Abstract
Electrospinning is a versatile strategy for creating nanofiber materials with various structures, which has broad application for a myriad of areas ranging from tissue engineering, energy harvesting, filtration and has become one of the most important academic and technical activities in the field of material science in recent years. In addition to playing a significant role in the construction of two-dimensional (2D) nanomaterials, electrospinning holds great promise as a robust method for producing three-dimensional (3D) aerogels and scaffolds. This article reviews and summarizes the recent advanced methods for fabricating electrospun three-dimensional nanofiber aerogels and scaffolds, including gas foaming, direct electrospinning of 3D nanofibrous scaffold, short nanofibers assembling into 3D aerogels/scaffolds, 3D printing, electrospray, origami and cell sheet engineering, centrifugal electrospinning, and other methods. Besides, intriguing formation process, crosslinking pathway, properties, and applications of 3D aerogels and scaffolds are also introduced. Taken together, these aerogels and scaffolds with various excellent features present tremendous potential in various fields.
Keywords: Electrospun nanofibers, Three-dimensional scaffolds, Aerogels, Tissue engineering
Graphical abstract

Highlights
-
•
Advanced fabricated methods and properties of three-dimensional(3D) nanofiber aerogels and scaffolds are summarized.
-
•
The existing problems and limitations of the 3D nanofiber aerogels and scaffolds are discussed.
-
•
The comparison among 3D electrospun scaffolds and aerogels fabrication technologies are discussed.
-
•
Prospects and challenges of 3D electrospun scaffolds and aerogels are proposed.
1. Introduction
Spider silk and nanocellulose are nanofibers found in nature, however, the low yield limits their application. To afford the preparation of artificial nanofibers, different types of techniques, such as drawing [1], self-assembly [2], template synthesis [3], and phase separation [4] have been introduced to achieve an array of nanofibers-based devices. Despite significant progress in the design of nanofibrous structures, there are yet significant challenges that need to be addressed for the clinical and industrial translation of nanofibers technology. Consequently, electrospinning technology has been pursued to develop nanofibrous scaffolds and devices for a myriad of clinical and industrial applications.
The formation of nanofibers through electrospinning is based on the uniaxial stretching of a viscoelastic solution [5]. Utilizing the high-voltage electrostatic field, polymer solution or melt is charged, forming a suspended cone-shaped droplet at the end of the nozzle. When the repulsion force exceeds its surface tension, the polymer micro liquid flow is ejected at a high speed. In a short period, these jets are stretched by the electric field. The solvent evaporates and the jet is deposited on the collector to form nanofibers.
Due to the high production efficiency of electrospinning, a library of natural and synthetic polymers have been electrospun. Natural materials such as chitosan [6], hyaluronic acid [7], silk fibroin [8], collagen [9], and gelatin [10] have been reported to enhance the biocompatibility of nanofibers. On the other hand, synthetic polymers such as poly(vinyl alcohol) (PVA) [11], poly(l-lactide) (PLLA) [12], polycaprolactone (PCL) [13], and poly(l-lactide-co-caprolactone) (PLCL) [14] have been investigated owing to their elasticity, biodegradability, and mechanical strength. Furthermore, the electrospinning of multi-component nanofibers has been pursued, which renders them amenable for overcoming the bottlenecks associated with the individual polymers and provide a window to realize nanofibers with the required properties such as, mechanical strength, biocompatibility, and degradation rate by finely adjusting the proportion of each component. In addition, electrospun fibers fabricated from inorganic nanoparticles such as silica can be prepared by the sol-gel method. As a versatile and robust micro/nanofabrication technique, electrospinning finds its potential application in a myriad of academic and industrial sectors including catalysis [15], drug delivery [[16], [17], [18]], air and liquid filtration [19,20], regenerative medicine and tissue engineering [21], biosensing [22], and food safety [23].
Conventional electrospinning processes employ a static plate collector placed at a certain distance from a charged nozzle containing a polymer solution. The limitation of the conventional electrospinning is that the scaffolds are made entirely of tightly packed layers of nanofibers, which only possess a surface porous structure due to the sheet-like assembly. This restriction may impede the applications of electrospun materials in certain fields, such as damping materials, sensors, battery, and 3D tissue repair. Thus, it is imperative to develop an innovative strategy capable of fabricating electrospun scaffolds with a stable 3D structure, while exhibiting nanofibrous morphologies and deep, interconnected pores [24]. In recent decades, various methods for preparing 3D electrospun nanofibrous scaffolds have been reported, such as multilayering electrospinning [25], liquid and template-assisted electrospinning [26], porogen-incorporated electrospinning [27], and the post-treated electrospunning [28]. Multilayer electrospinning involves the spinning of multilayers of scaffold materials to afford required mechanical properties or microstructure, which may also help fabricate hybrid electrospun scaffolds as well as achieve tissue/organ mimetic microstructure as in the case of tissue-engineered blood vessels. On the other hand, template-assisted electrospinning employs a sacrificial template, which can be leached out post-spinning. Conventionally, 3D scaffolds possess limited porosity and pore size, which can be improved by introducing an appropriate leachable porogen (e.g. salt or sugar). Similarly, structural and morphological properties of electrospun 3D scaffolds can be tuned by surface modification and or by using another technique such as femtosecond laser-assisted patterning. These methods are expected to enlarge the thickness, porosity, and specific surface area of the 3D scaffolds. For regenerative medicine and tissue engineering, 3D scaffolds can provide a better platform for cell growth and tissue morphogenesis than that of the traditional 2D cultures, which is due to the fact that the 3D scaffolds offer an additional direction for cell-cell interactions, cell migration, and cell morphogenesis, which is important in regulating the cell cycle and tissue functions [29].
In this review, we will briefly summarize recent works on 3D electrospun nanofibers assembled from various methods: (1) gas foaming, (2) direct electrospinning of 3D fibers, (3) short nanofibers assembling into 3D aerogels/scaffolds, (4) 3D printing, (5) electrospray, (6) origami and cell sheet engineering, (7) centrifugal electrospinning and other methods. The latest 3D nanofibrous scaffolds may find more potential and broad applications for biomedical applications, sensor development, thermal insulation, sound absorption, emulsion separation, and elasticity-responsive electrical conduction.
2. Gas foaming
Gas foaming processes utilize the nucleation and growth of gas bubbles that are generated in situ either via a chemical reaction or by adding inert gases into the polymer phase under different physical environments [30]. Electrospun membranes possess tightly packed nanofibers and interconnected micropores/nanopores. The aggregation of bubbles in the pores of the 2D scaffold might be a potential strategy to reassemble nanofibers into a fluffy 3D-like structure (Table 1).
Table 1.
Summary of the 3D scaffold fabrication by using the gas foaming method.
| Polymer | Foaming method | Foaming agent | Biological evaluation | Key findings | Ref |
|---|---|---|---|---|---|
| PCL, Nylon, Cellulose, PVDF | Gas foaming | NaBH4 | In vitro | Pore size & porosity↑; Cell infiltration↑ |
[31] |
| PCL membrane | Modified gas foaming | NaBH4 + Freeze-drying | In vitro | Anisotropic scaffold; Porosity↑; Nanotopgraphy maintained |
[32] |
| PCL membrane | Modified gas foaming | NaBH4 + Freeze drying | In vitro & subcutaneous (s.c.) implantation | Angiogenesis; M2/M1 macrophages↑ |
[33] |
| PCL membrane | Modified gas foaming | NaBH4 | In vitro | Complex-shaped scaffolds; Cell spreading & proliferation↑ |
[34] |
| Chitosan/PVA membrane | Gas foaming | NaBH4 | In vitro & wound healing assay | Sponge-like scaffold obtained; Hemostasis and ECM deposition↑; Scar formation↓ |
[35] |
| PLLA/silk fibroin membrane | Gas foaming | NaBH4 | In vitro & nerve regeneration | Schwann cell proliferation↑; Nerve regeneration↑ | [36] |
| PCL membrane ensconcing LL-37 & coumarin 6 | Gas foaming | Subcritical CO2 | Subcutaneous implantation | LL-37 & coumarin-6 bioactivity preserved; Cell infiltration↑; M2/M1 ratio↑ | [37] |
| PCL membrane | Gas foaming | CO2 saturated with ethanol | In vitro assay | Porosity↑; Cell migration & proliferation↑ | [38] |
2.1. Sodium borohydride as a gas foaming agent
The use of chemical foaming agents, such as sodium borohydride (NaBH4) to undergo in situ decompositions the gas release is an attractive approach for the generation of hydrogen gas bubbles at ambient temperature. The reaction equation is as follows: NaBH4 + 2H2O → NaBO2 + 4H2. The resulting 3D scaffolds exhibit high porosity as well as nanotopography. In addition, hydrogen is harmless to the material and NaBH4 can be dislodged via rinsing the scaffold with the deionized water several times.
Joshi et al. [31] fabricated electrospun membranes by using PCL, nylon, cellulose, and poly(vinylidene fluoride) (PVDF) and carried out an in-situ gas foaming process post electrospinning. Nanofibrous membranes were immersed in the NaBH4 solution. During the foaming process, the hydrogen nucleated the pores of the membranes and exerted pressure on the surrounding fibers, which ultimately underwent rearrangement forming a sponge-like structure (Fig. 1). As compared to the 2D membranes, scaffolds exhibited larger pores, macro-porosity, and 3D like structure. Although the 3D scaffold showed significant cell proliferation as compared to the 2D membrane, the infiltration of cells in the foamed scaffold was not demonstrated. Jiang et al. [32] reported a modified gas-foaming technique to afford PCL-based 3D anisotropic scaffolds and bi-layered tubular-shaped conduits with micron layered structure and controllable gap widths. In order to generate 3D nanofibrous scaffolds with a highly ordered and stable architecture, freeze-drying was carried out immediately after the gas foaming process. Compared with the 2D membrane, the 3D nanofibrous scaffolds possessed higher porosity as well as preserved nanotopographical cues. The increase of porosity and thickness of 3D scaffolds depended on the foaming time and the concentration of the foaming solution. In vitro evaluation demonstrated that the expanded nanofiber scaffold promoted the infiltration of cells, while the cells only grew on the surface on 2D membranes. Despite the obvious advantages in terms of cellular infiltration, the biocompatibility of scaffolds was not demonstrated in vivo.
Fig. 1.
Schematic illustration for the formation of low density, macroporous, spongy, and multi-layered 3D scaffolds. Reprinted with permission from Ref. [31].
To precisely control the size and the thickness of scaffolds as well as understand the biocompatibility of expanded 3D scaffolds in vivo, Jiang et al. [33] utilized a customized mold during the foaming process. 2D electrospun membranes were put into the mold (Fig. 2a) and foamed in NaBH4 aqueous solution (Fig. 2b). Then, the 3D scaffolds (thickness, 3 mm and 10 mm) were rinsed with deionized water (Fig. 2c). After freeze-drying, the 3D nanofibers with the precisely controlled thickness could be obtained (Fig. 2d). The biocompatibility of scaffolds was evaluated after subcutaneous implantation in rats for up to 8 weeks in vivo, which showed time-dependent increments in the numbers of CD68+, CCR7+, and CD163+ cells in the expanded 3D scaffolds as compared to 2D membranes. Moreover, 3D scaffolds also showed higher cell infiltration and blood vessel formation. In contrast, unexpanded membranes only showed cell accumulation on the surface of scaffolds. However, this method can only afford scaffolds with the simple shape and the fabrication of 3D scaffolds with complex geometry yet remains to be realized.
Fig. 2.
Schematic illustration of the fabrication of expanded PCL nanofiber scaffolds. a) A customized mold was made by a pair of glass sheets and spacers, which was assembled by glue. A piece of plasma-treated PCL fiber mat was placed at the center of the mold. b) The PCL fiber mat together with the mold was expanded in 1 M NaBH4 solution for 1 h at room temperature. c) The expanded PCL nanofiber scaffold was rinsed, vacuumed, and freeze-dried by using a lyophilizer. d) The glass mold was gently removed and the expanded PCL nanofiber scaffold was ready to be sterilized and used for implantation. Reprinted with permission from Ref. [33].
Despite improvements in the pore size and the morphology of electrospun membranes by using the foaming process, a lack of accurate control of the gas foaming method is a bottleneck for the design of 3D scaffolds. Gao et al. [34] combined 3D printing with electrospinning to fabricate customized shaped 3D scaffolds. First, a hemispherical shaped mold was fabricated by using 3D printing. The electrospun membrane was cut into a circle and placed in a circular groove at the bottom of the mold and covered with NaBH4 particles. Then, the assembled mold was soaked in methanol for the foaming process and it afforded a hemispherical shaped 3D scaffold (Fig. 3). The expansion process resulted in 3D expanded scaffolds, which lead to higher cell growth and a spreading type morphology. Intriguingly, the authors also bioprinted cell-encapsulated hydrogels onto expanded scaffolds, which were then instilled into scaffolds. Different types of geometrical shapes were formed. As an application attempt, a membrane composed of chitosan (CS) and PVA was immersed in the NaBH4 aqueous solution to afford a sponge-like scaffold for hemostasis and wound healing [35]. In order to improve the stability of the nanofibrous scaffold, membranes were first crosslinked by using glutaraldehyde vapors for up to 6 h. Due to an increase in the specific surface area of the 3D scaffolds, the interaction with the blood cells was improved, which accelerated hemostasis. Moreover, the scaffold exhibited good elasticity and suction capacity, which provided the tamponade to the deep wounds. Consequently, 3D scaffolds accelerated the wound healing with an improvement in the dermal function and a reduction in the scar area and collagen deposition in a mouse model. Similarly, 3D scaffolds have also been exploited as nerve guidance conduit (NGC) for peripheral nerve regeneration. Rao et al. [36] fabricated electrospun membranes consisting of PLLA and silk fibroin (SF) and treated those with NaBH4. Thus obtained 3D scaffolds were placed into a chitosan NGC to afford sponge containing NGC (SNGC). Conduits containing SNGC showed higher proliferation of Schwann cells and accelerated nerve regeneration and its function.
Fig. 3.
(a) Schematic illustration to fabricate 3D scaffolds with controllable shapes; (b) Foaming process of the 2D nanofibrous membrane to the 3D scaffold. Reprinted with permission from Ref. [34].
2.2. Carbon dioxide and subcritical CO2 fluid as gas foaming agents
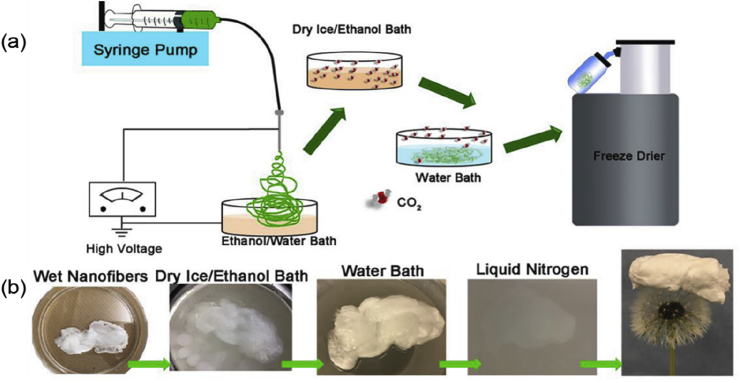
Sodium borohydride has been widely applied as a foaming agent, however, it needs to be dissolved in water to produce hydrogen, which poses a limitation for the processing of water-soluble materials. In addition, materials with weak mechanical properties may decompose during foaming due to the strong reactivity of NaBH4. Therefore, novel foaming approaches capable of avoiding the aforementioned shortcomings hold great promise of the research community. Carbon dioxide (CO2) acts as a favorable gas foaming agent owing to its several advantages, such as low cost, flame retardancy, and safety. Jiang et al. [37] expanded the 2D membranes into 3D scaffolds via depressurization of subcritical CO2 fluid. Expanded 3D scaffolds demonstrated layered structure and retained nanofibrous morphology. To demonstrate the potential of foaming technology to preserve the bioactivity of an antibacterial peptide ‘LL-37’ and coumarin 6, PCL membranes were fabricated and expanded into 3D scaffolds by using CO2. The results proved that 3D scaffolds maintained the fluorescence and antibacterial properties of coumarin 6 and LL-37 peptide, respectively. 3D scaffolds also promoted cell infiltration and blood vessel formation as well as lead to an increase in the M2/M1 ratio of macrophages as evaluated after subcutaneous implantation. Jing et al. [38] afforded 3D scaffolds by combining a foaming technique and electrospinning. Firstly, 70% ethanol was used as a collector to receive PCL membranes and mats were immersed into CO2-saturated ethanol for a few minutes. Afterward, membranes were placed into a water bath at room temperature followed by freeze-drying (Fig. 4). Due to the solubility difference of CO2 in ethanol and water, a lot of CO2 bubbles escaped from the inner pores of fibers and expanded scaffolds. Expanded scaffolds showed high porosity of 95.3%, which achieved cell migration into internal space and promoted cell proliferation. It is worth mentioning that the expansion of 2D membranes by using gas foaming technology and subcritical CO2 are different mechanistically. While in the gas foaming technique, a gas foaming agent that produces hydrogen (e.g. NaBH4) or carbon dioxide is employed and the 2D membrane is submerged into the foaming solution, which decomposes and produces the gas in situ thus producing a 3D-like structure. On the other hand, in the subcritical CO2 based method, the CO2 fluid penetrates the nanofibrous membrane and upon the depressurization changes into gaseous CO2, which ultimately releases off from the membrane leaving behind the porous structure. Since CO2 has different solubility in ethanol and water, submersion of 2D membranes into CO2-saturated ethanol followed by an immersion in the water also led to a muti-layer porous structure.
Fig. 4.
(a) Schematic to fabricate 3D nanofibrous scaffolds. (b) Representative photographs of the electrospun nanofibers in each step during the fabrication process. Reprinted with permission from Ref. [38].
3. Direct electrospinning of 3D nanofibrous scaffolds
Direct eletrospinning of 3D nanofibrous scaffold has been realized by using special collection equipment, self-assembly deposition, or liquid collection, which has the advantages of simple preparation steps and less cost. According to the different compositions of scaffolds and aerogels prepared by the above methods, herein, we summarize the direct electrospinning of inorganic and organic 3D nanofibrous scaffolds [Table 2].
Table 2.
Summary of the direct electrospinning of three-dimensional scaffolds.
| Polymer | Collection method | Biological evaluation | Key findings | Ref |
|---|---|---|---|---|
| PLCL, PLCL/SF | Dynamic liquid electrospinning | In vitro | Cell infiltration↑; Biomineralization↑ |
[26] |
| SiO2 nanofiber | Self-assembly electrospinning | ╳ | Fiber diameter↓; Surface area, 6.45 m2/g; Bulk density, 16 mg/cm3 |
[41] |
| SiO2/CaO nanofibers | Sol-gel assisted electrospinning | In vitro & s.c. implantation | Biomineralization↑ | [42] |
| Carbon nanofibers/SiO2 aerogel scaffold | Liquid-assisted collection | ╳ | Oil absorption↑ | [43] |
3.1. Direct electrospinning of 3D organic nanofibrous scaffolds
Polystyrene (PS) has been reported to be able to self-assemble via electrospinning into 3D scaffolds without the addition of a secondary phase or added environmental controls [39]. However, the weak structures and random shapes of PS based scaffolds limit their uses, especially in tissue engineering. By modifying the collector, such as replacing the traditional 2D flat collector with a 3D collection template, 3D controllable electrospun scaffolds can be successfully obtained. Sun et al. [26] directly collected 3D PLCL/SF nanofibers with a fixed shape by changing the collection template as shown in Fig. 5. In the first step, nanoyarns were fabricated by using a dynamic liquid electrospinning system [40]. A seven-pin plug-like roller rotating in the flowing water was used to collect the nanoyarns. Then, the prepared scaffold was transferred to a freeze-dryer and separated from the roller collector (Fig. 5b). Cells did not only accumulate on the surface of nanoyarns but also infiltrated into the interior of 3D scaffolds due to the porous structure. In addition, PLCL/SF scaffolds exhibited more biocompatibility as compared to PLCL based scaffolds and afforded the growth of hydroxyapatite through natural biomineralization.
Fig. 5.
Schematic of the electrospinning with a dynamic supporting system to fabricate nanoyarn scaffold. (a) nanoyarns fabrication by electrospinning. (b) fiber-reinforced scaffold fabrication and moving scaffold from the roller by freeze-drying technology. Reprinted from Ref. [26].
3.2. Direct electrospinning of 3D inorganic nanofibrous scaffolds
The combination of sol-gel technology and electrospinning has been developed to achieve a robust method for the preparation of inorganic nanofibers. Mi et al. [41] fabricated 3D silica-based fibrous scaffold using tetraethylorthosilicate (TEOS) and PVA colloidal solution by self-assembly electrospinning and subsequent calcination. The self-assembly deposition process is shown in Fig. 6A. When a high voltage was applied, the fibers were immediately deposited on the nail of a thumbtack and the fiber stack collapsed when the weight at the top became too heavy. Fig. 6B demonstrated that the 3D scaffold maintained its white color, while the average fiber diameter decreased from 2.5 μm to 1.9 μm due to the depletion of PVA content. The obtained scaffolds possessed a low bulk density of 16 mg/cm3 and a surface area of 6.45 m2/g.
Fig. 6.
(A). The electrospinning process of the as-spun fibers. (B) Photographs, SEM images, and fiber diameter of the as-spun fibers and silica fibers. Reprinted with permission from Ref. [41].
As mentioned previously, PVA was employed as a binder along with TEOS to afford 3D silica scaffolds, which required calcination post-electrospinning. To avoid the need for the binders, Poologasundarampillai et al. [42] modified processing conditions to realize a viscoelastic inorganic sol-gel solution that resulted in fibers by the entanglement of the intermolecularly-overlapped nano-silica species in the solution. In detail, a composition of 70 mol. % SiO2 and 30 mol. % CaO (70S30C) was employed as a precursor solution to cotton (or cotton candy-like) structures. These flexible 3D fibers possessed large inter-fiber space to promote angiogenesis and cell infiltration. After soaking in simulated body fluid for 12 h, the hydroxyapatite layer was deposited on the fiber and MC3T3-E1 osteoblasts cultured on the fiber showed no adverse cytotoxic effect, which indicated that the scaffold could promote bone tissue regeneration.
Lin et al. [43] reported a facile approach to fabricate mechanical strength enhanced 3D nanofibrous aerogel principally by employing liquid-assisted collection-electrospinning technology. As shown in Fig. 7, electrospun polyacrylonitrile (PAN) nanofibers were collected directly in a liquid collector containing graphene oxide (GO). The PAN nanofibers dispersion was freeze-dried to afford nanofibers/GO composite aerogel (NF/GOAs), which were then thermally treated to obtain fluffy carbon nanofibers/GO aerogels (CNF/GOAs). The resulting scaffold exhibited ultra-low density (2–3 mg/mL) and high compressibility (80%). CNF/GOAs aerogels showed a high absorption capacity (120–286 wt/wt) for different types of oils and high elasticity. After 10 cycles, the scaffolds still showed great absorption capacity, which provided feasibility for the large scale application.
Fig. 7.
Schematic diagram showing the synthesis procedure of CNF/GOAs aerogels: (1) continuous electrospun PAN nanofibers were collected by GO aqueous dispersion; (2) 3D NF/GOAs were prepared by freeze-drying the PAN nanofibers/GO sheets aqueous dispersions; (3) CNF/GOAs were prepared by the thermal treatment of NF/GOAs including pre-oxidation and carbonization. Reprinted with permission from Ref. [43].
4. Short nanofibers assembling into 3D aerogels/scaffolds
Electrospun nanofibers hold great promise as exceptional nanoscale building blocks for constructing 3D aerogels/scaffolds which can precisely control the physicochemical and mechanical properties due to their robust mechanical strength, low density, and flexibility. A usual fabrication method of short electrospun fibers is to cut the nanofibrous membrane into small pieces and then uniformly disperse the small pieces in the medium by using a homogenizer. In general, short fibers are introduced into aerogels/scaffolds to enhance their structural stability or serve as an ECM template to provide a suitable microenvironment for cell growth and proliferation. Short fibers must be crosslinked to form a 3D scaffold with a continuous fibrous structure that can substantially improve material utilization and the resultant properties. Therefore, different types of cross-linking strategies have been adopted to improve the performance of 3D aerogels/scaffolds. In the following section, we will focus on the composition, preparation, crosslinking method, and performance of 3D aerogels/scaffolds and divide the content into two parts: non-degradable 3D nanofiber aerogels/scaffolds and degradable 3D nanofiber scaffolds (Table 3).
Table 3.
Summary of assembly of short nanofibers into three-dimensional aerogel/scaffolds and 3D printing.
| Polymer | Fabrication method | Biological evaluation | Key findings | Ref |
|---|---|---|---|---|
| PAN/SiO2 scaffolds | Electrospinning | ╳ | Elastic resilience↑; Energy absorption↑ |
[44] |
| KGM/SiO2 scaffold | Electrospinning & freeze-drying | ╳ | Honeycomb-mimetic structure; Shape memory↑; Density, 0.14 mg/cm3 |
[45] |
| Alginate/SiO2 scaffolds | Electrospinning & ionic crosslinking | ╳ | Water content, 99.8%; Shape memory↑ |
[46] |
| Chitosan/SiO2–CaO scaffolds | Electrospinning & sol-gel synthesis | Calvarial defect model | Honeycomb-like structure; Self-deployment; Shape-memory; Elasticity & biomineralization↑ |
[47] |
| PLLA or PLLA/PCL sponges | 3D printed mold assisted electrospinning | In vitro | Shape memory↑; Cell proliferation↑ |
[48] |
| Gelatin/PLLA scaffold | Electrospinning | In vitro | Elasticity↑; Superabsorbent | [49] |
| BMP2-ensconced PLLA/GEL/HAP scaffold | Electrospinning | In vitro & in vivo | Osteogenesis↑; Biocompatibility↑ | [50] |
| PLGA/Col/Gel scaffold | Electrospinning | Cranial defect | Bone regeneration↑ | [51] |
| PLGA/Gel/Hap scaffold | Electrospinning & 3D printing | Cartilage defect | Shape memory↑; Cartilage regeneration↑ |
[52] |
| PLGA/Gel/CDM scaffold | Electrospinning & 3D printing | Cartilage defect | Cartilage regeneration↑ | [53] |
| PLLA/PEO scaffold | Stable jet electrospinning | In vitro | Cell proliferation & infiltration↑ | [54] |
| Silk fibroin | Stable jet electrospinning | In vitro | Cell adhesion & migration↑ | [55] |
4.1. Non-degradable 3D nanofiber aerogels/scaffolds
Inorganic nanofibers are the main components of non-degradable aerogels/scaffolds, which are obtained by the combination of sol-gel technology and electrospinning. Another approach is to calcine the aerogels/scaffolds containing organic components to afford carbonaceous aerogels/scaffolds. The main problems associated with 3D nanofiber aerogels/scaffolds are to construct homogeneous, continuous, and mechanically robust aerogels/scaffolds with 3D architecture. Si et al. [44] fabricated super-elastic nanofibrous aerogels (NFAs) with a hierarchical cellular structure. Bifunctional benzoxazine (BA-a) was employed as an in situ crosslinking agent, which afforded the formation of Mannich-bridged polybenzoxazine (PBZ) and cemented the adjacent nanofibers isotropically. The preparation method has been shown in Fig. 8. Polyacrylonitrile (PAN) nanofibers containing benzoxazine (PAN/BA-a) and silica nanofibers were first fabricated separately. After this, both PAN/BA-a and silica membranes were cut, homogenized in a medium, and freeze-dried to afford into an uncrosslinked NFA. The subsequent heating of uncrosslinked fibers at 240 °C for 1 h afforded crosslinked 3D fibrous networks, endowing the resultant NFAs with elastic resilience, efficient energy absorption, and multiple functionalities such as thermal insulation, sound absorption, emulsion separation, and elasticity-responsive electric conduction.
Fig. 8.
Design, processing, and cellular architectures of FIBER NFAs. (a) Schematic showing the synthesis steps. (1) Flexible PAN/BA-a and SiO2 membranes are produced by electrospinning. (2) Homogeneous nanofibrous dispersions are fabricated via high-speed homogenization. (3) Uncrosslinked NFAs are prepared by freeze-drying nanofibrous dispersions. (4) The resultant FIBER NFAs are prepared by the crosslinking treatment. (b) An optical photograph of FIBER NFAs with diverse shapes. (c–e) Microscopic architecture of FIBER NFAs at various magnifications, showing the hierarchical cellular fibrous structure. (f) Schematic representation of the dimensions of relevant structures. Scale bars, 20 mm(c), 5 mm (d) and 1 mm (e). Reprinted with permission from Ref. [44].
Ultralight carbonaceous aerogels exhibit the integrated properties of low apparent density, good electrical conductivity, and chemical inertness. However, these carbon units mainly come from non-renewable fossil resources which are affected by toxic reagents, complex equipment, technological requirements, and low production ability. Ding and coworkers demonstrated a sustainable strategy for creating super-elastic carbonaceous nanofibrous aerogels (CNFAs) with an ordered honeycomb-like structure by using a novel biomass-konjac glucomannan (KGM) and flexible SiO2 nanofibers [45]. As shown in Fig. 9, the SiO2 nanofibers and KGM powder were dispersed in sodium hydroxide solution to form a homogeneous nanofiber dispersion and then freeze-dried into KGM/SiO2 nanofiber composite aerogels (KNFAs). It was noticed that freeze-shaping led to a highly ordered structure, mimicking the honeycomb which possessed an apparent density similar to aerogels but was obviously structurally robust. In order to introduce further elastic bonding into the aerogels, the as-prepared KNFAs were heated at 90 °C to completely deacetylate the KGM, forming a thermosetting gel through strong hydrogen bonding and hydrophobic interaction and then carbonized at 850 °C to form carbonaceous nanofibrous networks composed of SiO2/carbon core-shell nanofibers. Being derived from abundant biomass, these honeycomb-mimetic carbonaceous aerogels exhibit potential to be used for a myriad of applications including wearable electronics such as health monitoring, human-machine interface, and artificial skin. Besides, these aerogels can be fabricated into different shapes and structures such as cylinder, triangle, prism, pentagonal prism, cuboid, and cone.
Fig. 9.
a) Schematic showing the synthesis steps. 1. The nanofibers dispersion is prepared through high-speed homogenization. 2. Frozen dispersions are prepared by freezing in liquid nitrogen. 3. KNFAs are fabricated via freeze-drying the frozen dispersions. 4. The resultant CNFAs are fabricated by the deacetylation and carbonization of KNFAs. b) An optical photograph of a KNFA. c–e) Microscopic structure of KNFAs at different magnifications. f) An optical photograph showing a 20 cm3 CNFA (ρ = 0.14 mg/cm3) standing on the tip of a konjac leaf. g–i) Microscopic structure of CNFAs at various magnifications, demonstrating the biomimetic honeycomb cellular fibrous architecture. j) Schematic showing the four levels of hierarchy at three distinct length scales. Reprinted from Ref. [45].
CNFAs were attractive for their features of extremely low density (minimum of 0.14 mg/cm3), super cyclable compressibility, zero Poisson's ratio, good thermal stability, elastic-responsive, conductivity, and high pressure-sensitivity. Since the lamellar deposition, high pack density, and anisotropic deposition of nanofibers poses a limitation for the design of mechanically robust and structurally efficient nanostructures, Ding and coworkers have also presented a robust strategy for creating high-water-containing and super-elastic nanofibrous hydrogels with ordered cellular structures by using sustainable alginate and silica nanofibers [46]. To realize elastic bonding in nanofiber networks, the NFAs were immersed in an aqueous solution of Al3+ to produce nanofibrous hydrogels (NFHs) with non-covalent ionic crosslinks in the alginate. These NFHs exhibit a combination of ultrahigh water content (99.8 wt%), complete recovery from 80% strain, shape-memory behavior owning to ionic crosslinks and pressure sensitivity (0.24 kPa−1), all of which are resulted by the synergistic effects of the ordered nanofibrous structure of silica and fully hydrated alginate-incorporated nanofibers.
As an attempt in the field of tissue engineering, Wang et al. [47] developed a scaffold composed of SiO2–CaO glass nanofibers and chitosan which were able to deform and fit into irregularly-shaped bone defects. The SiO2–CaO nanofibers were segmented and assembled into 3D porous scaffolds which were wrapped and bonded by natural polymer chitosan via homogenization and lyophilization processes. The SiO2–CaO nanofiber/chitosan (SiO2–CaO NF/CS) hybrid scaffolds possessed shape-recovery property, elasticity, and biomineralization as well as exhibited self-deploying feature and regenerative capability in a calvarial defect model in rats. The histological analysis revealed that the hybrid scaffolds containing mesenchymal stem cells (MSCs) were more effective in the new vascularized bone regeneration than that of the scaffold-only group. These mechanically-robust and self-deployable honey-comb shaped scaffolds containing entangled flexible networks were found to be of more effective than that of the conventional bioactive glass and hydroxyapatite in terms of the shape-recovery and mechanical properties and therefore hold great promise for the regeneration of osteoporotic bony defects.
4.2. Degradable 3D nanofiber scaffolds
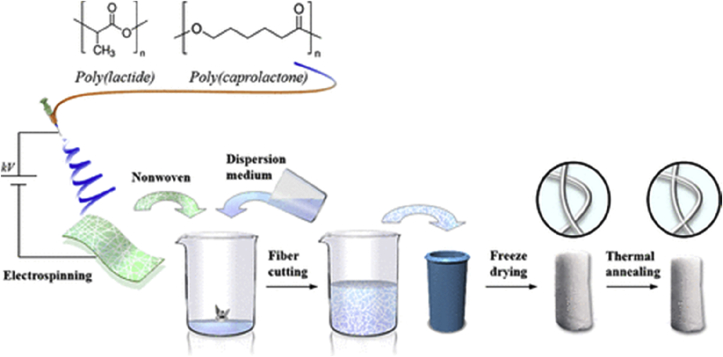
Compared with the non-degradable materials, degradable 3D nanofiber scaffolds have more extensive applications in the field of tissue engineering which resemble the structure of the 3D tissue while providing adhesion sites and a niche-like microenvironment to the cells. Degradability enables the 3D scaffold to enter into the body as a carrier and be absorbed by the human body after releasing drugs or growth factors as needed for the regenerative process. Short fibers from the synthetic polymers can be crosslinked by increasing the temperature of the dispersion of fiber without adding a crosslinking agent, thus maintaining the 3D structural stability. Mader et al. developed degradable, highly porous, open-cellular, and reversibly-compressible polymer fiber-based sponges (PFS) by using PLA [48]. As shown in Fig. 10, the prepared nanofibers were cut into short fibers which were dispersed and homogenized and then filled into a 3D printing mold and frozen. During the subsequent thermal annealing at 60 °C (PLA/PCL sponge) or 100 °C (PLA sponge), a physically crosslinked PFS with elastic properties could be realized. The degradable PFS showed excellent shape stability, reversible compressibility, shape-memory and can also be hydrophilized by the air-plasma treatment, which substantially improved the proliferation of cells in vitro.
Fig. 10.
Schematic to illustrate the general sponge fabrication steps. Reprinted from Ref. [48].
Synthetic materials, especially thermoplastic polymers possess excellent processability for the preparation of scaffolds, however, relatively long degradation time and more inflammation in vivo hamper their utilization. Consequently, natural polymers have received more attention due to their better biocompatibility and extensive sources, which show unique advantages in the preparation of 3D scaffolds. Chen et al. [49] prepared the porous 3D scaffold based on electrospun gelatin (GEL)/PLA nanofibers for cartilage tissue regeneration (Fig. 11). These scaffolds were either crosslinked by heating at high temperature or by using a solution containing hyaluronic acid (HA) and N-Ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride(EDC)/N-Hydroxysuccinimide (NHS). The scaffolds exhibited superabsorbent property, excellent cytocompatibility, and elasticity in the wet state as well as promoted cartilage regeneration.
Fig. 11.
Schematic illustration for 3D scaffold preparation. Reprinted from Ref. [49].
3D scaffolds exhibit hierarchical nanofibrous structures with interconnected macro-pores and superabsorbent properties, which is also suitable for bone repair. In bone tissue engineering, osteogenic growth factors play a crucial role in promoting new bone formation and regulating cell behaviors. Therefore, osteogenic growth factors can be physically adsorbed, covalently linked, or ensconced within 3D scaffolds to impart osteoinductive properties to the scaffolds. Ye et al. [50] fabricated electrospun 3D nanofibrous scaffolds to explore their potential application in bone tissue engineering. Pre-fabricated electrospun hydroxyapatite (nHA)/PLA/GEL nanofibers were processed into 3D scaffolds and then immobilized with synthetic bone morphogenic protein 2 (BMP-2)-derived peptides via a polydopamine (pDA)-assisted coating strategy to obtain nHA/PLA/GEL-PEP 3D nanofibrous scaffolds (Fig. 12).
Fig. 12.
Schematic of 3D nanofibrous scaffold preparation. Reprinted from Ref. [50].
In vitro and in vivo results demonstrated that the 3D nanofibrous scaffolds incorporated with nHA and BMP-2 peptides exhibited favorable biocompatibility and osteoinductivity. Weng et al. [51] developed ultralight 3D hybrid nanofiber aerogels composed of electrospun PLGA/collagen/gelatin. Strontium-copper (Sr–Cu) co-doped bioactive glass fibers, BMP-2 derived peptide, and a heptaglutamate domain (E7) molecule were encapsulated into scaffolds. The biocompatibility of scaffolds was evaluated in a cranial bone defect model. The results showed that the degradable hybrid aerogels significantly enhanced bone healing and defect closure as demonstrated 8 weeks after implantation as compared to unfilled defects, which was ascribed to the sustained release of E7‐BMP‐2 peptide.
5. 3D printing of nanofiber scaffolds
3D printing is a promising technology to precisely control the pore size and achieve the required shape of 3D scaffolds. However, most scaffolds based on 3D printing lack the surface structure to emulate the natural ECM. One pioneer study enabling the scaffold surface with fibrous structures has attempted to print 3D scaffolds with short fiber ink generated from electrospun nanofibers. Another way called stable jet electrospinning (SJES) which combines electrospinning and 3D printing to produce aligned ultrafine fibers via strengthening the control of jet instability in the electrospinning process presents a robust methodology for the fabrication of 3D printed nanofibrous scaffolds.
5.1. 3D printing with short fiber ink
3D nanofibrous scaffolds obtained by the assembly of short nanofibers face major challenges in the uncontrollable pore structures and inaccurate profiles that must be addressed. It is therefore pertinent to control the external geometry of scaffolds for the reconstruction of tissues with specific shapes. Appropriate pores have a significant effect on the distribution of cells and the infiltration of nutrients, thus affecting cell growth, ECM deposition, and tissue regeneration. 3D printing technology is a promising technique to accurately control the 3D shape and the aperture size of scaffolds. However, at present, most scaffolds based on 3D printing lack the ability to emulate the ECM microstructure. To address the aforementioned issue, Chen et al. [53] combined 3D printing and freeze-drying to afford 3D-printed (3DP) scaffolds with the precisely controlled shape made from electrospun fiber-based inks. In detail, they prepared gelatin/PLGA nanofiber membranes by using electrospinning, which was then dehydrated at 180 °C, cut into small pieces, and homogenized in butanol to disperse the fiber. After evaporation of the solvent, the short fibers were mixed with HA and dispersed into poly(ethylene oxide) (PEO) solution to afford bio-inks. Finally, 3DP scaffolds were fabricated and crosslinked by using EDC/NHS, which showed good elasticity, shape memory, and promoted cartilage regeneration in vivo (Fig. 13). Based on the same principle, Chen et al. [54] decellularized cow scapular cartilage to produce the cartilage decellularized matrix (CDM) and produced the bio-inks as described above. As compared to CDM and Gel/PLGA based 3DP scaffolds, hybrid scaffolds containing both CDM and Gel/PLGA showed not only improved mechanical properties but also high biocompatibility ascribed to the CDM and facilitated cartilage regeneration.
Fig. 13.
Schematic of various 3Delectrospun fiber scaffolds. Reprinted from Ref. [53].
5.2. 3D printing with stable jet electrospinning
Another way to combine electrospinning with 3D printing is to employ stable jet electrospinning (SJES), which uses high molecular weight PEO as a fiber-forming component to eliminate the whipping motion of jet during the electrostatic spinning process to fabricate high-strength fibers. Yuan et al. [55] dissolved PLLA and PEO in trifluoroethanol and formed a stable fiber jet by electrospinning. The collection device can realize the movement in the X–Y direction (Fig. 14A), thus forming the electrospun 3D scaffold with pre-determined patterns (Fig. 14B). In vitro cell culture showed that the 3D scaffolds promoted cell proliferation and infiltration. Based on the same principle, Yi et al. [56] prepared highly-oriented silk fibroin (SF) fibers with anisotropic structures by using SJES. In order to eliminate the whipping motion of jet in traditional electrospinning, the silk fibroin spinning solution was prepared by doping with high molecular weight PEO. The in vitro cell assay revealed that scaffolds can support the adhesion, migration, and growth of induced pluripotent stem cell-derived mesenchymal stem cells along with the fiber axis orientation.
Fig. 14.
(A) Schematic of the stable jet printing setup. (B) The patterns used to program the X–Y stage for printing the electrospun fibers into 3D structures. Reprinted from Ref. [55].
6. Electrospray
In addition to aerogel and scaffolds, microspheres have garnered the significant attention of the research community owing to their ability to deploy a myriad of growth factors, cells, and biologics for tissue engineering applications. While self-assembly and thermally-induced phase separation driven microspheres can afford the aforementioned advantages, they cannot be fabricated from a wide variety of materials. Consequently, alternative approaches have been pursued to realize microspheres including electrospinning driven methods (Table 4).
Table 4.
Summary of electrospray, origami, centrifugal electrospinning.
| Polymer | Fabrication method | Biological evaluation | Key findings | Ref |
|---|---|---|---|---|
| PCL/gelatin/GelMA nanofiber microspheres (NMs) | Electrospray and surface conjugation techniques | In vitro | Osteogenic differentiation↑; Microvascularization↑ |
[56] |
| PCL/gelatin/GelMA; PLGA/collagen/BG | Electrospray | In vitro & s.c. implantation | Cell infiltration↑; Host tissue integration↑ |
[57] |
| PLCL | Electrospinning and origami | In vitro & s.c. implantation | Cell infiltration↑; Collagen remodeling↑; Enhancing endothelialization | [58] |
| PLCL | Electrospinning and origami | In vitro | The mechanical property↑; Self-sealing property↑ | [59] |
| PCL/nHA | Electrospinning and origami | In vitro | Intricate architectures | [60] |
| PCL, PS, PVP, TPU | Centrifugal electrospinning | ╳ | Rapid release of ensconced antibacterial drug | [61] |
Indeed, it has been demonstrated that electrospinning can be annexed with electrospraying to afford nanofibers microspheres, which involves the assembly of electrospinning-mediated short nanofibers into microspheres by employing electrospraying. John et al. [57] fabricated nanofibers microspheres by using the combination of PCL, gelatin, and gelatin methacrylate (GelMA). Noticeably, GelMA was employed to afford the photo-driven conjugation of osteoinductive and vasculogenic peptides with microspheres, which facilitated osteogenic differentiation of MSCs and the tube formation of HUVECs. Despite the stabilized mechanical properties originating from the vapor phase crosslinking by the glutaraldehyde, these microspheres exhibited nonporous morphology, which impeded the cellular infiltration into microspheres.
To further leverage the potential of electrospinning-mediated electrospray technology to develop open porous microspheres, the same group [58] employed gas bubbles driven co-axial electrospraying of short nanofibers; the gas bubbles comprised the core whereas the short nanofibers comprised the shell of microspheres (Fig. 15). Indeed, a multitude of materials was employed to afford open porous nanofiber microspheres. Expectedly, open porous microspheres led to the complete cellular infiltration and vascularization into subcutaneous site 2 weeks after implantation. By contrast, cells resided superficially only in the case of nonporous microspheres.
Fig. 15.
Schematic illustration of the fabrication of NMs using coaxial electrospray and their potential applications. i) Coaxial electrospraying a nanofiber segment-containing solution in the shell and air in the core. ii) Freeze-drying NMs. iii) Cross-linking NMs. iv) Seeding cells to porous NMs. v) Using as a filler to the tissue defect. vi) Using as a carrier for cell delivery. Reprinted from Ref. [58].
7. Origami and cell sheet engineering
Similarly, efforts have also been made to fabricate intricate-shaped nanofibrous scaffolds which can better emulate the ECM, which include cell sheet engineering as well as origami-like methods. Lee et al. [59] fabricated 3D electrospun scaffolds by using cell sheet engineering. PLCL based electrospun membranes were co-seeded with human dermal fibroblasts (HDFs) and HUVECs on the opposite sides and then rolled along a tubular-shaped template to afford tubular scaffolds. In vitro analysis revealed capillary-like network formation in HDFs and HUVECs co-seeded grafts than that of the HDFs seeded grafts only. Similarly, ECM deposition was favored by the co-seeded scaffolds than that of the HDFs only group. The subcutaneous implantation of grafts for up to 14 days also revealed substantial improvements in the cell infiltration in the HDFs and HUVECs co-seeded vascular grafts. Mun et al. [60] have employed cell-sheet engineering to fabricate small-diameter vascular grafts, which showed vascular regeneration potential in vivo.
The same technique can also be extended to afford intricately shaped scaffolds with well-defined shapes and structures. Consequently, Song et al. [61] have employed the origami method to fabricate cell-laden 3D scaffolds. Electrospun membranes were fabricated by using PCL and hydroxyapatite, which were then seeded with the human fetal osteoblasts on both sides of membranes and stacked layer-by-layer by using a mold (Fig. 16). The origami-assisted culture of cell sheets afforded 3D like structure composed of cells and scaffold. However, the in vivo results were not demonstrated. Despite the obvious potential of cell sheet engineering and origami-like techniques to afford 3D electrospun scaffolds as well as intricately shaped structures, their clinical translation is impeded by the extensive in vitro manipulations.
Fig. 16.
Illustration of the construction process of the 3D nanofiber scaffold/cells complex. The inset is the nanofiber box prepared by origami. Reprinted from Ref. [61].
8. Centrifugal electrospinning and other methods
To better mimic the in vivo microenvironment, especially for bionic scaffolds including cardiovascular, ligaments, and tendons and to afford the alignment of nanofibers, centrifugal electrospinning has been put forward, which employs centrifugal force in addition to the electric force to drive the jet towards the collector. Wang et al. [62] have employed centrifugal electrospinning to fabricate hybrid scaffolds composed of PS and polyvinyl pyrrolidone (PVP), which permitted the rapid release of the ensconced antibacterial drug. The authors further employed centrifugal electrospinning to afford either individual or combination fibers of PCL, PS, PVP, and thermoplastic polyurethane (TPU).
Electrospinning can also enable fibers of different shapes and structures, which may permit tissue microenvironment-responsive drug release. Given the fact that hydrophilic therapeutics cannot be well incorporated into otherwise hydrophobic depots, a core-shell structured deploying agent may obviate these limitations. For instance, Zhao et al. [16] have demonstrated improved tendon healing with minimal adsorption owning to the controllable release of Mitomycin-C (MMC) from core-shell PLLA nanofibers. Hyaluronic acid was employed to form hydrosols containing MMC, which were then emulsified with PLLA solution to afford core-shell PLLA fibers consisting of MMC core and PLLA sheath. Owning to the distinct release profile of fast release at the initial time points and the steadier release at the later time points, nanofibrous scaffolds offered a unique platform for tendon healing while avoiding peritendinous adhesion through the inhibition of fibroblast's proliferation and lowering of the collagen secretion. Similarly, composite nanofibers can be fabricated to ascertain the stimuli-responsive drug release, which provides an amenable platform as compared to the protonation and deprotonation enabling coatings or co-solvent electrospinning plausibly due to the tedious experimental procedures. To afford, pH-responsive doxorubicin (DOX) delivery from mesoporous silica nanoparticles (MSNs) ensconced within PLLA nanofibers, MSNs were coated with calcium carbonate, which may give off CO2 at acidic pH. This in turn will enhance the penetration of water molecules into the nanofibers and further promote the release of the encapsulated drug for cancer elimination. In a seminal study, Zhao et al. [17] showed that nanofibrous scaffolds containing pH-activatable MSNs exhibited significantly higher potential for the cancer elimination in vitro and in vivo due to the sustained drug release from the CaCO3-gated MSNs. More intriguingly, these nanofibrous scaffolds were demonstrated to promote tumor necrosis in a subcutaneous site containing liver cancer. Similarly, the modification of nanofibrous scaffolds is widely pursued to achieve tunable growth factor delivery and biocompatibility. Whereas an array of functionalization approaches can be employed to instill the aforementioned properties into nanofibrous scaffolds, multiple chemical modification steps may compromise the physicochemical properties of nanofibrous scaffolds. Easily-operable methods permit not only precise surface modification but also help achieve tunable drug/growth factor delivery as well as achieve biocompatibility.
Mussel-inspired surface modification has proven to be a versatile technology for surface modification and has been employed to introduce a wide variety of growth factors and bioactive molecules on nanofibrous scaffolds. Cheng et al. [18] have modified PLLA nanofibers with different types of molecules, such as polyethylene glycol (PEG), RGD, and basic fibroblast growth factor (bFGF) by using poly(dopamine) coating and demonstrated that the structural and morphological properties of scaffolds remained preserved post-modification. These types of hybrid scaffolds could find applications for drug/growth factor delivery as well as enhancing cellular signaling and the biocompatibility.
9. Conclusions and future outlook
In summary, we introduced various advanced methods for fabricating electrospun 3D nanofiber aerogels and scaffolds (Table 5).
Table 5.
Comparison among 3D fabrication technologies.
| Gas foaming method | Mechanism | Gas bubbles generated in situ either via a chemical reaction or solubility difference of CO2 in ethanol and water and depressurization of subcritical CO2 fluid |
| Advantages | Increase the pore size and porosity of scaffold; Promote cell infiltration migration, proliferation, and angiogenesis |
|
| Disadvantages | Most scaffolds are restricted of weak mechanical strength; Imprecise thickness control and cause inflammatory; response in vivo; Hard to be used as a carrier of drugs or factors |
|
| Applications | Nerve regeneration; Hemostasis; Wound healing; In vitro tissue models | |
| Ref | [[31], [32], [33], [34], [35], [36], [37], [38]] | |
| Direct electrospinning | Mechanism | Collect scaffold directly with a special 3D shaped device; Use a thumbtack as collector producing 3D silica fibrous scaffold via self-assembly; Use the sol-gel solution to produce a bioactive 3D scaffold |
| Advantages | Promote biomineralization and cell infiltration; Reduce bulk density | |
| Disadvantages | Uncontrolled shape; Weak mechanical property | |
| Applications | Bone regeneration; Oils absorption | |
| Ref | [26,[41], [42], [43]] | |
| Short nanofibers assembling into 3D aerogels/scaffolds | Mechanism | Cut the nanofibrous membrane into small pieces, and then uniformly disperse the small pieces in the medium using a homogenizer. In general, short fibers are introduced into aerogels/scaffolds to enhance their structural stability or serve as an ECM template to provide a suitable microenvironment for cell growth and proliferation. |
| Advantages | Possess various excellent properties such as elastic resilience, energy absorption, shape memory, superabsorbent, and high-pressure sensitivity | |
| Disadvantages | Some scaffolds are non-biodegradable, hydrophobic and use toxic cross-linking agents; Complex preparation process | |
| Applications | Osteoporotic; Bone regeneration; Cartilage regeneration; Cranial bone regeneration; Thermal insulation; Sound absorption; Emulsion separation | |
| Ref | [[44], [45], [46], [47], [48], [49], [50], [51]] | |
| 3D printing | Mechanism | Print 3D scaffolds with short fiber ink enabling the scaffold surface with fibrous structures; Use stable jet electrospinning (SJES) to produce aligned ultrafine fibers via strengthening the control of jet instability |
| Advantages | Controlled shape; Promote cell proliferation, infiltration, adhesion and migration | |
| Disadvantages | Complex preparation process; Various parameters need to be controlled | |
| Applications | Cartilage regeneration; Anisotropic tissue regeneration | |
| Ref | [[52], [53], [54], [55]] | |
| Electrospray | Mechanism | Homogenize electrospun nanofiber mats to generate nanofiber segments and electrospray the crosslinker containing nanofiber segment solution into liquid nitrogen to obtained microspheres. |
| Advantages | Fabricate NMs from any material feasible for electrospinning | |
| Disadvantages | Complex preparation process; Crosslinking agent toxicity | |
| Applications | Biomimetic and injectable carrier; Osteogenesis; Angiogenesis; Tissue filling; Cell and drug delivery | |
| Ref | [56,57] | |
| Origami and cell sheet engineering | Mechanism | Cells were seeded on both two sides of the electrospun nanofiber film and use the co-cultured membrane to fabricate bio-tubular scaffolds or nanofiber boxes via origami. |
| Advantages | Cell infiltration; Intricate architectures | |
| Disadvantages | Complex preparation process; High requirements for operators | |
| Applications | 3D tissue construction; Vascular grafts regeneration | |
| Ref | [[58], [59], [60]] | |
| Centrifugal electrospinning | Mechanism | Use an electrospun rotating spinneret and obtain nanofiber from a conductive iron circular collector surrounding the spinneret. |
| Advantages | Promote production rates; High orientation | |
| Disadvantages | Require special equipment; Few types of the fiber structure | |
| Applications | Mass production of electrospun fibers; Drug release | |
| Ref | [61] |
A large number of synthetic and natural materials can be used in electrospinning and nanofibers found applications in various fields, such as catalysis, drug delivery, air, and liquid filtration, regenerative medicine and tissue engineering, biosensing, and food safety. Electrospinning is a simple fabrication technique, which permits the design of an array of scaffolds with different shapes and structures, whose physicochemical and morphological properties can be precisely tuned, besides core-shell type fibers, hybrid fibers, aligned fibers can be fabricated. The fiber surfaces can also be modified or combined with nanoparticles containing growth factors or drugs; thus further leveraging the potential of electrospinning technology for the tissue repair process.
Compared with the closely-packed 2D electrospun membranes, 3D aerogels and scaffolds feature larger surface area, controllable pore diameter, and inter-pore channels. Gas foaming as a simple and rapid method increases the pore size of the 2D electrospun membranes and affords highly porous 3D scaffolds which can then promote cell infiltration and nutrients exchange. Direct electrospinning of 3D fibers simplifies the process and could afford flexible 3D scaffolds to be applied for complex defects. Aerogels and scaffolds prepared by short fibers form micro/macro pores with adjustable density and controllable shape. Short fibers based printing solves the problem of lack of ECM emulation in 3D printed scaffolds. 3D aerogels and scaffolds prepared based on electrospinning technology have turned out to be superior in the field of functional materials, especially in biomedical materials, sensors, thermal insulation, sound absorption, emulsion separation, and other fields.
Though different types of methods can be employed for the fabrication of 3D scaffolds including gas foaming, short fibers self-assembly, direct electrospinning, 3D printing, electrospray, origami and cell sheet engineering, centrifugal electrospinning and other methods, every method has associated advantages and limitations. Whereas gas foaming methods do not allow for the reproducible fabrication of 3D scaffolds from 2D electrospun membranes, short-fiber based self-assembly requires additional processing steps post-electrospinning. Similarly, 3D printing processes require bio-ink and sophisticated printers as well as they are limited in terms of the selection of the material. Nonetheless, injectable and self-healing hydrogels may have obvious advantages for the printing of tissue-engineered scaffolds. These 3D materials prepared by electrospinning technology may face many problems to be solved urgently. The theoretical analysis of the formation process should be supplemented. For example, in the process of gas foaming, great efforts are still needed to probe the relationship between the concentration of the foaming agent and the physicochemical properties of 3D scaffolds including pore size, porosity, and surface roughness of scaffolds. Despite significant advances in the fabrication of electrospinning-based 3D aerogels and scaffolds, the limited output has yet to be overcome to achieve industrial-scale fabrication of nanofibrous materials, which limit the translation of electrospinning based products. Therefore, the development of production equipment for such fibers is necessary to realize a large-scale production in the future. Moreover, the application of the final products is still at its infancy, especially for regenerative medicine and tissue engineering applications. Well-executed in vitro and in vivo studies are warranted to decipher the long-term biological safety, stability, and application of nanofibrous scaffolds. In addition, the sudden release of biologics remains a recurring problem of electrospun 3D aerogels and scaffolds, which are employed as depots for drug/growth factors delivery. The limitation infiltration of host cells, as well as the mismatch of mechanical properties between the scaffolds and native vasculature, remains a roadblock for the in vivo applications of electrospinning based scaffolds. Moreover, the degradation rate of 3D scaffolds should commensurate with the tissue regenerative process and the degradation components should not cause an inflammatory response and be excreted from the body if the same is meant as a temporary scaffold for tissue regeneration, drug delivery, and cell transplantation.
Another problem that needs to be solved is to precisely control the macropores and architecture of the scaffold using 3D electrospinning. Since the cell infiltration and tissue regeneration depend upon the morphology and architecture of nano/microfibers, it is imperative to precisely control the fibers' thickness, pore dimensions, and porosity of electrospun scaffolds. The ECM architecture also depends upon the type of tissues. For instance, ligament, tendon, cardiac, muscle, and nerve tissues have been shown to possess aligned fibers' morphology [63]. Whereas electrospinning enables ECM-mimetic microstructure of scaffolds, small-sized pores hinder the cell infiltration and/or matrix deposition. Moreover, due to densely-spaced fibers’ architecture, electrospun scaffolds possess less porosity, which hampers tissue regeneration. A myriad of strategies has been pursued to increase the porosity and pore size of electrospun scaffolds including the use of leachable porogen, such as sugar crystals and salt particles, blending of sacrificial polymer along with the required polymer component, modification of collector apparatus, and post-treatment by laser irradiation [[64], [65], [66]]. It has also been demonstrated that the porosity and pore size of electrospun scaffolds can be increased by increasing the thickness of fibers, which then improved the cellular infiltration, neo-tissue regeneration as well as induced macrophages polarization [67,68]. To further emulate blood vessel morphology, layered scaffolds have been fabricated which offer precise control over the luminal or abluminal microstructure of fibers. Nanofibrous scaffolds exhibiting aligned nanofibers have also been prepared by increasing the collector speed in electrospinning and reported to improve the spreading of cardiomyocytes [69].
The precise engineering of the mechanical properties of scaffolds is also a very important consideration especially for the in vivo applications. For example, for bone and tendon tissue engineering, regenerative scaffolds require appropriate biomechanical properties to endure in vivo stress profile including the higher modulus large stress. If scaffolds are meant to be used for cartilage repair, they should possess good elasticity to withstand multiple cycles of compression and shape-recovery to maintain the stable morphology. On the other hand, for vascular reconstruction, scaffolds should possess sufficient mechanical properties as commensurate with the biomechanical properties of the native vasculature. Inherently, 3D scaffolds fabricated by using direct electrospinning possess weak mechanical properties and need precise engineering to well-fit the host tissue/organ. Nonetheless, these scaffolds can be suitable candidates for soft tissue regeneration including wound healing and/or homeostasis. Therefore, the authors are of the view that the mechanical properties of electrospun scaffolds should be precisely engineered keeping in view the in vivo microenvironment and the same can be tuned either by co-blending polymers or nanomaterials containing sufficient mechanical properties or by treating the scaffolds post-electrospinning such as crosslinking and/or another physical process. All of these bottlenecks are expected to be solved by novel technologies and approaches to facilitate the wider development and application of 3D nanofiber aerogels and scaffolds.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgments
This research was supported by the Fundamental Research Funds for the Central Universities (Grant No. 2232019A3-07), National Key Research Program of China (2016YFC1100202); National Natural Science Foundation of China (No.31771023); Science and Technology Commission of Shanghai Municipality (No. 19441902600), and a startup research grant of Higher Education Commission (HEC), Pakistan (Project No. 2089).
Biographies

Dr. Xiumei Mo is a professor at Donghua University. She once had two years of Postdoc experience at Kyoto University, three years of research fellow experience at the National University of Singapore, a one-year visiting professor experience at Aachen University of Applied Science and Technology. In Donghua University she was granted 30 more projects related to nanofiber fabrication for different tissue regeneration. She has published more than 300 papers. ISI Web of Science showed that she ranking No.7 in the world on electrospinning nanofiber publication. She got the Science Technical Invention Awards from Shanghai Municipality in 2008, Science and Technology Progress Awards from the State Department of People's Republic of China in 2009, Nature Science Awards from Shanghai Government in 2015. She is the committee member of China Biomaterials Society as well as the Biomedical Engineering Society Biomaterials Branch.

Yujie Chen, a Doctoral student, born in Huzhou, Zhejiang province. In 2018, he graduated from Jiaxing University with a bachelor's degree in Polymer Materials and Engineering. In the same year, he entered the Laboratory of Biomaterials and Tissue Engineering, Department of Bioengineering, Donghua University for his master's degree. The following year, he passed the examination of the master. His main topic is the research of three-dimensional electrospun nanofiber scaffolds and its application for cartilage repair.

Dr. Muhammad Shafiq has received his BS and MS degrees both from Pakistan. In 2018, Dr. Shafiq received his Ph.D. degree (summa cum laude) in Biomedical Engineering from University of Science & Technology (UST), South Korea while working under the mentorship of Dr. Soo Hyun Kim. He is now an Assistant Professor at the Department of Chemistry, PIEAS, Islamabad, Pakistan working in the field of polymeric biomaterials, self-assembled materials, and nanostructured materials for stem cell bioengineering, regenerative medicine, and tissue engineering as well as environmental remediation. Dr. Shafiq has authored over 30 peer-reviewed papers and 2 book chapters. He has been conferred with several prizes, awards, and honors including TERMIS-AP student travel award and Grand Prize from UST, academic excellence award from KIST, South Korea, and Research Productivity Award from Pakistan Council for Science & Technology.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Muhammad Shafiq, Email: shafiq@pieas.edu.pk.
Xiumei Mo, Email: xmm@dhu.edu.cn.
References
- 1.Sehaqui H., Ezekiel Mushi N., Morimune S., Salajkova M., Nishino T., Berglund L.A. Cellulose nanofiber orientation in nanopaper and nanocomposites by cold drawing. ACS Appl. Mater. Interfaces. 2012;4:1043–1049. doi: 10.1021/am2016766. [DOI] [PubMed] [Google Scholar]
- 2.Ma M., Kuang Y., Gao Y., Zhang Y., Gao P., Xu B. Aromatic-aromatic interactions induce the self-assembly of pentapeptidic derivatives in water to form nanofibers and supramolecular hydrogels. J. Am. Chem. Soc. 2010;132:2719–2728. doi: 10.1021/ja9088764. [DOI] [PubMed] [Google Scholar]
- 3.Yan C., Chen G., Zhou X., Sun J., Lv C. Template-based engineering of carbon-doped Co3O4 hollow nanofibers as anode materials for lithium-ion batteries. Adv. Funct. Mater. 2016;26:1428–1436. doi: 10.1002/adfm.201504695. [DOI] [Google Scholar]
- 4.Zhao J., Han W., Tu M., Huan S., Zeng R., Wu H., Cha Z., Zhou C. Preparation and properties of biomimetic porous nanofibrous poly(l-lactide) scaffold with chitosan nanofiber network by a dual thermally induced phase separation technique. Mater. Sci. Eng. C. 2012;32:1496–1502. doi: 10.1016/j.msec.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 5.Teo W.E., Ramakrishna S. A review on electrospinning design and nanofibre assemblies. Nanotechnology. 2006;17 doi: 10.1088/0957-4484/17/14/R01. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z.G., Wang P.W., Wei B., Mo X.M., Cui F.Z. Electrospun collagen-chitosan nanofiber: a biomimetic extracellular matrix for endothelial cell and smooth muscle cell. Acta Biomater. 2010;6:372–382. doi: 10.1016/j.actbio.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Brenner E.K., Schiffman J.D., Thompson E.A., Toth L.J., Schauer C.L. Electrospinning of hyaluronic acid nanofibers from aqueous ammonium solutions. Carbohydr. Polym. 2012;87:926–929. doi: 10.1016/j.carbpol.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 8.Jin D., Hu J., Xia D., Liu A., Kuang H., Du J., Mo X., Yin M. Evaluation of a simple off-the-shelf bi-layered vascular scaffold based on poly(L-lactide-co-ε-caprolactone)/silk fibroin in vitro and in vivo. Int. J. Nanomed. 2019;14:4261–4276. doi: 10.2147/IJN.S205569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu T., Zheng H., Chen J., Wang Y., Sun B., Morsi Y., El-Hamshary H., Al-Deyab S.S., Chen C., Mo X. Application of a bilayer tubular scaffold based on electrospun poly(l-lactide-co-caprolactone)/collagen fibers and yarns for tracheal tissue engineering. J. Mater. Chem. B. 2017;5:139–150. doi: 10.1039/c6tb02484j. [DOI] [PubMed] [Google Scholar]
- 10.Panzavolta S., Gioffrè M., Focarete M.L., Gualandi C., Foroni L., Bigi A. Electrospun gelatin nanofibers: optimization of genipin cross-linking to preserve fiber morphology after exposure to water. Acta Biomater. 2011;7:1702–1709. doi: 10.1016/j.actbio.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 11.Rad L.R., Momeni A., Ghazani B.F., Irani M., Mahmoudi M., Noghreh B. Removal of Ni2+ and Cd2+ ions from aqueous solutions using electrospun PVA/zeolite nanofibrous adsorbent. Chem. Eng. J. 2014;256:119–127. doi: 10.1016/j.cej.2014.06.066. [DOI] [Google Scholar]
- 12.Ma J., He Y., Liu X., Chen W., Wang A., Lin C.Y., Mo X., Ye X. A novel electrospun-aligned nanoyarn/three-dimensional porous nanofibrous hybrid scaffold for annulus fibrosus tissue engineering. Int. J. Nanomed. 2018;13:1553–1567. doi: 10.2147/IJN.S143990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang K., Fu Q., Yoo J., Chen X., Chandra P., Mo X., Song L., Atala A., Zhao W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: an in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017;50:154–164. doi: 10.1016/j.actbio.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Kuang H., Wang Y., Hu J., Wang C., Lu S., Mo X. A method for preparation of an internal layer of artificial vascular graft Co-modified with salvianolic acid B and heparin. ACS Appl. Mater. Interfaces. 2018;10:19365–19372. doi: 10.1021/acsami.8b02602. [DOI] [PubMed] [Google Scholar]
- 15.Duan G., Koehn-Serrano M., Greiner A. Highly efficient reusable sponge-type catalyst carriers based on short electrospun fibers. Macromol. Rapid Commun. 2017;38:1–6. doi: 10.1002/marc.201600511. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X., Jiang S., Liu S., Chen S., Lin Z.Y.W., Pan G., He F., Li F., Fan C., Cui W. Optimization of intrinsic and extrinsic tendon healing through controllable water-soluble mitomycin-C release from electrospun fibers by mediating adhesion-related gene expression. Biomaterials. 2015;61:61–74. doi: 10.1016/j.biomaterials.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Zhao X., Yuan Z., Yildirimer L., Zhao J., Lin Z.Y., Cao Z., Pan G., Cui W. Tumor-triggered controlled drug release from electrospun fibers using inorganic caps for inhibiting cancer relapse. Small. 2015;11:4284–4291. doi: 10.1002/smll.201500985. [DOI] [PubMed] [Google Scholar]
- 18.Cheng L., Sun X., Zhao X., Wang L., Yu J., Pan G., Li B., Yang H., Zhang Y., Cui W. Surface biofunctional drug-loaded electrospun fibrous scaffolds for comprehensive repairing hypertrophic scars. Biomaterials. 2016;83:169–181. doi: 10.1016/j.biomaterials.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Zhu M., Han J., Wang F., Shao W., Xiong R., Zhang Q., Pan H., Yang Y., Samal S.K., Zhang F., Huang C. Electrospun nanofibers membranes for effective air filtration. Macromol. Mater. Eng. 2017;302:1–27. doi: 10.1002/mame.201600353. [DOI] [Google Scholar]
- 20.Homaeigohar S.S., Buhr K., Ebert K. Polyethersulfone electrospun nanofibrous composite membrane for liquid filtration. J. Membr. Sci. 2010;365:68–77. doi: 10.1016/j.memsci.2010.08.041. [DOI] [Google Scholar]
- 21.Sun B., Zhou Z., Li D., Wu T., Zheng H., Liu J., Wang G., Yu Y., Mo X. Polypyrrole-coated poly(l-lactic acid-co-ε-caprolactone)/silk fibroin nanofibrous nerve guidance conduit induced nerve regeneration in rat. Mater. Sci. Eng. C. 2019;94:190–199. doi: 10.1016/j.msec.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Zheng B., Liu G., Yao A., Xiao Y., Du J., Guo Y., Xiao D., Hu Q., Choi M.M.F. A sensitive AgNPs/CuO nanofibers non-enzymatic glucose sensor based on electrospinning technology. Sensor. Actuator. B Chem. 2014;195:431–438. doi: 10.1016/j.snb.2014.01.046. [DOI] [Google Scholar]
- 23.Wen P., Zhu D.H., Wu H., Zong M.H., Jing Y.R., Han S.Y. Encapsulation of cinnamon essential oil in electrospun nanofibrous film for active food packaging. Food Contr. 2016;59:366–376. doi: 10.1016/j.foodcont.2015.06.005. [DOI] [Google Scholar]
- 24.Blakeney B.A., Tambralli A., Anderson J.M., Andukuri A., Lim D.J., Dean D.R., Jun H.W. Cell infiltration and growth in a low density, uncompressed three-dimensional electrospun nanofibrous scaffold. Biomaterials. 2011;32:1583–1590. doi: 10.1016/j.biomaterials.2010.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chainani A., Hippensteel K.J., Kishan A., Garrigues N.W., Ruch D.S., Guilak F., Little D. Multilayered electrospun scaffolds for tendon tissue engineering. Tissue Eng. A. 2013;19:2594–2604. doi: 10.1089/ten.tea.2013.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun B., Li J., Liu W., Aqeel B.M., El-Hamshary H., Al-Deyab S.S., Mo X. Fabrication and characterization of mineralized P(LLA-CL)/SF three-dimensional nanoyarn scaffolds, Iran. Polym. J. (English Ed. 2014;24:29–40. doi: 10.1007/s13726-014-0297-9. [DOI] [Google Scholar]
- 27.Kim T.G., Chung H.J., Park T.G. Macroporous and nanofibrous hyaluronic acid/collagen hybrid scaffold fabricated by concurrent electrospinning and deposition/leaching of salt particles. Acta Biomater. 2008;4:1611–1619. doi: 10.1016/j.actbio.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 28.In K.S., Won H.S., Sang Y.L., Sang H.L., Seong J.H., Myung C.L., Lee S.J. Chitosan nano-/microfibrous double-layered membrane with rolled-up three-dimensional structures for chondrocyte cultivation. J. Biomed. Mater. Res. 2009;90:595–602. doi: 10.1002/jbm.a.32109. [DOI] [PubMed] [Google Scholar]
- 29.Sun B., Long Y.Z., Zhang H.D., Li M.M., Duvail J.L., Jiang X.Y., Yin H.L. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014;39:862–890. doi: 10.1016/j.progpolymsci.2013.06.002. [DOI] [Google Scholar]
- 30.Dehghani F., Annabi N. Engineering porous scaffolds using gas-based techniques. Curr. Opin. Biotechnol. 2011;22:661–666. doi: 10.1016/j.copbio.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Joshi M.K., Pant H.R., Tiwari A.P., kim H.J., Park C.H., Kim C.S. Multi-layered macroporous three-dimensional nanofibrous scaffold via a novel gas foaming technique. Chem. Eng. J. 2015;275:79–88. doi: 10.1016/j.cej.2015.03.121. [DOI] [Google Scholar]
- 32.Jiang J., Carlson M.A., Teusink M.J., Wang H., MacEwan M.R., Xie J. Expanding two-dimensional electrospun nanofiber membranes in the third dimension by a modified gas-foaming technique. ACS Biomater. Sci. Eng. 2015;1:991–1001. doi: 10.1021/acsbiomaterials.5b00238. [DOI] [PubMed] [Google Scholar]
- 33.Jiang J., Li Z., Wang H., Wang Y., Carlson M.A., Teusink M.J., MacEwan M.R., Gu L., Xie J. Expanded 3D nanofiber scaffolds: cell penetration, neovascularization, and host response. Adv. Healthc. Mater. 2016;5:2993–3003. doi: 10.1002/adhm.201600808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Q., Gu H., Zhao P., Zhang C., Cao M., Fu J., He Y. Fabrication of electrospun nanofibrous scaffolds with 3D controllable geometric shapes. Mater. Des. 2018;157:159–169. doi: 10.1016/j.matdes.2018.07.042. [DOI] [Google Scholar]
- 35.Zhang K., Bai X., Yuan Z., Cao X., Jiao X., Li Y., Qin Y., Wen Y., Zhang X. Layered nanofiber sponge with an improved capacity for promoting blood coagulation and wound healing. Biomaterials. 2019;204:70–79. doi: 10.1016/j.biomaterials.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Rao F., Yuan Z., Li M., Yu F., Fang X., Jiang B., Wen Y., Zhang P. Expanded 3D nanofibre sponge scaffolds by gas-foaming technique enhance peripheral nerve regeneration, Artif. Cells. Nanomed. Biotechnol. 2019;47:491–500. doi: 10.1080/21691401.2018.1557669. [DOI] [PubMed] [Google Scholar]
- 37.Jiang J., Chen S., Wang H., Carlson M.A., Gombart A.F., Xie J. CO2-expanded nanofiber scaffolds maintain activity of encapsulated bioactive materials and promote cellular infiltration and positive host response. Acta Biomater. 2018;68:237–248. doi: 10.1016/j.actbio.2017.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jing X., Li H., Mi H.Y., Liu Y.J., Tan Y.M. Fabrication of fluffy shish-kebab structured nanofibers by electrospinning, CO2 escaping foaming and controlled crystallization for biomimetic tissue engineering scaffolds. Chem. Eng. J. 2019;372:785–795. doi: 10.1016/j.cej.2019.04.194. [DOI] [Google Scholar]
- 39.Sun B., Long Y., Yu F., Li M., Zhang H., Li W., Xu T. 2012. Nanoscale Self-Assembly Of A Three-Dimensional Fibrous Polymer Sponge By Electrospinning; pp. 2134–2137. [DOI] [PubMed] [Google Scholar]
- 40.Teo W.E., Gopal R., Ramaseshan R., Fujihara K., Ramakrishna S. A dynamic liquid support system for continuous electrospun yarn fabrication. Polymer. 2007;48:3400–3405. doi: 10.1016/j.polymer.2007.04.044. [DOI] [Google Scholar]
- 41.Mi H.Y., Jing X., Huang H.X., Turng L.S. Instantaneous self-assembly of three-dimensional silica fibers in electrospinning: insights into fiber deposition behavior. Mater. Lett. 2017;204:45–48. doi: 10.1016/j.matlet.2017.05.128. [DOI] [Google Scholar]
- 42.Poologasundarampillai G., Wang D., Li S., Nakamura J., Bradley R., Lee P.D., Stevens M.M., McPhail D.S., Kasuga T., Jones J.R. Cotton-wool-like bioactive glasses for bone regeneration. Acta Biomater. 2014;10:3733–3746. doi: 10.1016/j.actbio.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Lin Y.Z., Bin Zhong L., Dou S., Shao Z.D., Liu Q., Zheng Y.M. Facile synthesis of electrospun carbon nanofiber/graphene oxide composite aerogels for high efficiency oils absorption. Environ. Int. 2019;128:37–45. doi: 10.1016/j.envint.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 44.Si Y., Yu J., Tang X., Ge J., Ding B. Ultralight nanofibre-assembled cellular aerogels with superelasticity and multifunctionality. Nat. Commun. 2014;5 doi: 10.1038/ncomms6802. [DOI] [PubMed] [Google Scholar]
- 45.Si Y., Wang X., Yan C., Yang L., Yu J., Ding B. Ultralight biomass-derived carbonaceous nanofibrous aerogels with superelasticity and high pressure-sensitivity. Adv. Mater. 2016;28:9512–9518. doi: 10.1002/adma.201603143. [DOI] [PubMed] [Google Scholar]
- 46.Si Y., Wang L., Wang X., Tang N., Yu J., Ding B. Ultrahigh-water-content, superelastic, and shape-memory nanofiber-assembled hydrogels exhibiting pressure-responsive conductivity. Adv. Mater. 2017;29:1–7. doi: 10.1002/adma.201700339. [DOI] [PubMed] [Google Scholar]
- 47.Wang L., Qiu Y., Guo Y., Si Y., Liu L., Cao J., Yu J., Li X., Zhang Q., Ding B. Smart, elastic, and nanofiber-based 3D scaffolds with self-deploying capability for osteoporotic bone regeneration. Nano Lett. 2019;19:9112–9120. doi: 10.1021/acs.nanolett.9b04313. [DOI] [PubMed] [Google Scholar]
- 48.Mader M., Jérôme V., Freitag R., Agarwal S., Greiner A. Ultraporous, compressible, wettable polylactide/polycaprolactone sponges for tissue engineering. Biomacromolecules. 2018;19:1663–1673. doi: 10.1021/acs.biomac.8b00434. [DOI] [PubMed] [Google Scholar]
- 49.Chen W., Chen S., Morsi Y., El-Hamshary H., El-Newhy M., Fan C., Mo X. Superabsorbent 3D scaffold based on electrospun nanofibers for cartilage tissue engineering. ACS Appl. Mater. Interfaces. 2016;8:24415–24425. doi: 10.1021/acsami.6b06825. [DOI] [PubMed] [Google Scholar]
- 50.Ye K., Liu D., Kuang H., Cai J., Chen W., Sun B., Xia L., Fang B., Morsi Y., Mo X. Three-dimensional electrospun nanofibrous scaffolds displaying bone morphogenetic protein-2-derived peptides for the promotion of osteogenic differentiation of stem cells and bone regeneration. J. Colloid Interface Sci. 2019;534:625–636. doi: 10.1016/j.jcis.2018.09.071. [DOI] [PubMed] [Google Scholar]
- 51.Weng L., Boda S.K., Wang H., Teusink M.J., Shuler F.D., Xie J. Novel 3D hybrid nanofiber aerogels coupled with BMP-2 peptides for cranial bone regeneration. Adv. Healthc. Mater. 2018;7:1–16. doi: 10.1002/adhm.201701415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ye K., Liu D., Kuang H., Cai J., Chen W., Sun B., Xia L., Fang B., Morsi Y., Mo X. Three-dimensional electrospun nanofibrous scaffolds displaying bone morphogenetic protein-2-derived peptides for the promotion of osteogenic differentiation of stem cells and bone regeneration. J. Colloid Interface Sci. 2019;534:625–636. doi: 10.1016/j.jcis.2018.09.071. [DOI] [PubMed] [Google Scholar]
- 53.Chen W., Xu Y., Liu Y., Wang Z., Li Y., Jiang G., Mo X., Zhou G. Three-dimensional printed electrospun fiber-based scaffold for cartilage regeneration. Mater. Des. 2019 doi: 10.1016/j.matdes.2019.107886. [DOI] [Google Scholar]
- 54.Chen W., Xu Y., Li Y., Jia L., Mo X., Jiang G., Zhou G. 3D printing electrospinning fiber-reinforced decellularized extracellular matrix for cartilage regeneration. Chem. Eng. J. 2019 doi: 10.1016/j.cej.2019.122986. [DOI] [Google Scholar]
- 55.Yuan H., Zhou Q., Li B., Bao M., Lou X., Zhang Y. Direct printing of patterned three-dimensional ultrafine fibrous scaffolds by stable jet electrospinning for cellular ingrowth. Biofabrication. 2015;7 doi: 10.1088/1758-5090/7/4/045004. [DOI] [PubMed] [Google Scholar]
- 56.Yi B., Zhang H., Yu Z., Yuan H., Wang X., Zhang Y. Fabrication of high performance silk fibroin fibers: via stable jet electrospinning for potential use in anisotropic tissue regeneration. J. Mater. Chem. B. 2018;6:3934–3945. doi: 10.1039/c8tb00535d. [DOI] [PubMed] [Google Scholar]
- 57.John J.V., Choksi M., Chen S., Boda S.K., Su Y., McCarthy A., Teusink M.J., Reinhardt R.A., Xie J. Tethering peptides onto biomimetic and injectable nanofiber microspheres to direct cellular response. Nanomed. Nanotechnol. Biol. Med. 2019;22 doi: 10.1016/j.nano.2019.102081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.John J.V., McCarthy A., Wang H., Chen S., Su Y., Davis E., Li X., Park J.S., Reinhardt R.A., Xie J. Engineering biomimetic nanofiber microspheres with tailored size, predesigned structure, and desired composition via gas bubble–mediated coaxial electrospray. Small. 2020 doi: 10.1002/smll.201907393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee B., Shafiq M., Jung Y., Park J.C., Kim S.H. Characterization and preparation of bio-tubular scaffolds for fabricating artificial vascular grafts by combining electrospinning and a co-culture system. Macromol. Res. 2016;24:131–142. doi: 10.1007/s13233-016-4017-5. [DOI] [Google Scholar]
- 60.Mun C.H., Jung Y., Kim S.H., Lee S.H., Kim H.C., Kwon I.K., Kim S.H. Three-dimensional electrospun poly(Lactide-Co-ε-Caprolactone) for small-diameter vascular grafts. Tissue Eng. A. 2012;18:1608–1616. doi: 10.1089/ten.tea.2011.0695. [DOI] [PubMed] [Google Scholar]
- 61.Song J., Zhu G., Gao H., Wang L., Li N., Shi X., Wang Y. Origami meets electrospinning: a new strategy for 3D nanofiber scaffolds. Bio-Design Manuf. 2018;1:254–264. doi: 10.1007/s42242-018-0027-9. [DOI] [Google Scholar]
- 62.Wang L., Ahmad Z., Huang J., Li J.S., Chang M.W. Multi-compartment centrifugal electrospinning based composite fibers. Chem. Eng. J. 2017;330:541–549. doi: 10.1016/j.cej.2017.07.179. [DOI] [Google Scholar]
- 63.Pham Q.P., Sharma U., Mikos A.G. Electrospun poly (ε-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7:2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]
- 64.Rnjak-Kovacina J., Weiss A.S. Increasing the pore size of electrospun scaffolds. Tissue Eng. B Rev. 2011;17:365–372. doi: 10.1089/ten.teb.2011.0235. [DOI] [PubMed] [Google Scholar]
- 65.Zhong S., Zhang Y., Lim C.T. Fabrication of large pores in electrospun nanofibrous scaffolds for cellular infiltration: a review. Tissue Eng. B Rev. 2012;18:77–87. doi: 10.1089/ten.teb.2011.0390. [DOI] [PubMed] [Google Scholar]
- 66.Tara S., Kurobe H., Rocco K.A., Maxfield M.W., Best C.A., Yi T., Naito Y., Breuer C.K., Shinoka T. Well-organized neointima of large-pore poly(l-lactic acid) vascular graft coated with poly(l-lactic-co-ε-caprolactone) prevents calcific deposition compared to small-pore electrospun poly(l-lactic acid) graft in a mouse aortic implantation model. Atherosclerosis. 2014;237:684–691. doi: 10.1016/j.atherosclerosis.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Z., Cui Y., Wang J., Yang X., Wu Y., Wang K., Gao X., Li D., Li Y., Zheng X.L., Zhu Y., Kong D., Zhao Q. The effect of thick fibers and large pores of electrospun poly(ε-caprolactone) vascular grafts on macrophage polarization and arterial regeneration. Biomaterials. 2014;35:5700–5710. doi: 10.1016/j.biomaterials.2014.03.078. [DOI] [PubMed] [Google Scholar]
- 68.Shafiq M., Zhang Q., Zhi D., Wang K., Kong D., Kim D.H., Kim S.H. In situ blood vessel regeneration using SP (substance P) and SDF (stromal cell-derived factor)-1α peptide eluting vascular grafts, arterioscler. Thromb. Vasc. Biol. 2018;38:E117–E134. doi: 10.1161/ATVBAHA.118.310934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li J., Minami I., Shiozaki M., Yu L., Yajima S., Miyagawa S., Shiba Y., Morone N., Fukushima S., Yoshioka M., Li S., Qiao J., Li X., Wang L., Kotera H., Nakatsuji N., Sawa Y., Chen Y., Liu L. Human pluripotent stem cell-derived cardiac tissue-like constructs for repairing the infarcted myocardium. Stem Cell Rep. 2017;9:1546–1559. doi: 10.1016/j.stemcr.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]