Abstract
Purpose
To determine the effectiveness of dysphagia interventions compared to standard care in improving oral intake and reducing aspiration for adults in acute and critical care.
Methods
We searched electronic literature for randomised and quasi-randomised trials and bibliography lists of included studies to March 2020. Study screening, data extraction, risk of bias and quality assessments were conducted independently by two reviewers. Meta-analysis used fixed effects modelling. The systematic review protocol is registered and published.
Results
We identified 22 studies (19 stroke, 2 intensive care stroke and 1 general intensive care) testing 9 interventions and representing 1700 patients. Swallowing treatment showed no evidence of a difference in the time to return to oral intake (n = 33, MD (days) − 4.5, 95% CI − 10.6 to 1.6, 1 study, P = 0.15) (very low certainty) or in aspiration following treatment (n = 113, RR 0.79, 95% CI 0.44 to 1.45, 4 studies, I2 = 0%, P = 0.45) (low certainty). Swallowing treatment showed evidence of a reduced risk of pneumonia (n = 719, RR 0.71, 95% CI 0.56 to 0.89, 8 studies, I2 = 15%, P = 0.004) (low certainty) but no evidence of a difference in swallowing quality of life scores (n = 239, MD − 11.38, 95% CI − 23.83 to 1.08, I 2 = 78%, P = 0.07) (very low certainty).
Conclusion
There is limited evidence for the effectiveness of swallowing treatments in the acute and critical care setting. Clinical trials consistently measuring patient-centred outcomes are needed.
Electronic supplementary material
The online version of this article (10.1007/s00134-020-06126-y) contains supplementary material, which is available to authorized users.
Keywords: Dysphagia, Deglutition disorders, Intensive care, Critical care, Swallowing therapy, Dysphagia rehabilitation
Take-home message
| There is limited research on dysphagia interventions in acute and critical care settings and limited evidence to guide clinical practice in this area. Clinical trials consistently measuring patient-centred outcomes are needed. |
Introduction
Dysphagia in patients who are acutely and critically ill is often multi-factorial. Following acute stroke, dysphagia is in part caused by a loss of functional connectivity within the neural swallowing network. However, neuroplasticity results in the undamaged hemisphere compensating for lost functions from lesions in the affected hemisphere, with more than half of patients recovering swallow function in the first 3 weeks post-stroke [1]. In critical care, the pathogenesis of dysphagia may involve direct laryngeal trauma caused by endotracheal/tracheostomy tubes resulting in impairments in laryngeal closure, neuromyopathy resulting in weakness of oral, pharyngeal and laryngeal muscles and diminished laryngeal sensation secondary to prolonged endotracheal intubation [2, 3]. Up to 67% of patients intubated for prolonged periods can be affected [4, 5]. Studies using videofluoroscopy or endoscopy over clinical assessment report higher dysphagia incidence. This is because impaired physiology, resulting in symptoms such as silent aspiration (no cough response when food/fluids enter airway) and poor orol-pharyngeal secretion management can be visualised [6].
Consequences of dysphagia include delayed return to oral intake [7, 8], pneumonia, poor quality of life, longer intensive care and hospital stays [8–12] and is an independent predictor for 90-day mortality [13]. It remains an under-recognised but highly relevant clinical challenge with symptoms found to persist beyond hospital discharge for > 6 months in 23% of patients in a multicentre 5-year longitudinal study [14–17]. Sensory stimulation and muscle strengthening treatments may improve swallow function for such populations. The objective of this review was to determine the effectiveness of dysphagia interventions compared to standard care in improving oral intake and reducing aspiration for adults in acute and critical care settings.
Methods
We registered the protocol with the International Prospective Register of Systematic Reviews (PROSPERO CRD 42018116849) and published the review protocol in 2019 [18]. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines [19].
Study selection
We included randomised controlled trials (RCT) or quasi-RCT testing dysphagia interventions in adult patients in acute care or critical care settings. Cluster RCTs were excluded as we were not considering the group effect of a dysphagia intervention. We will consider their inclusion in future updates. Adult participants, 18 years or older of any sex, ethnicity and stage of illness were included. Acute care was defined as any acute hospital ward or unit (i.e. medical, respiratory, surgical, neurological or stroke units). Critical care was defined as intensive care units, with no limitation regarding intubation/ventilation times or the presence of tracheostomy in study participants. Studies conducted in rehabilitation, long-term care or outpatient settings were excluded.
We considered any dysphagia intervention delivered alone or in combination with traditional swallowing rehabilitation versus traditional swallowing rehabilitation, usual care or placebo (i.e. studies using a sham intervention). The primary outcomes were time taken to return to oral intake and aspiration incidence post-treatment (defined as score > 5 on the Penetration Aspiration Score [20]. Secondary outcomes included: incidence of pneumonia; quality of life (measured by Swallowing Quality of Life Scale [21]); length of hospital stay; change in secretion severity; change in pharyngeal residue severity; nutritional status; and intervention-related adverse events. Where studies report outcomes at different time-points, we will accept and report both.
Search strategy and data extraction
We searched Medline, CENTRAL, CINAHL, EMBASE, Web of Science, Clinicaltrials.gov, and the WHO International Clinical Trials Registry Platform from inception to March 2020 and included all languages. (Electronic Supplemental Material (ESM) Appendix A). Citations were imported to an online platform (www.covidence.org), and two reviewers (SD, JMcG) independently screened titles and abstracts for full-text review. Bibliography lists of included studies were searched in March 2020. We extracted information regarding setting, participant characteristics, intervention types and outcomes (ESM Appendix B). Intervention details were extracted using the Template for Intervention Description and Replication (TIDieR) checklist [22] (ESM Appendix C) and outcomes information gathered as per SPIRIT 2013 (i.e. specific measurement, analysis metric, method of aggregation and timepoint) [23] (ESM Appendix D). Two reviewers (SD, JMcG) independently extracted outcome data and assessed risk of bias using the Cochrane Collaboration Risk of Bias tool [24]. Disagreements were settled by consensus. Overall risk of bias for each study was then assigned low (all domains low); unclear (one or more domains unclear); high (one or more domains high) as per PROSPERO. We assigned an ‘unclear’ rating when the study did not report a specific domain in the published paper or protocol. We did not contact study authors for verbal clarification.
Data analysis and grading the evidence
We used RevMan software (Review Manager, version 5.3) for data analysis [25]. The following measures of treatment effect were used: risk ratio (RR) and 95% confidence intervals (CI) for the analysis of dichotomous outcomes, mean difference and standard mean difference and 95% CI for continuous outcomes. Studies reporting median/interquartile ranges were not converted to mean/standard deviation values for meta-analysis, as it was assumed the underlying data were skewed and such conversions are ill-advised by Cochrane [24]. Where different scales were used to measure an outcome, we ensured directionality of scales was uniform in meta-analysis. The number of participants analysed rather than number recruited per study was used.
Meta-analyses were performed if outcomes from two or more studies with similar interventions were available, and we used fixed effects models to calculate pooled estimates. By choosing a fixed effects model, we assumed that the true effect of dysphagia interventions (in size and direction) would be the same in every study and that observed differences among studies would be due to chance. Statistical heterogeneity was evaluated using the Chi-square test and the I2 statistic (I2 > 50%, substantial heterogeneity). If substantial heterogeneity existed, we repeated the meta-analysis using a random-effects model [24]. Subgroup analyses were planned for the following groups: acute versus critical care; younger age groups (< 65 years) versus older age groups (i.e. > 65 years) and types of interventions.
Applying Cochrane guidance, in three arm trials evaluating similar interventions, we statistically pooled the interventions rather than splitting control groups [24]. In three arm trials evaluating dissimilar interventions, control groups were split and compared to each intervention arm individually to avoid unit of analysis error [24]. Where additional unpublished outcome data were required, the review team contacted authors by email correspondence. If no author response was received, we contacted authors a second time, 4 weeks after initial correspondence.
We used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach to classify the certainty of evidence into high, moderate, low or very low for each outcome [26] and included a summary of findings table for the main outcomes (Table 1). As planned, publication bias will be evaluated using funnel plot asymmetry testing if a sufficient number of studies are identified (n > 10).
Table 1.
Summary of findings
| Swallowing therapy compared to standard care for oropharyngeal dysphagia in acute and critical care | ||||||
|---|---|---|---|---|---|---|
| Patient or population: adults with oropharyngeal dysphagia | ||||||
| Setting: Acute hospital wards and intensive care units | ||||||
| Intervention: swallowing therapy | ||||||
| Comparison: standard care |
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Risk with standard care | Risk with swallowing therapy | |||||
| Time (days) to return to oral intake | The mean time in days to return to oral intake was 11.9 days | MD 4.5 days lower (10.63 lower to 1.63 higher) | – | 33 (1 RCT) | ⨁◯◯◯ Very lowa,b | Oral intake was not defined (i.e. whether modified diet or pre-admission diet) |
| Aspiration incidence post-treatment assessed with: Penetration Aspiration Score rating 5 or greater on a 0–8 scale | 318 per 1000 | 251 per 1000 (140 to 461) | RR 0.79 (0.44 to 1.45) | 113 (4 RCTs) | ⨁⨁◯◯ Lowc | Variable time points: 3-months (32, 48); 2 weeks (40); not defined (33) |
| Incidence of pneumonia | 311 per 1000 | 221 per 1000 (174 to 276) | RR 0.71 (0.56 to 0.89) | 719 (8 RCTs) | ⨁⨁◯◯ Lowc | Variable time points used: during hospital admission (35, 44); post-randomisation (27); day 30 and hospital discharge (31); 2 months (45); 3 months (32); 6 month (28); and not defined (34) |
| Length of hospital stay (days) | MD 0.4 lower (3.6 lower to 2.8 higher) | – | 536 (4 RCTs) | ⨁◯◯◯ Very lowc,d | Defined as time from treatment to discharge (43); time from admission to discharge (27, 44) and not defined. (28) | |
| Quality of life post-treatment assessed with: Swallowing Quality of Life Scale: 0 to 200 | MD 11.38 SD lower (23.83 lower to 1.08 lower) | – | 239 (2 RCTs) | ⨁◯◯◯ Very lowc,e | Lower score indicates improved quality of life. Post-treatment timepoints not defined [39, 46] | |
| Intervention-related adverse events | 87 per 1000 | 151 per 1000 (50 to 458) | RR 1.74 (0.57 to 5.27) | 109 (2 RCTs) | ⨁⨁◯◯ Lowc | Following treatment, timepoints not defined [30, 31] |
| Change in pharyngeal residue severity assessed with: functional dysphagia scale; video fluoroscopy scoring scale; video fluoroscopic dysphagia scale | – | SMD 0.78 SD lower (1.3 lower to 0.26 lower) | – | 64 (3 RCTs) | ⨁◯◯◯ Very lowc,f | Lower scores indicates improvement in pharyngeal residue severity. Timepoint: following 4-week treatment period [38, 41, 42] |
| Nutritional status assessed with: (Albumin level g/L) | MD 0.9 higher (0.99 lower to 2.79 higher) | – | 141 (1 RCT) | ⨁⨁◯◯ Lowc | Higher scores indicates improved nutritional status | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| CI confidence interval, MD mean difference, RR risk ratio, SMD standardised mean difference | ||||||
| GRADE Working Group grades of evidence | ||||||
| High certainty: We are very confident that the true effect lies close to that of the estimate of the effect | ||||||
| Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different | ||||||
| Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect | ||||||
| Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one point due to serious inconsistency from wide CIs
bDowngraded two points due to very serious imprecision as number of participants did not reach optimal information size and only one study reported this outcome
cDowngraded two points due to very serious imprecision as number of participants did not reach optimal information size
dDowngraded one point due to serious inconsistency as a result of substantial heterogeneity (52%)
eDowngraded one point due to serious inconsistency as a result of substantial heterogeneity (78%)
fDowngraded one point due to serious risk of bias in both studies: allocation concealment and blinding were not clearly stated
Results
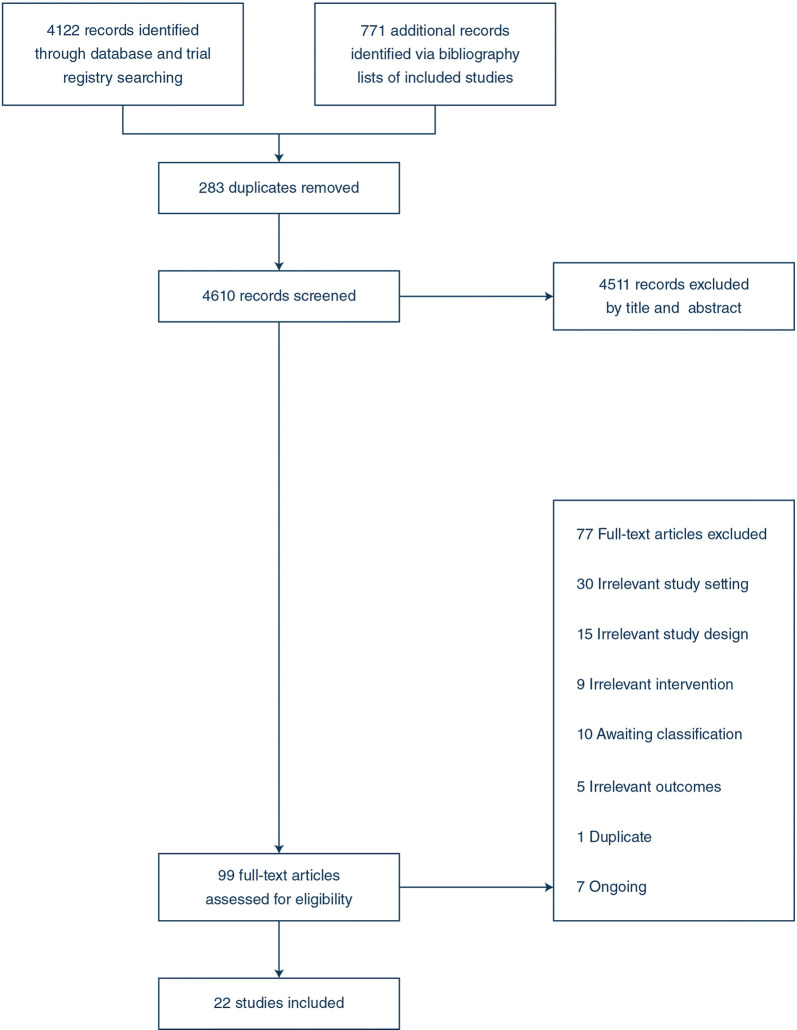
We identified 4893 studies. After removing duplicates and excluding irrelevant records, we assessed 99 full-text studies. We excluded a further 77 studies and included 22 trials (22 RCT, no quasi-RCT, and although not included, we found no cluster-RCTs). The trial populations included 19 stroke in acute care (n = 1568); 2 tracheostomised stroke in intensive care (n = 99); and 1 general intensive care (n = 33) [27–48] (Fig. 1) (ESM Appendix E and F). Twenty-three data sets were analysed in this review as one three-arm study compared two different interventions [32]. The majority of trials which were included were conducted in a single-centre setting (n = 19) with three multi-centre trials (Table 2). Twenty-one studies were published in English, and one study was published in Chinese and translated by a native Chinese researcher [46]
Fig. 1:
PRISMA flowchart
Table 2.
Details of included studies
| Study and country | Clinical setting/population/sample size | Intervention | Comparator |
|---|---|---|---|
| Du et al. [30] China | Neurology unit Stroke N = 40 | Transcranial magnetic stimulation | Sham stimulation |
| Park et al. [40] Korea | Stroke unit N = 18 | Transcranial magnetic stimulation | Sham stimulation |
| Kumar et al. [36] USA | Stroke unit N = 14 | Transcranial direct current stimulation | Sham stimulation and swallowing exercises/oral stimulation |
| Suntrup-Kreuger et al. [44] Germany | Stroke unit N = 60 | Transcranial direct current stimulation | Sham stimulation and swallowing exercises/oral stimulation |
| Yang et al. [48] Korea | Neurology unit Stroke N = 16 | Transcranial direct current stimulation | Sham stimulation and swallowing exercises |
| Moon et al. [39] Korea | Hospital ward Stroke N = 16 | Tongue palate resistance training | Swallowing exercises |
| Moon et al. [38] Korea | Stroke unit N = 18 | Respiratory muscle strength training | Swallowing exercises/thermal stimulation |
| Guillan-Sola et al. [32] Spain | Hospital ward Stroke N = 31 | Respiratory muscle strength training | Swallowing exercises |
| Bath et al. [27] Multi-national | Stroke unit N = 162 | Pharyngeal electrical stimulation | Sham stimulation |
| Dziewas et al. [31] Multi-national | Neuro intensive care stroke N = 69 | Pharyngeal electrical stimulation | Sham stimulation |
| Jayaskeren et al. [35] UK | Stroke unit N = 28 | Pharyngeal electrical stimulation | Sham stimulation |
| Suntrup et al. [43] Germany | Neuro intensive care stroke N = 30 | Pharyngeal electrical stimulation | Sham stimulation |
| Vasant et al. [45] UK | Stroke unit N = 36 | Pharyngeal electrical stimulation | Sham stimulation and swallowing exercises |
| Guillan-Sola et al. [32] Spain | Hospital ward Stroke N = 31 | Neuromuscular electrical stimulation | Swallowing exercises |
| Huang et al. [33] Taiwan | Hospital ward Stroke N = 29 | Neuromuscular electrical stimulation | Swallowing exercises/head postures |
| Li et al. [37] China | Stroke unit N = 118 | Neuromuscular electrical stimulation | Swallowing exercises with oral trials |
| Xia et al. [47] China | Neurology unit Stroke N = 120 | Neuromuscular electrical stimulation | Swallowing exercises |
| Park et al. [41] Korea | Hospital ward Stroke N = 22 | Chin tuck against resistance | Swallowing exercises/thermal stimulation |
| Chen et al. [29] China | Stroke unit N = 250 | Acupuncture | Swallowing exercises |
| Wu et al. [46] China | Stroke unit N = 229 | Acupuncture | Swallowing exercises/thermal stimulation |
| Carnaby et al. [28] Multi-national | Stroke unit N = 306 | Behavioural Intervention | Mealtime supervision/safe feeding practices |
| Hwang et al. [34] Korea | General intensive care population N = 33 | Behavioural Intervention | No therapy, oral hygiene |
| Park et al. [42] Korea | Stroke unit N = 24 | Effortful swallow training and traditional swallowing exercises | Traditional swallowing exercises |
The overall mean age of participants in both groups in all studies was 70 years. Disease severity for stroke was reported using National Institute of Health Stroke Scale [49], with average scores across experimental (11) and control (11.5) groups indicating moderate stroke disability. No studies included frailty assessments. Baseline dysphagia severity was measured using fourteen different assessment tools. Eight clinical assessments evaluated function based on bedside clinical signs and symptoms and clinicians recommended altering oral diet/fluid consistencies and feeding supervision. Six validated rating scales were used during videofluoroscopy/endoscopy assessments, to grade swallow physiology at oral, pharyngeal and upper oesophageal stages. Average baseline scores for experimental and control groups across all studies indicated moderate to severe dysphagia.
Seven trials involved three intervention arms [28, 30, 32, 33, 37, 46, 47]. In five trials, data from both intervention arms (same intervention delivered at different intensities) were combined and compared to the control group for both dichotomous and continuous outcomes. One trial tested two different interventions, and so the control group was split in half and compared to each intervention arm [32]. One trial descriptively reported on adverse events so no numerical data was available for meta-analysis [37].
At present, seven trials are ongoing in acute care [50–56], four of these in intensive care, testing swallowing exercises post-extubation [50] or sensory electrical stimulation during intubation or post-extubation [51–53]. Ten trials are unclassified testing interventions in stroke [57–66]. The review team were unable to obtain trial results or sufficient data from authors to confirm inclusion. (ESM Appendix G). An expert advisory group in the field of dysphagia and critical care research was consulted in June 2019 and confirmed that to the best of their knowledge there were no other completed or ongoing trials in intensive care at that time (ESM Appendix H).
Risk of bias in included studies
Twenty-two trials (23 data sets) were assessed for risk of bias (17 high risk; 6 unclear risk of bias). Low risk was assigned to the following domains: selection bias (random sequence generation: 18/23; 78%; allocation concealment: 14/23; 61%), detection bias (blinded outcome assessment) (19/23; 83%), attrition bias (20/23; 87%) and reporting bias (22/23; 96%). No studies clearly reported that personnel delivering the intervention were blinded and only 43% (10/23) of studies clearly reported that participants were blinded to the intervention. (ESM Appendix I).
Intervention reporting and replication
We assessed each study for the inclusion of twelve components involved in delivering an intervention as per TIDieR [22]. All studies reported rationale for treatment, materials and procedures used and the number, frequency, timing and intensity of treatment sessions. Thirteen studies reported who delivered the intervention [28, 29, 32–34, 37–39, 41, 42, 45, 47, 48] and seven detailed the training and/or experience of such personnel [27, 29, 33, 39, 41, 42, 45]. Thirteen studies provided information on whether treatments were tailored [27, 28, 30, 31, 33, 35, 37–40, 43, 45, 48], and no studies reported if an intervention was modified or whether intervention adherence assessment was completed. Actual adherence was calculated from the number of participants who completed an intervention. This was reported in all studies (ESM Appendix C).
Assessment of quality of the evidence
We present our assessment of the certainty of evidence for each outcome according to the GRADE approach in the summary of findings table (Table 1). We used a simple measure to assess publication bias based on the balance of published trials that showed an effect or not, as there was insufficient number of trials for funnel plot testing. Publication bias was not detected.
Subgroup analysis
Data were available to complete subgroup analysis of acute versus critical care for one outcome: pneumonia incidence and subgroup analysis of different interventions for five outcomes: aspiration incidence, quality of life, length of stay, change in pharyngeal residue severity and intervention-related adverse events.
Sensitivity analysis
We planned to investigate the influence of bias on results by undertaking a sensitivity analysis of primary outcomes excluding studies with a high risk of bias. This was not undertaken as relevant studies were assessed as having a high risk of bias.
Main outcomes
Time taken in days to return to oral intake
One trial [34] reported this outcome and compared a behavioural intervention (i.e. swallowing exercises and oral stimulation) to standard care (no swallowing related exercises) in an intensive care setting. Swallowing therapy showed no evidence of a difference in the time to return to oral intake (n = 33, MD (days) − 4.5, 95% CI − 10.6 to 1.6, 1 study, P = 0.15) (very low certainty).
Aspiration incidence post-intervention
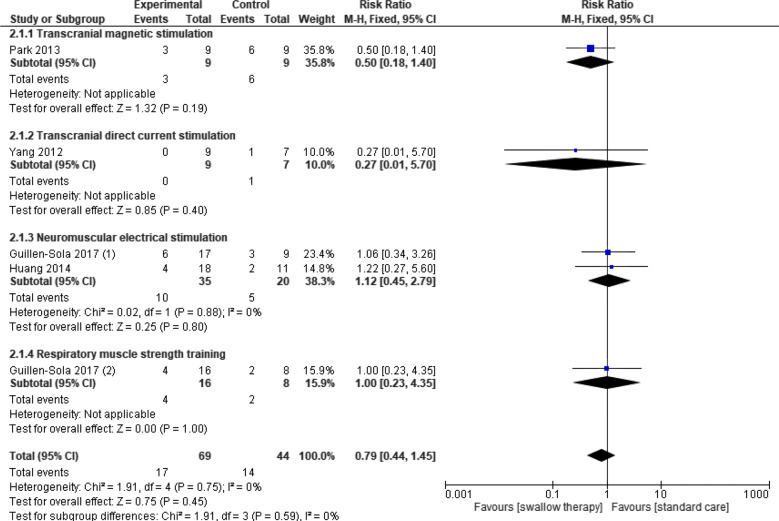
Four trials (5 data sets) reported this outcome for swallowing therapy versus standard care [32, 33, 40, 48]. Two studies provided unpublished data on request [33, 48]. Swallowing therapy showed no evidence of a difference in reducing aspiration post-intervention (n = 113, RR 0.79; 95% CI 0.44 to 1.45, I2 = 0%, P = 0.45) (low certainty). Subgroup analysis of intervention types showed no significant effects for transcranial magnetic stimulation; transcranial direct current stimulation (tDCS); neuromuscular electrical stimulation (NMES); or respiratory muscle strength training (RMST), with no significant subgroup interaction (P = 0.59, I2 = 0%) (Fig. 2). Data on the number of patients aspirating per group were requested from authors of eleven studies. Penetration aspiration scores (PAS) were reported as mean and SD per group but did not report data on number of patients aspirating (PAS > 5) versus not aspirating (PAS < 5). Two authors provided unpublished data [33, 48], three authors reported data were unavailable [40, 43, 44] and six authors provided no response [27, 29, 35, 37, 38, 45].
Fig. 2:
Swallowing therapy versus standard care: aspiration incidence post-intervention
Incidence of pneumonia
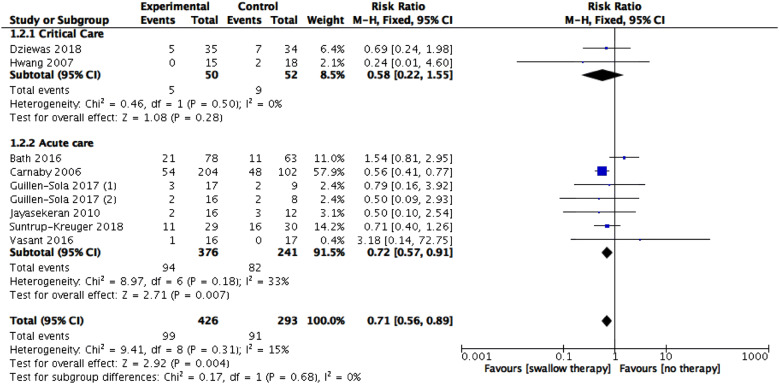
Eight trials (9 data sets) [27, 28, 31, 32, 34, 35, 44, 45] reported this outcome. The pooled results showed a beneficial effect for swallowing therapy (n = 719, RR 0.71, 95% CI 0.56 to 0.89, I2 = 15%, P = 0.004) (low certainty). Subgroup analysis of acute versus critical care showed a significant effect for acute care (7 datasets, 617 participants, RR 0.72, 95% CI 0.57 to 0.91, I2 = 33%, P = 0.007) and no evidence of a difference for critical care (2 trials, 102 participants, RR 0.58, 95% CI 0.22 to 1.55, I2 = 0%, P = 0.28) with no significant subgroup interaction (P = 0.68, I2 = 0%) (Fig. 3).
Fig. 3:
Swallowing therapy versus standard care: incidence of pneumonia
Quality of life post-intervention
Three trials reported this outcome [36, 42, 47]. Meta-analysis of data from two studies showed no evidence of a difference from swallowing therapy versus standard care (n = 239, MD − 11.38, 95% CI − 23.83 to 1.08, I2 = 78%, P = 0.07) (very low certainty). Subgroup analysis of intervention types found effect sizes were statistically significant for acupuncture but not for tongue-palate resistance training, with a significant subgroup interaction (P = 0.03, I2 = 77.9%) (ESM Appendix J: Fig. 1). A third study testing NMES [47] reported using the same scale but the direction of scoring was opposite to the other two studies, and scores were very different so it is reported individually. Swallowing therapy showed evidence of a difference (n = 120, MD − 166.00, 95% CI − 180.66, − 151.34, P < 0.00001).
Length of hospital stay
Five trials reported this outcome [27, 28, 43–45]. Meta-analysis of data from four studies showed no evidence of a difference from swallowing therapy versus standard care (n = 536, MD (days) − 0.4, 95% CI − 3.6 to 2.8, I 2 = 52%, P = 0.81) (very low certainty). Subgroup analysis of interventions showed no evidence of a difference for pharyngeal electrical stimulation, tDCS or behavioural interventions and a significant subgroup interaction (P = 0.04, I2 = 67.9%) (ESM Appendix J: Fig. 2). A fifth trial, testing pharyngeal electrical stimulation [45], reported median length of stay was 39 days and 52 days in the active and sham groups with no significant difference between arms observed by a stratified log-rank test (P = 0.62). Interquartile ranges were not reported.
Change in pharyngeal residue severity
Four studies reported this outcome [38, 40–42], using the Functional Dysphagia Scale (FDS) [67]; Videofluoroscopy Scoring Scale (VFSS) [68] and Videofluoroscopic Dysphagia Scale [69]. Meta-analysis of three studies reporting continuous outcomes found a beneficial effect from swallowing therapy (n = 64, SMD (FDS, VFSS, VDS scores) − 0.78, 95% CI − 1.3 to − 0.26, I2 = 39%, P = 0.003) (very low certainty). Subgroup analysis of interventions showed an individual effect for both RMST and chin tuck against resistance but no statistically significant difference for effortful swallowing training and no significant subgroup interaction (P = 0.19, I2 = 39.5%) (ESM Appendix J: Fig. 3). The fourth trial, testing transcranial magnetic stimulation, reported changes in residue severity as a dichotomous outcome but effect size was not statistically significant (n = 18, RR 0.5, 95% CI 0.18 to 1.40, P = 0.19 [40]. Residue scores were sometimes subsumed within overall swallowing assessment scores reported in studies. Authors of two trials included in this review were contacted for relevant raw data on this outcome, but this information was not provided [33, 48].
Nutritional status
One trial comparing pharyngeal electrical stimulation to standard care reported on nutritional status (measuring blood albumin g/L), but the effect size was not statistically significant (n = 141, MD 0.9, 95% CI − 0.99 to 2.79, P = 0.35 [27] (low certainty).
Change in oral-pharyngeal secretion severity
This outcome was not reported in any included studies in this review.
Intervention-related adverse events
Twelve studies reported on intervention-related adverse events [27, 29–32, 36, 37, 43–46, 48]. Ten studies testing pharyngeal electrical stimulation [27, 43, 45]; transcranial direct current stimulation [36, 44, 48]; neuromuscular electrical stimulation [32, 37]; and acupuncture [29, 46] descriptively reported no adverse events. Meta-analysis of data from two studies [30, 31] showed no significant difference between number of adverse events reported during swallowing therapy or standard care (n = 109, RR 1.74, 95% CI 0.57 to 5.27, I = 0%, P = 0.33 (low certainty). Subgroup analysis showed no significant difference for number of adverse events (ESM Appendix J: Fig. 4).
Discussion
Of the 22 studies included in this review, 19 were acute stroke patients (n = 1568), two tracheostomised stroke patients in intensive care (n = 99) and one general intensive care population (n = 33). Nine interventions, including electrical and magnetic neurostimulation approaches and muscle strengthening treatments were identified. Days taken to return to oral intake were considered an important patient-relevant outcome in this review but were reported in only one trial, and effect sizes were not statistically significant. Swallowing treatment was found to have a beneficial effect on another patient relevant outcome, pneumonia. A beneficial effect on pharyngeal residue severity was also found. While adverse event reporting was most common in studies testing electrical or magnetic stimulation, overall event rates were low (i.e. 9/63 vs 4/46 in experimental and control groups respectively) and not found to be statistically higher than controls. Effect sizes were not statistically significant for aspiration incidence post-treatment, quality of life, length of hospital stay or nutritional status. One review outcome, change in oral and pharyngeal secretion severity were not reported in any study.
Seven acute (n = 617) and two critical care studies (n = 102) reported on pneumonia incidence post-treatment (Fig. 2). A subgroup analysis of critical care studies revealed small sample sizes and wide confidence intervals, and therefore, we cannot be confident in finding a treatment effect. At present, it is unknown whether future, adequately powered trials will improve these findings.
As the populations included in this review were predominantly acute stroke with a very small number from intensive care; the resulting dysphagia in these populations will have different underlying mechanisms of impairment limiting generalisability of findings. Stroke patients present with neurogenic dysphagia resulting from cortical and/or sub-cortical damage to the swallowing network. Intensive care patients, however, present with dysphagia for a myriad of different reasons: mechanical injury due to pharyngeal and laryngeal trauma at intubation site; atrophy of skeletal muscle due to disuse during intubation; sensory deficits in swallowing due to disruption of sensory receptors during intubation and the sedating effects of medications in intensive care; and finally the presence and/or prolonged use of a tracheostomy tube [3]. Therefore, the tracheostomised, acute stroke patients in this review with have a complex dysphagia presentation. Their central swallowing network is disrupted due to the brain lesion, but the presence of a tracheostomy will also affect laryngeal sensory receptors necessary for safe swallowing, in the context of likely continuing skeletal muscle atrophy during their intensive care stay.
These tracheostomised, acute stroke patients were treated using pharyngeal electrical stimulation (PES) in two studies [31, 43]. Significant group differences were found in primary outcome: time to tracheostomy decannulation but no significant differences in length of stay, tube-feeding cessation or return to oral intake. Electrotherapies such as PES or neuromuscular electrical stimulation (NMES) provide sensory feedback via bulbar cranial nerves that innervate the pharynx. This increased sensory input has been shown to drive long-term changes in the cortical control of swallowing [70]. One could argue such sensory treatments tested in stroke populations could be used to target sensory deficits often observed among critically ill patients with dysphagia. The third completed study with a general ICU population involved swallowing exercises/oral stimulation delivered during intubation and found significant improvements in swallowing efficiency but no group differences in aspiration/pneumonia incidence or length of stay [34].
Four ongoing ICU studies were also identified. Apart from one [50], all studies are testing sensory electrical stimulation either during intubation or post-extubation [51–53]. As these studies are testing treatments at different timepoints during a patient’s ICU stay; they may provide valuable information on optimal treatment timing and its impact on patient relevant outcomes.
An expert advisory panel were consulted on the other interventions identified in this review and their use with intensive care patients. They questioned both the scientific rationale for using non-invasive brain stimulation treatments and the feasibility of using acupuncture for patients in intensive care. However, they suggested tongue-palate resistance training, chin tuck against resistance (CTAR) and respiratory muscle strength training (RMST) are all biologically plausible interventions that could target skeletal muscle atrophy of the swallowing mechanism commonly reported across both post-extubation and tracheostomised intensive care populations.
To date, no systematic review has evaluated dysphagia interventions conducted in intensive care. Our review’s findings were compared with a recent Cochrane review of interventions in acute care (stroke) [71]. Both reviews highlight wide variability in reported outcomes and their timepoints across studies; various subjective and objective assessment tools were used to measure swallow-related outcomes and moderate to very low study quality. The variability in outcome reporting in this review emphasises the need for a core outcome set for dysphagia intervention studies in intensive care.
In the interim, outcomes proposed in completed and ongoing ICU studies and recommended by an expert advisory group may be considered. They include: physiological outcomes (laryngeal closure times; pharyngo-laryngeal sensation; swallow biomechanics); functional outcomes (tracheostomy decannulation time; time to tube-feeding cessation; return to oral intake); psychological outcomes (patient comfort, pain and anxiety levels during intervention delivery). The strengths of our review are the high-quality systematic review Cochrane methodology used to screen, extract data and assess study quality independently by two reviewers. A comprehensive search strategy, including studies in all languages was developed with an independent medical librarian. A limitation of this review is that trial authors were not contacted directly to clarify unclear risk of bias ratings which may have resulted in trials being rated differently. A further limitation is the small number of underpowered studies available to provide reliable subgroup analyses. With such limited data, we have low certainty in subgroup findings and cannot confidently recommend specific interventions for acute and critical care populations at this time.
Conclusion
This review highlights the limited research on dysphagia interventions in the acute and critical care setting and the limited evidence to guide clinical practice in this area. Future studies testing interventions in this setting should consider patient relevant outcomes using similar, validated measurement tools.
Deviations from protocol
Nutritional status was not included as an outcome in original PROSPERO registration (28/11/18) but was added to updated version (8/01/19). PROSPERO registration outlined the following subgroup analyses: acute versus critical care and types of dysphagia interventions. The published protocol included an additional subgroup: younger age groups (< 65) versus older age groups (> 65). In the final review, data were only available and reported for subgroup analyses: acute versus critical care and types of dysphagia interventions. PROSPERO registration contained one primary outcome (time to oral intake). However, the published protocol and this review include a second primary outcome (aspiration incidence post-treatment) which we believe is important to consider in a critical care setting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The review team would like to acknowledge the expert advisory panel consulted to discuss review findings: Prof Martin Brodsky, Johns Hopkins University; Dr. Anna Miles, Auckland University; Prof Louise Rose, Kings College London; Dr. Anna Liisa Sutt and Dr. Alistair Proudfoot, St. Bart’s Trust, NHS, London; Dr. Jackie McRae, Kingston and St George’s University London; Dr. Bronwen Connolly, Queen’s University Belfast. The review team would also like to acknowledge Yue Su, doctoral researcher at Queen's University, Belfast, for translating all Chinese publications identified during this review into English language.
Author contributions
All authors contributed to this SR. As information specialist, RF led on developing a search strategy with SD and completing all database and clinical trial registry searches. All search terms were agreed with supervision team: BB, MW, DM. J McG and SD completed study screening, abstract and full-text viewing and data extraction and reviewed bibliography lists from included trials. SD entered and analysed data into RevMan, RA checked data entry and BB checked analysis. SD wrote review and all authors contributed. All authors read and approved the final manuscript.
Funding
This work is being conducted as part of a doctoral research fellowship awarded to SD and funded by the Health and Social Care Research and Development Division of the Public Health Agency in Northern Ireland, UK (Grant No. EAT/5382/17).
Compliance with ethical standards
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hamdy S, Aziz Q, Rothwell JC, Power M, Singh KD, Nicolson DA. Recovery of swallowing after dysphagic stroke relates to functional reorganization in the intact motor cortex. Gastroenterology. 1998;115(5):1104–1112. doi: 10.1016/s0016-5085(98)70081-2. [DOI] [PubMed] [Google Scholar]
- 2.Zuercher P, Moret CS, Dziewas R, Schefold JC. Dysphagia in the intensive care unit: epidemiology, mechanisms and clinical management. Crit Care. 2019;23:103–107. doi: 10.1186/s13054-019-2400-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macht M, Wimbish T, Bodine C. ICU-acquired swallowing disorders. Crit Care Med. 2013;41(10):2396–2405. doi: 10.1097/CCM.0b013e31829caf33. [DOI] [PubMed] [Google Scholar]
- 4.Barker J, Martino R, Reichardt B, Hickey EJ, Ralph-Edwards A. Incidence and impact of dysphagia in patients receiving prolonged endotracheal intubation after cardiac surgery. Can J Surg. 2009;52:119–124. [PMC free article] [PubMed] [Google Scholar]
- 5.Skoretz S, Yau TM, Ivanov J, Granton JT, Martino R. Dysphagia and associated risk factors following extubation in cardiovascular surgical patients. Dysphagia. 2014;6:647–654. doi: 10.1007/s00455-014-9555-4. [DOI] [PubMed] [Google Scholar]
- 6.Daly E, Miles A, Scott S, Gilham M. Finding the red flags: swallowing difficulties after cardiac surgery in patients with prolonged intubation. J Crit Care. 2016;31(1):119–124. doi: 10.1016/j.jcrc.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Tsai MH, Ku SC, Wang TG, Hsiao TY, Lee JJ, Chan DC, et al. Swallowing dysfunction following endotracheal intubation: age matters. Medicine (Baltimore) 2016;95:e3871. doi: 10.1097/MD.0000000000003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macht M, King CJ, Wimbish T, Clark BJ, Benson AB, Burnham EL, et al. Postextubation dysphagia is associated with longer hospitalization in survivors of critical illness with neurologic impairment. Crit Care. 2013;17:R119. doi: 10.1186/cc12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solh A, Okada M, Bhat A, Pietrantoni C. Swallowing disorders post orotracheal intubation in the elderly. Intensive Care Med. 2003;29:1451–1455. doi: 10.1007/s00134-003-1870-4. [DOI] [PubMed] [Google Scholar]
- 10.Ponfick M, Linden R, Nowak DA. Dysphagia-a common, transient symptom in critical illness polyneuropathy: a fiberoptic endoscopic evaluation of swallowing study. Crit Care Med. 2015;43(2):365–372. doi: 10.1097/CCM.0000000000000705. [DOI] [PubMed] [Google Scholar]
- 11.Kim MJ, Park YH, Park YS, Song YH. Associations between prolonged intubation and developing p2stextubation dysphagia and aspiration pneumonia in non-neurologic critically ill patients. Ann Rehabil Med. 2015;39:763–771. doi: 10.5535/arm.2015.39.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zielske J, Bohne S, Brunkhorst F, Axer H, Guntinas-Lichius O. Acute and long-term dysphagia in critically ill patients with severe sepsis: results of a prospective controlled observational study. Eur Arch Otorhinolaryngol. 2014;271(11):3085–3093. doi: 10.1007/s00405-014-3148-6. [DOI] [PubMed] [Google Scholar]
- 13.Schefold JC, Berger D, Zurcher P, Lensch M, Perren A, Jakob SM, et al. Dysphagia in mechanically ventilated ICU patients (DYnAMICS): a prospective observational trial. Crit Care Med. 2017;45(12):2061–2069. doi: 10.1097/CCM.0000000000002765. [DOI] [PubMed] [Google Scholar]
- 14.Marian T, Dunser M, Kokofer A, Dziewas R. Are intensive care physicians aware of dysphagia? The MADICU survey results. Intensive Care Med. 2018;44:973–975. doi: 10.1007/s00134-018-5181-1. [DOI] [PubMed] [Google Scholar]
- 15.Brodsky M, Pandian V, Needham D. Post-extubation dysphagia: a problem needing multidisciplinary efforts. Intensive Care Med. 2020;46:93–96. doi: 10.1007/s00134-019-05865-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuercher P, Dziewas R, Schefold C. Dysphagia in the intensive care unit: a (multidisciplinary) call to action. Intensive Care Med. 2020;46:554–556. doi: 10.1007/s00134-020-05937-3. [DOI] [PubMed] [Google Scholar]
- 17.Brodsky MB, Huang M, Shanholtz C, Mendez-Tellez PA, Palmer JB, Colantuoni E, et al. Recovery from dysphagia symptoms after oral endotracheal intubation in acute respiratory distress syndrome survivors. A 5-year longitudinal study. Ann Am Thorac Soc. 2017;14:376–383. doi: 10.1513/AnnalsATS.201606-455OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan S, Mc Gaughey J, Fallis R, McAuley DF, Walshe M, Blackwood B. Interventions for oropharyngeal dysphagia in acute and critical care: a protocol for a systematic review and meta-analysis. Syst Rev. 2019;8:283. doi: 10.1186/s13643-019-1196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart L, PRISMA-P Group Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g764. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 20.Rosenbek J, Robbins J, Roecker E, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- 21.Mc Horney CA, Robbins J, Lomax K, Rosenbek JC, Chignell K, Kramer AE, Bricker DE. The SWAL-QOL and SWAL-CARE outcomes tool for oropharyngeal dysphagia in adults: III Documentation of reliability and validity. Dysphagia. 2002;17(2):97–114. doi: 10.1007/s00455-001-0109-1. [DOI] [PubMed] [Google Scholar]
- 22.Hoffman T, Glasziou P, Boutron I, Milne R, Perera R, Moher D, Altman D, Barbour V, Macdonald H, Johnston M, Lamb S, Dixon-Woods M, McCullough P, Wyatt J, Chan A, Michie S. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:1687. doi: 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- 23.Chan A-W, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, Hróbjartsson A, Mann H, Dickersin K, Berlin J, Doré C, Parulekar W, Summerskill W, Groves T, Schulz K, Sox H, Rockhold FW, Rennie D, Moher D. SPIRIT 2013 Statement: Defining standard protocol items for clinical trials. Ann Intern Med. 2013;158:200–207. doi: 10.7326/0003-4819-158-3-201302050-00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Higgins JPT, Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration
- 25.Revman (2012) The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) Version 5.3. The Nordic Cochrane Centre, The Cochrane Collaboration
- 26.Balshem H, Helfand M, Schunemann H, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, Guyatt GH. GRADE guidelines: rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Bath PM, Scutt P, Love J, Clave P, Cohen D, Dziewas R, Iversen H, Ledl C, Ragab S, Soda H, Warusevitane A, Woisard V, Hamdy S. Pharyngeal electrical stimulation for treatment of dysphagia in subacute stroke: a randomised control trial. Stroke. 2016;47:1562–1570. doi: 10.1161/STROKEAHA.115.012455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carnaby G, Hankey GJ, Pizzi J. Behavioural intervention for dysphagia in acute stroke: a randomised controlled trial. Lancet Neurol. 2006;5:31–37. doi: 10.1016/S1474-4422(05)70252-0. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Fang J, Ma R, Gu X, Chen L, Li J, Xu S. Additional effects of acupuncture on early comprehensive rehabilitation in patients with mild to moderate acute ischaemic stroke: a multi-center randomised controlled trial. BMC Complement Altern Med. 2016;16:1–9. doi: 10.1186/s12906-016-1193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du J, Yang F, Liu L, Hu J, Cai B, Liu W, Xu G, Liu X. Repetitive transcranial magnetic stimulation for rehabilitation of poststroke dysphagia: a randomised double-blind clinical trial. Clin Neurophysiol. 2016;127:1907–1913. doi: 10.1016/j.clinph.2015.11.045. [DOI] [PubMed] [Google Scholar]
- 31.Dziewas R, Stellato R, van der Tweel I, Walther E, Werner CJ, Braun T, Citerio G, Jandl M, Friedrichs M, Notzel K, Vosko M, Mistry S, Hamdy S, McGowan S, Warnecke T, Zwittag P, Bath P. Pharyngeal electrical stimulation for early decannulation in tracheostomised patients with neurogenic dysphagia after stroke (PHAST-TRAC): a prospective, single-blinded, randomised trial. Lancet Neurol. 2018;17:849–859. doi: 10.1016/S1474-4422(18)30255-2. [DOI] [PubMed] [Google Scholar]
- 32.Guillen-Sola A, Sartor MM, Soler NB, Duarte E, Barrera MC, Marco E. Respiratory muscle strength training and neuromuscular electrical stimulation in subacute dysphagic stroke patients: a randomised controlled trial. Clin Rehabil. 2017;31(6):761–771. doi: 10.1177/0269215516652446. [DOI] [PubMed] [Google Scholar]
- 33.Huang K-L, Liu T-Y, Huang Y-C, Leong C-P, Lin W-C, Pong Y-P. Functional outcome in acute stroke patients with oropharyngeal dysphagia after swallowing therapy. J Stroke Cerebrovasc Dis. 2014;23(10):2547–2553. doi: 10.1016/j.jstrokecerebrovasdis.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 34.Hwang CH, Choi KH, Ko YS, Leem CM. Pre-emptive swallowing stimulation in long-term intubated patients. Clin Rehabil. 2007;21:41–46. doi: 10.1177/0269215506071286. [DOI] [PubMed] [Google Scholar]
- 35.Jayasekeran V, Singh S, Tyrrel P, Michou E, Jefferson S, Mistry S, Gamble E, Rothwell J, Thompson D, Hamdy S. Adjunctive functional pharyngeal electrical stimulation reverses swallowing disability after brain lesions. Gastroenterology. 2010;138:1737–1746. doi: 10.1053/j.gastro.2010.01.052. [DOI] [PubMed] [Google Scholar]
- 36.Kumar S, Wagner CW, Frayne C, Zhu L, Selim M, Feng W, Schlaug G. Noninvasive brain stimulation may improve stroke-related dysphagia: a pilot study. Stroke. 2011;42:1035–1040. doi: 10.1161/STROKEAHA.110.602128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Li Y, Wu X, Wang G, Yi X, Zhao Y, Guo M, Pan M, Tang C. The value of adding transcutaneous neuromuscular electrical stimulation (Vital Stim) to traditional therapy for poststroke dysphagia. Top Geriatr Rehabil. 2018;34(3):200–206. [Google Scholar]
- 38.Moon JH, Jung J-H, Won YS, Cho H-Y, Cho K. Effects of expiratory muscle strength training on swallowing function in acute stroke patients with dysphagia. J Phys Ther Sci. 2017;29:609–612. doi: 10.1589/jpts.29.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moon J-H, Hahm S-C, Won YS, Cho H-Y. The effects of tongue pressure strength and accuracy training on tongue pressure strength, swallowing function and quality of life in subacute stroke patients with dysphagia: a preliminary randomised clinical trial. Int J Rehabil Res. 2018;41(3):204–210. doi: 10.1097/MRR.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 40.Park J-W, Oh J-C, Lee J-W, Yeo J-S, Ryu KH. The effect of 5Hz high-frequency rTMS over contra-lesional pharyngeal motor cortex in post-stroke oropharyngeal dysphagia: a randomised controlled study. Neurogastroenterol Motil. 2013;25:324–e250. doi: 10.1111/nmo.12063. [DOI] [PubMed] [Google Scholar]
- 41.Park J-S, An D-H, Oh D-H, Chang M-Y. Effect of chin tuck against resistance exercise on patients with dysphagia following stroke: a randomised pilot trial. Neurorehabilitation. 2018;42:191–197. doi: 10.3233/NRE-172250. [DOI] [PubMed] [Google Scholar]
- 42.Park H-S, Oh D-H, Yoon T, Park J-S. Effect of effortful swallowing training on tongue strength and oropharyngeal swallowing function in stroke patients with dysphagia: a double-blind, randomised controlled trial. Int J Lang Commun Disord. 2019;54(3):479–484. doi: 10.1111/1460-6984.12453. [DOI] [PubMed] [Google Scholar]
- 43.Suntrup S, Marian T, Schroder JB, Suttrup I, Muhle P, Oelenberg S, Hamacher C, Minnerup J, Warnecke T, Dziewas R. Electrical pharyngeal stimulation for dysphagia treatment in tracheostomised stroke patients: a randomised controlled trial. Intensive Care Med. 2015;41:1629–1637. doi: 10.1007/s00134-015-3897-8. [DOI] [PubMed] [Google Scholar]
- 44.Suntrup-Kreuger S, Ringmaier C, Muhle P, Wollbrink A, Kemmling A, Hanning U, Claus I, Warnecke T, Teismann I, Pantev C, Dziewas R. Randomised trial of transcranial direct current stimulation for poststroke dysphagia. Ann Neurol. 2018;83:328–340. doi: 10.1002/ana.25151. [DOI] [PubMed] [Google Scholar]
- 45.Vasant DH, Michou E, O'Leary N, Vail A, Mistry S, Hamdy S. Pharyngeal electrical stimulation in dysphagia poststroke: a prospective, randomised single-blinded interventional study. Neurorehabil Neural Repair. 2016;30(9):866–875. doi: 10.1177/1545968316639129. [DOI] [PubMed] [Google Scholar]
- 46.Wu P, Liang F, Li Y, Yang L, Huang Y, Li A, Luo L, Tian W. Clinical observation on acupuncture plus rehabilitation training for dysphagia after stroke—a multi-centered randomised controlled trial. J Tradit Chin Med. 2011;52:45–48. [Google Scholar]
- 47.Xia W, Zheng C, Lei Q, Tang Z, Hua Q, Zhang Y, Zhu S. Treatment of post-stroke dysphagia by Vitalstim therapy coupled with conventional swallowing training. J Huazhong Univ Sci Technol. 2011;31(1):73–76. doi: 10.1007/s11596-011-0153-5. [DOI] [PubMed] [Google Scholar]
- 48.Yang EJ, Baek S-R, Shin J, Lim JY, Jang HJ, Kim YK, Paik N-J. Effects of transcranial direct current stimulation (tDCS) on post-stroke dysphagia. Restor Neurol Neurosci. 2012;30:303–311. doi: 10.3233/RNN-2012-110213. [DOI] [PubMed] [Google Scholar]
- 49.Brott T, Adams HP, Olinger CP, Marler JR, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 50.Menna S. The efficacy of speech therapy in post-intubated patients with swallowing disorder: clinical trial. RBR-9829jK
- 51.Brodsky M, Needham D. Understanding and improving dysphagia after mechanical ventilation. ClinicalTrials.gov: NCT02442102
- 52.Jakob S, Schefold J, Backlund M, Ala-Kokko T. The PhINEST study—Pharyngeal ICU Novel Electrical Stimulation Therapy. NCT 03840395 [DOI] [PMC free article] [PubMed]
- 53.Dziewas R. Pharyngeal electrical stimulation for the treatment of post-extubation dysphagia in acute stroke. ClinicalTrials.gov: NCT02470078
- 54.Hamdy S, Sasegbon S. The uility of cerebellar transcranial magnetic stimulation in the neurorehabilitation of dysphagia after stroke. NCT03274947
- 55.Macagnan FE. Brief and intensive therapy for dysphagia in patients with head and neck cancer. NCT03755921
- 56.Restivo D. tDCS for dysphagia associated to brainstem stroke. NCT04308733
- 57.Bulow M, Speyer R, Baijens L, Woisard V, Ekberg O. Neuromuscular electrical stimulation (NMES) in stroke patients with oral and pharyngeal dysfunction. Dysphagia. 2008;23:302–309. doi: 10.1007/s00455-007-9145-9. [DOI] [PubMed] [Google Scholar]
- 58.Carnaby G. Adjunctive neuromuscular electrical stimulation for the rehabilitation of swallowing (ANSRS). NCT0127982
- 59.de Fraga B, de Almeida S, Santana MG, Cassol M. Efficacy of myofunctional therapy associated with voice therapy in the rehabilitation of neurogenic oropharyngeal dysphagia: a pilot study. Int Arch Otorhinolaryngol. 2018;22:225–230. doi: 10.1055/s-0037-1605597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.El-Tamawy MS, Darwish MH, El-Azizi HS, Abdelalim AM, Taha SI. The influence of physical therapy on oropharyngeal dysphagia in acute stroke patients. Egypt J Neurol Psychiatry Neurosurg. 2015;52(3):201–205. [Google Scholar]
- 61.Eom M-J, Chang M-Y, Oh D-W, Kim H-D, Han N-M, Park J-S. Effects of resistance expiratory muscle strength training in elderly patients with dysphagic stroke. NeuroRehabilitation. 2017;41:747–752. doi: 10.3233/NRE-172192. [DOI] [PubMed] [Google Scholar]
- 62.Gao J, Zhang H-J. Effects of chin tuck against resistance exercise versus Shaker exercise on dysphagia and psychological state after cerebral infarction. Eur J Phys Rehabil Med. 2017;53(3):426–432. doi: 10.23736/S1973-9087.16.04346-X. [DOI] [PubMed] [Google Scholar]
- 63.Kim HD, Choi JB, Yoo SJ, Chang MY, Lee SW, Park JS. Tongue-to-palate resistance training improves tongue strength and oropharyngeal swallowing function in subacute stroke survivors with dysphagia. J Oral Rehabil. 2017;44:59–64. doi: 10.1111/joor.12461. [DOI] [PubMed] [Google Scholar]
- 64.Lim K-B, Lee H-J, Lim S-S, Choi Y-I. Neuromuscular electrical and Thermal-Tactile stimulation for dysphagia caused by stroke: a randomised controlled trial. J Rehabil Med. 2009;41:174–178. doi: 10.2340/16501977-0317. [DOI] [PubMed] [Google Scholar]
- 65.Park JS, Oh DH, Chang MY, Kim KM. Effects of expiratory muscle strength training on oropharyngeal dysphagia in subacute stroke patients: a randomised controlled trial. J Oral Rehabil. 2016;43:364–372. doi: 10.1111/joor.12382. [DOI] [PubMed] [Google Scholar]
- 66.Simonelli M, Ruoppolo G, Iosa M, Morone G, Fusco A, Grasso MG, Gallo A, Paolucci S. A stimulus for eating. The use of neuromuscular transcutaneous electrical stimulation in patients affected by severe dysphagia after subacute stroke: a pilot randomized controlled trial. NeuroRehabilitation. 2019;44:103–110. doi: 10.3233/NRE-182526. [DOI] [PubMed] [Google Scholar]
- 67.Han TR, Paik NJ, Park JW. Quantifying swallowing function after stroke: a functional dysphagia scale based on videofluoroscopic studies. Arch Phys Med Rehabil. 2001;82:677–682. doi: 10.1053/apmr.2001.21939. [DOI] [PubMed] [Google Scholar]
- 68.Eisenhuber E, Schima W, Schober E, et al. Videofluoroscopic assessment of patients with dysphagia: pharyngeal retention is a predictive factor for aspiration. Am J Roentgenol. 2002;178:393–398. doi: 10.2214/ajr.178.2.1780393. [DOI] [PubMed] [Google Scholar]
- 69.Han TR, Paik NJ, Park JW, Kwon BS. The prediction of persistent dysphagia beyond six months after stroke. Dysphagia. 2008;23:59–64. doi: 10.1007/s00455-007-9097-0. [DOI] [PubMed] [Google Scholar]
- 70.Hamdy S, Rothwell J, Azizm Q. Long term reorganisation of human motor cortex driven by short-term sensory stimulation. Nat Neurosci. 1998;1:64–68. doi: 10.1038/264. [DOI] [PubMed] [Google Scholar]
- 71.Bath PM, Lee HS, Everton LF. Swallowing therapy for dysphagia in acute and subacute stroke. Cochrane Database Syst Rev. 2018 doi: 10.1002/14651858. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.