Abstract
Background:
We sought to characterize recent prostate cancer incidence, distant stage diagnosis, and mortality rates by region, race/ethnicity, and age-group.
Methods:
In SEER*Stat, we examined age-specific and age-adjusted prostate cancer incidence, distant stage diagnosis, and mortality rates by race/ethnicity, Census region, and age group. Incidence and mortality analyses included men diagnosed with (n=723,269) and dying of (n=112,116) prostate cancer between 2012–2015.
Results:
Non-Hispanic black (NHB) and NH Asian/Pacific Islander (NHAPI) men had the highest and lowest rates, respectively, for each indicator across regions and age-groups. Hispanic men had lower incidence and mortality rates than non-Hispanic white (NHW) men in all regions except the Northeast where they had higher incidence (RR 1.16 (95%CI 1.14–1.19)) and similar mortality. Hispanics had higher distant stage rates in the Northeast (RR 1.18 (95%CI 1.08–1.28)) and South (RR 1.22 (95%CI 1.15–1.30)), but similar rates in other regions. Non-Hispanic American Indian/Alaskan Native (NHAIAN) men had higher distant stage rates than NHWs in the West (RR 1.38 (95%CI 1.15–1.65)). NHBs and Hispanics had higher distant stage rates than NHWs among those ages 55–69 years (RR 2.91 (95% CI 2.81–3.02) and 1.24 (95% CI 1.18–1.31) respectively), despite lower overall incidence for Hispanics in this age group.
Conclusions:
For Hispanic and NHAIAN men, prostate cancer indicators varied by region, while NHB and NHAPI men consistently had the highest and lowest rates, respectively, across regions.
Impact:
Regional and age-group differences in prostate cancer indicators between populations may improve understanding of prostate cancer risk and help inform screening decisions.
Keywords: prostate cancer, incidence, distant stage diagnosis, mortality, regional differences
Introduction
Prostate cancer remains the most commonly diagnosed non-skin cancer and the second leading cause of cancer mortality among men in the United States (1). Prostate cancer incidence has been highest in the Northeast United States and among non-Hispanic black (NHB) men (2–7). An analysis of prostate cancer incidence by region and race from 1999–2008 showed that incidence rates for white men were highest in the Northeast census divisions of New England and Middle Atlantic while incidence rates for black men were highest in the Middle Atlantic and South Atlantic census divisions (2). NHB men also have the highest prostate cancer mortality rate nationally (7–9). Evidence from 2002–2011 indicates that prostate cancer mortality rates were higher in West and North Central states among NHW men and higher in the South among NHB men (10). While regional differences in prostate cancer incidence and mortality have been examined for NHB and NHW men, they have not been as well characterized for other races/ethnicities.
Prostate cancer incidence peaked in 1992, then declined with an acceleration in the rate of decline from 2010–2014, as highlighted in the 2018 Annual Report to the Nation on the Status of Cancer (6). This accelerated rate of decline in recent years has been associated with changes to the United States Preventive Services Task Force (USPSTF) prostate-specific antigen (PSA) screening guidelines in 2012, which recommended against prostate cancer screening in all men (6, 11). While overall prostate cancer incidence has been declining, an increase in distant stage prostate cancer has been reported from 2010–2014, with some suggesting it may also be a result of the changes to the PSA screening guidelines (6). Less is known about regional variations in prostate cancer incidence by race/ethnicity since the 2012 change in USPSTF guidelines. Examining rates by region using more recent data would help determine whether these patterns are occurring similarly in all regions and for all groups. Characterizing regional differences in the incidence of distant stage prostate cancer by race/ethnicity may help identify populations experiencing these increases.
In addition to the increased burden of prostate cancer incidence and mortality noted above, NHB men have a higher rate of distant stage prostate cancer diagnoses and a younger age of prostate cancer diagnosis than NHW men (6, 12, 13). Though the most recent guidelines suggest discussing risks, harms and benefits of prostate cancer screening with NHB men, the USPSTF does not make separate screening recommendations for this higher risk population. Current USPSTF screening guidelines recommend that men between the ages of 55–69 years make an individual decision in conjunction with their healthcare provider regarding whether or not to be screened using the PSA test (14). An examination of the rates of distant-stage prostate cancer diagnosis by age group may better inform screening decisions based on age.
This paper examines regional variation in racial/ethnic differences in prostate cancer incidence and mortality from 2012–2015. Given recent findings of increasing distant stage prostate cancer diagnosis, we also characterize racial/ethnic differences in incidence of distant stage prostate cancer by region as well as by age group. Lastly, because rates for racial/ethnic groups other than NHB and NHW men have been reported less frequently in the literature, this paper characterizes these indicators for Hispanic, NH Asian-American/Pacific Islander (NHAPI), and NH American Indian/Alaska Native (NHAIAN) race/ethnicity as well.
Materials and Methods
We used incidence data from the United States Cancer Statistics (USCS) dataset, which is comprised of data from the Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries (NPCR) and National Cancer Institute’s (NCI) Surveillance, Epidemiology, and End Results (SEER) Program (15). USCS includes cancer incidence data from 51 population-based central cancer registries in the US. This report includes new cases of primary invasive prostate cancer diagnosed from 2012–2015. All statewide registries met USCS publication criteria for these years and were included in the analysis (15, 16). Thus for these years, cancer incidence data cover 100% of the United States population (15, 16). Prostate cancer mortality data were accessed through the SEER program’s SEER*Stat software with underlying data provided by the CDC’s National Center for Health Statistics (NCHS) (17).
Prostate cancer cases were defined using the International Classification of Diseases for Oncology, Third Edition topography code C61.9 (18). Cases identified only by autopsy or death certificate were excluded. We report prostate cancer incidence rates, rates of distant stage prostate cancer diagnoses, and prostate cancer mortality rates from 2012–2015. Rates (per 100,000 men) were reported by age group (all ages, < 55 years, 55 to 69 years, and ≥70 years), race/ethnicity (Non-Hispanic white (NHW), Non-Hispanic black (NHB), Hispanic, Non-Hispanic Asian/Pacific Islander (NHAPI), and Non-Hispanic American Indian/Alaska Native (NHAIAN)), year, and U.S. Census region (Northeast, Midwest, South, and West) (16). Additionally, we report prostate cancer incidence rates by SEER Summary stage (localized, regional, distant, or unstaged). Distant stage is defined by SEER as cancer that has metastasized to other organs or remote lymph nodes (19). We also examined race/ethnicity differences in rates of prostate cancer incidence, distant stage disease, and mortality by region and age group.
All analyses were performed in SEER*Stat, version 8.3.5 (20). Rates per 100,000 men were age-adjusted to the 2000 U.S. standard population with 19 age groups (Census P25–1130) (21). Population denominators are specific to race, ethnicity, and sex, and were obtained from SEER (22). Rate ratios (RR) and 95% confidence intervals (95% CI) were calculated using the Tiwari method to compare differences in rates by race/ethnicity (23, 24). Rate differences were considered statistically significant at p <0.05. Data were suppressed for any cell with less than 16 cases because of poor reliability and to maintain confidentiality of subjects (18). Data for figures have been submitted as supplemental tables.
Results
Table 1 presents age-adjusted rates of prostate cancer incidence, distant stage diagnosis, and mortality. From 2012 through 2015, 723,269 men were newly diagnosed with prostate cancer (age-adjusted rate 101.52 per 100,000 men). Of these cases, 65.6% occurred in men less than 70 years of age, although rates increased with age. Forty-four percent of distant stage diagnoses occurred in men less than 70 years of age. Compared to NHW men, rates for each indicator (incidence, distant stage diagnosis, and mortality) were higher for NHB men and lower for NHAPI men.
Table 1.
Age-adjusted Rates of Prostate Cancer Incidence, Distant-Stage Diagnosis, and Mortality, 2012–2015
| Incidence Rate | Distant Stage Rate | Mortality Rate | ||||
|---|---|---|---|---|---|---|
| Count (%) | Rate (95% CI) | Count (%) | Rate (95% CI) | Count (%) | Rate (95% CI) | |
| Total | 723,269 (100) | 101.52 (101.28, 101.76) | 46,800 (100) | 7.29 (7.22, 7.36) | 112,116 (100) | 19.21 (19.09, 19.32) |
| Age (Years) | ||||||
| <55 | 68,103 (9.42) | 14.54 (14.43, 14.65) | 2,828 (6.04) | 0.60 (0.58, 0.63) | 1,843 (1.64) | 0.39 (0.38, 0.41) |
| 55–69 | 406,155 (56.16) | 387.26 (386.07, 388.45) | 17,538 (37.47) | 16.72 (16.48, 16.97) | 21,191 (18.90) | 20.21 (19.93, 20.48) |
| 70+ | 249,011 (34.43) | 483.85 (481.95, 485.76) | 26,434 (56.48) | 51.36 (50.75, 51.99) | 89,082 (79.46) | 173.09 (171.96, 174.23) |
| Race/Ethnicity | ||||||
| NH White | 517,109 (73.74) | 94.52 (94.26, 94.79) | 32,853 (70.60) | 6.58 (6.51, 6.65) | 84,287 (75.18) | 17.99 (17.87, 18.11) |
| NH Black | 114,221 (16.29) | 166.75 (165.73, 167.78) | 8,386 (18.02) | 14.29 (13.96, 14.62) | 18,244 (16.27) | 39.90 (39.29, 40.51) |
| Hispanic | 51,260 (7.31) | 85.66 (84.88, 86.45) | 3,825 (8.22) | 7.30 (7.05, 7.55) | 6,800 (6.07) | 15.92 (15.53, 16.31) |
| NH API | 15,629 (2.23) | 52.18 (51.33, 53.03) | 1,194 (2.57) | 4.53 (4.27, 4.81) | 1,959 (1.74) | 8.69 (8.30, 9.09) |
| NH AIAN | 3,056 (0.44) | 69.42 (66.79, 72.12) | 279 (0.60) | 7.55 (6.61, 8.57) | 534 (0.48) | 18.03 (16.43, 19.73) |
| Diagnosis Year | ||||||
| 2012 | 185,990 (25.72) | 109.10 (108.59, 109.61) | 10,396 (22.21) | 6.76 (6.63, 6.89) | 27,244 (24.30) | 19.56 (19.33, 19.80) |
| 2013 | 181,849 (25.14) | 103.66 (103.17, 104.15) | 11,342 (24.24) | 7.20 (7.06, 7.33) | 27,681 (24.69) | 19.26 (19.03, 19.49) |
| 2014 | 174,160 (24.08) | 96.35 (95.89, 96.81) | 12,086 (25.82) | 7.40 (7.27, 7.54) | 28,343 (25.28) | 19.10 (18.88, 19.33) |
| 2015 | 181,270 (25.06) | 97.55 (97.09, 98.01) | 12,976 (27.73) | 7.76 (7.62, 7.89) | 28,848 (25.73) | 18.93 (18.71, 19.15) |
| Region | ||||||
| Northeast | 146,561 (20.26) | 112.36 (111.77, 112.95) | 9,354 (19.99) | 7.89 (7.72, 8.05) | 20,421 (18.21) | 18.48 (18.23, 18.74) |
| Midwest | 159,609 (22.07) | 102.08 (101.57, 102.59) | 10,659 (22.78) | 7.60 (7.46, 7.75) | 25,038 (22.33) | 19.42 (19.18, 19.67) |
| South | 271,611 (37.55) | 102.10 (101.71, 102.49) | 16,223 (34.66) | 6.76 (6.65, 6.86) | 41,198 (36.75) | 19.24 (19.05, 19.43) |
| West | 145,488 (20.12) | 91.34 (90.86, 91.82) | 10,564 25.57) | 7.35 (7.21, 7.50) | 25,459 (22.71) | 19.50 (19.26, 19.4) |
Incidence data are from population-based registries that participate in CDC’s National Program of Cancer Registries and/or NCI’s Surveillance, Epidemiology, and End Results Program and meet high-quality data criteria. These registries cover 100% of the U.S. population for 2012 to 2015.
Rates are per 100,000 men and are age-adjusted to the 2000 U.S. standard population (19 age groups – Census P25–1130). Rates by age group are not age-adjusted.
National Program of Cancer Registries and Surveillance, Epidemiology, and End Results SEER*Stat Database: NPCR and SEER Incidence – U.S. Cancer Statistics Public Use Research Database, Nov 2017 submission (2001–2015), United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Released June 2018, based on November 2017 submissions. Available at www.cdc.gov/cancer/public-use.
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, Aggregated With State, Total U.S. (1969–2015) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, released December 2017. Underlying mortality data provided by NCHS (www.cdc.gov/nchs)
Incidence rates were highest in the Northeast for NHW, NHB, and Hispanic men (Figure 1, Supplementary Table S1). For NHAIAN men, incidence was lower in the Northeast and West and higher in the Midwest and South, whereas for NHAPI men rates were similar across most regions. Compared with NHW men, rates for NHB men were higher across all regions. Hispanics had lower rates than NHW men in all regions except for the Northeast, where they had higher rates. NHAPI and NHAIAN men had lower rates in all regions compared to NHW men.
Figure 1.
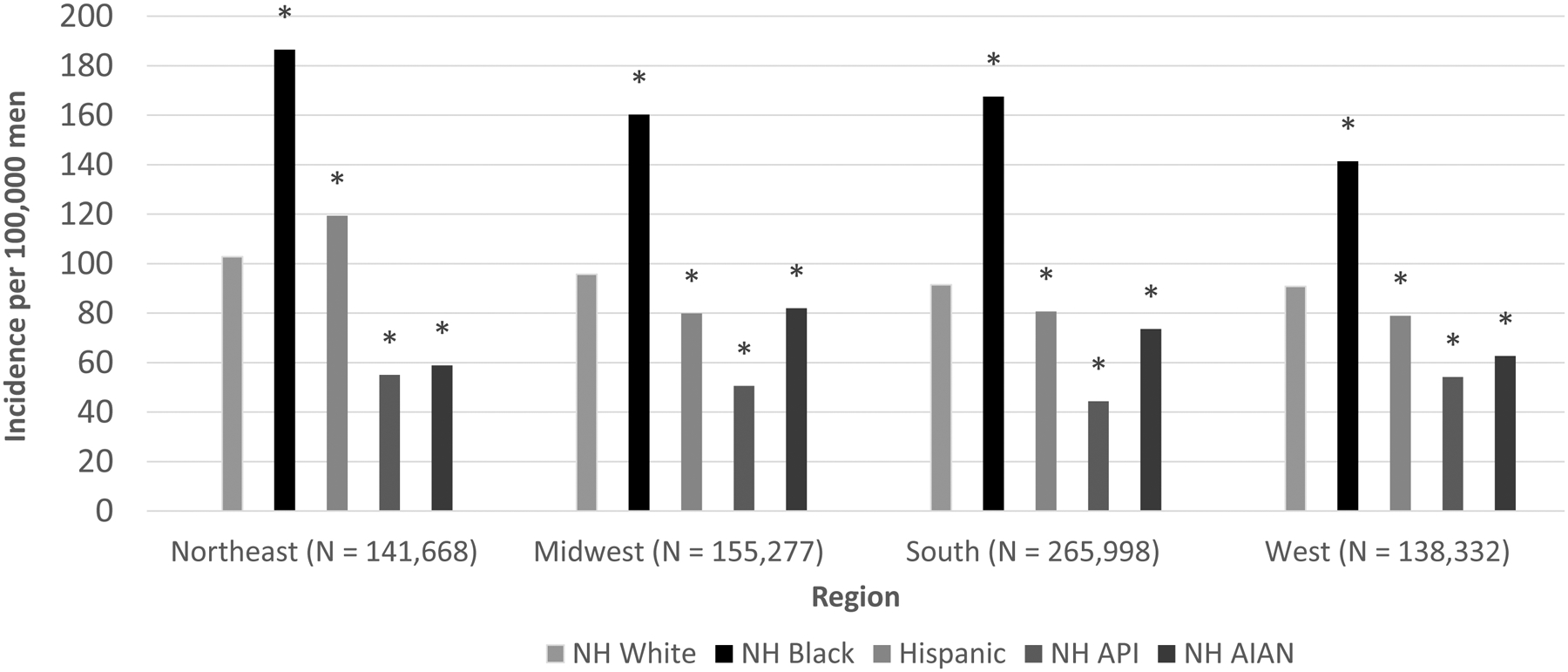
Age-adjusted Prostate Cancer Incidence Rates by Region and Race/Ethnicity, 2012–2015
NH = Non-Hispanic; API = Asian/Pacific Islander; AIAN = American Indian/Alaska Native
Asterisk(*) indicates statistically significant difference (P < 0.05) from Non-Hispanic white estimate.
Rates are per 100,000 men and are age-adjusted to the 2000 U.S. standard population (19 age groups – Census P25–1130).
Data are from population-based registries that participate in CDC’s National Program of Cancer Registries and/or NCI’s Surveillance, Epidemiology, and End Results Program and meet high-quality data criteria. These registries cover 100% of the U.S. population for 2012 to 2015.
National Program of Cancer Registries and Surveillance, Epidemiology, and End Results SEER*Stat Database: NPCR and SEER Incidence – U.S. Cancer Statistics Public Use Research Database, Nov 2017 submission (2001–2015), United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Released June 2018, based on November 2017 submissions. Available at www.cdc.gov/cancer/public-use.
In general, rates of distant stage diagnosis were lower in the South for all races/ethnicities (Figure 2, Supplementary Table S2). Despite lower overall incidence in most regions, rates of distant stage disease among Hispanic men were as high (Midwest and West) or higher (Northeast and South) than those of NHW men. Similarly, NHAIAN men had lower incidence rates compared with NHW men in all regions, but similar (Midwest and South) or higher (West) rates of distant stage diagnosis. NHB men had at least a twofold rate of distant stage diagnosis across all regions compared with NHW men.
Figure 2.
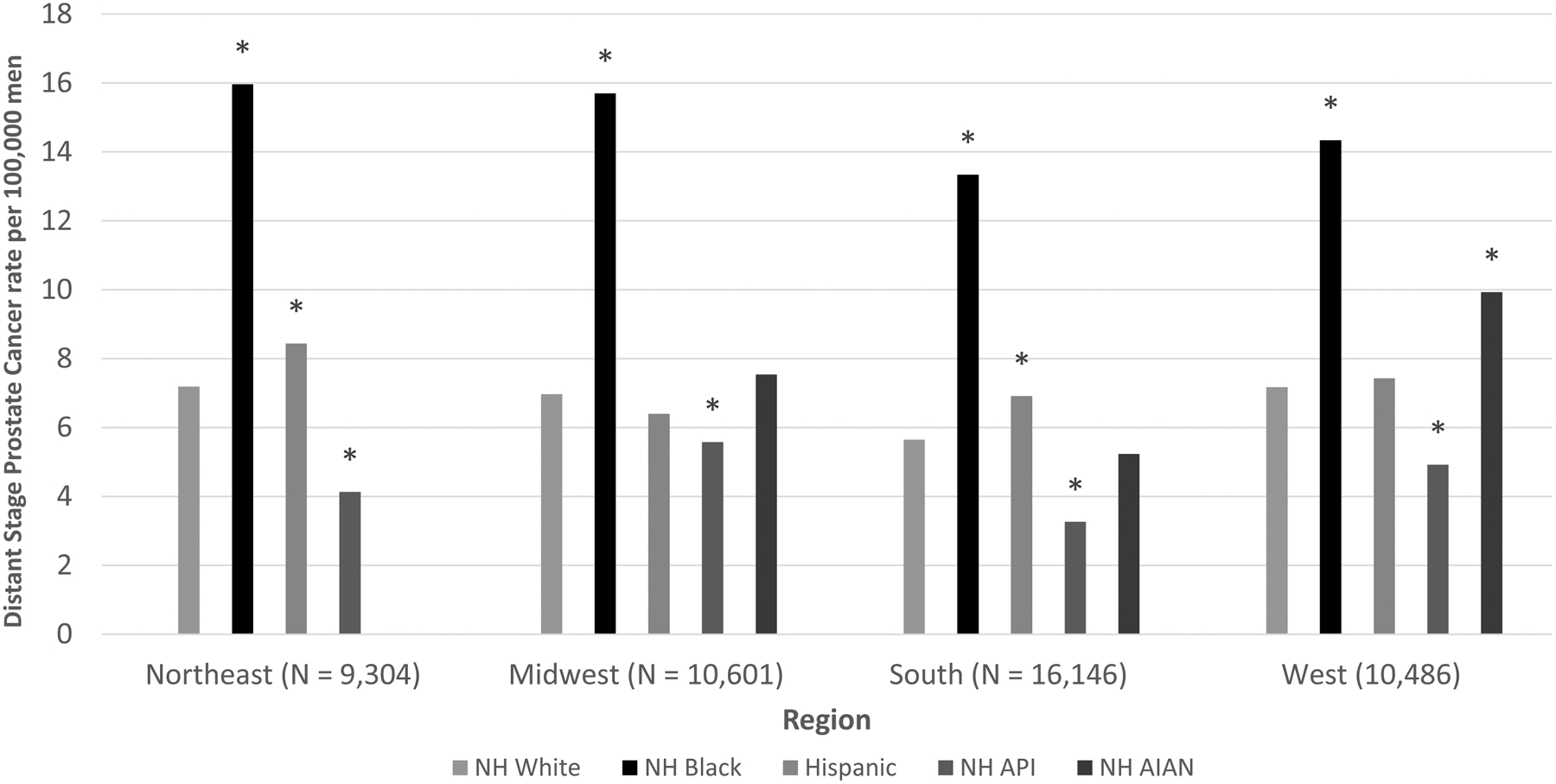
Age-adjusted Rates of Distant Stage Prostate Cancer by Region and Race/Ethnicity, 2012–2015
NH = Non-Hispanic; API = Asian/Pacific Islander; AIAN = American Indian/Alaska Native
Asterisk(*) indicates statistically significant difference (P < 0.05) from Non-Hispanic white estimate.
Data for NH AIAN in the Northeast suppressed due to low counts
Rates are per 100,000 men and are age-adjusted to the 2000 U.S. standard population (19 age groups – Census P25–1130).
Data are from population-based registries that participate in CDC’s National Program of Cancer Registries and/or NCI’s Surveillance, Epidemiology, and End Results Program and meet high-quality data criteria. These registries cover 100% of the U.S. population for 2012 to 2015.
National Program of Cancer Registries and Surveillance, Epidemiology, and End Results SEER*Stat Database: NPCR and SEER Incidence – U.S. Cancer Statistics Public Use Research Database, Nov 2017 submission (2001–2015), United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Released June 2018, based on November 2017 submissions. Available at www.cdc.gov/cancer/public-use.
Mortality rates in the West were among the highest for all races/ethnicities, except for NHB men, for whom rates were similar across regions (Figure 3, Supplementary Table S3). NHB men had the highest rates in each region, with the largest difference compared with NHW men in the South and smallest in the West. Hispanics had significantly lower mortality rates across all regions except the Northeast, where the rate was similar to NHW men. The lowest mortality rate among Hispanic men was in the Midwest. Mortality rates among NHAPI men were significantly lower than NHW men in each region, whereas rates for NHAIAN men were not significantly different from NHW men for any region.
Figure 3.
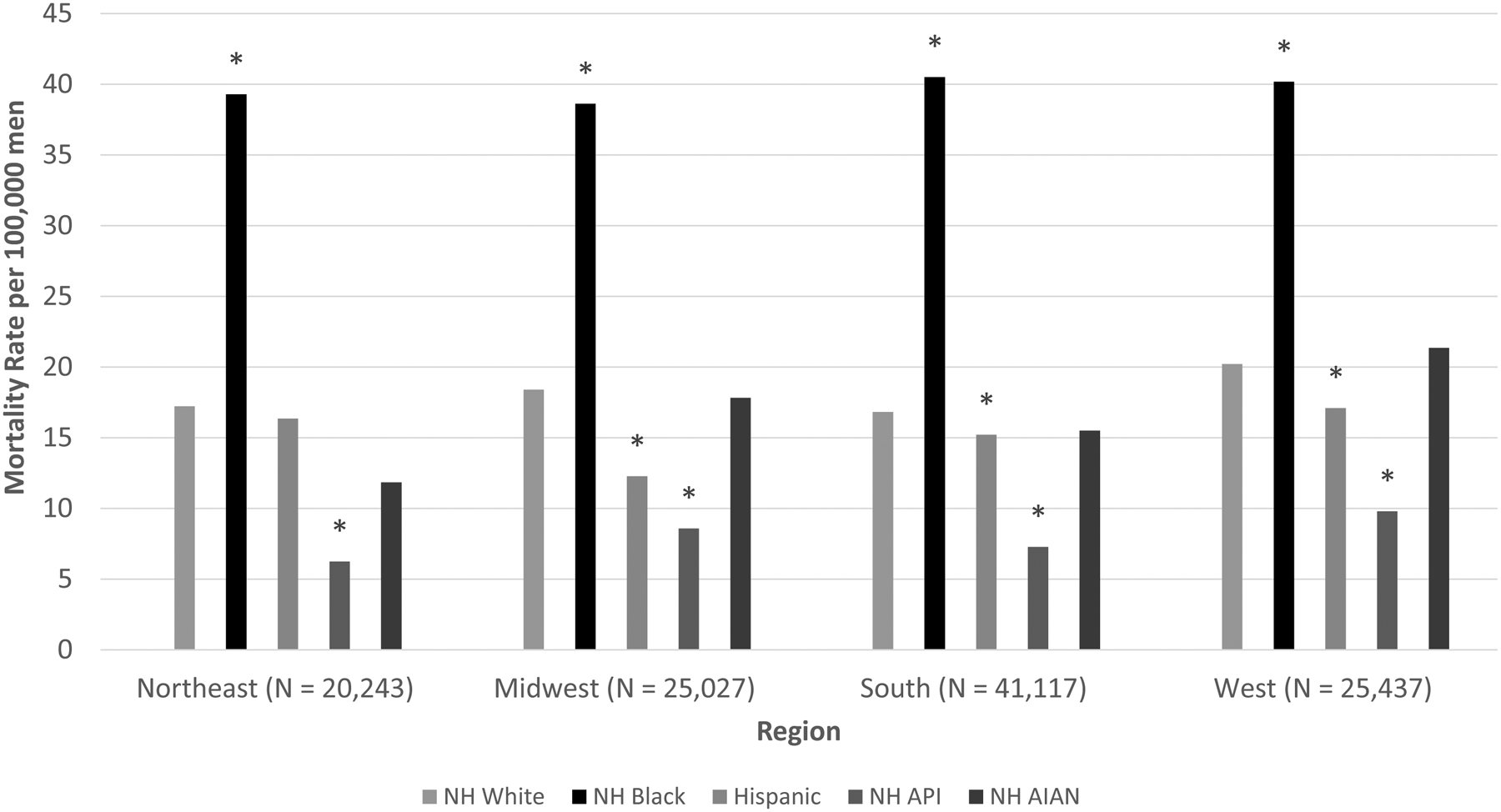
Age-adjusted Prostate Cancer Mortality Rates by Region and Race/Ethnicity, 2012–2015
NH = Non-Hispanic; API = Asian/Pacific Islander; AIAN = American Indian/Alaska Native
Asterisk(*) indicates statistically significant difference (P < 0.05) from Non-Hispanic white estimate.
Rates are per 100,000 men and are age-adjusted to the 2000 U.S. standard population (19 age groups – Census P25–1130).
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, Aggregated With State, Total U.S. (1969–2015) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, released December 2017. Underlying mortality data provided by NCHS (www.cdc.gov/nchs)
Table 2 presents age-adjusted rates (rates adjusted within each age-group to account for differences in age distribution by race/ethnicity) of prostate cancer incidence, distant stage diagnosis, and mortality by age and race/ethnicity. NHB men had the highest rates for each indicator across all age categories while NHAPI men had the lowest rates for each indicator across all age categories. For distant stage diagnosis, large differences in rates were observed between NHB men and NHW among men ages < 55 and between 55–69 years (RR 2.97 and RR 2.91, respectively). NHB men constituted 28.7% and 24.1% of men diagnosed with distant stage prostate cancer among those aged <55 years and aged 55–69 years, respectively. Hispanics had a similar rate of distant stage diagnosis compared with NHW men among men < 55 years, but a higher rate among men ages 55–69 years, despite lower overall incidence in this age group. Prostate cancer mortality was lower for Hispanic men in this age group than for NHW men however.
Table 2.
Age-Adjusted Rates of Prostate Cancer Incidence, Distant Stage Diagnosis, and Mortality by Age and Race/Ethnicity, 2012–2015
| RR (95% CI) | RR (95% CI) | RR (95% CI) | ||||
|---|---|---|---|---|---|---|
|
Incidence Race/Ethnicity | ||||||
| NH White | 11.87 (11.75, 11.98) | Reference | 360.06 (358.74, 361.38) | Reference | 449.82 (447.78, 451.86) | Reference |
| NH Black | 27.66 (27.24, 28.08)* | 2.33 (2.29, 2.37) | 674.07 (669.04, 679.13)* | 1.87 (1.86, 1.89) | 684.96 (676.82, 693.17)* | 1.52 (1.50, 1.54) |
| Hispanic | 8.00 (7.80, 8.21)* | 0.67 (0.66, 0.69) | 306.03 (302.44, 309.65)* | 0.85 (0.84, 0.86) | 457.90 (451.03, 464.86) | 1.02 (1.00, 1.03) |
| NH API | 3.87 (3.64, 4.11)* | 0.33 (0.31, 0.35) | 181.07 (177.19, 185.01)* | 0.50 (0.49, 0.51) | 294.52 (287.22, 301.96)* | 0.66 (0.64, 0.67) |
| NH AIAN | 8.79 (7.90, 9.75)* | 0.74 (0.67, 0.82) | 242.25 (230.92, 253.98)* | 0.67 (0.64, 0.71) | 358.95 (336.09, 383.01)* | 0.80 (0.75, 0.85) |
| Distant stage | ||||||
| NH White | 0.44 (0.42, 0.47) | Reference | 13.91 (13.65, 14.17) | Reference | 49.32 (48.64, 50.00) | Reference |
| NH Black | 1.32 (1.23, 1.41)* | 2.97 (2.73, 3.24) | 40.55 (39.32, 41.80)* | 2.91 (2.81, 3.02) | 90.36 (87.30, 93.50)* | 1.83 (1.77, 1.90) |
| Hispanic | 0.49 (0.44, 0.54) | 1.10 (0.98, 1.24) | 17.26 (16.42, 18.13)* | 1.24 (1.18, 1.31) | 52.26 (49.90, 54.70) | 1.06 (1.01, 1.11) |
| NH API | 0.19 (0.14, 0.25)* | 0.42 (0.31, 0.56) | 9.65 (8.77, 10.60)* | 0.69 (0.63, 0.76) | 34.89 (32.34, 37.59)* | 0.71 (0.65, 0.76) |
| NH AIAN | 0.73 (0.49, 1.03) | 1.64 (1.11, 2.34) | 16.84 (13.95, 20.15) | 1.21 (1.00, 1.45) | 53.51 (44.44, 63.88) | 1.08 (0.90, 1.30) |
| Mortality | ||||||
| NH White | 0.31 (0.29, 0.33) | Reference | 17.79 (17.49, 18.08) | Reference | 169.15 (167.89, 170.42) | Reference |
| NH Black | 0.81 (0.74, 0.89)* | 2.62 (2.35, 2.91) | 49.93 (48.55, 51.35)* | 2.81 (2.72, 2.90) | 360.30 (354.00, 366.68)* | 2.13 (2.09, 2.17) |
| Hispanic | 0.25 (0.22, 0.29) | 0.80 (0.69, 0.94) | 16.35 (15.52, 17.21)* | 0.92 (0.87, 0.97) | 149.09 (145.02, 153.24)* | 0.88 (0.86, 0.91) |
| NH API | 0.11 (0.07, 0.15)* | 0.34 (0.23, 0.50) | 7.34 (6.58, 8.18)* | 0.41 (0.37, 0.46) | 83.75 (79.67, 87.98)* | 0.50 (0.47, 0.52) |
| NH AIAN | ** | ** | 19.84 (16.69, 23.41) | 1.12 (0.94, 1.32) | 166.59 (149.86, 184.66) | 0.99 (0.89, 1.09) |
NH = Non-Hispanic; API = Asian/Pacific Islander; AIAN = American Indian/Alaska Native
Rates are per 100,000 men and are age-adjusted to the 2000 U.S. standard population (19 age groups – Census P25–1130).
Asterisk(*) indicates statistically significant difference (P < 0.05) from Non-Hispanic white estimate.
Double asterisk (**) indicates suppressed due to low counts
Data are from population-based registries that participate in CDC’s National Program of Cancer Registries and/or NCI’s Surveillance, Epidemiology, and End Results Program and meet high-quality data criteria. These registries cover 100% of the U.S. population for 2012 to 2015.
National Program of Cancer Registries and Surveillance, Epidemiology, and End Results SEER*Stat Database: NPCR and SEER Incidence – U.S. Cancer Statistics Public Use Research Database, Nov 2017 submission (2001–2015), United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Released June 2018, based on November 2017 submissions. Available at www.cdc.gov/cancer/public-use.
Discussion
This analysis of USCS data and mortality data from 2012–2015 revealed regional and age-group differences in prostate cancer incidence, distant stage diagnosis, and mortality by race/ethnicity. For Hispanic and NHAIAN men, prostate cancer incidence, distant stage diagnosis, and mortality varied by region, while NHB and NHAPI men consistently had the highest and lowest rates, respectively, across regions. Importantly, our study builds on the growing body of evidence by providing region-specific data for Hispanic, NHAPI, and NHAIAN populations, highlights regional differences in mortality by race/ethnicity, and examines distant-stage prostate cancer diagnoses by age and race/ethnicity. As previous data have shown regional differences in prostate cancer incidence and mortality between NHB and NHW men, our paper also examines such regional differences since the 2012 change in USPSTF guidelines for other race/ethnicity groups.
Race/Ethnicity
NHB men had a greater rate than white men of incident prostate cancer, being diagnosed with advanced stage disease, and dying from prostate cancer across all regions. Multiple reasons for black-white disparities in prostate cancer incidence have been explored. For differences in incidence, some have suggested a possible role of genetic and hormonal factors, such as circulating PSA and testosterone concentrations (2, 7, 25 – 27). Environmental factors such as diet and gene-environment interactions have also been suggested (2, 28). Prostate cancer mortality disparities have been examined with differences in treatment based on socioeconomic factors implicated (9, 29).
In contrast, NHAPI men had the lowest rate of developing prostate cancer, advanced stage disease, and dying from prostate cancer in every region and age group examined. Previous studies have postulated a possible role of hormonal, genetic, and dietary factors in lower prostate cancer incidence rates in certain NHAPI groups (30–34). However, despite the favorable prostate cancer indicators, prostate cancer remains the most commonly diagnosed cancer among NHAPI men (35). Notably, the finding that NHAPI men had lower rates of distant stage diagnosis than NHW men differed from previous reports, although differences could be attributable in part to variations across studies, such as in subpopulations reported (36–38).
Overall, our results showing decreased prostate cancer incidence and mortality but higher distant stage rates in Hispanic men compared to NHW men are consistent with previous reports (7–9, 39). However, it is uncertain why Hispanic men had increased incidence of prostate cancer in the Northeast compared to NHW men, and why rates of distant stage disease were at least as high as NHW men for each region despite lower incidence for most regions. While we did not examine this directly, one possible factor that may contribute to these findings is differences in subpopulations across regions. Previous evidence examining cancer mortality rates for 2008–2012 in Florida found that cancer mortality varied across Hispanic ethnic groups (40). Cancer screening use, including PSA testing, has also been reported to vary among these groups (41). Furthermore, Hispanic subgroups differ across geographic regions. For example, among Hispanic groups with a population of 1 million or more in 2010, most Dominicans and Puerto Ricans and 37% of South Americans resided in the Northeast, compared with fewer than 20% of other Hispanic subgroups (42). Dominicans have also been reported to have a higher percentage of black race than other Hispanic subgroups, at least in Florida (40). Increased participation in PSA screening results in higher disease detection rates. In many states, where estimates are available recent PSA screening data show that Hispanics have lower PSA screening rates than NHW men overall (43). Further analysis and disaggregation of Hispanic subgroups in the Northeast and other regions will help to elucidate these findings further.
Our findings of decreased prostate cancer incidence but not mortality for NHAIAN men compared to NHW men are consistent with a previous study comparing their prostate cancer incidence and mortality rates with NHW men (44). Factors that have been suggested to contribute to this pattern include lower socioeconomic status, decreased access to care, and increased rate of exposure to prostate cancer mortality risk factors, such as cigarette smoking (44–46). Similar to Hispanics, NHAIAN men showed a pattern of lower incidence rates but similar or higher rates of distant stage diagnosis than NHW men in each region reported. Differences across regions could reflect in part differences in subpopulations (44). Whether differences in PSA screening may have contributed to these patterns is unclear. Lower rates of PSA testing have been reported for Hispanic and NHAIAN men than NHW men nationally (45–48). Findings of lower incidence but similar or higher distant stage disease may have implications for men and their healthcare providers as they discuss whether to be screened.
Mortality by Region
Although the Northeast had the highest overall incidence and distant stage diagnosis rates, it also had the lowest mortality rate while the West had among the highest mortality rates despite having the lowest incidence rate. These findings raise questions about possible geographic differences in screening, access to treatment, and/or quality of care. Across all regions, mortality rates for NHB men were generally at least twice those of any other race/ethnicity group, consistent with previous evidence that rates are highest for NHB men (7–9). For Hispanic men, despite having similar or higher distant stage diagnosis rates in all regions, mortality rates were lower than NHW men in all regions except the Northeast where rates were similar. One factor possibly contributing to these inconsistencies between prostate cancer diagnoses and mortality could be that our cross-sectional study is not capturing the temporality of the relationship between prostate cancer diagnosis and mortality, with many men surviving for years after being diagnosed with prostate cancer (5). These regional differences in mortality, especially as they relate to prostate cancer incidence and distant stage diagnosis, provide interesting points for further study of possible differences in access to care and treatment patterns by region. Future monitoring of patterns will be important to determine whether observed differences in prostate cancer incidence and distant stage diagnosis by race/ethnicity and region lead to differences in mortality rates for these groups.
Distant Stage Prostate Cancer Diagnosis by Age and Race/Ethnicity
Overall, distant stage diagnosis rates were higher among NHB and Hispanic men than NHW men. By age, increases were more evident for NHB men < 55 years and those 55–69 years old although they had higher rates in all three age groups. A previous study concluded that prostate cancer may grow more aggressively in black than in white men, which could contribute to the increased distant stage diagnosis rate (49). Additionally, Hispanic men had a higher rate of distant stage diagnosis compared to NHW men among men ages 55–69 years, despite lower overall incidence in this age group. The USPSTF recommends that men aged 55–69 years have discussions with medical providers regarding the risks and benefits of being screened. Such discussions may be informed by findings of higher rates of distant stage at diagnosis for some groups such as NHB and Hispanic men.
Strengths and Limitations
Strengths of this study include high-quality data from a large, nationwide dataset that enables examination of differences by race/ethnicity, region, and age. As stated previously, one limitation to this cross-sectional study is that we are not able to capture temporality between prostate cancer diagnosis and mortality. Another limitation to this study is that the dataset does not include information about PSA screening for race/ethnic groups. It is possible that differences in PSA screening rates underlie some of the disease indicator patterns presented. Previous evidence indicates that PSA screening rates among white and black men are relatively similar by region, although more recent data would be informative (2). We were unable to explore patterns among Hispanics as PSA screening data for Hispanic men are unavailable for a large number of states, Studies have also reported that Hispanic, NHAPI, and NHAIAN men have lower PSA screening rates than NHW men (45–48, 50).. Also, counts for NHAPI/NHAIAN were smaller compared to other racial/ethnic groups so caution should be taken in interpretation of data for these populations. In addition, many AIAN self-identify as AIAN race and Hispanic ethnicity or multi-racial subjecting them to potential misclassification (51). Lastly, heterogeneity exists within race/ethnicity groups, and variation in rates within such groups may be masked (52).
Conclusion
Our findings demonstrate continued black-white disparities by geographic region in prostate cancer incidence, stage at diagnosis, and deaths while providing region-specific data for Hispanic, NHAPI, and NHAIAN men. These findings may inform conversations between men and their healthcare providers about PSA screening, by identifying not only men at increased risk for developing prostate cancer, but also those more likely to be diagnosed with distant stage disease or to die from it. Efforts to identify factors that drive differences in developing prostate cancer across regions and populations may prove useful to better understand the extent to which differences in screening and access to quality care may contribute to differences in outcomes. Furthermore, our findings highlight a role for additional disparities research, especially for groups relatively less studied such as Hispanic and NHAIAN men.
Supplementary Material
Acknowledgements
We thank Jessica King for guidance in use of SEER*Stat and orientation to USCS data.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The authors declare no potential conflicts of interest
References
- 1.U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on November 2017 submission data (1999–2015): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; www.cdc.gov/cancer/dataviz, June 2018. [Google Scholar]
- 2.Cook MB, Rosenberg PS, McCarty FA, et al. Racial disparities in prostate cancer incidence rates by census division in the United States, 1999–2008. Prostate. 2015;75(7):758–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devesa S, Grauman D, Blot W, Pennello G, Hoover RN, Fraumeni JF Jr. Atlas of cancer mortality in the United States, 1950–94. Vol. 99–4564: NIH; Washington (DC): U.S. Government Printing Office; 1999. [Google Scholar]
- 4.Jemal A, Ward E, Wu X, et al. Geographical patterns of prostate cancer mortality and variations in access to medical care in the United States. Cancer Epidemiol Biomarkers Prev 2005;14:590–5. doi: 10.1158/1055-9965.EPI-04-0522 [DOI] [PubMed] [Google Scholar]
- 5.Houston KA, King J, Li J, Jemal A. Trends in prostate cancer incidence rates and prevalence of prostate-specific antigen screening by socioeconomic status and regions in the US, 2004–2013. J Urol. 2018;199:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Negoita S, Feuer EJ, Mariotto M, et al. Annual report to the nation on the status of cancer, part II: recent changes in prostate cancer trends and disease characteristics. Cancer. 2018;124:2801‐2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brawley OW, Jani AB, Master V. Prostate cancer and race. Curr Probl Cancer. 2007;31:211–25. [DOI] [PubMed] [Google Scholar]
- 8.Singh GK, Jemal A. Socioeconomic and Racial/Ethnic Disparities in Cancer Mortality, Incidence, and Survival in the United States, 1950–2014: Over Six Decades of Changing Patterns and Widening Inequalities. J Environ Public Health. 2017;2017:2819372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brawley OW. Trends in prostate cancer in the United States. J Natl Cancer Inst Monogr. 2012;2012(45):152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegel RL, Sahar L, Portier KM, Ward EM, Jemal A Cancer death rates in US congressional districts. CA Cancer J Clin. 2015; 65: 339‐ 344. [DOI] [PubMed] [Google Scholar]
- 11.Moyer VA, on behalf of the U.S. Preventive Services Task Force. Screening for Prostate Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2012;157:120–134. doi: 10.7326/0003-4819-157-2-201207170-00459 [DOI] [PubMed] [Google Scholar]
- 12.Robbins HA, Engels EA, Pfeiffer RM, Shiels MS. Age at cancer diagnosis for blacks compared with whites in the United States. J Natl Cancer Inst. 2015;107(3):dju489. doi: 10.1093/jnci/dju489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karami S, Young HA, Henson DE.Earlier age at diagnosis: another dimension in cancer disparity? Cancer Detect Prev. 2007;31:29–34. [DOI] [PubMed] [Google Scholar]
- 14.US Preventive Services Task Force. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(18):1901–1913. doi: 10.1001/jama.2018.3710 [DOI] [PubMed] [Google Scholar]
- 15.National Program of Cancer Registries and Surveillance, Epidemiology, and End Results SEER*Stat Database: NPCR and SEER Incidence – U.S. Cancer Statistics 2001–2015 Public Use Research Database, United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; Released June 2018, based on the November 2017 submission. Accessed at www.cdc.gov/cancer/uscs/public-use. [Google Scholar]
- 16.NPCR and SEER Incidence – U.S. Cancer Statistics Public Use Database Data Standards and Data Dictionary. Centers for Disease Control and Prevention and National Cancer Institute; Released June 2018, based on the November 2017 submission. Accessed at www.cdc.gov/cancer/npcr/pdf/public-use/npcr-seer-public-use-database-data-dictionary-2001-2015-508.pdf. [Google Scholar]
- 17..Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, Aggregated With State, Total U.S. (1990–2015) <Katrina/Rita Population Adjustment>, National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, released December 2017. Underlying mortality data provided by National Center for Health Statistics; (www.cdc.gov/nchs). [Google Scholar]
- 18.Fritz A, Percy C, Jack A, Shanmugarathnam K, Sobin L, Parkin, et al. , editors. International Classification of Diseases for Oncology. 3rd edition Geneva, Switzerland: World Health Organization; 2000. Available at: http://www.who.int/classifications/icd/adaptations/oncology/en/. [Google Scholar]
- 19.Young JL Jr, Roffers SD, Ries LAG, Fritz AG, AA H, editors. SEER Summary Staging Manual - 2000: Codes and Coding Instructions. Bethesda, MD: National Cancer Institute; 2001. [Google Scholar]
- 20.National Cancer Institute. SEER*Stat software. Bethesda, MD: National Cancer Institute, Surveillance Research Program; 2019. Available at https://seer.cancer.gov/seerstat/. [Google Scholar]
- 21.Anderson R, Rosenberg H. Age standardization of death rates: implementation of the year 2000 standard. National Vital Statistics Report 1998;47:1–16. Available at www.ncbi.nlm.nih.gov/pubmed/9796247. [PubMed] [Google Scholar]
- 22.National Cancer Institute. Surveillance Epidemiology and End Results (SEER) Program. Population Estimates Used in NCI’s SEER*Stat Software Available at https://seer.cancer.gov/popdata/methods.html.
- 23.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Statistical Methods in Medical Research 2006;15:547–569. Available at www.ncbi.nlm.nih.gov/pubmed/17260923. [DOI] [PubMed] [Google Scholar]
- 24.Fay MP. Approximate confidence intervals for RRs from directly standardized rates with sparse data. Communications in Statistics: Theory and Methods 2007;28(9):2141–2160. [Google Scholar]
- 25.Richard A, Rohrmann S, Zhang L, et al. Racial variation in sex steroid hormone concentration in black and white men: a meta-analysis. Andrology. 2014;2(3):428–435. doi: 10.1111/j.2047-2927.2014.00206.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amundadottir LT, et al. A common variant associated with prostate cancer in European and African populations. Nature Genet. 2006;38:652–658 [DOI] [PubMed] [Google Scholar]
- 27.Powell IJ, Dyson G, Land S, et al. Genes Associated with Prostate Cancer Are Differentially Expressed in African American and European American Men. Cancer Epidemiology Biomarkers & Prevention. 2013; 22(5):891–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whittemore AS, Kolonel LN, Wu AH, et al. Prostate cancer in relation to diet, physical activity, and body size in blacks, whites, and Asians in the United States and Canada. J. Natl. Cancer Inst 1995; 87:652–661. [DOI] [PubMed] [Google Scholar]
- 29.Moses KA, Orom H, Brasel A, Gaddy J, Underwood W. Racial/Ethnic Disparity in Treatment for Prostate Cancer: Does Cancer Severity Matter? Urology. 2016; 99:76–83. doi: 10.1016/j.urology.2016.07.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004; 54:78 [PubMed: 15061598] [DOI] [PubMed] [Google Scholar]
- 31.Ross RK, Bernstein L, Lobo RA, Shimizu H, Stanczyk FZ, Pike MC, et al. 5-Alpha-reductase activity and risk of prostate cancer among Japanese and US white and black males. Lancet. 1992; 339:887–9. [DOI] [PubMed] [Google Scholar]
- 32.Ross RK,Pike MC, Coetzee GA, et al. Androgen metabolism and prostate cancer: establishing a model of genetic susceptibility. Cancer Res. 1998; 58:4497–450 [PubMed] [Google Scholar]
- 33.Cussenot O, Valeri A. Heterogeneity in genetic susceptibility to prostate cancer. Eur J Intern Med. 2001; 12:11–16. [DOI] [PubMed] [Google Scholar]
- 34.Applegate CC, Rowles JL, Ranard KM, Jeon S, Erdman JW. Soy Consumption and the Risk of Prostate Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients. 2018;10(1):40 Published 2018 Jan 4. doi: 10.3390/nu10010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torre LA, Sauer AM, Chen MS Jr, Kagawa-Singer M, Jemal A, Siegel RL. Cancer statistics for Asian Americans, Native Hawaiians, and Pacific Islanders, 2016: Converging incidence in males and females. CA Cancer J Clin. 2016;66(3):182–202. doi: 10.3322/caac.21335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robbins AS, Koppie TM, Gomez SL, Parikh-Patel A, Mills PK. Differences in prognostic factors and survival among White and Asian men with prostate cancer, California, 1995–2004. Cancer. 2007;110(6):1255–1263. [DOI] [PubMed] [Google Scholar]
- 37.Oakley-Girvan I, Kolonel LN, Gallagher RP, Wu AH, Felberg A, Whittemore AS. Stage at diagnosis and survival in a multiethnic cohort of prostate cancer patients. Am J Public Health. 2003;93(10):1753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chao GF, Krishna N, Aizer AA et al. (2016) Asian Americans and prostate cancer: a nationwide population-based analysis. Urol Oncol 34(5):233 e7–233.e15 [DOI] [PubMed] [Google Scholar]
- 39.Hoffman RM, Gilliland FD, Eley JW, et al. Racial and ethnic differences in advanced‐stage prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2001; 93: 388–395. [DOI] [PubMed] [Google Scholar]
- 40.Pinheiro PS, Callahan KE, Siegel RL, et al. Cancer Mortality in Hispanic Ethnic Groups. Cancer Epidemiol Biomarkers Prev. 2017;26(3):376–382. doi: 10.1158/1055-9965.EPI-16-0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorin SS, Heck JE. Cancer screening among Latino subgroups in the United States. Prev Med. 2005. May;40(5):515–26. [DOI] [PubMed] [Google Scholar]
- 42.Ennis SR, Rios-Vargas M, Albert NG. The Hispanic Population: 2010. U.S. Census Bureau. 2011. https://www.census.gov/prod/cen2010/briefs/c2010br-04.pdf [Google Scholar]
- 43.Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Population Health. BRFSS Prevalence & Trends Data [online]. 2015. https://www.cdc.gov/brfss/brfssprevalence/.
- 44.Hoffman RM, Li J, Henderson JA, Ajani UA, Wiggins C. Prostate cancer deaths and incident cases among American Indian/Alaska Native men, 1999–2009. Am J Public Health. 2014;104 Suppl 3(Suppl 3):S439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110(10):2119–2152. [DOI] [PubMed] [Google Scholar]
- 46.Melkonian SC, Jim MA, Haverkamp D, Wiggins CL, McCollum J, White MC, et al. Disparities in cancer incidence and trends among American Indians and Alaska Natives in the United States, 2010–2015. Cancer Epidemiology, Biomarkers & Prevention, 2019. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henderson JA, Espey DK, Jim MA, German RR, Shaw KM, Hoffman RM. Prostate cancer incidence among American Indian and Alaska Native men, US, 1999–2004. Cancer. 2008;113(5 suppl):1203–1212. [DOI] [PubMed] [Google Scholar]
- 48.Haile RW, John EM, Levine AJ, et al. A review of cancer in U.S. Hispanic populations. Cancer Prev Res (Phila). 2012;5(2):150–163. doi: 10.1158/1940-6207.CAPR-11-0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powell IJ, Bock CH, Ruterbusch JJ, Sakr W. Evidence Supports a Faster Growth Rate and/or Earlier Transformation to Clinically Significant Prostate Cancer in Black Than in White American Men, and Influences Racial Progression and Mortality Disparity. Journal of Urology. 2010; 183(5):1792–6. [PubMed: 20299055] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trinh QD, Li H, Meyer CP, et al. Determinants of cancer screening in Asian-Americans. Cancer Causes Control. 2016;27:989 10.1007/s10552-016-0776-8 [DOI] [PubMed] [Google Scholar]
- 51.Arias E, Schauman WS, Eschbach K, Sorlie PD, Backlund E. The validity of race and Hispanic origin reporting on death certificates in the United States. National Center for Health Statistics. Vital Health Stat 2(148). 2008. [PubMed] [Google Scholar]
- 52.Martinez Tyson D, Medina-Ramirez P, Flores AM, Siegel R, Aguado Loi C. Unpacking Hispanic Ethnicity-Cancer Mortality Differentials Among Hispanic Subgroups in the United States, 2004–2014. Front Public Health. 2018;6:219 Published 2018 August 31. doi: 10.3389/fpubh.2018.00219 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


