Abstract
Background:
Although there are considerable racial and ethnic disparities in prostate cancer incidence and mortality in the U.S. and globally, clinical trials often do not reflect disease incidence across racial and ethnic subgroups. This study aims to comprehensively review the reporting of race and ethnicity data and the representation of race and ethnicity across prostate cancer treatment-, prevention-, and screening-based clinical trials.
Methods:
Seventy-two global phase III and IV prevention, screening, and treatment prostate cancer clinical trials with enrollment start dates between 1987 and 2016 were analyzed in this study, representing a total of 893,378 individual trial participants. Availability and representation of race and ethnicity data by trial funding type, temporal changes in the racial/ethnic diversity of participants, and geographic representation of countries were assessed.
Results:
Of the 72 trials analyzed, 59 (81.9%) had available race data, and 11 (15.3%) of these trials additionally reported ethnicity. Of the trials reporting data, participants were overwhelmingly white men (with the highest proportion in U.S. non-publicly funded trials), comprising over 96% of the study population. The proportion of white participants in prostate cancer clinical trials has remained at over 80% since 1990. Geographically, Africa and the Caribbean were particularly underrepresented with only 3% of countries included.
Conclusions:
Trial participants continue to be majority white despite the known racial disparities in prostate cancer clinical outcomes.
Impact:
Current and future trials must use novel recruitment strategies to ensure enrollment of underrepresented men; targeting the inclusion of African and Caribbean medical centers is crucial to achieve equity in representation.
Keywords: prostate, cancer, disparities, trials, race
INTRODUCTION
While mortality rates of prostate cancer in the U.S. have recently been declining,1,2 significant disparities in prostate cancer care and outcomes remain among racial and ethnic groups in the U.S., especially among men of African descent. Compared to all other racial and ethnic groups, African American men have higher prostate cancer incidence and worse survival.1–3 In addition, the incidence of metastatic prostate cancer is now higher in Latino vs. white men in the U.S.4 Studies highlight that the underlying tumor biology of prostate cancer varies by race and ethnicity (defined by the Office of Management and Budget Categories described below), with a different prevalence of tumor alterations in black, white, and Asian men.5 Despite known racial and ethnic disparities in prostate cancer, research into the mechanisms underlying these disparities is lacking, in large part due to the lack of adequate collection of racial and ethnic data as well as the under-enrollment of African Americans and other U.S. minority populations in clinical trials.6,7
The collection of race and ethnicity data for federal purposes in the U.S. began in 1977 with the Office of Management and Budget (OMB) Directive 15, defining five racial and ethnic categories: White, Black, Asian or Pacific Islander, American Indian or Alaska Native, and Hispanic.8 To reflect increasing diversity in the U.S. population over the next two decades, the OMB revised the standards in 1997 to make Hispanic or Latino a separate ethnicity category, split the Asian or Pacific Islander category into two categories, and allow for the selection of more than one race category.9 To better capture the diversity of participants in clinical trials moving forward as the proportion of non-Hispanic White residents is expected to fall from 61.3% to 44.3% in the next 40 years,10 the U.S. Food and Drug Administration recently developed recommendations for more thorough and standardized collection of race and ethnicity data; however, these recommendations have yet to be accepted and implemented.11
Studies assessing diversity of participants in prostate cancer clinical trials under the 1997 OMB collection standards are sparse. A 2004 study examining participation in clinical trials for four types of cancer found that black and white patients had similar clinical trial enrollment fractions out of all cancer patients despite known racial disparities.12 Additionally, an analysis of castrate-resistant prostate cancer trials found low overall enrollment of black men (3.3%) in seven phase III clinical trials.13 Assessment of enrollment from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial found that black and Hispanic patients were under-enrolled despite specific efforts to enroll U.S. minority patients.14 Though this topic has been investigated more in recent years,15 a more comprehensive review of enrollment in prostate cancer clinical trials is needed to determine the extent of racial and ethnic diversity in trials and to determine where recruitment efforts should be targeted.
The objective of this study is to comprehensively review the reporting of race and ethnicity data and the representation of race and ethnicity across prostate cancer treatment-, prevention-, and screening-based clinical trials. It is important to identify benchmarks for trial enrollment at the outset in order to frame these analyses: as 56.3% of incident prostate cancer cases in the U.S. occur in non-Hispanic white men,16–17 we propose this as the maximum acceptable proportion of these men in trials. Additionally, 22.2% of incident prostate cancer cases occur in non-Hispanic black men;16–17 trials should strive to include at least this proportion of black men in their patient population as these men face the highest prostate cancer incidence and mortality of all races/ethnicities. At a bare minimum, it is important that each trial be sufficiently powered to be able to examine whether or not differences exist in effect of an intervention by race/ethnicity.
MATERIALS AND METHODS
Eligible trials for our comprehensive review were identified using PubMed and the U.S. National Library of Medicine’s Clinical Trials database (clinicaltrials.gov). “Prostate cancer” was used as the search term, and two (ER and LB) study investigators independently reviewed each of the study abstracts for eligibility criteria into the study. Any disagreements about eligibility were adjudicated by consensus. Global treatment-, prevention-, and screening-based clinical trials for primary and metastatic prostate cancer that have completed recruitment and have available results (as of August 18, 2019) were included for analysis.
Treatment trials were defined as completed phase III or IV trials aimed at treating the disease but not trials aimed at treating the side effects of treatment. Prevention trials were restricted to completed phase III or IV primary prevention trials aimed at preventing prostate cancer, not at preventing side effects of treatment. Screening trials were restricted to studies that evaluated prostate-specific antigen (PSA) screening and have concluded the enrollment phase. The trial selection process is outlined in Figure 1.
Figure 1. Inclusion/exclusion criteria for phase III and IV prostate cancer clinical trials.
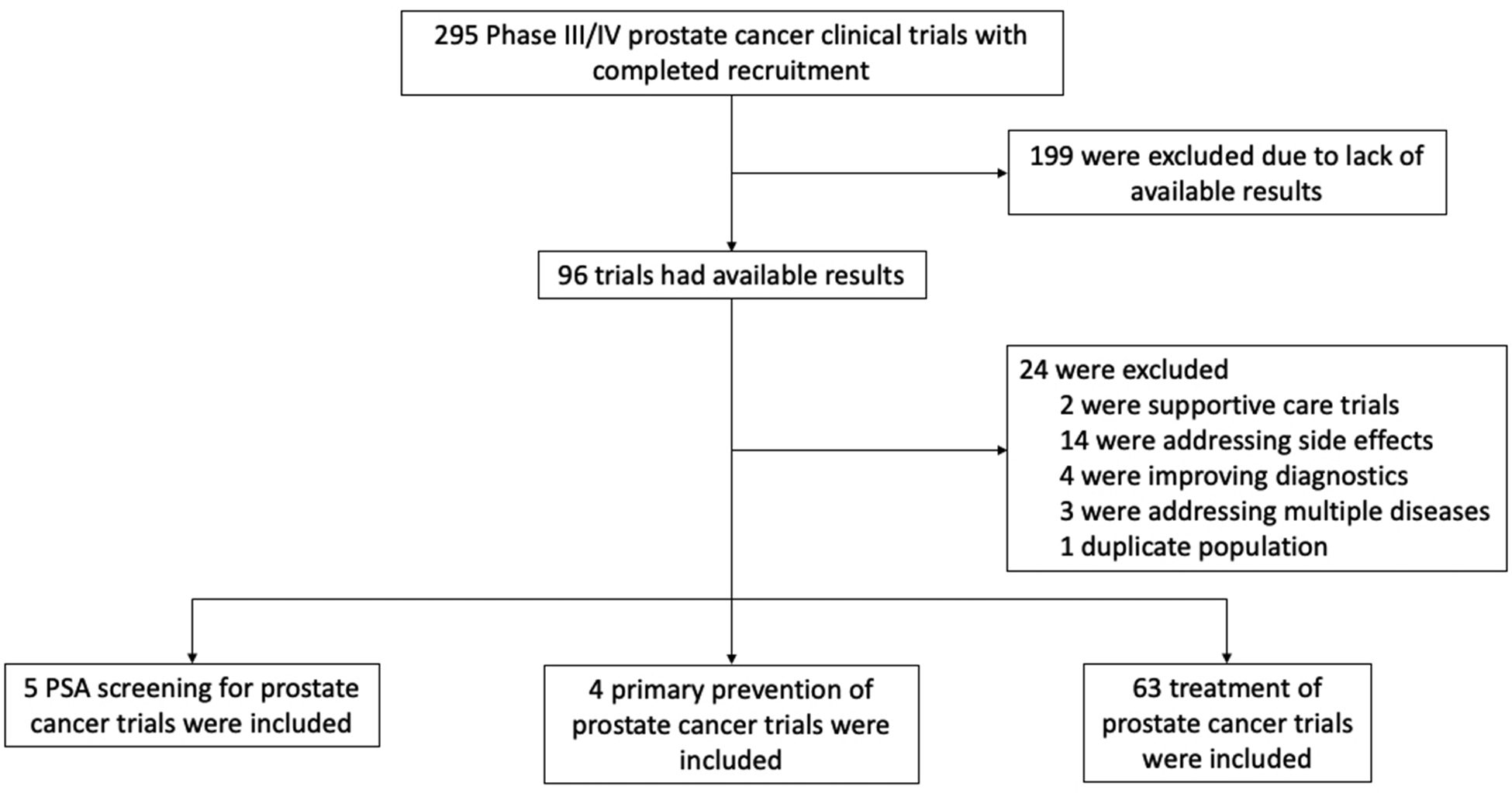
295 trials were initially identified; 72 were included in the analysis.
Information on recruitment start and end dates, number of participants, geographic location(s) of study centers, funding sources, availability of race and ethnicity data, and distribution of race and ethnicity of trial participants were collected from the clinical trials (Supplementary Table S1). Authors of studies without race/ethnicity data were contacted to request data if not available in the Clinical Trials Database or literature; all but six trials obtained responses. Descriptive statistics were calculated for each trial type and the total sample in relation to trial and participant information. All analyses were done using R version 3.4.3.
Funding Sources
Funding sources for each trial were identified through clinicaltrials.gov. Publicly funded trials included trials in which a U.S. government-sponsored institution (such as the National Institutes of Health or the Department of Veterans Affairs) was listed as either the study sponsor or a collaborator. All other sponsors/collaborators were categorized as “U.S. Non-Publicly Funded Trials.” The four European/Canadian-only screening trials not registered on clinicaltrials.gov were excluded from the funding categorization to focus specifically on the U.S. trial funding landscape.
Race/Ethnicity Representation
Representation of race and ethnicity categories was assessed using categories defined by the OMB as these designations are the current standardized recommendation for the collection of race/ethnicity data. The race and ethnicity categories reported in the clinical trials were consolidated under the OMB categories they best matched. The representation of each OMB race/ethnicity category was assessed across the three trial types by calculating the percentage of trials reporting at least one category aligning with each consolidated OMB category. The number of trials reporting each category and number of participants identified in each category were both used as units of analysis. Representation by race/ethnicity was additionally stratified by trial funding type.
Temporal Representation
Representation of race and ethnicity categories across time was examined by grouping the trials into five-year intervals from 1985–2019 based on their enrollment start date. All OMB race categories were used in the analysis; Hispanic or Latino ethnicity was combined with Hispanic or Latino race for the analysis. The “other” category for analysis included the participants identifying with the “Other” and “Other or No Answer” categories from the trials. Within each five-year interval, proportion of participants in each race/ethnicity category was calculated based on the total number of participants participating in the trials during that time period.
Geographic Representation
Geographic representation of participating study centers was examined using the trials that reported race and/or ethnicity. The geographic regions of representation for this study were defined using the subregions from the United Nations (U.N.) Statistics Division.18 The 195 countries that are recognized by the U.N. were included in the analysis. The countries represented in the clinical trials were identified and assigned their corresponding U.N. subregion. The proportion of countries represented by at least one study within each subregion was calculated by dividing the number of represented countries in each subregion by the total number of countries existing within that subregion. Results were plotted using MapChart.
RESULTS
Descriptive statistics for the treatment-, prevention-, and screening-based prostate cancer clinical trials analyzed are presented in Table 1. Seventy-two trials with a total of 893,378 pooled participants were included in the analyses. In all, 59 (81.9%) of the 72 total trials had available race data (from Clinical Trials Database, literature, FDA, or authors), and 11 (15.3%) of these 59 trials additionally reported ethnicity. Six (8.3%) of the trials either did not collect or do not share data on race/ethnicity (Supplementary Table S1). In regard to funding, 13 (19.1%) trials registered on clinicaltrials.gov were publicly funded; 55 (80.9%) were funded by pharmaceutical or biotechnology companies.
Table 1.
Descriptive Statistics of Analyzed Prostate Cancer Clinical Trials
| Trial Information | Treatment Trials (N=63) | Prevention Trials (N=4) | Screening Trials (N=5) | All Trials (N=72) |
|---|---|---|---|---|
| Earliest Recruitment Start Date | 1994 | 1993 | 1987 | 1987 |
| Latest Recruitment End Date | 2019 | 2012 | 2016 | 2019 |
| Availability of Race/Ethnicity Data, N (%): | ||||
| Trials with Available Race Data | 1 (81.0) | 4 (100) | 4 (80) | 59 (81.9) |
| Trials with Separate Ethnicity Data | 10 (15.9) | 1 (25) | 0 (0) | 11 (15.3) |
| U.S. Publicly Funded Trials | 9 (14.3) | 3 (75) | 1 (100)a | 13 (19.1)b |
| U.S. Non-Publicly Funded Trials | 54 (85.7) | 1 (25) | 0 (0) | 55 (80.9)b |
| Participant Information | Treatment Trials (N=38197) | Prevention Trials (N=62424) | Screening Trials (N=792757) | All Trials (N=893378) |
| Mean Number of Participants (SD) | 606 (538) | 15606 (14916) | 158551 (170776) | 12408 (57280) |
| Minimum Number of Participants | 10 | 423 | 9026 | 10 |
| Maximum Number of Participants | 1979 | 34888 | 419582 | 419582 |
| Availability of Race/Ethnicity Data, N (%): | ||||
| Participants with Available Race Data | 35014 (91.7) | 62424 (100) | 746564 (94.2) | 844002 (94.5) |
| Participants with Separate Ethnicity Data | 8091 (21.2) | 423 (0.7) | 0 (0) | 8514 (1.0) |
N=1 after exclusion of the four European/Canadian-only screening trials.
N=68 after exclusion of the four European/Canadian-only screening trials.
There was considerable variability in the representation of race and ethnicity categories represented in the analyzed trials as shown in Table 2. All trial categories represented were collected as race data specifically with the exception of the “Hispanic or Latino” categories which were collected as both race and ethnicity. Overall, 29 categories for race and ethnicity were reported across the trials.
Table 2.
Race/Ethnicity Categories Represented in Prostate Cancer Clinical Trials
| OMB Category | Trial Categories Represented |
|---|---|
| White | White |
| Non-Hispanic White | |
| Caucasians | |
| White - White/Caucasian/European Heritage | |
| Black or African American | Black or African American |
| Non-Hispanic Black | |
| African Americans | |
| African American/African Heritage | |
| Asian | Asian |
| Japanese | |
| Asian - Japanese Heritage | |
| Asian - South East Asian Heritage | |
| Asian/Oriental | |
| American Indian or Alaska Native | American Indian/Alaska Native |
| Pacific Islander or American Indiana | |
| Native Hawaiian or Other Pacific Islander | Native Hawaiian or Other Pacific Islander |
| Pacific Islander or American Indiana | |
| Hispanic or Latino (race) | Hispanic or Latino |
| Hispanic (non-African American) | |
| Hispanic (African American) | |
| More than one race | More than one race |
| Multiracial | |
| Coloured (mixed race) | |
| White and American Indian/Alaska Native | |
| Other and/or no answer | Other |
| Other or no answer | |
| Unknown or not reported | |
| Hispanic or Latino (ethnicity)b | Hispanic or Latino |
| Not Hispanic or Latino | |
| Unknown or not reported |
One study collected “Pacific Islander or American Indian” as a combined category.
Hispanic or Latino was the only category collected as an ethnicity. All other categories were collected as race.
The proportions of the treatment-, prevention-, and screening-based trials that included specific race and ethnicity categories are shown in the Table 3 “Trials” columns. Of the trials that reported information on race, all reported at least one category that falls under the white OMB category, and over 85% of trials also reported at least one category that falls under the Black or African American OMB category. Other categories were represented less frequently.
Table 3.
Representation of Race/Ethnicity in Prostate Cancer Clinical Trials (OMB Categories Reported)
| Treatment | Prevention | Screening | ||||
|---|---|---|---|---|---|---|
| Trials (N=51) | Participants (N=35014) | Trials (N=4) | Participants (N=62424) | Trials (N=4) | Participants (N=746564) | |
| Race groups represented, N (%): | ||||||
| White | 51 (100) | 29194 (83.4) | 4 (100) | 52785 (84.6) | 4 (100) | 727999 (97.5) |
| Black or African American | 46 (90.2) | 2329 (6.7) | 4 (100) | 5311 (8.5) | 1 (25) | 3375 (0.5) |
| Asian | 40 (78.4) | 1148 (3.3) | 2 (50) | 142 (0.2) | 1 (25) | 2991 (0.4) |
| American Indian or Alaska Native | 26 (51.0) | 193 (0.6) | 1 (25) | 3 (0.005) | 1 (25)a | 652 (0.09)a |
| Native Hawaiian or Other Pacific Islander | 25 (49.0) | 21 (0.06) | 1 (25) | 1 (0.002) | ||
| Hispanic or Latino (race) | 8 (15.7) | 300 (0.9) | 2 (50) | 2627 (4.2) | 1 (25) | 1611 (0.2) |
| More than one race | 19 (37.3) | 25 (0.07) | 1 (25) | 0 (0) | 0 (0) | 0 (0) |
| Other and/or no answer | 43 (84.3) | 1804 (5.2) | 4 (100) | 155 (2.5) | 4 (100) | 9913 (1.3) |
| Ethnicity categories represented, N (%): | ||||||
| Hispanic or Latino | 10 (19.6) | 547 (1.6) | 1 (25) | 17 (0.03) | 0 (0) | 0 (0) |
| Not Hispanic or Latino | 10 (19.6) | 6869 (19.6) | 1 (25) | 382 (0.6) | 0 (0) | 0 (0) |
| Unknown or not reported | 10 (19.6) | 675 (1.9) | 1 (25) | 24 (0.04) | 0 (0) | 0 (0) |
One screening trial analyzed reported “Pacific Islander or American Indian” as a combined category.
The proportion of participants in each race/ethnicity category by trial type is shown in the Table 3 “Participants” columns. Treatment, prevention, and screening trials all included majority white men (>83%). The Black or African American category had the second-highest representation of the race categories, ranging from 0.5% of participants in screening trials to 8.5% of participants in prevention trials. In total, 809978 (96.0%) of the 844002 participants with available race/ethnicity data were white. In U.S. publicly funded trials, 116171 (83.9%) of the 138457 participants were white; in U.S. non-publicly funded trials, 31005 (86.9%) of the 35660 participants were white (Supplementary Table S1).
The proportion of trial participants in each race/ethnicity category over time is shown in Figure 2. In each time period, in the trials with available race data, over 80% of participants were white with the exception of the trials with start dates between 1995 and 1999. Participation of Black or African American participants was highest at 11.3% between from 1995–1999 but has generally been below 5% for most time periods. All other categories have consistently represented less than 6% of participants each since 1985.
Figure 2. Representation of Races/Ethnicities in Prostate Cancer Clinical Trials over Time.
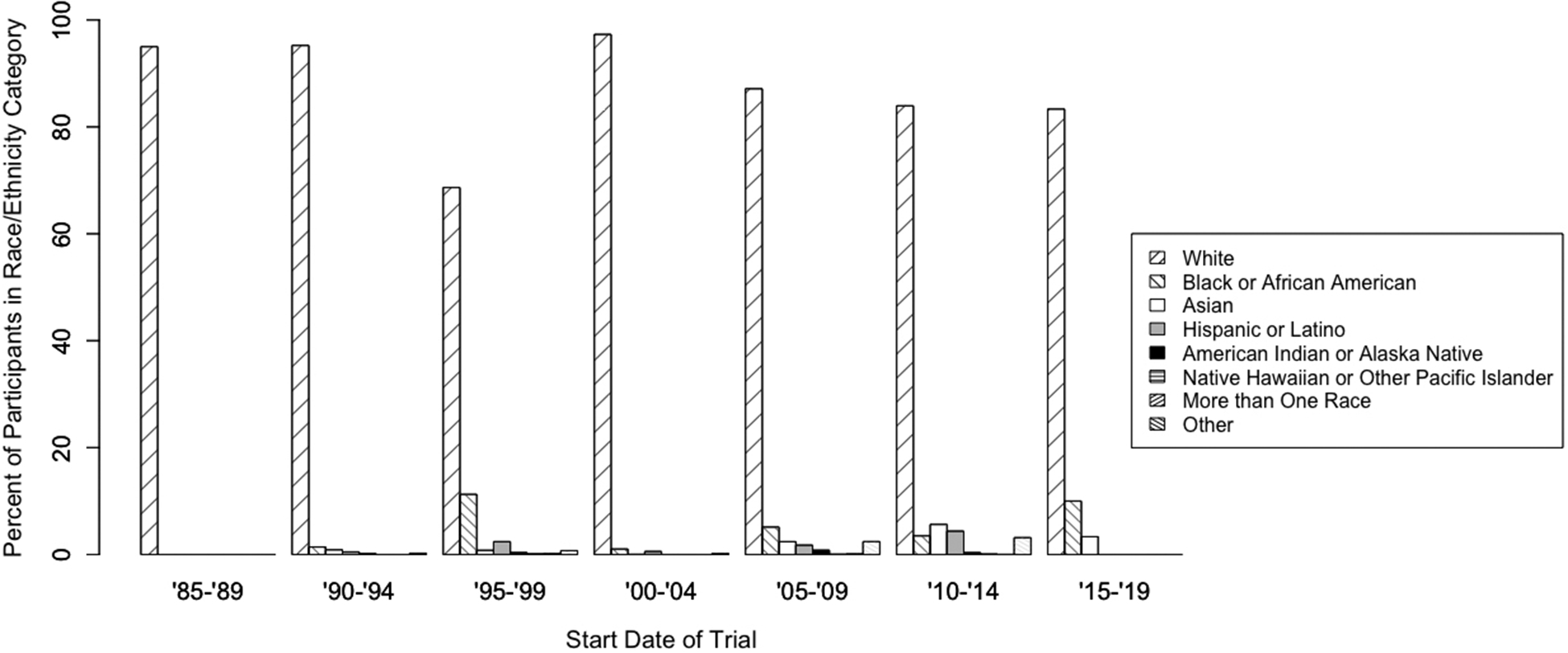
Individual patients categorized into OMB categories; proportion of patients in each category per 5-year range of trial start date.
The geographic representation of countries participating in the 59 trials with race/ethnicity data available is shown in Figure 3. Notably, the Caribbean, Eastern African, Central African, Western African, Central Asian, Polynesian, Melanesian, and Micronesian subregions all had 0% of countries represented.
Figure 3. Representation of UN Subregions in Prostate Cancer Clinical Trials.
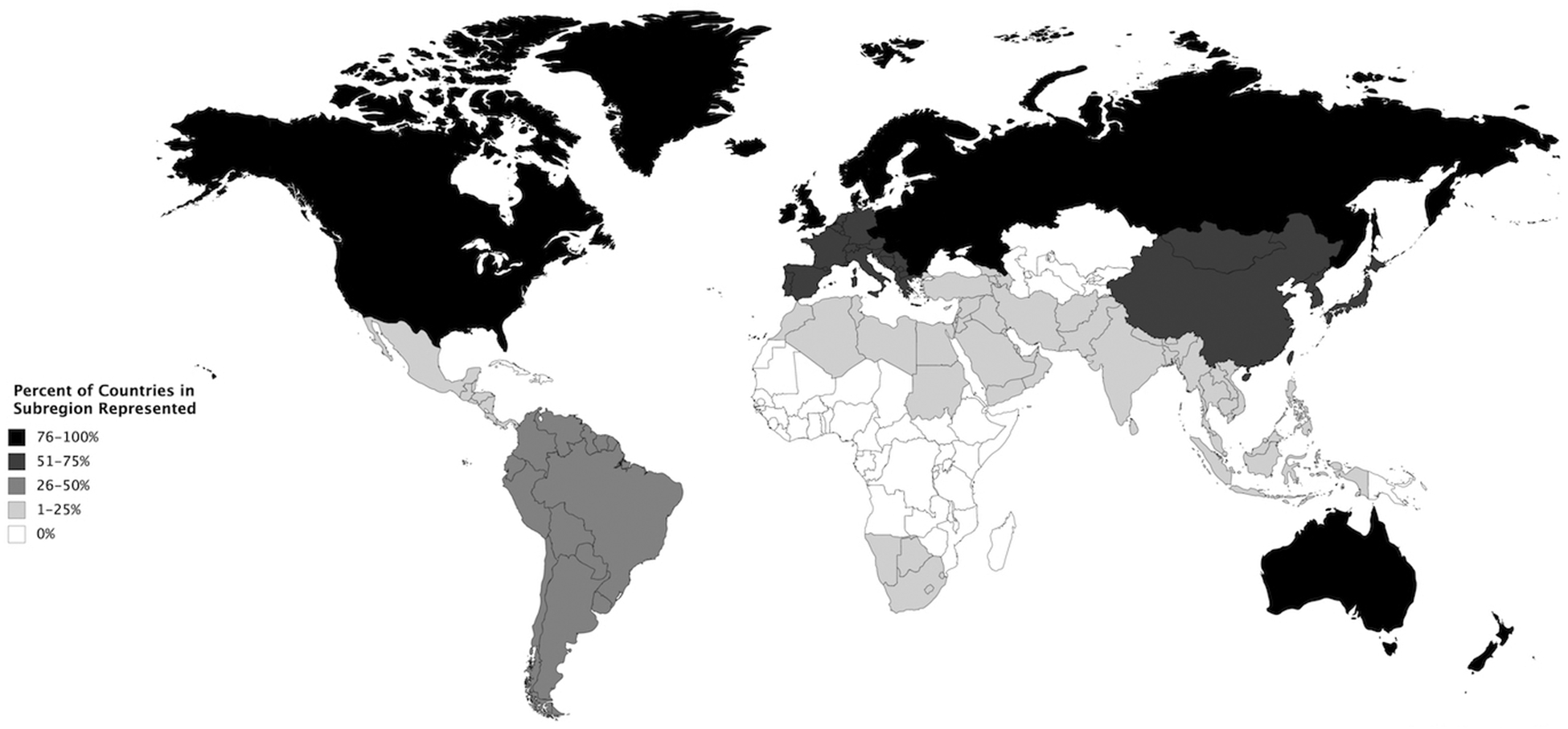
Proportion of countries in each UN subregion included in analyzed trials.
DISCUSSION
In this study, almost 30% of analyzed prostate cancer clinical trials did not explicitly report race or ethnicity, and the trials that did report race/ethnicity data did so inconsistently with twenty-nine different categories represented. Inconsistency in reporting of race/ethnicity in trials raises concerns around whether findings from trials will be generalizable to larger populations.
Perhaps most strikingly, 96% of all study participants with race/ethnicity data available were white. Though estimates of the racial/ethnic composition of the world are difficult to obtain, the percentage of people of significant European descent in the world is almost certainly less than 20% given the ethnic composition of countries around the world.19 Importantly, the screening trials included in this analysis were composed of 97.5% white men; screening guidelines for African American men have been debated and drawn partially from these data, but African American men represent only 0.5% of the screening trial population.20
Over 80% of the trials registered on clinicaltrials.gov were funded by pharmaceutical or biotechnology companies; these trials included a higher proportion of white men than the publicly funded trials. To our knowledge, no past studies have assessed the reporting of race/ethnicity in clinical trials by trial funding type. As non-government sponsored clinical trials are becoming more common,21 it is important to hold the private entities that sponsor the trials accountable for reporting race/ethnicity data and ensuring diversity of the trial participants.
Since 1990, white participants have continued to be enrolled in prostate cancer clinical trials at higher rates than all other races and have composed over 80% of the participant population for the past three decades despite increasing awareness of racial disparities in prostate cancer outcomes. From 1995–1999, the proportion of white participants was only 69%; however, approximately 18% of trial participants during this time period did not have race data available. Assuming these participants were also majority white, the proportion of white participants in prostate cancer clinical trials between 1995 and 1999 was above 80% as well.
Importantly, our results show a decrease in proportion of Black or African American men from 11.3% to 2.8% between 1995 and 2014. Only one trial (N=30 participants) was included in the 2015–2019 time period; more studies need to be included when results are released to determine current representative enrollment across broader trials. Some groups have recently shown a decline in the representation of U.S. racial/ethnic minorities in multi-cancer clinical trials;22 however, others have shown an increase in representation.15 Ongoing validation of this observation in prostate cancer clinical trials is necessary to determine the strength of and reasons for this decline.
Geographically, there is a large disparity in the countries represented in prostate cancer clinical trials. North American and European countries are very well-represented with the majority of countries in these regions participating in prostate cancer clinical trials. Despite African American men comprising almost 13% of the U.S. population, however, these trials are still predominantly reporting on white men. Notably, only 2 of the 67 Caribbean and African countries in the world were represented in the clinical trials analyzed in this study. As these regions remain amongst the highest for prostate cancer mortality despite their drastically lower incidence rates compared to the US and Europe,23 we believe that these regions should be prioritized for inclusion in prostate cancer clinical trials due to the significant burden of disease for these populations.
As mentioned in the introduction, we have proposed a benchmark of no more than 56.3% non-Hispanic white men and at least 22.2% non-Hispanic African American men to be included in prostate cancer clinical trials. It is difficult to determine a global standard for clinical trial enrollment by race as this construct is viewed differently by geographic region and country. We acknowledge the limitations of these benchmarks, such as the exclusion of other global regions and the use of a US-centric benchmark in a global analysis; however, we believe that these benchmarks are sufficient in this case as the overwhelming majority of trials were conducted in the U.S.
We specifically mention non-Hispanic white and African American men in our proposed benchmarks; regardless, the inclusion of U.S. minority groups other than African Americans should also be prioritized. It has been suggested that Hispanic mortality from prostate cancer has surpassed that of white patients in recent years.4 By enrolling more Hispanic patients into clinical trials, reasons for this relative increase in mortality could be investigated. Additionally, it is known that Asian men have drastically lower incidence of and mortality from prostate cancer compared to all other races.24 The inclusion of more Asian men in clinical trials will allow for the exploration of protective factors against prostate cancer with potential implications for prevention and treatment.
Barriers to the recruitment of minority participants into clinical trials are multifaceted. A recent systematic review of U.S. minority enrollment in cancer clinical trials proposed five key elements regarding the participation of African Americans in U.S. trials.25 Two of these elements include negative beliefs about participation in clinical trials and lack of knowledge surrounding clinical trials. Recent clinical trials implementing patient navigation programs and enhancing institutional clinical trial infrastructure have been shown to increase participation of U.S. minority patients.26–27
For prostate cancer specifically, current initiatives, such as the International Registry of Men with Advanced Prostate Cancer (IRONMAN), are using similar approaches to increase the enrollment of U.S. minority men into prostate cancer research. Regarding patient navigation, the IRONMAN Diversity Working Group and Advocacy Working Group aim to increase patient participation, particularly of U.S. minority men, in aspects of administration in the registry. IRONMAN hopes to support the agency of underrepresented participants in navigating their communities to bridge the gap between patients and prostate cancer clinical trials.
From the institutional perspective, IRONMAN and other groups are working to build clinical trial infrastructure in countries that have traditionally been excluded from clinical trials. IRONMAN has begun recruiting men with advanced prostate cancer in Brazil with plans to expand to South Africa. Additionally, IRONMAN has partnered with the African-Caribbean Cancer Consortium (AC3) and the Prostate Cancer Transatlantic Consortium (CaPTC), initiatives that focus specifically on investigating the racial disparities of prostate cancer. AC3 and CaPTC have been instrumental in establishing a strong prostate cancer research presence in African and Caribbean countries; the infrastructure built by these initiatives will provide a path to the recruitment of patients into clinical trials, including IRONMAN, in these regions. With more diverse participants enrolled into prostate cancer clinical trials through targeted recruitment efforts as in the studies mentioned above, men who are at the highest risk of developing and dying from prostate cancer will be better represented in research, allowing for investigation into treatments that best suit each diverse population.
Supplementary Material
ACKNOWLEDGMENTS
Emily M. Rencsok is supported by T32CA009001. Rana McKay, Franklin Huang, Jelani C. Zarif, and Lorelei A. Mucci are Young Investigators of the Prostate Cancer Foundation. We would additionally like to acknowledge all clinical trial authors for their correspondence regarding race/ethnicity data that has not been published.
Financial support: This project was supported in part by the Movember Foundation and T32CA009001.
Footnotes
Conflicts of interest: None
REFERENCES
- 1.Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J Natl Cancer Inst. 2017;109(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seigel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. [DOI] [PubMed] [Google Scholar]
- 3.Steele CB, Li J, Huang B, Weir HK. Prostate cancer survival in the United States by race and stage (2001–2009): Findings from the CONCORD-2 study. Cancer. 2017;123(S24): 5160–5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelly SP, Anderson WF, Rosenberg PS, Cook MB. Past, current, and future incidence rates and burden of metastatic prostate cancer in the United States. Eur Urol Focus. 2018;4(1):121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rayford W, Alger J, Takhar M, et al. PD56–01 Comparative genomic analysis of 1043 African American and non-African American prostate cancers: a report from the decipher grid collaborative. J of Urol. 2018;199(4): Suppl e1062. [Google Scholar]
- 6.Shanawani H, Dame L, Schwartz DA, Cook-Deegan R. Non-reporting and inconsistent reporting of race and ethnicity in articles that claim associations among genotype, outcome, and race or ethnicity. J Med Ethics. 2006;32(12):724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamel LM, Penner LA, Albrecht TL, Heath E, Gwede CK, Eggly S. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control. 2016;23(4):327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Office of Management and Budget. Statistical Policy Directive Number 15: Race and ethnic standards for federal statistics and administrative reporting. Washington, D.C. Adopted May; 12, 1977. [PubMed] [Google Scholar]
- 9.Office of Management and Budget. Revisions to the standards for the classification of federal data on race and ethnicity. Washington, D.C. Adopted October; 30, 1997. [Google Scholar]
- 10.Vespa J, Armstrong DM, Medina L. Demographic turning points for the United States: Population projections for 2020 to 2060 (Report P25–1144). Washington, D.C.: U.S. Census Bureau; 2018. [Google Scholar]
- 11.Food and Drug Administration. Collection of race and ethnicity data in clinical trials: Guidance for industry and Food and Drug Administration staff. Washington, D.C. Published October; 26, 2016. [Google Scholar]
- 12.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. [DOI] [PubMed] [Google Scholar]
- 13.Spratt DE, Osborne JE. Disparities in castration-resistant prostate cancer trials. J Clin Oncol. 2015;33(10):1101–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinsky PF, Ford M, Gamito E, et al. Enrollment of racial and ethnic minorities in the prostate, lung, colorectal, and ovarian cancer screening trial. J Natl Med Assoc. 2008;100(3):291–298. [DOI] [PubMed] [Google Scholar]
- 15.Balakrishnan AS, Palmer NR, Fergus KB, et al. Minority recruitment trends in phase III prostate cancer clinical trials (2003–2014): progress and critical areas for improvement. J Urol. 2019;201(2):259–267. [DOI] [PubMed] [Google Scholar]
- 16.American Cancer Society. Cancer Statistics Center. Prostate cancer at a glance. https://cancerstatisticscenter.cancer.org/#!/cancer-site/Prostate.
- 17.United States Census Bureau. QuickFacts: United States. https://www.census.gov/quickfacts/fact/table/US/PST045218
- 18.United Nations Statistics Division. Methodology: Standard country or area codes for statistical use (M49). https://unstats.un.org/unsd/methodology/m49/. Accessed August 18, 2018.
- 19.Central Intelligence Agency. The World Factbook: Ethnic Groups. https://www.cia.gov/library/publications/the-world-factbook/fields/400.html
- 20.Wallner LP. Prostate cancer in black men: Is it time for personalized screening approaches? Cancer. 2017;123(12):2312–2319. [DOI] [PubMed] [Google Scholar]
- 21.Ehrhardt S, Appel LJ, & Meinert CL. Trends in National Institutes of Health funding for clinical trials registered in clinicaltrials.gov. JAMA. 2015;314(23):2566–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duma N, Vera Aguilera J, Paludo J, et al. Representation of minorities and women in oncology clinical trials: Review of the past 14 years. J Oncol Pract. 2018;14(1):e1–e10. [DOI] [PubMed] [Google Scholar]
- 23.Taitt HE. Global trends and prostate cancer: A review of incidence, detection, and mortality as influenced by race, ethnicity, and geographic location. Am J Mens Health. 2018;12(6):1807–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.SEER Cancer Stat Facts: Prostate Cancer. National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/statfacts/html/prost.html [Google Scholar]
- 25.Rivers D, August EM, Sehovic I, et al. A systematic review of the factors influencing African Americans’ participation in cancer clinical trials. Contemp Clin Trials. 2013;35:13–32. [DOI] [PubMed] [Google Scholar]
- 26.Ghebre RG, Jones LA, Wenzel J, et al. State-of-the-science of patient navigation as a strategy for enhancing minority clinical trial accrual. Cancer. 2014;120(7):1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anwuri VV, Hall LE, Mathews K, et al. An institutional strategy to increase minority recruitment to therapeutic trials. Cancer Causes Control. 2013;24(10):1797–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


