 | 1.138‐4.768 | .021 | 2.330 | 1.138‐4.768 | 0.021 |
| Chemotherapy type | FOLFIRINOX/ Gem | 1.084 | 0.769‐1.530 | .644 | NI | 1.014 | 0.716‐1.438 | .936 | NI | ||||
Abbreviations: ALB, albumin; ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate aminotransferase; CA19‐9, carbohydrate antigen 19‐9; CEA, carcinoembryonic antigen; CRP, C‐reactive protein; GGT, glutamyl transpeptidase; HBsAg, hepatitis B surface antigen; HBsAg, hepatitis B surface antigen; IBIL, indirect bilirubin; LN, lymph node metastasis; PLT, platelet count; TBIL, total bilirubin; TNM, tumor‐node‐metastasis stage; WBC, white blood cell count.
3.4. Complications comparison
As shown in Table 4. No intra‐abdominal hemorrhage after treatment was observed in both groups. Drainage of seroperitoneum was conducted in one patient in the IRE group and two patients in the RFA group. In addition, acute pancreatitis occurred in two patients in the RFA group while no cases occurred in the IRE group. In terms of minor complications, fever and pain were the most common complications in both groups. Significantly more cases in the RFA group (9 of 18 patients) had fever than those in the IRE group (4 of 36 patients) while similar proportions of patients in both groups had pain after treatment and required analgesics. Notably, more patients in the RFA group had vomiting (4 of 18 patients) than those in IRE group (1 of 36 patients). All patients with complications received appropriate therapy and reached the discharge criteria.
Table 4.
Procedure‐related complications
| Variables | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| IRE (n = 36) | RFA (n = 22) | P | IRE (n = 36) | RFA (n = 18) | P | |
| Major complications | 2 | 7 | 2 | 7 | ||
| Seroperitoneum (require drainage) | 1 | 2 | .551 | 1 | 2 | .255 |
| Acute pancreatitis | 0 | 2 | .140 | 0 | 2 | .107 |
| Abdominal infection | 1 | 3 | .148 | 1 | 3 | .103 |
| Minor complications | 24 | 37 | 24 | 33 | ||
| Fever (axillary temperature > 38.5℃) | 4 | 11 | .002 | 4 | 9 | .005 |
| Pain (requiring analgesics) | 23 | 18 | .234 | 23 | 16 | .062 |
| Diarrhea | 2 | 3 | .357 | 2 | 3 | .319 |
| Vomiting | 1 | 4 | .063 | 1 | 4 | .038 |
4. DISCUSSION
Locally advanced pancreatic cancer, a devastating disease, owns relatively high mortalities and extremely low long‐term survival rates. 27 , 28 IRE and RFA can be used as local ablation methods after the induction chemotherapy for patients with LAPC, while the comparison of treatment efficacy of IRE and RFA remains unclear. The present study showed that although patients in the IRE group were associated with more advanced TNM stages, IRE was superior to RFA with respect to 1‐ and 2‐year OS and PFS for all patients and those whose tumor sizes were smaller than 4 cm. The survival differences were even more obvious when the baseline factors were balanced between two groups.
As a local thermal ablative method, RFA generates local high temperatures through the high‐frequency alternating current, leading to coagulative necrosis and protein denaturation inside neoplastic tissue. 22 The efficacy is partly limited by the heat sink effect in the heavily vascularized pancreas. Heat was dissipated by the blood vessels near ablation probes, leading to an area of lower temperature of the neighboring tumor cells. 29 , 30 Moreover, during the procedure of RFA, temperatures higher than 90℃ could induce thermal injuries and the injuries will increase with the elevation of temperatures. Therefore, the whole tumor ablation usually is avoided. The procedures of pull‐backs of the tips left a “security ring” at the periphery of the tumor, preventing high temperature diffusing to healthy surrounding tissues. 29 , 31 Therefore, to some extent, RFA can hardly achieve complete ablation in PDAC. 32 The incomplete ablation would result in rapid local recurrence and decreased long‐term survival. In contrast, relying on the application of short and high‐voltage current pulses through the tumor, IRE causes irreversible permeabilization in cell membrane integrity and induces subsequent apoptosis. 33 Therefore, IRE is not affected by the heat sink effect. The use of multiple needles allows bracketing the artery and leads to negligible amount of heat. Therefore, whole tumor can be surrounded by electric field of extremely high voltage without harming nearby important structure around pancreas. 22 Therefore, the application of IRE seems to be more appropriate than RFA for PDAC, which is characterized by encapsulating celiac axis or superior mesenteric artery. In addition, by disrupting the dense stroma of LAPC and reconstruction of microcirculation, 34 IRE contributed to the chemotherapy delivery to tumor, which also partly explained the survival benefit of IRE combined with systemic chemotherapy in patients with LAPC. Additionally, compared to other thermal ablative methods, IRE owns the nonthermal feature, which ensures the clinical effect is free of heat sink effect and leaves the supporting tissue largely unaffected. Considering the nature of preservation of vessels which is helpful for the transmission of immune molecules or cells, IRE may be more immunological sensitive than thermal ablations. Robert et al had shown that greater immune effect and therapeutic efficacy caused by IRE therapy in immunocompetent mice were observed than those in immunodeficient models, indicating that IRE could induce a systemic response beyond the targeted ablation region. 35 Previous studies have illustrated the immune‐stimulation effect induced by IRE was helpful for the survival elevation, 36 , 37 , 38 which would also act as the reason why IRE could work better than RFA in improving survival in LAPC patients.
A summary of studies which included a total of 106 LAPC patients after RFA treatment showed that the median postoperative complication rate and mortality were 28.3% and 7.5%, respectively. 32 The median survival was 6.5 months in that study, which was similar with that of our results. In this study, LAPC patients who were treated with IRE combined with induction chemotherapy had a median OS of 21.6 months, which was in accordance with the results from study of the largest cohort conducted by Martin et al 39 Moreover, Martin et al also reported a median PFS of 12.4 months, which was higher than that of our study. However, radical resection and margin accentuation by IRE were applied in nearly a quarter of all patients while our study only focused on LAPC patients who were not candidates for surgical resection. Similar with other study which showed that IRE could reduce local recurrence by allowing increased drug delivery to the tissue in the reversible electroporation zone, 40 IRE combined with chemotherapy, as a kind of multidiscipline approaches, could contribute to the elevated PFS rates. In this study, the median PFS was 7.7 months for patients in the IRE group, which was significantly longer than that of patients in the RFA group. Therefore, across‐study comparisons of long‐term survival consolidated the survival benefit of IRE over RFA for patients with LAPC. Moreover, it was reported that the complication rate and mortality of IRE were 29% and 2%, respectively, 39 , 41 indicating that compared with RFA, IRE was a more feasible and safe local ablation method.
In addition, the prognostic factors for patients with LAPC were explored and it was found that compared with IRE, RFA contributed to better OS and PFS. Also, low albumin level was an unfavorable prognostic factor for OS and PFS, whereas poorly differentiated tumor were negative prognostic factor for OS, consistent with results from previous studies. 42 , 43 Interestingly, when subgroup analyses stratified by tumor size were conducted, it was shown that patients had similar OS and PFS rates in both the IRE and RFA groups if tumor sizes were larger than 4 cm; although IRE displayed better in elevated long‐term survival rates in all patients or patients whose tumor sizes were smaller than 4 cm. Compared with a single ablation, multiple overlapping ablations may partly enlarge the possible ablation area and shrink the “security ring” around the tumor. Moreover, due to the presence of viable “security ring” of RFA at the periphery of tumor, the addition of IRE targeted at this area will make a complete ablation. Therefore, combining the advantages of IRE and RFA, it was suggested that maybe the combination of RFA ablation followed by tumor margin accentuation by IRE was a feasible local destructive method for the treatment of patients with LAPC. However, more appropriate randomized controlled studies are needed to evaluate the feasibility and efficacy of this new combination therapy.
There were several limitations which should be considered. The small sample size of patients and the potential patient selection bias kept us from drawing definitive conclusions. To improve the intergroup comparability, PSM analysis was applied to reduced selection bias. Large‐scale prospective randomized controlled studies are warranted to confirm results of this study.
In conclusion, it was the first time to show that compared with RFA, IRE resulted in better survival after the induction chemotherapy in LAPC patients and should be considered as the first‐line ablation modality. The efficacy of the combination therapy of IRE and induction chemotherapy is needed to be confirmed by randomized clinical trials.
CONFLICT OF INTERESTS
The authors declare that they have no conflict of interest.
AUTHORS CONTRIBUTIONS
Chaobin He and Shengping Li designed the study. Chaobin He, Jun Wang, and Yu Zhang analyzed and interpreted the data and wrote the manuscript. Zhiyuan Cai and Xiaojun Lin collected the data. Shengping Li revised the manuscript.
ACKNOWLEDGMENT
We thank Dr Zhanqiao Yuan for her assistance with the data collection.
He C, Wang J, Zhang Y, Cai Z, Lin X, Li S. Comparison of combination therapies in the management of locally advanced pancreatic cancer: Induction chemotherapy followed by irreversible electroporation vs radiofrequency ablation. Cancer Med. 2020;9:4699–4710. 10.1002/cam4.3119
Chaobin He, Jun Wang and Yu Zhang contributed equally to this work.
Funding information
This work was supported by grants from the National Natural Science Foundation of China (81171890; 81672390), the Major National Scientific Research Projects of China (NO. 2013CB910304).
DATA AVAILABILITY STATEMENT
The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (http://www.researchdata.org.cn), with the Approval Number as RDDA2019001060.
REFERENCES
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics 2020. CA: Cancer J Clin. 2020;70(1):7‐30. [DOI] [PubMed] [Google Scholar]
- 2. Ducreux M, Cuhna AS, Caramella C, et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals Oncol. 2015;26(Suppl 5):v56‐68. [DOI] [PubMed] [Google Scholar]
- 3. Rawla P, Sunkara T, Gaduputi V. Epidemiology of Pancreatic cancer: global trends, etiology and risk factors. World J Oncol. 2019;10(1):10‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nature Rev Clin Oncol. 2010;7(3):163‐172. [DOI] [PubMed] [Google Scholar]
- 5. Tempero MA, Malafa MP, Al‐Hawary M, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J National Comprehensive Cancer Network. 2017;15(8):1028‐1061.. [DOI] [PubMed] [Google Scholar]
- 6. Isaji S, Mizuno S, Windsor JA, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18(1):2‐11. [DOI] [PubMed] [Google Scholar]
- 7. Abrams RA, Lowy AM, O'Reilly EM, et al. Combined modality treatment of resectable and borderline resectable pancreas cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1751‐1756. [DOI] [PubMed] [Google Scholar]
- 8. Rocha Lima CM, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22(18):3776‐3783. [DOI] [PubMed] [Google Scholar]
- 9. Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination with oxaliplatin compared with gemcitabine alone in locally advanced or metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III trial. J Clin Oncol. 2005;23(15):3509‐3516. [DOI] [PubMed] [Google Scholar]
- 10. Heinemann V, Haas M, Boeck S. Neoadjuvant treatment of borderline resectable and non‐resectable pancreatic cancer. Annals Oncol. 2013;24(10):2484‐2492. [DOI] [PubMed] [Google Scholar]
- 11. Peixoto RD, Speers C, McGahan CE, et al. Prognostic factors and sites of metastasis in unresectable locally advanced pancreatic cancer. Cancer Med. 2015;4(8):1171‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Keane MG, Bramis K, Pereira SP, et al. Systematic review of novel ablative methods in locally advanced pancreatic cancer. World J Gastroenterol. 2014;20(9):2267‐2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rombouts SJ, Vogel JA, van Santvoort HC, et al. Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer. Br J Surgery. 2015;102(3):182‐193. [DOI] [PubMed] [Google Scholar]
- 14. Park BK, Gong IH, Kang MY, et al. RFA versus robotic partial nephrectomy for T1a renal cell carcinoma: a propensity score‐matched comparison of mid‐term outcome. Eur Radiol. 2018;28(7):2979‐2985. [DOI] [PubMed] [Google Scholar]
- 15. Xu XL, Liu XD, Liang M, et al. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinoma: systematic review of randomized controlled trials with meta‐analysis and trial sequential analysis. Radiology. 2018;287(2):461‐472. [DOI] [PubMed] [Google Scholar]
- 16. Girelli R, Frigerio I, Salvia R, et al. Feasibility and safety of radiofrequency ablation for locally advanced pancreatic cancer. Br J Surgery. 2010;97(2):220‐225. [DOI] [PubMed] [Google Scholar]
- 17. D'Onofrio M, Barbi E, Girelli R, et al. Radiofrequency ablation of locally advanced pancreatic adenocarcinoma: an overview. World J Gastroenterol. 2010;16(28):3478‐3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25(3):326‐331. [DOI] [PubMed] [Google Scholar]
- 19. Vogel JA, Rombouts SJ, de Rooij T, et al. Induction chemotherapy followed by resection or irreversible electroporation in locally advanced pancreatic cancer (IMPALA): a prospective cohort study. Ann Surg Oncol. 2017;24(9):2734‐2743. [DOI] [PubMed] [Google Scholar]
- 20. Huang KW, Yang PC, Pua U, et al. The efficacy of combination of induction chemotherapy and irreversible electroporation ablation for patients with locally advanced pancreatic adenocarcinoma. J Surg Oncol. 2018;118(1):31‐36. [DOI] [PubMed] [Google Scholar]
- 21. Geboers B, Scheffer HJ, Graybill PM, et al. High‐voltage electrical pulses in oncology: irreversible electroporation, electrochemotherapy, gene electrotransfer, electrofusion, and electroimmunotherapy. Radiology. 2020;295(2):254‐272. [DOI] [PubMed] [Google Scholar]
- 22. Paiella S, Salvia R, Ramera M, et al. Local ablative strategies for ductal pancreatic cancer (radiofrequency ablation, irreversible electroporation): a review. Gastroenterol Res Practice. 2016;2016:4508376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16(7):1727‐1733. [DOI] [PubMed] [Google Scholar]
- 24. Edge SBBD, Comptom CC, Fritz AG, Greene FL, Trotti A, eds. AJCC cancer staging manual. 7th edn New York, NY: Springer; 2010. [Google Scholar]
- 25. He C, Wang J, Sun S, et al. Irreversible electroporation versus radiotherapy after induction chemotherapy on survival in patients with locally advanced pancreatic cancer: a propensity score analysis. BMC Cancer. 2019;19(1):394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharmaceutical Statistics. 2011;10(2):150‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Worni M, Guller U, White RR, et al. Modest improvement in overall survival for patients with metastatic pancreatic cancer: a trend analysis using the surveillance, epidemiology, and end results registry from 1988 to 2008. Pancreas. 2013;42(7):1157‐1163. [DOI] [PubMed] [Google Scholar]
- 28. Balaban EP, Mangu PB, Khorana AA, et al. Locally advanced, unresectable pancreatic cancer: american society of clinical oncology clinical practice guideline. J Clin Oncol. 2016;34(22):2654‐2668. [DOI] [PubMed] [Google Scholar]
- 29. Girelli R, Frigerio I, Giardino A, et al. Results of 100 pancreatic radiofrequency ablations in the context of a multimodal strategy for stage III ductal adenocarcinoma. Langenbeck's Archives Surgery. 2013;398(1):63‐69. [DOI] [PubMed] [Google Scholar]
- 30. Spiliotis JD, Datsis AC, Michalopoulos NV, et al. Radiofrequency ablation combined with palliative surgery may prolong survival of patients with advanced cancer of the pancreas. Langenbeck's Archives of Surgery. 2007;392(1):55‐60. [DOI] [PubMed] [Google Scholar]
- 31. D'Onofrio M, Zamboni G, Faccioli N, et al. Ultrasonography of the pancreas. 4. Contrast‐enhanced imaging. Abdom Imaging. 2007;32(2):171‐181. [DOI] [PubMed] [Google Scholar]
- 32. Pezzilli R, Ricci C, Serra C, et al. The problems of radiofrequency ablation as an approach for advanced unresectable ductal pancreatic carcinoma. Cancers. 2010;2(3):1419‐1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Al Efishat M, Wolfgang CL, Weiss MJ. Stage III pancreatic cancer and the role of irreversible electroporation. BMJ (Clinical research ed). 2015;350:h521. [DOI] [PubMed] [Google Scholar]
- 34. Zhao J, Wen X, Tian L, et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat Commun. 2019;10(1):899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neal RE 2nd, Rossmeisl JH Jr, Robertson JL, et al. Improved local and systemic anti‐tumor efficacy for irreversible electroporation in immunocompetent versus immunodeficient mice. PLoS ONE. 2013;8(5):e64559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beitel‐White N, Martin RCG, Li Y, et al. Real‐time prediction of patient immune cell modulation during irreversible electroporation therapy. Sci Rep. 2019;9(1):17739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He C, Wang J, Sun S, et al. Immunomodulatory effect after irreversible electroporation in patients with locally advanced pancreatic cancer. J Oncol. 2019;2019:9346017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pandit H, Hong YK, Li Y, et al. Evaluating the regulatory immunomodulation effect of Irreversible Electroporation (IRE) in pancreatic adenocarcinoma. Ann Surg Oncol. 2019;26(3):800‐806. [DOI] [PubMed] [Google Scholar]
- 39. Martin RC 2nd, Kwon D, Chalikonda S, et al. Treatment of 200 locally advanced (stage III) pancreatic adenocarcinoma patients with irreversible electroporation: safety and efficacy. Annals Surgery. 2015;262(3):486‐494; discussion 92–4. [DOI] [PubMed] [Google Scholar]
- 40. Bhutiani N, Agle S, Li Y, et al. Irreversible electroporation enhances delivery of gemcitabine to pancreatic adenocarcinoma. J Surg Oncol. 2016;114(2):181‐186. [DOI] [PubMed] [Google Scholar]
- 41. Scheffer HJ, Nielsen K, de Jong MC, et al. Irreversible electroporation for nonthermal tumor ablation in the clinical setting: a systematic review of safety and efficacy. J Vascular Interventional Radiol. 2014;25(7):997‐1011; quiz 11. [DOI] [PubMed] [Google Scholar]
- 42. He C, Zhang Y, Cai Z, et al. Overall survival and cancer‐specific survival in patients with surgically resected pancreatic head adenocarcinoma: a competing risk nomogram analysis. Journal of Cancer. 2018;9(17):3156‐3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu Z, Jin K, Guo M, et al. Prognostic value of the CRP/Alb ratio, a novel inflammation‐based score in pancreatic cancer. Ann Surg Oncol. 2017;24(2):561‐568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (http://www.researchdata.org.cn), with the Approval Number as RDDA2019001060.


