Abstract
The COVID-19 pandemic has major implications for blood transfusion. There are uncertain patterns of demand, and transfusion institutions need to plan for reductions in donations and loss of crucial staff because of sickness and public health restrictions. We systematically searched for relevant studies addressing the transfusion chain—from donor, through collection and processing, to patients—to provide a synthesis of the published literature and guidance during times of potential or actual shortage. A reduction in donor numbers has largely been matched by reductions in demand for transfusion. Contingency planning includes prioritisation policies for patients in the event of predicted shortage. A range of strategies maintain ongoing equitable access to blood for transfusion during the pandemic, in addition to providing new therapies such as convalescent plasma. Sharing experience and developing expert consensus on the basis of evolving publications will help transfusion services and hospitals in countries at different stages in the pandemic.
Background
The ongoing COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is creating major disruption globally at all levels of health-care provision. In the UK, around a third of hospitalised patients with COVID-19 are estimated to die.1 Transfusion professionals are responding to uncertain patterns of demand for blood components, to reductions in the numbers of donations, and to loss of crucial staff because of sickness. A key activity for transfusion institutions during this period, whether hospital-based or separate blood transfusion services, is the monitoring of supply and demand so that sufficient blood stocks are maintained to support ongoing critical needs, for example, major trauma.
The objective of this Review is to provide a synthesis of the evolving published literature on COVID-19 and to provide expert opinion relevant to transfusion practice in times of potential or real shortage, addressing the entire transfusion chain from donor to patient. The search strategy that underpinned this Review has been regularly updated to incorporate new, relevant information. The focus is on providing practical guidance to support transfusion specialists worldwide at different stages in the pandemic, including as health services reopen for all activities. Further updates of searching will ensure that any new information is highlighted for readers.
Method
A systematic approach was taken to search and identify all published literature relevant to COVID-19. Searches were done using a comprehensive search strategy (appendix p 1). These searches were not limited by language or study type and were run daily by an information specialist. The following databases were searched: WHO COVID-19 global research database,2 PubMed, and Vox Sanguinis International Society for Blood Transfusion Science Series. In addition, a search was done for relevant general articles on blood and shortage, blood and contingency planning, and blood and major incident planning (appendix pp 1–2).
All identified references were screened by one person using predefined eligibility criteria (appendix pp 2–3). Each eligible reference was tagged with clinical key words, ranging in themed areas from donor to recipient. Any type of study or review was considered relevant. Outputs of searches were reviewed and incorporated by groups of clinicians into five key section themes defined at the onset of the project and described in the following sections of this Review. A table of registered, randomised controlled trials was created by weekly searches of ongoing trial registries, ClinicalTrials.gov, and the COVID-19 subset of the WHO International Clinical Trials Registry Platform database.3
Results
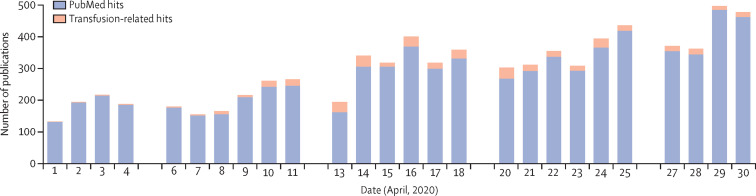
From March 23 to April 30, 2020, systematic searches identified over 9000 citations. During April, 2020, 7715 citations were screened for eligibility and 414 were included in the final citation list. Figure 1 shows the steady increase in citations during April and the proportion of citations relevant to the topic of transfusion chain from donor to recipient. The search narrative for emergency planning retrieved 1255 references after duplicates and irrelevant references were removed, from which 121 citations were included. A few ongoing systematic reviews were also identified.4
Figure 1.
The total number of COVID-19-related citations and the proportion of those relevant to transfusion
Theme 1: features of SARS-CoV-2 infection that affect patients' needs for transfusion
Characteristics of SARS-CoV-2 infection have been described by multiple reports.5, 6 Understanding these features informs the approaches required to address potential mismatches between blood supply (theme 2) and demand, including the activities of patient blood management implementation (theme 4). Anaemia is uncommon on admission. In patients admitted to intensive care, severe anaemia or platelet counts below 100 × 109 cells per L during the first 3 days are also uncommon.7 The severity of thrombocytopenia, when it does occur, appears to be a marker for poor outcomes.8, 9, 10 These publications support observations that many patients with COVID-19 do not require transfusion.11, 12 For example, data from Italy showed that 39% of patients required transfusion (median duration of hospitalisation of 15 days) for a main indication of anaemia (non-bleeding), with very few patients requiring platelets or plasma.13 In this study, direct antiglobulin test reactivity was common and anti-red blood cell antibodies were detected in 52 (46%) of 113 patients.13 Higher transfusion requirements are expected in patients who have extracorporeal membrane oxygenation than in those who do not, but this outcome is relevant to only a small number of patients.14
A distinct pattern of coagulation disturbance, including raised D-dimer concentration, has been identified as a poor prognostic marker.15 Many patients with COVID-19 have elevated fibrinogen, normal platelet counts, and often normal prothrombin time and activated partial thromboplastin time.16 Viscoelastic testing suggests that hypercoagulability changes are not consistent with a pattern of acute disseminated intravascular coagulation.17 Between late January and April, 2020, reports described a 25–31% incidence of thrombotic complications in patients admitted to intensive care units with COVID-19.18, 19 A study18 in France described 64 (43%) clinically relevant thrombotic complications from 150 patients, but only four (3%) patients presented with bleeding complications.20 Thrombotic changes have also been described postmortem.21
Plasma and cryoprecipitate transfusion to manage isolated abnormalities of coagulation in patients with no evidence of bleeding is not indicated for patients with COVID-19.22, 23 Given the risk of exacerbating any prothrombotic tendency, tranexamic acid is not indicated for patients with no bleeding.24 To date, bleeding complications that could increase transfusion requirements have not been frequently reported in patients with COVID-19, although this observation might require review should escalated schedules for heparin anticoagulation be applied.25
In the absence of specific transfusion trial data on patients with COVID-19, it is appropriate to follow general recommendations on restrictive thresholds for red blood cells and platelets.26, 27 Patients with COVID-19 might be elderly with comorbidities, such as cardiac disease. However, no data are available to inform whether patients with SARS-CoV-2 infection, with substantial respiratory symptoms and oxygen dependency, might benefit from red blood cell transfusion to maintain a haemoglobin concentration above 70 g/L. In addition, few data are available on the outcomes of women who develop COVID-19 during pregnancy, and some features might overlap with pre-eclampsia.28, 29, 30 Studies suggest that patients with blood group O have a lower risk of developing COVID-19.31, 32
Theme 2: what donor and donation factors need to be considered to maintain an adequate supply of blood during the COVID-19 pandemic?
When considering the broader issues for blood supply planning during the pandemic, a key consideration for transfusion services is maintaining the balance between supply and demand.33, 34, 35 Donor attendance might fall, as it did by 10–30% in the state of Washington, USA,36 and by 30% at Canadian Blood Services (Goldman M, unpublished). However, in the early stages of the pandemic this trend was compensated by a reduction in demand for blood because of a decrease in elective surgery and medical treatment.11, 12, 33, 34, 35, 36 Blood providers have planned to maintain or increase the inventory of fresh components, which are sustained with public appeals to donate and by ensuring that blood donation is regarded as a permitted activity during lockdown. Blood collection staff might be concerned about exposure to donors, become sick, or self-isolate through family exposure. These factors could lead to substantial reductions in staff availability to collect and process blood. Overall, many reports show that sufficiency of supply has been maintained to date, but in some areas shortages have been observed.37
Donor screening and testing strategies, the management of postdonation information for donors diagnosed with COVID-19, and changes in other transfusion practices are based on theoretical or confirmed risks of transmission. Because SARS-CoV-2 is a new virus, its potential for transfusion transmission, including by an asymptomatic viraemic donor, is uncertain.38 From experience, including with other coronaviruses, the risk is currently considered low.39, 40 Although SARS-CoV-2 RNA can be found in the bloodstream of infected individuals,41, 42, 43, 44 this might not equate to infectious viraemia. In one study, blood transfused from donors who were subsequently diagnosed with COVID-19 did not transmit SARS-CoV-2;39 however, data relating to SARS-CoV-2 are scarce and the number of blood donors with presymptomatic or asymptomatic COVID-19 when donating are not defined. To minimise risk of virus transmission, countries are developing guidance on the selection of donors, with precautionary deferral periods following infection and symptom reporting following donation. Haemovigilance systems should be in place to monitor any potential cases of transfusion transmission (WHO interim guidance, 2020).45
Table 1 provides additional information on the capacity to maintain and adjust access to blood donors and specific types of donations in the context of the COVID-19 pandemic. Actions to counteract the effects of the pandemic on blood availability might include changing practices that are applied to protect donors and recipients, including eligibility criteria. The risks incurred by relaxing some of these practices should be proportional to the benefit of sufficiency. Any changes need to be discussed with relevant stakeholders (regulatory bodies, and donor and patient representatives).
Table 1.
Donor and donation factors to consider for maintaining an adequate supply of blood during the COVID-19 pandemic
| Considerations | Possible actions | |
|---|---|---|
| Donor recruitment34, 46, 47, 48 | Donors tend to respond well to public appeals in situations of perceived exceptional need (eg, September 11 attacks and mass shooting events); a large influx of donors is to be expected, at least initially; donors are more tolerant to longer waiting times; platelet donations require close attention because of their short shelf life; some donors might be prevented from donating because of stay-at-home orders (eg, older, reliable donors) | Encourage appointments but discourage walk-ins; track donor characteristics (first-time donor vs repeat donor, as well as age, sex, etc); reinforce platelet aphaeresis donation programmes; consider increasing reliance on whole blood-derived platelets; more forcefully target first-time and reactivated donors for future donations |
| Donor eligibility40, 49, 50, 51, 52, 53 | Some donor-selection criteria could be relaxed without any meaningful effect on donor or product safety (for examples, see possible actions column); this approach can only be justified if supply cannot meet demand and changes need to be planned in advance because of their complexity (eg, regulatory aspects, IT system changes, and training of personnel); consideration should be given on the acceptability of reinstating pre-pandemic criteria after the pandemic is over (easier to explain to donors for some measures [eg, Haemoglobin concentrations] than others, such as reinstating permanent deferrals for variant Creutzfeldt–Jakob disease); some procedures can also be interrupted to increase compliance with public health recommendations, including social distancing; the COVID-19 situation might exacerbate criticisms over deferral policies for men who have sex with men, although shortening the deferral period will likely yield few additional donors | Discussions could be held with regulatory authorities regarding mechanisms for urgent implementation and expedited reviews; some procedures and criteria regarding donor safety could be considered for relaxation (eg, salty snacks on blood drives before and during donation, heart rate and blood pressure measurements, interdonation intervals, haemoglobin thresholds, and age restrictions); some procedures and criteria regarding recipient safety could be considered for relaxation (eg, deferral period for travel in a malaria-risk area; deferral period for tattoos, piercings, and needle-stick injuries; deferrals for men who have sex with men; and deferrals for variant Creutzfeldt-Jakob disease risk) |
| Blood drive planning36 | Decreasing and increasing demand; suitability of donation sites; public health recommendations and governmental communications regarding confinement; public appeals for donation; staff availability | Adjust the number and size of upcoming blood drives; review physical distancing requirements when choosing locations for mobile blood drives; consider expanding collections on fixed sites; work with public health advisors and government communicators to emphasise the importance of blood donation as a reason for travel; work with health authorities to coordinate public appeals for donation, if and when appropriate |
| Inventory management54 | Demand is hard to predict and might vary in different phases of the pandemic | Keep close contact with hospital customers, including regular updates of inventory; track system-wide inventory closely; monitor activities requiring increased blood use (eg, elective surgery and transplantation) |
| Protection of staff and donors36, 47, 55, 56, 57, 58 | Use of personal protective equipment for donors, staff, and volunteers; practice physical distancing; monitor COVID-19 illness among staff and donors; message donors before arrival on the blood drive regarding wellness; prescreening for COVID-19 signs and symptoms | Align practices with public health recommendations; review availability of personal protective equipment; implement a communication plan for occupational risk; disseminate guidelines for COVID-19 signs and symptoms among personnel, donors, and volunteers (quarantine and testing, etc); consider screening donors, personnel, and volunteers for symptoms and elevated temperature before entering facilities and donation sites |
| Availability of personnel36, 40, 59, 60 | Effect of COVID-19 on staff: illness, quarantine, and fear of disease | Prepare contingency plans for staff replacement (eg, reassignment and training of other non-essential staff); communicate clear supportive policies for sick leave; encourage staff to self-report illness or concerns; offer and strengthen psychological support for personnel |
| Plasma for fractionation61, 62 | The effect on supply of plasma for fractionation is uncertain, including the supply of immunoglobulins; blood providers might temporarily decrease their source plasma donation programmes to shift their capacity to whole blood donations | Efforts should be made to maintain or increase source plasma donations in the context of the pandemic; reconsider the need for certain procedures and criteria in donor screening, such as the annual physical exam; blood providers could take advantage of the influx of new and repeated donors to increase collections of source plasma collections |
| Product safety38, 39, 40, 41, 42, 43, 44, 45, 63 | To date, there is no evidence of SARS-CoV-2 transmission by transfusion; some infected people appear to have detectable RNA in their blood, even when they do not have severe symptoms; RNA has been found in a few blood donors, but the concentrations are low, and the results might represent false positives; RNA in blood does not necessarily represent infectious viral particles; the South Korean lookback study39 found no evidence of transmission | Do additional studies to establish the presence of virus in blood donors; do lookbacks and tracebacks when appropriate; reinforce postdonation information protocols; evaluate the availability and appropriateness of blood screening tests for donors; communicate risk assessments to relevant stakeholders; eligibility criteria should be applied to reduce the risk of collecting blood from infected donors; deferral periods should be applied for confirmed or suspected cases, for travel in countries or regions at high risk, and for exposure to confirmed cases (also important for safety of staff and other donors) |
SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
Theme 3: modifications to production, specification, and storage of blood components to help prevent blood shortage
Changes to processing and storage of blood components might contribute to maintaining the blood supply during a pandemic. Modification of donor and component testing criteria, including any additional safety measures for groups such as neonates, might have to be addressed. A more complete consideration of the different options should be based on factors such as the likely magnitude of gain, the perceived effect on clinical risk, the regulatory requirements, and the extent of complexity and ability to deliver change in the system. Changes that require substantial resources to implement might not be feasible during a pandemic, and therefore simplicity and forward planning is key. The panel describes modifications that could be considered, risk assessed, and discussed with stakeholders. These options should continue to be reviewed to account for the potential increase in demand for blood as non-COVID-19-related hospital activities are resumed (theme 4).
Panel. Strategies to modify production, specification, and storage of blood components to help prevent blood shortage.
Red blood cells
Extend shelf life if validated and within regulations
Review manufacturing process.64, 65
Platelets
Extend shelf life from 5 days to 7 days with appropriate bacterial testing or pathogen inactivation
Recovery and survival of platelets, as well as count increments following transfusion, decline with increasing storage duration.66, 67 Bacterial risk depends on the timing of sampling, sample volume, and the length of culture; delayed culture methods with 7 day storage have been shown to be effective.68 Depending on screening methodology, a further test at day 4 or at the end of storage might be required.
Extend shelf life to 8 days after review of internal laboratory data to guide feasibility
Review internal laboratory data to guide feasibility, and review data on bacterial risk. There is scant clinical data beyond day 7. At day 8, the recovery of fresh platelets manufactured from buffy-coats is nearly 70% and platelet survival is 45%.69, 70 Improved recovery and survival of platelets with prolonged storage has been observed with some types of additive solution.69, 70
Reduce dose for prophylactic transfusion (split products)
Some countries already issue split products for neonatal transfusion. Consider half doses, or methods to produce two-thirds to three-quarter doses, such as pooling fewer so-called buffy coats or splitting aphaeresis collections into more doses.71
Consider use of cold-stored platelets with 7–14-day shelf life for patients with bleeding only
Studies in healthy volunteers suggest that the survival of platelets from whole blood or platelet concentrates refrigerated for 10–15 days might maintain acceptable viability. Laboratory data suggest that platelets remain functional for 14–21 days without the need for agitation.72, 73, 74, 75, 76
Consider frozen platelets for bleeding patients only 77, 78
Plasma
Remove requirements to freeze plasma
Consider use of liquid (never frozen) plasma if freezer capacity or staff to freeze plasma are in short supply.79
Whole Blood
Use of whole blood
Consider if staff to manufacture components are in short supply or for massive transfusion.80, 81, 82, 83, 84
A first step is to review measures to minimise wastage. This strategy might support the temporary extension of component shelf life. Storage age for red blood cells and platelets is typically defined at a national level and varies across countries, with a red blood cell shelf life of 35–49 days for most. A shelf life extension for red blood cells should be considered early on, as once shortages occur the components will be used before reaching the maximum storage time. Randomised trials do not provide evidence of considerable adverse effects with longer storage for red blood cell transfusion.64, 65 Individual blood providers could review the flexibility of manufacturing processes to allow the extension of whole blood holding times, provided there is internal validation on component quality.
Most blood providers assign a shelf life of 5 days to platelets, or 7 days if they are either tested for bacteria or undergo pathogen inactivation treatment. Depending on the exact method of production and satisfactory validation data, platelet storage might be extended to 8 days with a considered assessment of the risks (eg, bacterial contamination and platelet viability). For relevant countries, consideration could also be given to stopping bacterial testing, with a possible concomitant reduction in shelf life, or testing platelets earlier in shelf life to release platelets to stock sooner. However, the latter will only alleviate short-term supply issues and might be less beneficial than in times of prolonged shortages. Cold storage of platelets at 2–6°C could also be considered, as this method might allow a shelf life of 7–14 days without the need for agitation.72 Stocks of frozen platelets, if available and expanded, might provide a haemostatic effect, in part because of the content of platelet microparticles.85
To increase platelet supply for prophylactic transfusions, one option could be to reduce the dose of platelets by splitting existing components. The PLADO trial71 reported no significant dose effect on the incidence of bleeding in patients with hypoproliferative thrombocytopenia, although more transfusions were given in the low-dose patient group. This option will require validation to ensure that platelet quality is maintained throughout storage, and education within hospitals.
Frozen plasma components have a long shelf life (several years) and therefore the ability to build and maintain stocks is more flexible than for cellular components. Liquid plasma (never frozen), which has a shelf life of 7–40 days, might be useful in the context of reduced freezer capacity, a shortage of staff to freeze plasma, or for the production of convalescent plasma (theme 5).
Whole blood was used for transfusion until the mid-1960s when its use ceased in civilian settings in favour of separated component parts—red blood cells, platelets, and plasma. International interest for the use of whole blood in the treatment of actively bleeding patients has been revived. In the context of the pandemic, whole blood is simple to manufacture and could be used if blood stocks are low or staff are in short supply.
Systems for pathogen inactivation of plasma and platelet (but not red blood cell) components are in routine use in some countries, but not all. These systems result in a 3–6 log reduction in infectivity of models of coronaviruses, such as severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), depending upon the technology used.38, 44 The decision to implement pathogen inactivation needs to take account the risk of not doing so, balanced against the cost and the resource required for implementation. For countries that do not already use pathogen inactivation, its rapid introduction is a large task. The risk of transmission through blood appears to be low, although our understanding will improve as the pandemic evolves. Similar considerations need to be given to pathogen inactivation of convalescent plasma.
Theme 4: prioritisation of blood use for patients in hospitals in the event of predicted shortage
A range of local mitigation strategies are required if blood shortages are anticipated (table 2 ).33 These policies might initially be based on national guidance documents for planning in the event of blood shortages.97 Each hospital should establish appropriate local structures to support responses. This strategy might include an Emergency Blood Management group with representation from clinical users, managers, and the hospital transfusion team. Actions will take into account shortage predictions by blood suppliers, inventory simplification, and changes to component shelf life or dose.
Table 2.
Strategies to prioritise blood use for patients in hospitals in the event of predicted shortage
| Considerations | Possible actions | |
|---|---|---|
| Red blood cell usage | Red blood cell shortages | Review the threshold of red blood cell transfusions for patients who are stable and low risk (eg, adults and children with mild symptomatic but not life-threatening anaemia)86, 87, 88 |
| Platelet usage | Platelet shortages for prophylactic transfusion | Use of platelets as prophylaxis should be restricted in patients with hypoproliferative thrombocytopenia without clinical bleeding, including autologous transplantation89 |
| Major bleeding | Blood shortages for patients with bleeding | Review local policies that are usually based on the use of blood components defined by ratio-driven therapy, preferably 1:1:1 for red blood cells, plasma, and platelets, or 1:1 for red blood cells and plasma if platelets are not available. If red blood cells are in short supply, consider giving plasma first or blood components at ratios of 1:2:1 (red blood cells, plasma, and platelets);90, 91 if platelets are scarce, consider cold-stored or frozen platelets, or whole blood;80, 92, 93 consider prothrombin complex concentrate and fibrinogen concentrates if frozen plasma or cryoprecipitate is in short supply for patients with bleeding;92 if type AB plasma is unavailable, consider use of type A plasma for massive transfusion93 |
| Alternatives for transfusion | Emphasising use of alternatives to transfusion at times of blood shortages | Ensure that alternative measures to increase haemoglobin are offered where appropriate (eg, parenteral iron and erythropoietin);94 tranexamic acid should be offered to patients with severe hypoproliferative thrombocytopenia or outpatients with chronic thrombocytopenia; desmopressin should also be considered for patients with uraemia or inherited platelet disorders who are at risk of bleeding, although few data exist for other patient populations95, 96 |
Key areas to address will be the use of blood for a non-urgent situation, such as elective surgery, although these activities might already have been restricted early in the pandemic to release staff and space. A pandemic infection leads not only to the deferral of non-urgent interventions, but also to the shielding of patients who are at increased risk of infection or having severe COVID-19. Patients might be reticent to attend health-care facilities, even for potentially serious symptoms. Increased thresholds for exposing patients to immunosuppressive therapy, such as consolidation chemotherapy and stem cell transplantation, have also been described.98 These changes in behaviour have resulted in a substantial reduction in demand for all blood components. Planning should address ongoing and unavoidable transfusion needs for selected patients with non-COVID-19-related health issues, such as trauma, emergency surgery, transfusion dependency (eg, for cancer, myelodysplastic syndrome, and thalassaemia),99, 100 and acute sickle chest crises requiring exchange transfusion.101, 102, 103
In the event of falling blood stocks, stringent implementation for all activities of patient blood management is required, which covers transfusion and preoperative anaemia management,94, 104 tightening local guidelines where possible. Audits of blood transfusion have consistently shown that around 20–30% of blood component use is outside guidance and hence probably unnecessary, reinforcing the need for patient blood management initiatives.105, 106 At more extreme levels of blood shortage, the release of blood components outside local guidelines should be reviewed by the blood transfusion laboratory with support from clinicians.107 Guidance for local concessionary release of components outside specifications should be developed if not already in place. Integral to the success of any local initiatives is a strong existing framework of accepted guidelines, agreed with clinicians from relevant departments. An ability to rapidly produce and disseminate authoritative national guidelines enables evidence to be assessed and changes updated consistently; for example, guidelines and evidence summaries from the National Institute for Health and Care Excellence.108
Finally, as the pandemic is controlled and the health-care system gradually returns to normal or, more probably, responds to increased rates of non-COVID-19-related activity (eg, delayed cancer treatments or postponed surgery), complications could increase following delays and planning for an increase in need for blood is therefore required.
Theme 5: use of convalescent plasma and immunoglobulins
Interest in the role of immunotherapies and convalescent plasma collected from patients who have recovered from COVID-19 is considerable.109, 110 The rationale for convalescent plasma is that patients who have developed neutralising antibodies against SARS-CoV-2 could lower or eliminate the viral load in patients with COVID-19.111 Convalescent plasma has been used in clinical studies for treating severe acute respiratory syndrome, including infections with SARS-CoV, MERS-CoV, avian influenza A (H5N1), and for Ebola, with some promising results.112, 113, 114, 115, 116 Case series of patients with COVID-19 have described a clinical benefit after receiving convalescent plasma,117, 118 but randomised trials are required given the potential risks associated with this treatment.119, 120
Many randomised trials of convalescent plasma have been registered and are ongoing in patients with COVID-19. However, there are differences in protocol design, including the use of convalescent plasma to treat adults or children admitted to the intensive care unit, to treat patients in settings other than intensive care, or even as prophylaxis (for people in close contact with those confirmed to have COVID-19).121 Convalescent plasma from donors could be collected at different stages of recovery (14–28 days after full recovery or >28 days after full recovery) using different products (aphaeresis plasma, whole blood-derived plasma) with various, or even unknown, anti-SARS-CoV-2 antibody titres within the product. Multiple organisational challenges need to be addressed to support and deliver a convalescent plasma programme, including policies for approving plasma donors and testing of donations. Another treatment option is passive immunisation by collecting plasma to extract hyperimmune immunoglobulins. This option will only become available when large amounts of plasma can be collected.
Discussion
This paper has collated information from multiple sources to provide a synthesis of the published literature to help inform the planning for critical imbalances in the blood supply chain during the COVID-19 pandemic. A key observation and challenge for clinicians is the expanding literature, reflected by the extensive number of citations identified. This issue raises considerable challenges for clinicians in keeping abreast of the published literature to identify studies with the most important effect on patient care. The degree to which specific changes are considered or implemented will depend on a variety of factors that apply locally and nationally. Recommendations have not been provided for each theme, although many of the actions described could be considered as best practice suggestions.
Early planning to review mitigation options is recommended; in particular, stock building and the extension of shelf life when stocks are good. Policy documents should include a hospital-based emergency management plan, ideally based on a national plan, integrated with monitoring across the blood component supply chain and rigorous application of the principles of patient blood management.
Transfusion requirements are low, even in patients who are critically ill with COVID-19. There are no robust data on the numbers of presymptomatic or asymptomatic donors who have subsequently seroconverted, or on the potential infectivity of blood with SARS-CoV-2,38, 122 although the risk of transfusion transmission is likely to be low. Recommendations for transfusion should conform to general messages of restrictive use of blood. In collaboration with public health agencies, blood services are well placed to contribute to epidemiological studies and biobanks evaluating the serology, features, and course of the COVID-19 pandemic.
A limitation to this project was the availability of only one individual to do the initial screening. The writing group considered the quality of much of the published literature to be insufficient. Many of the papers identified from searches were observational, including those describing results of primary research, and were open to all the limitations common to this design. However, formal methodological assessments were not done. As the quality of the publications strengthen, further updates of the search will incorporate more specific recommendations.123 A large number of ongoing randomised trials were identified, addressing areas of practice relevant to this Review (appendix pp 4–7). This progress is testament to the work of many researchers, although sharing protocols at early stages of development might be beneficial, exploring opportunities to collaborate, and ensuring consistency in outcome measures.119
Acknowledgments
Acknowledgments
We would like to acknowledge Kim Lacey (NHS Blood and Transplant, John Radcliffe Hospital, Oxford, UK) for administrative support and formatting.
Contributors
SJS, NS, and JT conceived of the idea for the Review. SJS wrote the first draft. SB and CD searched and screened the published literature. All authors contributed to the writing: theme 1 (SJS, DP, NS, JT), theme 2 (MGe, MGo, TOA), theme 3 (RC, HVN, TOA), theme 4 (EM, SJS, NS, DP), and theme 5 (CS-O).
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Wise J. A third of covid-19 patients admitted to UK hospitals die. BMJ. 2020;369 doi: 10.1136/bmj.m1794. [DOI] [PubMed] [Google Scholar]
- 2.WHO COVID-19 global literature on coronavirus disease. 2020. https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/
- 3.WHO International Clinical Trials Registry Platform (ICTRP) https://www.who.int/ictrp/en/
- 4.Rada G, Verdugo-Paiva F, Ávila C, et al. Evidence synthesis relevant to COVID-19: a protocol for multiple systematic reviews and overviews of systematic reviews. Medwave. 2020;20 doi: 10.5867/medwave.2020.03.7867. [DOI] [PubMed] [Google Scholar]
- 5.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in critically ill patients in the Seattle region—case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145–148. doi: 10.1016/j.cca.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu XM, Wang DQ. Consideration and suggestions on development of blood transfusion department under the epidemic situation of novel coronavirus pneumonia. Zhonghua Yi Xue Za Zhi. 2020;100:1041–1043. doi: 10.3760/cma.j.cn112137-20200221-00387. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 10.Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469–1472. doi: 10.1111/jth.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai X, Ren M, Chen F, Li L, Lei H, Wang X. Blood transfusion during the COVID-19 outbreak. Blood Transfus. 2020;18:79–82. doi: 10.2450/2020.0076-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan BE, Ong KH, Chan SSW, et al. Blood and blood product use during COVID-19 infection. Am J Hematol. 2020 doi: 10.1002/ajh.25823. published online April 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berzuini A, Bianco C, Paccapelo C, et al. Red cell bound antibodies and transfusion requirements in hospitalized patients with COVID-19. Blood. 2020 doi: 10.1182/blood.2020006695. published online June 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koeckerling D, Pan D, Mudalige NL, Oyefeso O, Barker J. Blood transfusion strategies and ECMO during the COVID-19 pandemic. Lancet Respir Med. 2020;8:E40. doi: 10.1016/S2213-2600(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost. 2020 doi: 10.1111/jth.14854. published online April 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panigada M, Bottino N, Tagliabue P, et al. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020 doi: 10.1111/jth.14850. published online April 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients in severe SARS-CoV-2 infection: a multicentre prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thachil J, Tang N, Gando S, et al. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Green L, Bolton-Maggs P, Beattie C, et al. British Society of Haematology Guidelines on the spectrum of fresh frozen plasma and cryoprecipitate products: their handling and use in various patient groups in the absence of major bleeding. Br J Haematol. 2018;181:54–67. doi: 10.1111/bjh.15167. [DOI] [PubMed] [Google Scholar]
- 24.Hunt BJ, Allard S, Keeling D, Norfolk D, Stanworth SJ, Pendry K. A practical guideline for the haematological management of major haemorrhage. Br J Haematol. 2015;170:788–803. doi: 10.1111/bjh.13580. [DOI] [PubMed] [Google Scholar]
- 25.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA. 2016;316:2025–2035. doi: 10.1001/jama.2016.9185. [DOI] [PubMed] [Google Scholar]
- 27.Estcourt LJ, Birchall J, Allard S, et al. Guidelines for the use of platelet transfusions. Br J Haematol. 2017;176:365–394. doi: 10.1111/bjh.14423. [DOI] [PubMed] [Google Scholar]
- 28.Vlachodimitropoulou Koumoutsea E, Vivanti AJ, Shehata N, et al. COVID-19 and acute coagulopathy in pregnancy. J Thromb Haemost. 2020 doi: 10.1111/jth.14856. published online April 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Della Gatta AN, Rizzo R, Pilu G, Simonazzi G. Coronavirus disease 2019 during pregnancy: a systematic review of reported cases. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.013. published online April 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breslin N, Baptiste C, Gyamfi-Bannerman C, et al. COVID-19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai X. ABO blood group predisposes to COVID-19 severity and cardiovascular diseases. Eur J Prev Cardiol. 2020 doi: 10.1177/2047487320922370. published online April 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellinghaus D, Degenhardt F, Bujanda L, et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med. 2020 doi: 10.1056/NEJMoa2020283. published online June 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yazer M, Pagano MB, Cho D, Lin Y. Vox Sanguinis International Forum on transfusion services about response to COVID-19. Vox Sang. 2020 doi: 10.1111/vox.12943. published online May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franchini M, Farrugia A, Velati C, et al. The impact of the SARS-CoV-2 outbreak on the safety and availability of blood transfusions in Italy. Vox Sang. 2020 doi: 10.1111/vox.12928. published online April 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohammadi S, Tabatabaei Yazdi SM, Eshghi P, Norooznezhad AH. Coronavirus disease 2019 (COVID-19) and decrease in blood donation: experience of Iranian Blood Transfusion Organization (IBTO) Vox Sang. 2020 doi: 10.1111/vox.12930. published online April 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pagano MB, Hess JR, Tsang HC, et al. Prepare to adapt: blood supply and transfusion support during the first 2 weeks of the 2019 novel coronavirus (COVID-19) pandemic affecting Washington state. Transfusion. 2020;60:908–911. doi: 10.1111/trf.15789. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Han W, Pan L, et al. Impact of COVID-19 on blood centres in Zhejiang province China. Vox Sang. 2020 doi: 10.1111/vox.12931. published online April 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang L, Yan Y, Wang L. Coronavirus disease 2019: coronaviruses and blood safety. Transfus Med Rev. 2020;34:75–80. doi: 10.1016/j.tmrv.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon SY, Kim EJ, Jung YS, Jang JS, Cho NS. Post-donation COVID-19 identification in blood donors. Vox Sang. 2020 doi: 10.1111/vox.12925. published online April 2. [DOI] [PubMed] [Google Scholar]
- 40.European Centre for Disease Prevention and Control Coronavirus disease 2019 (COVID-19) and supply of substances of human origin in the EU/EEA—first update. 2020. https://www.ecdc.europa.eu/sites/default/files/documents/COVID%2019-supply-substances-human-origin-first-update.pdf
- 41.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323:1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zheng S, Fan J, Yu F, et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang L, Zhao L, Gong H, Wang L, Wang L. Severe acute respiratory syndrome coronavirus 2 RNA detected in blood donations. Emerg Infect Dis. 2020 doi: 10.3201/eid2607.200839. published online April 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.WHO Maintaining a safe and adequate blood supply during the pandemic outbreak of coronavirus disease (COVID-19): interim guidance. March 20, 2020. https://apps.who.int/iris/handle/10665/331523
- 46.Lozada MJ, Cai S, Li M, Davidson SL, Nix J, Ramsey G. The Las Vegas mass shooting: an analysis of blood component administration and blood bank donations. J Trauma Acute Care Surg. 2019;86:128–133. doi: 10.1097/TA.0000000000002089. [DOI] [PubMed] [Google Scholar]
- 47.Leung JN, Lee CK. Impact of the COVID-19—a regional blood center's perspective. ISBT Science Series. 2020 doi: 10.1111/voxs.12558. published online April 17. [DOI] [Google Scholar]
- 48.Tran S, Lewalski EA, Dwyre DM, et al. Does donating blood for the first time during a national emergency create a better commitment to donating again? Vox Sang. 2010;98:e219–e224. doi: 10.1111/j.1423-0410.2009.01274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goldman M, Germain M, Grégoire Y, et al. Safety of blood donation by individuals over age 70 and their contribution to the blood supply in five developed countries: a BEST Collaborative group study. Transfusion. 2019;59:1267–1272. doi: 10.1111/trf.15132. [DOI] [PubMed] [Google Scholar]
- 50.Hoad VC, Guy RJ, Seed CR, Harley R. Tattoos, blood-borne viruses and blood donors: a blood donor cohort and risk assessment. Vox Sang. 2019;114:687–693. doi: 10.1111/vox.12832. [DOI] [PubMed] [Google Scholar]
- 51.Prinsze FJ, van de Laar T, Slot E, et al. No increased risk of transfusion-transmissible infections after tattooing, body piercing, or acupuncture among blood donors in the Netherlands. Transfusion. 2019;59:2575–2583. doi: 10.1111/trf.15421. [DOI] [PubMed] [Google Scholar]
- 52.Pillonel J, Pelat C, Tiberghien P, et al. The evolving blood donor deferral policy for men who have sex with men: impact on the risk of HIV transmission by transfusion in France. Transfusion. 2020;60:525–534. doi: 10.1111/trf.15677. [DOI] [PubMed] [Google Scholar]
- 53.Germain M. Men having sex with men and blood donation: is there a game changer on the horizon? Transfusion. 2020;60:437–440. doi: 10.1111/trf.15706. [DOI] [PubMed] [Google Scholar]
- 54.Abolghasemi H, Radfar MH, Tabatabaee M, Hosseini-Divkolayee NS, Burkle FM., Jr Revisiting blood transfusion preparedness: experience from the Bam earthquake response. Prehosp Disaster Med. 2008;23:391–394. doi: 10.1017/s1049023x00006117. [DOI] [PubMed] [Google Scholar]
- 55.Htun HL, Lim DW, Kyaw WM, et al. Responding to the COVID-19 outbreak in Singapore: staff protection and staff temperature and sickness surveillance systems. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa468. published online April 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Al-Tawfiq JA, Rothwell S, Mcgregor HA, Khouri ZA. A multi-faceted approach of a nursing led education in response to MERS-CoV infection. J Infect Public Health. 2018;11:260–264. doi: 10.1016/j.jiph.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gowing JR, Walker KN, Elmer SL, Cummings EA. Disaster preparedness among health professionals and support staff: what is effective? An integrative literature review. Prehosp Disaster Med. 2017;32:321–328. doi: 10.1017/S1049023X1700019X. [DOI] [PubMed] [Google Scholar]
- 58.Greenhalgh T, Schmid MB, Czypionka T, Bassler D, Gruer L. Face masks for the public during the covid-19 crisis. BMJ. 2020;369 doi: 10.1136/bmj.m1435. [DOI] [PubMed] [Google Scholar]
- 59.Brooks SK, Dunn R, Amlôt R, Rubin GJ, Greenberg N. Protecting the psychological wellbeing of staff exposed to disaster or emergency at work: a qualitative study. BMC Psychol. 2019;7:78. doi: 10.1186/s40359-019-0360-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khalid I, Khalid TJ, Qabajah MR, Barnard AG, Qushmaq IA. Healthcare workers emotions, perceived stressors and coping strategies during a MERS-CoV outbreak. Clin Med Res. 2016;14:7–14. doi: 10.3121/cmr.2016.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farrugia A, Grazzini G, Quinti I, Candura F, Profili S, Liumbruno GM. The growing importance of achieving national self-sufficiency in immunoglobulin in Italy. The emergence of a national imperative. Blood Transfus. 2019;17:449–458. doi: 10.2450/2019.0265-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Puig Rovira L. Plasma self-sufficiency in Spain. Transfus Apheresis Sci. 2020;59 doi: 10.1016/j.transci.2019.102700. [DOI] [PubMed] [Google Scholar]
- 63.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shah A, Brunskill SJ, Desborough MJ, Doree C, Trivella M, Stanworth SJ. Transfusion of red blood cells stored for shorter versus longer duration for all conditions. Cochrane Database Syst Rev. 2018;12 doi: 10.1002/14651858.CD010801.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trivella M, Stanworth SJ, Brunskill S, Dutton P, Altman DG. Can we be certain that storage duration of transfused red blood cells does not affect patient outcomes? BMJ. 2019;365 doi: 10.1136/bmj.l2320. [DOI] [PubMed] [Google Scholar]
- 66.Caram-Deelder C, Kreuger AL, Jacobse J, van der Bom JG, Middelburg RA. Effect of platelet storage time on platelet measurements: a systematic review and meta-analyses. Vox Sang. 2016;111:374–382. doi: 10.1111/vox.12443. [DOI] [PubMed] [Google Scholar]
- 67.MacLennan S, Harding K, Llewelyn C, et al. A randomized noninferiority crossover trial of corrected count increments and bleeding in thrombocytopenic hematology patients receiving 2- to 5- versus 6- or 7-day-stored platelets. Transfusion. 2015;55:1856–1865. doi: 10.1111/trf.13038. (quiz 1855). [DOI] [PubMed] [Google Scholar]
- 68.McDonald C, Allen J, Brailsford S, et al. Bacterial screening of platelet components by National Health Service Blood and Transplant, an effective risk reduction measure. Transfusion. 2017;57:1122–1131. doi: 10.1111/trf.14085. [DOI] [PubMed] [Google Scholar]
- 69.Slichter SJ, Bolgiano D, Corson J, et al. Extended storage of buffy coat platelet concentrates in plasma or a platelet additive solution. Transfusion. 2014;54:2283–2291. doi: 10.1111/trf.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Slichter SJ, Corson J, Jones MK, et al. Exploratory studies of extended storage of apheresis platelets in a platelet additive solution (PAS) Blood. 2014;123:271–280. doi: 10.1182/blood-2013-05-501247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Slichter SJ, Kaufman RM, Assmann SF, et al. Dose of prophylactic platelet transfusions and prevention of hemorrhage. N Engl J Med. 2010;362:600–613. doi: 10.1056/NEJMoa0904084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stolla M, Fitzpatrick L, Gettinger I, et al. In vivo viability of extended 4°C-stored autologous apheresis platelets. Transfusion. 2018;58:2407–2413. doi: 10.1111/trf.14833. [DOI] [PubMed] [Google Scholar]
- 73.Scorer T, Williams A, Reddoch-Cardenas K, Mumford A. Manufacturing variables and hemostatic function of cold-stored platelets: a systematic review of the literature. Transfusion. 2019;59:2722–2732. doi: 10.1111/trf.15396. [DOI] [PubMed] [Google Scholar]
- 74.Getz TM, Montgomery RK, Bynum JA, Aden JK, Pidcoke HF, Cap AP. Storage of platelets at 4°C in platelet additive solutions prevents aggregate formation and preserves platelet functional responses. Transfusion. 2016;56:1320–1328. doi: 10.1111/trf.13511. [DOI] [PubMed] [Google Scholar]
- 75.Johnson L, Tan S, Wood B, Davis A, Marks DC. Refrigeration and cryopreservation of platelets differentially affect platelet metabolism and function: a comparison with conventional platelet storage conditions. Transfusion. 2016;56:1807–1818. doi: 10.1111/trf.13630. [DOI] [PubMed] [Google Scholar]
- 76.Braathen H, Sivertsen J, Lunde THF, et al. In vitro quality and platelet function of cold and delayed cold storage of apheresis platelet concentrates in platelet additive solution for 21 days. Transfusion. 2019;59:2652–2661. doi: 10.1111/trf.15356. [DOI] [PubMed] [Google Scholar]
- 77.Reade MC, Marks DC, Bellomo R, et al. A randomized, controlled pilot clinical trial of cryopreserved platelets for perioperative surgical bleeding: the CLIP-I trial (Editorial, p. 2759) Transfusion. 2019;59:2794–2804. doi: 10.1111/trf.15423. [DOI] [PubMed] [Google Scholar]
- 78.Slichter SJ, Jones M, Ransom J, et al. Review of in vivo studies of dimethyl sulfoxide cryopreserved platelets. Transfus Med Rev. 2014;28:212–225. doi: 10.1016/j.tmrv.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 79.Backholer L, Green L, Huish S, et al. A paired comparison of thawed and liquid plasma. Transfusion. 2017;57:881–889. doi: 10.1111/trf.13915. [DOI] [PubMed] [Google Scholar]
- 80.Slichter SJ, Fitzpatrick L, Osborne B, et al. Platelets stored in whole blood at 4°C: in vivo posttransfusion platelet recoveries and survivals and in vitro hemostatic function. Transfusion. 2019;59:2084–2092. doi: 10.1111/trf.15302. [DOI] [PubMed] [Google Scholar]
- 81.Yazer MH, Cap AP, Spinella PC, Alarcon L, Triulzi DJ. How do I implement a whole blood program for massively bleeding patients? Transfusion. 2018;58:622–628. doi: 10.1111/trf.14474. [DOI] [PubMed] [Google Scholar]
- 82.Bahr M, Cap AP, Dishong D, Yazer MH. Practical considerations for a military whole blood program. Mil Med. 2020 doi: 10.1093/milmed/usz466. published online April 30. [DOI] [PubMed] [Google Scholar]
- 83.Yazer M, Spinella PC. Review of low titre group O whole blood use for massively bleeding patients around the world in 2019. ISBT Sci Ser. 2019;14:276–281. [Google Scholar]
- 84.Bjerkvig C, Sivertsen J, Braathen H, et al. Cold-stored whole blood in a Norwegian emergency helicopter service: an observational study on storage conditions and product quality. Transfusion. 2020 doi: 10.1111/trf.15802. published online April 22. [DOI] [PubMed] [Google Scholar]
- 85.Slichter SJ, Dumont LJ, Cancelas JA, et al. Safety and efficacy of cryopreserved platelets in bleeding patients with thrombocytopenia. Transfusion. 2018;58:2129–2138. doi: 10.1111/trf.14780. [DOI] [PubMed] [Google Scholar]
- 86.Maitland K, Kiguli S, Olupot-Olupot P, et al. Immediate transfusion in African children with uncomplicated severe anemia. N Engl J Med. 2019;381:407–419. doi: 10.1056/NEJMoa1900105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roy NB, Telfer P, Eleftheriou P, et al. Protecting vulnerable patients with inherited anaemias from unnecessary death during the COVID-19 pandemic. Br J Haematol. 2020;189:635–639. doi: 10.1111/bjh.16687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hadadi A, Mortezazadeh M, Kolahdouzan K, Alavian G. Does recombinant human erythropoietin administration in critically ill COVID-19 patients have miraculous therapeutic effects? J Med Virol. 2020;92:915–918. doi: 10.1002/jmv.25839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stanworth SJ, Estcourt LJ, Powter G, et al. A no-prophylaxis platelet-transfusion strategy for hematologic cancers. N Engl J Med. 2013;368:1771–1780. doi: 10.1056/NEJMoa1212772. [DOI] [PubMed] [Google Scholar]
- 90.Sperry JL, Guyette FX, Brown JB, et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 2018;379:315–326. doi: 10.1056/NEJMoa1802345. [DOI] [PubMed] [Google Scholar]
- 91.Pusateri AE, Moore EE, Moore HB, et al. Association of prehospital plasma transfusion with survival in trauma patients with hemorrhagic shock when transport times are longer than 20 minutes: a post hoc analysis of the PAMPer and COMBAT clinical trials. JAMA Surg. 2019;155 doi: 10.1001/jamasurg.2019.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fabes J, Brunskill SJ, Curry N, Doree C, Stanworth SJ. Pro-coagulant haemostatic factors for the prevention and treatment of bleeding in people without haemophilia. Cochrane Database Syst Rev. 2018;12 doi: 10.1002/14651858.CD010649.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dunbar NM, Yazer MH. Safety of the use of group A plasma in trauma: the STAT study. Transfusion. 2017;57:1879–1884. doi: 10.1111/trf.14139. [DOI] [PubMed] [Google Scholar]
- 94.Mueller MM, Van Remoortel H, Meybohm P, et al. Patient blood management: recommendations from the 2018 Frankfurt Consensus Conference. JAMA. 2019;321:983–997. doi: 10.1001/jama.2019.0554. [DOI] [PubMed] [Google Scholar]
- 95.Desborough MJ, Smethurst PA, Estcourt LJ, Stanworth SJ. Alternatives to allogeneic platelet transfusion. Br J Haematol. 2016;175:381–392. doi: 10.1111/bjh.14338. [DOI] [PubMed] [Google Scholar]
- 96.Desborough MJ, Oakland K, Brierley C, et al. Desmopressin use for minimising perioperative blood transfusion. Cochrane Database Syst Rev. 2017;7 doi: 10.1002/14651858.CD001884.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.National Advisory Committee on Blood and Blood Products Emergency framework for rationing of blood for massively bleeding patients during a red phase of a blood shortage. 2012. https://www.nacblood.ca/resources/shortages-plan/emergency-framework-final.pdf
- 98.Ljungman P, Mikulska M, de la Camara R, et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020 doi: 10.1038/s41409-020-0919-0. published online May 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.He W, Chen L, Chen L, et al. COVID-19 in persons with haematological cancers. Leukemia. 2020;34:1637–1645. doi: 10.1038/s41375-020-0836-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Willan J, King AJ, Hayes S, Collins GP, Peniket A. Care of haematology patients in a COVID-19 epidemic. Br J Haematol. 2020;189:241–243. doi: 10.1111/bjh.16620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hussain FA, Njoku FU, Saraf SL, Molokie RE, Gordeuk VR, Han J. COVID-19 infection in patients with sickle cell disease. Br J Haematol. 2020;189:851–852. doi: 10.1111/bjh.16734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nur E, Gaartman AE, van Tuijn CFJ, Tang MW, Biemond BJ. Vaso-occlusive crisis and acute chest syndrome in sickle cell disease due to 2019 novel coronavirus disease (COVID-19) Am J Hematol. 2020;95:725–726. doi: 10.1002/ajh.25821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dexter D, Simons D, Kiyaga C, et al. Mitigating the effect of the COVID-19 pandemic on sickle cell disease services in African countries. Lancet Haematol. 2020;7:e430–e432. doi: 10.1016/S2352-3026(20)30122-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shander A, Goobie SM, Warner MA, et al. The essential role of patient blood management in a pandemic: a call for action. Anesth Analg. 2020 doi: 10.1213/ANE.0000000000004844. published online March 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Murphy MF, Waters JH, Wood EM, Yazer MH. Transfusing blood safely and appropriately. BMJ. 2013;347 doi: 10.1136/bmj.f4303. [DOI] [PubMed] [Google Scholar]
- 106.Baron DM, Franchini M, Goobie SM, et al. Patient blood management during the COVID-19 pandemic: a narrative review. Anaesthesia. 2020 doi: 10.1111/anae.15095. published online April 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Doughty H, Green L, Callum J, Murphy MF. Triage tool for the rationing of blood for massively bleeding patients during a severe national blood shortage: guidance from the National Blood Transfusion Committee. Brit J Haem. 2020 doi: 10.1111/bjh.16736. published online May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.National Institute for Health and Care Excellence Improving health and social care through evidence-based guidance. 2020. https://www.nice.org.uk/covid-19
- 109.AminJafari A, Ghasemi S. The possible of immunotherapy for COVID-19: a systematic review. Int Immunopharmacol. 2020;83 doi: 10.1016/j.intimp.2020.106455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bloch EM, Shoham S, Casadevall A, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130:2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maxmen A. How blood from coronavirus survivors might save lives. Nature. 2020;580:16–17. doi: 10.1038/d41586-020-00895-8. [DOI] [PubMed] [Google Scholar]
- 112.Wong VW, Dai D, Wu AK, Sung JJ. Treatment of severe acute respiratory syndrome with convalescent plasma. Hong Kong Med J. 2003;9:199–201. [PubMed] [Google Scholar]
- 113.Arabi YM, Hajeer AH, Luke T, et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg Infect Dis. 2016;22:1554–1561. doi: 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357:1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- 115.Sahr F, Ansumana R, Massaquoi TA, et al. Evaluation of convalescent whole blood for treating Ebola virus disease in Freetown, Sierra Leone. J Infect. 2017;74:302–309. doi: 10.1016/j.jinf.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dzik S. COVID-19 convalescent plasma: now is the time for better science. Trans Med Rev. 2020 doi: 10.1016/j.tmrv.2020.04.002. published online April 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roback JD, Guarner J. Convalescent plasma to treat COVID-19: possibilities and challenges. JAMA. 2020 doi: 10.1001/jama.2020.4940. published online March 27. [DOI] [PubMed] [Google Scholar]
- 121.Tiberghien P, de Lambalerie X, Morel P, Gallian P, Lacombe K, Yazdanpanah Y. Collecting and evaluating convalescent plasma for COVID-19 treatment: why and how. Vox Sang. 2020 doi: 10.1111/vox.12926. published online April 2. [DOI] [PubMed] [Google Scholar]
- 122.Gandhi M, Yokoe DS, Havlir DV. Asymptomatic transmission, the achilles' heel of current strategies to control Covid-19. N Engl J Med. 2020;382:2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rome BN, Avorn J. Drug evaluation during the Covid-19 pandemic. N Engl J Med. 2020;382:2282–2284. doi: 10.1056/NEJMp2009457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.