Highlights
-
•
We purified a serine protease from Ficus carica latex.
-
•
Serine protease had a high tendency to hydrolyze fibrinogin.
-
•
Serine protease inhibited blood coagulation.
Keywords: Ficuscarica, Serine protease, Purification, Characterization, Anticoagulant
Abstract
Ficus carica is one of the most popular and edible plants. Its trees emanate latex of high medical importance. The well-studied procoagulant effect of ficin is a hallmark of this latex which protrudes an interesting question of how can the plant control this effect? In the present work, we purified and characterized a serine protease (FPIII) with fibrinolytic activity from F. carica latex and study the anticoagulant character of the latex. FPIII was inhibited by PMSF and its molecular weight was 48 kDa. The optimum pH and temperature of FPIII were detected at 8.5 and 60 °C, respectively. The activation energy of FPIII was 7 kcal/mol and was thermal stable up to 60 °C. FPIII tended to hydrolyze different protein substrates and showed a good catalytic efficiency (Kcat/Km). The anticoagulant effects and fibrinogenolytic activities of latex crude extract and FPIII were detected, which controls the procoagulant effect of ficin.
1. Introduction
Ficus genus consists of over 800 species and is one of about 40 genera from pantropic and subtropical origins [1]. The genus Ficus belongs to order Urticales and family Moraceae (the family of flowering plants) [2]. This family has trees and shrubs, which characteristically exude a milky juice (latex). This latex is also a main trait for Euphorbiaceae, Asclepiadaceae, Moraceae and Apocyanaceae [3]. In the ficus fruits, roots, leaves and lattices are used in the traditional medicine in various diseases such as gastrointestinal (colic, indigestion, loss of appetite and diarrhoea), respiratory (sore throats, coughs and bronchial problems), inflammatory, cardiovascular disorders, ulcerative diseases, and cancers [[3], [4], [5], [6]]. This medical importance comes from some proteins and polyphenols. The main categories of these proteins are proteases.
Proteases play crucial roles in plants. They have been identified from various plants parts, including fruits, stems, seeds and lattices [7]. Proteases are involved in numerous cellular and extracellular processes such as fruit growth and ripening [8], degradation of storage proteins in germinating seeds [9], activation of pro-enzymes and degradation of defective proteins [10]. Although the precise biological role of plant latex proteases remains speculative, they have been found to participate in defence mechanisms by protecting ripening fruits against plant pathogens like fungi and insects [11]. It was showed that they could act directly by executing the attack on the invading organisms [12]. in vitro experiments showed that cysteine proteases act by degrading the protective cuticle [13,14] or the peritrophic matrix [15].
Ficin (EC 3.4.22.3), as a significant component of F. carica latex, shares many common properties with papain concerning substrate specificity, esterase activity, transpeptidase reactions, and activation by reducing agents ; [[16], [17], [18]]. Devaraj [19] reported ficin from F. carica as a single polypeptide chain with a molecular mass of 23.1 kDa that is active at neutral pH while inactive at pH below 3.0.
In injury, mammalian tissues respond concurrently and quickly halting blood loss by coagulation. Damage to a blood vessel triggers activation of blood platelets and plasma coagulation system, leading to the formation of a blood clot containing platelets and fibrin. During these events, a cascade involving the sequential activation of plasma serine proteases takes place, culminating with the generation of thrombin, which converts plasma fibrinogen into a fibrin clot that prevents further bleeding [20]. Intriguingly, a similar phenomenon has been observed in latex-containing plants. Following fruit injury, bleeding proceeds until a clot form around the wounded area. During latex coagulation, several peptides are being processed in a non-random manner. Furthermore, peptide processing occurs concomitantly with the sequential activation of proteolytic enzymes, as in mammals [21]. After clot formation in mammals, there is a fibrin dissolved cascade that antagonist the coagulation cascade and prevent blood vessels from obstruction and this may happen in plants also.
As known that cysteine proteases as ficin are required for latex coagulation upon biotic or abiotic injuries [22]. Ficin also is a procoagulant that promotes blood coagulation by activation of factor x [23]. Here, we showed that Ficus carica latex contains a serine protease that has the ability to degrade fibrinogen and so could act as an anticoagulant. The anticoagulant behaviour of the latex might control its procoagulant character. This study lays the foundation for more studies to understand the latex characteristics as a plant defence mechanism. We are aiming here and in our previous studies [6,[24], [25], [26]], to investigate the robust characters of plant lattices enzymes for the biotechnological and pharmaceutical applications.
2. Materials and methods
2.1. Chemicals and reagents
Azocasein, albumin, fibrin, collagen, casein, cysteine, pepstatin A, β-mercaptoethanol (β-ME), ethylenediaminetetraacetic acid (EDTA), p-chloro-mercuribenzoic acid (p-CMB), soybean trypsin inhibitor ([2,27], phenylmethylsulfonyl fluoride (PMSF), iodoacetic acid (IAA), iodoacetamide [28], 110 phenanthroline, N-ethylmaleimide, ammonium persulphate, petroleum ether, trichloroacetic acid (TCA), diethyl aminoethyl (DEAE)-cellulose and all resins and reagents for electrophoresis were obtained from Sigma Chemical Co. (St Louis, MO).
2.2. Sample preparation
The latex was collected by breaking the end parts of the fig fruit. The latex was diluted two times with 20 mM sodium phosphate buffer, pH 7.0, and separated from rubber content by adding 0.5 mL of petroleum ether to every 1.0 mL of latex followed by centrifugation at 16,500 Xg for 15 min at room temperature. By centrifugation two layers were formed, the upper petroleum ether layer and lower aqueous layer that was removed and designated as a crude extract. The crude extract was stored at - 20 °C for further studies.
2.3. Enzyme activity assays
2.3.1. Azocasein assay
Protease activity with azocompounds as substrates was determined according to [29,30]. Crude extract or purified enzyme were incubated with 500 μL of 100 mM Tris−HCl buffer, pH 8.5 and 100 μL 2.5 % azocasein and distilled water to a total volume of 1 mL. Assays were carried out at 37 °C for 1 h and then stopped by the addition of 200 μl of 20 % (v / v) trichloroacetic acid. After removal of precipitated protein by centrifugation (12,000 Xg for 5 min at room temperature) the absorbance of the supernatant at 366 nm was determined. One unit of protease activity was defined as the amount of enzyme hydrolyzing 1 μg azocasein per hour under standard assay conditions.
2.3.2. Ninhydrin assay
Protease activity with other substrates gelatin, casein, collagen, fibrin, albumin and haemoglobin were determined according to Moore [31] by measuring the liberated α-amino nitrogen. Crude extract or purified enzyme were incubated with 500 μL of 100 mM Tris−HCl buffer, pH 8.5 and 100 μL of 3 % of each substrate and adjusted to 1 mL with distilled water. Assays were carried out at 37 °C for 1 h and then stopped by the addition of 200 μL of 20 % (v / v) trichloroacetic acid. After centrifugation, 0.5 mL of the supernatant was added to 1.0 mL of ninhydrin reagent (0.5 mL of 1 % ninhydrin in 0.5 M citrate buffer, pH 5.5, 0.2 mL of the same buffer, and 1.2 mL glycerol) [32] and heated for 10 min at 100 °C. Four millilitres of distilled water were added to each sample, and the absorbance at 570 nm was measured, and the increase in free amino groups was determined. Isoleucine was used for standard. One unit of proteolytic activity was defined as microgram of α-amino acid liberated per h under standard assay conditions.
2.4. Protein determination
Protein was determined by measuring the absorbance at 280 nm [33] for column fractions and by the method of Bradford [34] for pooled fractions using bovine serum albumin as a standard
2.5. Enzyme purification
2.5.1. Chromatography on DEAE- cellulose column
F. carica latex crude extract (13 mg total protein/mL) was applied on the top of DEAE-cellulose (10 × 1.6 cm i.d.) pre-equilibrated with 20 mM Tris-HCl buffer, pH 7.0. The exchanged materials were eluted stepwise with NaCl ranging from 0.0 to 0.25 M at a flow rate of 60 mL /h and collected in 3 mL fractions. Fractions exhibiting protease activity were collected and dialyzed against sucrose for concentration.
2.5.2. Chromatography on CM- cellulose column
The concentrated pooled fractions of DEAE-cellulose that have the highest proteolytic activity were applied on the top of CM-cellulose (10 × 1.6 cm i.d.) pre-equilibrated with 20 mM Tris-HCl buffer, pH 7.0. The exchanged materials were eluted stepwise with NaCl ranging from 0.0 to 0.35 M NaCl at a flow rate of 60 mL /h and collected in 3 mL fractions. Fractions exhibiting protease activity were collected and dialyzed against sucrose for concentration.
2.5.3. Chromatography on sephacryl S-200
The concentrated pooled fractions of CM-cellulose that have the proteolytic activity were applied on the top of a Sephacryl S-200 column (95 × 1.6 cm i.d.) equilibrated with 20 mM Tris-HCl buffer, pH 7.0 and developed at a flow rate of 20 mL / h and 2 mL fractions were collected.
2.6. Polyacrylamide gel electrophoresis
Electrophoresis under non-denaturing conditions was performed in 10 % (w / v) acrylamide slab gel according to the method of Davis [35] using a Tris-glycine buffer, pH 8.3. Protein bands were stained with Coomassie Brilliant Blue R-250.
2.7. Molecular weight determination
Molecular weight was determined by gel filtration on Sephacryl S-200. The column [13] (90 × 1.6 cm i.d.) was calibrated with cytochrome C (12.4 kDa), carbonic anhydrase (29 kDa), bovine serum albumin (67 kDa), alcohol dehydrogenase (150 kDa) and α-amylase (200 kDa). Dextran blue (2000 kDa) was used to determine the void volume (Vo). Subunit molecular weight was estimated by SDS-polyacrylamide gel electrophoresis [36]. SDS-denatured β-galactosidase (100 kDa), bovine serum albumin (65 kDa), ovalbumin (55 kDa), lactate dehydrogenase (35 kDa), restriction endonuclease Bsp98I (25 kDa), β-lactoglobulin (18 kDa), lysozyme (14 kDa) were used for the calibration curve [[37], [38], [39], [40], [41], [42]].
2.8. Enzyme characterization
Estimates of optimal temperature and pH were made by using a temperature range of 20–90 °C and a pH range of 3.5–9.0. The thermal stability was investigated by measuring the residual activity of the enzymes after 15 min of incubation at different temperatures prior to substrate addition. The Km values were determined from Lineweaver–Burk plots by using different substrate concentrations according to the method of [43]. The effect of metal cations was performed by incubating the enzyme for 15 min at 37 °C with 5 and 10 mM of cations prior to substrate addition. The effect of protease inhibitors was determined by incubation of the assay reaction mixture in the presence of inhibitors.
2.9. Clotting time assays
2.9.1. Bio-assay for the inhibition of the intrinsic blood coagulation pathway
The activated partial thromboplastin time (APTT) measured the clotting time of plasma at 37 °C in the presence of a platelet substitute (cephalin) and an activator (celite). This overall test evaluates the entire intrinsic pathway with the exception of the platelet factor [44]. Different concentrations of crude extract or purified enzyme were added to 100 μL of platelet-poor plasma (PPP) [fresh human blood was mixed with 0.11 M sodium citrate in the ratio of 4: 1 and the mixture was centrifuged for 15 min at 2700 Xg.] and incubated for 3 min at 37 °C. APTT reagent (100 μL) was added and the mixture was incubated for another 3 min at 37 °C. Finally, 100 μL of 0.025 M CaCl2 (pre-warmed at 37 °C) was added and the clotting time recorded. For determination of the control time, the experiment was performed by using 0.05 M Tris-HCl buffer, pH 8.5 (100 μL) instead of crude extract or purified enzyme [45].
2.9.2. Bio-assay for the inhibition of the extrinsic blood coagulation pathway
The prothrombin time (PT) studied the total extrinsic clotting system. It measured the clotting time of plasma at 37 °C in the presence of excess tissue thromboplastin and calcium [44]. Different concentrations of crude extract or purified enzyme were incubated with 100 μL of PPP and incubated for 3 min at 37 °C. Calcium- thromboplastin reagent (100 μL) pre-warmed at 37 °C, was added to PPP and the clotting time was recorded. For determination of the control time, the experiment was performed by using 0.05 M Tris−HCl buffer, pH 8.5 (100 μL) instead of crude extract or purified enzyme.
2.10. Fibrinogenolytic activity
Fibrinogenolytic hydrolyzing activity was measured according to the method of Ouyang and Teng [46]. A substrate solution of 2 mg of human fibrinogen in 5 mM of Tris-HCl buffer, pH 8.5 was mixed and incubated with crude extract or purified enzyme at 37 °C for various time intervals. In the meantime, 0.1 mL of the incubated solution was pipetted into a small test tube and added to 0.1 mL of stopping solution contained 10 M urea, 4% SDS, and 4% β-mercaptoethanol. This solution was incubated at 37 °C overnight and then applied to the top of 10 % SDS-PAGE and stained with Coomassie Brilliant Blue R-250.
3. Results and discussion
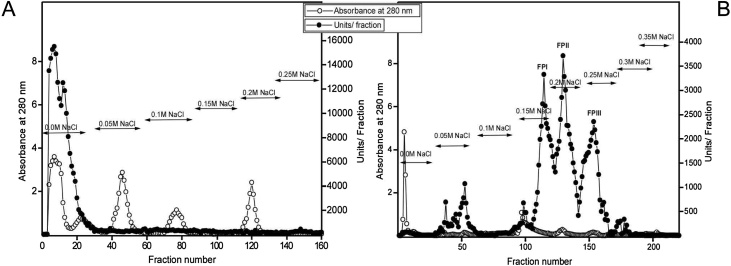
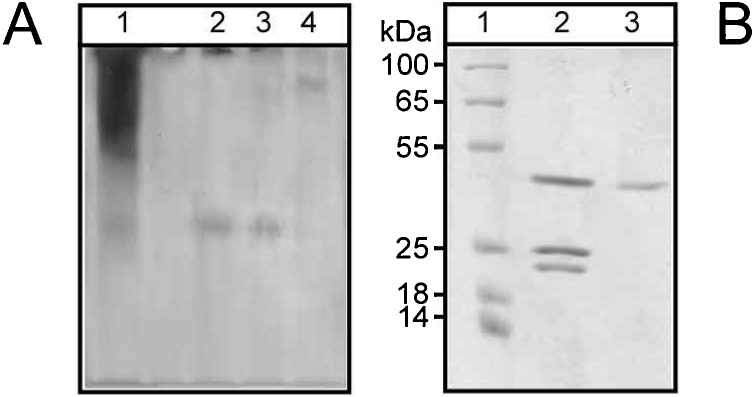
The purification of proteases from F. carica latex is summarized in Table 1. The elution profile of the chromatography on a DEAE-cellulose column (Fig. 1A) appeared that 60 % of applied protease activity was detected in one peak, which was the negative adsorbed fractions. The negative adsorbed fractions were applied to a CM-cellulose column, where it separated into three peaks PI, PII and PIII (Fig. 1B; Table 1). PI, PII and PIII were separately applied to a Sephacryl S-200 and designated as FPI, FPII and FPIII with specific activities of 2057, 2635 and 2430 units/mg protein, which were lower than that recorded for ficin from F. carica (Brunkswike) from China (3434 units/mg protein, [47], while were higher than the specific activity of streblin from latex of Streblus asper (6.92 units/mg protein, [48] and crinumin from latex of Crinum asiaticum (79.9 units/mg protein, [49]. The homogeneity of purified enzymes was demonstrated on PAGE (Fig. 2A; Table 1).
Table 1.
Purification scheme for F. carica latex proteases FP.
| Step | Total activitya | Total protein (mg) | S. A.b (U/mg protein) |
Fold purification | Recovery (%) |
|---|---|---|---|---|---|
| Crude extract | 15,300 | 13 | 1177 | 1 | 100 |
| DEAE-cellulose 0.0 M NaCl | 9300 | 5.7 | 1632 | 1.4 | 61 |
| CM-cellulose | |||||
| 0.15 M NaCl | 1818 | 0.75 | 2424 | 2.1 | 12 |
| 0.2 M NaCl (PII) | 2125 | 0.8 | 2656 | 2.3 | 14 |
| 0.25 M NaCl (PIII) | 1014 | 0.61 | 1662 | 1.4 | 7 |
| Sephacryl S200 | |||||
| FPI | 1255 | 0.61 | 2057 | 3.4 | 8.2 |
| FPII | 1370 | 0.52 | 2635 | 2.7 | 9 |
| FPIII | 486 | 0.2 | 2430 | 3.7 | 3.2 |
U: One unit of proteolytic activity was defined as μg azocasein hydrolyzed per hour under standard assay conditions.
S. A.: Specific activity.
Fig. 1.
Chromatographic profile of F. carica latex proteases.
(A) A typical elution profile for the chromatography of F. carica latex proteases on a DEAE-cellulose column (10×1.6 cm i.d.) equilibrated with 20 mM Tris−HCl buffer, pH 7.0 and eluted stepwise with NaCl (0.0 M to 0.25 M). Fractions of 3 mL were collected at 4oC and a flow rate of 60 mL/h.
(B) A typical elution profile for the chromatography of the non-adsorbed DEAE-cellulose protein fractions of F. carica latex proteases on a CM-cellulose column (10×1.6 cm i.d.) equilibrated with 20 mM Tris−HCl buffer, pH 7.0 and eluted stepwise with NaCl (0.0 M to 0.35 M). Fractions of 3 mL were collected at 4oC and a flow rate of 60 mL/h.
Fig. 2.
Homogenity of purified F. carica latex proteases.
(A) Native-PAGE of F. carica latex protease during purification steps; lane 1 = Crude extract; lane 2 = Sephacryl S200 FPI; lane 3= Sephacryl S200 FPII and lane 4= Sephacryl S200 FPIII.
(B) SDS-PAGE for molecular weight determination of F. carica latex proteases; lane 1 = Standard proteins; lane 2= DEAD cellulose fraction and lane 3 = Sephacryl S-200 FPIII. Molecular weight markers indicated: β-galactosidase (100 kDa), bovine serum albumin (65 kDa), ovalbumin (55 kDa), lactate dehydrogenase (35 kDa), restriction endonuclease Bsp98I (25 kDa), β-lactoglobulin (18 kDa), lysozyme (14 kDa).
The molecular weights of the purified F. carica FPI, FPII and FPIII were determined using a calibration curve of the Sephacryl S-200 column and then confirmed by SDS-PAGE, to be 25, 23 and 48 KDa, respectively (Fig. 2B).
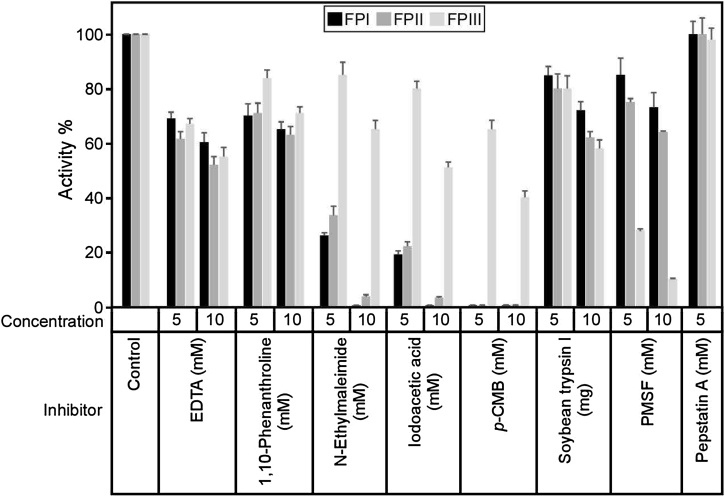
To estimate the family of the purified proteases F. carica FPI, FPII and FPIII, the sensitivity of these enzymes towards different inhibitors was investigated (Fig. 3). All the examined inhibitors had different inhibitory effects on FPI, FPII and FPIII (Fig. 3). N-ethylmaleimide, iodoacetic acid and p-CMB had the highest inhibitory effects on FPI and FPII, while PMSF had the highest inhibitory effect on FPIII (Fig. 3B). Therefore, these data suggested that FPI and FPII are cysteine protease, while FPIII is a serine protease. The bare effect of soybean trypsin inhibitor on FPIII (Fig. 3) is common on plant serine proteases [50]. The molecular weights and inhibitory effects observed for FPI and FPII, implying that these enzymes are types of ficins which were extensively studied [23,47,[51], [52], [53]]. On the same vein, FPIII is a serine protease and similar to previously described serine protease from F. carica var. brown Turkey [54].
Fig. 3.
Effect of protease inhibitors on purified F. carica proteases.
Effect of protease inhibitors on the caseinolytic activity of the purified F. carica protease FPI, FPII and FPIII. Purified proteases FPI, FPII and FPIII were pre-incubated for 15 min at 37 °C with 5 and 10 mM of the listed proteases inhibitors as a final concentration prior to substrate addition. The activity was measured using azocasein as a substrate. The activity of FPI, FPII and FPIII in absence of the inhibitors was taken as 100 % activity. n=3. The results are presented as the mean ± SD.
As cysteine proteases (ficin) were extensively studied, we focused on serine protease FPIII for further characterization.
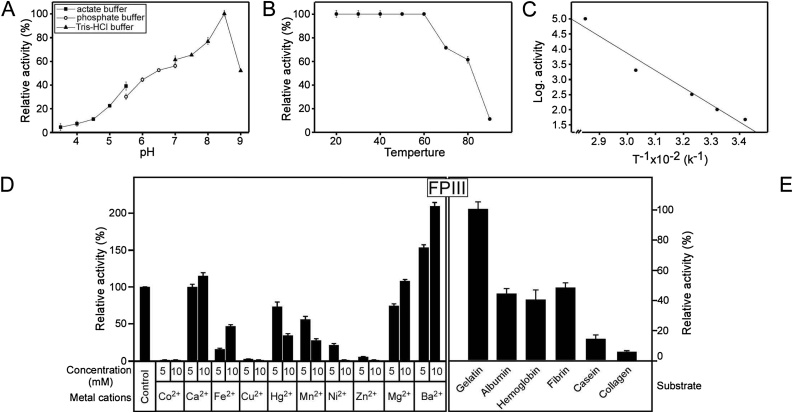
To determine the optimum pH for FPIII activity, FPIII activity was tested in different buffers systems with variable pHs (see material and methods). The FPIII exhibited a pH optimum at pH 8.5 using Tris-HCl buffer (Fig. 4A). The activity was sharply decreased around pH 8.5, where the enzyme recorded 77 % and 52 % of its activity at pH 8 and 9, respectively (Fig. 4A). This result was congruent with that reported for benghalensin from F. benghalensis (pH 8.0) [55] but higher than that recorded for ficin E from F. elastica (pH 6.0) [56].
Fig. 4.
Characterization of FPIII.
(A) Optimum pH of the F. carica protease FPIII. The reaction mixture contained in 1.0 mL: FPIII (5.6 units), 2 mg azocasein and 20 mM each of sodium acetate buffer (pH 3.5–5.5), sodium phosphate buffer (pH 5.5–7.5) and Tris−HCl buffer (pH 7.5–9.0). n=3. The results are presented as the mean ± SD.
Typical profiles for assessment (B) thermal stability (C) activation energy of the caseinolytic activity of F. carica protease FPIII using standard assays. n=3. The results are presented as the mean ± SD.
(D) Effect of selected metal cations on the caseinolytic activity of the purified F. carica protease FPIII. FPIII was pre-incubated for 15 min at 37 °C with 5 and 10 mM of the listed metal cations as a final concentration prior to substrate addition. The activity was measured using azocasein as a substrate. The activity of FPIII in absence of the metal cations was taken as 100 % activity. n=3. The results are presented as the mean ± SD.
(E) Relative activity of F. carica protease FPIII towards different native protein substrates. All assays were incubated at 37 ºC in 20 mM Tris−HCl buffer, pH 8.5, enzyme concentration of (5 μg/mL) and 5 mg substrate. Gelatin was taken as 100 % activity. n=3. The results are presented as the mean ± SD.
To estimate the optimum temperature for FPIII activity, the activity of FPIII was tested at different temperatures. FPIII showed the highest activity at 60 °C (data not shown), which was similar to some extent with that reported for benghalensin from F. benghalensis (55 °C) [55], religiosin from F. religiosa (55 °C) [57], carnein from Ipomoea carnea spp. (65 °C) [58] and streblin from Streblus asper (65 °C) [48]. Coincidently, FPIII was stable up to 60 °C, followed by a decrease in activity by 30 % and 40 % at 70 and 80 °C, respectively, while at 90 °C the enzyme retained only 10 % of its activity (Fig. 4B). This result was differing with that reported for Crinumin from Crinum asiaticum and artocarpin from Artocarpus heterophyllus that had stability against temperature up to 80 °C [49,59]. As a result, the thermal stability of FPIII may be attributed to its glycosylation which affects enzyme stability [60] as most of the latex proteases.
Using the activity of FPIII at a temperature range of (20−60 °C), the activation energy [10] of FPIII from F. carica latex was calculated to be 7 kcal /mol from the Arrhenius plot (Fig. 4C). This value was lower than that reported for serine proteases ‘a’ and ‘b’ from latex of Synadenium grantii Hook ‘f’ which were found to be 10.04 and 9.1 kcal/mol, respectively [61]. This low activation energy of FPIII suggested proper conformational changes, especially at the catalytic site, which improves affinity toward substrate binding with low energy needed. Serine proteases have the ability to overcome the activation energy by stabilization of the tetrahedral intermediate [62].
To examine the stimulatory and inhibitory effects of metal cations on FPIII, the activity of FPIII was tested using different metal cations (Fig. 4D). The FPIII was slightly activated by Ca2+ and Mg2+ at 10 mM as that recorded for euphorbains la1, la2 and la3 from E. lactea and E. lacteal cristata [63] and serine proteases from Holarrhena antidysenterica [64]. This non-inhibitory effect of Ca2+ and Mg2+ on FPIII activity, indicated that the protease wasn't a cysteine or aspartic [4]. In the same direction, Ba2+ elevated FPIII activity two times (Fig. 4D). On the other hand, metal cations (Mn2+, Hg2+, Ni2+ and Fe2+) inhibited FPIII activity by (45–90 %) (Fig. 4D). Similarly, FPIII showed almost no activity in the presence of Co2+, Cu2+ and Zn2+ (Fig. 4D). The inhibitory effect of Mn2+, Co2+, Cu2+ and Fe2+ on FPIII was comparable to that observed for artocarpin from Artocarpus heterophyllus [59], artocarpin from Artocarpus heterophyllus [59], and pertherain from Parthenium argentatum [65].
The effect of metal ions Zn2+, Cu2+, Ca2+ and Mg2+ was consistent with what happened with plasmin. The proteolytic active site of plasmin located in the C-terminal of β-chain and made up of His602, Asp645 and Ser740. This His residue is a suggestive target of oxidative inactivation caused by Cu2+[66]. Rather than, His, Asp and Ser other amino acid functional groups may be involved in metal binding, particularly the side chains of Cys and Glu. Metal ions are often bound to hydrophilic ligands surrounded by a hydrophobic shell [67]. For that, these results suggested that FPIII is a serine protease and depends on cysteine amino acid for its activity.
To determine the ability of FPIII to hydrolyze different proteins, we tested the activity of FPIII using different substrates (Fig. 4E). FPIII showed relative activities in order to gelatin > fibrin > albumin > hemoglobin > casein > collagen (Fig. 4E). This action differed from the action of serine protease from Holarrhena antidysenterica that hydrolyzed substrates with relative activities that arranged descendingly as casein > haemoglobin > bovine serum albumin > gelatin > azocasein [64] and crinumin from Crinum asiaticum that hydrolyzed casein, azocasein and haemoglobin with high efficiency [49].
The substrates affinities (Km) and (Vmax) of FPIII toward gelatin, albumin, haemoglobin, fibrin, casein and azocasein as substrates were estimated to be 3.9, 6, 5.1, 6.8, 2.7 and 0.82 mg substrate/mL with Vmax 3.45 × 10−3, 2.5 × 10−3, 1.88 × 10−3, 1.72 × 10−3, 1.63 × 10−3 and 0.72 × 10−3 (μmol. min-1. mg-1), respectively (Table 2). The catalytic efficiency (Kcat/Km) of FPIII toward gelatin was highest compared to the other substrates (Table 2).
Table 2.
The kinetic properties of F. carica protease FPIII.
| Substrates | Km (mg protein/mL) | V max (μmol. min−1. mg−1) | Kcat (s−1) | Kcat/Km (ml. s−1. mg−1) |
|---|---|---|---|---|
| Gelatin | 3.9 | 3.45 × 10−3 | 1200.0 | 307.7 |
| Albumin | 6 | 2.56 × 10−3 | 869.6 | 144.9 |
| Hemoglobin | 5.1 | 1.88 × 10−3 | 653.9 | 128.2 |
| Fibrin | 6.8 | 1.72 × 10−3 | 598.3 | 88.0 |
| Casein | 2.7 | 1.63 × 10−3 | 567.0 | 210.0 |
| Azocasein | 0.82 | 0.72 × 10−3 | 250.4 | 305.4 |
The procoagulant activity of ficin proteases was previously characterized. Here, we tested the effect on F. carica latex crude extract and FPIII on both intrinsic and extrinsic coagulation pathways using the activated partial thromboplastin time (APTT) and prothrombin time (PT), respectively (Table 3). F. carica crude extract showed both a coagulant and an anticoagulant behaviours in a concentration depending manner. At low total proteins concentration (13 μg), it decreased the APTT time and the PT time by 70 % and 50 %, respectively (Table 3). By elevating the total proteins concentrations of the crude extract up to (65 μg), it acted as an anticoagulant by prolonging the APTT time and the PT time by 134 % and 200 %, respectively (Table 3). On the other hand, FPIII could prolonged both the APTT and the PT time up to 300 s at a concentration of 5.6 μg (0.4 μM) (Table 3). These results disagreed to that recorded for crude extract of Jatropha crucas that acted as a procoagulant at a high concentration by shorten the PT and APTT time, while at a low concentration it performed as an anticoagulant by increasing both the PT and APTT times by 164 % and 565 %, respectively [68].
Table 3.
The prothrombin time (PT) and the activated partial thromboplastin time (APTT) of human plasma in presence of various concentrations of crude extract of F. carica latex proteases and purified FPIII.
| PT at different concentrations |
APTT at different concentrations |
||||||
|---|---|---|---|---|---|---|---|
| Crude extract of F. carica proteases |
FPIII |
Crude extract of F. carica proteases |
FPIII |
||||
| μg proteins | PT Sec. | μg proteins (0.4 μM) | PT Sec. | μg proteins | APTT Sec. | μg proteins (0.4 μM) | APTT Sec. |
| 13 | 15 ± 0.6 | 5.6 | >300 | 13 | 14 ± 0.5 | 5.6 | >300 |
| 26 | 18 ± 0.6 | 26 | 26 ± 1.0 | ||||
| 39 | 25 ± 1.0 | 39 | 38 ± 1.5 | ||||
| 52 | 35 ± 2.1 | 52 | 49 ± 2.0 | ||||
| 65 | 59 ± 1.5 | 65 | 63 ± 2.0 | ||||
| 78 | 82 ± 2.5 | 78 | 97 ± 3.0 | ||||
| 91 | 128 ± 3.0 | 91 | 175 ± 1.5 | ||||
| 104 | 200 ± 4.0 | 104 | 219 ± 3.0 | ||||
| 117 | 263 ± 3.2 | 117 | 270 ± 2.0 | ||||
| control | 30 | control | 47 | ||||
The different concentrations of crude extract of F. carica proteases and FPIII were pre-incubated with 10 mM BaCl2. The control is the clotting time of the human plasma contained 10 mM BaCl2 in absence of anticoagulant. n = 3. The results are presented as the mean ± SD.
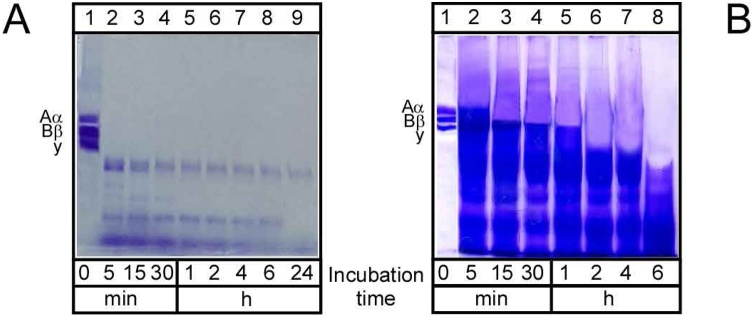
For further analysis, we tested the ability of F. carica crude extract and FPIII to digest fibrinogen as a key component of a blood clot [69]. The crude extract of F. carica showed an ability to degrade fibrinogen in 5 min (Fig. 5A). F. carica crude extract could digest the fibrinogen subunits (α, β and γ) completely in this short period leaving many bands of proteins (Fig. 5A). On the other hand, FPIII was able to digest the α subunit completely within 5 min and the β subunit in 15 min but the γ subunit takes a long time to be digested (about 2 h). These results are similar to that reported foreumiliin from Euphorbia milii [70] in the degradation of α-subunit of fibrinogen in 5 min. However, FPIII is considered to be more effective than eumiliin as the later degrades β subunit slowly and cannot degrade the γ-subunit.
Fig. 5.
Time-dependent hydrolysis of fibrinogen.
Time-dependent hydrolysis of fibrinogen by (A) crude extract of F. carica latex or (B) purified F. carica protease FPIII. Human fibrinogen (2 mg/mL) and FPIII (5.6 μg/mL) were co-incubated at 37˚C for different periods as indicated below each lane. The degradation of fibrinogen was analyzed by SDS-PAGE (10 %).
The procoagulant activity of F. carica crude extract could be explained by the presence of cysteine proteases FPI and FPII that are types of ficin. This procoagulants characters of cysteine proteases might be due to their ability to cleave the Aα and Bβ chains, but not γ chain of fibrinogen, releasing fibrinopeptide A and B, respectively forming fibrin clot that is soft and friable [71] or their ability to activate human factor X by cleave it to create FXa [23]. However, the anticoagulant activity of F. carica crude extract might be due to the complete digestion of fibrinogen or the presence of serine protease inhibitor [72] that inhibited coagulation factors or antiplatelet effect [73]. Collectively, the question here seems to be how is possible that F. carica latex has procoagulant and anticoagulant properties at the same time? It is not surprising to observe that plant usually contain a combination of constituents with opposing effect [73], precisely, it is possible that the same factor can act as a procoagulant and as an anticoagulant under different conditions [68]. For example, thrombin can act as a procoagulant when it cleaves fibrinogen and promotes the formation of a fibrin clot, while can work as an anticoagulant when it activates protein C in the presence of the cofactor thrombomodulin [74]. For plants that contain the contradictory behaviours, Fumaria indica has been shown to possess the combination of spasmogenic and spasmolytic activities thus explaining their use in constipation and diarrhoea [75]. Similarly, St. John’s wort has been shown to contain a combination of hypotensive and hypertensive constituents [76].
As a conclusion, our study reveals that F. carica latex can control its prominent coagulation stimulant nature caused by ficin (more than 5 isoenzymes) by a fibrinolytic serine proteases. The anticoagulant property of the latex at a higher protein concentration suggested the presence of other anti-proteases small peptides that may inhibit ficin.
4. Author contributions
MBH and ASF conceived the project; ASF and IHB supervised the project; MBH, MOE, EIK, IHB and ASF designed all the experiments and methods; MBH performed all the experiments and prepared all the figures; MBH, MOE and EIK did the data analysis; MBH and ASF wrote the original draft of the manuscript; MBH, MOE, EIK, IHB and ASF review and edited the final manuscript. All authors reviewed the manuscript.
Declaration of Competing Interest
The authors declare they have no competing financial interests or other conflicts of interest.
Acknowledgments
The authors gratefully acknowledge to National Research Centre, Cairo, Egypt, for providing the facilitations and equipment throughout the study.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00492.
Contributor Information
Mohamed B. Hamed, Email: drbelal999@hotmail.com.
Afaf S. Fahmy, Email: afafsfahmy@hotmail.com.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Joseph B., Raj S. Pharmacognostic and phytochemical properties of Ficus carica Linn –An overview. Int. J. Pharmtech Res. 2011;3(1):8–12. [Google Scholar]
- 2.Robbins B.H. A proteolytic enzyme in ficin, the anthelmintic principle of leche de higueron. J. Biol. Chem. 1930;87:251–257. [Google Scholar]
- 3.Rajesh R. Procoagulant activity of Calotropis gigantea latex associated with fibrin(ogen)olytic activity. Toxicon. 2005;46(1):84–92. doi: 10.1016/j.toxicon.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Asif-Ullah M., Kim K.S., Yu Y.G. Purification and characterization of a serine protease from Cucumis trigonus Roxburghi. Phytochemistry. 2006;67(9):870–875. doi: 10.1016/j.phytochem.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 5.McGovern T.W. The fig--Ficus carica L. Cutis. 2002;69(5):339–340. [PubMed] [Google Scholar]
- 6.Abdel-Aty A.M. Ficus carica, Ficus sycomorus and Euphorbia tirucalli latex extracts: Phytochemical screening, antioxidant and cytotoxic properties. Biocatal. Agric. Biotechnol. 2019;20:101199. [Google Scholar]
- 7.Boller T. CRC Press; 1986. Roles of Proteolytic Enzymes in Interaction of Plant and Other Organisms. [Google Scholar]
- 8.Brady C.J. Fruit ripening. Annu. Rev. Plant Physiol. 1985;38:155–178. [Google Scholar]
- 9.Kembhavi A.A. The two cysteine endopeptidases of legume seeds: purification and characterization by use of specific fluorometric assays. Arch. Biochem. Biophys. 1993;303(2):208–213. doi: 10.1006/abbi.1993.1274. [DOI] [PubMed] [Google Scholar]
- 10.Rudenskaya G.N. Taraxalisin -- a serine proteinase from dandelion Taraxacum officinale Webb s.l. FEBS Lett. 1998;437(3):237–240. doi: 10.1016/s0014-5793(98)01243-5. [DOI] [PubMed] [Google Scholar]
- 11.Baker E.N., Drenth J. Vol. 3. John Wiley and Sons; New York: 1987. (The Cysteine Proteinases Structure and Mechanism). [Google Scholar]
- 12.Konno K. Papain protects papaya trees from herbivorous insects: role of cysteine proteases in latex. Plant J. 2004;37(3):370–378. doi: 10.1046/j.1365-313x.2003.01968.x. [DOI] [PubMed] [Google Scholar]
- 13.Stepek G. Natural plant cysteine proteinases as anthelmintics? Trends Parasitol. 2004;20(7):322–327. doi: 10.1016/j.pt.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Stepek G. Assessment of the anthelmintic effect of natural plant cysteine proteinases against the gastrointestinal nematode, Heligmosomoides polygyrus, in vitro. Parasitology. 2005;130(Pt 2):203–211. doi: 10.1017/s0031182004006225. [DOI] [PubMed] [Google Scholar]
- 15.Mohan S. Degradation of the S. frugiperda peritrophic matrix by an inducible maize cysteine protease. J. Insect Physiol. 2006;52(1):21–28. doi: 10.1016/j.jinsphys.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Haq S.K., Rasheedi S., Khan R.H. Characterization of a partially folded intermediate of stem bromelain at low pH. Eur. J. Biochem. 2002;269(1):47–52. doi: 10.1046/j.0014-2956.2002.02620.x. [DOI] [PubMed] [Google Scholar]
- 17.Dubey V.K., Jagannadham M.V. Procerain, a stable cysteine protease from the latex of Calotropis procera. Phytochemistry. 2003;62(7):1057–1071. doi: 10.1016/s0031-9422(02)00676-3. [DOI] [PubMed] [Google Scholar]
- 18.Liener I.E., Friedenson B. Ficin. Methods Enzymol. 1970;19:261–273. [Google Scholar]
- 19.Devaraj K.B., Kumar P.R., Prakash V. Comparison of activity and conformational changes of ficin during denaturation by urea and guanidine hydrochloride. Process Biochem. 2011;46:458–464. [Google Scholar]
- 20.Macfarlane R.G. An enzyme cascade in the blood clotting mechanism, and its function as a biochemical amplifier. Nature. 1964;202:498–499. doi: 10.1038/202498a0. [DOI] [PubMed] [Google Scholar]
- 21.Moutim V. Spontaneous processing of peptides during coagulation of latex from Carica papaya. Plant Sci. 1999;142:115–121. [Google Scholar]
- 22.Azarkan M. Selective and reversible thiol-pegylation, an effective approach for purification and characterization of five fully active ficin (iso)forms from Ficus carica latex. Phytochemistry. 2011;72(14-15):1718–1731. doi: 10.1016/j.phytochem.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Richter G. Activation and inactivation of human factor X by proteases derived from Ficus carica. Br. J. Haematol. 2002;119(4):1042–1051. doi: 10.1046/j.1365-2141.2002.03954.x. [DOI] [PubMed] [Google Scholar]
- 24.Mohamed S.A. Ficus sycomorus latex: A thermostable peroxidase. Afr. J. Biotechnol. 2011;10(76):17532–17543. [Google Scholar]
- 25.Abdel-Aty A.M. Comparison of the potential of Ficus sycomorus latex and horseradish peroxidases in the decolorization of synthetic and natural dyes. J. Genet. Eng. Biotechnol. 2013;11(2):95–102. [Google Scholar]
- 26.Abdel-Aty A.M. Ficus sycomorus latex: An efficient alternative Egyptian source for horseradish peroxidase in labeling with antibodies for immunodiagnostic kits. Vet. World. 2018;11(10):1364–1370. doi: 10.14202/vetworld.2018.1364-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robbins B.H., Christie A. Sir William Osler the pediatrician. Am. J. Dis. Child. 1963;106:124–129. doi: 10.1001/archpedi.1963.02080050126002. [DOI] [PubMed] [Google Scholar]
- 28.Carucci J.A. Cutaneous anthrax management algorithm. J. Am. Acad. Dermatol. 2002;47(5):766–769. doi: 10.1067/mjd.2002.128381. [DOI] [PubMed] [Google Scholar]
- 29.Lemos F.J.A. Proteinases and amylases of larval midgut of Zabrotes subfasciatus reared on cowpea (Vigna unguiculata) seeds. Entomol. Exp. Appl. 1990;56(3):219–227. [Google Scholar]
- 30.Abdel-Aty A.M. Phenolic-antioxidant capacity of mango seed kernels: therapeutic effect against viper venoms. Rev. Bras. Farmacogn. 2018;28(5):594–601. [Google Scholar]
- 31.Moore S. Amino acid analysis: aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J. Biol. Chem. 1968;243(23):6281–6283. [PubMed] [Google Scholar]
- 32.Lee Y.P., Takahashi T. An improved colorimetric determination of amino acids with the use of ninhydrin. Anal. Biochem. 1966;14(1):71–77. [Google Scholar]
- 33.Warburg O., Christian W. Isolation and crystallization of enolase. Biochem. Z. 1942;310:386–421. [Google Scholar]
- 34.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 35.Davis B.J. DISC electrophoresis. II. method and application to human serum proteins. Ann. N. Y. Acad. Sci. 1964;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- 36.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 37.Tsolis K.C. Secretome dynamics in a gram-positive bacterial model. Mol. Cell Proteomics. 2019;18(3):423–436. doi: 10.1074/mcp.RA118.000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamed M.B. Monitoring protein secretion in Streptomyces Using fluorescent proteins. Front. Microbiol. 2018;9:3019. doi: 10.3389/fmicb.2018.03019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Busche T. Multi-omics and targeted approaches to determine the role of cellular proteases in Streptomyces protein secretion. Front. Microbiol. 2018;9:1174. doi: 10.3389/fmicb.2018.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamed M.B. Large-scale production of a thermostable Rhodothermus marinus cellulase by heterologous secretion from Streptomyces lividans. Microb. Cell Fact. 2017;16(1):232. doi: 10.1186/s12934-017-0847-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rebets Y. Characterization of sigma factor genes in streptomyces lividans TK24 using a genomic library-based approach for multiple gene deletions. Front. Microbiol. 2018;9:3033. doi: 10.3389/fmicb.2018.03033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Hakim A.E. Heterodimeric l-amino acid oxidase enzymes from Egyptian Cerastes cerastes venom: Purification, biochemical characterization and partial amino acid sequencing. J. Genet. Eng. Biotechnol. 2015;13(2):165–176. doi: 10.1016/j.jgeb.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lineweaver H., Burk D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934;56(3):658–666. [Google Scholar]
- 44.Becker U., Jering H., Roschlau P. Vol. 5. Academic press.; New York: 1984. (Coagulation Methods). [Google Scholar]
- 45.Gaspar A.R.M.D., Crause J.C., Neitz A.W.H. Identification of anticoagulant activities in the salivary glands of the soft tick, Ornithodoros savignyi. Exp. Appl. Acarol. 1995;19(2):117–127. doi: 10.1007/BF00052551. [DOI] [PubMed] [Google Scholar]
- 46.Ouyang C., Teng C.M. Fibrinogenolytic enzymes of Trimeresurus mucrosquamatus venom. Biochim. Biophys. Acta. 1976;420(2):298–308. doi: 10.1016/0005-2795(76)90321-4. [DOI] [PubMed] [Google Scholar]
- 47.Huang L. Purification and characterization of a proteolytic enzyme from fig latex. Chem. Res. Chin. Univ. 2008;24(3):348–352. [Google Scholar]
- 48.Tripathi P., Tomar R., Jagannadham M.V. Purification and biochemical characterization of a novel protease streblin. Food Chem. 2011;125(3):1005–1012. [Google Scholar]
- 49.Singh K.A. Crinumin, a chymotrypsin-like but glycosylated serine protease from Crinum asiaticum: Purification and physicochemical characterization. Food Chem. 2010;119(4):1352–1358. [Google Scholar]
- 50.Yadav S.C., Pande M., Jagannadham M.V. Highly stable glycosylated serine protease from the medicinal plant Euphorbia milii. Phytochemistry. 2006;67(14):1414–1426. doi: 10.1016/j.phytochem.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 51.Devaraj K.B., Kumar P.R., Prakash V. Purification, characterization, and solvent-induced thermal stabilization of ficin from Ficus carica. J. Agric. Food Chem. 2008;56(23):11417–11423. doi: 10.1021/jf802205a. [DOI] [PubMed] [Google Scholar]
- 52.Sgarbieri V.C. Ficus enzymes. I. Separation of the proteolytic enzymes of Ficus carica and Ficus glabrata latices. J. Biol. Chem. 1964;239:2170–2177. [PubMed] [Google Scholar]
- 53.Kramer D.E., Whitaker J.R. Ficus enzymes. II. Properties of the proteolytic enzymes from the latex of FICUS Carica Variety KADOTA. J. Biol. Chem. 1964;239:2178–2183. [PubMed] [Google Scholar]
- 54.Raskovic B. Identification, purification and characterization of a novel collagenolytic serine protease from fig (Ficus carica var. Brown Turkey) latex. J. Biosci. Bioeng. 2014;118(6):622–627. doi: 10.1016/j.jbiosc.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 55.Sharma A., Kumari M., Jagannadham M.V. Benghalensin, a highly stable serine protease from the latex of medicinal plant Ficus benghalensis. J. Agric. Food Chem. 2009;57(23):11120–11126. doi: 10.1021/jf902279u. [DOI] [PubMed] [Google Scholar]
- 56.Lynn K.R., Clevette-Radford N.A. Ficin E, a serine-centred protease from Ficus elastica. Phytochemistry. 1986;25(7):1559–1561. [Google Scholar]
- 57.Kumari M., Sharma A., Jagannadham M.V. Decolorization of crude latex by activated charcoal, purification and physico-chemical characterization of religiosin, a milk-clotting serine protease from the latex of Ficus religiosa. J. Agric. Food Chem. 2010;58(13):8027–8034. doi: 10.1021/jf101020u. [DOI] [PubMed] [Google Scholar]
- 58.Patel A.K., Singh V.K., Jagannadham M.V. Carnein, a serine protease from noxious plant weed Ipomoea carnea (morning glory) J. Agric. Food Chem. 2007;55(14):5809–5818. doi: 10.1021/jf063700h. [DOI] [PubMed] [Google Scholar]
- 59.Renuka Prasad K.M., Virupaksha T.K. Purification and characterization of a protease from jackfruit latex. Phytochemistry. 1990;29(6):1763–1766. [Google Scholar]
- 60.Thongsook T., Barrett D.M. Heat inactivation and reactivation of broccoli peroxidase. J. Agric. Food Chem. 2005;53(8):3215–3222. doi: 10.1021/jf0481610. [DOI] [PubMed] [Google Scholar]
- 61.Menon M. Isolation and characterization of proteolytic enzymes from the latex of Synadenium grantii Hook, ‘f’. Plant Sci. 2002;163(1):131–139. [Google Scholar]
- 62.Otto H.H., Schirmeister T. Cysteine proteases and their inhibitors. Chem. Rev. 1997;97(1):133–172. doi: 10.1021/cr950025u. [DOI] [PubMed] [Google Scholar]
- 63.Lynn K.R., Clevette-Radford N.A. Isolation and characterization of proteases from Euphorbia lactea and Euphorbia lactea cristata. Phytochemistry. 1986;25(4):807–810. [Google Scholar]
- 64.Khan H. Purification and characterization of serine protease from seeds of Holarrhena antidysenterica. Biotechnol. 2008;7:94–99. [Google Scholar]
- 65.Lynn K.R. Parthenain, a protease from Parthenium argentatum. Phytochemistry. 1988;27(7):1987–1991. [Google Scholar]
- 66.Lind S.E., McDonagh J.R., Smith C.J. Oxidative inactivation of plasmin and other serine proteases by copper and ascorbate. Blood. 1993;82(5):1522–1531. [PubMed] [Google Scholar]
- 67.Nowak P., Zgirski A. Effects of metal ions on activity of plasmin. Biol. Trace Elem. Res. 2003;93(1–3):87–94. doi: 10.1385/BTER:93:1-3:87. [DOI] [PubMed] [Google Scholar]
- 68.Osoniyi O., Onajobi F. Coagulant and anticoagulant activities in Jatropha curcas latex. J. Ethnopharmacol. 2003;89(1):101–105. doi: 10.1016/s0378-8741(03)00263-0. [DOI] [PubMed] [Google Scholar]
- 69.Kattula S., Byrnes J.R., Wolberg A.S. Fibrinogen and fibrin in Hemostasis and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2017;37(3):e13–e21. doi: 10.1161/ATVBAHA.117.308564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fonseca K.C. Purification and biochemical characterization of Eumiliin from Euphorbia milii var. hislopii latex. Phytochemistry. 2010;71(7):708–715. doi: 10.1016/j.phytochem.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 71.Shivaprasad H.V. Thrombin like activity of Asclepias curassavica L. latex: action of cysteine proteases. J. Ethnopharmacol. 2009;123(1):106–109. doi: 10.1016/j.jep.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 72.Hatano K. Purification and characterization of novel proteinase inhibitors from dried figs. J. Agric. Food Chem. 2006;54(2):562–567. doi: 10.1021/jf052123e. [DOI] [PubMed] [Google Scholar]
- 73.Gilani A.H. Ethnopharmacological studies on antispasmodic and antiplatelet activities of Ficus carica. J. Ethnopharmacol. 2008;119(1):1–5. doi: 10.1016/j.jep.2008.05.040. [DOI] [PubMed] [Google Scholar]
- 74.Cantwell A.M., Di Cera E. Rational design of a potent anticoagulant thrombin. J. Biol. Chem. 2000;275(51):39827–39830. doi: 10.1074/jbc.C000751200. [DOI] [PubMed] [Google Scholar]
- 75.Gilani A.H. Pharmacological basis for the use of Fumaria indica in constipation and diarrhea. J. Ethnopharmacol. 2005;96(3):585–589. doi: 10.1016/j.jep.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 76.Gilani A.H. Pharmacological basis for the use of turmeric in gastrointestinal and respiratory disorders. Life Sci. 2005;76(26):3089–3105. doi: 10.1016/j.lfs.2004.12.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.