Abstract
The half-billion-year history of animal evolution is characterized by decreasing rates of background extinction. Earth's increasing habitability for animals could result from several processes: (i) a decrease in the intensity of interactions among species that lead to extinctions; (ii) a decrease in the prevalence or intensity of geological triggers such as flood basalt eruptions and bolide impacts; (iii) a decrease in the sensitivity of animals to environmental disturbance; or (iv) an increase in the strength of stabilizing feedbacks within the climate system and biogeochemical cycles. There is no evidence that the prevalence or intensity of interactions among species or geological extinction triggers have decreased over time. There is, however, evidence from palaeontology, geochemistry and comparative physiology that animals have become more resilient to an environmental change and that the evolution of complex life has, on the whole, strengthened stabilizing feedbacks in the climate system. The differential success of certain phyla and classes appears to result, at least in part, from the anatomical solutions to the evolution of macroscopic size that were arrived at largely during Ediacaran and Cambrian time. Larger-bodied animals, enabled by increased anatomical complexity, were increasingly able to mix the marine sediment and water columns, thus promoting stability in biogeochemical cycles. In addition, body plans that also facilitated ecological differentiation have tended to be associated with lower rates of extinction. In this sense, Cambrian solutions to Cambrian problems have had a lasting impact on the trajectory of complex life and, in turn, fundamental properties of the Earth system.
Keywords: evolution, animals, biodiversity
1. Introduction
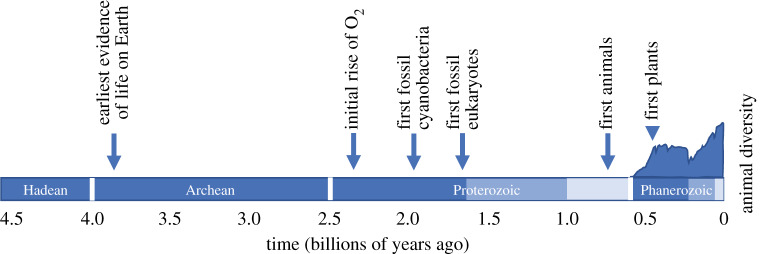
Animals are late arrivals to Earth, having originated nearly 4 billion years after the formation of the planet and at least 3 billion years after the origin of life (figure 1). Part of this delay results from the numerous evolutionary innovations that are required for the development of the eukaryotic cell and complex multicellularity [2,3], but geochemical evidence also indicates that, for the vast majority of Precambrian time (4.56–0.54 billion years ago), Earth's surface environments were not permissive to large, active, aerobic organisms (e.g. [4–6]). The evolution of animals appears to have occurred (geologically) soon after their survival was environmentally possible.
Figure 1.
Time scale for the history of the Earth, with key events in the history of life. Diversity of animal life (marine animal families) plotted on the vertical axis (from [1]). Colour differences in the Proterozoic and Phanerozoic bars indicate era boundaries within the eons. Figure inspired by an unpublished figure belonging to W. Fischer.
The Phanerozoic (542 Ma–present) fossil record provides numerous lines of evidence that the Precambrian/Cambrian boundary is not a simple threshold transition from a world uninhabitable to animals to one that is and has remained habitable. Rather, there is much evidence that Earth's surface environments have become increasingly habitable to animals across Phanerozoic time. Extinction rates have declined in the oceans and on land (figure 2a; [1,11]). Even the mass extinction events that eliminated large fractions of genus- and species-level diversity have caused comparatively little extinction above the level of family [12]. Animals have increased in size (figure 2b; [7]), activity levels [13], ecological complexity and differentiation (figure 2c; [8,14,15]), and, most likely, abundance [16,17] across time. The geological and geochemical records are also consistent with a trend towards increasing habitability of surface environments. The surface ocean has become increasingly oxygenated, as indicated by the greater abundance of iodine in the form of iodate incorporated into carbonate shells and sediments (figure 2d; [9]). The prevalence of unfossiliferous black shales in the marine sedimentary record has similarly declined, suggesting a reduction in the prevalence of anoxic bottom waters [18]. The magnitude of excursions in the carbon isotope record has declined systematically across the Phanerozoic (figure 2e), as confirmed by spectral decomposition ([19]: figure 2c therein). This indicates the long-term stabilization of the geological carbon cycle, a primary long-term control on Earth's climate.
Figure 2.
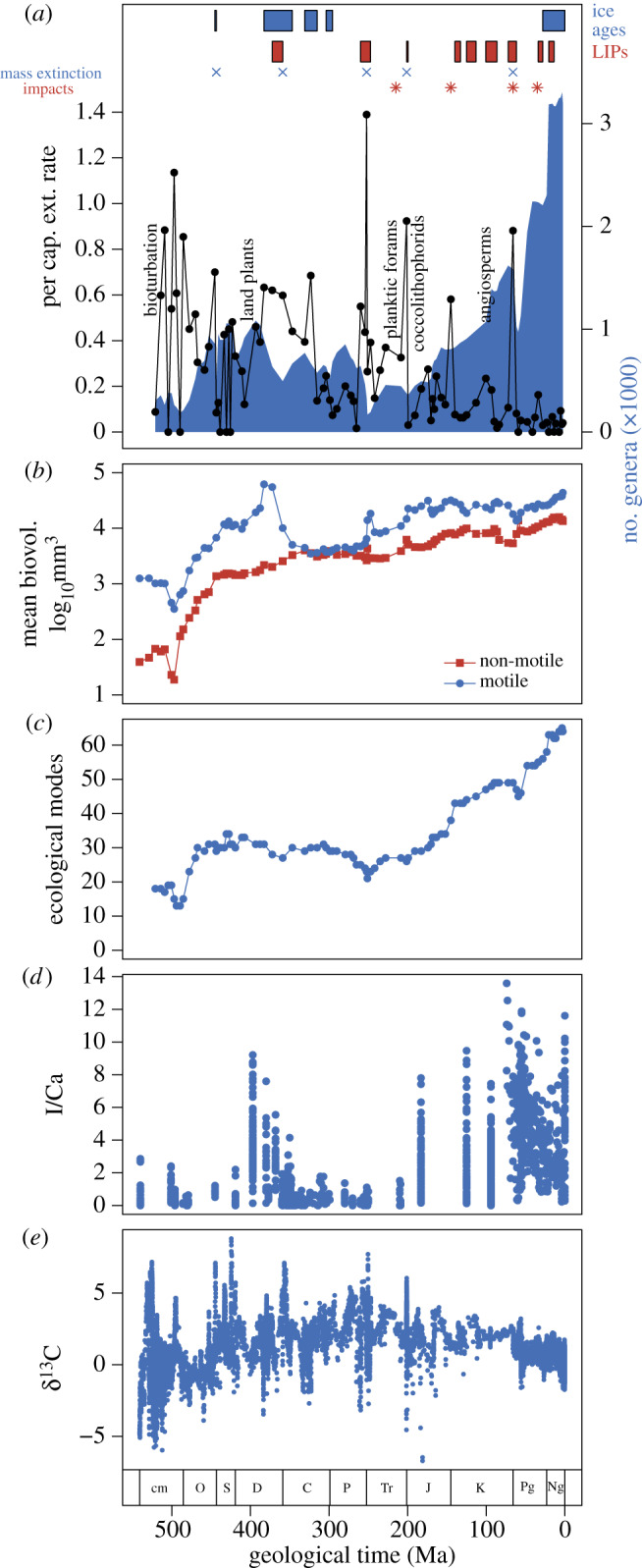
Phanerozoic records from the fossil record and geochemistry of marine sediments. (a) Per capita extinction rate, illustrating a long-term decline in the background extinction rate punctuated by mass extinction events (data from The Paleobiology Database). Also plotted are the timing of major ice ages, eruptions of large igneous provinces and the largest known impact events. (b) Mean (log-transformed) body size of marine animals, illustrating a long-term increase in mean body volume totals more than two orders of magnitude (replotted from [7]). (c) Total number of ecological modes occupied by marine animals, illustrating the long-term increase in the number of modes of life present in the oceans (replotted from [8]). (d) I/Ca ratio of marine sedimentary carbonates (replotted from [9]), illustrating a progressive increase in I/Ca of marine carbonates, implying an increase in oxygen concentrations in typical ocean surface waters. (e) Carbon isotope composition of marine carbonate sediments (replotted from [10]), illustrating a decrease in the magnitude of carbon isotope variation between the Cambrian and the present day.
If creating an Earth habitable to animals took so long, why has animal life in its entirety, and individual animal phyla and classes, been so persistent over the past half-billion years? And why has the habitability of Earth appeared to increase not only in terms of declining extinction rates [1] but also in terms of decreasing volatility in biogeochemical cycles (e.g. [19])? There are four broad mechanisms, not mutually exclusive, that could account for the decline in extinction rates and the associated evidence of increasing habitability: (i) ecological interactions among animals that lead to extinction have become less common across time; (ii) the geological triggers of extinction have decreased in frequency and/or intensity; (iii) animals have become more tolerant of environmental stress; or (iv) stabilizing feedbacks in the climate system have increased in strength across time, reducing the environmental stresses on animals associated with the same geological triggers.
2. Potential mechanisms
2.1. Ecological interactions
Interactions among species, directly or indirectly, can increase, decrease or leave the probability of extinction unchanged, depending on which process dominates. Darwin, in his famous ‘tangled bank’ metaphor [20], hypothesized that much extinction occurs gradually through the displacement of one species by another as they compete for resources. Under this scenario, one potential explanation for the decline in extinction rates across geological time is that antagonistic interactions among species have decreased in intensity across geological time. This scenario would run counter to the Red Queen hypothesis [21], which suggests that escalating antagonistic interactions among species leads to a changing selective landscape and time-constant risk of extinction, at least within a group of competing species.
The argument that competition for shared limiting resources is a strong driver of both diversification and extinction is supported by laboratory and field studies across a wide array of living species (e.g. [22–26]). While it is more difficult to identify competition in the fossil record, recent studies suggest that bivalves directly competed with brachiopods, contributing to the brachiopods' long-term decrease in ecological importance [27]. From a broader perspective, post-Palaeozoic (less than 252 Ma) taxonomic diversification of marine animals as a whole has been accomplished largely through increasingly even filling of both existing and newly evolved ecological modes of life (the combination of habitat tiering, motility and feeding mode niche axes; [28]), rather than a continued taxonomic diversity increase within already well-filled ecological modes of life [8], which suggests increased competitive interactions over time among taxa that fill broadly similar functional ecologies. While the terrestrial tetrapod fossil record similarly does not provide direct evidence for competition, and taxonomic expansion is best explained by unrestricted access to new ecospace [29], there is evidence for evolutionary priority effects where established groups within ecological modes can exclude competitors until the incumbents are removed, often by a major biotic crisis [29,30].
The fossil record also indicates that predator–prey interactions have increased in prevalence across evolutionary time. In the oceans, there have been long-term increases in the proportion of taxonomic diversity represented by predators [13], the prevalence of predatory shell drilling and shell crushing [31,32], the diversity of taxa capable of these predation styles and the frequency of anti-predatory morphologies in prey taxa, such as narrowed and reinforced apertures on the shells of gastropods as well as spines, ridges and knobs on the shells of bivalves and gastropods [33–35]. Similarly, the activity levels and behavioural sophistication of predators on land have generally increased, albeit not monotonically, from the Devonian to the present day, with dinosaurs, birds and mammals succeeding other vertebrate groups as the most diverse and ecologically important terrestrial predators. Thus, the available evidence of competitive interactions and predator–prey relationships runs counter to their predicted effect on extinction rates. That is, both competition and predation appear to have increased through time, yet extinction rates have declined.
Increased ecological interactions are also hypothesized to decrease extinction risk when positive interactions among species dominate or when diversity increases the stability or resilience of ecosystems. In the evolution of plant and animal ecosystems, there is also evidence that evolutionary innovations lead to the expansion of resources that open new ecological opportunities and increase the total carrying capacity of the system, such as the production of oxygen by cyanobacteria [36] and the enhanced recycling of nutrients by migrating animals [37]. Similarly, clear fossil evidence for mycorrhizal fungal associations with land plants date to the Devonian [38,39] and appear to be tightly linked to the taxonomic success of plants with the majority of described species believed to have active mutualistic fungal partners [40–42]. Further, coral reefs are now recognized to be composed of coral holobionts [43], with each polyp generally consisting of the cnidarian animal, a mutualistic photosynthetic symbiodinium dinoflagellate and an often diverse microbial community (e.g. [44]). The diversification and proliferation of corals since their origins in the Cambrian has led to some of the most biodiverse marine ecosystems in modern oceans [45]. Moreover, ecosystems with greater functional differentiation and interaction among functional groups tend to be more resilient to perturbation [46], generally display higher productivity [47,48] and can be more stable [49]. The fossil record indicates that marine communities have increased in the ecological complexity across time [14,15,28], particularly across the Permian–Triassic transition, 252 Ma [50]. Higher taxa that contribute most to the ecological complexity and taxonomic diversity are those that are most resistant to extinction [51]. The observation of declining extinction rates and increases in the taxonomic and functional diversity of ecosystems through the Phanerozoic is consistent with the hypothesis that increased ecological interactions have, in aggregate, decreased extinction probabilities through time.
2.2. Geological triggers
The processes most commonly hypothesized as triggers of extinction across geological time are bolide impact (e.g. [52,53]), flood basalt volcanism (e.g. [54,55]) and climate change (e.g. [56–59]). Many other potential triggers have also been put forward, some constituting potential downstream consequences of the aforementioned triggers; these include (but are not limited to) hydrogen sulfide build-up in the ocean and release to the atmosphere [60], ultraviolet radiation, potentially caused by the collapse of the ozone layer (e.g. [61]) and poisoning from heavy metals (e.g. [62]). Each of these triggers is thought to act by altering surface environments in ways that exceed the physiological tolerances of at least some species, often through changes in temperature or the availability of light or oxygen. Additional extinctions may occur through ecological processes, such as starvation following collapse of primary productivity [63,64]. The time distribution of each of these processes has become increasingly known over the past several decades.
There is no evidence that the terrestrial cratering rate has decreased across Phanerozoic time. If anything, there is evidence from both lunar and terrestrial data that cratering rates have increased by as much as 40% between the Proterozoic and Phanerozoic [65,66], and cratering data for the past 120 Myr do not indicate any decline in the cratering rate relative to the Phanerozoic as a whole [66]. Consequently, the hypothesis that all Phanerozoic mass extinctions, or perhaps even all extinctions, could have been triggered by impact events [52,53,67] is inconsistent with current data, at least under the assumption that the global biota has maintained a similar sensitivity to impact and therefore would be expected to follow a constant ‘kill curve’ mapping impactor size to extinction intensity. Furthermore, there is little evidence that major impact events correspond in age with major mass extinction events other than the end-Cretaceous. The largest known impact craters of Phanerozoic age, other than Chicxulub, do not correspond in age to major extinction events (Popigai Crater, 35 Ma; Manicouagan Crater, 214 Ma; [68]).
In contrast to impact, there is substantial evidence for flood basalt eruption coincident with many major and moderate extinction events across Phanerozoic time [54,55]. Similar to the impact record, however, the flood basalt record does not provide evidence for a decrease in frequency or magnitude across Phanerozoic time. There may be periodicity in the emplacement of flood basalts with a period of 62–65 Myr [69], similar in duration to cyclicity in biodiversity [70]. Even if flood basalt eruptions are triggers for many Phanerozoic extinctions, however, there is not a close correspondence between the volume of the basalt province and the magnitude of resulting extinction, at least in part because interactions between the basaltic magma and the intruded country rocks of the crust can substantially influence the magnitude, composition and rates of volatile release [55,71]. Furthermore, there is no decline in either the magnitude or frequency of flood basalt eruption that could account for the systematic decline in extinction rates across the Phanerozoic.
Earth has cycled between generally cold and generally warm climates across the past billion years. Glacial intervals associated with large continental ice sheets have occurred during Neoproterozoic, Ordovician, Carboniferous–Permian and Cenozoic time. Climate cooling has, furthermore, been hypothesized as a unifying cause of mass extinction [57]. Indeed, Late Ordovician glaciation is associated with mass extinction [17,72–74]. By contrast, the later Palaeozoic ice age is generally associated with lower rates of extinction [75], and the Cenozoic icehouse is not associated with any major extinctions prior to the origin and geographical spread of Homo sapiens [76,77]. There is no evidence that the secular decline in extinction rates can be accounted for simply by an increase or decrease in the prevalence of cold climate and continental ice sheets.
2.3. Animal physiology
A final potential factor in the decline of animal extinction rates across geological time is an increase in the tolerance of animals themselves to an environmental change. If animals have evolved more effective methods for tolerating the environmental change through behavioural or physiological adaptations, then one may expect lower rates of extinction across time even in the face of environmental stresses of similar magnitudes. One argument in favour of this scenario is that the geological record suggests that extinctions are caused by a limited range of environmental factors, primarily changes in climate, sunlight availability, oxygen availability and ocean pH. Therefore, one might expect that early extinctions would selectively remove lineages that lacked the capacity to survive such changes or evolve the necessary adaptations to endure them.
Many modern pelagic animals indeed have adaptations that allow them to survive in hypoxic or anoxic conditions, including the increased efficiency of O2 removal from seawater, the reduction of metabolic rates and the use of both aerobic and anaerobic metabolism, particularly for animals that can retreat to more oxygen-rich waters [78]. Of course, it is not possible to directly quantify metabolic rates in extinct animals. It is possible, however, to infer basic physiological traits for most fossil animals based on preserved skeletal morphology and analogy with living relatives. One useful proxy for overall metabolic efficiency, particularly in relation to environmental stress, is physiological buffering [79]. Physiologically buffered animals are those that can regulate their internal body chemistry and are thus more resistant to fluctuating environmental conditions, particularly O2 levels and pH. By contrast, unbuffered species are largely in equilibrium with seawater and are unlikely to persist for long periods of time in unfavourable conditions. A study of the end-Permian mass extinction demonstrated that surviving taxa tended to be highly buffered while most of the victims were poorly buffered [79]. Not only was physiological buffering an important factor during the end-Permian mass extinction, but it was also an important factor in selective extinctions throughout the Palaeozoic and Mesozoic [80]. However, extinction during the Cretaceous and Cenozoic does not appear to be selective with respect to physiological buffering [80], suggesting that the primary causes of extinction may have shifted across time. Additionally, animals that are both motile and physiologically buffered are at lower risk of extinction across the Phanerozoic as a whole [51], indicating that being able to move and having more control over internal body chemistry probably make species more resistant to common environmental stressors during extinction events, such as ocean warming and acidification.
2.4. Stabilizing feedbacks
The long-term habitability of Earth depends on the operation of stabilizing feedbacks that prevent the Earth's surface environments from shifting into states that are either uninhabitable or poorly habitable to animals. The two most important parameters for overall habitability are likely to be climate, especially surface temperatures, and oxygen availability in the atmosphere and oceans.
There is evidence in the Precambrian rock record for potentially extreme and persistent cooling associated with glaciation (e.g. [81]), but such ‘Snowball Earth’ glaciations have not occurred since the start of Cambrian time. The primary stabilizing feedback on Earth's climate across geological time is the silicate weathering feedback, by which chemical reactions between carbon dioxide and silicate minerals lead to the removal of CO2 from the atmosphere and its ultimate deposition as a carbonate sediment (e.g. [82,83]). The rate of the reaction is a function of the CO2 concentration in the atmosphere, the ambient temperature and the reactivity of the exposed bedrock. Increasing global temperature results in the acceleration of weathering reactions and an increased drawdown of CO2, thus stabilizing the climate of the planet. Similar reactions take place in off-axis hydrothermal vents near mid-ocean spreading ridges where warm water cycles through fresh basalt, resulting in the precipitation of carbonate in veins and pore spaces [84]. As global climate influences the temperature of ocean bottom water, hydrothermal alteration is an additional temperature-dependent negative feedback on Earth's climate.
The efficiency of this silicate weathering feedback has been widely hypothesized to have increased across Phanerozoic time. The evolution of land plants, in particular, is argued to have increased the efficiency of silicate weathering by causing more weathering to occur at lower levels of atmospheric CO2. In other words, plants are argued to have created a steeper relationship between pCO2 and weathering rate, leading to a stronger negative feedback on changes in pCO2 [85]. Specifically, plants affect chemical weathering in two ways: (i) by increasing the ambient pCO2 within soils and (ii) by increasing the transport of moisture into continental interiors [86]. Combining vapour transport and reactive transport models, Ibarra et al. [86] show that these two factors are subequal in magnitude. Their model suggests that the strengthening of the silicate weathering feedback through the evolution of land plants occurred in two major stages, the first (and most important) during Devonian time, when vascular plants first evolved, and the second during the Late Cretaceous and Cenozoic, when angiosperms diversified and increased in abundance. The evolution of plants was important not only for increasing the strength of the silicate weathering feedback but also for causing the strength of the feedback to become more stable over time. As plants caused weathering reactions to occur more efficiently, the relative influences of other factors, such as topographic relief, exposed lithologies across the land surface, climate, surface temperature and rainwater pH, are likely to have become less important.
Further feedback mechanisms may act to stabilize climate on shorter time scales. For example, the ocean can serve as a sink for carbon dioxide emitted directly into the atmosphere over time scales shorter than the ocean mixing time (ca. 1000 years; [87]), and the dissolution of fine-grained carbonate sediment on the deep-sea floor can buffer against ocean acidification and atmospheric CO2 build-up more rapidly than the silicate weathering feedback, but its capacity is limited by the eventual development of a layer of insoluble residue on the seafloor that will prevent further dissolution as well as the fact that carbonate dissolution increases the total amount of carbon in the ocean–atmosphere system, whose removal back into sediments will ultimately depend upon silicate weathering. An extremely large and rapid release of carbon into the ocean–atmosphere system does have the possibility of also overwhelming the silicate weathering feedback and may help to account for the severity of the end-Permian mass extinction [88]. Within the Phanerozoic, this carbonate buffering mechanism is likely to have been most important during the post-Palaeozoic. Since the Late Triassic evolution and the later Mesozoic diversification of planktonic foraminifera and coccolithophorid algae greatly increased carbonate sediment export to the seafloor and, thus, were not as available to protect against ocean acidification and climate change during earlier crises such as the end-Permian mass extinction [89,90]. Moreover, modelling has shown that the diversification and proliferation of species that secrete aragonitic exoskeletons were much less impacted by fluctuations in seawater temperature and Mg/Ca ratios starting in the Middle Jurassic, again associated with the diversification of planktonic calcifiers [91].
In parallel to weathering reactions that convert CO2 into carbonate minerals, the biological fixation of carbon into organic matter results in the net conversion of gaseous CO2 into a solid form that has no climatic effect. Currently, the vast majority of organic carbon produced by primary producers in the surface ocean is decomposed and oxidized as it sinks through the water column. Yet, in places where biological productivity exceeds the diffusion of oxidants to the site of degradation, or in places where organic matter is adsorbed onto sediments and buried rapidly, a much higher fraction of it escapes oxidation [92]. The solubility of oxygen in water is dependent on temperature, and hence climate. Black shales in otherwise well-oxygenated Cretaceous pelagic sediments [93] indicate that temporary warming episodes resulted in burial of large amounts of organic carbon. Many of these ocean anoxic events [94] were associated with massive volcanism [95]. These events may have been sustained or even enhanced by nutrient cycling feedbacks that allowed for the liberation of phosphate under anoxic bottom waters, further promoting the high primary productivity that tends to enhance local water column anoxia [96].
The prevalence of anoxia, and its negative impacts on benthic marine ecosystems, appears to have declined across Phanerozoic time. Because atmospheric oxygen levels appear to have reached or exceeded modern values by the Late Palaeozoic, the secular decline in ocean anoxia is not easily explained by a long-term increase in atmospheric pO2. Rather, it appears to mostly reflect a change in the distribution of oxygen demand within the water column. Specifically, more effective export of organic matter from the surface ocean into deep water can spread the same amount of oxygen demand across a larger volume of water, thereby reducing the volume of totally anoxic water [97]. Increasing sizes of phytoplankton cells across Phanerozoic time [98], the diversification of phytoplankton lineages with mineral ballast in the form of carbonate or silica shells and the evolution of zooplankton that repackage phytoplankton cells into larger faecal pellets could all have played a role in strengthening this ‘biological pump’ that spreads oxygen demand across a larger volume of ocean water. The reduction in the area of ocean anoxia would, in turn, reduce the influence of changes in climate or the sea level on the rate of organic matter burial, thereby stabilizing the carbon cycle, global climate and atmospheric oxygen levels (because oxygen accumulation in the atmosphere is ultimately a by-product of the burial of organic matter produced via oxygenic photosynthesis) [19,99].
3. Cambrian solutions
Geological evidence indicates that strengthening of stabilizing feedbacks in the Earth system, coupled with and enabled by the evolution of larger, more ecologically flexible and physiologically resilient lineages, is largely responsible for the long-term decline in overall extinction rates. Much of this process reflects the long-term consequences of evolutionary innovations that occurred during Ediacaran and Cambrian time.
3.1. Large body size
The evolution of large, complex animals requires systems for distributing the ingredients and products of metabolism across the entire body. While the earliest animals accomplished this task through diploblastic body plans where all cells are in close contact with the external environment, triploblastic animals required internal transport mechanisms to supply the cells of internal organs. Budd & Jensen [100] argue that the earliest bilaterian animals must have been large and possessed both a coelom and blood vascular system. The blood vascular system, a network of entirely or partially enclosed tubes distributed throughout the body, can only serve as a material transport system, and such a system would not be necessary in tiny (i.e. protist-sized) organisms that can rely on diffusion and convection. Some taxa evolved open circulatory systems, while others evolved closed circulatory systems. Both systems appear to function well at the typical sizes of Cambrian animals [101], but circulatory systems appear to have constrained the later body size evolution of different higher taxa. The greater velocity and efficiency associated with closed circulatory systems permitted closed circulatory system taxa (especially chordates and cephalopods) to evolve to much larger body sizes later in the Phanerozoic, much more often than their relatives with open circulatory systems [101].
3.2. Nutrient cycling
The large body sizes enabled by Cambrian innovations in animal body plans also enabled animals to play a new role in nutrient cycling. Although some Ediacaran animals were quite large, reaching maximum dimensions of a metre or more [102], they were, to a first approximation, ‘metabolically inert’ animals that fed through diffusion [103]. Few possessed internal circulatory systems or had the ability to move across the seafloor or to burrow within sediments. Many more Cambrian animals, by contrast, possessed complex anatomical systems for internal mass transport (circulatory system) and locomotion (coelom). Animal bioturbation of sediments may have provided a negative (i.e. stabilizing) feedback on atmospheric and oceanic oxygen concentrations [104] and thereby reduced the magnitude of carbon isotope excursions and associated effects on global climate [105]. Because larger animals move more sediment and may therefore be differentially important to the overall biomixing of the sediment column [106], they would have been differentially important to this stabilization of the Earth system.
In addition, the movement of animals through the water column would have had two potential impacts on nutrient cycling. First, animal motion through the water column causes physical mixing of the water, reducing the steepness of physical and chemical gradients in temperature, nutrient content and oxygen content. This mixing has been hypothesized to play a significant role in the modern oceans [107–110] and to have first become important during Cambrian time [111], although its importance has been questioned relative to that of the breaking of internal waves [112]. Even if the physical mixing of ocean water by animals is not a quantitatively important process, the direct movement of nutrients is. The movement of animals in the oceans and on land tends to counteract the directional movement of nutrients from land to rivers then the ocean, and finally into sediments through both directional migration such as the movement of anadromous fish up-river and through functionally random movement [37]. In certain cases, such as the recycling of nutrients to the sea surface via buoyant faecal plumes of whales, animal activity may directly accelerate the recycling of nutrients within ecosystems [113]. In either case, the use of metabolic energy to move nutrients tends to reduce the efficiency of nutrient removal from ecosystems through physical processes.
3.3. Ecological differentiation
Ecological differentiation during the Cambrian explosion further changed marine ecosystems. The advent of predator–prey interactions may have caused both the widespread evolution of exoskeletons as adaptations against predation and the diversification of predators and prey [114]. In particular, predator–prey interactions would have increased the number of different demands on individual organisms, which moved from an Ediacaran world in which body plans could be optimized for the uptake of dissolved nutrients into a Cambrian world in which survival depended upon success not only in feeding but also in avoiding predation and minimizing interaction with competitors, among other tasks [115]. This roughening of the fitness landscape would be expected to lead to an increase in biodiversity.
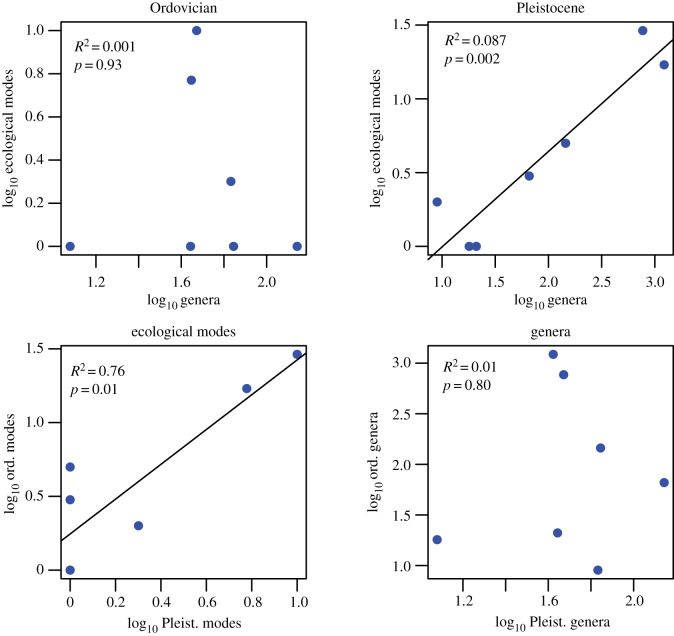
Broad functional differentiation of marine animals began during Cambrian time [14,28], and for phyla and classes with good fossil records, the higher taxa that achieved the greatest amount of functional differentiation by the end of Ordovician are also largely the clades that are the most taxonomically diverse in the modern oceans (figure 3; [8]). In fact, functional and taxonomic diversity within Linnaean classes were not significantly correlated during the Palaeozoic, but the number of ecological modes occupied by a class by the end of the Ordovician is a significant predictor of the number of genera in the class in the Pleistocene (444 Myr later) and the total Phanerozoic genus diversity of the class [8]. Therefore, the most taxonomically diverse classes in the modern marine fauna (e.g. bivalves, gastropods and teleost fishes) already had a higher propensity to explore ecospace in the Early Palaeozoic, but their taxonomic success was largely not realized until after the end-Permian and end-Cretaceous extinction events. In fact, ecologically diverse clades have come to dominate the modern oceans not by higher origination rates across the Phanerozoic, but rather by lower extinction rates, particularly during mass extinctions that primarily impacted ecologically homogeneous groups [51].
Figure 3.
Plots of genus richness versus the number of ecological modes of life for the Late Ordovician and Pleistocene. While the number of genera and modes within a Linnaean class were not correlated after the Cambrian and Ordovician radiations, they are correlated today. The strong correlation between Ordovician and Pleistocene ecological modes within classes indicates that classes that were able to diversify into many modes early in their histories have remained the most ecologically diverse and have also become the most taxonomically diverse [51] (plotted all classes with at least five genera in both the Ordovician and Pleistocene). Data from Knope et al. [8].
4. Later innovations
As noted above, the biologically driven stabilization of the Earth system did not end with Cambrian innovations. The evolution of land plants, in particular, caused additional, fundamental changes to the stabilizing feedbacks in the climate system through their impact on water transport and chemical weathering [85,86]. Similarly, later evolution of phytoplankton biomineralization and cell size distributions likely enhanced the stability of the climate system and ocean chemistry by creating a deep-sea carbonate sediment cover and by increasing the depth range over which organic respiration occurs in the ocean water column [90,97]. The processes initiated during Cambrian time, such as bioturbation and animal-driven transport of nutrients, continued to increase in degree, further stabilizing biogeochemical cycles [116]. Ecosystems continued to increase in complexity [14,29], potentially adding stability that outweighed any tendency of stronger antagonistic interactions to drive a greater risk of extinction, consistent with the ‘innate Gaia’ hypothesis that stabilizing feedbacks between life and its host planet should tend to evolve and strengthen over time [117]. And the selective processes of extinction, particularly during the major biotic crises, may have further filtered the global biota in ways that preferentially increased the proportion of biodiversity composed of taxa with greater ability to maintain homeostasis in the face of both short- and long-term environmental changes [79,80].
5. Conclusion
It has long been appreciated that the evolution of complex animal life required the earlier evolution of oxygenic photosynthesis and the subsequent accumulation of molecular oxygen in Earth's atmosphere [118,119]. The potential for biological innovation not only to create environmental opportunity but also to stabilize environmental conditions within the habitable range is also well known [120]. More recent work has continued to unveil the many ways in which innovations in animal and plant anatomy opened pathways to the ongoing stabilization of Earth's surface environments as well as to the evolution of organisms more tolerant of environmental change. It now appears that the increase in Earth's ability to support life across time and, especially, its increasing ability to support complex, multicellular life are largely a consequence of biological processes. These processes include natural selection for organisms with greater ability to survive in the face of environmental change but, more importantly, for ecosystems with greater complexity and stability as well as organisms that conduct activities that strengthen stabilizing feedbacks within the Earth system. Although animals first evolved during Neoproterozoic time, it was the anatomical and ecological innovations during Cambrian time that contributed most to this ongoing process of biologically driven improvement in Earth's habitability.
Acknowledgements
We thank numerous members of the Paleobiology Lab at Stanford University for many discussions on this topic over the past decade that helped to inspire this review.
Data accessibility
No new data were generated for this review paper. Sources for all data plotted in the figures are given in the figure captions.
Authors' contributions
All authors participated in the design of this review paper. All authors contributed to the writing and revision.
Competing interests
We declare we have no competing interests.
Funding
P.M.H. was funded by Stanford University through a Blaustein Visiting Professorship during the early design of this paper.
References
- 1.Raup DM, Sepkoski JJ. 1982. Mass extinctions in the marine fossil record. Science 215, 1501–1503. ( 10.1126/science.215.4539.1501) [DOI] [PubMed] [Google Scholar]
- 2.Szathmáry E, Smith JM. 1995. The major evolutionary transitions. Nature 374, 227–232. ( 10.1038/374227a0) [DOI] [PubMed] [Google Scholar]
- 3.Erwin DH, Laflamme M, Tweedt SM, Sperling EA, Pisani D, Peterson KJ. 2011. The Cambrian conundrum: early divergence and later ecological success in the early history of animals. Science 334, 1091–1097. ( 10.1126/science.1206375) [DOI] [PubMed] [Google Scholar]
- 4.Planavsky NJ, Reinhard CT, Wang X, Thomson D, McGoldrick P, Rainbird RH, Johnson T, Fischer WW, Lyons TW. 2014. Low mid-Proterozoic atmospheric oxygen levels and the delayed rise of animals. Science 346, 635–638. ( 10.1126/science.1258410) [DOI] [PubMed] [Google Scholar]
- 5.Sperling EA, Wolock CJ, Morgan AS, Gill BC, Kunzmann M, Halverson GP, Macdonald FA, Knoll AH, Johnston DT. 2015. Statistical analysis of iron geochemical data suggests limited late Proterozoic oxygenation. Nature 523, 451–454. ( 10.1038/nature14589) [DOI] [PubMed] [Google Scholar]
- 6.Reinhard CT, Planavsky NJ, Olson SL, Lyons TW, Erwin DH. 2016. Earth's oxygen cycle and the evolution of animal life. Proc. Natl Acad. Sci. USA 113, 8933–8938. ( 10.1073/pnas.1521544113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heim NA, Knope ML, Schaal EK, Wang SC, Payne JL. 2015. Cope's rule in the evolution of marine animals. Science 347, 867–870. ( 10.1126/science.1260065) [DOI] [PubMed] [Google Scholar]
- 8.Knope ML, Heim NA, Frishkoff LO, Payne JL. 2015. Limited role of functional differentiation in early diversification of animals. Nat. Commun. 6, 6455 ( 10.1038/ncomms7455) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu W, et al. 2018. Late inception of a resiliently oxygenated upper ocean. Science 361, 174–177. ( 10.1126/science.aar5372) [DOI] [PubMed] [Google Scholar]
- 10.Saltzman MR, Thomas E. 2012. Chapter 11. Carbon isotope stratigraphy. In The geologic time scale (eds Gradstein FM, Ogg JG, Schmitz M, Ogg G), pp. 207–232. Boston, MA: Elsevier. [Google Scholar]
- 11.Benton M. 1995. Diversification and extinction in the history of life. Science 268, 52–58. ( 10.1126/science.7701342) [DOI] [PubMed] [Google Scholar]
- 12.Valentine JW. 1969. Patterns of taxonomic and ecological structure of the shelf benthos during Phanerozoic time. Palaeontology 12, 684–709. [Google Scholar]
- 13.Bambach RK, Knoll AH, Sepkoski JJ. 2002. Anatomical and ecological constraints on Phanerozoic animal diversity in the marine realm. Proc. Natl Acad. Sci. USA 99, 6854–6859. ( 10.1073/pnas.092150999) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bambach RK, Bush AM, Erwin DH. 2007. Autecology and the filling of ecospace: key metazoan radiations. Palaeontology 50, 1–22. ( 10.1111/j.1475-4983.2006.00611.x) [DOI] [Google Scholar]
- 15.Novack-Gottshall PM. 2007. Using a theoretical ecospace to quantify the ecological diversity of Paleozoic and modern marine biotas. Paleobiology 33, 273–294. ( 10.1666/06054.1) [DOI] [Google Scholar]
- 16.Bambach RK. 1993. Seafood through time: changes in biomass, energetics, and productivity in the marine ecosystem. Paleobiology 19, 372–397. ( 10.1017/S0094837300000336) [DOI] [Google Scholar]
- 17.Finnegan S, et al. 2011. The magnitude and duration of Late Ordovician-Early Silurian glaciation. Science 331, 903–906. ( 10.1126/science.1200803) [DOI] [PubMed] [Google Scholar]
- 18.Peters SE. 2007. The problem with the Paleozoic. Paleobiology 33, 165–181. ( 10.1666/06067.1) [DOI] [Google Scholar]
- 19.Bachan A, Lau KV, Saltzman MR, Thomas E, Kump LR, Payne JL. 2017. A model for the decrease in amplitude of carbon isotope excursions across the Phanerozoic. Am. J. Sci. 317, 641–676. ( 10.2475/06.2017.01) [DOI] [Google Scholar]
- 20.Darwin C. 1964. On the origin of species: a facsimile of the first edition. Cambridge, MA: Harvard University Press. [Google Scholar]
- 21.Van Valen L. 1973. A new evolutionary law. Evol. Theory 1, 1–30. [Google Scholar]
- 22.Gause GF. 1934. Experimental analysis of Vito Volterra's mathematical theory of the struggle for existence. Science 79, 16–17. ( 10.1126/science.79.2036.16-a) [DOI] [PubMed] [Google Scholar]
- 23.MacArthur RH. 1958. Population ecology of some warblers of northeastern coniferous forests. Ecology 39, 599–619. ( 10.2307/1931600) [DOI] [Google Scholar]
- 24.Rainey PB, Travisano M. 1998. Adaptive radiation in a heterogeneous environment. Nature 394, 69–72. ( 10.1038/27900) [DOI] [PubMed] [Google Scholar]
- 25.Schluter D. 2000. The ecology of adaptive radiation. Oxford, UK: Oxford University Press. [Google Scholar]
- 26.Grant PR, Grant BR. 2006. Evolution of character displacement in Darwin's finches. Science 313, 224–226. ( 10.1126/science.1128374) [DOI] [PubMed] [Google Scholar]
- 27.Liow LH, Reitan T, Harnik PG. 2015. Ecological interactions on macroevolutionary time scales: clams and brachiopods are more than ships that pass in the night. Ecol. Lett. 18, 1030–1039. ( 10.1111/ele.12485) [DOI] [PubMed] [Google Scholar]
- 28.Bush AM, Bambach RK, Daley GM. 2007. Changes in theoretical ecospace utilization in marine fossil assemblages between the mid-Paleozoic and late Cenozoic. Paleobiology 33, 76–97. ( 10.1666/06013.1) [DOI] [Google Scholar]
- 29.Sahney S, Benton MJ, Ferry PA. 2010. Links between global taxonomic diversity, ecological diversity and the expansion of vertebrates on land. Biol. Lett. 6, 544–547. ( 10.1098/rsbl.2009.1024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenzweig ML, McCord RD. 1991. Incumbent replacement—evidence for long-term evolutionary progress. Paleobiology 17, 202–213. ( 10.1017/S0094837300010563) [DOI] [Google Scholar]
- 31.Kowalewski M, Dulai A, Fursich FT. 1998. A fossil record full of holes: the Phanerozoic history of drilling predation. Geology 26, 1091–1094. () [DOI] [Google Scholar]
- 32.Huntley JW, Kowalewski M. 2007. Strong coupling of predation intensity and diversity in the Phanerozoic fossil record. Proc. Natl Acad. Sci. USA 104, 15 006–15 010. ( 10.1073/pnas.0704960104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vermeij GJ. 1977. The Mesozoic marine revolution: evidence from snails, predators and grazers. Paleobiology 3, 245–258. ( 10.1017/S0094837300005352) [DOI] [Google Scholar]
- 34.Vermeij GJ. 1987. Evolution and escalation. Princeton, NJ: Princeton University Press. [Google Scholar]
- 35.Bambach RK. 1999. Energetics in the global marinefauna: a connection between terrestrial diversification and change in the marine biosphere. Geobios 32, 131–144. ( 10.1016/S0016-6995(99)80025-4) [DOI] [Google Scholar]
- 36.Erwin DH. 2007. Increasing returns, ecological feedback and the Early Triassic recovery. Palaeoworld 16, 9–15. ( 10.1016/j.palwor.2007.05.013) [DOI] [Google Scholar]
- 37.Doughty CE, et al. 2016. Global nutrient transport in a world of giants. Proc. Natl Acad. Sci. USA 113, 868–873. ( 10.1073/pnas.1502549112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Remy W, Taylor T, Hass H, Kerp H. 1994. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc. Natl Acad. Sci. USA 91, 11 841–11 843. ( 10.1073/pnas.91.25.11841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor TN, Klavins SD, Krings M, Taylor EL, Kerp H, Hass H. 2003. Fungi from the Rhynie chert: a view from the dark side. Earth Environment. Sci. Trans. R. Soc. Edinb. 94, 457–473. ( 10.1017/S026359330000081X) [DOI] [Google Scholar]
- 40.Trappe JM. 1987. Phylogenetic and ecologic aspects of mycotrophy in the angiosperms from an evolutionary standpoint. In Ecophysiology of VA mycorrhizal plants (ed. Safir GR.), pp. 5–25. Boca Raton, FL: CRC Press. [Google Scholar]
- 41.Simon L, Bousquet J, Levesque C, Lalonde M. 1993. Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants. Nature 363, 67–69. ( 10.1038/363067a0) [DOI] [Google Scholar]
- 42.Brundrett MC. 2002. Coevolution of roots and mycorrhizas of land plants. New Phytol. 154, 275–304. ( 10.1046/j.1469-8137.2002.00397.x) [DOI] [PubMed] [Google Scholar]
- 43.Rohwer F, Seguritan V, Farooq A, Knowlton N. 2002. Diversity and distribution of coral-associated bacteria. Mar. Ecol. Progr. Ser. 243, 1–10. ( 10.3354/meps243001) [DOI] [Google Scholar]
- 44.Grottoli AG, et al. 2018. Coral physiology and microbiome dynamics under combined warming and ocean acidification. PLoS One 13, e0191156 ( 10.1371/journal.pone.0191156) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spalding M, Ravilious C, Green E. 2001. World atlas of coral reefs. Berkeley, CA: University of California Press and UNEP/WCMC. [Google Scholar]
- 46.Roopnarine PD, Angielczyk KD. 2015. Community stability and selective extinction during the Permian-Triassic mass extinction. Science 350, 90–93. ( 10.1126/science.aab1371) [DOI] [PubMed] [Google Scholar]
- 47.Tilman D, Downing JA. 1994. Biodiversity and stability in grasslands. Nature 367, 363–365. ( 10.1038/367363a0) [DOI] [Google Scholar]
- 48.Tilman D, Knops J, Wedin D, Reich P, Ritchie M, Siemann E. 1997. The influence of functional diversity and composition on ecosystem processes. Science 277, 1300–1302. ( 10.1126/science.277.5330.1300) [DOI] [Google Scholar]
- 49.Tilman D, Reich PB, Knops JMH. 2006. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 441, 629–632. ( 10.1038/nature04742) [DOI] [PubMed] [Google Scholar]
- 50.Wagner PJ, Kosnik MA, Lidgard S. 2006. Abundance distributions imply elevated complexity of post-Paleozoic marine ecosystems. Science 314, 1289–1292. ( 10.1126/science.1133795) [DOI] [PubMed] [Google Scholar]
- 51.Knope ML, Bush AM, Frishkoff LO, Heim NA, Payne JL. 2020. Ecologically diverse clades dominate the oceans via extinction resistance. Science 367, 1035–1038. ( 10.1126/science.aax6398) [DOI] [PubMed] [Google Scholar]
- 52.Alvarez LW, Alvarez W, Asaro F, Michel HV. 1980. Extraterrestrial cause for the Cretaceous-Tertiary extinction. Science 208, 1095–1108. ( 10.1126/science.208.4448.1095) [DOI] [PubMed] [Google Scholar]
- 53.Raup DM. 1992. Large-body impact and extinction in the Phanerozoic. Paleobiology 18, 80–88. ( 10.1017/S0094837300012227) [DOI] [PubMed] [Google Scholar]
- 54.Courtillot VE, Renne PR. 2003. On the ages of flood basalt events. C.R. Geosci. 335, 113–140. ( 10.1016/S1631-0713(03)00006-3) [DOI] [Google Scholar]
- 55.Clapham ME, Renne PR. 2019. Flood basalts and mass extinctions. Annu. Rev. Earth Planet. Sci. 47, 275–303. ( 10.1146/annurev-earth-053018-060136) [DOI] [Google Scholar]
- 56.Stanley SM. 1988. Climatic cooling and mass extinction of Paleozoic reef communities. Palaios 3, 228–232. ( 10.2307/3514533) [DOI] [Google Scholar]
- 57.Stanley SM. 1988. Paleozoic mass extinctions; shared patterns suggest global cooling as a common cause. Am. J. Sci. 288, 334–352. ( 10.2475/ajs.288.4.334) [DOI] [Google Scholar]
- 58.Huey RB, Ward PD. 2005. Hypoxia, global warming, and terrestrial Late Permian extinctions. Science 308, 398–401. ( 10.1126/science.1108019) [DOI] [PubMed] [Google Scholar]
- 59.McElwain JC, Beerling DJ, Woodward FI. 1999. Fossil plants and global warming at the Triassic-Jurassic boundary. Science 285, 1386–1390. ( 10.1126/science.285.5432.1386) [DOI] [PubMed] [Google Scholar]
- 60.Kump LR, Pavlov A, Arthur MA. 2005. Massive release of hydrogen sulfide to the surface ocean and atmosphere during intervals of oceanic anoxia. Geology 33, 397–400. ( 10.1130/G21295.1) [DOI] [Google Scholar]
- 61.Cockell CS. 1999. Crises and extinction in the fossil record—a role for ultraviolet radiation? Paleobiology 25, 212–225. ( 10.1017/S0094837300026518) [DOI] [Google Scholar]
- 62.Grasby SE, Beauchamp B, Bond DPG, Wignall P, Talavera C, Galloway JM, Piepjohn K, Reinhardt L, Blomeier D. 2015. Progressive environmental deterioration in northwestern Pangea leading to the latest Permian extinction. Geol. Soc. Am. Bull. 127, 1331–1347. ( 10.1130/B31197.1) [DOI] [Google Scholar]
- 63.Tappan H. 1968. Primary production, isotopes, extinctions and the atmosphere. Palaeogeogr. Palaeoclimatol. Palaeoecol. 4, 187–210. ( 10.1016/0031-0182(68)90047-3) [DOI] [Google Scholar]
- 64.Rhodes MC, Thayer CW. 1991. Mass extinctions: ecological selectivity and primary production. Geology 19, 877–880. () [DOI] [Google Scholar]
- 65.McEwen AS, Moore JM, Shoemaker EM. 1997. The Phanerozoic impact cratering rate: evidence from the farside of the Moon. J. Geophys. Res. Plan. 102, 9231–9242. ( 10.1029/97JE00114) [DOI] [Google Scholar]
- 66.Shoemaker EM. 1998. Long-term variations in the impact cratering record on Earth. In Meteorites. Flux with time and impact effects, vol. 140 (eds Grady MM, Hutchinson R, McCall GJH, Rothery DA), pp. 7–10. London: Geological Society; Special Publications. [Google Scholar]
- 67.Raup DM. 1991. A kill curve for Phanerozoic marine species. Paleobiology 17, 37–48. ( 10.1017/S0094837300010332) [DOI] [PubMed] [Google Scholar]
- 68.Kring DA. 2003. Environmental consequences of impact cratering events as a function of ambient conditions on Earth. Astrobiology 3, 133–152. ( 10.1089/153110703321632471) [DOI] [PubMed] [Google Scholar]
- 69.Prokoph A, El Bilali H, Ernst R. 2013. Periodicities in the emplacement of large igneous provinces through the Phanerozoic: relations to ocean chemistry and marine biodiversity evolution. Geosci. Front. 4, 263–276. ( 10.1016/j.gsf.2012.08.001) [DOI] [Google Scholar]
- 70.Rohde RA, Muller RA. 2005. Cycles in fossil diversity. Nature 434, 208–210. ( 10.1038/nature03339) [DOI] [PubMed] [Google Scholar]
- 71.Ganino C, Arndt NT. 2009. Climate changes caused by degassing of sediments during the emplacement of large igneous provinces. Geology 37, 323–326. ( 10.1130/G25325A.1) [DOI] [Google Scholar]
- 72.Sheehan PM. 2001. The Late Ordovician mass extinction. Annu. Rev. Earth Planet. Sci. 29, 331–364. ( 10.1146/annurev.earth.29.1.331) [DOI] [Google Scholar]
- 73.Finnegan S, McClain CM, Kosnik MA, Payne JL. 2011. Escargots through time: an energetic comparison of marine gastropod assemblages before and after the Mesozoic Marine Revolution. Paleobiology 37, 252–269. ( 10.1666/09066.1) [DOI] [Google Scholar]
- 74.Finnegan S, Heim NA, Peters SE, Fischer WW. 2012. Climate change and the selective signature of the Late Ordovician mass extinction. Proc. Natl Acad. Sci. USA 109, 6829–6834. ( 10.1073/pnas.1117039109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Stanley SM, Powell MG. 2003. Depressed rates of origination and extinction during the late Paleozoic ice age: a new state for the global marine ecosystem. Geology 31, 877–880. ( 10.1130/G19654R.1) [DOI] [Google Scholar]
- 76.Smith FA, Elliott Smith RE, Lyons SK, Payne JL. 2018. Body size downgrading of mammals over the late Quaternary. Science 360, 310–313. ( 10.1126/science.aao5987) [DOI] [PubMed] [Google Scholar]
- 77.Smith FA, Elliott Smith RE, Lyons SK, Payne JL, Villaseñor A. 2019. The accelerating influence of humans on mammalian macroecological patterns over the late Quaternary. Quat. Sci. Rev. 211, 1–16. ( 10.1016/j.quascirev.2019.02.031) [DOI] [Google Scholar]
- 78.Childress J, Seibel B. 1998. Life at stable low oxygen levels: adaptations of animals to oceanic oxygen minimum layers. J. Exp. Biol. 201, 1223–1232. [DOI] [PubMed] [Google Scholar]
- 79.Knoll AH, Bambach RK, Payne JL, Pruss S, Fischer WW. 2007. Paleophysiology and end-Permian mass extinction. Earth Planet. Sci. Lett. 256, 295–313. ( 10.1016/j.epsl.2007.02.018) [DOI] [Google Scholar]
- 80.Payne JL, Bush AM, Chang E, Heim NA, Knope MA, Pruss S. 2016. Extinction intensity, selectivity and their combined macroevolutionary influence in the fossil record. Biol. Lett. 12, 20160202 ( 10.1098/rsbl.2016.0202) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoffman PF, Kaufman AJ, Halverson GP, Schrag DP. 1998. A Neoproterozoic snowball Earth. Science 281, 1342–1346. ( 10.1126/science.281.5381.1342) [DOI] [PubMed] [Google Scholar]
- 82.Walker JCG, Hays PB, Kasting JF. 1981. A negative feedback mechanism for the long-term stabilization of Earth's surface temperature. J. Geophys. Res. Oceans 86, 9776–9782. ( 10.1029/JC086iC10p09776) [DOI] [Google Scholar]
- 83.Berner RA, Lasaga AC, Garrels RM. 1983. The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide levels for the last 100 million years. Am. J. Sci. 289, 333–361. ( 10.2475/ajs.283.7.641) [DOI] [PubMed] [Google Scholar]
- 84.Coogan LA, Dosso SE. 2015. Alteration of ocean crust provides a strong temperature dependent feedback on the geological carbon cycle and is a primary driver of the Sr-isotopic composition of seawater. Earth Planet. Sci. Lett. 415, 38–46. ( 10.1016/j.epsl.2015.01.027) [DOI] [Google Scholar]
- 85.Berner RA. 1998. The carbon cycle and carbon dioxide over Phanerozoic time: the role of land plants. Phil. Trans. R. Soc. Lond. B 353, 75–82. ( 10.1098/rstb.1998.0192) [DOI] [Google Scholar]
- 86.Ibarra DE, Caves Rugenstein JK, Bachan A, Baresch A, Lau KV, Thomas DL, Lee J-E, Boyce CK, Chamberlain CP. 2019. Modeling the consequences of land plant evolution on silicate weathering. Am. J. Sci. 319, 1–43. ( 10.2475/01.2019.01) [DOI] [Google Scholar]
- 87.Archer D, Kheshgi H, Maier-Reimer E. 1997. Multiple timescales for neutralization of fossil fuel CO2. Geophys. Res. Lett. 24, 405–408. ( 10.1029/97GL00168) [DOI] [Google Scholar]
- 88.Kump LR. 2018. Prolonged Late Permian-Early Triassic hyperthermal: failure of climate regulation? Phil. Trans. R. Soc. A 376, 20170078 ( 10.1098/rsta.2017.0078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ridgwell A, Zeebe RE. 2005. The role of the global carbonate cycle in the regulation and evolution of the Earth system. Earth Planet. Sci. Lett. 234, 299–315. ( 10.1016/j.epsl.2005.03.006) [DOI] [Google Scholar]
- 90.Payne JL, Lehrmann DJ, Follett D, Seibel M, Kump LR, Riccardi A, Altiner D, Sano H, Wei J. 2007. Erosional truncation of uppermost Permian shallow-marine carbonates and implications for Permian-Triassic boundary events. Geol. Soc. Am. Bull. 119, 771–784. ( 10.1130/B26091.1) [DOI] [Google Scholar]
- 91.Eichenseer K, Balthasar U, Smart C, Stander J, Haaga K, Kiessling W. 2019. Jurassic shift from abiotic to biotic control on marine ecological success. Nat. Geosci. 12, 638–642. ( 10.1038/s41561-019-0392-9) [DOI] [Google Scholar]
- 92.Canfield DE. 1994. Factors influencing organic carbon preservation in marine sediments. Chem. Geol. 114, 315–329. ( 10.1016/0009-2541(94)90061-2) [DOI] [PubMed] [Google Scholar]
- 93.Scholle PA, Arthur MA. 1980. Carbon isotope fluctuations in Cretaceous pelagic limestones: potential stratigraphic and petroleum exploration tool. AAPG Bull. 64, 67–87. [Google Scholar]
- 94.Schlanger SO, Jenkyns HC. 1976. Cretaceous oceanic anoxic events: causes and consequences. Geol. Mijnbouw 55, 179–184. [Google Scholar]
- 95.Jenkyns HC. 2010. Geochemistry of oceanic anoxic events. Geochem. Geophys. Geosyst. 11, Q03004 ( 10.1029/2009GC002788) [DOI] [Google Scholar]
- 96.Van Cappellen P, Ingall ED. 1994. Benthic phosphorus regeneration, net primary production, and ocean anoxia: a model of the coupled marine biogeochemical cycles of carbon and phosphorus. Paleoceanography 9, 677–692. ( 10.1029/94PA01455) [DOI] [Google Scholar]
- 97.Meyer KM, Ridgwell A, Payne JL. 2016. The influence of the biological pump on ocean chemistry: implications for long-term trends in marine redox chemistry, the global carbon cycle, and marine animal ecosystems. Geobiology 14, 207–219. ( 10.1111/gbi.12176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Knoll AH, Follows MJ. 2016. A bottom-up perspective on ecosystem change in Mesozoic oceans. Proc. R. Soc. B 283, 20161755 ( 10.1098/rspb.2016.1755) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lau KV, et al. 2016. Marine anoxia and delayed Earth system recovery after the end-Permian extinction. Proc. Natl Acad. Sci. USA 113, 2360–2365. ( 10.1073/pnas.1515080113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Budd G, Jensen S. 2000. A critical reappraisal of the fossil record of the bilaterian phyla. Biol. Rev. Camb. Philos. Soc. 75, 253–295. ( 10.1111/j.1469-185x.1999.tb00046.x) [DOI] [PubMed] [Google Scholar]
- 101.Heim NA, et al. 2020. Respiratory medium and circulatory anatomy constrain size evolution in marine macrofauna. Paleobiology. ( 10.1017/pab.2020.16) [DOI] [Google Scholar]
- 102.Narbonne GM, Gehling JG. 2003. Life after snowball: the oldest complex Ediacaran fossils. Geology 31, 27–30. () [DOI] [Google Scholar]
- 103.Laflamme M, Xiao S, Kowalewski M. 2009. Osmotrophy in modular Ediacara organisms. Proc. Natl Acad. Sci. USA 106, 14 438–14 443. ( 10.1073/pnas.0904836106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Boyle RA, Dahl TW, Dale AW, Shields-Zhou GA, Zhu MY, Brasier MD, Canfield DE, Lenton TM. 2014. Stabilization of the coupled oxygen and phosphorus cycles by the evolution of bioturbation. Nat. Geosci. 7, 671–676. ( 10.1038/NGEO2213) [DOI] [Google Scholar]
- 105.Boyle RA, Dahl TW, Bjerrum CJ, Canfield DE. 2018. Bioturbation and directionality in Earth's carbon isotope record across the Neoproterozoic-Cambrian transition. Geobiology 16, 252–278. ( 10.1111/gbi.12277) [DOI] [PubMed] [Google Scholar]
- 106.Solan M, Cardinale BJ, Downing AL, Engelhardt KAM, Ruesink JL, Srivastava DS. 2004. Extinction and ecosystem function in the marine benthos. Science 306, 1177–1180. ( 10.1126/science.1103960) [DOI] [PubMed] [Google Scholar]
- 107.Kunze E, Dower J, Beveridge I, Dewey R, Bartlett K. 2006. Observations of biologically generated turbulence in a coastal inlet. Science 313, 1768–1770. ( 10.1126/science.1129378) [DOI] [PubMed] [Google Scholar]
- 108.Dabiri JO. 2010. Role of vertical migration in biogenic ocean mixing. Geophys. Res. Lett. 37, L11602 ( 10.1029/2010GL043556) [DOI] [Google Scholar]
- 109.Katija K. 2012. Biogenic inputs to ocean mixing. J. Exp. Biol. 215, 1040–1049. ( 10.1242/jeb.059279) [DOI] [PubMed] [Google Scholar]
- 110.Houghton I, Koseff J, Monismith S, Dabiri J. 2018. Vertically migrating swimmers generate aggregation-scale eddies in a stratified column. Nature 556, 497–500. ( 10.1038/s41586-018-0044-z) [DOI] [PubMed] [Google Scholar]
- 111.Butterfield NJ. 2018. Oxygen, animals and aquatic bioturbation: an updated account. Geobiology 16, 3–16. ( 10.1111/gbi.12267) [DOI] [PubMed] [Google Scholar]
- 112.Kunze E. 2019. Biologically generated mixing in the ocean. Annu. Rev. Mar. Sci. 11, 215–226. ( 10.1146/annurev-marine-010318-095047) [DOI] [PubMed] [Google Scholar]
- 113.Roman J, McCarthy JJ. 2010. The whale pump: marine mammals enhance primary productivity in a coastal basin. PLoS One 5, e13255 ( 10.1371/journal.pone.0013255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stanley SM. 1973. An ecological theory for the sudden origin of multicellular life in the late Precambrian. Proc. Natl Acad. Sci. USA 70, 1486–1489. ( 10.1073/pnas.70.5.1486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Marshall CR. 2006. Explaining the Cambrian ‘explosion’ of animals. Annu. Rev. Earth Planet. Sci. 34, 355–384. ( 10.1146/annurev.earth.33.031504.103001) [DOI] [Google Scholar]
- 116.Tarhan LG. 2018. The early Paleozoic development of bioturbation—evolutionary and geobiological consequences. Earth Sci. Rev. 178, 177–207. ( 10.1016/j.earscirev.2018.01.011) [DOI] [Google Scholar]
- 117.Lenton TM. 2002. Testing Gaia: the effect of life on Earth's habitability and regulation. Clim. Change 52, 409–422. 10.1023/A:1014201801949 [DOI] [Google Scholar]
- 118.Nursall JR. 1959. Oxygen as a prerequisite to the origin of the Metazoa. Nature 183, 1170–1172. ( 10.1038/1831170b0) [DOI] [Google Scholar]
- 119.Cloud PE. 1968. Atmospheric and hydrospheric evolution on the primitive Earth. Science 160, 729–736. ( 10.1126/science.160.3829.729) [DOI] [PubMed] [Google Scholar]
- 120.Watson AJ, Lovelock JE. 1983. Biological homeostasis of the global environment: the parable of Daisyworld. Tellus B 35, 284–289. ( 10.3402/tellusb.v35i4.14616) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were generated for this review paper. Sources for all data plotted in the figures are given in the figure captions.