Abstract
Background
HIV-1 sexual transmission occurs mostly through infected semen, which contains both free virions and infected leukocytes. Transmission initiated by infected cells has been shown by several in vitro and in vivo studies and a reduced capacity of broadly neutralizing antibodies (bNAbs) to inhibit cell-to-cell transmission has also been reported. However, due to limitations of available experimental models, there is yet no clarity to which extend bNAbs can prevent transmission mediated by semen leukocytes.
Methods
We developed a novel in vitro assay to measure cell-cell transmission that makes use of splenocytes or CD45+ semen leukocytes collected from acutely SHIV162P3-infected cynomolgus macaques. A panel of 11 bNAbs was used either alone or in combination to assess their inhibitory potential against both cell-free and cell-cell infection.
Findings
Splenocytes and semen leucocytes displayed a similar proportion of CD4+T-cell subsets. Either cell type transferred infection in vitro to target TZM-bl cells and PBMCs. Moreover, infection of macaques was achieved following intravaginal challenge with splenocytes. The anti-N-glycans/V3 loop bNAb 10–1074 was highly efficient against cell-associated transmission mediated by infected spleen cells and its potency was maintained when transmission was mediated by CD45+ semen leukocytes.
Interpretation
These results support the use of bNAbs in preventative or therapeutic studies aiming to block transmission events mediated not only by free viral particles but also by infected cells. Our experimental system could be used to predict in vivo efficacy of bNAbs.
Funding
This work was funded by the ANRS and the European Commission.
Keywords: Cell-to-cell transmission, Semen cells, bNAbs
Research in context.
Evidences before this study: HIV-1 transmission mediated by semen leukocytes plays a predominant role in infecting individuals and may represent a mechanism through which the virus can evade antibody-based immunity. When pursuing success in therapeutic antibody strategies, potency of bNAbs against both cell-free and infected cell-associated pathways should be considered.
Previous studies have reported that the ability of broad neutralizing antibodies (bNAbs) to interfere with cell-to-cell transmission depends on the cell type and the antibody used, however, there is no indication that bNAbs can prevent transmission mediated by semen leukocytes. Cell lines or even CD4+ T cells derived from blood have a different phenotype compared to semen cells. Thus, it is of utmost importance to establish more physiologically relevant in vitro systems which could predict the in vivo potency of bNAbs and inform immunoprophylaxis studies.
Added value of this study: Using the non-human primate model of SHIV162P3 infection, we describe a method for blocking cell-to-cell transmission with bNAbs using cells from spleen and semen from in vivo infected macaques. This assay could be used to down-select bNAbs displaying both high potency and efficacy against cell-to-cell transmission.
We provided evidences that bNAbs, including the anti-N-glycans/V3 loop bNAb 10–1074, inhibited with high efficiency in vitro cell-to-cell transmission mediated by both infected spleen cells and CD45+ semen leukocytes. This is the first study demonstrating that bNAbs could prevent transmission mediated by infected semen lymphocytes and the results support the use of bNAbs in clinical trials aiming to block cell-associated HIV-1.
Implications of all the available evidences: bNAbs represent a promising approach to HIV-1 prevention and treatment. Nevertheless challenges accompany the use of bNAbs, including sub-optimal efficacy in virus cell-to-cell transmission. Incomplete neutralization may allow HIV-1 to evade certain neutralizing responses by spreading through cell-cell pathway and favouring emergence of escape mutations. Current bNAbs may not be as broad and potent as predicted by in vitro assays. New screening methods that better predict in vivo bNAb sensitivity would help to select antibody candidates to be used in immunotherapy regiments.
Alt-text: Unlabelled box
1. Introduction
HIV-1 infection continues to be a major public health issue, with sexual transmission mediated by semen being responsible for more than 60% of new transmission events [1]. The virus is present in the semen as cell-free virions and also in lymphocytes [2], [3], [4]. Various in vitro and ex vivo studies have demonstrated that cell-associated virus (CAV) is transmitted 10- to 100-fold more efficiently than cell-free virus [2,5,6]. In addition, we and others have shown that systemic infection can be initiated in macaques in vivo following either intravaginal, intrarectal, or intravenous inoculation of SIV-infected cells [7], [8], [9]. Indeed, semen leucocytes are productively infected during all stages of SIVmac infection in cynomolgus macaques [10], similarly to those of HIV-1 infected humans [11,12]. Finally, several clinical studies have suggested a role for infected cells in sexual HIV-1 transmission.
An increasing number of studies have reported that broadly neutralizing antibodies (bNAbs) efficiently prevent in vivo intravenous and mucosal infection by cell-free HIV/SHIV [13], [14], [15], [16], [17], [18], [19], [20]. However, bNAb-mediated inhibition of CAV transmission has been largely overlooked. The partial efficacy of the PGT121 bNAb against cell-to-cell transmission in vivo in macaques [8] highlights the need to identify new Ab candidates against this mode of viral transmission. The few in vitro studies performed to date have yielded conflicting results, possibly due to the different experimental systems used [21], [22], [23], [24], [25], [26], [27], [28], [29]. Nevertheless, there is a large consensus that most bNAbs are less potent in vitro against cell-to-cell transmission than cell-free viral infection [21,24,25,29]. More importantly, studies performed thus far to predict the efficacy of bNAbs against CAV have not used cells infected in vivo and whether bNAbs can prevent CAV transmission mediated by semen leucocytes has not been addressed.
It would be ideal to have an in vitro assay which could accurately predict the capacity of bNAbs to inhibit cell-to-cell viral spread in vivo. Here, we assessed the efficacy of a panel of bNAbs to reduce SHIV162P3 cell-to-cell transmission. The source of cell-associated virus was semen leukocytes or splenocytes collected from SHIV162P3-infected cynomolgus macaques at the peak of infection. We demonstrate that bNAbs directed against the V3 loop and the bridging region (BR) of the envelope spike are highly effective against cell-associated transmission mediated by in-vivo infected spleen cells, even when used individually. Furthermore, the potency of the 10–1074 bNAb, targeting a carbohydrate-dependent epitope in the V3 loop of the HIV-1 envelope spike [30], was maintained when transmission was mediated by infected semen cells. This study supports the use of bNAbs to block cell-associated virus transmission mediated by semen cells in future in vivo studies.
2. Materials and methods
2.1. Ethics statement
This study used nonhuman primate models of HIV/AIDS in accordance with European Union guidelines for animal care (Journal Officiel des Communautés Européennes, L 358, December 18, 1986 and new directive 63/2010). All work related to animals was conducted in compliance with institutional guidelines and protocols approved by the local ethics committee (Comité d'Ethique en Experimentation Animale de la Direction des Sciences du Vivant au CEA under numbers A14_067 and APAFIS#373 2,015,032,511,332,650).
Healthy donor peripheral blood mononuclear cells (PBMCs) were obtained from buffy coats from the Établissement Français du sang (EFS) under informed donor consent. All procedures were in accordance with the ethical standards of the institutional and the regional ethical committee.
2.2. Animals, infection and sample collection
Adult cynomolgus macaques (Macaca fascicularis), weighing 5 to 11 kg, were imported from Mauritius and housed at the CEA/IDMIT infrastructure facilities according to European guidelines for animal care (Journal Officiel des Communautés Européennes, L 358, December 18, 1986 and new directive 63/2010). Animals (#AX450, #BA865F, #AX454 and #CI901) were infected by intravenous inoculation with a single dose of 1000 50% animal infectious doses (AID50) of SHIV162P3. #MF1414, #1103075 and #CA149F were infected by intrarectal inoculation with a single dose of 3 AID50 of SHIV162P3.
Semen and blood were collected from sedated animals following a 5 mg/kg intra-muscular injection of Zoletil®100 (Virbac, Carros, France). All animals were tested negative for SIV, SRV and STLV before enrolling into the study.
2.3. Semen collection
Semen was collected at days 11,12, 13, and 14 post-infection from macaques #BA865F,#1103075 #CA147F and #MF1414, respectively. The protocol of semen collection has been previously described [10]. Briefly, animals were sedated by a 5 mg/kg intramuscular injection of chlorhydrate Tiletamine (50 mg) combined with chlorhydrate Zolazepan (50 mg) (Virbac, Carros, France) during semen collection. Ejaculation was performed by intrarectal electrostimulation of nerve centers near the prostate with a probe (12.7 mm diameter) lubricated with conductor gel and an AC-1 electro-ejaculator (Beltron Instrument, Longmont, USA). Sequential stimulations were performed, with a pattern of six cycles, each cycle consisting of nine 2 s (s) stimulations followed by a tenth stimulation lasting 10 s. The voltage was increased every two cycles (1–3 V for the first two cycles, 2–4 V for the third and fourth cycle and 6–8 V for the last two cycles). If a complete ejaculate was not obtained after six cycles of stimulation, a 7th cycle at 7–10 V was performed. The complete ejaculate was immediately diluted in 1.2 ml phosphate-buffered saline (PBS).
2.4. Cells, antibodies and viruses
TZM-bl cells were obtained from the NIH AIDS Research and Reference Reagent Program (NIH ARRRP) and cultivated in DMEM containing 10% heat-inactivated fetal calf serum (FCS) and antibiotics. Human PBMCs from healthy donors were prepared from buffy coat and then cultivated in RPMI containing 10%FCS, 1% penicillin and streptomycin (PSN), 5 µg/ml phytoemoagglutinin (PHA) (Sigma-Aldrich), and 5 units/ml IL-2 (Roche) for three to four days before use. PBMCs were isolated from human healthy donors and collected using Lympholyte cell-separation media (Cedarlane) by centrifugation, aliquoted, and frozen at −80 °C. PBMCs from two donors were mixed to be used in the neutralization assay. The anti-N glycan 2G12, the anti-membrane proximal external region (MPER) 4E10 and 2F5, the anti-CD4 binding site (bs) b12 bNAbs and 5F3 (anti-gp41 non-neutralizing antibody (NNAb)) were obtained from Polymun Scientific, GmbH (Klosterneuburg, Austria). The anti-V3 10–1074 and PGT128, anti-bridging region (BR) PGT151, the anti-CD4bs N6 and 3BNC117, the anti- MPER 10E8, the anti-glycans V1/V2 PG16 and PGDM1400 bNAbs were provided by Dr. H. Mouquet, Institut Pasteur, Paris, France. The plasmid to produce the infectious molecular clone (IMC) of SHIV162P3 (pNL-LucR.T2A-SHIV-SF162P3.5.ecto) virus for in vitro studies of Ab inhibitory activity was kindly provided by Dr. C. Ochsenbauer, University of Alabama at Birmingham, USA.
2.5. Semen cell preparation
Seminal cells were separated from seminal plasma immediately after collection by centrifugation for 15 min at 775 x g, resuspended in 1 ml cold PBS supplemented with 10% FCS and 2 mM EDTA, and kept on ice for no more than 1 h. Cells were then centrifuged for 10 min at 1500 x g, filtered through a 70-µM sieve, and washed with 5 ml cold PBS supplemented with 10% FCS and 2 mM EDTA.
CD45+ semen cells from animals #BA865F, #1103075, #CA147F, and #MF1414 were enriched using CD45 magnetic beads and cell sorting with a BD FACS Aria Flow Cytometer. Total semen cells were incubated for 15 min at 4 °C with 20 μl anti-CD45 magnetic beads (Miltenyi Biotec) and washed once with 2 ml cold PBS supplemented with 0.5% BSA and 2 mM EDTA (sorting buffer). The CD45+ cell fraction was then enriched by magnetic-bead sorting, using LS columns (Miltenyi Biotec), according to the manufacturer's instructions. Cells were eluted in 4 ml sorting buffer. Following the magnetic bead-based enrichment process, cells were stained with amine-reactive blue dye (Life Technologies) to identify the dead cells, and anti-CD45 (clone D058-1283, BD Pharmingen). Cells were washed twice and stored at 4 °C in PBS/10% FCS. CD45+ cells were then sorted by simultaneous four-way sorting on a FACS Aria flow cytometer (BD Biosciences). The enriched cell fraction was used in the neutralization assay.
2.6. Splenocyte preparation
Spleen cells were mechanically dissociated and isolated by density-gradient separation with Lympholyte cell separation media (Cedarlane) from four animals (#AX450, #BA865F, #AX454, and #CI901) euthanized at the peak of viremia (between days 10 and 14 post-infection). Aliquots of cells were frozen in 10% dimethyl sulfoxide (DMSO).
2.7. Phenotypic characterization of splenocytes and semen leukocytes
Staining was performed on either thawed splenocytes or fresh semen. Amine-reactive blue dye (Live/dead Fixable, Life Technologies) was used to assess cell viability and exclude dead cells from the analysis. Cells were stained with monoclonal antibodies by incubation for 30 min at 4 °C, washed in PBS/10% FCS and fixed in commercial fixation solution (CellFIX, BD Biosciences). A list of antibodies used for staining of the splenocytes and semen leukocytes is shown in Supplementary Tables 1 and 2, respectively. Data were acquired on a FORTESSA instrument (BD Biosciences) using Diva software (BD Biosciences) and analyzed with Flowjo v10 (BD Biosciences).
2.8. In vivo cervico-vaginal exposure to SHIV162P3-infected splenocytes
Two female cynomolgus macaques (#CE468 and #CE470) were treated with 30 mg/kg medroxyprogesterone acetate (Depo-Provera) and then inoculated 30 days later by the vaginal route. Splenocytes from #AX450 were thawed 1 h before challenge and washed twice before being suspended in 1 mL RPMI medium for administration to macaques. The macaques were inoculated weekly with 10 million cells, corresponding to 4 × 106 viral DNA copies, until two consecutive detectable blood viral RNA loads were detected. Before inoculation, the lower female reproductive tract was inspected for signs of pre-existing inflammation. A lubricated nasogastric tube was used (Centravet) for the inoculation of cells into the vaginal vault. Animals were then kept in a prone position for 5 min. Animals were followed for two to three months after inoculation with the infected cells.
2.9. Cell-free virus titration and neutralization in the TZM-bl assay
Viral titrations were performed in TZM-bl cells as previously described [31]. The tissue culture infectious dose (TCID) 50 was defined as the reciprocal of the viral dilution resulting in 50% positive wells (Reed-Muench calculation). A cut-off value of 2.5 times background relative luminescence units (RLU) was used in the TCID and neutralization assays. A standard inoculum, corresponding to a virus dilution that yielded approximatively 300,000–500,000 RLU equivalents (± 15,000 RLU), was chosen for the neutralization assay to minimize virus-induced cytopathic effects while maintaining the ability to measure a 2-log reduction in virus infectivity. bNAbs were prepared by four serial three-fold dilutions, starting from a concentration of 8.32 µg/ml of a pool of three bNAbs (2G12+2F5+4E10) and 10 µg/ml for the single bNAb 2F5, 2G12, or b12. After 1 h of incubating the Ab with the viral supernatant (IMCs SHIV162P3), 104 TZM-bl cells were added to each well and the plates incubated for 48 h before luciferase activity was measured. Cell-free virus infections were carried out in culture medium containing 15 µg/ml of the polycation DEAE dextran (diethylaminoethyl; Amersham Biosciences, Fairfield, Connecticut, USA). Neutralization titers were defined as the Ab concentration at which the relative light unit (RLU) was reduced by 50% relative to the RLU of the virus control wells after subtraction of the background RLU in the control wells with only cells (IC50). By analogy, the IC75 and IC90 were defined as the concentration of Abs able to decrease the percentage of infected cells by 75% and 90%, respectively. The IC50, IC75 and IC90 were calculated using a linear interpolation method [32]. For calculating the mean, a value of greater than the highest Ab concentration used was recorded if 50% inhibition was not achieved. In contrast, if 50% inhibition was not achieved at the lowest Ab concentration used, a two-fold lower concentration than that value was recorded.
2.10. Assessment of cell-cell transmission and neutralization in the TZM-bl assay
Cell-cell transmission and inhibition were assessed by co-culturing splenocytes or CD45+ semen leukocytes with TZM-bl, in the presence/absence of bNAbs. A titration of infected splenocytes (input 10,000 – 200,000 cells) was performed to ensure infection of the target cells comparable to that with the free virus, corresponding to 150,000–200,000 RLU. Cells were pre-incubated with serial two-fold dilution of bNAbs, starting from a concentration of 132 µg/ml for 2F5, 4E10 and 2G12 and 50 µg/ml for b12 for 1 h at 37 °C before co-culturing with 10,000 TZM-bl per well. Forty thousand sorted semen cells were co-cultured with 10,000 TZM-bl in the presence/absence of 132 µg/ml of a pool of 2F5+2G12+4E10.
The co-culture was performed in 96 flat-well plates in DMEM medium containing 10% heat-inactivated FCS and antibiotics for 48 h at 37 °C in the absence of DEAE dextran. The inhibition activity was estimated by lysing the cells and measuring production of the luciferase reporter gene in TZM-bl upon the addition of firefly luciferase substrate (Promega, Madison Wisconsin, USA). The IC50, IC75 and IC90 were calculated as described above.
2.11. Cell-free viral titration and neutralization in the PBMC assay
Analogous to the TZM-bl assay, the TCID50 was first defined. Then, six steps of a two-fold dilution of bNAbs, starting from 10 µg/ml, were incubated with 20 and 40 TCID50 of viral supernatant (IMCs SHIV162P3) for 1 h. Then, 100,000 PHA-IL2 stimulated PBMCs from two donors were added to each well in 96 round-bottom well plates in RPMI medium containing 10% heat-inactivated FCS, antibiotics, and 20 units/ml IL-2 at 37 °C. Cells were washed on days 1 and 3 and the supernatant from each well collected on day 7 to detect SIVp27 Ag by ELISA using the RETRO-TEK SIV p27 Antigen ELISA (Helvetica Health Care, Geneva, Switzerland), according to manufacturer's recommendations. The IC50, IC75 and IC90 were calculated as described for the TZM-bl assay.
2.12. Assessment of cell-cell transmission and neutralization in the PBMC assay
The protocol was adapted from the cell-free neutralization assays. SHIV162P3-infected splenocytes or CD45+ semen leucocytes were used as donor cells. Each stock of splenocytes (input 40,000 – 160,000 cells) was titrated to define the number of cells allowing efficient cell-cell transmission. bNAbs were prepared and used as in the TZM-bl assay at a starting concentration of 132 µg/ml and 15 µg/ml for the 1st- and 2nd-generation bNAbs, respectively. A total of 5.0 × 105, 1.8 × 106, and 3.6 × 105 CD45+ leucocytes were isolated from 84, 315, and 52 µl of semen from #1103075, #CA147F and #MF1414, respectively and cells were used in three independent experiments. Forty thousand semen CD45+ cells were used for each condition and four three-fold dilutions were prepared starting from 5 µg/ml of the 10–1074 bNAb. For the experiment with semen cells in presence of 2F5+2G12+4E10 the Ab mix was used at a concentration of 132 µg/ml. Cells were incubated 1 h with the Ab before co-culture with 120,000 PHA-IL-2-activated PBMCs from two donors. The co-culture in 96 round-bottom well plates was performed in RPMI medium containing 10% heat-inactivated FCS, antibiotics, and 20 units/ml IL-2 at 37 °C. After seven days of culture, the supernatant was collected to detect viral replication by either RT-qPCR (Superscript III platinum one-step qPCR system) or p27-Ag detection (RETRO-TEK SIV p27 Antigen ELISA, Helvetica Health Care, Geneva, Switzerland), according to the manufacturer's recommendations. The IC50, IC75 and IC90 were calculated as described for the TZM-bl assay. Cells were collected and stained for p27 Ag.
2.13. Detection of SHIV-infected cells by intracellular p27 Ag staining
Staining was performed after seven days of splenocytes-PBMC co-culture. Dead cells were excluded from the analysis using an amine-reactive blue dye (Live/dead Fixable, Life Technologies). An anti-CD45 antibody (clone D058-1283, BD Pharmingen) that does not cross-react with human cells and an anti-CD3 antibody (clone UCHT1, BD Horizon) that does not cross-react with macaque cells were used to discriminate human PBMCs (CD45−CD3+) from macaque splenocytes (CD45+CD3−). A PE-conjugated anti-p24 antibody (clone KC57, displaying cross-reactivity with the p27 antigen) was used to estimate the transmission and inhibition activity. Cells were incubated with the monoclonal antibodies for 15 min at 4 °C, washed in PBS/10% FCS, permeabilized in Fixation/Permeabilization solution (BD Bioscience) for 20 min at 4 °C, and finally washed twice with Perm/Wash (BD Biosciences). The cells were next stained with KC57 antibody for 15 min at room temperature. Samples were washed twice with Perm/Wash and fixed (CellFIX, BD Biosciences). Data were acquired on a FORTESSA instrument (BD Biosciences) using Diva software (BD Biosciences) and analyzed with Flowjo v10 software (BD Biosciences).
2.14. Quantification of the viral load in plasma, seminal plasma, and culture supernatants
Blood plasma was isolated from EDTA-treated blood samples by centrifugation for 10 min at 1500 x g and stored frozen at −80 °C. Viral RNA (vRNA) was prepared from 200 µL cell-free plasma or culture supernatants using the Nucleospin 96 virus core kit (Machery-Nagel). Culture supernatants were collected at the indicated time points, centrifugated for 10 min at 1500 x g and stored frozen at −80 °C. Retro-transcription and cDNA amplification and quantification were performed in duplicate by RT-qPCR using the Superscript III Platinum one-step quantitative RT-PCR system (Invitrogen, Carlsbad, USA). RT-PCR was performed as previously described [33]. The quantification limit (QL) was estimated to be 111 and 1000 copies/ml and the detection limit (DL) 37 and 1000 copies/ml, when quantifying plasma and culture supernant respectively. Semen viral RNA was prepared from 500 µL seminal plasma using the QIAamp Ultrasens Virus kit (Qiagen, Courtaboeuf, France), according to the manufacturer's instructions. Quantitative RT-PCR was performed under the same conditions as above, with a QL of 37 copies/ml and a DL of 12.3 copies/ml. QL and DL were estimated to be 1 × 104 and 1 × 103 RNA copies/ml respectively when assaying cell-culture supernatants.
2.15. Viral DNA extraction from splenocytes and semen leucocytes, quantification and normalization
DNA was extracted from frozen cells using the NucleoSpin Tissue XS kit (Machery-Nagel), according to the manufacturer's instructions. DNA was analyzed in duplicate by real-time PCR Taqman assay using the Platinum qPCR SuperMix-UDG kit (Thermo Fisher Scientific) with SIV-gag primers and probe, as described elsewhere [34]. The SIVmac251 gag complementary DNA sequence, ligated into the pCR4-TOPO (Invitrogen) plasmid and purified with the HiSpeed Maxiprep Kit (Invitrogen), was used as a standard diluted in 5000 cells from uninfected macaques (serial 10-fold dilutions). The detection limit was 30 copies per 106 cells. The reaction, data acquisition, and analysis were performed with the iCycler real-time thermocycler (Bio-Rad).
We verified the number of cells in each unknown sample. DNA from 5000 cells from uninfected macaques was serially diluted 10-fold (up to 104). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene was then amplified simultaneously using a primer set and probe, as described elsewhere [34]. GAPDH-DNA levels in unknown samples were inferred by comparing the threshold cycle (Ct) value against a calibration curve. Unknown samples displayed levels of amplifiable complementary DNA or DNA equivalent to the levels obtained from 5000 cells. Absolute numbers of viral DNA were normalized to 5000 cells. Results are expressed as SIV DNA copy number per 106 cells.
2.16. Data visualization and statistical analysis
All data visualization and statistics analysis were performed using GraphPad Prism version 8.1 software (GraphPad Software, La Jolla, USA). Dose-response inhibition curves were plotted using sigmoid dose-response curves (variable slope). The non-parametric Mann Whitney was used to compare the infectivity of the various numbers of splenocytes used in the neutralization assay. P values of 0.05 or lower were considered significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3. Results
3.1. Splenocytes from infected macaques are phenotypically similar to semen leucocytes and transmit the infection to target cells in vitro and in vivo
We aimed to develop a robust system that allows direct quantification of cell-cell transmission using in vivo infected cells. The isolation of semen cells is highly challenging and the modest number of recovered cells does not allow extensive set-up experiments. We investigated whether SHIVI62P3 infected spleen cells resemble infected semen cells in vivo and could be used as a surrogate model in cell-to-cell transmission experiments.
We initially compared the composition of the immune cells in spleen cells with those of semen cells obtained from three SHIVI62P3-infected animals during primary infection (Table 1 and Supplementary Figure 1). The splenocytes were composed of 1.7 ± 2.5% CD14+ monocytes/macrophages, 6.8 ± 1.3% CD3+CD4+CD8− T cells, 7.5 ± 2.2% CD3+CD4−CD8+ T cells, and 29.1 ± 7.9% CD20+ cells. Most of the CD4+ T cells were CD28+CD95+ central memory (CM) cells (62.1 ± 8.7%). CD28−CD95+ effector memory (EM) and CD28+CD95− naive cells were present in smaller proportions (13.8 ± 3.2% and 18.2 ± 14.1%, respectively). The proportion of CD4+ T cells in semen was similar to that in spleen, representing 9.1% ± 2.5% of total lymphocytes. The proportion of CM and EM cells was also similar to that of splenocytes, whereas semen contained a lower proportion of monocytes/macrophages and a higher proportion of B cells than spleen. We previously reported an increase in the activation state of semen macrophages, CD4+ and CD8+ T cells during acute SIV infection [10,35]. A differential state of activation of spleen cells compared with semen cells might introduce a bias when evaluating cell-cell transmission. We thus analyzed the expression of the activation marker HLA-DR by T lymphocytes and of the differentiation marker CD69 by monocytes/macrophages and T cells. No significant differences were observed, with the exception of a slightly higher expression of CD69 by semen CD3+CD4+CD8− T cells (18.9 ± 17% vs 5.9 ± 0.5% of semen vs spleen cells, respectively) (Table 1). These results, further validated the choice to use splenocytes as surrogate of semen leukocytes.
Table 1.
Comparison of immune cell populations from the spleen and semen of three SHIV162P3-infected macaques.
| Cell type | Origin |
|
|---|---|---|
| Spleen | Semen | |
| B lymphocytes | 29.1 ± 7.9% | 0.7 ± 0.7% |
| Monocyte / macrophage | 1.7 ± 2.5% | 0.8 ± 0.7% |
| % CD69+ cells | 3.5 ± 3.1% | 2.1 ± 1.9% |
| CD4+ T lymphocytes | 6.8 ± 1.3% | 9.1 ± 2.5% |
| central memory | 62.1 ± 8.7% | 73.2 ± 5.7% |
| effector memory | 13.8 ± 3.2% | 4.2 ± 1.8% |
| naive | 18.2 ± 14.1% | 17.9 ± 5.6% |
| % CD69+ cells | 5.9 ± 0.5% | 18.9 ± 17% |
| % HLA-DR+ cells | 11 ± 4.1% | 18 ± 2.4% |
| CD8+ T lymphocytes | 7.5 ± 2.2% | 23.4 ± 11.1% |
| % CD69+ cells | 11.1 ± 5.2% | 15.5 ± 3.2% |
| % HLA-DR+ cells | 9.1 ± 6.7% | 13.2 ± 5.1% |
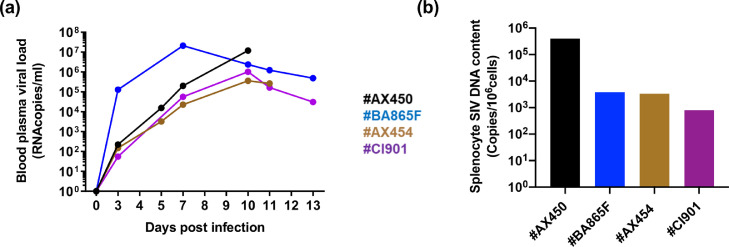
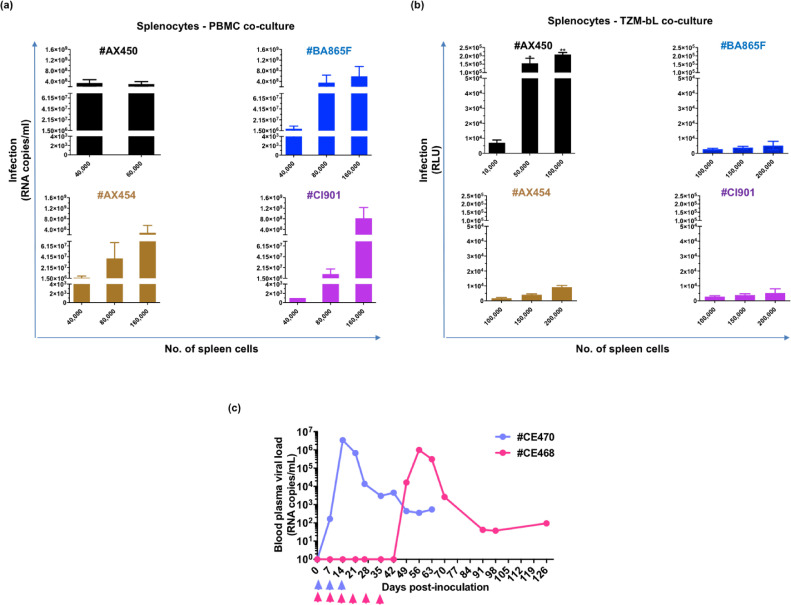
We prepared stocks of splenocytes from four macaques (#AX450, #AX454, #BA865F, and #CI901), sacrificed between days 10 and 13 post-infection, when the blood viral load (BVL) ranged from 105 to 107 copies/ml (Fig. 1a). The BVL was mirrored by the pro-viral load detected in the splenocytes, with cells from #AX450 containing the highest amount of integrated DNA (4 × 105, 3.8 × 103, 3.29 × 103, and 7.91 × 102 viral DNA copies per 106 cells for #AX450, #BA865F, #AX454, and #CI901, respectively) (Fig. 1b). We titrated the splenocytes using both PBMCs (Fig. 2a) and TZM-bl (Fig. 2b) as target cells and observed a linear increase in the transfer of cell-cell SHIV162P3 infection as the number of spleen cells was progressively increased (Fig. 2a, b). PBMCs were more sensitive to cell-mediated infection than TZM-bl cells, allowing viral transmission by spleen cells from the four donors, although with varying efficiency. Indeed, 40,000 splenocytes from #AX450 produced 3.4 × 108 ± 2.8 × 108 RNA copies/ml, whereas at least twice as many cells from the other three donors were needed to obtain a similar level of infection (Fig. 2a). Only splenocytes from #AX450 efficiently transmitted infection in co-cultures with TZM-bl (p < 0.05, non-parametric Mann Whitney test) (Fig. 2b). These results are consistent with the highest level of DNA detected in the splenocytes from #AX450 (Fig. 1b). Of note, among #AX450 splenocytes, naïve, EM, and CM CD4+ T cells were the major infected populations, with a DNA viral load 31-fold and 22-fold higher than in monocytes/macrophages (1 × 106 SIV DNA copies per 106 cells in naïve and EM, 7 × 105 in CM and 3.2 × 104 in monocytes/macrophages, respectively).
Fig. 1.
SHIV162P3 infection of macaques and detection of infected spleen cells. (a) Follow-up of blood viral RNA load in four macaques intravenously infected with SHIV162P3 up to the day of euthanasia. (b) SIV DNA content of SHIV162P3-infected spleen cells.
Fig. 2.
In vitro and in vivo infectivity of SHIV162P3-infected splenocytes. (a) Titration of splenocytes in coculture with PBMCs. Viral production was detected by RT-qPCR 7 days post-coculture. (b) Titration of splenocytes in coculture with TZM-bl cells. Infection was recorded as Relative Light Unit (RLU) at 48 h post-coculture after subtraction of the RLU of the control wells (TZM-bl only). Each bar represents the mean and standard error of the mean (SEM) of three or four independent experiments performed in triplicate. Each color represents a different animal. * p = 0.0385, ** p = 0.0039, non-parametric Mann Whitney test. (c) Follow-up of blood viral RNA load of two macaques (CE470 in violet and CE468 in pink) intravaginally exposed to 107 spleen cells from #AX450. Arrows represent weekly inoculations of splenocytes, performed for three weeks for CE470 and six weeks for CE468.
We then assessed whether splenocytes from #AX450 were infectious in vivo. Two female cynomolgus macaques were exposed weekly to 107 cells until an infection was clearly detected. Both macaques became persistently infected, with a peak blood plasma viral load of 3.5 × 106 and 1 × 106 viral RNA copies/ml, detected 14 and 56 days after the first intravaginal inoculation, respectively (Fig. 2c). Seroconversion was observed in both animals (Ab titer = 4000 three months post-infection).
These results prompted us to use splenocytes from #AX450 to assess bNAb-mediated inhibition of SHIV cell-to-cell transmission.
3.2. Inhibitory activity of a mixture of bNAbs against cell-free and cell-to-cell SHIV162P3 transmission
We compared the potency of bNAbs in neutralization assays against cell-free versus cell-associated SHIV transmission by initially determining the TCID50 of cell-free SHIV162P3 in TZM-bl cells. DEAE-dextran was used to distinguish cell-free from cell-associated virus infection [21,29]. A standard inoculum, corresponding to approximately 150,000–200,000 RLU equivalents, and an input of 105 spleen cells, which yielded comparable virus infectivity, was chosen for the neutralization assay. This allowed us to minimize virus-induced cytopathic effects while comparing the same input of virus in the two infection models (data not shown).
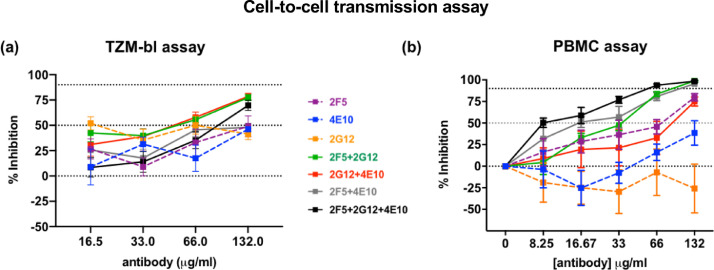
We previously successfully inhibited cell-free transmission of SHIV162P3 with the pool of 2F5, 2G12, and 4E10 bNAbs in vitro and in vivo [20]. Thus, we used the same panel of bNAbs to evaluate their inhibitory activity against cell-to-cell SHIV162P3 transmission. Coculture experiments were carried out in the presence of decreasing amounts of the bNAbs pool until 100% viral infectivity was regained. The pool inhibited SHIV162P3 cell-cell transmission with a mean IC50 value of 78.09 ± 36.85 µg/ml in the TZM-bl assay (Fig. 3a and Supplementary Table 3) and a mean IC90 value of 53.45 ± 12.65 µg/ml in the PBMC assay (Fig. 3b and Supplementary Table 3). Comparison of the inhibitory activity with the two transmission modes showed that the Ab mix had considerably less potency against cell-cell transmission than cell-free infection in TZM-bl and PBMC (132- and 190-fold lower IC50 and IC90, respectively) (Supplementary Table 3). Individual Abs 2F5, 2G12 and 4E10 blocked cell-free infection with higher potency, as reflected by fold change of IC50 (in TZM-bl assay) and IC90 (in PBMC assay) >1. In addition, bNAbs displayed higher efficacy in cell-free assays, as reflected by higher maximum inhibition at saturating concentrations of antibody (Supplementary Table 3). 2G12 did not inhibit both cell-free and cell-to-cell SHIV162P3 transmission at the concentration used in the assay (mean ± SD values from three independent TZM-bl-splenocytes coculture experiments were 297,000 ± 102,600 in absence of Ab and 175,800 ± 29,800, 148,000 ± 48,000, 194,000 ± 58,000 and 142,000 ± 35,000 in presence of 2G12 at 132 ug/ml, 66 ug/ml, 32 ug/ml and 16.5 ug/ml, respectively) (Fig. 3a), while it inhibited the parental HIV SF162 virus (IC50 = 4.62 ± 1.72 µg/ml in the TZM-bl assay).
Fig. 3.
Neutralization of SHIV162P3 cell-cell transmission by 2F5, 4E10, and 2G12 bNAbs used singly or in double or triple combinations. (a) TZM-bl neutralization assay. Results after 48 h of splenocyte/TZM-bl coculture are shown. (b) PBMC-based neutralization assay. Infection was analyzed by SIV p27 Ag ELISA seven days post-coculture. The mean and SEM of three to four independent experiments is shown. Dotted lines indicate 0, 50, and 90% inhibition viral transmission. Antibodies targeting the N-glycan of the gp120 (2G12) and the MPER (2F5 and 4E10) of the gp41 were used.
We then compared the efficiency of a triple versus double Ab pool by combining the Abs to target only the membrane-proximal external region (MPER) of gp41 (2F5 + 4E10) or both gp120 and the MPER (2G12 + 4E10 and 2G12 + 2F5). Double-Ab combinations were still active, although with some differences between the two assays. Although Abs targeting both gp120 and the MPER were as effective as the triple combination of Abs in the TZM-bl assay (IC50 = 48.93 ± 25.65 µg/ml for 2G12 + 4E10 and 36.64 ± 32.30 µg/ml for 2G12 + 2F5), in the PBMC assay the 2G12 + 4E10 combination failed to inhibit 90% of infection (IC90 and IC75 > 132 µg/ml and IC50 = 57.90 ± 58.80 µg/ml). In general, however, we observed no significant differences and the triple and double combinations displayed comparable IC values (Fig. 3 and Supplementary Table 3). The anti CD4bs IgG1 b12 and the non-neutralizing Ab 5F3 were used as positive and negative controls, respectively (Supplementary Table 3).
3.3. Antibodies targeting the V3-loop and bridging region of the viral envelope are the best candidates to inhibit SHIV162P3 cell-to-cell transmission
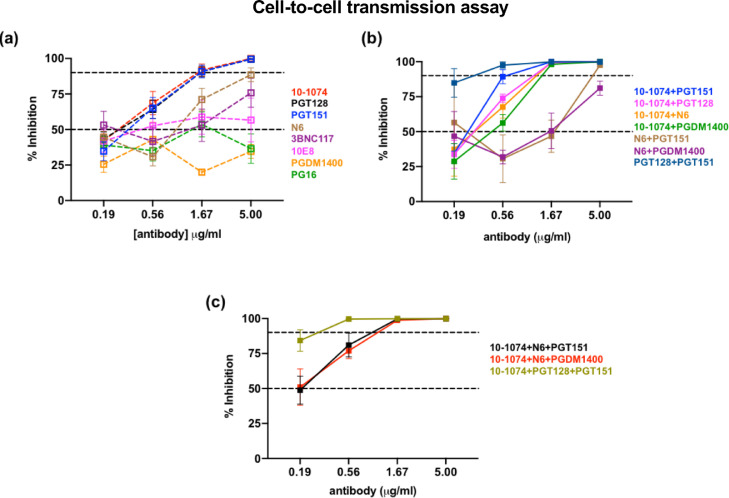
The identification of second-generation bNAbs with potencies 10 to 100-fold greater than the first-generation bNAbs has renewed interest in their potential clinical use. We investigated whether protection from cell-mediated transmission could be improved using second-generation bNAbs. The neutralization titers of eight individual and combined bNAbs were evaluated against SHIV162P3 in a PBMCs-based cell-free and cell-to-cell assay. PGDM1400 was predicted to be non-neutralizing before any experiment was performed. A maximum Ab concentration of 5 µg/ml was used versus 132 µg/ml for the first-generation bNAbs. The ICs of the bNAbs are shown in Supplementary Table 4. Among individual Abs, anti-V3 loop 10–1074 and PGT128 and anti-bridging region Ab PGT151 showed similar inhibitory potency against cell-to-cell transmission, with IC90s of 1.56 ± 1.09, 1.93 ± 0.9, and 1.71 ± 1.17 µg/ml, respectively (Fig. 4a). These concentrations were more than 50-fold lower than that of the 2F5+4E10+2G12 combination. Anti-CD4bs Ab N6 and 3BNC117 were less potent, with IC90 values of 4.45 ± 4.44 and > 15 µg/ml, respectively. 10E8 and PG16 did not neutralize SHIV162P3, analogously to PGDM1400 (Fig. 4a). Cell-free SHIV transmission was inhibited at Ab concentrations 11- to 33-fold lower than those for CAV transmission, as reflected by IC90 fold change (Supplementary Table 4). Interestingly, 10–1074, PGT128 and PGT151 were also the only bNAbs that completely neutralized cell-to-cell SHIV162P3 transmission, whereas the anti-CD4bs Abs displayed incomplete neutralization, with percentages of maximum inhibition in cell-to-cell infection of 89% for N6 and 76% for 3BNC117 (Supplementary Table 4).
Fig. 4.
Neutralization of SHIV162P3 cell-cell transmission by a panel of 2nd-generation bNAbs in a PBMC based assay. bNAbs were used singularly (a) or in combination (b, c). Infection was analyzed by SIV p27 Ag ELISA seven days post-coculture. The mean and SEM of three to four independent experiments is shown. Dotted lines indicate 0, 50 and 90% inhibition of viral transmission. Antibodies targeting the V3 (10–1074 and PGT128), the bridging region (PGT151), the CD4bs (N6 and 3BNC117), the MPER (10E8) and the V1/V2 (PG16 and PGDM1400) were used.
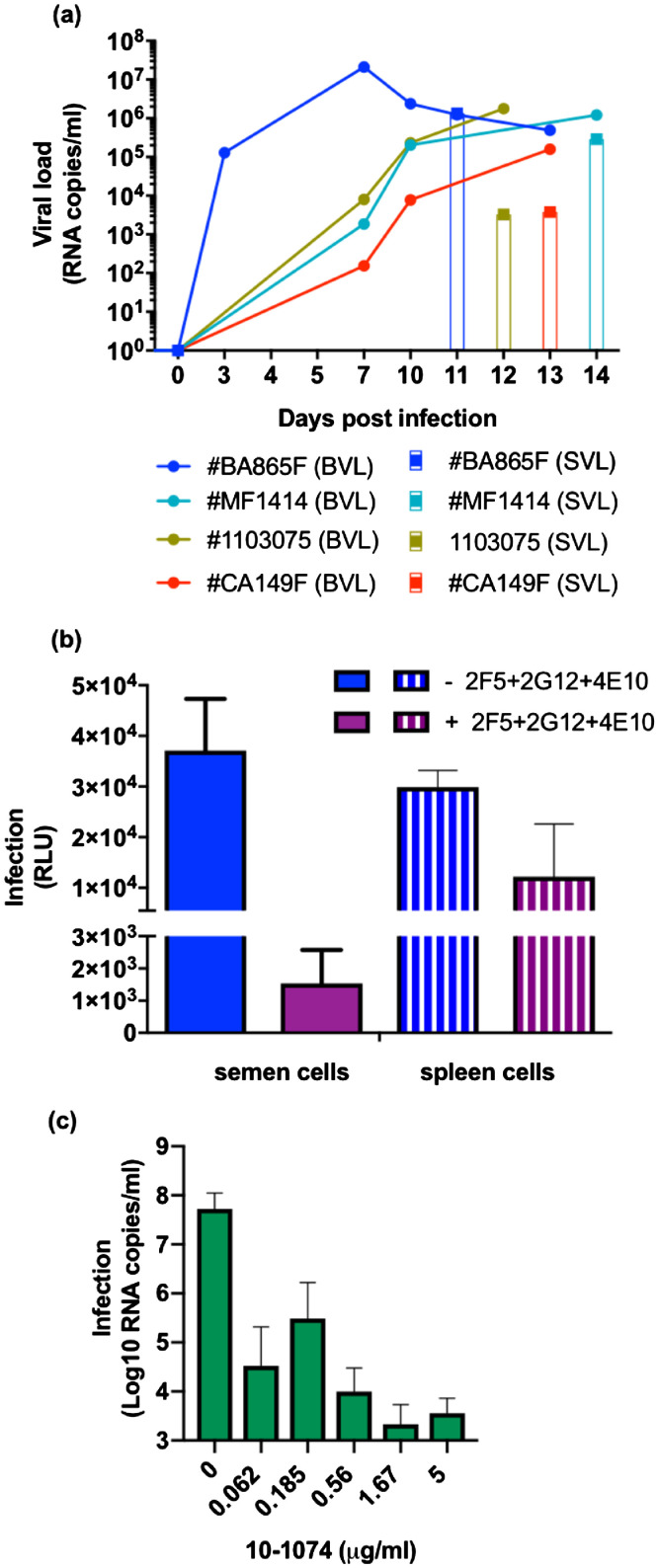
We then combined bNAbs to target different epitopes. Among seven double combinations (Fig. 4b) and three triple combinations (Fig. 4c) tested, the observed additive effect was moderated. Indeed, with the exception of the 10–1074+PGT128+PGT151 and PGT128+PGT151 combination which inhibited 90% of infection at concentration between 0.1 and 1.0 µg/ml, the IC90 values of triple and double Ab combination remained in the same order of magnitude as those of single Abs (between 1 and 10 µg/ml) (Supplementary Table 4). 10–1074, which showed considerable potency and efficacy when used alone, was selected to further test its potential to block SHIV transmission mediated by semen leucocytes.
We further confirmed the specific inhibition of cell-to-cell transmission by bNAbs by differentiating splenocytes from PBMCs in the coculture system by flow cytometry and assessing viral transfer to PBMCs by intracellular p27 Ag staining. This strategy allowed us to discriminate splenocytes (CD45+CD3−) from PBMCs (CD45−CD3+) (Supplementary Figure 2a). Among PBMCs, we identified CD3low and CD3high populations, of which both were infected, with values of p27+ cells among total live cells ranging from 1.08 ± 0.05% to 5.41± 1.11% for the CD3low population and 0.36 ± 0.03% to 0.39 ± 0.08% for the CD3high population. The percentage of infected cells was consistently lower in the presence of second-generation bNAbs, with the greatest potency relative to that of the 2F5+2G12+4E10 pool (Table 2). Among the various Ab/Ab combinations, 10–1074 used alone was among the most potent, showing a reduction of > 96% of infected p27+ cells when used at 5 µg/ml (Table 2 and Supplemenatry Fig. 2b). As expected, there was no inhibition in the presence of the NNAbs 5F3 or PDGM 1400 (Table 2 and Supplementary Figure 2c).
Table 2.
Percentage inhibition of p27+ cells in the presence of bNAbs.
 |
3.4. bNAbs inhibits transmission by SHIV162P3-infected semen cells
We sought to determine whether the Abs could still inhibit cell-mediated transmission when CD45+ semen leukocytes were used as the source of virus. Semen was collected from four macaques (#BA865F, #1103075, #CA147F, and #MF1414) when the blood viral load (BVL) ranged from 1.77E+06 to 1.58E+05 copies/ml and the seminal viral load (SVL) from 3.03E+03 to 1.34E+06 copies/ml (Fig. 5a).
Fig. 5.
bNAb-mediated inhibition of semen leukocyte SHIV162P3 transmission to target cells. (a) Follow-up of blood viral RNA load (BVL, lines) and semen viral load (SVL, histogram) on the day of euthanasia of four macaques infected with SHIV162P3. Each color represents a different macaque. (b) TZM-bl infection by either 40,000 CD45+ semen cells or 40,000 spleen cells in presence and absence of 132 µg/ml of 2F5+2G12+4E10 Ab pool (targeting the N-glycan of the gp120 and the MPER of the gp41). Mean and SEM of one representative experiment performed in duplicate is shown. (c) The anti-V3 10–1074 Ab mediated inhibition of transmission from CD45+ semen cells to PBMCs. Infection was analyzed by RT-qPCR seven days post-coculture. Each condition was assessed in duplicate. The mean and SEM of three independent experiments, with semen cells purified from three different donors is shown.
We initially compared the efficiency of the 2F5+2G12+4E10 Ab combination against SHIV162P3 transmission mediated by semen leukocytes and splenocytes from the same donor #BA865F, using a fix amount of 40.000 cells. Transmission by semen CD45+ cells to TZM-bl cells was inhibited more efficiently by 132 µg/ml Ab combination compared to splenocytes mediated transmission (Fig. 5b). Of note, semen cells from #BA865F were collected at day 11 whereas the spleen was harvest at day 13 post-infection, when the blood viral load had drop from 1.23E+06 to 4.93E+05 RNA copies/ml (Fig. 5a), yet inhibition of semen cells -mediated infection to TZM-bl was more efficient .
We then evaluated the potency of the anti-V3 bNAb 10–1074 Ab as representative of second-generation bNAbs in a PBMC assay. Three independent experiments were performed by co-cultivating semen CD45+ leucocytes collected from #1103075, #CA147F and #MF1414 respectively, with PBMCs in the presence of decreasing amounts of 10–1074. Semen CD45+ cells transferred infection to PBMCs (mean of 1.15 × 108 ± 1.21 × 108 RNA copies/ml at day 7 post-coculture) and 10–1074 bNAb inhibited transmission of the infection by up to 99% when used at a concentration as low as 0.062 μg/ml (Fig. 5c). Moreover, complete neutralization of SHIV162P3 cell-cell transmission was observed (100% of maximum inhibitory concentration when 10–1074 was used at 10 μg/ml). Thus, Ab 10–1074 strongly inhibited transmission mediated by semen cells, with an IC90 value (< 0.062 μg/ml) lower than those obtained when splenocytes were used as donor cells (Supplementary Table 4).
Our results support the potential use of bNAbs in vivo against cell-associated SHIV162P3 challenge.
4. Discussion
SHIV infection of macaques is the most suitable model for the preclinical evaluation of bNAbs inhibition but has been mainly used for studies of cell-free transmission and challenge with cell stocks remains unexplored [13,[15], [16], [17],19,20,36,37]. However, the selection of bNAbs that are able to suppress HIV-1 transmission, irrespective of the transmission mode, needs to be considered to ascertain their in vivo activity in therapeutic use and vaccines.
Here, we evaluated for the first time, the capability of a panel of bNAbs to inhibit cell-to-cell transmission of SHIV162P3 in a setting in which in vivo infected cells, notably semen cells, were used as the source of virus. HIV-1 transmission from infected to uninfected cells is likely to play a predominant role in infecting individuals [38] and several studies have shown that this mode of viral spread may represent a mechanism through which the virus can evade antibody-based immunity [21,24,29,39]. In this scenario, semen leukocytes may act as Trojan horses, protecting cell-associated virus from host immune defenses [40]. Previous studies have reported the inability of bNAbs to interfere with cell-to-cell transmission but have also demonstrated that these observations depend on the cell type and the Ab used. Moreover, these studies primarily focused on in vitro infected cells [21,24,25,29]. Due to the diversity between cell lines or even CD4+ T cells derived from blood and semen T cells, it is of utmost importance to establish more physiologically relevant in vitro systems. The cell-to-cell transmission system used here allowed us to evaluate bNAb-mediated inhibition in a setting in which both the percentage of infected cells and the proviral level reflect those found in vivo. Additionally, in vitro screening of bNAbs using a physiologically relevant cell-to-cell system is indispensable for the selection of the most promising candidates to be used in preclinical animal models.
Macaque semen has been found to be very similar to human semen, with a similar distribution and phenotype of semen leukocytes for the two species and expression of adhesion molecules involved in virological synapse formation and viral spreading [10], further validating the experimental system we used. In both human and macaques, infected cells in the semen are mostly represented by T lymphocytes and macrophages [10], [11], [12]. Here, we used spleen cells collected at a time of high plasma viremia of an acutely SHIV162P3-infected macaque as a surrogate of semen cells. We show that the major difference between the two cells types is represented by the percentage of macrophages, which are present in higher amounts in the spleens of infected macaques. However, the proportion of CD4+ T lymphocytes, both total and subsets of naïve, central memory and effective memory cells, were comparable. Moreover, similar activation level by monocytes/macrophages and both CD4+ and CD8+ lymphocytes in spleen and semen cells was observed, further highlighting the relevance of the experimental system we used. Our results showing that semen cells efficiently transmit the infection to target cells suggest that transmission is mostly mediated by lymphocytes. This was confirmed by the proviral load results we obtained, demonstrating that lymphocytes were the predominant infected cells among splenocytes. Accordingly, although both semen CD4+ T cells and macrophages contain viral DNA during primary and chronic SIV infection, infected cells are more frequently found in the CD4+ T-cell fraction [10]. However, in the context of chronic infection, CD4+ T cells are present in very low numbers and macrophages would therefore be major candidates for cell-to-cell transmission. Efficient HIV-1 transfer by infected macrophages and inhibition by bNAbs has been reported in several studies [20,22]. Our system might be further developed to mimic the chronic phase of infection by isolating cells (spleen and semen cells) from chronically infected macaques, when macrophages number would predominate over CD4+ T cells and would likely represent the major cell type transmitting infection [10]. Our results also suggest that cells carrying a high proviral content provide proof for a high local MOI, as previously reported [41]. Indeed, cell-to-cell transmission was more effective when the DNA content of splenocytes was above 104 copies per million cells. We could not systematically determine the proviral DNA content due to a limited amount of semen cells and the priority given to perform the transmission and neutralization assays. However, we observed that the seminal viral load possibly predicted the capacity of seminal leukocytes to transmit the infection in vitro. Indeed, transmission by semen leukocytes was not achieved when the semen viral load values were below 105 RNA copies/ml (data not shown).
Here, we also demonstrate that exposure of the intact mucosa of the vagina and cervix of macaques to spleen cells infected with SHIV162P3 results in the transmission of infection. In a previous study we estimated that approximately 105 copies of viral DNA would be needed to transmit infection to 50% of female macaques when using SIVmac251-infected cells [7]. In the present study with the less pathogenic SHIV162P3 strain we used a higher viral input, corresponding to 4 × 106 viral DNA copies. This finding is compatible with current knowledge on natural HIV-1 infection in humans [42].
We employed two systems to assess SHIV162P3 transmission from infected splenocytes and bNAb-dependent inhibition. One was the TZM-bl cell line, which has already been used for evaluating the neutralization of CAV transmission [21,29]. Although validated and widely used, TZM-bl cells express CD4 and CCR5 at higher than physiological levels [43] and are therefore not representative of CD4+ T lymphocytes in vivo. Moreover, the higher CCR5 level compared to PBMC was shown to reduce the sensitivity of the assay for certain Abs, including 2F5, 4E10 and 2G12, [44]. In agreement with this previous report, we showed that the PBMC assay was more sensitive for the detection of antibody-mediated inhibition of CAV transmission. The cell-type dependent differences in sensitivity we observed could also be attributed to different transition dynamics between conformational states and/or different maturation state of the envelope protein [23,29]. It is possible that neutralizing epitopes that are displayed on PBMC-associated envelope may be less accessible on a fraction of TZM-bl-associated envelope. The splenocyte/target-cell transmission assay allowed us to evaluate whether the mode of HIV transfer has an influence on the potency of neutralizing Abs. Indeed, it is recognized that only a subset of potent HIV-1 bNAbs are able to inhibit most of the steps involved in the lymphocyte-to-lymphocyte spread of HIV-1, including the formation of virological synapses and the transfer of viral material from infected cells to their targets [24]. Our results are in accordance with those of previous studies reporting lower activity of bNAbs against cell-cell transmission when compared to cell-free infection [21,24,29,39]. The importance to block cell-cell infection was highlighted by a mathematical analysis revealing that cell-to-cell transmission was more prone to give rise to escape mutations than cell-free virus spread [25]. Here, we also show that many of the 2nd generation bNAbs tested are effective against SHIV162P3 cell-to-cell transmission, with more than 50-fold higher potency when used individually over that of the triple combination of the 1st generation antibodies 2F5+4E10+2G12. One possible explanation for the differences in neutralization efficiency observed among antibodies may be due to differences in antibody binding avidity or differences in epitope accessibility [45]. Moreover, the difference in potency observed might be linked to the mechanism of action of the antibodies. Second generation bNAbs such as 10–1074 significantly decreased the formation of clusters and syncytia between uninfected and infected T cells, and the transfer of viral material through the virological synapse [24]. 2F5 or 4E10 (anti-MPER) rather act later, by inhibiting viral fusion [27,46]. Further studies aiming to visualize the establishment of the virological synapse between infected splenocytes (or ideally semen cells) and T cells and bNAbs inhibition would help to dissect the potential mechanism behind the different potency of 1st and 2nd generation Abs.
Among 2nd generation bNAbs, those of the V3-glycan class (10–1074 and PGT128) and the anti-bridging region PGT151 bNAb were the most efficient, whereas the anti CD4bs antibodies were slightly less effective against SHIV162P3 cell-to-cell transmission (IC90 values were in the range of 1 to 10 μg/ml for single antibodies, with the exception of 3BNC117). A combination of bNAbs that target different sites on the HIV-1 envelope glycoprotein are being considered for effective therapy and possibly prevention. The improved efficacy observed was dependent by the specific combination chosen [29]. We observed a moderate additive effect when antibodies targeting the V3 and the BR of the gp120 were combined in a triple or a double combination, which led to one log lower IC90 values compared to the other combinations and single Ab tested.
In addition to antibody potency, efficacy or maximum neutralization is a critical parameter to consider in antibody therapy against HIV-1. If a significant fraction of virus is resistant to neutralization even in presence of saturating Ab concentrations, this has important implications in terms of viral escape and might contribute to a lack of viral control. Consistent with a prior study [23], we observed that some bNAbs failed to completely block cell-to-cell transmission compared to cell-free transmission even when present at high titers. The diminished neutralization efficacy was especially evident with 1st generation bNAbs. Incomplete neutralization was observed also in case of cell-free transmission, which is consistent with what reported by others [47]. Interestingly, incomplete inhibition of both cell-free and cell-to-cell transmission was especially evident in the TZM-bl assay. These results further evidenced cell-type specific shift in neutralization potency and efficacy.
To proof antibody inhibition of transmission initiated by semen cells, we selected a triple combination of 1st generation bNAbs and a single highly potent 2nd generation antibody. The 2F5+2G12+4E10 combination completely prevented SHIV162P3 vaginal transmission in macaques [20] and inhibited semen-cell SHIV162P3 transmission when used at the relatively high concentration of 132 μg/ml. Among the 2nd generation bNAbs that revealed to be highly potent against cell-to-cell SHIV162P3 transmission mediated by infected splenocytes, 10–1074 has entered clinical evaluation [48]. Aside from being highly potent in vitro against a large panel of HIV-1 pseudoviruses and primary isolates, 10–1074 was shown to protect macaques from cell-free viral challenge and to suppress viremia in both non-human primates and HIV-1 infected patients [48], [49], [50], [51]. We observed a strong inhibition of cell-to-cell transmission mediated by seminal leukocytes by the 10–1074 Ab, as reflected by both Ab potency (IC90 < 0.062 μg/ml) and efficacy (100% of maximum neutralization capacity). The high efficiency of 10–1074 against CAV transmission may indicate a potential link between its inhibitory action in established infection in vivo and that during cell-cell transmission. Studies aiming to determine whether the efficiency of this Ab against cell-associated SHIV162P3 virus correlates with the ability to protect macaques exposed to infected cells are warranted.
Several studies provided evidences that bNAbs require Fc-mediated immunity for optimal efficacy in vivo [52], [53], [54]. Fc-mediated functions that eliminate infected cells may be particularly important for challenge systems involving cell-associated virus. Thus, even if maximal neutralization capacity fails to reach 100%, Fc-mediated functions represent an additional mechanism of action of the antibodies against infected T cells. Whether these additional functions also impact semen leukocyte-mediated infection of CD4+ T cells remains poorly characterized. The in vitro system we described here could be adapted to investigate the degree to which the efficacy of bNAbs is assisted by Fc-mediated functions.
A limitation of our study was that SHIV162P3 may not be representative of a diverse HIV-1 swarm and that a combination of bNAbs might be required to cover viral diversity and resistant variants within diverse viral populations, as suggested by other studies [29,55].
In conclusion, our results confirm previous observations that cell-to-cell transmission is more difficult to neutralize than that by cell-free virus and show that first-generation bNAbs may not inhibit SHIV162P3 cell-to-cell transmission when used alone. However, this was not true for certain second-generation bNAbs, including the V3 loop N332 glycan supersite 10–1074 Ab, which maintained efficacy even when used individually against SHIV162P3 transmission mediated by both spleen and semen cells. The splenocyte-PBMC co-culture system we developed offers experimental and analytical advantages over traditional models of HIV-1 cell-to-cell transmission for the study of bNAb-inhibition and may provide a useful platform and screening method to better predict in vivo bNAb sensitivity.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
Acknowledgments
We thank all members of the FlowCyTech, Animal Science and Welfare, and Infectiology Immunology Laboratory, L2I core facilities of the IDMIT infrastructure for their excellent expertise and outstanding contribution. We sincerely thank Dr. C. Gommet for the preparation of the AX450 spleen cells. We acknowledge the generosity of Polymuns Scientific, which provided the 1st generation broad neutralizing antibodies used in this study and that of Dr. C. Ochsenbauer, who provided the SHIV162P3 plasmid. The SHIV SF162P3 virus was obtained through the NIH AIDS Reagent Program, NIAID, NIH (cat# 6526).
Funding
This work was funded by the French Agence Nationale de Recherches sur le Sida et les Hépatites Virales (ANRS) and the European AIDS Vaccine Initiative 2020 (EAVI2020, grant agreement n° 681137) of the H2020 European program. MC was a beneficiary of a Marie Curie Individual fellowship (grant agreement n° 658277 for the project DCmucoHIV). KS was supported by fellowships from the ANRS (n° 2016–313) and the Franco-Thai Program (n° 849249B). This work was also supported by the “Programme Investissements d'Avenir” (PIA) managed by the ANR under reference ANR-11-INBS-0008, funding the Infectious Disease Models and Innovative Therapies (IDMIT, Fontenay-aux-Roses, France) infrastructure, and ANR-10-EQPX-02–01, funding the FlowCyTech facility (IDMIT, Fontenay-aux-Roses, France). It also benefits of the support of “Fond de Dotation Pierre Bergé” (SIDACTION, France). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ebiom.2020.102842.
Appendix. Supplementary materials
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS) 2018. UNAIDS Data 2018. [DOI] [PubMed] [Google Scholar]
- 2.Arien K.K., Jespers V., Vanham G. HIV sexual transmission and microbicides. Rev Med Virol. 2011;17:115–131. doi: 10.1002/rmv. [DOI] [PubMed] [Google Scholar]
- 3.Moir S., Chun T.-.W., Fauci A.S. Pathogenic mechanisms of HIV disease. Annu Rev Pathol Mech Dis. 2011;6:223–248. doi: 10.1146/annurev-pathol-011110-130254. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez S.M., Aguilar-Jimenez W., Su R.-.C., Rugeles M.T. Mucosa: key interactions determining sexual transmission of the HIV infection. Front Immunol. 2019;10:144. doi: 10.3389/FIMMU.2019.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ronen K., Sharma A., Overbaugh J. HIV transmission biology: translation for HIV prevention. AIDS. 2015;29:2219–2227. doi: 10.1097/QAD.0000000000000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sagar M. HIV-1 transmission biology: selection and characteristics of infecting viruses. J Infect Dis. 2010;202:S289–S296. doi: 10.1086/655656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sallé B., Brochard P., Bourry O., Mannioui A., Andrieu T., Prevot S. Infection of macaques after vaginal exposure to cell-associated simian immunodeficiency virus. J Infect Dis. 2010;202:337–344. doi: 10.1086/653619. [DOI] [PubMed] [Google Scholar]
- 8.Parsons M.S., Lloyd S.B., Lee W.S., Kristensen A.B., Amarasena T., Center R.J. Partial efficacy of a broadly neutralizing antibody against cell-associated SHIV infection. Sci Transl Med. 2017;9:eaaf1483. doi: 10.1126/scitranslmed.aaf1483. [DOI] [PubMed] [Google Scholar]
- 9.Kolodkin-Gal D., Hulot S.L., Korioth-Schmitz B., Gombos R.B., Zheng Y., Owuor J. Efficiency of cell-free and cell-associated virus in mucosal transmission of human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 2013;87:13589–13597. doi: 10.1128/jvi.03108-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernard-Stoecklin S., Gommet C., Corneau A.B., Guenounou S., Torres C., Dejucq-Rainsford N. Semen CD4+ T cells and macrophages are productively infected at all stages of SIV infection in Macaques. PLoS Pathog. 2013;9:1–13. doi: 10.1371/journal.ppat.1003810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H., Dornadula G., Beumont M., Livornese L., Van Uitert B., Henning K. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998;339:1803–1809. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]
- 12.Quayle A.J., Xu C., Mayer K.H., Anderson D.J. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J Infect Dis. 1997;176:960–968. doi: 10.1086/516541. [DOI] [PubMed] [Google Scholar]
- 13.Moldt B., Rakasz E.G., Schultz N., Chan-Hui P.-.Y., Swiderek K., Weisgrau K.L. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moldt B., Le K., Carnathan D.G., Whitney J.B., Schultz N., Lewis M.G. Neutralizing antibody affords comparable protection against vaginal and rectal SHIV challenge in macaques. AIDS. 2016;30:1543–1551. doi: 10.4172/2157-7633.1000305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pegu A., Yang Z.Y., Boyington J.C., Wu L., Ko S.Y., Schmidt S.D. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med. 2014:6. doi: 10.1126/scitranslmed.3008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shingai M., Donau O.K., Plishka R.J., Buckler-White A., Mascola J.R., Nabel G.J. Passive transfer of modest titers of potent and broadly neutralizing anti-HIV monoclonal antibodies block SHIV infection in macaques. J Exp Med. 2014;211:2061–2074. doi: 10.1084/jem.20132494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gautam R., Nishimura Y., Pegu A., Nason M.C., Klein F., Gazumyan A. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature. 2016;533:105–109. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rudicell R.S., Kwon Y.D., Ko S.-.Y., Pegu A., Louder M.K., Georgiev I.S. Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against Lentiviral infection in vivo. J Virol. 2014;88:12669–12682. doi: 10.1128/JVI.02213-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saunders K.O., Pegu A., Georgiev I.S., Zeng M., Joyce M.G., Yang Z.-.Y. Sustained delivery of a broadly neutralizing antibody in nonhuman primates confers long-term protection against simian/human immunodeficiency virus infection. J Virol. 2015;89:5895–5903. doi: 10.1128/jvi.00210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moog C., Dereuddre-Bosquet N., Teillaud J.-.L., Biedma M.E., Holl V., Van Ham G. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol. 2014;7:46–56. doi: 10.1038/mi.2013.23. [DOI] [PubMed] [Google Scholar]
- 21.a Abela I, L Berlinger, Schanz M., Reynell L., Günthard H.F., Rusert P. Cell-cell transmission enables HIV-1 to evade inhibition by potent CD4bs directed antibodies. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duncan C.J.A., Williams J.P., Schiffner T., Gärtner K., Ochsenbauer C., Kappes J. High-multiplicity HIV-1 infection and neutralizing antibody evasion mediated by the macrophage-T cell virological synapse. J Virol. 2014;88:2025–2034. doi: 10.1128/JVI.03245-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H., Zony C., Chen P., Chen B.K. Reduced potency and incomplete neutralization of broadly neutralizing antibodies against cell-to-cell transmission of HIV-1 with transmitted founder Envs. J Virol. 2017:91. doi: 10.1128/jvi.02425-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malbec M., Porrot F., Rua R., Horwitz J., Klein F., Halper-Stromberg A. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. J Exp Med. 2013;210:2813–2821. doi: 10.1084/jem.20131244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reh L., Magnus C., Schanz M., Weber J., Uhr T., Rusert P. Capacity of broadly neutralizing antibodies to inhibit HIV-1 cell-cell transmission is strain- and epitope-dependent. PLoS Pathog. 2015;11:1–34. doi: 10.1371/journal.ppat.1004966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schiffner T., Sattentau Q.J., Duncan C.J. Cell-to-cell spread of HIV-1 and evasion of neutralizing antibodies. Vaccine. 2013;31:5789–5797. doi: 10.1016/j.vaccine.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 27.Martin N., Welsch S., Jolly C., Briggs J.A.G., Vaux D., Sattentau Q.J. Virological synapse-mediated spread of human immunodeficiency virus type 1 between T cells is sensitive to entry inhibition. J Virol. 2010;84:3516–3527. doi: 10.1128/JVI.02651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCoy L.E., Groppelli E., Blanchetot C., de Haard H., Verrips T., Rutten L. Neutralisation of HIV-1 cell-cell spread by human and llama antibodies. Retrovirology. 2014;11:83. doi: 10.1186/s12977-014-0083-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gombos R.B., Kolodkin-Gal D., Eslamizar L., Owuor J.O., Mazzola E., Gonzalez A.M. Inhibitory effect of individual or combinations of broadly neutralizing antibodies and antiviral reagents against cell-free and cell-to-cell HIV-1 transmission. J Virol. 2015;89:7813–7828. doi: 10.1128/JVI.00783-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mouquet H., Scharf L., Euler Z., Liu Y., Eden C., Scheid J.F. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci USA. 2012:109. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montefiori D.C. Measuring HIV neutralization in a luciferase reporter gene assay. In: Prasad VR, Kalpana G V, Walker JM, editors. second. Vol. 485. Humana Press; 2009. pp. 359–374. (METHODS mol. biol. hiv protoc.). [DOI] [PubMed] [Google Scholar]
- 32.Heyndrickx L., Heath A., Sheik-Khalil E., Alcami J., Bongertz V., Jansson M. International network for comparison of HIV neutralization assays: the NeutNet report. PLoS One. 2012:7. doi: 10.1371/journal.pone.0004505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karlsson I., Malleret B., Brochard P., Delache B., Calvo J., Le Grand R. Dynamics of T-cell responses and memory T cells during primary simian immunodeficiency virus infection in cynomolgus Macaques. J Virol. 2007;81:13456–13468. doi: 10.1128/jvi.01619-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mannioui A., Bourry O., Sellier P., Delache B., Brochard P., Andrieu T. Dynamics of viral replication in blood and lymphoid tissues during SIVmac251 infection of macaques. Retrovirology. 2009;6:1–15. doi: 10.1186/1742-4690-6-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suphaphiphat K., Bernard-Stoecklin S., Gommet C., Delache B., Dereuddre‐Bosquet N., Kent S.J. Innate and adaptive anti-SIV responses in Macaque semen: implications for infectivity and risk of transmission. Front Immunol. 2020:11. doi: 10.3389/fimmu.2020.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Julg B., Tartaglia L.J., Keele B.F., Wagh K., Pegu A., Sok D. Broadly neutralizing antibodies targeting the HIV-1 envelope V2 apex confer protection against a clade C SHIV challenge. Sci Transl Med. 2017;9:eaal1321. doi: 10.1126/scitranslmed.aal1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hessell A.J., Rakasz E.G., Tehrani D.M., Huber M., Weisgrau K.L., Landucci G. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol. 2010;84:1302–1313. doi: 10.1128/JVI.01272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murooka T.T., Deruaz M., Marangoni F., Vrbanac V.D., Seung E., von Andrian U.H. HIV-infected T cells are migratory vehicles for viral dissemination. Nature. 2012;490:283–287. doi: 10.1038/nature11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganesh L., Leung K., Lore K., Levin R., Panet A., Schwartz O. Infection of specific dendritic cells by CCR5-tropic human immunodeficiency virus type 1 promotes cell-mediated transmission of virus resistant to broadly neutralizing antibodies. J Virol. 2004;78:11980–11987. doi: 10.1128/jvi.78.21.11980-11987.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson D.J., Politch J.A., Nadolski A.M., Blaskewicz C.D., Pudney J., Mayer K.H. Targetting Trojan Horse leukocytes for HIV prevention. AIIDS. 2010;24:163–187. doi: 10.1097/QAD.0b013e32833424c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Del Portillo A., Tripodi J., Najfeld V., Wodarz D., Levy D.N., Chen B.K. Multiploid inheritance of HIV-1 during cell-to-cell infection. J Virol. 2011;85:7169–7176. doi: 10.1128/jvi.00231-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernard-Stoecklin S., Gommet C., Cavarelli M., Le Grand R. Nonhuman primate models for cell-associated simian immunodeficiency virus transmission: the need to better understand the complexity of HIV mucosal transmission. J Infect Dis. 2014;210 Suppl 3:S660–S666. doi: 10.1093/infdis/jiu536. [DOI] [PubMed] [Google Scholar]
- 43.Montefiori D.C., Karnasuta C., Huang Y., Ahmed H., Gilbert P., De Souza M.S. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J Infect Dis. 2012;206:431–441. doi: 10.1093/infdis/jis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choudhry V., Zhang M.Y., Harris I., Sidorov I.A., Vu B., Dimitrov A.S. Increased efficacy of HIV-1 neutralization by antibodies at low CCR5 surface concentration. Biochem Biophys Res Commun. 2006;348:1107–1115. doi: 10.1016/j.bbrc.2006.07.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen P., Hubner W., Spinelli M.A., Chen B.K. Predominant mode of human immunodeficiency virus transfer between T cells is mediated by sustained env-dependent neutralization-resistant virological synapses. J Virol. 2007;81:12582–12595. doi: 10.1128/jvi.00381-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Massanella M., Puigdoménech I., Cabrera C., Fernandez-Figueras M.T., Aucher A., Gaibelet G. Antigp41 antibodies fail to block early events of virological synapses but inhibit HIV spread between T cells. AIDS. 2009;23:183–188. doi: 10.1097/QAD.0b013e32831ef1a3. [DOI] [PubMed] [Google Scholar]
- 47.McCoy L.E., Falkowska E., Doores K.J., Le K., Sok D., van Gils M.J. Incomplete neutralization and deviation from sigmoidal neutralization curves for HIV broadly neutralizing monoclonal antibodies. PLoS Pathog. 2015:11. doi: 10.1371/journal.ppat.1005110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caskey M., Schoofs T., Gruell H., Settler A., Karagounis T., Kreider E.F. Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat Med. 2017;23:185–191. doi: 10.1038/nm.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shingai M., Nishimura Y., Klein F., Mouquet H., Donau O.K., Plishka R. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mendoza P., Gruell H., Nogueira L., Pai J.A., Butler A.L., Millard K. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nat Med. 2018;561:479–484. doi: 10.1038/s41586-018-0531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bar-On Y., Gruell H., Schoofs T., Pai J.A., Nogueira L., Butler A.L. Safety and antiviral activity of combination HIV-1 broadly neutralizing antibodies in viremic individuals. Nat Med. 2018;24:1701–1707. doi: 10.1038/s41591-018-0186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hessell A.J., Hangartner L., Hunter M., Havenith C.E.G., Beurskens F.J., Bakker J.M. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 53.Bournazos S., Klein F., Pietzsch J., Seaman M.S., Nussenzweig M.C., Ravetch J.V. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell. 2014;158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horwitz J.A., Halper-Stromberg A., Mouquet H., Gitlin A.D., Tretiakova A., Eisenreich T.R. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci. 2013;110:16538–16543. doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Julg B., Liu P.-.T., Wagh K., Fischer W.M., Abbink P., Mercado N.B. Protection against a mixed SHIV challenge by a broadly neutralizing antibody cocktail. Sci Transl Med. 2017:9. doi: 10.1126/scitranslmed.aao4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.