Abstract
Cerebrovascular accident (CVA) is one of the leading causes of death and disability worldwide, as well as a major financial burden for health care systems. CVA rodent models provide experimental support to determine possible in vivo therapies to reduce brain injury and consequent sequelae. This study analyzed nociceptive, motor, cognitive and mood functions in mice submitted to distal middle cerebral artery (DMCA) occlusion. Male C57BL mice (n = 8) were randomly allocated to control or DMCA groups. Motor function was evaluated with the tests: grip force, rotarod and open field; and nociceptive threshold with von Frey and hot plate assessments. Cognitive function was evaluated with the inhibitory avoidance test, and mood with the tail suspension test. Evaluations were conducted on the seventh- and twenty-eighth-day post DMCA occlusion to assess medium- and long-term effects of the injury, respectively. DMCA occlusion significantly decreases muscle strength and spontaneous locomotion (p < 0.05) both medium- and long term; as well as increases immobility in the tail-suspension test (p < 0.05), suggesting a depressive-type behavior. However, DMCA occlusion did not affect nociceptive threshold nor cognitive functions (p > 0.05). These results suggest that, medium- and long-term effects of DMCA occlusion include motor function impairments, but no sensory dysfunction. Additionally, the injury affected mood but did not hinder cognitive function.
Keywords: Locomotor activity, Motor deficit, Sensory deficit, Neuroscience, Stroke
Introduction
Cerebral stroke (CS) models in rodents provide experimental support to determine possible in vivo therapies to reduce brain injury and consequent functional sequelae (Bacigaluppi et al. 2010). CS models in mice that affect the cerebral cortex are induced primarily by distal middle cerebral artery (DMCA) occlusion, which is a translational model of the most prevalent form of CS in clinical practice (approximately 87% of the cases of ischemic CS) (Mozzafarian et al. 2016). Additionally, the DMCA model has low mortality rates and allows for the investigation of medium and long term post-ischemic repercussions, the effects of neuroprotection and neuroplasticity, and/or the effectiveness of therapeutic and surgical strategies (Rosell et al. 2013).
Oliveira et al. (2014) analyzed cortical layers I through VI, in the motor areas, 48 h after mice were submitted to focal ischemia induced by DMCA occlusion. The authors demonstrated that cellular pathological alterations were most prominent in layer V, with nuclear pyknosis, chromatin fragmentation, degeneration—mainly in neurons—as well as morphological evidences of apoptosis. The cortical motor area was severely compromised by the ischemic insult, revealing neuroinflammation with proliferation and activation of astrocyte and microglial cells. However, the authors failed to analyze the possible functional deficits induced by DMCA occlusion warranting a more complete functional deficit characterization in this preclinical stroke model to yield more translationally relevant knowledge. In this context, the present study investigates possible functional alterations, such as: motor, sensorial (nociceptive), mood and cognitive in mice subjected to DMCA occlusion.
Methods
Animals
All animal care and experimental procedures were carried out in accordance with the National Institutes of Health Animal Care Guidelines (NIH publications number 80–23) and were approved by the Ethics Committee of the University of Southern Santa Catarina (protocol number 15.022.4.01.IV). All experiments were conducted using male C57/BL6 mice (25–35 g, n = 8 animals per group), housed at 22 ± 2 °C under a 12 h light/12 h dark cycle (lights on at 6:00 a.m.) and with free access to food and water. The animals were acclimated to the laboratory for at least 1 h before the experiments that were carried out between 8:00 and 12:00 h.
Surgery procedure: distal middle cerebral artery occlusion in mice
DMCA occlusion was performed as previously described by Oliveira et al. (2014), with a modified protocol from Kuraoka et al. (2009). Briefly, the mice were anesthetized by intraperitoneal (i.p.) injection of ketamine (50 mg/kg) and xylazine (5 mg/kg). After anesthesia the skin was incised and detached to expose the temporal muscle at the insertion of the temporal and zygomatic bone, allowing access to the temporal fossa. The temporal bone was thinned down with a micromotor (LB100, Beltec) and the left MCA distal portion was exposed. The occlusion was induced through electrocauterization with an electronic scalpel (BE 3000, KVN). The whole procedure was conducted with the help of a magnifying glass (SZ2-LGB, Olympus).
To evaluate functional repercussions of motor cortex ischemia induced by DMCA occlusion, the animals were submitted to different test protocols on the seventh (7) and twenty-eighth day (28) post DMCA (medium- and long-term effects induced by the injury, respectively). In each test, 16 animals allocated to two groups (n = 8), a control group (consisting of mice that did not undergo DMCA occlusion) and a DMCA occlusion group.
Assessment of sensitivity (sensory), muscle strength and locomotion (motor)
Sensorial assessment: von Frey and hot plate tests
Mechanical hyperalgesia was assessed using the von Frey test (Martins et al. 2016). Mice were placed individually in clear plexiglass boxes (9 cm × 7 cm × 11 cm) on elevated wire mesh platforms that provided access to the ventral surface of the right hind paw. A mechanical stimulus was applied perpendicularly to the plantar surface, with enough pressure to lightly curve the filament. Animals were evaluated only when all four paws were in contact with the wire mesh. Paw withdrawal responses were counted only when the animals fully removed the paw from the mesh. Mechanical hyperalgesia was assessed as the percentage of paw withdrawals in response to a series of 10 nonconsecutive stimuli (at 1-min intervals) with a 0.4 g and a 1.0 g von Frey monofilament (Stoelting, Chicago, IL, USA). The results are presented as a percentage of response, with 100% being 10/10 and 0% being 0/10 responses, as previously described (Martins et al. 2016).
Thermal hyperalgesia was assessed with a hot plate apparatus (Socrel, model-DS 37) maintained at 48 ± 2 °C. In this test the mice were placed inside an acrylic cylinder (20 cm in diameter) placed directly on the heated surface, and the amount of time (s) between placement and nociceptive behavior (licking the hindpaws or jumping—whichever occurred first) was recorded as response latency. To prevent tissue damage, a 30-s cutoff was used (Martins et al. 2016). The animals were evaluated at baseline and on days 7 and 28 following DMCA occlusion.
Muscle strength assessment: grip force test
Muscle strength was evaluated using the grip strength test conducted with a grip-force measurement system (Insight, Ribeirão Preto, SP, Brazil) as previously described (Martins et al. 2018). In this test, a wire grid measuring 10 cm × 12 cm was coupled to a dynamometer that recorded the force (g) measured by the animal’s paws (forepaws and hindpaws). Each animal was tested twice, in 2–3-min intervals, to obtain unilateral mean force. The mice were evaluated at baseline and on days 7 and 28 following DMCA occlusion.
Spontaneous locomotion: open field test
To evaluate the effect of DMCA occlusion on spontaneous locomotor activity, the mice were submitted to the open field test. The open-field apparatus consists of a wooden box measuring 40 × 60 × 50 cm. The floor of the arena is divided into 12 equal squares, and the number of squares crossed by the animal with all paws were counted in a 6 min session (Brooks and Dunnett 2009). The mice were evaluated at baseline and on days 7 and 28 following DMCA occlusion.
Forced locomotion: rota rod test
The rotarod apparatus, Rotamex-V-EE/85 (Columbus Instruments, USA) consists of an acrylic box divided into four compartments with a rotating shaft in-between.
Before DMCA occlusion, each mouse was trained in the rotarod apparatus (3 cm in diameter, 15 rpm) for up to 300 s. After the surgical procedure, mice were evaluated on the 7th and 28th day post-injury. The duration of time the animal stays on the rotating rod is a measure of their balance, coordination, physical condition, and motor-planning. Cut-off time was 300 s (Brooks and Dunnett 2009).
Mood and cognitive behavioral tests
Tail suspension test
The tail suspension test (TST) was performed according to the method previously described (Steru et al. 1985). Mice were suspended 50 cm above the floor by adhesive tape placed approximately 1 cm from the tip of the tail. Immobility time was recorded during a 6-min session. The animals are considered immobile when hanging passively and remaining completely motionless (Moretti et al. 2013). The mice were evaluated on days 7 and 28 following DMCA occlusion.
Step-down inhibitory avoidance task
A step-down inhibitory avoidance apparatus (Insight, Brazil) was used to evaluate aversive memory. In the training trial, animals were placed on a platform and received a footshock (0.2 mA, 2.0 s) after stepping down from the platform onto the grid. A retention test trial was performed 24 h after training (long-term memory) and was procedurally identical to the training, except that no foot shock was presented. The retention test step-down latency (maximum, 180 s) was used as a measure of inhibitory avoidance retention. Typically, animals with no cognitive impairment learn to remain longer on the platform than during the training session (Szapiro et al. 2002).
Statistical analysis
The results were analyzed in the GraphPad Prism program (version 6.0, La Jolla, California, USA). Initially, the Shapiro–Wilk normality test was applied to evaluate the normality of the data. Statistical analyses were carried by two-way ANOVA followed by Bonferroni’s test when appropriate, these analyses were presented as mean ± standard deviation (SD). The experiments designed to evaluate the involvement of mood and cognition were analyzed with unpaired Student’s t test. Data were presented as mean ± standard deviation. Values of p < 0.05 were considered as statistically significant.
Results
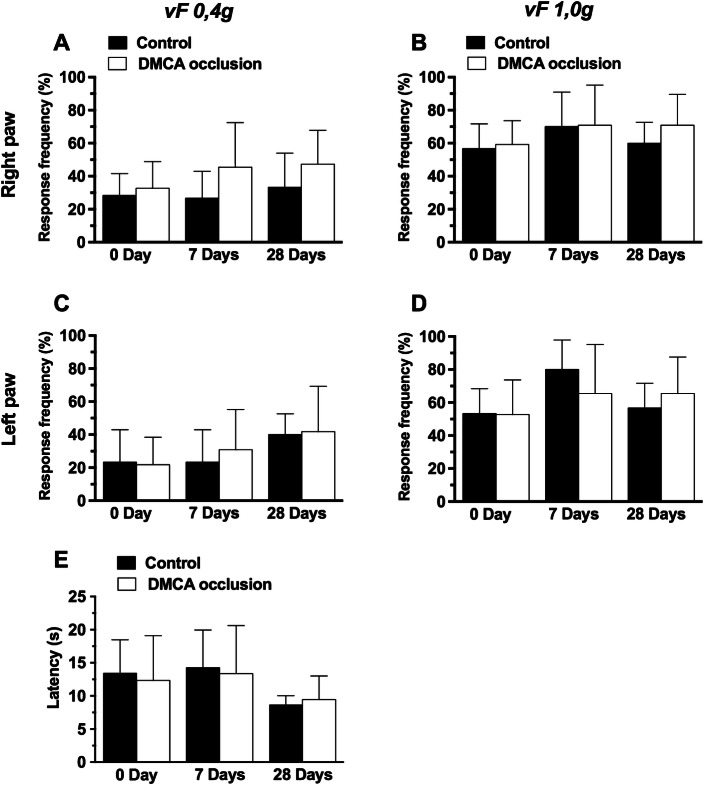
Results presented in Fig. 1a–d demonstrate that DMCA occlusion did not affect (p >0.05) hindpaw mechanical withdrawal frequency; neither (E) hindpaw thermal withdrawal latency (p >0.05), having no effect upon mechanical or thermal response thresholds.
Fig. 1.
Effect of distal middle cerebral artery (DMCA) occlusion on mechanical and thermal hyperalgesia in mice (a–e). Mechanical hyperalgesia assessment with a 0.4 g vF filament: right (a) and left hind paw (c). Mechanical hyperalgesia assessment with a 1.0 g vF filament: right (b) and left hind paw (f). e Thermal hyperalgesia assessment (48 °C). Each group represents the average of 8 animals and the vertical bars indicate the standard deviation. 7 days; 28 days: 7 or 28 days after DMCA occlusion. Two-way ANOVA followed by the Bonferroni test. vF: von Frey. *p < 0.05
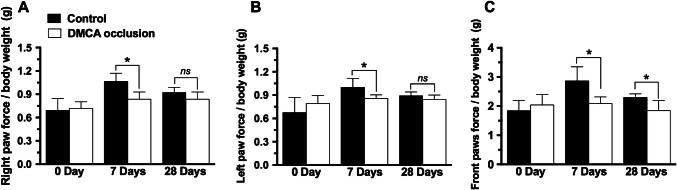
Results depicted on Fig. 2 show that CVA reduced right (panel a) as well as left (b) hindpaw grip force 7 days after ischemic injury (p <0.05). Twenty-eight days after DMCA occlusion there were no statistically significant differences in grip force between DMCA and control groups. Adversely, front paw grip force (panel c) was reduced on day seven (− 25%, p <0.05) as well on day twenty-eight (− 39.3%, p <0.05) after occlusion.
Fig. 2.
Effect of distal middle cerebral artery (DMCA) occlusion on grip force in mice (a–c). Right hind paw grip force (a), left hind paw grip force (b), front paws grip force (c). Each group represents the average of 8 animals and the vertical bars indicate the standard deviation. 7 days; 28 days: 7 or 28 days after DMCA occlusion. Two-way ANOVA followed by the Bonferroni test. *p < 0.05. ns not significant
Figure 3a demonstrates that DMCA occlusion significantly reduced spontaneous locomotive activity in the open field test at 7 days (p <0.05) but not 28 days (p >0.05) after the insult. Interestingly, there was no significant reduction in locomotor activity of DMCA animals in the period between day 7 and 28 post-DMCA, different from that observed in the control group. DMCA occlusion did not affect forced locomotion activity (p >0.05, panel b).
Fig. 3.
Effect of distal middle cerebral artery (DMCA) occlusion on locomotion of mice (a, b). Spontaneous locomotive activity in the Open Field test (a). Forced locomotive activity in the Rota-rod test (b). Each group represents the average of 8 animals and the vertical bars indicate the standard deviation. 7 days; 28 days: 7 or 28 days after DMCA occlusion. Two-way ANOVA followed by the Bonferroni test. vF: von Frey. *p < 0.05. ns not significant
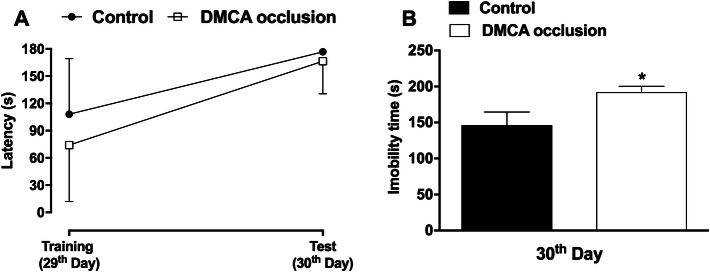
Results presented in Fig. 4a show that DMCA occlusion did not affect memory neither learning capacity (p >0.05). On the other hand, DMCA group animals were immobile for a longer period of time in the tail suspension test, an indication of depressive-like behavior (31.5%, p <0.05, panel b).
Fig. 4.
Effect of distal middle cerebral artery (DMCA) occlusion on mood and cognition tests in mice. Effect on learning ability and memory formation (a). Immobility time—behavior of the depressive type—(b). Each group represents the average of 8 animals and the vertical bars indicate the standard deviation. 29th Days; 30th Days: days after DMCA occlusion. Paired Student’s t test (a). Unpaired Student’s t test (b). *p < 0.05
Discussion
In the present study, we investigated medium- and long-term effects of DMCA occlusion on locomotive activity, nociception, cognition and depression-like behavior in mice. According to a study by Rosell et al. (2013), short-term analyses are those performed up to 48 h after an injury; medium-term up to 1 or 2 weeks; and long-term over that period. However, in the last update from the Stroke Treatment Academic Industry Roundtable (STAIR) (Fisher et al. 2009), (researchers in ischemic brain injury that hold meetings to determine consensus in the treatment of stroke patients), it was recommended that behavioral assessments in preclinical stroke models should be performed at least three or more weeks after the ischemic event, to demonstrate sustained benefits induced by the treatments (Braeuninger and Kleinschnitz 2009).
Motor-related deficits
The animals subjected to left DMCA occlusion developed medium and long-term marked motor deficits (Braeuninger and Kleinschnitz, 2009). These results were expected with this preclinical model of brain injury as it primarily affects the motor cortex as previously described by Oliveira et al. (2014). Motor deficits were more pronounced contralateral to the side of DMCA injury, as approximately 90% of the fibers of the corticospinal tract cross midline to form the lateral corticospinal tract (Machado and Haertel 2013) innervating the contralateral musculature. Even though a lesion in the left motor cortex normally produces motor changes in the right hemi-body of the patients, the animals subjected to left DMCA occlusion also presented ipsilateral (left) hindpaw motor deficits; although these were less marked when compared to the right paw. The 10% of the fibers of the corticospinal tract that do not cross midline likely account for these observed ipsilateral deficits (Machado and Haertel 2013).
Grip strength deficits
Other studies have addressed grip force loss in animal models of CS, with some achieving a similar outcomes (Molina 2011) as found in the current study, while others failing to identify any post-ischemia impairment (Craft et al. 2005). Rosell et al. (2013) found that hind limb grip strength was reduced in C57BL/6 mice 24 h after permanent ischemia induced by MCA electrocoagulation. However, the systematic use of grip force to evaluate motor sequelae following CVA has yet to become common practice (Molina 2011). In the present study, during post DMCA medium- and long-term evaluations (i.e., 1–3 weeks after the injury), the animals consistently presented decreases in grip force in all four limbs. However, after 4 weeks, this motor function returned, possibly due to innate regenerative capacity, neuronal plasticity and/or other compensatory mechanisms (Fisher et al. 2009).
Nociceptive effects
Studies have shown that injury or dysfunction in the motor cortex may underlie the pathophysiology of many painful conditions such as complex regional pain syndrome type 1 (CRPS-I), low back pain, or phantom limb pain (Gregory et al. 2013). To verify the presence/absence of mechanical/thermal allodynia or hyperalgesia in the present study, the animal’s sensory threshold to innocuous (0.4 g) or noxious (1.0 g) mechanical stimulus, as well as to thermal stimulation (heat at 48 °C) was evaluated. Contrary to expectations, we found no alterations in mechanical nor thermal response thresholds following DMCA occlusion suggesting that the DMCA-related motor cortex injury does not influence the nociceptive response threshold in animals.
Exploratory/balance deficits
Another important finding of this study was that DMCA occlusion significantly induced medium- but not long-term deficits to the animal’s exploratory behavior. These data are in line with the medium-term reduction of grip strength in both paws observed in the present study. However, there were no medium- nor long-term effects on coordination nor balance, as observed using the Rota rod test. As different areas of the brain are involved, loss of grip strength (controlled by the motor cortex) does not necessarily lead to balance impairments (mainly controlled by the cerebellum) (Bouët et al. 2007). Data obtained from the open field test suggests that control group animals, as expected, when re-exposed to the open field apparatus explored less, which is indicated by a significant reduction in the number of crosses. This finding may be associated with mnemonic impairment (habituation), although additional studies are needed to confirm this hypothesis (Zhoua et al. 2016). However, this finding was not observed in the animals submitted to DMCA occlusion, as they explored the apparatus as if they had not been previously exposed to it. Redish and Touretzky (1997) found that the hippocampus possesses ability of spacial memory associated with spatial navigation in rodent animal. Place cells in hippocampus are the biological foundation of cognitive map, and they were first found by O’Keefe and Nadel (1978) in the hippocampus with an electrophysiological method. Rodent animal can accomplish self-locating and path-finding task by forming a cognitive map in the hippocampus representing the environment. The study Wang et al. 2017, from the view of energy coding method, proves that synaptic plasticity plays an important role in spatial memory and mental exploration. The results show that the synaptic connections between place cells have a decisive influence on the distribution of the energy field, so as to affect the gradient of the energy field gradient-navigation vector. However, the change of navigation vector affects the path-finding of mental exploration, the repeat of the old path and the generation of the new path correspond to the certain firing patterns of place cells, which changes the synaptic connection strength in turn.
Depression-like and cognitive behavior
Longer immobility time in the tail suspension test, commonly classified as a depression-like behavior, was also induced by DMCA occlusion. Previous clinical trials have demonstrated a strong association between CS and the subsequent development of depression (Burvill et al. 1995; Robinson et al. 1997; Kimura et al. 2000; Hackett et al. 2005; Hussain, 2018; Lamti et al. 2019). Depression after CS is associated with cognitive dysfunction and worsening of functional impairment, as well as increased mortality (Johnson et al. 2007). Neural oscillations are known to show great variability and apparently random changes over time and brain conditions (Dimitriadis et al. 2015). Several studies have proven that neuronal oscillations in the human brain possess long-range temporal correlations (LRTC) in frequency, amplitude, duration and recurrence (Linkenkaer-Hansen et al. 2005). The analysis of LRTC which provides a quantitative index to dynamical oscillation of different time scales, have been applied to study electroencephalographic (EEG) oscillations of the human brain under different physiological and pathological states (Bornas et al. 2013; Lee et al. 2007). Hou et al. 2017, proposed to investigate the LRTC of EEG oscillation in depressive subjects after cerebral infarction. Statistical analysis was conducted to find the difference among different groups and correlation between the severity of depression and EEG oscillation, hoping to provide a novel insight into depression following cerebral infarction. Some studies have proposed that there is a higher risk of depression when the lesion is in the left hemisphere (Robison 2003). In a clinical study in which depression was defined by a score over 10 in the Beck Depression Inventory (BDI), the prevalence of the disease in the 11th month post-CS was 55% (Kotila et al. 1998); while in a study using the Present State Examination (PSE) and the Diagnostic and Statistical Manual of Mental Disorders (DSM-III) criteria, the prevalence of depression in the same post-CS period was of 33% (Robinson et al. 1987). Neither memory or learning capacity was adversely impacted by DMCA occlusion.
Cortical stroke preclinical model limitations
Mice models are well-characterized in genetics and molecular biology and have been increasingly used in the study of CS since the 1990s (Rosell et al. 2013, Howells et al. 2010). However, despite more than 20 different behavioral tests having been validated in animal models of CS (Lubjuhn et al. 2009), there still remains no consensus on which test is most appropriate for which CS model, thus each researcher must delineate selected tests according to their experimental design and preclinical model (Lubjuhn et al. 2009). While the rodent DMCA occlusion model is a valid model of CS for the study of cellular death and recovery, it presents challenges and certain limitations when used as a translational model to study functional recovery. For example, the occlusion process of a cerebral vessel in humans occurs generally as a result of long-term continuous injury to the cardiovascular system and is associated with the presence of comorbidities such as diabetes mellitus, systemic arterial hypertension and dyslipidemia—arising from obesity and sedentarism or even as a result of the natural aging process (Zhu and Auer 2004; O’Collins et al. 2006). In contrast, the animals that are typically used as a model of cerebral artery occlusion and subsequent ischemia/necrosis of the nervous tissue are previously healthy young animals with their biological and physiological capacities at their maximum levels of performance, a fact that likely gives them a greater probability of recovery (Duverger and MacKenzie 1988; Tamaki et al. 1995; Braeuninger and Kleinschnitz 2009). Unlike humans, these animals often present spontaneous recovery within a few days of the injury and exhibit a very low mortality rate (Braeuninger and Kleinschnitz 2009; Lubjuhn et al. 2009; Rosell et al. 2013; Tarai et al. 2019). To address this issue, greater consideration should be given to performing CS neurological injuries in animal models with comordity conditions. In addition, one must remain mindful of different anatomical proportions of white versus gray matter of mice when compared to humans, as well as functional differences of both brains (Rosell et al. 2013). With these limitations in mind, it becomes increasingly important that preclinical behavioral studies, similar to that of the present study, are being performed in order to develop and better refine long-term neurological impairments and behavioral function in rodents, similar to the National Institute of Health Stroke Scale (NIHSS) used in human neurological injury (Kasner 2006).
Conclusion
In conclusion, the present study reported that, in the medium- and long term, DMCA occlusion induced motor function deficits, but did not affect mechanical or thermal nociceptive threshold. In addition, the cortical injury altered exploratory and long-term depressive-like behavior but did not induce cognitive impairment. While this study provides new behavioral insights, additional behavioral and functional characterization using the DCMA occlusion model, as well as other preclinical stroke models, will be required prior to studies investigating the potential therapeutic effects of pharmacological and non-pharmacological interventions on behavior and function. This type of preclinical work becomes ever more crucial as functional independence remain the main recovery indicators following cortical stroke regardless of residual signs of brain damage (Lubjuhn et al. 2009).
Acknowledgements
Supported by grants from Universidade do Sul de Santa Catarina Curso de Medicina and Programa Unisul de Iniciação Científica (PUIC), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq—430556/2018-7 and 309407/2017-6), and Fundação de Amparo a Pesquisa e Inovação do Estado de Santa Catarina (FAPESC-2019TR73), Brazil.
Abbreviations
- CS
Cerebral stroke
- CVA
Cerebrovascular accident
- DMCA
Distal middle cerebral artery
- min
Minutes
- rpm
Revolutions per minute
- s
Seconds
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bacigaluppi M, Comi G, Hermann DM. Animal models of ischemic stroke. Part one: modeling risk factors. Open Neurol J. 2010;4:26–33. doi: 10.2174/1874205X01004020026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornas X, Noguera M, Balle M, et al. Long-range temporal correlations in resting EEG: its associations with depression related emotion regulation strategies. J Psychophysiol. 2013;27:60–66. doi: 10.1027/0269-8803/a000087. [DOI] [Google Scholar]
- Bouët V, Freret T, Toutain J, Divoux D, Boulouard M, SchumannBard P. Sensorimotor and cognitive deficits after transient middle cerebral artery occlusion in the mouse. Exp Neurol. 2007;203:555–567. doi: 10.1016/j.expneurol.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Braeuninger S, Kleinschnitz C. Rodent models of focal cerebral ischemia: procedural pitfalls and translational problems. Exp Transl Stroke Med. 2009;1:8. doi: 10.1186/2040-7378-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SP, Dunnett SB. Tests to assess motor phenotype in mice: a user’s guide. Nat Rev Neurosci. 2009;10:519–529. doi: 10.1038/nrn2652. [DOI] [PubMed] [Google Scholar]
- Burvill PW, Johnson GA, Jamrozik KD, Anderson CS, Stewart-Wynne EG, Chakera TM. Prevalence of depression after stroke: the Perth Community Stroke Study. Br J Psychiatry. 1995;166:320–327. doi: 10.1192/bjp.166.3.320. [DOI] [PubMed] [Google Scholar]
- Craft TK, Glasper ER, McCullough L, Zhang N, Sugo N, Otsuka T, et al. Social interaction improves experimental stroke outcome. Stroke. 2005;36:2006–2011. doi: 10.1161/01.STR.0000177538.17687.54. [DOI] [PubMed] [Google Scholar]
- Dimitriadis SI, Laskaris NA, Micheloyannis S. Transition dynamics of EEG-based network microstates during mental arithmetic and resting wakefulness reflects task-related modulations and developmental changes. Cogn Neurodyn. 2015;9:371–387. doi: 10.10007/s11571-015-9330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duverger D, Mackenzie ET. The quantification of cerebral infarction following focal ischemia in the rat: influence of strain, arterial pressure, blood glucose concentration, and age. J Cereb Blood Flow Metab. 1988;8:449–461. doi: 10.1038/jcbfm.1988.86. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SL, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory NS, Harris AL, Robinson CR, Dougherty PM, Fuchs PN, Sluka KA. An overview of animal models of pain: disease models and outcome measures. J Pain. 2013;14:1255–1269. doi: 10.1016/j.jpain.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett ML, Yapa C, Parag V, Anderson CS. Frequency of depression after stroke: a systematic review of observational studies. Stroke. 2005;36:1330–1340. doi: 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- Hou D, Wang C, Chen Y, Wang W, Du J. Long-range temporal correlations of broadband EEG oscillations for depressed subjects following different hemispheric cerebral infarction. Cogn Neurodyn. 2017;11:529–538. doi: 10.1007/s11571-017-9451-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells DW, Porritt MJ, Rewell SSJ, O’Collins V, Sena ES, Worp HBVD, et al. Different strokes for different folks: the rich diversity of animal models of focal cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1412–1431. doi: 10.1038/jcbfm.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain L. Detecting epileptic seizure with different feature extracting strategies using robust machine learning classification techniques by applying advance parameter optimization approach. Cogn Neurodyn. 2018;3:271–294. doi: 10.1007/s11571-018-9477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JK, Lui LY, Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J Gerontol A Biol Sci Med Sci. 2007;62:1134–1141. doi: 10.1093/gerona/62.10.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5:603–612. doi: 10.1016/S1474-4422(06)70495-1. [DOI] [PubMed] [Google Scholar]
- Kimura M, Robinson RG, Kosier JT. Treatment of cognitive impairment after poststroke depression: a double-blind treatment trial. Stroke. 2000;31:1482–1486. doi: 10.1161/01.STR.31.7.1482. [DOI] [PubMed] [Google Scholar]
- Kotila M, Numminen H, Waltimo O, Kaste M. Depression after stroke. Results of the FINNSTROKE Study. Stroke. 1998;29:68–72. doi: 10.1161/01.STR.29.2.368. [DOI] [PubMed] [Google Scholar]
- Kuraoka M, Furuta T, Matsuwaki T, Omatsu T, Ishii Y, Kyuwa S, Yoshikawa Y. Direct experimental occlusion of the distal middle cerebral artery induces high reproducibility of brain ischemia in mice. Exp Anim. 2009;58:19–29. doi: 10.1538/expanim.58.19. [DOI] [PubMed] [Google Scholar]
- Lamti HA, Ben Khelifa MM, Hugel V. Mental fatigue level detection based on event related and visual evoked potentials features fusion in virtual indoor environment. Cogn Neurodyn. 2019;3:271–285. doi: 10.1007/s11571-019-09523-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Yang BH, Lee JH, et al. Detrended fluctuation analysis of resting EEG in depressed outpatients and healthy controls. Clin Neurophysiol. 2007;118:2489–2496. doi: 10.1016/j.clinph.2007.08001. [DOI] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K, Monto S, Rytsala H, et al. Breakdown of long-range temporal correlations in theta oscillations in patients with major depressive disorder. J Neurosci. 2005;25:10131–10137. doi: 10.1523/JNEUROSCI.3244-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubjuhn J, Gastens A, Wilpert GV, Bargiotas P, Herrmann Murikinati OS, et al. Functional testing in a mouse stroke model induced by occlusion of the distal middle cerebral artery. J Neurosci Methods. 2009;184:95–103. doi: 10.1016/j.jneumeth.2009.07.029. [DOI] [PubMed] [Google Scholar]
- Machado A, Haertel LM. Neuroanatomia funcional. 3. São Paulo: Atheneu; 2013. [Google Scholar]
- Martins DF, Turnes BL, Cidral-Filho FJ, Bobinski F, Rosas RF, Danielski LG, Petronilho F, Santos AR. Light-emitting diode therapy reduces persistent inflammatory pain: role of interleukin 10 and antioxidant enzymes. Neuroscience. 2016;324:485–495. doi: 10.1016/j.neuroscience.2016.03035. [DOI] [PubMed] [Google Scholar]
- Martins DF, Martins TC, Batisti AP, Leonel LS, Bobinski F, Belmonte LAO, Martins LM, Ferreira EC, Santos ARS. Long-term regular eccentric exercise decreases neuropathic pain-like behavior and improves motor functional recovery in an axonotmesis mouse model: the role of insulin-like growth factor-1. Mol Neurobiol. 2018;55:6155–6168. doi: 10.1007/s12035-017-0829-3. [DOI] [PubMed] [Google Scholar]
- Molina CA. Reperfusion therapies for acute ischemic stroke: current pharmacological and mechanical approaches. Stroke. 2011;42:16–19. doi: 10.1161/STROKEAHA.110.598763. [DOI] [PubMed] [Google Scholar]
- Moretti M, Budni J, Freitas AE, Rosa PB, Rodrigues AL. Antidepressant-like effect of ascorbic acid is associated with the modulation of mammalian target of rapamycin pathway. J Psychiatr Res. 2013;48:16–24. doi: 10.1016/j.jpsychires.2013.10014. [DOI] [PubMed] [Google Scholar]
- Mozzafarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2016 update: a report from the American heart association. Circulation. 2016;133(4):e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- O’Collins VE, Macleod MR, Donnan GA, Horky LL, Worp van der BH, Howells DW (2006) 1026 experimental treatments in acute stroke. Ann Neurol 59:467–477, 62:1134–1141. 10.1002/ana.20741 [DOI] [PubMed]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Oxford University Press; 1978. [Google Scholar]
- Oliveira JL, Crispin PD, Duarte EC, Marloch GD, Gargioni R, Trentin AG, Silva MA. Histopathology of motor cortex in an experimental focal ischemic stroke in mouse model. J Chem Neuroanat. 2014;57–58:1–9. doi: 10.1016/j.jchemneu.2014.03002. [DOI] [PubMed] [Google Scholar]
- Redish AD, Touretzky DS (1997) Beyond the cognitive map. Ph.D. thesis, Department of Computer Science, Carnegie Mellon University, Pittsburg
- Robinson RG, Bolduc PL, Price TR. Two-year longitudinal study of poststroke mood disorders: diagnosis and outcome at one and two years. Stroke. 1987;18:837–843. doi: 10.1161/01.STR.18.5.837. [DOI] [PubMed] [Google Scholar]
- Robinson RG, et al. Neuropsychiatric consequences of stroke. Annu Rev Med. 1997;48:217–229. doi: 10.1146/annurev.med.48.1.217. [DOI] [PubMed] [Google Scholar]
- Robison R. Poststroke depression: prevalence, diagnosis, treatment, and disease progression. Biol Psychiatry. 2003;54:376–387. doi: 10.1016/S0006-3223(03)00423-2. [DOI] [PubMed] [Google Scholar]
- Rosell A, Agin V, Rahman M, Morancho A, Ali C, Koistinaho J, et al. Distal occlusion of the middle cerebral artery in mice: are we ready to assess long-term functional outcome? Transl Stroke Res. 2013;4:297–307. doi: 10.1007/s12975-012-0234-1. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Szapiro G, Galante JM, Barros DM, et al. Molecular mechanisms of memory retrieval. Neurochem Res. 2002;27:1491–1498. doi: 10.1023/A:1021648405461. [DOI] [PubMed] [Google Scholar]
- Tamaki K, Nakai M, Yokota T, Ogata J. Effects of aging and chronic hypertension on cerebral blood flow and cerebrovascular CO2 reactivity in the rat. Gerontology. 1995;41:11–17. doi: 10.1159/000213657. [DOI] [PubMed] [Google Scholar]
- Tarai S, Mukherjee R, Gupta S, Rizvanov AA, Palotás A, Chandrasekhar Pammi VS. Influence of pharmacological and epigenetic factors to suppress neurotrophic factors and enhance neural plasticity in stress and mood disorders. Cogn Neurodyn. 2019;3:219–237. doi: 10.1007/s11571-019-09522-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang R, Zhu Y. Optimal path-finding through mental exploration based on neural energy field gradients. Cogn Neurodyn. 2017;11:99–111. doi: 10.1007/s11571-016-9412-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhoua LYY, Wright TE, Clarkson AN. Prefrontal cortex stroke induces delayed impairment in spatial memory. Behav Brain Res. 2016;296:373–378. doi: 10.1016/j.bbr.2015.08.022. [DOI] [PubMed] [Google Scholar]
- Zhu CZ, Auer RN. Optimal blood glucose levels while using insulin to minimize the size of infarction in focal cerebral ischemia. J Neurosurg. 2004;101:664–668. doi: 10.3171/jns.2004.101.4.0664. [DOI] [PubMed] [Google Scholar]