Abstract
All biological processes, living organisms, and ecosystems have evolved with the Sun that confers a 24-hour periodicity to life on Earth. Circadian rhythms arose from evolutionary needs to maximize daily organismal fitness by enabling organisms to mount anticipatory and adaptive responses to recurrent light-dark cycles and associated environmental changes. The clock is a conserved feature in nearly all forms of life, ranging from prokaryotes to virtually every cell of multicellular eukaryotes. The mammalian clock comprises transcription factors interlocked in negative feedback loops, which generate circadian expression of genes that coordinate rhythmic physiology. In this review, we highlight previous and recent studies that have advanced our understanding of the transcriptional architecture of the mammalian clock, with a specific focus on epigenetic mechanisms, transcriptomics, and 3-dimensional chromatin architecture. In addition, we discuss reciprocal ways in which the clock and metabolism regulate each other to generate metabolic rhythms. We also highlight implications of circadian biology in human health, ranging from genetic and environment disruptions of the clock to novel therapeutic opportunities for circadian medicine. Finally, we explore remaining fundamental questions and future challenges to advancing the field forward.
Keywords: circadian rhythms, epigenetics, chromatin architecture, metabolism
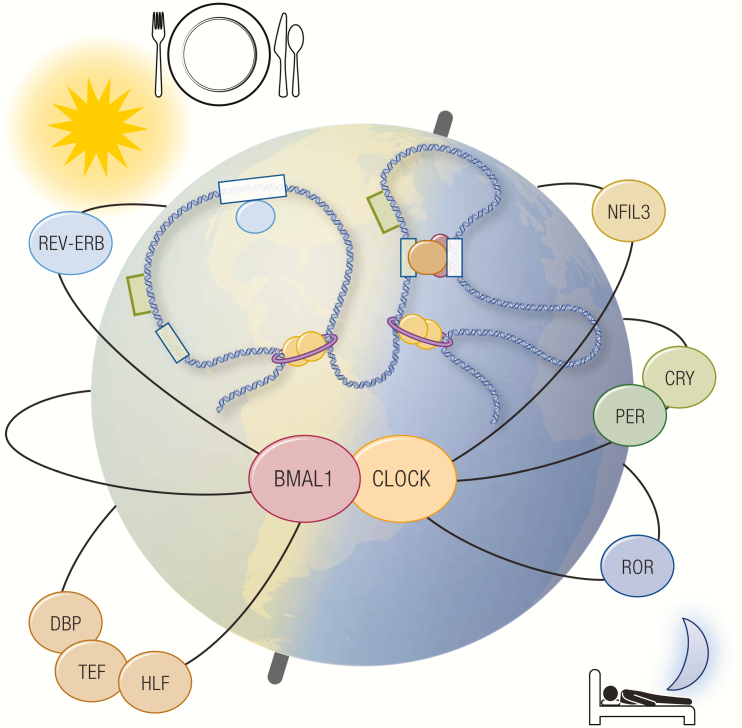
Graphical Abstract
Graphical Abstract.
Essential Points.
Circadian rhythms synchronize mammalian physiology with daily environmental variations.
Molecular clocks comprised of transcriptional-translational feedback loops generate oscillating rhythms that orchestrate rhythmic physiology.
Clock transcription factors modulate epigenomic rhythms, including histone modification and chromatin architectural oscillation.
Reciprocal crosstalk between the central clock and peripheral clocks aligns feeding behavior with circadian metabolic processes.
The clock serves a dual role as a driver and an integrator of metabolism.
Circadian medicine has the potential to leverage circadian physiology to add a new dimension to pharmacological treatments of disease.
The daily rotation of Earth around its axis creates an inherently dynamic ecosystem with recurrent light-dark cycles. In this geophysical framework, life emerged inextricably with the Sun, which delivers light to Earth’s surface with a periodicity of 24 hours. As a source of energy and genotoxic stress, light exerts a powerful evolutionary pressure to all photosensitive forms of life. Although light provides photic energy necessary for photosynthesis, it also poses formidable environmental insults in the form of damaging radiation and drastic oscillations in temperature through its light-dark cycles (1, 2). As a result, a myriad of life forms and their biological processes arose and evolved with circadian rhythms, which in Latin translates to “rhythms around (circa-) a day (-diem).”
From an evolutionary prospective, circadian rhythms confer distinct survival advantages. While irreversible environmental perturbations drive the selection of one or a few favorable phenotypes over generations of species, circadian variations of the environment select not only favorable phenotypes but also the time at which they should appear during the day. This evolutionary pressure gave rise to intrinsic timekeeping mechanisms that enabled circadian organization of phenotypic variations, which are temporally programmed to anticipate and adapt to the daily environmental cycle in order to maximize organismal fitness on a 24-hour time scale. It is on this evolutionary basis that nearly all fundamental biological processes are coupled with the rising and setting of the Sun (1, 2).
The notion that intrinsic timekeeping mechanisms coordinate circadian rhythms began with de Mairan’s observation in the 18th century that daily leaf movements of the Mimosa pudica plant occurred autonomously in darkness (3). A seminal study by Konopka and Benzer two centuries later demonstrated the genetic basis of rhythmic locomotor activity in fruit flies (4). This work inspired positional cloning experiments in the laboratories of Jeffrey Hall, Michael Rosbash, and Michael Young that ultimately identified the first clock gene period, for which they shared the 2017 Nobel Prize in Physiology or Medicine (5–8). Since the discovery of the Period gene in fruit flies, many clock genes encoding transcription factors (TFs) that compose the molecular clock have been cloned and characterized in mammalian species. However, even as the complexity of the clock field has grown, fundamental questions remain as to how these molecular cogs function together to keep the clock ticking at the systems level, ranging from transcriptional oscillations to cell autonomous rhythms and ultimately organismal physiology.
In the past decade, the advent of genome-wide techniques and genome-editing tools has offered a tangible opportunity to systemically untangle the clock at the magnitude of speed and complexity that has not been afforded in the past. In this review, we will focus on recent advances in our understanding of the transcriptional architecture of the mammalian clock and highlight its role in human physiology and pathology, with a specific emphasis on circadian metabolic homeostasis.
Mechanisms of the Mammalian Clock
Transcription factor network
The mammalian clock is orchestrated by activator and repressor TFs interlocked in cell-autonomous transcription-translational feedback loops with a delay, a fundamental governing principle of the molecular clock (9, 10). Nearly all clock TFs are embedded in negative feedback loops that lead to transcriptional repression of their own genes. With declining production, self-repression is abated, and transcription begins again, thereby generating oscillating levels of transcription and translation that recur every 24 hours. At the core, the mammalian clock consists of a forward limb that drives 3 regulatory limbs, which confer circadian rhythmicity to the forward limb in an interdependent manner (Fig. 1A).
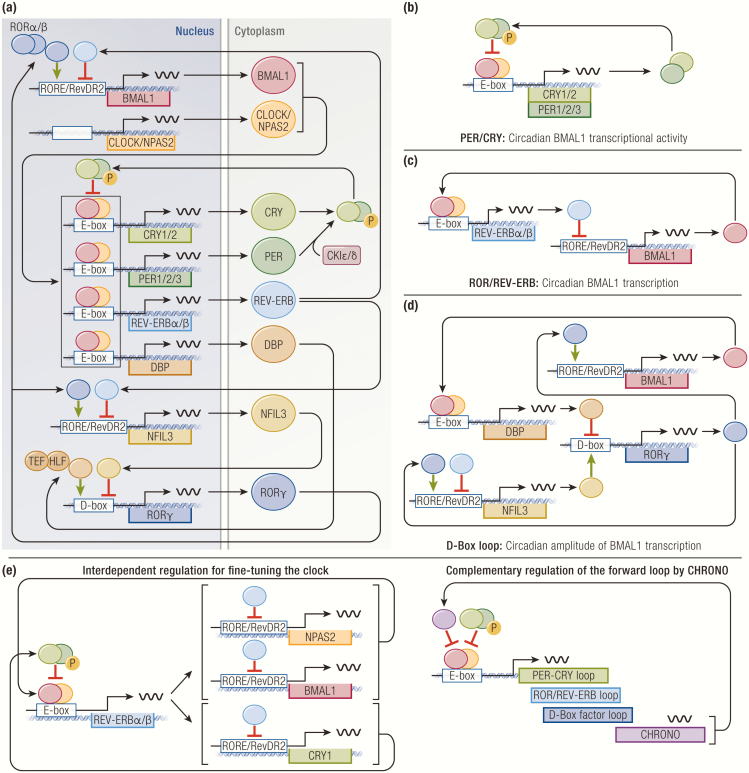
Figure 1.
Transcriptional network of core clock transcription factors (TFs). (A) A composite illustration of all major regulatory limbs. (B) PER/CRY regulate circadian BMAL1 transcriptional activity. (C) ROR and REV-ERB regulate circadian transcription of BMAL1. (D) D-box TFs enhance circadian amplitude of BMAL1 transcription. (E) Different regulatory limbs regulate one another to further fine tune the clock.
The forward limb of the mammalian clock is mainly driven by 2 basic helix-loop-helix PER-ARNT-SIM (PAS) domain-containing TFs, BMAL1 (brain and muscle ARNT-like 1, also referred to as MOP3, encoded by ARNTL) and CLOCK (circadian locomotor output cycles kaput, encoded by CLOCK) (11–14). BMAL1 and CLOCK heterodimerize to form a transcriptional activator complex, which binds E-box motifs at promoters and enhancers, including those of core clock genes that constitute the negative regulatory limbs. Although BMAL1 is an indispensable component of the mammalian clock, genetics studies demonstrated functional redundancy of CLOCK with its paralogue NPAS2 (neuronal PAS domain protein 2; encoded by NPAS2) in certain tissues, such as the forebrain and suprachiasmatic nucleus (15–18).
The first regulatory limb consists of PER1/2/3 (Period, encoded by PER1, PER2, PER3) and CRY1/2 (Cryptochrome, encoded by CRY1, CRY2). They are repressive TFs whose orthologues in fruit flies were first found to have clock functions, albeit working through divergent molecular mechanisms in the fruit fly and mammalian systems. (19–22). In the mammalian clock, the forward limb induces the expression of PER and CRY, which heterodimerize in the cytoplasm and translocate to the nucleus upon phosphorylation by casein kinase I (23–26). In the nucleus, PER-CRY heterodimers bind BMAL1-CLOCK heterodimers to form quaternary complexes that act as transcriptional repressors at BMAL1-target genes, including their own. As a result, this negative feedback loop confers a circadian rhythm to BMAL1/CLOCK transcriptional activity (27–30) (Fig. 1B). Of note, similar to CLOCK and NPAS2, PER and CRY paralogues share redundant and nonredundant functions in regulation of the clock because of their differential expression patterns across various tissues. This subject has recently been reviewed in greater detail (31).
The second regulatory limb is controlled by the nuclear hormone receptor TFs, RORα/β/γ (RAR-related orphan receptor, encoded by RORA, RORB, RORC), and REV-ERBα/β (reverse strand of c-erbα, encoded by NR1D1, NR1D2) (32–36). ROR and REV-ERB recognize and compete for RORE and RevDR2 motifs at promoters and enhancers, where they have opposing effects on transcription: ROR activates transcription, whereas REV-ERB represses transcription (37, 38). The forward limb drives the expression of REV-ERB, which then competes with ROR at cis-regulatory elements throughout the genome, including the BMAL1 promoter (39-42). As a result, rhythmic competition between REV-ERB and ROR establishes a negative feedback loop at the level of BMAL1 transcription, thereby augmenting circadian transcriptional activity of the forward limb (Fig. 1C). Of note, although both REV-ERBα and β show circadian expression in most tissues, expression patterns of RORα, β, and γ are considerably more complex, highly tissue-restricted, and not necessarily circadian in certain tissues (36, 43-48). For instance, RORβ is expressed in select regions of the brain and retina, whereas a specific γ-isoform RORγT is expressed during differentiation of naïve CD4+ T cells into T helper 17 cells where it serves as a critical lineage-determining factor (45, 46, 49–51). Such observations underscore the diverse biological roles of RORs in addition to its clock function that entail complex tissue-specific regulation [reviewed in (52)].
The third regulatory limb comprises the proline and acidic amino acid-rich basic leucine zipper TFs, including DBP (D-binding protein, encoded by DBP), TEF (thyrotroph embryonic factor, encoded by TEF), HLF (hepatic leukemic factor, encoded by HLF), and NFIL3/ E4BP4 (nuclear factor, interleukin-3 regulated/E4 promoter binding factor 4, encoded by NFIL3) (53-55). All of these factors recognize and compete for D-box motifs at promoters and enhancers, where they stimulate transcription in a redundant manner, except for NFIL3, which represses transcription (56, 57). The forward limb drives the circadian expression of DBP in the opposite phase of NFIL3, which is separately driven by the ROR-REV-ERB axis (58-63). The temporal opposition of DBP and NFIL3 activity generates circadian expression of their target genes, including RORγ. This cascade thus coordinates the peak expression of RORγ at the trough of REV-ERB expression, thereby enhancing the amplitude of BMAL1 transcription (62) (Fig. 1D).
Through their cognate genetic elements and combinations thereof, these regulatory limbs coordinate temporal expression patterns of clock genes as well as numerous clock-controlled genes that carry out circadian functions of the cell. Although this overview aims to simplify and highlight several key aspects of rhythm generation with an emphasis on transcriptional regulation of the forward limb, it is important to consider key questions that remain in the field. For instance, there is no clear consensus on what is required to be a core clock member because not all clock TFs have the same magnitude of effect on the forward loop. For example, mice lacking both REV-ERB α/β exhibit arrhythmic behavior and disrupted expression of many clock genes, including BMAL1 (39, 41). However, another study reported that inhibition of both REV-ERB α/β does not significantly impair BMAL1 transcriptional activity, despite the lack of rhythmic BMAL1 expression (64). Similarly, constitutive expression of BMAL1 in mice lacking endogenous BMAL1 has been shown to restore circadian behavior and transcriptional rhythms of the forward limb, which are likely generated by the intact PER-CRY axis (65). This finding that rhythmic BMAL1 expression may not be required for the forward limb suggests that the ROR-REV-ERB axis likely serves as an axillary limb that fine tunes the clock. Despite its mild effect on the forward limb, REV-ERB plays an indispensable role in the clock transcriptional network as a primary clock TF that is directly controlled by BMAL1 and intimately regulates expression of other clock genes and specific clock-controlled genes that coordinate cellular and organismal rhythms, as evidenced by the α/β knockout studies.
Extensive crosstalk among the regulatory limbs is also critical for orchestrating the clock. REV-ERB represses both BMAL1 and NPAS2, 2 positive regulators of the forward limb, while simultaneously repressing the negative regulator CRY1 by binding to its intronic enhancer (66-68) (Fig. 1E). Genetic deletion of this enhancer results in reduced rhythmicity of CRY1 expression and a shorter period in locomotor activity (68, 69). Adding more complexity to the simplified model of a single cognate genetic element controlling a clock gene, several clock TFs have been found to share overlapping genomic binding sites near clock genes, suggesting potential coregulation by more than one clock TF (39, 60). In addition, not all core clock genes are driven by the forward limb. In mice constitutively expressing REV-ERBα that abrogates BMAL1 expression, PER2 maintains robust circadian expression in the liver, which is abolished in ex vivo explants, implicating a role of systemic cues in direct regulation of the cell-autonomous clock (70).
Recent studies also identified the novel circadian TF CHRONO (computationally highlighted or chromatin immunoprecipitation-derived repressor of the network oscillator, encoded by GM129) (71–73). CHRONO is expressed in a circadian manner and interacts with BMAL1-CLOCK and PER to repress BMAL1-target genes, although it remains unknown how CHRONO complements the PER-CRY axis at the biochemical and transcriptional levels, which will require further investigation for a detailed functional characterization (Fig. 1E).
Epigenetic regulation of the clock
The observation that posttranslational modifications of histone tails regulate transcription provided the first molecular basis of epigenetics, which refers to heritable changes in gene expression without changes in genotype (74). In past decades, cloning of various epigenetic regulators spurred the rapid growth of epigenetics research dedicated to elucidating the biochemical basis of epigenetic modifications and understanding the epigenetic control of gene expression in numerous biological systems, including circadian rhythms (75, 76).
Broadly, epigenetic regulators are classified as “writers or erasers” and “readers.” Epigenetic writers and erasers modify histone tails with an array of posttranslational modifications that produce distinct chromatin states, which are recognized by epigenetic readers to generate specific transcriptional outputs. DNA methylation is another major branch of epigenetic regulation (77). Current evidence suggests DNA methylation is stable throughout circadian phases and likely not directly regulated by the clock (78, 79). Thus, this review focuses on histone modifications.
Histone acetylation is regulated by opposing actions of histone acetyl transferases (HAT) and histone deacetylases (HDAC). Acetylation of H3 lysine 9 and 27 (H3K9Ac and H3K27Ac) promotes an open and easily accessible euchromatin state for TFs to bind the genome to activate transcription (80, 81). Histone acetylations can also be recognized by a group of bromodomain and extraterminal motif proteins that participate in various aspects of transcription, such as recruitment of nucleosome remodeling complexes and elongation factors (82, 83).
Histone methylation is regulated by a large number of histone methyltransferases (HMT) and histone demethylases (84). Unlike acetylation, which is generally indicative of active transcription, histone methylation can be associated with either active or inactive transcription. Basic residues on histones can be mono-, di-, or tri-methylated, each with a discrete effect on transcription. For instance, H3 lysine 4 tri-methylation (H3K4me3) is associated with active promoters, whereas H3 lysine 4 mono-methylation (H3K4me1) is associated with active enhancers (85–87). In contrast, H3 lysine 27 tri-methylation (H3K27me3) catalyzed by polycomb repressive complex 2 is a repressive marker that serves as a scaffold for heterochromatin protein 1 (HP1), which causes local nucleosome condensation to promote a compact and transcriptionally silenced heterochromatin state (88, 89).
BMAL1-CLOCK.
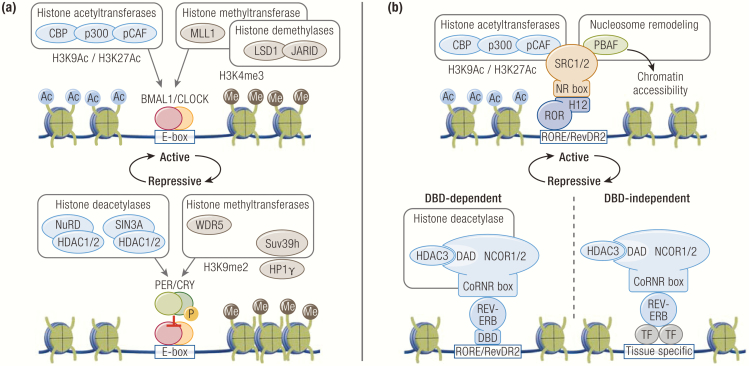
BMAL1-CLOCK/NPAS2 interact with the transcriptional co-activators p300, CREB-binding protein (CBP), and p300/CBP-associated factor (pCAF), all of which have HAT activity (90, 91) (Fig. 2A). In addition, CLOCK itself has been reported to have intrinsic HAT activity, which is enhanced by BMAL1 and functionally required to stimulate transcription at the PER and DBP promoters (92). BMAL-CLOCK also interact with the HMT mixed lineage leukemia 1 (MLL1) that catalyzes H3K4me3 at active promoters (93) (Fig. 2A). Interestingly, other members of the MLL family, MLL3 and MLL4, have also been shown to be rhythmically recruited to active promoters, although TFs mediating their recruitment remain unknown (94, 95). BMAL-CLOCK interact with lysine-specific demethylase 1 (LSD1) and another lysine demethylase JARID1A (Fig. 2A). Circadian phosphorylation of LSD1 by protein kinase Cα licenses LSD1 to bind BMAL1-CLOCK to activate transcription (96). Similarly, JARID1A is rhythmically recruited by BMAL-CLOCK to activate transcription (97). Intriguingly, both studies revealed that their catalytic activities are dispensable for transcriptional activation. Although the mechanisms of their catalytic-dependent and catalytic-independent functions remain unknown, an increasing number of epigenetic regulators have also been found to have catalytic-independent functions, with some studies demonstrating that certain epigenetic regulators, such as MLL3 and 4, coordinate transcription predominantly through their interactions with collaborative partners independently of their catalytic activity (98–102).
Figure 2.
Epigenetic regulators of the molecular clock. (A) Multiple epigenetic regulators associate with BMAL1/CLOCK and PER/CRY to facilitate transcriptional activation and repression, respectively. (B) ROR and REV-ERB recruit nuclear receptor coactivators and corepressors via mechanisms conserved in nuclear receptors. REV-ERB can also tether to tissue-specific transcription factors to regulate transcription in a DNA-binding domain (DBD)-independent manner.
PER-CRY.
During the repressive phase, PER-CRY interact with the Mi-2/nucleosome remodeling deacetylase (NuRD) and SIN3A corepressor complexes, both of which recruit HDAC1/2 to deacetylate histones and repress transcription (90, 103–108) (Fig. 2A). Approximately 4 hours later, PER recruits the HP1γ-Suv39h HMT complex to catalyze di- and tri-methylations on unmodified H3K9 residues to promote a local repressive chromatin state (105) (Fig. 2A). Sequential modifications of H3K9 in 2 consecutive circadian phases are mediated by 2 distinct PER complexes containing either HDAC1 or HP1γ, which are formed in a temporally discrete manner via an unknown mechanism. A separate study also demonstrated that HP1γ is recruited to these sites marked with H3K9me2, where it facilitates transcriptional repression by increasing local nucleosome density (63). In addition, PER interacts WD repeat-containing protein 5 (WDR5), a subunit of methyltransferase complexes, to augment PER-mediated transcriptional repression (107). Interestingly, WDR5 has been shown to complex with MLL1 that participates in the active phase with BMAL1, suggesting that the formation of epigenetic complexes is likely regulated by local chromatin and temporal contexts, as illustrated by the PER examples described previously (109, 110).
ROR and REV-ERB.
ROR and REV-ERB belong to the nuclear receptor (NR) superfamily consisting of TFs that share a conserved structural organization containing specific modular domains, such as DNA-binding domains (DBD) and ligand-binding domains (LBD) (111, 112). DBDs consist of 2 zinc-fingers that enable the recognition of and binding to specific DNA sequences (113, 114). LBDs consist of 12 α-helices that bind ligands with high affinity and specificity (115-117). In the classical model of NRs, ligand binding induces a conformational switch in LBDs that exposes a C-terminal helix 12 (H12) domain to recruit coactivators, whereas unliganded LBDs recruit corepressors (118, 119). Together, DBDs and LBDs serve as modular determinants of NR transcriptional activity, such that DBDs specify target genes and LBDs determine transcriptional outcomes through their interactions with ligands and coregulators.
ROR interacts with nuclear receptor coactivator (NCOA) 1 and 2 complexes (also known as steroid receptor complex or SRC1 and 2, among others names), which are members of the p160 coactivator family (120–123) (Fig. 2B). The C-terminal H12 of ROR binds short peptide leucine-x-x-leucine-leucine motifs within conserved NR interaction domains of the coactivators, termed NR boxes (124-126). This coordinated interaction recruits NCOA and its associated HAT CBP, p300, and pCAF to transactivate target genes (124, 127–130) (Fig. 2B). One interacting partner of SRC2 is the polybromo-associated BRG1/BRM-associated factor (PBAF) complex, which belongs to the ATP-dependent switch/sucrose non-fermentable chromatin remodeling complex family (121, 131–134) (Fig. 2B). Rhythmic recruitment of PBAF by ROR/SRC2 promotes chromatin accessibility, which is thought to facilitate loading REV-ERB onto chromatin during the repressive phase. In addition, another coactivator PGC1α has been shown to be rhythmically expressed in the liver and muscle tissues where it potentiates ROR transcriptional activity to coordinate the clock, although the generalizability of this interaction has not been established in other tissues (135). NCOA also interacts with coactivator-associated arginine methyltransferase 1 (also known as protein arginine N-methyltransferase 4) that has HMT activity, although its role in the clock has not been studied.
ROR is designated as an orphan NR without known endogenous ligands, although sterol derivatives have been proposed as possible candidates (136–139). Studies have yielded inconclusive results regarding ligand-dependent activation of ROR because ROR has been described as a constitutive activator that does not require ligands and also as a transcriptional repressor in certain contexts (138, 140, 141). Therefore, future studies are warranted to determine to what extent circadian ROR activity is regulated by endogenous ligands that are yet to be confirmed.
Among NRs, REV-ERB is unique in that it lacks a C-terminal H12 and thus acts as a constitutive transcriptional repressor that interacts with the nuclear receptor corepressor (NCOR) 1 and 2 complexes (NCOR2 is also known as silencing mediator of retinoid and thyroid receptor or SMRT) (37, 142–144) (Fig. 2B). For REV-ERB and other unliganded NRs, LBDs bind leucine-x-x-leucine-leucine motifs in short hydrophobic clefts of NCOR called corepressor nuclear receptor boxes or CoRNR boxes. The CoRNR boxes are analogous to the NR boxes of coactivators in that they both enable binding to NRs in a structurally similar mechanism (124). NCOR interacts with a cohort of factors that mediate transcriptional repression, including HDAC3 (119) (Fig. 2B). HDAC3 recognizes highly conserved deacetylase activating domains (DAD) of NCOR 1 and 2, which are also necessary for HDAC3 catalytic activity (145–147). Together, genomic recruitment of NCOR and HDAC3 by REV-ERB promotes histone deacetylation and clock gene repression (148-150). In support of this model, liver-specific deletion of either NCOR1 or HDAC3 de-represses BMAL1 expression (98, 148, 149). In addition, mice harboring point mutations that inactivate the DAD of NCOR1 or SMRT/NCOR2 (named N or S-DADm, respectively) exhibit a reduction in HDAC3 genomic recruitment and deacetylase activity, which results in perturbed clock gene expression and aberrant circadian behavior (151, 152). Interestingly, reconstitution of HDAC3 deletion with a catalytically inactive form of HDAC3 largely rescues repression of metabolic genes, as well as alterations in hepatic lipid metabolism, suggesting a deacetylase-independent role of HDAC3 (98).
Although ROR and REV-ERB are classically known to compete for their cognate motifs because of their highly similar DBDs, a hypomorphic form of REV-ERBα lacking its DBD has been found to be recruited to the genome in a DBD-independent manner by tethering to lineage-determining TFs (Fig. 2B). While DBD-dependent sites include clock genes coregulated by ROR and REV-ERB, DBD-independent sites are enriched for tissue-specific genes, revealing 2 discrete modes of action for REV-ERB in regulation of the clock and tissue-specific rhythms (149, 153).
Finally, heme has been identified as an endogenous ligand that potentiates RER-ERB repressive action by stabilizing its interaction with NCOR (154, 155). However, the extent to which heme dynamically controls REV-ERB activity in physiological contexts remains to be determined.
The complex epigenetic regulation of the clock revealed by these studies illuminate an extensive interplay between clock TFs and their coregulators while also raising a number of fundamental questions, particularly regarding the role of histone modifications and their respective epigenetic regulators in general transcription mechanisms. The finding that certain epigenetic regulators do not necessarily require catalytic activity challenges us to reconsider the notion that histone modifications have direct effects on transcription, a subject that remains to be intensely investigated (100–102, 156). Also, despite the growing list of known histone modifications, the biological significance and transcriptional function of these modifications have yet to withstand the test of time (157). Therefore, as our understanding of epigenetics continues to advance, biochemical and structural studies will become more imperative in unraveling the elusive details of catalytic-dependent and independent functions of epigenetic regulators that together coordinate their actions in various aspects of transcription.
From epigenomics to transcriptomics
Upon binding to the genome, clock TFs commence a temporal cascade of coregulator recruitment and epigenetic modifications that help initiate transcription. Several studies have used chromatin-immunopreciptation followed by sequencing (ChIP-seq) to delineate circadian dynamics of transcription at the genome-wide level. The first study that sought to explore this process performed ChIP-seq of REV-ERBα, which revealed that REV-ERBα binds thousands of cis-regulatory elements throughout the genome, collectively called cistrome (148). At the peak of REV-ERBα level, NCOR and HDAC3 are rhythmically recruited to REV-ERBα cistrome, which results in histone deacetylation and decreased RNA Pol II occupancy, demonstrating that REV-ERBα orchestrates an epigenomic rhythm of histone deacetylation and gene repression through NCOR and HDAC3.
A similar genome-wide study that focused on BMAL1/CLOCK and CRY/PER uncovered 3 distinct transcriptional phases of the forward limb: a poised state, a transcriptional activation state, and a repressed state (158). During the poised state, BMAL1/CLOCK and CRY1 are cobound without active transcription. The transcriptional activation state begins with declining CRY1 level, which is likely caused by decreased production or degradation mediated by the E3 ubiquitin ligase F-box/LRR-repeat protein 3 (FBXL3) (159–162). Subsequently, BMAL1/CLOCK occupancy rises, which recruits p300 and CBP and is accompanied by active marks H3K4me1 and H3K9Ac. Next, RNA Pol II is loaded into promoters to transcribe genes, which is accompanied by other active marks H3K4me3 and H3K27Ac. The active phase transitions to the repressive phase when PER1/2 and CRY2 co-occupy these sites to repress transcription, which is followed by delayed CRY1 binding that resets the cycle. Another study that measured circadian changes in histone modifications reported a related finding that oscillating levels of H3K4me3 at promoters and H3K27Ac at enhancers are correlated with rhythmic expression of nearby genes genome-wide (78).
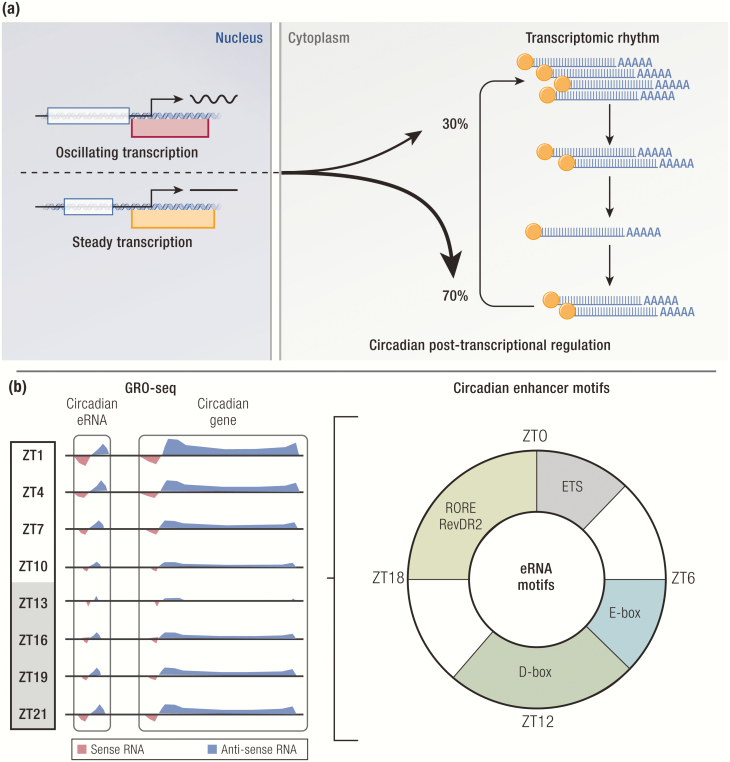
The same study that identified the distinct transcriptional phases of the clock also examined posttranscriptional regulation of circadian genes (158). Using whole transcriptomic RNA-seq, they identified 2 groups of genes, intron cycling versus exon cycling, that show circadian expression at the nascent transcription versus steady-state mRNA levels, respectively. Surprisingly, the vast majority of exon cycling genes do not have underlying transcriptional rhythms, leaving only 22% of exon cycling genes that are driven by de novo nascent transcription. These genes are enriched for the core clock genes and their known target genes with high rhythmic amplitudes. In a separate study, 15% of all detected genes are found to be transcribed rhythmically, 42% of which maintain comparable rhythms at the steady-state mRNA level (163). On the other hand, 22% of all detected genes show circadian rhythms at the steady-state mRNA level, but 70% of these genes do not exhibit underlying transcriptional rhythms. These studies demonstrated that nascent transcript rhythms do not necessarily correlate with steady-state mRNA rhythms and vice versa, revealing pervasive posttranscriptional regulation that likely plays a major role in shaping the rhythmic mRNA landscape (Fig. 3A).
Figure 3.
Epigenomics and transcriptomics of the molecular clock. (A) A majority of rhythmic mRNA transcripts do not exhibit underlying transcriptional rhythms. (B) Enhancers with circadian activity based on rhythmic enhancer RNA (eRNA) transcription show phase-specific enrichment of DNA binding motifs bound by both known and unknown clock transcription factors.
In another study, Global Run-On sequencing (GRO-seq) was used to not only measure nascent transcription genome-wide, but also to identify cis-regulatory elements that are marked by enhancer RNAs (eRNA), short noncoding and often bidirectional RNA species transcribed at active enhancers (60) (Fig. 3B). eRNA has been widely used as a robust marker of enhancer activity because its transcription level highly correlates with target gene expression (164). Although the functional significance of eRNA is debated, inhibition of eRNA synthesis by REV-ERBs has been shown to represses gene transcription in macrophages (165). Unbiased examination of eRNA expression in mouse livers collected over the course of 24 hours identified >5000 enhancers with circadian activity, as well as nearby target genes with oscillating expression in the same phases. In addition, de novo motif analyses of these enhancers revealed enrichment of DNA motifs, such as E-Box, RORE/RevDR2, and D-Box, in phases that correspond to maximal enhancer activity coordinated by the core clock TFs that recognize these motifs. Interestingly, E-twenty six (ETS) motifs were enriched in circadian enhancers with peak activity at zeitgeber time 0 to 3, implying a potential role of ETS TFs in regulating this phase of circadian transcription. Furthermore, correlation of eRNA expression levels with REV-ERBα activity enabled identification of a small fraction of its cistrome where REV-ERBα functionally represses transcription, which orthogonally confirmed the previous finding that NFIL3 is a direct target of REV-ERB (166, 167).
Despite such significant scientific strides made by the recent genome-wide studies, fundamental questions remain with regard to how clock TFs coordinate epigenomic rhythms in a tissue-specific manner. Although most core clock TFs are similarly expressed in nearly all cells except for embryonic stem cells that appear to lack a functional clock, it is unclear how clock TFs recognize and occupy different cistromes in different cell types (168, 169). One possible mechanism by which this is achieved is through the tethering of REV-ERB to tissue-specific, lineage-determining TFs in a DBD-independent manner, although it is unknown whether other clock TFs share a similar mechanism (149, 153). It is also possible that clock TFs can only bind enhancers that have been made accessible or “pioneered” by tissue-specific TFs that bind closed chromatin to loosen local nucleosome density. Interestingly, BMAL1/CLOCK have been reported to have a pioneering activity (170, 171). Another possibility is that genomic binding of clock TFs is determined by collaborative interactions with other TFs, though it is unclear what combinations of TFs are required to stably maintain enhancer activity (172).
While these genome-wide studies re-demonstrated the key importance of enhancer action in organizing epigenomic and transcriptional waves across the genome, they also unveiled the widespread prevalence of posttranscriptional regulation in setting transcriptomic rhythms. The finding that most rhythmic mRNA transcripts do not have underlying transcriptional rhythms alludes to possible circadian mechanisms that control mRNA stability and degradation. One regulator of this process is short noncoding microRNA, some of which show circadian expression and regulate the stability of their target transcripts, including several clock genes (173–177). Other aspects of mRNA processing, such as polyadenylation, can also regulate circadian accumulation and degradation of transcripts (178–180). Moreover, genome-wide ribosome profiling and proteomic approaches have uncovered new regulatory layers of the transcription-translational feedback loop that extend beyond transcription (181-184). Therefore, although this review focuses on transcriptional mechanisms, it is important to recognize that posttranscriptional and posttranslational mechanisms also help generate circadian rhythms, both in conjunction with and independently of rhythmic transcription.
Circadian gene expression in time and space
The observation that chromosomes occupy certain territories in the nucleus led to the first recognition that 3-dimensional (3D) organization of the genome is nonrandom (185–187). A century after this rudimentary microscopic finding was made, our understanding of 3D genome architecture has become much more sophisticated with development of cutting-edge chromatin conformation capture techniques that provided a magnified view into how the genome folds in 3D space to control gene transcription and genome function (188–191).
One major breakthrough was made by 3 independent laboratories that demonstrated that the genome is partitioned into highly conserved units called topologically associating domains (TAD) (192–194). TAD is on average 1 megabase pairs long and conserved in different tissues, as well as in certain syntenic regions shared between species (192). TAD boundaries are anchored together by a pair of CCCTC-binding factor (CTCF) that homodimerize in a convergent orientation to form a long-range chromatin loop, which is further stabilized by a ring-like protein complex called Cohesin (192, 195, 196). By spatially restraining a linear genomic region into a physically isolated loop, TAD insulates enhancers and genes to promote their interactions within the same TAD. As such, genetic disruption of TAD boundaries results in loss of insulation, leading to ectopic enhancer-promoter rewiring between neighboring TADs and aberrant gene expression (197, 198). TAD also demarcates regions of euchromatin and heterochromatin (190, 194, 195). TADs containing either euchromatin or heterochromatin segregate together in distinct higher order structures called compartment A or B, respectively. Compartment B is also enriched with lamin-associated domains, which contain repressed genes and associated with repressive histone mark H3K27me3 (199).
Although these studies provided an unprecedented insight into new mechanisms of gene regulation at the level of TAD and compartments, how enhancer and promoter (E-P) loops are formed and regulated continues to be an active field of investigation (200–205). In general, activator TF are thought to bind enhancers and recruit coactivators, such as the Mediator complex, which then interact with the preinitiation complex at promoters, thereby bringing enhancers to promoters to activate transcription (206, 207). Cohesin has been shown to be recruited to enhancers, particularly a long cluster of enhancers called super enhancers, but other studies also showed that not all enhancers are bound by Cohesin, thus leaving the exact role of Cohesin in regulation of E-P loops unclear (208-210).
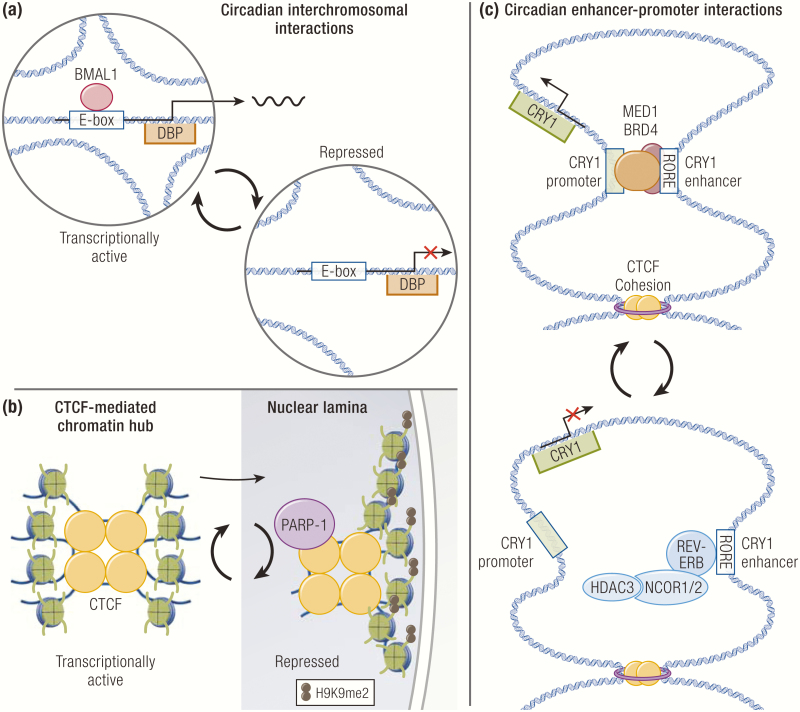
Consistent with the growing evidence that 3D chromatin architecture controls genome function, several studies have linked circadian transcription to spatiotemporal reorganization of the genome. For instance, the DBP promoter has been shown to undergo circadian interchromosomal interactions in a BMAL1-dependent manner, although the functional significance of trans-interactions in gene regulation remains controversial (211) (Fig. 4A). Depletion of Cohesin in cultured cells alters the local long-range topology of circadian genes and causes aberrant expression of several circadian genes (212). Nuclear positioning of genes has also been shown to impact transcription. Circadian interactions between CTCF and poly ADP-ribose polymerase 1 (PARP1) rhythmically bring CTCF-mediated interchromosomal hubs to the nuclear periphery containing lamin-associated domains, where genes acquire repressive H3K9me2 marks and become transcriptionally repressed (213) (Fig. 4B).
Figure 4.
Circadian regulation of 3-dimensional chromatin architecture. (A) The DBP promoter undergoes circadian interchromosomal interactions in a BMAL1-dependent manner. (B) Chromatin hubs organized by CCCTC-binding factors (CTCFs) are repositioned to the repressive nuclear laminar environment through circadian interaction with poly ADP-ribose polymerase-1 (PARP-1). (C) Enhancer-promoter loops undergo circadian reorganization. REV-ERB opposes functional enhancer-promoter formation to repress transcription.
In addition, comparison of 3D genome architecture at 2 opposite circadian phases revealed that although TAD boundaries are stable, interactions within TADs that contain circadian genes are dynamic and positively correlated with gene expression (214). One major type of such interactions is E-P loops, which are controlled by coordinated actions of TFs. In support of this, an intronic enhancer at the CRY1 locus has been shown to rhythmically loop to the promoter to drive circadian expression of CRY1 (69, 214) (Fig. 4C). Interestingly, formation of this E-P loop is opposed by REV-ERBα binding at the enhancer. Moreover, integrative analysis of REV-ERBα cistrome, nascent transcriptome, and 3D genome architecture data identified 2 groups of E-P loops, engaged versus passive, where REV-ERBα engages in transcriptional repression or passively binds without affecting transcription, respectively. At engaged sites, E-P loops are attenuated upon REV-ERBα binding, whereas those at passive sites are unaffected. Furthermore, engaged sites show stronger recruitment of NCOR1/HDAC3 and concordant histone deacetylation, which leads to eviction of the histone acetylation reader BRD4 and its interacting partner MED1, a component of the Mediator complex known to promote looping between enhancers and promoters (215–219) (Fig. 4C). This study revealed genome-wide 3D organizational plasticity that occurs in normal mammalian physiology and also demonstrated a novel role of transcriptional repressors in opposing E-P loops as a mechanism to repress gene transcription. To complement this finding, another study showed that BMAL1 rhythmically promotes chromatin interactions between enhancers that regulate the same genes, illustrating that dynamic chromatin loops underlie circadian gene transcription (220).
The governing principles of 3D genome organization unveiled a new regulatory layer of gene regulation, which generated new exciting questions and helped us revisit the unresolved from a renewed perspective. For instance, it remains unclear how enhancers specifically loop to their distal target promoters as opposed to interacting with the nearest promoters within the same TAD. Future studies that systemically compare molecular and topological characteristics of E-P loops may help identify emergent properties of these loops as well as regulatory logics behind enhancer-promoter pairing.
In addition to genome folding, mechanisms of nuclear positioning will improve our understanding of the spatiotemporal regulation of transcription. Interestingly, HDAC3 has been shown to interact with the nuclear envelope protein and transcriptional repressor LAP2β to repress transcription by tethering chromatin to the repressive nuclear lamina in a deacetylase-independent manner (221–223). Therefore, it will be important to examine whether circadian genes are regulated via this mechanism, and also whether clock TFs and their associated coregulators participate in this process.
Another important question to consider is why only a small subset binding sites are functional for any given TF. The observation that the repressive action of REV-ERBα is permitted at engaged sites but somehow buffered against at passive sites is perplexing (224). Although there is no definitive answer to this conundrum that has been observed with other TFs in numerous ChIP-seq studies, the composition of local chromatin landscapes at enhancers may play a role in determining transcriptional permissiveness.
Finally, there is an emerging appreciation that chromatin is biophysically compartmentalized into membraneless liquid states by phase separation (225). Several studies have reported that many TFs and coregulators contain a structural domain called intrinsically disordered regions that enable the formation of phase-separated compartments (226–229). Several groups have further extrapolated this finding to propose that phase separation drives and stabilizes the formation of E-P loops to activate transcription (230). Intriguingly, BRD4 and the Mediator complex have been implicated as critical components of phase-separated transcriptional compartments. It will thus be informative to examine how clock TFs and their associated coregulators control phase separation, which may have overarching implications in general mechanisms of transcriptional regulation.
Circadian Mammalian Physiology
Hierarchical structure of the organismal clock
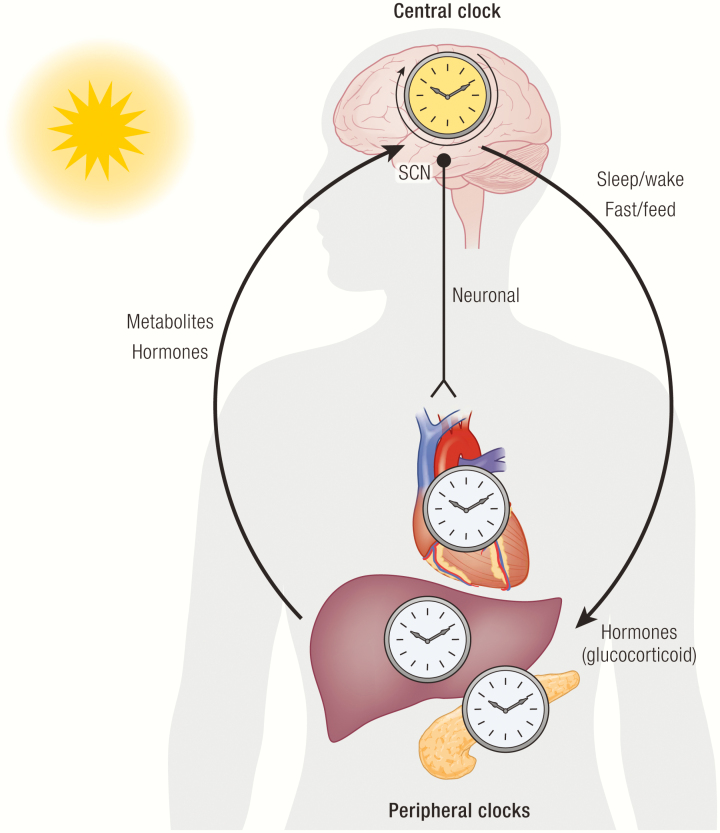
Self-sustaining cellular rhythms generated by complex transcriptional processes give rise to organismal rhythms. In the mammalian system, organismal rhythms are hierarchically compartmentalized into the central clock of the brain and the peripheral clocks of various organs. The central clock is largely controlled by the suprachiasmatic nucleus (SCN) of the hypothalamus, which is entrained by light detected by melanopsin-expressing retinal cells of the retinohypothalamic track (231, 232). The SCN projects to various output pathways in the brain and to peripheral organs via neuronal and humoral signals (233–236). Interestingly, a recent study demonstrated that the astrocytic clock in the SCN is necessary for rhythm generation, revealing an unexpected role of glial cells in supporting the central clock (237).
By coupling the circadian neurosensory circuit with the peripheral clocks, the SCN ensures proper timing of daily physiological processes and behavioral patterns, such as sleep-wake and fasting-feeding cycles. Indeed, mice lacking clock genes often exhibit damped circadian variations of food intake (238–242). The SCN also controls the synthesis and secretion of a number of hormones to entrain organismal rhythms. Diurnal secretion of glucocorticoids facilitates synchronization of the liver and kidney clocks with the central clock, and that timed administration of exogenous glucocorticoids in SCN-lesioned mice can restore 60% of circadian transcriptome of the liver (243, 244). In diurnal mammals such as humans, melatonin is produced from the pineal gland at night to help initiate sleep, among its many functions (245). Despite the wide use of melatonin as a supplemental sleep aid, its role in circadian rhythms and sleep has been studied only in limited contexts. This is largely because certain inbred rodent species do not produce melatonin, which poses a major experimental limitation and also restricts the generalizability of its function across mammalian species (246).
Through the hypothalamic control of behavior and endocrine hormones that synchronize the peripheral clocks, the central clock serves as the master clock that entrains organismal rhythms with the environmental cycle. However, although this whole-body entrainment is crucial for setting organismal rhythms, every tissue and organ can also be entrained by signals related to their functions (247–249). For instance, the liver clock is predominantly entrained by feeding and nutrient availability, such that feeding restricted to only certain hours of the day can rapidly shift the liver clock independently of the SCN clock that remains locked to the prevailing light-dark cycle (250, 251). The ability of the peripheral clocks to override entrainment by the central clock confers functional plasticity to peripheral organs so that they can rapidly adapt to stimuli and mount appropriate physiological responses at any time of the day.
The peripheral clocks also influence the central clock through hormones and metabolic signals. A high-fat diet (HFD) lengthens free-running periods and attenuates feeding and locomotor rhythms in mice (252). In addition, a variety of hormones that are tightly regulated by fasting and feeding, such as fibroblast growth factor 21 (FGF21), ghrelin, leptin, insulin, and glucagon-like peptide 1, can signal to the central clock and other food-entrainable clocks in the brain [reviewed in (253)]. For instance, FGF21 directly acts on the SCN to suppress physical activity and alter circadian behavior during the times of starvation (254). Such bidirectional crosstalk allows the central clock to entrain the peripheral clocks while simultaneously enabling the peripheral clocks to shift their own phases and feedback to the central clock to optimally meet tissue-specific demands (Fig. 5).
Figure 5.
Hierarchical structure of the organismal clock. The suprachiasmatic nucleus (SCN) of the hypothalamus serves as the central clock that entrains the organismal clock by synchronizing the peripheral clocks with the environment. The peripheral organs also have tissue-specific cues related to their functions that can entrain their own clocks independently of the central clock. Reciprocally, the peripheral clocks can feedback to the central clock to further fine tune the organismal clock.
Circadian regulation of metabolism
Metabolic rhythms arise within the framework of feeding and neuroendocrine rhythms coordinated by the central and peripheral clocks. Metabolism is regulated at multiple levels of organismal functions, ranging from cellular anabolism and catabolism of nutrients to systemic energy balance. In this review, we will specifically focus on the transcriptional control of metabolism, with an emphasis on how the clock transcriptional network generates metabolic output pathways, as well as reciprocal ways in which metabolic processes reprogram the clock. In support of the notion that metabolism is under circadian control, the vast majority of circadian transcriptome of the liver has been shown to belong to metabolic pathways (255). Moreover, many of cycling genes control key rate-limiting steps in their respective pathways, such as ALAS1 for heme biosynthesis and HMGCL for ketogenesis.
Mouse genetic studies provided early evidence that the core clock TFs play a critical role in circadian metabolism. Mice homozygous for a dominant-negative mutation in the CLOCK gene are hyperphagic and obese and exhibit profound metabolic derangements, including hyperleptinemia, hyperlipidemia, hepatic steatosis, hyperglycemia, and hypoinsulinemia (242, 256). Both CLOCK mutant mice and BMAL1 knockout mice exhibit loss of diurnal variations in triglyceride and glucose levels, reduced insulin secretion resulting from impaired gluconeogenesis and defective pancreatic islets development (257, 258). Further corroborating this finding, conditional ablation of BMAL1 in pancreatic β cells also causes hyperglycemia because of disrupted nutrient-responsive insulin secretion (258, 259). In addition, liver-specific deletion of BMAL1 causes hepatosteatosis and suppression of de novo lipogenesis (DNL) and fatty acid oxidation (260). In support of the fact that the positive regulators of the forward limb share overlapping transcriptional activity, NPAS2 knockout mice also show similar dysregulation of hepatic lipid metabolism genes (261). However, these knockout mice also display distinct physiological and metabolic phenotypes, implying TF-specific roles in organ function and development.
Consistent with cistromic and transcriptomic studies demonstrating that REV-ERB directly controls genes involved in lipid metabolism, mice lacking REV-ERBα/β have been shown to develop hepatosteatosis, along with elevated serum glucose and triglycerides, and reduced serum fatty acids (39, 41, 60, 148). Interestingly, another study reported a contrary finding that liver triglyceride levels are reduced in REV-ERBα knockout mice (262). This discrepancy could be due to different gene deletion strategies used in these studies, strain-specific genetic variations, or animal husbandry conditions affecting the microbiome, among other possibilities. Regulation of hepatic lipid metabolism by REV-ERB is in part mediated through the tethering mechanism, in which REV-ERB binds lineage-determining TFs, such as hepatocyte nuclear factor 6, and recruits the NCOR/HDAC3 complex to repress metabolic genes (149, 153). Indeed, mice with liver-specific deletion of NCOR or HDAC3 also develop hepatosteatosis, albeit to a greater extent than seen in REV-ERB knockout mice, which could be explained by the fact that NCOR and HDAC3 regulate other NRs in the liver, including hepatocyte nuclear factor 4α (98, 263–265).
Another means by which REV-ERB controls hepatic lipid metabolism is through its regulation of the NR peroxisome proliferator-activated receptor δ (PPARδ) (60, 214). At the trough of REV-ERB level, PPARδ drives the DNL biosynthetic pathway that produces the lipid species phosphatidylcholine 18:0/18:1 (266). This metabolite is secreted into the bloodstream and taken up by the muscle, where it serves as a ligand for another NR, PPARα, to promote fatty acid oxidation, thus simultaneously driving anabolism and catabolism in the 2 distant metabolic organs. Although ROR has also been implicated in hepatic lipid metabolism, studies have yielded conflicting results regarding the effects of ROR on hepatic triglyceride levels and lipogenic gene expression, which were speculated to be due to different experimental conditions, such as timing of feeding (267–271).
Although many of these studies focused on the role of clock TFs in major metabolic tissues, such as the liver and the pancreas, all metabolic organs act in concert to coordinate rhythmic metabolism and physiology. The diverse biology of REV-ERBα best illustrates how a single clock TF can orchestrate multiple metabolic processes in distant organs. In thermogenic brown adipose tissue, REV-ERBα directly represses the expression of uncoupling protein 1 to generate a circadian rhythm of body temperature (272, 273). In white adipose tissues, REV-ERBα represses the expression of β-KLOTHO, an essential coreceptor for FGF21, to modulate responsiveness to FGF21 signaling important for carbohydrate and lipid metabolism (274). In oxidative muscle tissues, REV-ERBα improves exercise capacity by promoting mitochondrial biogenesis through rhythmic activation of the PGC1α pathway (275). Beyond these organs, REV-ERBα also influences the gut microbiome through circadian regulation of Toll-like receptors in the intestinal epithelial cells, which have indirect effects on the intestinal clock, corticosterone synthesis, and systemic metabolism (276).
These in vivo studies demonstrate bona fide physiological significance of the clock in metabolic regulation. However, caution should be taken in interpreting metabolic studies involving whole-body knockout mice. For instance, whole-body knockout mice may exhibit arrhythmic behavior causing dyssynchrony between the central and peripheral clocks that may be responsible for the observed metabolic phenotypes. Tissue-specific and conditional knockout mice serve a particularly useful purpose in this regard because they provide an amendable experimental tool that can help dissociate primary metabolic derangements from confounding behavioral effects. Another challenge in interpreting metabolic studies is that different primary metabolic changes may ultimately converge into similar metabolic outcomes, which may occur through different combinations of disrupted anabolic and catabolic pathways or systemic decompensation. For example, despite the finding that CRY opposes the transcriptional activity of BMAL1 and CLOCK, liver-specific deletion of CRY1/2 also results in hyperglycemia, as seen in BMAL1 knockout and CLOCK mutant mice (277). Future in vivo studies could combine unbiased cistromic and transcriptomic analyses with quantitative metabolic tracing in an integrative approach to identify and link transcriptional changes to tissue-specific metabolic alterations (278–281).
Metabolic regulation of the clock
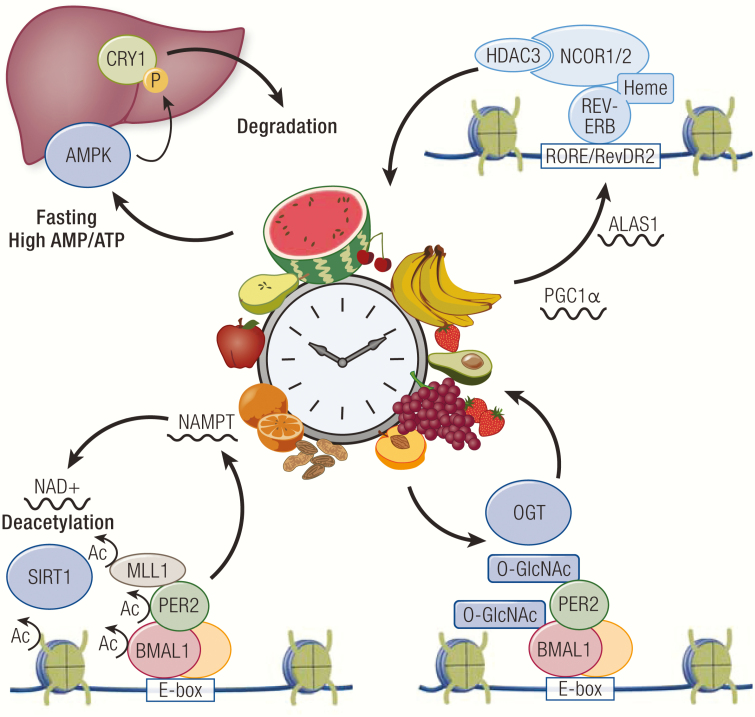
Mirroring the key role of the clock in metabolic homeostasis, numerous metabolic pathways have been shown to have reciprocal feedback mechanisms to reprogram the clock. For example, clock TFs can directly interact with signaling factors of metabolic pathways. AMP-activated protein kinase (AMPK) is a nutrient-responsive modulator of metabolic signals (282, 283). AMPK is activated by phosphorylation from upstream signal factors during nutrient restriction that produces high AMP to ATP ratios (284). Activated AMPK phosphorylates a number of target proteins, including CRY1 in the liver. Phosphorylation of CRY1 causes protein destabilization that leads to rapid degradation and a phase shift of the liver clock (285) (Fig. 6). AMPK also activates casein kinase I via phosphorylation, which then phosphorylates PER2 for degradation to rewire the peripheral clock (286).
Figure 6.
Metabolic regulation of the clock. Activated AMP-activated protein kinase (AMPK) phosphorylates CRY1 to induce its degradation, which results in reprogramming of the liver clock. Many metabolic pathways such as heme biosynthesis and NAD+ production, are regulated in a circadian manner at the transcriptional level. NAD+ regulates activity of sirtuin 1 (SIRT1) that deacetylates clock TFs and their associated factors and histones. Heme serves as an endogenous ligand that potentiates REV-ERB activity. The hexosamine biosynthesis pathway regulates O-GlcNAc transferase (OGT) activity, which controls the stability and transcriptional function of clock TFs.
The intracellular NAD+/NADH redox state also has a broad impact on the clock. Reduced forms of the redox cofactors, NAD(H) and NADP(H), can directly bind BMAL1/CLOCK and NPAS2 heterodimers to enhance DNA binding. In contrast, their oxidized forms, NAD+ and NADP+, counteract this effect (287). In addition, NAD+ can serve as a cofactor for sirtuin 1 (SIRT1), which is an HDAC that has been shown to rhythmically bind CLOCK-BMAL1 and deacetylate PER2 and BMAL1 (288, 289) (Fig. 6). Deacetylation of PER2 promotes its degradation, whereas deacetylation of BMAL1 reverses CLOCK-mediated acetylation that promotes interaction with CRY1 (289, 290). SIRT1 can also deacetylate MLL1 bound to BMAL1/CLOCK, which results in suppression of MLL1 catalytic activity and transcriptional activity (291) (Fig. 6). Interestingly, BMAL1/CLOCK directly controls circadian expression of nicotinamide phosphoribosyltransferase (NAMPT), a rate-limiting enzyme in the NAD+ biosynthetic pathway (292, 293). This generates an oscillating level of NAD+ that drives circadian SIRT1 activity, thereby establishing a metabolic feedback loop tied to the clock transcriptional network. Furthermore, AMPK increases the intracellular NAD+/NADH ratio during fasting, which activates SIRT1 to deacetylate PGC1α and turn on its transcriptional activity, suggesting a possible convergence of the two metabolic pathways in regulation of the clock (294, 295).
The intracellular glucose state can affect clock TF activity via addition of O-linked β-N-acetylglucosamine (O-GlcNAcylation), a nutrient-driven posttranslational modification regulated by the hexosamine biosynthesis pathway (296). PER reversibly undergoes O-GlcNAcylation catalyzed by O-GlcNAc transferase at serine residues, which competitively prevents phosphorylation and potentiates its repressive activity (297) (Fig. 6). Moreover, BMAL1/CLOCK also rhythmically undergo O-GlcNAcylation, which augments their transcriptional activity by increasing protein stability through competitive inhibition of ubiquitin-mediated degradation (298) (Fig. 6).
Other metabolites also have direct effects on the clock. Many small lipophilic metabolites induce transcriptional changes by serving as ligands for NRs, which regulate metabolic pathways related to their respective ligands. Heme is a known ligand for REV-ERB that potentiates its repressive activity (299) (Fig. 6). The intracellular redox state has been shown to regulate the interaction between REV-ERB and heme by multiple mechanisms. Reduction of a thiol-disulfide redox switch in the LBD of REV-ERBβ to a dithiol state increases heme binding in the LBD, thus linking oxidative stress to circadian rhythms and metabolic imbalance (300). Similarly, the REV-ERBβ-heme complex can be regulated by nitric oxide and carbon monoxide gases, which induce at least 6 distinct heme-binding conformational states, though it remains unclear whether these states modulate REV-ERB transcriptional activity in vivo (301).
NPAS2 has also been shown to bind heme via its 2 PAS domains, where heme functions as a redox sensor. Carbon monoxide can bind to this redox sensor to hinder heterodimerization with BMAL1 and reduce DNA binding in vitro (302). Interestingly, heme metabolism has been shown to be regulated by both REV-ERB and NPAS2. REV-ERB inhibits heme biosynthesis by repressing the expression of PGC1α, a potent inducer of ALAS1 expression, whereas BMAL1/NPAS2 promote heme biosynthesis by driving circadian expression of ALAS1 (303, 304). Interestingly, heme is synthesized from glycine and succinyl-CoA, raising the possibility that amino acid biosynthesis and the citric acid cycle may indirectly influence the clock through heme and its modulatory effects on these clock TFs.
Although much less is known about ligand-mediated regulation of ROR, both ROR and REV-ERB have been shown to regulate steroid and bile acid metabolism in the liver, which may produce endogenous ligands that can form a transcriptional feedback loop with ROR (48, 167, 262, 270, 305). In support of this speculation, many NRs show circadian expression across multiple metabolic tissues, suggesting that NRs and their ligands could broadly link rhythmic metabolism to the clock, and vice versa (44).
Although these studies highlight the intricate reciprocal relationship between metabolism and the clock, metabolic processes can also generate transcriptional rhythms that are functionally uncoupled from the clock. A combined transcriptomic and metabolomic study revealed HFD causes both phase shifts and abolition of metabolite and transcriptional rhythms in mice (306). Interestingly, HFD also induces new oscillations of metabolites and transcripts in the liver. Motif analysis of promoter regions of the genes that gained new rhythms revealed enrichment of DNA sequences recognized by 2 major regulators of lipid metabolism, PPARγ and sterol regulatory element-binding protein (SREBP). Consistent with this analysis, the study found that circadian expression of PPARγ was induced by HFD.
Another study revisited the same question with an unbiased approach using GRO-seq to identify HFD-specific circadian enhancers based on eRNA transcription (307). Motif analysis confirmed that DNA binding motifs for PPARγ and SREBP are indeed enriched in circadian enhancers induced by HFD. However, liver-specific deletion of PPARγ failed to abrogate activity of these enhancers, which led to the identification of PPARα as the culprit factor with the same DNA binding motif that orchestrated the HFD-induced transcriptional rhythm. Furthermore, a genetic loss of function experiment showed that SREBP-dependent DNL drives rhythmic PPARα activity likely through circadian production of endogenous PPARα ligands, unraveling a new mechanism by which metabolic processes generate transcriptional rhythms without redirecting the core clock TFs.
Although these examples focus how metabolites and energetic states are integrated through regulation of TF activity, there are also indirect ways in which metabolism can influence circadian transcription. For instance, various metabolites serve as substrates for histone modifications catalyzed by metabolic enzymes [reviewed in (308)]. Global histone acetylation levels are under the control of glucose availability and the intracellular acetyl-CoA level (309, 310). Despite the growing evidence that many metabolite-derived modifications are present on chromatin and facilitate gene regulation, it remains unknown whether these modifications regulate specific metabolic or clock genes in a transcriptional feedback loop to control their respective metabolic pathways. In the future, it will be exciting to explore how metabolic processes affect other aspects of gene regulation, some of which were discussed in this review. For instance, PARP1 activity is highly dependent on NAD+. It is possible that nuclear positioning mediated by PARP1 and CTCF is controlled by the intracellular redox state. PARP1 has also been shown to participate in phase separation, suggesting possible redox-dependent regulation of E-P loops by PARP1 (311–314). Such studies will not only yield a new biological insight into the complex interplay between metabolism and circadian rhythms, but also open up novel therapeutic opportunities for treating metabolic and circadian disorders.
Implications of circadian rhythms in human health
The recognition that circadian rhythms play a central role in human physiology is founded upon numerous studies demonstrating that both genetic and environmental perturbations of the clock result in human pathologies. In particular, human genetics studies have significantly advanced our understanding of circadian rhythms and sleep. These seminal studies not only identified causal mutations in clock genes underlying different chronotypes, such as familial advanced sleep-phase syndrome and delayed sleep phase disorder, but they also illuminated fundamental genetic and biochemical mechanisms of the mammalian clock (23, 315–323).
Epidemiological studies linked environmental circadian disruptions to overall poor health outcomes. The Nurses’ Health Study, a large prospective cohort study of the long-term effect of lifestyle and environmental factors, demonstrated association between night shift work and an increased risk of type 2 diabetes, a finding that has been consistently reproduced by other studies (324–331). Modern lifestyle is a common cause of circadian misalignment because of altering bedtime on weekends and frequent exposure to disruptive blue light from electronics at night. This condition, commonly referred to as social jetlag, has been associated with detrimental effects on sleep and metabolism (332–334). To mitigate such adverse effects, dynamic lighting that simulates circadian variations of light has been implemented in nursing homes and intensive care units as a preventive strategy to improve sleep parameters and well-being, though its benefits have been limited (335–337). In parallel with these epidemiological observations, laboratory studies demonstrated a causal role of circadian misalignment in metabolic dysregulation. Healthy individuals who were subjected to controlled circadian disruption exhibited signs of prediabetes, characterized by poor glucose homeostasis, attenuated insulin function, and an overall decrease in energy expenditure (338–340). A genome-wide association study found a genetic link between metabolic syndrome and single nucleotide polymorphisms in the clock genes NPAS2 and PER2 (341). In addition, a single nucleotide polymorphism in the human melatonin receptor B1 gene (MTNR1B) has been associated with type 2 diabetes and obesity, suggesting complex genetic interactions among the clock, sleep, and metabolism (245, 342–344).
The growing appreciation of circadian rhythms in human health spearheaded clinical efforts to translate circadian biology into “chronotherapies,” novel therapeutic interventions that target the clock or leverage circadian physiology to improve pharmacodynamics and efficacy of existing medications (Fig. 7). The field of cardiovascular medicine in particular has longstanding interests in implementing circadian medicine. The observation that myocardial infarction (MI) occurs more frequently in the morning provided early evidence that circadian rhythms are important for cardiovascular health, which is further corroborated by a related finding that the incidence of MI increases around shifts to and from daylight savings time (345, 346). The higher incidence of MI in the morning is thought to be caused by rhythmic expression of the major fibrolytic inhibitor plasminogen activator inhibitor type 1, whose activity generates a nadir in net fibrinolysis in the early morning that coincides with the period of elevated MI risk (347, 348). A recent study demonstrated that, like plasminogen activator inhibitor type 1, many targets of common cardiovascular medications, such as beta blockers, calcium channel blockers, and nonsteroidal anti-inflammatory drugs, are expressed in a circadian manner along with other proteins involved in drug transport and metabolism, supporting the notion that timing is an important determinant of therapeutic efficacy (349). Indeed, small randomized trials suggested that antihypertensive medications, such as angiotensin-converting enzyme inhibitors and angiotensin-receptor blockers, are more effective when administered in the evening, and this time-dependent effect was observed with other pharmacologic agents as well (350–354).
Figure 7.
Promise of circadian medicine. Understanding circadian physiology will enable development of novel therapeutic approaches that will help improve human health in the future.
Timing of operation has also been shown to impact perioperative surgical risks. A single-centered randomized study demonstrated that patients who underwent aortic valve replacement in the afternoon were less likely to experience major adverse cardiac events compared with the morning group (355). Ex vivo analysis of human myocardial biopsy samples and animal experiments implicated that high REV-ERBα expression is associated with an elevated risk for hypoxia–reoxygenation injury, likely resulting from REV-ERBα-mediated repression of the ischemia–reperfusion injury modulator CDKN1α/p21. Moreover, inhibition of REV-ERBα, either by genetic deletion or pharmacologic intervention with a putative REV-ERBα antagonist SR8278, ameliorated the hypoxia–reoxygenation injury noted at the peak of REV-ERBα expression, although this observation should be interpreted with caution in the light of a recent study suggesting that REV-ERBα modulators may have nonspecific effects (356).
Last, intermittent fasting has recently garnered significant clinical interest for its broad benefits in metabolic health (357, 358). One form of intermittent fasting is time-restricted feeding (TRF), which refers to limitation of daily food intake to a consistent interval of less than 12 hours without reduction in caloric intake. Several studies in mice demonstrated that TRF provides protection against a hypernutritive challenge and reverses metabolic imbalances, resulting in improved glycemic control, decreased hepatosteatosis, improved serum lipid profiles, and attenuated inflammatory responses (359–361). In support of these rodent studies, small human cohort studies reported similar trends (362, 363). These beneficial effects are presumed to be in part mediated by harmonization of circadian rhythms and feeding patterns that favorably optimizes energy utilization and storage (364). However, another study suggested that rhythmic food intake alone can significantly remodel rhythmic transcriptome of the mouse liver without affecting the core clock genes (365). Similarly, mice lacking the liver clock still show improved metabolic parameters on TRF, suggesting that the liver clock may be dispensable for the therapeutic benefits of TRF (366). Therefore, future studies are required to not only gain a mechanistic insight into how TRF improves metabolic health, but also to rigorously investigate its promising benefits in humans in large controlled cohorts.
Concluding Remarks
In this review, we have explored various transcriptional and epigenomic mechanisms by which the core clock TFs orchestrate circadian gene expression. We revisited the complex biology of epigenetic regulation that challenged our previous thinking, and discussed the emerging role of 3D chromatin architecture in genome function. We highlighted recent studies that examined transcriptional regulation in circadian time and genome space, which have not only advanced our understanding of the transcriptional architecture of the clock, but also generated new exciting questions and hypotheses aforementioned. In addition, we focused on how the clock transcriptionally regulates metabolism through reciprocal crosstalk mechanisms that allow the clock to serve a dual role as a driver and an integrator of metabolism.
Implications of circadian biology in mammalian physiology informed by these animal studies were further advanced by human genetics, epidemiological, and clinical studies that linked circadian misalignment to various human diseases, including metabolic syndrome and cardiovascular diseases. Indeed, time is certainly ripe for circadian medicine that will begin to translate our accumulated knowledge into novel therapeutic opportunities. However, equally imperative are rigorous clinical investigations in humans and parallel mechanistic studies in animals that will help discover the molecular underpinning of circadian pathophysiology at the systems level spanning from transcriptional rhythms to circadian behavior, which will unravel more dimensions of the molecular timekeeper that has existed within us as an integral component of life on Earth.
Acknowledgments
We thank members of the Lazar laboratory (Dongyin Guan, Yang Xiao, Pieterjan Dierickx, and Marine Adlanmerini) for their constructive feedback and careful reading of the manuscript. Figures were created with BioRender.
Financial Support: This work was supported by the JPB Foundation (M.A.L.) and National Institutes of Health grants R01 DK45586 (M.A.L.), P30 DK19525 (M.A.L.), T32 GM007170 (Y.H.K.), T32 GM008216 (Y.H.K.), and F30 DK112507 (Y.H.K.).
Glossary
Abbreviations
- 3D
3-dimensional
- AMPK
AMP-activated protein kinase
- BMAL
brain and muscle ARNT-like 1
- CBP
CREB-binding protein
- ChIP
chromatin immunoprecipitation
- CHRONO
computationally highlighted or chromatin immunoprecipitation-derived repressor of the network oscillator
- CLOCK
circadian locomotor output cycles kaput
- CRY1/2
cryptochrome 1/2
- CTCF
CCCTC-binding factor
- DAD
deacetylase activating domain
- DBD
DNA-binding domain
- DBP
D-binding protein
- DNL
de novo lipogenesis
- E-P
enhancer and promoter
- eRNA
enhancer RNA
- FBXL
F-box/LRR-repeat protein
- FGF
fibroblast growth factor
- HAT
histone acetyl transferase
- HDAC
histone deacetylase
- HFD
high-fat diet
- HMT
histone methyltransferase
- HP1
heterochromatin protein 1
- LBD
ligand-binding domain
- LSD
lysine-specific demethylase
- MLL
mixed lineage leukemia
- NCOA
nuclear receptor coactivator
- NCOR
nuclear receptor corepressor
- NFIL
nuclear factor, interleukin
- NPAS2
neuronal PAS domain protein 2
- NR
nuclear receptor
- MI
myocardial infarction
- PARP
poly ADP-ribose polymerase
- PPAR
peroxisome proliferator-activated receptor
- REV-ERB
reverse strand of c-erbα
- ROR
RAR-related orphan receptor
- SCN
suprachiasmatic nucleus
- SIRT
sirtuin
- SREBP
sterol regulatory element-binding protein
- TAD
topologically associating domain
- TF
transcription factor
- TRF
time-restricted feeding
Additional Information
Disclosure Summary: M.A.L. is consultant to Pfizer, Novartis, Madrigal Pharmaceuticals, and Calico, and receives research support from Pfizer.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References and Notes
- 1. Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16-54. [DOI] [PubMed] [Google Scholar]
- 2. Rosbash M. The implications of multiple circadian clock origins. PLoS Biol. 2009;7(3):e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Mairan J-J D. Observation botanique. Hist Acad Roy Sci. 1792:35-36. [Google Scholar]
- 4. Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971;68(9):2112-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bargiello TA, Young MW. Molecular-genetics of a biological clock in drosophila. Proc Natl Acad Sci U S A. 1984;81(7):2142-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bargiello TA, Jackson FR, Young MW. Restoration of circadian behavioural rhythms by gene transfer in Drosophila. Nature. 1984;312(5996):752-754. [DOI] [PubMed] [Google Scholar]
- 7. Reddy P, Zehring WA, Wheeler DA, et al. Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell. 1984;38(3):701-710. [DOI] [PubMed] [Google Scholar]
- 8. Zehring WA, Wheeler DA, Reddy P, et al. P-element transformation with period locus DNA restores rhythmicity to mutant, arrhythmic Drosophila melanogaster. Cell. 1984;39(2 Pt 1):369-376. [DOI] [PubMed] [Google Scholar]
- 9. Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18(3):164-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Alon U. Network motifs: theory and experimental approaches. Nat Rev Genet. 2007;8(6):450-461. [DOI] [PubMed] [Google Scholar]
- 11. Vitaterna MH, King DP, Chang AM, et al. Mutagenesis and mapping of a mouse gene, clock, essential for circadian behavior. Science. 1994;264(5159):719-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. King DP, Zhao Y, Sangoram AM, et al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89(4):641-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bunger MK, Wilsbacher LD, Moran SM, et al. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell. 2000;103(7):1009-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hogenesch JB, Chan WK, Jackiw VH, et al. Characterization of a subset of the basic-helix-loop-helix-PAS superfamily that interacts with components of the dioxin signaling pathway. J Biol Chem. 1997;272(13):8581-8593. [DOI] [PubMed] [Google Scholar]
- 15. Reick M, Garcia JA, Dudley C, McKnight SL. NPAS2: an analog of clock operative in the mammalian forebrain. Science. 2001;293(5529):506-509. [DOI] [PubMed] [Google Scholar]
- 16. DeBruyne JP, Weaver DR, Reppert SM. CLOCK and NPAS2 have overlapping roles in the suprachiasmatic circadian clock. Nat Neurosci. 2007;10(5):543-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou Y-D, Barnard M, Tian H, et al. Molecular characterization of two mammalian bHLH-PAS domain. Proc Natl Acad Sci U S A. 1997;94:713-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Landgraf D, Wang LL, Diemer T, Welsh DK. NPAS2 compensates for loss of CLOCK in peripheral circadian oscillators. PLoS Genet. 2016;12(2): e1005882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. MPer1 and mper2 are essential for normal resetting of the circadian clock. J Biol Rhythms. 2001;16(2):100-104. [DOI] [PubMed] [Google Scholar]
- 20. Zheng B, Larkin DW, Albrecht U, et al. The mPer2 gene encodes a functional component of the mammalian circadian clock. Nature. 1999;400(6740): 169-173. [DOI] [PubMed] [Google Scholar]
- 21. Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30(2):525-536. [DOI] [PubMed] [Google Scholar]
- 22. Emery P, So WV, Kaneko M, Hall JC, Rosbash M. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell. 1998;95(5):669-679. [DOI] [PubMed] [Google Scholar]
- 23. Xu Y, Padiath QS, Shapiro RE, et al. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434(7033):640-644. [DOI] [PubMed] [Google Scholar]
- 24. Lee HM, Chen R, Kim H, Etchegaray JP, Weaver DR, Lee C. The period of the circadian oscillator is primarily determined by the balance between casein kinase 1 and protein phosphatase 1. Proc Natl Acad Sci U S A. 2011;108(39):16451-16456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Etchegaray JP, Machida KK, Noton E, et al. Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Mol Cell Biol. 2009;29(14):3853-3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aryal RP, Kwak PB, Tamayo AG, et al. Macromolecular assemblies of the mammalian circadian clock. Mol Cell. 2017;67(5):770-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Griffin EA Jr, Staknis D, Weitz CJ. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science. 1999;286(5440):768-771. [DOI] [PubMed] [Google Scholar]
- 28. Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91(7):1055-1064. [DOI] [PubMed] [Google Scholar]
- 29. Sun ZS, Albrecht U, Zhuchenko O, Bailey J, Eichele G, Lee CC. RIGUI, a putative mammalian ortholog of the Drosophila period gene. Cell. 1997;90(6):1003-1011. [DOI] [PubMed] [Google Scholar]
- 30. Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107(7):855-867. [DOI] [PubMed] [Google Scholar]
- 31. Kim P, Oster H, Lehnert H, et al. Coupling the circadian clock to homeostasis: the role of period in timing physiology. Endocr Rev. 2019;40(1):66-95. [DOI] [PubMed] [Google Scholar]
- 32. Lazar MA, Hodin RA, Darling DS, Chin WW. A novel member of the thyroid/steroid hormone receptor family is encoded by the opposite strand of the rat c-erbA alpha transcriptional unit. Mol Cell Biol. 1989;9(3):1128-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dumas B, Harding HP, Choi HS, et al. A new orphan member of the nuclear hormone receptor superfamily closely related to Rev-Erb. Mol Endocrinol. 1994;8(8):996-1005. [DOI] [PubMed] [Google Scholar]
- 34. Giguère V, Tini M, Flock G, Ong E, Evans RM, Otulakowski G. Isoform-specific amino-terminal domains dictate DNA-binding properties of ROR alpha, a novel family of orphan hormone nuclear receptors. Genes Dev. 1994;8(5):538-553. [DOI] [PubMed] [Google Scholar]
- 35. Carlberg C, Hooft van Huijsduijnen R, Staple JK, DeLamarter JF, Becker-André M. RZRs, a new family of retinoid-related orphan receptors that function as both monomers and homodimers. Mol Endocrinol. 1994;8(6):757-770. [DOI] [PubMed] [Google Scholar]
- 36. Akashi M, Takumi T. The orphan nuclear receptor RORalpha regulates circadian transcription of the mammalian core-clock Bmal1. Nat Struct Mol Biol. 2005;12(5):441-448. [DOI] [PubMed] [Google Scholar]
- 37. Harding HP, Lazar MA. The monomer-binding orphan receptor Rev-Erb represses transcription as a dimer on a novel direct repeat. Mol Cell Biol. 1995;15(9):4791-4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Giguère V, McBroom LD, Flock G. Determinants of target gene specificity for ROR alpha 1: monomeric DNA binding by an orphan nuclear receptor. Mol Cell Biol. 1995;15(5):2517-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cho H, Zhao X, Hatori M, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485(7396):123-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Preitner N, Damiola F, Lopez-Molina L, et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110(2):251-260. [DOI] [PubMed] [Google Scholar]
- 41. Bugge A, Feng D, Everett LJ, et al. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26(7):657-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sato TK, Panda S, Miraglia LJ, et al. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron. 2004;43(4):527-537. [DOI] [PubMed] [Google Scholar]
- 43. Ueda E, Kurebayashi S, Sakaue M, Backlund M, Koller B, Jetten AM. High incidence of T-cell lymphomas in mice deficient in the retinoid-related orphan receptor RORgamma. Cancer Res. 2002;62(3):901-909. [PubMed] [Google Scholar]
- 44. Yang X, Downes M, Yu RT, et al. Nuclear receptor expression links the circadian clock to metabolism. Cell. 2006;126(4):801-810. [DOI] [PubMed] [Google Scholar]
- 45. Schaeren-Wiemers N, André E, Kapfhammer JP, Becker-André M. The expression pattern of the orphan nuclear receptor RORbeta in the developing and adult rat nervous system suggests a role in the processing of sensory information and in circadian rhythm. Eur J Neurosci. 1997;9(12):2687-2701. [DOI] [PubMed] [Google Scholar]
- 46. André E, Conquet F, Steinmayr M, Stratton SC, Porciatti V, Becker-André M. Disruption of retinoid-related orphan receptor beta changes circadian behavior, causes retinal degeneration and leads to vacillans phenotype in mice. EMBO J. 1998;17(14):3867-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Guillaumond F, Dardente H, Giguère V, Cermakian N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms. 2005;20(5):391-403. [DOI] [PubMed] [Google Scholar]
- 48. Kang HS, Angers M, Beak JY, et al. Gene expression profiling reveals a regulatory role for ROR alpha and ROR gamma in phase I and phase II metabolism. Physiol Genomics. 2007;31(2):281-294. [DOI] [PubMed] [Google Scholar]
- 49. Nakagawa Y, O’Leary DD. Dynamic patterned expression of orphan nuclear receptor genes RORalpha and RORbeta in developing mouse forebrain. Dev Neurosci. 2003;25(2-4):234-244. [DOI] [PubMed] [Google Scholar]
- 50. Ivanov II, McKenzie BS, Zhou L, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126(6):1121-1133. [DOI] [PubMed] [Google Scholar]
- 51. Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9(6):641-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jetten AM. Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal. 2007;7(1):1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mueller CR, Maire P, Schibler U. DBP, a liver-enriched transcriptional activator, is expressed late in ontogeny and its tissue specificity is determined posttranscriptionally. Cell. 1990;61(2):279-291. [DOI] [PubMed] [Google Scholar]
- 54. Drolet DW, Scully KM, Simmons DM, et al. TEF, a transcription factor expressed specifically in the anterior pituitary during embryogenesis, defines a new class of leucine zipper proteins. Genes Dev. 1991;5(10):1739-1753. [DOI] [PubMed] [Google Scholar]
- 55. Wuarin J, Schibler U. Expression of the liver-enriched transcriptional activator protein DBP follows a stringent circadian rhythm. Cell. 1990;63(6):1257-1266. [DOI] [PubMed] [Google Scholar]
- 56. Gachon F, Fonjallaz P, Damiola F, et al. The loss of circadian PAR bZip transcription factors results in epilepsy. Genes Dev. 2004;18(12):1397-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mitsui S, Yamaguchi S, Matsuo T, Ishida Y, Okamura H. Antagonistic role of E4BP4 and PAR proteins in the circadian oscillatory mechanism. Genes Dev. 2001;15(8):995-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stratmann M, Suter DM, Molina N, Naef F, Schibler U. Circadian Dbp transcription relies on highly dynamic BMAL1-CLOCK interaction with E boxes and requires the proteasome. Mol Cell. 2012;48(2):277-287. [DOI] [PubMed] [Google Scholar]
- 59. Ripperger JA, Shearman LP, Reppert SM, Schibler U. CLOCK, an essential pacemaker component, controls expression of the circadian transcription factor DBP. Genes Dev. 2000;14(6):679-689. [PMC free article] [PubMed] [Google Scholar]
- 60. Fang B, Everett LJ, Jager J, et al. Circadian enhancers coordinate multiple phases of rhythmic gene transcription in vivo. Cell. 2014;159(5):1140-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lopez-Molina L, Conquet F, Dubois-Dauphin M, Schibler U. The DBP gene is expressed according to a circadian rhythm in the suprachiasmatic nucleus and influences circadian behavior. Embo J. 1997;16(22):6762-6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yu X, Rollins D, Ruhn KA, et al. TH17 cell differentiation is regulated by the circadian clock. Science. 2013;342(6159):727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ripperger JA, Schibler U. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet. 2006;38(3):369-374. [DOI] [PubMed] [Google Scholar]
- 64. Liu AC, Tran HG, Zhang EE, Priest AA, Welsh DK, Kay SA. Redundant function of REV-ERBalpha and beta and non-essential role for Bmal1 cycling in transcriptional regulation of intracellular circadian rhythms. PLoS Genet. 2008;4(2):e1000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McDearmon EL, Patel KN, Ko CH, et al. Dissecting the functions of the mammalian clock protein BMAL1 by tissue-specific rescue in mice. Science. 2006;314(5803):1304-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Takeda Y, Kang HS, Angers M, Jetten AM. Retinoic acid-related orphan receptor γ directly regulates neuronal PAS domain protein 2 transcription in vivo. Nucleic Acids Res. 2011;39(11):4769-4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Crumbley C, Wang Y, Kojetin DJ, Burris TP. Characterization of the core mammalian clock component, NPAS2, as a REV-ERBalpha/RORalpha target gene. J Biol Chem. 2010;285(46):35386-35392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ukai-Tadenuma M, Yamada RG, Xu H, Ripperger JA, Liu AC, Ueda HR. Delay in feedback repression by cryptochrome 1 is required for circadian clock function. Cell. 2011;144(2):268-281. [DOI] [PubMed] [Google Scholar]
- 69. Mermet J, Yeung J, Hurni C, et al. Clock-dependent chromatin topology modulates circadian transcription and behavior. Genes Dev. 2018;32(5-6):347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kornmann B, Schaad O, Bujard H, Takahashi JS, Schibler U. System-driven and oscillator-dependent circadian transcription in mice with a conditionally active liver clock. PLoS Biol. 2007;5(2):e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Anafi RC, Lee Y, Sato TK, et al. Machine learning helps identify CHRONO as a circadian clock component. PLoS Biol. 2014;12(4):e1001840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Goriki A, Hatanaka F, Myung J, et al. A novel protein, CHRONO, functions as a core component of the mammalian circadian clock. PLoS Biol. 2014;12(4):e1001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Annayev Y, Adar S, Chiou YY, Lieb JD, Sancar A, Ye R. Gene model 129 (Gm129) encodes a novel transcriptional repressor that modulates circadian gene expression. J Biol Chem. 2014;289(8):5013-5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Allis CD, Jenuwein T. The molecular hallmarks of epigenetic control. Nat Rev Genet. 2016;17(8):487-500. [DOI] [PubMed] [Google Scholar]
- 75. Haberland M, Montgomery RL, Olson EN. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat Rev Genet. 2009;10(1):32-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lee KK, Workman JL. Histone acetyltransferase complexes: one size doesn’t fit all. Nat Rev Mol Cell Biol. 2007;8(4):284-295. [DOI] [PubMed] [Google Scholar]
- 77. Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13(7):484-492. [DOI] [PubMed] [Google Scholar]
- 78. Vollmers C, Schmitz RJ, Nathanson J, Yeo G, Ecker JR, Panda S. Circadian oscillations of protein-coding and regulatory RNAs in a highly dynamic mammalian liver epigenome. Cell Metab. 2012;16(6):833-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Law JA, Jacobsen SE. Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat Rev Genet. 2010;11(3):204-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rada-Iglesias A, Bajpai R, Swigut T, Brugmann SA, Flynn RA, Wysocka J. A unique chromatin signature uncovers early developmental enhancers in humans. Nature. 2011;470(7333):279-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Creyghton MP, Cheng AW, Welstead GG, et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc Natl Acad Sci U S A. 2010;107(50):21931-21936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fujisawa T, Filippakopoulos P. Functions of bromodomain-containing proteins and their roles in homeostasis and cancer. Nat Rev Mol Cell Biol. 2017;18(4):246-262. [DOI] [PubMed] [Google Scholar]
- 83. Belkina AC, Denis GV. BET domain co-regulators in obesity, inflammation and cancer. Nat Rev Cancer. 2012;12(7):465-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Greer EL, Shi Y. Histone methylation: a dynamic mark in health, disease and inheritance. Nat Rev Genet. 2012;13(5):343-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Heintzman ND, Hon GC, Hawkins RD, et al. Histone modifications at human enhancers reflect global cell-type-specific gene expression. Nature. 2009;459(7243):108-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130(1):77-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Barski A, Cuddapah S, Cui K, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129(4):823-837. [DOI] [PubMed] [Google Scholar]
- 88. Lachner M, O’Carroll D, Rea S, Mechtler K, Jenuwein T. Methylation of histone H3 lysine 9 creates a binding site for HP1 proteins. Nature. 2001;410(6824):116-120. [DOI] [PubMed] [Google Scholar]
- 89. Bannister AJ, Zegerman P, Partridge JF, et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature. 2001;410(6824):120-124. [DOI] [PubMed] [Google Scholar]
- 90. Curtis AM, Seo SB, Westgate EJ, et al. Histone acetyltransferase-dependent chromatin remodeling and the vascular clock. J Biol Chem. 2004;279(8):7091-7097. [DOI] [PubMed] [Google Scholar]
- 91. Etchegaray JP, Lee C, Wade PA, Reppert SM. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature. 2003;421(6919):177-182. [DOI] [PubMed] [Google Scholar]
- 92. Doi M, Hirayama J, Sassone-Corsi P. Circadian regulator CLOCK is a histone acetyltransferase. Cell. 2006;125(3):497-508. [DOI] [PubMed] [Google Scholar]
- 93. Katada S, Sassone-Corsi P. The histone methyltransferase MLL1 permits the oscillation of circadian gene expression. Nat Struct Mol Biol. 2010;17(12):1414-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Valekunja UK, Edgar RS, Oklejewicz M, et al. Histone methyltransferase MLL3 contributes to genome-scale circadian transcription. Proc Natl Acad Sci U S A. 2013;110(4):1554-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kim DH, Rhee JC, Yeo S, et al. Crucial roles of mixed-lineage leukemia 3 and 4 as epigenetic switches of the hepatic circadian clock controlling bile acid homeostasis in mice. Hepatology. 2015;61(3):1012-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nam HJ, Boo K, Kim D, et al. Phosphorylation of LSD1 by PKCα is crucial for circadian rhythmicity and phase resetting. Mol Cell. 2014;53(5):791-805. [DOI] [PubMed] [Google Scholar]
- 97. DiTacchio L, Le HD, Vollmers C, et al. Histone lysine demethylase JARID1a activates CLOCK-BMAL1 and influences the circadian clock. Science. 2011;333(6051):1881-1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sun Z, Feng D, Fang B, et al. Deacetylase-independent function of HDAC3 in transcription and metabolism requires nuclear receptor corepressor. Mol Cell. 2013;52(6):769-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mullican SE, Gaddis CA, Alenghat T, et al. Histone deacetylase 3 is an epigenomic brake in macrophage alternative activation. Genes Dev. 2011;25(23):2480-2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Local A, Huang H, Albuquerque CP, et al. Identification of H3K4me1-associated proteins at mammalian enhancers. Nat Genet. 2018;50(1):73-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Dorighi KM, Swigut T, Henriques T, et al. Mll3 and Mll4 facilitate enhancer RNA synthesis and transcription from promoters independently of H3K4 monomethylation. Mol Cell. 2017;66(4):568-576.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rickels R, Herz HM, Sze CC, et al. Histone H3K4 monomethylation catalyzed by Trr and mammalian COMPASS-like proteins at enhancers is dispensable for development and viability. Nat Genet. 2017;49(11):1647-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Naruse Y, Oh-hashi K, Iijima N, Naruse M, Yoshioka H, Tanaka M. Circadian and light-induced transcription of clock gene Per1 depends on histone acetylation and deacetylation. Mol Cell Biol. 2004;24(14):6278-6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Duong HA, Robles MS, Knutti D, Weitz CJ. A molecular mechanism for circadian clock negative feedback. Science. 2011;332(6036):1436-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Duong HA, Weitz CJ. Temporal orchestration of repressive chromatin modifiers by circadian clock Period complexes. Nat Struct Mol Biol. 2014;21(2):126-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Padmanabhan K, Robles MS, Westerling T, Weitz CJ. Feedback regulation of transcriptional termination by the mammalian circadian clock PERIOD complex. Science. 2012;337(6094):599-602. [DOI] [PubMed] [Google Scholar]
- 107. Brown SA, Ripperger J, Kadener S, et al. PERIOD1-associated proteins modulate the negative limb of the mammalian circadian oscillator. Science. 2005;308(5722):693-696. [DOI] [PubMed] [Google Scholar]
- 108. Kim JY, Kwak PB, Weitz CJ. Specificity in circadian clock feedback from targeted reconstitution of the NuRD corepressor. Mol Cell. 2014;56(6):738-748. [DOI] [PubMed] [Google Scholar]
- 109. Wysocka J, Swigut T, Milne TA, et al. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell. 2005;121(6):859-872. [DOI] [PubMed] [Google Scholar]
- 110. Wallach T, Schellenberg K, Maier B, et al. Dynamic circadian protein-protein interaction networks predict temporal organization of cellular functions. PLoS Genet. 2013;9(3):e1003398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mangelsdorf DJ, Thummel C, Beato M, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83(6):835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Nuclear Receptors Nomenclature Committee, Auwerx J, Baulieu E, et al. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97(2):161-163. [DOI] [PubMed] [Google Scholar]
- 113. Freedman LP, Luisi BF, Korszun ZR, Basavappa R, Sigler PB, Yamamoto KR. The function and structure of the metal coordination sites within the glucocorticoid receptor DNA binding domain. Nature. 1988;336:403-405. [DOI] [PubMed] [Google Scholar]
- 114. Green S, Kumar V, Theulaz I, Wahli W, Chambon P. The N-terminal DNA-binding ‘zinc finger’ of the oestrogen and glucocorticoid receptors determines target gene specificity. EMBO J. 1988;7(10):3037-3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wagner RL, Apriletti JW, McGrath ME, West BL, Baxter JD, Fletterick RJ. A structural role for hormone in the thyroid hormone receptor. Nature. 1995;378(6558):690-697. [DOI] [PubMed] [Google Scholar]
- 116. Shiau AK, Barstad D, Loria PM, et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95(7):927-937. [DOI] [PubMed] [Google Scholar]
- 117. Bourguet W, Ruff M, Chambon P, Gronemeyer H, Moras D. Crystal structure of the ligand-binding domain of the human nuclear receptor RXR-alpha. Nature. 1995;375(6530):377-382. [DOI] [PubMed] [Google Scholar]
- 118. McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108(4):465-474. [DOI] [PubMed] [Google Scholar]
- 119. Lazar MA. Nuclear receptor corepressors. Nucl Recept Signal. 2003;1:e001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Stashi E, Lanz RB, Mao J, et al. SRC-2 is an essential coactivator for orchestrating metabolism and circadian rhythm. Cell Rep. 2014;6(4):633-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhu B, Gates LA, Stashi E, et al. Coactivator-dependent oscillation of chromatin accessibility dictates circadian gene amplitude via REV-ERB loading. Mol Cell. 2015;60(5):769-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Atkins GB, Hu X, Guenther MG, Rachez C, Freedman LP, Lazar MA. Coactivators for the orphan nuclear receptor RORalpha. Mol Endocrinol. 1999;13(9):1550-1557. [DOI] [PubMed] [Google Scholar]
- 123. Xie H, Sadim MS, Sun Z. RORgammat recruits steroid receptor coactivators to ensure thymocyte survival. J Immunol. 2005;175(6):3800-3809. [DOI] [PubMed] [Google Scholar]
- 124. Heery DM, Kalkhoven E, Hoare S, Parker MG. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387(6634):733-736. [DOI] [PubMed] [Google Scholar]
- 125. Feng W, Ribeiro RC, Wagner RL, et al. Hormone-dependent coactivator binding to a hydrophobic cleft on nuclear receptors. Science. 1998;280(5370):1747-1749. [DOI] [PubMed] [Google Scholar]
- 126. Nolte RT, Wisely GB, Westin S, et al. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature. 1998;395(6698):137-143. [DOI] [PubMed] [Google Scholar]
- 127. Torchia J, Rose DW, Inostroza J, et al. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387(6634):677-684. [DOI] [PubMed] [Google Scholar]
- 128. Chen H, Lin RJ, Schiltz RL, et al. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with P/CAF and CBP/p300. Cell. 1997;90(3):569-580. [DOI] [PubMed] [Google Scholar]
- 129. Hanstein B, Eckner R, DiRenzo J, et al. p300 is a component of an estrogen receptor coactivator complex. Proc Natl Acad Sci U S A. 1996;93(October):11540-11545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Yao TP, Ku G, Zhou N, Scully R, Livingston DM. The nuclear hormone receptor coactivator SRC-1 is a specific target of p300. Proc Natl Acad Sci U S A. 1996;93(20):10626-10631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Lemon B, Inouye C, King DS, Tjian R. Selectivity of chromatin-remodelling cofactors for ligand-activated transcription. Nature. 2001;414(6866):924-928. [DOI] [PubMed] [Google Scholar]
- 132. Sif S, Saurin AJ, Imbalzano AN, Kingston RE. Purification and characterization of mSin3A-containing Brg1 and hBrm chromatin remodeling complexes. Genes Dev. 2001;15(5):603-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang W, Côté J, Xue Y, et al. Purification and biochemical heterogeneity of the mammalian SWI-SNF complex. Embo J. 1996;15(19):5370-5382. [PMC free article] [PubMed] [Google Scholar]
- 134. Kwon H, Imbalzano AN, Khavari PA, Kingston RE, Green MR. Nucleosome disruption and enhancement of activator binding by a human SW1/SNF complex. Nature. 1994;370(6489):477-481. [DOI] [PubMed] [Google Scholar]
- 135. Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447(7143):477-481. [DOI] [PubMed] [Google Scholar]
- 136. Kallen J, Schlaeppi JM, Bitsch F, Delhon I, Fournier B. Crystal structure of the human RORalpha Ligand binding domain in complex with cholesterol sulfate at 2.2 A. J Biol Chem. 2004;279(14):14033-14038. [DOI] [PubMed] [Google Scholar]
- 137. Kallen JA, Schlaeppi JM, Bitsch F, et al. X-ray structure of the hRORalpha LBD at 1.63 A: structural and functional data that cholesterol or a cholesterol derivative is the natural ligand of RORalpha. Structure. 2002;10(12):1697-1707. [DOI] [PubMed] [Google Scholar]
- 138. Stehlin-Gaon C, Willmann D, Zeyer D, et al. All-trans retinoic acid is a ligand for the orphan nuclear receptor ROR beta. Nat Struct Biol. 2003;10(10):820-825. [DOI] [PubMed] [Google Scholar]
- 139. Solt LA, Burris TP. Action of RORs and their ligands in (patho)physiology. Trends Endocrinol Metab. 2012;23(12):619-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Harding HP, Atkins GB, Jaffe AB, Seo WJ, Lazar MA. Transcriptional activation and repression by RORalpha, an orphan nuclear receptor required for cerebellar development. Mol Endocrinol. 1997;11(11):1737-1746. [DOI] [PubMed] [Google Scholar]
- 141. Harris JM, Lau P, Chen SL, Muscat GE. Characterization of the retinoid orphan-related receptor-alpha coactivator binding interface: a structural basis for ligand-independent transcription. Mol Endocrinol. 2002;16(5):998-1012. [DOI] [PubMed] [Google Scholar]
- 142. Chen JD, Evans RM. A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature. 1995;377(6548):454-457. [DOI] [PubMed] [Google Scholar]
- 143. Zamir I, Zhang J, Lazar MA. Stoichiometric and steric principles governing repression by nuclear hormone receptors. Genes Dev. 1997;11(7):835-846. [DOI] [PubMed] [Google Scholar]
- 144. Hörlein AJ, Näär AM, Heinzel T, et al. Ligand-independent repression by the thyroid hormone receptor mediated by a nuclear receptor co-repressor. Nature. 1995;377(6548):397-404. [DOI] [PubMed] [Google Scholar]
- 145. Watson PJ, Fairall L, Santos GM, Schwabe JW. Structure of HDAC3 bound to co-repressor and inositol tetraphosphate. Nature. 2012;481(7381):335-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Guenther MG, Barak O, Lazar MA. The SMRT and N-CoR corepressors are activating cofactors for histone deacetylase 3. Mol Cell Biol. 2001;21(18):6091-6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Guenther MG, Lane WS, Fischle W, Verdin E, Lazar MA, Shiekhattar R. A core SMRT corepressor complex containing HDAC3 and TBL1, a WD40-repeat protein linked to deafness. Genes Dev. 2000;14(9):1048-1057. [PMC free article] [PubMed] [Google Scholar]
- 148. Feng D, Liu T, Sun Z, et al. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science. 2011;331(6022):1315-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zhang Y, Fang B, Emmett MJ, et al. Gene regulation. Discrete functions of nuclear receptor Rev-erbα couple metabolism to the clock. Science. 2015;348(6242):1488-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Yin L, Lazar MA. The orphan nuclear receptor Rev-erbalpha recruits the N-CoR/histone deacetylase 3 corepressor to regulate the circadian Bmal1 gene. Mol Endocrinol. 2005;19(6):1452-1459. [DOI] [PubMed] [Google Scholar]
- 151. You SH, Lim HW, Sun Z, Broache M, Won KJ, Lazar MA. Nuclear receptor co-repressors are required for the histone-deacetylase activity of HDAC3 in vivo. Nat Struct Mol Biol. 2013;20(2):182-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Alenghat T, Meyers K, Mullican SE, et al. Nuclear receptor corepressor and histone deacetylase 3 govern circadian metabolic physiology. Nature. 2008;456(7224):997-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zhang Y, Fang B, Damle M, et al. HNF6 and Rev-erbα integrate hepatic lipid metabolism by overlapping and distinct transcriptional mechanisms. Genes Dev. 2016;30(14):1636-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Yin L, Wu N, Curtin JC, et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318(5857):1786-1789. [DOI] [PubMed] [Google Scholar]
- 155. Raghuram S, Stayrook KR, Huang P, et al. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat Struct Mol Biol. 2007;14(12):1207-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Rada-Iglesias A. Is H3K4me1 at enhancers correlative or causative? Nat Genet. 2018;50(1):4-5. [DOI] [PubMed] [Google Scholar]
- 157. Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693-705. [DOI] [PubMed] [Google Scholar]
- 158. Koike N, Yoo SH, Huang HC, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338(6105):349-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Yoo SH, Mohawk JA, Siepka SM, et al. Competing E3 ubiquitin ligases govern circadian periodicity by degradation of CRY in nucleus and cytoplasm. Cell. 2013;152(5):1091-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Siepka SM, Yoo SH, Park J, et al. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell. 2007;129(5):1011-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Godinho SI, Maywood ES, Shaw L, et al. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science. 2007;316(5826):897-900. [DOI] [PubMed] [Google Scholar]
- 162. Busino L, Bassermann F, Maiolica A, et al. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science. 2007;316(5826):900-904. [DOI] [PubMed] [Google Scholar]
- 163. Menet JS, Rodriguez J, Abruzzi KC, Rosbash M. Nascent-Seq reveals novel features of mouse circadian transcriptional regulation. Elife. 2012;1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Li W, Notani D, Rosenfeld MG. Enhancers as non-coding RNA transcription units: recent insights and future perspectives. Nat Rev Genet. 2016;17(4):207-223. [DOI] [PubMed] [Google Scholar]
- 165. Lam MT, Cho H, Lesch HP, et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013;498(7455):511-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ueda HR, Hayashi S, Chen W, et al. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat Genet. 2005;37(2):187-192. [DOI] [PubMed] [Google Scholar]
- 167. Duez H, van der Veen JN, Duhem C, et al. Regulation of bile acid synthesis by the nuclear receptor Rev-erbalpha. Gastroenterology. 2008;135(2):689-698. [DOI] [PubMed] [Google Scholar]
- 168. Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111(45):16219-16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Dierickx P, Vermunt MW, Muraro MJ, et al. Circadian networks in human embryonic stem cell-derived cardiomyocytes. EMBO Rep. 2017;18(7):1199-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Menet JS, Pescatore S, Rosbash M. CLOCK:BMAL1 is a pioneer-like transcription factor. Genes Dev. 2014;28(1):8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Zaret KS, Carroll JS. Pioneer transcription factors: establishing competence for gene expression. Genes Dev. 2011;25(21):2227-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Heinz S, Romanoski CE, Benner C, Glass CK. The selection and function of cell type-specific enhancers. Nat Rev Mol Cell Biol. 2015;16(3):144-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Du NH, Arpat AB, De Matos M, Gatfield D. MicroRNAs shape circadian hepatic gene expression on a transcriptome-wide scale. Elife. 2014;2014(3): 1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Wang H, Fan Z, Zhao M, et al. Oscillating primary transcripts harbor miRNAs with circadian functions. Sci Rep. 2016;6(October 2015):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Na YJ, Sung JH, Lee SC, et al. Comprehensive analysis of microRNA-mRNA co-expression in circadian rhythm. Exp Mol Med. 2009;41(9):638-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Cheng HY, Papp JW, Varlamova O, et al. microRNA modulation of circadian-clock period and entrainment. Neuron. 2007;54(5):813-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Gatfield D, Le Martelot G, Vejnar CE, et al. Integration of microRNA miR-122 in hepatic circadian gene expression. Genes Dev. 2009;23(11):1313-1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Kojima S, Sher-Chen EL, Green CB. Circadian control of mRNA polyadenylation dynamics regulates rhythmic protein expression. Genes Dev. 2012;26(24):2724-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Wang J, Symul L, Yeung J, et al. Circadian clock-dependent and -independent posttranscriptional regulation underlies temporal mRNA accumulation in mouse liver. Proc Natl Acad Sci U S A. 2018;115(8):E1916-E1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Gendreau KL, Unruh BA, Zhou C, Kojima S. Identification and characterization of transcripts regulated by circadian alternative polyadenylation in mouse liver. G3 (Bethesda). 2018;8(11):3539-3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Jang C, Lahens NF, Hogenesch JB, Sehgal A. Ribosome profiling reveals an important role for translational control in circadian gene expression. Genome Res. 2015;25(12):1836-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Wang J, Mauvoisin D, Martin E, et al. Nuclear proteomics uncovers diurnal regulatory landscapes in mouse liver. Cell Metab. 2017;25(1):102-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Robles MS, Humphrey SJ, Mann M. Phosphorylation is a central mechanism for circadian control of metabolism and physiology. Cell Metab. 2017;25(1):118-127. [DOI] [PubMed] [Google Scholar]
- 184. Hurley JM, Jankowski MS, De Los Santos H, et al. Circadian proteomic analysis uncovers mechanisms of post-transcriptional regulation in metabolic pathways. Cell Syst. 2018;7(6):613-626.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Boveri T. Die Blastomerenkerne von Ascaris megalo ephala und die Theorie der Chromosomenindividualität. Arch für Zellforsch. 1909;3: 181-268. [Google Scholar]
- 186. Rabl C. Uber Zellteilung. Morphol Jahrb. 1885;10:214-330. [Google Scholar]
- 187. Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet. 2001;2(4):292-301. [DOI] [PubMed] [Google Scholar]
- 188. de Wit E, de Laat W. A decade of 3C technologies: insights into nuclear organization. Genes Dev. 2012;26(1):11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Denker A, de Laat W. The second decade of 3C technologies: detailed insights into nuclear organization. Genes Dev. 2016;30(12):1357-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Lieberman-Aiden E, van Berkum NL, Williams L, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326(5950):289-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Kempfer R, Pombo A. Methods for mapping 3D chromosome architecture. Nat Rev Genet. 2020;21(4):207-226. [DOI] [PubMed] [Google Scholar]
- 192. Dixon JR, Selvaraj S, Yue F, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485(7398):376-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Nora EP, Lajoie BR, Schulz EG, et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485(7398):381-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Sexton T, Yaffe E, Kenigsberg E, et al. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell. 2012;148(3):458-472. [DOI] [PubMed] [Google Scholar]
- 195. Rao SS, Huntley MH, Durand NC, et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159(7):1665-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Phillips-Cremins JE, Sauria ME, Sanyal A, et al. Architectural protein subclasses shape 3D organization of genomes during lineage commitment. Cell. 2013;153(6):1281-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Lupiáñez DG, Kraft K, Heinrich V, et al. Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell. 2015;161(5):1012-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Lupiáñez DG, Spielmann M, Mundlos S. Breaking TADs: how alterations of chromatin domains result in disease. Trends Genet. 2016;32(4):225-237. [DOI] [PubMed] [Google Scholar]
- 199. van Steensel B, Belmont AS. Lamina-associated domains: links with chromosome architecture, heterochromatin, and gene repression. Cell. 2017;169(5): 780-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Bulger M, Groudine M. Looping versus linking: toward a model for long-distance gene activation. Genes Dev. 1999;13(19):2465-2477. [DOI] [PubMed] [Google Scholar]
- 201. Chambeyron S, Bickmore WA. Does looping and clustering in the nucleus regulate gene expression? Curr Opin Cell Biol. 2004;16(3):256-262. [DOI] [PubMed] [Google Scholar]
- 202. Engel JD, Tanimoto K. Looping, linking, and chromatin activity: new insights into beta-globin locus regulation. Cell. 2000;100(5):499-502. [DOI] [PubMed] [Google Scholar]
- 203. Kleinjan DA, van Heyningen V. Long-range control of gene expression: emerging mechanisms and disruption in disease. Am J Hum Genet. 2005;76(1):8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Ong CT, Corces VG. CTCF: an architectural protein bridging genome topology and function. Nat Rev Genet. 2014;15(4):234-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. West AG, Fraser P. Remote control of gene transcription. Hum Mol Genet. 2005;14 Spec No 1:R101-R111. [DOI] [PubMed] [Google Scholar]
- 206. Malik S, Roeder RG. The metazoan Mediator co-activator complex as an integrative hub for transcriptional regulation. Nat Rev Genet. 2010;11(11):761-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Soutourina J. Transcription regulation by the Mediator complex. Nat Rev Mol Cell Biol. 2018;19(4): 262-274. [DOI] [PubMed] [Google Scholar]
- 208. Dowen JM, Fan ZP, Hnisz D, et al. Control of cell identity genes occurs in insulated neighborhoods in mammalian chromosomes. Cell. 2014;159(2):374-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Rubin AJ, Barajas BC, Furlan-Magaril M, et al. Lineage-specific dynamic and pre-established enhancer-promoter contacts cooperate in terminal differentiation. Nat Genet. 2017;49(10):1522-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Kagey MH, Newman JJ, Bilodeau S, et al. Mediator and cohesin connect gene expression and chromatin architecture. Nature. 2010;467(7314):430-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Aguilar-Arnal L, Hakim O, Patel VR, Baldi P, Hager GL, Sassone-Corsi P. Cycles in spatial and temporal chromosomal organization driven by the circadian clock. Nat Struct Mol Biol. 2013;20(10):1206-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Xu Y, Guo W, Li P, et al. Long-range chromosome interactions mediated by cohesin shape circadian gene expression. PLoS Genet. 2016;12(5):1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Zhao H, Sifakis EG, Sumida N, et al. PARP1- and CTCF-mediated interactions between active and repressed chromatin at the lamina promote oscillating transcription. Mol Cell. 2015;59(6): 984-997. [DOI] [PubMed] [Google Scholar]
- 214. Kim YH, Marhon SA, Zhang Y, Steger DJ, Won KJ, Lazar MA. Rev-erbα dynamically modulates chromatin looping to control circadian gene transcription. Science. 2018;359(6381):1274-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Dey A, Chitsaz F, Abbasi A, Misteli T, Ozato K. The double bromodomain protein Brd4 binds to acetylated chromatin during interphase and mitosis. Proc Natl Acad Sci U S A. 2003;100(15):8758-8763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Roe JS, Mercan F, Rivera K, Pappin DJ, Vakoc CR. BET bromodomain inhibition suppresses the function of hematopoietic transcription factors in acute myeloid leukemia. Mol Cell. 2015;58(6):1028-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Jiang YW, Veschambre P, Erdjument-Bromage H, et al. Mammalian mediator of transcriptional regulation and its possible role as an end-point of signal transduction pathways. Proc Natl Acad Sci U S A. 1998;95(15):8538-8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Lovén J, Hoke HA, Lin CY, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013;153(2):320-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Bhagwat AS, Roe JS, Mok BYL, Hohmann AF, Shi J, Vakoc CR. BET bromodomain inhibition releases the mediator complex from select cis-regulatory elements. Cell Rep. 2016;15(3):519-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Beytebiere JR, Trott AJ, Greenwell BJ, et al. Tissue-specific BMAL1 cistromes reveal that rhythmic transcription is associated with rhythmic enhancer-enhancer interactions. Genes Dev. 2019;33(5-6): 294-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Zullo JM, Demarco IA, Piqué-Regi R, et al. DNA sequence-dependent compartmentalization and silencing of chromatin at the nuclear lamina. Cell. 2012;149(7):1474-1487. [DOI] [PubMed] [Google Scholar]
- 222. Poleshko A, Shah PP, Gupta M, et al. Genome-nuclear lamina interactions regulate cardiac stem cell lineage restriction. Cell. 2017;171(3):573-587.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Somech R, Shaklai S, Geller O, et al. The nuclear-envelope protein and transcriptional repressor LAP2beta interacts with HDAC3 at the nuclear periphery, and induces histone H4 deacetylation. J Cell Sci. 2005;118(Pt 17):4017-4025. [DOI] [PubMed] [Google Scholar]
- 224. de Lima CDM, Göndör A. Circadian organization of the genome. Science. 2018;359(6381):1212-1213. [DOI] [PubMed] [Google Scholar]
- 225. Hyman AA, Weber CA, Jülicher F. Liquid-liquid phase separation in biology. Annu Rev Cell Dev Biol. 2014;30:39-58. [DOI] [PubMed] [Google Scholar]
- 226. Banani SF, Lee HO, Hyman AA, Rosen MK. Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol. 2017;18(5):285-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Harlen KM, Churchman LS. The code and beyond: transcription regulation by the RNA polymerase II carboxy-terminal domain. Nat Rev Mol Cell Biol. 2017;18(4):263-273. [DOI] [PubMed] [Google Scholar]
- 228. Tatarakis A, Behrouzi R, Moazed D. Evolving models of heterochromatin: from foci to liquid droplets. Mol Cell. 2017;67(5):725-727. [DOI] [PubMed] [Google Scholar]
- 229. Wright PE, Dyson HJ. Intrinsically disordered proteins in cellular signalling and regulation. Nat Rev Mol Cell Biol. 2015;16(1):18-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Hnisz D, Shrinivas K, Young RA, Chakraborty AK, Sharp PA. A phase separation model for transcriptional control. Cell. 2017;169(1):13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: an opsin in melanophores, brain, and eye. Proc Natl Acad Sci U S A. 1998;95(1):340-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Hattar S, Lucas RJ, Mrosovsky N, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424(6944):76-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. J Neurosci. 2003;23(33):10691-10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Gallardo CM, Darvas M, Oviatt M, et al. Dopamine receptor 1 neurons in the dorsal striatum regulate food anticipatory circadian activity rhythms in mice. Elife. 2014;3:e03781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Orozco-Solis R, Aguilar-Arnal L, Murakami M, et al. The circadian clock in the ventromedial hypothalamus controls cyclic energy expenditure. Cell Metab. 2016;23(3):467-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Hastings MH, Maywood ES, Brancaccio M. Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci. 2018;19(8):453-469. [DOI] [PubMed] [Google Scholar]
- 237. Brancaccio M, Edwards MD, Patton AP, et al. Cell-autonomous clock of astrocytes drives circadian behavior in mammals. Science. 2019;363(6423):187-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Yang S, Liu A, Weidenhammer A, et al. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology. 2009;150(5):2153-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Sen S, Dumont S, Sage-Ciocca D, et al. Expression of the clock gene Rev-erbα in the brain controls the circadian organisation of food intake and locomotor activity, but not daily variations of energy metabolism. J Neuroendocrinol. 2018;30(1):1-11. [DOI] [PubMed] [Google Scholar]
- 240. Adamovich Y, Rousso-Noori L, Zwighaft Z, et al. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014;19(2):319-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Dudley CA, Erbel-Sieler C, Estill SJ, et al. Altered patterns of sleep and behavioral adaptability in NPAS2-deficient mice. Science. 2003;301(5631):379-383. [DOI] [PubMed] [Google Scholar]
- 242. Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Le Minh N, Damiola F, Tronche F, Schütz G, Schibler U. Glucocorticoid hormones inhibit food-induced phase-shifting of peripheral circadian oscillators. Embo J. 2001;20(24):7128-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Reddy AB, Maywood ES, Karp NA, et al. Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology. 2007;45(6):1478-1488. [DOI] [PubMed] [Google Scholar]
- 245. Karamitri A, Jockers R. Melatonin in type 2 diabetes mellitus and obesity. Nat Rev Endocrinol. 2019;15(2):105-125. [DOI] [PubMed] [Google Scholar]
- 246. Kennaway DJ. Melatonin research in mice: a review. Chronobiol Int. 2019;36(9):1167-1183. [DOI] [PubMed] [Google Scholar]
- 247. Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119(5):693-705. [DOI] [PubMed] [Google Scholar]
- 248. Miller BH, McDearmon EL, Panda S, et al. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci U S A. 2007;104(9):3342-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Mure LS, Le HD, Benegiamo G, et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 2018;1232:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291(5503):490-493. [DOI] [PubMed] [Google Scholar]
- 251. Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950-2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Kohsaka A, Laposky AD, Ramsey KM, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414-421. [DOI] [PubMed] [Google Scholar]
- 253. Challet E. The circadian regulation of food intake. Nat Rev Endocrinol. 2019;15(7):393-405. [DOI] [PubMed] [Google Scholar]
- 254. Bookout AL, de Groot MH, Owen BM, et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med. 2013;19(9):1147-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Panda S, Antoch MP, Miller BH, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109(3):307-320. [DOI] [PubMed] [Google Scholar]
- 256. Pan X, Jiang XC, Hussain MM. Impaired cholesterol metabolism and enhanced atherosclerosis in clock mutant mice. Circulation. 2013;128(16):1758-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Rudic RD, McNamara P, Curtis AM, et al. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2(11):e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Marcheva B, Ramsey KM, Buhr ED, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466(7306):627-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Perelis M, Marcheva B, Ramsey KM, et al. Pancreatic β cell enhancers regulate rhythmic transcription of genes controlling insulin secretion. Science. 2015;350(6261):aac4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Zhang D, Tong X, Nelson BB, et al. The hepatic BMAL1/AKT/lipogenesis axis protects against alcoholic liver disease in mice via promoting PPARα pathway. Hepatology. 2018;68(3):883-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. O’Neil D, Mendez-Figueroa H, Mistretta TA, Su C, Lane RH, Aagaard KM. Dysregulation of Npas2 leads to altered metabolic pathways in a murine knockout model. Mol Genet Metab. 2013;110(3):378-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Le Martelot G, Claudel T, Gatfield D, et al. REV-ERBalpha participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009;7(9):e1000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Armour SM, Remsberg JR, Damle M, et al. An HDAC3-PROX1 corepressor module acts on HNF4α to control hepatic triglycerides. Nat Commun. 2017;8(1):549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Sun Z, Miller RA, Patel RT, et al. Hepatic Hdac3 promotes gluconeogenesis by repressing lipid synthesis and sequestration. Nat Med. 2012;18(6):934-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Papazyan R, Sun Z, Kim YH, et al. Physiological suppression of lipotoxic liver damage by complementary actions of HDAC3 and SCAP/SREBP. Cell Metab. 2016;24(6):863-874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Liu S, Brown JD, Stanya KJ, et al. A diurnal serum lipid integrates hepatic lipogenesis and peripheral fatty acid use. Nature. 2013;502(7472):550-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Kang HS, Okamoto K, Takeda Y, et al. Transcriptional profiling reveals a role for RORalpha in regulating gene expression in obesity-associated inflammation and hepatic steatosis. Physiol Genomics. 2011;43(13):818-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Takeda Y, Kang HS, Freudenberg J, DeGraff LM, Jothi R, Jetten AM. Retinoic acid-related orphan receptor γ (RORγ): a novel participant in the diurnal regulation of hepatic gluconeogenesis and insulin sensitivity. PLoS Genet. 2014;10(5):e1004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Lau P, Fitzsimmons RL, Raichur S, Wang SC, Lechtken A, Muscat GE. The orphan nuclear receptor, RORalpha, regulates gene expression that controls lipid metabolism: staggerer (SG/SG) mice are resistant to diet-induced obesity. J Biol Chem. 2008;283(26):18411-18421. [DOI] [PubMed] [Google Scholar]
- 270. Wada T, Kang HS, Angers M, et al. Identification of oxysterol 7alpha-hydroxylase (Cyp7b1) as a novel retinoid-related orphan receptor alpha (RORalpha) (NR1F1) target gene and a functional cross-talk between RORalpha and liver X receptor (NR1H3). Mol Pharmacol. 2008;73(3):891-899. [DOI] [PubMed] [Google Scholar]
- 271. Zhang Y, Papazyan R, Damle M, et al. The hepatic circadian clock fine-tunes the lipogenic response to feeding through RORα/γ. Genes Dev. 2017;31(12):1202-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Gerhart-Hines Z, Feng D, Emmett MJ, et al. The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature. 2013;503(7476):410-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Lee P, Bova R, Schofield L, et al. Brown adipose tissue exhibits a glucose-responsive thermogenic biorhythm in humans. Cell Metab. 2016;23(4):602-609. [DOI] [PubMed] [Google Scholar]
- 274. Jager J, Wang F, Fang B, et al. The nuclear receptor Rev-erbα regulates adipose tissue-specific FGF21 signaling. J Biol Chem. 2016;291(20):10867-10875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Woldt E, Sebti Y, Solt LA, et al. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med. 2013;19(8):1039-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Mukherji A, Kobiita A, Ye T, Chambon P. Homeostasis in intestinal epithelium is orchestrated by the circadian clock and microbiota cues transduced by TLRs. Cell. 2013;153(4):812-827. [DOI] [PubMed] [Google Scholar]
- 277. Zhang EE, Liu Y, Dentin R, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16(10):1152-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Dyar KA, Lutter D, Artati A, et al. Atlas of circadian metabolism reveals system-wide coordination and communication between clocks. Cell. 2018;174(6):1571-1585.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Neinast MD, Jang C, Hui S, et al. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab. 2019;29(2):417-429.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Jang C, Hui S, Zeng X, et al. Metabolite exchange between mammalian organs quantified in pigs. Cell Metab. 2019;30(3):594-606.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Estrella MA, Du J, Chen L, et al. The metabolites NADP+ and NADPH are the targets of the circadian protein Nocturnin (curled). Nat Commun. 2019;10(1):2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Cantó C, Auwerx J. Calorie restriction: is AMPK a key sensor and effector? Physiology (Bethesda). 2011;26(4):214-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8(10):774-785. [DOI] [PubMed] [Google Scholar]
- 284. Davies SP, Carling D, Munday MR, Hardie DG. Diurnal rhythm of phosphorylation of rat liver acetyl-CoA carboxylase by the AMP-activated protein kinase, demonstrated using freeze-clamping. Effects of high fat diets. Eur J Biochem. 1992;203(3):615-623. [DOI] [PubMed] [Google Scholar]
- 285. Lamia KA, Sachdeva UM, DiTacchio L, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326(5951):437-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Um JH, Yang S, Yamazaki S, et al. Activation of 5’-AMP-activated kinase with diabetes drug metformin induces casein kinase Iepsilon (CKIepsilon)-dependent degradation of clock protein mPer2. J Biol Chem. 2007;282(29):20794-20798. [DOI] [PubMed] [Google Scholar]
- 287. Rutter J, Reick M, Wu LC, McKnight SL. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science. 2001;293(5529):510-514. [DOI] [PubMed] [Google Scholar]
- 288. Asher G, Gatfield D, Stratmann M, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134(2):317-328. [DOI] [PubMed] [Google Scholar]
- 289. Nakahata Y, Kaluzova M, Grimaldi B, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134(2):329-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Hirayama J, Sahar S, Grimaldi B, et al. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature. 2007;450(7172):1086-1090. [DOI] [PubMed] [Google Scholar]
- 291. Aguilar-Arnal L, Katada S, Orozco-Solis R, Sassone-Corsi P. NAD(+)-SIRT1 control of H3K4 trimethylation through circadian deacetylation of MLL1. Nat Struct Mol Biol. 2015;22(4):312-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Ramsey KM, Yoshino J, Brace CS, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324(5927):651-654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324(5927):654-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Cantó C, Gerhart-Hines Z, Feige JN, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113-118. [DOI] [PubMed] [Google Scholar]
- 296. Hanover JA, Krause MW, Love DC. Bittersweet memories: linking metabolism to epigenetics through O-GlcNAcylation. Nat Rev Mol Cell Biol. 2012;13(5):312-321. [DOI] [PubMed] [Google Scholar]
- 297. Kaasik K, Kivimäe S, Allen JJ, et al. Glucose sensor O-GlcNAcylation coordinates with phosphorylation to regulate circadian clock. Cell Metab. 2013;17(2):291-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Li MD, Ruan HB, Hughes ME, et al. O-GlcNAc signaling entrains the circadian clock by inhibiting BMAL1/CLOCK ubiquitination. Cell Metab. 2013;17(2):303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Phelan CA, Gampe RT Jr, Lambert MH, et al. Structure of Rev-erbalpha bound to N-CoR reveals a unique mechanism of nuclear receptor-co-repressor interaction. Nat Struct Mol Biol. 2010;17(7):808-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Gupta N, Ragsdale SW. Thiol-disulfide redox dependence of heme binding and heme ligand switching in nuclear hormone receptor rev-erb{beta}. J Biol Chem. 2011;286(6):4392-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Pardee KI, Xu X, Reinking J, et al. The structural basis of gas-responsive transcription by the human nuclear hormone receptor REV-ERBbeta. PLoS Biol. 2009;7(2):e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Dioum EM, Rutter J, Tuckerman JR, Gonzalez G, Gilles-Gonzalez MA, McKnight SL. NPAS2: a gas-responsive transcription factor. Science. 2002;298(5602):2385-2387. [DOI] [PubMed] [Google Scholar]
- 303. Kaasik K, Lee CC. Reciprocal regulation of haem biosynthesis and the circadian clock in mammals. Nature. 2004;430(6998):467-471. [DOI] [PubMed] [Google Scholar]
- 304. Wu N, Yin L, Hanniman EA, Joshi S, Lazar MA. Negative feedback maintenance of heme homeostasis by its receptor, Rev-erba. Genes Dev. 2009;23(18):2201-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Pathak P, Li T, Chiang JY. Retinoic acid-related orphan receptor α regulates diurnal rhythm and fasting induction of sterol 12α-hydroxylase in bile acid synthesis. J Biol Chem. 2013;288(52):37154-37165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Eckel-Mahan KL, Patel VR, de Mateo S, et al. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155(7):1464-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Guan D, Xiong Y, Borck PC, et al. Diet-induced circadian enhancer remodeling synchronizes opposing hepatic lipid metabolic processes. Cell. 2018;174(4):831-842.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Li X, Egervari G, Wang Y, Berger SL, Lu Z. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat Rev Mol Cell Biol. 2018;19(9):563-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324(5930):1076-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Lee JV, Carrer A, Shah S, et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab. 2014;20(2):306-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. McGurk L, Gomes E, Guo L, et al. Poly(ADP-ribose) prevents pathological phase separation of TDP-43 by promoting liquid demixing and stress granule localization. Mol Cell. 2018;71(5):703-717.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Altmeyer M, Neelsen KJ, Teloni F, et al. Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose). Nat Commun. 2015;6:8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Duan Y, Du A, Gu J, et al. PARylation regulates stress granule dynamics, phase separation, and neurotoxicity of disease-related RNA-binding proteins. Cell Res. 2019;29(3):233-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Singatulina AS, Hamon L, Sukhanova MV, et al. PARP-1 activation directs FUS to DNA damage sites to form PARG-reversible compartments enriched in damaged DNA. Cell Rep. 2019;27(6):1809-1821.e5. [DOI] [PubMed] [Google Scholar]
- 315. Jones CR, Campbell SS, Zone SE, et al. Familial advanced sleep-phase syndrome: a short-period circadian rhythm variant in humans. Nat Med. 1999;5(9):1062-1065. [DOI] [PubMed] [Google Scholar]
- 316. Reid KJ, Chang AM, Dubocovich ML, Turek FW, Takahashi JS, Zee PC. Familial advanced sleep phase syndrome. Arch Neurol. 2001;58(7):1089-1094. [DOI] [PubMed] [Google Scholar]
- 317. Toh KL, Jones CR, He Y, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291(5506):1040-1043. [DOI] [PubMed] [Google Scholar]
- 318. Vanselow K, Vanselow JT, Westermark PO, et al. Differential effects of PER2 phosphorylation: molecular basis for the human familial advanced sleep phase syndrome (FASPS). Genes Dev. 2006;20(19):2660-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Xu Y, Toh KL, Jones CR, Shin JY, Fu YH, Ptácek LJ. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell. 2007;128(1):59-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Iwase T, Kajimura N, Uchiyama M, et al. Mutation screening of the human Clock gene in circadian rhythm sleep disorders. Psychiatry Res. 2002;109(2):121-128. [DOI] [PubMed] [Google Scholar]
- 321. Mishima K, Tozawa T, Satoh K, Saitoh H, Mishima Y. The 3111T/C polymorphism of hClock is associated with evening preference and delayed sleep timing in a Japanese population sample. Am J Med Genet B Neuropsychiatr Genet. 2005;133B(1):101-104. [DOI] [PubMed] [Google Scholar]
- 322. Carpen JD, Archer SN, Skene DJ, Smits M, von Schantz M. A single-nucleotide polymorphism in the 5′-untranslated region of the hPER2 gene is associated with diurnal preference. J Sleep Res. 2005;14(3):293-297. [DOI] [PubMed] [Google Scholar]
- 323. Patke A, Murphy PJ, Onat OE, et al. Mutation of the human circadian clock gene CRY1 in familial delayed sleep phase disorder. Cell. 2017;169(2):203-215.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Pan A, Schernhammer ES, Sun Q, Hu FB. Rotating night shift work and risk of type 2 diabetes: two prospective cohort studies in women. PLoS Med. 2011;8(12):e1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Antunes LC, Levandovski R, Dantas G, Caumo W, Hidalgo MP. Obesity and shift work: chronobiological aspects. Nutr Res Rev. 2010;23(1):155-168. [DOI] [PubMed] [Google Scholar]
- 326. De Bacquer D, Van Risseghem M, Clays E, Kittel F, De Backer G, Braeckman L. Rotating shift work and the metabolic syndrome: a prospective study. Int J Epidemiol. 2009;38(3):848-854. [DOI] [PubMed] [Google Scholar]
- 327. Suwazono Y, Dochi M, Oishi M, Tanaka K, Kobayashi E, Sakata K. Shiftwork and impaired glucose metabolism: a 14-year cohort study on 7104 male workers. Chronobiol Int. 2009;26(5):926-941. [DOI] [PubMed] [Google Scholar]
- 328. Suwazono Y, Sakata K, Okubo Y, et al. Long-term longitudinal study on the relationship between alternating shift work and the onset of diabetes mellitus in male Japanese workers. J Occup Environ Med. 2006;48(5):455-461. [DOI] [PubMed] [Google Scholar]
- 329. Kroenke CH, Spiegelman D, Manson J, Schernhammer ES, Colditz GA, Kawachi I. Work characteristics and incidence of type 2 diabetes in women. Am J Epidemiol. 2007;165(2):175-183. [DOI] [PubMed] [Google Scholar]
- 330. Lin YC, Hsiao TJ, Chen PC. Persistent rotating shift-work exposure accelerates development of metabolic syndrome among middle-aged female employees: a five-year follow-up. Chronobiol Int. 2009;26(4):740-755. [DOI] [PubMed] [Google Scholar]
- 331. Shan Z, Li Y, Zong G, et al. Rotating night shift work and adherence to unhealthy lifestyle in predicting risk of type 2 diabetes: results from two large US cohorts of female nurses. BMJ. 2018;363:k4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22(10):939-943. [DOI] [PubMed] [Google Scholar]
- 333. Chang AM, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci U S A. 2015;112(4):1232-1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Chinoy ED, Duffy JF, Czeisler CA. Unrestricted evening use of light-emitting tablet computers delays self-selected bedtime and disrupts circadian timing and alertness. Physiol Rep. 2018;6(10):e13692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Ancoli-Israel S, Schnierow B, Kelsoe J, Fink R. A pedigree of one family with delayed sleep phase syndrome. J Biol Med Rhythm Res. 2001;18(5):831-840. [DOI] [PubMed] [Google Scholar]
- 336. Morag I, Ohlsson A. Cycled light in the intensive care unit for preterm and low birth weight infants. Cochrane Database Syst Rev. 2016;2016(8):1-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Simons KS, Laheij RJ, van den Boogaard M, et al. Dynamic light application therapy to reduce the incidence and duration of delirium in intensive-care patients: a randomised controlled trial. Lancet Respir Med. 2016;4(3):194-202. [DOI] [PubMed] [Google Scholar]
- 338. McHill AW, Melanson EL, Higgins J, et al. Impact of circadian misalignment on energy metabolism during simulated nightshift work. Proc Natl Acad Sci U S A. 2014;111(48):17302-17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339. Buxton OM, Cain SW, O’Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4(129):129ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci U S A. 2009;106(11):4453-4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Englund A, Kovanen L, Saarikoski ST, et al. NPAS2 and PER2 are linked to risk factors of the metabolic syndrome. J Circadian Rhythms. 2009;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Bouatia-Naji N, Bonnefond A, Cavalcanti-Proença C, et al. A variant near MTNR1B is associated with increased fasting plasma glucose levels and type 2 diabetes risk. Nat Genet. 2009;41(1):89-94. [DOI] [PubMed] [Google Scholar]
- 343. Lyssenko V, Nagorny CL, Erdos MR, et al. Common variant in MTNR1B associated with increased risk of type 2 diabetes and impaired early insulin secretion. Nat Genet. 2009;41(1):82-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Prokopenko I, Langenberg C, Florez JC, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41(1):77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Muller JE, Stone PH, Turi ZG, et al. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med. 1985;313(21):1315-1322. [DOI] [PubMed] [Google Scholar]
- 346. Jiddou MR, Pica M, Boura J, Qu L, Franklin BA. Incidence of myocardial infarction with shifts to and from daylight savings time. Am J Cardiol. 2013;111(5):631-635. [DOI] [PubMed] [Google Scholar]
- 347. Wang J, Yin L, Lazar MA. The orphan nuclear receptor Rev-erb alpha regulates circadian expression of plasminogen activator inhibitor type 1. J Biol Chem. 2006;281(45):33842-33848. [DOI] [PubMed] [Google Scholar]
- 348. Andreotti F, Davies GJ, Hackett DR, et al. Major circadian fluctuations in fibrinolytic factors and possible relevance to time of onset of myocardial infarction, sudden cardiac death and stroke. Am J Cardiol. 1988;62(9):635-637. [DOI] [PubMed] [Google Scholar]
- 349. Ruben MD, Wu G, Smith DF, et al. A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. Sci Transl Med. 2018;10(458):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Hermida RC, Ayala DE. Chronotherapy with the angiotensin-converting enzyme inhibitor ramipril in essential hypertension: improved blood pressure control with bedtime dosing. Hypertension. 2009;54(1):40-46. [DOI] [PubMed] [Google Scholar]
- 351. Hermida RC, Ayala DE, Fernández JR, Calvo C. Comparison of the efficacy of morning versus evening administration of telmisartan in essential hypertension. Hypertension. 2007;50(4):715-722. [DOI] [PubMed] [Google Scholar]
- 352. Hermida RC, Ayala DE, Chayan L, Mojon A, Fernandez JR. Administration-time-dependent effects of olmesartan on the ambulatory blood pressure of essential hypertension patients. Chronobiol Int. 2009;26(1):61-79. [DOI] [PubMed] [Google Scholar]
- 353. Cederroth CR, Albrecht U, Bass J, et al. Medicine in the Fourth Dimension. Cell Metab. 2019;30(2):238-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Ruben MD, Smith DF, FitzGerald GA, Hogenesch JB. Dosing time matters. Science. 2019;365(6453):547-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Montaigne D, Marechal X, Modine T, et al. Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbα antagonism: a single-centre propensity-matched cohort study and a randomised study. Lancet. 2018;391(10115):59-69. [DOI] [PubMed] [Google Scholar]
- 356. Dierickx P, Emmett MJ, Jiang C, et al. SR9009 has REV-ERB-independent effects on cell proliferation and metabolism. Proc Natl Acad Sci U S A. 2019;116(25):12147-12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Di Francesco A, Di Germanio C, Bernier M, de Cabo R. A time to fast. Science. 2018;362(6416):770-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381(26):2541-2551. [DOI] [PubMed] [Google Scholar]
- 359. Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20(6):991-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Hatori M, Vollmers C, Zarrinpar A, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Mitchell SJ, Bernier M, Mattison JA, et al. Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab. 2019;29(1):221-228.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212-1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Wilkinson MJ, Manoogian ENC, Zadourian A, et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31(1):92-104.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Panda S. Circadian physiology of metabolism. Science. 2016;354(6315):1008-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Greenwell BJ, Trott AJ, Beytebiere JR, et al. Rhythmic food intake drives rhythmic gene expression more potently than the hepatic circadian clock in mice. Cell Rep. 2019;27(3):649-657.e5. [DOI] [PubMed] [Google Scholar]
- 366. Chaix A, Lin T, Le HD, Chang MW, Panda S. Time-restricted feeding prevents obesity and metabolic syndrome in mice lacking a circadian clock. Cell Metab. 2019;29(2):303-319.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]