Abstract
The ketone functional group has a unique reactivity in organic chemistry and is associated with a number of useful reactions. Catalytic methods for ketone synthesis are continually being developed. Here, we report a photoredox, nickel and phosphoranyl radical synergistic cross-electrophile coupling of commercially available chemicals, aromatic acids and aryl/alkyl bromides. This allows for concise synthesis of highly functionalized ketones directly, without the preparation of activated carbonyl intermediates or organometallic compounds, and thus complements the conventional Weinreb ketone synthesis. Use of the appropriate photocatalyst, ligand amount and solvents can match the reaction rate required by any simple catalytic cycle. The practicality and synthetic robustness of the reaction are illustrated by the facile synthesis of complex ketones from readily available feedstock chemicals.
Subject terms: Catalytic mechanisms, Homogeneous catalysis, Synthetic chemistry methodology
Due to their abundance and importance in organic chemistry, development of methods for ketone synthesis is essential. Here, the authors report a photoredox, nickel and phosphoranyl radical synergistic cross-electrophile coupling of aromatic acids and aryl/alkyl bromides to directly synthesise ketones.
Introduction
Ketones play a prominent role in organic chemistry. The ketone moiety is extremely common in natural products and pharmaceuticals1 and in dyes, fragrancies and flavors2. It is also a versatile reaction center in organic synthesis3. Many frequently used reactions, including the Mannich reaction, Wittig reaction, Grignard reaction, Passerini reaction, Baeyer–Villiger oxidation, and Wolff–Kishner–Huang reduction describe a wide array of transformations of ketones. The development of a practical route to ketones from feedstock chemicals has long been a subject of interest4–12. Carboxylic acids and organohalides are commercially abundant and structurally diverse, bench-stable feedstock chemicals commonly used in organic synthesis (Fig. 1a). When producing ketones from carboxylic acids and organohalides, the stoichiometric approach requires preparation of necessary intermediates such as amides or aldehydes and Grignard reagents13,14. If aldehydes are employed, reoxidation is necessary15. Catalytic strategies for production of ketones rely on transition metal-catalyzed carbon–carbon coupling between activated carbonyls such as acid chlorides or anhydrides with organometallic reagents (Fig. 1b)16–19. However, activated carbonyls are generally prepared in as many as three steps from carboxylic acids and organometallics are obtained typically by metalation of organohalides20, which can often lead to poor functional group compatibility or a lengthy functional group protection/deprotection process.
Fig. 1. Catalytic cross-electrophile coupling between carboxylic acids and organohalides.
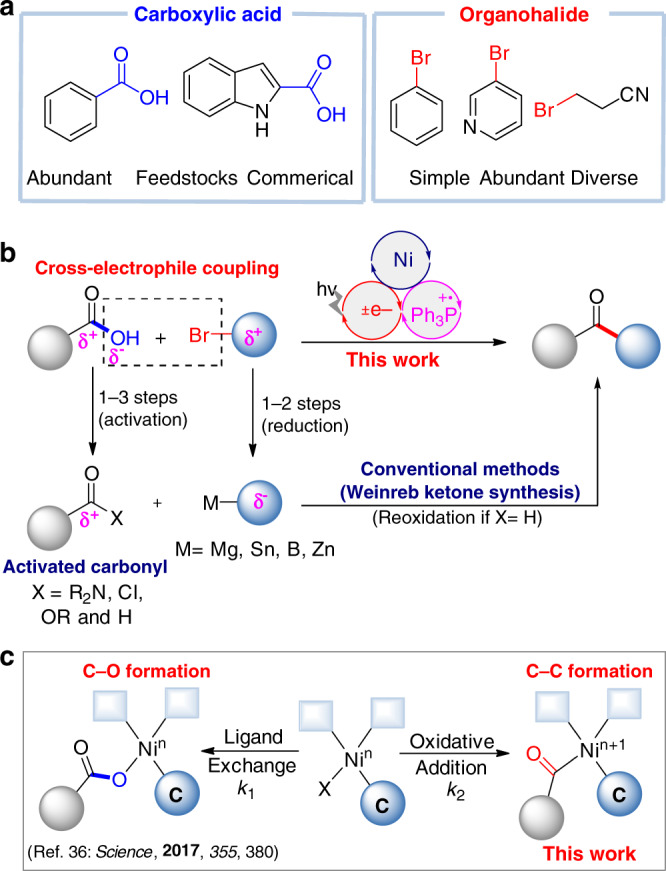
a The abundant feedstock chemicals in synthetic lab. b Direct cross-electrophile coupling of acid and organohalides. c Key challenge: C–O versus C–C formation.
In recent years, nickel-catalyzed cross-electrophile coupling has attracted considerable attention21–30. The carbon–carbon coupling arises from two different electrophiles in the presence of stoichiometric reductant. We posited that a carboxylic acid could be directly used as a latent electrophile (C-terminus) rather than a nucleophile (O-terminus) in cross-coupling. This could simplify and upgrade ketone synthesis from carboxylic acids and organic halides13. Very recently, our group and Doyle et al. reported an elegant photoredox-promoted mild deoxygenation of carboxylic acids generating acyl radicals31–35. Since photoredox and nickel-catalyzed C–O bond formation between carboxylic acids and aromatic bromides has been reported36 to achieve the desired cross-electrophile coupling, acyl radical oxidative addition by a metallaphotoredox pathway37–41 is essential to suppress the C–O bond formation (Fig. 1c). Alternatively, a judicious strategy reported by Gong et al. is conversion of carboxylic acid into anhydrides in situ to suppress the C–O coupling22,23. However, the use of free carboxylic acids as acyl radical precursors is a challenge in the oxidative addition step as a result of the strong bond dissociation energy of the C–O bond (106 kcal mol−1)33.
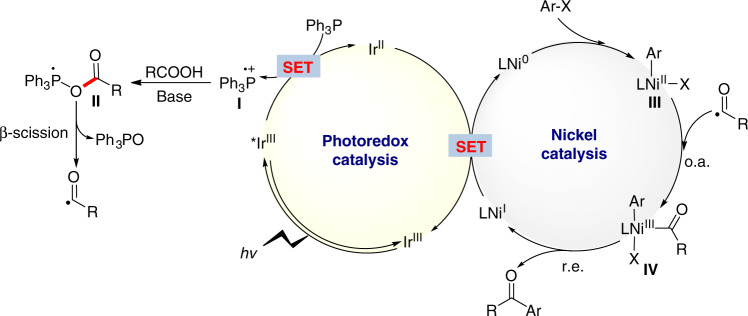
A proposed mechanism for the designed metallaphotoredox cross-electrophile coupling is shown in Fig. 2. The photoexcited *[Ir(dF(CF3)ppy)2(dtbbpy)]PF6 [1/2Ered (*IrIII/IrII) = +1.21 V vs SCE, τ = 2.3 μs]38 causes single-electron transfer (SET) oxidation of triphenylphosphine (Ered = +0.98V vs SCE)31, as indicated by our Stern–Volmer experimental results (see Supplementary Fig. 7). The triphenylphosphine radical cation (I) generated recombines with the carboxylate anion to form a phosphoranyl radical intermediate (II). Owing to the strong affinity between the phosphoranyl radical and oxygen, a facile β-scission of the radical species (II) occurs, giving rise to a nucleophilic acyl radical42, which can undergo oxidative addition to the resulting aryl-NiII species (III) giving the NiIII species (IV)43–45. Finally, reductive elimination from the NiIII intermediate (IV) can generate the desired cross-electrophile coupling product, R-CO-Ar. A second SET event from IrII ([IrIII/IrII] = −1.37 V vs SCE), leading to an NiI species completes both catalytic cycles. The synergistic combination of photoredox with nickel catalysis proposed in Fig. 2 is a subject of continuing research46–58, and several challenges remain in this cross-electrophile coupling and must be addressed: (1) Efficient control of the matching of the C–O bond cleavage and the radical addition to the nickel center; (2) weakening of the interference of stoichiometric triphenylphosphine in the nickel catalytic unit; and (3) use of appropriate ligands and solvents to significantly suppress the C–O bond formation. Herein, we report a formal cross-electrophile coupling of carboxylic acids with aryl or alkyl halides enabled by photoredox and nickel catalysis, and phosphoranyl radical synergistic chemistry, leading to concise synthesis of ketones (Fig. 1b).
Fig. 2. Proposed mechanism.
Mechanistic proposal for cross-electrophile coupling of acid and aryl bromides. o.a. oxidative addition, r.e. reductive elimination.
Results
Reaction optimization
Our investigation of this cross-electrophile coupling began with the reaction of 4-methyl-benzoic acid (1) with 5-bromo-2-(trifluoromethyl) pyridine (2), and the representative results are presented in Table 1. The optimized reaction conditions include 2 mol% [Ir{dF(CF3)ppy}2{dtbbpy}]PF6, 3 mol% NiBr2.dme together with 5 mol% 4,4′-di-tert-butyl-2,2′-bipyridine (L1, Fig. 3) and 1.5 equiv Ph3P with a mixed DMF-CH3CN solvent (entry 1, Table 1). Under the standard conditions, the desired cross-electrophile coupling product (3) can be obtained in 82% yield while the yield of the C–O coupling process, giving 3′ is suppressed to 16%. We found the loading amount of ligand plays an important role in the control of the C–C and C–O bond formation (entries 2–4, Table 1). An increased or decreased loading amount of 4,4′-di-tert-butyl-2,2’-bipyridine can facilitate the formation of the C–O coupling by-product (3′). Screening of different ligands and solvents indicated that both a ligand effect and a solvent effect are crucial for a successful cross-electrophile coupling (entries 5–11, Table 1). We speculated that only compatible consecutive steps with well-matched rates would benefit this cross-electrophile coupling. In the absence of either photocatalyst, NiBr2·dme, triphenylphosphine or light irradiation, the model reaction failed to occur. The quantum yield of the model reaction was determined to be 0.35, arguing against a radical chain pathway.
Table 1.
Optimization of the reaction conditions.
 | ||
|---|---|---|
| Entry | Variation of standard conditions | Isolated yield: 3 (3′) |
| 1 | None | 82% (16%) |
| 2 | 10 mol% L1 | 46% (37%) |
| 3 | 15 mol% L1 | 23% (53%) |
| 4 | 3 mol% of L1 | 19% (10%) |
| 5 | 5 mol% L2 | 31% (33%) |
| 6 | 5 mol% L3 | 42% (28%) |
| 7 | 5 mol% L4 | 35% (40%) |
| 8 | 5 mol% L5 | nd |
| 9 | 5 mol% L6 | nd |
| 10 | DCM instead of DMF/MeCN | nd |
| 11 | DMA instead of DMF/MeCN | 25% (17%) |
| 12 | no PC or NiBr2 or Ph3P or light | nd |
Standard conditions: photocatalyst (2 mol%), NiBr2·dme (3 mol%), L1 (5 mol%), 1a (0.2 mmol), 2a (0.4 mmol), Ph3P (0.3 mmol), K3PO4 (0.2 mmol), Cs2CO3 (0.2 mmol), DMF-CH3CN (2.0 mL, v/v = 1:1), blue LEDs, ambient temperature, 20 h.
DMF N,N-dimethylformamide; DMA N,N-dimethylacetamide, DCM dichloromethane, DME 1,2-dimethoxyethane, nd not detected.
Fig. 3. Photocatalysts and ligands.
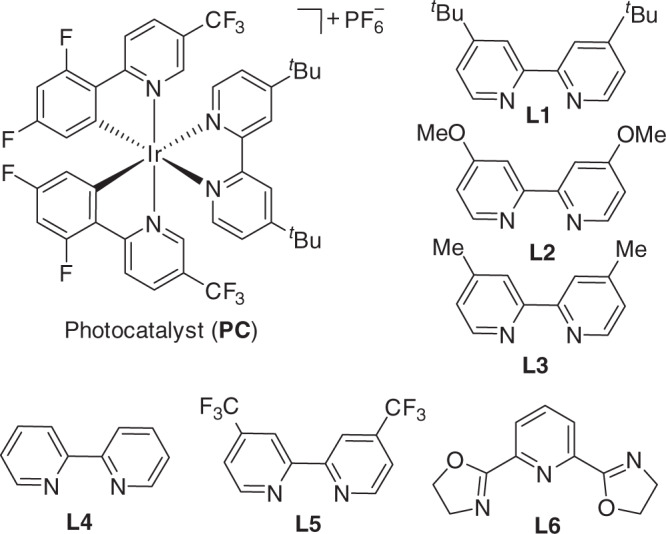
Catalysts and ligands for cross-electrophile coupling of acid and aryl bromides.
Substrate scope
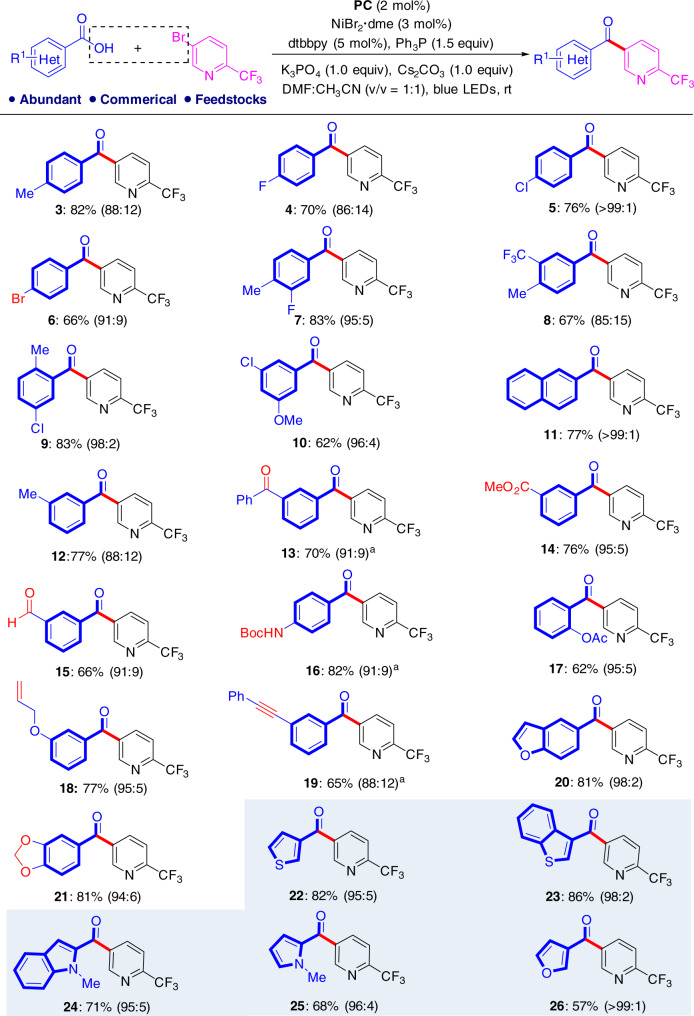
With the optimized conditions in hand, we investigated the scope of the cross-electrophile coupling reaction with regard to aromatic carboxylic acids, and obtained the results in Fig. 4. In general, this protocol is highly efficient and has a broad substrate scope. The electron-rich and electron-poor functional groups on the ortho-, meta-, and para-positions on the phenyl groups of the aromatic acids have little influence on the coupling process and the desired ketones (3–21) are formed in 62–83% yield. A series of useful functional groups, such as bromine (6), reactive carbonyl groups (13–16), a terminal alkene (18), an internal alkyne (19) and an acetal (21) tolerate the reaction conditions well. Some of these functional groups have difficulty surviving the conventional Weinreb ketone synthesis method toward Grignard reagents. Hetero-aromatic carboxylic acids are satisfactory starting materials and can uniformly produce the synthetically valuable diheteroaromatic ketones (22–26) with moderate to good yields. However, examination of the reaction of aliphatic carboxylic acids under these standard conditions showed that only a trace amount of the desired product can be produced while both decarboxylative C–C coupling and direct C–O coupling can occur.
Fig. 4. Carboxylic acid scope at a 0.2 mmol scale under standard conditions.
The isolated yield of ketone is given for product and the GC ratio of ketone and ester is given in parenthesis. aThe ratio of ketone and ester is calculated based on isolation.
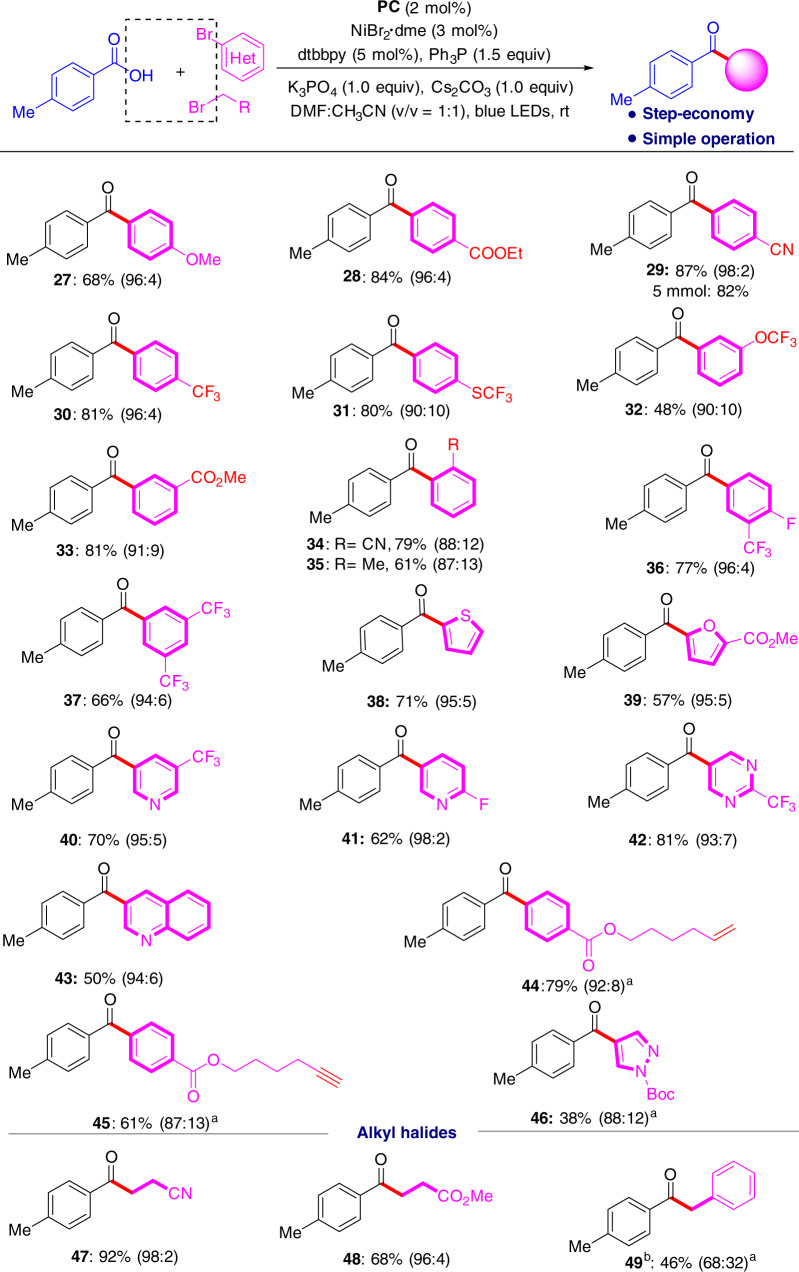
Subsequently, we studied the substrate scope of aromatic bromides (Fig. 5) and found that many commercially available aromatic bromides can be used to deliver the desired ketones (27–46) in good yields. The excellent functional group tolerance of –COOR (28, 33, 44, 45), –CN (29, 34), terminal unsaturated chemical bonds (43, 44), and heteroarenes (38–43, 46) support the practicality of the reaction. With this strategy, it is also very easy to construct fluorine- and fluoroalkyl-containing diaryl ketones (30–32, 36, 37, 39–42) with acceptable yields. Several alkyl halides also serve as coupling partners in this cross-electrophile coupling reaction, leading to functionalized ketones (47–49) in good yields (up to 92%). When benzyl chloride was employed, 46% yield of ketone (49) was obtained and a significant amount of by-product ester was formed possibly because of the nucleophilic substitution side reaction. This coupling reaction can allow for the construction of highly functionalized ketones in an operationally simple, step-economical and gram-scale reaction (29, 5 mmol scale).
Fig. 5. Organohalide scope on a 0.2 mmol scale under standard conditions.
The isolated yield of ketone is given and the GC ratio of ketone and ester is given in parenthesis. aThe ratio of ketone and ester is calculated based on isolation. bBenzyl chloride was used.
Synthetic application
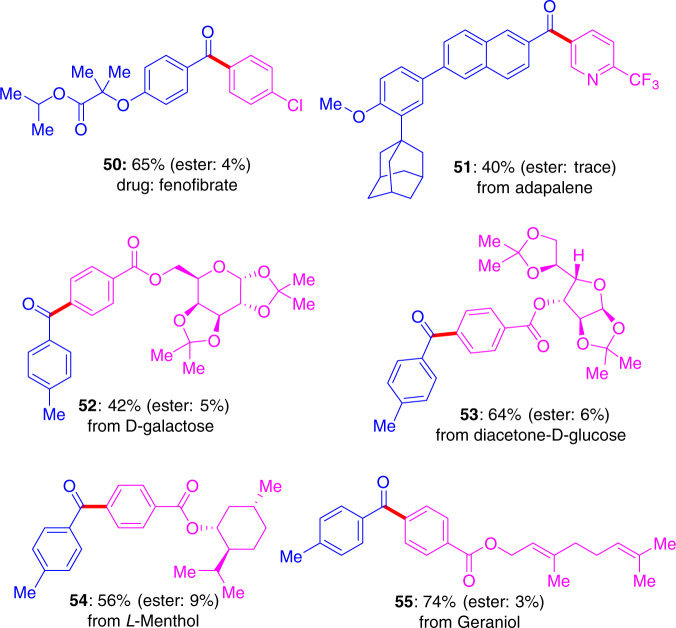
To further demonstrate the synthetic robustness of the reaction, we applied the strategy for the construction of a series of complex ketones from carboxylic acids and aromatic bromides (Fig. 6). Fenofibrate (50) is a drug used to adjust lipid levels and blood viscosity and it could be prepared in one step in 65% yield. The complex ketones (50–55) can be obtained in synthetically useful yields. The precise cross-electrophile coupling also allows for introduction of functional groups at an early synthetic stage to limit the number of synthetic steps thus improving the efficiency.
Fig. 6. Concise synthesis of complex ketones.
Carboxylic acids are blue and aromatic bromides are pink. The isolated yield of by-product ester is given in parenthesis.
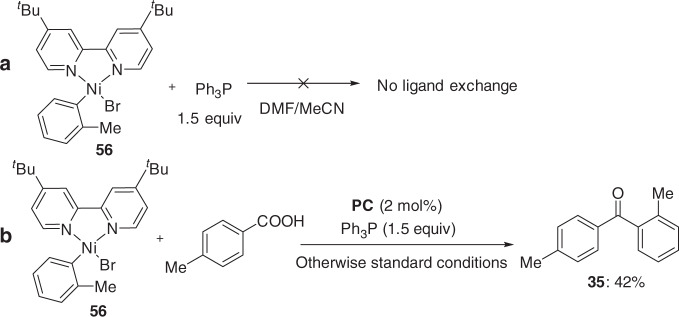
Mechanism of stoichiometric reactions
We performed the stoichiometric reactions of Ar–Ni(II) intermediate (56) with 1.5 equiv. Ph3P in DMF/MeCN. Interestingly, no ligand exchange was observed by 31P NMR analysis (Fig. 7, also see Supplementary Fig. 10). Treatment of Ar–Ni(II) intermediate (56) under the photoredox conditions, led to the desired deoxygenative C–C coupling product (35), which was obtained in 42% yield, further supporting the proposed mechanism (Fig. 7).
Fig. 7. Mechanistic studies.
a The reaction of Ph3P with Ar–Ni(II) intermediates. b Stoichiometric reactions of Ar–Ni(II) intermediates.
Discussion
We have developed a cross-electrophile coupling between aromatic carboxylic acids and organic bromides, inexpensive and abundant feedstock chemicals, enabled by photoredox and a nickel and phosphoranyl radical synergistic combination, affording a wide array of structurally diverse ketones with excellent functional group compatibility. This strategy for ketone synthesis can significantly improve the synthetic efficiency and step-economy, and it also opens a door to construct highly functionalized or complex ketones which are still difficult to prepare by a conventional Weinreb ketone synthesis. We found that the use of appropriate ligand loading amount (5 mol% of 4,4′-di-tert-butyl-2,2′-bipyridine), mixed solvents (DMF/CH3CN) and combined inorganic bases (K3PO4 and Cs2CO3) is crucial to achieve the desired C–C bond formation reactions, affording the desired ketone products. The employment of more or less of ligand results in a sharply decreased yield of the ketone product and an increased yield of the ester by-product. Use of combined bases and mixed solvents would improve the deprotonation of carboxylic acids to expedite the acyl radical generation and use of the precise amount of a ligand would promote the acyl radical oxidative addition to the arylnickel (II) species. We speculated that a facile C–O bond cleavage and subsequent rapid acyl radical oxidative addition rate can control the selective C–C bond formation. We believe this cross-electrophile coupling strategy of carboxylic acids and organic halides will upgrade the synthesis of ketones with great potential application in organic synthesis, drug discovery and optochemical biology given the importance and ubiquity of ketones.
Methods
General procedure for cross-electrophile coupling of carboxylic acids and organohalides
Preparation of Ni-based catalyst solution: In the nitrogen-filled glove box, a stirring bar, NiBr2·dme (1.9 mg, 3.0 mol%), 4,4′-di-tert-butyl-2,2′-bipyridine (2.7 mg, 5.0 mol%) and CH3CN/DMF (2.0 mL, V/V = 1:1) were successively added to an oven-dried vial (8 mL screw-cap threaded). The vial was then sealed with a Teflon-lined plastic screw-cap and stirred until the resulting mixture become homogeneous (about 20 min).
Photocatalyst Ir[dF(CF3)ppy]2(dtbbpy)PF6 (4.5 mg, 2 mol%), aromatic carboxylic acid (0.2 mmol, 1.0 equiv), aryl bromide (0.4 mmol, 2.0 equiv), Ph3P (78.6 mg, 0.3 mmol, 1.5 equiv), anhydrous powder K3PO4 (42.4 mg, 0.2 mmol, 1.0 equiv), and anhydrous powder Cs2CO3 (65.0 mg, 0.2 mmol, 1.0 equiv) were added to an oven-dried 10 mL Schlenk tube equipped with a magnetic stirring bar. The tube was evacuated and backfilled with argon three times. Subsequently, the nickel-catalyst solution was transferred into this Schlenk tube under argon. The tube was then sealed and placed ~5 cm from 2 × 45 W blue LEDs. The reaction mixture was stirred for 20–36 h at room temperature (air-condition was used to keep the temperature is 25 °C or so). After completion, the reaction mixture was removed from the light, diluted with water and the aqueous layer was extracted with EtOAc (3 × 2.0 mL). The combined organic layers were washed with brine, dried over anhydrous Na2SO4, filtered, and concentrated. The residue was purified by flash chromatography on silica gel to afford the corresponding ketone products.
Supplementary information
Acknowledgements
We thank the National Natural Science Foundation of China (21971108, 21971111, 21702098, 21732003, and 21672099), the Natural Science Foundation of Jiangsu Province (Grant No. BK20190006), Fundamental Research Funds for the Central Universities (020514380214), “Innovation & Entrepreneurship Talents Plan” of Jiangsu Province, “1000‐Youth Talents Plan”, “Jiangsu Six Peak Talent Project”, and start-up funds from Nanjing University for financial support. Y.L., K.L., C.Z., and W.X. are warmly acknowledged to reproduce experimental procedures for products (3, 23, 24 and 34).
Author contributions
J.X. conceived and supervised the whole project and wrote the paper with input from all authors. R.R., C.Z., and J.X. designed and discussed the experiments; R.R. and K.L. performed and analyzed the experiments.
Data availability
The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon reasonable request.
Competing interests
J.X., R.R., and K.L. declare the following competing interests that one Chinese patent has been registered (201910828905.0). All other authors declare no competing interest.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Rehanguli Ruzi, Kai Liu.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-17224-2.
References
- 1.McDaniel R, et al. Multiple genetic modifications of the erythromycin polyketide synthase to produce a library of novel “unnatural” natural products. Proc. Natl Acad. Sci. USA. 1999;96:1846–1851. doi: 10.1073/pnas.96.5.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tan Y, Siebert KJ. Quantitative structure-activity relationship modeling of alcohol, ester, aldehyde, and ketone flavor thresholds in beer from molecular features. J. Agric. Food Chem. 2004;52:3057–3064. doi: 10.1021/jf035149j. [DOI] [PubMed] [Google Scholar]
- 3.Otera, J. (ed.) Modern Carbonyl Chemistry (Wiley-VCH, 2000).
- 4.Brunet JJ, Chauvin R. Synthesis of diarylketones through carbonylative coupling. Chem. Soc. Rev. 1995;24:89–95. [Google Scholar]
- 5.Li Y, Hu Y, Wu XF. Non-noble metal-catalysed carbonylative transformations. Chem. Soc. Rev. 2018;47:172–194. doi: 10.1039/c7cs00529f. [DOI] [PubMed] [Google Scholar]
- 6.Ruan J, Saidi O, Iggo JA, Xiao J. Direct acylation of aryl bromides with aldehydes by palladium catalysis. J. Am. Chem. Soc. 2008;130:10510–10511. doi: 10.1021/ja804351z. [DOI] [PubMed] [Google Scholar]
- 7.Jiang X, Zhang M-M, Xiong W, Lu L-Q, Xiao W-J. Deaminative (carbonylative) alkyl-Heck-type reactions enabled by photocatalytic C-N bond activation. Angew. Chem. Int. Ed. 2019;58:2402–2406. doi: 10.1002/anie.201813689. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Cary BP, Beyer PD, Gellman SH, Weix DJ. Ketones from nickel-catalyzed decarboxylative, non-symmetric cross-electrophile coupling of carboxylic acid esters. Angew. Chem. Int. Ed. 2019;58:12081–12085. doi: 10.1002/anie.201906000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergonzini G, Cassani C, Wallentin C-J. Acyl radicals from aromatic carboxylic acids by means of visible-light photoredox catalysis. Angew. Chem. Int. Ed. 2015;54:14066–14069. doi: 10.1002/anie.201506432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong Z, Wang J, Ren Z, Dong G. Ortho C-H acylation of aryl iodides by palladium/norbornene catalysis. Angew. Chem. Int. Ed. 2015;54:12664–12668. doi: 10.1002/anie.201506397. [DOI] [PubMed] [Google Scholar]
- 11.Fu MC, Shang R, Zhao B, Wang B, Fu Y. Photocatalytic decarboxylative alkylations mediated by triphenylphosphine and sodium iodide. Science. 2019;363:1429–1434. doi: 10.1126/science.aav3200. [DOI] [PubMed] [Google Scholar]
- 12.Shi R, Hu X. From alkyl halides to ketones: nickel-catalyzed reductive carbonylation utilizing ethyl chloroformate as the carbonyl source. Angew. Chem. Int. Ed. 2019;58:7454–7458. doi: 10.1002/anie.201903330. [DOI] [PubMed] [Google Scholar]
- 13.Nahm S, Weinreb SM. N-methoxy-N-methylamides as effective acylating agents. Tetrahedron Lett. 1981;22:3815–3818. [Google Scholar]
- 14.Bechara WS, Pelletier G, Charette AB. Chemoselective synthesis of ketones and ketimines by addition of organometallic reagents to secondary amides. Nat. Chem. 2012;4:228–234. doi: 10.1038/nchem.1268. [DOI] [PubMed] [Google Scholar]
- 15.Steves JE, Stahl SS. Copper(I)/ABNO-catalyzed aerobic alcohol oxidation: alleviating steric and electronic constraints of Cu/TEMPO catalyst systems. J. Am. Chem. Soc. 2013;135:15742–15745. doi: 10.1021/ja409241h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu XF, Neumann H, Beller M. Palladium-catalyzed carbonylative coupling reactions between Ar-X and carbon nucleophiles. Chem. Soc. Rev. 2011;40:4986–5009. doi: 10.1039/c1cs15109f. [DOI] [PubMed] [Google Scholar]
- 17.Guo L, Rueping M. Decarbonylative cross-couplings: nickel catalyzed functional group interconversion strategies for the construction of complex organic molecules. Acc. Chem. Res. 2018;51:1185–1195. doi: 10.1021/acs.accounts.8b00023. [DOI] [PubMed] [Google Scholar]
- 18.Wang D, Zhang Z. Palladium-catalyzed cross-coupling reactions of carboxylic anhydrides with organozinc reagents. Org. Lett. 2003;5:4645–4648. doi: 10.1021/ol035801w. [DOI] [PubMed] [Google Scholar]
- 19.Ben Halima T, et al. Palladium-catalyzed Suzuki-Miyaura coupling of aryl esters. J. Am. Chem. Soc. 2017;139:1311–1318. doi: 10.1021/jacs.6b12329. [DOI] [PubMed] [Google Scholar]
- 20.Hartwig, J. F. In Organotransition Metal Chemistry: from Bonding to Catalysis (University Science Books, Sausalito, 2010).
- 21.Biswas S, Weix DJ. Mechanism and selectivity in nickel-catalyzed cross-electrophile coupling of aryl halides with alkyl halides. J. Am. Chem. Soc. 2013;135:16192–16197. doi: 10.1021/ja407589e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao C, Jia X, Wang X, Gong H. Ni-catalyzed reductive coupling of alkyl acids with unactivated tertiary alkyl and glycosyl halides. J. Am. Chem. Soc. 2014;136:17645–17651. doi: 10.1021/ja510653n. [DOI] [PubMed] [Google Scholar]
- 23.Yin H, Zhao C, You H, Lin K, Gong H. Mild ketone formation via Ni-catalyzed reductive coupling of unactivated alkyl halides with acid anhydrides. Chem. Commun. 2012;48:7034–7036. doi: 10.1039/c2cc33232a. [DOI] [PubMed] [Google Scholar]
- 24.Ackerman LK, Lovell MM, Weix DJ. Multimetallic catalysed cross-coupling of aryl bromides with aryl triflates. Nature. 2015;524:454–457. doi: 10.1038/nature14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Wang S, Xue W, Gong H. Nickel-catalyzed reductive coupling of aryl bromides with tertiary alkyl halides. J. Am. Chem. Soc. 2015;137:11562–11565. doi: 10.1021/jacs.5b06255. [DOI] [PubMed] [Google Scholar]
- 26.Jin Y, Wang C. Nickel‐catalyzed asymmetric reductive arylalkylation of unactivated alkenes. Angew. Chem. Int. Ed. 2019;131:6794–6798. doi: 10.1002/anie.201901067. [DOI] [PubMed] [Google Scholar]
- 27.Ye Y, Chen H, Sessler JL, Gong H. Zn-mediated fragmentation of tertiary alkyl oxalates enabling formation of alkylated and arylated quaternary carbon centers. J. Am. Chem. Soc. 2019;141:820–824. doi: 10.1021/jacs.8b12801. [DOI] [PubMed] [Google Scholar]
- 28.Ni S, et al. Ni-catalyzed deaminative cross-electrophile coupling of Katritzky salts with halides via C horizontal line N bond activation. Sci. Adv. 2019;5:eaaw9516. doi: 10.1126/sciadv.aaw9516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poremba KE, Kadunce NT, Suzuki N, Cherney AH, Reisman SE. Nickel-catalyzed asymmetric reductive cross-coupling to access 1,1-diarylalkanes. J. Am. Chem. Soc. 2017;139:5684–5687. doi: 10.1021/jacs.7b01705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He R-D, et al. Reductive coupling between C–N and C–O electrophiles. J. Am. Chem. Soc. 2019;141:12481–12486. doi: 10.1021/jacs.9b05224. [DOI] [PubMed] [Google Scholar]
- 31.Zhang M, Xie J, Zhu C. A general deoxygenation approach for synthesis of ketones from aromatic carboxylic acids and alkenes. Nat. Commun. 2018;9:3517. doi: 10.1038/s41467-018-06019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M, Yuan X-A, Zhu C, Xie J. Deoxygenative deuteration of carboxylic acids with D2O. Angew. Chem. Int. Ed. 2019;58:312–316. doi: 10.1002/anie.201811522. [DOI] [PubMed] [Google Scholar]
- 33.Ruzi R, et al. Deoxygenative arylation of carboxylic acids by aryl migration. Chem. Eur. J. 2019;25:12724–12729. doi: 10.1002/chem.201903816. [DOI] [PubMed] [Google Scholar]
- 34.Stache EE, Ertel AB, Tomislav R, Doyle AG. Generation of phosphoranyl radicals via photoredox catalysis enables voltage-independent activation of strong C-O bonds. Acs. Catal. 2018;8:11134–11139. doi: 10.1021/acscatal.8b03592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao R, Bera S, Cheseaux A, Hu X. Deoxygenative trifluoromethylthiolation of carboxylic acids. Chem. Sci. 2019;10:9555–9559. doi: 10.1039/c9sc03396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welin ER, Le C, Arias-Rotondo DM, McCusker JK, MacMillan DW. Photosensitized, energy transfer-mediated organometallic catalysis through electronically excited nickel(II) Science. 2017;355:380–385. doi: 10.1126/science.aal2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Twilton J, et al. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 2017;1:0052. [Google Scholar]
- 38.Prier CK, Rankic DA, MacMillan DW. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milligan JA, Phelan JP, Badir SO, Molander GA. Alkyl carbon-carbon bond formation by nickel/photoredox cross-coupling. Angew. Chem. Int. Ed. 2019;58:6152–6163. doi: 10.1002/anie.201809431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skubi KL, Blum TR, Yoon TP. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 2016;116:10035–10074. doi: 10.1021/acs.chemrev.6b00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang CS, Dixneuf PH, Soule JF. Photoredox catalysis for building C-C bonds from C(sp2)-H bonds. Chem. Rev. 2018;118:7532–7585. doi: 10.1021/acs.chemrev.8b00077. [DOI] [PubMed] [Google Scholar]
- 42.Schiavon G, Zecchin S, Cogoni G, Bontempelli G. Anodic oxidation of triphenylphosphine at a platinum electrode in acetonitrile medium. J. Electroanal. Chem. Interfac. 1973;48:425–431. [Google Scholar]
- 43.Zhang X, MacMillan DWC. Direct aldehyde C-H arylation and alkylation via the combination of nickel, hydrogen atom transfer, and photoredox catalysis. J. Am. Chem. Soc. 2017;139:11353–11356. doi: 10.1021/jacs.7b07078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schirmer TE, Wimmer A, Weinzierl FWC, König B. Photo–nickel dual catalytic benzoylation of aryl bromides. Chem. Commun. 2019;55:10796–10799. doi: 10.1039/c9cc04726c. [DOI] [PubMed] [Google Scholar]
- 45.Chu L, Lipshultz JM, MacMillan DW. Merging Photoredox and nickel catalysis: the direct synthesis of ketones by the decarboxylative arylation of alpha-oxo acids. Angew. Chem. Int. Ed. 2015;54:7929–7933. doi: 10.1002/anie.201501908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith RT, et al. Metallaphotoredox-catalyzed cross-electrophile Csp(3)-Csp(3) coupling of aliphatic bromides. J. Am. Chem. Soc. 2018;140:17433–17438. doi: 10.1021/jacs.8b12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konev MO, McTeague TA, Johannes JW. Nickel-catalyzed photoredox-mediated cross-coupling of aryl electrophiles and aryl azides. ACS Catal. 2018;8:9120–9124. [Google Scholar]
- 48.Ackerman LKG, Martinez Alvarado JI, Doyle AG. Direct C-C bond formation from alkanes using Ni-photoredox catalysis. J. Am. Chem. Soc. 2018;140:14059–14063. doi: 10.1021/jacs.8b09191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu C, et al. A multicomponent synthesis of stereodefined olefins via nickel catalysis and single electron/triplet energy transfer. Nat. Catal. 2019;2:678–687. [Google Scholar]
- 50.Yan D-M, Xiao C, Chen J-R. Strong C(sp3)-H arylation by synergistic decatungstate photo-HAT and nickel. Catal. Chem. 2018;4:2496–2498. [Google Scholar]
- 51.Meng Q-Y, Wang S, Huff GS, König B. Ligand-controlled regioselective hydrocarboxylation of styrenes with CO2 by combining visible light and nickel catalysis. J. Am. Chem. Soc. 2018;140:3198–3201. doi: 10.1021/jacs.7b13448. [DOI] [PubMed] [Google Scholar]
- 52.Johnston CP, Smith RT, Allmendinger S, MacMillan DW. Metallaphotoredox-catalysed sp(3)-sp(3) cross-coupling of carboxylic acids with alkyl halides. Nature. 2016;536:322–325. doi: 10.1038/nature19056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, Luo Y, Cheo HW, Lan Y, Wu J. Photoredox-catalysis-modulated, nickel-catalyzed divergent difunctionalization of ethylene. Chemistry. 2019;5:192–203. [Google Scholar]
- 54.Le CC, MacMillan DW. Fragment couplings via CO2 extrusion-recombination: expansion of a classic bond-forming strategy via metallaphotoredox. J. Am. Chem. Soc. 2015;137:11938–11941. doi: 10.1021/jacs.5b08304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joe CL, Doyle AG. Direct acylation of Csp(3)-H bonds enabled by nickel and photoredox catalysis. Angew. Chem. Int. Ed. 2016;55:4040–4043. doi: 10.1002/anie.201511438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leeuwen T, Buzzetti L, Perego LA, Melchiorre P. A redox-active nickel complex that acts as an electron mediator in photochemical Giese reactions. Angew. Chem. Int. Ed. 2019;58:4953–4957. doi: 10.1002/anie.201814497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang L, Hashmi ASK. The combination of benzaldehyde and nickel-catalyzed photoredox alkylation/arylation. Angew. Chem. Int. Ed. 2019;58:1823–1827. doi: 10.1002/anie.201810526. [DOI] [PubMed] [Google Scholar]
- 58.Si X, Zhang L, Hashmi ASK. Benzaldehyde- and nickel-catalyzed photoredox C(sp3)-H alkylation/arylation with amides and thioethers. Org. Lett. 2019;21:6329–6332. doi: 10.1021/acs.orglett.9b02226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all other data supporting the findings of this study are available within the article and Supplementary Information files, and also are available from the corresponding author upon reasonable request.