Abstract
Type 2 diabetes (T2D) has been rising in prevalence in the United States and worldwide over the past few decades and contributes to significant morbidity and premature mortality, primarily due to cardiovascular disease (CVD). Cardiorespiratory fitness (CRF) is a modifiable cardiovascular (CV) risk factor in the general population and in people with T2D. Young people and adults with T2D have reduced CRF when compared with their peers without T2D who are similarly active and of similar body mass index. Furthermore, the impairment in CRF conferred by T2D is greater in women than in men. Various factors may contribute to this abnormality in people with T2D, including insulin resistance and mitochondrial, vascular, and cardiac dysfunction. As proof of concept that understanding the mediators of impaired CRF in T2D can inform intervention, we previously demonstrated that an insulin sensitizer improved CRF in adults with T2D. This review focuses on how contributing factors influence CRF and why they may be compromised in T2D. Functional exercise capacity is a measure of interrelated systems biology; as such, the contribution of derangement in each of these factors to T2D-mediated impairment in CRF is complex and varied. Therefore, successful approaches to improve CRF in T2D should be multifaceted and individually designed. The current status of this research and future directions are outlined.
Keywords: cardiorespiratory fitness, type 2 diabetes, cardiovascular disease, microvascular, mitochondria, endothelium
The prevalence of diabetes mellitus (DM) in the United States has been rising since 1990. In 2018, 34.2 million people, around 10.5% of the US population, had DM (11% of men, 9.5% of women) [1]. In 2016, it was estimated that 91.2% of adults with DM have type 2 diabetes (T2D) [2] and the prevalence of youth-onset T2D is also rising [1]. DM and its related health complications, including cardiovascular disease (CVD), chronic kidney disease (CKD), and congestive heart failure (CHF), are associated with significant morbidity and mortality. People with DM accounted for 7.8 million hospital discharges in 2016 with 1.7 million of these hospitalizations due to major CVD events [1]. Additionally, premature mortality from CVD is 2- to 6-fold greater in people with DM than for people without diabetes [3]. Lower levels of cardiorespiratory fitness (CRF), measured as maximal oxygen consumption (VO2max), are predictive of greater short-term mortality risk in adults and children with DM [4–6], as in the general population [7–9]. Lower CRF in people with DM has also been associated with future CVD events and as such is an important modifiable CV risk factor [10–12]. In a study by Seyoum et al., men and women with diabetes who developed CVD events within 5 years had lower baseline peak VO2 than those who did not have CVD events during this follow-up period [10].
Compared with healthy individuals without diabetes, CRF is lower in adults and children with T2D and in children with type 1 diabetes, even in the absence of clinically apparent CVD in either group. These findings were observed in individuals with similar habitual physical activity levels, age, pubertal stage (for youth), and body mass index (BMI) (Table 1) [13–19]. Additionally, the presence of T2D confers a greater CRF deficit in women than in men [15, 20]. Not only does lower CRF predict premature mortality, it may lead to barriers to exercise recommendations by raising the relative intensity of a given work rate [21]. Potential physiological mechanisms that may contribute to lower CRF in people with T2D include insulin resistance and mitochondrial, vascular, and cardiac dysfunction. This review will focus on how each of these factors may contribute to CRF impairment in T2D and conclude with an assessment of the current state of knowledge about sex differences in CRF in people with T2D.
Table 1.
Mean cardiorespiratory fitness (CRF) before graded exercise training in women with and without type 2 diabetes (T2D).
| Lean participants (control) | Overweight participants (control) | T2D participants | |
|---|---|---|---|
| VO2max (mL/kg/min) | 25.1 ±4.7 | 21.8 ±2.9 | 17.7 ±4.0a |
Values are means ±SD.
a P < .05 for difference between the T2D group and the other 2 groups.
Adapted from Brandenburg SL, Reusch JE, Bauer TA, Jeffers BW, Hiatt WR, Regensteiner JG. Effects of exercise training on oxygen uptake kinetic responses in women with type 2 diabetes. Diabetes Care. 1999;22(10):1640–1646 [17].
1. Search Methods
Articles included in this narrative review were compiled from original research articles, societal guidelines, and reviews from peer-reviewed journals included in the PubMed database as of 9 April 2020. Search terms included “exercise capacity” OR “cardiorespiratory fitness” PLUS “diabetes AND either “endothelial,” “mitochondria,” “blood flow,” “fibrinolysis,” OR “NOS.” We additionally searched references within publications relevant to the topic.
2. Insulin Action and CRF
Insulin resistance, a defining feature of T2D, correlates with decreased CRF. For example, we demonstrated that insulin resistance measured by the hyperinsulinemic euglycemic clamp strongly and independently correlated with peak VO2 in adolescents with T2D [18, 19]. In a proof of concept study, we therefore tested the impact of the insulin sensitizer rosiglitazone on CRF in people with T2D. We observed a significant 7% increase in peak VO2 with rosiglitazone alone compared with a placebo (Table 2) [22]. This finding was corroborated by Kadoglou et al., suggesting that targeting insulin action can improve functional status in people with T2D [23, 24]. Insulin action is associated with cardiac and skeletal muscle function and metabolic flexibility with exercise [25]. In addition, our current work supports a relationship between insulin action and factors related to CRF: cardiac function and skeletal muscle microvascular perfusion [20, 26]. As a vasoactive hormone, insulin has been shown in both rodents and healthy human participants to increase skeletal and cardiac muscle perfusion [27–29]. This perfusion response is blunted in insulin-resistant states [30, 31]. Decreased microvascular blood flow has been shown during insulin infusions in both insulin-resistant animal models and human participants [32]. Moreover, recent studies indicate that decreased microvascular blood flow may limit muscle glucose uptake by limiting the delivery of glucose to the myocyte [33]. Glucose and oxygen are both perfusion limited in their delivery to muscle, suggesting that any underlying microvascular defects are likely to have similar effects on both oxygen and glucose [34]. Even in people with obesity and no family history of DM, insulin-mediated microvascular perfusion in heart and skeletal muscle is decreased [35]. This is important as the microvasculature provides endothelial surface area needed for tissue uptake of oxygen and substrates that are vital to tissue health and function [36]. Indeed, multiple studies have shown that improved skeletal muscle microvascular perfusion via insulin sensitizers/enhancers and agents that cause vasodilation, such as glucagon-like peptide 1 receptor agonists (GLP-1), is associated with improved muscle oxygenation regardless of insulin sensitivity, suggesting that targeting microvascular insulin resistance and overall skeletal muscle and heart perfusion may result in both improved muscle oxygenation and glucose delivery during exercise [22, 37–39]. Taken together, these data support a role for insulin action in the heart, skeletal muscle, and the microvasculature as contributors to CRF.
Table 2.
Mean cardiorespiratory fitness (CRF) before and after exercise when treated with thiazolidinedione (Rosiglitazone) vs placebo.
| Placebo | Rosiglitazone | |
|---|---|---|
| VO2max (mL/kg/min) | ||
| Before | 19.4 ± 5.2 | 19.8 ± 5.3 |
| After | 18.1 ± 5.3 | 21.2 ± 5.1a |
Values are means ±SD.
a P < 0.05 difference within groups before and after treatment.
Adapted from Regensteiner JG, Bauer TA, Reusch JE. Rosiglitazone improves exercise capacity in individuals with type 2 diabetes. Diabetes care. 2005;28(12):2877–2883 [22]. Copyright 2005 by the American Diabetes Association.
3. Insulin Resistance and Oxidative Capacity
Insulin resistance is correlated with mitochondrial dysfunction, but the cause and effect relationship is unclear—whether this is a cause or consequence of insulin resistance is a matter of active investigation. For example, skeletal muscle oxidative enzymes are lower in adults with T2D, while glycolytic enzymes are elevated, compared with adults without T2D, suggesting a relationship between mitochondrial dysregulation and insulin resistance [40]. Insulin-resistant adult offspring of people with T2D also have lower mitochondrial activity in vivo and lower expression of mitochondrial and mitochondrial biogenesis genes and proteins than insulin-sensitive adults matched for physical activity, height, weight, and age [41–44]. In a study on healthy participants, a lipid infusion resulted in lower glucose oxidation and muscle glycogen synthesis, as well as a lower glucose-6-phosphate and intracellular glucose concentration, suggesting less glucose transport [45]. Evidence also demonstrates that lipid infusion induces insulin resistance by altering skeletal muscle microvascular perfusion [28]. Lower oxidative capacity has been previously shown in skeletal muscle with decreased NADH:O2 oxidoreductase activity (measure of respiratory chain activity, when normalized to citrate synthase and creatine kinase activity) in adults with T2D compared with both obese and lean adults [46]. Lower oxidative capacity has also been demonstrated in adolescents with T2D in vivo using in-MRI exercise. In particular, skeletal muscle adenosine diphosphate time constant, a blood flow–dependent mitochondrial function measure, was slowed and oxidative phosphorylation rates lower in the adolescents with T2D, linking skeletal muscle blood flow and mitochondrial dysfunction in these young people. Moreover, lack of suppression free fatty acids and longer skeletal muscle adenosine diphosphate time constant were independently associated with insulin resistance [47]. Therefore, the interplay between insulin resistance in skeletal muscle and mitochondrial dysfunction may be an adaptation to a chronically hyperglycemic and/or hyperlipemic state and is likely mediated by both microvascular and skeletal muscle changes [25, 48].
Mitochondrial dysfunction is also observed in the myocardium of people with T2D [49]. In human adults with well-controlled, uncomplicated T2D, there is a lower ratio of cardiac phosphocreatine to adenosine triphosphate than in healthy controls. Furthermore, young people and adults with T2D have lower indices of diastolic function, possibly suggestive of early diastolic dysfunction [18, 50]. These findings are similar to those seen in people with clinical heart failure [49, 51, 52]. Peterson et al. found that participants with T2D had lower fractional glucose uptake and oxidation, glycolysis, and glycogen deposition in myocardial tissue [53]. Impaired myocardial mitochondrial function in DM may correlate with future mortality. For instance, in people with dilated cardiomyopathy, the myocardial phosphocreatine-to-adenosine triphosphate ratio has prognostic value predicting total and CV mortality [54]. Our understanding of the complex interaction between insulin resistance, cardiac mitochondrial dysfunction, and CRF is still evolving [55].
4. Glucose, Endothelium, and Vascular Regulation
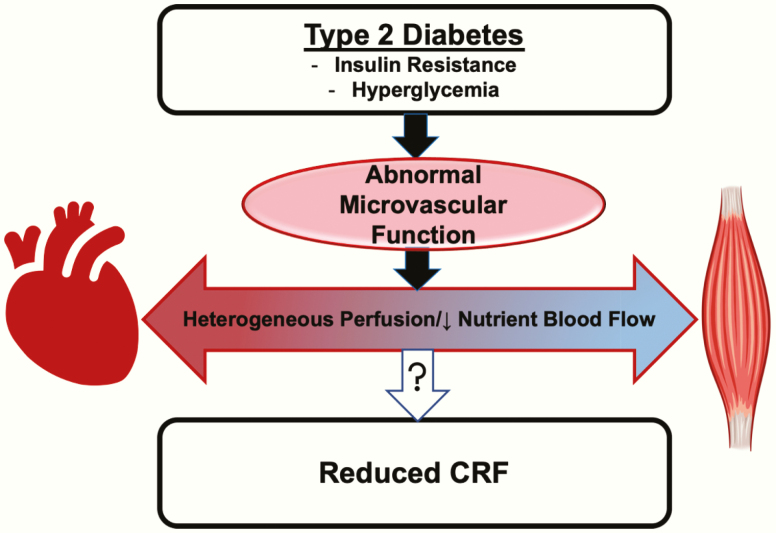
Hyperglycemia also leads to vascular dysfunction (Fig. 1). The endothelium, or lining of the vasculature, is the first defense of the tissue against toxic metabolites and inflammatory cytokines and is also responsible for moderating oxygen and nutrient access to tissue; both roles are imperative for tissue health and function [28, 56]. The endothelium fine tunes vascular tone via its production of nitric oxide (NO) [57], which leads to calcium-mediated vasodilation and increased perfusion of vital structures [57]. Insulin’s vasodilatory effect is NO mediated [58], and insulin increases NO production via Akt phosphorylation of endothelial nitric oxide synthase (eNOS) [59]. In T2D, insulin regulation of nitric oxide synthase (NOS) is impaired [59, 60].
Figure 1.
Insulin resistance and hyperglycemia of T2D lead to abnormal microvascular function and heterogeneous microvascular perfusion with lower nutrient blood flow, which may contribute to reduced CRF in people with T2D.
In addition to insulin regulation of perfusion, DM impacts the macrovasculature and microvasculature in many ways. For example, elevation in glucose and fatty acids lead to inflammation and glucose-mediated production of advanced glycation end products (AGEs), activation of the renin–angiotensin activating system (RAAS) pathway, and aldosterone regulation [61]. Hyperglycemia leads to production of AGEs [62]. These accrue in the wall of vessels, leading to integral loss of the structure of the vessel wall and underlying basement membrane, as well as proinflammatory signaling contributing to systemic vascular dysfunction [63–65]. Additionally, in the Multiethnic Study of Arthrosclerosis (MESA) study population, higher aldosterone levels were associated with higher fasting glucose, insulin resistance, and risk of incident DM, suggesting a role for RAAS activation in DM [66]. Previous studies have shown that RAAS modulation with angiotensin II receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACEi) lower production of AGEs [67, 68]. Olmesartan, an ARB, has been shown to inhibit AGE-related inflammation in endothelial cells [69]. In the Heart Outcomes Prevention Evaluation (HOPE) study, treatment of participants with DM with ACEi led to lower risk of CVD events, mortality, and nephropathy [70]. Furthermore, Parving et al. showed the renoprotective effect of irbesartan, an ARB, on participants with DM and hypertension [71]. In the IRMA 2 study, inflammatory markers and endothelial dysfunction predicted progression to diabetic nephropathy in T2D [72]; participants on irbesartan, an ARB, had significantly lower markers of inflammation (high sensitivity c-reactive protein, interleukin-6, and fibrinogen) [73]. These findings suggest a role of AGEs and RAAS activation in vascular dysfunction and progression of microvascular disease in diabetes, which are likely contributors to impaired CRF as well.
Vascular dysfunction is present prior to overt CVD and is even detectable in youth with T2D. Decreased limb blood flow correlated with reduced peak VO2 in young people with T2D [18]. Additionally, arterial stiffness was seen in around half of the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) cohort [74]. These data support a conceptual model wherein vascular insulin resistance, impaired eNOS regulation, and glucotoxicity- and lipotoxicity-mediated dysregulation of AGEs and vasoactive hormones could each contribute to decreased CRF in T2D.
5. The Role of NOS and NO in DM
Previous studies in healthy participants have shown that aerobic exercise attenuates mitochondrial damage and improves both vascular and skeletal muscle mitochondrial function, both of which are be impaired in DM [75–77]. However, when rat models of DM and hypertension (Spontaneously Hypertensive Heart Failure [SHHF] obese) underwent an exercise intervention, there was no induction of vascular mitochondrial content as expected and as seen with control rats [78]. NOS/NO are known upstream regulators of mitochondrial biogenesis [79–81]. Animal models have demonstrated that NOS/NO are pivotal to an expression of vascular mitochondrial content and adaptive response to exercise (Table 3) [38, 39, 78, 82–86]. Lack of NOS expression in these animal studies led to decreased vascular mitochondrial content and impaired vascular mitochondrial response to exercise [84] Therefore, eNOS has a role in mitochondrial adaptation to exercise, and dysregulation of eNOS in DM may explain the decreased response to exercise.
Table 3.
Summary of animal studies investigating role of nitric oxide synthase (NOS) and nitric oxide (NO) in vascular mitochondrial adaptation to exercise, muscle perfusion and oxygenation, and muscle glucose extraction.
| Vascular mitochondria | Systemic response | |||||
|---|---|---|---|---|---|---|
| Animal study | Intervention | Content | Respiration | Adaptation | Endurance | Metabolism |
| Goto-Kakizaki (GK) rata [78] | High glucose | ↓ | ↓ | none | ±↑ | — |
| eNOS +/– and –/– null mice [84] | None | ↓ | — | — | — | — |
| Sprague Dawley ratb [84] | NOS-inhibitor/exercise | ↓ | — | ↓ | — | — |
| GK rata [83] | Saxagliptin/exercise | ↑ | — | ↑ | ↑ | ↑ |
| Wistar ratc [85] | GLP-1 receptor antagonist/exercise | — | ↓ | ↓ | ↓ | ↓ |
| Wistar ratc [87] | BH4 precursor | ↑ | — | ↑ | — | ↑ |
| Skeletal muscle | ||||||
| Mitochondria | Perfusion/Oxygenation | |||||
| Sprague Dawley ratb [39] | High-fat diet/Liraglutide | ↑ | ↑ | |||
| Sprague Dawley ratb [38] | Continuous GLP-1 infusion | ↑ | ↑ | |||
| Streptozotocin (STZ)-induced mousea [82] | BH4 | ↑ | — | |||
| eNOS and nNOS null mouse [86] | Acute and chronic exercise | ↑±training response | — | |||
aNonobese DM model.
bMetabolic syndrome model.
cControl model.
GLP-1 has been shown to stimulate NOS and cyclic AMP via G-protein–coupled receptor signaling, leading to increased glucose uptake in tissues and insulin-independent vasodilation [88]. In insulin-resistant rat models, treatment with liraglutide, a GLP-1 receptor agonist, rescued insulin-mediated increases in muscle perfusion and oxygenation [39]. Continuous GLP-1 infusion led to increased muscle microvascular blood perfusion, plasma NO, muscle insulin uptake, and muscle glucose extraction [38]. Furthermore, treatment with saxagliptin, a dipeptidyl peptidase 4 inhibitor that inhibits GLP-1 degradation, plus exercise training in an insulin-resistant rat model restored exercise-mediated vascular mitochondrial response and led to increased exercise capacity as measured by endurance [83]. Finally, rats treated with a GLP-1 receptor antagonist had decreased CRF, with and without exercise training. They also had attenuated vascular adaptation to exercise training [85]. However, in people with T2D, sitagliptin improved diastolic cardiac function, but not CRF, in the absence of formal exercise training [89]. Similarly, in people with T2D, treatment with exenatide, a GLP-1 agonist, improved diastolic heart function and arterial stiffness, but did not improve CRF in the absence of formal exercise training [90]. These data suggest a role for GLP-1 in improving insulin-mediated defects in mitochondrial function and endothelial function, as well as in mediating adaptive effects of exercise training; to date, these benefits have only been clearly demonstrated in animal models (summarized in Table 3).
It has been reported that eNOS, as 1 of 3 NOS isoforms, generates NO only if a key cofactor, tetrahydrobiopterin (BH4) is present [65]. NO originates from L-arginine catabolism by NOS. Reports have demonstrated that intravenous infusion of low-dose L-arginine improved insulin-mediated vasodilation in obese and T2D human participants and improved insulin sensitivity in all participants (healthy, obese, and T2D) [91]. Furthermore, our group treated participants with uncomplicated T2D with oral L-arginine for 7 days and demonstrated an increase in brachial artery diameter response and increased hyperemic forearm blood flow. There was no significant change in response noted in controls after 7 days of L-arginine [92]. In diabetes, oxidation of BH4 to 7,8-dihydrobiopterin leads to eNOS uncoupling and dysfunction [93]. In a metabolic syndrome mouse model, BH4 administration lowered glucose by acting as an insulin sensitizer and attenuating eNOS dysfunction [82]. Therefore, both defective insulin signaling to NOS and NOS uncoupling in diabetes can lead to abnormal vasomotion and vascular insulin resistance.
While NOS certainly has a role in vascular adaptation to exercise, it is not fully responsible for the lack of adaptive response to exercise training in animal models. eNOS and nNOS null mice subjected to acute (60 minutes) and chronic (9 days of 60 minutes) exercise training had increases in mitochondrial biogenesis markers during both short-term and long-term exercise training, demonstrating that while eNOS and nNOS are involved in mitochondrial adaptation, there are additional pathways necessary for mitochondrial response in the skeletal muscle to exercise training in nondiabetic models [86]. Sjøberg et al. have shown that these muscular mitochondrial adaptations to exercise are dependent on increased microvascular perfusion and molecular signaling, both induced by insulin, suggesting a role for blood flow–dependent insulin delivery and intact molecular signaling in the endothelium by insulin in exercise adaptive response and consequently decreased CRF in T2D [94].
6. Blood Flow and Muscle Oxygenation in T2D
Adequate perfusion of skeletal and cardiac muscle is necessary for appropriate function during a bout of exercise. Impaired blood flow distribution with exercise in T2D appears to contribute to decreased oxidative capacity by limiting skeletal muscle oxygen for oxidative flux. In a study by Bauer et al. [95], there was a rise in muscle deoxygenation (deoxygenated hemoglobin/myoglobin) that exceeded the rate of oxygen extraction after a bout of exercise in participants with T2D, suggesting a mismatch of supply to demand. There was also a delayed increase in blood flow after onset of exercise in these participants, consistent with perfusion limiting the exercise response [95]. Muscle deoxygenation during exercise in people with T2D was further studied by assessing maximal reactive hyperemic blood flow, peak oxygen utilization during exercise, and assessment of skeletal muscle oxygenation and its relation to blood volume and hematocrit. In control participants, muscle deoxyhemoglobin accumulation correlated inversely with peak VO2. In contrast, in people with uncomplicated T2D, this correlation was not present; we postulated that this difference in T2D was due to heterogeneous muscle blood flow distribution. In this study, participants with T2D had similar total limb blood flow and tissue hemoglobin content after a bout of exercise as those without T2D, suggesting that the oxygen extraction impairment is independent of limb total blood flow [26].
The role of heterogeneous microvascular perfusion in decreased skeletal muscle oxygen extraction in T2D was first tested in a metabolic syndrome animal model. We demonstrated that heterogeneous blood flow could predict oxygen extraction and that oxygen extraction could be restored with acute antioxidant treatment in obese Zucker rats [34, 96]. The observation that this defect could be corrected led to our next series of clinical studies. In sedentary obese participants with and without T2D, we examined in vivo oxidative flux using 31P-magnetic resonance spectroscopy. As with previous reports, we observed lower oxidative flux in people with T2D. We tested whether the difference in oxidative flux was due to lower oxygen availability using supplemental oxygen and observed that the supplemental oxygen improved oxidative flux in people with T2D, but not in control participants [97]. These data support the overall working model that perfusion heterogeneity and local muscle hypoxia contribute to in vivo impaired mitochondrial function in people with T2D.
7. Potential Mediators of Heterogeneous and Lower Nutrient Blood Flow in T2D
Heterogeneous blood flow in T2D may be in part due to degradation of the endothelial glycocalyx (Table 4), a semipermeable layer of glycoproteins and proteoglycans at the blood vessel luminal surface [104]. People with DM have been shown to have a decrease in glycocalyx in both acute and chronic exposure of their vessels to hyperglycemia. When there is loss of the glycocalyx, protective enzymes on the surface of the endothelium are lost, leaving endothelial cells vulnerable to oxidative stress and inflammation [98].
Table 4.
Potential mediators of heterogeneous and lower nutrient blood flow.
DM is also associated with premature atherosclerosis [105] and a prothrombotic state. Increased thrombosis is in part due to augmented platelet activation and creation of compact fibrin networks that are lysis resistant [99]. In our earlier work, we reported that premenopausal women with T2D lose their fibrinolytic potential [100]. Inadequate dynamic resolution of microthrombi is a plausible contributor to heterogeneous blood distribution. Additionally, people with DM have more neutrophil extracellular traps formation. Neutrophil extracellular traps are composed of histones and DNA, which interact with fibrinogen to form thicker fibrin fibers leading to prolongation of clot lysis. This association leads to augmentation of thrombin generation, reduction in permeability of the clot, and consequent decreased fibrinolysis. These complexes are correlated with glycemic control and increased inflammatory state (elevated interleukin-6) [101]. Taken together, these abnormalities are plausible contributors to heterogeneous blood flow in DM.
Lower capillary density, sometimes termed capillary rarefaction, is also present in DM. Reports by Prior et al. have demonstrated increased skeletal muscle capillarization with exercise training in elderly participants and people with impaired glucose tolerance [102, 103]. In a rodent model, we examined the relative contribution of capillary density versus heterogeneous tissue perfusion to muscle oxygen extraction. Only 20% of the decreased muscle oxygen extraction observed in the Zucker rat model could be explained by the lower skeletal muscle capillary density [96]. There is currently not a robust human literature on capillary density changes in uncomplicated T2D so more research is required.
8. Cardiac Dysfunction
We have reported abnormal cardiac function in the setting of exercise in people with uncomplicated T2D. Specifically, women with uncomplicated, recently diagnosed T2D have a significantly and disproportionately greater rise in pulmonary capillary wedge pressure during exercise than in healthy control female participants [20, 106]. This finding suggests a stiff heart, as is also seen with diastolic dysfunction. The augmentation of pulmonary capillary wedge pressure in response to exercise correlated with poorer myocardial perfusion across all regions of the heart [20]. Similarly, adolescents with T2D have greater left ventricular mass and abnormal cardiac circumferential strain, along with the finding of lower CRF than in lean and obese healthy control participants. Circumferential strain and CRF correlated with low adiponectin and fat mass, suggestive of obesity factoring into cardiac dysfunction and lower exercise capacity [19]. These findings of subclinical cardiac dysfunction with preserved cardiac output suggest that the noted decline in CRF in people with T2D may be independent of cardiac output and yet still have a cardiac contributor to its multifactorial etiology. Specifically, cardiac microvascular perfusion abnormalities contributing to cardiac stiffness may be implicated, but this question requires further evaluation [20].
9. Sex Differences in CVD Risk and CRF
There are similar absolute risks for CVD among men and women with DM. However, there is a much higher relative risk for CVD conferred by DM in women than in men with DM, despite a similar DM incidence [1, 107–110]. This difference in CV morbidity and mortality risk may be due to T2D dampening the cardioprotection thought to occur in premenopausal women [111–113]. It may also be due to a greater CVD risk factor burden in women with DM than in men with DM—conversely, it may also relate to a lower CVD risk factor burden in women without DM than in men without DM. The multifaceted underlying mechanisms that may account for the excess CVD risk seen in women with T2D are complex and are reviewed more comprehensively elsewhere [111, 114]. Furthermore, as described in the introduction, the presence of T2D confers a greater reduction in CRF in women than in men when compared with their nondiabetic counterparts [15, 20, 115]. Women of all ages have lower physical activity levels than their male counterparts [116, 117]. Women report more barriers to exercise, which may contribute to this difference in physical activity between the sexes [118, 119]. Women with T2D also perceive greater effort during exercise at the same work rate than women without T2D [21, 120]. To date, the physiological explanation for this sex difference is not well understood. Previous work demonstrated that female sex is associated with increased stiffness of the left ventricle during exercise among adults with T2D [121]. Additionally, in the HERITAGE study, women with T2D who underwent aerobic exercise training had a 3-fold smaller improvement in insulin sensitivity than men with T2D who completed the same intensity and frequency of exercise training [122]. It is unknown why these sex differences exist; further investigation is needed to assess underlying mechanisms that may contribute to lower CRF and higher relative risk of CVD in women with T2D.
10. Summary
DM prevalence continues to rise in the United States with associated increased CVD morbidity and mortality. People with T2D have reduced CRF compared with healthy participants, a factor that is associated with increased CV mortality. The mechanisms of lower CRF in T2D are multifaceted and involve interrelated defects in insulin action, mitochondrial dysfunction, skeletal muscle microvasculature, and cardiac dysfunction. Various interventions have been studied to counteract these abnormalities; however, further human research is needed to evaluate the effect of these therapies on CRF in T2D (Table 5). Additionally, there is variable response to intervention [123], perhaps due to the multifaceted complexity of exercise training and its effect on the heart, vasculature, and skeletal muscle. Regular exercise typically improves CRF and insulin resistance in healthy controls. However, exercise can have inconsistent therapeutic effect in people with T2D compared with otherwise similar healthy participants. Additionally, there appears to be a greater deficit in CRF and associated physiological dysfunction in women with DM. These sex differences suggest that more research should be done on the role of sex as a moderator of the response to exercise therapy. Therefore, interventions must focus on tackling these mechanisms contributing to reduced CRF, as well as augmenting the beneficial effects of exercise in people with T2D.
Table 5.
Factors contributing to reduced cardiorespiratory fitness (CRF) in type 2 diabetes (T2D) and therapies that may improve these factors.
| Factors | Potential interventions |
|---|---|
| Abnormal insulin action Lower oxidative capacity Impaired vascular regulation by nitric oxide synthase and nitric oxide Lower nutrient blood flow Heterogeneous microvascular perfusion Cardiac dysfunction Sex | Exercise [17, 123–125] Thiazolidinedione (rosiglitazone) [22, 23] Angiotensin II receptor blockers [67–69, 71, 73] Glucagon-like peptide 1 receptor agonists [36, 38, 39, 85, 88]Dipeptidyl peptidase 4 inhibitor [83]L-arginine (intravenous [91] or oral [92]) |
Acknowledgments
The authors wish to acknowledge all our study participants and all of the students, fellows, and technicians within our laboratories.
Financial Support: J.E.B.R. reports funding from the U.S. Department of Veterans Affairs (BX002046, CX001532) and AstraZeneca. J.G.R. reports funding from Merck, the Office of Research on Women’s Health (HD057022), and the American Diabetes Association.
Glossary
Abbreviations
- ACEi
angiotensin-converting enzyme inhibitor
- AGE
advanced glycation end product
- ARB
angiotensin II receptor blocker
- BH4
tetrahydrobiopterin
- BMI
body mass index
- CHF
congestive heart failure
- CKD
chronic kidney disease
- CV
cardiovascular
- CVD
cardiovascular disease
- CRF
cardiorespiratory fitness
- DM
diabetes mellitus
- eNOS
endothelial nitric oxide synthase
- GLP-1
glucagon-like peptide 1 receptor agonists
- NO
nitric oxide
- NOS
nitric oxide synthase
- RAAS
renin–angiotensin activating system
- T2D
type 2 diabetes
- VO2max
maximal oxygen consumption
Contributor Information
Layla A Abushamat, Email: layla.abushamat@cuanschutz.edu.
Jane E B Reusch, Email: jane.reusch@cuanschutz.edu.
Additional Information
Disclosure Summary: J.E.B.R. and J.G.R. disclose AstraZeneca IIT and Merck IIT.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. CDC. National Diabetes Statistics Report. Atlanta, GA: Centers for Disease Control and Prevention; 2020. [Google Scholar]
- 2. Xu G, Liu B, Sun Y, et al. . Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: population based study. BMJ. 2018;362:k1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia. 2001;44(Suppl (2):S14-S21. [DOI] [PubMed] [Google Scholar]
- 4. Church TS, Cheng YJ, Earnest CP, et al. . Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes Care. 2004;27(1):83-88. [DOI] [PubMed] [Google Scholar]
- 5. Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med. 2000;132(8):605-611. [DOI] [PubMed] [Google Scholar]
- 6. Lyerly GW, Sui X, Lavie CJ, Church TS, Hand GA, Blair SN. The association between cardiorespiratory fitness and risk of all-cause mortality among women with impaired fasting glucose or undiagnosed diabetes mellitus. Mayo Clin Proc. 2009;84(9):780-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346(11):793-801. [DOI] [PubMed] [Google Scholar]
- 8. Shah RV, Murthy VL, Colangelo LA, et al. . Association of fitness in young adulthood with survival and cardiovascular risk: the coronary artery risk development in young adults (CARDIA) study. JAMA Intern Med. 2016;176(1):87-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farrell SW, Finley CE, Jackson AW, Vega GL, Morrow JR Jr. Association of multiple adiposity exposures and cardiorespiratory fitness with all-cause mortality in men: the Cooper Center Longitudinal Study. Mayo Clin Proc. 2014;89(6):772-780. [DOI] [PubMed] [Google Scholar]
- 10. Seyoum B, Estacio RO, Berhanu P, Schrier RW. Exercise capacity is a predictor of cardiovascular events in patients with type 2 diabetes mellitus. Diab Vasc Dis Res. 2006;3(3):197-201. [DOI] [PubMed] [Google Scholar]
- 11. Booth FW, Gordon SE, Carlson CJ, Hamilton MT. Waging war on modern chronic diseases: primary prevention through exercise biology. J Appl Physiol (1985). 2000;88(2):774-787. [DOI] [PubMed] [Google Scholar]
- 12. Booth FW, Laye MJ, Roberts MD. Lifetime sedentary living accelerates some aspects of secondary aging. J Appl Physiol (1985). 2011;111(5):1497-1504. [DOI] [PubMed] [Google Scholar]
- 13. Komatsu WR, Gabbay MA, Castro ML, et al. . Aerobic exercise capacity in normal adolescents and those with type 1 diabetes mellitus. Pediatr Diabetes. 2005;6(3):145-149. [DOI] [PubMed] [Google Scholar]
- 14. Nadeau KJ, Regensteiner JG, Bauer TA, et al. . Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95(2):513-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Regensteiner JG, Sippel J, McFarling ET, Wolfel EE, Hiatt WR. Effects of non-insulin-dependent diabetes on oxygen consumption during treadmill exercise. Med Sci Sports Exerc. 1995;27(5):661-667. [PubMed] [Google Scholar]
- 16. Regensteiner JG, Bauer TA, Reusch JE, et al. . Abnormal oxygen uptake kinetic responses in women with type II diabetes mellitus. J Appl Physiol (1985). 1998;85(1):310-317. [DOI] [PubMed] [Google Scholar]
- 17. Brandenburg SL, Reusch JE, Bauer TA, Jeffers BW, Hiatt WR, Regensteiner JG. Effects of exercise training on oxygen uptake kinetic responses in women with type 2 diabetes. Diabetes Care. 1999;22(10):1640-1646. [DOI] [PubMed] [Google Scholar]
- 18. Nadeau KJ, Zeitler PS, Bauer TA, et al. . Insulin resistance in adolescents with type 2 diabetes is associated with impaired exercise capacity. J Clin Endocrinol Metab. 2009;94(10):3687-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bjornstad P, Truong U, Dorosz JL, et al. . Cardiopulmonary dysfunction and adiponectin in adolescents with type 2 diabetes. J Am Heart Assoc. 2016;5(3):e002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Regensteiner JG, Bauer TA, Reusch JE, et al. . Cardiac dysfunction during exercise in uncomplicated type 2 diabetes. Med Sci Sports Exerc. 2009;41(5):977-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huebschmann AG, Reis EN, Emsermann C, et al. . Women with type 2 diabetes perceive harder effort during exercise than nondiabetic women. Appl Physiol Nutr Metab. 2009;34(5):851-857. [DOI] [PubMed] [Google Scholar]
- 22. Regensteiner JG, Bauer TA, Reusch JE. Rosiglitazone improves exercise capacity in individuals with type 2 diabetes. Diabetes Care. 2005;28(12):2877-2883. [DOI] [PubMed] [Google Scholar]
- 23. Kadoglou NP, Iliadis F, Angelopoulou N, Perrea D, Liapis CD, Alevizos M. Beneficial effects of rosiglitazone on novel cardiovascular risk factors in patients with type 2 diabetes mellitus. Diabet Med. 2008;25(3):333-340. [DOI] [PubMed] [Google Scholar]
- 24. Kadoglou NP, Iliadis F, Sailer N, et al. . Exercise training ameliorates the effects of rosiglitazone on traditional and novel cardiovascular risk factors in patients with type 2 diabetes mellitus. Metabolism. 2010;59(4):599-607. [DOI] [PubMed] [Google Scholar]
- 25. Goodpaster BH, Sparks LM. Metabolic flexibility in health and disease. Cell Metab. 2017;25(5):1027-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mason McClatchey P, Bauer TA, Regensteiner JG, Schauer IE, Huebschmann AG, Reusch JEB. Dissociation of local and global skeletal muscle oxygen transport metrics in type 2 diabetes. J Diabetes Complications. 2017;31(8):1311-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab. 2011;301(2):E252-E263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu Z, Liu J, Jahn LA, Fowler DE, Barrett EJ. Infusing lipid raises plasma free fatty acids and induces insulin resistance in muscle microvasculature. J Clin Endocrinol Metab. 2009;94(9):3543-3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kusters YH, Barrett EJ. Muscle microvasculature’s structural and functional specializations facilitate muscle metabolism. Am J Physiol Endocrinol Metab. 2016;310(6):E379-E387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baron AD, Tarshoby M, Hook G, et al. . Interaction between insulin sensitivity and muscle perfusion on glucose uptake in human skeletal muscle: evidence for capillary recruitment. Diabetes. 2000;49(5):768-774. [DOI] [PubMed] [Google Scholar]
- 31. Clerk LH, Vincent MA, Barrett EJ, Lankford MF, Lindner JR. Skeletal muscle capillary responses to insulin are abnormal in late-stage diabetes and are restored by angiotensin-converting enzyme inhibition. Am J Physiol Endocrinol Metab. 2007;293(6):E1804-E1809. [DOI] [PubMed] [Google Scholar]
- 32. Keske MA, Premilovac D, Bradley EA, Dwyer RM, Richards SM, Rattigan S. Muscle microvascular blood flow responses in insulin resistance and ageing. J Physiol. 2016;594(8):2223-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McClatchey PM, Williams IM, Xu Z, et al. . Perfusion controls muscle glucose uptake by altering the rate of glucose dispersion in vivo. Am J Physiol Endocrinol Metab. 2019;317(6):E1022-E1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McClatchey PM, Frisbee JC, Reusch JEB. A conceptual framework for predicting and addressing the consequences of disease-related microvascular dysfunction. Microcirculation. 2017;24(6):e12359. doi: 10.1111/micc.12359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang N, Tan AWK, Jahn LA, et al. . Vasodilatory actions of glucagon-like peptide 1 are preserved in skeletal and cardiac muscle microvasculature but not in conduit artery in obese humans with vascular insulin resistance. Diabetes Care. 2020;43(3):634-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang N, Alvin T, Jahn LA, et al. . Glucagon-like peptide 1’s vasodilatory actions are preserved in skeletal and cardiac muscle microvasculature but not in conduit artery in obese humans with vascular insulin resistance [Published online ahead of December 30, 2019]. Diabetes Care. 2019. doi: 10.2337/dc19-1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chai W, Wang W, Liu J, et al. . Angiotensin II type 1 and type 2 receptors regulate basal skeletal muscle microvascular volume and glucose use. Hypertension. 2010;55(2):523-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chai W, Dong Z, Wang N, et al. . Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes. 2012;61(4):888-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chai W, Fu Z, Aylor KW, Barrett EJ, Liu Z. Liraglutide prevents microvascular insulin resistance and preserves muscle capillary density in high-fat diet-fed rats. Am J Physiol Endocrinol Metab. 2016;311(3):E640-E648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simoneau JA, Kelley DE. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. J Appl Physiol (1985). 1997;83(1):166-171. [DOI] [PubMed] [Google Scholar]
- 41. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350(7):664-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patti ME. Gene expression in humans with diabetes and prediabetes: what have we learned about diabetes pathophysiology? Curr Opin Clin Nutr Metab Care. 2004;7(4):383-390. [DOI] [PubMed] [Google Scholar]
- 43. Patti ME, Butte AJ, Crunkhorn S, et al. . Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100(14):8466-8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mootha VK, Lindgren CM, Eriksson KF, et al. . PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267-273. [DOI] [PubMed] [Google Scholar]
- 45. Dresner A, Laurent D, Marcucci M, et al. . Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103(2):253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944-2950. [DOI] [PubMed] [Google Scholar]
- 47. Cree-Green M, Gupta A, Coe GV, et al. . Insulin resistance in type 2 diabetes youth relates to serum free fatty acids and muscle mitochondrial dysfunction. J Diabetes Complications. 2017;31(1):141-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barrett EJ, Eggleston EM, Inyard AC, et al. . The vascular actions of insulin control its delivery to muscle and regulate the rate-limiting step in skeletal muscle insulin action. Diabetologia. 2009;52(5):752-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bugger H, Abel ED. Mitochondria in the diabetic heart. Cardiovasc Res. 2010;88(2):229-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Diamant M, Lamb HJ, Groeneveld Y, et al. . Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol. 2003;42(2):328-335. [DOI] [PubMed] [Google Scholar]
- 51. Casademont J, Miró O. Electron transport chain defects in heart failure. Heart Fail Rev. 2002;7(2):131-139. [DOI] [PubMed] [Google Scholar]
- 52. Kadkhodayan A, Lin CH, Coggan AR, et al. . Sex affects myocardial blood flow and fatty acid substrate metabolism in humans with nonischemic heart failure. J Nucl Cardiol. 2017;24(4):1226-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Peterson LR, Herrero P, Coggan AR, et al. . Type 2 diabetes, obesity, and sex difference affect the fate of glucose in the human heart. Am J Physiol Heart Circ Physiol. 2015;308(12):H1510-H1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Neubauer S, Horn M, Cramer M, et al. . Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96(7):2190-2196. [DOI] [PubMed] [Google Scholar]
- 55. Vega RB, Konhilas JP, Kelly DP, Leinwand LA. Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metab. 2017;25(5):1012-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang H, Wang AX, Liu Z, Chai W, Barrett EJ. The trafficking/interaction of eNOS and caveolin-1 induced by insulin modulates endothelial nitric oxide production. Mol Endocrinol. 2009;23(10):1613-1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Davidson SM. Endothelial mitochondria and heart disease. Cardiovasc Res. 2010;88(1):58-66. [DOI] [PubMed] [Google Scholar]
- 58. Scherrer U, Randin D, Vollenweider P, Vollenweider L, Nicod P. Nitric oxide release accounts for insulin’s vascular effects in humans. J Clin Invest. 1994;94(6):2511-2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179). J Biol Chem. 2001;276(32):30392-30398. [DOI] [PubMed] [Google Scholar]
- 60. Xing W, Li Y, Zhang H, et al. . Improvement of vascular insulin sensitivity by downregulation of GRK2 mediates exercise-induced alleviation of hypertension in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2013;305(8):H1111-H1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lim HS, MacFadyen RJ, Lip GY. Diabetes mellitus, the renin-angiotensin-aldosterone system, and the heart. Arch Intern Med. 2004;164(16):1737-1748. [DOI] [PubMed] [Google Scholar]
- 62. Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep. 2014;14(1):453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res. 2010;106(5):842-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huebschmann AG, Regensteiner JG, Vlassara H, Reusch JE. Diabetes and advanced glycoxidation end products. Diabetes Care. 2006;29(6):1420-1432. [DOI] [PubMed] [Google Scholar]
- 65. Avogaro A, Fadini GP, Gallo A, Pagnin E, de Kreutzenberg S. Endothelial dysfunction in type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2006;16(Suppl (1):S39-S45. [DOI] [PubMed] [Google Scholar]
- 66. Joseph JJ, Echouffo Tcheugui JB, Effoe VS, Hsueh WA, Allison MA, Golden SH. Renin-angiotensin-aldosterone system, glucose metabolism and incident type 2 diabetes mellitus: MESA. J Am Heart Assoc. 2018;7(17):e009890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Miyata T, van Ypersele de Strihou C, Ueda Y, et al. . Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: biochemical mechanisms. J Am Soc Nephrol. 2002;13(10):2478-2487. [DOI] [PubMed] [Google Scholar]
- 68. Matsui T, Nishino Y, Maeda S, Takeuchi M, Yamagishi S. Irbesartan inhibits advanced glycation end product (AGE)-induced up-regulation of vascular cell adhesion molecule-1 (VCAM-1) mRNA levels in glomerular endothelial cells. Microvasc Res. 2011;81(3):269-273. [DOI] [PubMed] [Google Scholar]
- 69. Yamagishi S, Matsui T, Nakamura K, et al. . Olmesartan blocks inflammatory reactions in endothelial cells evoked by advanced glycation end products by suppressing generation of reactive oxygen species. Ophthalmic Res. 2008;40(1):10-15. [DOI] [PubMed] [Google Scholar]
- 70. Heart Outcomes Prevention Evaluation (HOPE) Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000;355(9200):253-259. [PubMed] [Google Scholar]
- 71. Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P; Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med. 2001;345(12):870-878. [DOI] [PubMed] [Google Scholar]
- 72. Persson F, Rossing P, Hovind P, et al. . Endothelial dysfunction and inflammation predict development of diabetic nephropathy in the Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria (IRMA 2) study. Scand J Clin Lab Invest. 2008;68(8):731-738. [DOI] [PubMed] [Google Scholar]
- 73. Persson F, Rossing P, Hovind P, et al. . Irbesartan treatment reduces biomarkers of inflammatory activity in patients with type 2 diabetes and microalbuminuria: an IRMA 2 substudy. Diabetes. 2006;55(12):3550-3555. [DOI] [PubMed] [Google Scholar]
- 74. Shah AS, El Ghormli L, Gidding SS, et al. . Prevalence of arterial stiffness in adolescents with type 2 diabetes in the TODAY cohort: relationships to glycemic control and other risk factors. J Diabetes Complications. 2018;32(8):740-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Starritt EC, Angus D, Hargreaves M. Effect of short-term training on mitochondrial ATP production rate in human skeletal muscle. J Appl Physiol (1985). 1999;86(2):450-454. [DOI] [PubMed] [Google Scholar]
- 76. Spina RJ, Chi MM, Hopkins MG, Nemeth PM, Lowry OH, Holloszy JO. Mitochondrial enzymes increase in muscle in response to 7-10 days of cycle exercise. J Appl Physiol (1985). 1996;80(6):2250-2254. [DOI] [PubMed] [Google Scholar]
- 77. Bishop DJ, Granata C, Eynon N. Can we optimise the exercise training prescription to maximise improvements in mitochondria function and content? Biochim Biophys Acta. 2014;1840(4):1266-1275. [DOI] [PubMed] [Google Scholar]
- 78. Knaub LA, McCune S, Chicco AJ, et al. . Impaired response to exercise intervention in the vasculature in metabolic syndrome. Diab Vasc Dis Res. 2013;10(3):222-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nisoli E, Clementi E, Paolucci C, et al. . Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299(5608):896-899. [DOI] [PubMed] [Google Scholar]
- 80. Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci. 2006;119(Pt 14):2855-2862. [DOI] [PubMed] [Google Scholar]
- 81. Nisoli E, Tonello C, Cardile A, et al. . Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310(5746):314-317. [DOI] [PubMed] [Google Scholar]
- 82. Abudukadier A, Fujita Y, Obara A, et al. . Tetrahydrobiopterin has a glucose-lowering effect by suppressing hepatic gluconeogenesis in an endothelial nitric oxide synthase-dependent manner in diabetic mice. Diabetes. 2013;62(9):3033-3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Keller AC, Knaub LA, Miller MW, Birdsey N, Klemm DJ, Reusch JE. Saxagliptin restores vascular mitochondrial exercise response in the Goto-Kakizaki rat. J Cardiovasc Pharmacol. 2015;65(2):137-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Miller MW, Knaub LA, Olivera-Fragoso LF, et al. . Nitric oxide regulates vascular adaptive mitochondrial dynamics. Am J Physiol Heart Circ Physiol. 2013;304(12):H1624-H1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Scalzo RL, Knaub LA, Hull SE, et al. . Glucagon-like peptide-1 receptor antagonism impairs basal exercise capacity and vascular adaptation to aerobic exercise training in rats. Physiol Rep. 2018;6(13):e13754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wadley GD, Choate J, McConell GK. NOS isoform-specific regulation of basal but not exercise-induced mitochondrial biogenesis in mouse skeletal muscle. J Physiol. 2007;585(Pt 1):253-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Keller AC, Knaub LA, Scalzo RL, et al. . Sepiapterin improves vascular reactivity and insulin-stimulated glucose in Wistar rats. Oxid Med Cell Longev. 2018;2018:7363485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117(18):2340-2350. [DOI] [PubMed] [Google Scholar]
- 89. Scalzo RL, Rafferty D, Schauer I, et al. . Sitagliptin improves diastolic cardiac function but not cardiorespiratory fitness in adults with type 2 diabetes. J Diabetes Complications. 2019;33(8):561-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Scalzo RL, Moreau KL, Ozemek C, et al. . Exenatide improves diastolic function and attenuates arterial stiffness but does not alter exercise capacity in individuals with type 2 diabetes. J Diabetes Complications. 2017;31(2):449-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wascher TC, Graier WF, Dittrich P, et al. . Effects of low-dose L-arginine on insulin-mediated vasodilatation and insulin sensitivity. Eur J Clin Invest. 1997;27(8):690-695. [DOI] [PubMed] [Google Scholar]
- 92. Regensteiner JG, Popylisen S, Bauer TA, et al. . Oral L-arginine and vitamins E and C improve endothelial function in women with type 2 diabetes. Vasc Med. 2003;8(3):169-175. [DOI] [PubMed] [Google Scholar]
- 93. Crabtree MJ, Channon KM. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide. 2011;25(2):81-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sjøberg KA, Frøsig C, Kjøbsted R, et al. . Exercise increases human skeletal muscle insulin sensitivity via coordinated increases in microvascular perfusion and molecular signaling. Diabetes. 2017;66(6):1501-1510. [DOI] [PubMed] [Google Scholar]
- 95. Bauer TA, Reusch JE, Levi M, Regensteiner JG. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care. 2007;30(11):2880-2885. [DOI] [PubMed] [Google Scholar]
- 96. Mason McClatchey P, Wu F, Olfert IM, et al. . Impaired tissue oxygenation in metabolic syndrome requires increased microvascular perfusion heterogeneity. J Cardiovasc Transl Res. 2017;10(1):69-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cree-Green M, Scalzo RL, Harrall K, et al. . Supplemental oxygen improves in vivo mitochondrial oxidative phosphorylation flux in sedentary obese adults with type 2 diabetes. Diabetes. 2018;67(7):1369-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454(3):345-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kearney K, Tomlinson D, Smith K, Ajjan R. Hypofibrinolysis in diabetes: a therapeutic target for the reduction of cardiovascular risk. Cardiovasc Diabetol. 2017;16(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Brandenburg SL, Reusch JE, Felder KK, et al. . Impaired fibrinolysis in premenopausal women and age-matched men with Type 2 diabetes mellitus: a pilot study. J Investig Med. 2002;50(2):110-115. [DOI] [PubMed] [Google Scholar]
- 101. Bryk AH, Prior SM, Plens K, et al. . Predictors of neutrophil extracellular traps markers in type 2 diabetes mellitus: associations with a prothrombotic state and hypofibrinolysis. Cardiovasc Diabetol. 2019;18(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Prior SJ, Goldberg AP, Ortmeyer HK, et al. . Increased skeletal muscle capillarization independently enhances insulin sensitivity in older adults after exercise training and detraining. Diabetes. 2015;64(10):3386-3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Prior SJ, Blumenthal JB, Katzel LI, Goldberg AP, Ryan AS. Increased skeletal muscle capillarization after aerobic exercise training and weight loss improves insulin sensitivity in adults with IGT. Diabetes Care. 2014;37(5):1469-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. McClatchey PM, Schafer M, Hunter KS, Reusch JE. The endothelial glycocalyx promotes homogenous blood flow distribution within the microvasculature. Am J Physiol Heart Circ Physiol. 2016;311(1):H168-H176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Stout R. Diabetes, atherosclerosis and aging. Diabetes Care. 1990;13:20-23. [Google Scholar]
- 106. Regensteiner JG, Bauer TA, Huebschmann AG, et al. . Sex differences in the effects of type 2 diabetes on exercise performance. Med Sci Sports Exerc. 2015;47(1):58-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. Jama. 1979;241(19):2035-2038. [DOI] [PubMed] [Google Scholar]
- 108. Regensteiner JG, Golden S, Huebschmann AG, et al. ; American Heart Association Diabetes Committee of the Council on Lifestyle and Cardiometabolic Health, Council on Epidemiology and Prevention, Council on Functional Genomics and Translational Biology, and Council on Hypertension Sex differences in the cardiovascular consequences of diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2015;132(25):2424-2447. [DOI] [PubMed] [Google Scholar]
- 109. Prospective Studies Collaboration, Asia Pacific Cohort Studies Collaboration. Sex-specific relevance of diabetes to occlusive vascular and other mortality: a collaborative meta-analysis of individual data from 980 793 adults from 68 prospective studies. Lancet Diabetes Endocrinol. 2018;6(7):538-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kalyani RR, Lazo M, Ouyang P, et al. . Sex differences in diabetes and risk of incident coronary artery disease in healthy young and middle-aged adults. Diabetes Care. 2014;37(3):830-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Huebschmann AG, Huxley RR, Kohrt WM, Zeitler P, Regensteiner JG, Reusch JEB. Sex differences in the burden of type 2 diabetes and cardiovascular risk across the life course. Diabetologia. 2019;62(10):1761-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kanaya AM, Grady D, Barrett-Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med. 2002;162(15):1737-1745. [DOI] [PubMed] [Google Scholar]
- 113. Wenger NK. Coronary heart disease in women: highlights of the past 2 years–stepping stones, milestones and obstructing boulders. Nat Clin Pract Cardiovasc Med. 2006;3(4):194-202. [DOI] [PubMed] [Google Scholar]
- 114. Sattar N. Gender aspects in type 2 diabetes mellitus and cardiometabolic risk. Best Pract Res Clin Endocrinol Metab. 2013;27(4):501-507. [DOI] [PubMed] [Google Scholar]
- 115. Fang ZY, Sharman J, Prins JB, Marwick TH. Determinants of exercise capacity in patients with type 2 diabetes. Diabetes Care. 2005;28(7):1643-1648. [DOI] [PubMed] [Google Scholar]
- 116. Scholes S, Bann D. Education-related disparities in reported physical activity during leisure-time, active transportation, and work among US adults: repeated cross-sectional analysis from the National Health and Nutrition Examination Surveys, 2007 to 2016. BMC Public Health. 2018;18(1):926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Sisson SB, Camhi SM, Church TS, et al. . Leisure time sedentary behavior, occupational/domestic physical activity, and metabolic syndrome in U.S. men and women. Metab Syndr Relat Disord. 2009;7(6):529-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Barrett JE, Plotnikoff RC, Courneya KS, Raine KD. Physical activity and type 2 diabetes: exploring the role of gender and income. Diabetes Educ. 2007;33(1):128-143. [DOI] [PubMed] [Google Scholar]
- 119. Edwards ES, Sackett SC. Psychosocial variables related to why women are less active than men and related health implications. Clin Med Insights Womens Health. 2016;9(Suppl 1):47-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Huebschmann AG, Kohrt WM, Herlache L, et al. . Type 2 diabetes exaggerates exercise effort and impairs exercise performance in older women. BMJ Open Diabetes Res Care. 2015;3(1):e000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ha JW, Lee HC, Park S, et al. . Gender-related difference in left ventricular diastolic elastance during exercise in patients with diabetes mellitus. Circ J. 2008;72(9):1443-1448. [DOI] [PubMed] [Google Scholar]
- 122. Boulé NG, Weisnagel SJ, Lakka TA, et al. ; HERITAGE Family Study Effects of exercise training on glucose homeostasis: the HERITAGE Family Study. Diabetes Care. 2005;28(1):108-114. [DOI] [PubMed] [Google Scholar]
- 123. Ross R, Goodpaster BH, Koch LG, et al. . Precision exercise medicine: understanding exercise response variability. Br J Sports Med. 2019;53(18):1141-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Bouchard C, An P, Rice T, et al. . Familial aggregation of VO(2max) response to exercise training: results from the HERITAGE Family Study. J Appl Physiol (1985). 1999;87(3):1003-1008. [DOI] [PubMed] [Google Scholar]
- 125. Pandey A, Johnson JL, Slentz CA, et al. . Short-term changes in cardiorespiratory fitness in response to exercise training and the association with long-term cardiorespiratory fitness decline: the STRRIDE Reunion Study. J Am Heart Assoc. 2019;8(20):e012876. [DOI] [PMC free article] [PubMed] [Google Scholar]