Abstract
Background
Perform a systematic review and meta-analysis of SARS-CoV-2 infection and pregnancy.
Methods
Databases (Medline, Embase, Clinicaltrials.gov, Cochrane Library) were searched electronically on 6th April and updated regularly until 8th June 2020. Reports of pregnant women with reverse transcription PCR (RT-PCR) confirmed COVID-19 were included. Meta-analytical proportion summaries and meta-regression analyses for key clinical outcomes are provided.
Findings
86 studies were included, 17 studies (2567 pregnancies) in the quantitative synthesis; other small case series and case reports were used to extract rarely-reported events and outcome. Most women (73.9%) were in the third trimester; 52.4% have delivered, half by caesarean section (48.3%). The proportion of Black, Asian or minority ethnic group membership (50.8%); obesity (38.2%), and chronic co-morbidities (32.5%) were high. The most commonly reported clinical symptoms were fever (63.3%), cough (71.4%) and dyspnoea (34.4%). The commonest laboratory abnormalities were raised CRP or procalcitonin (54.0%), lymphopenia (34.2%) and elevated transaminases (16.0%). Preterm birth before 37 weeks’ gestation was common (21.8%), usually medically-indicated (18.4%). Maternal intensive care unit admission was required in 7.0%, with intubation in 3.4%. Maternal mortality was uncommon (~1%). Maternal intensive care admission was higher in cohorts with higher rates of co-morbidities (beta=0.007, p<0.05) and maternal age over 35 years (beta=0.007, p<0.01). Maternal mortality was higher in cohorts with higher rates of antiviral drug use (beta=0.03, p<0.001), likely due to residual confounding. Neonatal nasopharyngeal swab RT-PCR was positive in 1.4%.
Interpretation
The risk of iatrogenic preterm birth and caesarean delivery was increased. The available evidence is reassuring, suggesting that maternal morbidity is similar to that of women of reproductive age. Vertical transmission of the virus probably occurs, albeit in a small proportion of cases.
Funding
N/A
Research in context.
Evidence before this study
Large studies of pregnancy outcomes following SARS-CoV-2 infection in pregnancy are lacking. These studies are unlikely to be available in the near future, as each centre has small numbers of confirmed infections in pregnancy and networks will report only in the fullness of time. A recent systematic review reported an aggregate summary of all coronavirus-related illnesses, concluding that the risks of miscarriage, pre-eclampsia, preterm birth, and perinatal death are higher; however, similar pregnancy outcomes across the coronavirus family were assumed. Other systematic reviews have reported data from less than 500 pregnancies and lacked methodological robustness.
Added value of this study
In this systematic review, we searched multiple databases (Medline, Embase, Clinicaltrials.gov, Cochrane Library) to 8th June 2020. We summarized the available evidence on the clinical features, laboratory and radiological findings, as well as the pregnancy and neonatal outcomes; 86 studies were included, 17 studies (2567 pregnancies) in the quantitative synthesis; other small case series and case reports were used to extract rarely-reported events and outcomes. Most women (73.9%) were in the third trimester; 52.4% have delivered, half by caesarean section (48.3%). The proportion of Black, Asian or minority ethnic group membership (50.8%); obesity (38.2%), and chronic co-morbidities (32.5%) were high. The most commonly reported clinical symptoms were fever (63.3%), cough (71.4%) and dyspnoea (34.4%). The commonest laboratory abnormalities were raised CRP or procalcitonin (54.0%), lymphopenia (34.2%) and elevated transaminases (16.0%). Preterm birth before 37 weeks’ gestation was common (21.8%), usually medically-indicated (18.4%). Maternal intensive care unit admission was required in 7.0%, with intubation in 3.4%. Maternal mortality was uncommon (~1%). Maternal intensive care admission was higher in cohorts with higher rates of co-morbidities and maternal age over 35 years. Neonatal nasopharyngeal swab RT-PCR was positive in 1.4%.
Implications of all the available evidence
This meta-analysis includes the largest number of pregnancies with SARS-CoV-2 reported to date. The study has raised concerns about heightened risks of iatrogenic preterm birth, Caesarean delivery, and possibly, transmission to the newborn before or after birth, while providing reassuring evidence that women and babies are not at increased risk of serious illness. These data are important to inform counselling of women during the pandemic, and encourage them to seek both antenatal care, routine or related to specific problems. While further information about the direct and indirect implications of the pandemic will become available from SARS-CoV-2 networks in the future and may inform care in future pandemics, the data presented here are needed to inform current care throughout the current pandemic and future waves.
Alt-text: Unlabelled box
1. Introduction
The outbreak of a novel coronavirus (SARS-CoV-2 which causes COVID-19) was first reported in Wuhan, China in December 2019, although it is likely that cases appeared in mid-November. Within a few weeks, the virus had spread rapidly throughout China, and within a month, worldwide. On the 11th of March 2020, this SARS-CoV-2 outbreak was recognised as a pandemic by the World Health Organization (WHO) [1]. As of the 1st June 2020, over 6 million cases have been diagnosed in 210 countries, with a total of 379,728 deaths.
Previous smaller coronavirus outbreaks (i.e., Severe Acute Respiratory Syndrome [SARS] and Middle East Respiratory Syndrome [MERS] [2], [3], [4]) have associated infection in pregnancy with more serious illness and preterm birth, although information has been primarily from small observational studies and case series [2], [3], [4]. Concern about the implications of COVID-19 in pregnancy is further fuelled by its reproductive capacity, the scale of the outbreak, and the societal response.
A recent systematic review has reported an aggregate summary of all coronavirus-related illnesses (SARS and MERS included) and concluded that the risk of miscarriage, preeclampsia, preterm birth, and perinatal death is higher [5]. However, this review included few data for outcomes related to SARS-CoV-2 and emphasised the rapid rate of accumulating evidence and the need for updates.
We aimed to systematically review the available literature on COVID-19 and pregnancy, to provide comprehensive data to care-providers, pregnant women and their families, inform national and international guidelines, and direct the course of ongoing and future studies [6,7].
2. Methods
This review was performed according to established methodology for systematic reviews and meta-analysis. Medline, Embase, Clinicaltrials.gov and Cochrane Library databases were searched electronically (April 6th 2020) and updated on April 13th 2020 and May 7th 2020, utilising combinations of the relevant medical subject heading (MeSH) terms, key words, and word variants for “COVID-19” and “pregnancy” (Supplementary Table 1). After May 7th 2020, the literature update was performed manually on a weekly basis until June 8st 2020. Grey literature was not searched as double-reporting could not be ascertained. Reference lists of relevant articles and reviews were hand-searched for additional reports. PRISMA and MOOSE guidelines were followed [8,9]. The study was registered with the PROSPERO database (Registration number: CRD42020178004).
2.1. Study selection and data collection
Maternal outcomes included the clinical COVID-19 presentation and associated laboratory findings, maternal complications, and treatment received. Obstetric complications included preterm birth, stillbirth, mode of delivery and fetal distress. Perinatal outcomes included perinatal death and evidence of vertical transmission. Any outcome not explicitly mentioned was considered not to have been reported. For laboratory parameters, reported local reference ranges were used to define abnormal.
Case reports, case series, cohort, case-control studies and randomised controlled trials were eligible for inclusion. Studies that reported on women with PCR confirmed SARS-CoV-2 infection were eligible for inclusion. Clinically diagnosed cases, conference abstracts, expert opinions, and critical appraisals were excluded. Two authors (EK, CB) reviewed all abstracts independently. Agreement regarding potential relevance was reached by consensus (AK, EK, CB); full text copies of those papers were obtained and the same two reviewers independently extracted relevant data regarding study characteristics and maternal and perinatal outcomes. Inconsistencies were discussed by the reviewers and consensus reached or by discussion with a third author (AK). If more than one study was published on the same patients with identical endpoints, the report containing the most comprehensive (larger patient numbers) information was included. Individual cases were cross-checked with reports from the same hospital, whenever possible. If there was suspicion that a case report or series was subject to double-reporting, and this could not be confirmed, the publication was excluded; this included aggregate reports from multiple centres which previously published case series.
2.2. Quality assessment
Quality assessment of included studies was performed using the Newcastle-Ottawa Scale (NOS) or modified NOS for case-control, cohort studies or case series, [10,11] according to selection of the study groups (score 0-4), comparability of the groups (score 0-2), and ascertainment of outcomes (score 0-3). Two authors (EK, CB) scored all studies independently. Agreement was reached by consensus and a third author was involved (AK) in disputed cases.
2.3. Statistical Analysis
For women infected with SARS-CoV-2 in pregnancy, demographic and disease characteristics were analysed descriptively. A random-effect meta-analysis of proportions was used to estimate the pooled rates of each outcome in all pregnancies, and a random-intercept logistic regression meta-analysis with continuity correction used for outcomes where there was at least one event in available studies. Proportion meta-analysis was performed for studies (sample size >15) that reported on all available cases over a defined time period. A narrative synthesis was undertaken when few events were reported from small case series. Between-study heterogeneity was explored using the I2 statistic, with >50% reflecting substantial heterogeneity. In some studies reporting only summary statistics (mean and standard deviation or median and interquartile range), Monte-Carlo simulations were employed to estimate the proportion of the outcome according to the desired cut-off. Normality was assumed if the data were reported as mean and standard deviation. Normality was assumed for data reported as median and interquartile range only when the distribution was symmetrical. Meta-regression analyses were conducted for a few key clinical outcomes (maternal intensive care unit admission, maternal death, perinatal death, neonatal PCR positivity), if meta-analytical summaries indicated significant heterogeneity. All potential confounders were tested and those that explain the heterogeneity (p value for beta <0.05 and p value for residual heterogeneity >0.05) were reported. Potential publication bias was assessed using Egger's test and the creation of funnel plots for visual inspection when and if enough studies (N>10) were available.[11] Analyses were conducted using R for statistical computing software and meta package.
3. Results
3.1. General characteristics
Of 283 articles identified, 114 full manuscripts were assessed further for eligibility and 86 studies were included (Figure 1, Supplementary Table 2) [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93]. Of those, 17 studies (2567 pregnancies) that report on all available cases over a definite time period and sufficiently large sample size (N>15) were included in the quantitative synthesis [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28]. Other small case series and case reports were used to extract rarely reported events and outcome such as maternal or neonatal mortality, evidence for vertical transmission or placental pathologies [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79], [80], [81], [82], [83], [84], [85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97]. Quality was moderate to high for the included studies (Table 1). The main weaknesses were retrospective design and inconsistent definition and reporting of outcomes. There were five reports from national registries (UK, Netherlands, China, France and Brazil), while the rest were regional reports from other countries (Italy, USA, Spain) (Table 1). One study strictly included only women with severe and critical COVID-19 [20]. Uptake of antiviral treatments and the drug regimens used varied significantly among the countries (Table 1). The included outcomes from individual studies can be found in Table 1. Publication bias was assessed for outcome with more than 10 included studies (Supplementary Table 3). The funnel plot asymmetry tests suggested possible publication bias in maternal intensive care unit admission (bias -2.61, p=0.013) and maternal death (bias -2.85, p=0.001). Two reports from China had risk of overlap and the study with more comprehensive reporting or larger numbers was used for each outcome [16,17].
Fig. 1.
PRISMA 2009 flow diagram.
Table 1.
Summary and quality assessment of studies included in the quantitative synthesis.
| Author, year | Region/Country | Women with COVID-19 | Included outcomes | Severe COVID-19* | Proportion receiving antiviral treatment and used regimen (combinations of listed drugs) | Maternal mortality | Newcastle-Ottawa Scale score and/or overall judgement |
|---|---|---|---|---|---|---|---|
| Pereira A, 2020 | Madrid, Spain | 60 | Symptoms, maternal characteristics, laboratory parameters, pregnancy and neonatal outcomes | 2/60 | Hydroxychloroquine, lopinavir, ritonavir, darunavir, tocilizumab, cobicistat 35%, (21/60) | 0/60 | ⋆⋆ ⋆ ⋆/⋆⋆/⋆⋆⋆ High |
| Duffy C, 2020 | Boston, USA | 15 | Laboratory parameters | Not extractable | Not reported | Not reported | Moderate |
| Lokken E, 2020 | Washington, USA | 46 | Symptoms, maternal characteristics, laboratory parameters, pregnancy outcomes | 6/46 | Remdesivir, hydroxychloroquine, 6.5% (3/46) | 0/46 | High |
| Qadri F, 2020 | Michigan, USA | 16 | Maternal characteristics, laboratory parameters, pregnancy and neonatal outcomes | 0/16 | Remdesivir, hydroxychloroquine, 12.5% (2/16) | 0/16 | High |
| Yan J, 2020 | Within and outside of Hubei Province, China | 65 | Pregnancy and neonatal outcomes | 6/65 | Oseltamivir, ganciclovir, arbidol, peramivir, interferon, aciclovir, ribavirin, 9.2% (6/65) | 0/65 | High |
| Chen L, 2020 | Hubei Province, Wuhan, China | 84 | Symptoms, maternal characteristics | Not extractable | Not reported | 0/84 | High |
| Andrikopoulou M, 2020 | New York, USA | 158 | Maternal characteristics, laboratory parameters, maternal outcomes | Not extractable | Not reported | 0/158 | High |
| Knight M, 2020 | National, UK | 427 | Symptoms, maternal characteristics, pregnancy and neonatal outcomes | Not reported | Oseltamivir, lopinavir, ritonavir 2.1% (9/427) | 5/427 | ⋆ ⋆ ⋆/⋆⋆/⋆⋆⋆ High |
| Williams R, 2020 | New York, Philadelphia, New Jersey, Ohio, USA | 64 | Maternal characteristics, pregnancy and neonatal outcomes | 64/64 | Remdesivir, hydroxychloroquine, ~81% (52/64) | 0/64 | ⋆ ⋆ ⋆/⋆⋆/⋆⋆⋆ High |
| Savasi M, 2020 | Lombardi, Italy | 77 | Symptoms, maternal characteristics, laboratory parameters, pregnancy and neonatal outcomes | 14/77 | Lopinavir, ritonavir, remdesivir, darunavir, 32.5% (25/77) | 0/77 | ⋆ ⋆ ⋆/⋆⋆/⋆⋆ High |
| London V, 2020 | New York, USA | 68 | Symptoms, maternal characteristics, laboratory parameters, pregnancy and neonatal outcomes | Not extractable | Hydroxychloroquine, 20.6% (16/68) | 0/68 | ⋆ ⋆ ⋆/⋆⋆/⋆⋆ High |
| Goldfarb I, 2020 | Massachusetts, USA | 61 | Maternal characteristics, pregnancy and neonatal outcomes | Not reported | Not reported | 0/61 | ⋆ ⋆ ⋆/⋆⋆/⋆⋆ High |
| Kayer G, 2020 | National, France | 617 | Symptoms, maternal characteristics, pregnancy and neonatal outcomes | 128/617 | Not reported | 1/617 | ⋆⋆ ⋆ ⋆/⋆⋆/⋆⋆⋆ High |
| Romagano M,2020 | New Jersey, USA | 73 | Maternal outcomes | Not extractable | Not extractable | 0/73 | Moderate |
| Mendoza M, 2020 | Barcelona, Spain | 42 | Maternal characteristics, maternal outcomes | 8/42 | Not reported | 0/42 | ⋆ ⋆ ⋆/⋆⋆/⋆⋆ High |
| NethOSS registry, 2020 | National,Netherlands | 210 | Symptoms, pregnancy and neonatal outcomes | Not reported | Remdesivir, Oseltamivir, 1.4% (3/210) | 1/210 | High |
| Brazilian registry, 2020 | National, Brazil | 484 | Symptoms, maternal characteristics, maternal outcomes | Not reported | Not specified, 42% (121/288) | 36/484 | High |
PCR: polymerase chain reaction, COVID-19: coronavirus disease 2019.
*As defined by individual studies.
3.2. Clinical symptoms, laboratory and imaging findings
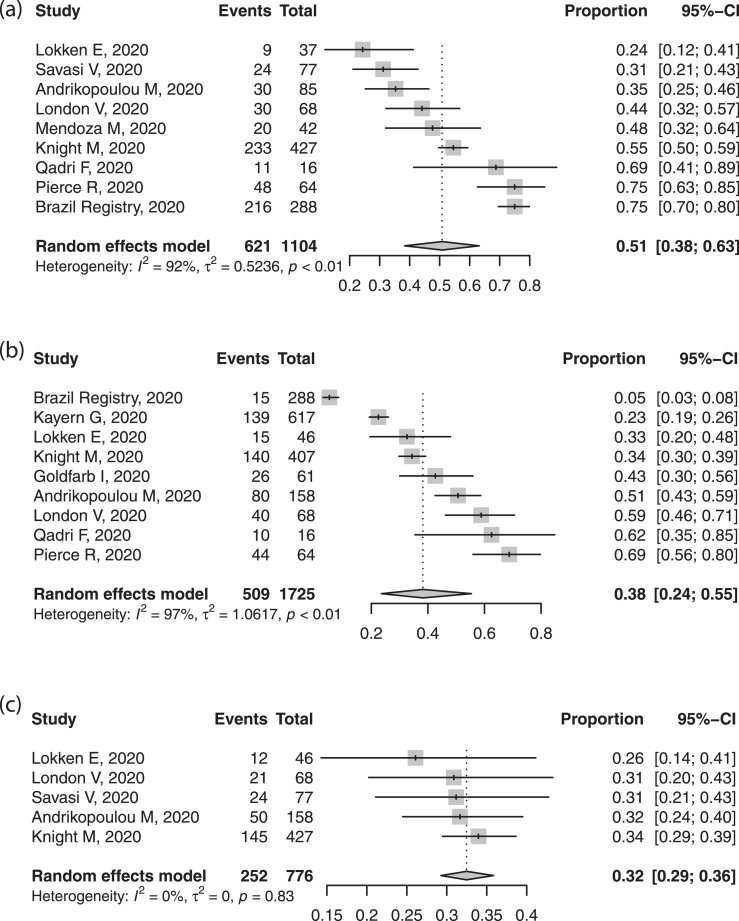
Table 2 presents pooled proportions for our primary analysis of 17 reports. Most women presented with fever and cough, although estimates varied substantially between reports. Some women had dyspnoea (≈35%), had the characteristic anosmia, or non-specific complaints of myalgia, fatigue, or headache (~15-20%). Less than one fifth were asymptomatic, although this too varied substantially between reports. Black, Asian or ethnic minorities (BAME) were common in the included reports (~50%). The rates of obesity (38.2%) and comorbidities were also high (32.5%) (Figure 2). Most women (~75%) were in the third trimester or postpartum at the time of reporting. Investigations revealed that most had raised inflammatory markers of C-reactive protein or procalcitonin, and around one third had lymphopenia; raised liver enzymes complicated 16.0%. Thrombocytopenia was rare. Raised D-Dimer levels were very common (~85%) but reports did not include pregnancy-specific normal ranges.
Table 2.
Pooled proportions of presenting symptoms, maternal characteristics, laboratory findings, treatments and clinical outcomes in pregnant women with reverse transcription polymerase chain reaction (RT-PCR) confirmed SARS-CoV-2 infection.
| Studies (N) | Cases (n/N) | Proportion (95% CI)* | I[2] | |
|---|---|---|---|---|
| Symptoms at presentation | ||||
| Asymptomatic | 10 | 253/1205 | 14.5% (5.6–32.5%) | 97.0% |
| Fever | 10 | 1292/1987 | 63.3% (54.7–71.2%) | 91.7% |
| Cough | 10 | 1391/1987 | 71.4% (66.2–76.2%) | 79.4% |
| Loss of taste or smell | 3 | 194/635 | 22.9% (11.6–40.1%) | 87.2% |
| Myalgia, limb or joint pain | 4 | 104/640 | 18.9% (9.7–33.5%) | 89.9% |
| Fatigue, tiredness | 3 | 101/545 | 18.5% (15.5–22.0%) | 0.0% |
| Headache | 4 | 92/640 | 15.0% (8.9–24.1%) | 78.4% |
| Shortness of breath | 9 | 789/1941 | 34.4% (25.7–44.4%) | 93.9% |
| Chest pain, tightness | 2 | 30/170 | 17.5% (9.8–29.2%) | 62.5% |
| Diarrhoea | 6 | 126/1621 | 7.4% (5.4–10.0%) | 58.8% |
| Maternal and pregnancy characteristics | ||||
| Maternal age >35 years | 8 | 504/1524 | 30.6% (25.1–36.6%) | 77.5% |
| Black, Asian or minority ethnic | 9 | 621/1104 | 50.8% (38.3–63.2%) | 92.3% |
| Obesity (BMI >30 kg/m2) | 9 | 509/1725 | 38.2% (23.6–55.4%) | 97.0% |
| Smoker | 4 | 39/1155 | 3.3% (2.2–4.9%) | 21.9% |
| Asthma | 7 | 126/1661 | 8.8% (5.9–13.1%) | 79.4% |
| Chronic hypertension | 8 | 68/1710 | 4.2% (2.5–6.9%) | 72.3% |
| Cardiac disease | 2 | 26/715 | 3.2% (1.0–9.7%) | 84.4% |
| Any comorbidity | 5 | 252/776 | 32.5% (29.3–35.8%) | 0.0% |
| Third trimester | 8 | 900/1223 | 73.9% (63.7–82.1%) | 91.4% |
| Laboratory findings† | ||||
| Raised D-dimer | 2 | 77/91 | 84.6% (75.7–90.7%) | 0.0% |
| Raised C-reactive protein or procalcitonin | 6 | 144/351 | 54.0% (16.5–87.5%) | 97.5% |
| Lymphopenia | 7 | 143/444 | 34.2% (24.9–44.8%) | 75.1% |
| Thrombocytopenia | 3 | 7/259 | 3.2% (0.9–10.7%) | 62.9% |
| Elevated AST levels | 4 | 48/318 | 16.0% (10.7–23.2%) | 48.8% |
| Treatments received | ||||
| Antiviral treatment | 10 | 291/1321 | 21.1% (7.9–45.5%) | 97.6% |
| Anticoagulation | 2 | 69/141 | 51.1% (21.2–80.3%) | 92.5% |
| Maternal complications | ||||
| Bacteria or viral co-infection¶ | 1 | 5/60 | 8.3% (3.5–18.5%) | – |
| Maternal intensive care unit admission | 13 | 159/1591 | 7.0% (4.4–10.9%) | 81.7% |
| Oxygen support (nasal or non-invasive ventilation) | 10 | 295/1623 | 18.2% (9.8–31.1%) | 95.5% |
| Intubation and mechanical ventilation | 11 | 92/1680 | 3.4% (1.5–7.7%) | 90.2% |
| Maternal ECMO | 12 | 13/1896 | 0.7% (0.4–1.2%) | 0.0% |
| Maternal death | 15 | 43/2468 | 0.9% (0.4–2.3%) | 73.4% |
| Obstetric outcomes | ||||
| Delivered cases | 10 | 746/1650 | 52.4% (37.9–66.5%) | 96.1% |
| Delivery due to COVID-19 related reasons | 8 | 95/497 | 19.0% (8.9–36.0%) | 89.4% |
| Delivery due to fetal distress | 6 | 15/238 | 5.3% (2.3–11.8%) | 40.2% |
| Preterm birth (any) | 10 | 183/746 | 21.8% (14.6–31.3%) | 82.3% |
| Spontaneous preterm birth | 7 | 22/440 | 5.0% (3.3–7.5%) | 0.0% |
| Medically indicated preterm birth | 6 | 92/430 | 18.4% (8.3–35.8%) | 87.4% |
| Preterm birth < 34+0 weeks | 4 | 13/147 | 3.3% (0.2–31.9%) | 87.0% |
| Caesarean delivery | 10 | 390/746 | 48.3 (34.1–62.7%) | 91.5% |
| Perinatal outcomes | ||||
| Perinatal death | ||||
| Stillbirth†† | 8 | 12/1362 | 0.9% (0.5–1.5%) | 0.0% |
| Neonatal death | 8 | 4/688 | 0.6% (0.2–1.5%) | 0.0% |
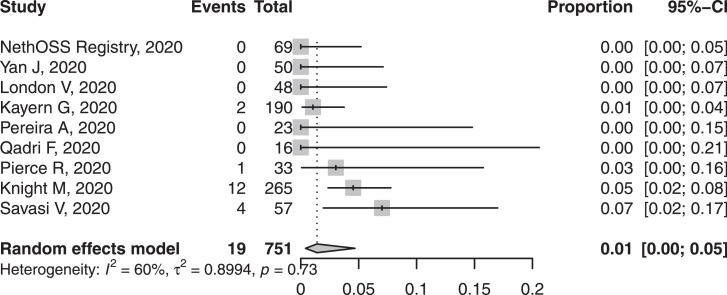
| SARS-CoV-2 PCR positivity after delivery | 9 | 19/751 | 1.4% (0.4–4.7%) | 59.8% |
AST: Aspartate Transaminase, ECMO: Extra-Corporeal Membrane Oxygenation, RT-PCR: reverse-transcription polymerase chain reaction, CI: confidence interval, n: numerator, N: denominator.
*Random-intercept logistic regression meta-analysis with continuity correction.
† Abnormal according to local reference range.
¶Any bacterial or viral respiratory pathogen (mycoplasma, influenza, respiratory syncytial virus, etc.).
††When total births were used as the denominator, the rate of stillbirth was 16.1 per 1000 births.
Fig. 2.
Forest plot of pooled proportion of (a) black, Asian or minority ethnic, (b) obesity and (c) chronic co-morbidities in pregnant women with confirmed COVID-19.
3.3. Maternal complications
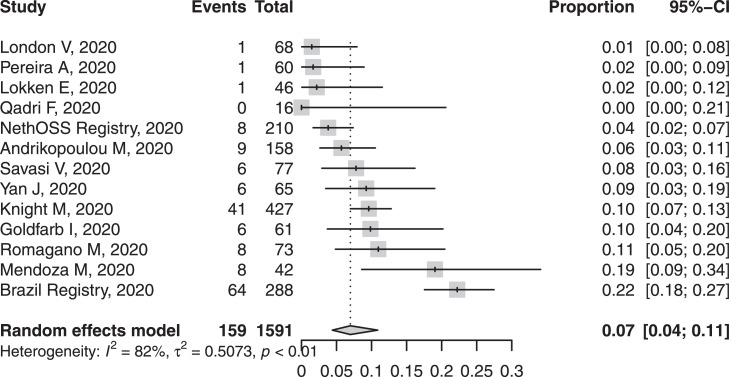
Few women had evidence of co-infection, bacterial or viral. Admission to intensive care unit occurred in ~7% of women, with or without respiratory support, with the upper 95% CI 11% (Figure 3). 43 of 2468 women died with a pooled proportion of 0.9% according to inverse variance proportion regression meta-analysis. Invasive mechanical ventilation support and extracorporeal membrane oxygenation were uncommon (3.4% and 0.7%, respectively).
Fig. 3.
Forest plot of pooled proportion of maternal intensive care unit admission in pregnant women with confirmed COVID-19.
3.4. Obstetric and perinatal outcomes
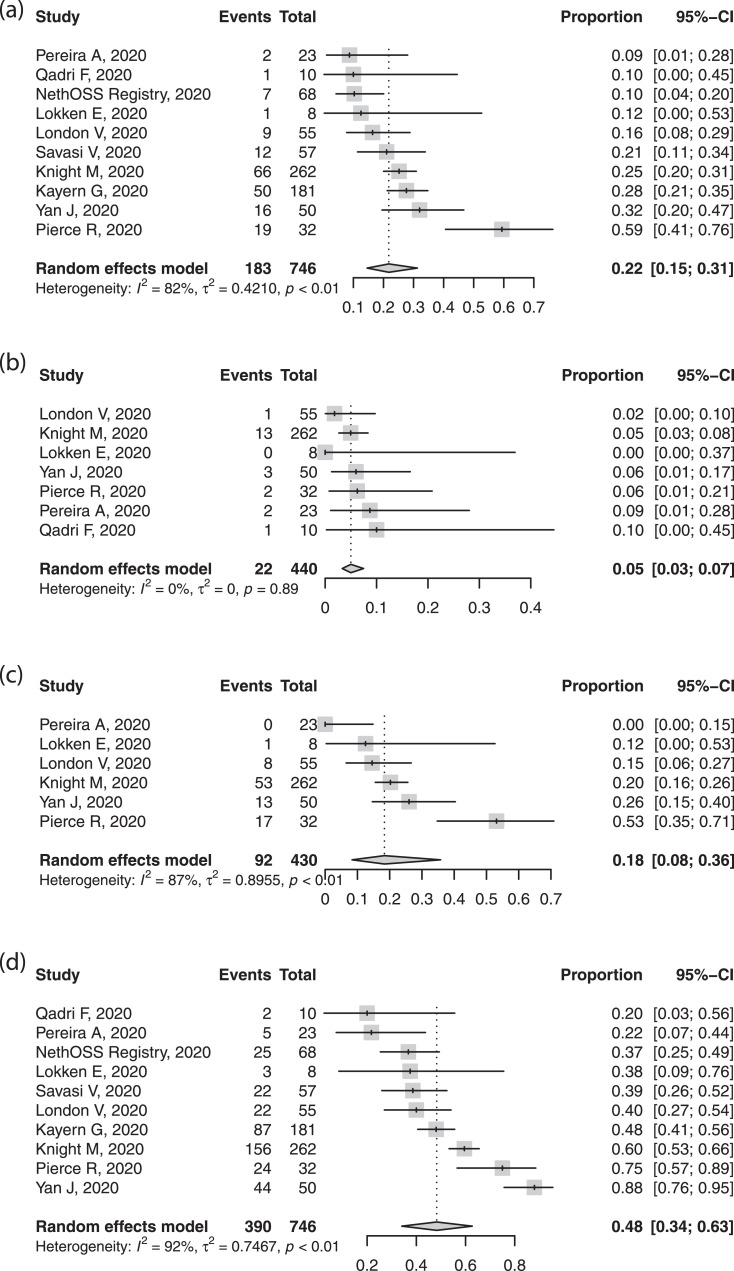
Preterm birth, primarily iatrogenic, was common, in around one fifth of cases, with variability between studies (Figure 4). Half of births were by caesarean section (Figure 4). While few studies reported indications, the most common indication was not fetal distress, but related to COVID-19, severe maternal pneumonia, or fear of sudden maternal decompensation. The spontaneous preterm delivery rate was within expected ranges. Perinatal deaths occurred in less than 1%; there were twelve stillbirths and four neonatal deaths. The rate of neonatal SARS-CoV-2 positivity was ~1-2% (Figure 5).
Fig. 4.
Forest plots of pooled proportion of (a) preterm birth, (b) spontaneous and (c) medically indicated preterm birth, and (d) caesarean section in pregnant women with confirmed SARS-CoV-2 infection.
Fig. 5.
Forest plot of pooled proportion of neonatal SARS-CoV-2 positivity in babies born to women with confirmed COVID-19.
3.5. Meta-regression analysis
Significant heterogeneity was observed in three of the four critical outcomes (maternal intensive care unit admission, maternal death, neonatal PCR positivity). Meta-regression analysis showed that the statistical heterogeneity in maternal intensive care unit admission was explained by the presence of maternal co-morbidities (beta=0.007, p<0.05) and maternal age over 35 years (beta=0.007, p<0.01). Heterogeneity in maternal death was explained by the use of antiviral treatment (beta=0.026, p<0.001). Neonatal SARS-CoV-2 PCR positivity was explained by spontaneous preterm delivery (beta=0.19, p<0.05) (Supplementary Table 4). Maternal admission to intensive care unit was more common in cohorts with a higher rate of maternal co-morbidities and older maternal age. Neonatal SARS-CoV-2 positivity was higher in cohorts with higher spontaneous preterm birth rate. Maternal mortality appeared to be higher in cohorts with more reported use of antiviral treatment. However, this is likely to be explained by a residual confounder not accounted for in this analysis. Third trimester pregnancy, BAME background and obesity did not adequately explain the heterogeneity in maternal intensive care unit admission, maternal death or neonatal SARS-CoV-2 positivity.
4. Rare outcome events reported in small case series and case reports
Analysis of conception products that may be associated with vertical transmission were reported in a minority of cases (placenta: 10.7%, amniotic fluid: 5.5%, cord blood: 6.2%; Supplementary Table 5). Maternal bodily fluid PCR positivity was rare (vaginal swab: 0%, stool: 12.5%, breast milk: 6.7%). Neonatal and maternal mortality figures were higher (2-3%) in case reports owing to reporting bias. There were 65 placental pathology reports available, with inflammatory changes documented in one fifth (Supplementary Table 6). 42 placental pathology reports were available for maternal or fetal malperfusion findings, which were very common (78.6%).
5. Discussion
In this systematic review, we summarise the published, peer-reviewed information about women with confirmed COVID-19 in pregnancy. Women usually presented with fever and cough. The prevalence of BAME background and maternal comorbidities was high. Most show biochemical evidence of inflammation, mainly lymphopenia. Many received antiviral therapy and few have been admitted to intensive care. Pregnancy outcomes were generally good, although there is little information about early pregnancy exposure, but iatrogenic preterm birth was common. Perinatal and maternal mortality were rare (<1%). Neonatal SARS-CoV-2 PCR positivity was rare but vertical transmission seems probable as the information on the SARS-CoV-2 positivity of other biological samples (maternal, conception products, fetal or neonatal) accrues.
This comprehensive search was without language restriction, yielding a large number of included studies with 2567 pregnancies in the quantitative synthesis. We strove to identify potential duplicate reports by cross-checking cases reported from the same centres and, when there was doubt, excluded those studies. We performed proportion meta-analysis for outcomes with sufficiently large cohorts, while we provided narrative summaries for rare outcomes from small case series and reports. Finally, we performed meta-regression analyses to explore the heterogeneity in critical clinical outcomes
The major limitations of this review are the retrospective design (especially case series and reports), the lack of universal testing for SARS-CoV-2 (given that up to 90% of pregnant women who are infected with SARS-CoV-2 are asymptomatic [98]), and the lack of standardised antenatal surveillance, management, timing and mode of delivery of women with COVID-19. The lack of standardised symptom and outcome queries and reporting did not allow for reporting mutually exclusive categories. The transformation of maternity care services worldwide may result in indirect effects of COVID-19 on maternal and perinatal outcomes, and these are currently unmeasured. A significant proportion of pregnancies were affected by COVID-19 in the third trimester and then delivered, so we were unable to comment meaningfully on outcomes related to early pregnancy exposure or those that take time to develop, such as fetal growth restriction. Despite detecting significant publication bias for only two investigated outcomes, it is likely that publication bias is a problem for all reported outcomes. However, there is no methodological or statistical way of preventing such bias at this time, and this must be acknowledged as a limitation. Finally, there are press reports of adverse outcomes that have not yet appeared in the peer reviewed literature, so serious complications such as maternal death or stillbirth are likely currently under-reported; however, we excluded press reports because we would be unable to ascertain duplicate cases.
Data from non-pregnant adults in China have described the most common presenting symptoms of COVID-19 as fever and cough, [99] as in our review. However, in the UK, patient-reported outcomes from confirmed cases have highlighted greater prominence of fatigue (80%) and loss of taste and smell (59%), along with persistent cough (58%) and dyspnoea (49%); fever was less prominent (32%) [100]. These differences could relate to unstructured assessment of symptoms, varying criteria for formal SARS-CoV-2 testing, or pregnancy-induced changes in the immune system.
The need for intensive care in pregnant women with COVID-19 in our review is slightly higher than that for infected non-pregnant women of reproductive age, thought to be no higher than 4.2% [101]. While reassuring, the proportion of women admitted to an intensive care unit was affected by the presence of maternal comorbidities and possibly by additional confounders not accounted for in this review. Furthermore, there was significant publication bias in the analysis, intensive care unit admission criteria was not defined and may vary between studies. Therefore, it is not possible to verify or refute an increased risk of intensive care unit admission for pregnant women. While national programs investigating maternal deaths will report the relevant data, this will take time and will not inform care during the current pandemic.
This review provides information required to guide current care during the SARS-CoV-2 pandemic. Key knowledge gaps include the relationship between infection and outcomes for all pregnant women, based on universal (not symptoms-based) testing and structured enquiry about symptoms. We lack information about meaningful numbers of women infected in early pregnancy and about the indications for iatrogenic preterm delivery and caesarean section, particularly in relation to local practice. We lack information on the indirect effects of COVID-19 on pregnancy outcomes; given the dramatic changes seen in maternity care services in order to deliver government-mandated social distancing and lockdown for non-essential services, these effects on pregnancy outcomes may be as great, if not greater, than the direct effects of the virus. The relative increase in stillbirth could be related to direct effects of SARS-CoV-2 and/or indirect effects of COVID-19 (i.e., maternal care-seeking and/or changes in maternity care service delivery). Finally, we need to support comprehensive assessment of potential vertical transmission to understand whether it occurs and, if so, how and with what frequency. A new study sponsored by Public Health England to address these questions is ongoing (www.pericovid.com); whenever possible, serum samples from neonates born to SARS-CoV-2 positive mothers should be stored until robust immunological testing is available, as advised by the Royal College of Paediatrics and Child Health, UK. Knowledge of safety and protective immunity will inform the role for COVID-19 vaccines in pregnant women. It is also necessary to put in place mechanisms for long-term follow-up of babies of infected mothers.
The evidence to date is reassuring with regards to the risks of COVID-19 in pregnancy. The risk of maternal intensive care unit admission is likely to be similar to that in other women of reproductive age when summary aggregates are compared to available data from Centers for Disease Control and Prevention from the United States [101]. While pregnancy outcomes are generally good, iatrogenic preterm birth appears to be increased, and vertical transmission is a possibility for which we need more evidence. Importantly, a critical gap in our knowledge is the impact on pregnancy outcomes of the COVID-19-related transformation of maternity care services.
Funding
No funding received
Contribution of Each Author
AK, EK, CB conducted the literature search, study selection and data extraction and analysis. All authors contributed to the data interpretation, writing and editing of the manuscript.
All authors have read and agreed to the published version of the manuscript.
Declaration of Competing Interest
Dr. Morris is the President and Trustee of the Royal College of Obstetricians and Gynaecologists. He is a Trustee of the British Menopause Society and the Chairman of the Baby Lifeline Multiprofessional Advisory Panel.
Acknowledgements
None
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100446.
Supplementary Material
References
- 1.World Health Organization. WHO Timeline - COVID-19. 2020. [online] Who.int. Available at: <https://www.who.int/news-room/detail/08-04-2020-who-timeline—covid-19>[Accessed 10 April 2020].
- 2.Wong S.F., Chow K.M., Leung T.N. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191:292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park M.H., Kim H.R., Choi D.H., Sung J.H., Kim J.H. Emergency cesarean section in an epidemic of the middle east respiratory syndrome: a case report. Korean J Anesthesiol. 2016;69:287–291. doi: 10.4097/kjae.2016.69.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam C.M., Wong S.F., Leung T.N. A case-controlled study comparing clinical course and outcomes of pregnant and non-pregnant women with severe acute respiratory syndrome. BJOG. 2004;111:771–774. doi: 10.1111/j.1471-0528.2004.00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Mascio D., Khalil A., Saccone G. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ Coronavirus disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. 2020. Am J Obstet Gynecol doi:10.1016/j.ajog.2020.02.017 [DOI] [PMC free article] [PubMed]
- 7.Poon L.C., Yang H., Lee J.C.S. ISUOG interim guidance on 2019 novel coronavirus infection during pregnancy and puerperium: information for healthcare professionals. Ultrasound Obstet Gynecol. 2020 doi: 10.1002/uog.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shamseer L., Moher D., Clarke M. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 9.Stroup D.F., Berlin J.A., Morton S.C. et al: Group ftM-aOOSiE. Meta-analysis of observational studies in epidemiology - a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 10.Ohri.ca. 2020. Ottawa Hospital Research Institute. [online] Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp[Accessed 10 April 2020].
- 11.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira A., Cruz-Melguizo S., Adrien M., Fuentes L., Marin E., Perez-Medina T. Clinical course of coronavirus disease-2019 (covid-19) in pregnancy. Acta Obstet Gynecol Scand. 2020 doi: 10.1111/aogs.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duffy C.R., Hart J.M., Modest A.M., Hacker M.R., Golen T., Li Y., Zera C., Shainker S.A., Mehrotra P., Zash R., Wylie B.J. Lymphopenia and severe acute respiratory syndrome coronavirus 2 (sars-cov-2) infection among hospitalized obstetric patients. Obstet Gynecol. 2020 doi: 10.1097/AOG.0000000000003984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lokken E.M., Walker C.L., Delaney S., Kachikis A., Kretzer N.M., Erickson A., Resnick R., Vanderhoeven J., Hwang J.K., Barnhart N., Rah J., McCartney S.A., Ma K.K., Huebner E.M., Thomas C., Sheng J.S., Paek B.W., Retzlaff K., Kline C.R., Munson J., Blain M., Lacourse S.M., Deutsch G., Adams Waldorf K. Clinical characteristics of 46 pregnant women with a sars-cov-2 infection in washington state. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qadri F., Mariona F.Pregnancy affected by sars-cov-2 infection: a flash report from michigan. J Matern Fetal Neonatal Med. 2020:1–3. doi:10.1080/14767058.2020.1765334 [DOI] [PubMed]
- 16.Yan J., Guo J., Fan C., Juan J., Yu X., Li J., Feng L., Li C., Chen H., Qiao Y., Lei D., Wang C., Xiong G., Xiao F., He W., Pang Q., Hu X., Wang S., Chen D., Zhang Y., Poon L.C., Yang H. Coronavirus disease 2019 in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L., Li Q., Zheng D., Jiang H., Wei Y., Zou L., Feng L., Xiong G., Sun G., Wang H., Zhao Y., Qiao J. Clinical characteristics of pregnant women with COVID-19 in Wuhan, China. N Engl J Med. 2020 doi: 10.1056/NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrikopoulou M., Madden N., Wen T., Aubey J.J., Aziz A., Baptiste C.D., Breslin N., D'Alton M.E., Fuchs K.M., Goffman D., Gyamfi-Bannerman C., Matseoane-Peterssen D.N., Miller R.S., Sheen J.J., Simpson L.L., Sutton D., Zork N., Friedman A.M. Symptoms and critical illness among obstetric patients with coronavirus disease 2019 (covid-19) infection. Obstet Gynecol. 2020 doi: 10.1097/AOG.0000000000003996. [DOI] [PubMed] [Google Scholar]
- 19.Knight M., Bunch K., Vousden N., Morris E., Simpson N., Gale C., O'Brien P., Quigley M., Brocklehurst P., Kurinczuk J. Characteristics and outcoems of pregnant women hospitalised with confirmed SARS-CoV-2 infection in the UK: a national cohort study using the UK Obstetric Surveillance System (UKOSS) BMJ. 2020 [Accepted, In press] [Google Scholar]
- 20.Pierce-Williams R.A.M., Burd J., Felder L., Khoury R., Bernstein P.S., Avila K., Penfield C.A., Roman A.S., DeBolt C.A., Stone J.L., Bianco A., Kern-Goldberger A.R., Hirshberg A., Srinivas S.K., Jayakumaran J.S., Brandt J.S., Anastasio H., Birsner M., O'Brien D.S., Sedev H.M., Dolin C.D., Schnettler W.T., Suhag A., Ahluwalia S., Navathe R.S., Khalifeh A., Anderson K., Berghella V. Clinical course of severe and critical covid-19 in hospitalized pregnancies: a us cohort study. Am J Obstet Gynecol MFM. 2020:100134. doi:10.1016/j.ajogmf.2020.100134 [DOI] [PMC free article] [PubMed]
- 21.Savasi V.M., Parisi F., Patane L., Ferrazzi E., Frigerio L., Pellegrino A., Spinillo A., Tateo S., Ottoboni M., Veronese P., Petraglia F., Vergani P., Facchinetti F., Spazzini D., Cetin I. Clinical findings and disease severity in hospitalized pregnant women with coronavirus disease 2019 (covid-19) Obstet Gynecol. 2020 doi: 10.1097/AOG.0000000000003979. [DOI] [PubMed] [Google Scholar]
- 22.London V., McLaren R., Jr., Atallah F., Cepeda C., McCalla S., Fisher N., Stein J.L., Haberman S., Minkoff H. The relationship between status at presentation and outcomes among pregnant women with covid-19. Am J Perinatol. 2020 doi: 10.1055/s-0040-1712164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NVOG. 2020. Update Registratie COVID-19 Positieve Zwangeren In Nethoss | NVOG. [online] Available at: <https://www.nvog.nl/actueel/registratie-van-covid-19-positieve-zwangeren-in-nethoss/>[Accessed 7 June 2020]. [In Dutch]
- 24.Saude.gov.br. 2020. [online] Available at: <https://www.saude.gov.br/images/pdf/2020/May/29/2020-05-25—BEE17—Boletim-do-COE.pdf>[Accessed 29 May 2020]. [In Portuguese]
- 25.Kayem G., Alessandrini V., Azria E., Blanc J., Bohec C., Bornes M., Bretelle F., Ceccaldi P.-.F., Chalet Y., Chauleur C., Cordier A.-.G., Deruelle P., Desbrière R., Doret M., Dreyfus M., Driessen M., Fermaut M., Gallot D., Garabédian C., Huissoud C., Lecarpentier E., Luton D., Morel O., Perrotin F., Picone O., Rozenberg P., Schmitz T., Sentilhes L., Sroussi J., Vayssière C., Verspyck E., Vivanti A.J., Winer N. A snapshot of the covid-19 pandemic among pregnant women in France. J Gynecol Obstetr Hum Reprod. 2020 doi: 10.1016/j.jogoh.2020.101826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romagano M.P., Guerrero K., Spillane N., Kayaalp E., Smilen S.W., Alvarez M., Alvarez-Perez J., Francis Kim A., Aschner J., Al-Khan A. Perinatal outcomes in critically ill pregnant women with covid-19. Am J Obstetr Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100151. [DOI] [Google Scholar]
- 27.Mendoza M., Garcia-Ruiz I., Maiz N., Rodo C., Garcia-Manau P., Serrano B., Lopez-Martinez R.M., Balcells J., Fernandez-Hidalgo N., Carreras E., Suy A. Preeclampsia-like syndrome induced by severe covid-19: a prospective observational study. BJOG Int J Obstetr Gynaecol. 2020 doi: 10.1111/1471-0528.16339. n/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldfarb I.T., Clapp M.A., Soffer M.D., Shook L.L., Rushfirth K., Edlow A.G., Boatin A.A., Kaimal A.J., Barth W.H., Jr., Bryant A.S. Prevalence and severity of coronavirus disease 2019 (covid-19) illness in symptomatic pregnant and postpartum women stratified by hispanic ethnicity. Obstet Gynecol. 2020 doi: 10.1097/aog.0000000000004005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed I., Azhar A., Eltaweel N., Tan B.K. First Covid‐19 maternal mortality in the UK associated with thrombotic complications. Br J Haematol. 2020 doi: 10.1111/bjh.16849. https://onlinelibrary.wiley.com/doi/abs/10.1111/bjh.16849 bjh.16849. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alzamora M.C., Paredes T., Caceres D., Webb C.M., Valdez L.M., La Rosa M. Severe COVID-19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020;1(212) doi: 10.1055/s-0040-1710050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson J., Schauer J., Bryant S., Graves C.R. The use of convalescent plasma therapy and remdesivir in the successful management of a critically ill obstetric patient with novel coronavirus 2019 infection: a case report. Case Rep Women's Heal. 2020 doi: 10.1016/j.crwh.2020.e00221. e00221. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baergen R.N., Heller D.S. Placental pathology in Covid-19 positive mothers: preliminary findings. Pediatr Dev Pathol. 2020;23(3):177–180. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baud D., Greub G., Favre G., Gengler C., Jaton K., Dubruc E. Second-trimester miscarriage in a pregnant woman with SARS-CoV-2 infection. JAMA J Am Med Assoc. 2020:1–3. doi: 10.1001/jama.2020.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Browne P.C., Linfert J.B., Perez-Jorge E. Successful Treatment of Preterm Labor in Association with Acute COVID-19 Infection [published online ahead of print, 2020 Apr 24] Am J Perinatol. 2020 doi: 10.1055/s-0040-1709993. 10.1055/s-0040-1709993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blauvelt C.A., Chiu C., Donovan A.L., Prahl M., Shimotake T.K., George R.B. Acute respiratory distress syndrome in a preterm pregnant patient with coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2020;00(00) doi: 10.1097/AOG.0000000000003949. [DOI] [PubMed] [Google Scholar]
- 36.Buonsenso D., Raffaelli F., Tamburrini E., Biasucci D.G., Salvi S., Smargiassi A. Clinical role of lung ultrasound for the diagnosis and monitoring of COVID-19 pneumonia in pregnant women. Ultrasound Obstet Gynecol. 2020 Apr 26 doi: 10.1002/uog.22055. https://onlinelibrary.wiley.com/doi/abs/10.1002/uog.22055 uog.22055. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buonsenso D., Costa S., Sanguinetti M., Cattani P., Posteraro B., Marchetti S. Neonatal late onset infection with severe acute respiratory syndrome coronavirus 2. Am J Perinatol. 2020;1(212) doi: 10.1055/s-0040-1710541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carosso A., Cosma S., Borella F., Marozio L., Coscia A., Ghisetti V. Pre-labor anorectal swab for SARS-CoV-2 in COVID-19 pregnant patients: is it time to think about it? Eur J Obstet Gynecol Reprod Biol. 2020 doi: 10.1016/j.ejogrb.2020.04.023. (2019):2019–20. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020 Mar;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3. [Internet]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen S., Huang B., Luo D.J., Li X., Yang F., Zhao Y. [Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases] Zhonghua bing li xue za zhi = Chin J Pathol. 2020;49(0):E005. doi: 10.3760/cma.j.cn112151-20200225-00138. http://www.ncbi.nlm.nih.gov/pubmed/32114744 [Internet]Available from: [DOI] [PubMed] [Google Scholar]
- 41.Chen S., Liao E., Cao D., Gao Y., Sun G., Shao Y. Clinical analysis of pregnant women with 2019 novel coronavirus pneumonia. J Med Virol. 2020 doi: 10.1002/jmv.25789. [Internet]0–2. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooke W.R., Billett A., Gleeson S., Jacques A., Place K., Siddall J. SARS-CoV-2 infection in very preterm pregnancy: experiences from two cases. Eur J Obstet Gynecol Reprod Biol. 2020;2115(20) doi: 10.1016/j.ejogrb.2020.05.025. [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong L., Tian J., He S., Zhu C., Wang J., Liu C. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020 Mar 26 doi: 10.1001/jama.2020.4621. https://jamanetwork.com/journals/jama/fullarticle/2763853 [Internet]E1–3. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doria M., Peixinho C., Laranjo M., Varejao A.M., Silva P.T. Covid-19 during pregnancy: a case series from an universally tested population from the north of Portugal. Eur J Obstet Gynecol. 2020;May 15 doi: 10.1016/j.ejogrb.2020.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dumpa V., Kamity R., Vinci A.N., Noyola E., Noor A. Neonatal coronavirus 2019 (COVID-19) infection: a case report and review of literature. Cureus. 2020;2019(5) doi: 10.7759/cureus.8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fan C., Lei D., Fang C., Li C., Wang M., Liu Y. Perinatal transmission of COVID-19 associated SARS-CoV-2: should we worry? Intergovernmental panel on climate change, editor. Clin Infect Dis. 2020 Mar 17;53(9):1–30. [Internet]Available from: file:///C:/Users/User/Downloads/fvm939e.pdf. [Google Scholar]
- 47.Gidlöf S., Savchenko J., Brune T., Josefsson H. COVID-19 in pregnancy with comorbidities: More liberal testing strategy is needed. Acta Obstet Gynecol Scand. 2020 Apr 17 doi: 10.1111/aogs.13862. https://ejournal3.undip.ac.id/index.php/jamt/article/view/5101 [Internet]aogs.13862. Available from: [DOI] [PubMed] [Google Scholar]
- 48.González Romero D., Ocampo Pérez J., González Bautista L., Santana-Cabrera L. Pregnancy and perinatal outcome of a woman with COVID-19 infection. Rev Clin Esp. 2020 doi: 10.1016/j.rce.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Govind A., Essien S., Karthikeyan A., Fakokunde A., Janga D., Yoong W. Re: novel coronavirus COVID-19 in late pregnancy: outcomes of first nine cases in an inner city London hospital. Eur J Obstet Gynecol Reprod Biol. 2019;2020:3–5. doi: 10.1016/j.ejogrb.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groß R., Conzelmann C., Müller J.A., Stenger S., Steinhart K., Kirchhoff F. Detection of SARS-CoV-2 in human breastmilk. Lancet (Lond, Engl) 2020;6736(20) doi: 10.1016/S0140-6736(20)31181-8. http://www.ncbi.nlm.nih.gov/pubmed/32446324%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC7241971 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A. Maternal death due to COVID-19 [published online ahead of print, 2020 Apr 28] Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.030. S0002-9378(20)30516-0. [DOI] [Google Scholar]
- 52.Hirshberg A., Kern-Goldberger A.R., Levine L.D., Pierce-Williams R., Short W.R., Parry S. Care of critically ill pregnant patients with coronavirus disease 2019: a case series. Am J Obstet Gynecol. 2020 May;3(2):54–67. doi: 10.1016/j.ajog.2020.04.029. http://repositorio.unan.edu.ni/2986/1/5624.pdf [Internet]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hong L., Smith N., Keerthy M., Lee-Griffith M., Garcia R., Shaman M. Severe COVID-19 infection in pregnancy requiring intubation without preterm delivery: a case report. Case Reports Women's Heal. 2020;27:e00217. doi: 10.1016/j.crwh.2020.e00217. [Internet]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Inchingolo R., Smargiassi A., Moro F., Buonsenso D., Salvi S., Del Giacomo P. The diagnosis of pneumonia in a pregnant woman with COVID-19 using maternal lung ultrasound. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.020. [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iqbal S.N., Overcash R., Mokhtari N., Saeed H., Gold S., Auguste T. An uncomplicated delivery in a patient with Covid-19 in the United States. N Engl J Med. 2020;382(16):1–3. doi: 10.1056/NEJMc2007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Joudi N., Henkel A., Lock W.S., Lyell D. Preeclampsia Treatment in SARS-CoV-2. Am J Obstet Gynecol MFM. 2020 May doi: 10.1016/j.ajogmf.2020.100146. https://linkinghub.elsevier.com/retrieve/pii/S2589933320300902 [Internet](925):100146. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Juusela A., Nazir M., Gimovsky M. Two Cases of COVID-19 Related Cardiomyopathy in Pregnancy. Am J Obstet Gynecol MFM. 2020 Apr doi: 10.1016/j.ajogmf.2020.100113. [Internet]100113. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalafat E., Yaprak E., Cinar G., Varli B., Ozisik S., Uzun C. Lung ultrasound and computed tomographic findings in pregnant woman with COVID-19. Ultrasound Obstet Gynecol. 2020:1–12. doi: 10.1002/uog.22034. [DOI] [PubMed] [Google Scholar]
- 59.Khan S., Peng L., Siddique R., Nabi G., Nawsherwan Xue M. Impact of COVID-19 infection on pregnancy outcomes and the risk of maternal-to-neonatal intrapartum transmission of COVID-19 during natural birth. Infect Control Hosp Epidemiol. 2020 Mar 19 doi: 10.1017/ice.2020.84. https://www.cambridge.org/core/product/identifier/S0899823X20000847/type/journal_article [Internet](March):1–9. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kirtsman M., Diambomba Y., Poutanen S.M., Malinowski A.K., Vlachodimitropoulou E., Parks W.T. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. Can Med Assoc J. 2020 doi: 10.1503/cmaj.200821. cmaj.200821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kuhrt K., McMicking J., Nanda S., Nelson-Piercy C., Shennan A. Placental abruption in a twin pregnancy at 32 weeks’ gestation complicated by coronavirus disease 2019 without vertical transmission to the babies. Am J Obstet Gynecol MFM. 2020 May doi: 10.1016/j.ajogmf.2020.100135. https://linkinghub.elsevier.com/retrieve/pii/S2589933320300781 [Internet]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee D.H., Lee J., Kim E., Woo K., Park H.Y., An J. Emergency cesarean section on severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2) confirmed patient [published online ahead of print, 2020 Mar 31] Korean J Anesthesiol. 2020 doi: 10.4097/kja.20116. 10.4097/kja.20116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li J., Wang Y., Zeng Y., Song T., Pan X., Jia M. Critically ill pregnant patient with COVID-19 and neonatal death within two hours of birth. Int J Gynaecol Obstet. 2020:19–21. doi: 10.1002/ijgo.13189. (May) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li Y., Zhao R., Zheng S., Chen X., Wang J., Sheng X. Lack of Vertical Transmission of Severe Acute Respiratory Syndrome Coronavirus 2, China. Emerg Infect Dis. 2020;26(6):4–7. doi: 10.3201/eid2606.200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liao X., Yang H., Kong J., Yang H. Chest CT findings in a pregnant patient with 2019 novel coronavirus disease. Balkan Med J. 2020 Mar 26:2019–2021. doi: 10.4274/balkanmedj.galenos.2020.2020.3.89. http://balkanmedicaljournal.org/pdf.php?&id=2196 [Internet](March)Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu D., Li L., Wu X., Zheng D., Wang J., Yang L. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. Am J Roentgenol. 2020 Mar 18:1–6. doi: 10.2214/AJR.20.23072. https://www.ajronline.org/doi/10.2214/AJR.20.23072 [Internet](July)Available from: [DOI] [PubMed] [Google Scholar]
- 67.Liu Y., Chen H., Tang K., Guo Y. Clinical manifestations and outcome of SARS-CoV-2 infection during pregnancy. J Infect. 2020 Mar doi: 10.1016/j.jinf.2020.02.028. [Internet]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lowe B., Bopp B. COVID-19 vaginal delivery – A case report. Aust New Zeal J Obstet Gynaecol. 2020 May 28 doi: 10.1111/ajo.13173. https://onlinelibrary.wiley.com/doi/abs/10.1111/ajo.13173 [Internet]ajo.13173. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu D., Sang L., Du S., Li T., Chang Y., Yang X.A. Asymptomatic COVID-19 infection in late pregnancy indicated no vertical transmission. J Med Virol. 2020:1–5. doi: 10.1002/jmv.25927. (March) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lyra J., Valente R., Rosário M., Guimarães M. Cesarean section in a pregnant woman with COVID-19: first case in Portugal. Acta Med Port. 2020;33:1–3. doi: 10.20344/amp.13883. [DOI] [PubMed] [Google Scholar]
- 71.Mehta H., Ivanovic S., Cronin A., VanBrunt L., Mistry N., Miller R. Novel coronavirus-related acute respiratory distress syndrome in a patient with twin pregnancy: A case report. Case Reports Women's Heal. 2020 doi: 10.1016/j.crwh.2020.e00220. [Internet]e00220. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Panichaya P., Thaweerat W., Uthaisan J. Prolonged viral persistence in COVID-19 s trimester pregnant patient. Eur J Obstet Gynecol Reprod Biol. 2020 doi: 10.1016/j.ejogrb.2020.05.030. http://www.ncbi.nlm.nih.gov/pubmed/32425299%0Ahttp://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=PMC7233222 [Internet]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patanè L., Morotti D., Giunta M.R., Sigismondi C., Piccoli M.G., Frigerio L. Vertical transmission of COVID-19: SARS-CoV-2 RNA on the fetal side of the placenta in pregnancies with COVID-19 positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020 May doi: 10.1016/j.ajogmf.2020.100145. https://linkinghub.elsevier.com/retrieve/pii/S2589933320300896 [Internet]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Perrone S., Deolmi M., Giordano M., D'Alvano T., Gambini L., Corradi M. Report of a series of healthy term newborns from convalescent mothers with COVID-19. Acta Bio Medica Atenei Parm. 2020;91(2):251–255. doi: 10.23750/abm.v91i2.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piersigilli F., Carkeek K., Hocq C., van Grambezen B., Hubinont C., Chatzis O. COVID-19 in a 26-week preterm neonate. Lancet Child Adolesc Heal. 2020;4642(20):19–21. doi: 10.1016/S2352-4642(20)30140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Polónia-Valente R., Moucho M., Tavares M., Vilan A., Montenegro N., Rodrigues T. Vaginal delivery in a woman infected with SARS-CoV-2 – the first case reported in Portugal. Eur J Obstet Gynecol Reprod Biol. 2019;2020:2019–2020. doi: 10.1016/j.ejogrb.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rosen M.H., Axelrad J., Hudesman D., Rubin D.T., Chang S. Management of acute severe ulcerative colitis in a pregnant woman with COVID-19 infection: a case report and review of the literature. Inflamm Bowel Dis. 2020:1–3. doi: 10.1093/ibd/izaa109. XX(Xx) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schnettler W.T., Al Ahwel Y., Suhag A. Severe ARDS in COVID-19-infected pregnancy: obstetric and intensive care considerations. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100120. [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID-19. Am J Clin Pathol. 2020 May 22:1–10. doi: 10.1093/ajcp/aqaa089. https://academic.oup.com/ajcp/advance-article/doi/10.1093/ajcp/aqaa089/5842018 [Internet]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song L., Xiao W., Ling K., Yao S., Chen X. Anesthetic management for emergent cesarean delivery in a parturient with recent diagnosis of coronavirus disease 2019 (COVID-19): a case report. Transl Perioper Pain Med. 2020;7(3):234–237. [Google Scholar]
- 81.Taghizadieh A., Mikaeili H., Ahmadi M., Valizadeh H. Acute kidney injury in pregnant women following SARS-CoV-2 infection: a case report from Iran. Respir Med Case Rep. 2020;30 doi: 10.1016/j.rmcr.2020.101090. [Internet]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tang M.W., Nur E., Biemond B.J. Immune Thrombocytopenia during Pregnancy due to COVID-19. Am J Hematol. 2020 doi: 10.1002/ajh.25877. http://www.ncbi.nlm.nih.gov/pubmed/32445584 [Internet]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vallejo V., Ilagan J.G. A postpartum death due to coronavirus disease 2019 (COVID-19) in the United States. Obstet Gynecol. 2020;00(00):1–4. doi: 10.1097/AOG.0000000000003950. [DOI] [PubMed] [Google Scholar]
- 84.Vibert F., Kretz M., Thuet V., Barthel F., De Marcillac F., Deruelle P. Prone positioning and high-flow oxygen improved respiratory function in a 25-week pregnant woman with COVID-19. Eur J Obstet Gynecol Reprod Biol. 2019;2020:2019–2020. doi: 10.1016/j.ejogrb.2020.05.022. [Internet]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vlachodimitropoulou Koumoutsea E., Vivanti A.J., Shehata N., Benachi A., Le Gouez A., Desconclois C. COVID19 and acute coagulopathy in pregnancy. J Thromb Haemost. 2020 doi: 10.1111/jth.14856. (4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X., Zhou Z., Zhang J., Zhu F., Tang Y., Shen X. A case of 2019 Novel Coronavirus in a pregnant woman with preterm delivery [published online ahead of print, 2020 Feb 28] Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa200. ciaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wen R., Sun Y., Xing Q.S. A patient with SARS-CoV-2 infection during pregnancy in Qingdao, China. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.03.004. [Internet](xxxx):3–4. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu Y., Liu C., Dong L., Zhang C., Chen Y., Liu J. Viral shedding of COVID-19 in pregnant women. SSRN Electron J. 2020 https://www.ssrn.com/abstract=3562059 [Internet]Available from: [Google Scholar]
- 89.Xia H., Zhao S., Wu Z., Luo H., Zhou C., Chen X. Emergency Caesarean delivery in a patient with confirmed coronavirus disease 2019 under spinal anaesthesia. Br J Anaesth. 2020 Mar doi: 10.1016/j.bja.2020.02.016. [Internet];(xxx):8–10. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang P., Wang X., Liu P., Wei C., He B., Zheng J. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J Clin Virol. 2020;127(March) doi: 10.1016/j.jcv.2020.104356. [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yassa M., Birol P., Mutlu A.M., Tekin A.B., Sandal K., Tug N. Lung ultrasound can influence the clinical treatment of pregnant women With COVID-19. J Ultrasound Med. 2020 doi: 10.1002/jum.15367. http://www.ncbi.nlm.nih.gov/pubmed/32478445 [Internet]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu N., Li W., Kang Q., Xiong Z., Wang S., Lin X. Clinical features and obstetric and neonatal outcomes of pregnant patients with COVID-19 in Wuhan, China: a retrospective, single-centre, descriptive study. Lancet Infect Dis. 2020;20(5):559–564. doi: 10.1016/S1473-3099(20)30176-6. [Internet]Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yu N., Li W., Kang Q., Zeng W., Feng L., Wu J. No SARS-CoV-2 detected in amniotic fluid in mid-pregnancy. Lancet Infect Dis. 2020;3099(20):19–20. doi: 10.1016/S1473-3099(20)30320-0. [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zamaniyan M., Ebadi A., Aghajanpoor Mir S., Rahmani Z., Haghshenas M., Azizi S. Preterm delivery in pregnant woman with critical COVID-19 pneumonia and vertical transmission. Prenat Diagn. 2020 doi: 10.1002/pd.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zambrano L.I., Fuentes-Barahona I.C., Bejarano-Torres D.A., Bustillo C., Gonzales G., Vallecillo-Chinchilla G. A pregnant woman with COVID-19 in central America. Travel Med Infect Dis. 2020 Mar;3099(March) doi: 10.1016/j.tmaid.2020.101639. [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zeng L., Xia S., Yuan W., Yan K., Xiao F., Shao J. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;23(77):4–6. doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zeng H., Xu C., Fan J. Antibodies in Infants Born to Mothers With COVID-19 Pneumonia [published online ahead of print, 2020 Mar 26] JAMA. 2020;323(18):1848‐1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sutton D., Fuchs K., D'Alton M., Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N Engl J Med. 2020;382:2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. China medical treatment expert group for C. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Menni C., Valdes A., Freydin M.B. Loss of smell and taste in combination with other symptoms is a strong predictor of COVID-19 infection. medRxiv. 2020 doi: 10.1101/2020.04.05.20048421. 2020.2004.2005.20048421. [DOI] [Google Scholar]
- 101.Severe outcomes among patients with coronovirus disease 2019 (COVID-19) – United States. MMWR Mornb Mortal Wkly Rp. 2020 doi: 10.15585/mmwr.mm6912e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.