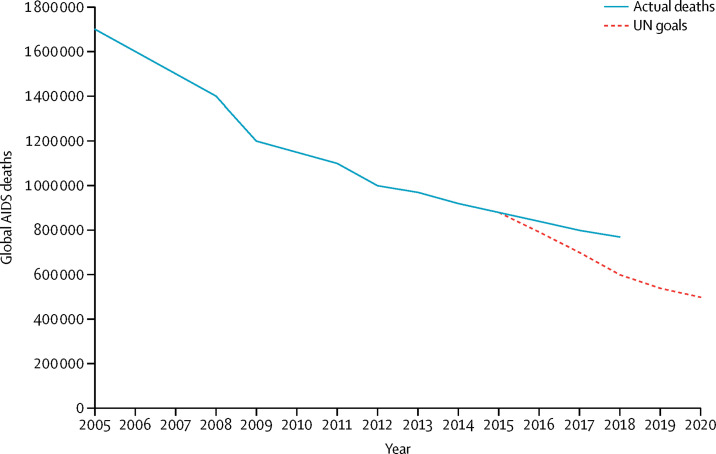
Eliminating unnecessary deaths is at the core of global health efforts, from responses to COVID-19 to HIV, non-communicable diseases, and maternal mortality. However, experience with HIV shows that reducing mortality requires a more robust approach to tracking and intervening than has been used to date. 38 million people are living with HIV,1 a consequence of a pandemic that spread worldwide. The multinational AIDS response grew out of a global concern for the catastrophic loss of life, as HIV devastated communities in highly burdened countries. From a time when it seemed impossible for interventions to reach people globally, today about 79% of all people living with HIV know their status, and more than half of all people living with HIV have achieved viral suppression using advanced antiretroviral therapy.1 This historic response has saved more than 11 million lives in the past decade alone.1 However, progress against mortality has slowed considerably, and the goals set by world leaders at the UN General Assembly in 2016—due to be accomplished in 2020—are not likely to be achieved, even after a change in the underlying mathematical models in 2019 meant estimated global mortality figures were lowered by nearly 200 000 deaths. AIDS-related illness remains the leading cause of death globally among people aged 15–49 years.2 Although the 2019 figures for global mortality represent a 45% decline since 2005, they are far off-track from the globally agreed goal of fewer than 500 000 HIV-related deaths by 2020 (figure ).1 As global health efforts mature, the HIV experience can provide lessons for tackling mortality from other causes.
Figure.
Global AIDS mortality
UN fast track goals versus current trajectory. Data from UNAIDS.3
The politics of data, including the type of data collected, for what use, and by whom, is part of the challenge.4 Funding and political attention in national and global health systems are inherently constrained, often flowing to meet the needs of powerful actors and address questions framed by them.5 The few resources available for monitoring mortality are directed primarily towards estimating deaths due to AIDS at a high level. As many countries do not have systems for tracking actual mortality and causes of mortality, well-financed efforts compensate by triangulating multiple data sources to model estimates at the national level. The idea at the heart of this approach is to show the pandemic's magnitude and global distribution—crucial, at the early stages of the pandemic, in generating the political will to scale up new treatment programmes.
These estimates address the interests of international funders, politicians, UN agencies, academics, global activists, and the international media. The target audience is similar for many global health datasets, including, for example, the Institute for Health Metrics and Evaluation's Global Burden of Disease studies.6 These estimates allow aid agencies, such as the US President's Emergency Plan for AIDS Relief (PEPFAR) and the Global Fund, to justify their financing, and national politicians in both donor governments and implementing governments to take (often deservedly) credit for any progress.
However, these estimates do little to help local leaders, programme managers, and clinicians, all of whom exercise far less power over budgets, to assess the effectiveness of HIV services. Mature HIV programmes face the complex task of accelerating enrolment while simultaneously improving the quality of services and addressing long-term retention of people who start treatment when still asymptomatic. Monitoring systems could track actual deaths and their causes, feeding these data back for use throughout the health system, but these systems have not been prioritised. The existing priorities perpetuate the long-standing challenge in global health of missing mortality data for programmatic and research purposes.7, 8 For example, South Africa's success in establishing a system for registering deaths makes it the only country in sub-Saharan Africa with a classification higher than very low in vital registration capacity.9
The absence of data on actual mortality at the programme and clinic level, whether all-cause mortality or AIDS-related mortality, has undermined the AIDS response on several fronts. Most fundamentally, it has led to death being undervalued as an outcome in HIV programmes. Many clinics and programmes track individuals in care but do not have a clear understanding of which of their patients could have died and of what cause, making them prone to underestimating mortality. In this context, a broad set of patients have for years been categorised as lost to follow-up, conflating all individuals who transfer to another clinic or disengage from care and stop treatment with individuals who have actually died. This amalgamation of different groups is a problem not just in HIV but in multiple efforts to fight disease, from tuberculosis to cardiovascular disease and diabetes.10, 11
One study that traced a sample of people lost to follow-up in Uganda, Kenya, and Tanzania found that 27% of these individuals had actually died, three times more than clinic-level data suggested.12 In another sample of people who initiated antiretroviral therapy in 22 African countries, about 15% had died within 5 years, 2·5 times the rate shown in clinic records.13 Although distinguishing AIDS mortality from non-AIDS mortality is difficult in low-resource settings, tracking all-cause mortality among people living with HIV at a granular level will yield actionable insights, even as greater diagnostic capacity is built. These measures can also support integration efforts by increasing awareness of the numerous so-called silent deaths due to non-HIV causes, such as cardiovascular disease.
One of the most important implications of the paucity of data of local mortality is that programmes do not sufficiently prioritise interventions for people with advanced disease. Studies in South Africa, Kenya, Zambia, and the Democratic Republic of the Congo have shown that most patients with HIV admitted to hospital have already been on antiretroviral therapy (often for years) but they either stop treatment or are on a treatment regimen that is not effectively suppressing the virus.14, 15, 16 A high proportion of patients die because of HIV-related illnesses that could have been prevented. A set of evidence-based clinical interventions has been recommended by WHO to prevent this mortality, including point-of-care cryptococcal screening and tuberculosis urine lipoarabinomannan screening, along with prophylactic and preventive treatment. However, uptake has been slow and insufficient in a context of scarce resources.17 Data on localised and specific mortality could provide a basis for programme managers to target resources to clinics, regions, or populations where these interventions are most needed.
Without granular mortality data, comparisons between different sites, regions, and subpopulations cannot be made. A study across four provinces in Zambia, for example, found that some clinics had mortality rates greater than ten times those of the best performing clinics; a degree of heterogeneity that could be related to clinical, structural, or other factors.18 Health leaders who have access to reliable mortality information can identify and learn from successful programmes and apply these findings to programmes that underperform.
Generating information to be used at the low levels of the health-care system, by clinic managers, district-level public health officials, and local community groups, has not been a political priority. Generating this kind of information is also not simple. Medical record systems in low-income and middle-income countries remain weak. Many deaths among people living with HIV occur outside the health-care system. Yet action is even more urgent in situations where deaths are concentrated among the individuals who are hardest to reach.1 We see at least three opportunities to address these challenges and enable timely mortality tracking, alongside efforts to prevent unnecessary deaths.
First, progress in countries as diverse as South Africa, Malaysia, Nicaragua, and Fiji shows that developing more robust vital registries is possible within a few years and with relatively small amounts of funding. In South Africa, in particular, tracking the mortality of young people using systems at the local level helped monitor the effectiveness of HIV programmes. Low-cost efforts to create sample vital registration systems with verbal autopsy also show promise.8 Importantly, these efforts have the advantage of supporting all health programmes. HIV funders, including PEPFAR and the Global Fund, should partner with national governments and health funders, such as the UK Department for International Development and World Bank, to develop and strengthen vital registries, building on momentum from WHO, UNICEF, and others.19
Second, robust patient-tracing activities should be incorporated as core objectives of all HIV programmes and funded accordingly. Actual mortality rates should be key programme indicators, made easier by investments in vital registration. PEPFAR took the bold step, in 2019, of requiring the programmes it funds to report on HIV mortality.20 Hopefully, this step will improve patient outcomes by incentivising effective interventions for advanced HIV disease and support for people who have stopped treatment to re-enter care.17
Third, we can move towards a variety of outcome-oriented global health programmes beyond HIV, for which measures of success move from the number of patients receiving services to explicit reductions in mortality rates. Some maternal health efforts have shown it is possible to uncover and address the causes of maternal deaths, but many have struggled to have a widespread effect. These approaches face similar challenges to HIV with regard to data limitations, adequate scaling, sufficient resources, and a high number of deaths outside facilities.21 Other compelling examples, such as the public–private Saving Mothers Giving Life programme, show how focusing on maternal mortality rates and leveraging systems developed for the HIV response could improve facilities and increase demand. Initiated by the Obama administration, the programme helped decrease maternal mortality by 41% in regions of Uganda, and 44% in regions of Zambia before it was discontinued by the Trump administration.22 These programmes should be revived and expanded.
Broader global health efforts can learn from the HIV experience. Mortality estimates based on aggregate national modelling can be influential in drawing political attention to these issues, and in encouraging collective action and making high-level decisions on the allocation of resources. However, as effective programmes are scaled up, mortality is likely to become more localised, heterogeneous, and less susceptible to single interventions. From emerging infectious diseases to cancer and mental health diseases, this path is likely to be similar. As efforts to address non-communicable diseases in low-income and middle-income countries gain momentum, they are likely to include provision of long-term biomedical interventions such as anti-hypertensives for high blood pressure and oral hypoglycaemic medications for diabetes. Tracking actual mortality and building the necessary capacity to identify the causes of death will be key for programmes to fully account for the progress that is made and motivate filling gaps in the quality of services.
Making the shift to reporting on and addressing actual mortality will require a political reorientation by funders towards prioritising information that might be less useful to them than high-level modelled estimates but essential for the people designing and implementing frontline programmes. The HIV response could lead this change by committing resources as part of an effort to regain the momentum against preventable deaths. This commitment will require a collective effort to mobilise the political will needed to improve and empower decision-making and a renewed focus on what ultimately matters most to the individuals in the pandemic's path—avoiding unnecessary deaths.
Contributors
All authors contributed to conceptualising, drafting, and editing the manuscript.
Declaration of interests
MMK received a grant for a joint policy mapping project with UNAIDS. All other authors declare no competing interests.
References
- 1.UNAIDS . UNAIDS; Geneva: 2019. 2019 Global AIDS update: communities at the centre. [Google Scholar]
- 2.Institute for Health Metrics and Evaluation Global Burden of Disease Compare. 2019. https://vizhub.healthdata.org/gbd-compare/
- 3.UNAIDS AIDS Info. 2019. https://aidsinfo.unaids.org/
- 4.Jerven M. Beyond precision: embracing the politics of global health numbers. Lancet. 2018;392:468–469. doi: 10.1016/S0140-6736(18)31700-8. [DOI] [PubMed] [Google Scholar]
- 5.Shiffman J, Smith S. Generation of political priority for global health initiatives: a framework and case study of maternal mortality. Lancet. 2007;370:1370–1379. doi: 10.1016/S0140-6736(07)61579-7. [DOI] [PubMed] [Google Scholar]
- 6.Tichenor M, Sridhar D. Metric partnerships: global burden of disease estimates within the World Bank, the World Health Organisation, and the Institute for Health Metrics and Evaluation. Wellcome Open Res. 2019;4:35. doi: 10.12688/wellcomeopenres.15011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathers CD, Fat DM, Inoue M, Rao C, Lopez AD. Counting the dead and what they died from: an assessment of the global status of cause of death data. Bull World Health Organ. 2005;83:171–177. [PMC free article] [PubMed] [Google Scholar]
- 8.Jha P. Reliable direct measurement of causes of death in low- and middle-income countries. BMC Med. 2014;12:19. doi: 10.1186/1741-7015-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikkelsen L, Phillips DE, AbouZahr C, et al. A global assessment of civil registration and vital statistics systems: monitoring data quality and progress. Lancet. 2015;386:1395–1406. doi: 10.1016/S0140-6736(15)60171-4. [DOI] [PubMed] [Google Scholar]
- 10.Labhardt ND, Balo JR, Ndam M, Manga E, Stoll B. Improved retention rates with low-cost interventions in hypertension and diabetes management in a rural African environment of nurse-led care: a cluster-randomised trial. Trop Med Int Health. 2011;16:1276–1284. doi: 10.1111/j.1365-3156.2011.02827.x. [DOI] [PubMed] [Google Scholar]
- 11.Shringarpure KS, Isaakidis P, Sagili KD, Baxi RK. Loss-to-follow-up on multidrug resistant tuberculosis treatment in Gujarat, India: the when and who of it. PLoS One. 2015;10 doi: 10.1371/journal.pone.0132543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng EH, Odeny TA, Lyamuya RE, et al. Estimation of mortality among HIV-infected people on antiretroviral treatment in East Africa: a sampling based approach in an observational, multisite, cohort study. Lancet HIV. 2015;2:e107–e116. doi: 10.1016/S2352-3018(15)00002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haas AD, Zaniewski E, Anderegg N, et al. Retention and mortality on antiretroviral therapy in sub-Saharan Africa: collaborative analyses of HIV treatment programmes. J Int AIDS Soc. 2018;21 doi: 10.1002/jia2.25084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ousley J, Niyibizi AA, Wanjala S, et al. High proportions of patients with advanced HIV are antiretroviral therapy experienced: hospitalization outcomes from 2 sub-Saharan African sites. Clin Infect Dis. 2018;66(suppl 2):S126–S131. doi: 10.1093/cid/ciy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meintjes G, Kerkhoff AD, Burton R, et al. HIV-related medical admissions to a South African district hospital remain frequent despite effective antiretroviral therapy scale-up. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000002269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haachambwa L, Kandiwo N, Zulu PM, et al. Care continuum and postdischarge outcomes among HIV-infected adults admitted to the hospital in Zambia. Open Forum Infect Dis. 2019;6 doi: 10.1093/ofid/ofz336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO . World Health Organization; Geneva: 2017. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. [PubMed] [Google Scholar]
- 18.Holmes CB, Sikazwe I, Sikombe K, et al. Estimated mortality on HIV treatment among active patients and patients lost to follow-up in 4 provinces of Zambia: Findings from a multistage sampling-based survey. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UNICEF The future for women and children: UNICEF and WHO joint statement on strengthening Civil Registration and Vital Statistics (CRVS) 2018. https://www.who.int/healthinfo/civil_registration/WHO_UNICEF_Statement_CRVS_2018.pdf?ua=1
- 20.President's Emergency Plan For AIDS Relief Monitoring, evaluation, and reporting indicator reference guide. 2019. https://www.state.gov/wp-content/uploads/2019/10/PEPFAR-MER-Indicator-Reference-Guide-Version-2.4-FY20.pdf
- 21.Koblinsky M. Maternal death surveillance and response: a tall order for effectiveness in resource-poor settings. Glob Health Sci Pract. 2017;5:333–337. doi: 10.9745/GHSP-D-17-00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serbanescu F, Clark TA, Goodwin MM, et al. Impact of the Saving Mothers, Giving Life approach on decreasing maternal and perinatal deaths in Uganda and Zambia. Glob Health Sci Pract. 2019;7(suppl 1):S27–S47. doi: 10.9745/GHSP-D-18-00428. [DOI] [PMC free article] [PubMed] [Google Scholar]