Abstract
Proper chromosome segregation during cell division is essential in all domains of life. In the majority of bacterial species, faithful chromosome segregation is mediated by the tripartite ParABS system, consisting of an ATPase protein ParA, a CTPase and DNA-binding protein ParB, and a centromere-like parS site. The parS site is most often located near the origin of replication and is segregated first after chromosome replication. ParB nucleates on parS before binding to adjacent non-specific DNA to form a multimeric nucleoprotein complex. ParA interacts with ParB to drive the higher-order ParB–DNA complex, and hence the replicating chromosomes, to each daughter cell. Here, we review the various models for the formation of the ParABS complex and describe its role in segregating the origin-proximal region of the chromosome. Additionally, we discuss outstanding questions and challenges in understanding bacterial chromosome segregation.
Keywords: chromosome organization, chromosome segregation, chromosome maintenance, ParA–ParB–parS, spreading, SMC
1. Introduction
Faithful chromosome segregation is essential to ensure each daughter cell inherits a full copy of the genetic information of the parent. Chromosome segregation is not a trivial process, especially in bacteria, because DNA must be maintained in a compacted state to fit within the limited volume of the cells, and chromosome segregation often occurs concomitantly with DNA replication rather than being separated temporally, as in eukaryotes. Bacterial chromosome segregation can be divided into multiple overlapping steps: (i) segregation of DNA proximal to the origin of replication, (ii) segregation of the bulk of the chromosome, and (iii) segregation of DNA near the terminus of replication. In this review, we focus on progress towards understanding the molecular basis for segregating the origin-proximal region, specifically by the tripartite ParA–ParB–parS system.
The par locus was first discovered in low-copy-number plasmids, and was shown to be essential for their stable inheritance [1–4]. A functionally equivalent par locus was later found to be important for chromosome segregation in Bacillus subtilis [5–7]. In Caulobacter crescentus, Hyphomonas neptunium and Myxococcus xanthus, genes encoded in the par locus (ParABS) were found to be essential for cell viability [8–11], whereas in other bacterial species engineered strains lacking ParABS were viable but had an elevated number of anucleate cells owing to defects in chromosome segregation [12–27]. A comparative genomic study suggested that the chromosomal ParABS system is conserved in two-thirds of bacterial species [28]. In most bacteria, one or multiple parS sites are commonly found near the origin of replication [28]. The parS site is the first DNA locus to be segregated after chromosome replication [7,11,13,29]. ParB is a DNA-binding protein that nucleates on parS to recruit additional ParB molecules to adjacent non-specific DNA to form a network of protein–DNA complexes [30]. The ParB–DNA nucleoprotein complex stimulates the ATPase activity of ParA, creating a gradient of ParA–ATP that drives the movement of the origin-proximal region of the chromosome (and subsequently, the whole chromosome) along this gradient to the opposite pole of the cell [31–39]. ParB also recruits the structural maintenance of chromosome (SMC) complex onto the chromosome to reduce DNA entanglement, thereby promoting the individualization of replicated chromosomes [16,40–46].
Since the discovery of the ParABS system over 35 years ago, tremendous progress has been made towards answering some of the key questions about how this system works:
-
—
How does ParB recruit tens to hundreds more ParB proteins to assemble a higher-order nucleoprotein complex? (Discussed in §2.)
-
—
What is the molecular mechanism of ParA-mediated DNA segregation? (Discussed in §3.)
-
—
How does ParB recruits SMC and other protein partners to coordinate chromosome segregation with chromosome organization? (Discussed in §4.)
-
—
How does evolution shape factors that are involved in bacterial chromosome segregation and maintenance? (Discussed in §5.)
In this review, we summarize recent progress and compare the competing models for addressing these key questions, before highlighting outstanding questions and challenges for fully understanding the ParABS system and chromosome segregation in bacteria.
2. ParB–parS interaction and the assembly of a higher-order nucleoprotein complex
ParB binding to parS nucleates the recruitment of additional ParB molecules which associate with neighbouring DNA, a process known as spreading, to form a higher-order ParB-DNA nucleoprotein complex [30]. The purpose of this higher-order complex, whether to strengthen the physical link between DNA and ParA or to provide a specific DNA topology to facilitate DNA segregation, is still under debate. However, since bacterial strains harbouring nucleation competent but spreading-defective mutants of parB are either unviable or have elevated number of anucleate cells, it is clear that a higher-order nucleoprotein complex is a prerequisite for faithful chromosome segregation [7,47–50]. In this section, we describe and discuss the current and emerging models for the assembly of this essential nucleoprotein complex.
2.1. Domain organization and shared features of chromosomal ParB protein family
Chromosomal ParB proteins share a common domain architecture, consisting of an N-terminal domain (NTD), a central DNA-binding domain (DBD) and a C-terminal domain (CTD) (figure 1a). A highly conserved arginine-rich motif (GERRxRA) resides in the NTD and mediates protein–protein and protein–ligand interactions [30,51,52] (figure 1a). The DBD contains a helix–turn–helix motif that enables ParB to nucleate on parS specifically [30]. The CTD, which is the least conserved domain among ParB homologs, contains a leucine zipper motif that allows ParB to homodimerize [30] (figure 1a). The CTD of Bacillus subtilis ParB also has a lysine-rich amino acid patch that provides additional non-specific DNA-binding and DNA condensation activities [53]. Currently, the structure of a full-length chromosomal ParB is not available. The flexibility of ParB, endowed by amino acid linkers that connect consecutive domains, has hindered the effort to crystallize and solve the structure of a full-length protein. Nevertheless, structure-function insights have been gained from X-ray crystallography/NMR studies using a single-domain or domain-truncated variants of ParB from various bacterial species [52–58]. Structural comparisons suggested that ParB, especially its NTD, can adopt multiple alternative conformations that might facilitate the assembly of a higher-order nucleoprotein complex.
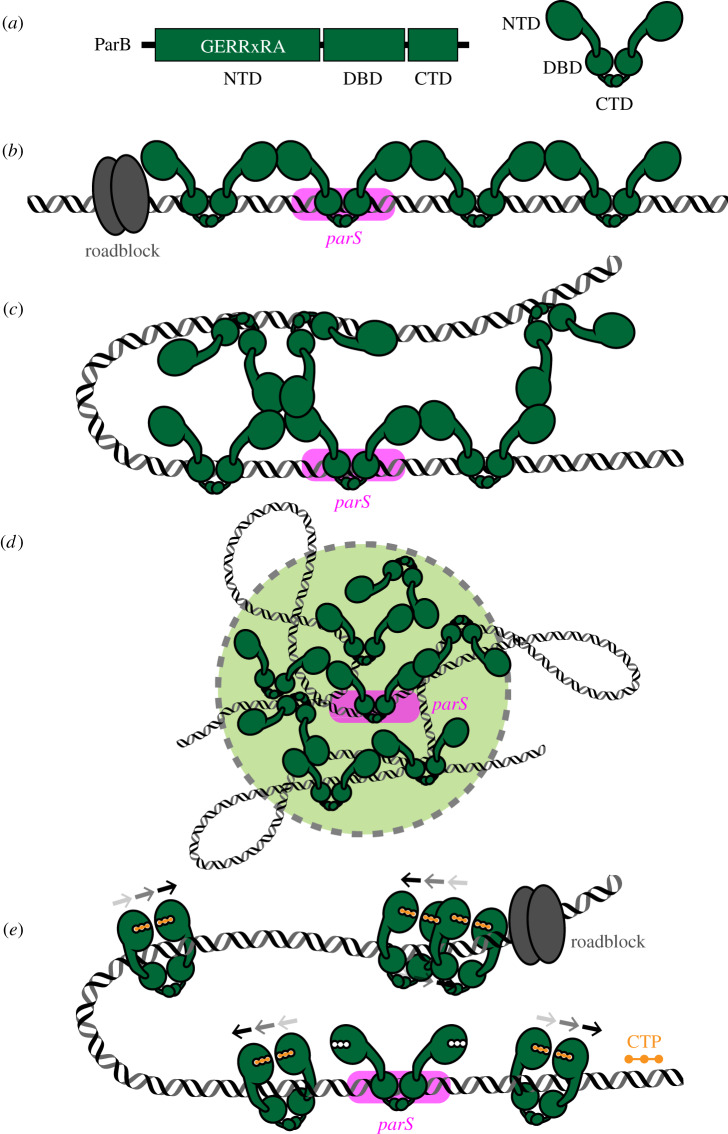
Figure 1.
The assembly of a higher-order ParB–DNA nucleoprotein complex. (a) Chromosomal ParB proteins share a common domain architecture, consisting of an N-terminal domain (NTD), a central DNA-binding domain (DBD) and a C-terminal domain (CTD). The NTD harbours a conserved arginine-rich motif (GERRxRA) that mediates ParB–ParB and ParB–cytidine triphosphate (CTP) interactions. (b) Model 1: ParB spreading by a one-dimensional filamentation. (c) Model 2: ParB spreading by bridging and condensing DNA. (d) Model 3: ParB spreading by caging DNA. (e) Model 4: ParB spreading by sliding on DNA. ParB switches from an open to a closed clamp upon binding to CTP (orange). ParB and parS are coloured green and magenta, respectively. The arrows above the ParB–CTP complexes (e) indicate their progressive sliding on DNA. A tight DNA-binding protein (grey) can unidirectionally block the one-dimensional filamentation or the sliding of ParB on DNA.
Four models have been proposed relating to the assembly of a higher-order ParB–DNA nucleoprotein complex. Here we assess the evidence for and against each model.
2.2. Model 1—one-dimensional filamentation of ParB
The earliest evidence of a higher-order ParB-DNA nucleoprotein complex came from studies of a plasmid-borne ParB. Overexpression of an F-plasmid ParB protein (ParBF or SopB) was observed to repress the expression of antibiotic resistance genes several kilobases away from the parS (sopC) site on the plasmid [59]. Moreover, ParBF overexpression also prevents DNA gyrase and restriction enzyme access to DNA regions neighbouring the parS site [59]. Similarly, a P1-plasmid ParB (ParBP1) also silences the expression of genes adjacent to parS in both directions for several kilobases, with the efficiency of gene silencing decreasing as the genomic distance from parS increases [60]. A direct association of ParBP1 with the silenced DNA was demonstrated by chromatin immunoprecipitation PCR (ChIP-PCR) assay [60]. Based on these observations, it was proposed the growth of a filament of ParB proteins nucleated at parS and then spread outward to neighbouring DNA (figure 1b). This model was further supported by the observation that a site-specific DNA-binding protein, RepA, could attenuate the ParBP1-mediated gene silencing effect, presumably by acting as a roadblock to partially stop the filamentation of ParB [60] (figure 1b). Multiple chromosomal ParBs have subsequently been observed by ChIP-chip/seq to associate with an extended DNA region beyond parS [13,16,19,44,47,48,61,62], hence chromosomal ParBs were also thought to oligomerize to form a nucleoprotein filament. The highly conserved arginine-rich patch (GERRxRA) at the NTD has been implicated in mediating ParB filamentation, as mutations in this region impair the ability of ParB to associate extensively with DNA beyond parS [47–49,62,63]. This early model of ParB spreading is straightforward and attractive; however, later studies have argued that the intracellular concentration of ParB is too low to support such an extensive one-dimensional filamentation in vivo [62,64]. Moreover, at native expression levels, B. subtilis ParB (Spo0 J) does not silence genes adjacent to parS [48], suggesting that the ParB-DNA nucleoprotein complex might be more dynamic than can be explained by the one-dimensional filamentation model.
2.3. Model 2—bridging and condensing DNA
A combination of quantitative immunoblotting and immunofluorescence microscopy approaches led to the estimate that approximately 20 ParB dimers are associated with each parS site in B. subtilis, allowing for maximally approximately 500 bp of DNA to be covered by a continuous filament of ParB [62]. This is substantially lower than the approximately 10–20 kb of ParB-bound DNA observed by ChIP-chip [48,61], arguing against the one-dimensional filamentation model. Instead, a new model was proposed based on the observation that B. subtilis ParB can bridge different segments of DNA (figure 1c). In a single-molecule microscopy-based assay, bacteriophage λ DNA (approx. 50 kb) was tethered at one end to a microscope slide and stretched out by a buffer flow. The introduction of purified B. subtilis ParB compacted the flow-extended DNA, demonstrating that ParB can form bridges and condense bound DNA [62]. Moreover, mutations in the arginine-rich patch which eliminate the extensive in vivo ChIP-seq profile of B. subtilis ParB also impair in vitro DNA-bridging activity [62].
ParB-mediated DNA bridging was also observed using magnetic-tweezers assays [65]. The additional non-specific DNA-binding activity owing to a surface-exposed lysine-rich patch at the CTD of B. subtilis ParB was found to be essential for this function [53,66]. Mutations in these lysine residues eliminate DNA bridging and condensation in vitro and reduce ParB-DNA nucleoprotein formation in vivo, as assessed by the less extensive ChIP-qPCR profile and by the dimmer and fuzzier appearance of fluorescently labelled ParB foci [53]. It is important to emphasize that interactions among NTD of B. subtilis ParB are also necessary for bridging DNA (figure 1c); neither CTD alone nor ParB with mutations at the arginine-rich patch (at the NTD) can condense DNA in vitro [53,66]. The non-specific DNA-binding activity of the CTD is thought to provide multiple anchors on DNA that can be brought spatially close together by the NTD–NTD interactions (figure 1c). Insights into the molecular mechanism of NTD–NTD interactions were provided by the co-crystal structure of a CTD-truncated Helicobacter pylori ParB in complex with a parS DNA duplex [54]. This structure shows H. pylori ParB interacting with an adjacent ParB on a pseudo-continuous DNA in the crystal lattice (in cis interactions or one-dimensional filamentation) and also with ParB on a disconnected DNA duplex (in trans interactions or three-dimensional bridging) (figure 1c), with the arginine-rich patch at the core of the NTD–NTD interaction interface [54]. By comparison with the Thermus thermophilus apo-ParB structure, it was proposed that the nucleation of ParB onto parS induces a conformational change at the NTD that exposes the arginine-rich patch for the NTD–NTD interactions [49,54,56].
In sum, it has been proposed that DNA-bridging activity allows a limited number of ParB molecules to bring regions of DNA that are several kilobases apart together in three-dimensional space to form a compacted nucleoprotein complex (figure 1c). Nevertheless, a computational modelling study has suggested that a combination of both one-dimensional filamentation and three-dimensional bridges are required to recreate the condensed ParB–DNA nucleoprotein complex observed in vivo [67]. Thus, while the DNA-bridging model is an important step towards understanding the assembly of the ParB–DNA nucleoprotein complex, it is unlikely to be the final say. The main caveat is that B. subtilis ParB can bridge to condense DNA in vitro regardless of the presence of parS [53,62,65]. This contradicts in vivo data showing parS is absolutely required for the clustering of fluorescently labelled ParB molecules into a tight focus [62,68]. Moreover, the lysine-rich patch (at the CTD of B. subtilis ParB) is not highly conserved; for example, ParB from Caulobacter crescentus lacks the equivalent lysine residues and does not bridge/condense DNA in vitro [55]. As such, it is not yet clear how prevalent DNA-bridging activity is among chromosomal ParB homologs.
2.4. Model 3—caging ParB and DNA
A model broadly similar to bridging and condensing DNA that aims to better explain the observed parS-dependent confinement of ParB in vivo has been proposed [69]. In this nucleation and caging model, the parS site acts as a ParB nucleation centre, while weak but synergistic protein–protein and protein–DNA interactions cage ParB spatially into a confined volume inside the cells [69] (figure 1d). Supporting this model, single-molecule super-resolution microscopy demonstrated that the binding of ParBF to parS results in a very high local concentration of protein in vivo, where greater than 90% of ParBF in the cell are confined in clusters at parS [69]. Similarly, the local concentration of C. crescentus ParB near parS has been estimated to reach approximately 500 µM (500 times more concentrated than typically used for in vitro experiments) [31]. Despite ParBF (or C. crescentus ParB) having expectedly low-affinity interactions with non-specific DNA, these interactions may occur stochastically at very high frequency, especially at the extreme local concentration of ParB in vivo, to create a cage of dynamically exchanged ParB–DNA complexes (figure 1d). Fluorescence recovery after photo-bleaching (FRAP) experiments have shown that ParBF molecules rapidly exchange between different clusters, further highlighting the dynamic nature of cages of ParB-DNA in vivo [70]. The nucleation and caging model has also been shown applicable to the Vibrio cholerae chromosomal ParB–parS system, suggesting that this dynamic self-assembly mechanism might be conserved from plasmids to chromosomes [70].
2.5. Model 4—lateral sliding of a ParB–CTP clamp on DNA
Recent studies have uncovered a new cofactor of ParB [51,52]. Various plasmid- and chromosome-encoded ParB and ParB-like proteins have been found to bind and hydrolyse cytidine triphosphate (CTP) to cytidine di-phosphate (CDP) and inorganic phosphate [51,52,71]. A co-crystal structure showed CDP binding to the arginine-rich patch at the NTD of B. subtilis ParB (CTP was hydrolysed to CDP during crystallization) [52]. At the same time, another co-crystal structure showed a M. xanthus ParB-like protein (PadC) in complex with CTP [51]. CTP (or CDP) is sandwiched between two NTDs, thus promoting a new NTD self-dimerization interface that has not been observed previously [51,52]. Employing site-specific cross-linking assays and single-molecule imaging, it was demonstrated that CTP-induced self-dimerization creates a clamp-like ParB that entraps DNA within its central cavity [52] (figure 1e). A comparison between the B. subtilis ParB–CDP structure and the H. pylori ParB–parS structure suggested that CTP binding induces a conformational change at the central DNA-binding domain that is incompatible with parS binding [52,54]. Studies with C. crescentus and M. xanthus ParBs further showed that CTP binding reduces ParB nucleation at parS and/or liberates pre-bound ParB from parS [51,71], thereby facilitating the escape of ParB from a high-affinity nucleation site to a low-affinity neighbouring DNA. Therefore, CTP probably serves to switch ParB from a nucleating to a sliding mode (figure 1e). Overall, it was suggested that ParB clamp can self-load at parS, without the need of a dedicated loading factor, and spreads by sliding to the neighbouring DNA while still entrapping DNA [52,71] (figure 1e). The interpretation of a sliding ParB–CTP clamp on DNA is further backed up by several lines of evidence: (i) tight DNA-binding proteins, such as a catalytic-dead EcoRI (E111Q) variant or TetR, can block the spreading of B. subtilis and C. crescentus ParB–CTP on DNA in vitro [52,71] (figure 1e), and (ii) C. crescentus ParB only accumulates on DNA that has both ends blocked (by a bulky biotin-streptavidin complex) to prevent a run-off [71]. However, it is not yet clear whether the translocation of ParB–CTP on DNA is entirely a passive one-dimensional diffusion process or whether it is facilitated by unknown interactions between the protein and DNA. CTP hydrolysis is unlikely to provide energy for ParB translocation since its hydrolysis rate is extremely low, ranging from approximately 3 to approximately 36 CTP molecules per hour [51,52,71]. Moreover, ParB in complex with a non-hydrolysable CTPγS analog can still self-load and accumulate on DNA, albeit with a reduced stability [52,71]. It has been speculated that CTP hydrolysis might contribute to recycling of ParB between the nucleation and translocation modes [52,71]. Mutant proteins (N112S and N172A of B. subtilis and M. xanthus ParB, respectively), which bind CTP but are deficient for hydrolysis, fail to form tight foci inside the cells [40,51,52]; however, this is weak evidence for the in vivo role of CTP hydrolysis since B. subtilis ParB (N112S) is already impaired at forming a protein clamp [52]. A better understanding of the CTPase mechanism that enables the design of a mutation at the catalytic site to eliminate CTP hydrolysis while allowing NTD self-dimerization is likely to provide a key insight into the role of CTP hydrolysis.
2.6. Reconciliation of different models: outstanding questions and challenges
The unexpected finding of the ParB–CTP interaction has fundamentally changed thinking on the assembly of a higher-order nucleoprotein complex and bacterial chromosome segregation by the ParABS system. But does the ‘ParB spreading by sliding’ model supersede previously proposed models? It is too early to answer this question adequately, given that many mechanistic details are still missing. For example, an alternative view has been proposed wherein parS binding stimulates the CTPase activity to switch M. xanthus ParB from a CTP-bound closed conformation to an apo/CDP-bound open conformation, liberating the NTD to engage in DNA-bridging/caging interactions [51]. It is possible that there are two different modes of action of ParB inside the cells: one for bridging/caging DNA together, and another for the lateral sliding of ParB on DNA. Investigating the relative contribution of the two different modes of action to chromosome segregation, especially in vivo, is an important challenge. Some of other immediate questions to which answers can help refine or reconcile different models include:
-
—
How dynamic is the ParB clamp opening and closing when bound to parS and/or to CTP?
-
—
Can the ParB clamp entrap two or more DNA segments together [52], thereby contributing to DNA bridging and condensation?
-
—
What is the mechanism of CTP hydrolysis?
-
—
Does the translocation of ParB supercoil DNA, thereby compacting parS-proximal DNA?
-
—
Is there a variation in CTP-binding affinity and CTP hydrolysis rate among ParB orthologs, and how does this natural variation impact chromosome segregation in different bacterial species?
Whether CTP plays a regulatory role in chromosome segregation, in addition to being a co-factor of ParB, is also unknown. The concentration of nucleoside triphosphate (NTP) ranges from approximately 0.3 to approximately 3 mM inside bacterial cells [72]. Their concentrations can decrease by ∼tenfold as cells enter the stationary phase [72] but it is unlikely to impact ParB–CTP binding significantly. Indeed, foci of a fluorescently tagged ParB do not disappear when C. crescentus cells enter the stationary phase or during starvation [73]. For these reasons, we speculate that the assembly of ParB–DNA nucleoprotein complex is not regulated by varying the intracellular concentration of CTP. However, there is a formal possibility that other NTP-related small molecules, whose diversity has only been realized recently [74], could have a regulatory impact. Future work will undoubtedly continue to provide important new insights into the assembly of the ParB–DNA nucleoprotein complex and its roles in chromosome segregation.
3. ParB–DNA interaction with ParA and segregation of the origin-proximal chromosomal region
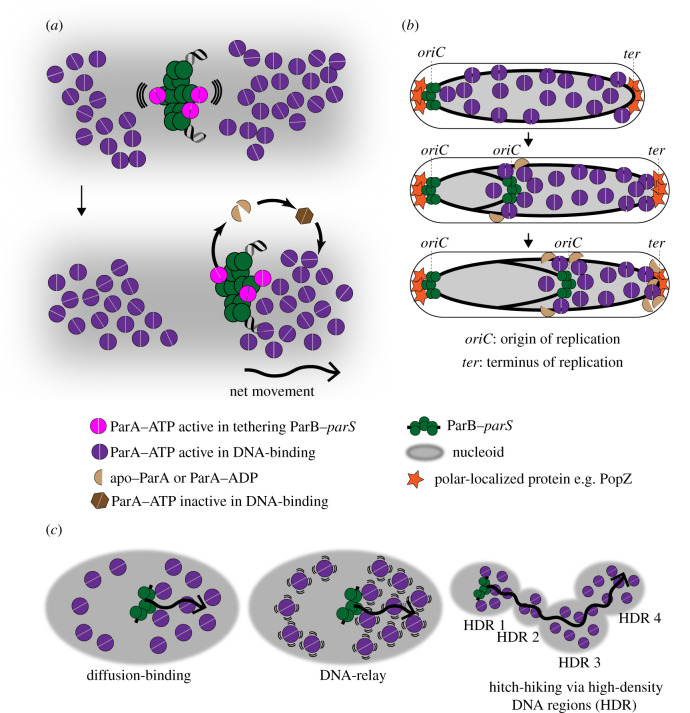
ParA is a deviant Walker A ATPase protein [75] that enables a directional movement of ParB-bound DNA. Early studies of plasmid and chromosome segregation proposed a mechanism for DNA-pulling by either a linear or a helical ParA filament [76–83], akin to the mitotic spindle apparatus in eukaryotes. According to this model, ParA–ATP polymerizes into a filamentous structure along the cell length, with the edge of the filament capturing the ParB–DNA nucleoprotein complex. ParB binds ParA and stimulates its ATPase activity to hydrolyse ATP, thereby depolymerizing the ParA filament and concomitantly pulling the ParB–DNA complex (hence, the plasmid/chromosome) along the retracting filament to the opposite cell pole [76,83,84]. While purified ParA from various bacterial species could self-aggregate into filament-like structures in the presence of ATP/ADP [34,76,79,82,85–91], no such continuous polymer was seen in recent co-crystal structures of ParA with DNA, even at the high concentration of protein and DNA used to generate crystals. Furthermore, the spatial distribution of an F-plasmid ParA and C. crescentus ParA in vivo is inconsistent with a continuous filamentous structure, instead they form small patches or a cloud-like gradient of sparsely distributed molecules inside the cells, as observed by super-resolution microscopy [31,37]. As such, it is uncertain whether a DNA-pulling mechanism by a ParA filament is operating in vivo.
It has been proposed that a ParA filament is not necessary for DNA segregation, and that a diffusion-ratchet mechanism can also explain the directional movement of segregating DNA [33,35,36,38,92,93] (figure 2a,b). In this model, ParA binds ATP to homodimerize and to associate with non-specific DNA. X-ray crystallographic and hydrogen/deuterium exchange mass spectrometry analysis of ParA with nucleotides and DNA have revealed the dimerization interface and a multifaced DNA-binding surface [94–96]. ParB, via its N-terminal peptide, binds ParA directly and stimulates the ATPase activity of ParA, thereby dissociating ParA dimer into individual monomers that no longer bind DNA [34,36,97,98] (figure 2a). This stimulation in the ATPase activity creates a local gradient of ParA–ATP with the least DNA-bound ParA–ATP near the ParB–DNA complex (figure 2a). The ParB–DNA complex then diffuses up the gradient, by Brownian motion, to rebind ParA–ATP, resulting in a net movement of the ParB-anchored DNA (figure 2a). The initial movement of the ParB–DNA complex in one chosen direction enforces the continued movement in the same direction, resulting in a long-range directional movement of the DNA (figure 2a,b). The released monomeric apo–ParA/ParA–ADP can rebind ATP to homodimerize and later regains its non-specific DNA-binding activity (figure 2a). It is worth noting that the released apo–ParA/ParA–ADP can rebind ATP but cannot immediately bind DNA until a transition occurs in the ParA–ATP structure (figure 2a); this transitional state presumably introduces a time delay mechanism to ensure the existence of a ParA–ATP gradient surrounding the ParB–DNA complex [38,94]. Without this delay, regenerated ParA–ATP will instantly rebind DNA in the same location, thus dissipating the gradient. Other organism-specific factors, for example, the polarly localized proteins PopZ and TipN in C. crescentus, may also contribute to maintain the ParA–ATP gradient by sequestering apo–ParA/ParA–ADP away from the nucleoid and to regenerate ParA–ATP only at the cell pole [99,100] (figure 2b). Based on computational modelling it has been argued that the short-range diffusion of a ParB–DNA complex up the gradient of ParA–ATP might not be sufficient to explain a robust unidirectional segregation of chromosome towards the new cell pole (figure 2b) and that the diffusion-ratchet model should be extended to incorporate a component of DNA elasticity. In this model, DNA-bound ParA–ATP complexes can harness the elastic dynamics of the chromosome to relay the partition complex over a long distance from one DNA location to another [31] (figure 2c). Similarly, it has also been proposed that partition complexes can also hitchhike from one high-density DNA region to another on the chromosome to move the ParB-bound DNA progressively [37] (figure 2c). High-density DNA regions have been observed in B. subtilis and Escherichia coli by super-resolution microscopy and may represent highly compacted domains of the chromosomes [37,101]. The preferred association of ParA–ATP with high-density DNA regions, via its non-specific DNA-binding activity, might create the required directional bias in the movement of the ParABS complex (figure 2c).
Figure 2.
ParA drives the movement of ParB-bound DNA to segregate plasmids and chromosomes. (a) A diffusion-ratchet model for ParA-mediated transport of ParB-bound DNA. A ParB–DNA complex (green) interacts with ParA–ATP (violet) to tether to the nucleoid (grey), and to stimulate the ATPase activity of ParA. ParA–ATP dimers (violet) bind the nucleoid non-specifically. After ATP hydrolysis, monomers of apo–ParA/ParA–ADP (light brown) no longer bind DNA, thus creating a zone of depletion of ParA–ATP surrounding the ParB–DNA complex. By thermal fluctuation (wavy lines), the ParB–DNA complex moves to the edge of the zone of depletion to rebind ParA–ATP. The initial movement of the ParB–DNA complex in one chosen direction enforces the continued movement in the same direction, resulting in a long-range directional movement of the DNA (see b). The released apo–ParA/ParA–ADP (light brown) rebinds ATP but cannot immediately bind DNA (the dark brown hexagon) until a transition occurs in the ParA–ATP structure. (b) The segregation of the origin-proximal region of the chromosome by the ParABS system. For example, in C. crescentus, one ParB–DNA complex remains at the pole after chromosome replication, while the other moves along the gradient of ParA–ATP, via the diffusion-ratchet mechanism, to the opposite cell pole. The polarly localized proteins (e.g. PopZ, orange) contribute to maintaining the ParA–ATP gradient by sequestering apo–ParA/ParA–ADP away from the nucleoid and to regenerate them at the pole. (c) Other variations of the diffusion-ratchet model have been proposed to include an element of DNA elasticity (i.e. the DNA-relay model) or high-density DNA regions (HDR) (i.e. the hitch-hiking model). A wavy arrow indicates the directional movement of the partition complex.
The diffusion-ratchet model emphasizes the crucial role of ParB in stimulating the ATPase activity of ParA to create the ParA–ATP gradient. However, an alternative view on the ATPase-stimulating role of ParB, at least for the F-plasmid ParAB system (SopAB) suggested that the stimulation of ParAF ATPase activity mainly serves to spatially separate F-plasmid clusters following replication and to prevent them from re-forming later [102]. The directional movement of replicated F plasmids might depend on a basal ATPase activity of ParAF but does not need further stimulation by ParBF [102]. Finally, the recent discovery of CTP as a cofactor of both plasmid- and chromosome-encoded ParB raises many important questions. Does ParB–CTP further stimulate the ATPase activity of ParA, and conversely, does ParA accelerate the CTP hydrolysis rate of ParB? Early evidence suggested that CTP can modulate ParA–ParB interaction; mutations at the CTP-binding pocket of a ParB-like protein PadC were shown to impair PadC–ParA binding in vitro (i.e. ParA preferentially binds to PadC–CTP, rather than to apo-PadC, and gave rise to aberrant ParA localization patterns in vivo [51]). Future works, especially with the canonical ParABS system, will provide important insights to refine current models for the ParA-directed DNA segregation.
4. The ParB-DNA and SMC coordinate chromosome segregation with chromosome organization
In addition to its role in DNA segregation, ParB also participates in other biological processes such as chromosome organization, nucleoid occlusion, regulation of DNA replication initiation and regulation of gene expression [16,24,40,41,100,103–113]. The wide range of ParB-interacting partners reflects (i) the central role of the ParB-DNA nucleoprotein as a hub to couple chromosome segregation with other biological processes and (ii) the capacity of ParB to evolve additional functions. For a further discussion, we refer the reader to recent reviews [114,115]. In this section, we instead focus on the interaction between ParB and the SMC complex that is directly relevant to the segregation of the origin-proximal region of the chromosome.
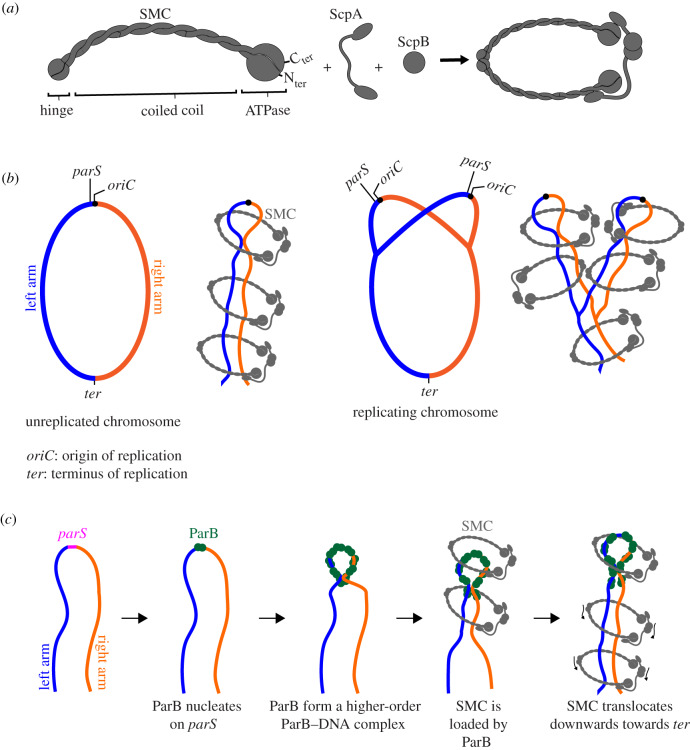
A canonical bacterial SMC is composed of an ATPase domain (the head), a dimerization domain (the hinge) and an extended antiparallel coiled-coil region in the middle [116] (figure 3a). Two SMC monomers homodimerize together with the accessory proteins (ScpA and ScpB) to form a ring-like protein complex that can bring distal DNA segments close together spatially to organize the chromosome [116–118] (figure 3a). This entrapment of DNA has been shown for B. subtilis SMC [119] and for eukaryotic SMC homologs such as cohesin and condensin [120–123]. Application of chromosome conformation capture assays (Hi-C/3C-seq) to cells from a range of bacterial species lacking SMC have revealed a reduced interaction between opposite arms of the chromosome, suggesting that SMC entraps and tethers the two chromosome arms together [43,44,101,124–126] (figure 3b). SMC is recruited onto the chromosome by ParB at the origin-proximal parS sites [16,40–42,44] (figure 3c). After loading, SMC redistributes directionally away from parS towards the replication terminus (ter) while maintaining the tethers between the parS-proximal regions of the chromosome arms [42,125] (figure 3c). Given that parS sites are often found near the origin of replication, parS-loaded SMCs preferably condense newly replicated DNA to package them into individual entities and away from each other (figure 3b). This DNA-unlinking activity is independent of topoisomerase IV, at least in B. subtilis, and might help to prevent catenation between replicated chromosomes at the replication fork or promote their resolution behind the fork [45,46]. If replicated chromosomes are not resolved, their entanglement might hinder movement of individual chromosomes to opposite cell poles by the ParABS system. In C. crescentus, segregation of origin-proximal DNA occurs in two steps; the duplicated origins are released from the pole and separate slightly from one another first before one of the origins is moved unidirectionally by ParABS to the opposite cell pole [127]. While the initial separation does not require ParA [127,128], it might be facilitated by the DNA-unlinking activity of SMC.
Figure 3.
The ParB–DNA nucleoprotein complex recruits SMC to coordinate chromosome segregation and chromosome organization. (a) Components of the bacterial SMC complex. (b) SMC (grey) tethers the two arms (blue and orange) of a circular chromosome together. An SMC–ScpA–ScpB complex can either hold both the left and the right arm of the chromosome within its lumen or two SMC complexes, each encircles one chromosome arm, can handcuff to tether both chromosome arms together. For simplicity, only SMCs entrapping both chromosome arms are shown. SMC probably packages sister chromosomes into individual entities and away from each other, thus minimizing DNA entanglement between replicating chromosomes. (c) A schematic model of how SMC is loaded at parS by ParB and translocates on the chromosome towards the replication terminus (ter). For simplicity, alternative conformations of SMC (ring or rod) are not illustrated; the SMC complex is shown as a generic ring that entraps DNA. Schematic pictures are not drawn to scale.
Precisely how SMC translocates on the chromosome is not yet clear; several models have been proposed, and we refer the reader to a recent review [129] for an in-depth discussion. How ParB loads SMC onto the chromosome is also not fully understood; the weak and transient interaction between ParB and SMC has made efforts to study their interactions by traditional methodologies (such as bacterial two-hybrid or co-immunoprecipitation) difficult [41,42,44]. However, it was suggested that DNA-bound ParB probably interacts directly with SMC to recruit it to the DNA [40]. Indeed, a ParB-interacting area has been identified in the neck region in between the ATPase head domain and the coiled coil of B. subtilis SMC [130], while mutations that eliminate SMC recruitment have been mapped onto the N-terminal domain of B. subtilis ParB [40,43]. Those same mutations also impair the ability of ParB to assemble into a higher-order nucleoprotein complex, hence it is tempting to speculate that either (i) a high local concentration of DNA-bound ParB is necessary to recruit sufficient SMC molecules or (ii) the DNA-bridging/clamping activity of ParB ensures SMC entraps DNA correctly at the loading step. Future experiments, particularly a cell-free reconstitution of a ParB-dependent SMC recruitment and translocation, will provide further insights into the mechanism of actions of bacterial SMC and its contribution to chromosome segregation.
5. The evolution of the ParABS system and bacterial chromosome segregation
Research in multiple model species and an ever-increasing number of sequenced bacterial genomes has highlighted variations in the mechanism for bacterial chromosome segregation. Approximately 25% of bacterial species lack ParABS homologs entirely [28] and thus probably employ other systems to facilitate their chromosome segregation [131–133]. In some species, for example, Streptococcus pneumoniae or Staphylococcus aureus, only ParB–parS and SMC are present while a ParA homolog is missing [28]. Even in species with the canonical ParABS system, there exists a wide variation in the number of parS sites; for example, Xanthomonas campestris has a single parS site while Streptomyces coelicolor and Listeria innocua accumulated up to 20–23 parS sites near the origin of replication [28,134]. Why is the number of parS sites variable when a single parS site is often sufficient for chromosome segregation [12,13,62]? How does this variation in the number of parS sites impact chromosome segregation in different bacterial species in their niches? Why do parS sites position closely on the genome and what drives their clustering over evolutionary time? For the last question, a transposon-based saturated insertion of a parS site on the Pseudomonas aeruginosa and C. crescentus chromosome offered some insights; it was discovered that the insertion of a de novo or a second parS site is only tolerable in approximately 600 kb region surrounding the native parS locus or the origin of replication without severely affecting cell fitness [13,47]. These results suggest a self-reinforcing mechanism for the expansion of the bacterial centromere region by restricting the multiplication of parS to a narrow region near the original site.
Another noteworthy example of the evolution of the ParABS system is the gene duplication and neo-functionalization event that generated a nucleoid occlusion factor (Noc) in Firmicutes [135–139]. Noc, a ParB-like protein, plays a role in preserving the integrity of the chromosome; it does so by preventing the cell division machinery from assembling in the vicinity of the segregating chromosome, which might be otherwise guillotined, thereby damaging the DNA [136,140,141]. An amphipathic helix is present at the N-terminus of Noc instead of the ParA–ATPase-stimulating peptide commonly found in ParB [141]. Mutations that perturbed the amphipathicity of this helix also eliminated the nucleoid occlusion function, while replacing the native helix with one from the hepatitis C virus protein NS4B restored the nucleoid occlusion activity [141,142]. A mutational event that resulted in the grafting of an amphipathic helix might have been the evolutionary mechanism that once granted a novel function to a ParB protein [137,141]. Furthermore, in contrast to ParB, Noc does not bind parS but recognizes a different DNA-binding sequence called NBS (Noc-Binding Site) [140]. NBS differs from parS by only two bases but Noc and ParB recognize and bind them with exquisite specificity [140,143]. X-ray crystallography and systematic scanning mutagenesis identified a minimal set of just four amino acids that mediate ParB-parS/Noc-NBS binding specificity [143]. Deep mutational scanning of these four specificity residues enabled an in silico reconstitution of possible evolutionary paths that reprogramed DNA-binding specificity from parS to NBS [143]. A small number of required mutations and the large number of mutational paths to reprogram DNA-binding specificity illustrates the evolvability of the ParABS system.
The existence of various ParA homologs with diverse functions is also intriguing. In Rhodobacter sphaeroides, an orphan ParA-like protein (PpfA) uses non-specific nucleoid binding to separate cytoplasmic clusters of chemotaxis proteins [144]. Similar to the canonical ParABS system, the ATPase activity of PpfA is modulated by the N terminus of a ParB analog (TlpT) [144]. In C. crescentus, another ParA homolog (MipZ) coordinates chromosome segregation with cell division by directly interfering with FtsZ polymerization [109]. MipZ binds DNA non-specifically and also interacts with ParB to create a bipolar protein gradient in the cells that restricts FtsZ ring formation to the mid cell, where the concentration of MipZ is lowest [95,109,145]. In V. cholerae, three ParA-like ATPases (ParA1, FlhG and ParC) interact with a polar transmembrane protein HubP to control polar localization of the chromosome origin, the chemotactic machinery and the flagellum [111]. These examples illustrate how diverse functions in biology can evolve from a general mechanism and are therefore interesting from both evolutionary and mechanistic standpoints.
Last but not least, a DNA segregation system that combines bacterial ParAB-like and eukaryotic histone-like components has been identified in the archaea Sulfolobus [146,147]. This system consists of an ATPase ParA, an atypical ParB adaptor and a novel centromere-binding protein AspA. The N-terminal domain of the archaeal ParB is similar to the bacterial ParB NTD; however, its C-terminal domain resembles an eukaryotic histone protein CenpA [146]. A long amino acid linker that connects the two domains of the archaeal ParB interacts with ParA, while its N-terminal domain binds AspA. AspA binds the centromere, thereby serves as a physical link between the archaeal ParA–ParB and the segregating DNA [146]. The hybrid nature of the archaeal DNA segregation machinery demonstrates how evolution has diversified DNA segregation systems, possibly to adapt to the specific needs of each organism, while keeping the general mechanism conserved across the three domains of life.
6. Final perspectives
Over 35 years of research has led to tremendous progress in understanding the molecular mechanism of the ParABS system and its roles in DNA segregation. Nevertheless, many mechanistic details are missing or only now starting to emerge. The recent discovery of cytidine triphosphate as a cofactor of ParB illustrates this point perfectly. Research with bacterial systems has already benefited tremendously from the recent explosion of interest and technological advances from the eukaryotic chromosome field. We predict that novel high–throughput sequencing-based methodologies, single-molecule imaging, single-molecule biophysics, and traditional biochemistry and genetics will continue to provide further insights into the mechanisms of chromosome segregation in bacteria. Finally, various orthogonal ParB–parS systems have been exploited to label and image DNA loci in vivo, in both bacteria and eukaryotes [148–151]. Recent studies have also expanded the utilization of the ParABS system in synthetic biology, for example, as part of a genetic circuit to enable asymmetric cell division in E. coli [152,153]. Such exciting developments will benefit from ongoing research into the mechanistic details of the ParABS system and its evolvability to acquire new functions.
Supplementary Material
Data accessibility
This article does not contain any additional data.
Authors' contributions
Both authors have contributed to the writing of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by the Royal Society University Research Fellowship no. (UF140053) to T.B.K.L. and the Royal Society Research grant no. (RG150448) to A.S.B.J and T.B.K.L.
References
- 1.Abeles AL, Friedman SA, Austin SJ. 1985. Partition of unit-copy miniplasmids to daughter cells. III. The DNA sequence and functional organization of the P1 partition region. J. Mol. Biol. 185, 261–272. ( 10.1016/0022-2836(85)90402-4) [DOI] [PubMed] [Google Scholar]
- 2.Austin S, Abeles A. 1983. Partition of unit-copy miniplasmids to daughter cells. II. The partition region of miniplasmid P1 encodes an essential protein and a centromere-like site at which it acts. J. Mol. Biol. 169, 373–387. ( 10.1016/s0022-2836(83)80056-4) [DOI] [PubMed] [Google Scholar]
- 3.Austin S, Abeles A. 1983. Partition of unit-copy miniplasmids to daughter cells. I. P1 and F miniplasmids contain discrete, interchangeable sequences sufficient to promote equipartition. J. Mol. Biol. 169, 353–372. ( 10.1016/s0022-2836(83)80055-2) [DOI] [PubMed] [Google Scholar]
- 4.Mori H, Kondo A, Ohshima A, Ogura T, Hiraga S. 1986. Structure and function of the F plasmid genes essential for partitioning. J. Mol. Biol. 192, 1–15. ( 10.1016/0022-2836(86)90459-6) [DOI] [PubMed] [Google Scholar]
- 5.Ireton K, Gunther NW, Grossman AD. 1994. spo0 J is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J. Bacteriol. 176, 5320–5329. ( 10.1128/jb.176.17.5320-5329.1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mysliwiec TH, Errington J, Vaidya AB, Bramucci MG. 1991. The Bacillus subtilis spo0 J gene: evidence for involvement in catabolite repression of sporulation. J. Bacteriol. 173, 1911–1919. ( 10.1128/jb.173.6.1911-1919.1991) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin DC, Grossman AD. 1998. Identification and characterization of a bacterial chromosome partitioning site. Cell 92, 675–685. ( 10.1016/s0092-8674(00)81135-6) [DOI] [PubMed] [Google Scholar]
- 8.Mohl DA, Easter J, Gober JW. 2001. The chromosome partitioning protein, ParB, is required for cytokinesis in Caulobacter crescentus. Mol. Microbiol. 42, 741–755. ( 10.1046/j.1365-2958.2001.02643.x) [DOI] [PubMed] [Google Scholar]
- 9.Jung A, Raßbach A, Pulpetta RL, van Teeseling MCF, Heinrich K, Sobetzko P, Serrania J, Becker A, Thanbichler M. 2019. Two-step chromosome segregation in the stalked budding bacterium Hyphomonas neptunium. Nat. Commun. 10, 3290 ( 10.1038/s41467-019-11242-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iniesta AA. 2014. ParABS system in chromosome partitioning in the bacterium Myxococcus xanthus. PLoS ONE 9, e86897 ( 10.1371/journal.pone.0086897) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harms A, Treuner-Lange A, Schumacher D, Sogaard-Andersen L. 2013. Tracking of chromosome and replisome dynamics in Myxococcus xanthus reveals a novel chromosome arrangement. PLoS Genet 9, e1003802 ( 10.1371/journal.pgen.1003802) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jecz P, Bartosik AA, Glabski K, Jagura-Burdzy G. 2015. A single parS sequence from the cluster of four sites closest to oriC is necessary and sufficient for proper chromosome segregation in Pseudomonas aeruginosa. PLoS ONE 10, e0120867 ( 10.1371/journal.pone.0120867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagage V, Boccard F, Vallet-Gely I. 2016. Regional control of chromosome segregation in Pseudomonas aeruginosa. PLoS Genetics 12, e1006428 ( 10.1371/journal.pgen.1006428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu W, Herbert S, Graumann PL, Götz F. 2010. Contribution of SMC (Structural Maintenance of Chromosomes) and SpoIIIE to chromosome segregation in staphylococci. J. Bacteriol. 192, 4067–4073. ( 10.1128/JB.00010-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee PS, Grossman AD. 2006. The chromosome partitioning proteins Soj (ParA) and Spo0 J (ParB) contribute to accurate chromosome partitioning, separation of replicated sister origins, and regulation of replication initiation in Bacillus subtilis. Mol. Microbiol. 60, 853–869. ( 10.1111/j.1365-2958.2006.05140.x) [DOI] [PubMed] [Google Scholar]
- 16.Minnen A, Attaiech L, Thon M, Gruber S, Veening J-W. 2011. SMC is recruited to oriC by ParB and promotes chromosome segregation in Streptococcus pneumoniae. Mol. Microbiol. 81, 676–688. ( 10.1111/j.1365-2958.2011.07722.x) [DOI] [PubMed] [Google Scholar]
- 17.Donovan C, Schwaiger A, Krämer R, Bramkamp M. 2010. Subcellular localization and characterization of the ParAB system from Corynebacterium glutamicum. J. Bacteriol. 192, 3441–3451. ( 10.1128/JB.00214-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santi I, McKinney JD. 2015. Chromosome organization and replisome dynamics in Mycobacterium smegmatis. mBio 6, e01999-14 ( 10.1128/mBio.01999-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donczew M, Mackiewicz P, Wróbel A, Flärdh K, Zakrzewska-Czerwińska J, Jakimowicz D. 2016. ParA and ParB coordinate chromosome segregation with cell elongation and division during Streptomyces sporulation. Open Biol. 6, 150263 ( 10.1098/rsob.150263) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du W-L, Dubarry N, Passot FM, Kamgoué A, Murray H, Lane D, Pasta F.. 2016. Orderly Replication and Segregation of the Four Replicons of Burkholderia cenocepacia J2315. PLoS Genet. 12, e1006172 ( 10.1371/journal.pgen.1006172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartosik AA, Mierzejewska J, Thomas CM, Jagura-Burdzy G. 2009. ParB deficiency in Pseudomonas aeruginosa destabilizes the partner protein ParA and affects a variety of physiological parameters. Microbiology (Reading, Engl.) 155, 1080–1092. ( 10.1099/mic.0.024661-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis RA, Bignell CR, Zeng W, Jones AC, Thomas CM. 2002. Chromosome loss from par mutants of Pseudomonas putida depends on growth medium and phase of growth. Microbiology (Reading, Engl.) 148, 537–548. ( 10.1099/00221287-148-2-537) [DOI] [PubMed] [Google Scholar]
- 23.Yamaichi Y, Fogel MA, McLeod SM, Hui MP, Waldor MK. 2007. Distinct centromere-like parS sites on the two chromosomes of Vibrio spp. J. Bacteriol. 189, 5314–5324. ( 10.1128/JB.00416-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadoya R, Baek JH, Sarker A, Chattoraj DK. 2011. Participation of chromosome segregation protein ParAI of Vibrio cholerae in chromosome replication. J. Bacteriol. 193, 1504–1514. ( 10.1128/JB.01067-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charaka VK, Misra HS. 2012. Functional characterization of the role of the chromosome I partitioning system in genome segregation in Deinococcus radiodurans. J. Bacteriol. 194, 5739–5748. ( 10.1128/JB.00610-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Angelov A, Pham VTT, Leis B, Liebl W. 2015. Characterization of chromosomal and megaplasmid partitioning loci in Thermus thermophilus HB27. BMC Genomics 16, 317 ( 10.1186/s12864-015-1523-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H. 2019. Random chromosome partitioning in the polyploid bacterium Thermus thermophilus HB27. G3 (Bethesda) 9, 1249–1261. ( 10.1534/g3.119.400086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livny J, Yamaichi Y, Waldor MK. 2007. Distribution of centromere-like parS sites in bacteria: insights from comparative genomics. J. Bacteriol. 189, 8693–8703. ( 10.1128/JB.01239-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toro E, Hong S-H, McAdams HH, Shapiro L. 2008. Caulobacter requires a dedicated mechanism to initiate chromosome segregation. PNAS 105, 15 435–15 440. ( 10.1073/pnas.0807448105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funnell BE. 2016. ParB partition proteins: complex formation and spreading at bacterial and plasmid centromeres. Front. Mol. Biosci. 3, 44 ( 10.3389/fmolb.2016.00044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim HC, Surovtsev IV, Beltran BG, Huang F, Bewersdorf J, Jacobs-Wagner C. 2014. Evidence for a DNA-relay mechanism in ParABS-mediated chromosome segregation. Elife 3, e02758 ( 10.7554/eLife.02758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohl DA, Gober JW. 1997. Cell cycle-dependent polar localization of chromosome partitioning proteins in Caulobacter crescentus. Cell 88, 675–684. ( 10.1016/s0092-8674(00)81910-8) [DOI] [PubMed] [Google Scholar]
- 33.Hwang LC, Vecchiarelli AG, Han Y-W, Mizuuchi M, Harada Y, Funnell BE, Mizuuchi K. 2013. ParA-mediated plasmid partition driven by protein pattern self-organization. The EMBO Journal 32, 1238–1249. ( 10.1038/emboj.2013.34) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leonard TA, Butler PJ, Löwe J. 2005. Bacterial chromosome segregation: structure and DNA binding of the Soj dimer—a conserved biological switch. EMBO J. 24, 270–282. ( 10.1038/sj.emboj.7600530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vecchiarelli AG, Neuman KC, Mizuuchi K. 2014. A propagating ATPase gradient drives transport of surface-confined cellular cargo. PNAS 111, 4880–4885. ( 10.1073/pnas.1401025111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vecchiarelli AG, Hwang LC, Mizuuchi K. 2013. Cell-free study of F plasmid partition provides evidence for cargo transport by a diffusion-ratchet mechanism. Proc. Natl Acad. Sci. USA 110, E1390-E1397. ( 10.1073/pnas.1302745110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gall AL, et al. 2016. Bacterial partition complexes segregate within the volume of the nucleoid. Nat. Commun. 7, 1–10. ( 10.1038/ncomms12107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vecchiarelli AG, Han Y-W, Tan X, Mizuuchi M, Ghirlando R, Biertümpfel C, Funnell BE, Mizuuchi K. 2010. ATP control of dynamic P1 ParA–DNA interactions: a key role for the nucleoid in plasmid partition. Mol. Microbiol. 78, 78–91. ( 10.1111/j.1365-2958.2010.07314.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Badrinarayanan A, Le TBK, Laub MT.. 2015. Bacterial chromosome organization and segregation. Annu. Rev. Cell Dev. Biol. 31, 171–199. ( 10.1146/annurev-cellbio-100814-125211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gruber S, Errington J. 2009. Recruitment of condensin to replication origin regions by ParB/SpoOJ promotes chromosome segregation in B. subtilis. Cell 137, 685–696. ( 10.1016/j.cell.2009.02.035) [DOI] [PubMed] [Google Scholar]
- 41.Sullivan NL, Marquis KA, Rudner DZ. 2009. Recruitment of SMC by ParB-parS organizes the origin region and promotes efficient chromosome segregation. Cell 137, 697–707. ( 10.1016/j.cell.2009.04.044) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tran NT, Laub MT, Le TBK. 2017. SMC progressively aligns chromosomal arms in Caulobacter crescentus but is antagonized by convergent transcription. Cell Rep. 20, 2057–2071. ( 10.1016/j.celrep.2017.08.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, Le TBK, Lajoie BR, Dekker J, Laub MT, Rudner DZ. 2015. Condensin promotes the juxtaposition of DNA flanking its loading site in Bacillus subtilis. Genes Dev. 29, 1661–1675. ( 10.1101/gad.265876.115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Böhm K, Giacomelli G, Schmidt A, Imhof A, Koszul R, Marbouty M, Bramkamp M. 2020. Chromosome organization by a conserved condensin-ParB system in the actinobacterium Corynebacterium glutamicum. Nat. Commun. 11, 1485 ( 10.1038/s41467-020-15238-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Tang OW, Riley EP, Rudner DZ. 2014. The SMC condensin complex is required for origin segregation in Bacillus subtilis. Curr. Biol. 24, 287–292. ( 10.1016/j.cub.2013.11.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gruber S, Veening J-W, Bach J, Blettinger M, Bramkamp M, Errington J. 2014. Interlinked sister chromosomes arise in the absence of condensin during fast replication in B. subtilis. Curr. Biol. 24, 293–298. ( 10.1016/j.cub.2013.12.049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran NT, Stevenson CE, Som NF, Thanapipatsiri A, Jalal ASB, Le TBK. 2018. Permissive zones for the centromere-binding protein ParB on the Caulobacter crescentus chromosome. Nucleic Acids Res. 46, 1196–1209. ( 10.1093/nar/gkx1192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Breier AM, Grossman AD. 2007. Whole-genome analysis of the chromosome partitioning and sporulation protein Spo0 J (ParB) reveals spreading and origin-distal sites on the Bacillus subtilis chromosome. Mol. Microbiol. 64, 703–718. ( 10.1111/j.1365-2958.2007.05690.x) [DOI] [PubMed] [Google Scholar]
- 49.Song D, Rodrigues K, Graham TGW, Loparo JJ. 2017. A network of cis and trans interactions is required for ParB spreading. Nucleic Acids Res. 45, 7106–7117. ( 10.1093/nar/gkx271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kusiak M, Gapczyńska A, Płochocka D, Thomas CM, Jagura-Burdzy G. 2011. Binding and spreading of ParB on DNA determine its biological function in Pseudomonas aeruginosa. J. Bacteriol. 193, 3342–3355. ( 10.1128/JB.00328-11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Osorio-Valeriano M, Altegoer F, Steinchen W, Urban S, Liu Y, Bange G, Thanbichler M. 2019. ParB-type DNA segregation proteins are CTP-dependent molecular switches. Cell 179, 1512–1524. ( 10.1016/j.cell.2019.11.015) [DOI] [PubMed] [Google Scholar]
- 52.Soh Y-M, et al. 2019. Self-organization of parS centromeres by the ParB CTP hydrolase. Science 366, 1129–1133. ( 10.1126/science.aay3965) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisher GL, et al. 2017. The structural basis for dynamic DNA binding and bridging interactions which condense the bacterial centromere. Elife 6, e28086 ( 10.7554/eLife.28086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen B-W, Lin M-H, Chu C-H, Hsu C-E, Sun Y-J. 2015. Insights into ParB spreading from the complex structure of Spo0 J and parS. Proc. Natl. Acad. Sci. USA 112, 6613–6618. ( 10.1073/pnas.1421927112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jalal ASB, Pastrana CL, Tran NT, Stevenson CE, Lawson DM, Moreno-Herrero F, Le TBK. 2019. Structural and biochemical analyses of Caulobacter crescentus ParB reveal the role of its N-terminal domain in chromosome segregation. bioRxiv, 816959 ( 10.1101/816959) [DOI]
- 56.Leonard TA, Butler PJG, Löwe J. 2004. Structural analysis of the chromosome segregation protein Spo0 J from Thermus thermophilus. Mol. Microbiol. 53, 419–432. ( 10.1111/j.1365-2958.2004.04133.x) [DOI] [PubMed] [Google Scholar]
- 57.Schumacher MA, Piro KM, Xu W. 2010. Insight into F plasmid DNA segregation revealed by structures of SopB and SopB–DNA complexes. Nucleic Acids Res 38, 4514–4526. ( 10.1093/nar/gkq161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schumacher MA, Funnell BE. 2005. Structures of ParB bound to DNA reveal mechanism of partition complex formation. Nature 438, 516–519. ( 10.1038/nature04149) [DOI] [PubMed] [Google Scholar]
- 59.Lynch AS, Wang JC. 1995. SopB protein-mediated silencing of genes linked to the sopC locus of Escherichia coli F plasmid. PNAS 92, 1896–1900. ( 10.1073/pnas.92.6.1896) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rodionov O, Lobocka M, Yarmolinsky M. 1999. Silencing of genes flanking the P1 plasmid centromere. Science 283, 546–549. ( 10.1126/science.283.5401.546) [DOI] [PubMed] [Google Scholar]
- 61.Murray H, Ferreira H, Errington J. 2006. The bacterial chromosome segregation protein Spo0 J spreads along DNA from parS nucleation sites. Mol. Microbiol. 61, 1352–1361. ( 10.1111/j.1365-2958.2006.05316.x) [DOI] [PubMed] [Google Scholar]
- 62.Graham TGW, Wang X, Song D, Etson CM, van Oijen AM, Rudner DZ, Loparo JJ. 2014. ParB spreading requires DNA bridging. Genes Dev. 28, 1228–1238. ( 10.1101/gad.242206.114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawalek A, Bartosik AA, Glabski K, Jagura-Burdzy G. 2018. Pseudomonas aeruginosa partitioning protein ParB acts as a nucleoid-associated protein binding to multiple copies of a parS-related motif. Nucleic Acids Res. 46, 4592–4606. ( 10.1093/nar/gky257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bingle LEH, Macartney DP, Fantozzi A, Manzoor SE, Thomas CM. 2005. Flexibility in repression and cooperativity by KorB of broad host range IncP-1 plasmid RK2. J. Mol. Biol. 349, 302–316. ( 10.1016/j.jmb.2005.03.062) [DOI] [PubMed] [Google Scholar]
- 65.Taylor JA, Pastrana CL, Butterer A, Pernstich C, Gwynn EJ, Sobott F, Moreno-Herrero F, Dillingham MS. 2015. Specific and non-specific interactions of ParB with DNA: implications for chromosome segregation. Nucleic Acids Res. 43, 719–731. ( 10.1093/nar/gku1295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Madariaga-Marcos J, Pastrana CL, Fisher GL, Dillingham MS, Moreno-Herrero F. 2019. ParB dynamics and the critical role of the CTD in DNA condensation unveiled by combined force-fluorescence measurements. Elife 8, e43812 ( 10.7554/eLife.43812) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Broedersz CP, Wang X, Meir Y, Loparo JJ, Rudner DZ, Wingreen NS. 2014. Condensation and localization of the partitioning protein ParB on the bacterial chromosome. PNAS 111, 8809–8814. ( 10.1073/pnas.1402529111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Erdmann N, Petroff T, Funnell BE. 1999. Intracellular localization of P1 ParB protein depends on ParA and parS. Proc. Natl Acad. Sci. USA 96, 14 905–14 910. ( 10.1073/pnas.96.26.14905) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanchez A, Cattoni DI, Walter J-C, Rech J, Parmeggiani A, Nollmann M, Bouet J-Y. 2015. Stochastic self-assembly of ParB proteins builds the bacterial DNA segregation apparatus. Cell Syst. 1, 163–173. ( 10.1016/j.cels.2015.07.013) [DOI] [PubMed] [Google Scholar]
- 70.Debaugny RE, et al. 2018. A conserved mechanism drives partition complex assembly on bacterial chromosomes and plasmids. Mol. Syst. Biol. 14, e8516 ( 10.15252/msb.20188516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jalal AS, Tran NT, Le TB.. 2020. ParB spreading on DNA requires cytidine triphosphate in vitro. eLife 9, e53515 ( 10.7554/eLife.53515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Buckstein MH, He J, Rubin H. 2008. Characterization of nucleotide pools as a function of physiological state in Escherichia coli. J. Bacteriol. 190, 718–726. ( 10.1128/JB.01020-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Britos L, Abeliuk E, Taverner T, Lipton M, McAdams H, Shapiro L. 2011. Regulatory response to carbon starvation in Caulobacter crescentus. PLoS ONE 6, e18179 ( 10.1371/journal.pone.0018179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whiteley AT, et al. 2019. Bacterial cGAS-like enzymes synthesize diverse nucleotide signals. Nature 567, 194–199. ( 10.1038/s41586-019-0953-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Koonin EV. 1993. A superfamily of ATPases with diverse functions containing either classical or deviant ATP-binding motif. J. Mol. Biol. 229, 1165–1174. ( 10.1006/jmbi.1993.1115) [DOI] [PubMed] [Google Scholar]
- 76.Ptacin JL, Lee SF, Garner EC, Toro E, Eckart M, Comolli LR, Moerner WE, Shapiro L. 2010. A spindle-like apparatus guides bacterial chromosome segregation. Nat. Cell Biol. 12, 791–798. ( 10.1038/ncb2083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ebersbach G, Gerdes K. 2004. Bacterial mitosis: partitioning protein ParA oscillates in spiral-shaped structures and positions plasmids at mid-cell. Mol. Microbiol. 52, 385–398. ( 10.1111/j.1365-2958.2004.04002.x) [DOI] [PubMed] [Google Scholar]
- 78.Adachi S, Hori K, Hiraga S. 2006. Subcellular positioning of F plasmid mediated by dynamic localization of SopA and SopB. J. Mol. Biol. 356, 850–863. ( 10.1016/j.jmb.2005.11.088) [DOI] [PubMed] [Google Scholar]
- 79.Ebersbach G, Ringgaard S, Møller-Jensen J, Wang Q, Sherratt DJ, Gerdes K. 2006. Regular cellular distribution of plasmids by oscillating and filament-forming ParA ATPase of plasmid pB171. Mol. Microbiol. 61, 1428–1442. ( 10.1111/j.1365-2958.2006.05322.x) [DOI] [PubMed] [Google Scholar]
- 80.Fogel MA, Waldor MK. 2006. A dynamic, mitotic-like mechanism for bacterial chromosome segregation. Genes Dev. 20, 3269–3282. ( 10.1101/gad.1496506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hatano T, Yamaichi Y, Niki H. 2007. Oscillating focus of SopA associated with filamentous structure guides partitioning of F plasmid. Mol. Microbiol. 64, 1198–1213. ( 10.1111/j.1365-2958.2007.05728.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pratto F, Cicek A, Weihofen WA, Lurz R, Saenger W, Alonso JC. 2008. Streptococcus pyogenes pSM19035 requires dynamic assembly of ATP-bound ParA and ParB on parS DNA during plasmid segregation. Nucleic Acids Res. 36, 3676–3689. ( 10.1093/nar/gkn170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ringgaard S, van Zon J, Howard M, Gerdes K.. 2009. Movement and equipositioning of plasmids by ParA filament disassembly. Proc. Natl Acad. Sci. USA 106, 19 369–19 374. ( 10.1073/pnas.0908347106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gerdes K, Howard M, Szardenings F. 2010. Pushing and pulling in prokaryotic DNA segregation. Cell 141, 927–942. ( 10.1016/j.cell.2010.05.033) [DOI] [PubMed] [Google Scholar]
- 85.Barillà D, Rosenberg MF, Nobbmann U, Hayes F. 2005. Bacterial DNA segregation dynamics mediated by the polymerizing protein ParF. EMBO J. 24, 1453–1464. ( 10.1038/sj.emboj.7600619) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bouet J-Y, Ah-Seng Y, Benmeradi N, Lane D. 2007. Polymerization of SopA partition ATPase: regulation by DNA binding and SopB. Mol. Microbiol. 63, 468–481. ( 10.1111/j.1365-2958.2006.05537.x) [DOI] [PubMed] [Google Scholar]
- 87.Havey JC, Vecchiarelli AG, Funnell BE. 2012. ATP-regulated interactions between P1 ParA, ParB and non-specific DNA that are stabilized by the plasmid partition site, parS. Nucleic Acids Res. 40, 801–812. ( 10.1093/nar/gkr747) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dunham TD, Xu W, Funnell BE, Schumacher MA. 2009. Structural basis for ADP-mediated transcriptional regulation by P1 and P7 ParA. EMBO J. 28, 1792–1802. ( 10.1038/emboj.2009.120) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Machón C, Fothergill TJG, Barillà D, Hayes F. 2007. Promiscuous stimulation of ParF protein polymerization by heterogeneous centromere binding factors. J. Mol. Biol. 374, 1–8. ( 10.1016/j.jmb.2007.09.025) [DOI] [PubMed] [Google Scholar]
- 90.Lim GE, Derman AI, Pogliano J. 2005. Bacterial DNA segregation by dynamic SopA polymers. Proc. Natl Acad. Sci. USA 102, 17 658–17 663. ( 10.1073/pnas.0507222102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hui MP, Galkin VE, Yu X, Stasiak AZ, Stasiak A, Waldor MK, Egelman EH. 2010. ParA2, a Vibrio cholerae chromosome partitioning protein, forms left-handed helical filaments on DNA. Proc. Nat. Acad. Sci. USA 107, 4590–4595. ( 10.1073/pnas.0913060107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vecchiarelli AG, Mizuuchi K, Funnell BE. 2012. Surfing biological surfaces: exploiting the nucleoid for partition and transport in bacteria. Mol. Microbiol. 86, 513–523. ( 10.1111/mmi.12017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hu L, Vecchiarelli AG, Mizuuchi K, Neuman KC, Liu J. 2017. Brownian ratchet mechanisms of ParA-mediated partitioning. Plasmid 92, 12–16. ( 10.1016/j.plasmid.2017.05.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang H, Schumacher MA. 2017. Structures of partition protein ParA with nonspecific DNA and ParB effector reveal molecular insights into principles governing Walker-box DNA segregation. Genes Dev. 31, 481–492. ( 10.1101/gad.296319.117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Corrales-Guerrero L, He B, Refes Y, Panis G, Bange G, Viollier PH, Steinchen W, Thanbichler M. 2020. Molecular architecture of the DNA-binding sites of the P-loop ATPases MipZ and ParA from Caulobacter crescentus. Nucleic Acids Res. 48, 4769–4779. ( 10.1093/nar/gkaa192) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chu C-H, Yen C-Y, Chen B-W, Lin M-G, Wang L-H, Tang K-Z, Hsiao C-D, Sun Y-J. 2019. Crystal structures of HpSoj–DNA complexes and the nucleoid-adaptor complex formation in chromosome segregation. Nucleic Acids Res. 47, 2113–2129. ( 10.1093/nar/gky1251) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Barillà D, Carmelo E, Hayes F. 2007. The tail of the ParG DNA segregation protein remodels ParF polymers and enhances ATP hydrolysis via an arginine finger-like motif. Proc. Natl Acad. Sci. USA 104, 1811–1816. ( 10.1073/pnas.0607216104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Volante A, Alonso JC. 2015. Molecular anatomy of ParA-ParA and ParA-ParB interactions during plasmid partitioning. J. Biol. Chem. 290, 18 782–18 795. ( 10.1074/jbc.M115.649632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ptacin JL, Gahlmann A, Bowman GR, Perez AM, von Diezmann ARS, Eckart MR, Moerner WE, Shapiro L. 2014. Bacterial scaffold directs pole-specific centromere segregation. PNAS 111, E2046–E2055. ( 10.1073/pnas.1405188111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schofield WB, Lim HC, Jacobs-Wagner C. 2010. Cell cycle coordination and regulation of bacterial chromosome segregation dynamics by polarly localized proteins. EMBO J. 29, 3068–3081. ( 10.1038/emboj.2010.207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marbouty M, et al. 2015. Condensin- and replication-mediated bacterial chromosome folding and origin condensation revealed by Hi-C and super-resolution imaging. Mol. Cell 59, 588–602. ( 10.1016/j.molcel.2015.07.020) [DOI] [PubMed] [Google Scholar]
- 102.Ah-Seng Y, Rech J, Lane D, Bouet J-Y. 2013. Defining the role of ATP hydrolysis in mitotic segregation of bacterial plasmids. PLoS Genet. 9, e1003956 ( 10.1371/journal.pgen.1003956) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Attaiech L, Minnen A, Kjos M, Gruber S, Veening J-W. 2015. The ParB-parS chromosome segregation system modulates competence development in Streptococcus pneumoniae. mBio 6, e00662-15 ( 10.1128/mBio.00662-15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murray H, Errington J. 2008. Dynamic control of the DNA replication initiation protein DnaA by Soj/ParA. Cell 135, 74–84. ( 10.1016/j.cell.2008.07.044) [DOI] [PubMed] [Google Scholar]
- 105.Pióro M, Małecki T, Portas M, Magierowska I, Trojanowski D, Sherratt D, Zakrzewska-Czerwińska J, Ginda K, Jakimowicz D. 2019. Competition between DivIVA and the nucleoid for ParA binding promotes segrosome separation and modulates mycobacterial cell elongation. Mol. Microbiol. 111, 204–220. ( 10.1111/mmi.14149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nourikyan J, et al. 2015. Autophosphorylation of the bacterial tyrosine-kinase CpsD connects capsule synthesis with the cell cycle in Streptococcus pneumoniae. PLoS Genet. 11, e1005518 ( 10.1371/journal.pgen.1005518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mercy C, et al. 2019. RocS drives chromosome segregation and nucleoid protection in Streptococcus pneumoniae. Nat. Microbiol. 4, 1661–1670. ( 10.1038/s41564-019-0472-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Szafran M, Skut P, Ditkowski B, Ginda K, Chandra G, Zakrzewska-Czerwińska J, Jakimowicz D. 2013. Topoisomerase I (TopA) is recruited to ParB complexes and is required for proper chromosome organization during Streptomyces coelicolor sporulation. J. Bacteriol. 195, 4445–4455. ( 10.1128/JB.00798-13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Thanbichler M, Shapiro L. 2006. MipZ, a spatial regulator coordinating chromosome segregation with cell division in Caulobacter. Cell 126, 147–162. ( 10.1016/j.cell.2006.05.038) [DOI] [PubMed] [Google Scholar]
- 110.Maurya GK, Modi K, Misra HS. 2016. Divisome and segrosome components of Deinococcus radiodurans interact through cell division regulatory proteins. Microbiology (Reading, Engl.) 162, 1321–1334. ( 10.1099/mic.0.000330) [DOI] [PubMed] [Google Scholar]
- 111.Yamaichi Y, Bruckner R, Ringgaard S, Möll A, Cameron DE, Briegel A, Jensen GJ, Davis BM, Waldor MK. 2012. A multidomain hub anchors the chromosome segregation and chemotactic machinery to the bacterial pole. Genes Dev. 26, 2348–2360. ( 10.1101/gad.199869.112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dubarry N, Willis CR, Ball G, Lesterlin C, Armitage JP. 2019. In vivo imaging of the segregation of the 2 chromosomes and the cell division proteins of Rhodobacter sphaeroides reveals an unexpected role for MipZ. mBio 10, e02515-18 ( 10.1128/mBio.02515-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Toro-Nahuelpan M, Corrales-Guerrero L, Zwiener T, Osorio-Valeriano M, Müller F-D, Plitzko JM, Bramkamp M, Thanbichler M, Schüler D. 2019. A gradient-forming MipZ protein mediating the control of cell division in the magnetotactic bacterium Magnetospirillum gryphiswaldense. Mol. Microbiol. 112, 1423–1439. ( 10.1111/mmi.14369) [DOI] [PubMed] [Google Scholar]
- 114.Kawalek A, Wawrzyniak P, Bartosik AA, Jagura-Burdzy G. 2020. Rules and exceptions: the role of chromosomal ParB in DNA segregation and other cellular processes. Microorganisms 8, 0. ( 10.3390/microorganisms8010105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pióro M, Jakimowicz D. 2020. Chromosome segregation proteins as coordinators of cell cycle in response to environmental conditions. Front. Microbiol. 11, 588 ( 10.3389/fmicb.2020.00588) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nolivos S, Sherratt D. 2014. The bacterial chromosome: architecture and action of bacterial SMC and SMC-like complexes. FEMS Microbiol. Rev. 38, 380–392. ( 10.1111/1574-6976.12045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mascarenhas J, Soppa J, Strunnikov AV, Graumann PL. 2002. Cell cycle-dependent localization of two novel prokaryotic chromosome segregation and condensation proteins in Bacillus subtilis that interact with SMC protein. EMBO J. 21, 3108–3118. ( 10.1093/emboj/cdf314) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Soppa J, Kobayashi K, Noirot-Gros M-F, Oesterhelt D, Ehrlich SD, Dervyn E, Ogasawara N, Moriya S. 2002. Discovery of two novel families of proteins that are proposed to interact with prokaryotic SMC proteins, and characterization of the Bacillus subtilis family members ScpA and ScpB. Mol. Microbiol. 45, 59–71. ( 10.1046/j.1365-2958.2002.03012.x) [DOI] [PubMed] [Google Scholar]
- 119.Wilhelm L, Bürmann F, Minnen A, Shin H-C, Toseland CP, Oh B-H, Gruber S. 2015. SMC condensin entraps chromosomal DNA by an ATP hydrolysis dependent loading mechanism in Bacillus subtilis. eLife 4, e06659 ( 10.7554/eLife.06659) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cuylen S, Metz J, Haering CH. 2011. Condensin structures chromosomal DNA through topological links. Nat. Struct. Mol. Biol. 18, 894–901. ( 10.1038/nsmb.2087) [DOI] [PubMed] [Google Scholar]
- 121.Haering CH, Farcas A-M, Arumugam P, Metson J, Nasmyth K. 2008. The cohesin ring concatenates sister DNA molecules. Nature 454, 297–301. ( 10.1038/nature07098) [DOI] [PubMed] [Google Scholar]
- 122.Murayama Y, Uhlmann F. 2014. Biochemical reconstitution of topological DNA binding by the cohesin ring. Nature 505, 367–371. ( 10.1038/nature12867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kanno T, Berta DG, Sjögren C. 2015. The Smc5/6 complex is an ATP-dependent intermolecular DNA linker. Cell Rep 12, 1471–1482. ( 10.1016/j.celrep.2015.07.048) [DOI] [PubMed] [Google Scholar]
- 124.Le TB, Imakaev MV, Mirny LA, Laub MT.. 2013. High-resolution mapping of the spatial organization of a bacterial chromosome. Science 342, 731–734. ( 10.1126/science.1242059) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang X, Brandão HB, Le TBK, Laub MT, Rudner DZ.. 2017. Bacillus subtilis SMC complexes juxtapose chromosome arms as they travel from origin to terminus. Science 355, 524–527. ( 10.1126/science.aai8982) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Val M-E, et al. 2016. A checkpoint control orchestrates the replication of the two chromosomes of Vibrio cholerae. Sci. Adv. 2, e1501914 ( 10.1126/sciadv.1501914) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shebelut CW, Guberman JM, van Teeffelen S, Yakhnina AA, Gitai Z.. 2010. Caulobacter chromosome segregation is an ordered multistep process. Proc. Natl Acad. Sci. USA 107, 14 194–14 198. ( 10.1073/pnas.1005274107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Taylor JA, Panis G, Viollier PH, Marczynski GT. 2017. A novel nucleoid-associated protein coordinates chromosome replication and chromosome partition. Nucleic Acids Res. 45, 8916–8929. ( 10.1093/nar/gkx596) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yatskevich S, Rhodes J, Nasmyth K. 2019. Organization of chromosomal DNA by SMC complexes. Annu. Rev. Genet. 53, 445–482. ( 10.1146/annurev-genet-112618-043633) [DOI] [PubMed] [Google Scholar]
- 130.Minnen A, Bürmann F, Wilhelm L, Anchimiuk A, Diebold-Durand M-L, Gruber S. 2016. Control of SMC coiled coil architecture by the ATPase heads facilitates targeting to chromosomal ParB/parS and Release onto Flanking DNA. Cell Reports 14, 2003–2016. ( 10.1016/j.celrep.2016.01.066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fekete RA, Chattoraj DK. 2005. A cis-acting sequence involved in chromosome segregation in Escherichia coli. Mol. Microbiol. 55, 175–183. ( 10.1111/j.1365-2958.2004.04392.x) [DOI] [PubMed] [Google Scholar]
- 132.Wang X, Sherratt DJ. 2010. Independent segregation of the two arms of the Escherichia coli ori region requires neither RNA synthesis nor MreB dynamics. J. Bacteriol. 192, 6143–6153. ( 10.1128/JB.00861-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yamaichi Y, Niki H. 2004. migS, a cis-acting site that affects bipolar positioning of oriC on the Escherichia coli chromosome. EMBO J. 23, 221–233. ( 10.1038/sj.emboj.7600028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jakimowicz D, Chater K, Zakrzewska-Czerwínska J. 2002. The ParB protein of Streptomyces coelicolor A3(2) recognizes a cluster of parS sequences within the origin-proximal region of the linear chromosome. Mol. Microbiol. 45, 1365–1377. ( 10.1046/j.1365-2958.2002.03102.x) [DOI] [PubMed] [Google Scholar]
- 135.Sievers J, Raether B, Perego M, Errington J. 2002. Characterization of the parB-Like yyaA Gene of Bacillus subtilis. J. Bacteriol. 184, 1102–1111. ( 10.1128/jb.184.4.1102-1111.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu LJ, Errington J. 2004. Coordination of cell division and chromosome segregation by a nucleoid occlusion protein in Bacillus subtilis. Cell 117, 915–925. ( 10.1016/j.cell.2004.06.002) [DOI] [PubMed] [Google Scholar]
- 137.Wu LJ, Errington J. 2011. Nucleoid occlusion and bacterial cell division. Nat. Rev. Microbiol. 10, 8–12. ( 10.1038/nrmicro2671) [DOI] [PubMed] [Google Scholar]
- 138.Schumacher MA. 2017. Bacterial nucleoid occlusion: multiple mechanisms for preventing chromosome bisection during cell division. Subcell. Biochem. 84, 267–298. ( 10.1007/978-3-319-53047-5_9) [DOI] [PubMed] [Google Scholar]
- 139.Pang T, Wang X, Lim HC, Bernhardt TG, Rudner DZ. 2017. The nucleoid occlusion factor Noc controls DNA replication initiation in Staphylococcus aureus. PLoS Genetics 13, e1006908 ( 10.1371/journal.pgen.1006908) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu LJ, Ishikawa S, Kawai Y, Oshima T, Ogasawara N, Errington J. 2009. Noc protein binds to specific DNA sequences to coordinate cell division with chromosome segregation. EMBO J. 28, 1940–1952. ( 10.1038/emboj.2009.144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Adams DW, Wu LJ, Errington J. 2015. Nucleoid occlusion protein Noc recruits DNA to the bacterial cell membrane. EMBO J. 34, 491–501. ( 10.15252/embj.201490177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lundin M, Monné M, Widell A, von Heijne G, Persson MAA. 2003. Topology of the membrane-associated hepatitis C virus protein NS4B. J. Virol. 77, 5428–5438. ( 10.1128/JVI.77.9.5428-5438.2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jalal ASB, Tran NT, Stevenson CE, Tan X, Lawson DM, Le TBK. 2019. Evolving a new protein-DNA interface via sequential introduction of permissive and specificity-switching mutations. bioRxiv, 724823 ( 10.1101/724823) [DOI]
- 144.Roberts MAJ, Wadhams GH, Hadfield KA, Tickner S, Armitage JP. 2012. ParA-like protein uses nonspecific chromosomal DNA binding to partition protein complexes. Proc. Natl Acad. Sci. USA 109, 6698–6703. ( 10.1073/pnas.1114000109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kiekebusch D, Michie KA, Essen L-O, Löwe J, Thanbichler M. 2012. Localized dimerization and nucleoid binding drive gradient formation by the bacterial cell division inhibitor MipZ. Mol. Cell 46, 245–259. ( 10.1016/j.molcel.2012.03.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Schumacher MA, et al. 2015. Structures of archaeal DNA segregation machinery reveal bacterial and eukaryotic linkages. Science 349, 1120–1124. ( 10.1126/science.aaa9046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Barillà D. 2016. Driving apart and segregating genomes in Archaea. Trends Microbiol. 24, 957–967. ( 10.1016/j.tim.2016.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Le TB, Laub MT.. 2014. New approaches to understanding the spatial organization of bacterial genomes. Current Opin. Microbiol. 22, 15–21. ( 10.1016/j.mib.2014.09.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mariamé B, Kappler-Gratias S, Kappler M, Balor S, Gallardo F, Bystricky K. 2018. Real-time visualization and quantification of human cytomegalovirus replication in living cells using the ANCHOR DNA labeling technology. J. Virol. 92, e00571-18. ( 10.1128/JVI.00571-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Germier T, et al. 2017. Real-time imaging of a single gene reveals transcription-initiated local confinement. Biophys. J. 113, 1383–1394. ( 10.1016/j.bpj.2017.08.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Germier T, Audibert S, Kocanova S, Lane D, Bystricky K. 2018. Real-time imaging of specific genomic loci in eukaryotic cells using the ANCHOR DNA labelling system. Methods 142, 16–23. ( 10.1016/j.ymeth.2018.04.008) [DOI] [PubMed] [Google Scholar]
- 152.Molinari S, Shis DL, Bhakta SP, Chappell J, Igoshin OA, Bennett MR. 2019. A synthetic system for asymmetric cell division in Escherichia coli. Nat. Chem. Biol. 15, 917–924. ( 10.1038/s41589-019-0339-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mushnikov NV, Fomicheva A, Gomelsky M, Bowman GR. 2019. Inducible asymmetric cell division and cell differentiation in a bacterium. Nat. Chem. Biol. 15, 925–931. ( 10.1038/s41589-019-0340-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article does not contain any additional data.