Abstract
Purpose
To report a case of cicatricial ectropion and madarosis with the use of anti-epidermal growth factor receptor medication panitumumab.
Observations
An 82-year-old man with metastatic colorectal cancer presented with cicatricial ectropion and madarosis after starting panitumumab, an anti-epidermal growth factor receptor medication used to treat metastatic colorectal cancer. His findings resolved several weeks after discontinuation of panitumumab and treatment with lubrication and antibiotic/steroid ointment.
Conclusion
This case demonstrates the importance to consider potential medication side effects when treating periocular conditions in patients taking anti-epidermal growth factor receptor (anti-EGFR) agents.
Keywords: Anti-epidermal growth factor receptor (anti-EGFR), Cicatricial ectropion, Madarosis, Panitumumab
1. Introduction
Panitumumab is a monoclonal antibody targeting the epidermal growth factor receptor (EGFR) and is FDA-approved for use in metastatic colorectal cancer. It has been shown to improve the overall survival in patients with wild-type RAS metastatic colorectal cancer.1 Normal epithelial cells and cancer cells both express EGFR; therefore, common side effects of EGFR inhibitors include adverse skin reactions such as redness, acneiform dermatitis, itching, and rash. Ocular and periocular side effects are less common; however, dry eye syndrome, blepharitis, trichomegaly, and eyelid rash have been reported.2 There are very few reported cases of EGFR-related ectropion.3, 4, 5, 6 We report a case of panitumumab-induced madarosis and cicatricial ectropion.
2. Case report
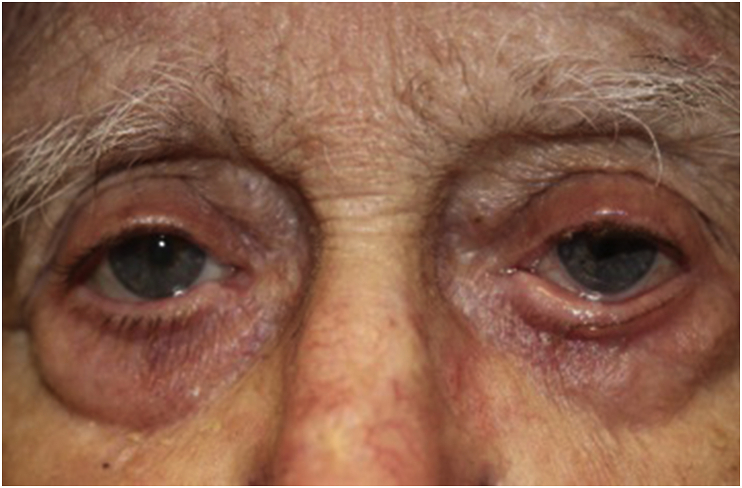
An 82-year-old Caucasian male with metastatic colon cancer presented with 3-month history of progressively worsening severe eye irritation, bilateral lower eyelid changes and loss of eyelashes. His symptoms began 2 months after his oncologist started him on the chemotherapy regimen FOLFIRI (leucoverin calcium, fluorouracil, and irinotecan hydrochloride) and panitumumab. The patient was subsequently referred for ophthalmic evaluation by his oncologist due to ongoing eye symptoms. On examination, best corrected visual acuity was 20/50 in the right eye and 20/80 in the left eye. Pupillary response, intraocular pressure, motility, and confrontation visual fields were normal. External examination revealed scalp excoriations, cicatricial ectropion of the bilateral lower eyelids, madarosis of bilateral upper and lower eyelids, bilateral upper eyelid ptosis, as well as severe eyelid margin inflammation (Fig. 1). Slit-lamp examination showed trace bilateral conjunctival hyperemia, inferior corneal staining, and nuclear sclerotic cataracts.
Fig. 1.
Initial visit; bilateral periocular response to panitumumab included cicatricial ectropion, atrophy of orbital fat, lateral canthal tendons disinsertion, madarosis, upper eyelid ptosis, as well as eyelid margin inflammation.
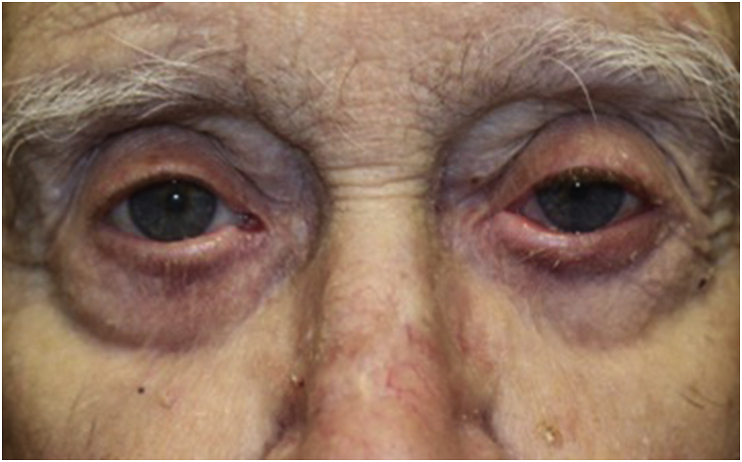
The patient was started on neomycin/polymyxin B/dexamethasone ophthalmic ointment three-times a day, and suspicion of panitumumab as the causative agent was communicated with the patient's oncologist and it was subsequently held. Two weeks after the initial visit, the patient reported improvement in periocular irritation. Eight weeks after the initial visit, there was complete and near-complete resolution of cicatricial ectropion of the right and left lower lids respectively with bilateral significant improvement of the previously noted madarosis and lid margin inflammation (Fig. 2). Three months after initial visit, patient had complete resolution of cicatricial ectropion and his madarosis continued to improve on the left side (Fig. 3). Best-corrected visual acuity improved to 20/25 in the right eye and 20/40 in the left eye. Mild residual senile ectropion remained but was well-managed with artificial tears, warm compresses, and lubricating ointment as needed.
Fig. 2.
Eight weeks after initial visit and 12 weeks after cessation of panitumumab; complete resolution of cicatricial ectropion of the right lower lid, significant improvement of madarosis, and near-complete resolution of left lower lid ectropion and lid margin inflammation.
Fig. 3.
Three months after initial visit, patient's madarosis improved on the left side, and complete resolution of cicatricial ectropion.
3. Discussion
Cicatricial ectropion occurs when there is abnormal contraction or scarring of skin secondary to infectious, inflammatory, chemical, thermal, immunological, or post-surgical changes to the eyelids. The most common adverse reactions caused by anti-EGFR medications are skin reactions, gastrointestinal disorders, fatigue, pyrexia, hypomagnesia, and paronychia.7 Adverse skin reactions associated with EGFR inhibitors are thought to be caused by direct inhibition of the EGFR in keratinocytes and hair follicles, which interferes with cell survival, proliferation, differentiation, and migration.7,8
Panitumumab is a fully human monoclonal antibody that binds and inhibits EFGRs of both normal epidermal cells and that of tumor cells. It is currently used either as monotherapy or in combination with first- and second-line chemotherapy to treat metastatic wild-type RAS colorectal cancer.1,9
Cicatricial ectropion and trichomegaly have been reported in older-generation anti-EGFR agents such as cetuximab and erlotinib.3,6, 10, 11, 12 Ocular adnexal side effects such as cicatricial ectropion and eyelid dermatitis have also been reported with the use fluoropyrimidine and docetaxel.13,14 Panitumumab-induced trichomegaly, poliosis, and cicatricial ectropion are rare.5,15 However, the combination of cicatricial ectropion and madarosis associated with panitumumab has not been previously described to our knowledge, although is not unexpected given that this has been previously reported with cetuximab.2,3,6
The use of oral doxycycline and topical ophthalmic steroid/antibiotic ointment have been tried to treat EGFR-inhibitor induced cicatricial ectropion 5; however, results from randomized clinical trials on the effects of various interventions (i.e. sunscreen, skin moisturizer, topical steroid, oral tetracycline) in the management of EGFR inhibitor-induced skin and periocular toxicity have been equivocal.8 In our case, the patient had been previously treated with FOLFIRI without experiencing periocular side effects. After a careful discussion of the risks and benefits of discontinuing panitumumab, the patient and his oncologist elected to indefinitely defer this therapy and we managed him with lubrication and topical antibiotic/steroid ointment until complete resolution of his symptoms. We believe that both the discontinuation of panitumumab and aggressive surface lubrication played significant roles in our patient's recovery.
4. Conclusion
In summary, this case serves as an example of a careful review of medications’ side effects can avoid unnecessary and premature surgical intervention in the treatment of cicatricial ectropion associated with anti-EFGR medications. Oncologists, ophthalmologists, and optometrists should be aware of the potential side effects of these medications in order to undertake collaborative management strategies, including altering chemotherapeutic regimens and conservative treatment options when necessary.
Patient consent
The patient consented to publication of the case in writing.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Declaration of competing interest
The authors have no financial disclosure.
Acknowledgements
None.
References
- 1.Lee M.S., Kopetz S. Current and future approaches to target the epidermal growth factor receptor and its downstream signaling in metastatic colorectal cancer. Clin Colorectal Canc. 2015;14(4):203–218. doi: 10.1016/j.clcc.2015.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Borkar D.S., Lacouture M.E., Basti S. Spectrum of ocular toxicities from epidermal growth factor receptor inhibitors and their intermediate-term follow-up: a five-year review. Support Care Canc. 2013;21(4):1167–1174. doi: 10.1007/s00520-012-1645-y. [DOI] [PubMed] [Google Scholar]
- 3.Garibaldi D.C., Adler R.A. Cicatricial ectropion associated with treatment of metastatic colorectal cancer with cetuximab. Ophthalmic Plast Reconstr Surg. 2007;23(1):62–63. doi: 10.1097/IOP.0b013e31802d9025. [DOI] [PubMed] [Google Scholar]
- 4.Saint-Jean A., Sainz de la Maza M., Morral M. Ocular adverse events of systemic inhibitors of the epidermal growth factor receptor: report of 5 cases. Ophthalmology. 2012 Sep;119(9):1798–1802. doi: 10.1016/j.ophtha.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Scofield-Kaplan S., Todaro J., Winn B.J. Reversible cicatricial ectropion associated with EGFR inhibitors. Orbit. 2018;37(5):364–367. doi: 10.1080/01676830.2017.1423342. [DOI] [PubMed] [Google Scholar]
- 6.Vinod K., Diaz V. Use of amniotic membrane graft in the surgical management of cicatricial ectropion associated with cetuximab therapy. J Surg Case Rep. 2015;2015(1) doi: 10.1093/jscr/rju143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ketzer S., Schimmel K., Koopman M., Guchelaar H.J. Clinical pharmacokinetics and pharmacodynamics of the epidermal growth factor receptor inhibitor panitumumab in the treatment of colorectal cancer. Clin Pharmacokinet. 2018;57(4):455–473. doi: 10.1007/s40262-017-0590-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baas J.M., Krens L.L., Guchelaar H.J. Recommendations on management of EGFR inhibitor-induced skin toxicity: a systematic review. Canc Treat Rev. 2012;38(5):505–514. doi: 10.1016/j.ctrv.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Battaglin F., Dadduzio V., Bergamo F. Anti-EGFR monoclonal antibody panitumumab for the treatment of patients with metastatic colorectal cancer: an overview of current practice and future perspectives. Expet Opin Biol Ther. 2017;17(10):1297–1308. doi: 10.1080/14712598.2017.1356815. [DOI] [PubMed] [Google Scholar]
- 10.Frankfort B.J., Garibaldi D.C. Periocular cutaneous toxicity and cicatricial ectropion: a potential class effect of antineoplastic agents that inhibit EGFR signaling. Ophthalmic Plast Reconstr Surg. 2007;23(6):496–497. doi: 10.1097/IOP.0b013e31815a124b. [DOI] [PubMed] [Google Scholar]
- 11.Lane K., Goldstein S.M. Erlotinib-associated trichomegaly. Ophthalmic Plast Reconstr Surg. 2007;23(1):65–66. doi: 10.1097/IOP.0b013e31802d9802. [DOI] [PubMed] [Google Scholar]
- 12.Methvin A.B., Gausas R.E. Newly recognized ocular side effects of erlotinib. Ophthalmic Plast Reconstr Surg. 2007;23(1):63–65. doi: 10.1097/IOP.0b013e31802d97f0. [DOI] [PubMed] [Google Scholar]
- 13.Eiseman A.S., Flanagan J.C., Brooks A.B., Mitchell E.P., Pemberton C.H. Ocular surface, ocular adnexal, and lacrimal complications associated with the use of systemic 5-fluorouracil. Ophthalmic Plast Reconstr Surg. 2003;19(3):216–224. doi: 10.1097/01.iop.0000066648.33513.3d. [DOI] [PubMed] [Google Scholar]
- 14.Kaya A.O., Buyukberber S., Coskun U. Acute erythema and edematous skin reaction and ectropion following docetaxel in a patient with non-small cell lung cancer. Cutan Ocul Toxicol. 2008;27(4):327–331. doi: 10.1080/15569520802431369. [DOI] [PubMed] [Google Scholar]
- 15.Goyal S., Uwaydat S.H. Epidermal growth factor receptor inhibitor induced trichomegaly and poliosis. Ophthalmology. 2018;125(2):294. doi: 10.1016/j.ophtha.2017.11.004. [DOI] [PubMed] [Google Scholar]