Abstract
Mechanical cues are delivered to resident cells by the extracellular matrix and play an important role in directing cell processes, ranging from embryonic development and cancer metastasis to stem cell differentiation. Recently, cellular responses to viscoelastic and elastic mechanical cues have been studied; however, questions remain as to how cells identify and transduce these cues differently. We present a synthetic cell culture substrate with viscoelastic properties based on thioester exchange chemistry that can be modulated in situ with the photoinitiated thiol-ene ‘click’ reaction. With this method, stress relaxation in thioester hydrogels with an average relaxation time of 740,000 s can be switched off in the presence of cells without change to the elastic modulus. NIH 3T3 fibroblasts, cultured for 48 hr on viscoelastic compared to elastic thioester substrates, displayed increased cell area (660–560 μm2) and increased nuclear to cytoplasmic YAP/TAZ ratios (2.4 to 2.2) when cultured on elastic compared to viscoelastic hydrogels, respectively. Next, when the viscoelasticity was switched off after 24 hr, the fibroblasts responded to this change and exhibited an average cell area of 540 μm2, and nuclear to cytoplasmic YAP/TAZ ratio of 2.1, approaching that of the control elastic gels. Phototunable viscoelastic thioester hydrogels provide a tunable materials system to investigate time-dependent cellular responses to viscoelasticity and should prove useful for understanding the dynamics of mechanoresponsive cellular pathways.
Keywords: NIH 3T3 Fibroblasts, Covalent adaptable network, mechanotransduction, Tunable viscoelasticity, photoresponsive biomaterial
Introduction
The extracellular matrix (ECM) is more than a fibrous protein-based material in which cells reside; it is a conduit for a multitude of signals that cells can recognize and to which they can respond. ECM mechanics are one such signal. Specifically, the effect of tissue elasticity on cell behavior has been well studied in the literature [e.g., Engler et al.1 and reviewed by Guilak et al.2]. When independently manipulated, tissue elasticity plays an important role in key events in embryonic development,3 the progression of fibrosis,4 and cancer metastasis.5 However, the view that mechanically active cells only respond to elasticity has been complicated by the discovery that cells respond to other mechanical properties too.
For example, the time-dependent, or viscoelastic, mechanical properties of the ECM and biomaterial mimics have been shown to be an equally important driver of cell processes as time-independent properties (e.g., elastic modulus). In some cases, evidence suggests that viscoelasticity and elastic modulus are independent control knobs that can elicit similar trends, including the extent of cell spreading67, nuclear localization of mechanically sensitive transcription regulators (e.g., YAP/TAZ),86 and bias of human mesenchymal stem cell (hMSC) fate decisions9. However, when Darnell et al. examined the transcriptional changes that occurred when hMSCs were cultured on soft vs. stiff materials, and compared them to the transcriptional changes of hMSCs cultured on slow vs. fast stress relaxing substrates, many of the changes in gene transcription were not the same; there were in fact differences in how the cells sensed these cues.10 In the case of secreted immunomodulatory factors by cells, matrix elasticity has been shown to change how hMSCs respond to viscoelastic mechanical cues.11 Understanding and deconvoluting this nuanced relationship between elastic and viscoelastic cues is of broad interest to the biomaterials field. This information will not only provide fundamental insight about the time scales of cellular responses during mechanotransduction, but may further inform design principles for the development of the next generation of biomaterials for cell-based therapies.
The ability of the field to interrogate the interplay between viscoelastic and elastic cues is driven by the development of new materials. Among the early studies, Cameron et al. tuned the loss modulus of polyacrylamide hydrogels between 1–130 [Pa] by changing the length of encapsulated linear polyacrylamide chains, while leaving the storage modulus constant; they observed increased spreading, proliferation and differentiation potential of hMSCs with increasing substrate creep.12 These findings were later corroborated when Chaudhuri et al. used ionically crosslinked alginate hydrogels with stress relaxation properties inherently decoupled from elastic modulus and found that faster rates of stress relaxation led to not only increased cell spreading and proliferation of hMSCs,6 but also biased them towards an osteogenic phenotype.13
Motivated by these initial studies that showcased the importance of viscoelastic properties, many in the field became interested in developing covalent adaptable chemistries for use in hydrogels. In these materials, viscoelastic properties arise from the kinetics of the selected covalent adaptable chemistry allowing for viscoelastic properties to be built into a wide array of materials and architectures. McKinnon et al. developed PEG hydrogels with reversible hydrazone bonds that could be engineered with aliphatic aldehydes to relax rapidly or benzaldehydes to relax more slowly , and demonstrated that encapsulated C2C12s adopted extended morphologies in fast relaxing materials.14 In a similar vein, Tang et al. demonstrated how orthogonal boronic ester and dibenzocyclooctyne azide crosslinking chemistries could be used to investigate the viscoelastic and elastic effects of covalent chemistries on hMSC morphology.15 These works that showed how dynamic chemistries could be used to form materials of varied viscoelastic properties, and we became interested in how covalent adaptable chemistries could be used to manipulate viscoelastic properties in situ. In one recent example, Marozas et al. used an allyl sulfide functionalized hydrogel and a photoinitiator catalyzed allyl sulfide exchange to resolve relaxation of cytoskeletal tension in individual cell processes by patterning viscoelastic regions into the material with light at high resolution. Building from this concept, we sought to exploit on the versatility of thioester hydrogels16 and their viscoelastic properties to develop a cell culture system where viscoelasticity could be turned off on demand.
In thioester containing materials, free thiol species react with thioester groups to drive the exchange of crosslinks that gives rise to viscoelasticity.16 By controlling the concentration of pendant thiols in a thioester crosslinked hydrogel, viscoelastic properties can be tuned over a broad range of timescales. With the aid of a ‘click’ reaction, like the photoinitiated thiol-ene reaction, thiol groups can be efficiently consumed, and thioester viscoelasticity reduced, through a means that is cytocompatible and spatiotemporally controlled with light.17
In this contribution, experiments demonstrated how thioester hydrogels can be formulated to modulate viscoelastic material properties in situ. Results show that control of the concentration of tethered thiols in thioester materials gives rise to lasting temporal control over viscoelastic properties. In combination with a photoinitiated thiol-ene reaction, the viscoelastic properties of cell laden materials were manipulated in situ in a cytocompatible way that left the elastic modulus unchanged. Selected materials were used to measure the response of NIH 3T3 fibroblast cells to a switch from a viscoelastic environment to an elastic environment. By measuring cell area and the nuclear translocation of yes-associated protein (YAP) and transcriptional co-activator with PDZ binding motif (TAZ), we confirmed cells responded to a switch from a viscoelastic to an elastic microenviroment. The control over viscoelastic properties that switchable thioester materials allow researcher to explore new fundamental questions related viscoelasticity and the cellular processes involved.
Materials and Methods
Materials
Unless otherwise noted, all reagents were purchased from Sigma Aldrich or Fisher Scientific and used without further purification. 5-norbornene-2-carboxylic acid (97 %, predominately endo isomer) was purchased from Alfa Aesar and used without further purification. Dulbecco’s modified Eagle’s media (DMEM), phosphate buffered saline (PBS), fetal bovine serum (FBS), penicillin–streptomycin, Amphotericin B, and trypsin/EDTA were all purchased from ThermoFisher. 8-arm 20 kDa poly(ethylene glycol) thiol was purchased from JenKem Technologies and used without further purification. The norbornene functionalized cell adhesion peptide Norborne-RGDSG was synthesized according to a previously published protocol.16 NIH/3T3 fibroblasts were acquired from the American Type Culture Collection (ATCC CRL1658).
Synthesis of 8-arm 20kDa Poly(ethylene glycol) Thioester Norbornene (TENB)
An oven dried 100 ml round bottomed flask was charged with 0.3 ml of 5-norbornene-2-carboxylic acid (338 mg, 2.45 mol), 760 mg of N-[(Dimethylamino)-1H-1,2,3-triazolo-[4,5-b]pyridin-1-ylmethylene]-N-methylmethanaminium hexafluorophosphate N-oxide (HATU, 2.0 mmol ), and 0.5 ml 4-methylmorpholine (460 mg, 4.5 mmol) were combined with 20 ml of anhydrous dimethylformamide. The reaction was purged for 15 min, 100 mg of 8-arm 20 kDa poly(ethylene glycol) (PEG) thiol (0.05 mmol) was added and the reaction was purged for an additional 20 min. The reaction was stirred at room temperature for 20 hr under an argon environment. The reaction was concentrated in vacuo and precipitated in cold diethyl ether twice. The resulting white powder purified by dialysis (8 kDa molecular weight cutoff). The purified product was lyophilized to yield 900 mg (84 % yield with 85 % functionalization verified by NMR according to previously published protocols18,19 (SI Figure S1). 1H NMR (400 MHz, CDCl3) δ 6.29–5.82 (m, 2H), 3.99–3.42 (m, 1818H) Functionalization was later confirmed to be 97% by rheology (SI Figure S2).
Synthesis of PEG Thioester Hydrogels
8-arm PEG thiol (Mw = 20 kDa) and 8-arm PEG thioester norbornene (Mw = 21 kDa) were combined in a pre-gel mixture with a thiol to norbornene function group ratio of 2:1 and a total polymer content of 4 wt% by mass. Lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) was used as a photoinitiator and was synthesized as previously described.20 LAP and norbornene-RGDSG were incorporated at a final concentration of 1 mM. Viscoelastic thioester hydrogels formed readily when exposed to 10 [mW][cm]−2 of 365 nm UV light for 60 s.
Elastic hydrogels were then formed by swelling viscoelastic thioester hydrogels (described above) in a solution containing 1mM LAP, 10 mM 5-norbornene-2-carboxylic acid, and 200 mM MOPS buffer titrated to a pH of 7.4, for 30 minutes. The hydrogels were then exposed to 100 s of 10 [mW][cm]−2 of 365 nm UV light. Swollen hydrogels were then either swollen in PBS for 20 min and characterized or swollen overnight and seeded with cells.
Material Characterization
Hydrogel formation was characterized using oscillatory shear rheology on a TA Instruments stress-controlled rheometer. The pre-hydrogel mixture was loaded onto the instrument and subjected to 60 s of 10 [mW][cm]−2 365 nm UV light followed by the application of a ring of mineral oil to prevent dehydration. Evolution of shear elastic and loss moduli was recorded using a time sweep at 1% strain and a frequency of 1 [s]−1. Stress relaxation was then characterized by a stress relaxation at a fixed gap width; a 10% strain was applied to each sample, and the resulting stress was monitored for 5400 s.
Characterization of swollen hydrogels was conducted using a constant axial force of 0.2 N and a PBS bath to prevent dehydration. Shear elastic and loss moduli were quantified with a time sweep at 10 % strain and 0.05 [s]−1. Stress relaxation was then characterized by a stress relaxation at a constant axial force of 0.2 N; a 10 % strain was applied to each sample over a period of 5 s, and the resulting stress was monitored for 5400 s. For longer experiments an oil ring was used to prevent dehydration.
NIH-3T3 Culture on Hydrogel and Expansion on Tissue Culture Plastic
NIH-3T3 were cultured in experimental media (high glucose Dulbecco’s Modified Eagle Medium 4.5 [g][L]−1 glucose) supplemented with 10 % FBS, 50 [μg][mL]−1 penicillin, 50 [μg][mL]−1 streptomycin, and 0.5 [μg][mL]−1 of Amphotericin B. NIH-3T3s were then trypsinized and seeded on the hydrogels at a density of 16,000 cells/cm2 for immunostaining analysis.
Switch off Viscoelasticity in Culture
After 24 hours of culture on 2D thioester substrates, experimental media was replaced with 500 μL fluorobrite media (ThermoFisher) containing 10 [mmol][L]−1 5-norbornene-2-carboxylic acid, and 1 [mmol][L]−1 LAP. To ensure non-switch conditions would not be biased, non-switch conditions had media replaced with 500 μL of fluorobrite media. Following 30 min of incubation, all wells were irradiated with 100 s of 10 [mW][cm]−2 of 365 nm UV light followed immediate replacement of media with 1 mL of experimental media.
Immunostaining
Hydrogels were fixed by treatment with 2% paraformaldehyde (PFA) for 15 min and subsequently fixed with 4% PFA for 30 min. Samples were washed three times with PBS for 10 min at RT and then permeabilized and blocked with 0.1% Triton X100 in PBS and 5 % bovine serum albumin (BSA) in PBS for 1 hr at RT respectively. Samples were incubated with primary anti-YAP antibody (1:400, mouse, Santa Cruz Biotech) overnight at 4 °C and with secondary antibodies Goat anti-rabbit Alexaflour 488 (1:400, Invitrogen), DAPI (1:500, Sigma), and HCS Cell Mask Orange (1:5000 Thermo Fisher) for 1 hr in the dark at RT. After incubation the samples were washed three times (10 min each) with PBST (0.5 wt% Tween-20 in PBS) at RT. Hydrogels were imaged with a spinning disk confocal microscope (Operetta High Con- tent Imaging System, Perkin Elmer). Analysis was conducted using Harmony software (Perkin Elmer).
Cell Area and YAP Nuclear Localization Quantification
Harmony software (Perkin Elmer) was used to identify the nuclear (DAPI) and cytoplasmic region (HCS Cells Mask) of each cell in a single imaging plane. These parameters were then used to calculate the cell area and the average YAP intensity in the nuclear and cytoplasmic. Next, the YAP nuclear to cytoplasmic ratio was calculated as the average YAP intensity in the nucleus divided by the average YAP intensity in the cytoplasm. Representative images were taken with a Nikon Laser Scanning Confocal microscope with a 20 x water objective.
Statistics
Unless stated otherwise, all data were treated with Gaussian statistics. For single comparisons an F-test was first used to validate that the standard deviation of the two data sets were not different, followed by an unpaired t-test. In cases of multiple comparisons, Brown-Forsythe and Bartlett’s tests were used to confirm that the standard deviations of each data set were not different and were followed by a one-way ANOVA with a Tukey multiple comparison post-hoc with an alpha of 0.05.
For cell area and YAP analysis, 40–100 cells were analyzed per hydrogel, then 10 cells were chosen at random using a random number generator and combined to form a representative population of 50 or 60 cells on which statistical conclusions were made. Cell area and YAP data were characterized with non-parametric statistical tests. For these data, the Mann-Whitney test was used for single comparisons, and the Kruskal-Wallis test with a post Hoc of Dunn’s multiple comparisons was used for multiple comparisons also with an alpha of 0.05. Finally, it should be noted that outliers were removed from cell area and YAP localization data using route’s test with a threshold of Q=1%.
Results
Photopolymerization of Thioester Crosslinked Hydrogels
Poly(ethylene glycol) (PEG) (8-arm 20 kDa) was functionalized with thioester-norbornene (TENB) end groups using a HATU coupling reaction (Figure 1a). Macromer functionalization was measured as ~85% with nuclear magnetic resonance spectroscopy (SI Figure S3), however due to the importance of precise stoichiometry in future experiments we elected to calculate functionalization of the PEG TENB with a rheological method which indicated a true functionalization of 97%(SI Figure S1). 8-arm 20 kDa PEG TENB was then combined with 8-arm 20kDa PEG thiol in a 1 to 1 ratio in the presence of lithium phenyl-2,4,6-trimethylbenzoylphosphinate (LAP) and 365nm UV light to give rise to fully formed hydrogel networks by the thiol-ene click reaction (Figure 1b). A light intensity of 10 [mW][cm]−2 was chosen to initiate photopolymerization, as it both facilitates a rapid and spatiotemporally controlled reaction and is known to be cytocompatible.21 Shear oscillatory rheology was used to track gel formation in-situ, and at 10 [mW][cm]−2 a fully formed hydrogel with a shear elastic modulus of 3500 Pa was generated after 60s of irradiation (Figure 1c). This time was further used to polymerize all materials in this work.
FIGURE 1.
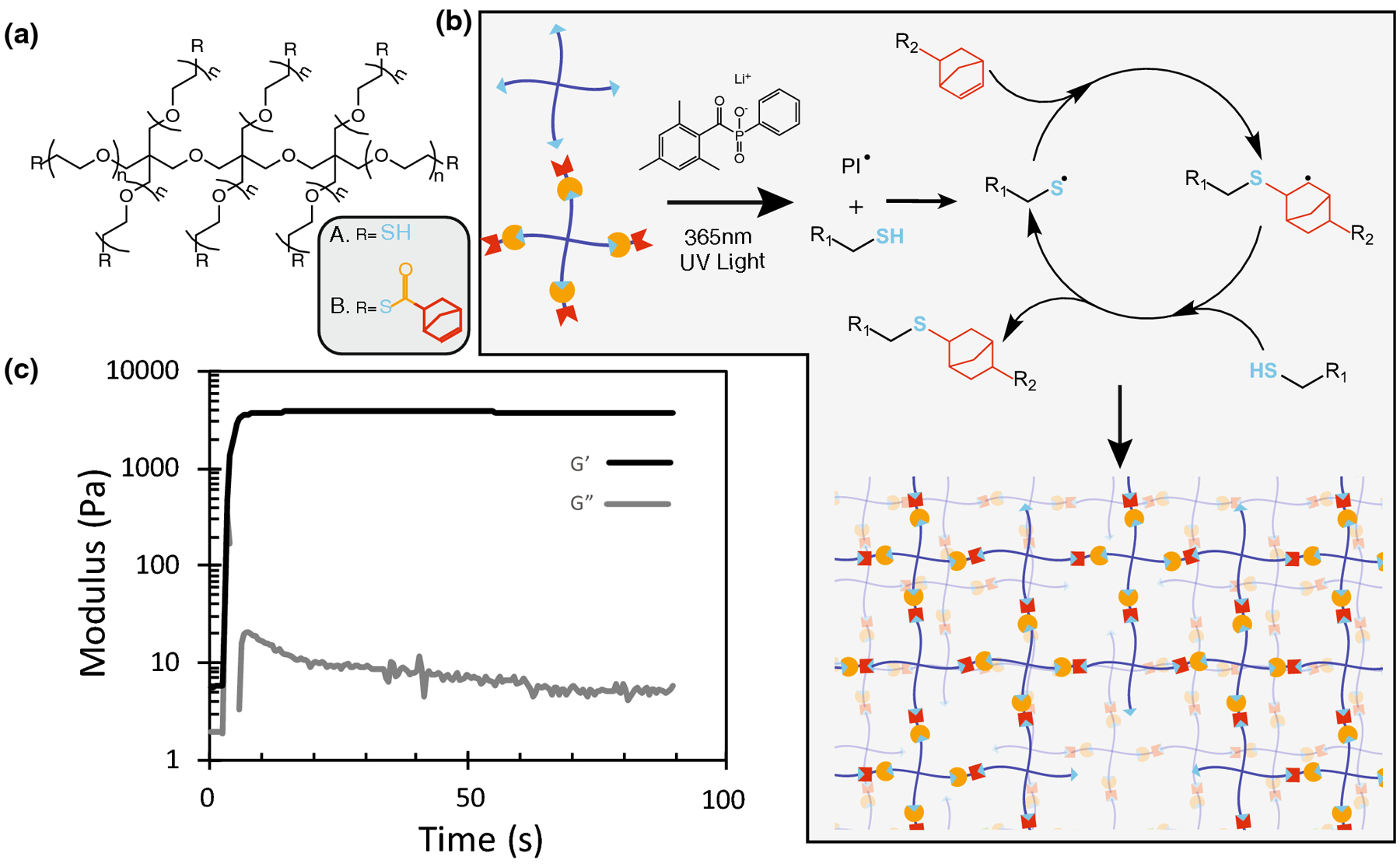
Multifunctional PEG thiol and thioester norbornene macromers rapidly form hydrogels via the photoinitiated thiol-ene polymerization. (a) Molecular structure of 8 arm 20 kDa PEG Thiol and 8 arm 20 kDa PEG Thioester norbornene. (b) Macromers participate in the radical mediated thiol-ene ‘click’ reaction catalyzed by the photoinitiator LAP to give rise to a fully formed hydrogel network. (c) A 3 wt% stoichiometric mixture of the two PEG macromers containing 1mM LAP rapidly polymerizes to a final modulus of 3500 Pa after 60s of irradiation with 10[mW][cm]−2 365nm UV light.
Tuning viscoelasticity through thioester exchange
After characterizing network formation, we assessed the range of viscoelastic properties attainable with this material. Thioester exchange is known to be catalyzed by free thiol species.16 Knowing this, we explored the effect of the pendant thiol concentration within the thioester hydrogels on the exchange rate of individual crosslinks and, in turn, tune the viscoelastic properties of the hydrogels (Figure 2a). By tuning the stoichiometry of the gel precursor molecules at a constant initial concentration of 3 wt% polymer, the excess of thiol groups after gel formation was tuned from 0–200% excess thiol relative to the initial concentration of norbornene groups. After each hydrogel was formed in situ, the final, equilibrium swollen modulus of the network was measured with a time-sweep at 1% strain and 1[s]−1 frequency. Results show that the final modulus of the hydrogel decreased as the thiol excess increased, consistent with the expectation that the presence of unreacted thiols correlates with fewer crosslinks formed (Figure 2b). Next, stress relaxation was measured by applying a 10% strain and recording how the resulting stress decreased over time. Overall, higher concentrations of thiol exhibited stress profiles with more relaxation (Figure 2c), and each profile was fit to a stretched exponential (Equation 1) to obtain the characteristic time constant τk and stretching exponent β. These fit constants can be found in supplementary Table S1.
FIGURE 2.
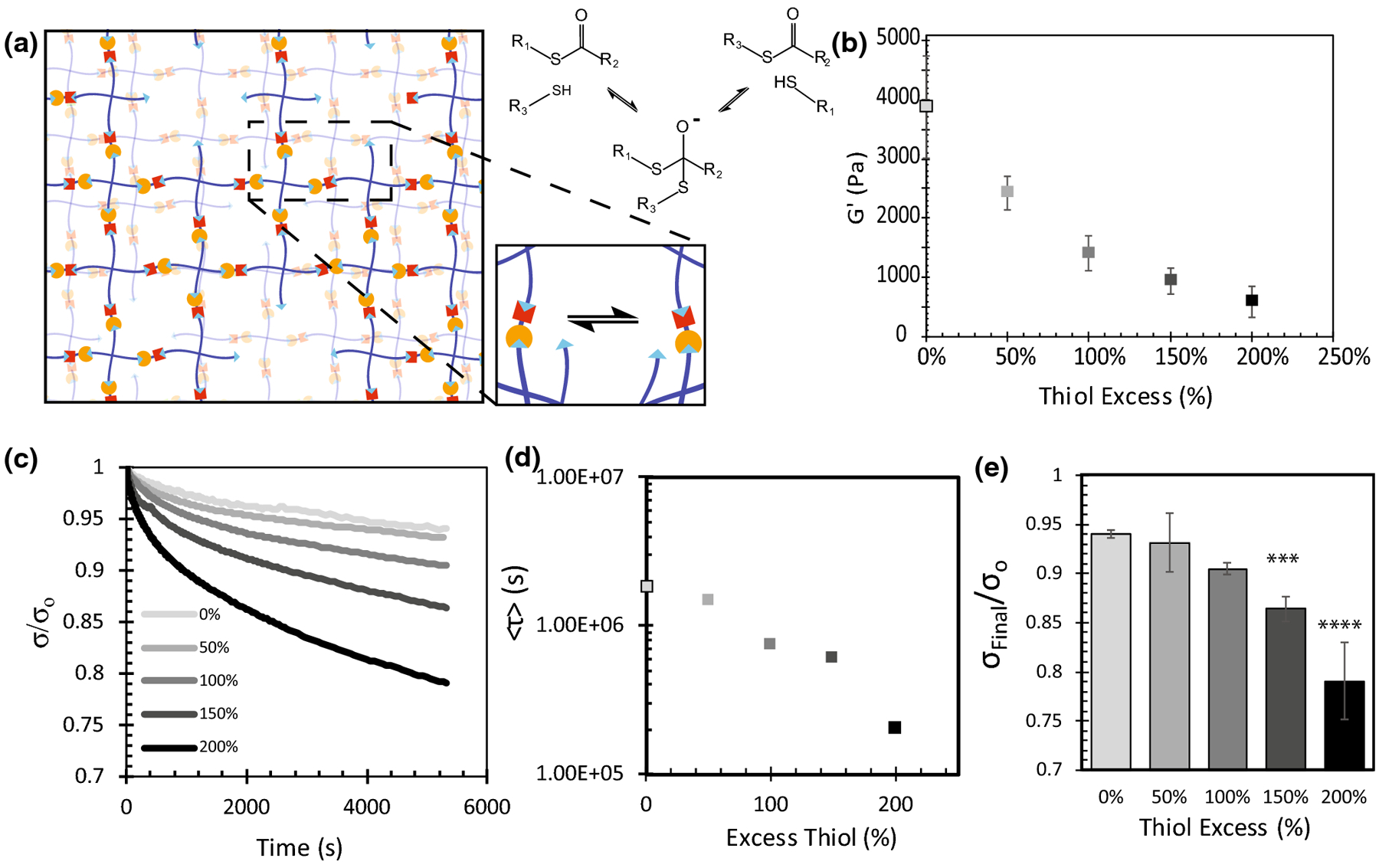
Viscoelastic properties of thioester crosslinked hydrogels depend on the concentration of unreacted thiols in the network. (a) Gel stoichiometry was tuned to ensure unreacted thiol species remained after network formation. These thiols then facilitated the exchange of crosslinks via the thioester exchange reaction. (b) In-situ shear elastic modulus of thioester hydrogels with a stoichiometric thiol excess ranging from 0–200%. (c) In-situ stress relaxation properties of thioester gels with a stoichiometric thiol excess ranging from 0–200%. Each profile is an average of n=3 replicates. (d) Average stress relaxation profiles (2c) were fitted to a stretched exponential equation (1) <τ> was calculated using equation (2) and the fitted parameters τk and β. (e) Final fraction of stress remaining after 5400s of relaxation. Reported error bars are the standard deviation, (***) indicates p < 0.001, (****) indicates p<0.0001, n=3.
| (1) |
From these data, the average relaxation time constant <τ> was calculated using
| (2) |
Here, σ is the shear stress measured at time t, σo is the initial stress, and Γ is the gamma function. From this analysis, we observed a range of <τ> values stretching from 200,000 to 2,000,000 s (Figure 2d). Over the 1.5 hr experiment, significant increases were observed in the total amount of stress relaxed as the concentration of thiols in the material increased (Figure 2e). The 100% excess thiol condition, with a shear elastic modulus of 1.4 kPa that translates to a Young’s modulus of ~4.2 kPa assuming linear elasticity, was selected as for further cell studies related to mechanotransduction, and henceforth will be referred to as the viscoelastic thioester condition.
Turning Off Viscoelasticity with the Thiol-ene ‘Click’ Reaction
Given the strong connection between thiol concentration and viscoelastic properties, we next sought to exploit the efficient photoinitiated thiol-ene click reaction to consume pendent thiols in the network, allowing viscoelastic properties of these materials to be modified with light in situ. First, viscoelastic thioester materials containing an excess of thiol were fabricated, swollen in a solution containing a photoinitiator and an alkene, and then finally irradiated with 365 nm light at 10 [mW][cm]−2 (Figure 3a). For the 100% excess thiol condition of interest, the final concentration of pendent thiols after network formation was approximately 5 [mmol][L]−1 based on stoichiometry calculations. To ensure all thiols would be reacted to completion, we elected to swell in a water-soluble and commercially available alkene 5-norbornene-2-carboxylic acid at a concentration of 10 [mmol][L]−1 and the photoinitiator LAP at a concentration of 1mM. The sample was subsequently irradiated with 365nm UV light at an intensity of 10 [mW][cm]−2. To ensure cytocompatibility, we confirmed that treatment of NIH 3T3 fibroblasts with 10 mM NB-COOH did not affect their viability by testing their metabolic activity using an Alamar’s assay (SI Figure S3).
FIGURE 3.
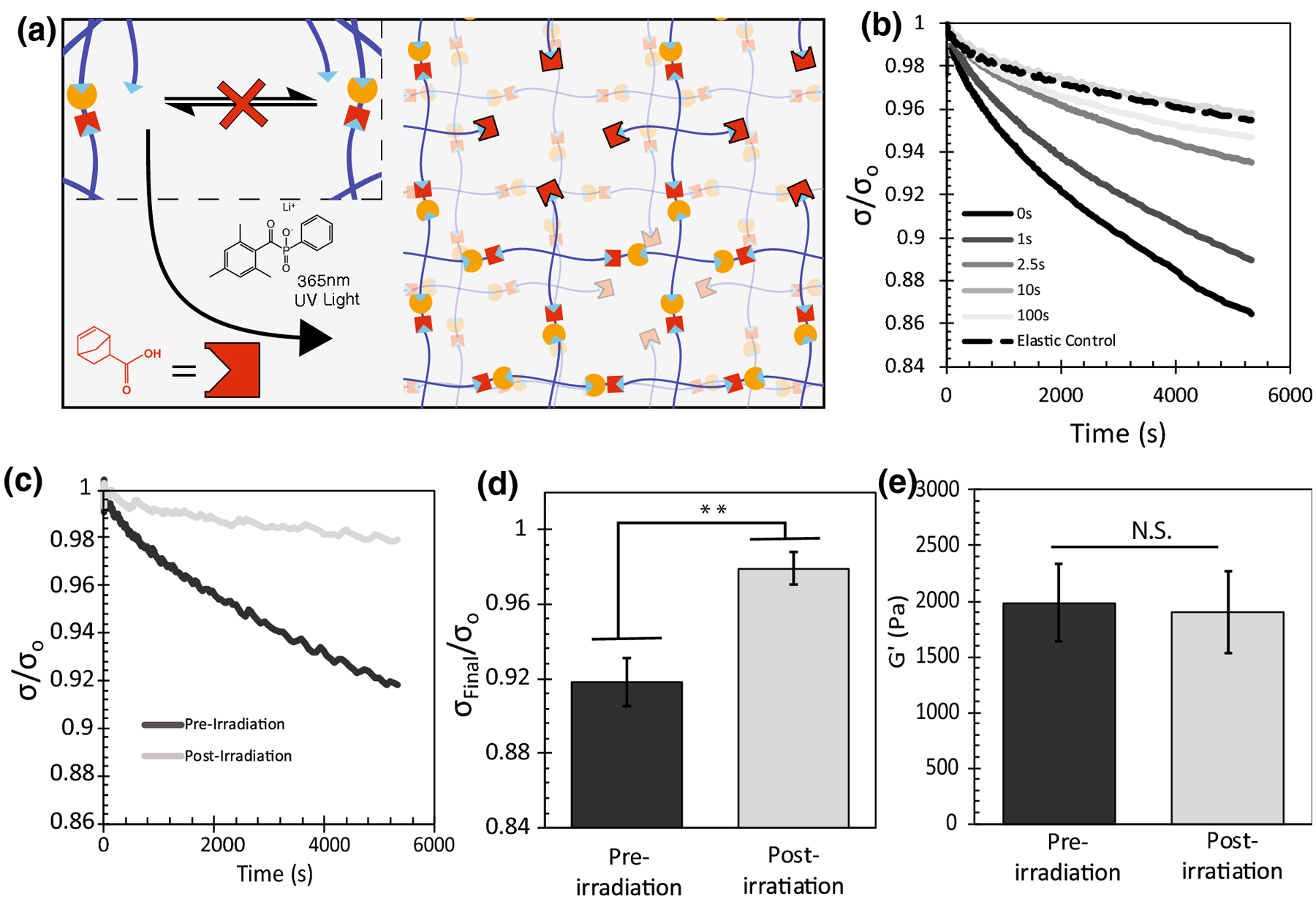
The photoinitiated thiol-ene reaction can be used to consume thiol function groups in the thioester network to tune stress relaxation with light. (a) By swelling LAP and norbornene COOH, the photoinitiated thiol-ene reaction can consume all network thiol species, interrupting the thioester exchange and bringing viscoelastic behavior to a halt. (b) Swollen stress relaxation properties of hydrogels swollen with 1mM LAP and 10mM Norbornene but irradiated with 10[mW][cm]−2 365nm UV light for different amounts of time. (n=1) (c) Swollen stress relaxation properties of swollen hydrogels before and after 100s of irradiation with 10[mW][cm]−2 365nm UV light. (n=3) (d) Final fraction of stress remaining after 5400s of relaxation. (n=3) (e) Swollen shear elastic modulus of swollen hydrogels before and after 100s of irradiation with 10[mW][cm]−2 365nm UV light. (n=3) Reported error bars are the standard deviation, (**) indicates p < 0.01, (N.S.) indicates no significance.
Next, 100% excess thiol thioester hydrogels were swollen for 30 min in PBS, irradiated for 0–100s, and characterized by stress relaxation. Hydrogel conditions irradiated for longer times exhibited slower rates of stress relaxation. By 10s of irradiation, the amount of network relaxation was comparable to a purely elastic non-adaptable PEG hydrogel control (Figure 3b). The result that the 10 s condition exhibited lower stress relaxation than the 100 s condition is likely a result of errors associated with attempting to measure viscoelastic properties of an elastic material. Given that these hydrogels are compressed a small axial force, nominal differences in modulus could account for these measurable differences in relaxation due to poroelasticity deforming less in response to the same axial force (SI Figure S4), however these differences are an artifact of the measurement and would have no effect on cell behavior. For this reason, the 100 s condition was selected as the chosen irradiation time necessary to effectively turn off stress relaxation properties in a thioester gel. To investigate the properties of viscoelastic thioester hydrogels and their treated elastic counterparts, a paired test was conducted in which viscoelastic gels swollen with norbornene and LAP were mechanically tested, irradiated in-situ, and tested again. As predicted, we observed a significant reduction (p<0.01) of stress relaxation between the viscoelastic and elastic conditions (Figure 3c–d) and no change in the mechanical storage modulus (Fig 3e). These data show that the photoinitiated thiol-ene reaction can be used to essentially turn off viscoelastic behavior in thioester hydrogels without any observed change to the stiffness of the hydrogel.
Thioester Mechanics Unaffected by Swelling Over Time
To characterize thioester hydrogel properties in a state more relevant to cell culture, the equilibrium modulus and stress relaxation properties of both elastic and viscoelastic thioester hydrogels were quantified over time while swelling in a phosphate buffered saline solution. First, equilibrium swollen viscoelastic hydrogels were prepared and the viscoelasticity was turned off by exposing half of the population to LAP, norbornene and 100 s of 10 [mW][cm]−2 365 nm UV light. All gels were allowed to swell in phosphate buffered saline. Samples were collected at 1, 24 and 48 hours and subjected to elastic modulus and stress relaxation measurements. The average swollen modulus for both the elastic and viscoelastic condition was approximately 1800 Pa and showed no significant decrease over the course of the 48 hour experiment (Figure 4a). As expected, when subjected to stress relaxation, the elastic hydrogels (Figure 4c) showed markedly less relaxation compared to the viscoelastic condition (Figure 4d). After closer inspection of the total amount of relaxation over the course of the 1.5hr experiment, no changes in relaxation were observed for the elastic condition over 48 hours (Figure 4e) and a modest increase was observed for the viscoelastic condition. In summary, once in a swollen environment, both the elastic and viscoelastic properties of these thioester hydrogels remained constant over a period of 48 hours.
FIGURE 4.
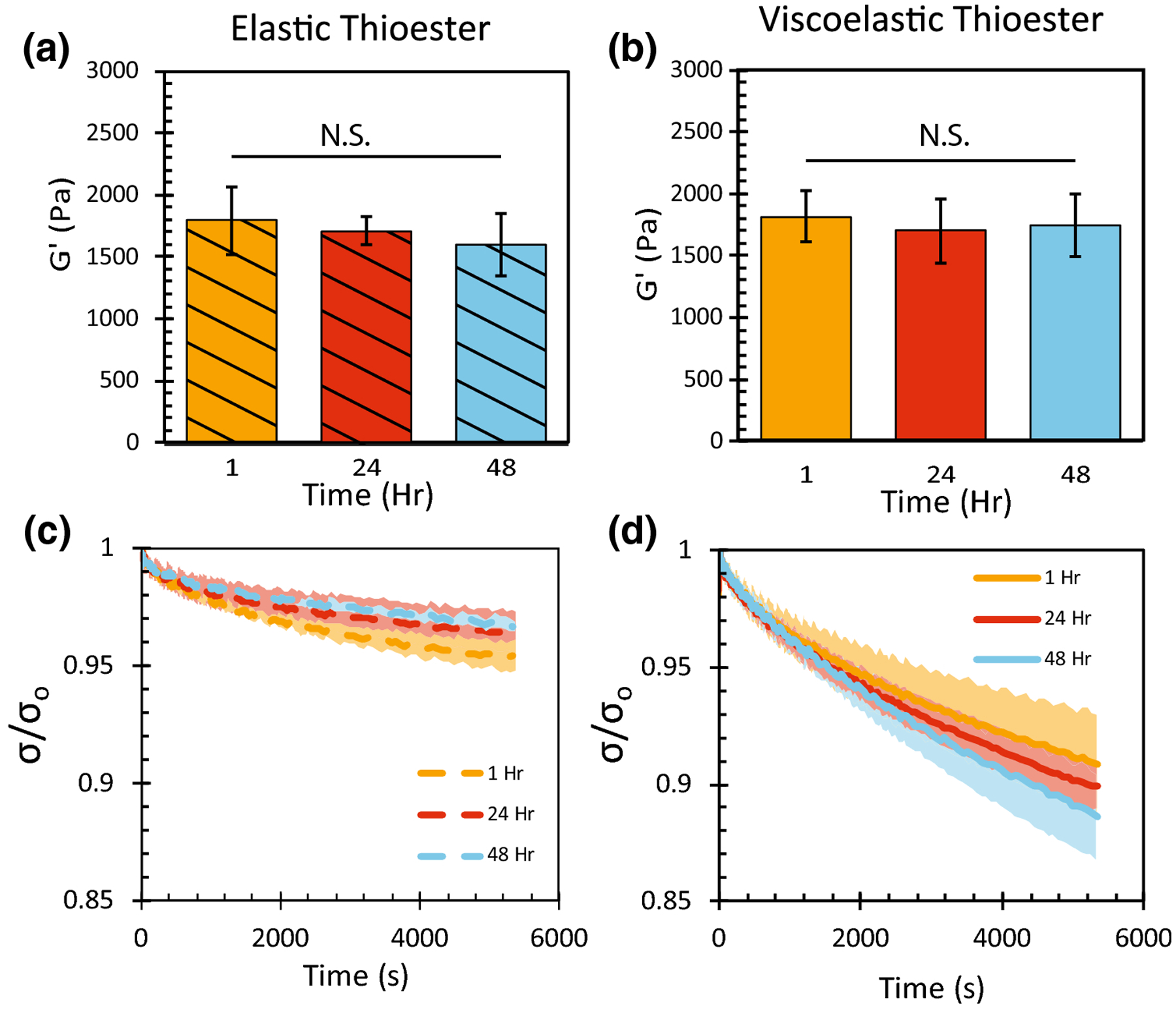
Swollen thioester hydrogels containing a 100% excess of network thiols were either treated with norbornene, LAP and light to be made purely elastic (striped/dashed), or left untreated as viscoelastic hydrogels (solid). (a,b) Shear elastic modulus of thioester hydrogels swollen in phosphate buffered saline. (c,d) Stress relaxation profile of swollen thioester hydrogels measured over 5400s. Final fraction of stress remaining after 5400s of relaxation. Each curve represents an n of 3 and the reported shaded region is the standard deviation, (N.S.) indicates no significance.
NIH 3T3 Fibroblasts Respond to Changes in Stress Relaxation
When studying mechanosensing of cells using viscoelastic material substrates, cell area and nuclear localization of YAP/TAZ are common outputs. In prior work6,13,24 mechanosensing cells have been observed to exert stronger traction forces on viscoelastic substrates, translating to increased cell area and cytoskeletal tension, leading to the nuclear localization of the transcription regulators, YAP/TAZ. NIH 3T3 fibroblasts were seeded on thioester hydrogels, fixed after 48 hours of culture and assessed for changes in cell area and the ratio of YAP/TAZ in the nucleus compared to the cytoplasm. Cells were seeded on a purely viscoelastic thioester condition, a purely elastic thioester condition, and on viscoelastic thioester gels in which viscoelasticity was switched off after 24 hr (Figure 5a). Control experiments demonstrated that cell viability and YAP localization were not affected by treatment with LAP, NB and light. Cell area increased slightly in response to treatment, however since cell area was observed to be smaller on the switch condition this effect is clearly not as important as changes in substrate mechanics (SI FigureS5, SI FigureS6). Cells were stained for YAP/TAZ (AF-488), nuclei (DAPI), and cytoplasm (HCS cell mask orange) (Figure 5b). The observed area of spread cells was 660 ± 250 [μm]2 (reported error is the standard deviation) on the viscoelastic condition and was larger than the average cell area of 560 ±170 [μm]2 observed on the elastic condition (Figure 5c). This trend agrees with literature and indicates that the cells are responding to the differences in the viscoelasticity between the two hydrogels microenvironments. Next, the average cell area for the switch hydrogel condition was characterized and found to be between the viscoelastic and elastic conditions at a value 540 ± 160 [μm]2, and was significantly smaller than the elastic condition. Images were quantified by dividing the ratio of the YAP staining intensity in the nucleus by the intensity in the cytoplasm. The average nuclear to cytoplasmic YAP/TAZ ratio was higher on the viscoelastic condition of 2.4 ± 0.5 compared to 2.2 ± 0.4 reported on the elastic substrate ,indicating once more that cells can sense the difference between these two substrates (Figure 5d). The YAP/TAZ ratio on the switch condition was 2.1 ± 0.3 statistically the same as the elastic substrate and decreased compared to its original viscoelastic condition.
FIGURE 5.
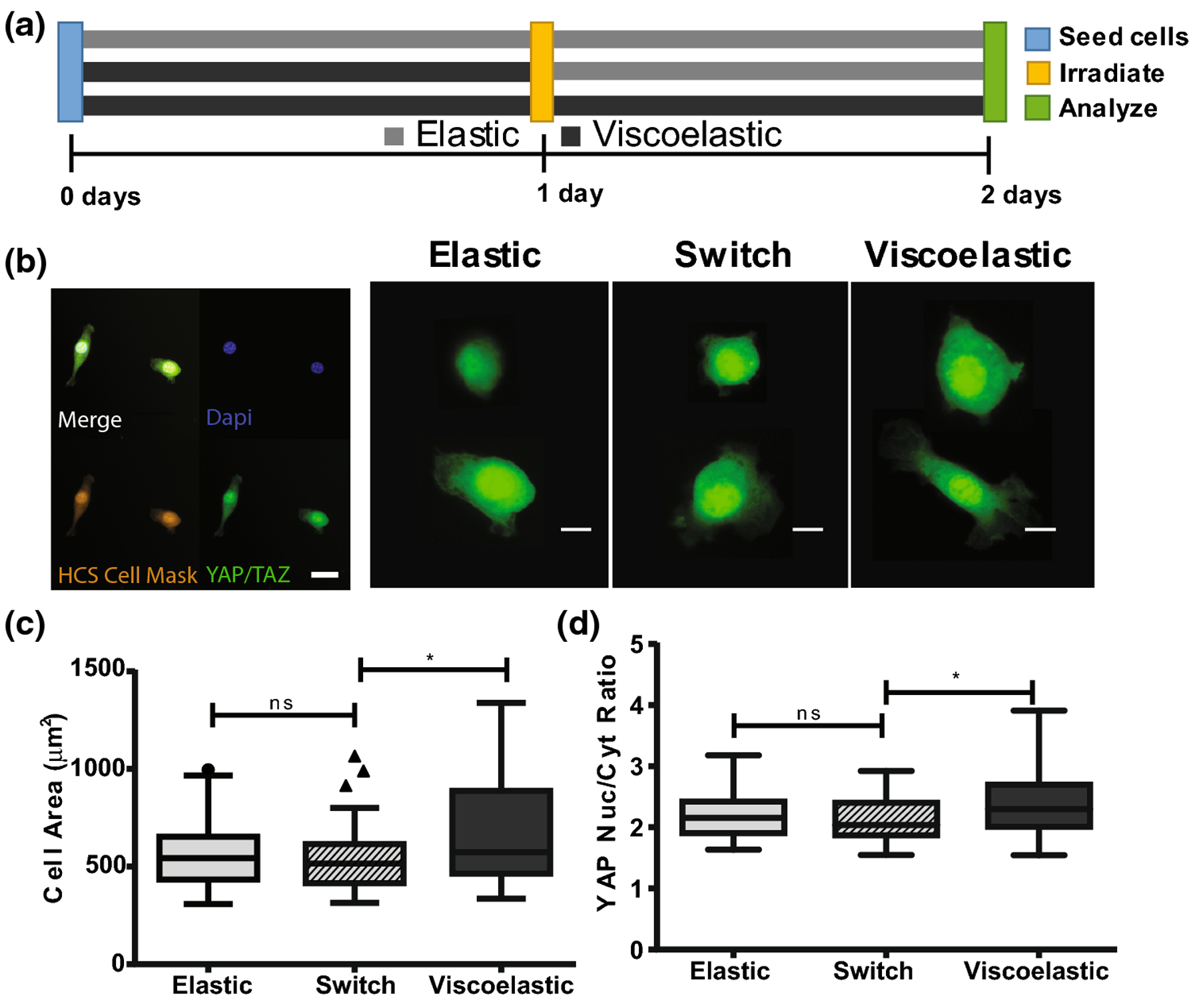
(a) NIH 3T3 fibroblasts were seeded onto swollen 2D thioester hydrogels and cultured for 48 hours. Cells were seeded onto an elastic thioester condition, a viscoelastic thioester condition and a switch condition where viscoelastic hydrogels were made elastic after 24 hours of culture. (b) Cells were fixed at 48 hours and stained for HCS Cell Mask (orange), DAPI (blue) and YAP/TAZ (green); pictured scalebar is 30 microns. YAP/TAZ staining for select cells is shown; the red circle indicates the outline of the nucleus as identified by DAPI staining. Scale bar for single cell images is 10 microns. (c) Spread cell area is visualized by a Tukey boxplot. (d) Nuclear to cytoplasmic ratio of YAP. Reported statistics were calculated using a one tailed student’s t-test, and a population of n=50–60 cells for each condition. Greater than 40 cells were quantified per hydrogel, then 10 cells were selected at random and added to the final population sampling for that condition. (*p<0.05, **p<0.01, ns nonsignificant)
Discussion
As the biomaterials field has advanced the understanding as to how cells transduce time-independent mechanical signals [e.g., elastic modulus] and time-dependent mechanical signals [e.g., stress relaxation], a question of how cells differentiate between these stimuli has come into focus.24 Materials where viscoelasticity can be changed in situ have the potential to provide insight into mechanotransduction, because the cell response to a creep or stress relaxation on multiple time scales can be studied. Though design of modular viscoelastic materials remains challenging, some examples already exist in the literature. Marozas et al. developed the first cell culture platform where viscoelastic properties could be altered in the presence of cells during a period of light irradiation, and demonstrated how changes in viscoelasticity on short timescales can lead to a decrease in cytoskeletal tension.17 Inspired by the types of questions this material facilitated, we designed a viscoelastic material based on thioester exchange that allows for permanent changes in viscoelasticity by reacting away thiol species with light. This complementary material system can naturally facilitate the study of cell processes that happen on longer timescales and allow switching from viscoelastic to elastic microenvironments in a spatiotemporal controlled manner. Through the use of a water-soluble monofunctional norbornene, viscoelastic properties were completely switched off (Figure 3b) without any changes to the elastic modulus (Figure 3e).
We then demonstrated that mechanical properties of both elastic and viscoelastic thioester hydrogels remained constant over the course of 48 hours (Figure 4). However, that is not to say that mechanics would not change after 48 hours and at this point there are two important issues to note. The first is that viscoelastic hydrogels based on covalent adaptable chemistries fundamentally swell in solution at the same rate at which they relax stress. As a result, hydrogels that stress relax faster reach reverse gelation quicker. There are some strategies that have been used in the literature to address this shortcoming, Tang et al. for instance used a percolating network of non-adaptable crosslinks to extend cell culture up to 7 days.15 Though the thioester network had only adaptable crosslinks in this work, significant swelling was not observed over the course of 2 days, as shown by the constant equilibrium modulus plotted in Figure 4b. This result is explained by the fact that the relaxation profiles of this material, when fit to a stretched exponential, had a β value between 0.4 and 0.5 (SI Table S1), which is highly heterogeneous compared to an ideal network where β is 1. Functionally, this means that although 10% of stress is relaxed in just 1.5 hrs, 20% of the stress is not relaxed until 8 hrs, and by the end of one week, only 60% of bonds in the material have exchanged. The second issue that was addressed was that over time all thiol function groups in a biological system can oxidize to form disulfide bonds, which no-longer participate in the thioester exchange. Though the timescale of this oxidation can be tuned by choosing thiol species with a higher pKa value25, ultimately this phenomena limits how long thioester materials can remain viscoelastic. In this work however, no significant disulfide bond formation was observed over 48 hours because no reduction of stress relaxation over time occurred (Figure 4f).
NIH 3T3 fibroblasts were cultured for 48 hours on thioester hydrogels that were purely elastic, purely viscoelastic, or switched from viscoelastic to elastic after 24 hours. Cells cultured on purely viscoelastic substrates showed increased cell spreading and increased nuclear YAP/TAZ compared to the purely elastic thioester substrates, demonstrating that even cells responded to changes in material relaxation, even at timescales longer than once thought. Recent efforts in the literature have focused on viscoelastic substrates with fast relaxation timescales on the order of 10–1000 s. These fast relaxing substrates have been shown to elicit large increases in cell spreading and nuclear localized YAP/TAZ across multiple cell types.6,13,15 These responses are thought to be driven by ligand clustering that leads to stronger focal adhesions and occurs on a similar timescale.26 Of course the effect of stress relaxation over longer time scales on cell phenomena [e.g. the differentiation of hMSCs, cell secretory properties] has also been studied, but these phenomena are also thought to be driven by increased ligand clustering that occurs on the 10–1000 s time scale.13 With this context, the fact that we observe increased cell spreading and nuclear localization of YAP/TAZ even at much slower relaxation rates could suggest that other cell-matrix interactions (i.e., outside-in signaling) exist that occur over timescales much longer than originally thought, perhaps even that ligand clustering events are not necessarily independent from one another Further experimentation would be needed to confirm whether this is the case.
Observation of NIH 3T3 fibroblasts cultured on the switch thioester condition showed that after 24 hours of culture on a viscoelastic substrate followed by 24 hours of culture on an elastic substrate resulted in cells that behaved more closely to the elastic control than to the viscoelastic control. Though this experiment is rudimentary in nature, it showcases a new class of questions that can now be asked. How would cell spreading and YAP/TAZ be impacted if the point in time when the switch occurred was changed? As a tool, thioester substrates have a limited amount of time over which they can remain viscoelastic, but even the time course shown here is sufficient to investigate changes in other downstream phenomena such as epigenetics and RNA expression. Such information could prove valuable in deconvoluting how cells fundamentally process elastic and viscoelastic matrix cues, and how viscoelastic properties could be engineered to yield particular cell responses.
In conclusion, we have presented a viscoelastic hydrogel material based on thioester exchange covalent adaptable chemistry that rapidly forms a gel using the photoinitiated thiol-ene reaction. It was shown that viscoelastic properties like stress relaxation in this material depend on the concentration of unreacted thiol groups in the network. Once a network is formed, results demonstrated that LAP and 5-norbornene-2-carboxylic acid could be swollen in the material and used to initiate a second thiol-ene reaction that consumes the thiol species in the network, effectively turning off viscoelasticity without any change to the swollen modulus. Each of these reactions is cytocompatible and can be conducted in the presence of cells in 2D or 3D cultures. Lastly, we demonstrated that this well-defined tool with switchable viscoelastic properties could be used to gain information on the timescales in which NIH 3T3 fibroblasts respond to viscoelastic cues. Switchable viscoelastic thioester materials add to a growing collection of highly tunable viscoelastic cell culture platforms already being used to understand interactions between cells and the extracellular matrix.
Supplementary Material
Acknowledgements:
This work was supported by a grant from the National Institutes of Health (DE016523), and BJC also gratefully acknowledges support via a GAANN fellowship from the Department of Education. The authors would also like to gratefully acknowledge Dr. Kemal Arda Günay and Francis Yavitt for thoughtful conversation and critical reviews in the preparation of this manuscript. The authors do not have any conflicts of interest to declare.
Abbreviations
- ECM
Extracellular matrix
- hMSC
Human mesenchymal stem cell
- YAP
Yes-associated protein
- TAZ
Transcriptional co-activator with PDZ binding motif
- DMEM
Dulbecco’s modified Eagle’s media
- NB COOH
5-norbornene-2-carboxylic acid (Norbornene COOH)
- PBS
Phosphate buffered saline
- FBS
Fetal bovine serum
- kDa
Kilodalton
- PEG
Poly(ethylene glycol)
- TENB
Thioester Norbornene
- LAP
Phenyl-2,4,6-trimethylbenzoylphosphinate
- PFA
Paraformaldehyde
- BSA
Bovine serum albumin
- HATU
N-[(Dimethylamino)-1H-1,2,3-triazolo-[4,5-b]pyridin-1-ylmethylene]-N-methylmethanaminium hexafluorophosphate N-oxide
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References:
- 1.Engler AJ, Sen S, Sweeney HL & Discher DE Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 126, 677–689 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Guilak F et al. Control of Stem Cell Fate by Physical Interactions with the Extracellular Matrix. Cell Stem Cell 5, 17–26 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ingber DE Mechanical control of tissue morphogenesis during embryological development. Int. J. Dev. Biol 50, 255–266 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Liu F et al. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am. J. Physiol. Cell. Mol. Physiol 308, L344–L357 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanconato F, Cordenonsi M & Piccolo S YAP/TAZ at the Roots of Cancer. Cancer Cell 29, 783–803 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhuri O et al. Substrate stress relaxation regulates cell spreading. Nat. Commun (2015). doi: 10.1038/ncomms7365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cameron AR, Frith JE & Cooper-White JJ The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials 32, 5979–93 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Dupont S et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Chaudhuri O et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater 15, 326–34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darnell M et al. Material microenvironmental properties couple to induce distinct transcriptional programs in mammalian stem cells. Proc. Natl. Acad. Sci (2018). doi: 10.1073/pnas.1802568115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vining KH, Stafford A & Mooney DJ Sequential modes of crosslinking tune viscoelasticity of cell-instructive hydrogels. Biomaterials (2019). doi: 10.1016/j.biomaterials.2018.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cameron AR, Frith JE & Cooper-White JJ The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials 32, 5979–5993 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Chaudhuri O et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater (2016). doi: 10.1038/nmat4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKinnon DD, Domaille DW, Cha JN & Anseth KS Biophysically defined and cytocompatible covalently adaptable networks as viscoelastic 3D cell culture systems. Adv. Mater 26, 865–72 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang S et al. Adaptable Fast Relaxing Boronate-Based Hydrogels for Probing Cell-Matrix Interactions. Adv. Sci 5, 1800638 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown TE et al. Photopolymerized dynamic hydrogels with tunable viscoelastic properties through thioester exchange. Biomaterials 178, 496–503 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marozas IA, Cooper-White JJ & Anseth KS Photo-induced viscoelasticity in cytocompatible hydrogel substrates. New J. Phys 21, 045004 (2019). [Google Scholar]
- 18.Fairbanks BD et al. A Versatile Synthetic Extracellular Matrix Mimic via Thiol-Norbornene Photopolymerization. Adv. Mater 21, 5005–5010 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aimetti AA, Machen AJ & Anseth KS Poly(ethylene glycol) hydrogels formed by thiol-ene photopolymerization for enzyme-responsive protein delivery. Biomaterials 30, 6048–6054 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fairbanks BD, Schwartz MP, Bowman CN & Anseth KS Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials 30, 6702–6707 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang C, Tibbitt MW, Basta L & Anseth KS Mechanical memory and dosing influence stem cell fate. Nat. Mater 13, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rammensee S, Huemmerich D, Hermanson KD, Scheibel T & Bausch AR Rheological characterization of hydrogels formed by recombinantly produced spider silk. Appl. Phys. A Mater. Sci. Process 82, 261–264 (2006). [Google Scholar]
- 23.Richardson BM, Wilcox DG, Randolph MA & Anseth KS Hydrazone covalent adaptable networks modulate extracellular matrix deposition for cartilage tissue engineering. Acta Biomater. 83, 71–82 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dey K, Agnelli S & Sartore L Dynamic freedom: Substrate stress relaxation stimulates cell responses. Biomater. Sci 7, 836–842 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Poole LB The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med 80, 148–157 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaudhuri O Viscoelastic hydrogels for 3D cell culture. Biomater. Sci 5, (2017). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


