Summary
Cell cycle progression is controlled by the interplay of established cell cycle regulators. Changes in these regulators' activity underpin differences in cell cycle kinetics between cell types. We investigated whether long intergenic noncoding RNAs (lincRNAs) contribute to embryonic stem cell cycle adaptations. Using single-cell RNA sequencing data for mouse embryonic stem cells (mESCs) staged as G1, S, or G2/M we found differentially expressed lincRNAs are enriched among cell cycle-regulated genes. These lincRNAs (CC-lincRNAs) are co-expressed with genes involved in cell cycle regulation. We tested the impact of two CC-lincRNA candidates and show using CRISPR activation that increasing their expression is associated with deregulated cell cycle progression. Interestingly, CC-lincRNAs are often differentially expressed between G1 and S, their promoters are enriched in pluripotency transcription factor (TF) binding sites, and their transcripts are frequently co-regulated with genes involved in the maintenance of pluripotency, suggesting a contribution of CC-lincRNAs to mESC cell cycle adaptations.
Subject Areas: Molecular Biology, Cell Biology
Highlights
-
•
Genes differentially expressed between mESC cell cycle stages are enriched in lincRNAs
-
•
CC-lincRNAs are co-expressed with cell cycle and pluripotency genes
-
•
CC-lincRNAs are often mESC specific and their promoters enriched in pluripotency TFs
-
•
Upregulation of two CC-lincRNAs results in deregulated mESC cell cycle progression
Molecular Biology; Cell Biology
Introduction
The cell cycle is a dynamic sequence of events leading from one parent cell to two daughter cells. This requires replication of chromosomes during the Synthesis phase (S phase) and their segregation into two daughter cells during Mitosis (M phase). M and S phases are separated by two Gap phases, G1 and G2, that act as checkpoints to prevent cell division without genome replication and aberrant polyploidy (Murray and Hunt, 1993). Progression through cell cycle is regulated by cell cycle stage-specific activation and repression of numerous proteins including Cyclin Dependent Kinases (CDKs) and proteins from the Cyclin family (Morgan, 1995). In most somatic cells, the oscillatory expression or activity of distinct Cyclin-Cdk complexes allows the activation and repression of cell cycle regulators and promotes cell cycle transitions (Morgan, 1995). One key regulator of this process is the retinoblastoma protein (RB) that controls G1 and prevents entry into S phase. Upon entrance in G1, RB is unphosphorylated (active) and blocks the expression of genes required for G1/S transition. During G1, RB is phosphorylated and becomes inactive, allowing cells to progress to S phase (Weinberg, 1995). In embryonic stem cells (ESCs), RB is hyperphosphorylated, resulting in suppression of the G1-S checkpoint and thereby rapid shuttling between DNA synthesis and mitosis, decreasing the average duration of the ESC cell cycle (reviewed in Zaveri and Dhawan [2018]).
These significant adaptations in the ESC cell cycle are important for the maintenance of the embryonic stem cell state and cell fate decisions, as highlighted by the partial overlap between the gene regulatory networks that control the two processes (Soufi and Dalton, 2016). For example, both Oct4 and Nanog, two core pluripotency factors, control genes involved in cell cycle regulation: in mouse embryonic stem cells (mESCs), Oct4 represses the expression of p21, a Cyclin-dependent kinase inhibitor that is expressed in somatic cells but not in embryonic stem cells (Lee et al., 2010); NANOG, whose expression is cell cycle regulated, controls S-phase entry by regulating the expression of Cdc25C and CDK6 in human ESCs (hESCs) (Zhang et al., 2009). Although the association between cell cycle dynamics and cell state is well established, the molecular mechanisms underlying this connection remain uncharacterized (Soufi and Dalton, 2016).
In addition to proteins, noncoding RNAs, including long intergenic noncoding RNAs (lincRNAs), have also been shown to contribute to cell cycle progression (Kitagawa et al., 2013). An example of this is MALAT1, a lincRNA that is frequently upregulated in multiple human cancers (Sun and Ma, 2019). In human fibroblasts, depletion of MALAT1 leads to decreased expression of several genes involved in cell cycle progression and results in G1 arrest. MALAT1 is also involved in splicing of B-Myb, a gene involved in the transcriptional regulation of several mitotic proteins (Tripathi et al., 2013). More recently, the cohesion regulator long noncoding RNA (CONCR) has been found to be necessary for cell cycle progression and DNA replication. CONCR expression is activated by the transcription factor MYC and is upregulated in multiple cancer types (Marchese et al., 2016). Silencing of CONCR leads to a significant decrease in DNA synthesis. At the molecular level, CONCR physically interacts with DDEA/H-box helicase 11, which ensures the proper separation of sister chromatids during the cell division process. The absence of CONCR leads to the loss of sister chromatid cohesion and affects metaphase (Marchese et al., 2016). Finally, lincRNA expression is often dysregulated in cancer and the characterization of subsets of cancer-associated lincRNAs highlights their potential roles as cell cycle progression modulators (Schmitt and Chang, 2016; Notzold et al., 2017).
LincRNAs are also part of the network controlling stem cell fate maintenance and differentiation (Yan et al., 2017). Because lincRNA expression is often tissue specific (Derrien et al., 2012; Tuck et al., 2018), in contrast to proteins, we hypothesized they can support cell type-specific activity of ubiquitously expressed genes and act at the intersection of cell cycle and mESC cell state regulation. The ability of tissues-specific noncoding RNAs to modulate the activity of ubiquitously expressed gene was already exemplified by lncSCA7. This lincRNA regulates ATXN7 levels in retinal and cerebellar neurons and contributes to specific degeneration of these cells in SCA7 patients (Tan et al., 2014). Additionally, the relative short half-lives of lincRNAs (Clark et al., 2012; Mukherjee et al., 2017) further underscores their potential as modulators of temporally resolved processes such as cell cycle progression.
Here, we investigate the contributions of lincRNAs to embryonic stem cell cycle adaptations.
Results
LincRNA Expression Is Often Cell Cycle Regulated
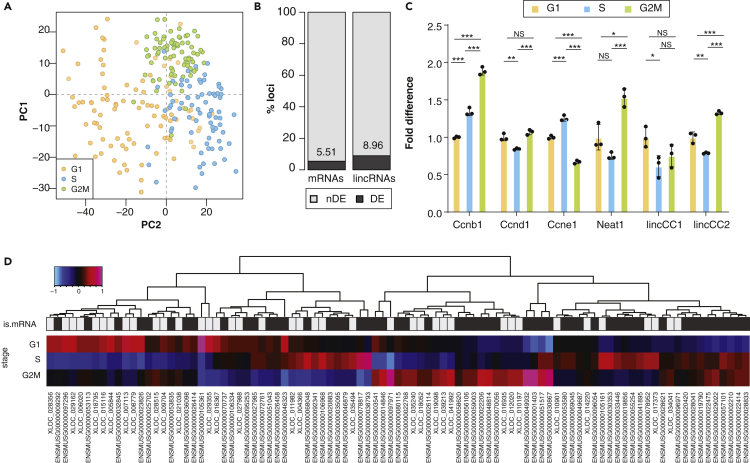
We used publicly available single-cell RNA sequencing (scRNAseq) data for 279 mESCs with known cell cycle stage (Buettner et al., 2015) to assess the extent of cell cycle-regulated lincRNA expression in mESCs. We estimated the expression of protein-coding transcripts and lincRNAs in each of these cells. After excluding cells and genes that failed quality control (Figure S1) we identified 10,487 genes, including 781 lincRNAs, whose expression can be robustly detected in 246 cells. As previously shown (Buettner et al., 2015), gene expression patterns in this dataset reflect the cell cycle stages of the individual cells (Figure 1A), supporting its use to identify transcripts whose expression are cell cycle dependent. Using DEseq2, we identified 638 genes (6.1%) whose expression is significantly different between at least two cell cycle stages (Table S1). The proportion of differentially expressed lincRNAs (n = 70, 8.96%) is significantly higher than that found for protein-coding genes (n = 501, 5.51%) (proportions test p value = 7 × 10−10, Figure 1B, Table S1), indicating that lincRNA expression is more dynamic throughout the mESC cell cycle than is the expression of protein-coding genes (Figure 1D).
Figure 1.
LincRNA Expression Is Often Cell Cycle Regulated
(A) Principal component analysis of gene expression for all loci passing the technical noise filter. The first two principal components (PC1 on the x axis and PC2 on the y axis) together explain 5.15% of the total variability, and separate cells in different cell cycle stages (G1: orange, S: blue; G2/M: green).
(B) Percentage of protein-coding genes (left) and lincRNAs (right) that are differentially expressed (dark gray, numbers indicate the percentage) between at least two cell cycle stages.
(C) Fold difference in normalized expression (relative to G1) of known cell cycle-regulated genes (Ccnb1, Ccnd1, Ccne1, Neat1) and two differentially expressed novel lincRNAs (lincCC1 and lincCC2) in mESCs at different cell cycle stages (G1: orange, S: blue, G2/M: green).
(D) Heatmap representing the median Z score of the shifted log expression level across all cells in each of the three stages (G1, S and G2M, rows) for each differentially expressed lincRNA and mRNA (columns). Rectangles in “is.mRNA” row are colored gray and black for lincRNA and mRNA, respectively. Color scale top right. Significance (two-tailed unpaired t test p value) between cell cycle stage expression is indicated as NS p > 0.05, ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
The median expression of mRNAs is roughly 14 times higher than that of lincRNAs (Figure S2A) and steady-state abundance can impact the ability to detect differential gene expression, as highlighted by the significantly higher expression (two-tailed Wilcoxon test, p value = 3 × 10−43) of genes identified as differentially expressed (Figure S2A). To assess whether lincRNAs' relatively low expression impacts our differential gene expression analysis results, we repeated the analysis by re-quantifying protein-coding gene expression using a subset (1/14) of randomly sampled reads from each library. At this sequencing depth, the median mRNA expression is comparable with that of lincRNAs in the full data (Figure S2B). We repeated expression quantification, quality control, normalization, filtering, and differential gene expression analyses using these randomly sampled reads and found that only 60% of the mRNAs found to be differentially expressed using the full data are also differentially expressed when using the down-sampled libraries. This result indicates that the higher proportion of differentially expressed lincRNAs is likely an underestimate owing to the limited power to measure their expression using single cell RNA sequencing data.
To validate our in silico differential gene expression predictions we used DNA content to sort mESC cells into G1, S, and G2/M stages and measured the cell cycle stage expression of twelve differentially expressed lincRNAs, including Neat1, and three mESC cell cycle protein-coding genes (Ccnb1, Ccnd1 and Ccne1) by quantitative PCR (Figures 1C and S3). The cell cycle protein-coding gene and lincRNA expression patterns measured by qPCR were generally consistent with what was estimated using scRNA sequencing data. The results of this analysis support that despite the relatively low expression of lincRNAs that complicates the accurate estimation of their expression, our differential expression predictions are generally robust.
Differentially Expressed lincRNAs Contribute to the Regulation of Cell Cycle Progression
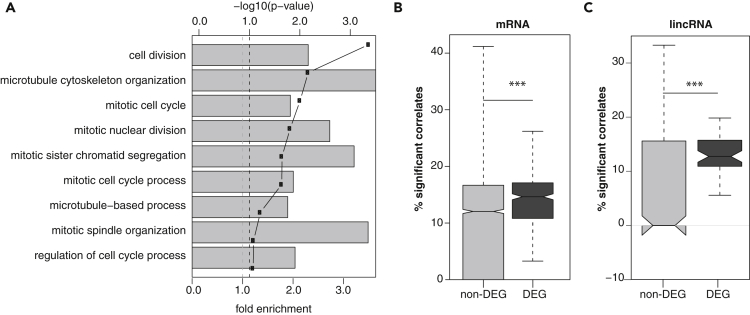
Consistent with the role of differentially expressed protein-coding genes in the regulation of cell cycle progression, these genes are significantly enriched (hypergeometric test adjusted p value < 0,05) in annotations with gene ontology terms such as “cell division” and other related cell cycle processes (Figure 2A, Table S2), as previously described (Dominguez et al., 2016; Liu et al., 2017; Pena-Diaz et al., 2013). The lower sensitivity of scRNAseq together with the differences in embryonic and adult cell cycle dynamics and experimental design likely explain the relatively smaller enrichment in “cell division”-related terms among differentially expressed genes relative to previous studies (Dominguez et al., 2016; Pena-Diaz et al., 2013; Liu et al., 2017). Furthermore, 8.8% (44 of 501) of differentially expressed protein-coding loci are orthologous to genes previously shown to have a periodic expression throughout the human cell cycle (Santos et al., 2015), a significant 3.1-fold enrichment relative to all mESC expressed protein-coding loci (214, hypergeometric test p value = 1 × 10−11), in cell cycle-related terms. These results support that genes differentially expressed between cell cycle stages are indeed enriched in cell cycle regulators.
Figure 2.
Genes Differentially Expressed between Cell Cycle Stages Have Cell Cycle-Related Functions
(A) Results of the GO term enrichment analysis for cell cycle stage differentially expressed genes. Gray bars indicate the fold enrichment, and points the associated -log10 of the p value. Dashed line indicates the 10% FDR cutoff. Distribution of the percentage of genes cell cycle regulators that are significantly correlated in expression with (B) protein-coding genes and (C) lincRNAs. Differentially or non-differentially expressed genes from each biotope are represented in dark and light gray, respectively. Significance of the distribution comparison (two-tailed Mann-Whitney-Wilcoxon rank-sum test) are indicated as ∗∗∗ p value < 0.001.
The enrichment of cell cycle-related genes among those differentially expressed between cell cycle stages suggests that some of the lincRNAs identified here as differentially expressed might also contribute to mESC cell cycle regulation. Most lincRNAs differentially expressed between cell cycle stages were annotated de novo (53 de novo lincRNAs) using mESC RNA sequencing data (Tan et al., 2015). We searched the literature for functions of the remaining seventeen differentially expressed lincRNAs annotated by ENSEMBL (Table S3). Of these, only four have been previously characterized: Neat1, Malat1, the host transcript for snord93, and miRNA-24 pri-miRNA transcript. Malat1 is a cancer-associated lincRNA and has been previously implicated in cell cycle progression. For example, Malat1 is highly expressed during the S and M phases of human fibroblasts where it controls cell cycle-related processes (Tripathi et al., 2013). Neat1, whose knockdown was recently shown to impair S phase transition (Adriaens et al., 2016), has also been frequently associated with cancer and cell cycle progression (Yu et al., 2017). Relatively little is known about snord93, but changes in this RNA's levels impact cell proliferation (Patterson et al., 2017), which would be consistent with a role in cell cycle progression. Finally, miRNA-24 post-transcriptionally regulates MYC and E2F2, two known cell cycle genes, and changes in its levels impair G1 transition (Lal et al., 2009). In conclusion, all of the four annotated and characterized lincRNAs we identified as being differentially expressed either have established roles or have been associated with cell cycle progression.
Given the paucity of functional annotations for lincRNAs, and to assess broadly the contributions of differentially expressed lincRNAs to the cell cycle, we reasoned that genes that functionally participate in cell cycle regulation should genetically interact with known cell cycle regulators. To first validate this idea, we considered cell cycle phase differentially expressed protein-coding genes not annotated as cell cycle regulators and found their expression is more often significantly (p value = 1 × 10−8, Wilcoxon rank-sum test) correlated with the expression of annotated cell cycle genes than non-differentially expressed protein-coding loci (Figure 2B). Similarly, we found that the expression of differentially expressed lincRNAs is also significantly more often correlated with the levels of cell cycle genes than non-differentially expressed lincRNAs (Figure 2C, p value = 2 × 10−4 Wilcoxon rank-sum test), supporting their contributions to mESC cell cycle progression. Hereafter, we refer to differentially expressed lincRNAs and protein-coding genes as cell cycle-associated lincRNAs and protein-coding genes, or CC-lincRNAs and CC-PCGs, respectively.
Cell Cycle lincRNAs Are Associated with Stem Cell Cycle Adaptations
The cell cycle is a ubiquitous process, yet most lincRNAs are expressed in cell type-specific manner (Ulitsky and Bartel, 2013). Do the CC-lincRNAs identified here contribute to cell cycle regulation ubiquitously, or are their functions restricted to mESCs? To gain initial insights into this question we used publicly available transcriptome-wide data (Yue et al., 2014) to estimate the tissue specificity of lincRNAs and protein-coding genes across 29 adult and developing mouse tissue and cell lines. We estimated Tau, a measure of tissue specificity, for CC-lincRNAs, CC-PCGs, and known cell cycle regulators. Tau varies between 0% for “ubiquitously expressed” to 100% for “tissue-specifically expressed” genes (Kryuchkova-Mostacci and Robinson-Rechavi, 2017). As expected, CC-PCGs, as well as established cell cycle genes, are broadly expressed (median tau = 24.8%). In contrast, CC-lincRNAs are significantly more tissue specific (median tau = 42%, Wilcox rank-sum test p value = 7 × 10−13, Figure 3A) than their protein-coding counterparts and are as tissue specific as other mESC-expressed lincRNAs (data not shown).
Figure 3.
CC-lincRNAs Are Associated with Stem Cell Cycle Adaptations
(A–C) (A) Distribution of tissue specificity (measured as Tau) for CC-lincRNAs, CC-PCGs, and annotated cell cycle genes. Distribution of the fold difference in expression of (B) lincRNAs and (C) protein-coding genes during a 216-h neuronal commitment of mESCs. Fold difference in expression is relative to the median fold difference at time = 8 h.
(D) Fold enrichment in binding (bars, x axis) of pluripotency transcription factors (y axis) for CC-PCGs (dark gray) and CC-mRNAs (light gray).
(E) Cell cycle stage-average (G1: orange, S: blue, G2/M: green) of the expression Z scores of CC-PCGs (left panel) and CC-lincRNAs (right panel).
(F) Proportional Euler diagram of the number of lincRNAs (left) and protein-coding genes (right) differentially expressed between G1 and S (orange), G1 and G2M (blue), or S and G2/M (green).
(G) Distribution of the correlations between pairs of pluripotency-associated genes (black) or known pluripotency genes, and differentially expressed (CC, gray) or non-differentially expressed (nonCC, white) protein-coding genes and lincRNAs. Significance (two-tailed Mann-Whitney-Wilcoxon rank-sum test) for the comparison between pluripotency-associated genes and the different gene classes is indicated on top of the boxplot as NS p > 0.05, ∗∗p < 0.01, ∗∗∗p < 0.00.
To further test if CC-lincRNA expression is often restricted to mESCs, we investigated changes in their transcript abundance upon neuronal differentiation of mESCs. In vitro neural differentiation is a well-defined and highly efficient process (>80% of differentiated cells). We took advantage of publicly available transcriptome-wide expression for a time course of neuronal commitment of mESC (Sun et al., 2017) to investigate the changes in noncoding and coding gene expression. Consistent with their tissue-specific expression (Figure 3A), CC-lincRNA expression decreases rapidly upon differentiation (Figure 3B), supporting their contribution to cell cycle regulation being restricted to mESCs. In contrast, CC-PCGs are expressed at similar levels throughout neuronal commitment (Figure 3C).
The transcriptional network controlling mESC cell state and function is regulated by a set of mESC core transcription factors (Chambers and Tomlinson, 2009). The short G1 phase that characterizes the embryonic stem cell cycle, which is critical to ensure maintenance of pluripotency, is in part orchestrated by stem cell-specific factors (Boward et al., 2016). We took advantage of publicly available chromatin immunoprecipitation sequencing (ChIP-seq) data for pluripotency transcription factors in mESCs (Chen et al., 2008) to assess the extent of these factors' binding to cell cycle-regulated lincRNA promoters. We found that CC-lincRNA promoters are enriched in mESC core transcription factors (TFs) supporting their role in the network underlying mESC cell state. Specifically, promoters of CC-lincRNAs are significantly enriched, relative to all expressed lincRNAs, in the binding of pluripotency transcription factors, including Nanog, Oct4, and Sox2 (false discovery rate [FDR]<0.05, permutation test) (Figure 3D). We found no significant enrichment by most pluripotency TFs at the promotes of CC-PCGs (Figure 3D).
To assess what aspect of mESC cell cycle progression might be more often modulated by CC-lincRNAs, we investigated their relative expression across different cell cycle phases. Consistent with previous observations that mRNA expression peaks at G2/M phase in human cells (Cho et al., 2001; Whitfield et al., 2002) we found that CC-protein-coding genes were highly expressed in S and G2/M (Figure 3E) and were often differentially expressed between G1 and G2/M (Figure 3F). In contrast, the levels of CC-lincRNAs were higher in G1 relative to all other cell cycle stages (Figure 3E) and most were differentially expressed between G1 and S phases (Figure 3F).
Given the significantly elevated expression of CC-lincRNAs in G1, their tissue-specific expression and evidence that their transcription is regulated by stem cell-specific transcription factors, we hypothesized that CC-lincRNAs may contribute to the interplay between cell cycle and maintenance of pluripotency in mESCs. If CC-lincRNAs participate in the network controlling maintenance of mESC cell state, they should be co-expressed with genes involved in maintenance of pluripotency. To test this hypothesis, we investigated the correlation, during mESC neuronal commitment, between CC-genes and genes implicated in maintenance of pluripotency (Xu et al., 2013). First, we found that, consistent with the interplay between cell cycle control and maintenance of mESC cell state, the median pairwise correlation between CC-PCGs and pluripotency genes (Spearman's r = 0.06) is similar to what is found for pairs of genes implicated in pluripotency (r = 0.10, two-tailed Wilcoxon test p value = 0.3, Figure 3G). Consistent with our hypothesis that CC-lincRNAs contribute to maintenance of mESC cell state, we found that the extent of their association with pluripotency genes (r = 0.05) is also similar to what we estimate for pairs of genes implicated in pluripotency (two-tailed Wilcoxon test p value = 0.7, Figure 3G). For comparison, we also estimated the strength of the association with genes involved in pluripotency for non-cell cycle differentially expressed mESC coding (r = 0.03) and non-coding (r = −0.01) genes and found this to be significantly lower than that of CC-lincRNAs or CC-mRNAs (two-tailed Wilcoxon test p values: nonCC-mRNA = 0.002, nonCC-lncRNA = 0.0001, Figure 3G). Similar results are obtained when considering only significant correlations (correlation test p < 0.05) between pluripotency gene and coding or noncoding transcripts.
These results support the hypothesis that some CC-lincRNAs modulate specific aspects of mESC cell cycle and may contribute to the regulation of stem cell cycle adaptions.
Candidate CC-lincRNA Analysis Supports lincRNA Roles in Cell Cycle Regulation
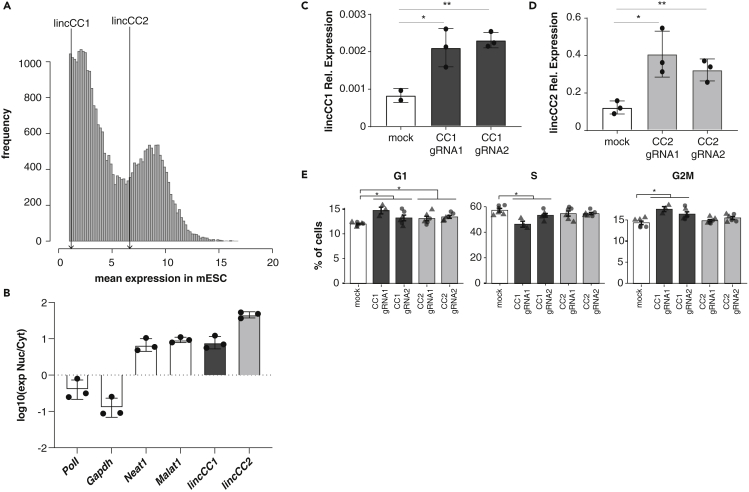
Next, we aimed to experimentally validate the contributions of previously uncharacterized CC-lincRNAs to mESC cell cycle regulation. Considering that relatively low transcript abundance may limit individual lincRNA's ability to regulate cell cycle progression, we selected one modestly expressed CC-lincRNA (XLOC_018328, hereafter lincCC1, Figure S4A) and one CC-lincRNA that is relatively highly expressed (Gm26853, hereafter lincCC2, Figure S4B) in mESCs (Figure 4A). We determined subcellular localization of these transcripts in mESCs by subcellular fractionation followed by qPCR and found that both tested CC-lincRNAs are predominantly nuclear (Figure 4B).
Figure 4.
Candidate lincRNA Overexpression Impacts Cell Cycle Progression
(A) Histogram of the mean cell expression of all considered loci in mESCs. Lines indicate the expression of lincCC1 and lincCC2.
(B–D) (B) Ratio between the nuclear and cytoplasmic expression (log10) for two cytosolic mRNAs (PolII and Gapdh), two nuclearly retained lincRNAs (Malat1 and Neat1), and lincCC1 and lincCC2. Each point corresponds to the results for one biological replicate. Relative (C) lincCC1 and (D) lincCC2 expression 72 h after co-transfection of Cas9-VP160 with scramble-, gRNA1-, or gRNA2-expressing constructs for three independent biological replicates.
(E) Percentage of mESCs at G1, S, and G2/M stages of the cell cycle 72 h post transfection of mESCs with scramble (white), lincCC1 (dark gray), or lincCC2 (light gray) targeting gRNAs. Technical replicates for two different biological replicates represented as either triangles or circles. Significance of comparisons is indicated as NS p > 0.05, ∗p < 0.05, ∗∗p < 0.01.
We employed CRISPR activation (CRISPRa) to increase endogenous lincRNAs' transcription. For each lincRNA, we designed two guide RNAs (gRNA) to target VP160-fused dead-Cas9 (dCas9-VP160) (Cheng et al., 2013) to the vicinity of lincRNA promoters. As a control, we designed a non-targeting scrambled gRNA. We transiently co-transfected each of the gRNA-expressing constructs with a dCas9-VP160 expressing vector. Seventy-two hours post transfection we observed an average 2-fold upregulation (two-tailed unpaired t test p value CC1 gRNA1 = 0.02, CC1 gRNA2 = 0.0002, CC2 gRNA1 = 0.0001, CC2 gRNA2 = 0.0001) of candidate lincRNAs expression (Figures 4C and 4D) in mESCs treated with targeting gRNA, relative to control.
To assess the impact of changes in candidate lincRNA expression upon cell cycle progression in mESC, we determined the proportions of cells in G1, S, and G2/M phases using fluorescence-activated cell sorting (FACS). We used EdU incorporation and DNA content to identify the proportion of single cells in each cell cycle phase following CC-lincRNA overexpression (Figure S4C). We found that, consistently with the proposed role of lincCC1 and lincCC2 in the modulation of cell cycle progression, upregulation of these lincRNAs is associated with significant changes in the proportion of cells within different cell cycle stages. LincCC1 upregulation is associated with a significant increase (p value = 0.0002) in the number of cells in G2/M and small, yet significant changes in the proportion of cells in G1 (p = 0.005) and S phases (p value = 0.002) (Figure 4E). LincCC2 mostly affects the proportion of cells in G1 (p value = 0.02).
These observations are consistent with the role of these CC-lincRNAs in the regulation of cell cycle progression.
Discussion
The cell cycle is central to the development of a multicellular organism from a single cell zygote and is required for cell renewal. Dysregulation of this process can cause disease, most notably cancer. Progression through the cell cycle requires dynamic and tightly regulated transitions between well-defined cell cycle stages, which are controlled by changes in cell cycle regulators' activity. Although control of cell cycle progression has been primarily assigned to protein-coding genes, several lincRNAs have recently been shown to contribute to this process (Kitagawa et al., 2013). For example, a recent siRNA screen in HeLa cells revealed that knockdown of 26 lincRNAs, whose expression is deregulated in cancer, impacts different aspects of cell cycle progression (Notzold et al., 2017). The role of lincRNAs in this process is further supported by a more recent comprehensive high-content RNAi screen in the same cells (Stojic et al., 2020). In parallel, characterization of individual lincRNAs has also illustrated how these noncoding RNAs contribute to the regulation of different aspects of cell cycle (Kitagawa et al., 2013).
In contrast to most cell cycle protein-coding regulators, lincRNA expression is often restricted in time and space, as supported by the analysis of their expression across adult and embryonic tissues and in single cells (for example, Derrien et al., 2012; Tuck et al., 2018; Ulitsky and Bartel, 2013). This suggests that most lincRNA-encoded functions are tissue specific. Although lincRNAs have been previously implicated in the regulation of cell cycle progression (Adriaens et al., 2016; Tripathi et al., 2013; Marchese et al., 2016), their role, if any, in cell type-specific modulations of this central biological process remains understudied. Here, we used mESC cycle, which is characterized by a truncated G1 phase, as a model system to assess the contributions of lincRNAs expressed in mESCs to the modulation of this stem cell adaptation.
To identify lincRNAs that are putative cell cycle regulators, we reasoned that for noncoding genes the functional moiety is the transcript; thus, differential noncoding gene expression between cell cycle stages would result in differential activity and enrich for lincRNAs with roles in cell cycle regulation. Consistent with this hypothesis, our genome-wide analyses and experimental validations support the role of lincRNAs differentially expressed between mESC cell cycle stages as modulators of cell cycle progression, likely through interaction with other cell cycle regulators.
Interestingly and relative to protein-coding genes, whose activity during cell cycle is often modulated by post-translational modifications, lincRNAs are enriched among differentially expressed transcripts, supporting that, in mESCs, their expression is more frequently dynamic throughout cell cycle progression. The fraction of cell cycle-regulated lincRNAs is similar to a previous estimate based on HeLa cells bulk RNA sequencing (~9% [Dominguez et al., 2016]). However, the dynamics of lincRNA expression throughout the cell cycle differs between cell types, as in contrast with this earlier study in HeLa (Dominguez et al., 2016) that revealed no preferential cell stage-specific expression in mESCs, we found most lincRNAs are highly expressed in G1 and differentially expressed between G1 and S phases. Given the critical importance of the G1-S transition in maintenance of embryonic stem cell state (Boward et al., 2016), we hypothesize that a subset of lincRNAs with roles in mESC cell cycle progression also have roles in maintenance of stem cell state. Supporting this hypothesis is the observation that these lincRNAs' expression is often restricted to pluripotent cells and rapidly decreases upon exit from pluripotency and entry into neural commitment. Furthermore, the expression of these lincRNAs is often regulated by core pluripotency transcription factors, including Nanog, Sox2, and Oct4. Finally, we provide preliminary evidence that cell cycle lincRNAs are part of the network underlying pluripotency.
Although further work is now required to disentangle how individual lincRNAs modulate cell cycle progression in mESCs, our results suggest that tissue-specific regulation by lincRNAs contributes to cell type-specific adaptations of ubiquitous processes.
Limitations of the Study
The present study aimed to identify the contributions of lincRNAs, which are generally tissue specifically expressed, to embryonic stem cell type-specific adaptations. Toward this end we explored publicly available cell cycle-staged single-cell RNA sequencing data and analyzed the impact of candidate lincRNA expression on cell cycle progression.
Our computational analysis was limited by the absence of an alternative dataset that could be used to replicate our observations. One consequence of this limitation is that analysis presented in Figure 2 uses the same data as that used to identify differential expressed genes and is therefore not independent.
The candidate analysis we reported is limited by the absence of efficient approaches to deplete nuclear lincRNA expression in mESCs. As a consequence and despite several attempts, using either shRNAs or CRISRPi, we were unable to transiently or constitutively deplete lincCC1 or CC2. Establishing and characterizing mESCs where candidate lincRNA function is lost would have allowed us to understand the biological and molecular contributions of lincRNAs to mESC cell cycle adaptations.
Resource Availability
Lead Contact
Ana Claudia Marques, anaclaudia.marques@unil.ch.
Data and Code Availability
All code used is available upon requestMaterials availability Is not relevant because no new materials were generated in the context of this project.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
pKLV-U6gRNA-EF(BbsI)-PGKpuro2ABFP was a gift from Hiroshi Ochiai (Addgene plasmid # 62348; http://n2t.net/addgene:62348; RRID:Addgene_62348); pAC95-pmax-dCas9VP160-2A-neo was a gift from Rudolf Jaenisch (Addgene plasmid # 48227; http://n2t.net/addgene:48227; RRID:Addgene_48227).
We would like to thank the University of Lausanne Flow Cytometry Platform for help with FACS and sorting analysis. The computations were performed at the Vital-IT (https://www.vital-it.ch) Center for high-performance computing of the SIB Swiss Institute of Bioinformatics. We would like to thank Dario Bottinelli for technical support during the early stages of this project and Vincent Dion and Constance Ciaudo for reading and commenting on early versions of this manuscript. This work was funded by the Swiss National Science Foundation (grant PP00P3_150667 to A.C.M.) and the NCCR in RNA & Disease (A.C.M.).
Authors Contributions
A.A.T.S., A.B., and A.C.M. contributed to the study design. A.A.T.S. and J.Y.T. performed the in silico analysis. A.A.T.S., A.B., M.F.d.S., B.A., and A.C.M. performed the in vitro analysis. A.C.M. supervised the study. A.C.M. and A.A.T.S. wrote the manuscript. All coauthors read and approved the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: July 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101291.
Supplemental Information
References
- Adriaens C., Standaert L., Barra J., Latil M., Verfaillie A., Kalev P., Boeckx B., Wijnhoven P.W., Radaelli E., Vermi W. p53 induces formation of NEAT1 lncRNA-containing paraspeckles that modulate replication stress response and chemosensitivity. Nat. Med. 2016;22:861–868. doi: 10.1038/nm.4135. [DOI] [PubMed] [Google Scholar]
- Boward B., Wu T., Dalton S. Concise review: control of cell fate through cell cycle and pluripotency networks. Stem Cells. 2016;34:1427–1436. doi: 10.1002/stem.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner F., Natarajan K.N., Casale F.P., Proserpio V., Scialdone A., Theis F.J., Teichmann S.A., Marioni J.C., Stegle O. Computational analysis of cell-to-cell heterogeneity in single-cell RNA-sequencing data reveals hidden subpopulations of cells. Nat. Biotechnol. 2015;33:155–160. doi: 10.1038/nbt.3102. [DOI] [PubMed] [Google Scholar]
- Chambers I., Tomlinson S.R. The transcriptional foundation of pluripotency. Development. 2009;136:2311–2322. doi: 10.1242/dev.024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Xu H., Yuan P., Fang F., Huss M., Vega V.B., Wong E., Orlov Y.L., Zhang W., Jiang J. Integration of external signaling pathways with the core transcriptional network in embryonic stem cells. Cell. 2008;133:1106–1117. doi: 10.1016/j.cell.2008.04.043. [DOI] [PubMed] [Google Scholar]
- Cheng A.W., Wang H., Yang H., Shi L., Katz Y., Theunissen T.W., Rangarajan S., Shivalila C.S., Dadon D.B., Jaenisch R. Multiplexed activation of endogenous genes by CRISPR-on, an RNA-guided transcriptional activator system. Cell Res. 2013;23:1163–1171. doi: 10.1038/cr.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho R.J., Huang M., Campbell M.J., Dong H., Steinmetz L., Sapinoso L., Hampton G., Elledge S.J., Davis R.W., Lockhart D.J. Transcriptional regulation and function during the human cell cycle. Nat. Genet. 2001;27:48–54. doi: 10.1038/83751. [DOI] [PubMed] [Google Scholar]
- Clark M.B., Johnston R.L., Inostroza-Ponta M., Fox A.H., Fortini E., Moscato P., Dinger M.E., Mattick J.S. Genome-wide analysis of long noncoding RNA stability. Genome Res. 2012;22:885–898. doi: 10.1101/gr.131037.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrien T., Johnson R., Bussotti G., Tanzer A., Djebali S., Tilgner H., Guernec G., Martin D., Merkel A., Knowles D.G. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22:1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez D., Tsai Y.H., Gomez N., Jha D.K., Davis I., Wang Z. A high-resolution transcriptome map of cell cycle reveals novel connections between periodic genes and cancer. Cell Res. 2016;26:946–962. doi: 10.1038/cr.2016.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M., Kitagawa K., Kotake Y., Niida H., Ohhata T. Cell cycle regulation by long non-coding RNAs. Cell Mol. Life Sci. 2013;70:4785–4794. doi: 10.1007/s00018-013-1423-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryuchkova-Mostacci N., Robinson-Rechavi M. A benchmark of gene expression tissue-specificity metrics. Brief Bioinform. 2017;18:205–214. doi: 10.1093/bib/bbw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A., Navarro F., Maher C.A., Maliszewski L.E., Yan N., O'day E., Chowdhury D., Dykxhoorn D.M., Tsai P., Hofmann O. miR-24 Inhibits cell proliferation by targeting E2F2, MYC, and other cell-cycle genes via binding to "seedless" 3'UTR microRNA recognition elements. Mol. Cell. 2009;35:610–625. doi: 10.1016/j.molcel.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Go Y., Kang I., Han Y.M., Kim J. Oct-4 controls cell-cycle progression of embryonic stem cells. Biochem. J. 2010;426:171–181. doi: 10.1042/BJ20091439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Chen S., Wang S., Soares F., Fischer M., Meng F., Du Z., Lin C., Meyer C., Decaprio J.A. Transcriptional landscape of the human cell cycle. Proc. Natl. Acad. Sci. U S A. 2017;114:3473–3478. doi: 10.1073/pnas.1617636114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese F.P., Grossi E., Marin-Bejar O., Bharti S.K., Raimondi I., Gonzalez J., Martinez-Herrera D.J., Athie A., Amadoz A., Brosh R.M., Jr., Huarte M. A long noncoding RNA regulates sister chromatid cohesion. Mol. Cell. 2016;63:397–407. doi: 10.1016/j.molcel.2016.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D.O. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- Mukherjee N., Calviello L., Hirsekorn A., De Pretis S., Pelizzola M., Ohler U. Integrative classification of human coding and noncoding genes through RNA metabolism profiles. Nat. Struct. Mol. Biol. 2017;24:86–96. doi: 10.1038/nsmb.3325. [DOI] [PubMed] [Google Scholar]
- Murray A., Hunt T. Oxford University Press; 1993. The Cell Cycle, an Introduction. [Google Scholar]
- Notzold L., Frank L., Gandhi M., Polycarpou-Schwarz M., Gross M., Gunkel M., Beil N., Erfle H., Harder N., Rohr K. The long non-coding RNA LINC00152 is essential for cell cycle progression through mitosis in HeLa cells. Sci. Rep. 2017;7:2265. doi: 10.1038/s41598-017-02357-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson D.G., Roberts J.T., King V.M., Houserova D., Barnhill E.C., Crucello A., Polska C.J., Brantley L.W., Kaufman G.C., Nguyen M. Human snoRNA-93 is processed into a microRNA-like RNA that promotes breast cancer cell invasion. NPJ Breast Cancer. 2017;3:25. doi: 10.1038/s41523-017-0032-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Diaz J., Hegre S.A., Anderssen E., Aas P.A., Mjelle R., Gilfillan G.D., Lyle R., Drablos F., Krokan H.E., Saetrom P. Transcription profiling during the cell cycle shows that a subset of Polycomb-targeted genes is upregulated during DNA replication. Nucleic Acids Res. 2013;41:2846–2856. doi: 10.1093/nar/gks1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos A., Wernersson R., Jensen L.J. Cyclebase 3.0: a multi-organism database on cell-cycle regulation and phenotypes. Nucleic Acids Res. 2015;43:D1140–D1144. doi: 10.1093/nar/gku1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A.M., Chang H.Y. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29:452–463. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soufi A., Dalton S. Cycling through developmental decisions: how cell cycle dynamics control pluripotency, differentiation and reprogramming. Development. 2016;143:4301–4311. doi: 10.1242/dev.142075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojic L., Lun A.T.L., Mascalchi P., Ernst C., Redmond A.M., Mangei J., Barr A.R., Bousgouni V., Bakal C., Marioni J.C. A high-content RNAi screen reveals multiple roles for long noncoding RNAs in cell division. Nat. Commun. 2020;11:1851. doi: 10.1038/s41467-020-14978-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Ma L. New insights into long non-coding RNA MALAT1 in cancer and metastasis. Cancers (Basel) 2019;11:216. doi: 10.3390/cancers11020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N., Yu X., Li F., Liu D., Suo S., Chen W., Chen S., Song L., Green C.D., McDermott J. Inference of differentiation time for single cell transcriptomes using cell population reference data. Nat. Commun. 2017;8:1856. doi: 10.1038/s41467-017-01860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J.Y., Vance K.W., Varela M.A., Sirey T., Watson L.M., Curtis H.J., Marinello M., Alves S., Steinkraus B., Cooper S. Cross-talking noncoding RNAs contribute to cell-specific neurodegeneration in SCA7. Nat. Struct. Mol. Biol. 2014;21:955–961. doi: 10.1038/nsmb.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J.Y., Sirey T., Honti F., Graham B., Piovesan A., Merkenschlager M., Webber C., Ponting C.P., Marques A.C. Extensive microRNA-mediated crosstalk between lncRNAs and mRNAs in mouse embryonic stem cells. Genome Res. 2015;25:655–666. doi: 10.1101/gr.181974.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi V., Shen Z., Chakraborty A., Giri S., Freier S.M., Wu X., Zhang Y., Gorospe M., Prasanth S.G., Lal A., Prasanth K.V. Long noncoding RNA MALAT1 controls cell cycle progression by regulating the expression of oncogenic transcription factor B-MYB. PLoS Genet. 2013;9:e1003368. doi: 10.1371/journal.pgen.1003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuck A.C., Natarajan K.N., Rice G.M., Borawski J., Mohn F., Rankova A., Flemr M., Wenger A., Nutiu R., Teichmann S., Buhler M. Distinctive features of lincRNA gene expression suggest widespread RNA-independent functions. Life Sci. Alliance. 2018;1:e201800124. doi: 10.26508/lsa.201800124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulitsky I., Bartel D.P. lincRNAs: genomics, evolution, and mechanisms. Cell. 2013;154:26–46. doi: 10.1016/j.cell.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R.A. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Whitfield M.L., Sherlock G., Saldanha A.J., Murray J.I., Ball C.A., Alexander K.E., Matese J.C., Perou C.M., Hurt M.M., Brown P.O., Botstein D. Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol. Biol. Cell. 2002;13:1977–2000. doi: 10.1091/mbc.02-02-0030.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H., Baroukh C., Dannenfelser R., Chen E.Y., Tan C.M., Kou Y., Kim Y.E., Lemischka I.R., Ma'ayan A. ESCAPE: database for integrating high-content published data collected from human and mouse embryonic stem cells. Database (Oxford) 2013;2013:bat045. doi: 10.1093/database/bat045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan P., Luo S., Lu J.Y., Shen X. Cis- and trans-acting lncRNAs in pluripotency and reprogramming. Curr. Opin. Genet. Dev. 2017;46:170–178. doi: 10.1016/j.gde.2017.07.009. [DOI] [PubMed] [Google Scholar]
- Yu X., Li Z., Zheng H., Chan M.T., Wu W.K. NEAT1: a novel cancer-related long non-coding RNA. Cell Prolif. 2017;50:e12329. doi: 10.1111/cpr.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue F., Cheng Y., Breschi A., Vierstra J., Wu W., Ryba T., Sandstrom R., Ma Z., Davis C., Pope B.D. A comparative encyclopedia of DNA elements in the mouse genome. Nature. 2014;515:355–364. doi: 10.1038/nature13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaveri L., Dhawan J. Cycling to meet fate: connecting pluripotency to the cell cycle. Front. Cell Dev. Biol. 2018;6:57. doi: 10.3389/fcell.2018.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Neganova I., Przyborski S., Yang C., Cooke M., Atkinson S.P., Anyfantis G., Fenyk S., Keith W.N., Hoare S.F. A role for NANOG in G1 to S transition in human embryonic stem cells through direct binding of CDK6 and CDC25A. J. Cell Biol. 2009;184:67–82. doi: 10.1083/jcb.200801009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All code used is available upon requestMaterials availability Is not relevant because no new materials were generated in the context of this project.