Abstract
Background:
The four-kallikrein (4K) panel has been demonstrated to improve prediction of aggressive prostate cancer (PCa) compared to prostate-specific antigen (PSA) among men with moderately elevated PSA levels. However, the development and testing of the 4K panel has been conducted primarily in White men, with limited data in African Americans and no studies in other racial and ethnic groups.
Methods:
We evaluated the 4K panel in a nested case-control study among African American, Latino, Japanese, Native Hawaiian, and White men in the Multiethnic Cohort (MEC). Pre-diagnostic blood levels of free, intact, and total PSA and human kallikrein-related peptidase 2 were measured among 1,667 incident PCa cases and 691 controls with PSA≥2 ng/mL. We evaluated the discriminative ability of the 4K panel within and across all racial/ethnic groups.
Results:
The 4K panel enhanced discrimination of overall PCa compared to free plus total PSA and total PSA alone (AUC 0.748 versus 0.711 and 0.669, respectively). Discrimination was further enhanced for Gleason 8+ PCa, aggressive PCa, and death due to PCa, and to a lesser degree for non-aggressive PCa. Improvement of the 4K panel over PSA was observed in each population. Adding a PCa polygenic risk score slightly improved upon the discriminative ability of the 4K panel.
Conclusions:
The superior discriminative ability of the 4K panel over PSA for overall and aggressive PCa across multiethnic populations indicates the broad clinical applicability of the 4K panel.
Impact:
Our multiethnic investigation suggests potential for the 4K panel to improve current PCa screening practices.
Keywords: Aggressive prostate cancer, Four-kallikrein panel, Multiethnic populations, Polygenic risk score, Prostate specific antigen
INTRODUCTION
Prostate-specific antigen (PSA) is a prognostic factor for prostate cancer (PCa), and PSA levels during midlife have been shown to be predictive of subsequent aggressive and lethal disease in men of European and African ancestry(1,2). However, studies have found that PSA screening leads to considerable over-diagnosis of indolent disease that is unlikely to progress and a large number of men undergoing biopsies and unnecessary invasive treatments that lower quality of life(3–6). Given these limitations, most guidelines recommend against systematic population-based PSA screening(7). Screening tools with improved discriminative ability for aggressive and lethal disease than PSA could greatly enhance clinical decision making and reduce the number of unnecessary biopsies performed and treatments received.
The four-kallikrein (4K) panel, commercially available as the 4Kscore test (OPKO Health Inc.), which consists of blood measures of total, free, and intact PSA and human kallikrein-related peptidase 2 (hK2), has been demonstrated to improve detection of aggressive PCa compared to PSA among men with moderately elevated PSA levels(8–16). A meta-analysis of 11,134 predominantly White participants across 12 studies reported that the 4K panel predicted high grade PCa with an area under the curve (AUC) of 0.81 (95% CI 0.79–0.83), with AUC increments of improvement ranging from 0.03–0.12 compared to PSA(17). However, only three previous 4K investigations included men of African descent(12,13,18) (390 men total or 3.5% of the 11,134 men in the meta-analysis), and none included substantial numbers of participants from other non-White populations. It is of particular importance to comprehensively understand the 4K panel effectiveness in multiethnic populations, as PCa risk differs widely by ancestral backgrounds. Among US men, African Americans have an approximately two-fold higher PCa mortality rate compared to Whites and Hispanics (38.9 per 100,000 men versus 18.1 and 15.8, respectively), whereas Asians/Pacific Islanders have the lowest (8.6 per 100,000)(19). Further, it has been suggested that prostate tumors progress more quickly in men of African ancestry(20).
In this investigation, we evaluated the discriminative performance of the previously established 4K model(10) in a nested case-control PCa study among African American (AA), Latino (LA), Japanese (JA), Native Hawaiian (NH), and White (WH) men in the Multiethnic Cohort (MEC) with moderately elevated PSA levels, as these men are the target population for clinical use of the 4K panel. In secondary analyses, we assessed whether the inclusion of a PCa polygenic risk score (PRS) enhances the discriminative ability of the 4K panel.
MATERIALS AND METHODS
Participants
The MEC is a large multiethnic prospective cohort established between 1993–1996 to investigate risk of cancer and other chronic conditions among individuals living in Hawaii and Los Angeles(21). Between 2001–2006, blood samples were collected from ~67,000 participants for nested case-control cancer studies. For this investigation, pre-diagnostic plasma levels of free, intact, and total PSA and hK2 were measured among 2,224 incident PCa cases and 2,230 controls, of which 1,667 cases and 691 controls had elevated total PSA (≥2 ng/mL). Cancer cases were identified through linkage to cancer Surveillance, Epidemiology, and End Results (SEER) registries in Hawaii and California, while cancer mortality was determined though routine linkages to death certificates through state death files and the National Death Index for deaths that occurred outside of Hawaii and California. With the exception of death due to PCa, disease aggressiveness was defined at the time of diagnosis based on histological status of disease (e.g., Gleason score, stage), which was obtained from SEER registries. Controls were matched to cases using incidence density sampling based on race and ethnicity, birth year, area (Hawaii or Los Angeles), hours of fasting, and year and time of collection. Controls were alive and PCa free at the age of the case diagnosis (see Supplement). Participants included AA, LA, JA, NH, and WH men, with race/ethnicity acquired from a questionnaire completed by participants at baseline(22).
Five primary outcomes were assessed: overall PCa, Gleason 8+ PCa, aggressive PCa, PCa death, and non-aggressive PCa. Gleason 8+ cases were those with a Gleason score≥8. Aggressive cases were those with either a Gleason score≥8, non-localized disease, or who later died due to PCa. Non-aggressive cases were defined as cases with a Gleason score≤7, localized disease, and who did not die due to PCa. The outcomes of aggressive PCa and PCa death excluded 19 ineligible controls who died prior to the matched case’s PCa death. We additionally compared aggressive cases to non-aggressive cases. Because of changes in the SEER grading system over time we were unable to differentiate Gleason 7 tumors for a substantial proportion of cases, and thus, we focused the analysis on men with Gleason 8+ tumors.
We obtained informed written consent from all participants, and study protocols were approved by the Institutional Review Boards at the University of Hawaii and the University of Southern California.
Laboratory Methods
Laboratory methods have been previously described(12) (see Supplement). Samples were processed in two batches, with 38 samples processed in both. Measurements between batches for these 38 samples were highly correlated (using all 38 samples, not limited to those with PSA≥2 ng/mL): Pearson r(total PSA)=0.99, r(free PSA)=0.98, r(intact PSA)=0.94, and r(hK2)=0.79 (after excluding one outlying sample with discordant hK2 levels, correlation for hK2 became r=0.90). In the combined batches, the coefficient of variation was 49.9% for total PSA at the median 2.15 ng/mL, 40.5% for free PSA at the median 0.51 ng/mL, 43.8% for intact PSA at the median 0.28 ng/mL, and 43.3% for hK2 at the median 0.05 ng/mL. Coefficients of variation within batches were comparable (Table S1). 4K markers were positively correlated (Table S2).
Statistical Analyses
Comparisons of Individual 4K Marker Levels and 4K Score
Ethnic-specific differences in 4K marker levels and associations between 4K marker levels and potential PCa risk factors were assessed using the full sample (not limited those with PSA≥2 ng/mL). The 4K score was calculated by combining the four biomarkers into a single score, reflecting each participant’s predicted probability of receiving a positive biopsy, using the pre-specified model(10) equivalent to the commercially available 4Kscore (OPKO Health Inc.). Linear regression models including each of the 4K markers and the 4K score as separate outcomes were used to assess ethnic-specific differences in marker levels, stratifying by case-control status and controlling for matching factors, body-mass index (BMI) at blood draw, and laboratory batch. Similar linear models were used to test associations between 4K markers and type 2 diabetes (T2D), BMI, and age at blood draw.
Discriminative Ability of 4K Panel
We evaluated the ability of the 4K panel to discriminate between PCa cases and controls by calculating AUCs, implemented in the pROC R package(23), of logistic regression models with covariates included for matching factors, BMI at blood draw, and batch. Model AUCs were compared between models that additionally included 1) total PSA, 2) total and free PSA, and 3) the 4K score as variables, focusing on relative AUCs. Analyses were repeated within racial/ethnic groups to assess ethnic-specific AUCs. Ethnic-specific differences in the discriminative ability of the 4K panel were investigated by evaluating model fit using a likelihood ratio test (LRT), comparing the 4K model to a model adding an interaction term for 4K score*ethnicity. Theoretical considerations suggest that the 4K panel would not be of value to men with low PSA due to the difficulty of measuring extremely low levels of PSA-forms (e.g., intact PSA), and indeed, previous studies have shown that this model does not enhance the discriminative ability of PSA in this subgroup(24); thus, primary analyses were restricted to individuals with PSA≥2 ng/mL. Since this PSA restriction breaks the matching of case and controls, we ran sensitivity analyses limited to sets of matched cases and controls within each outcome, where each participant had PSA≥2 ng/mL. Sensitivity analyses were also performed excluding cases diagnosed <1 year from the blood draw (n=148). Analyses were additionally performed stratified by the median age at blood draw (69 years).
Polygenic Risk Scores
In secondary analyses, we investigated whether a PCa PRS could improve the discriminative ability of the 4K panel. Of the 2,358 MEC participants with PSA≥2 ng/mL, 1,776 had genome-wide imputed genotype data. A weighted PRS was calculated for each participant as the sum of the number of risk alleles carried by an individual, weighted by previously estimated variant-specific effects for 135 PCa-associated variants (see Supplement). AUCs were recalculated for all models in this subset of participants with PRS and for a fourth model adding the PRS as a covariate to the 4K model, along with genetic ancestry using the first 10 principal components from a principal component analysis (see Supplement).
RESULTS
Participants
Participant characteristics for those with PSA≥2 ng/mL used in the primary analysis to assess the discriminative ability of the 4K panel are described in Table 1. The mean ages of cases and controls at blood draw were 69 (range: 47–86) and 72 (54–87), respectively. Samples were drawn an average of 4.1 years (range: <1–18 years) prior to a PCa diagnosis. Characteristics for the full sample used to compare individual 4K marker levels are in Table S3 (note that the distribution of participants by race/ethnicity was similar before and after applying the PSA exclusion criterion).
Table 1.
Participant characteristics (N=2,358). Unadjusted mean (SD) or N (%).
| Characteristic | No Cancer (n=691) | Cancer (n=1,667) | Gleason 8+ PCa (n=370) | Aggressive PCa (n=543) | Death due to PCa (n=93) | Non-Aggressive PCa (n=1,011) |
|---|---|---|---|---|---|---|
| Demographic/Clinical Characteristics | ||||||
| Age at Diagnosis | -- | 73.0 (7.6) | 75.5 (8.0) | 74.1 (8.2) | 75.1 (7.9) | 72.2 (7.2) |
| Birth Year | 1931 (7.0) | 1933 (7.8) | 1931 (7.4) | 1933 (7.8) | 1929 (7.1) | 1934 (7.7) |
| Blood Draw Year | 2002 (2.3) | 2002 (2.4) | 2002 (2.3) | 2002 (2.3) | 2001 (2.4) | 2002 (2.4) |
| Age at Blood Draw | 71.6 (6.6) | 68.9 (7.5) | 71.1 (7.3) | 69.9 (7.6) | 71.9 (6.9) | 68.3 (7.5) |
| BMI at Blood Draw | 26.4 (4.2) | 26.5 (4.0) | 26.5 (3.9) | 26.7 (4.0) | 26.6 (4.0) | 26.5 (4.0) |
| Area | ||||||
| Hawaii | 331 (48) | 836 (50) | 220 (59) | 296 (55) | 27 (29) | 510 (50) |
| Los Angeles | 360 (52) | 831 (50) | 150 (41) | 247 (45) | 66 (71) | 501 (50) |
| Ethnicity | ||||||
| Japanese | 218 (32) | 539 (32) | 144 (39) | 184 (34) | 11 (12) | 333 (33) |
| African American | 156 (23) | 363 (22) | 64 (17) | 108 (20) | 37 (40) | 216 (21) |
| Latino | 153 (22) | 347 (21) | 53 (14) | 97 (18) | 24 (26) | 214 (21) |
| White | 131 (19) | 325 (19) | 84 (23) | 118 (22) | 20 (22) | 192 (19) |
| Native Hawaiian | 33 (5) | 93 (6) | 25 (7) | 36 (7) | 1 (1) | 56 (6) |
| Tumor Characteristics | ||||||
| Gleason Score | ||||||
| ≤7 | -- | 1,231 (74) | 0 | 145 (27) | 35 (38) | 1,011 (100) |
| ≥8 | -- | 370 (22) | 370 (100) | 370 (68) | 42 (45) | 0 |
| NA | 691 (100) | 66 (4) | 0 | 28 (5) | 16 (17) | 0 |
| Stage | ||||||
| Localized | -- | 1,308 (78) | 252 (68) | 279 (51) | 39 (42) | 1,011 (100) |
| Regional | -- | 153 (9) | 49 (13) | 153 (28) | 8 (9) | 0 |
| Metastatic | -- | 79 (5) | 43 (12) | 79 (15) | 38 (41) | 0 |
| NA | 691 (100) | 127 (8) | 26 (7) | 32 (6) | 8 (9) | 0 |
| 4K Markers | ||||||
| Total PSA (ng/mL) | 4.60 (4.94) | 7.58 (16.15) | 10.23 (23.74) | 10.32 (22.73) | 21.49 (38.16) | 5.62 (5.56) |
| Free PSA (ng/mL) | 1.13 (0.88) | 1.31 (3.16) | 1.74 (6.05) | 1.72 (5.30) | 3.65 (9.04) | 1.07 (0.84) |
| Intact PSA (ng/mL) | 0.55 (0.43) | 0.74 (2.21) | 1.06 (4.25) | 1.05 (3.75) | 2.47 (6.66) | 0.57 (0.50) |
| hK2 (ng/mL) | 0.09 (0.14) | 0.11 (0.18) | 0.15 (0.29) | 0.15 (0.28) | 0.29 (0.43) | 0.10 (0.10) |
| 4K Score (%) | 15.3 (15.5) | 27.5 (24.2) | 35.7 (26.9) | 34.7 (27.3) | 50.1 (32.5) | 24.7 (21.1) |
Comparisons of Individual 4K Marker Levels and 4K Score
4K marker levels and 4K scores at time of the blood draw were significantly higher in men subsequently diagnosed with PCa compared to those who did not develop PCa (Table S4 and Figure S1). Among PCa controls, individuals with T2D had slightly lower total PSA and 4K scores than those without T2D (Table S5). Older age was associated with higher individual 4K marker levels and 4K scores (Table S6). Among cases, African Americans had higher levels of total, free, and intact PSA than Latinos and lower levels of hK2 than Native Hawaiians (Figure 1), potentially influenced by slightly different distributions of aggressive disease between ethnicities (Table 1). Among controls, 4K scores were slightly higher in African Americans than Japanese, Latinos, and Native Hawaiians (Figure 1).
Figure 1.
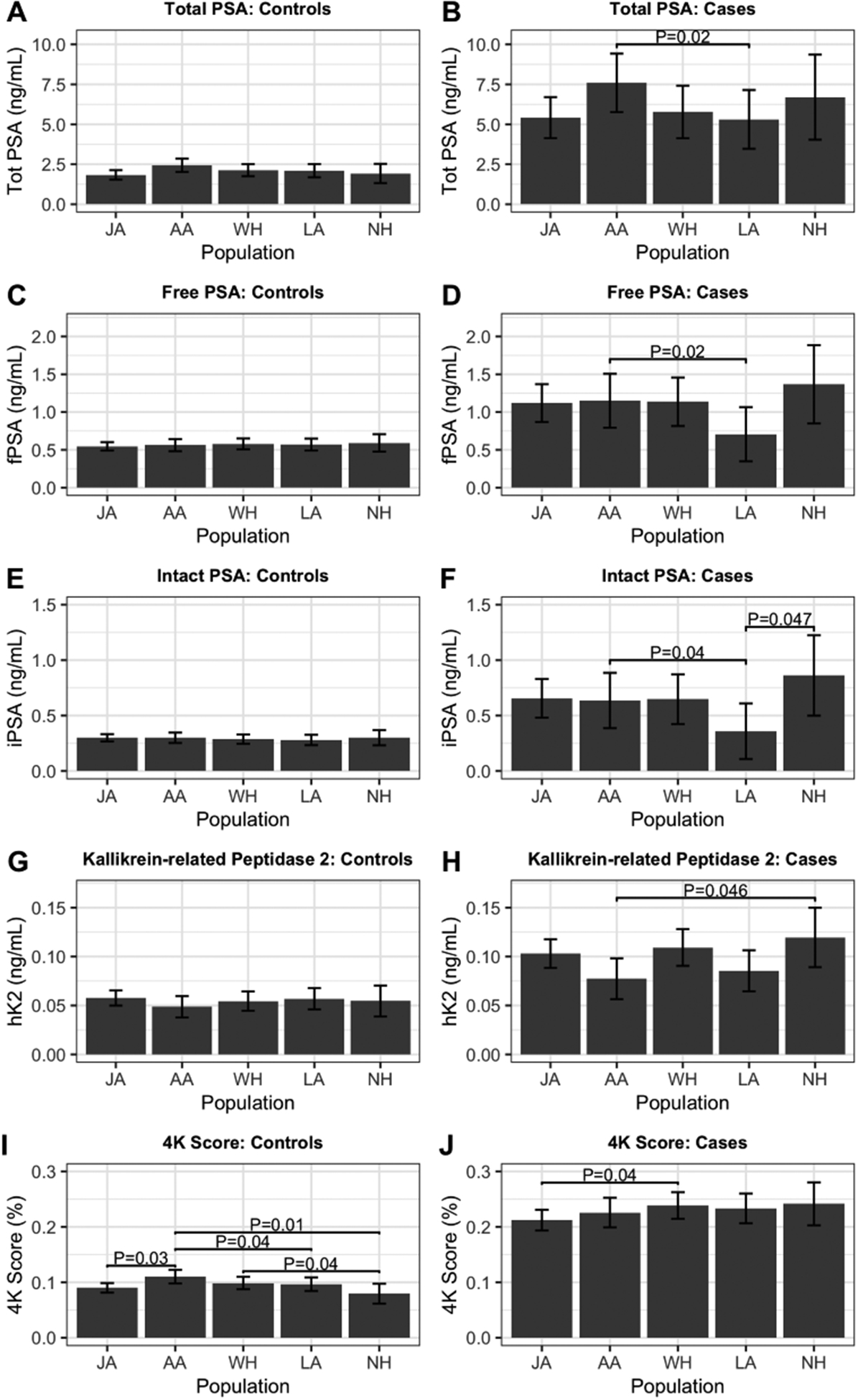
Levels of 4K markers and 4K score by ethnicity. Mean levels are based on linear regression models adjusted for matching factors (race and ethnicity, age at blood draw, area [Hawaii or Los Angeles], hours of fasting, year of collection, and time of collection), body-mass index (BMI) at blood draw, and laboratory batch. Unadjusted P-value indicated when P<0.05. JA: Japanese, AA: African American, LA: Latino, WH: White, NH: Native Hawaiian.
Discriminative Ability of 4K Panel
Model fit for overall PCa was significantly improved when adding an interaction term for 4K*ethnicity (LRT P<0.001), suggesting differences in 4K model performance by ethnicity. Thus, we report overall and ethnic-specific results. Among men with elevated PSA (≥2.0 ng/mL; 1,667 cases and 691 controls), the AUC for overall PCa was 0.748 for the 4K panel compared to 0.711 for free plus total PSA and 0.669 for total PSA alone (Figure 2 and Table S7). Discrimination was similarly enhanced for the 4K panel when comparing controls to aggressive disease subsets for cases: the AUC was 0.777 for Gleason 8+ PCa (versus 0.739 for free plus total PSA and 0.685 for total PSA alone) and 0.782 for aggressive PCa (versus 0.739 for free plus total PSA and 0.678 for total PSA alone). We observed improved discriminative ability of the 4K panel over PSA when comparing non-aggressive PCa cases to controls (4K AUC of 0.747 versus 0.726 for free plus total PSA and 0.693 for total PSA alone); however, the magnitude of these improvements was lower for non-aggressive PCa than aggressive outcomes (AUC increment comparing 4K to total PSA: +0.104 and +0.054 for aggressive and non-aggressive PCa, respectively; Figure 2 and Table S7). Overall, the 4K panel was less predictive of non-aggressive PCa (AUC=0.747) than aggressive PCa (AUC=0.782). When comparing aggressive to non-aggressive cases, the AUC for the 4K panel had slightly improved discriminative ability over PSA, with an AUC of 0.645 for the 4K panel compared to 0.639 for free plus total PSA and 0.632 for total PSA alone (Figure 2 and Table S7).
Figure 2.
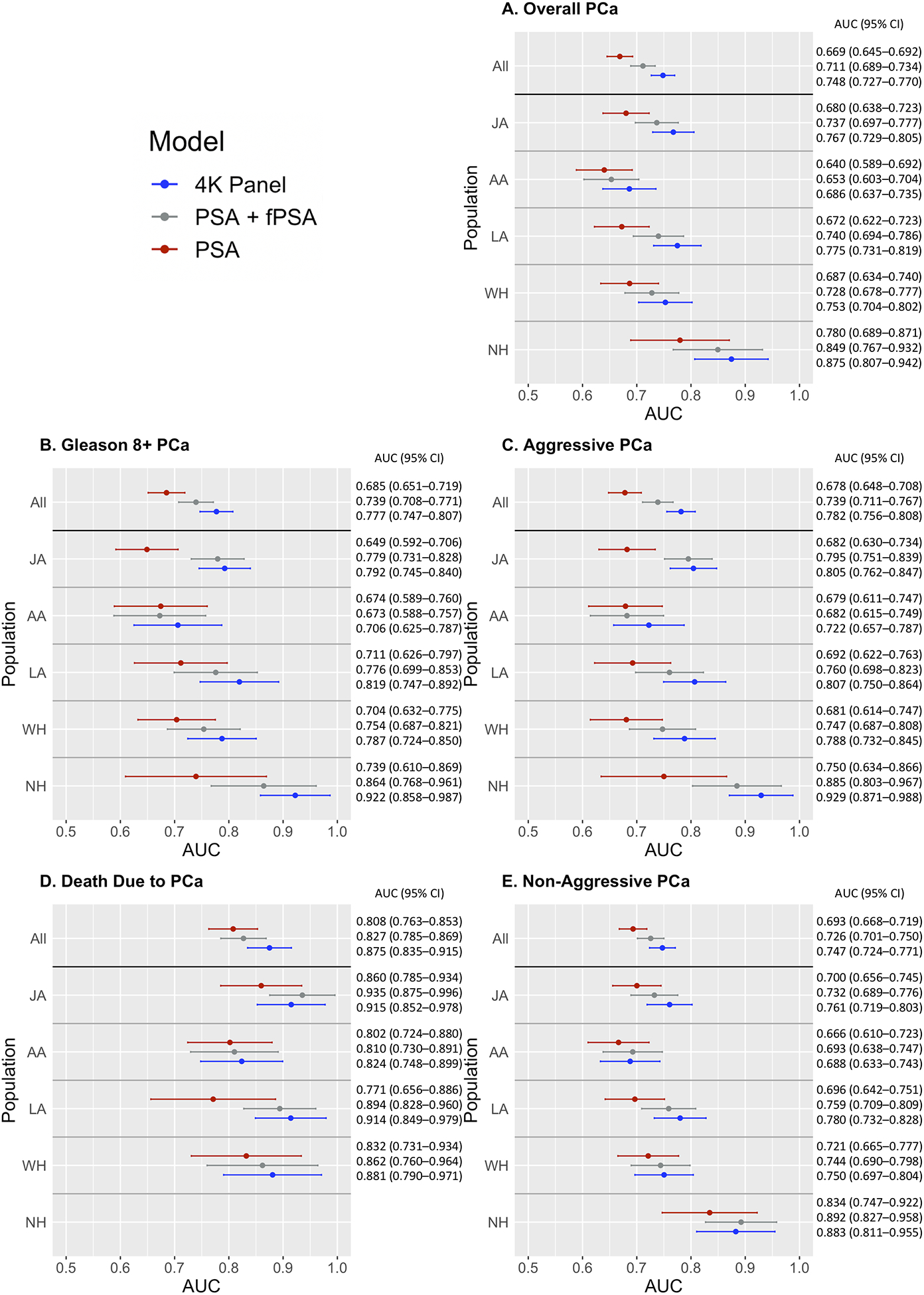
AUC results by outcome, ethnicity, and model (PSA, PSA + free PSA, 4K Panel) among men with total PSA≥2 ng/mL. A. Overall PCa, B. High Grade PCa, C. Aggressive PCa, D. Death Due to PCa, E. Non-Aggressive PCa. Analyses are adjusted for matching factors (race and ethnicity [“All” analyses only], age at blood draw, area [Hawaii or Los Angeles], hours of fasting, year of collection, and time of collection), body-mass index (BMI) at blood draw, and laboratory batch. The 95% confidence interval for each AUC is indicated by horizontal bars. JA: Japanese, AA: African American, LA: Latino, WH: White, NH: Native Hawaiian.
Improvements of the 4K panel over PSA were consistently observed in each racial/ethnic group for overall, high grade, and aggressive PCa, and PCa death; however, PCa death AUCs within populations were unstable due to the smaller number of events. AUC improvements between the 4K and free plus total PSA models were similar between populations, ranging from +0.009 to +0.058 across overall, Gleason 8+, and aggressive PCa. AUC improvements between the 4K and total PSA only models ranged from +0.032 to +0.183, with African Americans having the lowest and Native Hawaiians the highest AUC improvement. Japanese and Latino men consistently had large AUC improvements (Figure 2 and Table S7). The 4K model had the highest discriminative ability in Native Hawaiians, followed by Latinos, Japanese, Whites, and African Americans. Similar to the free plus total PSA model, the 4K model consistently had the lowest discriminative ability in African Americans.
AUCs calculated in sensitivity analyses limited to matched cases and controls with PSA≤2 ng/mL for each outcome were extremely similar to those observed in the complete sample; results stratified by population were highly similar as well, suggesting that our results are robust to the inclusion of all controls in analyses of disease aggressiveness, although some sample sizes were too small to calculate reliable AUC estimates (Table S8). Upon removing cases diagnosed less than one year after blood draw, results were similar, and the interpretation was identical (results not shown). As expected and consistent with previous findings(24), the 4K model did not notably enhance the discriminative ability of PSA when including individuals with PSA<2 ng/mL (Tables S9 and S10).
In analyses stratified by median age at blood draw, the 4K panel typically had improved performance compared to PSA and the combined total plus free PSA across outcomes and populations in men providing a blood draw at >69 and ≤69 years of age. For overall PCa, the discriminative ability of all three models (total PSA, total plus free PSA, and the 4K panel) was higher in older individuals, and the AUC improvement of the 4K panel (compared to PSA and total plus free PSA) was greater in older individuals. This was also observed for the more aggressive categories when including all populations and less consistently within populations. Among Japanese men providing a blood draw at younger ages, the 4K panel had lower AUCs compared to total plus free PSA for overall PCa, Gleason 8+, and aggressive PCa (Table S11).
Polygenic Risk Score
When including the PRS in the 4K model among men with elevated PSA (≥2 ng/mL) and genomic data (1,252 cases and 524 controls), the AUC increased by ~0.01 in all subgroups (to 0.766 for overall PCa, 0.789 for Gleason 8+ PCa, 0.761 for non-aggressive PCa, and 0.801 for aggressive PCa). Improvements were greater within Native Hawaiians and minimal among Japanese (Figure S2 and Table S12).
In sensitivity analyses, AUCs were recalculated using 10-fold cross-validation on the full sample. Results were similar and the interpretation was identical (results not shown).
DISCUSSION
In this prospective investigation, we demonstrate that the 4K panel can be extended to non-White men and that it effectively discriminates overall and aggressive PCa cases from controls among men with PSA≥2 ng/mL in multiethnic populations. It also discriminates non-aggressive PCa cases from controls, although to a lesser degree than observed for aggressive PCa. The 4K panel performed consistently better than free plus total PSA and total PSA alone in all racial and ethnic populations tested. Compared to total PSA alone, the 4K panel had the least amount of improvement among African American men, with the AUC increment of improvement being about half the increment observed in the full sample across outcomes. We also noted slight but consistent improvements in the discriminative ability of the 4K model with the inclusion of a PCa PRS.
Improved PCa discrimination is of particular importance among African American men, as they have the highest PCa incidence and mortality rates in the US(19,21,25) and tumors with more aggressive characteristics(20,26). Despite the lower performance of the 4K panel in African Americans compared to other populations, our results suggest that African American men are likely to benefit from the 4K panel versus PSA alone. Three previous 4K investigations that included African American men did not identify significant differences between the performance of the 4K panel in African Americans versus Whites, potentially due to limited sample sizes and the use of samples collected closer to the time of diagnosis (12,13,18).
Among the five populations tested, Japanese and Native Hawaiians have the lowest PCa incidence and mortality(27). Despite the 4K model being based on estimates from European men, in our investigation, it had slightly better discriminative ability in Native Hawaiians, Japanese, and Latinos than Whites. However, in analyses stratified by the median age at blood draw, the 4K panel had improved discriminative ability in older Japanese men but did not have improved performance compared to the combined total plus free PSA in younger Japanese men for overall, Gleason 8+, and aggressive PCa. The 4K panel performed particularly well in Native Hawaiians, with AUCs ranging from 0.875–0.929 among the four outcomes and large AUC improvements compared to PSA alone. Although this is the largest multiethnic investigation of the 4K panel to date, replication with larger sample sizes, particularly among Native Hawaiians, is needed to verify the comparative performance of the 4K panel between racial and ethnic populations.
Our multiethnic 4K findings are consistent with other largely-European 4K investigations. A study of 4,765 biopsied European ProtecT participants with PSA≥3 ng/mL reported a high grade PCa (defined as Gleason score≥7) AUC of 0.82 (95% CI 0.80–0.84) and overall PCa AUC of 0.72 (95% CI 0.70–0.73)(10), comparable to the MEC Gleason 8+ PCa AUC of 0.78 (95% CI 0.75–0.81) and overall PCa AUC of 0.75 (95% CI 0.73–0.77). The AUC increment of the 4K panel compared to free plus total PSA and PSA alone in this previous investigation is also comparable to our results.
This study was not without limitations. The PSA exclusion criterion greatly reduced our sample size and power for within population analyses, particularly for Native Hawaiians. Only 93 participants in our sample died due to PCa, limiting our power to assess the 4K panel’s ethnic-specific ability to discriminate between controls and PCa cases who likely have the most lethal tumors and whose prognosis cannot be determined by stage or grade. Although PSA screening rates were comparable between ethnicities (61% of WH, 59% of AA, 51% of JA, 51% of LA, and 32% of NH, reported undergoing clinical PSA screening), lower rates in Native Hawaiians could contribute to the higher 4K performance in this group. The 4K panel may not have performed as well in African Americans because the 4K model was developed within a predominantly White sample(10) and Whites have dissimilar PCa risk from African Americans. This could potentially be improved by developing a new model using a large African American population. Since participants were not all biopsied and we relied on SEER registries and death certificates for PCa outcomes, we cannot eliminate the possibility of misclassification bias.
Given the high mortality burden of PCa, excessive false positive rates resulting from standard PCa screening measures, and the huge health disparity of this disease, a screening tool that can be effectively and universally applied across racial and ethnic populations would be valuable. Our multiethnic prospective investigation suggests that the 4K panel has superior discriminative ability over total and free PSA to detect PCa, especially for more aggressive disease and to a lesser degree for non-aggressive disease, within and across racial and ethnic populations, implicating the broad clinical applicability of the 4K panel and its potential to improve current PCa screening practices. Future investigations to improve the performance of the 4K model for African American men should be a priority. Until then, race and ethnicity should be taken into consideration when the 4K panel is utilized clinically.
Supplementary Material
Acknowledgements
We would like to thank all participants from the Multiethnic Cohort study. We also thank Anqi Wang for support with manuscript preparation.
Financial Information:
Dr. Darst was supported in part by an award from the Achievement Rewards for College Scientists Foundation Los Angeles Founder Chapter. This work was supported in part by the National Institutes of Health/National Cancer Institute (NIH/NCI) with a Cancer Center Support Grant to Memorial Sloan Kettering Cancer Center [P30 CA008748], a SPORE grant in Prostate Cancer to Dr. H. Scher [P50-CA92629], R01CA160816 to Drs. Lilja and Vickers, the Sidney Kimmel Center for Prostate and Urologic Cancers, David H. Koch through the Prostate Cancer Foundation, and K99CA246063 to Dr. Darst. This work was also supported in part by the Swedish Cancer Society (CAN 2017/559) and the Swedish Research Council (VR-MH project no. 2016-02974). The MEC is supported by NIH/NCI grant U01 CA164973.
Footnotes
Conflict of interest:
Hans Lilja hold patents on assays for free PSA, intact PSA, and hK2. Andrew Vickers and Hans Lilja are named on a patent for a statistical method to detect prostate cancer that has been commercialized by OPKO Health. Andrew Vickers and Hans Lilja receive royalties from sales of the test. Hans Lilja has stock and Andrew Vickers has stock options in OPKO Health.
REFERENCES
- 1.Preston MA, Gerke T, Carlsson SV, Signorello L, Sjoberg DD, Markt SC, et al. Baseline Prostate-specific Antigen Level in Midlife and Aggressive Prostate Cancer in Black Men. Eur Urol 2019;75(3):399–407 doi 10.1016/j.eururo.2018.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preston MA, Batista JL, Wilson KM, Carlsson SV, Gerke T, Sjoberg DD, et al. Baseline Prostate-Specific Antigen Levels in Midlife Predict Lethal Prostate Cancer. J Clin Oncol 2016;34(23):2705–11 doi 10.1200/JCO.2016.66.7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst 2009;101(6):374–83 doi 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin RM, Donovan JL, Turner EL, Metcalfe C, Young GJ, Walsh EI, et al. Effect of a Low-Intensity PSA-Based Screening Intervention on Prostate Cancer Mortality: The CAP Randomized Clinical Trial. JAMA 2018;319(9):883–95 doi 10.1001/jama.2018.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor KL, Luta G, Miller AB, Church TR, Kelly SP, Muenz LR, et al. Long-term disease-specific functioning among prostate cancer survivors and noncancer controls in the prostate, lung, colorectal, and ovarian cancer screening trial. J Clin Oncol 2012;30(22):2768–75 doi 10.1200/JCO.2011.41.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986–2005. J Natl Cancer Inst 2009;101(19):1325–9 doi 10.1093/jnci/djp278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tikkinen KAO, Dahm P, Lytvyn L, Heen AF, Vernooij RWM, Siemieniuk RAC, et al. Prostate cancer screening with prostate-specific antigen (PSA) test: a clinical practice guideline. BMJ 2018;362:k3581 doi 10.1136/bmj.k3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vickers A, Cronin A, Roobol M, Savage C, Peltola M, Pettersson K, et al. Reducing unnecessary biopsy during prostate cancer screening using a four-kallikrein panel: an independent replication. J Clin Oncol 2010;28(15):2493–8 doi 10.1200/JCO.2009.24.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta A, Roobol MJ, Savage CJ, Peltola M, Pettersson K, Scardino PT, et al. A four-kallikrein panel for the prediction of repeat prostate biopsy: data from the European Randomized Study of Prostate Cancer screening in Rotterdam, Netherlands. Br J Cancer 2010;103(5):708–14 doi 10.1038/sj.bjc.6605815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bryant RJ, Sjoberg DD, Vickers AJ, Robinson MC, Kumar R, Marsden L, et al. Predicting high-grade cancer at ten-core prostate biopsy using four kallikrein markers measured in blood in the ProtecT study. J Natl Cancer Inst 2015;107(7) doi 10.1093/jnci/djv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nordstrom T, Vickers A, Assel M, Lilja H, Gronberg H, Eklund M. Comparison Between the Four-kallikrein Panel and Prostate Health Index for Predicting Prostate Cancer. Eur Urol 2015;68(1):139–46 doi 10.1016/j.eururo.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim EH, Andriole GL, Crawford ED, Sjoberg DD, Assel M, Vickers AJ, et al. Detection of High Grade Prostate Cancer among PLCO Participants Using a Prespecified 4-Kallikrein Marker Panel. J Urol 2017;197(4):1041–7 doi 10.1016/j.juro.2016.10.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Punnen S, Freedland SJ, Polascik TJ, Loeb S, Risk MC, Savage S, et al. A Multi-Institutional Prospective Trial Confirms Noninvasive Blood Test Maintains Predictive Value in African American Men. J Urol 2018;199(6):1459–63 doi 10.1016/j.juro.2017.11.113. [DOI] [PubMed] [Google Scholar]
- 14.Vickers AJ, Cronin AM, Aus G, Pihl CG, Becker C, Pettersson K, et al. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European Randomized Study of Prostate Cancer Screening in Goteborg, Sweden. BMC Med 2008;6:19 doi 10.1186/1741-7015-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vickers AJ, Cronin AM, Roobol MJ, Savage CJ, Peltola M, Pettersson K, et al. A four-kallikrein panel predicts prostate cancer in men with recent screening: data from the European Randomized Study of Screening for Prostate Cancer, Rotterdam. Clin Cancer Res 2010;16(12):3232–9 doi 10.1158/1078-0432.CCR-10-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Assel M, Sjoblom L, Murtola TJ, Talala K, Kujala P, Stenman UH, et al. A Four-kallikrein Panel and beta-Microseminoprotein in Predicting High-grade Prostate Cancer on Biopsy: An Independent Replication from the Finnish Section of the European Randomized Study of Screening for Prostate Cancer. Eur Urol Focus 2017. doi 10.1016/j.euf.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zappala SM, Scardino PT, Okrongly D, Linder V, Dong Y. Clinical performance of the 4Kscore Test to predict high-grade prostate cancer at biopsy: A meta-analysis of us and European clinical validation study results. Rev Urol 2017;19(3):149–55 doi 10.3909/riu0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parekh DJ, Punnen S, Sjoberg DD, Asroff SW, Bailen JL, Cochran JS, et al. A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer. Eur Urol 2015;68(3):464–70 doi 10.1016/j.eururo.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 19.Group USCSW. 2018 U.S. Cancer Statisitcs Visualizations Tool, based on November 2017 submission data (1999–2015). U.S. Department of Health and Human Services, Center for Disease Control and Prevention and National Cancer Institute <www.cdc.gov/cancer/dataviz>. [Google Scholar]
- 20.Powell IJ, Bock CH, Ruterbusch JJ, Sakr W. Evidence supports a faster growth rate and/or earlier transformation to clinically significant prostate cancer in black than in white American men, and influences racial progression and mortality disparity. J Urol 2010;183(5):1792–6 doi 10.1016/j.juro.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolonel LN, Henderson BE, Hankin JH, Nomura AM, Wilkens LR, Pike MC, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000;151(4):346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H, Haiman CA, Kolonel LN, Henderson BE, Wilkens LR, Le Marchand L, et al. Self-reported ethnicity, genetic structure and the impact of population stratification in a multiethnic study. Hum Genet 2010;128(2):165–77 doi 10.1007/s00439-010-0841-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC bioinformatics 2011;12:77 doi 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stattin P, Vickers AJ, Sjoberg DD, Johansson R, Granfors T, Johansson M, et al. Improving the Specificity of Screening for Lethal Prostate Cancer Using Prostate-specific Antigen and a Panel of Kallikrein Markers: A Nested Case-Control Study. Eur Urol 2015;68(2):207–13 doi 10.1016/j.eururo.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Lee SJ, Teunissen CE, Pool R, Shipley MJ, Teumer A, Chouraki V, et al. Circulating metabolites and general cognitive ability and dementia: Evidence from 11 cohort studies. Alzheimers Dement 2018;14(6):707–22 doi 10.1016/j.jalz.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Sundi D, Ross AE, Humphreys EB, Han M, Partin AW, Carter HB, et al. African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: should active surveillance still be an option for them? J Clin Oncol 2013;31(24):2991–7 doi 10.1200/JCO.2012.47.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SY, Haiman CA, Cheng I, Park SL, Wilkens LR, Kolonel LN, et al. Racial/ethnic differences in lifestyle-related factors and prostate cancer risk: the Multiethnic Cohort Study. Cancer Causes Control 2015;26(10):1507–15 doi 10.1007/s10552-015-0644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


