Abstract
Alcohol use disorder (AUD) and posttraumatic stress disorder (PTSD) are two prevalent psychiatric conditions in the U.S. The co-occurrence of AUD and PTSD is also common, and associated with a more severe clinical presentation and worse treatment outcomes across the biopsychosocial spectrum (e.g., social and vocational functioning, physical health) as compared to either disorder alone. Despite the high co-occurrence and negative outcomes, research on effective medications for AUD/PTSD is sparse and there is little empirical evidence to guide treatment decisions. The study described in this paper addresses this knowledge gap by testing the efficacy of N-acetylcysteine (NAC) in reducing alcohol use and PTSD symptoms. Animal studies and prior clinical research suggest a role for NAC in the treatment of substance use disorders and PTSD via glutamate modulation. NAC is a cysteine pro-drug that stimulates the cystine-glutamate exchanger, normalizes glial glutamate transporters, and restores glutamatergic tone on presynaptic receptors in reward regions of the brain. Moreover, NAC is available over-the-counter, has a long-established safety record, and does not require titration to achieve the target dose. This paper describes the rationale, study design, and methodology of a 12-week, randomized, double-blind, placebo-controlled trial of NAC (2400 mg/day) among adults with co-occurring AUD and PTSD. Functional magnetic resonance imaging (fMRI) and proton magnetic resonance spectroscopy (1H-MRS) are utilized to investigate the neural circuitry and neurochemistry underlying comorbid AUD/PTSD and identify predictors of treatment outcome. This study is designed to determine the efficacy of NAC in the treatment of co-occurring AUD/PTSD and provide new information regarding mechanisms of action implicated in co-occurring AUD/PTSD.
Keywords: Alcohol, Alcohol use disorder, Posttraumatic stress disorder, PTSD, N-acetylcysteine
1. Introduction
Alcohol use disorder (AUD) and post-traumatic stress disorder (PTSD) are two common and debilitating psychiatric conditions. Data from national epidemiological studies indicate that the lifetime prevalence rates of AUD and PTSD are 29.1% and 7.8%, respectively [21,28,29]. Moreover, AUD and PTSD frequently co-occur [60,71]. Among individuals seeking treatment for substance use disorders, 30%–59% meet criteria for PTSD [4,19,39,49,71]. This is of considerable clinical importance, as comorbid substance use disorder and PTSD is associated with a more severe presentation and worse treatment outcomes as compared to either disorder alone [40,60,71].
Research on effective treatments for comorbid AUD/PTSD is sparse and no medications, to date, have conclusively been shown to improve symptoms of co-occurring AUD and PTSD [50]. Emerging evidence regarding shared neurobiologic links between AUD and PTSD suggests potential treatment targets. In particular, preclinical and clinical studies indicate that glutamate dysfunction plays an important role in addictive processes across multiple substances of abuse, including alcohol [3,17,43,47] as well as PTSD [48]. As such, medications that normalize glutamatergic functioning, such as N-acetylcysteine (NAC), are potential candidate pharmacotherapies ([27,50,66,67,70].
NAC is an antioxidant that has been used in the treatment of acetaminophen overdose for more than three decades [64]. In animal models, NAC reduces reinstatement to alcohol and drug use, purportedly via glial glutamate transporter (GLT-1) dependent mechanisms [16,18,20,31,55,58]. In human studies, NAC has been shown to reduce craving and substance use, although the findings are mixed [22,23,32,35,57,75]. In a recent, 8-week randomized controlled trial (RCT) of 35 individuals with comorbid substance use disorders and PTSD, NAC significantly reduced craving and PTSD symptoms [5]. Moreover, NAC was well tolerated in this sample with minimal, transient side effects. Given the effects of NAC on shared neurobiological impairments associated with both AUD and PTSD, as well as promising preliminary data, the current study was designed to examine NAC in a larger sample of individuals (N = 200) with co-occurring AUD/PTSD. This paper describes the study design and methodology of the ongoing RCT, which is the first study to our knowledge to evaluate NAC among civilians with co-occurring AUD/PTSD.
1.1. Research objectives and hypotheses
The primary objective of the current study is to evaluate NAC in reducing alcohol use and PTSD symptomatology by comparing NAC (2400 mg/day) to placebo among individuals with current AUD/PTSD. Secondary objectives are to evaluate the effects of NAC on impairment in associated areas of functioning (e.g., depression). In addition, we are utilizing functional magnetic resonance imaging (fMRI) and proton magnetic resonance spectroscopy (1H-MRS) to investigate the underlying pathophysiology of AUD/PTSD and identify predictors of treatment response.
There are three main hypotheses regarding changes in outcomes of interest during the treatment phase (weeks 1–12). Hypothesis 1 proposes that participants who receive NAC, as compared to placebo, will evidence significantly greater reductions in alcohol use and craving at the end of treatment. Hypothesis 2 is that NAC, as compared to placebo, will result in significantly greater reduction in PTSD symptoms at the end of treatment. Finally, Hypothesis 3 centers on the functional neuroimaging aims and proposes that (a) prefrontal cortex-amygdala connectivity at rest and in response to alcohol vs. neutral cues during the baseline scan will predict reduction in alcohol use, and (b) prefrontal cortex-amygdala connectivity at rest and in response to trauma vs. neutral cues at baseline scan will predict reduction in PTSD symptoms. Exploratory analyses will examine the effects of NAC vs. placebo on (a) changes in glutamate concentrations in the dorsal anterior cingulate cortex (dACC), and (b) the relationship between changes in glutamate concentrations and clinical outcomes.
2. Materials and methods
2.1. Research design
This study is a phase II, double-blind, 12-week RCT to evaluate the efficacy of NAC (2400 mg/day) in reducing alcohol use and PTSD symptomatology among treatment-seeking individuals with current AUD and PTSD. Following the informed consent process and baseline assessment, eligible subjects are randomized to receive NAC or placebo. All participants, regardless of treatment arm, receive weekly individual cognitive-behavioral therapy (CBT) for AUD. Following completion of the treatment phase of the study, participants complete follow-up assessment visits at 3, 6, and 12 months post-treatment. Baseline neuroimaging (scan 1) is completed prior to medication initiation and repeated (scan 2) prior to medication discontinuation. Fig. 1 illustrates the study design.
Fig. 1. Study design overview.
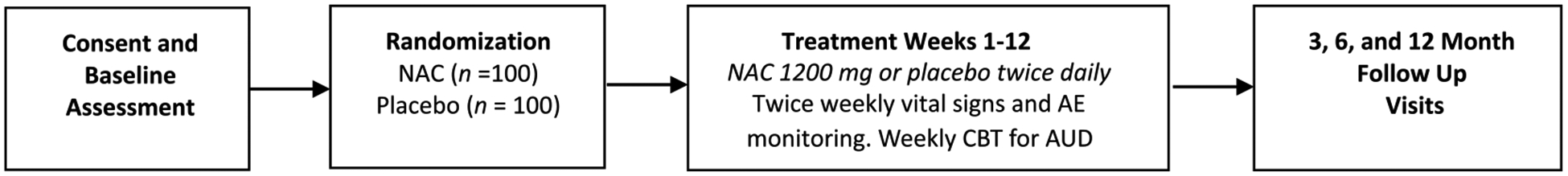
Note. Participants receive 12, 60-min sessions of cognitive-behavioral therapy (CBT) for alcohol use disorder (AUD) and either NAC (2400 mg/day, dosed as 1200 mg twice daily) or placebo. Twice weekly visits occur during the treatment phase (weeks 1–12). Follow-up visits occur at 3, 6 and 12 months post-treatment.
2.2. Participants
Participants (N = 200) include adults of all genders, races, and ethnicities, ages 18–65 with current AUD and PTSD. The vast majority of participants are anticipated to be civilians and roughly half of the sample female. Inclusion criteria include: 1) meets DSM-5 criteria for current AUD as assessed by the Mini International Neuropsychiatric Interview (MINI; [59]) and endorses some alcohol use within the past 60 days, and 2) meets DSM-5 criteria for current PTSD as assessed by the Clinician Administered PTSD Scale (CAPS-5; [72,73]). Participants may meet DSM-5 criteria for another substance use disorder as long as alcohol is the primary substance of choice. Participants taking psychotropic medications are required to be maintained on a stable dose for at least four weeks before treatment initiation. Exclusion criteria for the overall study include: 1) history of or current psychotic disorder or bipolar disorder, 2) pregnant or current nursing status, 3) evidence of liver damage; alanine aminotransferase (ALT) or aspartate aminotransferase (AST) levels greater than 3 times the upper limit of normal; 4) asthma or any clinically significant medical condition that in the opinion of the investigator would adversely affect safety or study participation; 5) use of medications that may have an interaction if taken with NAC (e.g., nitroglycerin, carbamazepine); 6) history of childhood or adult seizures of any cause; or 7) any clinically significant medical condition that in the opinion of the investigator would adversely affect safety or study participation. Individuals presenting with significant withdrawal symptoms, as evidenced by a score of ≥10 on the Clinical Institute Withdrawal Assessment of Alcohol – Revised (CIWA; [69]), are referred for clinical detoxification and may be re-assessed for study eligibility after medically supervised detoxification has been completed. Individuals considered an immediate suicide risk or who are likely to require hospitalization during the course of the study for suicidality are excluded. Exclusion criteria specific to the neuroimaging component include claustrophobia; metal fragments in the face, eyes or skin; non-removable hearing aids; shunts/stents; metal mesh/coil implants; metal plate/pin/screws/wires; or any other metal implants.
2.3. Procedures
This study was reviewed and approved by the Institutional Review Board (IRB) of the Medical University of South Carolina (MUSC). The study is being carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). Potential participants are given a full description of the study and asked to read and sign IRB-approved informed consent forms before any study procedures take place. Interested individuals are initially screened for eligibility and those who meet inclusion criteria are invited to come into the office for a comprehensive baseline assessment (see Table 1 for measures). Following the baseline visit, eligible participants complete a randomization and medication initiation, enter the 12-week RCT, and return for post-treatment follow-up at 3, 6, and 12 months. At week 6 (mid-treatment), some assessments are repeated (e.g., CAPS-5, pregnancy test for women, Columbia Suicide Severity Rating Scale) as shown in Table 1. Eligible participants also complete the initial neuroimaging visit prior to medication initiation and the second neuroimaging session during week 8 of treatment phase. Ineligible individuals are referred clinically for treatment.
Table 1.
Assessment Instruments and Timeline.
| Instrument name | Purpose/domain | Baseline | Weekly | End of treatment | Follow up |
|---|---|---|---|---|---|
| Informed consent | Obtain informed consent | X | |||
| Demographics form | Characterize sample | X | |||
| History and physical examination | Assess medical problems & eligibility | X | |||
| MINI interview for DSM-5 | Assess AUD and other DSM-5 psychiatric disorders | X | |||
| Columbia-suicide severity rating scale: CSSRSa | Assess suicidality & eligibility | X | Week 6 | X | X |
| Pregnancy test for female subjectsb | Assess for pregnancy | X | Week 6 | ||
| Clinical institute withdrawal assessment of alcohol-revised: CIWA | Study eligibility, safety | X | |||
| Urine drug screen tests: UDSa | Assess drug use | X | Week 6 | X | X |
| Breathalyzer | Assess alcohol use (biological) | X | X | X | X |
| Adverse events | Monitor AEs and safety | X | X | X | X |
| Vital signs | Blood pressure and heart rate | X | X | X | |
| Concurrent medications form | Monitor medications | X | X | X | X |
| Medication adherence log | Assess medication compliance | X | X | ||
| Urine riboflavin testa | Biomarker of medication compliance | Week 6 | X | ||
| Clinician administered PTSD scale: CAPS-5a | PTSD diagnosis and symptoms (clinician rated) | X | Week 6 | X | X |
| PTSD checklist: PCL-5 | PTSD symptoms (self-report) | X | X | X | X |
| Timeline follow-back: TLFB | Assess alcohol use (amount and frequency) | X | X | X | X |
| Obsessive compulsive drinking scale: OCDS | Measure alcohol craving | X | X | X | X |
| Visual analog scale: VAS | Assess alcohol craving | X | X | X | X |
| Alcohol use disorders identification test: AUDIT | Assess alcohol use and related problems | X | Month 12 | ||
| Ethyl Glucuronide: EtGa | Biomarker of alcohol use | Week 6 | X | X | |
| Beck depression inventory-II: BDI-lI | Measure depression | X | X | X | X |
| Satisfaction questionnaire | Asses overall satisfaction | X | Month 12 | ||
| Treatment services review | Monitor services utilization | X | X | X | X |
Note. Follow up = 3, 6, and 12 months following treatment.
Repeated at week 6.
Pregnancy test at baseline, before medication initiation, before neuroimaging sessions, and at week 6.
2.4. Study medication, dosage, administration, and randomization
The dose of NAC (2400 mg/day) and length of treatment (12 weeks) are based on previous research [23,24,32,57]. NAC is initiated at 2400 mg/day and does not require upward or downward titration, which is an advantage to this medication. Placebo capsules are matched and visually identical to the NAC capsules. All NAC and placebo capsules contain 25 mg riboflavin, which serves as a biomarker to assess medication compliance. Medication adherence is monitored through use of a riboflavin assay. Study participants who request multi-vitamin supplementation during the treatment phase of the study are provided with a specific multi-vitamin formulation (Tri-Vi-Sol) that does not contain riboflavin. Weekly pill counts and documentation of missed doses are carefully recorded at each visit. Research staff administer the study medication or placebo at the weekly visits, and participants are given take-home doses to self-administer on the days in between study visits. Participants are maintained at the target dose for 12 weeks. Side effects and adverse events are evaluated weekly.
Participants are randomly assigned (1:1) to receive NAC or matched placebo. The double-blind design is further preserved by treatment assignment according to a pre-arranged randomization scheme. Stratified block randomization is used to balance the randomization assignment with respect to alcohol use and PTSD symptoms. For this study, high AUD is defined as ≥16 on the AUDIT at baseline and high PTSD is defined as ≥48 on the PCL-5 at baseline. Randomization is carried out by a pharmacist who is not involved in the clinical management of participants.
2.5. Cognitive behavioral therapy (CBT)
All participants receive 12, individual, weekly 60-min sessions of manualized CBT for AUD [26]. Examples of session topics include: assessing high-risk situations, coping with cravings and urges to drink, managing thoughts about drinking, drink refusal skills, and seemingly irrelevant decisions. Receipt of weekly CBT during the treatment phase is designed to help facilitate retention and medication adherence, and ensure that all participants receive adequate psychosocial support, regardless of medication arm. It also serves to provide a standardized behavioral platform, which may enhance statistical power by restricting variability resulting from varied types of interventions (error or “noise” variance) [10].
Therapist Training, Supervision and Fidelity Monitoring: All therapists are masters- or doctoral-level clinicians with experience delivering CBT. Therapists receive extensive training in the CBT protocol by the investigators, including didactic review of intervention-specific theory and manual review. Throughout the study, therapists receive weekly supervision focusing on manual adherence, and any clinical concerns about particular patients. Supervision also includes monitoring therapists to assure that they are not delivering trauma-specific therapy. Patients needing PTSD treatment post intervention are referred for specialized treatment.
2.6. Assessments
Table 1 includes the list and timeline of assessments used in this study. Measures are included to capture information related to demographics, alcohol use disorder, trauma exposure, PTSD, and depression and mood [6,51,69]. The measures were selected because many are standardized, have good psychometric properties, and are widely used in research on AUD and PTSD.
The MINI International Neuropsychiatric Interview for DSM-5 [59] is used to assess diagnosis of AUD. Alcohol use is assessed using the Timeline Follow-back measure (TLFB; [65]), a calendar-based self report measure that yields consistently high test-retest reliability and convergent validity with other self-reports and collateral reports [9]. Craving is measured using the Obsessive Compulsive Drinking Scale (OCDS; [2]), a 14-item self-report measure of craving with high internal consistency and concurrent validity and good predictive validity of future drinking [2]. The standard 12-month version of the Alcohol Use Disorder Identification Test (AUDIT; [56]) is given at baseline and used as a measure of AUD severity. The AUDIT is repeated at the 12-month follow-up visit. Ethyl glucuronide (EtG), a serum biomarker which correlates with the level of recent alcohol consumption, is collected at baseline, week 6, week 12, and all follow-up time points (i.e., 3, 6, and 12 months).
PTSD diagnosis and severity are assessed using the CAPS-5 [72], and the PCL-5 is used to measure self-report PTSD symptomatology [73]. The CAPS-5 is a 30-item structured diagnostic interview and is considered the gold standard for assessing PTSD. The CAPS-5 also captures the participant’s trauma exposure and global perception of distress and functional impairment. Symptom clusters are scored on a 5-point severity scale with a total severity score ranging from 0 to 80. The CAPS-5 is administered at baseline, mid-treatment, end-of-treatment, and follow-up visits. The PCL-5 is a 20-item self-report measure that assesses PTSD severity and has excellent psychometric characteristics [36]. The PCL-5 is administered at baseline, weekly during the treatment phase, and at follow-up visits.
2.7. Neuroimaging procedures
Given the severe negative outcomes associated with comorbid AUD/PTSD and lack of effective treatments, investigation of the neural circuitry and neurochemistry underlying AUD/PTSD comorbidity could be important in guiding future studies. The current study employs fMRI and 1H-MRS at two time points: immediately prior to medication initiation and during week 8 of the treatment phase. Week 8 was selected as the second scan time point because a) we hypothesize medication effects will occur by this time based on our previous research [5], and b) scanning at week 8 may increase sample size and power as compared to waiting to week 12. Fig. 2 illustrates the components of the neuroimaging visits. Each neuroimaging visit is 60–90 min in duration. Prior to the first neuroimaging scan, personalized imagery scripts pertaining to the participant’s index trauma, alcohol cues, and a neutral event are developed according to standard procedures outlined by Sinha and colleagues [61–63]. During the neuroimaging visits, participants are placed in the scanner wearing headphones to listen to the audio-recorded imagery script cues. To minimize potential carry-over effects, the scripts are counterbalanced so that half of the subjects in the placebo group and half of the subjects in the NAC group are exposed to the alcohol cue first and the remaining participants in each group are exposed to the trauma cue first. As illustrated in Fig. 2, the neutral cue always precedes the alcohol and trauma cues.
Fig. 2. Overview of neuroimaging procedures.
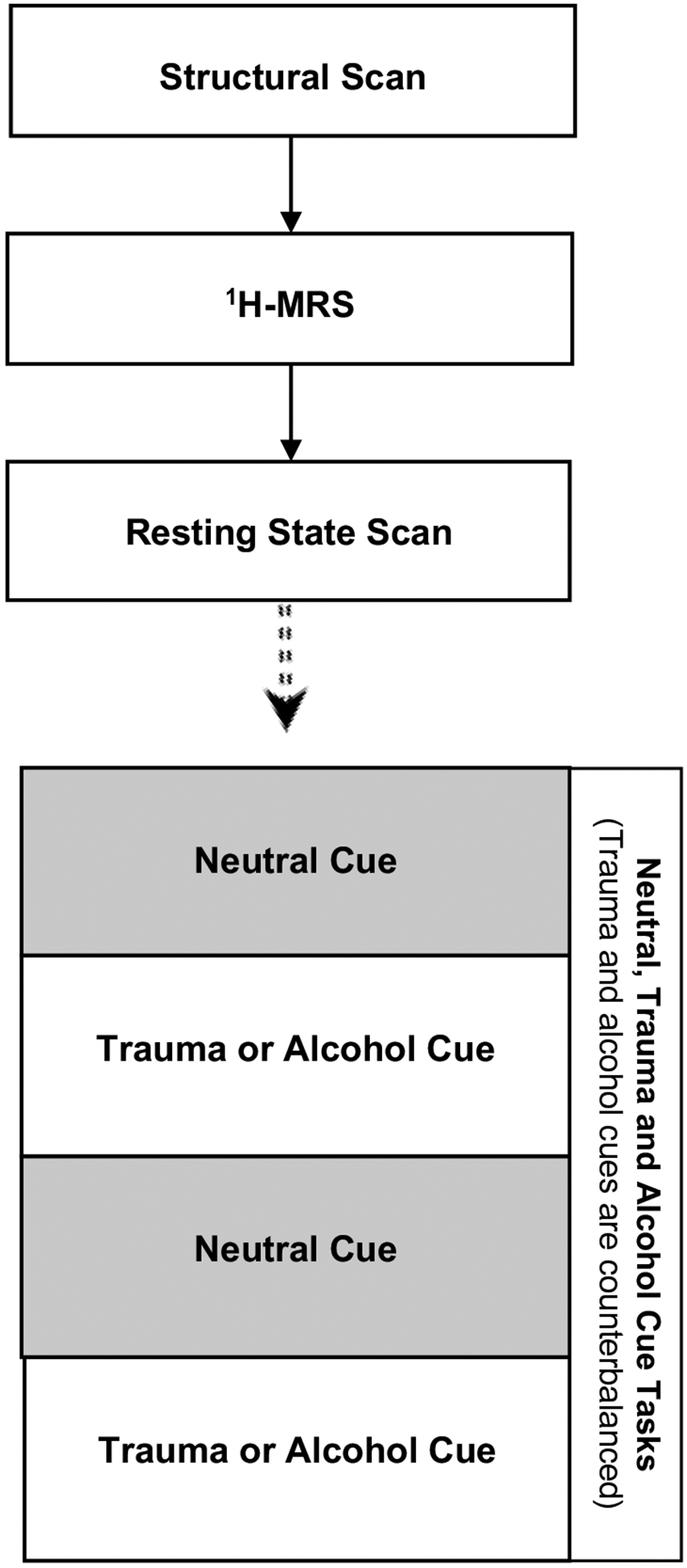
Note. Structural scan followed by assessment of dorsal anterior cingulate cortex (dACC) using 1H-MRS, resting state connectivity, and response to alcohol, trauma and neutral cues.
Participants are screened for metal using a handheld metal detector and a self-report instrument about metal or devices in the body which is reviewed by the MRI technician with each participant. During initial scanner tuning, localizing, and structural scanning, participants are shown “relaxing” images (i.e., 20 scenic pictures, each displayed for 30 s). For co-registration and normalization of functional images, as well as 1H-MRS voxel-placement and tissue-segmentation, a high resolution T1-weighted MPRAGE anatomical image is acquired with the following parameters: TR = 2300 ms, TE = 2.26 ms, flip angle = 8°, field of view = 256 mm, slice thickness 1.0 mm. The scanning planes are oriented parallel to the anterior commissure–posterior commissure line. The ACC voxel for 1H-MRS is placed on midsagittal T1-weighted images, anterior to the genu of the corpus callosum, with the ventral edge of the voxel aligned with the dorsal edge of the genu and a voxel size of 3 × 2.5 × 2.5 cm3 selected. Following auto-shimming, single-voxel water-suppressed 1H-MRS spectra are acquired using a Point Resolved Spectroscopy (PRESS) sequence: Repetition Time (TR) = 2000 ms; Echo Time (TE) = 40 ms; number of averages = 128); an unsuppressed water spectrum will be co-acquired (TE = 40 ms, number of averages = 16), scaled for partial volume effects and relaxation, and used as a concentration reference. For the resting state scans, participants are asked to relax and keep their eyes opened and fixed on a cross-hair while two 6-min resting state scans are collected (320 brain volumes per scan). For both resting state and task runs, T2*-weighted gradient-echo planar images (EPI) are acquired with the following parameters: TR = 1100 ms, TE = 30 ms, flip angle = 65°, acceleration factor = 3, matrix 64 × 64, field of view = 19.2 cm, slice thickness = 3.0 mm with no gap, with 51 slices, T > C −15 orientation, to cover the entire brain. Field maps are collected for each scanning session. All scans are conducted on a Siemens 3 T Prisma MRI scanner (Siemens Medical, Erlangen, Germany).
2.8. Data analytic plan
2.8.1. General considerations and power for the RCT
Statistical analyses will be conducted on the intent-to-treat (ITT) sample consisting of all randomized participants. Contrasts between treatment groups will assess differences on demographic and clinical baseline characteristics. This study is powered to estimate the efficacy of NAC on reduction in alcohol use, alcohol craving, and PTSD severity at the end of the treatment phase (Hypotheses 1 and 2). Specifically, the primary outcomes are a) number of standard drink units as measured by the TLFB, b) craving as measured by the OCDS, and c) PTSD symptoms as measured by the CAPS-5 and PCL-5. A pilot trial of NAC among veterans with substance use disorders and PTSD found that reductions in craving were significantly greater in the NAC vs. placebo treated group (change = 3.0 vs. change = 1.3) [5]. A randomized sample size of 100 participants per treatment arm provides > 95% power with a type 1 error rate of 5% to detect this effect. Further, with this sample size, a 25% decrease in the consumption of standard drinks can be detected with at least 80% power between the NAC and placebo treated arms (risk ratio = 0.75). In the pilot study, a clinically significant decrease in CAPS-5 total scores following 8 weeks of treatment (change = 26.8) while the placebo response was attenuated (change = 17.1) [5]. Assuming a similar difference in treatment effects in a larger population, the study is powered at 0.80 with a type 1 error rate of 5% to detect this difference with 75 participants randomized to each of the two treatment arms (total N = 150). In the same pilot trial, 77% of subjects completed the 8-week trial; similar retention is anticipated for the current study. Therefore, a randomized sample size of 100 participants per treatment arm (total N = 200) would maintain power to detect the clinically significant difference stated above in the presence of 25–30% attrition during the study.
To test the hypothesis that NAC will result in significant reductions in alcohol use (standard drink units) and craving compared to placebo, a mixed effects modeling framework is specified. A Poisson distribution is assumed with a logarithm link function to assess the effects of treatment group (NAC or placebo). Baseline alcohol use and craving are included in pertinent models as covariates. In addition to the primary outcomes, the multimodal assessment of alcohol use will allow us to examine secondary outcomes of drinking behavior (e.g., heavy drinking days) and the proportion of participants who reduce alcohol use based on the World Health Organization (WHO) risk levels (measured as grams of pure ethanol per day) [12,74].
Generalized linear mixed effects regression models with a Gaussian distribution and adjusted for baseline CAPS-5 scores (or PCL-5 scores) will be used to test the hypothesis that NAC versus placebo will result in significantly greater reduction in PTSD symptoms as measured by the CAPS-5 and PCL-5. Assumptions of residual normality and homoscedasticity are checked using statistical tests and graphical methods (i.e., residual and Q-Q plots, and Shapiro-Wilk tests) and transformation is done as necessary. Restricted maximum likelihood (REML) methods will estimate the fixed effects and variance components.
2.8.2. General considerations and power for the neuroimaging component
Power calculations for the neuroimaging component are based on previous studies of individuals with PTSD or substance use disorders. One study of 29 individuals (PTSD = 14, controls = 15) observed significant differences in PFC-amygdala (AMY) resting state connectivity [68]. The effect size for this difference was 0.80 (Cohen’s d). Thus, power reaches 0.80 with a total sample size of N = 26 (two-tailed, alpha = 0.05). Another study of 45 cocaine-dependent individuals found that individuals who relapsed (n = 24) had significantly lower PFC-AMY connectivity at baseline as compared to individuals who did not relapse (n = 21) [37]. The effect size for this reduction was 1.0 (Cohen’s d). Power reaches 0.80 with N = 34 subjects (two-tailed, alpha = 0.05). One study examining the effects of NAC on brain glutamate found that one dose of NAC vs. placebo administered 1 h prior to scanning, led to a significant modification in ACC glutamate with an effect size of d = 2.6 in individuals with cocaine dependence [57]. Although the present study will be examining the effects of chronic NAC treatment in individuals with AUD and PTSD, an effect of similar magnitude would provide power > 0.99.
Functional connectivity is measured using a psychophysiologic interaction (PPI) seed-based approach [13]. PPI is a method for examining task-specific changes in the correlation between time series in various brain areas [46]. Following acquisition of the imaging data, preprocessing corrects for geometric distortion and head motion, with spatial smoothing (FWHM = 6 mm). A mask of the two seed regions is made using a 12-mm diameter sphere located in the center of the left and right AMY using the Montreal Neurological Institute coordinates (x, y, z = ± 22, 0, −22). The mean corrected and high pass filtered time series of the blood‑oxygen-level dependent (BOLD) signal in each AMY sphere is extracted for each participant and used in a single subject whole brain PPI analysis. Statistical analysis is performed at the individual-subject level using FEAT (FMRI Expert Analysis Tool), with six head motion parameters and motion outliers as covariates. The interactions of interest include: alcohol (vs. neutral) x (left or right) AMY time series, and trauma (vs. neutral) x (left or right) AMY. For resting state fMRI analysis, a seed-based approach is used with the same AMY seed regions applied in the PPI analysis and head motion parameters and outliers as covariates.
To test hypotheses 3A and 3B that connectivity between the PFC and the AMY at rest and in response to cues at baseline will predict change in alcohol use and PTSD symptoms due to treatment, separate linear regression tests are used. Changes in total standard drinks and the CAPS-5 total score are regressed against the parameter estimate obtained from the voxel with the maximum Z-score from each PFC cluster exhibiting a significant association with the AMY time series at rest, and a significant task × seed interaction with the AMY in response to the alcohol cue (Hypothesis 3A) and trauma cue (Hypothesis 3B). Further exploratory analyses examine change in PFC-AMY connectivity from pre- to post-treatment and associations with alcohol and PTSD outcomes, both within and between medication groups. In addition, group-level exploratory fMRI analyses use a mixed effects approach (FLAME1 in FSL), with separate group analyses performed for left and right AMY.
1H-MRS spectra data are analyzed using LC Model 6.3 [52], an operator-independent curve-fitting software package that uses least-squares estimation for quantifying metabolite concentrations; the basis set for TE = 40 ms is provided by the vendor and includes a number of metabolites including glutamate, creatine, glutamine, N-acetylaspartate, and phosphocholine. Only metabolites with fitting uncertainties (Cramer-Rao Lower Bound values) < 20% of SD in the LC Model output are retained for analysis. LC Model includes standardized zero filling, Fourier transformation, and automated phase, baseline and eddy current correction. To address variability in within-voxel tissue composition we extract and segment T1-weighted images into partial volume maps of gray matter (GM), white matter (WM), and CSF using FSL tools, match the coordinates and size of the 1H-MRS voxel with the segmented images and extract the tissue fractions within the voxel, correct the raw values obtained from the LC model (scaled to water) for CSF and coil loading, and calculate each participant’s GM to brain matter (GM/[GM + WM]) ratio for use as a covariate in the analyses. Change in glutamate levels is analyzed as a mixed model with visit as a within participant variable and medication group as between participant variables. Variables known to influence ACC glutamate levels (e.g., age, smoking status, within-voxel tissue composition, days since last drink) are considered as covariates. Further exploratory regression analyses examine associations between glutamate difference scores and clinical outcomes (e.g., change in total standard drinks and CAPS-5 total score) both within and between medication groups.
3. Discussion
AUD and PTSD are highly prevalent psychiatric conditions that frequently co-occur and are associated with an array of related mental, physical, and social problems. Despite the frequent co-occurrence and deleterious consequences associated with AUD/PTSD, there are substantial gaps in the evidence base regarding the treatment of AUD/PTSD and no medications have been proven effective for this comorbidity. This paper describes the study design and methodology of an ongoing RCT to test the efficacy of N-acetylcysteine (NAC), an antioxidant with glutamatergic modulationproperties, in reducing alcohol use and PTSD symptomatology.
A confluence of findings suggest that glutamate dysfunction plays an important role in the underlying neuropathology of both AUD and PTSD. Glutamate is the main excitatory neurotransmitter in the brain and it is highly involved in learning and memory [45]. AUD and other substance use disorders have been characterized by reduced accumbens glial glutamate transport (GLT-1) ([54,76]. In animal models, pharmacological restoration of GLT-1 has been shown to inhibit relapse ([30,42,54,54,77]). An acute stressor was shown to produce an enduring reduction in GLT-1, and these animals acquired cocaine self-administration more readily than sham stress rats [15]. Importantly, pharmacologic restoration of GLT-1 prevented the stress-induced facilitation of substance use, suggesting GLT-1 as a potential pharmacotherapeutic target in treating comorbid AUD and PTSD. Furthermore, glutamatergic neurotransmission in the limbic region mediates the acquisition and extinction of fear conditioning and plays a pivotal role in the pathophysiology of PTSD [44]. Glutamate also modulates the release of other neurotransmitters involved in AUD and PTSD, such as serotonin, dopamine, and GABA ([25,45].
In order to address these gaps in the literature, the current study examines the efficacy of NAC (2400 mg/day) among individuals with current AUD and PTSD. NAC is a cysteine pro-drug that stimulates the cystine-glutamate exchanger and normalizes glial glutamate transporters, which are reduced in both substance use disorders and PTSD, and restores glutamatergic tone on presynaptic receptors in the nucleus accumbens [41,70]. Some preclinical and clinical studies indicate NAC reduces substance use and craving, purportedly via GLT-1 dependent mechanisms (([1,11,14,18,20,31,38,55,58]. Gray and colleagues conducted an 8-week, double-blind, placebo-controlled trial of NAC in individuals with cannabis use disorders (N = 116) and found significantly higher rates of negative urine cannabinoid tests among participants in the NAC as compared to the placebo group [22]. LaRowe and colleagues conducted a double-blind, placebo-controlled trial among individuals with cocaine use disorder (N = 111) and found that, among patients entering the trial abstinent, NAC was associated with significantly longer time to relapse and lower craving as compared to placebo [32]. In a small human laboratory cue reactivity study of in-patients with cocaine use disorder (N = 15), NAC resulted in reduced desire to use cocaine and reduced cue viewing time, as compared to placebo [33]. Despite these positive findings, some studies of NAC among patients with substance use disorders or other mental health conditions (e.g., depression) have found negative or mixed results ([7,8,23,70,75].
Specific to comorbid AUD and PTSD, our group recently completed the first investigation of NAC among veterans with co-occurring substance use disorders and PTSD [5]. This 8-week, double-blind, RCT pilot trial evaluated NAC (2400 mg/day) vs. placebo among 35 veterans. The most common substance use disorder was AUD (85.1%). NAC treatment was associated with significantly reduced self-report and clinician- rated PTSD symptoms as compared to placebo. The CAPS total score was reduced by 46% in the NAC group vs. 25% in the placebo group (d = 1.27, p < .001). NAC reduced PCL scores 32% in the NAC group vs. 3% in the placebo group (d = 1.30, p < .001). Moreover, depression was significantly reduced in the NAC group but not the placebo group. Among patients treated with NAC, craving was reduced 81% vs. only 32% in the placebo group (p < .05). A trend was observed for the NAC group to evidence fewer positive UDS tests during treatment (p = .07). Finally, retention in the trial was excellent and did not differ by group: 77.1% completed all 8 weeks. Medication compliance (measured via riboflavin) was 82.9% and did not differ by group. NAC was well tolerated and side effects were mild. Taken together, the results from preclinical and clinical studies suggest that NAC is a promising candidate pharmacotherapy for the treatment of comorbid AUD/PTSD. Unlike other potential candidate medications for comorbid AUD/PTSD, NAC is inexpensive, available over-the-counter, does not require a titration upward to the target dose nor titration downward at the end of treatment, and has a long-established safety record in adults and children [34], all of which confer ease of transferability from research to clinical practice if the results of this study support efficacy. Thus, the current study was designed to build on our previous work by testing NAC in a larger sample with sufficient statistical power.
The current study utilizes a double-blind, placebo-controlled randomized design, measures functioning in related areas, such as depression, and incorporates multi-modal neuroimaging. In comparison with the majority of clinical pharmacotherapy trials, the current study has the unique advantage of simultaneously assessing clinical, biological, neurochemical and neural connectivity indices of both AUD and PTSD symptoms. The design pairs rigorous traditional RCT methodology with an array of human laboratory and novel neuroimaging procedures to elucidate behavioral and neurobiological mechanisms underlying comorbid AUD/PTSD. These neurobiological data will not only provide new knowledge on mechanisms of change for alcohol use and PTSD symptoms, but also related features such as depression symptomatology. Furthermore, one potential benefit of using imaging measures is that not all patients may respond to NAC or they may respond in different ways, so understanding the neurobiological pathways can inform the development of more targeted, precision medicine approaches. As such, regardless of the overall efficacy of NAC, the study is poised to advance scientific understanding in the field and inform effective clinical management of individuals with comorbid AUD and PTSD.
Funding
This research was supported by the National Institute on Alcohol Abuse and Alcoholism (R01AA025086 and R01AA025086-03S1, K23 AA023845, T32AA007474) and the National Institute on Drug Abuse (K02 DA039229, R25 DA020537, T32 DA007288) and the Veterans Administration BX004727.
Role of the funding source
The funding sources had no involvement in the study design, the collection, analysis and interpretation of data, the writing of this manuscript, or the decision to submit this manuscript for publication.
Abbreviations:
- 1H-MRS
proton magnetic resonance spectroscopy
- ACC
anterior cingulate cortex
- AMY
amygdala
- AUD
alcohol use disorder
- BOLD
blood‑oxygen-level dependent
- CAPS-5
Clinician-Administered PTSD Scale for DSM-5
- CIWA-Ar
Revised Clinician Institute Withdrawal Assessment of Alcohol
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
- EPI
gradient-echo planar images
- FDA
U.S. Food and Drug Administration
- FEAT
FMRI Expert Analysis Tool
- fMRI
functional magnetic resonance imaging
- FMRIB
functional magnetic resonance imaging of the brain
- FSL
FMRIB Software Library
- GM
gray matter
- HIV
human immunodeficiency virus
- IRB
Institutional Review Board
- MINI
Mini International Neuropsychiatric Interview
- MPRAGE
magnetization-prepared rapid gradient-echo
- MRI
magnetic resonance imaging
- MUSC
Medical University of South Carolina
- OCDS
Obsessive Compulsive Drinking Scale
- PCL-5
PTSD Checklist for DSM-5
- PFC
prefrontal cortex
- PPI
psychophysiological interaction
- PTSD
posttraumatic stress disorder
- RCT
randomized controlled trial
- TLFB
Timeline Follow-back
- USP-grade
meets or exceeds requirements of the United States Pharmacopeia
- U.S.
United States
- VA
U.S. Department of Veterans Affairs
- VAS
Visual Analogue Scale
- WHO
World Health Organization
- WM
white matter
Footnotes
Publisher's Disclaimer: Disclaimer
The views expressed herein are solely those of the authors and do not reflect an endorsement by or the official policy or position of the Ralph H. Johnson VA, the Department of Veterans Affairs, or the U.S. Government.
References
- [1].Amen SL, Piacentine LB, Ahmad ME, Li S, Mantsch JR, Risinger RC, Baker DA, Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans, Neuropsychopharmacology 36 (4) (2011) 871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anton RF, Moak DH, Latham P, The obsessive compulsive drinking scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior, Alcohol. Clin. Exp. Res 19 (1) (1995) 92–99, 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- [3].Averill LA, Purohit P, Averill CL, Boesl MA, Krystal JH, Abdallah CG, Glutamate dysregulation and glutamatergic therapeutics for PTSD: evidence from human studies, Neurosci. Lett 649 (2017) 147–155, 10.1016/j.neulet.2016.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Back SE, Dansky BS, Coffey SF, Saladin ME, Sonne S, Brady KT, Cocaine dependence with and without posttraumatic stress disorder: a comparison of substance use, trauma history and psychiatric comorbidity, Am. J. Addict 9 (1) (2000) 51–62. [DOI] [PubMed] [Google Scholar]
- [5].Back SE, McCauley JL, Korte KJ, Gros DF, Leavitt V, Gray KM, … Kalivas PW, A double-blind, randomized, controlled pilot trial of n-acetylcysteine in veterans with posttraumatic stress disorder and substance use disorders, Journal of Clinical Psychiatry 77 (11) (2016) e1439–e1446, 10.4088/JCP.15m10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Beck AT, Steer RA, Brown GK, Beck Depression Inventory-II (BDI-II), Psychological Corporation, San Antonio, TX, 1996. [Google Scholar]
- [7].Berk M, Dean OM, Cotton SM, Jeavons S, Tanious M, Kohlmann K, Hewitt K, Moss K, Allwang C, Schapkaitz I, Robbins J, Cobb H, Ng F, Dodd S, Bush A, Malhi GS, The efficacy of adjunctive N-acetylcysteine in major depressive disorder: a double-blind, randomized, placebo-controlled trial, J. Clin. Psychiatry 75 (6) (2014) 628–636. [DOI] [PubMed] [Google Scholar]
- [8].Berk M, Turner A, Malhi GS, Ng CH, Cotton SM, Dodd S, Samuni Y, Tanious M, McAulay C, Dowling N, Sarris J, Owen L, Waterdrinker A, Smith D, Dean OM, A randomised controlled trial of a mitochondrial therapeutic target for bipolar depression: mitochondrial agents, N-acetylcysteine, and placebo, BMC Med. 17 (1) (2019) 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Carey KB, Reliability and validity of the time-line follow-back interview among psychiatric outpatients: a preliminary report, Psychol. Addict. Behav 11 (1) (1997) 26–33. [Google Scholar]
- [10].Carroll KM, Kosten TR, Rounsaville BJ, Choosing a behavioral therapy platform for pharmacotherapy of substance users, Drug Alcohol Depend. 75 (2) (2004) 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dean O, Giorlando F, Berk M, N-acetylcysteine in psychiatry: current therapeutic evidence and potential mechanisms of action, J. Psychiatry Neurosci 36 (2) (2011) 78–86, 10.1503/jpn.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Falk DE, O’Malley SS, Witkiewitz K, Anton R, Litten RZ, Slater M, Kranzler HR, Mann KF, Hason DS, Johnson B, Meulien D, Ryan M, Fertig J, For the Alcohol Clinical Trials Initiative (ACTIVE) Workgroup, Evaluation of drinking risk levels as outcomes in alcohol pharmacotherapy trials: a secondary analysis of 3 randomized clinical trials, JAMA Psychiatry 76 (4) (2019) 374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ, Psychophysiological and modulatory interactions in neuroimaging, NeuroImage 6 (3) (1997) 218–229, 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- [14].Froeliger B, McConnell PA, Stankeviciute N, McClure EA, Kalivas PW, Gray KM, The effects of N-Acetylcysteine on frontostriatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: a double-blind, placebo-controlled fMRI pilot study, Drug Alcohol Depend. 156 (2015) 234–242, 10.1016/j.drugalcdep.2015.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Garcia-Keller C, Kupchik YM, Gipson C, Brown RM, Spencer S, Bollati F, Esparza MA, Roberts-Wolfe D, Heinsbroek J, Bobadilla A, Cancela LM, Kalivas PW, Glutamatergic mechanisms of comorbidity between stress disorders and cocaine addiction, Mol. Psychiatry 21 (8) (2016) 1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Garcia-Keller C, Martinez SA, Esparza MA, Bollati F, Kalivas PW, Cancela LM, Cross-sensitization between cocaine and acute restraint stress is associated with sensitized dopamine but not glutamate release in the nucleus accumbens, Eur. J. Neurosci 37 (2013) 982–995, 10.1111/ejn.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gass JT, Olive MF, Glutamatergic substrates of drug addiction and alcoholism, Biochem. Pharmacol 75 (2008) 218–265, 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gass JT, Sinclair CM, Cleva RM, Widholm JJ, Olive MF, Alcohol-seeking behavior is associated with increased glutamate transmission in basolateral amygdala and nucleus accumbens as measured by glutamate-oxidase-coated biosensors, Addict. Biol 16 (2) (2011) 215–228, 10.1111/j.1369-1600.2010.00262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gielen N, Havermans RC, Tekelenburg M, Jansen A, Prevalence of post-traumatic stress disorder among patients with substance use disorder: it is higher than clinicians think it is, Eur. J. Psychotraumatol 3 (2012), 10.3402/ejpt.v3i0.17734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gipson CD, Reissner KJ, Kupchik YM, Smith AW, Stankeviciute N, Hensley-Simon ME, Kalivas PW, Reinstatement of nicotine seeking is mediated by glutamatergic plasticity, Proc. Natl. Acad. Sci 110 (22) (2013) 9124–9129, 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS, Epidemiology of DSM-5 alcohol use disorder: results from the National epidemiologic survey on alcohol and related conditions III, JAMA Psychiatry 72 (8) (2015) 757–766, 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, McRae-Clark AL, Brady KT, A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents, Am. J. Psychiatr 169 (8) (2012) 805–812, 10.1176/appi.ajp.2012.12010055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Gray KM, Sonne SC, McClure EA, Ghitza UE, Matthews AG, McRae-Clark AL, Carroll KM, Potter JS, Wiest K, Mooney LJ, Hasson A, Walsh SL, Lofwall MR, Babalonis S, Lindblad RW, Sparenborg S, Wahle A, King JS, Baker NL, Tomko RL, Haynes LF, Vandrey RG, Levin FR, A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults, Drug Alcohol Depend. 177 (2017) 249–257, 10.1016/j.drugalcdep.2017.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gray KM, Watson NL, Carpenter MJ, LaRowe SD, N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study, Am. J. Addict 19 (2010) 187–189, 10.1111/j.1521-0391.2009.00027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jedema HP, Moghddam B, Characterization of excitatory amino acid modulation of dopamine release in the prefrontal cortex of conscious rats, J. Neurochem 66 (1996) 1448–1453. [DOI] [PubMed] [Google Scholar]
- [26].Kadden RP, Carroll K, Donovan D, Cooney N, Monti P, Abrams D, Hester R, Cognitive-Behavioral Coping Skills Therapy Manual: A Clinical Research Guide for Therapists Treating Individuals with Alcohol Abuse and Dependence (DHHS Pub. No. ADM 92–1995), US Department of Health and Human Services/National Institute on Alcohol Abuse and Alcoholism, Rockville, MD, 1995. [Google Scholar]
- [27].Kalivas BC, Kalivas PW, Corticostriatal circuitry in regulating diseases characterized by intrusive thinking, Dialogues Clin. Neurosci 18 (2016) 65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kessler RC, Chiu WT, Demler O, Walters EE, Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication, Arch. Gen. Psychiatry 62 (6) (2005) 617–627, 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB, Posttraumatic stress disorder in the National Comorbidity Survey, Arch. Gen. Psychiatry 52 (1995) 1048–1060, 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- [30].Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW, The role of cystine-glutamate exchange in nicotine dependence in rats and humans, Biol. Psychiatry 65 (10) (2009) 841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW, Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking, J. Neurosci 30 (23) (2010) 7984–7992, 10.1523/jneurosci.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].LaRowe SD, Kalivas PW, Nicholas JS, Randall PK, Mardikian PN, Malcolm RJ, A double-blind placebo-controlled trial of N-acetylcysteine in the treatment of cocaine dependence, Am. J. Addict 22 (5) (2013) 443–452, 10.1111/j.1521-0391.2013.12034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, Brady K, Kalivas P, Malcolm R, Is cocaine desire reduced by n-acetylcysteine? Am. J. Psychiatr 164 (2007) 1115–1117. [DOI] [PubMed] [Google Scholar]
- [34].Marzullo L, An update of N-acetylcysteine treatment for acute acetaminophen toxicity in children, Curr. Opin. Pediatr 17 (2005) 239–245. [DOI] [PubMed] [Google Scholar]
- [35].McClure EA, Baker NL, Gipson CD, Carpenter MJ, Roper AP, Froeliger BE, Gray KM, An open-label pilot trial of N-acetylcysteine and varenicline in adult cigarette smokers, Am. J. Drug Alcohol Abuse 41 (1) (2015) 52–56, 10.3109/00952990.2014.933839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].McDonald SD, Calhoun PS, The diagnostic accuracy of the PTSD checklist: a critical review, Clin. Psychol. Rev 30 (8) (2010) 976–987. [DOI] [PubMed] [Google Scholar]
- [37].McHugh MJ, Demers CH, Salmeron BJ, Devous MD Sr., Stein EA, Adinoff B, Cortico-amygdala coupling as a marker of early relapse risk in cocaine-addicted individuals, Front. Psychiatry 5 (2014) 16, 10.3389/fpsyt.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].McKetin R, Dean OM, Baker AL, Carter G, Turner A, Kelly PJ, Berk M, A potential role for N-acetylcysteine in the management of methamphetamine dependence, Drug Alcohol Rev. 36 (2) (2017) 153–159. [DOI] [PubMed] [Google Scholar]
- [39].Meshberg-Cohen S, Black AC, DeViva JC, Petrakis IL, Rosen MI, Trauma treatment for veterans in buprenorphine maintenance treatment for opioid use disorder, Addict. Behav 89 (2019) 29–34, 10.1016/j.addbeh.2018.09.010. [DOI] [PubMed] [Google Scholar]
- [40].Mills KL, Teesson M, Ross J, Peters L, Trauma, PTSD, and substance use disorders: findings from the Australian National Survey of mental health and well-being, Am. J. Psychiatr 163 (4) (2006) 652–658, 10.1176/appi.ajp.163.4.652. [DOI] [PubMed] [Google Scholar]
- [41].Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK, Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking, J. Neurosci 25 (27) (2005) 6389–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, et al. , Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse, Proce. Nat. Acad. Sci. USA 108 (2011) 385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Mulholland PJ, Chandler LJ, Kalivas PW, Signals from the fourth dimension regulate drug relapse, Trends Neurosci. 39 (2016) 472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nair J, Ajit SS, The role of the glutamatergic system in posttraumatic stress disorder, CNS Spectrums 13 (7) (2008) 585–591. [DOI] [PubMed] [Google Scholar]
- [45].Niciu MJ, Kelmendi B, Sanacora G, Overview of glutamatergic neurotransmission in the nervous system, Pharmacol. Biochem. Behav 100 (4) (2012) 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H, Tools of the trade: psychophysiological interactions and functional connectivity, Soc. Cogn. Affect. Neurosci 7 (2012) 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Oscar-Berman M, Marinkovic K, Alcohol: effects on neurobehavioral functions and the brain, Neuropsychol. Rev 17 (3) (2007) 239–257, 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Pennington DL, Abé C, Batki SL, Meyerhoff DJ, A preliminary examination of cortical neurotransmitter levels associated with heavy drinking in posttraumatic stress disorder, Psychiatry Res. Neuroimaging 224 (3) (2014) 281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Petrakis IL, Rosenheck R, Desai R, Substance use comorbidity among veterans with posttraumatic stress disorder and other psychiatric illness, Am. J. Addict 20 (3) (2011) 185–189, 10.1111/j.1521-0391.2011.00126.x. [DOI] [PubMed] [Google Scholar]
- [50].Petrakis IL, Simpson TL, Posttraumatic stress disorder and alcohol use disorder: a critical review of pharmacologic treatments, Alcohol. Clin. Exp. Res 41 (2) (2017) 226–237, 10.1111/acer.13297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Posner K, Brent D, Lucas C, Gould M, Stanley B, Brown G, Fisher P, Zelazny J, Burke A, Oquendo M, Mann J, Columbia-Suicide Severity Rating Scale (C-SSRS), Columbia University Medical Center, New York, NY, 2008. [Google Scholar]
- [52].Provencher SW, Estimation of metabolite concentrations from localized in vivo proton NMR spectra, Magn. Reson. Med 30 (6) (1993) 672–679. [DOI] [PubMed] [Google Scholar]
- [53].Ramirez-Niño AM, D’Souza MS, Markou A, N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking, Psychopharmacology 225 (2) (2013) 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW, Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement, Addict. Biol 20 (2) (2015) 316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Roberts-Wolfe DJ, Kalivas PW, Glutamate transporter GLT-1 as a therapeutic target for substance use disorders, CNS Neurol. Disord. Drug Target 14 (2015) 745–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M, Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II, Addiction 88 (1993) 791–804. [DOI] [PubMed] [Google Scholar]
- [57].Schmaal L, Veltman DJ, Nederveen A, van den Brink W, Goudriaan AE, N-acetylcysteine normalized glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study, Neuropsychopharmacology 37 (9) (2012) 2143–2152, 10.1038/npp.2012.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Schneider RJ, Santos CF, Clarimundo V, Dalmaz C, Elisabetsky E, Gomez R, N-acetylcysteine prevents behavioral and biochemical changes induced by alcohol cessation in rats, Alcohol 49 (3) (2015) 259–263, 10.1016/j.alcohol.2015.01.009. [DOI] [PubMed] [Google Scholar]
- [59].Sheehan D, The MINI International Neuropsychiatric Interview, (Version 7.0. 2) for DSM-5, (2016). [Google Scholar]
- [60].Simpson TL, Rise P, Browne KC, Lehavot K, Kaysen D, Clinical presentations, social functioning, and treatment receipt among individuals with comorbid lifetime PTSD and alcohol use disorders versus drug use disorders: findings from NESARC-III, Addiction 114 (6) (2019) 983–993, 10.1111/add.14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sinha R, Li CR, Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications, Drug Alcohol Rev. 26 (1) (2007) 25–31, 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- [62].Sinha R, Talih M, Malison R, Anderson GA, Cooney N, Kreek M, Hypothalamic-pituitary-adrenal axis and sympathoadreno-medullary responses during stress-induced and drug cue-induced cocaine craving states, Psychopharmacology 170 (2003) 62–72. [DOI] [PubMed] [Google Scholar]
- [63].Sinha R, Tuit K, Imagery Script Development Procedures Manual, Yale University School of Medicine, 2 Church Street South Suite 209, New Haven, CT: 06519, 2012 [Google Scholar]
- [64].Smilkstein MJ, Knapp GL, Kulig KW, Rumack BH, Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985), N. Engl. J. Med 319 (1988) 1557–1562. [DOI] [PubMed] [Google Scholar]
- [65].Sobell LC, Sobell MB, Timeline Follow-Back Measuring Alcohol Consumption, Springer, 1992. [Google Scholar]
- [66].Spencer S, Kalivas PW, Glutamate transport: a new bench to bedside mechanism for treating drug abuse, Int. J. Neuropsychopharmacol 20 (10) (2017) 797–812, 10.1093/ijnp/pyx050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Squeglia LM, Baker NL, McClure EA, Tomko RL, Adisetiyo V, Gray KM, Alcohol use during a trial of N-acetylcysteine for adolescent marijuana cessation, Addict. Behav 63 (2016) 172–177, 10.1016/j.addbeh.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I, Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder, J. Psychiatry Neurosci 37 (4) (2012) 241–249, 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM, Assessment alcohol withdrawal: the revised clinical institute withdrawal assessment for scale (CIWA-Ar), Br. J. Addict 84 (11) (1989) 1353–1357, 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- [70].Tomko RL, Jones JL, Gilmore AK, Brady KT, Back SE, Gray KM, N-acetylcysteine: a potential treatment for substance use disorders, Curr. Psychiatr 17 (6) (2018) 30–36, 41–42, 55. [PMC free article] [PubMed] [Google Scholar]
- [71].Vujanovic AA, Back SE, Posttraumatic Stress and Substance Use Disorders: A Comprehensive Clinical Handbook, Routledge, New York, NY, 2019. [Google Scholar]
- [72].Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, Keane TM, Clinician-Administered PTSD Scale for DSM-5 (CAPS-5), Available from, 2013. www.ptsd.va.gov. [DOI] [PMC free article] [PubMed]
- [73].Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, Schnurr PP, The PTSD Checklist for DSM-5 (PCL-5), Available from the National Center for PTSD 2013. www.ptsd.va.gov. [Google Scholar]
- [74].Witkiewitz K, Finney JW, Harris AH, Kivlahan DR, Kranzler HR, Recommendations for the design and analysis of treatment trials for alcohol use disorders, Alcohol. Clin. Exp. Res 39 (9) (2015) 1557–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Zheng W, Zhang QE, Cai DB, Yang XH, Qiu Y, Ungvari GS, Ng CH, Ning MYP, Xiang YT, N-acetylcysteine for major mental disorders: a systematic review and meta-analysis of randomized controlled trials, Acta Psychiatr. Scand 137 (5) (2018) 391–400. [DOI] [PubMed] [Google Scholar]
- [76].Roberts-Wolfe DJ, Kalivas PW, Glutamate transporter GLT-1 as a therapeutic target for substance use disorders, CNS & Neurological Disorders-Drug Targets (6) (2015) 745–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Sari Y, et al. , Glutamate transporter 1: potential target for the treatment ofdependence, Alcoholism: Clinical & Experimental Research 35 (2011). [Google Scholar]


