Abstract
The development of novel 3D tissue culture systems has enabled the in vitro study of in vivo processes, thereby overcoming many of the limitations of previous 2D tissue culture systems. Advances in biomaterials, including the discovery of novel synthetic polymers has allowed for the generation of physiologically relevant in vitro 3D culture models. A large number of 3D culture systems, aided by novel organ-on-a-chip and bioreactor technologies have been developed to improve reproducibility and scalability of in vitro organ models. The discovery of induced pluripotent stem cells (iPSCs) and the increasing number of protocols to generate iPSC-derived cell types has allowed for the generation of novel 3D models with minimal ethical limitations. The production of iPSC-derived 3D cultures has revolutionized the field of developmental biology and in particular, the study of fetal brain development. Furthermore, physiologically relevant 3D cultures generated from PSCs or adult stem cells (ASCs) have greatly advanced in vitro disease modelling and drug discovery. This review focuses on advances in 3D culture systems over the past years to model fetal development, disease pathology and support drug discovery in vitro, with a specific focus on the enabling role of biomaterials.
1. Introduction
Historically, in vitro tissue culture has been conducted primarily in a 2D setting with at most a co-culture of two to three different cell types. Although informative and easily accessible, this method provides an oversimplified understanding of what is naturally a multiplex system. Models cultured in static conditions on hard plastic surfaces cannot fully recapitulate the chemical and mechanical cues to promote similar cell-cell and cell-ECM interactions in vivo. Studying a complex system such as the human body in discrete sections may neglect biophysical and 3D matrix related factors that will undoubtedly impact the crosstalk between different cell types or alter critical signaling pathways. Additionally, to run such experiments, only a select number of variables could be simultaneously compared, requiring a well-defined and attributive experimental hypothesis. Regardless, due to the conventional methods for 2D culture and the breadth of research available for benchmarking, little has been done to expand the boundaries of in vitro modelling.
Given the challenge in translating research findings from in vitro experiments and animal models to clinical trials, many have expressed the need for an intermediate stage where novel findings can be tested against human-specific models. The integration of engineering principles with fundamental biological knowledge has further led to significant developments in 3D culturing techniques, enhancing the ability of researchers to reproduce physiologically relevant environments in vitro. This review will provide an overview of commonly used biomaterials and novel three-dimensional culture systems such as organoids and organ-on-a-chip systems, as well as the applications of these technologies in developmental and disease models and high-throughput drug testing. With a vast range of novel biomaterials at our disposal, researchers are rapidly moving away from plastic culture and transitioning into intricate microfabricated 3D models that capture specific tissue functions, as seen in the development of organ-on-a-chip systems1. Likewise, the availability of the organ-specific cells has increased tremendously, due to the improved understanding of developmental biology and advancement in stem cell technology. These critical steps allow for successes in 3D tissue development and the creation of functional engineered tissues2. Here, we defined organoids as spherical aggregates of cells that occur due to the spontaneous differentiation of either adult or pluripotent stem cells. Organs-on-a-chip are defined as we stated previously, as cell culture methods that have a significant engineering component, such as guided spatial confinement of cells or incorporation of sensors and microfluidic channels32. Significant advancements have occurred in models for cardiac, pulmonary, renal as well as hepatic tissues but more recently there has been a vested interest in examining 3D culture techniques for the development of long-term neural models3–6. The authors will also address the drawbacks of existing models and propose strategies for enhancing their accuracy.
With the development of more complex 3D models and the ability to customize a system on a patient-specific basis, such models may be better predictors of human physiology than animal models. Additionally, with the increasing accessibility of genetic sequencing tools, these systems will likely be more economical and provide faster results. However, with the present number of competing 3D models, further testing will be necessary to standardize these systems and improve the reproducibility of experimental outcomes. 3D culture modelling is indisputably superior to its predecessors but will require a substantial amount of additional investigation prior to its adaptation for widespread clinical use.
2. Sources of Cells for In Vitro 3D Culture Models
3D culture models can be defined as the growth and interaction of cells in vitro in a three-dimensional space. Although a range of cell types can be grown in vitro under 3D conditions, this review focuses on culture models derived from stem and stem-like cells. Pluripotent stem cells (PSCs), including embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), are commonly used to generate 3D tissues in vitro through regulation of differentiation cues (Figure 1A). Alternatively, adult stem cells can be isolated from volunteers and grown in vitro under conditions that support the formation of 3D tissues (Figure 1B). 3D cancer models generated in vitro using cancer cells isolated from patients are referred to as tumour organoids.
Figure 1. Sources of cells for in vitro 3D culture models.
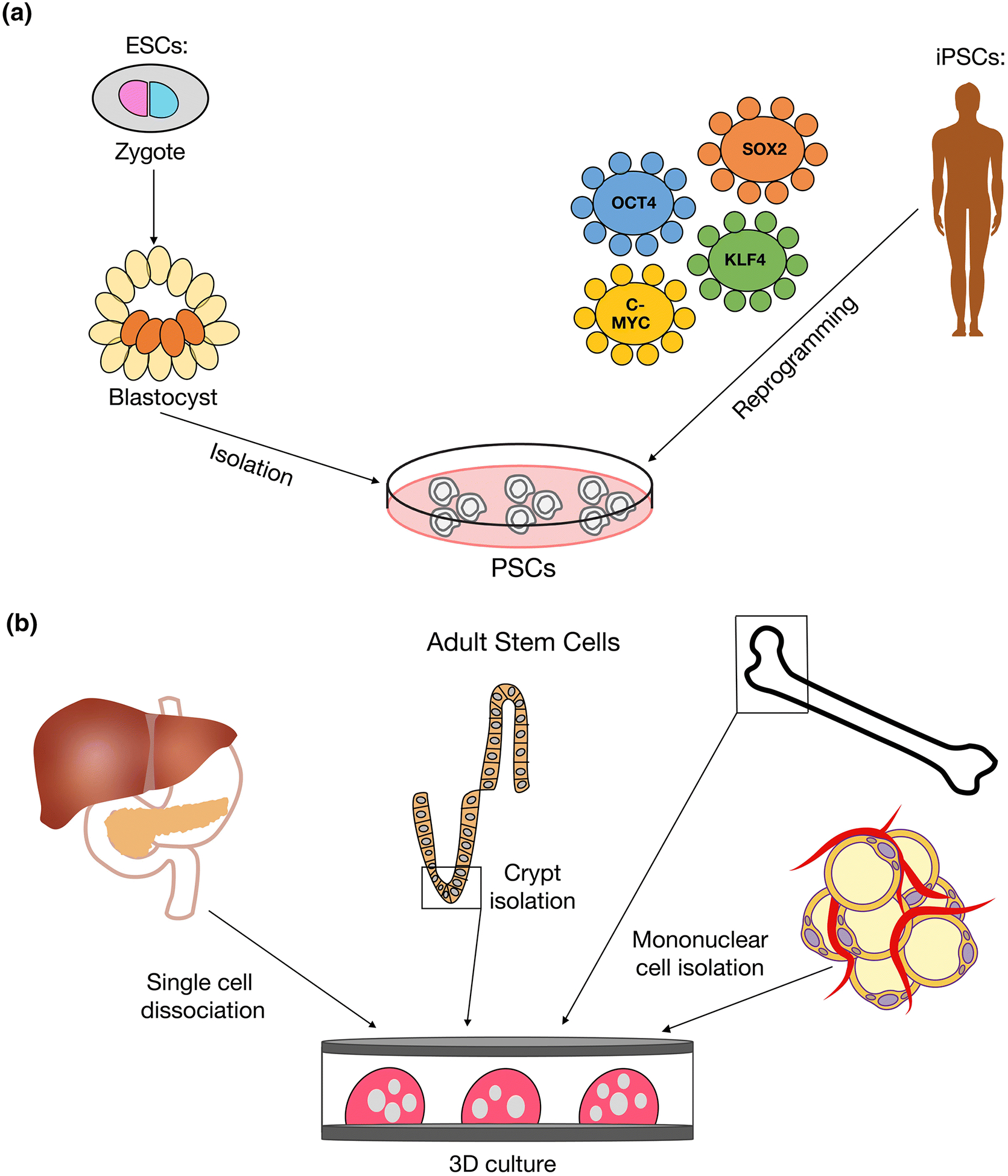
A. Human PSCs are comprised of ESCs and iPSCs. ESCs are generated from pre-implantation embryos by isolating the inner cell mass of a blastocyst and culturing these cells in vitro under the appropriate conditions to maintain pluripotency. Alternatively, iPSCs are generated by the direct reprogramming of adult cells into a pluripotent state. A viral vector encoding the transcription factors OCT4, SOX2, KLF4 and c-MYC, known as the Yamanaka factors, is used to deliver the reprogramming factors to the cells. B. Adult stem cells (ASCs) isolated from healthy volunteers or patients are grown in vitro under optimized conditions to support the growth and expansion of 3D culture models such as organoids and spheroids. Examples of tissues where ASCs have been successfully isolated from include liver, pancreas, intestinal crypt, bone marrow and fat15,31,37,39,40,93.
A specific sub-set of 3D cell culture are organoids. Organoids are self-organizing in vitro organ-like 3D structures that are formed from PSC-derived (Figure 2) or adult stem cell-derived progenitors (Figure 1B) and contain multiple cell types that resemble the in vivo organ and retain some physiological function7. These systems are most often generated relying on self-aggregation of differentiating cells. However, they can be formed by embedding cells in hydrogels containing polymers or extracellular matrix components8. The predominantly used biomaterials for culturing organoids are Matrigel and collagen. Garnier and colleagues enhanced and stabilized culture conditions for human hepatocytes in Matrigel hydrogels9. Qian and colleagues have also used Matrigel to culture specific regions of the brain by using embryoid bodies10. Furthermore, this study grew brain organoids in spinning bioreactors to generate parts of the forebrain from iPSCs. Synthetic polymers were explored using polyethylene glycol, a synthetic polymer for organoid formation as demonstrated by Blondel and colleagues for mouse and human intestinal modelling11. Furthermore, functionalizing polyethylene glycol with a peptide, RGDSP, allowed for enhanced adherence of mouse intestinal stem cells. Considering the range of biomaterials available, organoid cultures are able to replicate various tissue types including brain, heart, kidney, retinal, intestinal and colon among others12–17
Figure 2. Technologies to support in vitro 3D culture models.
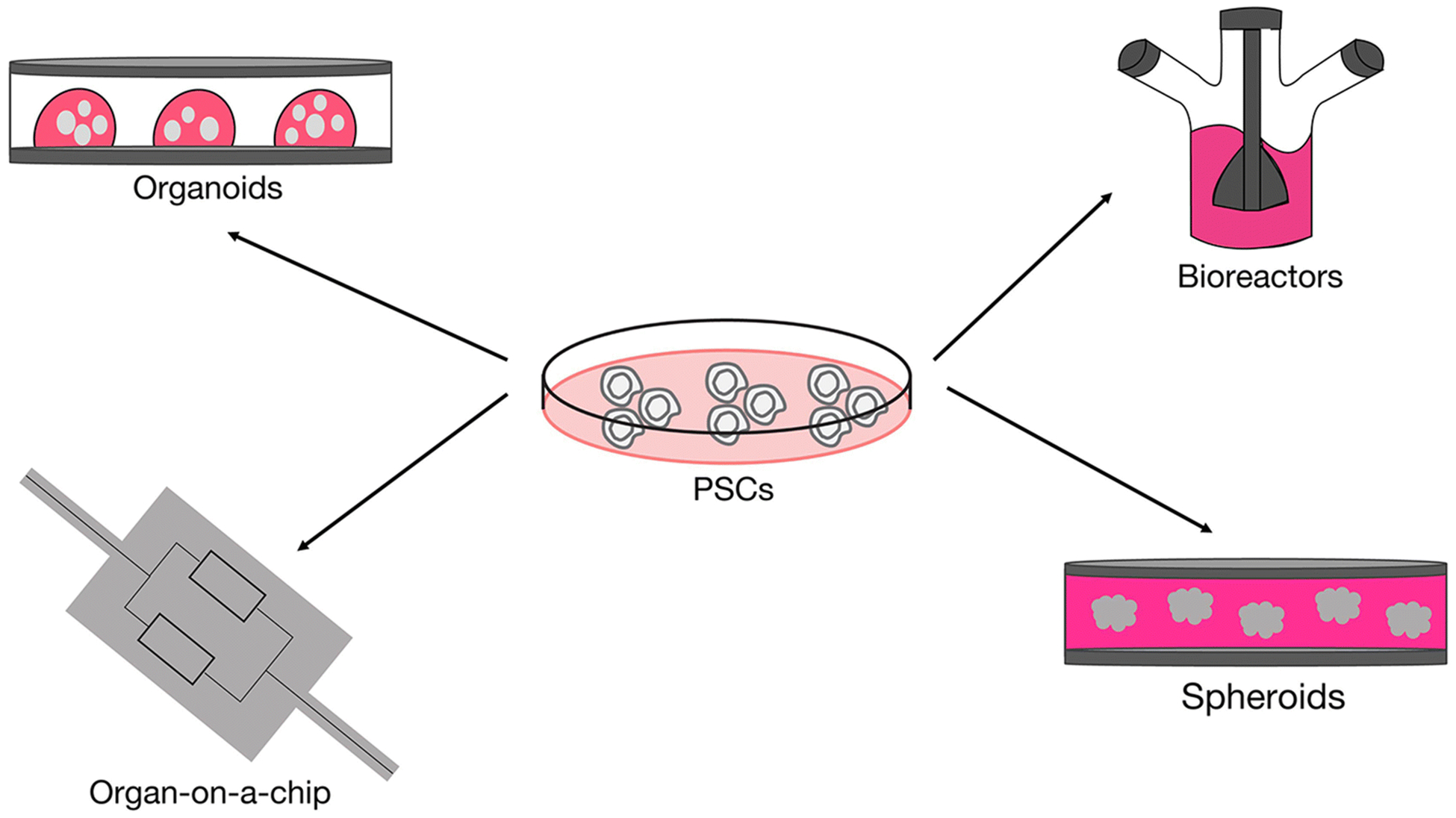
PSCs can be used to generate a range of different 3D culture models by combining differentiation protocols with technologies to support cell growth under 3D conditions. The simplest method involves culturing differentiated cells under suspension conditions to form spheroids. Organoids are generated by embedding differentiated cells in an extracellular matrix such as Matrigel. Bioreactors are used to scale up organoid and spheroid cultures and to allow for long-term growth of these tissues due to increased circulation of nutrients and waste products. Organ-on-a-chip systems make use of microfabricated devices with micrometer-sized chambers and continuous perfusion of culture medium. Multiple chips can be linked fluidically to join several organ models and recapitulate in vivo physiology.
2.1. Pluripotent Stem Cell (PSC)-derived 3D Culture Models
The most commonly used technique to generate in vitro 3D culture models begins with PSCs, either iPSCs or ESCs, that are differentiated into specific cell types of interest using a defined protocol. PSCs can initially be differentiated under 2D or 3D culture conditions and later combined with other differentiated cell types or commercially available cell lines to generate 3D co-cultures. 3D tissue models from all three germ layers – ectoderm, endoderm and mesoderm have been generated using this workflow (Table 1) with cerebral, pancreatic and kidney organoids as examples for each germ layer, respectively18–20. These 3D models can be used to study tissue development as well as congenital conditions and developmental defects in vitro, ultimately leading to the establishment of more efficacious treatments. The main limitation of PSC-derived 3D models is that the tissues generated resemble embryonic or fetal rather than adult tissues. This raises the question of whether 3D tissues generated in vitro from PSCs will be useful for studying adult diseases or in the development of transplantation therapies, emphasizing the need for bioengineering approaches that can generate mature tissue.
Table 1.
Summary of PSC-derived 3D culture models.
| Germ Layer | Organ | Cell Types Required | 3D Model | Reference |
|---|---|---|---|---|
| Endoderm | Pancreas | Pancreatic progenitors | Pancreatic spheroids/organoids | 20,23,200 |
| Liver | Hepatocyte progenitors, endothelial cells (HUVECs), hMSCs | Liver bud organoids | 92 | |
| Intestine | Intestinal progenitors | Intestinal organoids | 16 | |
| Stomach | Gastric progenitors | Gastric organoids | 201 | |
| Lung | Lung progenitors | Lung organoids | 158,202 | |
| Mesoderm | Heart | Cardiomyocyte progenitors | Cardiospheres | 99 |
| Kidney | Renal progenitors | Kidney/ureteric bud organoids | 17,19,100 | |
| Ureteric bud progenitors, mouse metanephric mesenchymal cells | Chimeric kidney organoid | 203 | ||
| Blood | Megakaryocytes | Megakaryocyte spheroids | 98 | |
| Ectoderm | Retina | Retinal progenitors | Optic cup organoid | 14 |
| Brain | Neural progenitors | Cerebral organoids | 10,18,91 | |
| Neural progenitors | Midbrain organoids | 204 | ||
| Neural progenitors | Neurospheres | 6 |
Organoid models can be derived from PSCs by first inducing differentiation into a specific germ layer, followed by further differentiation into lineage-specific progenitors upon aggregation21. For example, a model of neural tube formation was generated by culturing mouse ESCs under neural induction conditions to form 3D neuroepithelia, which could be further patterned through the addition of Sonic Hedgehog or retinoic acid22. The addition of retinoic acid to these 3D neuroepithelial cultures spontaneously formed a floor plate, which resulted in the generation of various dorsal and ventral neural subtypes. Pancreatic organoids were generated from iPSC-derived pancreatic progenitors, which resulted in mature pancreatic cell types upon prolonged culture, including differentiated β-cells that secreted insulin in response to glucose treatment23. Cerebral organoids grown from iPSC-derived neural progenitors have been used to generate 3D tissue to model brain development in vitro18. However, a major limitation of PSC-derived organoids is the resemblance to fetal organs and the requirement of extended in vitro culture to generate more mature fates. For example, PSC-derived cardiac organoids present an immature action potential profile, calcium transients and glucose-dependent metabolic activity similar to the human fetal heart12,24–26. Human cerebral organoids also share similar proteomic profiles with fetal brain tissues.27,28
2.2. Adult Stem Cell-derived 3D Culture Models
An alternative to PSC-derived 3D culture models is the use of adult stem cells isolated from healthy volunteers or patients (Figure 1B). These adult stem cells, such as hematopoietic stem cells, bone marrow stem cells, adipose-derived stem cells and intestinal stem cells, can be isolated from a patient biopsy, surgical resection or donated healthy and diseased organs, and can serve as cell sources for both normal and tumour organoids29–31. These cells can be obtained without ethical limitations related to the use of ESCs and have the potency to differentiate into many cell types in vitro. However, they are typically only multipotent and do not have the same differentiation potential as PSCs32–34. The advantage of using adult stem cells over PSCs is the ability to generate 3D models of adult tissue in vitro, due to the fetal-like phenotypes reported from PSCs differentiation35–38. Therefore, this system is useful for studying tissue homeostasis, organ injury and repair, cellular aging and the mechanisms of age-related diseases such as cancer. These cell types are also well suited as a source for autologous transplantation therapies. Some organs for which these models have been developed are shown in Table 2, including the small intestine, colon, liver, pancreas and lung15,37,39–41. However, the acquisition of adult stem cells is more invasive, expensive and relies on the presence of stem cell pools within the tissue of interest. Thus, obtaining adult stem cells can be a limiting factor depending on the invasiveness of the surgery required. Another challenge is the longevity of the cultures, since some developed models cannot be cultured for long periods of time. However, this can often be resolved through optimization of the in vitro culture conditions.
Table 2.
Summary of adult stem cell-derived 3D culture models
| Organ | Isolated Cell Type | 3D Model | Reference |
|---|---|---|---|
| Bone | Human articular chondrocytes (hACs), hMSCs | Chondrocyte spheroids | 93 |
| Human osteoblasts | Bone spheroids | 205 | |
| Small Intestine | Intestinal stem cells | Small intestinal organoids | 15,40 |
| Colon | Intestinal stem cells | Colonic organoids | 15 |
| Lung | Alveolar epithelial progenitor (AEP), human lung fibroblasts (CCL-171) | Lung organoids | 41 |
| Liver | Mouse liver cells | Liver organoids | 37 |
| Stomach | Mouse gastric stem cells | Gastric organoids | 206 |
| Pancreas | Mouse pancreatic progenitors | Pancreatic organoids | 39 |
| Prostate | Prostate stem cells | Prostate organoids | 207 |
| Ovary | Fallopian tube stem cells | Fallopian tube organoids | 208 |
As previously described, patient-derived adult stem cells can be used to generate organoids resembling healthy adult organs. Various epithelial organoid models have been generated using this methodology. The first adult stem cell-derived organoid models were generated by embedding isolated intestinal crypts or sorted epithelial cells in Matrigel, which expanded to form intestinal and colonic organoids15.
A common problem of static 3D cultures, including both spheroids and organoids, is the presence of a necrotic interior due to the nutrient diffusion limit and a lack of vasculature, though this can be partially solved by the use of bioreactors that better circulate nutrients. Further development of organoid culture models through co-culture of additional cell types such as stromal, immune or vascular cells is also required to create models that better mimic the in vivo organ30. Since organoids are generated by aggregation and self-organization of stem and progenitor cells, this affects the reproducibility of the system. These shortcomings are essential to the downstream applications discussed in this review.
2.3. Tumour Organoids
The growth of patient-derived tumour samples in vitro under 3D conditions is referred to as tumour organoids. In these culture systems, cancer cells isolated from patients are grown under optimized growth conditions resulting in a 3D aggregate of tumour cells. Tumour organoids are increasingly being used as models to study tumorigenesis and disease progression in vitro and as models for drug discovery and personalized medicine. The benefit of such models over traditional cancer cell lines is that tumour organoids provide a physiologically relevant system that maintains the heterogeneity of the original tumour42. Additionally, many tumour organoid models consist of different cell types, allowing the study of niche-tumour interactions in vitro. Examples of patient-derived tumour organoid models developed thus far include models of colorectal cancer (CRC), glioblastoma multiforme (GBM), breast and lung cancer29,43–46. The previously developed tumour organoid models have shown the benefit of using patient-derived primary cells combined with 3D culture systems to more accurately model the tumour environment compared to traditional cancer cell models. Advancements in these tumour organoid models will allow for improved drug discovery and personalized medicine.
3. Biomaterials Facilitating 3D Tissue Models
A key difference between 2D and 3D tissue models is the use of biomaterials, in which cells reside, in order to better simulate in vivo physiological conditions. The development of novel in vitro 3D models has largely been possible due to the advancement and discovery of biomaterials. These biomaterials can be designed to form various 3D scaffolds with functional groups, i.e. through photo-polymerization, to achieve the optimal microenvironment for cell growth. As an example, stereolithography technology utilizes UV energy to 3D print scaffolds, thereby creating custom designs to match numerous applications.
Biomaterials are classified as either synthetic, naturally derived or hybrid composites of the former two. Each biomaterial has its own unique properties that must meet specific requirements to be considered for use in 3D tissue modelling. For in vitro uses, the mechanical and chemical properties of the biomaterials should be tailored for optimal cellular attachment and growth. The recapitulation of tissue-specific architecture is also preferred. For in vivo applications, the biomaterial must have a minimal immune response while simultaneously supporting cell growth. Additional factors for consideration include compatibility of the biomaterial by-products, manufacturing ease and scaffold permeability to allow for exchange of nutrients and waste metabolites47. During the process of material selection, it is also critical to identify a biomaterial that can support the growth of the cell line chosen for the in vitro 3D model. Examples include the usage of fibrin and collagen to develop calcified tissue models or calcium phosphate and collagen for bone modelling48,49. The section below will highlight various polymers and their respective fabrication techniques.
3.1. Natural Biomaterials
The advantages of using 3D scaffolds composed of natural polymers include structural properties similar to the in vivo extracellular matrix (ECM), the presence of pre-existing ligands for cell surface receptors allowing for cell attachment and communication, as well as ease of fabrication. These properties are critical for cultured cells to proliferate and assemble a tissue in a biocompatible scaffold. Furthermore, natural polymers do not require post-chemical modification or synthesis to incorporate ligands or peptides, unlike synthetic polymers. However, there are a few disadvantages including weak mechanical properties, poor immunogenicity and uncontrollable degradation rates50,51. Examples of natural scaffolds include collagen, fibrin, gelatin, laminin, and Matrigel52–55.
Matrigel is a commonly used natural ECM derived from the basement membrane of a mouse sarcoma. Therefore it resembles in vivo basement membrane and is composed of laminin, collagen IV and heparin sulfate proteoglycan as well as many growth factors, such as basic fibroblast growth factor (FGF), epidermal growth factor and insulin-like growth factor 156–59, with a limitation that the exact composition varies batch to batch. It has additional applications such as xenografting, invasion assays and angiogenesis tube assays56,60. Matrigel has been widely used for 3D culture for a variety of cells including breast cancer cells, human dermal papilla and hepatocellular carcinoma cells46,61–63. Despite successful application of this polymer, some disadvantages include high cost and the potential for xenogeneic by-products64–67. With these considerations, it is difficult to proceed with clinical applications since Matrigel is animal derived, incompatible with good manufacturing practices (GMP) and its batch-to-batch variability may influence disease modelling and drug testing results.
Another widely explored natural polymer is collagen gel derived from either rodent tails or bovine dermis, which recapitulates the in vivo ECM similar to Matrigel68. The advantages of this polymer include biocompatibility and tunable factors such as porosity and degradation rates69. Three examples of applications of collagen gels include the sandwiching of endothelial cells with other cells to fabricate capillary networks, smooth muscle-actin in fibroblasts or embryonic cardiomyocytes to build a new heart muscle model system70–72.
3.2. Synthetic Biomaterials
Examples of commonly used synthetic polymers include polydimethylsiloxane (PDMS), poly(lactic-co-glycolic acid), poly(ethylene glycol) and polyurethanes. Synthetic polymers are highly tunable to provide desired mechanical properties and degradation rates with improved reproducibility. They can also conjugate proteins or drugs for targeted delivery. The disadvantages include the lack of specific ligands for cell surface receptors, as well as potential toxic by-products produced by polymer backbone degradation51. To compensate for this, prior to crosslinking, the polymer backbone can undergo chemical modification with peptides such as RGD (arg-gly-asp) to enhance cell-specific adhesion or GPQG-AGQ to enhance proteolytic degradation or signalling ligands like vascular endothelial growth factor (VEGF) and bone morphogenetic protein (BMP) to regulate cellular activity73–76.
PDMS is commonly used for the fabrication of microfluidic devices and organ-on-a-chip systems. Studies from a number of research groups have demonstrated the benefits to using this synthetic polymer in microfabricated devices due to its flexibility, low-cost, chemical inertness and relatively simple chemistry77. Despite advantages in microfabrication, there are a number of limitations of this material in 3D cell culture. One of the limitations is the affinity of PDMS for hydrophobic molecules which would hinder drug testing. One study proposed to coat PDMS with parylene or paraffin wax, however it is not the common practice in organ-on-a-chip devices78. Alternative materials with low absorption and adequate castability are still under investigation79–82.
The focus of biomaterial research has shifted from natural to synthetic polymers that can match the biomimetic characteristics of natural polymers while allowing for greater reproducibility and control over the physical and chemical properties of the material. Hybridized synthetic and natural polymer scaffolds allow for researchers to draw on the strengths of both materials.
4. Modulating Growth Conditions to Support In vitro 3D Culture Systems
Decades of research on the embryonic development of several model systems has resulted in the ability to differentiate PSCs into lineages comprising all three germ layers. This developmental knowledge was employed to support the growth and expansion of patient-derived 3D culture systems such as spheroids and organoids. The precise control of cell signalling pathways known to regulate the fate of stem cells is a critical aspect in the development of in vitro 3D model systems. The development of novel technologies (Figure 2) such as bioreactors has allowed for the expansion of 3D tissues in vitro while facilitating the scaling up and long-term maintenance of cultures. In addition, novel organ-on-a-chip technologies (Figure 2) have allowed for multiple tissue systems to be connected in vitro, a step towards recapitulating in vivo physiological conditions.
4.1. Regulation of Developmental Signalling Pathways to Support In Vitro 3D Culture Systems
Several signalling pathways such as Notch, Wnt, Hedgehog and transforming growth factor β (TGF-β) are tightly regulated and play an important role during embryonic development83–86. Each of these pathways is known to regulate stem cell maintenance, differentiation and organogenesis in a context-dependent manner. During development, the activity of these pathways controls various key processes such as axis formation, germ-layer specification and left-right asymmetry. Nodal, a member of the TGF-β superfamily, is highly important as it regulates all three of these events86. TGF-β signalling, which includes the BMP and Activin subfamilies, is also critically important for organogenesis, since at least one ligand from the TGF-β superfamily is involved in the development of most organs86. The activity of these pathways is also significant for the homeostasis of many adult tissues, by regulating the proliferation and self-renewal of tissue stem cells as well as promoting injury-dependent organ regeneration.
It has become clear that these developmental pathways are connected, with activation of one pathway regulating the ligand expression of another. For example, Hedgehog signalling often induces the expression of Wnt ligands and in some cases BMP ligands83. Since these signalling pathways play important roles in stem cell maintenance and organogenesis, it is unsurprising that the use of stem cells as in vitro 3D model systems relies on the precise control of these pathways. Though some models rely on endogenous signals secreted by the cells in culture, often through co-culture of niche cells with stem cells, most in vitro 3D models utilize the addition of exogenous growth factors and small molecules. For example, the growth of small intestinal and colonic organoids requires the exogenous addition of Noggin, R-spondin and Wnt3A to inhibit TGF-β signalling and activate Wnt signalling15. Additionally, PSC-derived cerebral organoids resemble a dorsal forebrain fate if left untreated or can be patterned into a ventral forebrain fate upon the addition of SHH agonists and Wnt antagonists87.
4.2. The Use of Suspension Cultures and Spheroids for In Vitro 3D Culture
The in vitro culture of non-adherent multi-cellular aggregates, or spheroids, is a commonly used example of a 3D culture model (Figure 2). Spheroids can be formed through several methods with the hanging drop, forced aggregation and centrifugation methods being the most known. The hanging drop method relies on the self-aggregation of cells within droplets, suspended from the surface of a well plate88. Commercial products such as AggreWell can be used to assist with initial spheroid formation within a standardized multi-well setting89. Alternatively, the liquid overlay technique forms spheroids by stirring the cell culture medium, usually within a bioreactor. Spheroid models have been generated for several tissue types including cartilage, pancreatic, muscle, retinal and brain88. Although spheroids are usually formed through aggregation of a single cell type, co-culturing different cell types can create more complex systems to better mimic in vivo tissues. Spheroid formation is reproducible and can be scaled up using high-throughput fabrication techniques90. One of the most common applications of spheroid cultures is the formation of neurospheres. Neurospheres can be generated from iPSC-derived neural progenitor cells, with the resulting spheroids resembling human fetal neural progenitor cells based on morphology and marker expression6. Spheroids can be used to model astrocyte maturation and development as demonstrated by the formation of human cortical spheroids from iPSCs, which were cultured for up to 590 days in vitro91. Liver bud spheroids were formed through co-culture of iPSC-derived hepatocyte progenitors with commercially available endothelial cells and mesenchymal stem cells92. These liver spheroids were shown to model in vivo fetal liver development based on transcriptome analysis. The co-culture of articular chondrocytes and mesenchymal stem cells isolated from osteoarthritic patients was used to develop an in vitro 3D model of cartilage93.
4.3. The Use of Bioreactors to Support In Vitro 3D Culture Systems
Bioreactors are used for suspension cultures of various cell types and systems (Figure 2), including spheroids and organoids, allowing for improved nutrient and oxygen distribution that supports the expansion of 3D tissues in vitro94. Tutoplast, alginate and Matrigel are some of the most common polymers used due to their ability to mimic in vivo physiological properties including the presence of pre-existing ligands for cell adherence, communication via spatial and temporal signals, and structural complexity. Matrigel and alginate can crosslink to form hydrogels at various polymer concentrations, recapitulating the physical properties and structure of a human breast, used to study the cancer cell line MDA-MB-23195.
Alternatively, synthetic polymers are advantageous as they are highly tunable to provide desired mechanical properties and degradation rates, are highly reproducible and can conjugate proteins or drugs for targeted delivery. Tillman et al., have published their work on electrospun scaffolds used for vascular reconstruction composed of polycaprolactone and collagen. These scaffolds were advantageous due to their ability to support the growth of endothelial and muscle cells under constant pulsatile flow conditions and prevent in vivo inflammatory response for the duration of a month while maintaining biomechanical strength and integrity96.
Bioreactors have been used for in vitro organoid systems such as retinal, brain and kidney in addition to suspension cultures of megakaryocytes and cardiomyocytes10,97–100. The use of 50mL rotating-wall vessel bioreactors to culture retinal organoids derived from mouse PSCs allowed for the development and maturation of retinal tissue that was not possible under static conditions97. Cerebral organoids were generated from iPSC-derived embryoid bodies embedded in Matrigel before transferring to spinning bioreactors18. The organoids were cultured for up to 10 months and expressed markers from all major areas of the brain as well as specific cortical sub-regions.
A 3D printed mini spinning bioreactor was developed to culture forebrain, midbrain or hypothalamic organoids in vitro10,101. This reusable device was smaller than most commercially available spin flasks, consisting of 12 chambers. However, the mini spinning bioreactor is not commercially available and requires in-house 3D printing to construct. A model of PSC-derived kidney organoids resembling the human fetal kidney was also developed using a 125mL spinner flask bioreactor100. The kidney organoids were comprised of nephrons containing podocytes, proximal and distal tubules, collecting ducts, endothelial cells and interstitial cells100. Spinner flask bioreactors can also be used to develop efficient, xeno-free and scalable culture systems, one example being the differentiation of iPSCs into megakaryocytes as a potential transfusion therapy for diseases such as thrombocytopenia98. Additionally, a 40mL bioreactor system was used to produce iPSC-derived cardiomyocytes in suspension culture99.
4.4. The Use of Microfluidics and Organ-on-a-chip Systems for In Vitro 3D Culture
An organ-on-a-chip is a type of microfabricated device used for culturing cells in micrometer-sized chambers with continuous perfusion of culture medium (Figure 2). The goal of organ-on-a-chip systems is to form a functional unit that replicates some aspects of in vivo tissues or organs. Multiple organ-on-a-chip systems can be linked fluidically to model the interface between tissues and the physiological interaction between different organs. Organ-on-a-chip systems can be used to quantify in vivo physiological functions of organs such as absorption of drugs and secretion of metabolites and enzymes4,102,103. Unlike static culture models, microfluidic systems incorporate physiological mechanical cues and fluid flow, which better support the growth of 3D tissues in vitro and help overcome the diffusion limit104.
PDMS, as previously described, can be used for soft lithography to simulate vessels by forming narrow or wide channels while microcontact printing allows the cells to differentiate into the shape of choice. Using microcontact printing for drug screening, Khadpekar and colleagues demonstrated how C2C12 myoblast cells differentiate into circular or rectangular shapes and increase or decrease in size depending on the scaffold pattern105.
A novel platform developed by the Radisic group called Biowire, incorporates PDMS channel templates which remodel type I collagen gels and seeded cells106. This method, in conjunction with electrical stimulation, assisted PSC-derived cardiomyocytes and supporting cells to develop electrophysiological properties and recapitulate cardiac tissues. In Biowire, cells were seeded into a microfabricated well containing a collagen gel surrounding a surgical suture. The cells were electrically stimulated with increasing frequency, which resulted in the generation of 3D aligned cardiac tissues with frequent striations. Due to the previously described limitations of PDMS with regard to drug testing, the next generation Biowire II platform, involved two parallel poly(octamethylene maleate (anhydride citrate)) (POMaC) polymer wires between which cardiac tissue would self-assemble107. This system allowed for continuous, non-invasive measurements of force and calcium transients. The addition of PSC-derived atrial and ventricular cardiomyocytes generated heteropolar Biowires that better mimicked in vivo tissue, allowing the system’s application for disease modelling and drug. In addition, the platform supports the scale up production of therapeutically relevant cells for transplantation108.
A microfluidic system was designed to model the blood-brain barrier in vitro through the culture of cerebral endothelial cells with neurons or astrocytes grown in separated compartments109. Furthermore, a microfluidic array system was used to co-culture human neural stem cells with human mesenchymal stem cells to investigate the paracrine signalling effects of mesenchymal stem cells on neuronal differentiation110. A model to study axon fascicles in vitro was created by culturing PSC-derived motor neurons in a microchannel device such that axonal growth was directionally restricted111. Morphological, electrical and physical analyses were performed using this system and hydrogen peroxide treatment was used as a proof-of-principle to model oxidative stress-mediated neurodegenerative disease.
An example of vasculature-on-a-chip is the AngioChip system, developed by the Radisic group, composed of a multidimensional scaffold containing parenchymal cells and a built-in endothelialized vascular network112. The AngioChip consists of a network of microchannels which are each 100 μm in diameter, distributed 10–20 μm apart, in order to overcome the diffusion limit of oxygen and nutrients within the parenchymal spaces. The use of AngioChip allowed for improved viability of parenchymal cells and vascular sprouting of endothelial cells from the lumen into the parenchymal space. An example of a multi-organ chip was created by connecting four human organ models using commercially available cell lines for the purpose of in vitro drug toxicity testing113. The multi-organ chip was composed of a 3D small intestine, skin biopsy, 3D liver spheroids and kidney proximal tubule compartments113.
5. Developmental Models
In order to understand embryonic development, lineage specification and tissue homeostasis, developmental models of various organs are required. Furthermore, such models will provide insights into how certain diseases and drugs affect the developing organ.
In 2012, Sasai and colleagues asked whether an in vitro system of differentiated mouse ESCs could recapitulate some of the regulatory systems of organogenesis and form layered neural structures114. To guide self-assembly, this group explored the possibility of regulating spatial patterning and morphogenesis by employing developmental signals, developing methods to generate brain structures and the retina in vitro. More recent advancements to developmental models of the brain and retina will be described in the proceeding sections. Other developmental models, derived from endoderm and mesoderm fates, will also be discussed in brief.
5.1. Brain
Developmental models of the brain, in the form of 3D organoids and spheroids, have allowed researchers to investigate different elements of human brain development and pathology. Furthermore, such models can be used for testing novel stem cell and regenerative therapies of the central nervous system. The organization of the cerebral cortex is one of the characteristic features of cortical tissue architecture, including the formation of a polarized cortical plate and radially aligned neurons115. A goal of 3D culture modelling is to reproducibly generate human cortical tissue while maintaining this self-organizing capacity. Such models will allow for the study of early cortical development and neuronal migration. Signal modulation and scaffold design guide the differentiation of PSCs into neural tissue.
Different methods have been employed to recreate the detailed structural features of the brain, specifically during developmental stages. Silk-collagen scaffolds have allowed researchers to recapitulate the compartmentalized architecture and basic structural features formed during the development of the forebrain cerebral cortex, designing scaffold architectures that provided spatial separation of cell bodies and neural processes116. This structural foundation allowed for the development of robust neuronal projections and neural network maturation, when seeded with primary cortical neurons. Aside from structural stability, other properties of silk protein, such as its inherent negative charge that protects neurons from excitotoxicity, contribute to the longevity of the constructs. Alternatively, synthetic hydrogels have been a popular choice of scaffold in organoid development, as previously mentioned. Wu et al. investigated the role of hydrogel rigidity in the behaviour of human iPSC-derived neural progenitor cells (hiPSC-NPCs)117. Spontaneous migration and neurite out-growth were compared between soft and hard methacrylated hyaluronic acid (Me-HA) hydrogels. Soft hydrogels enhanced the neural differentiation of progenitor cells by upregulating the expression of neural maturation markers.
Despite the relative success of self-organizing in vitro cerebral organoids in investigating neural migration and network formation, limitations include high variability between batches, random tissue identity and incomplete morphological differentiation118,119. A recent study has suggested that inconsistent neural induction may be a main cause of organoid variability115. Neural induction refers to the process by which ESCs become committed to the ectodermal lineage and subsequently to a neural fate. Most recognized developmental models of the brain, published from 2008–2013, started from spherical embryoid bodies, which developed neuroectoderm on the exterior of the embryoid body18,120,121. To achieve consistent neural induction and improve the efficiency of neuroectoderm formation, a method was developed to increase the surface area to volume ratio of the engineered tissue115. Rather than spherical embryoid bodies, elongated embryoid bodies were generated from hPSCs seeded onto poly(lactide-co-glycolide) (PLGA) microfilaments as a floating scaffold. In contrast to spherical organoids, which displayed highly variable amounts of all germ layers, microfilament-engineered embryoid bodies were able to reproducibly form neuroectoderm with an almost complete lack of non-neural tissue115.
Beyond the first report of neural rosette formation from human ESCs in 2001, current techniques are able to generate neural tissue which resemble the 3D organization of specific regions of the brain7,122,123. In 2008, Eiraku et. al established a method of generating self-organizing cortical spheroids using embryonic stem cells, mimicking early corticogenesis120. More recently, the possibility of generating neural tissue from human PSCs and fate-restricted neural stem cells have been explored. Although human iPSCs have been able to generate structures such as the cerebellum and cerebral cortex, the generation of more highly specialized structures seems to require a higher degree of pre-patterning. In an effort to address this challenge, human neuroepithelial stem cells have been cultured on Matrigel droplets and differentiated under dynamic conditions into human midbrain-specific organoids124. This method allowed for differentiation of neuroepithelial stem cell derived organoids into spatially organized midbrain dopaminergic neurons, forming clearly specified clusters within the human midbrain-specific organoids (Figure 2). Furthermore, a ventral midbrain identity was evidenced by the expression of midbrain specific transcriptional factors (e.g. OTX2) and nuclear factors (e.g. NURR1).
Advancements in 3D culture techniques have allowed researchers to model complex interactions and investigate the relationships between various subdomains of the developing human brain. Birey and colleagues modelled the saltatory migration of GABAergic neurons from the ventral to dorsal forebrain and their integration into cortical circuits, thereby monitoring interregional interactions with human cells125. As part of the study, dorsal and ventral forebrain subdomains derived from human iPSCs were fused in vitro to identify the transcriptional changes associated with interneuron migration and model diseases associated with migration defects, such as Timothy Syndrome. In another study, the concept of organoid fusion allowed for the generation of a dorsal-ventral axis by fusing independently patterned organoids of ventral and dorsal forebrain identities87. As was previously described, inconsistent neural induction often results in organoid variability. In order to promote a ventral forebrain identity, a combination of Wnt inhibition and activation of SHH signalling were applied to the human iPSCs during differentiation, to mimic in vivo embryonic development. Alternatively, a recent study examined interneuron migration by fusing human medial ganglionic eminence organoids and human cortical organoids126. To date, other models have lacked this complexity, rather focusing on generating brain organoids which model cortical development in isolation. The generation of a system to model human medial ganglionic eminence development is significant in that cortical interneurons are derived primarily from the medial ganglionic eminence of the subpallium.
The greatest challenge to be addressed in 3D developmental models of the brain is the generation of diverse cell types to mimic more closely the cell-cell interactions and circuit connectivity of the central nervous system. Furthermore, although methods have been developed to culture cerebral organoids for hundreds of days, the field requires robust methods for producing more mature tissue, beyond the fetal stages.
5.2. Retina
Many forms of blindness are caused by the loss or dysfunction of retinal photoreceptors. In order to better understand the development of such conditions, the key structural and functional features of the native retina are modelled in vitro. Recent developments in retinal organoids, primarily based on PSCs, contain not only physiological retinal cell subtypes but also model the in vivo layered morphology.
In vertebrates, the retina is developed from the neuroectoderm, specifically the anterior neural tube127. The optic vesicles are formed during the late stages of anterior neural tube formation. The distal region of the optic vesicles generate neural progenitor cells whereas the dorsal region gives rise to the retinal pigment epithelium128. The optic cup is formed by the invagination of the apical optic vesicles, such that it is brought into close proximity to the distal retinal pigment epithelium. Over time, the neural retinal progenitor cells give rise to all retinal neurons, including the cone and rod photoreceptors. The in vitro differentiation strategy is based on the idea of mimicking embryonic development by regulating similar signalling cues and growth factors as present in vivo. Differentiation requires neural induction of PSCs and neural patterning into the direction of the anterior neural plate.
Zhong and colleagues demonstrated, for the first time, the beginning of photoreceptor outer segment maturation, as well as some degree of photoreceptor functionality as evidenced by electrical response upon light exposure129. Human iPSC-derived free-floating aggregates are reported to successfully recapitulate the main steps of retinal development observed in vivo and form 3D retinal cups that contain all major retinal cell types with proper morphological arrangement. As shown in previous studies, Notch pathway inhibition is employed during the early to mid-stages of retinal development to increase the proportion of photoreceptors within laminated organoids, through a process of lamination in which neurons migrate towards their final layer within the developing tissue130,131.
Despite taking initial steps to advance photoreceptor differentiation, the robustness of the photoreceptor outer segment was still lacking in the above study. The development of retinal organoids which give rise to photoreceptors with robust outer segments was first reported in 2017132. Through optimization of growth medium composition, oxygen concentration, and aggregate size, this study was able to form floating 3D optic vesicle organoids which supported long term culture and allowed for advanced photoreceptor development. Employing hypoxic conditions during culture reduced cellular stress, increased proliferation and enhanced chromosomal integrity, as previously demonstrated by the hypoxic generation of optic vesicles and optic cups as well as previous studies using 2D systems133.
Researchers have also been working towards increasing the yield of PSC-derived retinal cells. In 2017, it was reported that 3D cultures with a biomaterial scaffold could improve the generation of retinal tissue from human PSCs. The use of hyaluronic acid or alginate-based hydrogels (0.5% RGD-alginate hydrogel) was able to enhance the yield of retinal organoids and the expression of neural retina and retinal pigment epithelium expression markers134. Although many studies to date have focused on the generation of retinal pigment epithelium and photoreceptors from PSCs, the derivation of retinal ganglionic cells has been largely limited due to the shortage of reliable markers for these cells. To overcome this limitation, Meyer’s group undertook the comprehensive analysis of retinal ganglionic cell differentiation in order to enhance yield, by directing hPSCs to differentiate in a step-wise fashion towards a retinal lineage and identifying a specific subtype of retinal ganglionic cells, intrinsically photosensitive retinal ganglion cells135. This study was the first to demonstrate the development of intrinsically photosensitive retinal ganglion cells from human PSCs, as identified by the expression of the phototransduction protein melanopsin. Appropriate morphological and physiological features were also observed in the derived retinal ganglion cells at developmentally appropriate time points.
Despite the advancements that have been made in establishing physiologically relevant developmental models of the retina, there are a few challenges that still need to be addressed. Future research will work towards reducing culture time, developing photoreceptor outer segments with more mature morphologies, and the introduction of vasculature and immune cells
5.3. Endodermal and Mesodermal Models
Beyond cortical and retinal developmental models which are based on the ectoderm, PSCs are able to differentiate into endoderm and mesoderm germ layers, allowing for the development of organoid models of the intestine and kidney, respectively. Induction of PSCs towards endodermal or mesodermal lineages requires the Nodal signalling pathway; defective signalling compromises mesoendoderm development across all species studied136. Nodal activity determines the fate of the mesoendoderm, where high levels of signaling promote endoderm development and low levels promote the generation of the mesoderm. To mimic Nodal signalling, developmental models have utilized the TGF- β ligand, Activin A, to promote differentiation of PSCs137.
Activin A signaling results in expression of transcription factors Sox-17 and HNG-3- β, leading to posterior endoderm patterning, which gives rise to the small intestine. In 2011, Well’s group constructed the first developmental model of the intestine by differentiating iPSCs into 3D human intestinal organoids138. Human intestinal organoids are capable of some transport functions and exhibit essential morphological features including the luminal brush border. However, a limitation of current models is that they fail to express proteins for specific segments of the small intestine (i.e. duodenum, jejunum, ileum and colon) due to their lack of maturity139. Rather, a mixed population of distal and proximal intestinal cells are produced. Efforts have been made to produce more mature intestinal models. In 2017, researchers developed a cross-linked collagen hydrogel which permitted stem cell maintenance and proliferation, as well as the formation of differentiated lineages of the small intestine140.
Renal progenitors originate from the intermediate mesoderm and give rise to the various cell types of the kidney, such as pronephros, mesonephros and metanephros5. As per the developmental models described above, methods have been developed to generate differentiated progenitor cells from human PSCs. Due to the tissue complexity, researchers have been working towards identifying the developmental mechanisms responsible for specific cell types, each of which have distinct spatiotemporal origins from the intermediate mesoderm. Takasato and colleagues developed a method of selectively differentiating PSCs towards a collecting duct lineage, rather than kidney mesenchymal progenitor cells141. Using this methodology, anterior-posterior patterning of the intermediate mesoderm allowed for the formation of complex organoids with fully segmented nephrons, surrounded by endothelial and renal interstitium, effectively recapitulating first-trimester fetal kidneys19,142.
Moreover, Takebe’s group has contributed to the formation of vascularized 3D cultures and stable endodermal lineages. Although iPSCs are responsive to proliferative stimuli and ensure organ growth, chromosomal instability and variations in genetics or epigenetics makes them susceptible to tumorigenesis. Through reproducible generation of human posterior gut endoderm cells and utilization of FGF, TGF and Wnt signalling, progenitor cells formed multiple endodermal lineages with greater stability than iPSCs143. Large scale organoid production has been limited due to the high cost of developmental reagents required for nephron progenitor specification in vitro141,144. To alleviate this issue, a method has been recently developed to generate kidney organoids from iPSCs by substituting Fibroblast growth factor 9 with a more readily available reagent, Knockout Serum Replacement100. A challenge to be addressed in future studies is meeting mass transport limitations and organoid maturity.
The lateral mesoderm gives rise to several cell lineages, including cardiac progenitor cells. A crucial balance between Nodal and BMP2 signaling, regulated by morphogen gradients, allows for the mesendoderm to be segregated into a cardiac mesoderm145. However, the signals that trigger the migration of cardiogenic mesodermal cells and determine early cell fate decisions – from epiblast to specific cardiac cell – remain unclear. Recently, genome editing has allowed for the study of human cardiogenesis: the identification of networks of genes that drive specific differentiation events146.
6. 3D Cultures for Disease Modelling
The advent of commercially available stem cell lines and molecular biology techniques such as single-cell RNA sequencing, cryo-electron microscopy and the CRISPR/Cas9 technology have resulted in a paradigm shift in bioengineering. Researchers are now capable of recreating patient-specific pathologies in vitro to understand disease etiology and progression, while additionally testing drug candidates for treatment. The limitations associated with animal models and immortalized cell lines such as cost, ethical concerns and poor recapitulation in clinical trials will likely no longer be obstacles in the future of medical research. These 3D culture models will allow clinicians to come closer to determining the molecular basis of a disease rather than relying on established signs and symptoms for diagnostic purposes.
The current gold standard for preclinical testing and disease modelling is animal studies. However, such testing is liable of inadequate clinical translation since mechanistic findings differ considerably across species. Additionally, the conditions of animal testing often cause distress as well as uncontrollable and abnormal changes in behaviour, raising a number of ethical concerns147. To address this need, complex control and monitoring systems have been engineered to recapitulate a physiological microenvironment providing stimuli to induce targeted effects in ex vivo models as summarized in Table 3148. Genomic tools have elucidated key pathways that can trigger the onset of disease in healthy tissues, narrowing down the search for therapeutic targets and effectively reversing a disease state.
Table 3.
Summary of existing in vitro 3D disease models
| Organ | Associated diseases | Reference |
|---|---|---|
| Brain | Alzheimer’s | 150 |
| Neuropsychiatric diseases (autism, schizophrenia, bipolar disorder) | 149 | |
| Microcephaly | 18 | |
| Kidney | Polycystic Kidney Disease, Fibrosis | 163,165 |
| Nephronophthisis | 164 | |
| Eye | Retinal Degeneration | 153 |
| Glaucoma | 152 | |
| Stomach | Infection with H. pylori | 209 |
| Pancreas | Cystic fibrosis | 166 |
| Pancreatic Cancer | 192 | |
| Liver | Hepatocellular carcinoma and cholangiocarcinoma | 190 |
| Hepatitis B virus | 162 | |
| Heart | Dilated cardiomyopathy | 154 |
| Cardiac Hypertrophy and Heart Failure | 107,155 | |
| Cardiac sodium channel disease and related cardiac arrhythmias | 157,210 | |
| Lungs | Influenza | 160 |
| Congenital surfactant deficiency syndrome | 158 | |
| Asthma | 211 | |
| Pulmonary Edema | 4 | |
| Multiple organs | Systemic toxicity | 113,169 |
6.1. Central Nervous System and Eyes
Most of the recent studies in 3D culture have focused on the central nervous system. The complexity of the human neural network and the tight regulation under which it functions have left researchers with many unanswered questions. In the past, ethical limitations have restricted scientists, solely permitting the analysis of patients post-mortem and animal models with inherently simpler cerebral systems. The need for a more accurate human model has driven many to create both in vitro and in silico models for testing149.
By reprogramming stromal tissues gathered from a patient with severe microcephaly, researchers have conclusively demonstrated that mutations in the CDK5RAP2 protein cause premature neural differentiation and a loss of key progenitor cells18. Additionally, deviations observed in the orientation of radial glial spindles resulted in a lack of symmetry which stunted neural stem cell growth.
The high incidence of neuropsychiatric diseases such as autism spectrum disorder, bipolar disorder and schizophrenia present a serious burden to modern medicine. Current findings indicate that there is a substantial overlap in causative mechanisms for these disorders, but discrepancies in different risk-associated genes and sensitizing environmental effects result in a variety of disease phenotypes. By harnessing the computational power of large-scale molecular profiling techniques, such as single-nucleotide polymorphism typing and exosome sequencing, a comparison of cell types and their hierarchical organization can be assessed for key disorder-specific determinants.
However, the maturation of neural circuits and heterogeneous cell networks has yet to be validated against adult tissues and will require further investigation149. In 2014, Choi et al. developed the first human neural model which demonstrated both critical hallmarks of Alzheimer’s disease: β-amyloid (Aβ) plaques and neurofibrillary tangles. This was utilized to understand the molecular mechanisms of p-tau pathologies that would generate Aβ species even in the absence of mutations in the frontotemporal lobe. FAD mutations in the β-amyloid precursor protein and Presenilin-1 gene were tied to the attenuation of plaque formation and could prove to be a viable target for therapeutic intervention150.
It has been nearly a decade since the first 3D self-organizing optic cup was developed by Eiraku and colleagues using floating aggregates of ESCs cultured in serum free, low growth-factor conditions151. This resulted in the formation of a two-walled cup morphology with an invaginated distal section that expressed neural retinal markers (Chx10, Rx, Pax6 and Six3), within a culture period of only 8–10 days. Breakthroughs such as this have spurred scientists to explore other aspects of ophthalmic architecture such as the human trabecular meshwork (HTM), which is a primary risk factor in glaucoma due to its ability to regulate intraocular pressure. Cultures of patient-derived HTM cells were subjected to 7 days of prednisolone acetate treatment, a corticosteroid that is administered to treat swelling within the eye, followed by perfusion and immunohistological studies152. An increased expression of myocilin and ECM proteins was observed, resulting in the formation of fibrous plaques. This coupled with higher resistance due to structural changes in the trabecular meshwork and the accumulation of ECM by-products linked to the downregulation of matrix metalloproteinases, suggests a possible mode for disease progression. 3D culturing techniques have allowed for the development of structured retinal sheets for transplantation to help treat patients suffering from retinitis pigmentosa (RP), which leads to blindness due to the loss of photoreceptor cells. Although this disease is genetic in nature, the complex pathology and the large array of mutations associated with RP have ruled out the use of gene therapies as a primary treatment option. However, recent work conducted in the RIKEN Center for Developmental Biology demonstrated the successful subretinal transplantation of iPSC-derived 3D retinal tissues. These constructs, containing mature photoreceptors integrated with the host bipolar cells, could have a potential application in studying disease progression ex vivo and investigating the genes responsible for retinal degeneration153.
6.2. The Heart and Lungs
As discussed throughout the review, 3D cell culture systems allow for cells to be exposed to specific biomechanical stimuli necessary for expression of physiologically relevant phenotypes, given their tight control mechanisms and capacity for longitudinal study. One dynamic study combined genetic manipulation as well as chemical and mechanical stresses to investigate the pathology of dilated cardiomyopathy. Findings indicated that titin-truncating variants and related missense mutations can diminish contractility in cells, while associated protein mutations interfere with regeneration and remodelling of cardiac tissues. This can lead to reduced responses to myocardial stresses, which are critical for maintaining cardiac health154.
The functional maturation of engineered tissues is a fundamental challenge of tissue engineering. A study conducted by Tiburcy et al. was capable of not only displaying key characteristics of mature myocardium but also producing a response similar to cardiac failure (cardiomyocyte hypertrophy, contractile dysfunction and N-terminal pro B-type natriuretic peptide release) when experiencing long term catecholamine toxicity155. Given that the cells utilized in this study were patient-derived, it is highly probable that patient specific myocardium models could be generated for personalized drug testing.
Biowire II, as described above, has demonstrated the capacity for the controlled assessment of mechanical, electrophysical and polygenic disease modelling. A study comparing the profiles of RNA expression from Biowire II models of both diseased and healthy patients displayed a consistent upregulation of 25 pathways linked to the pathological remodelling of cardiac tissues. Significant differences were also observed in the contractile function of the engineered tissues3,156. Likewise, studies have demonstrated that PSC-derived human cardiomyocytes can display SCN5A and Na+ channel repression mutations which may be used to model cardiac Na+ channel disease157. These are just some examples of the many research findings that could be harnessed to develop functional disease models for human cardiology.
In the last decade, organ-on-a-chip technologies have heavily contributed to our understanding of cellular interactions and behaviours under defined environmental conditions. A model of the alveolar-capillary interface containing human pulmonary epithelial and endothelial cells was able to recreate breathing motions through changes in air and fluid flow as well as induced mechanical strain. This was later used to test for pulmonary edema induced by drug toxicity through the administration of clinically relevant doses of interleukin-2 (IL-2), an FDA approved oncological drug. The combined effect of IL-2 and simulated cyclic mechanical strain from breathing lead to measurable disruption of the alveolar epithelium and capillary endothelium, substantiating the toxic effects of such a treatment.
Protocols published in 2014 by researchers from the Columbia Center for Translational Immunology provided detailed accounts of the agents necessary to convert human iPSCs into basal, goblet, club, ciliated as well as type I and II alveolar epithelial cells. With access to this large catalogue of cells, a number of unique studies could be proposed to assess different co-cultures and cellular interactions in relation to critical pulmonary diseases158. Another study which provided a better understanding of the synergistic relationship between the epithelial and mesenchymal linings of the lungs, involved the co-culture of bronchial epithelial cells and lung fibroblasts on a collagen gel matrix. This was used to study tissue and ECM remodelling upon the delivery of transforming growth factor β1 (TGF-β1) which is known to promote remodelling of connective tissues and plays a critical role in maintaining the epithelial defenses against viral infections, allergens and pollutants159. Astonishingly, there is no robust in vitro model for measuring the infectivity of viral agents in humans. Such models would greatly help to accelerate therapeutic discoveries as they could unearth the mechanism of infection and replication of the virus in humans. Work carried out by Zhou and colleagues at the University of Hong Kong allowed for the development of long-term expanding 3D lung organoids, which replicated a human airway inclusive of four cell types: goblet, ciliated, club and basal cells160. A cell culture medium, consisting of CHIR99021, a Wnt agonist, and FGF10 was employed to increase the number of ciliated cells and the level of serine proteases in the system, necessary to induce viral infection. Thus, such a model could be applied in future studies to investigate differences between strains of the influenza virus and other pulmonary infectious agents.
6.3. Abdominal Organs
Similar approaches, as discussed above, have been applied to study larger organs in the abdominal cavity. Human iPSCs were programmed into hepatocyte-like cells and cholangiocytes to develop liver organoids161. The advantage of this technique is that the cells were sourced from skin fibroblasts and mononuclear blood cells, which can be collected in a relatively non-invasive manner. The use of gene editing would make these models robust tools for the study of monogenic liver diseases, while integration with other organ models may help to determine how dysfunction in the liver can directly affect other organs.
To this purpose, work carried out at Imperial College London lead to the development of a 3D microfluidic primary human hepatocyte culture, capable of maturing over 40 days, which was a period long enough to model the life cycle of Hepatitis B virus (HBV) infection and replication162. This helped elucidate the mechanisms by which HBV suppresses the innate immune system and identified novel biomarkers that may correlate to the diseased state. This novel culture system can be combined with viral agents isolated from patients for the ex vivo investigation of potential therapeutics.
Similarly, Freedman et al, demonstrated that human iPSCs are capable of recapitulating renal tissues through the development of self-organized hollow spheroids163. The inhibition of the enzyme glycogen synthase kinase-3 resulted in segmentation within the spheroids to create cells characteristic of podocytes, endothelium and proximal tubules. The organoids expressed appropriate biomarkers such as kidney injury molecule-1 (KIM-1) upon the induction of nephrotoxic chemical injury due to the administration of cisplatin or gentamicin. Additionally, a number of genetic studies have recently surfaced due to the increased accessibility of gene sequencing tools.
Forbes et al. examined a kidney organoid platform developed from a patient identified with nephronophthisis, a disease that impairs renal function in children causing premature kidney failure164. This study identified a mutation in IFT140, a gene tied to retrograde intraflagellar transport and determined that diseased individuals expressed significantly lower levels of genes for cell-cell junction, dynein motor assembly and apicobasal polarity. Recently, an in vitro co-culture model of the interface between tubular epithelial cells and neighbouring fibroblasts was used to examine, in real time, the compounds that could modulate intercellular crosstalk in patients suffering from kidney fibrosis165. Though limitations in replicating the synergistic and complex effects of the native environment remain, the novelty of this approach lies in the high-throughput manner by which it can record related findings that may conceivably be extended to pulmonary fibrosis.
Pancreatic organoids developed from human iPSCs have generated tissues containing acinar and ductal cells which exhibit appropriate marker profiles, gene expression and functional hallmarks. Upon implantation into mice, these tissues have shown no evidence of tumourigenic capability and have further been used to study the effects of cystic fibrosis on pancreatic function166. This research was able to correlate cystic fibrosis transmembrane conductance regulator (CFTR) activation levels in functional assays to global expression in the body and develop an mRNA-based gene therapy for the treatment of diseased tissues.
6.4. Multi-Organ Systems
Although much progress has occurred in disease modelling, one major challenge is developing models of systemic diseases such as Lyme disease, atherosclerosis, malaria and diabetes167. In systemic diseases, no effect is isolated, but rather has a cascading impact in related organ systems. To address this, some researchers have developed culture systems with multiple organs modelled in sequence168. Although the scale of the network developed may be limited, novel interactions between tissues can be elucidated.
Maschmeyer et al., presented a functional four organ system consisting of human intestine, skin, liver and kidney counterparts, held in co-culture for 28 days113. The biological barriers of each organ were tested for sustained integrity over the duration of the study and the model was used to test drug uptake and delivery. Such a system could also be applicable for studying the chronic effects of systemic diseases or the spread of viral agents throughout the body. Likewise, researchers at the University of Central Florida developed a system containing cardiac, muscle, neuronal and liver modules with pumpless recirculation of media and continual functionality over 14 days169. This arrangement allowed for the testing of mechanical, chemical and electrical responses to therapeutic agents in order to assess their toxicity. Although such models are currently utilized for drug testing, they may have a significant potential in the assessment of disease progression in patients. Such models will provide a better understanding of complex diseases and allow for a multifaceted therapeutic approach.
Even in these advanced bioengineered systems, challenges related to the accurate recapitulation of physiological conditions still exist. One such task is the design of a blood substitute that can circulate through the system carrying nutrients, biomolecules and waste to support crosstalk between tissues. This material would likely have to be renewable with a carrying capacity similar to human blood. The use of synthetic materials in these models may also be a source of concern as their long-term effects on the cells in culture and the possible drugs or pathogens introduced would need to be assessed for clinical relevance.
7. Drug Testing
The importance of 3D cell culture models in drug testing has increased over the past decade due to the ability to recapitulate some characteristics of in vivo conditions. 3D cultures can act as a preclinical model to determine cellular responses to drugs or for personalized medicine with patient derived cells. The use of 3D cultures for in vitro drug testing has a potential to better translate to patient findings than the previously used animal models, improving the success rate of future clinical trials. For example, Theralizumab (also known as TGN1412), a novel monoclonal antibody drug, was used to stimulate and expand T cells independently of the receptors. The first phase I clinical trials took place with six healthy patients where they were intravenously injected to assess the effects. Within 90 minutes, all six patients had systemic inflammations that led to the failure of several organs. Luckily, after intensive organ support, all six patients survived170. If this drug could have been tested for side effects using an in vitro 3D model, the severe complications that occurred to those six patients could have been prevented. This section will focus on the validation of 3D culture models and monitoring drug responses for disease models.
There is a particular interest from the standpoint for drug discovery in developing a blood brain (BBB) barrier models, as permeability of this barrier critically determines central nervous system toxicity of new drug candidates. In addition, with the prevalence of cancer, there is an urgent need to develop safer and more effective cancer drugs. Thus, current research efforts have focused on developing new BBB and cancer models. In addition, cardiac toxicity remains the predominant reason for the withdrawal of already approved drugs. This paper will focus on reviewing the development of these models from human cells in the context of drug discovery.
7.1. Blood Brain Barrier
3D cell culture models must be validated by observing the interaction between drugs of known effects and cells, in order to determine if these models recapitulate human physiology. There have been several well-established 3D models recapitulating many parts of the body including the human lung, pancreas, liver, intestine and bone77,171–174. A part of the body that has yet to be completely replicated and understood is the blood-brain barrier (BBB), the most complex neuroprotective barrier between the blood and the brain. The BBB allows only specific molecules to pass through the membrane, which prevents the entry of many therapeutic candidates developed to treat central nervous system (CNS) disorders. Many studies have attempted to recapitulate the BBB, yet limitations remain. One of these limitations includes the loss of human brain microvascular endothelial cell characteristics in culture, due to the use of pump systems to deliver nutrients in microfluidic devices. Additional constraints include models with high fluid-to-tissue ratio and the utilization of animal brain tissues (i.e. murine), which do not accurately represent the human BBB.
Wang et al., developed a pumpless microfluidic BBB model which incorporated a permeable layer, with minimal applied shear stress175. This model co-cultures rat primary astrocytes and hiPSC-derived brain microvascular endothelial cells on silicone sheets, sandwiched between porous polycarbonate membranes. The study used FITC-dextran, doxorubicin, cimetidine and caffeine to validate their BBB model to determine if they resembled in vivo conditions175.
Campisi et al., was the first to design a BBB microfluidic model from PDMS using a tri-culture of primary brain cells, human primary astrocytes and microvascular cells derived from human iPSC-derived endothelial cells. These cells contribute to the overall integrity of the BBB and facilitate the passage of molecules across the barrier. The incorporation of these cells was validated by staining for specific junctions (ZO-1, occluding and calduin-5), laminin and collagen IV captured by confocal images. To further validate the model, the permeability coefficient for 40 kDa and 10 kDa FITC-dextran was compared to in vivo rat conditions176.
Lastly, Wevers et al., developed a high-throughput microfluidic platform that cultures endothelial cells, pericytes and astrocytes on a two lane OrganoPlate along with a collagen-I gel177. Similar to the papers previously mentioned, the model was validated via immunostaining of tight junction proteins and incorporating FITC-dextran to determine the permeability coefficient. The authors proceeded to test for MEM-189, an antibody that binds the human transferrin receptor which is expressed by endothelial cells, with anti-hen egg lysozyme serving as the negative control due to its inability to bind to human cells178,179. Results obtained from flow cytometry demonstrated anti-human transferrin receptor bound to the endothelial cells as expected from in vivo studies. This model has potential for the discovery of CNS-penetrant antibodies177. As these novel techniques are published, this will bring us closer to recapitulating the complex structure of the BBB in order to understand which drugs can be effectively used to treat diseases like Parkinson’s disease, Alzheimer’s or brain cancer.
Once a 3D cell culture model has been validated as mentioned above, drug screening can be completed to determine the cellular response. The goal of drug screening is to test multiple drugs to find a candidate that can decrease the viability or proliferation of diseased cells. These candidates can then be further tested with additional 3D in vitro or in vivo animal models to confirm the effects of the drug prior to clinical trials.
7.2. Brain and Eye Disease
Recent work has contributed to the development of 3D models capable of replicating Alzheimer’s disease. Clinical trials for docosahexaenoic acid (DHA) as a potential treatment for Alzheimer’s disease (AD) have shown variable results180,181. A study by Kondo et al. utilized iPSCs from AD patients with either an autosomal-recessive mutation of the APP-E693 gene or sporadic AD cells. The iPSCs were differentiated into neural cells using a 3D floating culture, which was further differentiated in Matrigel. The results showed that higher concentrations of DHA decreased the levels of proteins prevalent in AD like BiP, cleaved caspase-4 and peroxiredoxin-4. In addition, stress oxidation with the CellROX™ assay, decreased the progression of AD, as previously demonstrated182–184. Finally, the LDH assay showed >80% viability for up to 16 days when exposed to the drug. Despite the promising results, DHA treatment did not decrease the levels of Aβ. Therefore, the authors concluded that evaluating more patient-derived AD cells at various stages of disease was required to understand why the Aβ levels remained constant after DHA treatment185. This model will help clinicians understand the complex mechanisms of AD development and potentially find a candidate to treat this disease.
In terms of developing 3D models of eye disease, Torrejon et al., focused on understanding the effects of rho-associated kinase (ROCK) inhibition on patients with glaucoma, as this drug is currently in clinical trials to determine if the drug action disrupts the actin filaments in the human trabecular meshwork (HTM) of the eye. By adding ROCK inhibitors, the levels of collagen IV, myocilin and fibronectin substantially decreased supporting the use of this drug in clinical trials. Although this paper did not discover a novel drug, the ability to recapitulate the glaucoma with steroid treatment is novel and can be used as a platform to test other drug candidates186.
The eye and brain models selected for this review focused solely on glaucoma and Alzheimer’s disease. What is missing is the ability to simulate multiple diseases under one model. Despite the success in developing these novel models, one current limitation is to determine and evaluate which specific model most faithfully recapitulates the cellular response upon drug treatment. Once these organ models are fully validated, the next step for drug testing is to connect multiple organs together, an advance which will require a thorough understanding of scaling laws between different organs. Recent reviews highlight the challenges and potential solutions to achieving this task187,188.
7.3. Cancer Models
Cancer has been an ongoing issue that patients encounter with few to no solutions, leading to an abundance of cancer models that have been published within the past 5 years. These models tested drugs which showed promising results in clinical trials and animal models, providing another platform to re-evaluate previous findings and elucidate the mechanisms of action. In the proceeding sections, we will highlight various oncology, neurologic and cardiac cancer models.
A brain cancer chip was established by developing a 3D microfluidic device comprised of a photopolymerizable hydrogel composed of poly(ethylene) glycol diacrylate (PEGDA) because of its ability to release drugs through diffusion189. A commercially available glioblastoma cell line, U87, was grown in a 3D spheroid and cultured in the PEGDA hydrogels to form cancer spheroids which were tested with Pitavastatin and Irinotecan. These FDA-approved drugs are used to treat glioblastoma multiforme (GBM). This unique microfluidic system can administer these drugs together through separate channels where each channel receives a different concentration of the drug in a system. The results indicated equal concentration of Pitavastatin and Irinotecan injected together had the lowest cell viability after 1, 4 and 7 days administered in comparison to the drugs individually.
A 3D organoid culture system was generated using donor-derived liver tissues to simulate near-physiological conditions that retained liver functionality190. This model was used to study primary liver cancer (PLC) and tested 29 anti-cancer compounds in a dilution series of each drug, where a dose response curve was completed to determine the viability utilizing luminescent single curve191. Of the 29 compounds, the drugs with promising results were Taselisib, Gemcitabine, SCH772984 and Dasatanib based on reduced cancer cell viability. Specifically, Taselisib inhibited the growth of the tumoroids, while Dasatinib suppressed tumoroid formation. The remaining drugs tested did not affect PLC viability190. Thus, the next step is to use 3D culture models to evaluate combination therapies to look for synergistic effects.
A 3D pancreatic cancer model grown in Matrigel was used to test six chemotherapeutic drugs: gemcitabine, paclitaxel, docetaxel, doxorubicin, epirubicin and vinorelbine192. Gemcitabine is the current standard treatment for pancreatic cancer, however it has limited efficacy193. To determine the efficacy of each drug, a resazurin assay and cell viability staining (calcein-AM) were applied to the cells to determine the survival of cells treated after 6 days at a drug concentration of 200μM and 0.2 nM. Treatment with gemcitabine decreased cell viability as shown in the resazurin and cell viability assays as predicted, while none of the remaining drugs tested showed similar results. This indicates the importance of finding novel drugs to treat pancreatic cancer, with additional options including combining multiple therapeutic agents to further decrease the viability of cancer cells192.
A developed microfluidic lung-on-a-chip model was simulated by culturing non-small-cell lung carcinoma (NSCLC) adenocarcinoma cells with primary lung alveolar cells or small airway epithelial cells on the top channel, while the bottom channel contained endothelial cells. Adjacent to the endothelialepithelial complex were vacuum channels to replicate the cyclic mechanical strain or breathing patterns. Next, two known drugs, rociletinib and erlotinib used in clinical trials for NSCLC were exposed to this model to determine cell viability via a MTT assay194–196. In this specific paper, the rociletinib-treated cells showed almost no survival at a concentration of 1μM while erlotinib treatment decreased survival by 40% at 10 μM. These drugs were then introduced to the microfluidic device to replicate these results, but there was no decrease in tumour viability. These results contradicted the expected outcomes from previous work and more importantly from clinical trials, thus, emphasising the importance of critical evaluation of results obtained from 3D cell culture systems197.
7.4. Cardiac models
Cardiac models have been heavily studied for drug modelling as there are numerous diseases affecting the heart, as previously mentioned. An example of a 3D drug screening model is the three dimensional cardiac model fabricated by Lu et al., that tested antibiotics (ampicillin, erythromycin and Trovafloxacin), anticancer (Vandetanib and Tamoxifen) and drugs for treating diabetes (Metformin, Rosiglitazone and Troglitazone)198. The ATP activity of the cells and tissue contractibility determined the overall effect of the drug and was compared to clinical results. This model demonstrates how the tissue’s contractility could detect cardiotoxicity more effectively than the ATP activity of the cells. In fact, six of the eight drugs showed results similar to clinical observations, a sign of promising preliminary results for the model.
The Biowire platforms, described in the previous section, can be used for drug screening3,106. This was shown by exposing the model to known drugs with chamber-specific responses, including Ranolazine for decreasing conduction velocity and serotonin for increasing the Ca2+ transients in the atrial regions while not affecting ventricular regions. More importantly, Biowire II can be used to understand if the drugs tested can have a synergistic or independent effect on the atrial and ventricular regions3. It has also been used for screening of small molecule kinase inhibitors for cardiotoxicity.199
8. Summary
2D cell culture models generally lack the architecture of in vivo tissues. Intense research over the past decade has resulted in the development of many 3D culture models that recapitulate aspects of in vivo organ structure and function. In particular, the development of organoids has revolutionized developmental biology, disease modelling and drug discovery as organoid models are capable of generating complex 3D structures that contain multiple cell types and retain some functions of the in vivo organ.
The introduction of novel biomaterials has been critical to the development of in vitro 3D models. Further studies in the field of biomaterials should focus on the generation of hybrid polymers, combining the advantages of both natural and synthetic polymers, to replace the use of common materials such as PDMS and Matrigel. The use of PSCs and ASCs has allowed for the generation of models that span all three germ layers and most organs. However, the current challenge with most directed differentiation protocols is the fact that they result in cells and tissues that resemble embryonic and fetal tissue rather than the mature cells required for transplantation. Furthermore, the utilization of bioreactors allows for scaling of production, which is needed for the translation of these 3D systems into cell therapies. Additional research is required to connect multiple organ models for in vitro disease modelling and drug testing in a body-on-a-chip system. Another important challenge is to improve the reproducibility of current models, especially organoids, which can be achieved through applying engineering methods of microfabrication to control the cellular microenvironment.
The generation of 3D culture systems has greatly enabled the study of human development in vitro while minimizing the ethical considerations, allowing for the investigation of early embryonic development, lineage specification and organogenesis. 3D culture models have been critical in studying aspects of human development which have been previously limited by interspecies differences, providing a better understanding of congenital conditions, developmental disorders, and human diseases. Current disease models have mainly focused on examining the effects of a disease on an individual organ. Additional research is required to integrate the various organ models through a body-on-a-chip approach to study the systemic effects of disease. The generation of 3D culture systems has also revolutionized in vitro drug testing. 3D systems will replace traditional 2D culture systems for high-throughput screening of compound libraries and allow for greater clinical translation due to the improved physiological relevance of these models. The development of 3D models that allow for in vitro testing of drug absorption, metabolism and toxicity will minimize the need for expensive pre-clinical animal studies. Importantly, the use of iPSC-derived 3D systems enables personalized medicine by performing drug screens with patient-specific cells to inform the treatment plan for that patient.
The generation of in vitro 3D models that mimic in vivo organs has great potential for the discovery of novel therapeutics, including small molecules and cell therapies, as well as understanding developmental mechanisms of diseased and healthy tissue. There is also a potential to commercialize these models, in particular for the various organ-on-a-chip and body-on-a-chip systems that can be mass produced for both academic and industrial purposes. By developing more representative culture systems, clinicians and scientists will be better able to diagnose, treat and mitigate disease.
Figure 3. Neuronal Differentiation and Self-organizing in HMOs.
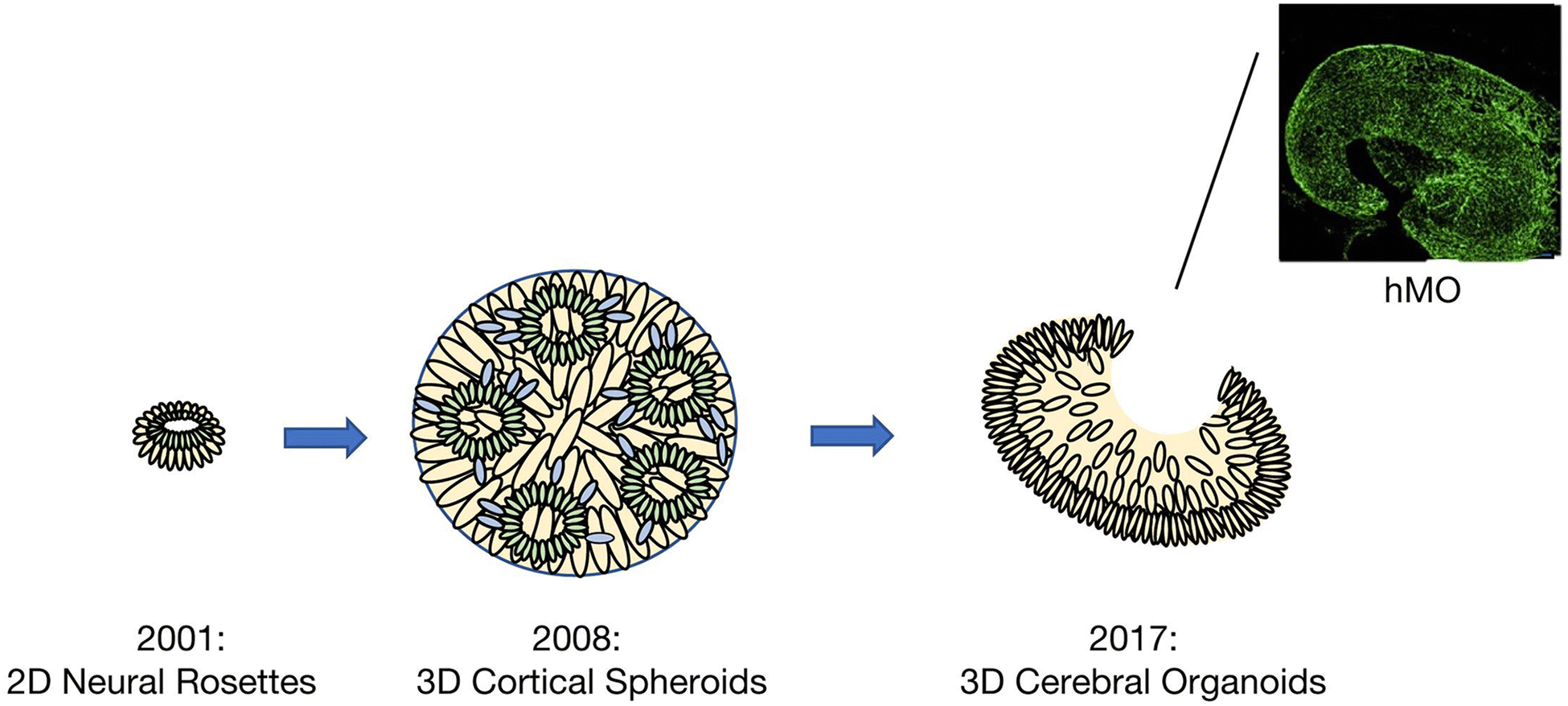
Starting from the initial development of neural rosettes in 2001, developmental models of the brain have become more complex and representative of corticogenesis. In 2008, the development of cortical spheroids allowed for the modelling of both deep and superficial cortical neurons, which then self-organized in a manner that resembled early corticogenesis. More recently, human pluripotent stem cell (hPSC) derived cerebral organoids allow for self-organization and self-patterning of several brain regions. Cerebral organoids have also enabled the development of more specialized structures, such as human midbrain-specific organoids (hMOs). Immunostaining for midbrain dopaminergic neuron (mDN) markers TUJ1 (green) reveals clearly specified clusters of mDNs within hMOs124*. In the schematic, green represents neural stem cells, cream represents intermediate progenitors and blue represents migrating or mature neurons.
*This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. To view a copy of this license, visit http://creativecommons.org/licenses/by-ncnd/4.0/ or send a letter to Creative Commons, PO Box 1866, Mountain View, CA 94042, USA. .US
Acknowledgements
We wish to thank Dr. Penney Gilbert for providing us with guidance in the preparation of this review. Radisic lab is supported by the Canadian Institutes of Health Research (CIHR) Operating Grants (MOP-126027 and MOP-137107), Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (RGPIN 326982-10), NSERC-CIHR Collaborative Health Research Grant (CHRP 493737-16) and National Institutes of Health Grant 2R01 HL076485.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Competing financial interests:
The authors declare no competing financial interests.
References:
- 1.Ahadian S et al. Organ-On-A-Chip Platforms: A Convergence of Advanced Materials, Cells, and Microscale Technologies. Adv Healthc Mater 7, doi: 10.1002/adhm.201800734 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Clevers H Modeling Development and Disease with Organoids. Cell 165, 1586–1597, doi: 10.1016/j.cell.2016.05.082 (2016). [DOI] [PubMed] [Google Scholar]
- 3.Zhao Y et al. A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 176, 913–927. e918 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huh D et al. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med 4, 159ra147, doi: 10.1126/scitranslmed.3004249 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davidson AJ, Lewis P, Przepiorski A & Sander V Turning mesoderm into kidney. Semin Cell Dev Biol 91, 86–93, doi: 10.1016/j.semcdb.2018.08.016 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Hofrichter M et al. Comparative performance analysis of human iPSC-derived and primary neural progenitor cells (NPC) grown as neurospheres in vitro. Stem Cell Research 25, 72–82, doi: 10.1016/j.scr.2017.10.013 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Lancaster MA & Knoblich JA Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125, doi: 10.1126/science.1247125 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Devarasetty M, Mazzocchi AR & Skardal A Applications of Bioengineered 3D Tissue and Tumor Organoids in Drug Development and Precision Medicine: Current and Future. BioDrugs 32, 53–68, doi: 10.1007/s40259-017-0258-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garnier D et al. Expansion of human primary hepatocytes in vitro through their amplification as liver progenitors in a 3D organoid system. Scientific reports 8, 8222 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qian X et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 165, 1238–1254, doi: 10.1016/j.cell.2016.04.032 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blondel D & Lutolf MP Bioinspired Hydrogels for 3D Organoid Culture. Chimia (Aarau) 73, 81–85, doi: 10.2533/chimia.2019.81 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Mills RJ et al. Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest. Proceedings of the National Academy of Sciences of the United States of America 114, E8372–E8381, doi: 10.1073/pnas.1707316114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancaster MA et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakano T et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785, doi: 10.1016/j.stem.2012.05.009 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Sato T et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772, doi: 10.1053/j.gastro.2011.07.050 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Spence JR et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109, doi: 10.1038/nature09691 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taguchi A & Nishinakamura R Higher-Order Kidney Organogenesis from Pluripotent Stem Cells. Cell Stem Cell 21, 730–746 e736, doi: 10.1016/j.stem.2017.10.011 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Lancaster MA et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379, doi: 10.1038/nature12517 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takasato M et al. Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526, 564–568, doi: 10.1038/nature15695 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Huang L et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell-and patient-derived tumor organoids. Nat Med 21, 1364–1371, doi: 10.1038/nm.3973 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho BX, Pek NMQ & Soh BS Disease Modeling Using 3D Organoids Derived from Human Induced Pluripotent Stem Cells. Int J Mol Sci 19, doi: 10.3390/ijms19040936 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meinhardt A et al. 3D reconstitution of the patterned neural tube from embryonic stem cells. Stem Cell Rep 3, 987–999, doi: 10.1016/j.stemcr.2014.09.020 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mihara Y et al. Production of pancreatic progenitor cells from human induced pluripotent stem cells using a three-dimensional suspension bioreactor system. J Tissue Eng Regen Med 11, 3193–3201, doi: 10.1002/term.2228 (2017). [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y et al. Towards chamber specific heart-on-a-chip for drug testing applications. Advanced drug delivery reviews, doi: 10.1016/j.addr.2019.12.002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao Y, Korolj A, Feric N & Radisic M Human pluripotent stem cell-derived cardiomyocyte based models for cardiotoxicity and drug discovery. Expert opinion on drug safety 15, 1455–1458, doi: 10.1080/14740338.2016.1223624 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Devalla HD & Passier R Cardiac differentiation of pluripotent stem cells and implications for modeling the heart in health and disease. Science translational medicine 10, doi: 10.1126/scitranslmed.aah5457 (2018). [DOI] [PubMed] [Google Scholar]
- 27.McCauley HA & Wells JM Pluripotent stem cell-derived organoids: using principles of developmental biology to grow human tissues in a dish. Development 144, 958–962, doi: 10.1242/dev.140731 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nascimento JM et al. Human Cerebral Organoids and Fetal Brain Tissue Share Proteomic Similarities. Frontiers in cell and developmental biology 7, 303, doi: 10.3389/fcell.2019.00303 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van de Wetering M et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945, doi: 10.1016/j.cell.2015.03.053 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dutta D, Heo I & Clevers H Disease Modeling in Stem Cell-Derived 3D Organoid Systems. Trends Mol Med 23, 393–410, doi: 10.1016/j.molmed.2017.02.007 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Muller S et al. Human adipose stromal-vascular fraction self-organizes to form vascularized adipose tissue in 3D cultures. Scientific reports 9, 7250, doi: 10.1038/s41598-019-43624-6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bergmann O et al. Evidence for Cardiomyocyte Renewal in Humans. Science 324, 98, doi: 10.1126/science.1164680 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kretzschmar K et al. Profiling proliferative cells and their progeny in damaged murine hearts. Proceedings of the National Academy of Sciences 115, E12245, doi: 10.1073/pnas.1805829115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kretzschmar K & Clevers H Organoids: Modeling Development and the Stem Cell Niche in a Dish. Dev Cell 38, 590–600, doi: 10.1016/j.devcel.2016.08.014 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Batalov I & Feinberg AW Differentiation of Cardiomyocytes from Human Pluripotent Stem Cells Using Monolayer Culture. Biomark Insights 10, 71–76 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronaldson-Bouchard K et al. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 556, 239–243, doi: 10.1038/s41586-018-0016-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huch M et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 494, 247–250, doi: 10.1038/nature11826 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broutier L et al. Culture and establishment of self-renewing human and mouse adult liver and pancreas 3D organoids and their genetic manipulation. Nat Protoc 11, 1724–1743, doi: 10.1038/nprot.2016.097 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Huch M et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J 32, 2708–2721, doi: 10.1038/emboj.2013.204 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato T et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265, doi: 10.1038/nature07935 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Zacharias WJ et al. Regeneration of the lung alveolus by an evolutionarily conserved epithelial progenitor. Nature 555, 251–255, doi: 10.1038/nature25786 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weeber F, Ooft SN, Dijkstra KK & Voest EE Tumor Organoids as a Pre-clinical Cancer Model for Drug Discovery. Cell Chemical Biology 24, 1092–1100, doi: 10.1016/j.chembiol.2017.06.012 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Finnberg NK et al. Application of 3D tumoroid systems to define immune and cytotoxic therapeutic responses based on tumoroid and tissue slice culture molecular signatures. Oncotarget 8, 66747–66757, doi: 10.18632/oncotarget.19965 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akay M et al. Drug Screening of Human GBM Spheroids in Brain Cancer Chip. Sci Rep 8, 15423, doi: 10.1038/s41598-018-33641-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujii M et al. A Colorectal Tumor Organoid Library Demonstrates Progressive Loss of Niche Factor Requirements during Tumorigenesis. Cell Stem Cell 18, 827–838, doi: 10.1016/j.stem.2016.04.003 (2016). [DOI] [PubMed] [Google Scholar]
- 46.Sachs N et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 172, 373–386 e310, doi: 10.1016/j.cell.2017.11.010 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Pampaloni F, Reynaud EG & Stelzer EH The third dimension bridges the gap between cell culture and live tissue. Nature reviews Molecular cell biology 8, 839 (2007). [DOI] [PubMed] [Google Scholar]
- 48.Inzana JA et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 35, 4026–4034 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tibbitt MW & Anseth KS Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnology and bioengineering 103, 655–663 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.des Rieux A, Shikanov A & Shea LD Fibrin hydrogels for non-viral vector delivery in vitro. J Control Release 136, 148–154, doi: 10.1016/j.jconrel.2009.02.004 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lutolf MP & Hubbell JA Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 23, 47–55, doi: 10.1038/nbt1055 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Godugu C et al. AlgiMatrix based 3D cell culture system as an in-vitro tumor model for anticancer studies. PLoS One 8, e53708, doi: 10.1371/journal.pone.0053708 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grinnell F Fibroblast biology in three-dimensional collagen matrices. Trends Cell Biol 13, 264–269, doi: 10.1016/s0962-8924(03)00057-6 (2003). [DOI] [PubMed] [Google Scholar]
- 54.Ravi M, Paramesh V, Kaviya SR, Anuradha E & Solomon FD 3D cell culture systems: advantages and applications. J Cell Physiol 230, 16–26, doi: 10.1002/jcp.24683 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Li ML et al. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proceedings of the National Academy of Sciences of the United States of America 84, 136–140, doi: 10.1073/pnas.84.1.136 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benton G, Arnaoutova I, George J, Kleinman HK & Koblinski J Matrigel: from discovery and ECM mimicry to assays and models for cancer research. Adv Drug Deliv Rev 79–80, 3–18, doi: 10.1016/j.addr.2014.06.005 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Kleinman HK & Martin GR Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol 15, 378–386, doi: 10.1016/j.semcancer.2005.05.004 (2005). [DOI] [PubMed] [Google Scholar]
- 58.Vukicevic S et al. Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res 202, 1–8, doi: 10.1016/0014-4827(92)90397-q (1992). [DOI] [PubMed] [Google Scholar]
- 59.Hughes CS, Postovit LM & Lajoie GA Matrigel: a complex protein mixture required for optimal growth of cell culture. Proteomics 10, 1886–1890, doi: 10.1002/pmic.200900758 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Pabst A, Ziebart T, Ackermann M, Konerding M & Walter C Bisphosphonates’ antiangiogenic potency in the development of bisphosphonate-associated osteonecrosis of the jaws: influence on microvessel sprouting in an in vivo 3D Matrigel assay. Clinical oral investigations 18, 1015–1022 (2014). [DOI] [PubMed] [Google Scholar]
- 61.Sun T et al. Expression and functional significance of Twist1 in hepatocellular carcinoma: its role in vasculogenic mimicry. Hepatology 51, 545–556 (2010). [DOI] [PubMed] [Google Scholar]
- 62.Poincloux R et al. Contractility of the cell rear drives invasion of breast tumor cells in 3D Matrigel. Proceedings of the National Academy of Sciences 108, 1943–1948 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miao Y, Sun YB, Liu BC, Jiang JD & Hu ZQ Controllable production of transplantable adult human high-passage dermal papilla spheroids using 3D matrigel culture. Tissue Engineering Part A 20, 2329–2338 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Debnath J, Muthuswamy SK & Brugge JS Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30, 256–268 (2003). [DOI] [PubMed] [Google Scholar]
- 65.Debnath J, Muthuswamy SK & Brugge JS Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30, 256–268, doi: 10.1016/s1046-2023(03)00032-x (2003). [DOI] [PubMed] [Google Scholar]
- 66.Staton CA et al. Current methods for assaying angiogenesis in vitro and in vivo. International journal of experimental pathology 85, 233–248 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mallon BS, Park K-Y, Chen KG, Hamilton RS & McKay RD Toward xeno-free culture of human embryonic stem cells. The international journal of biochemistry & cell biology 38, 1063–1075 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolf K et al. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol 20, 931–941, doi: 10.1016/j.semcdb.2009.08.005 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Szot CS, Buchanan CF, Freeman JW & Rylander MN 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials 32, 7905–7912 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Eschenhagen T et al. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 11, 683–694, doi: 10.1096/fasebj.11.8.9240969 (1997). [DOI] [PubMed] [Google Scholar]
- 71.Yang S, Graham J, Kahn JW, Schwartz EA & Gerritsen ME Functional roles for PECAM-1 (CD31) and VE-cadherin (CD144) in tube assembly and lumen formation in three-dimensional collagen gels. Am J Pathol 155, 887–895, doi: 10.1016/S0002-9440(10)65188-7 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arora PD, Narani N & McCulloch CA The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. Am J Pathol 154, 871–882 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hersel U, Dahmen C & Kessler H RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials 24, 4385–4415 (2003). [DOI] [PubMed] [Google Scholar]
- 74.Seliktar D, Zisch AH, Lutolf MP, Wrana JL & Hubbell JA MMP-2 sensitive, VEGF-bearing bioactive hydrogels for promotion of vascular healing. J Biomed Mater Res A 68, 704–716, doi: 10.1002/jbm.a.20091 (2004). [DOI] [PubMed] [Google Scholar]
- 75.Tae G, Scatena M, Stayton PS & Hoffman AS PEG-cross-linked heparin is an affinity hydrogel for sustained release of vascular endothelial growth factor. J Biomater Sci Polym Ed 17, 187–197 (2006). [DOI] [PubMed] [Google Scholar]
- 76.Lutolf MP et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nature biotechnology 21, 513–518, doi: 10.1038/nbt818 (2003). [DOI] [PubMed] [Google Scholar]
- 77.Bein A et al. Microfluidic organ-on-a-chip models of human intestine. Cellular and molecular gastroenterology and hepatology 5, 659–668 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berthier E, Young EW & Beebe D Engineers are from PDMS-land, Biologists are from Polystyrenia. Lab Chip 12, 1224–1237, doi: 10.1039/c2lc20982a (2012). [DOI] [PubMed] [Google Scholar]
- 79.Domansky K et al. Clear castable polyurethane elastomer for fabrication of microfluidic devices. Lab on a chip 13, 3956–3964, doi: 10.1039/c3lc50558h (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao Y et al. A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 176, 913–927 e918, doi: 10.1016/j.cell.2018.11.042 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao Y et al. A Multimaterial Microphysiological Platform Enabled by Rapid Casting of Elastic Microwires. Advanced healthcare materials 8, e1801187, doi: 10.1002/adhm.201801187 (2019). [DOI] [PubMed] [Google Scholar]
- 82.Choe A, Ha SK, Choi I, Choi N & Sung JH Microfluidic Gut-liver chip for reproducing the first pass metabolism. Biomed Microdevices 19, 4, doi: 10.1007/s10544-016-0143-2 (2017). [DOI] [PubMed] [Google Scholar]
- 83.Briscoe J & Therond PP The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol 14, 416–429, doi: 10.1038/nrm3598 (2013). [DOI] [PubMed] [Google Scholar]
- 84.Siebel C & Lendahl U Notch Signaling in Development, Tissue Homeostasis, and Disease. Physiological Reviews 97, 1235–1294, doi: 10.1152/physrev.00005.2017 (2017). [DOI] [PubMed] [Google Scholar]
- 85.Steinhart Z & Angers S Wnt signaling in development and tissue homeostasis. Development 145, doi: 10.1242/dev.146589 (2018). [DOI] [PubMed] [Google Scholar]
- 86.Wu MY & Hill CS Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev Cell 16, 329–343, doi: 10.1016/j.devcel.2009.02.012 (2009). [DOI] [PubMed] [Google Scholar]
- 87.Bagley JA, Reumann D, Bian S, Levi-Strauss J & Knoblich JA Fused cerebral organoids model interactions between brain regions. Nat Methods 14, 743–751, doi: 10.1038/nmeth.4304 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fennema E, Rivron N, Rouwkema J, van Blitterswijk C & de Boer J Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol 31, 108–115, doi: 10.1016/j.tibtech.2012.12.003 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Yoon SJ et al. Reliability of human cortical organoid generation. Nat Methods 16, 75–78, doi: 10.1038/s41592-018-0255-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Laschke MW & Menger MD Life is 3D: Boosting Spheroid Function for Tissue Engineering. Trends Biotechnol 35, 133–144, doi: 10.1016/j.tibtech.2016.08.004 (2017). [DOI] [PubMed] [Google Scholar]
- 91.Sloan SA et al. Human Astrocyte Maturation Captured in 3D Cerebral Cortical Spheroids Derived from Pluripotent Stem Cells. Neuron 95, 779–790 e776, doi: 10.1016/j.neuron.2017.07.035 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Camp JG et al. Multilineage communication regulates human liver bud development from pluripotency. Nature 546, 533–538, doi: 10.1038/nature22796 (2017). [DOI] [PubMed] [Google Scholar]
- 93.Khurshid M, Mulet-Sierra A, Adesida A & Sen A Osteoarthritic human chondrocytes proliferate in 3D co-culture with mesenchymal stem cells in suspension bioreactors. J Tissue Eng Regen Med 12, e1418–e1432, doi: 10.1002/term.2531 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yin X et al. Engineering Stem Cell Organoids. Cell Stem Cell 18, 25–38, doi: 10.1016/j.stem.2015.12.005 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cavo M et al. A new cell-laden 3D Alginate-Matrigel hydrogel resembles human breast cancer cell malignant morphology, spread and invasion capability observed “in vivo”. Sci Rep 8, 5333, doi: 10.1038/s41598-018-23250-4 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tillman BW et al. The in vivo stability of electrospun polycaprolactone-collagen scaffolds in vascular reconstruction. Biomaterials 30, 583–588, doi: 10.1016/j.biomaterials.2008.10.006 (2009). [DOI] [PubMed] [Google Scholar]
- 97.DiStefano T et al. Accelerated and Improved Differentiation of Retinal Organoids from Pluripotent Stem Cells in Rotating-Wall Vessel Bioreactors. Stem Cell Rep 10, 300–313, doi: 10.1016/j.stemcr.2017.11.001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Eicke D et al. Large-scale production of megakaryocytes in microcarrier-supported stirred suspension bioreactors. Scientific Reports 8, doi: 10.1038/s41598-018-28459-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fischer B et al. A complete workflow for the differentiation and the dissociation of hiPSC-derived cardiospheres. Stem Cell Res 32, 65–72, doi: 10.1016/j.scr.2018.08.015 (2018). [DOI] [PubMed] [Google Scholar]
- 100.Przepiorski A et al. A Simple Bioreactor-Based Method to Generate Kidney Organoids from Pluripotent Stem Cells. Stem Cell Rep 11, 470–484, doi: 10.1016/j.stemcr.2018.06.018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qian X et al. Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nat Protoc 13, 565–580, doi: 10.1038/nprot.2017.152 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jalili-Firoozinezhad S et al. Modeling radiation injury-induced cell death and countermeasure drug responses in a human Gut-on-a-Chip. Cell Death Dis 9, 223, doi: 10.1038/s41419-018-0304-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou M et al. Development of a Functional Glomerulus at the Organ Level on a Chip to Mimic Hypertensive Nephropathy. Sci Rep 6, 31771, doi: 10.1038/srep31771 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bhatia SN & Ingber DE Microfluidic organs-on-chips. Nat Biotechnol 32, 760–772, doi: 10.1038/nbt.2989 (2014). [DOI] [PubMed] [Google Scholar]
- 105.Khadpekar AJ, Khan M, Sose A & Majumder A Low Cost and Lithography-free stamp fabrication for Microcontact printing. Scientific reports 9, 1024 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nunes SS et al. Biowire: a platform for maturation of human pluripotent stem cell–derived cardiomyocytes. Nature Methods 10, 781, doi:https://www.nature.com/articles/nmeth.2524#supplementary-information (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhao Y et al. A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling. Cell 176, 913–927.e918, doi: 10.1016/j.cell.2018.11.042 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao Y et al. A Multimaterial Microphysiological Platform Enabled by Rapid Casting of Elastic Microwires. Advanced healthcare materials, e1801187, doi: 10.1002/adhm.201801187 (2019). [DOI] [PubMed] [Google Scholar]
- 109.Adriani G, Ma D, Pavesi A, Kamm RD & Goh ELA 3D neurovascular microfluidic model consisting of neurons, astrocytes and cerebral endothelial cells as a blood-brain barrier. Lab Chip 17, 448–459, doi: 10.1039/c6lc00638h (2017). [DOI] [PubMed] [Google Scholar]
- 110.Yang K et al. Recapitulation of in vivo-like paracrine signals of human mesenchymal stem cells for functional neuronal differentiation of human neural stem cells in a 3D microfluidic system. Biomaterials 63, 177–188, doi: 10.1016/j.biomaterials.2015.06.011 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Kawada J et al. Generation of a Motor Nerve Organoid with Human Stem Cell-Derived Neurons. Stem Cell Rep 9, 1441–1449, doi: 10.1016/j.stemcr.2017.09.021 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang B et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis. Nature Materials 15, 669, doi:https://www.nature.com/articles/nmat4570#supplementary-information (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Maschmeyer I et al. A four-organ-chip for interconnected long-term co-culture of human intestine, liver, skin and kidney equivalents. Lab Chip 15, 2688–2699, doi: 10.1039/c5lc00392j (2015). [DOI] [PubMed] [Google Scholar]
- 114.Eiraku M & Sasai Y Self-formation of layered neural structures in three-dimensional culture of ES cells. Curr Opin Neurobiol 22, 768–777, doi: 10.1016/j.conb.2012.02.005 (2012). [DOI] [PubMed] [Google Scholar]
- 115.Lancaster MA et al. Guided self-organization and cortical plate formation in human brain organoids. Nature Biotechnology 35, 659–+, doi: 10.1038/nbt.3906 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chwalek K et al. Providing the right cues in nerve guidance conduits: Biofunctionalization versus fiber profile to facilitate oriented neuronal outgrowth. Mater Sci Eng C Mater Biol Appl 61, 466–472, doi: 10.1016/j.msec.2015.12.059 (2016). [DOI] [PubMed] [Google Scholar]
- 117.Wu S, Xu R, Duan B & Jiang P Three-Dimensional Hyaluronic Acid Hydrogel-Based Models for In Vitro Human iPSC-Derived NPC Culture and Differentiation. J Mater Chem B 5, 3870–3878, doi: 10.1039/C7TB00721C (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Langer R & Vacanti J Advances in tissue engineering. Journal of pediatric surgery 51, 8–12 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xinaris C, Brizi V & Remuzzi G Organoid Models and Applications in Biomedical Research. Nephron 130, 191–199, doi: 10.1159/000433566 (2015). [DOI] [PubMed] [Google Scholar]
- 120.Eiraku M et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell stem cell 3, 519–532 (2008). [DOI] [PubMed] [Google Scholar]
- 121.Mariani J et al. Modeling human cortical development in vitro using induced pluripotent stem cells. Proceedings of the National Academy of Sciences 109, 12770–12775 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kelava I & Lancaster MA Dishing out mini-brains: Current progress and future prospects in brain organoid research. Dev Biol 420, 199–209, doi: 10.1016/j.ydbio.2016.06.037 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang SC, Wernig M, Duncan ID, Brustle O & Thomson JA In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol 19, 1129–1133, doi: 10.1038/nbt1201-1129 (2001). [DOI] [PubMed] [Google Scholar]
- 124.Monzel AS et al. Derivation of Human Midbrain-Specific Organoids from Neuroepithelial Stem Cells. Stem Cell Rep 8, 1144–1154, doi: 10.1016/j.stemcr.2017.03.010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Birey F et al. Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59, doi: 10.1038/nature22330 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xiang Y et al. Fusion of Regionally Specified hPSC-Derived Organoids Models Human Brain Development and Interneuron Migration. Cell Stem Cell 21, 383–398 e387, doi: 10.1016/j.stem.2017.07.007 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fuhrmann S Eye morphogenesis and patterning of the optic vesicle. Curr Top Dev Biol 93, 61–84, doi: 10.1016/B978-0-12-385044-7.00003-5 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nguyen M & Arnheiter H Signaling and transcriptional regulation in early mammalian eye development: a link between FGF and MITF. Development 127, 3581–3591 (2000). [DOI] [PubMed] [Google Scholar]
- 129.Zhong X et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun 5, 4047, doi: 10.1038/ncomms5047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mellough CB, Sernagor E, Moreno-Gimeno I, Steel DH & Lako M Efficient stage-specific differentiation of human pluripotent stem cells toward retinal photoreceptor cells. Stem Cells 30, 673–686, doi: 10.1002/stem.1037 (2012). [DOI] [PubMed] [Google Scholar]
- 131.Tucker BA et al. Patient-specific iPSC-derived photoreceptor precursor cells as a means to investigate retinitis pigmentosa. Elife 2, e00824 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wahlin KJ et al. Photoreceptor Outer Segment-like Structures in Long-Term 3D Retinas from Human Pluripotent Stem Cells. Scientific Reports 7, doi:ARTN 766 10.1038/s41598-017-00774-9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen HY, Kaya KD, Dong L & Swaroop A Three-dimensional retinal organoids from mouse pluripotent stem cells mimic in vivo development with enhanced stratification and rod photoreceptor differentiation. Mol Vis 22, 1077–1094 (2016). [PMC free article] [PubMed] [Google Scholar]
- 134.Hunt NC et al. 3D culture of human pluripotent stem cells in RGD-alginate hydrogel improves retinal tissue development. Acta Biomater 49, 329–343, doi: 10.1016/j.actbio.2016.11.016 (2017). [DOI] [PubMed] [Google Scholar]
- 135.Ohlemacher SK et al. Stepwise Differentiation of Retinal Ganglion Cells from Human Pluripotent Stem Cells Enables Analysis of Glaucomatous Neurodegeneration. Stem Cells 34, 1553–1562, doi: 10.1002/stem.2356 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shen MM Nodal signaling: developmental roles and regulation. Development 134, 1023–1034, doi: 10.1242/dev.000166 (2007). [DOI] [PubMed] [Google Scholar]
- 137.D’Amour KA et al. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 23, 1534–1541, doi: 10.1038/nbt1163 (2005). [DOI] [PubMed] [Google Scholar]
- 138.McCracken KW, Howell JC, Wells JM & Spence JR Generating human intestinal tissue from pluripotent stem cells in vitro. Nat Protoc 6, 1920–1928, doi: 10.1038/nprot.2011.410 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.In JG et al. Human mini-guts: new insights into intestinal physiology and host-pathogen interactions. Nat Rev Gastroenterol Hepatol 13, 633–642, doi: 10.1038/nrgastro.2016.142 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang YL et al. A microengineered collagen scaffold for generating a polarized crypt-villus architecture of human small intestinal epithelium. Biomaterials 128, 44–55, doi: 10.1016/j.biomaterials.2017.03.005 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Takasato M et al. Directing human embryonic stem cell differentiation towards a renal lineage generates a self-organizing kidney. Nature cell biology 16, 118 (2014). [DOI] [PubMed] [Google Scholar]
- 142.Takasato M, Pei XE, Chiu HS & Little MH Generation of kidney organoids from human pluripotent stem cells. Nature protocols 11, 1681 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang B et al. Microfabrication of AngioChip, a biodegradable polymer scaffold with microfluidic vasculature. Nature Protocols 13, 1793–1813, doi: 10.1038/s41596-018-0015-8 (2018). [DOI] [PubMed] [Google Scholar]
- 144.Barak H et al. FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Developmental cell 22, 1191–1207 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sumi T, Tsuneyoshi N, Nakatsuji N & Suemori H Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development 135, 2969–2979, doi: 10.1242/dev.021121 (2008). [DOI] [PubMed] [Google Scholar]
- 146.Lian X, Xu J, Li J & Chien KR Next-generation models of human cardiogenesis via genome editing. Cold Spring Harb Perspect Med 4, a013920, doi: 10.1101/cshperspect.a013920 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Akhtar A The flaws and human harms of animal experimentation. Camb Q Healthc Ethics 24, 407–419, doi: 10.1017/S0963180115000079 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Benam KH et al. Engineered In Vitro Disease Models. Annu Rev Pathol-Mech 10, 195–262, doi: 10.1146/annurev-pathol-012414-040418 (2015). [DOI] [PubMed] [Google Scholar]
- 149.Quadrato G, Brown J & Arlotta P The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nat Med 22, 1220–1228, doi: 10.1038/nm.4214 (2016). [DOI] [PubMed] [Google Scholar]
- 150.Choi SH et al. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature 515, 274–278, doi: 10.1038/nature13800 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Eiraku M et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56, doi: 10.1038/nature09941 (2011). [DOI] [PubMed] [Google Scholar]
- 152.Torrejon KY et al. Bioengineered glaucomatous 3D human trabecular meshwork as an in vitro disease model. Biotechnol Bioeng 113, 1357–1368, doi: 10.1002/bit.25899 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Assawachananont J et al. Transplantation of Embryonic and Induced Pluripotent Stem Cell-Derived 3D Retinal Sheets into Retinal Degenerative Mice. Stem Cell Rep 2, 662–674, doi: 10.1016/j.stemcr.2014.03.011 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hinson JT et al. HEART DISEASE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy. Science 349, 982–986, doi: 10.1126/science.aaa5458 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tiburcy M et al. Defined Engineered Human Myocardium With Advanced Maturation for Applications in Heart Failure Modeling and Repair. Circulation 135, 1832–1847, doi: 10.1161/CIRCULATIONAHA.116.024145 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhao Y et al. Engineering microenvironment for human cardiac tissue assembly in heart-on-a-chip platform. Matrix biology : journal of the International Society for Matrix Biology, doi: 10.1016/j.matbio.2019.04.001 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Davis RP et al. Cardiomyocytes derived from pluripotent stem cells recapitulate electrophysiological characteristics of an overlap syndrome of cardiac sodium channel disease. Circulation 125, 3079–3091, doi: 10.1161/CIRCULATIONAHA.111.066092 (2012). [DOI] [PubMed] [Google Scholar]
- 158.Huang SX et al. Efficient generation of lung and airway epithelial cells from human pluripotent stem cells. Nat Biotechnol 32, 84–91, doi: 10.1038/nbt.2754 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ishikawa S, Ishimori K & Ito SA 3D epithelial-mesenchymal co-culture model of human bronchial tissue recapitulates multiple features of airway tissue remodeling by TGF-beta1 treatment. Respir Res 18, 195, doi: 10.1186/s12931-017-0680-0 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhou J et al. Differentiated human airway organoids to assess infectivity of emerging influenza virus. Proc Natl Acad Sci U S A 115, 6822–6827, doi: 10.1073/pnas.1806308115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fiorotto R et al. Liver diseases in the dish: iPSC and organoids as a new approach to modeling liver diseases. Biochim Biophys Acta Mol Basis Dis 1865, 920–928, doi: 10.1016/j.bbadis.2018.08.038 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ortega-Prieto AM et al. 3D microfluidic liver cultures as a physiological preclinical tool for hepatitis B virus infection. Nat Commun 9, 682, doi: 10.1038/s41467-018-02969-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Freedman BS et al. Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun 6, 8715, doi: 10.1038/ncomms9715 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Forbes TA et al. Patient-iPSC-Derived Kidney Organoids Show Functional Validation of a Ciliopathic Renal Phenotype and Reveal Underlying Pathogenetic Mechanisms. Am J Hum Genet 102, 816–831, doi: 10.1016/j.ajhg.2018.03.014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nugraha B et al. Monitoring and manipulating cellular crosstalk during kidney fibrosis inside a 3D in vitro co-culture. Sci Rep 7, 14490, doi: 10.1038/s41598-017-12683-y (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hohwieler M et al. Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut 66, 473–486 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rathnayake N et al. Salivary biomarkers for detection of systemic diseases. PLoS One 8, e61356, doi: 10.1371/journal.pone.0061356 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang YI, Carmona C, Hickman JJ & Shuler ML Multiorgan Microphysiological Systems for Drug Development: Strategies, Advances, and Challenges. Adv Healthc Mater 7, doi:ARTN 170100010.1002/adhm.201701000 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Oleaga C et al. Multi-Organ toxicity demonstration in a functional human in vitro system composed of four organs. Sci Rep 6, 20030, doi: 10.1038/srep20030 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Suntharalingam G et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 355, 1018–1028, doi: 10.1056/NEJMoa063842 (2006). [DOI] [PubMed] [Google Scholar]
- 171.Belloni D et al. Modeling multiple myeloma-bone marrow interactions and response to drugs in a 3D surrogate microenvironment. haematologica, haematol. 2017.167486 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang X et al. Nanofiber membrane supported lung-on-a-chip microdevice for anti-cancer drug testing. Lab on a Chip 18, 486–495 (2018). [DOI] [PubMed] [Google Scholar]
- 173.Bell CC et al. Comparison of Hepatic 2D Sandwich Cultures and 3D Spheroids for Long-term Toxicity Applications: A Multicenter Study. Toxicological Sciences 162, 655–666 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Boj SF et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 160, 324–338, doi: 10.1016/j.cell.2014.12.021 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang YI, Abaci HE & Shuler ML Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol Bioeng 114, 184–194, doi: 10.1002/bit.26045 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Campisi M et al. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 180, 117–129, doi: 10.1016/j.biomaterials.2018.07.014 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wevers NR et al. A perfused human blood-brain barrier on-a-chip for high-throughput assessment of barrier function and antibody transport. Fluids Barriers CNS 15, 23, doi: 10.1186/s12987-018-0108-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Terasaki PI Transplant Antigens: A Brief History of HLA. Textbook of Organ Transplantation, 30–35, doi:Book_Doi 10.1002/9781118873434 (2014). [Google Scholar]
- 179.Sade H et al. A human blood-brain barrier transcytosis assay reveals antibody transcytosis influenced by pH-dependent receptor binding. PLoS One 9, e96340, doi: 10.1371/journal.pone.0096340 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Freund-Levi Y et al. Omega-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: a randomized double-blind trial. Arch Neurol 63, 1402–1408, doi: 10.1001/archneur.63.10.1402 (2006). [DOI] [PubMed] [Google Scholar]
- 181.Quinn JF et al. Docosahexaenoic acid supplementation and cognitive decline in Alzheimer disease: a randomized trial. JAMA 304, 1903–1911, doi: 10.1001/jama.2010.1510 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Perry G et al. Comparative biology and pathology of oxidative stress in Alzheimer and other neurodegenerative diseases: beyond damage and response. Comp Biochem Physiol C Toxicol Pharmacol 133, 507–513, doi: 10.1016/s1532-0456(02)00119-9 (2002). [DOI] [PubMed] [Google Scholar]
- 183.Perry G et al. Reactive oxygen: its sources and significance in Alzheimer disease. J Neural Transm Suppl, 69–75, doi: 10.1007/978-3-7091-6139-5_7 (2002). [DOI] [PubMed] [Google Scholar]
- 184.Perry G, Cash AD & Smith MA Alzheimer Disease and Oxidative Stress. J Biomed Biotechnol 2, 120–123, doi: 10.1155/S1110724302203010 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kondo T et al. Modeling Alzheimer’s disease with iPSCs reveals stress phenotypes associated with intracellular Abeta and differential drug responsiveness. Cell Stem Cell 12, 487–496, doi: 10.1016/j.stem.2013.01.009 (2013). [DOI] [PubMed] [Google Scholar]
- 186.Torrejon KY et al. Bioengineered glaucomatous 3D human trabecular meshwork as an in vitro disease model. Biotechnology and bioengineering 113, 1357–1368 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Skardal A, Shupe T & Atala A in Principles of Regenerative Medicine 769–786 (Elsevier, 2019). [Google Scholar]
- 188.Esch MB & Mahler GJ in Microfluidic Cell Culture Systems 323–350 (Elsevier, 2019). [Google Scholar]
- 189.Fan Y, Nguyen DT, Akay Y, Xu F & Akay M Engineering a Brain Cancer Chip for High-throughput Drug Screening. Sci Rep 6, 25062, doi: 10.1038/srep25062 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Broutier L et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat Med 23, 1424–1435, doi: 10.1038/nm.4438 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vis DJ et al. Multilevel models improve precision and speed of IC50 estimates. Pharmacogenomics 17, 691–700, doi: 10.2217/pgs.16.15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shelper TB, Lovitt CJ & Avery VM Assessing Drug Efficacy in a Miniaturized Pancreatic Cancer In Vitro 3D Cell Culture Model. Assay Drug Dev Technol 14, 367–380, doi: 10.1089/adt.2016.737 (2016). [DOI] [PubMed] [Google Scholar]
- 193.Michl P & Gress TM Current concepts and novel targets in advanced pancreatic cancer. Gut 62, 317–326, doi: 10.1136/gutjnl-2012-303588 (2013). [DOI] [PubMed] [Google Scholar]
- 194.Sequist LV et al. Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J Med 372, 1700–1709, doi: 10.1056/NEJMoa1413654 (2015). [DOI] [PubMed] [Google Scholar]
- 195.Bean J et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Molecular Cancer Therapeutics 6, 3333s–3334s (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang S, Cang S & Liu D Third-generation inhibitors targeting EGFR T790M mutation in advanced non-small cell lung cancer. J Hematol Oncol 9, 34, doi: 10.1186/s13045-016-0268-z (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Hassell BA et al. Human Organ Chip Models Recapitulate Orthotopic Lung Cancer Growth, Therapeutic Responses, and Tumor Dormancy In Vitro. Cell Rep 21, 508–516, doi: 10.1016/j.celrep.2017.09.043 (2017). [DOI] [PubMed] [Google Scholar]
- 198.Lu HF et al. Engineering a functional three-dimensional human cardiac tissue model for drug toxicity screening. Biofabrication 9, 025011 (2017). [DOI] [PubMed] [Google Scholar]
- 199.Conant G, Ahadian S, Zhao Y & Radisic M Kinase inhibitor screening using artificial neural networks and engineered cardiac biowires. Scientific reports 7, 11807, doi: 10.1038/s41598-017-12048-5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Greggio C et al. Artificial three-dimensional niches deconstruct pancreas development in vitro. Development 140, 4452–4462, doi: 10.1242/dev.096628 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.McCracken KW et al. Modelling human development and disease in pluripotent stem-cell-derived gastric organoids. Nature 516, 400–404, doi: 10.1038/nature13863 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dye BR et al. In vitro generation of human pluripotent stem cell derived lung organoids. Elife 4, doi: 10.7554/eLife.05098 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Xia Y et al. Directed differentiation of human pluripotent cells to ureteric bud kidney progenitor-like cells. Nat Cell Biol 15, 1507–1515, doi: 10.1038/ncb2872 (2013). [DOI] [PubMed] [Google Scholar]
- 204.Jo J et al. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell 19, 248–257, doi: 10.1016/j.stem.2016.07.005 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kale S et al. Three-dimensional cellular development is essential for ex vivo formation of human bone. Nat Biotechnol 18, 954–958, doi: 10.1038/79439 (2000). [DOI] [PubMed] [Google Scholar]
- 206.Barker N et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36, doi: 10.1016/j.stem.2009.11.013 (2010). [DOI] [PubMed] [Google Scholar]
- 207.Karthaus WR et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell 159, 163–175, doi: 10.1016/j.cell.2014.08.017 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kessler M et al. The Notch and Wnt pathways regulate stemness and differentiation in human fallopian tube organoids. Nat Commun 6, 8989, doi: 10.1038/ncomms9989 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Bartfeld S & Clevers H Organoids as Model for Infectious Diseases: Culture of Human and Murine Stomach Organoids and Microinjection of Helicobacter Pylori. J Vis Exp, doi: 10.3791/53359 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Desplantez T et al. Connexin43 ablation in foetal atrial myocytes decreases electrical coupling, partner connexins, and sodium current. Cardiovasc Res 94, 58–65, doi: 10.1093/cvr/cvs025 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ingram JL et al. Airway fibroblasts in asthma manifest an invasive phenotype. Am J Respir Crit Care Med 183, 1625–1632, doi: 10.1164/rccm.201009-1452OC (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]


