Abstract
The complement system is a vital component of the innate immune system, though its role in bacteremia is poorly understood. We present complement levels in Staphylococcus aureus bacteremia (SAB) and Gram-negative bacteremia (GNB) and describe observed associations of complement levels with clinical outcomes. Complement and cytokine levels were measured in serum samples from 20 hospitalized patients with SAB, 20 hospitalized patients with GNB, 10 non-infected hospitalized patients, and 10 community controls. C5a levels were significantly higher in patients with SAB as compared to patients with GNB. Low C4 and C3 levels were associated with septic shock and 30-day mortality in patients with GNB, and elevated C3 was associated with a desirable outcome defined as absence of (1) septic shock, (2) acute renal failure, and (3) death within 30 days of bacteremia. Low levels of C9 were associated with septic shock in patients with GNB but not SAB. Elevated IL-10 was associated with increased 30-day mortality in patients with SAB. Complement profiles differ in patients with SAB and those with GNB. Measurement of IL-10 in patients with SAB and of C4, C3, and C9 in patients with GNB may help to identify those at higher risk for poor outcomes.
Electronic supplementary material
The online version of this article (10.1007/s10096-020-03955-z) contains supplementary material, which is available to authorized users.
Keywords: Complement, Staphylococcus aureus bacteremia, Gram-negative bacteremia, Cytokine, Sepsis, Septic shock
Introduction
Bloodstream infections are associated with substantial morbidity and mortality worldwide [1, 2]. It is estimated that approximately 2 million episodes and 250,000 deaths from bloodstream infections occur annually in Europe and North America combined [2]. A consistent predictor of mortality for patients with S. aureus bacteremia (SAB) [3, 4] and Gram-negative bacteremia (GNB) is the presence of septic shock [5]. Septic shock is thought to involve a dysregulated immune response to infection, the mechanisms of which are incompletely understood. The complement system is a critical component of innate immunity and has been implicated as a significant player in the pathogenesis of sepsis and septic shock [6–8]. Acute phase cytokine expression has also been explored in sepsis in hopes of understanding the immune dysregulation during infection [9]. Despite these studies, significant gaps remain in understanding the immune dysregulation that occurs during bacteremia and septic shock. The prognostic implications of acute phase inflammatory markers in patients with bacteremia are also poorly understood.
In the current report, we undertook an exploratory study to address these unknowns. We evaluated complement and cytokine levels in patients with Staphylococcus aureus and Gram-negative bacteremia as compared to non-bacteremic hospitalized patients and community controls and explored potential associations between levels of these immune components and clinical outcome of the source patients.
Methods
Study population
This prospective cohort study was conducted at two medical centers in North Carolina: Duke University Hospital (a tertiary referral center with 957 beds) and Duke Regional Hospital (a community hospital with 369 beds). The patient sample was identified using an existing protocol active at both sites known as the Bloodstream Infections Registry (BSIR). The BSIR collects and stores both clinical data and biological specimens from non-neutropenic hospitalized patients aged 18 years or older who have culture-confirmed monomicrobial bloodstream infections caused by either Staphylococcus aureus or Gram-negative bacteria.
All patients with either monomicrobial S. aureus bacteremia (SAB) or Gram-negative bacteremia (GNB) enrolled in the BSIR during a 10-month period between August 2014 and May 2018 were eligible for inclusion in the current study. The study was approved by the Duke Institutional Review Board, and written informed consent was obtained from all participants or their legally authorized representatives prior to their enrollment in this study. Patients were classified as SAB or GNB based on the results of their index microbiological cultures. Participants from each of these two groups were then randomly selected to form the final study sample. A third group of hospitalized patients was comprised of patients who did not have a bloodstream infection but met all other BSIR inclusion criteria from April 2018 to May 2018. These controls were approached and enrolled sequentially until a target of 10 patients, 5 from critical care units and 5 from non-critical care units, was met. An additional 10 samples from non-infected, non-hospitalized adult community controls were added by the sponsor as a quality control measure.
Clinical data abstraction
Clinical data were collected from the electronic medical record of each participant using a standardized case report form (CRF). Information including demographics, comorbidities, hospitalization, and clinical outcomes was obtained for all in-patient participants. Sepsis was defined as having a positive blood culture and having met at least two of the SIRS criteria: [1] temperature > 38 °C or < 36 °C, [2] heart rate > 90 beats per minute, [3] respiratory rate > 20 or partial pressure of carbon dioxide < 32, [4] white blood cell count > 12,000 cells/mm3 or < 4000 cells/mm3 or having > 10% immature (neutrophil bands) forms [10]. Septic shock was defined as sepsis with hypotension (systolic blood pressure ≤ 90 mmHg) and perfusion abnormalities as previously described [10]. Acute renal failure was defined as serum creatinine > 1.5 higher than baseline or increasing by > 0.3 mg/dL within 48 hours [11]. Persistent bacteremia was defined as the presence of repeat positive blood cultures following appropriate antimicrobial therapy after ≥ 5 days for SAB patients [12] and ≥ 3 days for patients with GNB [13]. A patient was classified as taking an immunosuppressive agent if taking a medication from any one or more of the following pharmacologic classes: calcineurin inhibitors, mTOR inhibitors, anti-proliferative agents, monoclonal antibodies, or corticosteroids. A desirable outcome was achieved if a patient did not experience septic shock or acute renal failure and was alive at 30 days after bloodstream infection.
Biological specimen
Blood samples from were collected for all participants at a single time point, within 3 days following the index culture. Cell-free plasma and serum from each sample, including controls, were obtained and stored at − 80 °C. Complement and cytokine levels were then measured as outlined below.
Complement level measurement
C1q, C2, C5, and C9 concentrations were measured by radial immunodiffusion with their respective polyclonal anti-complement molecules (Complement Laboratory, National Jewish Hospital). Plasma specimens were aliquoted into duplicate wells in agarose and allowed to diffuse into the gel to form immunoprecipitation rings around the application well. The gels were washed to remove unprecipitated proteins, dried and stained. The outside diameter of the ring precipitated antigen was measured and used to calculate the area of the outer ring. Then the diameter of the inner ring was measured and used to calculate the area of the inner ring. The total area of the precipitation was determined by subtracting the area of the inner ring from the area of the outer ring: (Aprecipitation) = (Aouter ring) − (Ainner ring).
C5a concentrations were determined using the C5a MicroVue ELISA Assay (C5a Quidel ELISA, San Diego). Methods were carried out according to manufacturer’s instructions. Briefly, plasma specimens were added to a 96-well plate and coated with a murine monoclonal antibody to C5a, incubated, and then washed. Horseradish peroxidase conjugated anti-C5a was added to each well to bind immobilized C5a captured in the first step, incubated, and then washed. Last, the chromogenic substance 3,3′,5,5′ tetramethylbenzidine (TMB) was added to the wells and incubated. Spectrophotometry at A450 was used to determine the color intensity, which was proportional to the concentration of C5a present in the specimens.
C4 and C3 concentrations were determined by using the Human C4 Kit and C3 kit, respectively, for use on the Binding Site SPAPLUS analyzer (Binding Site, San Diego), a turbidity analysis, according to the manufacturer’s instructions. Briefly, human C4 antiserum or C3 antiserum was added to the serum samples to form insoluble complexes. The turbidity of the specimens was measured using the SPAPLUS analyzer. Concentrations were calculated automatically by reference to a calibration curve stored within the instrument.
To determine the normal range of complement levels, EDTA plasma samples from self-reported healthy individuals were collected and measured in accordance with the laboratory standard operating procedures and National Jewish Health IRB policies. The measurements were made on seven different assays by two different technologists. The normal range was defined as the mean for the normal distribution of the donor samples plus or minus two standard deviations. The normal ranges were determined as follows; C1q (83–125 μg/mL), C2 (22.2–39.8 μg/mL), C4 (0.170–0.415 mg/mL), C3 (0.938–1.661 mg/mL), C5 (55–113 μg/mL), C5a (0.2–12.6 ng/mL), and C9 (33–95 μg/mL).
Cytokine measurement
Cytokines including IL-2, IL-4, IL-5, IL-6, IL-10, IL-12p70, IFNγ, and TNFα were determined using MILLIPLEX® MAP based on the Luminex® xMAP® technology according to the manufacturer’s instructions. Briefly, cytokine levels were determined using immunoassays on the surface of MagPlex® -C microspheres, fluorescent-coded magnetic beads, which were coated with specific capture antibodies. After analyte from each test sample was captured by the bead, the mixture was incubated with the reporter molecule Streptavidin-PE conjugate, and the fluorescent reporter signals were analyzed on the Luminex® analyzer (MAGPIX®) to determine the concentration of cytokine present in each sample. The normal ranges for cytokines were validated per the Clinical Laboratory Standards Institute (CLSI) guidelines [14] and were determined as follows: IL-2 (3.2–60.6 pg/mL), IL-4 (3.2–4.1 pg/mL), IL-5 (3.2–4.1 pg/mL), IL-6 (3.2–11.9 pg/mL), IL-10 (3.2–19.0 pg/mL), IL-12p70 (3.2–8.4 pg/mL), IFNγ (3.2–24.1 pg/mL), and TNFα (3.2–22.3 pg/mL). The lower limit of quantification (LLOQ) for all above cytokines was 3.2 pg/mL. Values below the LLOQ were reported as < 3.2 pg/mL.
Statistical analysis
The distributions of continuous measures are presented as medians and quartiles and categorical variables are evaluated using counts and percentages. Statistical comparisons between groups were made with the Kruskal–Wallis test for continuous variables and Fisher’s exact test for categorical variables. Statistical significance was set at p < 0.05.
Results
Study population
A total of 60 patients were included in the study: 20 hospitalized patients with SAB, 20 hospitalized patients with GNB, 10 hospitalized non-infected patients, and 10 non-infected, non-hospitalized community controls. Of the patients with SAB, 5 (25%) were due to methicillin resistant S. aureus (MRSA). Of the patients with GNB, 6 (30%) had Escherichia coli, 6 (30%) had Klebsiella pneumoniae, 4 (20%) had Serratia marcescens, 2 (10%) had Pseudomonas aeruginosa, 1 (5%) had Morganella morganii, and 1 (5%) had Enterobacter cloacae.
Patient demographics are reported in Table 1. For the 10 non-infected, non-hospitalized community controls, only demographic data were available. Median age of patients did not differ significantly between any of the four study groups (median age [IQR]: GNB 64.5 years [48–74.5], SAB 62 years, [57.5–73], hospitalized controls 56 years [48–64], and community controls 52 years [30–59]; p = 0.1272). Sex and race differed significantly between the four groups (p = 0.02866 and p < 0.001, respectively).
Table 1.
Demographics and clinical data
| Bacteremic population | Control population | ||||
|---|---|---|---|---|---|
| No. (%) of patients | |||||
| GNB (n = 20) | SAB (n = 20) | Hospitalized noninfected controls (n = 10) | Community controls (n = 10) | P value | |
| Age, median (IQR) | 64.5 (48–74.5) | 62 (57.5–73) | 56 (48–64) | 52 (30–59) | 0.1272 |
| Sex | |||||
| Female | 11 (55) | 3 (15) | 2 (20) | 2 (20) | 0.02866 |
| Race | < 0.001 | ||||
| Black | 7 (35) | 4(20) | 4 (40) | 3 (30) | |
| White | 13 (65) | 15 (75) | 6 (60) | 0 (0) | |
| Other | 0 (0) | 1 (5) | 0 (0) | 7 (70) | |
GNB Gram-negative bacteremia, SAB S. aureus bacteremia, IQR interquartile range
Clinical characteristics of the bacteremic patients and hospitalized controls are reported in Table 2. There was a greater incidence of neoplasm in the GNB group than the SAB group and hospitalized, noninfected control group (55% vs 15% vs 10%, p = 0.00791). Median creatinine was higher in the SAB group than the GNB group (median [IQR]: GNB 1.05 [0.8–2.0] vs SAB 2.2 [1.4–3.5], p = 0.010); however, incidence of acute renal failure was not significantly different (GNB 35% vs SAB 20%, p = 0.480). Nine (45%) of the patients with SAB and 1 (5%) patient with GNB had persistent bacteremia (p = 0.008). Thirty-day all-cause mortality, septic shock, and acute respiratory distress syndrome were not significantly different between patients with GNB vs SAB (p = 0.661, p = 1.000, and p = 1.000, respectively).
Table 2.
Clinical characteristics of the bacteremic patients and hospitalized controls
| Bacteremic population | Control population | ||||
|---|---|---|---|---|---|
| No. (%) of patients | |||||
| GNB (n = 20) | SAB (n = 20) | P value (GNB, SAB) | Hospitalized, noninfected controls (n = 10) | P value (GNB, SAB, controls) | |
| Comorbidity | |||||
| BMI, median (IQR) | 28.3 (24.9–34.0) | 30.1 (24.4–35.3) | 0.6263 | 36.1 (24.1–37.7) | 0.6256 |
| Diabetes | 2 (10) | 7 (35) | 0.127 | 5 (50) | 0.04333 |
| Hemodialysis-dependent prior to Infection | 0 (0) | 3 (15) | 0.231 | 0 (0) | 0.21429 |
| Neoplasm | 11 (55) | 3 (15) | 0.019 | 1 (10) | 0.00791 |
| Rheumatoid arthritis | 0 (0) | 1 (5) | 1.000 | 1 (10) | 0.67347 |
| Immunosuppressive therapy | 8 (40) | 8 (40) | 1.000 | 2 (20) | 0.865 |
| Transplant recipient | 2 (10) | 2 (10) | 1.000 | 0 (0) | 0.67000 |
| HIV+ | 1 (5) | 1 (5) | 1.000 | 0 (0) | 1.00000 |
| Median APACHE II Score (IQR) | 15.0 (11.5–17.0) | 15.5 (10–19.5) | 0.735 | 13.5 (6.0–24.0) | 0.5881 |
| Disease manifestations | |||||
| Fever | 14 (70) | 14 (70) | 1.000 | 0 (0) | 0.00021 |
| Malaise | 7 (35) | 13 (65) | 0.113 | 1 (10) | 0.01097 |
| Edema | 4 (20) | 8 (40) | 0.301 | 1 (10) | 0.19657 |
| WBC (IQR) | 13.9 (7.7–19.2) | 11.3 (7.1–20.0) | 0.695 | 6.0 (3.5–8.1) | 0.0452 |
| Lactate (IQR) | 2.0 (0.8–4.4) | 1.6 (0.8–1.8) | 0.169 | 1.7 (1.2–1.9) | 0.2948 |
| Creatinine (IQR) | 1.05 (0.8–2.0) | 2.2 (1.4–3.5) | 0.010 | 1.25 (0.9–2.7) | 0.0285 |
| Source of Infection (%) | 0.022 | ||||
| Endovascular | 4 (20) | 9 (45) | |||
| Skin/soft tissue | 3 (15) | 8 (40) | |||
| GU/GI | 8 (40) | 2 (10) | |||
| Other | 2 (10) | 0 (0) | |||
| Unknown primary | 3 (15) | 1 (5) | |||
| Outcomes | |||||
| Death (all cause) 30 days | 4 (20) | 2 (10) | 0.661 | ||
| Septic shock | 2 (10) | 1 (5) | 1.000 | ||
| Acute respiratory distress syndrome | 0 (0) | 1 (5) | 1.000 | ||
| Acute renal failure | 7 (35) | 4 (20) | 0.480 | ||
| Persistent bacteremia | 1 (5) | 9 (45) | 0.008 | ||
GNB Gram-negative bacteremia, SAB S. aureus bacteremia, IQR interquartile range, BMI body mass index, HIV+ human immunodeficiency virus positive, WBC white blood cell, GU genitourinary, GI gastrointestinal
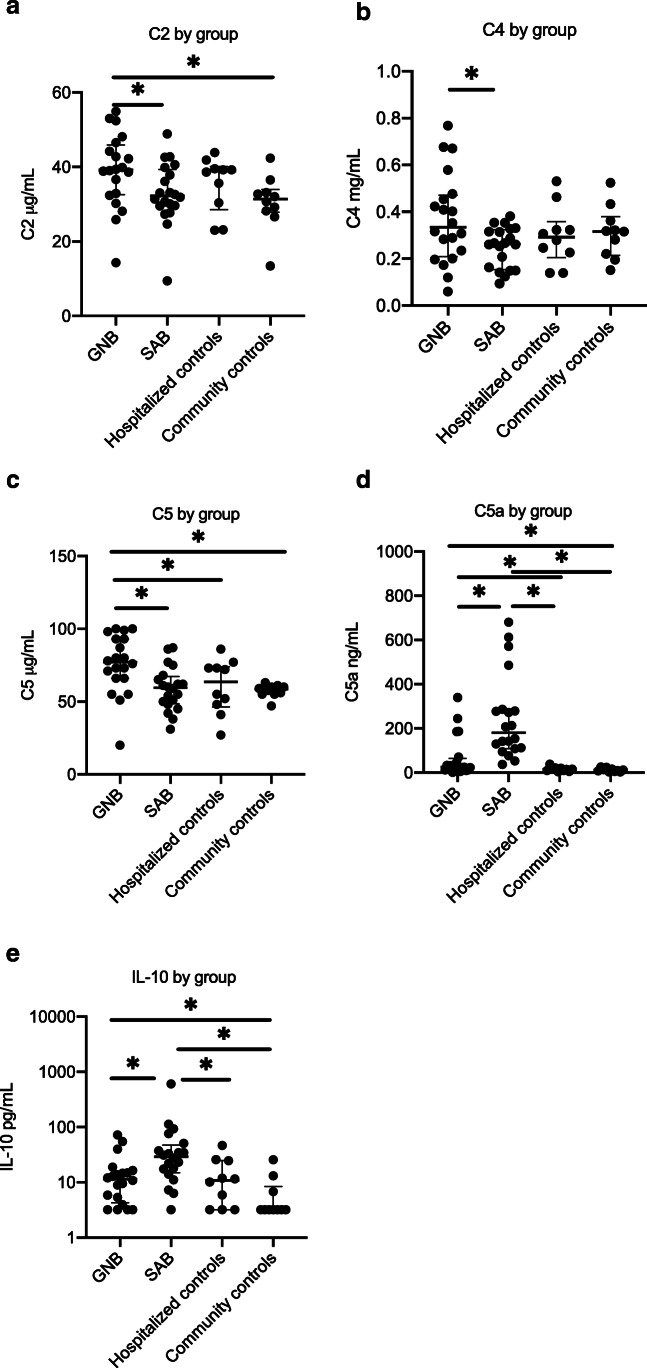
Complement and cytokine levels compared across groups
Complement and cytokine levels were compared across each group (Fig. 1A–E). The median level of C2 in the GNB group was 39.2 μg/mL and was significantly greater than the SAB and community control groups where the median levels were 32.25 μg/mL and 31.4 μg/mL, respectively (p = 0.0360 and p = 0.0175). Levels of C2 in the SAB group did not differ significantly from that of either control group (non-infected, hospitalized controls: p = 0.4284 and community controls: p = 0.4544).
Fig. 1.
A–E Complement and cytokine concentrations during bacteremia vary by pathogen. GNB Gram-negative bacteremia, SAB S. aureus bacteremia; the asterisk denotes a statistically significant difference. The horizontal lines compare medians across groups
The median level of C4 in the GNB group was 0.334 mg/mL and was significantly greater than in the SAB group where the median level was 0.263 mg/mL (p = 0.0398), but was not significantly different from median C4 levels in either the hospitalized control or community control groups (0.293 mg/mL, p = 0.3788 and 0.316 mg/mL, p = 0.5379, respectively).
The median level of C5 in GNB was 77.0 μg/mL and was greater than median C5 level in SAB, hospitalized controls and community controls where the median levels of C5 were 59.5 μg/mL (p = 0.0024), 63.5 μg/mL (p = 0.0426), and 58.5 μg/mL (p = 0.0042), respectively.
Median level of C9 in the GNB group was 124.5 μg/mL and was significantly greater than the median C9 levels in the hospitalized controls and community controls which were 76 μg/mL (p = 0.0477) and 62.5 μg/mL (p = 0.0007), respectively. Median level of C9 in the SAB group was 88.0 μg/mL and was also significantly greater in the SAB group as compared to the community controls (p = 0.0018).
The median C5a level in the SAB group was 180.3 ng/mL, and was significantly higher than the median C5a levels in the GNB, hospitalized control and community control groups which were 25.25 ng/mL (p < 0.0001), 15.1 ng/mL (p < 0.0001) and 11 ng/mL (p < 0.0001), respectively. The median C5a levels were also significantly higher in the GNB group as compared to the hospitalized controls and community controls (p = 0.0477 and p = 0.0209, respectively).
Median levels of C1q and C3 did not significantly differ between any of the four groups. Complement levels did not significantly differ by immunosuppressive therapy.
The median IL-10 level in the SAB population was 29.11 pg/mL, which was significantly higher than the median IL-10 level in the GNB group, hospitalized controls, and community controls, which were 11.45 pg/mL (p = 0.0045), 10.81 pg/mL (p = 0.0185), and 2.26 pg/mL (p = 0.0003), respectively. All other cytokine levels were similar among the bacteremic groups and are reported in the supplementary data. Median levels of IL-6, IL-8, IL-1RA, and TNF-α were significantly higher in the GNB group as compared to the community controls (IL-6 22.05 pg/mL vs 2.26 pg/mL, p < 0.0001; IL-8 13.42 pg/mL vs 9.39 pg/mL, p = 0.0094; TNF- α 38.57 pg/mL vs 23.72 pg/mL, p < 0.0001; IL-1RA 68.53 pg/mL vs 34.37 pg/mL, p = 0.0042) (Supplemental Figure). Median levels of IL-6, IL-8 and TNF- α in the SAB group were 39.04 pg/mL, 34.20 pg/mL, and 45.47 pg/mL, respectively, and were significantly higher than the median levels in the community control group (p = 0.004, p = 0.0013 and p = 0.0004, respectively).
Association of complement and cytokine levels with outcomes
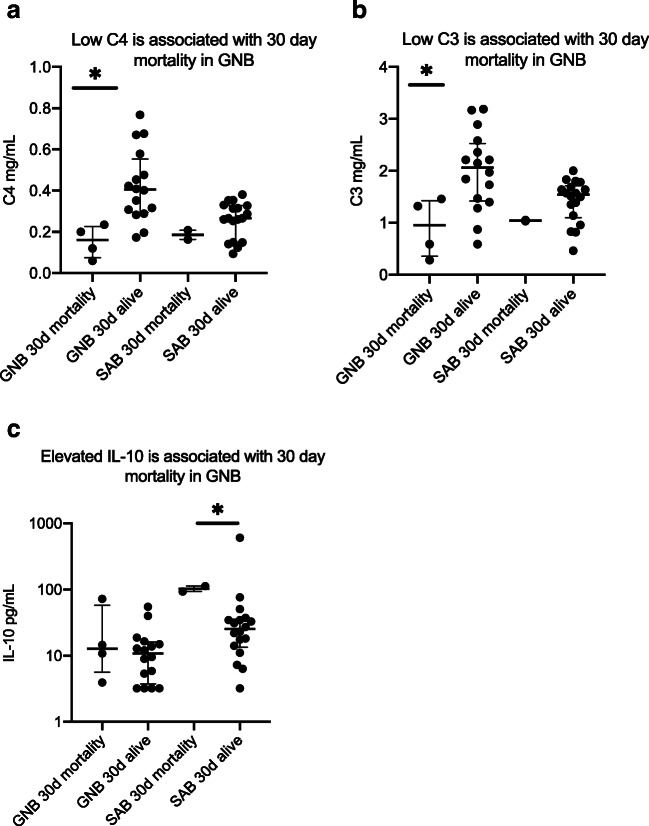
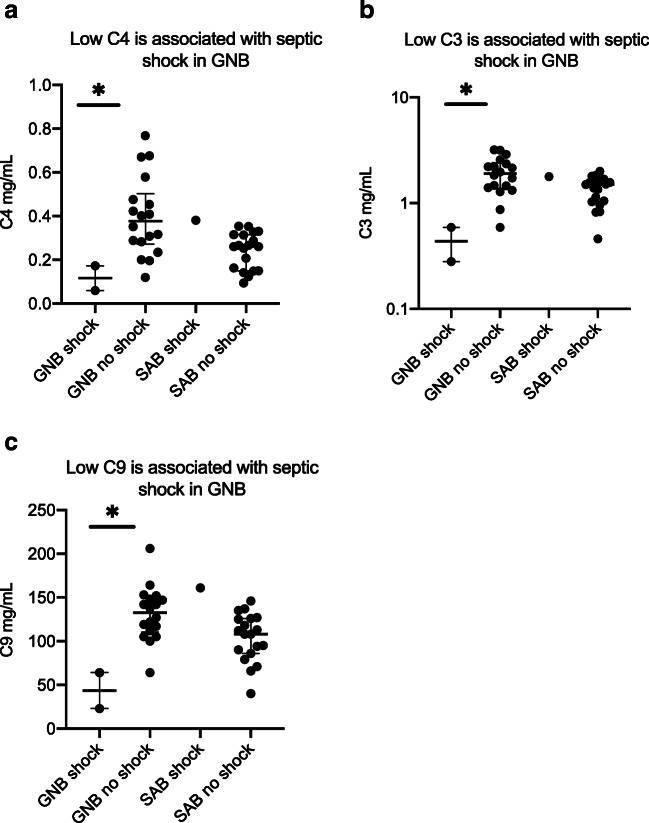
In patients with GNB, low levels of C4 (Figs. 2A and 3A) and C3 (Figs. 2B and 3B) were associated with 30 day mortality and septic shock while decreased C9 (Fig. 3C) was associated with septic shock alone. C3 levels were similar among SAB patients with or without acute renal failure, but C3 levels were significantly lower in patients with GNB with acute renal failure compared to those without renal failure. Patients with GNB who experienced desirable outcomes had elevated C3 levels. None of the complement molecules were associated with the development of sepsis, septic shock, or 30-day mortality in patients with SAB (Fig. 4).
Fig. 2.
A–C Biomarkers of mortality in bacteremic patients. GNB Gram-negative bacteremia, SAB S. aureus bacteremia; the asterisk denotes statistically significant difference. The horizontal lines compare medians across groups
Fig. 3.
A–C Biomarkers of septic shock in patients with bloodstream infections. GNB Gram-negative bacteremia, SAB S. aureus bacteremia; the asterisk denotes a statistically significant difference. The horizontal lines compare medians across groups
Fig. 4.
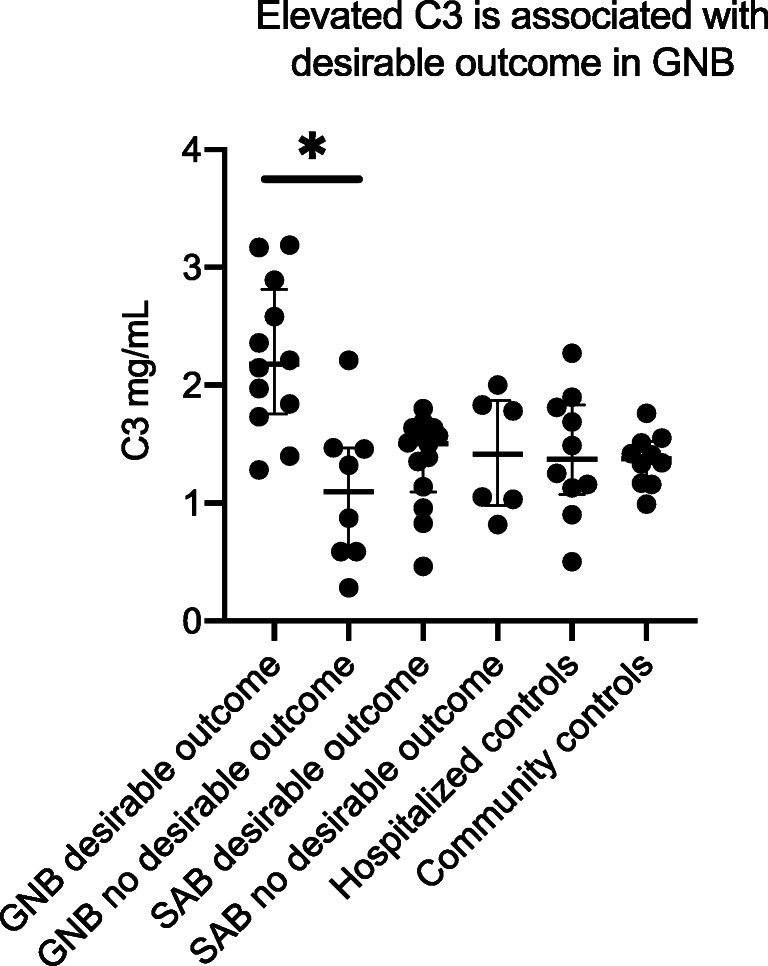
High C3 is associated with a desirable outcome in GNB. GNB Gram-negative bacteremia, SAB S. aureus bacteremai, ARF acute renal failure; the asterisk denotes statistically significant difference. The horizontal lines compare medians across groups. Desirable outcome is defined as the absence of acute renal failure, septic shock, or death within 30 days of first positive blood culture
Levels of IL-10 (Fig. 2C) were significantly higher in SAB patients who experienced mortality at 30-days as compared to those who survived. None of the complement or cytokine levels were associated with persistent bacteremia in the GNB or SAB groups.
Discussion
In this exploratory study our findings suggest that complement profiles among patients with SAB differ from patients with GNB. During a bacterial infection, the complement cascade may be activated through either the classical, alternative, or mannose-binding lectin (MBL) pathway. Upon initiation of the pathway through engaging C1, C3, or MBL, a serine proteolytic cascade leads to cleavage of complement proteins into smaller active compounds [4], ultimately converging on C3 activation and downstream target deposition of the membrane attack complex and release of anaphylatoxins C3a and C5a [15]. These anaphylatoxins have garnered the most attention in animal models and observational human studies of sepsis, with several studies reporting increased levels of C3a and C5a in sepsis and shock [16–19]. In the present study, C5a levels were significantly higher in the bacteremic groups as compared to the non-infected and community control groups, likely reflecting the greater degree of complement cascade activation in bacteremic patients, with ongoing cleavage of C5 in the terminal pathway to produce C5a and C5b [20]. Interestingly, C5a levels were significantly higher in the SAB cohort as compared to GNB, indicating that there are marked differences in the way S. aureus and Gram-negative pathogens stimulate the innate immune system. The high levels of C5a in the SAB group may reflect an underlying immune evasion mechanism that enables S. aureus to establish a chronic infection. A previously characterized protein called chemotaxis inhibitory protein of S. aureus (CHIPS) is a molecule unique to S. aureus that competes with C5a to bind to the C5a receptor (C5aR), preventing neutrophil and monocyte chemotaxis and T cell activation [21]. We postulate that the elevated C5a levels in our SAB cohort may reflect high levels of unbound C5a in the serum due to the competitive binding of CHIPS to the C5aR, and may provide an opportunity for further understanding of how S. aureus manipulates the innate immune system.
Notably, there was no association of elevated C5a with septic shock or increased 30-day mortality in any of the patients with either SAB or GNB. This was an unexpected finding as elevated C5a in experimental models of sepsis leads to increased mortality due to neutrophil dysfunction and damage to the vasculature [22]. It is possible that this association was not detected due to the limitations of sample size. Alternatively, perhaps CHIPS blocking C5aR may prevent the anaphylatoxic effects of C5a that can lead to shock during SAB. Some studies also suggest a protective role for C5a in animal models of SAB [23, 24]. Larger studies are needed to confirm these findings. Conversely, there is no evidence to support a protective role of C5a during GNB. In fact, the presence of C5a has been directly correlated with the development of shock, multi-organ failure, and death in murine models undergoing cecal ligation and puncture (CLP) [25]. It may be that our GNB cohort was too small to detect such a difference, or perhaps the effects of C5a are not quite as detrimental in humans as they are in mice. We conclude that the role of C5a in bacteremia is controversial, incompletely understood, and needs further investigation.
Low levels of C3 and C4 were significantly associated with septic shock and with 30-day mortality in patients with GNB in our sample. These findings suggest a protective role of C3 and C4. In murine models of cecal ligation and puncture (CLP), C3 and C4 deficient mice are more likely to experience septic shock, and the absence of C3 is associated with increased mortality in sepsis [6]. Low C3 has also been associated with mortality in infected humans in a handful of small observational studies [26–28]. To our knowledge, this is the first study to report an association between low C4 levels and mortality during gram-negative bloodstream infection, and further studies are needed to validate this finding. Importantly, C3 and C4 levels were not associated with any adverse outcomes in patients with SAB. Collectively, these observations provide insight into possible differences in immune dysregulation that occur during GNB versus SAB. We submit that low levels of these C3 and C4 are in large part due to LPS mediated overconsumption of these key molecules in GNB, as supported by murine models of C3- and C4-deficient mice challenged with endotoxin [29]. We conclude that depletion of C3 and C4 may lead to uncontrolled infection, septic shock and subsequently death.
Low levels of C9 were significantly associated with septic shock in our patients with GNB. To our knowledge this association has also not previously been established. C9 is a key component of the membrane attack complex that inserts on GNB outer membrane and leads to bacterial lysis. It is recognized that neonates are deficient in C9 and are highly susceptible to E. coli sepsis. Supplementation of C9 to neonatal serum enhances the capacity of neonatal serum to kill E. coli [30]. Hence, the association of low C9 with septic shock in GNB patients is plausible in this regard, with low levels likely reflecting over-consumption of this key complement protein.
Levels of C5 and C9 were significantly higher in GNB as compared to hospitalized, non-infected controls and community controls, and levels of C9 were significantly higher in SAB patients as compared to community controls. These were unexpected results as C5 and C9, members of the terminal complement pathway, were expected to be cleaved and consumed, respectively, during an active infection [31]. However, a cross-sectional study investigating complement levels in healthy adults recently reported that aging may be associated with enhanced functioning of the alternative and classical pathways, leading to increased levels of the terminal pathway components [32]. We hypothesize that the aforementioned elevated levels of C5 and C9 in the bacteremic groups may in part be a function of the older age in the GNB and SAB groups (i.e. > 60 years). Indeed, the median age > 60 years in our SAB and GNB groups should be recognized as a potential confounder and a possible explanation for the elevated C9 in both SAB and GNB, and C5 in GNB, as compared to community controls.
We found that C3 levels were significantly lower in patients who develop acute kidney injury (AKI) during GNB as compared to those who did not develop AKI. While the role of complement in glomerulonephropathies such as ANCA vasculitis, hemolytic uremic syndrome and anti-glomerular basement membrane syndrome is well characterized [33], the role of complement in sepsis-related AKI is poorly understood. In porcine models of sepsis, terminal complement complexes (C5b-C9) concentrate in the kidney [34]. Perhaps lower levels of C3 in patients with GNB are indicative of excessive terminal complement system activation with C5b-9 renal deposition and subsequent AKI. Alternatively, lower levels of C3 may instead reflect renal ischemia during GNB septic shock. Whether C3 is involved in the pathogenesis of AKI during GNB or whether it is simply a harbinger of end-organ damage during sepsis is yet to be determined.
As an important corollary, we found that elevated levels of C3 were associated with a desirable outcome in patients with GNB. Due to the small GNB sample size and the highly variable measurements of C3 within this sample, further studies are needed to validate this finding. However, based on our results, we submit that measurement of C3 in patients with GNB to assist in illness severity stratification may prove to be an informative subject for future studies, eventually providing a platform to begin investigating the role of recombinant C3 as an adjunctive therapy in patients with GNB septic shock.
Patients with SAB had greater levels of IL-10 than patients without SAB (i.e., controls, GNB). This may be because Staphylococcal cell wall peptidoglycan lipopeptides and glycopolymers employ toll-like receptor 2 signaling (TLR2) on antigen-presenting cells to generate increased IL-10 expression [35]. Notably, within the SAB group, elevated levels of IL-10 were associated with mortality. Our findings validate those by Rose et al. who found that an elevated level of IL-10 is independently associated with mortality in patients with SAB (OR, 1.05; p = 0.014) [36]. IL-10 is an anti-inflammatory cytokine which regulates the pro-inflammatory response during infection by downregulating the expression of proinflammatory cytokines and induces apoptosis of antigen presenting cells and inhibits CD4+ T cell activation. We hypothesize that elevated IL-10 in patients with SAB reflects an overly suppressed inflammatory state, or “immunoparalysis” as suggested by Rose et al. and Chau et al. [36, 37], whereby the host immune system cannot control the infection. This association was not found in the GNB population. We suspect this association of elevated IL-10 and mortality is unique to S. aureus and alludes to a unique mechanism employed by S. aureus to exploit the host immune system. Measuring IL-10 levels in patients with SAB may assist in identifying patients at increased risk for mortality from their bloodstream infection, and raises the question of whether an IL-10 monoclonal antibody can aide in the treatment of patients with SAB and high IL-10 levels. Lastly, patients with GNB and SAB had greater levels of IL-6, IL-8 and TNF-α than community controls. All three of these pro-inflammatory cytokines are critical components of acute phase response to infection [38], and have been reported to be elevated in bacteremic individuals [39–41].
As anticipated, complement levels in bacteremic patients did not differ by immunosuppressive therapy use. Medications targeting T-cells (i.e., calcineurin inhibitors, Belatacept), B-cells (i.e., Rituximab, Atacicept), and specific cytokine inhibitors (i.e., IL-2 receptor antagonist Basiliximab, IL-6 receptor antagonist Tocilizumab), do not appear to affect the complement cascade [42]. Similarly, despite their wide range of effects on the immune system, corticosteroids do not appear to affect the complement cascade [42]. Hence, use of immunosuppressive agents in 40% of the bacteremic population would be unlikely to affect the complement concentration.
The power of our study is limited owing to the small sample size, the heterogenous GNB group that includes six different pathogenic organisms, and the single time point of collection of biological specimens. While all biological specimens were collected within 72 hours of initial positive blood culture, it is possible that there is variability of complement and cytokine concentrations within this initial 72 hour time interval, and that timing of antimicrobial therapy may influence concentrations. Another limitation is that, given the substantial number of markers we examined, the use of p < 0.05 for statistical significance can be called into question. However, as this is an exploratory analysis, this limitation is of less concern than would be the case for an inferential study.
Our results indicate that the immunologic biosignature of SAB is fundamentally distinct from that of GNB. Additionally, our findings add to the limited data regarding complement levels and the corresponding immune dysregulation in sepsis. We hypothesize that measurement of C3, C4 and C9 levels at the time of admission in patients with GNB may help to risk stratify patients who are at increased risk for septic shock, and measurement of C3 and C4 specifically may help to stratify those GNB patients at increased risk for mortality. Similarly, we suspect that measurement of IL-10 levels in SAB may stratify those at increased risk for mortality. Finally, we postulate that our observations that normal to elevated C3 levels in patients correlate with a desirable outcome could provide the rational for an interventional trial of C3 supplementation in C3 deplete patients with GNB.
Electronic supplementary material
(DOCX 674 kb).
Acknowledgements
The authors would like to thank Alida Coppi for help with the cytokine data and reading the manuscript.
Funding information
This work was funded by a grant from Regeneron to Dr. Vance G. Fowler Jr. Dr. Fowler was supported by NIH grant K24-AI093969. Dr. Eichenberger was supported by NIH grant T32-AI100851.
Compliance with ethical standards
Conflicts of interest
Dr. Vance Fowler reports personal fees from Novartis, Novadigm, Durata, Debiopharm, Genentech, Achaogen, Affinium, Medicines Co., Cerexa, Tetraphase, Trius, MedImmune, Bayer, Theravance, Basilea, Affinergy, Janssen, xBiotech, Contrafect, Regeneron, Basilea, Destiny, and Amphliphi Biosciences. Integrated Biotherapeutics; C3J, grants from NIH, MedImmune, Cerexa/Forest/Actavis/Allergan, Pfizer, Advanced Liquid Logics, Theravance, Novartis, and Cubist/Merck; Medical Biosurfaces; Locus; Affinergy; Contrafect; Karius; Genentech, Regeneron, Basilea, and Janssen, from Green Cross, Cubist, Cerexa, Durata, Theravance; Debiopharm, Royalties from UpToDate; and a patent sepsis diagnostics pending.
Ethical approval
The study was approved by the Duke Institutional Review Board.
Informed consent
Written informed consent was obtained from all participants or their legally authorized representatives prior to their enrollment in this study
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pliakos EE, Andreatos N, Shehadeh F, Ziakas PD, Mylonakis E (2018) The cost-effectiveness of rapid diagnostic testing for the diagnosis of bloodstream infections with or without antimicrobial stewardship. Clin Microbiol Rev 31(3):e00095–17 [DOI] [PMC free article] [PubMed]
- 2.Goto M, Al-Hasan MN. Overall burden of bloodstream infection and nosocomial bloodstream infection in North America and Europe. Clin Microbiol Infect. 2013;19:501–509. doi: 10.1111/1469-0691.12195. [DOI] [PubMed] [Google Scholar]
- 3.van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev. 2012;25:362–386. doi: 10.1128/CMR.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ammerlaan H, Seifert H, Harbarth S, Brun-Buisson C, Torres A, Antonelli M, Kluytmans J, Bonten M. Adequacy of antimicrobial treatment and outcome of Staphylococcus aureus bacteremia in 9 Western European countries. Clin Infect Dis. 2009;49:997–1005. doi: 10.1086/605555. [DOI] [PubMed] [Google Scholar]
- 5.Ackerman AL, Parameshwar PS, Anger JT. Diagnosis and treatment of patients with prostatic abscess in the post-antibiotic era. Int J Urol. 2018;25:103–110. doi: 10.1111/iju.13451. [DOI] [PubMed] [Google Scholar]
- 6.Flierl MA, Rittirsch D, Nadeau BA, Day DE, Zetoune FS, Sarma JV, Huber-Lang MS, Ward PA. Functions of the complement components C3 and C5 during sepsis. FASEB J. 2008;22:3483–3490. doi: 10.1096/fj.08-110595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward PA, Gao H. Sepsis, complement and the dysregulated inflammatory response. J Cell Mol Med. 2009;13:4154–4160. doi: 10.1111/j.1582-4934.2009.00893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charchaflieh J, Wei J, Labaze G, Hou YJ, Babarsh B, Stutz H, Lee H, Worah S, Zhang M. The role of complement system in septic shock. Clin Dev Immunol. 2012;2012:407324. doi: 10.1155/2012/407324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chousterman BG, Swirski FK, Weber GF. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 10.Anonymous American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. doi: 10.1097/00003246-199206000-00025. [DOI] [PubMed] [Google Scholar]
- 11.Kellum JA, Lameire N. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Souli M, Ruffin F, Choi SH, Park LP, Gao S, Lent NC, Sharma-Kuinkel BK, Thaden JT, Maskarinec SA, Wanda L, Hill-Rorie J, Warren B, Hansen B, Fowler VG (2019) Changing characteristics of Staphylococcus aureus bacteremia: results from a 21-year, prospective, Longitudinal Study. Clin Infect Dis. 10.1093/cid/ciz112 [DOI] [PMC free article] [PubMed]
- 13.Harris JA, Cobbs CG. Persistent gram-negative bacteremia. Observations in twenty patients. Am J Surg. 1973;125:705–717. doi: 10.1016/0002-9610(73)90169-4. [DOI] [PubMed] [Google Scholar]
- 14.CLSI . Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline—third edition. Wayne: Clinical Lab Standards Institute; 2010. [Google Scholar]
- 15.Varela JC, Tomlinson S. Complement: an overview for the clinician. Hematol Oncol Clin North Am. 2015;29:409–427. doi: 10.1016/j.hoc.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu R, Lin F, Bao C, Wang FS. Mechanism of C5a-induced immunologic derangement in sepsis. Cell Mol Immunol. 2017;14:792–793. doi: 10.1038/cmi.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber-Lang M, Sarma VJ, Lu KT, McGuire SR, Padgaonkar VA, Guo RF, Younkin EM, Kunkel RG, Ding J, Erickson R, Curnutte JT, Ward PA. Role of C5a in multiorgan failure during sepsis. J Immunol. 2001;166:1193–1199. doi: 10.4049/jimmunol.166.2.1193. [DOI] [PubMed] [Google Scholar]
- 18.Hack CE, Nuijens JH, Felt-Bersma RJ, Schreuder WO, Eerenberg-Belmer AJ, Paardekooper J, Bronsveld W, Thijs LG. Elevated plasma levels of the anaphylatoxins C3a and C4a are associated with a fatal outcome in sepsis. Am J Med. 1989;86:20–26. doi: 10.1016/0002-9343(89)90224-6. [DOI] [PubMed] [Google Scholar]
- 19.Groeneveld AB, Tacx AN, Bossink AW, van Mierlo GJ, Hack CE. Circulating inflammatory mediators predict shock and mortality in febrile patients with microbial infection. Clin Immunol. 2003;106:106–115. doi: 10.1016/S1521-6616(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 20.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 21.Rooijakkers SH, Ruyken M, van Roon J, van Kessel KP, van Strijp JA, van Wamel WJ. Early expression of SCIN and CHIPS drives instant immune evasion by Staphylococcus aureus. Cell Microbiol. 2006;8:1282–1293. doi: 10.1111/j.1462-5822.2006.00709.x. [DOI] [PubMed] [Google Scholar]
- 22.Ward PA. The dark side of C5a in sepsis. Nat Rev Immunol. 2004;4:133–142. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 23.von Kockritz-Blickwede M, Konrad S, Foster S, Gessner JE, Medina E. Protective role of complement C5a in an experimental model of Staphylococcus aureus bacteremia. J Innate Immun. 2010;2:87–92. doi: 10.1159/000247157. [DOI] [PubMed] [Google Scholar]
- 24.Georgoutsou-Spyridonos M, Ricklin D, Pratsinis H, Perivolioti E, Pirmettis I, Garcia BL, Geisbrecht BV, Foukas PG, Lambris JD, Mastellos DC, Sfyroera G. Attenuation of Staphylococcus aureus-induced bacteremia by human mini-antibodies targeting the complement inhibitory protein Efb. J Immunol. 2015;195:3946–3958. doi: 10.4049/jimmunol.1500966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czermak BJ, Sarma V, Pierson CL, Warner RL, Huber-Lang M, Bless NM, Schmal H, Friedl HP, Ward PA. Protective effects of C5a blockade in sepsis. Nat Med. 1999;5:788–792. doi: 10.1038/10512. [DOI] [PubMed] [Google Scholar]
- 26.Ren J, Zhao Y, Yuan Y, Han G, Li W, Huang Q, Tong Z, Li J. Complement depletion deteriorates clinical outcomes of severe abdominal sepsis: a conspirator of infection and coagulopathy in crime? PLoS One. 2012;7:e47095. doi: 10.1371/journal.pone.0047095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Younger JG, Bracho DO, Chung-Esaki HM, Lee M, Rana GK, Sen A, Jones AE. Complement activation in emergency department patients with severe sepsis. Acad Emerg Med. 2010;17:353–359. doi: 10.1111/j.1553-2712.2010.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modi S, Rashid M, Malik A, Shahid M. Study of complement activation, C3 and interleukin-6 levels in burn patients and their role as prognostic markers. Indian J Med Microbiol. 2014;32:137–142. doi: 10.4103/0255-0857.129793. [DOI] [PubMed] [Google Scholar]
- 29.Fischer MB, Prodeus AP, Nicholson-Weller A, Ma M, Murrow J, Reid RR, Warren HB, Lage AL, Moore FD, Jr, Rosen FS, Carroll MC. Increased susceptibility to endotoxin shock in complement C3- and C4-deficient mice is corrected by C1 inhibitor replacement. J Immunol. 1997;159:976–982. [PubMed] [Google Scholar]
- 30.Lassiter HA, Wilson JL, Feldhoff RC, Hoffpauir JM, Klueber KM. Supplemental complement component C9 enhances the capacity of neonatal serum to kill multiple isolates of pathogenic Escherichia coli. Pediatr Res. 1994;35:389–396. doi: 10.1203/00006450-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Coonrod JD, Rylko-Bauer B. Complement levels in pneumococcal pneumonia. Infect Immun. 1977;18:14–22. doi: 10.1128/IAI.18.1.14-22.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gaya da Costa M, Poppelaars F, van Kooten C, Mollnes TE, Tedesco F, Würzner R, Trouw LA, Truedsson L, Daha MR, Roos A, Seelen MA. Age and sex-associated changes of complement activity and complement levels in a healthy Caucasian population. Front Immunol. 2018;9:2664. doi: 10.3389/fimmu.2018.02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCullough JW, Renner B, Thurman JM. The role of the complement system in acute kidney injury. Semin Nephrol. 2013;33:543–556. doi: 10.1016/j.semnephrol.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuerholz T, Leuwer M, Cobas-Meyer M, Vangerow B, Kube F, Kirschfink M, Marx G. Terminal complement complex in septic shock with capillary leakage: marker of complement activation? Eur J Anaesthesiol. 2005;22:541–547. doi: 10.1017/S0265021505000931. [DOI] [PubMed] [Google Scholar]
- 35.Frodermann V, Chau TA, Sayedyahossein S, Toth JM, Heinrichs DE, Madrenas J. A modulatory interleukin-10 response to staphylococcal peptidoglycan prevents Th1/Th17 adaptive immunity to Staphylococcus aureus. J Infect Dis. 2011;204:253–262. doi: 10.1093/infdis/jir276. [DOI] [PubMed] [Google Scholar]
- 36.Rose WE, Eickhoff JC, Shukla SK, Pantrangi M, Rooijakkers S, Cosgrove SE, Nizet V, Sakoulas G. Elevated serum interleukin-10 at time of hospital admission is predictive of mortality in patients with Staphylococcus aureus bacteremia. J Infect Dis. 2012;206:1604–1611. doi: 10.1093/infdis/jis552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chau TA, McCully ML, Brintnell W, An G, Kasper KJ, Vines ED, Kubes P, Haeryfar SM, McCormick JK, Cairns E, Heinrichs DE, Madrenas J. Toll-like receptor 2 ligands on the staphylococcal cell wall downregulate superantigen-induced T cell activation and prevent toxic shock syndrome. Nat Med. 2009;15:641–648. doi: 10.1038/nm.1965. [DOI] [PubMed] [Google Scholar]
- 38.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 39.Daef EA, Elsherbiny NM, Agban MN, Riad KF, Mohammed LF. Bloodstream infections in febrile neutropenic pediatric Cancer patients: microbiological and sepsis biomarkers insight. Egypt J Immunol. 2018;25:21–34. [PubMed] [Google Scholar]
- 40.Miedema KG, Vermont CL, Ball LM, de Bont ES, Kamps WA, van Tol MJ, Jol-van der Zijde CM, Tissing WJ. The diagnostic value of interleukin-8 for the detection of bacteremia in pediatric hematopoietic stem cell recipients with febrile neutropenia. Transplantation. 2014;98:e80–e81. doi: 10.1097/TP.0000000000000434. [DOI] [PubMed] [Google Scholar]
- 41.Spooner CE, Markowitz NP, Saravolatz LD. The role of tumor necrosis factor in sepsis. Clin Immunol Immunopathol. 1992;62:S11–S17. doi: 10.1016/0090-1229(92)90036-N. [DOI] [PubMed] [Google Scholar]
- 42.Wiseman AC. Immunosuppressive medications. Clin J Am Soc Nephrol. 2016;11:332–343. doi: 10.2215/CJN.08570814. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 674 kb).