Abstract
Mudstone-hosted microfossils are a major component of the Proterozoic fossil record, particularly dominating the record of early eukaryotic life. Early organisms possessed no biomineralized parts to resist decay and controls on their fossilization in mudstones are poorly understood. Consequently, the Proterozoic fossil record is compromised—we do not know whether changing temporal/spatial patterns of microfossil occurrences reflect evolution or the distribution of favourable fossilization conditions. We investigated fossilization within the approximately 1000 Ma Lakhanda Group (Russia) and the approximately 800 Ma Svanbergfjellet and Wynniatt formations (Svalbard and Arctic Canada). Vertical sections of microfossils and surrounding matrices were extracted from thin sections by focused ion beam milling. Elemental mapping and synchrotron-based infrared microspectroscopy revealed that microfossils are surrounded by haloes rich in aluminium, probably hosted in kaolinite. Kaolinite has been implicated in Cambrian Burgess Shale-type (BST) fossilization and is known to slow the growth of degraders. The Neoproterozoic mudstone microfossil record may be biased to tropical settings conducive to kaolinite formation. These deposits lack metazoan fossils even though they share fossilization conditions with younger BST deposits that are capable of preserving non-mineralizing metazoans. Thus metazoans, at least those typically preserved in BST deposits, were probably absent from sedimentary environments before approximately 800 Ma.
Keywords: Proterozoic eon, early eukaryotes, clay minerals, complex life, metazoan antiquity, taphonomy
1. Introduction
Due to a lack of organisms with readily fossilized hard parts, it has long been observed that the pre-Cambrian fossil record is impoverished compared with its Phanerozoic counterpart [1]. However, over the last approximately 70 years palaeontologists have discovered a number of exceptionally preserved assemblages of diverse microfossils in Proterozoic rocks [2,3]. Three principal lithologies record microfossils: early diagenetic cherts and phosphates, and mudstones [4]. Evidence from the record of eukaryotic microfossils suggests that the mudstone record dominates—47 of 59 fossiliferous assemblages in a recent compilation are mudstone hosted [4]. We know little of the taphonomic processes that operated in mudstones [5]. What factors promoted fossilization? What determines which mudstones preserve non-biomineralized microfossils? Understanding taphonomy in Proterozoic mudstones is vital to our efforts to chart Proterozoic evolution. Furthermore, we need to understand whether there are temporal or spatial biases to the Proterozoic microfossil record. Patterns of microfossil diversity may represent real ecological patterns, but they could also represent spatial/temporal changes in the availability of environmental conditions conducive to fossilization. Our inability to unravel these scenarios fundamentally compromises the use of mudstone-hosted microfossils to reveal Proterozoic evolutionary history.
Examples of soft tissue fossilization [6] in Phanerozoic mudstones provide a basis for taphonomic hypotheses that can be tested on Proterozoic deposits. Cambrian Burgess Shale-type (BST) deposits are the most studied, preserving early metazoans including diverse organisms that lacked mineralized skeletons [7]. A variety of factors have been proposed to influence BST fossilization, including oxidant supply, sediment composition, the propensity of soft tissues for replication in authigenic minerals (e.g. pyrite, phosphate and clay minerals) and cementation of the overlying sediment (see [8] for a review). A role for clay minerals has been posited more recently: experiments have shown that the aluminium- and iron-rich clays kaolinite and berthierine, which are major constituents of a large proportion of Cambrian strata hosting BST fossils [9,10], slow the growth of decay bacteria [11] and increase decay resistance through clay–organic matter interactions [12–18]. Evidence is also emerging that kaolinite, in particular, may attach to or precipitate on some tissues early in diagenesis, helping to conserve them by providing a protective coating and/or facilitating polymerization [14,19,20]. Intriguingly, elements indicative of these clays (e.g. aluminium and iron) have also been reported adjacent to some Proterozoic fossils, but their host phases are difficult to constrain [21–23]. Do aluminium- and/or iron-rich clay minerals promote fossilization in Proterozoic strata? Do they promote fossilization not only of metazoans but also of other phylogenetic groups such as eukaryotic algae, or even cyanobacteria, which have diverse biopolymer compositions?
We investigated the taphonomy of microfossils (both probable eukaryotes and cyanobacteria; table 1) from three exceptional Tonian (1000–717 Ma) deposits: the approximately 1000 Ma Lakhanda Group (Russia) [24,25], and the approximately 800 Ma Svanbergfjellet (Svalbard) [26] and Wynniatt (Canada) formations [29]. These assemblages include some of the best preserved and most diverse pre-Ediacaran fossils and often include microfossils that are more fragile than the decay-resistant spheroidal acritarchs commonly recovered from Proterozoic strata [2,4]. More fragile forms include relatively large (up to millimetre scale) multicellular morphologies and those that possess intricate spines or processes [7,24–26,29]. For example, microfossils from the Svanbergfjellet Formation may represent some of the oldest examples of multicellular eukaryotic green algae (chlorophytes) [26,27]. We used a novel combination of microanalytical techniques to probe sediment mineralogy immediately adjacent to microfossil cell walls. These data illuminate the role of clay minerals in Proterozoic taphonomy, allowing comparisons with BST fossilization.
Table 1.
Interpreted phylogenetic affinities and associated biopolymers of the studied microfossils: Lakhanda [24,25], Svanbergfjellet [2,26–28] and Wynniatt [29]. Biopolymers taken from [30–34].
| phylogenetic affinity | principal biopolymer | thin section, university collection, England Finder coordinates | |
|---|---|---|---|
| Lakhanda | |||
| Siphonophycus | ?cyanobacterium | sheaths of carbohydrate fibrils | LK67, Cambridge, UK, N32/2 |
| Svanbergfjellet | |||
| Proterocladus major | chlorophyte | carbohydrates including cellulose | 86-G-62–52, Harvard, MA, N47/2 |
| Germinosphaera fibrilla | ?eukaryote (possible vaucheriacean alga or fungus) | likely aliphatic composition similar to sporopollenin or algaenan | 86-G-62–68, Harvard, MA, Q48/4 |
| microfossil fragment | ?eukaryote | undetermined | 86-G-62-54, Harvard, MA, L26/2 |
| Wynniatt | |||
| ?Ostiana (or possibly Palaeastrum) | ?cyanobacterium (or chlorophyte) | lipids and proteins with possible sheaths of carbohydrate fibrils (or, in the case of chlorophyte, carbohydrates including cellulose) | 88-KL-131-2, Cambridge, UK, D35/2 |
| Siphonophycus | ?cyanobacterium | sheaths of carbohydrate fibrils | 88-KL-131-2, Cambridge, UK, Q46/2 |
2. Material and methods
Microfossils were identified in thin sections cut sub-parallel to sedimentary laminae that were obtained for previous studies (as in BST fossilization [7], fossils are compressed into two dimensions parallel to laminae). Thin section number, university collection and England Finder coordinates are given in table 1 for each microfossil/population studied. Thin sections from Harvard University, Cambridge, MA, are deposited in the Paleobotanical Collections of the Harvard University Herbaria, while the sections from the University of Cambridge, Cambridge, UK, are in the collections of N. Butterfield. A filamentous microfossil (sheathed cyanobacterium), Siphonophycus, was examined from the Lakhanda Group, whereas three microfossils were analysed from the Svanbergfjellet Formation: the chlorophyte Proterocladus major, the acanthomorphic acritarch (eukaryote) Germinosphaera fibrilla and an unidentified fragment of a larger microfossil (eukaryote). A monostromatic population of spheroids, possibly Ostiana (cyanobacterium) or the chlorophyte Palaeastrum (we refer to this population as Ostiana), and another Siphonophycus specimen were analysed from the Wynniatt Formation. For details on phylogenetic assignments, see table 1. The microfossils from each deposit were derived from single rock samples, one per deposit (Lakhanda, LK67, Cambridge, UK; Svanbergfjellet, 86-G-62, Harvard, MA; Wynniatt, 88-KL-131, Cambridge, UK). Vertical sections (approx. 30 µm × 10 µm × 1 µm) of microfossils and adjoining matrices were extracted perpendicular (or sub-perpendicular) to sedimentary laminae from the thin sections using focused ion beam (FIB) milling at the Harvard Center for Nanoscale Systems (CNS), Cambridge, MA, and attached to copper transmission electron microscopy (TEM) grids. Milling was performed on an FEI Helios 660 Dual-Beam FIB/SEM (scanning electron microscope) equipped with an Autoprobe 400 micromanipulator.
Vertical sections were imaged using the SEM on the FEI Helios 660 at CNS and also on a Carl Zeiss Merlin SEM equipped with an Oxford Instruments X-MaxN 150 mm2 X-ray detector at the Department of Materials, University of Oxford (ODM), Oxford, UK. Energy dispersive X-ray spectroscopy (EDS) at ODM was used to map elemental distributions across vertical sections for all samples except Germinosphaera (which was destroyed in sample manipulation before maps could be generated). In order to reduce sampling volume (thereby increasing spatial resolution) and reduce charging effects, EDS was carried out at voltages below 10 kV, with higher energy analysis to confirm the identity of elements when required (see electronic supplementary material, table S1 for configurations). Maps were processed using AZtec v3.3 and the TruMap function. Synchrotron-based Fourier transform infrared (FTIR) microspectroscopy at the MIRIAM beamline of Diamond Light Source, Didcot, UK, was used to identify the mineral hosts of elemental variations in all vertical sections. The beamline was coupled to a Bruker Vertex 80 V FTIR spectrometer and Hyperion 3000 microscope equipped with a 50 µm, LN2 cooled, midband mercury--cadmium--telluride detector. About 256 scans were coded at 4 cm−1 spectral resolution per point. The infrared focal spot was confined to a diffraction limited area using slits of effective aperture 3 × 3 µm at the sample, via × 36 optics in transmission geometry with numerical aperture (NA) = 0.5. The sample was mapped across this aperture with step size 1 µm, oversampling with respect to the aperture size. In these conditions, the resulting spatial resolution is expected to be diffraction limited and wavelength (λ) dependent, approximately at the Abbe resolution limit = λ/2NA = λ (at NA 0.5). The spatial resolution is about 2.8 µm for the M–OH spectral region of interest.
Finally, powder X-ray diffraction (XRD) was performed on each rock sample using a PANalytical Empyrean diffractometer at the Department of Earth Sciences, University of Oxford, Oxford, UK, employing a Co Kα source and a PIXcel-1D detector. A substitute for the Svanbergfjellet sample, from the same locality/stratigraphic horizon, was obtained from the Cambridge, UK, collections (sample 99-L-15) as no 86-G-62 material remains. Mineral identifications were confirmed using the International Centre for Diffraction Data (ICDD) Powder Diffraction File-4+ database (http://www.icdd.com/products/pdf4.htm) and the reference intensity ratio method [35]. Clay mineral species were distinguished by weak but diagnostic peaks commonly manifested as a composite reflection from 060 and/or 33-1 [36].
3. Results
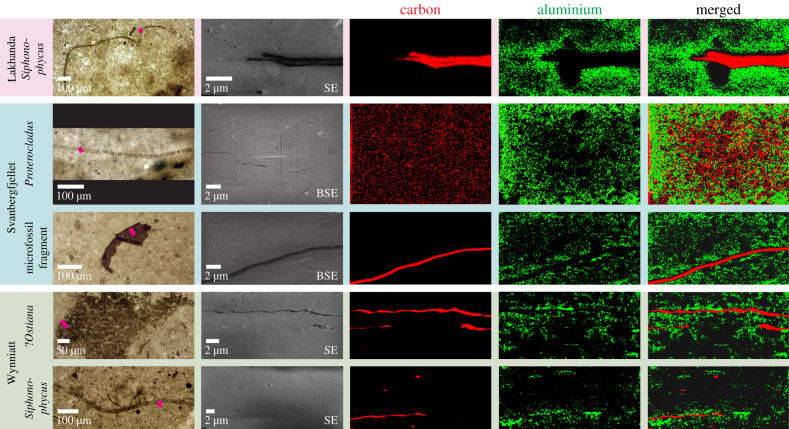
SEM imaging of the vertical sections revealed the cross-sectional structure of the microfossils (figure 1), which varies in thickness. The Svanbergfjellet microfossil Proterocladus is only tens of nanometres thick and hardly perceptible, while the Lakhanda Siphonophycus specimen and both the Germinosphaera specimen and the unidentified microfossil fragment from Svanbergfjellet are up to approximately 0.5 µm thick. All specimens from the Wynniatt Formation are intermediate in thickness (approx. 0.2–0.4 µm). In some cases, microfossil walls have split and infilled with matrix, as in some of the Wynniatt Ostiana individuals, but the majority are intact. The Wynniatt Ostiana cluster is evident on two sedimentary laminae in the sample and thus may represent multiple colonies or one colony that has been infiltrated by sediment during deposition (figure 1). The Siphonophycus specimen from Lakhanda may be split into two or represents two superimposed filaments (figure 1). SEM images confirmed that microfossils are compressed sub-parallel to sedimentary laminae. The lithologies of the samples are similar, with most sediment grains ≪1 µm and only sparse grains approximately 1 µm, around which microfossils may be deflected: e.g. the vertical section of the Lakhanda Siphonophycus (figure 1). Larger grains are normally rounded, although some oblong sub-angular grains, which can reach greater than 5 µm in maximum dimension (figure 1) with long axes parallel to sedimentary laminae, are associated with the Svanbergfjellet microfossils.
Figure 1.
Studied microfossils with elemental distributions. Light photomicrographs of microfossils in thin section. Locations of extracted vertical sections shown by pink bars. SEM micrographs of extracted vertical sections and EDS maps showing carbon and aluminium distributions. Carbon constitutes the microfossils which are surrounded by a concentration of aluminium. Secondary electron (SE) or backscatter electron (BSE) SEM images were used to minimize charging effects. Specimen details are listed in table 1. SEM and EDS operating conditions for each map/image are listed in electronic supplementary material, table S1. Light and SEM images of the Svanbergfjellet Germinosphaera specimen/vertical section are given in electronic supplementary material, figure S1.
EDS analysis revealed that the microfossils are composed of carbon (figure 1), although the result was ambiguous for the Svanbergfjellet Proterocladus specimen, probably reflecting its very thin cross-section and the relatively large volume producing X-rays which, even at 5–10 kV, can have a masking effect on local elemental enrichments. The Lakhanda Siphonophycus specimen and the Wynniatt Ostiana specimens are enriched in sulfur. The sediment surrounding all the microfossils is largely similar (see electronic supplementary material, figure S2), dominated by silicon and oxygen with minor aluminium, carbon, chlorine, iron, magnesium, nitrogen, phosphorus, potassium, sodium and sulfur, reflecting a siliciclastic matrix with contributions from both organic matter and diagenetic minerals. Calcium is also present but sparsely distributed, probably reflecting a minor admixture of carbonate minerals. Larger rounded quartz grains are present (identified by a dominantly silicon and oxygen composition). Larger sub-angular grains in Svanbergfjellet vertical sections are enriched in magnesium and iron, indicative of chlorite.
Aluminium is generally enriched adjacent to microfossil carbon compared with the matrix, often forming a halo (figure 1). Aluminium haloes with a thickness less than 3 µm are particularly apparent surrounding the Svanbergfjellet unidentified microfossil fragment and the Lakhanda Siphonophycus specimen. None of the Wynniatt vertical sections shows a contiguous halo, but there are discontinuous aluminium enrichments adjacent to microfossils. The only vertical section for which aluminium enrichments adjacent to microfossils were not identified unambiguously is that of the Svanbergfjellet Proterocladus specimen; however, as noted regarding its carbon composition, this may be due to its thin cross-section and EDS masking effects.
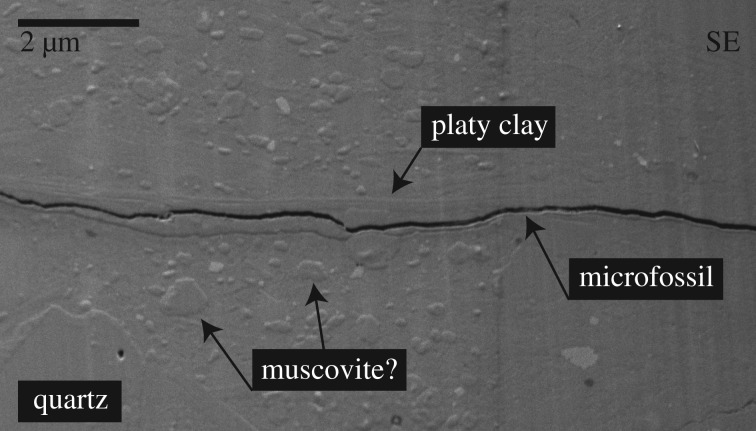
SEM imaging provides evidence that the aluminium enrichments occur in platy materials, presumably clays. SEM of the Wynniatt Siphonophycus vertical section shows a halo of platy material, parallel to sedimentary laminae, extending less than 0.5 µm around the microfossil (figure 2). A layer of matrix characterized by a lack of the larger grains or pseudo-hexagonal crystals that are common elsewhere (possibly muscovite) extends less than 1 µm beyond this layer. Coarser crystals often display planar interlocking boundaries, suggesting a phase of overgrowth after physical deposition. These observations indicate differential chemistry and mineralogy adjacent to the microfossil compared with that of the matrix. The finely crystalline and platy nature of the material hosting the aluminium enrichments identified by EDS suggests that aluminium may be bound largely in a clay mineral structure.
Figure 2.
SEM secondary electron (SE) micrograph of the vertical section of Siphonophycus from the Wynniatt Formation. Microfossil cross-section is surrounded by a halo of a platy mineral likely to be kaolinite. Coarser crystals with planar interlocking boundaries (likely to be muscovite) and large quartz grains are also highlighted. SEM operating conditions are listed in electronic supplementary material, table S1.
Synchrotron-based FTIR microspectroscopy confirmed that the most likely clay mineral host of these aluminium enrichments is kaolinite and its metamorphic products. Kaolinite can be identified using FTIR by a series of three M–OH bands at approximately 3620, approximately 3652 and approximately 3694 cm−1 [37]. For randomly oriented powders, the approximately 3620 and approximately 3694 cm−1 bands are more intense than the approximately 3652 cm−1 band; their frequencies are also sufficiently distinct from illite-group and chlorite-group minerals to allow unambiguous identification of kaolinite. FTIR microspectra from all Lakhanda and Svanbergfjellet vertical sections clearly differentiated these bands (figure 3). Vertical sections of the Wynniatt Formation microfossils showed a broad M–OH band between approximately 3500 and approximately 3700 cm−1, with no observable kaolinite bands (figure 3). This probably reflects a dominance of illite/muscovite in Wynniatt clay mineralogy [37]. Any kaolinite, if present, is diluted beyond the detection limit in FTIR microspectra. Mild contact metamorphism of Wynniatt strata [38] may have transformed kaolinite to illite/muscovite [39]. Microfossil carbon is not recorded in our FTIR microspectra. Other Proterozoic microfossils are known to produce FTIR spectra indicative of their carbon composition [40]. Its absence in our analysis probably reflects a drowning of any organic signature given the limited amount of microfossil material compared with the mineral matrix.
Figure 3.
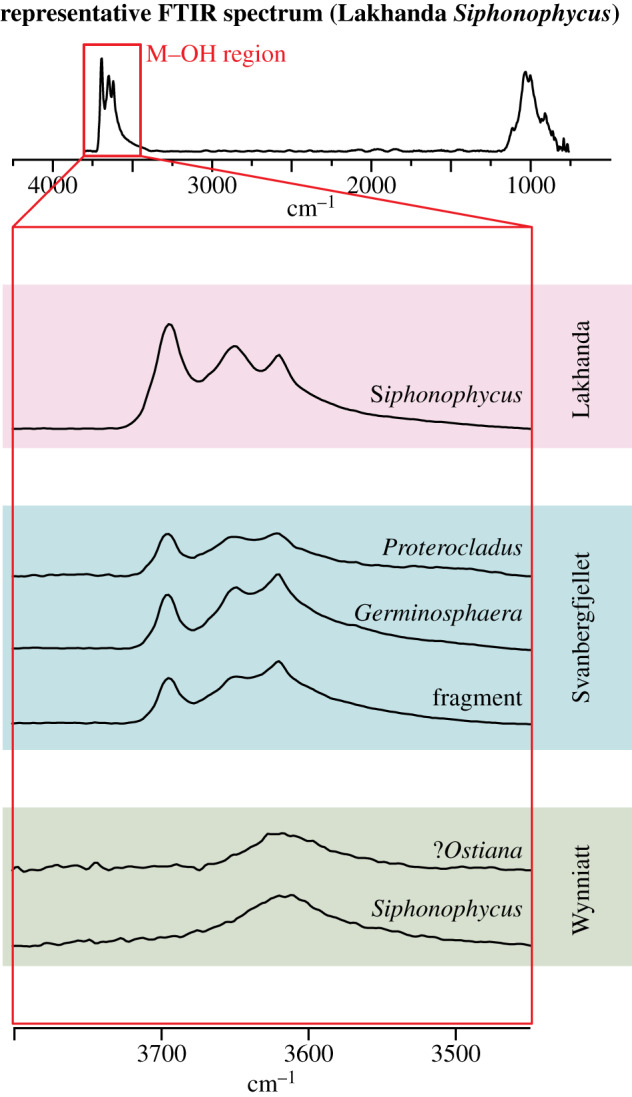
Representative synchrotron-based FTIR microspectra. Top: Representative total spectrum showing an average of Lakhanda Siphonophycus spectra: numbers 188, 189, 190, 203, 204, 205, 218, 219, 220. Both M–OH bands are present plus a broad silicate peak at approximately 1000 cm−1 which is not resolved in sufficient detail to interpret its mineralogical components. Below are representative spectra from the M–OH region for each vertical section plotted on the same vertical scale. Characteristic bands at approximately 3620, approximately 3652 and approximately 3694 cm−1 are recorded for the vertical sections from Lakhanda and Svanbergfjellet, while a single broad band between approximately 3500 and approximately 3700 cm−1 is recorded for the vertical sections from Wynniatt. Representative spectra: Lakhanda Siphonophycus number 237; Svanbergfjellet Proterocladus number 150, Germinosphaera number 150 and microfossil fragment number 263; and Wynniatt Ostiana number 240 and Siphonophycus number 204. See electronic supplementary material, table S2 for FTIR data.
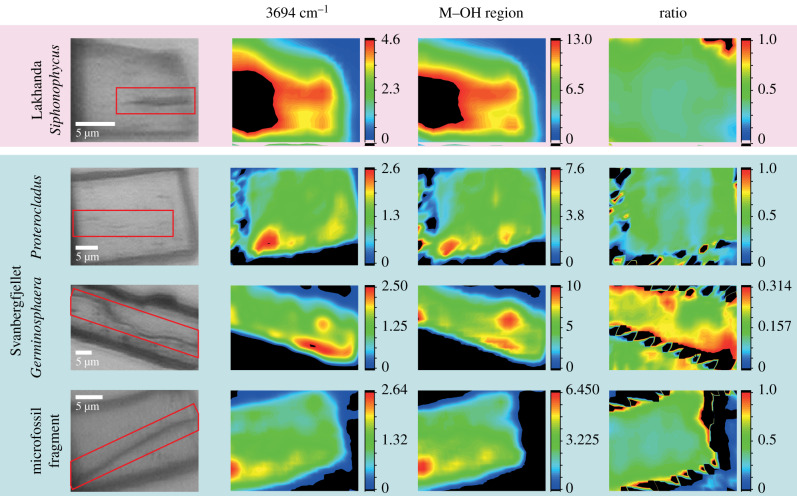
The total area under each band provides a proxy for the distribution of minerals. When mapped across the vertical sections, the areas under the approximately 3694 cm−1 band and the entire M–OH region show some variations between specimens (figure 4) but cannot be fully matched to the EDS maps (figure 1; electronic supplementary material, figure S2). In the Lakhanda Siphonophycus and Svanbergfjellet vertical sections, both FTIR bands are weaker on and around the microfossils, a contrast that is especially evident near hotspots (red) where the bands are locally strong. This is indicative of a lack of OH within microfossils at the approximately 2.8 µm resolution of the FTIR map, rather than a lack of OH groups around microfossils. In the Lakhanda Siphonophycus vertical section, the region where OH bands are weaker extends down vertically, perhaps corresponding to the larger quartz grain below the microfossil (electronic supplementary material, figure S2). The source of the FTIR band hotspots is not evident in light microscopy or EDS images (figure 1; electronic supplementary material, figure S2), but overall we can conclude that kaolinite is present across the bulk of the mineral phase of the four FTIR maps of the vertical sections in figure 4. The large black region on the left side of the Lakhanda Siphonophycus FTIR maps is where the band strength is high and saturated with respect to the colour scale in the image (greater than 4.6 or greater than 13 for the 3694 cm−1 and full M–OH band, respectively). This apparent increase in band strength may include optical effects of the interaction between the infrared beam and the metallic weld close (less than 5 µm) to the attachment of the sample to the TEM grid. The ratio of the approximately 3694 cm−1 band to the entire M–OH region reflects the distribution of kaolinite with respect to total illite [37]. At the approximately 2.8 µm spatial resolution available, a fairly constant abundance of kaolinite, with respect to total illite, across each vertical section is implied by the similar ratios, with no clear correspondence to the microfossils. The black zig-zag patterns along the edges of the vertical section are artefacts resulting from optical scattering from the vertical section. These biomineralogical data from synchrotron-based FTIR microspectroscopy confirm that kaolinite and/or its metamorphic products are present in all vertical sections. Although the FTIR maps do not fully resolve the distribution of minerals with the necessary spatial resolution, in concert with EDS maps they provide a compelling case for kaolinite enrichment adjacent to microfossils.
Figure 4.
Synchrotron-based FTIR microspectroscopy maps of compositional variation indicated by the areas below the approximately 3694 cm−1 band, the entire M–OH region (integrated band intensities in arbitrary units), and the ratio of these two areas, for Lakhanda and Svanbergfjellet vertical sections which show clearly defined M–OH bands. The light microscopy images of vertical sections (left) indicate the position of the microfossils (red boxes). See text for further details.
Further confirmation that kaolinite represents a component of the fossiliferous rocks is evident in bulk powder XRD analyses of hand samples from each locality (table 2), although these do not sample individual laminae. All samples are dominated by muscovite (34–74%, mean = 57.7, s.d. = 21, n=3) and quartz (26–58%, mean = 37, s.d. = 18.2, n = 3); the Svanbergfjellet sample contains minor chlorite (7%) in addition. The reference intensity ratio method detected kaolinite (8%) only in the Lakhanda sample. However, weak but diagnostic peaks manifested as a composite reflection from 060 and/or 33-1 showed that kaolinite is also present in the Svanbergfjellet sample. Relative abundance of clay species can be obtained as a linear function of the area underlying these peaks [36]. This method shows that kaolinite represents 30.7% of total clay (glauconite + illite type 1 + illite type 2 + kaolinite) in the Svanbergfjellet sample and 66.2% in the Lakhanda sample. The high kaolinite content of the Lakhanda sample is consistent with mineralogical studies of these strata [41]. The absence of kaolinite from powder XRD and the high illite content (90%) of the Wynniatt sample is consistent with the broad M–OH band in the FTIR data, perhaps reflecting complete transformation of any precursor kaolinite to illite/muscovite during contact metamorphism [38]. 060 and/or 33-1 composite reflections suggest that glauconite (10%) is also present in the Wynniatt sample.
Table 2.
Bulk powder XRD results. Percentage of each rock sample composed of different minerals is calculated by the reference intensity ratio method [35]. Clay speciation is also presented as a percentage of total clay (glauconite + illite 1 + illite 2 + kaolinite) using the relative areas of weak but diagnostic peaks from 060/33-1 [36]. Qtz = quartz, Musc = muscovite, Chl = chlorite, Kaol = kaolinite, Gl = glauconite, and Il1 and Il2 = two varieties of illite. Lakhanda sample LK67, Svanbergfjellet sample 99-L-15, and Wynniatt sample 88-KL-131.
| reference intensity ratio % |
060/33-1 % total clay |
|||||||
|---|---|---|---|---|---|---|---|---|
| Qtz | Musc | Chl | Kaol | Gl | Il1 | Il2 | Kaol | |
| Lakhanda | 58 | 34 | 0 | 8 | 0 | 0 | 33.8 | 66.2 |
| Svanbergfjellet | 27 | 65 | 7 | 0 | 0 | 6.6 | 62.6 | 30.7 |
| Wynniatt | 26 | 74 | 0 | 0 | 10 | 90 | 0 | 0 |
4. Discussion
4.1. Kaolinite and Proterozoic fossilization
The identification of aluminium haloes surrounding microfossils, hosted in kaolinite and its metamorphic products illite/muscovite, prompts comparisons with Cambrian BST fossilization. Strata with BST fossilization globally are enriched in berthierine (a diagenetic product of kaolinite when iron is present) [9,10]. Recently, it has also been observed that metazoan fossils from the Burgess Shale at the Walcott Quarry (Canada) are themselves enriched in kaolinite compared with their surrounding mudstone matrix [19,20]. The kaolinite enrichment on Burgess Shale fossils is attributed to bonding between the fossil organic matter and the mineral during organism decay, which is inferred to have slowed or arrested subsequent transformation of fossil-associated kaolinite to other minerals during greenschist facies metamorphism [20,42]. This interpretation implies that local decreases in pH induced by decay [43] result in positively charged carcase organic matter, facilitating bonding with negatively charged edge sites on kaolinite [e.g. 44–46]. These edge sites, which account for 10–20% of the surface area of the mineral [45], are known to be relatively acidic, increasing the likelihood that they would persist in a negatively charged state at relatively low pH, maximizing the probability of binding to organic matter [47,48]. It is unclear whether kaolinite attached to fossil organic matter was from the sediment, or whether it precipitated in situ. It has been argued that the chemical properties of kaolinite were a significant factor in the fossilization of Burgess Shale metazoans [19,20]. Kaolinite is known to promote polymerization and adsorption of a variety of organic molecules [44,45,49] and to trigger kerogen maturation upon pyrolysis [50]. It can stabilize pre-existing organic cross-links via the donation of electrons, reducing double bonds [51], as well as inhibit the growth of bacteria that promote decay [11]. Experimental studies have shown that kaolinite–organic interactions often promote the conservation of morphology in decaying metazoan tissues [12–18].
The aluminosilicate haloes surrounding the microfossils studied here suggest that kaolinite attachment and/or in situ precipitation onto microbial cell walls or enclosing sheaths, via a similar process to BST fossilization, was a significant factor in the preservation of microfossils in Proterozoic mudstones. These data add to recent studies that report concentrations of elements indicative of clays (e.g. aluminium and iron) adjacent to other Proterozoic fossils [21–23]. The organisms studied here probably represent a variety of evolutionary clades, including eukaryotic chlorophyte algae and cyanobacteria [24–27,29]. The cell walls and extracellular sheaths of these organisms were composed of different biomolecules (table 1) from those of the metazoans preserved in BST deposits, with variable resistance to decay [30,31]. Cyanobacteria cells are commonly enclosed by sheaths composed of chemically distinct carbohydrate fibrils which are relatively resistant to decay [32]—in the case of Siphonophycus, for example. Ostiana, on the other hand, may not possess a sheath; the cell walls probably comprised lipids and proteins [33]. Chlorophytes are dominated by carbohydrates, including cellulose, which make up the cell walls. In addition, acanthomorphic acritarchs, such as Germinosphaera, are commonly characterized by highly resistant walls of aliphatic composition similar to sporopollenin or algaenan [34]. By contrast, metazoans, like those represented in BST deposits, are composed of a range of biomolecules including proteins, carbohydrates and lipids, although sclerotized and cuticularized tissues are preferentially preserved [52]. The similarity of kaolinite enrichments in fossils representing these different clades suggests that this type of fossilization is not biopolymer specific. Nor is it morphology dependent—the fossils studied here range in morphology from filaments and spheroids to multicellular remains and, in the case of Burgess Shale fossils, macroscopic forms. Indeed, numerous studies have already shown that clay minerals commonly attach to the organics of microbial organisms such as cyanobacteria in natural and experimental systems [53–58]. The enrichments of kaolinite adjacent to microfossil cell walls argue not only for a role for kaolinite in fossilization but, through organism–mineral interactions, a role for incipient microfossils in facilitating kaolinite enrichment in the first place. A similar process has also been used as an explanation for silica enrichments surrounding microfossils [59].
4.2. Biases of the Proterozoic shale-hosted microfossil record
Identifying a taphonomic role for kaolinite in Proterozoic mudstones, the dominant lithology for early microfossils [4], indicates that the Proterozoic mudstone-hosted fossil record may be biased to environmental settings that were rich in kaolinite or hosted conditions conducive to its formation, giving us pause for thought when considering temporal/spatial patterns in the Proterozoic microfossil record. Kaolinite today is primarily sourced from tropical weathering regimes where drainage is high and soil pH low [39]. Palaeogeographic reconstructions suggest that each of the studied deposits (Lakhanda Group––Siberia, Svanbergfjellet Formation––East Svalbard and Wynniatt Formation––Laurentia) formed in tropical to mid-palaeolatitudes [60], consistent with this interpretation. Moreover, reconstruction of seawater pH through geological time suggests that Neoproterozoic oceans were characterized by lower pH than their modern counterparts [61]. Such a palaeoenvironmental bias to fossilization is likely to be more acute for organisms where preservation of delicate morphology is required. The three assemblages studied include rarely preserved morphologies that were presumably more fragile (e.g. multi-celled, slender spines/processes) than those from other Proterozoic localities with microfossils [24–26,29]. It is not clear from our work whether the presence of kaolinite in the local environment is sufficient or kaolinite is required at a specific concentration.
4.3. Implications for our understanding of the antiquity of metazoans
Our results have specific implications for understanding the emergence of metazoan life. There is a disconnect between molecular clock estimates for the antiquity of metazoans and their earliest body fossils. A recent molecular analysis placed the last common ancestor of extant metazoans at 833–650 Ma [62], yet unambiguous body fossil evidence extends only as far as approximately 580 Ma (for example [63]). Where are the missing pre-580 Ma metazoan fossils? Can the gap be explained by taphonomic bias [64]? BST fossilization provides an unusually comprehensive picture of Cambrian metazoan diversity [62,65–67]; even microscopic metazoans are represented [68,69]. The demonstration that some Neoproterozoic mudstones share taphonomic pathways with BST deposits identifies at least three Tonian targets for early metazoan fossils. However, the microfossils in these assemblages represent microbial eukaryotes and bacteria [2,24–29,70,71]. Some large morphologically complex microfossils in Ediacaran mudstones have been interpreted as egg and/or diapause cysts of metazoans [72] and a similar interpretation has been advanced for a population of Tonian microfossils [73], but other interpretations have been proposed [74]. Macroscopic fossils, e.g. Chuaria and Tawuia, occur in two of the deposits (Svanbergfjellet and Wynniatt) [24–26,29], and although recent evidence suggests that some of these may be multicellular there is no evidence that they represent metazoans [75]. The lack of metazoan fossils in these deposits despite BST conditions conducive to their fossilization may indicate that metazoans had not evolved by approximately 800 Ma. If so, this provides a soft maximum age (a maximum age based on the probability that fossils of this age are not metazoans) for their appearance, as suggested previously [68] and employed in some molecular clocks [65] but without consideration of taphonomy. It could be argued that, even though metazoans are not a component of the three assemblages that we investigated, they could be present in others of similar age, or that small early metazoans are not preserved even by BST fossilization. Nonetheless this soft maximum age can now be applied with greater confidence.
4.4. Clay mineral–organic interactions and the search for fossils on Mars
Our results may also be relevant to the search for fossilized life on Mars [76]. A variety of clay minerals have been identified on Mars on the basis of orbital infrared spectroscopy, including illite, kaolinite, smectites, chlorites and serpentine minerals [77–79]. Clay minerals have been identified in crustal rocks that may encompass a wide range of palaeoenvironmental conditions. These deposits have been variably interpreted as in situ weathering profiles, fine-grained clastic sediments and hydrothermally altered crust [77–79]. Our data suggest that specific interactions between kaolinite mineral surfaces and solution may have facilitated the polymerization of organic molecules––a key step in promoting fossilization. These reactions, however, are dependent on the pH of the solution relative to the acidity of clay edge sites. It is reasonable to assume that these same principles apply to the early Martian surface; in suitable chemical conditions, kaolinite may have served a similar taphonomic role to that envisaged for samples described here. However, available data indicate that clay-rich deposits on early Mars experienced a much broader range in pore/bottom water pH, variable contact times with liquid water and large fluctuations in ionic strength [79,80]. Thus, taphonomically favourable clay mineral assemblages on Mars are best diagnosed in light of depositional and diagenetic constraints on fluid chemistry and a mechanistic consideration of clay mineral–organic interactions.
5. Conclusion
An understanding of taphonomy is critical to the interpretation of morphology and the environmental/temporal ranges of fossil taxa, and is particularly important for Proterozoic palaeobiology where biomineralized tissues are absent. Kaolinite or its metamorphic equivalents are associated with all microfossils in the three Tonian deposits we studied, despite their phylogenetic, compositional and stratigraphic diversity. Our search for new microfossils, particularly fragile metazoans, in Proterozoic strata should focus on sites where kaolinite is likely to be present. These search criteria may also be valid when exploring other planets, such as Mars [76], for evidence of past life. Further studies should expand this investigation to a wider variety of Proterozoic fossiliferous mudstones, such as those of the Ediacaran Doushantuo Formation which do not contain complex metazoans [81], in order to determine the prevalence of appropriate taphonomic conditions across time and palaeoenvironments.
Supplementary Material
Supplementary Material
Acknowledgements
We are grateful to N. Butterfield for access to fossils, valuable discussion and comments on a draft of this paper. R.P.A. thanks the organizers of the Royal Society Discussion Meeting for the invitation to present at the meeting, in addition to P. Donoghue for valuable discussion and H. Bechtel, E. Clark, K. Clayton, B. Johnson, W. Samela and R. Tostevin for assistance with sample preparation and data collection. Diamond Light Source is acknowledged for beamtime SM15975-1 and SM21059-1 at MIRIAM beamline B22. Two anonymous reviewers provided constructive comments.
Data accessibility
Synchrotron-based Fourier transform infrared microspectroscopy data collected at the Diamond Light Source, Didcot, UK, and used to build figure 4 are available in the electronic supplementary material.
Authors' contributions
R.P.A., N.J.T., K.D.B. and D.E.G.B. designed the research. A.H.K. provided fossils. A.A. prepared vertical sections. R.P.A. and G.M.H. analysed vertical sections using SEM and EDS. R.P.A., G.C., M.D.F. and I.L. analysed vertical sections using synchrotron-based FTIR microspectroscopy. R.P.A. and N.J.T. analysed fossiliferous rocks using XRD. R.P.A. wrote the manuscript with discussion and input from all authors.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by All Souls College, Diamond Light Source (SM15975-1 and SM21059-1), Geological Society of America, Geological Society of London, NASA Astrobiology Institute (NNA13AA90A), Yale Institute for Biospheric Studies and Yale Peabody Museum of Natural History. R.P.A. was supported by All Souls College and NASA (NNX14AP10H). This work was performed in part at the Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Coordinated Infrastructure Network (NNCI), which is supported by the National Science Foundation under NSF award no. 1541959. CNS is part of Harvard University.
References
- 1.Knoll AH. 2003. Life on a young planet: the first 3 billion years of evolution on earth. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Butterfield NJ. 2015. Early evolution of the Eukaryota. Palaeontology 58, 5–17. ( 10.1111/pala.12139) [DOI] [Google Scholar]
- 3.Butterfield NJ. 2015. Proterozoic photosynthesis—a critical review. Palaeontology 58, 953–972. ( 10.1111/pala.12211) [DOI] [Google Scholar]
- 4.Cohen PA, Macdonald FA. 2015. The Proterozoic record of eukaryotes. Paleobiology 41, 610–632. ( 10.1017/pab.2015.25) [DOI] [Google Scholar]
- 5.Knoll AH. 2014. Paleobiological perspectives on early eukaryotic evolution. Cold Spring Harb. Perspect. Biol. 6, a016121 ( 10.1101/cshperspect.a016121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muscente AD, et al. 2017. Exceptionally preserved fossil assemblages through geologic time and space. Gondwana Res. 48, 164–188. ( 10.1016/j.gr.2017.04.020) [DOI] [Google Scholar]
- 7.Butterfield NJ. 1995. Secular distribution of Burgess-Shale-type preservation. Lethaia 28, 1–13. ( 10.1111/j.1502-3931.1995.tb01587.x) [DOI] [Google Scholar]
- 8.Gaines RR. 2014. Burgess Shale-type preservation and its distribution in space and time. Paleontol. Soc. Pap. 20, 123–146. ( 10.1017/S1089332600002837) [DOI] [Google Scholar]
- 9.Anderson RP, Tosca NJ, Gaines RR, Mongiardino Koch N, Briggs DEG. 2018. A mineralogical signature for Burgess Shale-type fossilization. Geology 46, 347–350. ( 10.1130/G39941.1) [DOI] [Google Scholar]
- 10.Saleh F, Pittet B, Perrillat J-P, Lefebvre B. 2019. Orbital control on exceptional fossil preservation. Geology 47, 103–106. ( 10.1130/G45598.1) [DOI] [Google Scholar]
- 11.McMahon S, Anderson RP, Saupe EE, Briggs DEG. 2016. Experimental evidence that clay inhibits bacterial decomposers: implications for preservation of organic fossils. Geology 44, 867–870. ( 10.1130/G38454.1) [DOI] [Google Scholar]
- 12.Naimark E, Kalinina M, Shokurov A, Boeva N, Markov A, Zaytseva L. 2016. Decaying in different clays: implications for soft-tissue preservation. Palaeontology 59, 583–595. ( 10.1111/pala.12246) [DOI] [Google Scholar]
- 13.Naimark EB, Kalinina MA, Shokurov AV, Markov AV, Boeva NM. 2016. Decaying of Artemia salina in clay colloids: 14-month experimental formation of subfossils. J. Paleontol. 90, 472–484. ( 10.1017/jpa.2016.23) [DOI] [Google Scholar]
- 14.Wilson LA, Butterfield NJ. 2014. Sediment effects on the preservation of Burgess Shale-type compression fossils. Palaios 29, 145–154. ( 10.2110/palo.2013.075) [DOI] [Google Scholar]
- 15.Martin D, Briggs DEG, Parkes RJ. 2003. Experimental mineralization of invertebrate eggs and the preservation of Neoproterozoic embryos. Geology 31, 39–42. () [DOI] [Google Scholar]
- 16.Naimark E, Kalinina M, Boeva N. 2018. Persistence of external anatomy of small crustaceans in a long term taphonomic experiment. Palaios 33, 154–163. ( 10.2110/palo.2017.083) [DOI] [Google Scholar]
- 17.Naimark E, Kalinina M, Shokurov A, Markov A, Zaytseva L, Boeva N. 2018. Mineral composition of host sediments influences the fossilization of soft tissues. Can. J. Earth Sci. 55, 1271–1283. ( 10.1139/cjes-2017-0237) [DOI] [Google Scholar]
- 18.Newman S, Daye M, Fakra SC, Marcus MA, Pajusalu M, Pruss SB, Smith EF, Bosak T. 2019. Experimental preservation of muscle tissue in quartz sand and kaolinite. Palaios 34, 437–451. ( 10.2110/palo.2019.030) [DOI] [Google Scholar]
- 19.Orr PJ, Briggs DEG, Kearns SL. 1998. Cambrian Burgess Shale animals replicated in clay minerals. Science 281, 1173–1175. ( 10.1126/science.281.5380.1173) [DOI] [PubMed] [Google Scholar]
- 20.Anderson RP. 2019. Clay minerals and the fossilisation of early complex life. In The origin and rise of complex life: integrating models, geochemical and palaeontological data (eds Wood R, Liu AG, Lenton TM, Poulton SW, Donoghue PCJ). London, UK: The Royal Society. [Google Scholar]
- 21.Anderson EP, Schiffbauer JD, Xiao SH. 2011. Taphonomic study of Ediacaran organic-walled fossils confirms the importance of clay minerals and pyrite in Burgess Shale-type preservation. Geology 39, 643–646. ( 10.1130/G31969.1) [DOI] [Google Scholar]
- 22.Wacey D. et al 2016. Contrasting microfossil preservation and lake chemistries within the 1200–1000 Ma Torridonian Supergroup of NW Scoland. In Earth system evolution and early life: a celebration of the work of Martin Brasier (eds Brasier AT, McIlroy D, McLoughlin N). London, UK: The Geological Society. [Google Scholar]
- 23.Wacey D, Saunders M, Roberts M, Menon S, Green L, Kong C, Culwick T, Strother P, Brasier MD. 2014. Enhanced cellular preservation by clay minerals in 1 billion-year-old lakes. Sci. Rep. 4, 5841 ( 10.1038/srep05841) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermann TN, Podkovyrov VN. 2010. A discovery of Riphean heterotrophs in the Lakhanda Group of Siberia. Paleontol. J. 44, 374–383. [Google Scholar]
- 25.Hermann TN. 1990. Organic world one billion years ago. Leningrad, Russia: Nauka. [Google Scholar]
- 26.Butterfield NJ, Knoll AH, Swett K. 1994. Paleobiology of the Neoproterozoic Svanbergfjellet Formation, Spitsbergen. Foss. Strata 34, 1–84. ( 10.1111/j.1502-3931.1994.tb01558.x) [DOI] [Google Scholar]
- 27.Tang Q, Pang K, Yuan X, Xiao S. 2020. A one-billion-year-old multicellular chlorophyte. Nat. Ecol. Evol. 4, 543–549. ( 10.1038/s41559-020-1122-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Butterfield NJ. 2005. Probable Proterozoic fungi. Paleobiology 31, 165–182. () [DOI] [Google Scholar]
- 29.Butterfield NJ, Rainbird RH. 1998. Diverse organic-walled fossils, including ‘possible dinoflagellates,’ from the early Neoproterozoic of arctic Canada. Geology 26, 963–966. () [DOI] [Google Scholar]
- 30.Briggs DEG, Summons RE. 2014. Ancient biomolecules: their origins, fossilization, and role in revealing the history of life. BioEssays 36, 482–490. ( 10.1002/bies.201400010) [DOI] [PubMed] [Google Scholar]
- 31.Tegelaar EW, de Leeuw JW, Derenne S, Largeau C.. 1989. A reappraisal of kerogen formation. Geochim. Cosmochim. Acta 53, 3103–3106. ( 10.1016/0016-7037(89)90191-9) [DOI] [Google Scholar]
- 32.Hoiczyk E. 1998. Structural and biochemical analysis of the sheath of Phormidium uncinatum. J. Bacteriol. 180, 3923–3932. ( 10.1128/JB.180.15.3923-3932.1998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh K, Jones GJ, Dunstan RH. 1997. Effect of irradiance on fatty acid, carotenoid, total protein composition and growth of Microcystis aeruginosa. Photochemistry 44, 817–824. ( 10.1016/S0031-9422(96)00573-0) [DOI] [Google Scholar]
- 34.Bernard S, Benzerara K, Beyssac O, Balan E, Brown GE Jr. 2015. Evolution of the macromolecular structure of sporopollenin during thermal degradation. Heliyon 1, e00034 ( 10.1016/j.heliyon.2015.e00034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Snyder RL, Bish DL. 1989. Quantitative analysis. Rev. Mineral. Geochem. 20, 101–144. [Google Scholar]
- 36.Środoń J, Drits VA, McCarty DK, Hsieh JCC, Eberl DD. 2001. Quantitative X-ray diffraction analysis of clay-bearing rocks from random preparations. Clays Clay Miner. 49, 514–528. ( 10.1346/CCMN.2001.0490604) [DOI] [Google Scholar]
- 37.Russell JD, Fraser AR. 1994. Infrared methods. In Clay mineralogy: spectroscopic and chemical determinative methods (ed. Wilson MJ.), pp. 11–67. London, UK: Champman and Hall. [Google Scholar]
- 38.Thomson D, Rainbird RH, Dix G. 2014. Architecture of a Neoproterozoic intracratonic carbonate ramp succession: Wynniatt Formation, Amundsen Basin, Arctic Canada. Sediment. Geol. 299, 119–138. ( 10.1016/j.sedgeo.2013.11.005) [DOI] [Google Scholar]
- 39.Wilson MJ. 2013. Rock forming minerals, 3C: clay minerals. London, UK: The Geological Society. [Google Scholar]
- 40.Marshall CP, Javaux EJ, Knoll AH, Walter MR. 2005. Combined micro-Fourier transform infrared (FTIR) spectroscopy and micro-Raman spectroscopy of Proterozoic acritarchs: a new approach to Palaeobiology. Precambrian Res. 138, 208–224. ( 10.1016/j.precamres.2005.05.006) [DOI] [Google Scholar]
- 41.Podkovyrov VN. 2009. Mesoproterozoic Lakhanda Lagerstätte, Siberia: Paleoecology and taphonomy of the microbiota. Precambrian Res. 173, 146–153. ( 10.1016/j.precamres.2009.04.006) [DOI] [Google Scholar]
- 42.Powell W. 2003. Greenschist-facies metamorphism of the Burgess Shale and its implications for models of fossil formation and preservation. Can. J. Earth Sci. 40, 13–25. ( 10.1139/e02-103) [DOI] [Google Scholar]
- 43.Briggs DEG, Kear AJ. 1993. Fossilization of soft tissue in the laboratory. Science 259, 1439–1442. ( 10.1126/science.259.5100.1439) [DOI] [PubMed] [Google Scholar]
- 44.Skujinš J, Pukite A, McLaren AD. 1974. Adsorption and activity of chitinase on kaolinite. Soil Biol. Biochem. 6, 179–182. ( 10.1016/00380717(74)90024-8) [DOI] [Google Scholar]
- 45.Theng BKG. 1974. The chemistry of clay-organic reactions. New York, NY: John Wiley & Sons. [Google Scholar]
- 46.Yu WH, Li N, Tong DS, Zhou CH, Lin CX, Xu CY. 2013. Adsorption of proteins and nucleic acids on clay minerals and their interactions: a review. Appl. Clay Sci. 80–81, 443–452. ( 10.1016/j.clay.2013.06.003) [DOI] [Google Scholar]
- 47.Sposito G. 1984. The surface chemistry of soils. Oxford, UK: Oxford University Press. [Google Scholar]
- 48.Liu X, Lu X, Sprik M, Cheng J, Meijer EJ, Wang R. 2013. Acidity of edge surface sites of montmorillonite and kaolinite. Geochim. Cosmochim. Acta 117, 180–190. ( 10.1016/j.gca.2013.04.008) [DOI] [Google Scholar]
- 49.Solomon DH, Rosser MJ. 1965. Reactions catalyzed by minerals. Part 1. Polymerization of styrene. J. App. Polym. Sci. 9, 1261–1271. ( 10.1002/app/1965.070090407) [DOI] [Google Scholar]
- 50.Pan C, Geng A, Zhong N, Liu J. 2010. Kerogen pyrolysis in the presence and absence of water and minerals: steranes and triterpenoids. Fuel 89, 336–345. ( 10.1016/j.fuel.2009.06.032) [DOI] [Google Scholar]
- 51.Stimler NP, Tanzer ML. 1977. Location of the intermolecular cross linking sites in collagen. In Protein crosslinking (ed. Friedman M.), pp. 675–697. Boston, MA: Springer. [DOI] [PubMed] [Google Scholar]
- 52.Saleh F, Antcliffe JB, Lefebvre B, Pittet B, Laibl L, Perez F, Lustri L, Gueriau P, Daley AC. 2020. Taphonomic bias in exceptionally preserved biotas. Earth Planet. Sci. Lett. 529, 115873 ( 10.1016/j.epsl.2019.115873) [DOI] [Google Scholar]
- 53.Konhauser KO, Fisher QJ, Fyfe WS, Longstaffe FJ, Powell MA. 1998. Authigenic mineralization and detrital clay binding by freshwater biofilms: the Brahmani River, India. Geomicrobiol. J. 15, 209–222. ( 10.1080/01490459809378077) [DOI] [Google Scholar]
- 54.Konhauser KO, Fyfe WS, Ferris FG, Beveridge TJ. 1993. Metal sorption and mineral precipitation by bacteria in two Amazonian river systems: Rio Solimoes and Rio Negro, Brazil. Geology 161, 399–413. [Google Scholar]
- 55.Konhauser KO, Urrutia MO. 1999. Bacterial clay authigenesis: a common biogeochemical process. Chem. Geol. 161, 399–413. ( 10.1016/S0009-2541(99)00118-7) [DOI] [Google Scholar]
- 56.Ferris FG, Fyfe WS, Beveridge TJ. 1988. Metallic ion binding by Bacillus subtilis: implications for the fossilization of microorganisms. Geology 16, 149–152. () [DOI] [Google Scholar]
- 57.Newman SA, Klepac-Ceraj V, Mariotti G, Pruss SB, Watson N, Bosak T. 2017. Experimental fossilization of mat-forming cyanobacteria in coarse-grained siliciclastic sediments. Geobiology 15, 484–498. ( 10.1111/gbi.12229) [DOI] [PubMed] [Google Scholar]
- 58.Newman SA, Mariotti G, Pruss SB, Bosak T. 2016. Insights into cyanobacterial fossilization in Ediacaran siliciclastic environments. Geology 44, 579–582. ( 10.1130/G37791.1) [DOI] [Google Scholar]
- 59.Knoll AH. 1985. Exceptional preservation of photosynthetic organisms in silicified carbonates and silicified peats. Phil. Trans. R. Soc. Lond. B 311, 111–122. ( 10.1098/rstb.1985.0143) [DOI] [Google Scholar]
- 60.Li Z-X, Evans DAD, Halverson GP. 2013. Neoproterozoic glaciations in a revised global palaeogeography from the breakup of Rodinia to the assembly of Gondwanaland. Sediment. Geol. 294, 219–232. ( 10.1016/j.sedgeo.2013.05.016) [DOI] [Google Scholar]
- 61.Halevy I, Bachan A. 2017. The geologic history of seawater pH. Science 355, 1069–1071. ( 10.1126/science.aal4151) [DOI] [PubMed] [Google Scholar]
- 62.dos Reis M, Thawornwattana Y, Angelis K, Telford MJ, Donoghue PCJ, Yang Z. 2015. Uncertainty in the timing of origin of animals and the limits of precision in molecular timescales. Curr. Biol. 25, 2939–2950. ( 10.1016/j.cub.2015.09.066) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bobrovskiy I, Hope JM, Ivantsov A, Nettersheim BJ, Hallmann C, Brocks JJ. 2018. Ancient steroids establish the Ediacaran fossil Dickinsonia as one of the earliest animals. Science 361, 1246–1249. ( 10.1126/science.aat7228) [DOI] [PubMed] [Google Scholar]
- 64.Runnegar B. 1982. The Cambrian explosion: animals or fossils? J. Geol. Soc. Aust. 29, 395–411. ( 10.1080/00167618208729222) [DOI] [Google Scholar]
- 65.Betts HC, Puttick MN, Clark JW, Williams TA, Donoghue PCJ, Pisani D. 2018. Integrated genomic and fossil evidence illuminates life's early evolution and eukaryote origin. Nature Ecology and Evolution 2, 1556–1562. ( 10.1038/s41559-018-0644-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Briggs DEG, Erwin DH, Collier FJ. 1994. Fossils of the Burgess Shale. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 67.Hou X, Siveter DJ, Siveter DJ, Aldridge RJ, Cong P, Gabbott SE, Ma X, Purnell MA, Williams M. 2017. The Cambrian fossils of Chengjiang, China: the flowering of early animal life, 2nd edn. Oxford, UK: John Wiley & Sons Ltd. [Google Scholar]
- 68.Butterfield NJ, Harvey THP. 2012. Small carbonaceous fossils (SCFs): a new measure of early Paleozoic paleobiology. Geology 40, 71–74. ( 10.1130/g32580.1) [DOI] [Google Scholar]
- 69.Harvey THP, Butterfield NJ. 2017. Exceptionally preserved Cambrian loriciferans and the early animal invasion of the meiobenthos. Nat. Ecol. Evol. 1, 0022 ( 10.1038/s41559-016-0022) [DOI] [PubMed] [Google Scholar]
- 70.Butterfield NJ. 2004. A vaucheriacean alga from the middle Neoproterozoic of Spitsbergen: implication for the evolution of Proterozoic eukaryotes and the Cambrian explosion. Paleobiology 30, 231–252. () [DOI] [Google Scholar]
- 71.Butterfield NJ. 2009. Modes of pre-Ediacaran multicellularity. Precambrian Res. 173, 201–211. ( 10.1016/j.precamres.2009.01.008) [DOI] [Google Scholar]
- 72.Cohen PA, Knoll AH, Kodner RB. 2009. Large spinose microfossils in Ediacaran rocks as resting stages of early animals. Proc. Natl Acad. Sci. USA 106, 6519–6524. ( 10.1073/pnas.0902322106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cornet Y, François C, Compère P, Callec Y, Roberty S, Plumier JC, Javaux EJ. 2019. New insights on the paleobiology, biostratigraphy, and paleogeography of the pre-Sturtian microfossil index taxon Cerebrosphaera. Precambrian Res. 322, 105410 ( 10.1016/j.precamres.2019.105410) [DOI] [Google Scholar]
- 74.Shang X, Liu P, Moczydłowksa M. 2019. Acritarchs from the Doushantuo Formation at Liujing section in Songlin area of Guizhou Province, South China: implications for early-middle Ediacaran biostratigraphy. Precambrian Res. 334, 105453 ( 10.1016/j.precamres.2019.105453) [DOI] [Google Scholar]
- 75.Tang Q, Pang T, Yuan X, Xiao S. 2017. Electron microscopy reveals evidence for simple multicellularity in the Proterozoic fossil Chuaria. Geology 45, 75–78. ( 10.1130/G38680.1) [DOI] [Google Scholar]
- 76.McMahon S, Bosak T, Grotzinger JP, Milliken RE, Summons RE, Newman S, Fraeman A, Williford KH, Briggs DEG. 2018. A field guide to finding fossils on Mars. J. Geophys. Res. Planets 123, 1012–1040. ( 10.1029/2017JE005478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ehlmann BL, Mustard JF, Murchie SL, Bibring J-P, Meunier A, Fraeman AA, Langevin Y. 2011. Subsurface water and clay mineral formation during the early history of Mars. Nature 479, 53–60. ( 10.1038/nature10582) [DOI] [PubMed] [Google Scholar]
- 78.Ehlmann BL, et al. 2009. Identification of hydrated silicate minerals on Mars using MRO-CRISM: geologic context near Nili Fossae and implications for aqueous alteration. J. Geophys. Res. Planets 114, E00D08 ( 10.1029/2009JE003339) [DOI] [Google Scholar]
- 79.Cino CD, Dehouck E, McLennan SM. 2017. Geochemical constraints on the presence of clay minerals in the Burns formation, Meridiani Planum, Mars. Icarus 281, 137–150. ( 10.1016/j.icarus.2016.08.029) [DOI] [Google Scholar]
- 80.Cuadros J. 2015. Clays are messy—also on Mars. Am. Mineral. 100, 669–670. ( 10.2138/am-2015-5229) [DOI] [Google Scholar]
- 81.Xiao S, Yuan X, Steiner M, Knoll AH. 2002. Macroscopic carbonaceous compressions in a terminal Proterozoic shale: a systematic reassessment of the Miaohe biota, South China. J. Paleontol. 76, 347–376. ( 10.1017/S0022336000041743) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Synchrotron-based Fourier transform infrared microspectroscopy data collected at the Diamond Light Source, Didcot, UK, and used to build figure 4 are available in the electronic supplementary material.