Abstract
Background
Melanoma is a very malignant skin cancer with high mortality and unsatisfactory prognosis. Many long noncoding RNAs (lncRNAs) have been reported to be aberrantly expressed in melanoma. How lncRNA regulates melanoma progression is poorly defined. LncRNA CAR10 has been shown to regulate the progression of several cancers and its role in melanoma remains unclear. This study aims to determine the role and mechanism of lncRNA CAR10 in the regulation of melanoma progression.
Methods
qRT-PCR was utilized to analyze CAR10 in melanoma human tissues and cell lines while Kaplan–Meier curve was used to examine the survival rate. CCK8 assay and EdU assay were used to assess cell proliferation when Transwell assay was conducted to determine migration and invasion. And tumor xenograft assay was performed to evaluate tumor growth in vivo. Additionally, luciferase assay and RNA pulldown assay were performed to analyze the interactions among CAR10, miR-125b-5p and RAB3D.
Results
LncRNA CAR10 was upregulated in melanoma tissues and cell lines. Upregulation of CAR10 predicted a poor prognosis in patients with melanoma. CAR10 knockdown suppressed proliferation, migration and invasion of melanoma cells in vitro. CAR10 silencing attenuated tumor growth in vivo. CAR10 inhibited miR-125b-5p activity to upregulate RAB3D expression. And miR-125b-5p/RAB3D signaling is crucial for CAR10-dependent melanoma progression.
Conclusion
Our work suggests that lncRNA CAR10 promotes melanoma growth and metastasis through modulating miR-125b-5p/RAB3D axis.
Keywords: melanoma, CAR10, progression, miR-125b-5p, RAB3D
Introduction
Melanoma is a kind of very aggressive skin cancer and has a high rate of mortality.1 Furthermore, the incidence of melanoma is increasing steadily around the world.2 Despite some advances achieved in the past decades, patients with melanoma still show an unsatisfactory prognosis due to metastasis and resistance to chemotherapy or radiotherapy.3 Recently, targeted and immunotherapy has been developed for the treatment of advanced melanoma.4 However, it is urgently required to understand the underlying molecular mechanism of melanoma progression.
Long noncoding RNAs (lncRNAs) are a novel type of noncoding RNAs and characterized by over 200 nucleotides in length and no protein-coding ability.5,6 Increasing references have indicated a crucial role of lncRNA in cancers through regulating many biological processes, such as proliferation, differentiation and metastasis.7,8 Besides, many lncRNAs may serve as biomarkers for diagnosis or prognosis in the tumor.8 For example, lncRNA RUNX1-IT1 suppresses liver cancer growth and impairs stemness.9 LncRNA WT1-As inhibits the proliferation of thyroid cancer cells via upregulating miR-203.10 LncRNA LINC01783 upregulation predicts a poor prognosis and enhances cervical tumor cell growth, migration and invasion through inhibiting miR-199b-5p and promoting GBP1 expression.11 Additionally, lncRNA CASC9 is upregulated in tongue squamous cell cancer tissues and positively regulates growth and invasiveness by modulating miR-423/SOX12 axis.12 Several lncRNAs have been reported to participate in melanoma.13–15 However, there are still so many lncRNAs whose functions are largely unknown in melanoma.
LncRNA CAR1 is involved in lung adenocarcinoma and cervical cancer.16,17 However, the role of CAR1 in melanoma remains unclear. References have indicated that lncRNAs may regulate the proliferation, metastasis and apoptosis of melanoma.18,19 LncRNA could be a miRNA sponge to regulate melanoma progression.19 In this study, we demonstrated that CAR10/miR-125b-5p/RAB3D axis plays crucial roles in regulating melanoma development and CAR10 may be a potential therapeutic target.
Materials and Methods
Patient Tissues
Thirty-nine melanoma patient tissues were obtained from The First Affiliated Hospital of Wenzhou Medical University by surgery. Histopathological diagnosis was performed by at least two pathologists. All patients received no chemotherapy or radiotherapy before surgery. Tissues were frozen in liquid nitrogen until use. This study was approved by the Ethics Committee of The First Affiliated Hospital of Wenzhou Medical University. We obtained all signed informed consents. All experiments were performed in accordance with the ethical guidelines of the Declaration of Helsinki.
Cell Culture and Transfection
Human melanoma cell lines and human epidermal melanocytes HEMa-LP were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cell lines were cultured in DMEM medium (Gibco, USA; cat. No. 11995065) containing 10% fetal bovine serum (FBS; Gibco, USA; cat. No. 16140071) and 2.5% penicillin and streptomycin. Short hairpin RNA (shRNA) targeting CAR10, miR-125b-5p mimics and miR-125b-5p inhibitor were from GenePharma (Shanghai, China). Transfection was performed by using Lipofectamine 3000 (Invitrogen, USA).
CCK8
Proliferation was measured through the CCK8 kit (Dojindo Molecular Technologies, Japan) according to the manufacturer’s instructions. In brief, transfected cells were seeded into 96-well plates and incubated for 24h, 48h or 72h. Then, 10 μL of CCK8 was added and incubated for 2h. The optical density (OD) value at 450 nm was measured via using a microplate spectrophotometer (BioTek Instruments, USA).
Transwell Assay
Transwell assay was performed through Corning costar (8-μm pore size; Cambridge, USA). The lower chamber was added with a complete medium (DMEM medium containing 10% FBS) and the upper chamber was added with serum-free medium containing transfected cells. After cultured for 48 h, the migrated or invaded cells were fixed with methanol and stained with 0.1% crystal violet. Then, cells were photographed under a microscope.
qRT-PCR
After isolation of total RNA using TRIzol (Invitrogen, Carlsbad, USA; cat. no. IS10007), cDNA was synthesized using reverse transcription kit (Applied Biosystems, CA). Then, qPCR was completed by using the SYBR Green Mix (Promega) based on 2−ΔΔCt method. U6 and GAPDH were endogenous control genes.
Cell Cycle Analysis
Cells were harvested and treated with pre-cooled 75% ethanol at 4°C overnight. Then, cells were washed and stained with PI/RNase staining solution (BD Biosciences) for 15 min at 25°C in the dark, followed by flow cytometric analysis.
Bioinformatics Analysis
miRDB (http://mirdb.org/) was used to predict the interaction between CAR10 and miR-125b-5p. TargetScan (http://www.targetscan.org/vert_71/) was used to predict the interaction between miR-125b-5p and RAB3D.
Luciferase Reporter Assay
Wild-type or mutant sequence was inserted into pmiR-RB-REPORORT TMVector (Ribobio, China). Then, the luciferase reporter and miR-125b-5p were transfected into cells via Lipofectamine3000 according to the manufacturer’s instructions (Invitrogen, USA). Forty-eight hours later, the relative luciferase activities were measured through the Dual-Luciferase Reporter Assay System (Promega, WI, USA).
RNA Pulldown Assay
Biotinylated WT or Mut CAR10 was bought from GenePharma (Shanghai, China). Biotinylated vectors were transfected into cells and cell lysates were incubated with M-280 streptavidin magnetic beads (Invitrogen, USA). The precipitated RNA was detected by using qRT-PCR.
Xenograft Assay
Stably shCAR10 or shNC (negative control) transfected cells (1.5 × 106) were injected subcutaneously into the flanks of female BALB/c nude mice (n=5 for each group). Then, tumor size was determined every week. Tumor weight was measured after 5 weeks post-injection. The animal experiments were approved by the Ethics Committee of The First Affiliated Hospital of Wenzhou Medical University and in accordance with the National Institute of Health’s (NIH) Animal Care and Use Committee guidelines.
Statistical Analysis
The data was analyzed using Graphpad Prism 6 software and expressed as the mean ± standard deviation. The statistical significance of the data was analyzed via t-test or one-way ANOVA. Survival plots were determined by Kaplan−Meier analysis and log-rank test. P < 0.05 was considered to have statistical significance.
Results
LncRNA CAR10 Expression in Melanoma
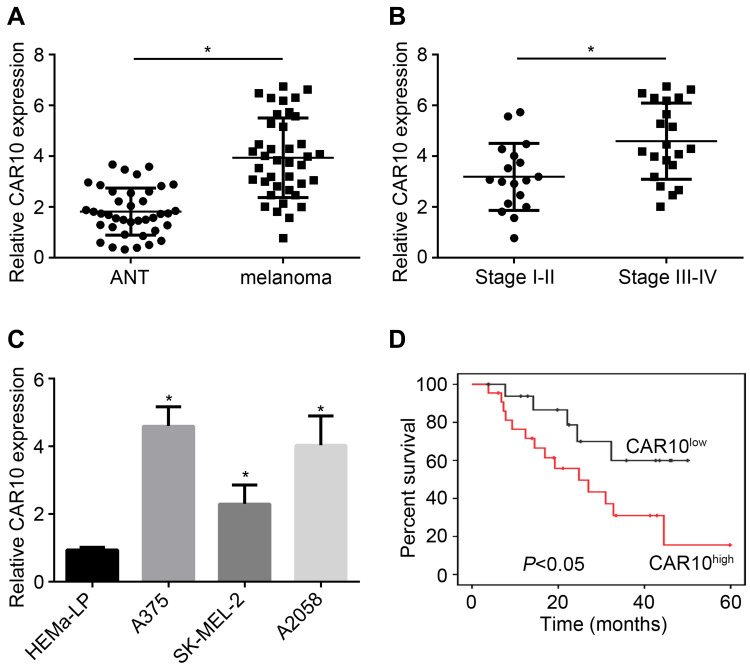
To investigate the role of CAR10 in melanoma, we carried out qRT-PCR analysis to determine the expression of CAR10 in tumor tissues and adjacent normal tissues. CAR10 expression in melanoma tissues was higher (Figure 1A). Moreover, the CAR10 level in melanoma tissues with stage III/IV was upregulated (Figure 1B). Compared with HEMa-LP cells, melanoma cell lines also showed higher CAR10 expression levels (Figure 1C). Besides, CAR10 low expression in melanoma patients is correlated with a high survival rate (Figure 1D).
Figure 1.
LncRNA CAR10 expression in melanoma. (A) CAR10 was upregulated in melanoma tissues compared to adjacent normal tissues (ANT). (B) CAR10 expression was increased in advanced melanoma tissues. (C) CAR10 expression in melanoma cell lines was tested by qRT-PCR. (D) The relationship between CAR10 expression and survival rate was analyzed. *P<0.05.
CAR10 Downregulation Inhibited Proliferation, Migration and Invasion
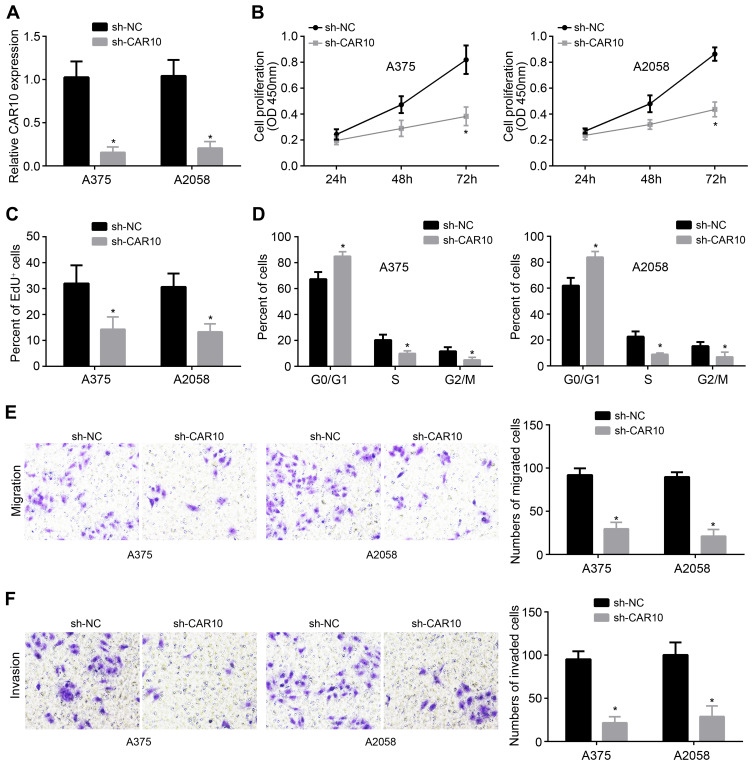
CAR10 expression was the highest in A375 and A2058 cells among all detected cells. Therefore, A375 and A2058 cells were used for functional experiments. To examine the effects of CAR10 on melanoma, we knocked it down in A375 and A2058 cells (Figure 2A). Then, CCK8 assay was performed to analyze cell proliferation. We found that CAR10 knockdown gradually inhibited cell proliferation (Figure 2B). Furthermore, EdU incorporation assay showed a similar trend with CCK8 assay (Figure 2C). Besides, we found that CAR10 knockdown induced more cells arrested in G0/G1 phase (Figure 2D), suggesting that CAR10 silencing inhibits cell cycle progression. Finally, transwell assay was used to observe the effects of CAR10 on metastasis. We found that CAR10 knockdown led to a decrease of migrated and invaded cell numbers (Figure 2E and F).
Figure 2.
CAR10 downregulation inhibited proliferation, migration and invasion. (A) Relative expression of CAR10 was measured after shRNA transfection. (B) CCK8 assay was used to analyze proliferation. (C) EdU incorporation assay was performed to determine cell proliferation. (D) Cell cycle was detected after CAR10 silencing. (E and F) Transwell assay was performed to test cell migration and invasion. *P<0.05.
Confirmation of the Relationships Among CAR10, miR-125b-5p and RAB3D
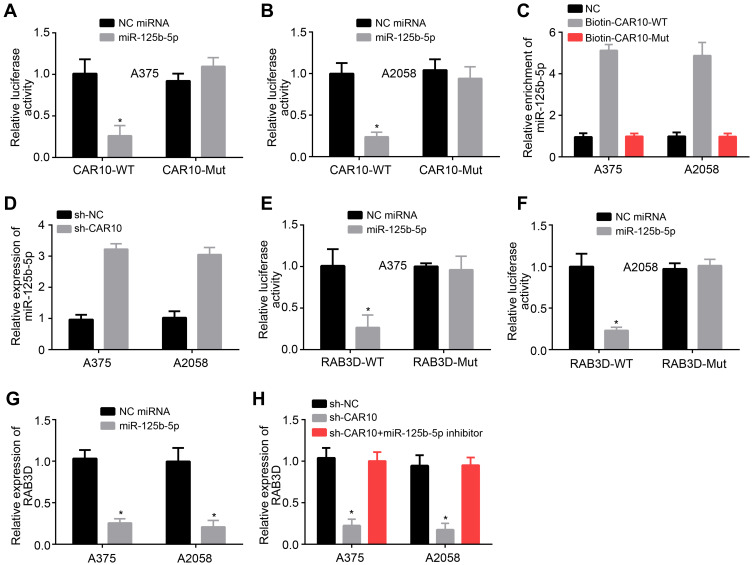
Through bioinformatics analysis (miRDB), we identified that CAR10 may interact with miR-125b-5p, which is consistent with a recent study.17 To validate it, luciferase reporter assay was performed. We found that miR-125b-5p mimics inhibited the activity of wild-type (WT) CAR10 reporter in both A375 and A2058 cells (Figure 3A and B). RNA pulldown assay also indicated that CAR10 could directly precipitated miR-125b-5p (Figure 3C). And miR-125b-5p expression was increased after CAR10 knockdown (Figure 3D). We then predicted the potential targets of miR-125b-5p through TargetScan7 and identified RAB3D. Through luciferase reporter assay, we also validated the interaction between miR-125b-5p and RAB3D (Figure 3E and F). Notably, either miR-125b-5p mimics or CAR10 silencing inhibited the expression of RAB3D (Figure 3G and H). However, RAB3D expression could be rescued by miR-125b-5p inhibitors (Figure 3H), indicating CAR10 promotes RAB3D expression through modulating miR-125b-5p.
Figure 3.
Confirmation of the relationships among CAR10, miR-125b-5p and RAB3D. (A and B) Dual-luciferase reporter assay was performed to analyze the interaction between CAR10 and miR-125b-5p. (C) RNA pulldown was conducted to examine the association between CAR10 and miR-125b-5p. (D) miR-125b-5p level was monitored after CAR10 silencing. (E and F) Dual-luciferase reporter assay was performed to analyze the interaction between miR-125b-5p and RAB3D. (G and H) RAB3D expression was monitored after transfection of miR-125b-5p mimics, sh-CAR10 or indicated plasmids. *P<0.05.
CAR10 Promotes Melanoma Progression Through miR-125b-5p/RAB3D Axis
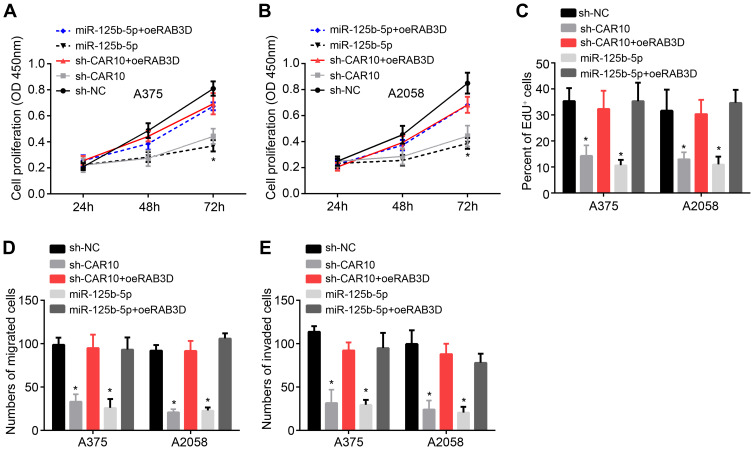
To validate the physiological role of CAR10/miR-125b-5p/RAB3D axis, we restored RAB3D expression. Through CCK8 and EdU incorporation assays, we found that either CAR10 knockdown or miR-125b-5p mimics inhibited the proliferation of A375 and A2058 cells (Figure 4A–C). Similarly, the potential for migration and invasion was also suppressed by CAR10 knockdown and miR-125b-5p mimics (Figure 4D and E). However, restoration of RAB3D rescued proliferation, migration and invasion of melanoma (Figure 4A–E).
Figure 4.
CAR10 promotes melanoma progression through miR-125b-5p/RAB3D axis. (A and B) CCK8 assay was performed to test proliferation. (C) EdU incorporation assay was conducted to analyze melanoma proliferation. (D and E) Transwell assay was utilized to determine migration and invasion. *P<0.05.
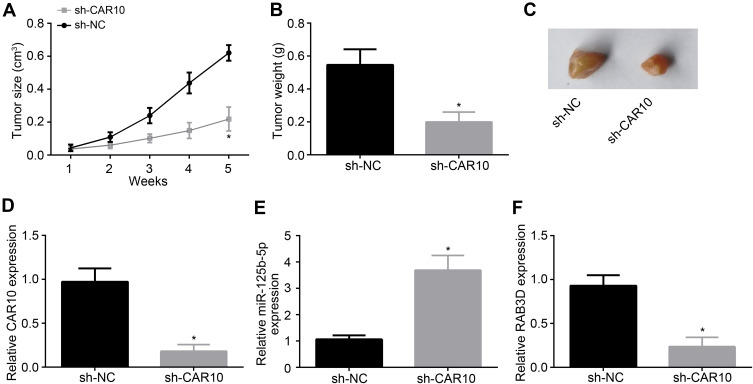
CAR10 Knockdown Inhibits Melanoma Growth in vivo
To further confirm the role of CAR10 in vivo, Xenograft assay was conducted. Tumor size and weight were measured at indicated time points. We found that CAR10 knockdown suppressed the volume and weight of tumor tissues (Figure 5A–C). Moreover, in tumor tissues, the expression levels of CAR10 and RAB3D were downregulated while miR-125b-5p expression was increased (Figure 5D–F), indicating that CAR10 positively affects melanoma growth in vivo through miR-125b-5p/RAB3D axis.
Figure 5.
CAR10 knockdown inhibits melanoma growth in vivo. (A) Tumor size was monitored every 1 week. (B and C) Tumor weight was determined after 5 weeks. (D–F) Relative expression of CAR10, miR-125b-5p and RAB3D was analyzed through qRT-PCR in tumor tissues. *P<0.05.
Discussion
Melanoma is an aggressive cutaneous cancer and characterized by high metastatic potential.20 Over 70% of deaths from skin cancer are induced by melanoma.1 Recently, targeted and immunotherapy was suggested to be a potential effective method of advanced melanoma treatment.4 In this study, we found that lncRNA CAR10 was upregulated in melanoma tissues. And CAR10 high expression predicted unsatisfactory prognosis in melanoma patients. We also indicated that CAR10 knockdown suppressed the proliferation, migration and invasion of melanoma in vitro and in vivo through regulating miR-125b-5p/RAB3D pathway.
Previous works have validated that lncRNAs have important functions in regulating malignant behaviors of melanoma.19,21 For instance, lncRNA MEG3 is reported to promote melanoma proliferation and invasion by targeting miR_21/E-cadherin signaling.19 LINC00459 is downregulated in melanoma and suppresses melanoma growth and metastasis via miR-218/DKK3 axis.21 LncRNA LINC00518 elevates the abilities of proliferation and metastasis through modulating miR-204-5p/AP1S2 pathway in melanoma.22 Recently, CAR10 function is not fully researched. Only two studies suggest it is involved in the tumorigenesis of lung cancer and cervical cancer.16,17 In this study, we found that CAR10 expression was significantly elevated in melanoma tissues. CAR10 level was positively linked with poor prognosis in patients with melanoma. Furthermore, we illustrated that CAR10 knockdown inhibited proliferation, migration and invasion of melanoma in vitro. And CAR10 downregulation impaired tumor growth in vivo. Thus, CAR10 is a key oncogene in melanoma by regulating tumor cell growth and metastasis.
Extensive researches have proven that lncRNA may work as ceRNAs in cancers, including bladder cancer, glioblastoma and melanoma.22–24 LncRNAs could sponge miRNAs and inhibit the binding of miRNAs to target mRNAs, leading to target gene upregulation.22 For example, lncRNA JPX sponges miR-33a to upregulate Twist1 expression and enhance tumorigenesis and invasion of lung tumor.25 LncRNA ROR1-AS1 overexpression contributes to proliferation, migration and invasion of bladder tumor cells through targeting miR-504.26 As previously identified, we also found that CAR10 sponges miR-125b-5p.17 We demonstrated that miR-125b-5p was directly targeted by CAR10 in melanoma. MiR-125b-5p has been shown to regulate tumor development. For example, several references indicated that miR-125b-5p inhibits the progression of cervical cancer, colorectal cancer and bladder cancer.17,27,28 Yet, the potential role of miR-125b-5p in melanoma is unclear. Here, we found that miR-125b-5p upregulation markedly suppressed the proliferation, migration and invasion of melanoma cells. Therefore, our findings for the first time indicate miR-125b-5p as an anti-cancer miRNA.
Afterwards, we identified the downstream target of miR-125b-5p through bioinformatics method. We demonstrated miR-125b-5p directly targeted RAB3D and inhibited its expression. Previous work suggests that Rab3D has critical roles in colorectal cancer, esophageal squamous cell carcinoma and osteosarcoma.29–32 RAB3D function in melanoma is unknown. Here, we showed that RAB3D restoration could significantly rescue the abilities of proliferation, migration and invasion of melanoma cells. Our results support that RAB3D contributes to melanoma development.
In summary, we demonstrated that CAR10 plays an important function in regulating melanoma progression. Our findings provide a molecular biological basis of melanoma aggravation and suggest that CAR10/miR-125b-5p/RAB3D signaling may be a potential therapeutic target. Besides, whether biological or pharmacological substances that interfere with this axis could be of potential clinical appliance remains more investigation.
Acknowledgment
We thank all patients involved in this study.
Data Sharing Statement
All data are made available from the corresponding author upon reasonable request.
Disclosure
All authors declare no conflicts of interest.
References
- 1.Alqathama A. BRAF in malignant melanoma progression and metastasis: potentials and challenges. Am J Cancer Res. 2020;10(4):1103–1114. [PMC free article] [PubMed] [Google Scholar]
- 2.Bedogni B, Paus R. Hair(y) matters in melanoma biology. Trends Mol Med. 2020;26(5):441–449. doi: 10.1016/j.molmed.2020.02.005 [DOI] [PubMed] [Google Scholar]
- 3.Rishi A, Yu HM. Current treatment of melanoma brain metastasis. Curr Treat Options Oncol. 2020;21(6):45. doi: 10.1007/s11864-020-00733-z [DOI] [PubMed] [Google Scholar]
- 4.da Silveira Nogueira Lima JP, Georgieva M, Haaland B, de Lima Lopes G. A systematic review and network meta-analysis of immunotherapy and targeted therapy for advanced melanoma. Cancer Med. 2017;6(6):1143–1153. doi: 10.1002/cam4.1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu B, Ye B, Yang L, et al. Long noncoding RNA lncKdm2b is required for ILC3 maintenance by initiation of Zfp292 expression. Nat Immunol. 2017;18(5):499–508. doi: 10.1038/ni.3712 [DOI] [PubMed] [Google Scholar]
- 6.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904–914. doi: 10.1016/j.molcel.2011.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng WX, Koirala P, Mo YY. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36(41):5661–5667. doi: 10.1038/onc.2017.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–1076. doi: 10.1038/nature08975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun L, Wang L, Chen T, et al. LncRNA RUNX1-IT1 which is downregulated by hypoxia-driven histone deacetylase 3 represses proliferation and cancer stem-like properties in hepatocellular carcinoma cells. Cell Death Dis. 2020;11(2):95. doi: 10.1038/s41419-020-2274-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le F, Luo P, Ouyang Q, Zhong X. LncRNA WT1-AS downregulates survivin by upregulating miR-203 in papillary thyroid carcinoma. Cancer Manag Res. 2020;12:443–449. doi: 10.2147/CMAR.S232294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen WJ, Xiong L, Yang L, et al. Long non-coding RNA LINC01783 promotes the progression of cervical cancer by sponging miR-199b-5p to mediate GBP1 expression. Cancer Manag Res. 2020;12:363–373. doi: 10.2147/CMAR.S230171 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Chen X, Xu H, Sun G, Zhang Y. LncRNA CASC9 affects cell proliferation, migration, and invasion of tongue squamous cell carcinoma via regulating miR-423-5p/SOX12 axes. Cancer Manag Res. 2020;12:277–287. doi: 10.2147/CMAR.S220351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Gao J, Yu Y, Zhao Z, Pan Y. LncRNA FOXD3-AS1 promotes proliferation, invasion and migration of cutaneous malignant melanoma via regulating miR-325/MAP3K2. Biomed Pharmacother. 2019;120:109438. doi: 10.1016/j.biopha.2019.109438 [DOI] [PubMed] [Google Scholar]
- 14.Schmidt K, Weidmann CA, Hilimire TA, et al. Targeting the oncogenic long non-coding RNA SLNCR1 by blocking its sequence-specific binding to the androgen receptor. Cell Rep. 2020;30(2):541–554 e545. doi: 10.1016/j.celrep.2019.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coe EA, Tan JY, Shapiro M, et al. The MITF-SOX10 regulated long non-coding RNA DIRC3 is a melanoma tumour suppressor. PLoS Genet. 2019;15(12):e1008501. doi: 10.1371/journal.pgen.1008501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge X, Li GY, Jiang L, et al. Long noncoding RNA CAR10 promotes lung adenocarcinoma metastasis via miR-203/30/SNAI axis. Oncogene. 2019;38(16):3061–3076. doi: 10.1038/s41388-018-0645-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu T, Zhang Q, Gao L. LncRNA CAR10 upregulates PDPK1 to promote cervical cancer development by sponging miR-125b-5p. Biomed Res Int. 2020;2020:4351671. doi: 10.1155/2020/4351671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Tang D, Wu T, Sun F. ELF1-mediated LUCAT1 promotes choroidal melanoma by modulating RBX1 expression. Cancer Med. 2020;9(6):2160–2170. doi: 10.1002/cam4.2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu L, Zhu L, Li Y, Zheng Z, Lin X, Yang C. LncRNA MEG3 promotes melanoma growth, metastasis and formation through modulating miR-21/E-cadherin axis. Cancer Cell Int. 2020;20:12. doi: 10.1186/s12935-019-1087-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Read J, Wadt KA, Hayward NK. Melanoma genetics. J Med Genet. 2016;53(1):1–14. doi: 10.1136/jmedgenet-2015-103150 [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Xu W, Zheng Z, Cao Z. LINC00459 sponging miR-218 to elevate DKK3 inhibits proliferation and invasion in melanoma. Sci Rep. 2019;9(1):19139. doi: 10.1038/s41598-019-55701-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luan W, Ding Y, Ma S, Ruan H, Wang J, Lu F. Long noncoding RNA LINC00518 acts as a competing endogenous RNA to promote the metastasis of malignant melanoma via miR-204-5p/AP1S2 axis. Cell Death Dis. 2019;10(11):855. doi: 10.1038/s41419-019-2090-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Du L, Yang X, et al. A nomogram combining long non-coding RNA expression profiles and clinical factors predicts survival in patients with bladder cancer. Aging. 2020;12:2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu C, Wei Y, Wang X, et al. DNA-methylation-mediated activating of lncRNA SNHG12 promotes temozolomide resistance in glioblastoma. Mol Cancer. 2020;19(1):28. doi: 10.1186/s12943-020-1137-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan J, Fang S, Tian H, et al. lncRNA JPX/miR-33a-5p/Twist1 axis regulates tumorigenesis and metastasis of lung cancer by activating Wnt/β-catenin signaling. Mol Cancer. 2020;19(1):9. doi: 10.1186/s12943-020-1133-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Q, Fu L, Tu W-J. Upregulation of long non-coding RNA ROR1-AS1 promotes cell growth and migration in bladder cancer by regulation of miR-504. PLoS One. 2020;15(1):e0227568. doi: 10.1371/journal.pone.0227568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park GB, Jeong JY, Kim D. Modified TLR-mediated downregulation of miR-125b-5p enhances CD248 (endosialin)-induced metastasis and drug resistance in colorectal cancer cells. Mol Carcinog. 2020;59(2):154–167. doi: 10.1002/mc.23137 [DOI] [PubMed] [Google Scholar]
- 28.Liu S, Chen Q, Wang Y. MiR-125b-5p suppresses the bladder cancer progression via targeting HK2 and suppressing PI3K/AKT pathway. Hum Cell. 2020;33(1):185–194. doi: 10.1007/s13577-019-00285-x [DOI] [PubMed] [Google Scholar]
- 29.Luo Y, Ye GY, Qin SL, et al. High expression of Rab3D predicts poor prognosis and associates with tumor progression in colorectal cancer. Int J Biochem Cell Biol. 2016;75:53–62. doi: 10.1016/j.biocel.2016.03.017 [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Kong R, Sun L. Silencing of Rab3D suppresses the proliferation and invasion of esophageal squamous cell carcinoma cells. Biomed Pharmacother. 2017;91:402–407. doi: 10.1016/j.biopha.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 31.Jiashi W, Chuang Q, Zhenjun Z, Guangbin W, Bin L, Ming H. MicroRNA-506-3p inhibits osteosarcoma cell proliferation and metastasis by suppressing RAB3D expression. Aging. 2018;10(6):1294–1305. doi: 10.18632/aging.101468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao K, Fang Y, Wang H, Jiang Z, Guo L, Hu Y. The lncRNA HOXA11-AS regulates Rab3D expression by sponging miR-125a-5p promoting metastasis of osteosarcoma. Cancer Manag Res. 2019;11:4505–4518. doi: 10.2147/CMAR.S196025 [DOI] [PMC free article] [PubMed] [Google Scholar]