Abstract
Background
Robust data reporting the survival of cancer patients on home parenteral nutrition (HPN) are lacking. The aim of this prospective, cohort study was to investigate clinical characteristics, predictive factors, and overall survival (OS) of adult‐malnourished cancer patients eligible for HPN according to the European guideline recommendations.
Methods
During the study period, 1658 cancer patients were consecutively evaluated in a tertiary university hospital. Of these, 761 who received HPN were grouped into four cohorts according to the provision of supplemental PN (SPN) or total (TPN) and whether they received chemotherapy (CT+ or CT‐): SPN/CT+ (n = 376), TPN/CT+ (n = 99), SPN/CT‐ (n = 191), and TPN/CT‐ (n = 95). Patient demographics, nutritional status, cancer‐related characteristics, and prognostic scores assessed at HPN start. The primary outcome was OS.
Results
Median OS was 8.9, 4.3, 5.7, and 2.2 months for the SPN/CT+, TPN/CT+, SPN/CT‐, and TPN/CT‐ cohorts, respectively. In multivariable analysis, predictors showing significant association with decreased survival were patient cohorts, modified Glasgow Prognostic Score (1 and 2 scores), weight loss (>15%) in the 3 months before HPN start, and TNM IV stage while protective factors of survival were Karnofsky Performance Status (>50), albumin level (>3.5 g/dL), oral protein intake, BMI (>20.5), and weight at HPN start.
Conclusion
For the first time, in four different cohorts of cancer patients on HPN, clinical characteristics and survival were compared. This large study showed that survival is significantly correlated with patient characteristics at HPN start and that the presence of favorable factors may determine even a fourfold increase in survival. These data are expected to assist physicians in the appropriate prescription of HPN.
Keywords: cancer survival, cohort study, home care, medical nutrition therapy, oncologic treatment, supportive care
This is the largest, prospective, clinical study investigating survival exclusively of adult malnourished cancer patients receiving home parenteral nutrition (HPN). This study showed that survival is significantly correlated with patient characteristics at HPN start and that the presence of favorable factors may determine even a 4‐fold increase in survival.

1. INTRODUCTION
In cancer patients, an impaired nutritional status impacts negatively on functional status, quality of life, tolerance to oncologic treatments, rates of hospitalization, length of stay, and survival. 1 International guidelines recommend to regularly screen all cancer patients for nutrition impact symptoms and clinical signs of malnutrition and, if found at risk, to design personalized nutritional interventions. 2 , 3 Nutritional support in oncologic patients is a step‐by‐step intervention, 1 starting from dietary counseling and oral nutritional supplements (ONS) to Medical Nutrition therapy (MNT), either enteral nutrition (EN) or parenteral nutrition (PN). 4 , 5 In case that oral nutrition remains inadequate despite counseling and ONS and EN are not feasible, insufficient, or contraindicated, home PN (HPN) ensures that patients receive adequate nutritional therapy. 6 This is the case in patients with chronic severe enteral food intolerance (untreatable nausea, vomiting, abdominal pain, malabsorption, or diarrhea) or with severe intestinal insufficiency due to malignant inoperable bowel obstruction (peritoneal carcinomatosis, intra‐abdominal recurrences), short bowel syndrome, radiation enteritis, and high‐output ileostomy or fistulas. 1 , 7 HPN can be total (TPN) when patients have no or negligible ONS/EN nutrition (<200 kcal/day) 8 or supplemental PN (SPN). Generally, SPN at home provides 1000‐1250 kcal per day, from three to six times per week in patients with residual—but insufficient—oral food intake.
Prevalence of HPN in cancer patients throughout the world reflects differing practices, with this variability possibly attributable to different reimbursement policies and economic resources allocation as well as to cultural, ethical, and social aspects among countries. 9 , 10 , 11 , 12 , 13 Additionally, the low level of the available evidence due to lack of randomized controlled trials (RCTs), 14 together with the limited knowledge and diffusion of guidelines on nutrition in cancer patients, 15 , 16 , 17 may contribute as well to this heterogeneity. In 2005, a survey to determine the prevalence of home MNT in Italy reported that in adults the greatest prevalence of HPN was observed in oncologic patients; in particular, a positive association was found between the number of years since the regulation was issued and home MNT prevalence. 18 Since 1986, in the Piedmont Region both home EN and PN have been regulated by specific laws that stated the need of consistent screening of cancer patient nutritional status and their eligibility for HPN is assessed according to specific clinical practice guidelines. For years our Unit has been collecting data with the goal to determine factors affecting clinical practice, complications, and outcomes of cancer patients receiving HPN, in collaboration with the Regional Health Council, oncology units, and general practitioners. 19 , 20 , 21 , 22 , 23 , 24 , 25
HPN cannot be studied in a randomized design, due to the fact that the control arm would be no feeding and that would be considered unethical in persons with severely compromised food intake or intestinal failure. Therefore, alternative study designs are required to interpret the potential benefit of this form of nutritional therapy.
The aim of this prospectively conducted study was to increase the knowledge of real‐world data on the use of HPN in cancer patients reporting a 7‐year experience of our Unit. In particular, we provided a detailed description of the pathway leading to the delivery of HPN in oncologic patients accordingly to daily clinical practice, clinical characteristics, predictive factors of survival at the time of HPN start, and survival since HPN start.
2. METHODS
2.1. Study design
This was a prospective, cohort study conducted in a 1200‐bed tertiary university hospital. From 1 June 2008 through 31 May 2015, all consecutive adult cancer patients who were candidates for HPN were eligible for enrollment. Study methods were conducted and reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Our criteria for accepting patients in the HPN program followed the European guideline recommendations for eligibility 5 and included the following: proven and prolonged failure to meet nutritional requirements by oral or enteral route (no food for more than 1 week or less than 60% of requirement for more than 1‐2 weeks), with a potential risk of earlier death due to malnutrition rather than from cancer progression; life expectancy > 2 months; Karnofsky Performance Status (KPS) ≥50; adequate control of pain and other severe symptoms (dyspnea, vomiting); absence of severe organ dysfunctions; written informed consent confirming that the patient accepted this modality of nutritional support; having a central venous catheter (CVC); approval by the physician responsible for HPN, the oncologist and the general practitioner; presence of environmental conditions compatible with HPN; availability of an in‐home caregiver; and availability of a specifically trained nursing team dedicated to the patient home care, as provided by the Public Health Service. 23 Exclusion criteria for HPN were as follows: capability to meet the nutritional requirements by oral or enteral route; KPS < 50; uncontrolled symptoms; severe organ dysfunctions (heart, respiratory, liver, and renal); lack of an in‐home caregiver and HPN refusal by the patient.
The evaluation of eligibility of a cancer patient for the HPN program was requested to our Unit mainly by oncologists, as also surgeons, internal medicine physicians, and general practitioners. Inpatients were assessed during the consultations carried out in the wards before discharge, while ambulatory patients were assessed in a dedicated hospital outpatient department in the Comprehensive Cancer Center. All patients were assessed for eligibility by the physician (PC) and the dietician (TM) responsible for HPN. At baseline (at HPN start), data recorded included anthropometric (actual body weight, body mass index [BMI], weight loss in the last 3 months) and clinical‐oncological assessments (tumor site and stage, anticancer treatments), and assessment of residual oral feeding, if present. Performance status was graded using the WHO/Eastern Cooperative Oncology Group (ECOG) scale 26 and the KPS. 27 Systemic inflammation was estimated using serum C‐reactive protein (CRP) and albumin, and inflammatory response was graded according to the modified Glasgow Prognostic Score (mGPS; 0 = CRP ≤10 mg/L + any albumin; 1 = CRP >10 mg/L + albumin ≥3.5 g/dL; 2 = CRP >10 mg/L + albumin <3.5 g/dL). 28 Nutritional status was assessed using the Patient‐Generated Subjective Global Assessment (PG‐SGA), which combines qualitative and semi‐quantitative data to yield a comprehensive malnutrition score (A = well‐nourished; B = suspected malnutrition or moderate malnutrition; or C = severely malnourished. 29 Three days was the mean time between eligibility for HPN and PN start at home.
After HPN start, all patients were closely monitored by the physician responsible for HPN (PC) through regularly scheduled and structured telephone interviews (at least every 15 days) and home visits by the nursing team and general practitioner (initially daily for 2‐3 weeks, and at least every 7 days thereafter). After adequate training, home caregivers administered HPN. Telephone assistance was available for patients as well as their caregivers and health‐care providers at all times. HPN was delivered to 98.4% of patients using standard commercially manufactured ready‐to‐use bags containing amino acids, electrolytes, glucose, and lipids, overnight for 10‐14 hours per day through a CVC. HPN regimen was individually designed to meet protein, calorie, and fluid requirements; generally, HPN was prescribed to provide 25‐30 kcal/kg/day, depending on the patient activity of daily living, and an amino acid supply of 1‐1.5 g/kg/day. Every 30 days from HPN start (± 5 days), an outpatient re‐evaluation by both the physician and the dietitian (including a 24‐hour food recall) was performed.
Eligible patients without an in‐home caregiver were admitted to hospice facilities, while patients who refused HPN, but with an in‐home caregiver, were assisted at home by palliative care teams (nurse and physician). All these patients received artificial hydration (balanced salt solutions) through a CVC according to their needs.
All patients were followed up until withdrawal of HPN or death. HPN was withdrawn in case of worsening clinical state (onset of severe organ dysfunction or uncontrolled symptoms; downgrading of performance status; estimated life expectancy of hours to days and patient will). 30 Overall survival (OS) was calculated as the number of days between the date of HPN start and the date of patient death from any cause, with censoring at the date of last follow‐up assessment in alive subjects (on 28 February 2019).
Ethics committee approval was obtained and written informed consent was obtained from each patient prior to any procedures. The consent to participate was obtained from the chief investigator (PC). The latter submitted an update report monthly to the regional competent authority and annually or on request to the approving research ethics committee.
2.2. Statistical Analysis
All analyses were tabulated and a description of patient clinical characteristics was provided on the overall population as well as selected cohorts: patients were grouped into four mutually exclusive cohorts depending on type of HPN (SPN or TPN) and whether they received chemotherapy (CT+ or CT‐). Qualitative variables were described in terms of frequencies and percentages, while quantitative variables were reported as mean value and standard deviation (SD) or median and 95% confidence interval (CI). The primary outcome was OS measured in months between the date of HPN start and the date of death. Patients still alive at the end of the observation period were censored. Kaplan‐Meier curves plotting time to death since HPN start were also presented for the four cohorts with log‐rank tests. Study variables were fitted into a Cox proportional hazards model to estimated hazard ratios (HRs) and the associate 95% CI. A stepwise variable selection approach was used to enter only significant predictors into the final model (threshold 0.1). Results were considered significant if p value <0.05. All the analyses were performed using SAS software (Version 9.4).
3. RESULTS
3.1. Assessment of eligibility for HPN
During the study period, 1658 patients were consecutively evaluated. About 897 (54.1%) were excluded as they did not meet the inclusion criteria (Figure 1). In particular, 314 (18.9%) were judged to be eligible for a program providing dietary counseling and ONS; 64 (3.9%) were eligible for tube feeding (home EN); 213 (12.8%) had a KPS < 50; 153 (9.2%) patients presented with severe organ dysfunction. Other reasons for non‐administration were admission to hospice facility due to lack of an in‐home caregiver (119, 7.2%) or HPN refusal by the patient (34, 2.1%). Finally, 761 (45.9%) received HPN. Among patients who started HPN, 376 (49.4%) received SPN and CT (SPN/CT+); 99 (13.0%) received TPN and CT (TPN/CT+); 191 (25.1%) received SPN but not CT (SPN/CT‐); and 95 (12.5%) received TPN but not CT (TPN/CT‐).
Figure 1.
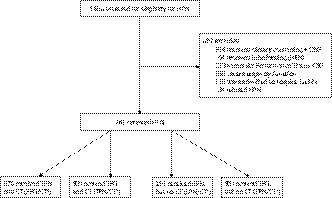
Study flow chart. HPN: home parenteral nutrition; ONS: oral nutritional supplements; HEN: home enteral nutrition; SPN: supplemental parenteral nutrition; TPN: total parenteral nutrition; CT+: chemotherapy received; CT‐: no chemotherapy received
The overall population required HPN mainly because the oral food intake was severely compromised by the presence of peritoneal carcinomatosis (30%) or intra‐abdominal recurrences (58%); less frequently (12%) because of short bowel syndrome, high‐output ileostomy or fistulas, and not feasible or tolerated EN.
The rate of catheter‐related bloodstream infections (CRBSIs) was 0.29 per 1000 catheter‐days while the rate of overall other catheter‐related complications (CRCs; local infection, mechanical, and venous thrombosis) was 0.79 per 1000 catheter‐days. Eight patients required hospitalization due to CRBSI and one of them died. No clinically relevant HPN‐related metabolic complications occurred. There were no significant differences in complications or adverse events among the four cohorts.
3.2. Patient characteristics
In Table 1, the characteristics of patients at HPN start. Regarding age, patients who did not receive chemotherapy (CT‐) were older compared with those in the SPN/CT+ group, with more patients in the ≥ 70 years category. The cohorts of patients who did not receive chemotherapy presented a proportion of underweight patients of 50% or above. In particular, the SPN/CT‐ group had lower weight and BMI compared with SPN/CT+ (Table 1). Patient distribution in weight loss and BMI‐adjusted weight loss categories (Figure 2A) was different between SPN/CT+ and the other cohorts, suggesting that patients in the SPN/CT+ group are less likely to be in the highest categories of weight loss (> 15%), even when adjusted for BMI (BMI‐adjusted weight loss grade 4). Similarly, severely malnourished patients (PG‐SGA rating of C) were more present in the TPN/CT+, SPN/CT‐, TPN/CT‐ groups when compared with SPN/CT+ (Figure 2B). When we looked at residual oral food intake at the start of HPN, the number of patients reporting better oral calorie and protein intake was higher in the SPN/CT+ group compared with SPN/CT‐ (Table 1).
Table 1.
Patient characteristics by type of parenteral nutrition and whether they received chemotherapy during the study period
| Overall (N = 761) | Chemotherapy received | No chemotherapy received | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPN/CT+ (N = 376) | TPN/CT+ (N = 99) | SPN/CT‐ (N = 191) | TPN/CT‐ (N = 95) | ||||||||||||
| N | % | N | % | N | % | P1 | N | % | P2 | N | % | P3 | |||
| Sex | |||||||||||||||
| Female | 380 | 49.9 | 197 | 52.4 | 41 | 41.4 | 102 | 53.4 | 40 | 42.1 | |||||
| Age (years) | |||||||||||||||
| Mean (SD) | 62.90 | (10.28) | 60.07 | (9.15) | 62.07 | (9.89) | 66.12 | (10.84) | <.01 | 68.49 | (9.73) | <.01 | |||
| Age categories (years) | |||||||||||||||
| <70 | 555 | 72.9 | 325 | 86.4 | 75 | 75.8 | 113 | 59.2 | 42 | 44.2 | |||||
| ≥70 | 206 | 27.1 | 51 | 13.6 | 24 | 24.2 | <.01 | 78 | 40.8 | <.01 | 53 | 55.8 | <.01 | ||
| Actual weight (kg) | |||||||||||||||
| Mean (SD) | 58.42 | (10.92) | 59.56 | (11.32) | 58.74 | (10.12) | 56.11 | (10.23) | <.01 | 58.21 | (10.92) | ||||
| BMI (kg/m2) | |||||||||||||||
| Mean (SD) | 21.37 | (3.76) | 21.75 | (4.01) | 21.62 | (3.60) | 20.54 | (3.12) | <.01 | 21.23 | (3.84) | ||||
| BMI class | |||||||||||||||
| <20.5 kg/m2 | 334 | 43.9 | 149 | 39.6 | 37 | 37.4 | 101 | 52.9 | 47 | 49.5 | |||||
| ≥ 20.5 kg/m2 | 427 | 56.1 | 227 | 60.4 | 62 | 62.6 | 90 | 47.1 | <.01 | 48 | 50.5 | ||||
| Weight loss (%) categories a | |||||||||||||||
| ≤10.0% | 233 | 30.6 | 162 | 43.1 | 12 | 12.1 | 52 | 27.2 | 7 | 7.4 | |||||
| 10.1%‐15% | 187 | 24.6 | 109 | 29.0 | 21 | 21.2 | 40 | 20.9 | 17 | 17.9 | |||||
| 15.1%‐20% | 181 | 23.8 | 59 | 15.7 | 35 | 35.4 | 49 | 25.7 | 38 | 40.0 | |||||
| >20% | 160 | 21.0 | 46 | 12.2 | 31 | 31.3 | <.01 | 50 | 26.2 | <.01 | 33 | 34.7 | <.01 | ||
| BMI‐adjusted weight loss | |||||||||||||||
| Grade 2 | 26 | 3.4 | 18 | 4.8 | 2 | 2.0 | 5 | 2.6 | 1 | 1.1 | |||||
| Grade 3 | 264 | 34.7 | 177 | 47.1 | 24 | 24.2 | 49 | 25.7 | 14 | 14.7 | |||||
| Grade 4 | 471 | 61.9 | 181 | 48.1 | 73 | 73.7 | <.01 | 137 | 71.7 | <.01 | 80 | 84.2 | <.01 | ||
| PG‐SGA | |||||||||||||||
| B b | 242 | 31.8 | 171 | 45.5 | 12 | 12.1 | 52 | 27.2 | 7 | 7.4 | |||||
| C c | 519 | 68.2 | 205 | 54.5 | 87 | 87.9 | <.01 | 139 | 72.8 | <.01 | 88 | 92.6 | <.01 | ||
| Oral calorie intake (kcal/day) | |||||||||||||||
| <500 | 322 | 42.3 | 65 | 17.3 | — | — | 63 | 33.0 | — | — | |||||
| 500‐1000 | 409 | 53.8 | 291 | 77.4 | — | — | 118 | 61.8 | — | — | |||||
| >1000 | 30 | 3.9 | 20 | 5.3 | — | — | 10 | 5.2 | <.01 | — | — | ||||
| Oral protein intake (g/day) | |||||||||||||||
| <20 | 353 | 46.39 | 84 | 22.3 | — | — | 75 | 39.3 | — | — | |||||
| ≥20 | 408 | 53.61 | 292 | 77.7 | — | — | 116 | 60.7 | <.01 | — | — | ||||
| Tumor site | |||||||||||||||
| Ovary | 47 | 6.2 | 24 | 6.4 | 6 | 6.1 | 11 | 5.8 | 6 | 6.3 | |||||
| Gastrointestinal | 564 | 74.1 | 293 | 77.9 | 72 | 72.7 | 134 | 70.2 | 65 | 68.4 | |||||
| Other | 150 | 19.7 | 59 | 15.7 | 21 | 21.2 | 46 | 24.1 | 24 | 25.3 | |||||
| Metastatic cancer | |||||||||||||||
| No | 301 | 39.6 | 174 | 46.3 | 27 | 27.3 | 67 | 35.1 | 33 | 34.7 | |||||
| Yes | 460 | 60.5 | 202 | 53.7 | 72 | 72.7 | <.01 | 124 | 64.9 | <.05 | 62 | 65.3 | <.05 | ||
| Peritoneal carcinomatosis | |||||||||||||||
| No | 533 | 70.0 | 288 | 76.6 | 54 | 54.6 | 126 | 66.0 | 65 | 68.4 | |||||
| Yes | 228 | 30.0 | 88 | 23.4 | 45 | 45.5 | <.01 | 65 | 34.0 | <.01 | 30 | 31.6 | |||
| Cancer staging | |||||||||||||||
| II/III | 220 | 28.9 | 137 | 36.4 | 19 | 19.2 | 47 | 24.6 | 17 | 17.9 | |||||
| IV | 541 | 71.1 | 239 | 63.6 | 80 | 80.8 | <.01 | 144 | 75.4 | <.01 | 78 | 82.1 | <.01 | ||
| KPS | |||||||||||||||
| 50 | 102 | 13.4 | 8 | 2.1 | 11 | 11.1 | 43 | 22.5 | 40 | 42.1 | |||||
| 60 | 222 | 29.2 | 95 | 25.3 | 33 | 33.3 | 65 | 34.0 | 29 | 30.5 | |||||
| 70 | 387 | 50.9 | 230 | 61.2 | 55 | 55.6 | 77 | 40.3 | 25 | 26.3 | |||||
| 80‐90 | 50 | 6.6 | 43 | 11.4 | . | . | <.01 | 6 | 3.1 | <.01 | 1 | 1.1 | <.01 | ||
| Albumin | |||||||||||||||
| <3.5 g/dL | 430 | 56.5 | 163 | 43.4 | 62 | 62.6 | 123 | 64.4 | 82 | 86.3 | |||||
| ≥3.5 g/dL | 331 | 43.5 | 213 | 56.7 | 37 | 37.4 | <.01 | 68 | 35.6 | <.01 | 13 | 13.7 | <.01 | ||
| CRP | |||||||||||||||
| ≤10 mg/L | 390 | 51.2 | 261 | 69.4 | 33 | 33.3 | 78 | 40.8 | 18 | 18.9 | |||||
| >10 mg/L | 371 | 48.8 | 115 | 30.6 | 66 | 66.7 | <.01 | 113 | 59.2 | <.01 | 77 | 81.1 | <.01 | ||
| mGPS | |||||||||||||||
| 0 | 390 | 51.3 | 261 | 69.4 | 33 | 33.3 | 78 | 40.8 | 18 | 19.0 | |||||
| 1 | 99 | 13.0 | 45 | 12.0 | 18 | 18.2 | 28 | 14.7 | 8 | 8.4 | |||||
| 2 | 272 | 35.7 | 70 | 18.6 | 48 | 48.5 | <.01 | 85 | 44.5 | <.01 | 69 | 72.6 | <.01 | ||
All patient characteristics were at the time of home parenteral nutrition start.
Abbreviations: BMI, body mass index; CRP, C‐reactive protein; CT−, no chemotherapy received; CT+, chemotherapy received; ECOG, Eastern Cooperative Oncology Group; KPS, Karnofsky Performance Status; mGPS, modified Glasgow Prognostic Score; PG‐SGA, Patient‐Generated Subjective Global Assessment; SD, standard deviation; SPN, supplemental parenteral nutrition; TPN, total parenteral nutrition.
P 1: the groups compared were SPN/CT+ vs. TPN/CT+; P 2: SPN/CT+ vs. SPN/CT‐; P 3: SPN/CT+ vs. TPN/CT‐. Where the comparison was significant, P‐value is reported.
In the last 3 months before home parenteral nutrition (HPN) start.
Moderately malnourished or suspected malnutrition.
Severely malnourished.
Figure 2.
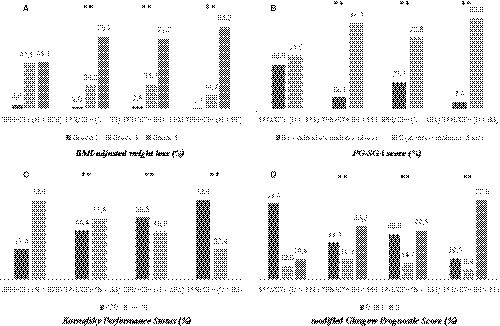
Differences in patient distribution (%) for the four groups identified (by the type of parenteral nutrition and whether they received chemotherapy during the study period). Differences are according to (a) BMI‐adjusted weight loss categories, (b) Patient‐Generated Subjective Global Assessment (PG‐SGA) score, (c) Karnofsky Performance Status, and (d) modified Glasgow Prognostic Score. ** P < .01 (SPN/CT + was the reference group when compared with TPN/CT+; SPN/CT‐; TPN/CT‐).SPN: supplemental parenteral nutrition; TPN: total parenteral nutrition; CT+: chemotherapy received; CT‐: no chemotherapy received; BMI: body mass index
Overall, most frequent tumors observed among patients starting HPN were those involving the digestive system. In particular, stomach cancer was the most frequently observed tumor for all cohorts (36%, 37%, 20%, and 24% for SPN/CT+, TPN/CT+, SPN/CT‐, and TPN/CT‐, respectively), followed by pancreatic and colon cancers. The number of patients with metastatic cancer, peritoneal carcinomatosis, and cancer stage IV was lower in the SPN/CT+ compared with the other three cohorts (Table 1). A similar difference in patient distribution was observed for the KPS (measured in four categories, Table 1, or as binary variable, Figure 2C) and the mGPS (Figure 2D). Patients who did not undergo chemotherapy and received TPN (TPN/CT‐) had a lower KPS (73% had KPS 50 or 60), lower albumin levels (86% had an albumin value < 3.5 g/dL), higher CRP levels (81% had a CRP value > 10 mg/L), and a worse mGPS (73% had an mGPS of 2). In contrast, patients who underwent chemotherapy and received SPN (SPN/CT+) had a higher KPS, higher albumin levels, lower CRP levels, and a better mGPS (Table 1).
3.3. Patient survival
Maximum follow‐up time was just over 6 years and no patients were lost to follow‐up. In Figure 3, Kaplan‐Meier curves for survival since HPN start in the different cohorts of treatment show a sharp decline in the first 12 months. The proportion of patients who were alive after the first 6 months of follow‐up since HPN start went from 77.9% for the SPN/CT+ group to 26.3%, 48.7%, and 18.9% (TPN/CT+, SPN/CT‐, and TPN/CT‐, respectively). Median OS in the whole population was 6.7 months (95% CI, 6.4 to 7.1), but was significantly different in the four cohorts analyzed: patients in the SPN/CT+ group survived significantly longer than those in the other cohorts (Figure 2). When comparing the other three cohorts between them, median OS differences were statistically significant in log‐rank test analysis (Table of Figure 3).
Figure 3.
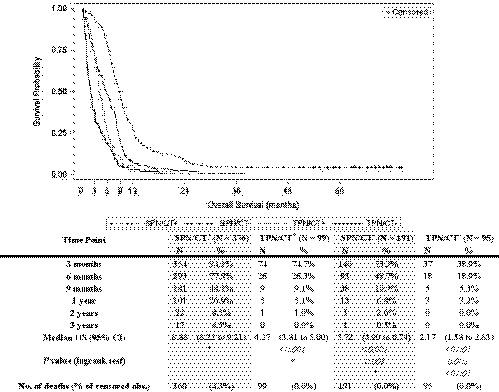
Overall survival. Overall survival (in months) since home parenteral nutrition start reported as Kaplan‐Meier curves that identify the different cohorts; percent of patients alive at different time‐points; median survival and 95% CI. * Reference group for the log‐rank test. SPN: supplemental parenteral nutrition; TPN: total parenteral nutrition; CT+: chemotherapy received; CT‐: no chemotherapy received; CI: confidence interval
All variables were analyzed to identify independent predictors of survival in univariate and multivariable analysis with the exception of peritoneal carcinomatosis that was excluded in the final model due to collinearity (Table 2). Differences in survival between SPN/CT+ and the other cohorts were confirmed in univariate and fully adjusted analysis with the TPN/CT‐ group having the highest HR. Similarly, the protective factors of survival at HPN start were as follows: a higher KPS (>50), higher oral protein intake (>20 g/day), normal albumin levels (≥3.5 g/dL), BMI (>20.5), and weight, while the predictors that decreased survival at HPN start were as follows: mGPS of 1 or 2, weight loss in the last 3 months (>15%), and being on cancer stage IV compared with II/III. Conversely, age (≥70), weight 3 months before HPN start, decreased oral calorie intake, BMI‐adjusted weight loss grade 4, PG‐SGA score rating of C, ECOG scale of 2, and CRP > 10 mg/L were significant only in univariate analysis.
Table 2.
Univariate analysis and Cox's proportional hazards models to assess the effect on patient survival of the variables considered in the study
| Univariate | Stepwise variables selection | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Cohort subgroups | ||||||
| SPN/CT+ (N = 376) | 1 | 1 | ||||
| TPN/CT+ (N = 99) | 2.99 | 2.38 to 3.76 | <.001 | 1.35 | 1.03 to 1.77 | .028 |
| SPN/CT‐ (N = 191) | 2.01 | 1.68 to 2.40 | <.001 | 1.46 | 1.20 to 1.77 | <.001 |
| TPN/CT‐ (N = 95) | 4.57 | 3.62 to 5.77 | <.001 | 1.71 | 1.28 to 2.29 | <.001 |
| Sex | ||||||
| Male | 1 | 1 | ||||
| Female | 0.89 | 0.77 to 1.03 | .117 | 0.64 | 0.54 to 0.76 | <.001 |
| Age categories (years) | ||||||
| <70 | 1 | |||||
| ≥70 | 1.77 | 1.50 to 2.08 | <.001 | |||
| Actual weight (kg, continuous) | 0.98 | 0.98 to 0.99 | <.001 | 0.97 | 0.96 to 0.98 | <.001 |
| BMI class | ||||||
| <20.5 kg/m a | 1 | 1 | ||||
| ≥20.5 kg/m2 | 0.71 | 0.61 to 0.82 | <.001 | 1.25 | 1.02 to 1.54 | .033 |
| Weight loss a (%) categories | ||||||
| ≤10.0% | 1 | 1 | ||||
| 10.1%‐15% | 1.56 | 1.28 to 1.90 | <.001 | 1.11 | 0.89 to 1.37 | .355 |
| 15.1%‐20% | 6.91 | 5.53 to 8.65 | <.001 | 2.45 | 1.87 to 3.21 | <.001 |
| >20% | 9.42 | 7.45 to 11.91 | <.001 | 2.94 | 2.22 to 3.90 | <.001 |
| Oral calorie intake (kcal/day) | ||||||
| >1000 | 1 | |||||
| 500‐1000 | 1.86 | 1.24 to 2.80 | .003 | |||
| <500 | 4.97 | 3.28 to 7.52 | <.001 | |||
| Oral protein intake (g/day) | ||||||
| <20 | 1 | 1 | ||||
| ≥20 | 0.37 | 0.32 to 0.43 | <.001 | 0.71 | 0.58 to 0.86 | <.001 |
| Tumor site | ||||||
| Ovary | 1 | |||||
| Gastrointestinal | 0.88 | 0.65 to 1.18 | .392 | |||
| Other | 1.18 | 0.85 to 1.63 | .336 | |||
| Cancer staging | ||||||
| II/III | 1 | 1 | ||||
| IV | 2.80 | 2.36 to 3.33 | <.001 | 1.34 | 1.01 to 1.78 | .044 |
| KPS | ||||||
| 50 | 1 | 1 | ||||
| 60 | 0.58 | 0.46 to 0.74 | <.001 | 0.69 | 0.53 to 0.89 | .005 |
| 70 | 0.23 | 0.18 to 0.29 | <.001 | 0.63 | 0.49 to 0.82 | <.001 |
| 80‐90 | 0.08 | 0.06 to 0.12 | <.001 | 0.33 | 0.22 to 0.50 | <.001 |
| ECOG scale | ||||||
| 1 | 1 | |||||
| 2 | 3.35 | 2.87 to 3.92 | <.001 | |||
| Albumin | ||||||
| <3.5 g/dL | 1 | 1 | ||||
| ≥3.5 g/dL | 0.37 | 0.32 to 0.43 | <.001 | 0.69 | 0.55 to 0.87 | .001 |
| mGPS | ||||||
| 0 | 1 | 1 | ||||
| 1 | 3.63 | 2.87 to 4.57 | <.001 | 3.17 | 2.39 to 4.22 | <.001 |
| 2 | 19.69 | 15.74 to 24.63 | <.001 | 8.35 | 6.35 to 10.97 | <.001 |
All patient characteristics were at the time of home parenteral nutrition start.
Abbreviations: BMI, body mass index; CI, confidence interval; CT‐, no chemotherapy received; CT+, chemotherapy received; ECOG, Eastern Cooperative Oncology Group; KPS, Karnofsky Performance Status; mGPS, modified Glasgow Prognostic Score; SPN, supplemental parenteral nutrition; TPN, total parenteral nutrition.
In the last 3 months before home parenteral nutrition (HPN) start.
4. DISCUSSION
In the last 20 years, survival in cancer outpatients on PN has been investigated in a number of observational studies. 8 , 11 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 Some studies reported that a survival longer than expected in aphagic or severely hypophagic cancer patients can be achieved through PN. 8 , 11 , 32 This especially occurred in patients presenting favorable prognostic factors (KPS, mGPS, and tumor stage) 8 , 31 , 34 , 35 , 36 or responding to chemotherapy. 33 In these studies, median OS varied widely: 45 days, 33 , 35 3‐4 months, 8 , 34 , 37 , 38 or 5‐6 months. 11 , 31 , 32 , 36
However, all these papers have one or more of the following limitations: patient inclusion criteria did not comply with guideline recommendations for PN administration (KPS < 50) 8 , 31 , 34 , 35 , 36 or short life expectancy 33 , 35 ; a small sample size of 50‐150 patients 11 , 31 , 32 , 33 , 34 , 35 , 37 or less 36 , 38 ; design of the study (retrospective instead of prospective) 32 , 34 , 35 , 38 ; inclusion of patients with both cancer and noncancer diseases 37 , 38 ; inclusion of patients with different oncologic approaches (treated and nontreated patients) 11 , 33 , 36 , 38 ; and different setting of PN administration (hospital or hospice instead of home). 32 , 33 Therefore, it has not been possible to highlight a possible survival advantage induced by HPN so far.
To our knowledge, this is the largest, prospective, clinical study investigating survival exclusively of adult‐malnourished cancer patients receiving PN at home. Moreover, the originality of our study is that we investigated the OS of four cohorts of cancer patients on HPN grouped according to the provision of SPN or TPN and whether they received chemotherapy. Despite the differences in median OS reported between cohorts, the aim of this study was not to draw inference on the benefit of the type of PN (SPN or TPN) or chemotherapy, given the differences between the four cohorts at HPN start. For instance, the longer median OS in the SPN/CT+ group was not surprising as patients in this cohort were younger, suffered less weight loss and cancer progression in the months leading to HPN start, had better nutritional status and prognostic scores (KPS and mGPS). Therefore, instead of focusing on comparison, results from this observational study should be considered as a detailed description of each cohort.
Interestingly, the cohorts of patients who showed a higher median survival (SPN groups) are often considered the ones less likely to receive HPN because of the presence of residual oral food intake. 17 , 39 , 40 Given the median OS of nearly 9 months, this study adds to the discussion around the benefit of SPN in advanced cancer patients receiving chemotherapy. 20 , 41 , 42 Of course, no direct inference on the benefit of SPN can be drawn from this study given that no control group without PN was available.
Indeed, RCTs remain the gold standard for this comparison. Regarding survival outcome, two underpowered RCTs reported that PN was neither superior to fluid administration in patients with days or a few weeks of expected survival 43 nor to dietary counseling in patients with oral energy intake above 75% of estimated needs and receiving chemotherapy. 44 However, the major limitation of both RCTs was that patient inclusion criteria did not comply with guideline recommendations for PN administration. Actually, PN is neither indicated in patients with short life expectancy nor in patients with sufficient ONS or EN intake. 2 , 45
Indications that higher KPS scores increase the probability of survival have been reported in observational 8 , 34 , 35 and survival prediction studies, 19 although KPS scores were reported as lower or equal/higher than 50. Differently, in this study we included only patients with KPS ≥ 50 according to the guideline recommendations for eligibility for HPN. Consequently, given the large sample of patients, survival analyses in this study were able to highlight how even small increases in KPS score (10‐point increases) could significantly have a protective effect on survival.
The mGPS is an inflammation‐based prognostic score widely used in predicting survival in cancer patients. 28 , 46 , 47 Specifically, when measured at HPN start, elevated mGPS scores have been associated with a higher risk of mortality 8 , 34 and has also been incorporated in a nomogram to predict survival in cancer patients on HPN. 19 Our study confirmed the value of mGPS as a strong predictor of survival while PG‐SGA, ECOG scale, and BMI‐adjusted weight loss 48 failed to provide prognostic contribution in the multivariable analysis. Similarly, we confirmed that tumor spread (TNM stage IV at HPN start) was a predictor of reduced survival in these patients. 19
In multivariable analysis, other predictors of survival were higher oral protein intake and normal albumin levels that showed protective effect on mortality. The role of albumin as prognostic factor in patients with advanced cancer is known, 46 although little indications that albumin levels could be a significant predictor in HPN patients have been reported. 31 , 34 , 35
In this study, we also reported how body weight at HPN start was a significant factor and each kilogram of weight could potentially reduce the risk of death by 3%. Indeed, the suggestion that in cancer patients, especially those exposed to cancer therapy, a higher body weight could be beneficial to survival has already been proposed. 49 However, a number of variables associated with body weight and weight loss are similar and therefore unlikely be significant in the multivariate analysis.
In our study, some patients died before the estimated life expectancy of 2 months; therefore these "estimates" are inadequate. Our survival analysis lends itself to the potential development of a prognostic index, which including all the key variables could be extremely powerful in predicting short survival probabilities (few weeks). Indeed, since June 2015 we adopted a nomogram to predict survival in cancer patients and replace the estimated life expectancy as inclusion criteria for HPN eligibility. 19
Some physicians are concerned that initiating HPN may lead to onset of CRCs, especially in patients receiving chemotherapy. An important message for oncologists is that, as in our early experience, 22 , 23 we reported that HPN can be safely carried out (low rate of CRBSI and other CRCs) in cancer patients, even if receiving chemotherapy, consistent with the current practice of HPN.
However, HPN safety is mainly assured if the evaluation of eligibility for HPN program is performed by physicians and dieticians expert in nutrition in oncology as well as if patients and caregivers are adequately trained at home by a specialized nursing staff and are carefully monitored in the follow‐up. 50 In this study, eligibility for HPN program was assessed according to a set of criteria following the European guidelines 4 , 5 that assured a regulated access to this nutritional intervention. As a result is noteworthy that the cohort of patients who received HPN represents only the 46% of the initial 1658 patients referred for eligibility for HPN to our Unit.
When compared with previous studies in this research field, our study has many strengths: [1] was a prospectively conducted study; [2] data were collected through a clinical observation carried out over a 7‐year period; [3] the study population consisted of 761 adult patients; [4] all patients were evaluated as eligible for HPN program according to the guideline recommendations; [5] only cancer patients were included; [6] all patients were malnourished; [7] only patients receiving PN at home were included; [8] two thirds of patients were receiving chemotherapy during the study period; [9] there were no missing data; and [10] no patients were lost at follow‐up.
In our Region, institution of HPN is the standard of care in these cancer patients, and furthermore it has been instituted by established criteria according to oncology nutrition clinical practice guidelines. 4 , 5 This gives us an opportunity to determine which factors may be associated with survival in patients fed intravenously. This study could not be done in other countries where, for cultural (not scientific) reasons, HPN is not that standard of care.
The main limitation of this study is the lack of randomization; however, an RCT is ethically unacceptable because a control group of patients who are aphagic or severely hypophagic and do not get any nutritional support is at risk of earlier death due to malnutrition rather than from cancer progression. Besides, this is a single‐center study; however, our results may be generalizable to other populations of cancer patients on HPN according to guideline recommendations. Finally, patients’ cancer treatment plans were not reported; however, a detailed description of the chemotherapy and radiotherapy regimens adopted in the different sites and stages of the tumors was beyond the aims of this paper.
5. CONCLUSION
With the increasing accessibility to effective anticancer treatments and their ability to transform the trajectory of the advanced cancer into a chronic condition, a growing number of patients are expected to be in need of HPN. However, there is a lack of robust data reporting the survival of these patients on HPN.
In summary, this prospective study in a large population of cancer patients on HPN showed that survival is significantly correlated with patient characteristics at HPN start and that the presence of favorable factors may determine even a fourfold increase in survival. These data reporting real‐world data on the use of HPN are expected to assist physicians in appropriate prescription of HPN, patients and their home caregivers to make better‐informed treatment decisions, and health‐care professionals and institutions to develop best practices for HPN.
CONFLICT OF INTEREST
PC reported honoraria for speaking and teaching from Baxter. The other authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
PC was the chief investigator, designed the study and developed the protocol. PC, TM, and LB participated in recruitment of patients. PC and RP developed and carried out the statistical analysis plan. PC, TM, and ADF coordinated the data collection and regulatory and governance requirements. PC and RP interpreted the data. PC wrote the draft manuscript. All authors contributed to the review and amendments of the manuscript for important intellectual content and approved this final version for submission.
ACKNOWLEDGEMENTS
The authors are grateful to all the patients and their caregivers whose willingness to participate made this study possible. We thank all physicians, nurses, and dietitians involved in the Public Health Program of Parenteral Nutrition in Oncology in Piedmont, which made this study possible.
Cotogni P, Monge T, Passera R, Brossa L, De Francesco A. Clinical characteristics and predictive factors of survival of 761 cancer patients on home parenteral nutrition: A prospective, cohort study. Cancer Med. 2020;9:4686–4698. 10.1002/cam4.3064
Funding information
This study was partially supported by grants from the Regional Public Healthcare Office, Piedmont Region, Italy (19700/27.001, 1837/27.001 to PC).
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bozzetti F. Nutritional support of the oncology patient. Crit Rev Oncol Hematol. 2013;87(2):172‐200. [DOI] [PubMed] [Google Scholar]
- 2. Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36(1):11‐48. [DOI] [PubMed] [Google Scholar]
- 3. August DA, Huhmann MB. American Society for Parenteral and Enteral Nutrition (ASPEN) board of directors. ASPEN clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enter Nutr. 2009;33(5):472‐500. [DOI] [PubMed] [Google Scholar]
- 4. Arends J, Bodoky G, Bozzetti F, et al. ESPEN guidelines on enteral nutrition: non‐surgical oncology. Clin Nutr. 2006;25(2):245‐259. [DOI] [PubMed] [Google Scholar]
- 5. Bozzetti F, Arends J, Lundholm K, et al. ESPEN Guidelines on Parenteral Nutrition: non‐surgical oncology. Clin Nutr. 2009;28(4):445‐454. [DOI] [PubMed] [Google Scholar]
- 6. Staun M, Pironi L, Bozzetti F, et al. ESPEN Guidelines on parenteral nutrition: home parenteral nutrition (HPN) in adult patients. Clin Nutr. 2009;28(4):467‐479. [DOI] [PubMed] [Google Scholar]
- 7. Muscaritoli M, Molfino A, Laviano A, et al. Parenteral nutrition in advanced cancer patients. Crit Rev Oncol Hematol. 2012;84(1):26‐36. [DOI] [PubMed] [Google Scholar]
- 8. Bozzetti F, Santarpia L, Pironi L, et al. The prognosis of incurable cachectic cancer patients on home parenteral nutrition: a multi‐centre observational study with prospective follow‐up of 414 patients. Ann Oncol. 2014;25(2):487‐493. [DOI] [PubMed] [Google Scholar]
- 9. Bozzetti F. Nutrition, hydration, and patient’s preferences at the end of life. Support Care Cancer. 2015;23(6):1487‐1488. [DOI] [PubMed] [Google Scholar]
- 10. Druml C, Ballmer PE, Druml W, et al. ESPEN guideline on ethical aspects of artificial nutrition and hydration. Clin Nutr. 2016;35(3):545‐556. [DOI] [PubMed] [Google Scholar]
- 11. Hoda D, Jatoi A, Burnes J, et al. Should patients with advanced, incurable cancers ever be sent home with total parenteral nutrition? A single institution’s 20‐year experience. Cancer. 2005;103(4):863‐868. [DOI] [PubMed] [Google Scholar]
- 12. Martin L, de van der Schueren MAE, Blauwhoff‐Buskermolen S, et al. Identifying the barriers and enablers to nutrition care in head and neck and esophageal cancers: an international qualitative study. JPEN J Parenter Enter Nutr. 2016;40(3):355‐366. [DOI] [PubMed] [Google Scholar]
- 13. Naghibi M, Smith TR, Elia M. A systematic review with meta‐analysis of survival, quality of life and cost‐effectiveness of home parenteral nutrition in patients with inoperable malignant bowel obstruction. Clin Nutr. 2015;34(5):825‐837. [DOI] [PubMed] [Google Scholar]
- 14. Sowerbutts AM, Lal S, Sremanakova J, et al. Home parenteral nutrition for people with inoperable malignant bowel obstruction. Cochrane Database Syst Rev. 2018;8:1‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spiro A, Baldwin C, Patterson A, et al. The views and practice of oncologists towards nutritional support in patients receiving chemotherapy. Br J Cancer. 2006;95(4):431‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Caccialanza R, Cereda E, Pinto C, et al. Awareness and consideration of malnutrition among oncologists: insights from an exploratory survey. Nutrition. 2016;32(9):1028‐1032. [DOI] [PubMed] [Google Scholar]
- 17. Cotogni P, Pedrazzoli P, De Waele E, et al. Nutritional therapy in cancer patients receiving chemoradiotherapy: Should we need stronger recommendations to act for improving outcomes? J Cancer. 2019;10(18):4318‐4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pironi L, Candusso M, Biondo A, et al. Prevalence of home artificial nutrition in Italy in 2005: a survey by the Italian Society for Parenteral and Enteral Nutrition (SINPE). Clin Nutr. 2007;26(1):123‐132. [DOI] [PubMed] [Google Scholar]
- 19. Bozzetti F, Cotogni P, Lo Vullo S, et al. Development and validation of a nomogram to predict survival in incurable cachectic cancer patients on home parenteral nutrition. Ann Oncol. 2015;26(11):2335‐2340. [DOI] [PubMed] [Google Scholar]
- 20. Cotogni P, Monge T, Fadda M, et al. Bioelectrical impedance analysis for monitoring cancer patients receiving chemotherapy and home parenteral nutrition. BMC Cancer. 2018;18(1):990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cotogni P, De Carli L, Passera R, et al. Longitudinal study of quality of life in advanced cancer patients on home parenteral nutrition. Cancer Med. 2017;6(7):1799‐1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cotogni P, Barbero C, Garrino C, et al. Peripherally inserted central catheters in non‐hospitalized cancer patients: 5‐year results of a prospective study. Support Care Cancer. 2015;23(2):403‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cotogni P, Pittiruti M, Barbero C, et al. Catheter‐related complications in cancer patients on home parenteral nutrition: a prospective study of over 51,000 catheter days. JPEN J Parenter Enter Nutr. 2013;37(3):375‐383. [DOI] [PubMed] [Google Scholar]
- 24. De Francesco A, Fadda M, Malfi G, et al. Home parenteral nutrition in Italy: Data from the Italian National Register. Clin Nutr. 1995;14:6‐9. [DOI] [PubMed] [Google Scholar]
- 25. Finocchiaro E, Rahimi F, Agnello E, et al. Home parenteral nutrition in advanced cancer patients: a four‐years multicenter prospective observational study. Nutr Ther Metab. 2007;25(1):31‐39. [Google Scholar]
- 26. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern cooperative oncology group. Am J Clin Oncol. 1982;5:649‐655. [PubMed] [Google Scholar]
- 27. Karnofsky DA, Burchenal JH. The clinical evaluation of chemotherapeutic agents in cancer In: MacLeod CM, ed. Evaluation of Chemotherapeutic Agents. New York, NY: Columbia University Press; 1949:191‐205. [Google Scholar]
- 28. McMillan DC. An inflammation‐based prognostic score and its role in the nutrition‐based management of patients with cancer. Proc Nutr Soc. 2008;67:257‐262. [DOI] [PubMed] [Google Scholar]
- 29. Ottery FD. Definition of standardized nutritional assessment and interventional pathways in oncology. Nutrition. 1996;12(1):S15‐S19. [DOI] [PubMed] [Google Scholar]
- 30. Cotogni P. Enteral versus parenteral nutrition in cancer patients: evidences and controversies. Ann Palliat Med. 2016;5(1):42‐49. [DOI] [PubMed] [Google Scholar]
- 31. Chermesh I, Mashiach T, Amit A, et al. Home parenteral nutrition (HTPN) for incurable patients with cancer with gastrointestinal obstruction: do the benefits outweigh the risks? Med Oncol. 2011;28(1):83‐88. [DOI] [PubMed] [Google Scholar]
- 32. Fan B‐G. Parenteral nutrition prolongs the survival of patients associated with malignant gastrointestinal obstruction. JPEN J Parenter Enter Nutr. 2007;31(6):508‐510. [DOI] [PubMed] [Google Scholar]
- 33. Guerra EM, Cortés‐Salgado A, Mateo‐Lobo R, et al. Role of parenteral nutrition in oncologic patients with intestinal occlusion and peritoneal carcinomatosis. Nutr Hosp. 2015;32(3):1222‐1227. [DOI] [PubMed] [Google Scholar]
- 34. Keane N, Fragkos KC, Patel PS, et al. Performance status, prognostic scoring, and parenteral nutrition requirements predict survival in patients with advanced cancer receiving home parenteral nutrition. Nutr Cancer. 2018;70(1):73‐82. [DOI] [PubMed] [Google Scholar]
- 35. Santarpia L, Alfonsi L, Pasanisi F, et al. Predictive factors of survival in patients with peritoneal carcinomatosis on home parenteral nutrition. Nutrition. 2006;22(4):355‐360. [DOI] [PubMed] [Google Scholar]
- 36. Soo I, Gramlich L. Use of parenteral nutrition in patients with advanced cancer. Appl Physiol Nutr Metab. 2008;33(1):102‐106. [DOI] [PubMed] [Google Scholar]
- 37. Theilla M, Cohen J, Kagan I, et al. Home parenteral nutrition for advanced cancer patients: contributes to survival? Nutrition. 2018;2018(54):197‐200. [DOI] [PubMed] [Google Scholar]
- 38. Wang M‐Y, Wu M‐H, Hsieh D‐Y, et al. Home parenteral nutrition support in adults: experience of a medical center in Asia. JPEN J Parenter Enter Nutr. 2007;31(4):306‐310. [DOI] [PubMed] [Google Scholar]
- 39. Caccialanza R, De Lorenzo F, Gianotti L, et al. Nutritional support for cancer patients: still a neglected right? Support Care Cancer. 2017;25(10):3001‐3004. [DOI] [PubMed] [Google Scholar]
- 40. Prado CM, Sawyer MB, Ghosh S, et al. Central tenet of cancer cachexia therapy: do patients with advanced cancer have exploitable anabolic potential? Am J Clin Nutr. 2013;98(4):1012‐1019. [DOI] [PubMed] [Google Scholar]
- 41. Aapro M, Arends J, Bozzetti F, et al. Early recognition of malnutrition and cachexia in the cancer patient: a position paper of a European School of Oncology Task Force. Ann Oncol. 2014;25(8):1492‐1499. [DOI] [PubMed] [Google Scholar]
- 42. Durán‐Poveda M, Jimenez‐Fonseca P, Sirvent‐Ochando M, et al. Integral nutritional approach to the care of cancer patients: results from a Delphi panel. Clin Transl Oncol. 2018;20(9):1202‐1211. [DOI] [PubMed] [Google Scholar]
- 43. Oh SY, Jun HJ, Park SJ, et al. A randomized phase II study to assess the effectiveness of fluid therapy or intensive nutritional support on survival in patients with advanced cancer who cannot be nourished via enteral route. J Palliat Med. 2014;17(11):1266‐1270. [DOI] [PubMed] [Google Scholar]
- 44. Obling SR, Wilson BV, Pfeiffer P, et al. Home parenteral nutrition increases fat free mass in patients with incurable gastrointestinal cancer. Results of a randomized controlled trial. Clin Nutr. 2017;38(1):182‐190. [DOI] [PubMed] [Google Scholar]
- 45. Tobberup R, Thoresen L, Falkmer UG, et al. Effects of current parenteral nutrition treatment on health‐related quality of life, physical function, nutritional status, survival and adverse events exclusively in patients with advanced cancer: a systematic literature review. Crit Rev Oncol Hematol. 2019;139(1):96‐107. [DOI] [PubMed] [Google Scholar]
- 46. Dolan RD, McSorley ST, Horgan PG, et al. The role of the systemic inflammatory response in predicting outcomes in patients with advanced inoperable cancer: systematic review and meta‐analysis. Crit Rev Oncol Hematol. 2017;116:134‐146. [DOI] [PubMed] [Google Scholar]
- 47. Proctor MJ, Morrison DS, Talwar D, et al. An inflammation‐based prognostic score (mGPS) predicts cancer survival independent of tumour site: a glasgow inflammation outcome study. Br J Cancer. 2011;104(4):726‐734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Martin L, Senesse P, Gioulbasanis I, et al. Diagnostic criteria for the classification of cancer‐associated weight loss. J Clin Oncol. 2014;33(1):90‐99. [DOI] [PubMed] [Google Scholar]
- 49. Tsang NM, Pai PC, Chuang CC, et al. Overweight and obesity predict better overall survival rates in cancer patients with distant metastases. Cancer Med. 2016;5(4):665‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dreesen M, Foulon V, Hiele M, et al. Quality of care for cancer patients on home parenteral nutrition: development of key interventions and outcome indicators using a two‐round Delphi approach. Support Care Cancer. 2013;21(5):1373‐1381. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


