Abstract
Atlantic salmon smolts (approx. 20-months old) were fed experimental diets with different combinations of omega-6:omega-3 fatty acids (FAs) (high-ω6, high-ω3, or balanced) and eicosapentaenoic acid plus docosahexaenoic acid (EPA + DHA) levels (0.3, 1.0 or 1.4%) for 12 weeks. Muscle FA (% total FA) reflected dietary C18-polyunsaturated FA; however, muscle EPA per cent and content (mg g−1) were not different in salmon fed high-ω3 or balanced diets. Muscle DHA per cent was similar among treatments, while DHA content increased in fish fed 1.4% EPA + DHA, compared with those fed 0.3–1.0% EPA + DHA combined with high-ω6 FA. Muscle 20:3ω6 (DGLA) content was highest in those fed high-ω6 with 0.3% EPA + DHA. Quantitative polymerase chain reaction analyses on liver RNA showed that the monounsaturated FA synthesis-related gene, scdb, was upregulated in fish fed 1.0% EPA + DHA with high-ω6 compared to those fed 0.3% EPA + DHA. In high-ω3-fed salmon, liver elovl2 transcript levels were higher with 0.3% EPA + DHA than with 1.0% EPA + DHA. In high-ω6-fed fish, elovl2 did not vary with EPA + DHA levels, but it was positively correlated with muscle ARA, 22:4ω3 and DGLA. These results suggest dietary 18:3ω3 elongation contributed to maintaining muscle EPA + DHA levels despite a two- to threefold change in dietary proportions, while 18:2ω6 with 0.3% EPA + DHA increased muscle DGLA more than arachidonic acid (ARA). Positive correlations between hepatic elovl2 and fabp10a with muscle ω6:ω3 and EPA + DHA + ARA, respectively, were confirmed by reanalysing data from a previous salmon trial with lower variations in dietary EPA + DHA and ω6:ω3 ratios.
This article is part of the theme issue ‘The next horizons for lipids as ‘trophic biomarkers’: evidence and significance of consumer modification of dietary fatty acids’.
Keywords: diet, EPA and DHA, ω6:ω3 ratio, lipid metabolism biomarkers
1. Introduction
Fish are important sources of eicosapentaenoic acid (EPA, 20:5ω3) and docosahexaenoic acid (DHA, 22:6ω3), which are physiologically necessary fatty acids (FAs) for humans. There is a growing gap between the supply of traditional fish oil (FO) sources of EPA and DHA, and demand by both the aquafeed and the human omega-3 (ω3) capsule industries [1]. This has led to the current trend of maximizing replacement of FO with vegetable alternatives in farmed salmon diets which affects fillet nutritional value, and has increased the risk of reducing salmon flesh EPA + DHA levels for consumers [2]. Thus, the study of molecular mechanisms involved in the modification of dietary FA and consequences for fish muscle FA composition remain a current priority in aquaculture research [3–5]. Fatty acyl desaturases and elongases are the main enzymes contributing to long-chain polyunsaturated FA (LC-PUFA) synthesis from FA precursors. The activity of these enzymes increases by replacing FO with vegetable oils (VO), which are very low in LC-PUFA but are rich in C18 unsaturated FA [6,7]. Furthermore, different C18 PUFA (e.g. 18:2ω6 and 18:3ω3) might have different roles in activating the elongation and desaturation pathways [8]. The ability of teleosts to elongate and desaturate C18 PUFA may be driven by trophic availability which differs in freshwater and marine environments [9]. Salmonids may have evolved to elongate C18 PUFA just enough to satisfy their physiological requirements, and only store extra LC-PUFA in muscle tissue when provided in excess through the diet [10,11]. Combining both dietary regulators (i.e. lower levels of EPA + DHA with the inclusion of different levels of 18:2ω6 and 18:3ω3) of the elongation and desaturation pathway is a current practice in the aquafeed industry; however, most existing studies on farmed fish have investigated the nutritional effects of either the dietary ω6:ω3 ratio or EPA + DHA levels, rather than their interaction (e.g. [2,4,5,12]). We hypothesized that various combinations of EPA + DHA levels with either high dietary 18:2ω6 or 18:3ω3 would reveal FA desaturation and elongation capacity in Atlantic salmon fed diets with low FO inclusion levels, which in turn would help industry formulate low FO diets with minimized impact on fillet EPA and DHA levels.
Here we examined the impact of different dietary combinations of ω6:ω3 ratios (range: 0.6–4.5) and EPA + DHA levels (0.3, 1.0 and 1.4%) on the transcript levels of FA metabolism-related genes in the liver, and the muscle lipid composition (%) and content (mg g−1) in Atlantic salmon. To support observed patterns between muscle lipid composition (%) and hepatic transcripts, a new statistical analysis was performed using data from a previous salmon feeding trial (1.0–1.3%EPA+DHA) [5] in which levels of EPA + DHA and ratios of ω6:ω3 (range: 0.4–2.7) varied less among the diets used.
2. Material and methods
(a). Diets and animals in the 0.3–1.4%EPA+DHA feeding trial
Five experimental diets were formulated to share all primary sources of macronutrients except for the lipids (electronic supplementary material, table S1). Different sources of dietary lipids were employed so that the experimental diets had contrasting levels of EPA + DHA, and ω6:ω3 ratios. The list comprised lipid sources of animal (i.e. FO and poultry fat) and plant origin (i.e. soy oil, linseed oil and rapeseed oil). Two dietary EPA+DHA levels (0.3 or 1.0% (i.e. 2.9 or 6.7% of total FA)) were combined with two ω6:ω3 ratios (high-ω6 (up to 43.4% of total FAs) or high-ω3 (up to 27.3%)), resulting in four diets: 0.3% EPA+DHA with higher ω6 (0.3%EPA+DHA↑ω6), 0.3% EPA+DHA with higher ω3 (0.3%EPA+DHA↑ω3), 1.0% EPA+DHA with higher ω6 (1%EPA+DHA↑ω6) and 1.0% EPA+DHA with higher ω3 (1%EPA+DHA↑ω3). A fifth diet containing the highest level of EPA and DHA (1.4%) and a more balanced ω6:ω3 ratio (16.4% ω3 FA versus approximately 14.2% ω6 FA) was used as control. All diets contained similar levels of arachidonic acid (ARA; 20:4ω6). Diets were formulated to be isoenergetic, isolipidic and isoproteic, and to supply the fish with the necessary nutritional requirements for salmonids [13]. Basal diets––with no oil addition––were manufactured by EWOS Canada (Surrey, BC, Canada) and top-coated with the different oil mixes (electronic supplementary material, table S1) at the Chute Animal Nutrition Centre (Dalhousie University, Truro, NS, Canada).
A 12-week feeding trial was conducted in the Dr Joe Brown Aquatic Research Building (JBARB; Memorial University, St. John's, NL, Canada). All procedures involving live fish were carried out in compliance with guidelines of the Canadian Council of Animal Care (MUN Animal Care Protocol #16-75-MR). Smolts were transported from a regional salmon farm to the JBARB, where they were PIT (Passive Integrated Transponder)-tagged and kept in a 3800 L tank until experimentation (October 2016–March 2017). Subsequently, 810 salmon of 210 ± 44 g (mean ± standard deviation (s.d.); approx. 20-month old) were randomly distributed into twenty 620 L tanks (40–41 fish per tank; 4 tanks per diet) and allowed to acclimate for eight weeks before switching from a commercial feed (EWOS Dynamic S, Cargill Inc., MN, USA) to the experimental diets. At all stages, fish were fed to satiation overnight using automatic feeders (AVF6 Vibrating Feeders, Pentair Aquatic Eco-Systems, Inc., Florida, USA). The uneaten pellets were collected every morning, and daily feed ration was adjusted based on the number of uneaten pellets. All tanks were exposed to 24 h light and connected to a flow-through seawater system, and water quality (e.g. temperature (approx. 12°C) and oxygen saturation (approx. 10 mg ml−1)) were monitored daily.
(b). Sampling
Ten fish in total (each from a different tank) at the beginning of the trial (i.e. initial) and five fish/tank at the end of the trial (i.e. week 12) were euthanized and dissected for tissue sample collection. Fish were fasted 24 h before euthanasia by MS-222 overdose (400 mg l−1 in seawater; Syndel Laboratories, Vancouver, BC, Canada). At each sampling, weight and fork length were recorded, and dorsal white muscle and liver tissues were sampled. Dorsal muscle samples for lipid analyses were collected in lipid-clean 15 ml glass test tubes. After sampling, 2 ml of chloroform were added to the tubes and the headspace-air was replaced with nitrogen before storing at −20°C until analysis. For gene expression analysis, liver samples (approx. 100 mg of tissue per sample) were placed in 1.5 ml RNase-free tubes, then flash-frozen in liquid nitrogen and stored at −80°C until RNA extraction.
(c). Fatty acid and lipid class analyses
Lipid extraction, lipid class separation and FA derivatization were performed as previously described [5]. Briefly, all tools were lipid-clean, the homogenization process was conducted on ice and all samples were covered with nitrogen after all steps. Extraction was done using chloroform:methanol:water (8 : 4 : 3). Lipid class determination was done in a three-step separation on silica gel Chromarods, and lipid class quantity was determined using Iatroscan Mark VI flame ionization detection (Mitsubishi Kagaku Iatron, Inc., Tokyo, Japan). Data were processed using Peak Simple software (SRI Instruments, version 3.67, Torrance, CA, USA).
FAs in 50 µl of lipid extracts were derivatized using Hilditch reagent (1.5 H2SO4:98.5 anhydrous MeOH) for 1 h at 100°C. Transesterified samples were analysed in an HP 6890 gas chromatograph on a Zebron ZB-WAX plus™ (30 m × 0.32 mm × 0.25 µm) column. Derivatized samples were injected at 65°C, and the temperature was increased at a rate of 40°C min−1 to 195°C; then it was increased to 220°C at a rate of 2°C min−1. Hydrogen carrier gas flow was 2 ml min−1, and the starting temperature of the injector was 150°C with an increase of 120°C min−1 to 250°C. The detector temperature was held at 260°C. Peaks were compared to those obtained using standards from Supelco (Bellefonte, PA, USA): 37 component FAME mix (Product number 47885-U), PUFA 3 (product number 47085-U) and PUFA 1 (product number 47033-U). Chromatograms were integrated using Chromatography Data Systems Open Laboratory CDS, and the FA data were calculated as area per cent of FAME. FA proportions were calculated as a per cent of total FAs. FA content (mg g−1) was calculated based on the acyl-lipid mg g−1 amounts obtained by Iatroscan.
(d). RNA extraction, DNase treatment, column purification and cDNA synthesis
Liver samples were homogenized in TRIzol reagent (Invitrogen, Carlsbad, CA, USA) using steel beads and QIAshredder spin-columns, following manufacturer instructions. Extracted RNA was re-extracted using a phenol-chloroform method [14], DNase-treated using RNase-free DNase Set (QIAGEN, Mississauga, ON, Canada) and column-cleaned using RNeasy Mini Clean-up Kit (QIAGEN) as described [15,16]. RNA integrity and purity were assessed using 1.0% agarose gel electrophoresis and NanoDrop spectrophotometry (Thermo Fisher, Mississauga, ON, Canada), respectively. A260/280 ratios were between 2.0 and 2.2, and A260/230 ratios were 1.8–2.3. Each cDNA synthesis was conducted in 20 µl reaction from 1.0 µg of DNase-treated, column-purified total RNA, using random primers (250 ng; Invitrogen/Life Technologies) and M-MLV reverse transcriptase (200 U; Invitrogen/Life Technologies) with the manufacturer's first strand buffer (1X final concentration), dNTPs (0.5 mM final concentration) and DTT (10 mM final concentration) at 37°C for 50 min [15].
(e). Real-time quantitative polymerase chain reaction analysis
The list of genes of interest (GOI) for the quantitative polymerase chain reaction (qPCR) study included genes related to de novo FA synthesis, FA elongation and desaturation, and FA oxidation, and are shown in electronic supplementary material, table S3. Paralogues of those genes were included if detected following procedures described in Caballero-Solare et al. [16]. For the newly designed primers, we used the PrimerQuest design tool (https://www.idtdna.com/Primerquest/Home/Index). Primer pair quality control was performed as described [16]. This included a five-point 1:3 dilution series (starting with cDNA representing 10 ng of input total RNA) used for standard curve generation to check amplification efficiency [17] and single-product amplification (dissociation curve analysis; [15,16]). Amplification efficiencies and amplicon sizes are shown in electronic supplementary material, table S3. Nine candidate normalizer genes were tested on two individuals from each dietary group (randomly selected), and their stability was analysed using GeNorm [18]. Since none complied with GeNorm stability cut-off level (M-value < 0.5), we re-analysed the three most stable (i.e. actb, rpl32, and polr2a) using four individuals/group (one fish/tank). Rpl32 and polr2a showed the highest stability (i.e. GeNorm M-values of 0.38 and 0.4) and were selected as normalizers. The mRNA levels of the GOIs and two normalizer genes were qPCR-assessed using eight individuals (2 fish per quadruplicate tank) from each dietary group. Each reaction contained 4 µl of cDNA (RNA input: 5 ng), 50 nM forward and reverse primers and 1 × Power SYBR Green Master Mix (Applied Biosystems/Life Technologies). qPCR reactions were carried out in triplicate for eight biological replicates from each treatment, following procedures previously described [15]. A ViiA 7 real-time PCR system (Applied Biosystems/Life Technologies) was used to run the qPCR analyses (cycling parameters; melting curves and no-template controls were included [15]). The relative quantity (RQ) of each GOI was calculated using the ViiA™ 7 Software v1.2 (Applied Biosystems) for ΔΔCT analysis [19] and incorporating amplification efficiencies (electronic supplementary material, table S3) [17]. For each GOI, the sample with the lowest normalized expression was used as a calibrator (i.e. RQ value of 1.0). All RQs are presented as mean ± s.d.
(f). Comparison with data from the 1–1.3%EPA+DHA feeding trial
Muscle FA profiles and liver RQ data from a previous study [5] with 1.0–1.3% EPA + DHA were analysed for Pearson's correlations. In that trial, five experimental diets with similar EPA + DHA levels (approx. 1.0–1.3%) but varying ω6:ω3 ratios were tested. The dietary ω6:ω3 ratios were: 1:3 (high-ω3), 1:2 (medium-ω3), 1:1 (balanced), 2:1 (medium-ω6) and 3:1 (high-ω6) [5]. The lipid composition of these diets [5] is also shown in electronic supplementary material, table S7. In that study, PIT-tagged smolts (203 ± 24 g and approx. 20-month age) were randomly distributed into twenty 620 L tanks in the JBARB, fed a commercial diet (Winter EP20, Skretting Canada, NB, Canada) during an 18-day acclimation period and then fed the experimental diets for 12 weeks. Holding conditions (e.g. 24 h light photoperiod, 11°C seawater temperature) [5] were similar to those of the 0.3–1.4%EPA+DHA feeding trial.
(g). Statistical methods
General linear models were used to analyse diet and tank effects in the muscle lipid composition and liver qPCR data. The models included tanks and diets as a random and fixed factor, respectively; and this was followed by Tukey pairwise comparisons. A one-way ANOVA followed by Tukey post-hoc tests were used for the comparison of initial (i.e. week 0) and week 12 lipid profiles. To compare the effect of EPA + DHA levels (i.e. 0.3 and 1.0%) factor and/or ω6 or ω3 (i.e. high-ω6 and high-ω3) factor, a two-way ANOVA followed by Tukey post-hoc test was used. All residuals were examined for normality and homoscedasticity (i.e. Shapiro–Wilk and Levene's tests, respectively). Pearson's correlation analyses were performed to relate muscle lipid composition with liver gene expression data. All the above statistical analyses were performed using IBM SPSS (IBM SPSS Statistics, Version 25, Armonk, New York, USA) and Minitab (Minitab 16 Statistical Software, State College, PA, USA) statistical software. PRIMER 7 (PRIMER-E Ltd., Auckland, New Zealand) was used to run principal coordinates analysis (PCoA) using similar muscle FA and hepatic transcripts from the two studies separately to illustrate common clustering patterns across studies. PRIMER 7 was also used to run a permutational analysis of variance (PERMANOVA) and similarity of percentages analysis (SIMPER) for the 0.3–1.4%EPA+DHA trial data. FAs and lipid classes that accounted for < 1.0% across groups were not included in the analyses. The significance level was p < 0.05 for all statistical analyses.
3. Results
(a). Growth performance
As found previously [5], diets in the 0.3–1.4%EPA+DHA trial did not affect final body weight (606.43 ± 33.6 g) nor weight gain (331.4 ± 23.1 g) significantly (p ≥ 0.9 for body weight and weight gain; F-values = 0.15 and 0.26, respectively) after the 12-week feeding trial.
(b). Muscle tissue lipid composition and content
Muscle lipid class composition (%) or content (mg g−1) showed no significant differences among dietary groups at the end of the feeding trial (p = 0.05–0.89) (table 1; electronic supplementary material, table S2, respectively). Sterol (ST) proportion (%) decreased at the end of the feeding trial compared with the initial lipid composition (p ≤ 0.005, F = 11.83). Conversely, total lipid (mg g−1) increased in all groups over time (i.e. week 12 versus week 0; p ≤ 0.005, F-value = 9.3; table 1).
Table 1.
Lipid and fatty acid composition1 (% total and content mg g−1) of Atlantic salmon muscle tissue after 12 weeks of feeding experimental diets. Italicized values are significantly different from initial ones and different letters indicate significantly different values across dietary treatments.
| initial | 0.3%EPA + DHA ↑ω6 | 0.3%EPA + DHA↑ω3 | 1%EPA + DHA↑ω6 | 1%EPA + DHA↑ω3 | control2 | p-value | |
|---|---|---|---|---|---|---|---|
| lipid class composition (% of total lipid) | |||||||
| hydrocarbons | ND | 1.5 ± 4.9 | 0.8 ± 2.2 | 1.5 ± 3.1 | 1.4 ± 2.3 | 1.1 ± 2.5 | 0.89 |
| triacylglycerols | 48.1 ± 17.6 | 53.3 ± 17.3 | 58.5 ± 9.1 | 62.0 ± 19.4 | 57.2 ± 17.2 | 64.2 ± 13.3 | 0.52 |
| sterols | 14.9 ± 5.8 | 3.8 ± 3.9 | 2.8 ± 0.9 | 1.9 ± 3.3 | 4.2 ± 5.6 | 5.7 ± 6.5 | 0.21 |
| phospholipids | 36.9 ± 13.2 | 40.9 ± 14.6 | 34.2 ± 12.2 | 29.6 ± 17.9 | 35.9 ± 13.5 | 26.6 ± 12.1 | 0.25 |
| total lipids (mg g−1 wet weight) | 2.8 ± 1.5 | 12.1 ± 5.1 | 11.2 ± 2.9 | 7.3 ± 4.6 | 10.8 ± 4.3 | 13.3 ± 5.8 | 0.90 |
| FA composition (% total FAs) | |||||||
| 14:0 | 1.7 ± 0.4 | 0.9 ± 0.2c | 0.9 ± 0.2c | 1.5 ± 0.3b | 1.5 ± 0.4b | 1.9 ± 0.4a | p ≤ 0.01 |
| 16:0 | 16.8 ± 1.04 | 14.7 ± 1.6 | 14.4 ± 1.01 | 14.6 ± 0.9 | 14.0 ± 0.7 | 13.8 ± 1 | 0.10 |
| 16:1ω7 | 3.4 ± 1 | 1.9 ± 0.3c | 2.4 ± 0.4abc | 2.3 ± 0.7bc | 2.8 ± 0.5ab | 2.9 ± 0.8a | p ≤ 0.01 |
| 18:0 | 4.6 ± 0.18 | 4.7 ± 0.5a | 4.4 ± 0.3ab | 4.2 ± 0.5bc | 4.2 ± 0.5bc | 3.8 ± 0.3c | p ≤ 0.01 |
| 18:1ω7 | 2.9 ± 0.2 | 1.9 ± 0.5d | 2.1 ± 0.1cd | 2.2 ± 0.2b | 2.3 ± 0.1bc | 3.0 ± 0.1a | p ≤ 0.01 |
| 18:1ω9 | 19.4 ± 4.8 | 20.9 ± 5.4bc | 23.6 ± 2.4b | 19.3 ± 2.3c | 21.5 ± 2.7bc | 27.1 ± 3.2a | p ≤ 0.01 |
| 18:2ω6 (LNA) | 7.2 ± 1.7 | 25.5 ± 2a | 12.1 ± 1.1c | 19.7 ± 3.4b | 11.0 ± 1.2c | 10.4 ± 1.1c | p ≤ 0.01 |
| 18:3ω3 (ALA) | 1.5 ± 0.3 | 2.9 ± 0.3c | 9.7 ± 0.9a | 2.7 ± 0.3c | 9.0 ± 0.8b | 2.4 ± 0.3cd | p ≤ 0.01 |
| 18:3ω6 | 0.3 ± 0.07 | 1.2 ± 0.3a | 0.6 ± 0.1b | 0.6 ± 0.2b | 0.4 ± 0.1c | 0.3 ± 0.1c | p ≤ 0.01 |
| 18:4ω3 | 0.7 ± 0.2 | 0.9 ± 0.2d | 2.4 ± 0.5a | 1.0 ± 0.2cd | 2.0 ± 0.3b | 1.2 ± 0.19c | p ≤ 0.01 |
| 20:1ω11 | ND | 0.3 ± 0.1c | 0.3 ± 0.1c | 0.1 ± 0.1c | 1.5 ± 0.2b | 2.5 ± 0.49a | p ≤ 0.01 |
| 20:1ω7 | ND | 0.1 ± 0.05b | 0.1 ± 0.02b | 1.3 ± 0.2a | 0.1 ± 0.02b | 0.1 ± 0.03b | p ≤ 0.01 |
| 20:1ω9 | 1.4 ± 0.5 | 0.8 ± 0.2c | 0.9 ± 0.2c | 1.4 ± 0.3b | 1.3 ± 0.3b | 1.9 ± 0.2a | p ≤ 0.01 |
| 20:2ω6 | 0.6 ± 0.09 | 1.6 ± 0.3a | 0.7 ± 0.1c | 1.4 ± 0.3a | 0.7 ± 0.1bc | 0.8 ± 0.1b | p ≤ 0.01 |
| 20:3ω6 (DGLA) | 0.6 ± 0.07 | 1.9 ± 0.3a | 0.8 ± 0.1c | 1.1 ± 0.1b | 0.6 ± 0.06d | 0.6 ± 0.06d | p ≤ 0.01 |
| 20:4ω3 | 0.7 ± 0.09 | 0.5 ± 0.1c | 1.2 ± 0.2ab | 0.8 ± 0.1bc | 1.2 ± 0.1a | 1.0 ± 0.12ab | p ≤ 0.01 |
| 20:4ω6 (ARA) | 1.8 ± 0.33 | 1.4 ± 0.7a | 1.2 ± 0.2ab | 1.1 ± 0.3abc | 1.0 ± 0.2bc | 0.8 ± 0.4c | p ≤ 0.01 |
| 20:5ω3 (EPA) | 5.9 ± 1.05 | 2.3 ± 0.7b | 3.4 ± 0.7a | 3.4 ± 0.8a | 3.7 ± 0.7a | 3.6 ± 0.8a | p ≤ 0.01 |
| 22:1ω11 | ND | 0.4 ± 0.2c | 0.1 ± 0.03d | 0.1 ± 0.04d | 1.4 ± 0.3b | 2.4 ± 0.4a | p ≤ 0.01 |
| 22:1ω7 | 0.04 ± 0.02 | 0.1 ± 0.2ab | 0.1 ± 0.08ab | 0.2 ± 0.3a | 0.1 ± 0.07b | 0.1 ± 0.04b | p ≤ 0.01 |
| 22:1ω9 | 0.2 ± 0.08 | 0.1 ± 0.19c | 0.3 ± 0.2b | 1.4 ± 0.4a | 0.2 ± 0.08c | 0.3 ± 0.09bc | p ≤ 0.01 |
| 22:4ω3 | ND | 0.1 ± 0.1b | 0.2 ± 0.08a | 0.2 ± 0.08a | 0.1 ± 0.06b | 0.1 ± 0.2b | p ≤ 0.01 |
| 22:4ω6 | ND | 0.1 ± 0.04 | 0.2 ± 0.2 | 0.2 ± 0.4 | 0.1 ± 0.1 | 0.1 ± 0.03 | 0.61 |
| 22:5ω3 | 1.8 ± 0.2 | 0.9 ± 0.6b | 1.0 ± 0.2a | 1.1 ± 0.2a | 1.1 ± 0.1a | 1.2 ± 0.2a | 0.02 |
| 22:5ω6 | 0.5 ± 0.09 | 0.3 ± 0.2a | 0.0 ± 0.02b | 0.2 ± 0.2ab | 0.2 ± 0.05ab | 0.2 ± 0.06ab | p ≤ 0.01 |
| 22:6ω3 (DHA) | 25.7 ± 7.1 | 10.2 ± 3.6 | 14.0 ± 3.06 | 13.7 ± 5 | 14.0 ± 4.2 | 13.21 ± 4.4 | 0.21 |
| ΣSFA3 | 23.7 ± 0.6 | 21.5 ± 2.1 | 19.3 ± 4.8 | 21.9 ± 1.9 | 20.5 ± 0.6 | 20.2 ± 1.1 | 0.10 |
| ΣMUFA4 | 27.9 ± 6.6 | 27.2 ± 6.1b | 29.3 ± 7.7b | 29.5 ± 3.6b | 32.0 ± 4.1b | 41.5 ± 4.6a | p ≤ 0.01 |
| ΣPUFA5 | 48.5 ± 6.1 | 51.0 ± 4.5a | 45.7 ± 11.4a | 48.2 ± 3.3a | 47.0 ± 3.9a | 37.9 ± 4.5b | p ≤ 0.01 |
| Σω3 | 36.5 ± 7.7 | 18.2 ± 4.2c | 30.5 ± 8a | 23.3 ± 5.5bc | 31.9 ± 4.7a | 23.2 ± 5.08b | p ≤ 0.01 |
| Σω6 | 10.9 ± 1.5 | 32.0 ± 2.2a | 15.6 ± 0.9c | 24.3 ± 3.5b | 13.9 ± 1cd | 13.2 ± 0.9d | p ≤ 0.01 |
| ω6:ω3 | 0.3 ± 0.1 | 1.9 ± 0.4a | 0.4 ± 0.05c | 1.2 ± 0.3b | 0.4 ± 0.06c | 0.6 ± 0.07 | p ≤ 0.01 |
| 18:3ω3 + 18:2ω6 | 8.8 ± 1.9 | 28.4 ± 2.2a | 20.7 ± 5.3b | 22.4 ± 3.7ab | 20.0 ± 1.7b | 12.8 ± 1.3c | p ≤ 0.01 |
| 20:5ω3 (EPA) (mg g−1 wet weight) | 0.1 ± 0.07 | 0.2 ± 0.04b | 0.4 ± 0.1a | 0.2 ± 0.06b | 0.3 ± 0.1a | 0.4 ± 0.1a | p ≤ 0.01 |
| 22:6ω3 (DHA) (mg g−1 wet weight) | 0.5 ± 0.2 | 0.9 ± 0.2bc | 1.4 ± 0.5ab | 0.7 ± 0.2c | 1.2 ± 0.3abc | 1.4 ± 0.5a | p ≤ 0.01 |
| Total FA (mg g−1 wet weight) | 2.1 ± 1.2 | 9.8 ± 4.05ab | 9.3 ± 2.5ab | 6.1 ± 4.4bc | 9.2 ± 4.3ab | 11.2 ± 4.3a | 0.21 |
1mean ± s.d. (n = 16–21).
2control diet: 1.4% EPA + DHA + more balanced levels of ω3 and ω6 fatty acid.
3saturated fatty acids.
4monounsaturated fatty acids.
5polyunsaturated fatty acids.
Comparing dietary treatments with the initial samples (table 1), ΣSFA (% of total FAs) decreased significantly after 12 weeks of feeding high-ω3 (i.e. 0.3%EPA+DHA↑ω3 and 1%EPA+DHA↑ω3) and control diets (table 1). Although some individual MUFA and PUFA did show significant changes over time (initial versus experimental groups), fish fed the control diet showed a significant increase in ΣMUFA and a decrease in ΣPUFA over time (table 1). Σω3 showed a significant reduction over time except in those fish fed high-ω3 diets. Σω6, 18:2ω6, 18:3ω3 and 18:3ω3+18:2ω6 increased throughout the trial in all dietary groups. After 12 weeks, muscle EPA and DHA showed a significant decrease in all groups (table 1).
At week 12 (table 1), the muscle FA % of 18:2ω6 and 18:3ω3 (LC ω6 and LC ω3-precursors) reflected those of the diets (i.e. higher 18:2ω6 in the high-ω6-fed salmon; higher 18:3ω3 in the high-ω3-fed salmon). Muscle ARA levels (%) in salmon fed 0.3% EPA+DHA (i.e. 0.3%EPA+DHA↑ω6 and 0.3%EPA+DHA↑ω3) were significantly higher compared to the control. Muscle 20:3ω6 (DGLA; dihomo-γ-linolenic acid) levels in salmon fed 0.3%EPA+DHA↑ω6 was significantly higher than all other groups. EPA levels in salmon fed 0.3%EPA+DHA↑ω6 were significantly lower than in the other groups (table 1). Muscle DHA showed no significant differences among diets. Muscle ΣSFA was similar across the experimental groups. Muscle ΣMUFA was higher in salmon fed the control diet than in the other groups. By contrast, ΣPUFA was significantly lower in fish fed the control diet compared to the other treatments. Muscle Σω3 was higher with high-ω3 diets than with the rest and was higher with control diet than with 0.3%EPA+DHA↑ω6 diet. Conversely, muscle Σω6 was higher in those fed high-ω6 than in those fed the other diets. Also, salmon fed the control diet showed lower muscle Σω6 than those fed 0.3%EPA+DHA↑ω3 but similar to those fed 1%EPA+DHA↑ω3.
At week 12 (electronic supplementary material, table S2), the muscle tissue content (mg g−1) of 18:2ω6 was significantly higher in 0.3%EPA+DHA↑ω6 compared with all other dietary treatments (electronic supplementary material, table S2). The muscle content of 18:3ω3 (mg g−1) reflected dietary intake (i.e. higher 18:3ω3 in 0.3%EPA+DHA↑ω3 and 1%EPA+DHA↑ω3) (electronic supplementary material, table S2). DHA content (mg g−1) was higher in fish fed control diet than in those fed 0.3%EPA+DHA↑ω6 and 1%EPA+DHA↑ω6 (table 1), and was not different between groups fed high-ω3 (i.e. 0.3%EPA+DHA↑ω3, 1%EPA+DHA↑ω3; table 1; electronic supplementary material, table S2). EPA content (mg g−1) was significantly higher in both high-ω3 and control-fed salmon (1.4% EPA+DHA) compared with those fed high-ω6 (table 1 and electronic supplementary material, table S2). There were no significant diet effects on ΣSFA (mg g−1) or ΣPUFA (mg g−1) (electronic supplementary material, table S2). Muscle ΣMUFA (mg g−1) was significantly higher with control diet than with the other diets except for 1%EPA+DHA↑ω3 (electronic supplementary material, table S2). Σω3 (mg g−1) reflected the composition of the diets (i.e. higher in 0.3%EPA+DHA↑ω3 and 1%EPA+DHA↑ω3; electronic supplementary material, table S2). Σω6 was highest in those salmon fed 0.3%EPA+DHA↑ω6 (electronic supplementary material, table S2) as compared with those fed the other diets. EPA+DHA was higher with 0.3%EPA+DHA↑ω3 and control than with high-ω6 diets (electronic supplementary material, table S2).
(c). Liver quantitative polymerase chain reaction analysis
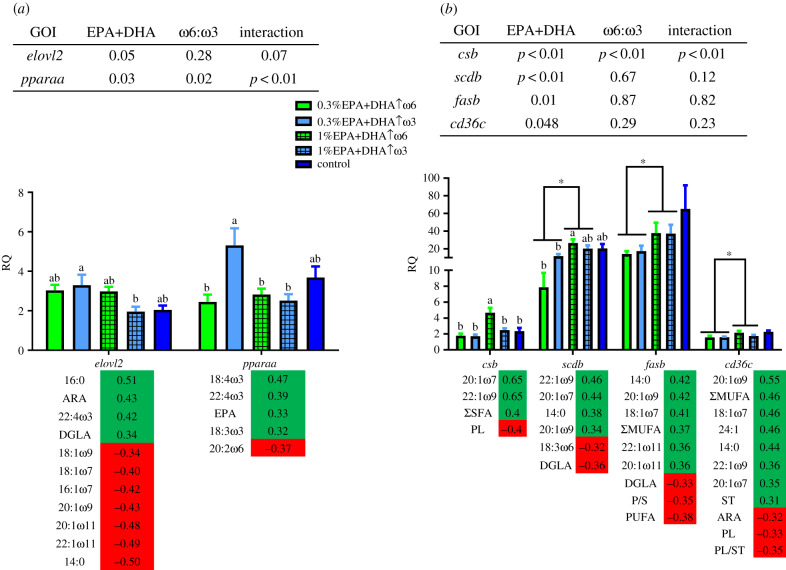
Most of the targeted biomarkers did not display significant differences in the transcript expression response to dietary treatments (electronic supplementary material, table S4). However, salmon fed 0.3%EPA+DHA↑ω3 showed upregulated hepatic elovl2 mRNA levels compared with those fed 1%EPA+DHA↑ω3 (figure 1a, electronic supplementary material, table S4). Diet 1%EPA+DHA↑ω3 also promoted higher pparaa mRNA levels than the other diets except for the control (figure 1a). Fish fed 1%EPA+DHA↑ω6 showed significant upregulation of csb as compared with those fed 0.3% EPA+DHA levels, 1%EPA+DHA↑ω3 and control diet (figure 1b). Feeding 1.0% EPA+DHA resulted in higher transcript levels of scdb, fasb and cd36c compared with 0.3% EPA+DHA (figure 1b).
Figure 1.
Effect of diets on the transcript levels of lipid-related biomarkers. (a) Transcripts with putative roles in C18-polyunsaturated fatty acid elongation (elovl2) and the master regulator transcription factor pparaa. (b) Transcripts related to the Krebs cycle (csb), de novo fatty acid synthesis (scdb and fasb) and fatty acid transport (cd36c). The transcript RQs (relative quantities) are shown as mean ± s.e. Bars with different letters are significantly different for a one-way ANOVA across dietary treatments. P-values from a two-way ANOVA are given in the upper table (significance is shown with asterisks (*) on the figure). The listed fatty acids (lower table) are significantly correlated with the corresponding biomarker and placed to show the correlation direction (i.e. green for positive and red for negative). Diets fed: 0.3%EPA+DHA↑ω6, 0.3%EPA+DHA↑ω3, 1%EPA+DHA↑ω6, 1%EPA+DHA↑ω3 and the control diet (1.4% EPA+DHA+balanced levels of ω3+ω6).
(d). Correlation and multivariate analyses of hepatic transcript expression and muscle lipid composition and content
(i). Correlation of hepatic transcript expression and muscle lipid composition
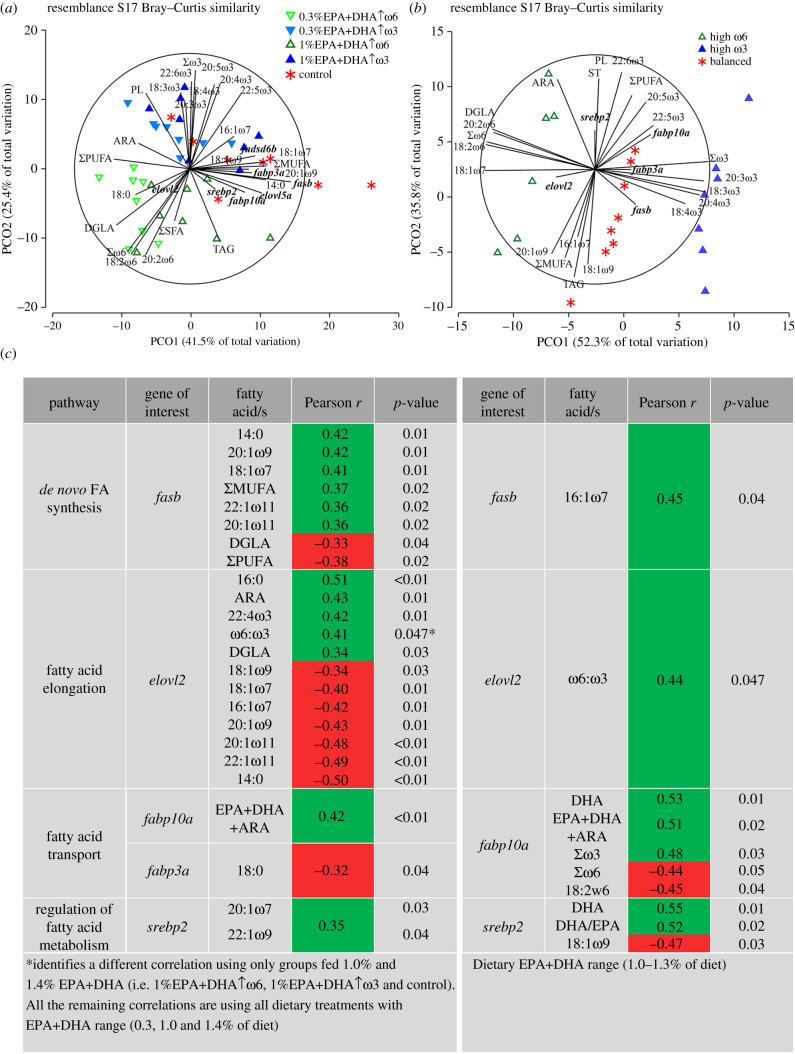
Liver csb expression levels were positively correlated with 20:1ω7, 22:1ω9 and ΣSFA, and negatively correlated with phospholipid (PL) (figure 1b; electronic supplementary material, table S5). The transcript levels of acac and acly were positively correlated with 14:0, 18:1ω7 and 20:1ω9 (electronic supplementary material, table S5). Acac was negatively correlated with polyunsaturated/saturated FAs (P/S) and ΣPUFA (electronic supplementary material, table S5). Acly was positively correlated with ΣMUFA, and negatively with DGLA and ΣPUFA (electronic supplementary material, table S5). Scdb correlated positively with 14:0, 20:1ω7, 20:1ω9, 22:1ω9, and negatively with 18:3ω6 and DGLA (figure 1b, electronic supplementary material, table S5). The mRNA levels of fasb were positively correlated with 14:0, 18:1ω7, 20:1ω9, 20:1ω11, 22:1ω11 and ΣMUFA; and negatively with DGLA, P/S and ΣPUFA (figure 1b; electronic supplementary material, table S5). Cd36c transcript levels were positively correlated with 14:0, 20:1ω9, 18:1ω7, 24:1, 22:1ω9, 20:1ω7 and ST; and negatively with ARA, PL and PL/ST (figure 1b; electronic supplementary material, table S5). Elovl2 was positively correlated with 16:0, ARA, DGLA and 22:4ω3, and negatively with 14:0, 16:1ω7, 18:1ω9, 18:1ω7, 20:1ω9, 20:1ω11 and 22:1ω11 (figure 1b; electronic supplementary material, table S5). Elovl5a was positively correlated with 20:1ω7 and 22:1ω9 (electronic supplementary material, table S5), and elovl5b was negatively correlated with 22:5ω6 and 22:4ω6 (electronic supplementary material, table S5). Cpt1a was positively correlated with 16:0, whereas acox1 was negatively correlated with 22:5ω6 (electronic supplementary material, table S5). Fabp3a mRNA levels were negatively correlated with 18:0, and fabp10a correlated positively with EPA+DHA+ARA (figure 2c; electronic supplementary material, table S5). Pparaa was correlated positively with 18:3ω3, 18:4ω3, EPA and 22:4ω3; and negatively with 20:2ω6 (figure 1b; electronic supplementary material, table S5). Pparab was correlated positively with 20:1ω7, 20:1ω9 and 22:1ω9, and negatively with 18:4ω3 (electronic supplementary material, table S5). The transcript levels of pparg were positively correlated with 22:4ω3 (electronic supplementary material, table S5). Srebp2 was positively correlated with 20:1ω7 and 22:1ω9 (electronic supplementary material, table S5).
Figure 2.
Principal coordinates analysis (PCoA) of the same transcripts and muscle fatty acids from the two trials showing vectors with Pearson's r > 0.45. (a) PCoA and significant bivariate correlations (below on the left in (c)) between muscle fatty acids percent and liver transcript levels for the 0.3–1.4%EPA+DHA trial. (b) PCoA and significant bivariate correlations (below on the right in (c)) between muscle fatty acids percent and liver transcript levels for the 1–1.3%EPA+DHA trial. Transcripts are bolded on both plots. (c) Fatty acid (%) significant correlations with liver transcript levels; an asterisk (*) identifies the results of a different correlation using only groups fed 1.0% and 1.4% EPA+DHA in the 0.3–1.4%EPA+DHA trial.
(ii). Correlation analysis of hepatic transcript expression and muscle lipid content
The significant correlation results of hepatic transcript expression and lipid content (mg g−1) were largely similar to those observed between the hepatic transcript expression and lipid per cent (electronic supplementary material, table S5); however, additional correlations were observed with gene expression and lipid content (mg g−1). Liver csb transcript levels were positively correlated with 22:1ω7. The transcript levels of scdb were negatively correlated with 18:3ω3+18:2ω6. The mRNA levels of fasb were positively correlated with 16:1ω7 and 22:5ω3. Cd36c was negatively correlated with P/S. Additional positive correlations were observed with cpt1a transcript levels and 17:1, 23:0 and ARA. Pparg was negatively correlated with 20:2ω6. The transcript levels of srebp2 were negatively correlated with ΣPUFA, 18:3ω3+18:2ω6 and individual PUFAs (i.e. 18:3ω6, 18:4ω3 and ARA). Also, srebp2 was negatively correlated with ΣSFA and individual SFA (i.e. 18:0 and 16:0).
(iii). Multivariate analyses of hepatic transcript expression and muscle lipid composition
PCoA illustrated the separation among the three dietary EPA+DHA levels along the x-axis (PCO1; from left to right: 0.3%, 1.0% and 1.4%), and between high-ω3/high-ω6 along the y-axis (i.e. PCO2; high-ω3 on top, high-ω6 below; figure 2a). PCO1 and PCO2 explained 41.5% and 25.4% of the total variation, respectively. PCoA analyses associated ΣMUFA and fasb with higher dietary EPA+DHA levels, whereas the segregation between high-ω3 and high-ω6 diets was driven by muscle ω3 and ω6 FAs levels. PL also associated with high-ω3 diets, and TAG (triacylglycerols) with individuals fed 1%EPA+DHA↑ω6.
The SIMPER analysis showed that the 0.3%EPA+DHA↑ω6 and control groups were the most different (25.83% dissimilar), while the 0.3%EPA+DHA↑ω3 and 1%EPA+DHA↑ω3 groups were the least different (13.99% dissimilar). Also, it showed that fasb transcript level was the main driver of dissimilarity between different dietary EPA+DHA groups, regardless of dietary ω6:ω3 (electronic supplementary material, table S6). Besides fasb, the dissimilarities for 0.3% EPA+DHA versus 1.0% EPA+DHA and 1.0% EPA+DHA versus control (i.e. 1.4% EPA+DHA) were caused mainly by scdb, TAG and acac; and by ΣMUFA for the comparison 0.3% EPA+DHA versus control. Among the list of main contributors to the dissimilarities between high-ω6 and high-ω3, we mainly identified fasb, TAG, PL, Σω6 and Σω3. PERMANOVA pairwise comparisons showed significant differences (p(perm) < 0.05) among dietary treatments, except when comparing fish fed diets 0.3%EPA+DHA↑ω3 and 1%EPA+DHA↑ω3 (p(perm) = 0.08).
(e). Comparison with reanalysed data from the 1–1.3%EPA+DHA feeding trial
The PCoA plot of data from the study with more narrow EPA+DHA and ω6:ω3 ranges [5] showed a clear separation among the dietary treatments (figure 2b). High-ω6-fed fish loaded on the left side of the plot (negatively on PCO1) and associated with ω6 FA and elovl2 (despite its low contribution). The high-ω3-fed fish were loaded on the right side of the plot (positively on PCO1), and associated with ω3 FA, as well as fasb and fabp3a (although they contributed less). As expected, the balanced-diet-fed salmon clustered between the high-ω6- and high-ω3-fed salmon. PERMANOVA pairwise tests showed that all treatments were significantly different from each other (p(perm) = 0.0001–0.0011]). PCO1 and PCO2 accounted for 52.3 and 35.8% of the variability, respectively.
In the study with narrower ranges of EPA+DHA and ω6:ω3 [5], the mRNA level of hepatic fasb was positively correlated with 16:1ω7, while elovl2 was positively correlated with ω6:ω3 (figure 2c). By restricting the correlation analysis of the 0.3–1.4%EPA+DHA trial to EPA+DHA levels of 1.0–1.4% EPA+DHA (i.e. removing the 0.3% diets), we also found a positive correlation between elovl2 and ω6:ω3 (figure 2c). Fabp10a showed positive correlations with DHA, EPA + DHA+ ARA and Σω3, and negative correlations with 18:2ω6 and Σω6. This transcript also showed a positive correlation with muscle EPA+DHA+ARA in the 0.3–1.4%EPA+DHA trial (figure 2c). Finally, srebp2 correlated positively with DHA and DHA/EPA, and negatively with 18:1ω9 (figure 2c; electronic supplementary material, table S5).
4. Discussion
(a). Growth performance
A 12-week feeding trial was conducted with Atlantic salmon (Salmo salar) smolts to investigate growth performance and trophic modification of FA. The interaction of varying dietary EPA+DHA levels with either high 18:2ω6 or high 18:3ω3 induced changes in expression of hepatic lipid metabolism relevant transcripts and white muscle lipid composition of Atlantic salmon (approx. 20-month old). These metabolic adjustments––namely the promotion of LC-PUFA synthesis––may have prevented significant negative effects on growth at dietary EPA+DHA levels below 1% (optimal % estimated in [20]). These findings underline the nutritional importance of LC-PUFA precursors (i.e. 18:3ω3 and 18:2ω6) for farmed Atlantic salmon fed plant-based diets.
(b). Muscle tissue fatty acids (composition and content) at week 12 and liver quantitative polymerase chain reaction analysis
The aquaculture industry depends on FO as the main source of the highly valuable FAs EPA and DHA. The experimental diets were made following the industry practice of formulating with EPA and DHA together instead of independently. However, each FA was determined separately in the diets and tissues; each is discussed separately. Muscle DHA proportion (%) did not reflect the dietary input and showed no significant variation among dietary groups. On the other hand, muscle DHA content (mg g−1) was higher in fish fed the control diet than in those fish fed high-ω6, but not in those fed high-ω3 (table 1). Interestingly, dietary EPA+DHA levels did not seem to affect muscle DHA content within the groups of fish fed high-ω6 and high-ω3 diets. The latter suggests higher DHA retention (especially, in the high-ω6 groups fed lower ω3 precursor levels) and/or activation of the elongation and desaturation pathway (mainly, in the high-ω3 groups fed high-ω3 precursor levels). In a previous study, EPA+DHA retention was found to increase with a gradual decrease in dietary EPA+DHA [21]. This potential DHA retention could relate to the sparing effect of other FAs such as SFAs [22]. As found in previous studies [20,23], gene expression analysis did not show significant diet-related changes in the FA β-oxidation pathway. Yet, hepatic cpt1a transcript levels (i.e. mitochondrial β-oxidation) correlated positively with 16:0 (%), and the content of 17:1 and 23:0 (mg g−1; electronic supplementary material, table S5). The levels of another less abundant SFA in the muscle (i.e. 18:0) correlated negatively with fabp3a (i.e. plasma membrane-to-mitochondria FA transport; electronic supplementary material, table S5) and is in agreement with a previous study [23].
Atlantic salmon have some capacity to metabolize 18:3ω3 to EPA and further to DHA via reactions catalysed by elongases such as Elovl2 and Elovl5, among other enzymes [5,15,20,24,25]. In the current study, lower dietary EPA+DHA levels reduced muscle EPA (%) only when lower 18:3ω3 levels were supplied to the fish (i.e. 0.3%EPA+DHA↑ω6 diet). At higher dietary 18:3ω3 levels, the metabolism of salmon fed 0.3%EPA+DHA↑ω3 seemed to have promoted EPA synthesis given their relatively high muscle EPA content (i.e. similar to that of fish fed 1%EPA+DHA↑ω3 and control diet) and upregulated hepatic elovl2 transcript levels (as compared with those of fish fed 1%EPA+DHA↑ω3). EPA is inefficiently retained in the tissue compared to DHA in Atlantic salmon [22]. Hence, the ability of salmon fed 0.3%EPA+DHA↑ω3 to compensate for lower dietary EPA levels is less likely to be due to enhanced EPA retention than to increased EPA synthesis. However, an inefficient retention and low dietary supply of 18:3ω3 could be the cause behind the similarly low muscle EPA contents in fish fed high-ω6 regardless of dietary EPA+DHA levels (i.e. 0.3%EPA+DHA↑ω6 versus 1%EPA+DHA↑ω6). Lastly, the similar elovl2 transcript levels found in fish fed high-ω6, regardless of the dietary EPA+DHA, diverge from the induction by low dietary EPA+DHA observed in fish fed high-ω3 and suggests that ω3 precursors might play a more prominent role in the regulation of elovl2 transcript expression.
Interestingly, cpt1a (carnitine palmitoyl transferase, a FA oxidation biomarker) was positively correlated with muscle content of ARA but not DGLA. Indeed, salmon fed 0.3%EPA+DHA↑ω6 showed a higher concentration of DGLA than ARA in the muscle tissue. Prostaglandins (PGs) derived from the metabolism of DGLA have been reported as anti-inflammatory lipid mediators, in contrast with ARA-derived PGs, which are known to be pro-inflammatory [26,27]. Therefore, our correlations might suggest the preference of salmon liver to oxidize ARA and favour DGLA retention, in what might be a strategy to prevent accumulation of pro-inflammatory compounds in the body.
Transcription factor Ppara regulates various pathways in lipid metabolism, and different studies suggest the ability of Ppara to regulate LC-PUFA biosynthesis [28,29]. Pparaa upregulation in salmon fed 0.3%EPA+DHA↑ω3 compared to 1%EPA+DHA↑ω3 concomitant with that of elovl2 (figure 1a; electronic supplementary material, table S4) might suggest a regulatory role in ω3 FA elongation and desaturation processes.
The transcript expression profiles of fasb and scdb suggest the promotion of de novo FA synthesis and SFA-MUFA conversion, respectively, with increased dietary EPA+DHA levels (i.e. 1.0% versus 0.3% DHA+EPA). These two processes have been found to respond to dietary fats in mammals [30]. Scdb upregulation was the strongest in salmon fed 1%EPA+DHA↑ω6, which coincided with the higher transcript level of csb, a biomarker for the Krebs cycle––and therefore for the supply of carbon precursors for de novo FA synthesis. Moreover, the present study found positive correlations between biomarker genes related to de novo FA synthesis and MUFA synthesis (i.e. csb, acac, acly, scdb, fasb) and several monounsaturated FAs in both per cent and content (mg g−1). In multivariate analyses, fasb was the main contributor to the dissimilarities among the different dietary EPA+DHA groups (electronic supplementary material, table S6). Taken together, our results indicate that feeding higher dietary EPA+DHA levels (i.e. higher dietary FO inclusion) shifted Atlantic salmon metabolism towards increased MUFA synthesis. MUFA has been associated with immunity regulation in mammals [31]. Further research is required to determine the role of MUFA in salmon immune regulation.
(c). Fillet DHA and EPA contents and ω6:ω3 ratios
Over the course of the 0.3–1.4%EPA+DHA trial, animals fed high-ω3 diets increased their DHA content 2.5- to 3-fold, which was as much as those fed the control diet. DHA content increased to 1.2–1.4 mg g−1 for all three treatments (i.e. 0.3%EPA+DHA↑ω3, 1%EPA+DHA↑ω3 and control diet) despite a 2.5-fold difference in dietary DHA proportions. In addition, high-ω3 diets increased the EPA content by 3-fold which, again, was as much as the control. EPA content increased to 0.3–0.4 mg g−1 for the 3 treatments (i.e. 0.3%EPA+DHA↑ω3, 1%EPA+DHA↑ω3 and control diet) despite a 3.5-fold difference in dietary EPA proportions. The high-ω3 diets contributed to improving the fillet quality compared to the high-ω6 diets, as it decreased the ω6:ω3 ratio in the fillets. The fillet ω6:ω3 ratio was 0.4 for those fed high-ω3 diets regardless of the EPA+DHA levels, while the high-ω6 diets ranged from 1 to 2. The control diet had a 0.6 ω6:ω3 ratio in the fillet. An ω6:ω3 ratio of 2–3 in human diets has been shown to be beneficial for rheumatoid arthritis and colorectal cancer [32], while a ratio of 1–2 is one of the most important dietary factors in the prevention of obesity [33].
(d). Comparison with the 1–1.3%EPA+DHA trial
In both studies, elovl2 transcript levels were associated with high-ω6-fed fish (figure 2a,b). In the 0.3–1.4%EPA+DHA trial, elovl2 was positively correlated with ARA, DGLA and 22:4ω3 (%), while in the 1–1.3%EPA+DHA trial [5], it was positively correlated with muscle ω6:ω3 (figure 2b) and this result was confirmed by a second correlation using the upper range of EPA+DHA diets (1–1.4%) for the 0.3–1.4%EPA+DHA trial (figure 2a). Similarly, in a previous study with Atlantic salmon, hepatic elovl2 transcript expression level showed a positive correlation with muscle 18:2ω6 and Σω6 [19]. These data and the shared correlation between both studies suggest that elovl2 encodes an enzyme that can elongate both ω6 and ω3 precursors.
Srebp2 showed a contrasting correlation with individual MUFAs between the two studies. When EPA+DHA ≥ 1.0% (1–1.3%EPA+DHA trial) [5], srebp2 was negatively correlated with 18:1ω9 (figure 2b). By contrast, with EPA+DHA between 0.3 and 1.4%, srebp2 was positively correlated with MUFA (20:1ω7 and 22:1ω9) (figure 2a). Srebp showed a response to LC-PUFA in the salmon cell line, SHK-1 [34]. The upregulation of genes related to de novo FA synthesis and SFA-MUFA conversion (i.e. fasb and scdb, respectively; when comparing 1.0% to 0.3% EPA+DHA) may explain the contradictory srebp2-MUFA correlations across the two studies. In other words, the wider range of EPA+DHA (LC-PUFA) in the 0.3–1.4%EPA+DHA trial may contribute to the lack of agreement.
The 0.3–1.4% (figure 2a) and the 1–1.3% EPA+DHA studies (figure 2b) showed an association of fabp10a transcript levels with higher levels of EPA+DHA and high-ω3, respectively. In the 1–1.3%EPA+DHA study, fabp10a transcript expression was negatively correlated with muscle 18:2ω6 and Σω6 and positively correlated with DHA and Σω3. Interestingly, fabp10a was positively correlated with EPA+DHA+ARA in both studies. Fabp10 is a biomarker for liver intracellular FA transport with a broad binding capacity [35], and a decreased FA uptake in hepatocytes from salmon fed 75% VO as compared with FO-fed fish was previously reported [36]. Altogether, these data suggest that longer chain PUFA were preferentially transported in the cell over C18 PUFA by Fabp10, which might have contributed to the deposition of EPA, DHA and ARA in the Atlantic salmon muscle. Further research on Fabp10 affinity for its different ligands is needed to understand its role in the mobilization of LC-PUFA.
5. Conclusion
Twelve-week feeding trials were conducted to investigate how dietary FAs affect muscle lipid composition and the expression of hepatic genes related to FA modification. Varying dietary levels of EPA+DHA (0.3, 1.0 and 1.4%) in combination with different dietary levels of PUFA precursors (i.e. high-ω6, high-ω3 and balanced) did not alter salmon growth performance but provoked significant changes in white muscle lipid composition and the expression of key liver metabolism biomarker genes. White muscle reflected diet composition with respect to C18 PUFA%. These changes correlated with gene expression profiles (e.g. elovl2) that suggested a promotion of LC-PUFA precursor elongation in fish fed low EPA+DHA (0.3%) levels. This promotion resulted in similar levels of EPA+DHA in the muscle of the 0.3%EPA+DHA↑ω3 and those fed 1%EPA+DHA↑ω3 and control diets. Salmon fed high-ω3 diets increased their DHA and EPA contents 2.5–3-fold which was as much as those fed the control diet. This was despite a 2.5–3-fold difference in dietary DHA and EPA proportions.
Although the highest response of elovl2 was with low EPA+DHA and high 18:3ω3, elovl2 transcript expression was, overall, associated with fish fed high-ω6 and lower ω3 as shown by multivariate analysis in both studies. Fish fed high levels of ω6 (lower ω3), regardless of the EPA+DHA level (i.e. 0.3 or 1.0%), showed a similar response in the elongation biomarker elovl2, suggesting fish attempt to elongate any available C18 PUFA. Stable isotope analysis is required to confirm the participation of elongation and desaturation in tissue DHA. The current study revealed that fish fed 1.0% EPA+DHA in combination with a high level of ω6 upregulated the de novo FA-synthesis pathway transcripts compared to those fed 0.3% EPA+DHA, a trend continued in salmon fed higher EPA+DHA levels. In summary, varying dietary C18–C22 PUFA by up to fivefold affected de novo synthesis of SFA and MUFA and elongation and desaturation of PUFA.
Supplementary Material
Acknowledgements
The authors would like to thank Chute Animal Nutrition Center team (Dalhousie University, Truro, NS, Canada), Dr Joe Brown Aquatic Research Building (JBARB) staff for fish husbandry, and Jeanette Wells and Xi Xue for providing training, helping during sampling and laboratory support. We are also grateful to Cara Kirkpatrick (Genome Atlantic, Halifax, Canada) for all help in program management.
Ethics
Canadian Council of Animal Care (MUN Animal Care Protocol #16-75-MR).
Data accessibility
This article has no additional data.
Authors' contributions
M.E. performed analyses, discussed the results and did the major contribution in writing the manuscript. T.K. contributed text to the manuscript and comparison data from a previous study. K.S.P. did RNA preparation. A.C.S., R.G.T., M.L.R. and C.C.P. contributed to the experimental design and manuscript writing.
Competing interests
We declare we have no competing interests.
Funding
This work was part of the Integrated Pathogen Management of Co-infection in Atlantic Salmon (IPMC) project, funded by the Government of Canada through Genome Canada and Genome Atlantic. The project was also funded by the Government of Newfoundland and Labrador through the Department of Tourism, Culture, Industry and Innovation; Mitacs, through the Mitacs Accelerate program; and Cargill Innovation (formerly EWOS Innovation). Additional funding to M.L.R. is provided by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant and by the Ocean Frontier Institute (OFI).
References
- 1.Tocher DR. 2015. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture 449, 94–107. ( 10.1016/j.aquaculture.2015.01.010) [DOI] [Google Scholar]
- 2.Sprague M, Dick JR, Tocher DR. 2016. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006–2015. Sci. Rep. 6, 21892 ( 10.1038/srep21892) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bou M, Todorčević M, Torgersen J, Škugor S, Navarro I, Ruyter B. 2016. De novo lipogenesis in Atlantic salmon adipocytes. Biochim. Biophys. Acta Gen. Subjects 1860, 86–96. ( 10.1016/j.bbagen.2015.10.022) [DOI] [PubMed] [Google Scholar]
- 4.Tocher DR. 2003. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 11, 107–184. ( 10.1080/713610925) [DOI] [Google Scholar]
- 5.Katan T, Caballero-Solares A, Taylor RG, Rise ML, Parrish CC. 2019. Effect of plant-based diets with varying ratios of ω6 to ω3 fatty acids on growth performance, tissue composition, fatty acid biosynthesis and lipid-related gene expression in Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. D 30, 290–304. ( 10.1016/j.cbd.2019.03.004) [DOI] [PubMed] [Google Scholar]
- 6.Bell JG, McEvoy J, Tocher DR, McGhee F, Campbell PJ, Sargent JR. 2001. Replacement of fish oil with rapeseed oil in diets of Atlantic salmon (Salmo salar) affects tissue lipid compositions and hepatocyte fatty acid metabolism. J. Nutr. 131, 1535–1543. ( 10.1093/jn/131.5.1535) [DOI] [PubMed] [Google Scholar]
- 7.Bell JG, Henderson RJ, Tocher DR, McGhee F, Dick JR, Porter A, Smullen RP, Sargent JR. 2002. Substituting fish oil with crude palm oil in the diet of Atlantic salmon (Salmo salar) affects muscle fatty acid composition and hepatic fatty acid metabolism. J. Nutr. 132, 222–230. ( 10.1093/jn/132.2.222) [DOI] [PubMed] [Google Scholar]
- 8.Cleveland BJ, Francis DS, Turchini GM. 2012. Echium oil provides no benefit over linseed oil for (n-3) long-chain PUFA biosynthesis in rainbow trout. J. Nutr. 142, 1449–1455. ( 10.3945/jn.112.161497) [DOI] [PubMed] [Google Scholar]
- 9.Agaba MK, Tocher DR, Zheng X, Dickson CA, Dick JR, Teale AJ. 2005. Cloning and functional characterisation of polyunsaturated fatty acid elongases of marine and freshwater teleost fish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 142, 342–352. ( 10.1016/j.cbpb.2005.08.005) [DOI] [PubMed] [Google Scholar]
- 10.Castro LFC, Monroig Ó, Leaver MJ, Wilson J, Cunha I, Tocher DR. 2012. Functional desaturase Fads1 (Δ5) and Fads2 (Δ6) orthologues evolved before the origin of jawed vertebrates. PLoS ONE 7, e31950 ( 10.1371/journal.pone.0031950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leaver MJ, Bautista JM, Björnsson BT, Jönsson E, Krey G, Tocher DR, Torstensen BE. 2008. Towards fish lipid nutrigenomics: current state and prospects for fin-fish aquaculture. Rev. Fish. Sci. 16, 73–94. ( 10.1080/10641260802325278) [DOI] [Google Scholar]
- 12.Qian C, Hart B, Colombo SM. 2020. Re-evaluating the dietary requirement of EPA and DHA for Atlantic salmon in freshwater. Aquaculture 518, 734870 ( 10.1016/j.aquaculture.2019.734870) [DOI] [Google Scholar]
- 13.National Research Council. 2011. Nutrient requirements of fish and shrimp. Washington, DC: National Academic Press. [Google Scholar]
- 14.Xu Q, Feng CY, Hori TS, Plouffe DA, Buchanan JT, Rise ML. 2013. Family-specific differences in growth rate and hepatic gene expression in juvenile triploid growth hormone (GH) transgenic Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. Part D 8, 317–333. ( 10.1016/j.cbd.2013.09.002) [DOI] [PubMed] [Google Scholar]
- 15.Xue X, Hixson SM, Hori TS, Booman M, Parrish CC, Anderson DM, Rise ML. 2015. Atlantic salmon (Salmo salar) liver transcriptome response to diets containing Camelina sativa products. Comp. Biochem. Physiol. Part D 14, 1–15. ( 10.1016/j.cbd.2015.01.005) [DOI] [PubMed] [Google Scholar]
- 16.Caballero-Solares A, Hall JR, Xue X, Eslamloo K, Taylor RG, Parrish CC, Rise ML. 2017. The dietary replacement of marine ingredients by terrestrial animal and plant alternatives modulates the antiviral immune response of Atlantic salmon (Salmo salar). Fish Shellfish Immunol. 64, 24–38. ( 10.1016/j.fsi.2017.02.040) [DOI] [PubMed] [Google Scholar]
- 17.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 29, e45 ( 10.1093/nar/29.9.e45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F.. 2002. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, research0034 ( 10.1186/gb-2002-3-7-research0034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. ( 10.1006/meth.2001.1262) [DOI] [PubMed] [Google Scholar]
- 20.Hixson SM, Parrish CC, Xue X, Wells JS, Collins SA, Anderson DM, Rise ML. 2017. Growth performance, tissue composition, and gene expression responses in Atlantic salmon (Salmo salar) fed varying levels of different lipid sources. Aquaculture 467, 76–88. ( 10.1016/j.aquaculture.2016.04.011) [DOI] [Google Scholar]
- 21.Bou M, et al. 2017. Requirements of n-3 very long-chain PUFA in Atlantic salmon (Salmo salar L): effects of different dietary levels of EPA and DHA on fish performance and tissue composition and integrity. Br. J. Nutr. 117, 30–47. ( 10.1017/S0007114516004396) [DOI] [PubMed] [Google Scholar]
- 22.Emery JA, Norambuena F, Trushenski J, Turchini GM. 2016. Uncoupling EPA and DHA in fish nutrition: dietary demand is limited in Atlantic salmon and effectively met by DHA alone. Lipids 51, 399–412. ( 10.1007/s11745-016-4136-y) [DOI] [PubMed] [Google Scholar]
- 23.Caballero-Solares A, Xue X, Parrish CC, Foroutani MB, Taylor RG, Rise ML. 2018. Changes in the liver transcriptome of farmed Atlantic salmon (Salmo salar) fed experimental diets based on terrestrial alternatives to fish meal and fish oil. BMC Genomics 19, 796 ( 10.1186/s12864-018-5188-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morais S, Monroig Ó, Zheng X, Leaver M, Tocher D. 2009. Highly unsaturated fatty acid synthesis in Atlantic salmon: characterization of ELOVL5- and ELOVL2-like elongases. Mar. Biotechnol. 11, 627–639. ( 10.1007/s10126-009-9179-0) [DOI] [PubMed] [Google Scholar]
- 25.Vagner M, Santigosa E. 2011. Characterization and modulation of gene expression and enzymatic activity of delta-6 desaturase in teleosts: a review. Aquaculture 315, 131–143. ( 10.1016/j.aquaculture.2010.11.031) [DOI] [Google Scholar]
- 26.Nagy K, Tiuca I-D. 2017. Importance of fatty acids in physiopathology of human body. In Fatty acids (eds Tiuca I-D, Catala A), chapter 1. Rijeka, Croatia: IntechOpen. ( 10.5772/67407) [DOI] [Google Scholar]
- 27.Innes JK, Calder PC. 2018. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids 132, 41–48. ( 10.1016/j.plefa.2018.03.004) [DOI] [PubMed] [Google Scholar]
- 28.Dong X, et al. 2017. Regulation of FADS2 transcription by SREBP-1 and PPAR-α influences LC-PUFA biosynthesis in fish . Sci. Rep. 7, 40024 ( 10.1038/srep40024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu K-C, Song L, Guo H-Y, Guo L, Zhang N, Liu B-S, Jiang S-G, Zhang D-C. 2019. Elovl4a participates in LC-PUFA biosynthesis and is regulated by PPARαβ in golden pompano Trachinotus ovatus (Linnaeus 1758). Sci. Rep. 9, 1–11. ( 10.1038/s41598-019-41288-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jump DB. 2011. Fatty acid regulation of hepatic lipid metabolism. Curr. Opin. Clin. Nutr. Metab. Care 14, 115–120. ( 10.1097/MCO.0b013e328342991c) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yaqoob P. 2002. Monounsaturated fatty acids and immune function. Eur. J. Clin. Nutr. 56(Suppl. 3), S9–S13. ( 10.1038/sj.ejcn.1601477) [DOI] [PubMed] [Google Scholar]
- 32.Simopoulos AP. 2002. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 56, 365–379. ( 10.1016/S0753-3322(02)00253-6) [DOI] [PubMed] [Google Scholar]
- 33.Simopoulos AP. 2016. An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 8, 128 ( 10.3390/nu8030128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minghetti M, Leaver MJ, Tocher DR. 2011. Transcriptional control mechanisms of genes of lipid and fatty acid metabolism in the Atlantic salmon (Salmo salar L.) established cell line, SHK-1. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1811, 194–202. ( 10.1016/j.bbalip.2010.12.008) [DOI] [PubMed] [Google Scholar]
- 35.Jordal A-EO, Hordvik I, Pelsers M, Bernlohr DA, Torstensen BE. 2006. FABP3 and FABP10 in Atlantic salmon (Salmo salar L.)—general effects of dietary fatty acid composition and life cycle variations. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 145, 147–158. ( 10.1016/j.cbpb.2006.05.007) [DOI] [PubMed] [Google Scholar]
- 36.Stubhaug I, Tocher DR, Bell JG, Dick JR, Torstensen BE. 2005. Fatty acid metabolism in Atlantic salmon (Salmo salar L.) hepatocytes and influence of dietary vegetable oil. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1734, 277–288. ( 10.1016/j.bbalip.2005.04.003) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This article has no additional data.