Graphical abstract
Keywords: Crude glycerol, Conversion route, Value-added products, Economic aspects, Environmental benefits and limitations
Highlights
-
•
Glycerol can be converted to value-added products using different catalytic processes.
-
•
Optimum process technology for glycerol conversion depends on specific reaction conditions.
-
•
Sustainability of the products developed depend upon economic output and chemical conversion is preferred.
-
•
Environmental benefits encourage biochemical routes over chemical routes.
Abstract
The enormous production of glycerol, a waste stream from biodiesel industries, as a low-value product has been causing a threat to both the environment and the economy. Therefore, it needs to be transformed effectively and efficiently into valued products for contributing positively towards the biodiesel economy. It can either be converted directly into competent chemicals or can be used as a feedstock/precursor for deriving valuable derivatives. In this review article, a technical evaluation has been stirred up, various factors and technologies used for producing value-added products from crude glycerol, Environmental and economic aspects of different conversion routes, cost factors and challenges of integration of the different routes for biorefinery have been reviewed and elaborated. There are tremendous environmental benefits in the conversion of crude glycerol via the biochemical route, the product and residue become eco-friendly. However, chemical conversions are faster processes, and economically viable if environmental aspects are partially ignored.
1. Introduction
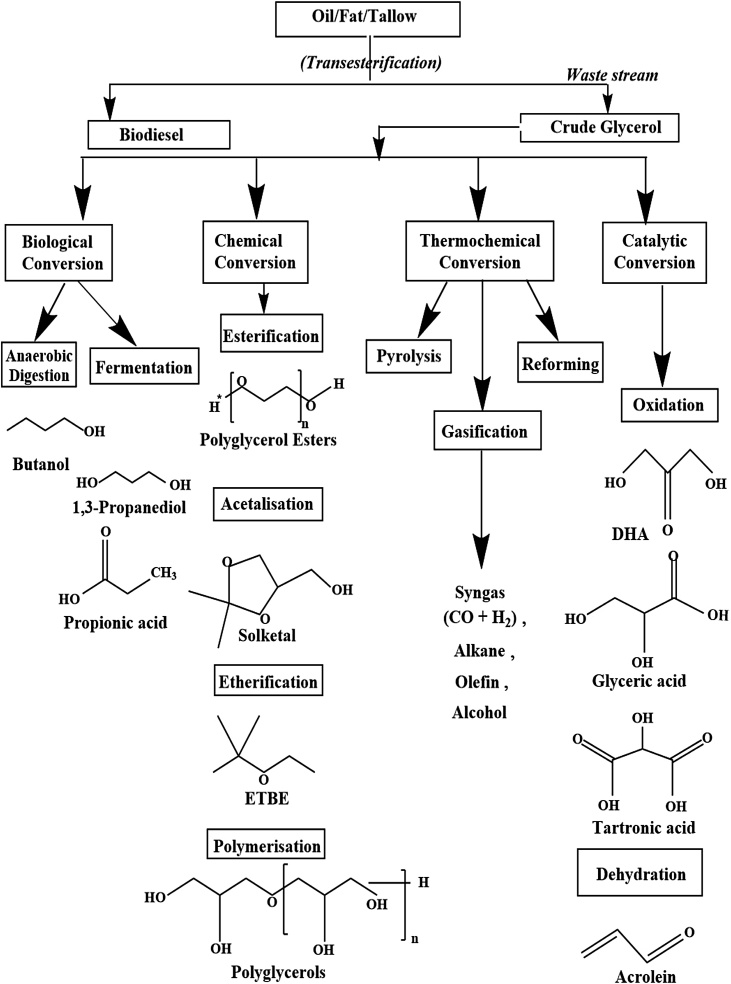
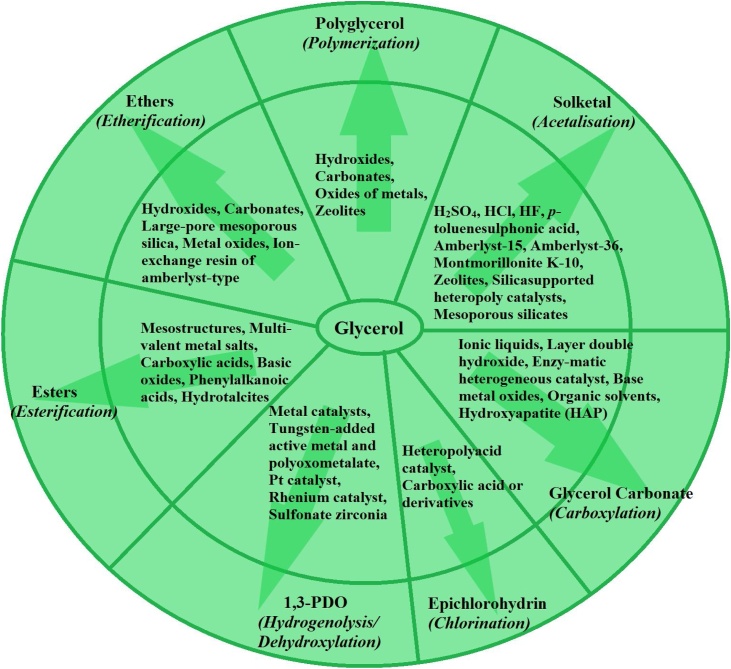
The raised petroleum prices, fuel crisis due to increasing population and unfavorable effects of emission of hazardous chemicals and greenhouse gases in the environment have stimulated the researcher for searching biofuel(s) as the alternative to fossil fuels [1]. Vegetable oilseed mainly consists of four components, viz. Shell, husk, oil (lipid) and oil-cake of which the nature of the shell and husk is identical to other loose lignocellulosic biomass; oil is a triglyceride in the liquid form while oil-cake is a cellulosic and protein-rich component [2]. The processing of lignocellulosic biomass can be either for direct combustion and power generation, 2G-bioethanol production, while oil-cake can be a feed material for animals and fish or can be used as feedstock for biogas generation. Oil if non-edible, is mostly used for biodiesel production, pharmaceuticals or for other chemical industry applications. Many research articles and reviews are available in the literature for biomass combustion, biogas production from oilcake, and for biodiesel production via transesterification [[3], [4], [5]]. Biodiesel is one such renewable biofuel derived from lipids with easily accessible conditions and chemical reactions [6]. It has the competence to overcome environmental and ecological disturbances. Biodiesel, popularly termed as fatty acid methyl ester (FAME), is basically derived from the process called transesterification of triglycerides (vegetable oils, animal tallow/fats, waste cooking oils, edible plants, biomass, microalgae) with an alcohol either methanol or ethanol using a suitable basic or acidic catalyst [[7], [8], [9]]. The reaction can be catalyzed either homogeneously or heterogeneously [9]. During this production process, two phases, i.e. upper phase called biodiesel (methyl or ethyl esters) and the lower phase called crude glycerol are formed, which are then separated easily after resting the reaction mixture for a few hours at neutral pH [7]. Different pathways through which crude glycerol can be processed into different valuable products are as shown in Fig. 1.
Fig. 1.
Derivatives and chemicals produced from glycerol.
Jatropha oil in South-East Asian countries, Soybean oil in USA, Rapeseed oil in Europe are the main potential sources for biodiesel production due to their availability [3,10]. The hypothetical concept of biorefinery plant is also linked via biodiesel production [11]. The term “bio-refinery,” supplemented recently to describe for the provision of converting biomass to food, fuel, and valuable chemicals [12]. After transesterification reaction, almost equal volume of biodiesel and about 1/10th volume of glycerol is obtained in a well-set biodiesel industry. However, mostly the glycerol obtained is the crude one which needs further purification for the generation of other value-added chemicals or derivatives. These chemicals are further coupled with the biodiesel industry to potentially realize a biorefinery set up.
Glycerol, also known as 1,2,3-propanetriol, is a compound and a resource of the oleo-chemical industry. Glycerol is commonly called glycerine. It is the primary co-product synthesized from the biodiesel production and its production is about 10 wt. % of the amount of biodiesel produced, i.e. around 0.10 kg of crude glycerol is generated per kg of biodiesel [13]. Also, it can be produced from saponification (soap production) and hydrolysis of fatty acids. In the current scenario, 10 % of glycerol is generated from hydrolysis, 12 % glycerol from saponification and 50–80 % glycerol from the transesterification process [13]. Thus, the report states that 66 % of world’s glycerol comes only from the biodiesel industry. The world-wide production of glycerol will be approximately 41.9 billion liters [14]. Therefore, utilization of the glycerol as a raw material using sustainable processes is of vital importance. As a means for literature review for conversion of glycerol into value added products, recently published articles in the topic are as listed in Table 1.
Table 1.
List and scope of review articles published for glycerol valorisation to value added products.
| S. No. | Title of article | Scope and attribute | Authors |
|---|---|---|---|
| 1. | Glycerol acetals and ketals as possible diesel additives. A review of their synthesis protocols | Different approaches and techniques used to obtain glycerol acetals and ketals | Ancuţa Roxana Trifoi, Paul Şerban Agachi, Timea Pap (2016) |
| 2. | Catalytic vaporization of raw glycerol derived from biodiesel: a review |
Role of heterogeneous catalyst on the valorisation of glycerol | Sravanthi Veluturla, Narula Archna, D. Subba Rao, N. Hezil, I.S. Indraja & S. Spoorthi (2017) |
| 3. | Glycerol Production and Transformation: A Critical Review with Particular Emphasis on Glycerol Reforming Reaction for Producing Hydrogen in Conventional and Membrane Reactors | Focus on glycerol reforming reactions, catalysts developed for use, membrane catalytic reactors | Giuseppe Bagnato, Adolfo Iulianelli, Aimaro Sanna and Angelo Basile (2017) |
| 4. | Oxygenated fuel additives from glycerol valorization. Main production pathways and effects on fuel properties and engine performance: A critical review | Transformation of glycerol into oxygenated fuel additives and discussion of fuel properties and other characteristics | A. Cornejo, I. Barrio, M. Campoy, J. Lázaro, B. Navarrete (2017) |
| 5. | Review on enzymatic synthesis of value added products of glycerol, a by-product derived from biodiesel production | Different bioconversion technologies of crude-glycerol, relevant approach for the production of various chemicals from bio-glycerol over enzyme and chemical catalysts | J. Pradima, M. Rajeswari Kulkarni, Archna (2017) |
| 6. | Continuous Flow Conversion of Glycerol into Chemicals: An Overview | Glycerol valorization in liquid phase continuous flow systems using different types of catalysts and processes |
Christophe Len, Frederic Delbecq, Cristobal Cara Corpas, Encarnacion Ruiz Ramos (2017) |
| 7. | Environmental and economical perspectives of a glycerol Biorefinery | Chemical research and process design in the development of CO2- and other biorefineries | Giacomo M. Lari, Giorgio Pastore, Moritz Haus, Yiyu Ding, Stavros Papadokonstantakis, Cecilia Mondelli and Javier Pérez-Ramírez (2018) |
| 8. | Techno-Economic Analysis of a Glycerol Biorefinery | Environmental and economic assessment of a glycerol biorefinery by including operating costs |
Sebastiano C. D’Angelo, Agostino Dall’Ara, Cecilia Mondelli, Javier Pérez-Ramírez and Stavros Papadokonstantakis (2018) |
| 9. | A Review on the Catalytic Acetalization of Bio-renewable Glycerol to Fuel Additives | Focus on innovative and potential technologies for sustainable production of solketal | Amin Talebian-Kiakalaieh, Nor Aishah Saidina Amin, Neda Najaafi and Sara Tarighi (2018) |
| 10. | Glycerol from biodiesel production: Technological paths for sustainability | Analysis of patents related to the use of glycerol in the period from 1993 to 2015 | Marcos Roberto Monteiro, Cristie Luis Kugelmeier, Rafael Sanaiotte Pinheiro, Mario Otávio Batalha, Aldara da Silva César (2018) |
| 11. | Glycerol valorization under continuous flow conditions-Recent advances | Glycerol valorization to valuable products under liquid phase continuous flow systems using different types of catalysts and processes | Rajender S. Varma, Christophe Len (2018) |
| 12. | Spotlight on biodiversity of microbial cell factories for glycerol conversion | Biodiversity of naturally glycerol consuming Microorganisms - impact and importance | Hannes Russmayer, Michael Egermeier, Denis Kalemasi, Michael Sauer (2019) |
| 13. | Sustainable value-added C3 chemicals from glycerol transformations: A mini review for heterogeneous catalytic processes | Progress on sustainable C3 chemical production from catalytic glycerol transformations | Yuan Wang, Yang Xiao, Guomin Xiao (2019) |
The hygroscopicity and water-soluble nature of glycerol are due to its three-backbone containing carbon atoms along with hydrophilic hydroxyl groups as shown in (Fig. 2) [15]. This unique structure of glycerol is responsible for its properties, making it a prominent material for numerous applications [16]. Glycerol without any chemical treatment and purification is in crude form, while after purification it is in pure form. Pure and commercially synthesized glycerol has almost the same qualities because of their applications [17]. The purity of crude glycerol is 60–80 %, while pure or synthesized one has 100 % pure. Crude glycerol obtained contains a lot of impurities such as alcohol, organic and inorganic salts, heavy metals, water, soap, mono, and di-glyceride traces, matter organic non-glycerol (MONG), fatty acid methyl esters (FAME), fatty acid esters (FAE), free fatty acids (FFA), polyol, and ash [13]. Different quality and grades of glycerol are commercially available throughout the globe. Analytical grade glycerol is the highest purity glycerol having >98 % purity without any contaminants. USP (United States Pharmacopeia) grade is 96–99.5 % pure, whose generic use is in food, pharmaceuticals, personal care and cosmetics [18]. A comparison of the physicochemical properties of crude and pure glycerol is shown in Table 2.
Fig. 2.
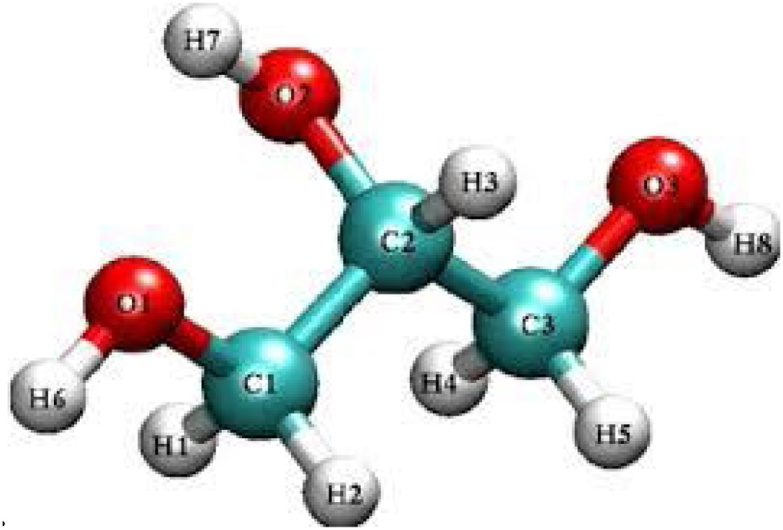
Molecular structure of glycerol.
Table 2.
Comparison of the properties of crude and pure glycerol [19].
| Common properties |
Uncommon properties |
||||
|---|---|---|---|---|---|
| Property | Crude glycerol | Pure glycerol | Property | Crude glycerol | Pure glycerol |
| Density (g/cm3) | 1.01to 1.20 | 1.31 | Odour | – | Odourless |
| pH | 2.0 to 10.8 | 6.4 | Methanol content | 6.2–12.6% | – |
| Viscosity (mPa.s) | 1213 | 930 | FFA content | 35.7–96.4 % | – |
| Colour | Dark brown | Colourless | FAME content | 5.2–51.6 % | – |
| Glycerol content | 22.9–63 % | > 98.7 % | Soap content | 20.5–31.4 % | – |
| Water content | 1 to 28.7 % | – | |||
| Ash content | 2.7 to 5.7 % | – | |||
| Melting point (o C) | – | 17.8 | |||
| Boiling point (o C) | – | 290 | |||
| Flash point (o C) | – | >400 | |||
| Vapor pressure (mmHg) | – | 0.13 | |||
| Solubility | – | Miscible in water | |||
Pure glycerol is commercially the chief feedstock such as for the production of food additives, surfactants, lubricants, beverages, pharmaceuticals, cosmetics, textiles and many more. However, on both small as well as large scale, the purification process of crude glycerol is a very exorbitant [7,20. In addition to pure/high-quality glycerol, an abundance of impure glycerol is available in the market at low prices and that can be potentially used as a valued feedstock [21]. Although pure glycerol is a highly valued chemical escorted by numerous uses, but the impurities present in the crude glycerol make the purification techniques costly escalating the price of glycerol [21]. Therefore, current research is a matter of interest that lies in the valorisation of crude glycerol via directly converting it into value-added products using sustainable production processes. Among the several recently published review articles for valorisation of glycerol as listed in Table 1, none have been found which describes fully the techno economic aspects for optimum yield of product, economic analysis of the processes and products and environmental aspects. Therefore, this review article considers the techno economic aspects of different processes and products for glycerol utilization to value added products including environmental aspects. There are numerous reported techniques for the transformation of crude glycerol into valuable products via biological conversion, catalytic conversion, enzymatic conversion and thermo chemical conversion [22]. Several varieties of important products and chemicals produced by processing glycerol are citric acid, DHA, GTBE, MTBE, esters, biopolymers, lactic acid, hydrogen, synthesis gas, etc.
2. Different technologies for glycerol conversion
The conventional conversion of glycerol mainly includes the reaction between glycerol and another molecule for the production of a new valuable chemical. The catalytic pathways include usage of crude glycerol as a chief feedstock using different types of catalysts which differ in their nature.
2.1. Biological conversion process
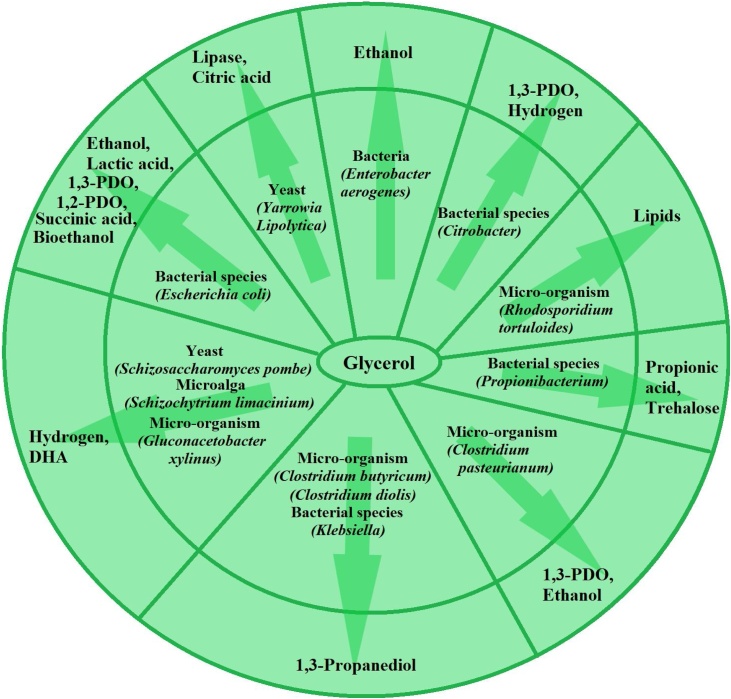
Biological routes utilize microorganisms and enzymes for the conversion glycerol either by aerobic or anaerobic metabolism. The conversion of glycerol into useful products with the aid of microbes such as fungi, microalgae, and bacteria is termed as biological conversion [23]. These biological reactions are carried out in a large bio-reactor under aerobic, anaerobic or micro aerobic conditions as per the demand of different microorganism [22]. It is realistically difficult to evaluate the bioconversion of glycerol due to the limited studies and commercial scale unit has not yet been reported, but one can get numerous products by bioconversion [24] and many other organic acids by fermentation as depicted in Fig. 3 and Table 3, respectively. The utility of the end products obtained from such conversion is another important factor that can lead to the commercialization of technology.
Fig. 3.
Bio-catalytic pathways for conversion of crude glycerol to value added products [[28], [29], [30], [31], [32], [33], [34], [35]].
Table 3.
Classification of process and products of biological conversion pathways.
| S. No |
Reactants (Molar ratio) | Pathway | Catalyst | Optimal Reaction Condition(s) | Product (s) -yield | Reference |
|---|---|---|---|---|---|---|
| 1. | Glycerol | Fermentation | Escherichia coli AC-521 | 42 ◦C,pH-6.5, 88 hour | Lactic acid - 85.8 g/L | [25] |
| 2. | Crude glycerol | Fermentation | G. Oxydans cells immobilised over polyurethane foam | 31 °C, glycerol concentration – 20 g/L, pH – 4.7 | 1,3-Dihydroxyacetone - 17.83 g/L | [26] |
| 3. | Glycerol | Fermentation | Gluconacetobacter xylinus cells | 25(w/v), 60 hour, 30 ◦C, pH - 6 | Dihydroxyacetone -6.3 g/L | [30] |
| 4. | Glycerol | Fermentation | Rhodosporidium toruloides | 30−60 hour, C/N ratio – 300 | Lipids | [32] |
| 5. | Pure and recovered glycerol | Ferementation | Enterobacter aerogenes | Glycerol conc. – 39 g/L, 120 hour, pH - 7 | Ethanol – 20 g/L | [33] |
| 6. | Glycerol | Anaerobic batch fermentation | Escherichia coli SS1 | 37 °C,96 hour, glycerol conc. – 20 g/L | Bioethanol (ethyl alcohol) – 9.23 g/L | [34] |
| 7. | Crude glycerol and crustacean waste | Fermentation | Yarrowia lipolytica | 30 °C,pH – 6 | Lipase | [35] |
| 8. | Glycerol | Batch fermentation |
Clostridium butyricum DSP1 |
37 ◦C, pH- 7, 140 g/L | 1,3-propandiol – 67 g/L | [36] |
| 9. | Crude glycerol | Fermentation | Schizochytrium limacinum | 19.2 ◦C,100 g/L | Docosahexaenoic acid (DHA) – 4.91 /L | [37] |
The principal utilities are:
-
•
1,3-propanediol (PDO) - It is an organic chemical obtained via anaerobic fermentation or micro-aerobic fermentation by bacteria. It is used in the solvents and adhesives [[1,22].
-
•
Lactic acid (2-Hydroxypropanoic acid) - It is produced by the microbial conversion of the glycerol i.e. via a fermentation process using several microorganisms. Lactic acid along with its salts and esters has the potential to use in the food, cosmetic, pharmaceutical and agricultural industries [25].
-
•
Hydrogen - It is produced using crude glycerol through microbial fermentation. It is an energy carrier in fuel cells [1,26].
-
•
Trehalose - It is a reducing sugar which is used as a stabilizer in therapeutic products [26].
-
•
Glyceric acid (GA) - GA is an organic acid also named as 2,3-dihydroxy propionic acid. It is produced by bioconversion of glycerol. It is a multifunctional monomer [23].
-
•
Citric acid – It is produced by fermentation. This organic acid is weakest in nature among all. It is an intermediate of tricarboxylic acid is widely used as a preservative in the food industry [23].
-
•
Succinic acid - This dicarboxylic acid having four carbon atoms is produced by anaerobic metabolism pathway using different microorganisms. It is used as an intermediate for producing chemicals used in pharmaceutical and food industry [1,23].
-
•
Docosahexaenoic acid (DHA) – Different uses of DHA includes sun tanning agent in cosmetic industry, the feedstock for pharmaceuticals and the main element for producing fine chemicals, synthesis of new biodegradable polymers [27].
-
•
Eicosapentaenoic acid (EPA) - It is produced by fungal fermentation of glycerol [22].
-
•
Polyhydroxyalkanoates(PHA) - PHA is a biopolymer produced by microbial fermentation of glycerol using several bacterial strains. Glycerol is a carbon source used for producing PHA [26].
-
•
Biogas - It is produced by acidogenesis followed by methanogenesis in anaerobic digestion of glycerol [1].
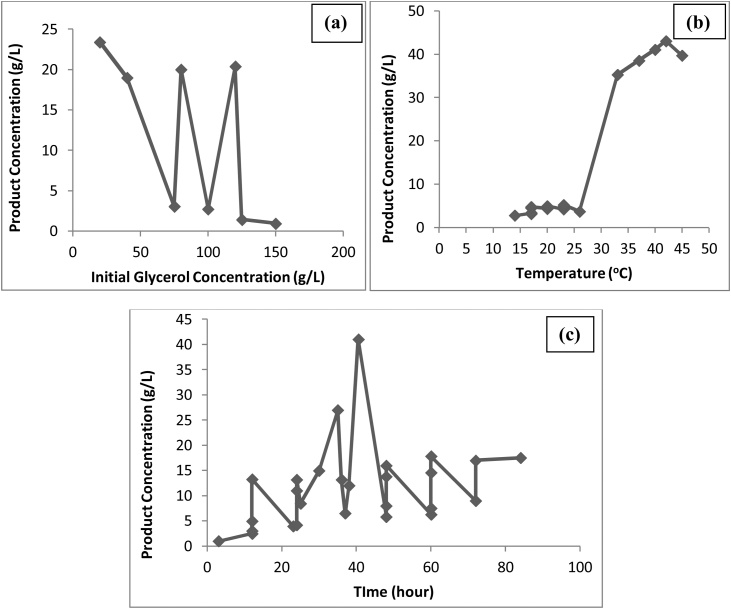
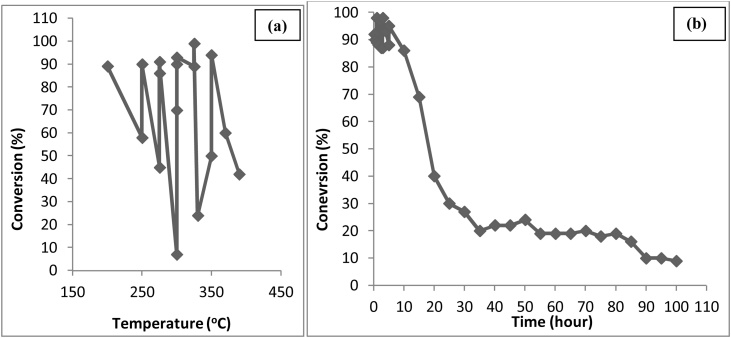
A comparative technical assessment of the different biological conversion processes with respect to initial glycerol concentration, reaction temperature and reaction time leading to maximum yield of end products are depicted in Fig. 4(a), (b), and (c) [25,26,30,[32], [33], [34], [35], [36], [37]]. From the Fig. 4(a), the maximum product concentration is 23.2 g/L corresponding to glycerol concentration of 20 g/L, as evident that higher substrate concentration inhibits the reactivity of organism, (b) maximum product concentration is 43.1 g/L at 42 °C as higher temperatures may not support the growth of organism [24,38] and (c) maximum product concentration is 41 g/L at 40.5 h as with the increase in time, there might be depletion of some factor(s) that adversely affect the reactivity of the enzyme/organism [35,38].
Fig. 4.
Effect of (a) Initial Glycerol Concentration, (b) Temperature and (c) Time on bioconversion of glycerol.
However, crude glycerol has many impurities in it that can have adverse impact on the growth of cell and product formation. They inhibit the metabolism of the organisms [39]. Sometimes, the presence of impurity in excess amount adheres the synthetic pathway of the product [39]. The presence of soap can cause negative effects on cell growth and lipid accumulation. It decreases motility of cells, impacts their orientation and changes their morphology [40]. The presence of methanol in the reaction mixture in excess amount decreases the lipid concentration due the change in the membrane fluidity and activity of enzymes [40]. If salts are present in excess, they increase the production of carbohydrates and lipids in the cell [41]. Thus, one should focus on the development of micro-organism that can put up with the impurities present in crude glycerol or remove the impurities of crude glycerol either by evaporation or via acid treatment [40].
2.1.1. Challenges in the crude glycerol valorisation via biochemical routes
Though biochemical pathway is a promising route for the conversion of glycerol, but there are some challenges associated with it. Catalyst selectivity towards some products, the low reusability of the catalyst, harsh conditions for the catalysts, difficulty in separation of catalyst from the products formed and low product yield even after long reaction times are some of the challenges related to catalyst [42]. These are due to the impure nature of the crude glycerol. Therefore, it is a difficult task for industries for using crude glycerol directly along with its impurities. High cost of production and low stability of the enzymes produced are also obstacles that are challenging and feed stock availability [43,44]. Therefore, it is necessary to use the microorganisms that are tolerant to impurities in crude glycerol [45]. Detail aspects has been discussed in the subsequent sections.
2.2. Chemical conversion processes
Chemical conversion is the most common method studied for the conversion of glycerol. Various studies are available for the chemical transformation of glycerol [[46], [47], [48], [49], [50]]. Different useful products obtained from the chemical processes with various industrial applications via different pathways are listed below:
-
•
Polyglycerols - Polymerization of glycerol produces polyglycerols. These are non-ionic surfactants used in the food, detergents and cosmetics industries [46].
-
•
Solketal - Acetalization of glycerol provides solketal. It can also be obtained by condensation of glycerol. It is an effective oxygenated fuel additive, surfactant, and flavoring agent [47].
-
•
Ethers - Etherification of glycerol produces methyl tert-butyl ether (MTBE), di-tert-butyl glycerol ether (DTBG), tri-tert-butyl glycerol ether (TTBG), ethyl tertiary butyl ether (ETBE) [48].
-
•
Esters - Esterification of glycerol gives polyglycerol esters, acylated esters (mono-, di-, tri-acetin), Glyceryl diacetate (DAG), Glyceryl triacetate (TAG). These are used as oxygenated fuel additives [49].
-
•
1,3-propanediol - It is produced by dehydroxylation of glycerol. It is mainly used for the generation of polytrimethylene terephthalate (PTT) polymer [50]. The global production of 1,3-propanediol already reached to 45,300 tons and has been growing continuously [14].
-
•
Ketals – Ketals are obtained from ketalisation of glycerol that are good ignition accelerators and anti-knock additives in combustion engines [51].
-
•
Malleated glycerides – Mallenization of glycerol produces malleated glycerides. These are the renewable precursors used for the production of plastics [22].
-
•
Epichlorohydrin (ECH) - Epicerol process produces ECH. In Epicerol process, glycerol is reacted with a chlorinating agent like HCl. Mainly, epoxy resins are produced from ECH [52].
-
•
Glycerol carbonate (GC) – GC can be synthesized from glycerol from various carboxylation sources. GC is used as an electrolyte and solvent in batteries [53].
-
•
C8 chain ethers - Telomerisation of glycerol produces C8 chain ethers of glycerol, which are used as surfactants [52].
A schematic diagram for the conversion of crude glycerol to value-added products via chemical reaction pathways using different catalysts is depicted in Fig. 5. Some of the important products obtained by chemical conversion and the effect of various parameters on their yield are elaborated looking at the potential for industrial applications.
Fig. 5.
Chemical reaction pathways for conversion of crude glycerol to value added products [[7,18,36,37,[53], [54], [55], [56]].
2.2.1. Epichlorohydrin
1-chloro-2,3-epoxy propane (ECH) is an organochlorine compound and an epoxide. Epoxy resins, elastomers, pesticides, synthetic glycerol, plasticizers, chlorohydrins rubbers, etc. are some of the products that can be produced via ECH [7]. In the Asia-Pacific region, it is used for the production of wind turbine blades. It helps in the reinforcement of paper which is used for making tea bags. It is produced by the epicerol process. The epicerol process produces epichlorohydrin from the glycerol obtained from the biodiesel Industry, while in the conventional process, ECH is produced from the reaction between propylene and chlorine. The reaction conditions reviewed from different literature for epichlorohydrin production from crude glycerol are tabulated in Table 4. The Dow Chemical Company produces ECH globally [7].
Table 4.
Production of Epichlorohydrin from glycerol.
| S. No. | Reactants (Molar ratio) | Catalyst (amount) | Optimal Reaction condition(s) | Product(s) | Reference |
|---|---|---|---|---|---|
| 1. | Glycerol:HCl gas | Hydrotalcite-derived mixed oxides of Al and Mg (HTIcs) | 150 ◦C, 0.5 hour | ECH | [57] |
| 2. | Glycerol:HCl | Carboxylic acids (8 % by moles) | 100 ◦C, 3 hours, | ECH | [58] |
| 3. | Dichloropropanol:NaOH (1.05:1) | – | 50 ◦C, 15 seconds | ECH | [59] |
| 4. | Glycerol:Hydrogen Chloride | Carboxylic acid (2 wt.%) | 200 ◦C,4 hour | ECH | [60] |
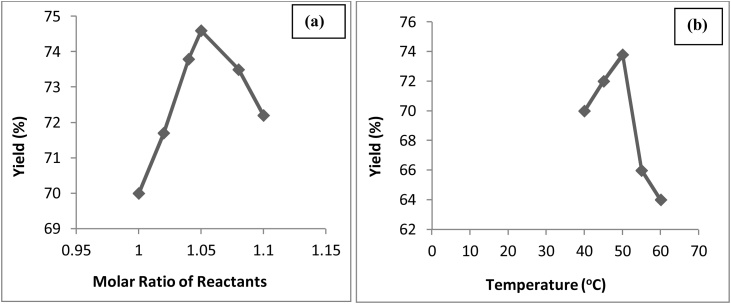
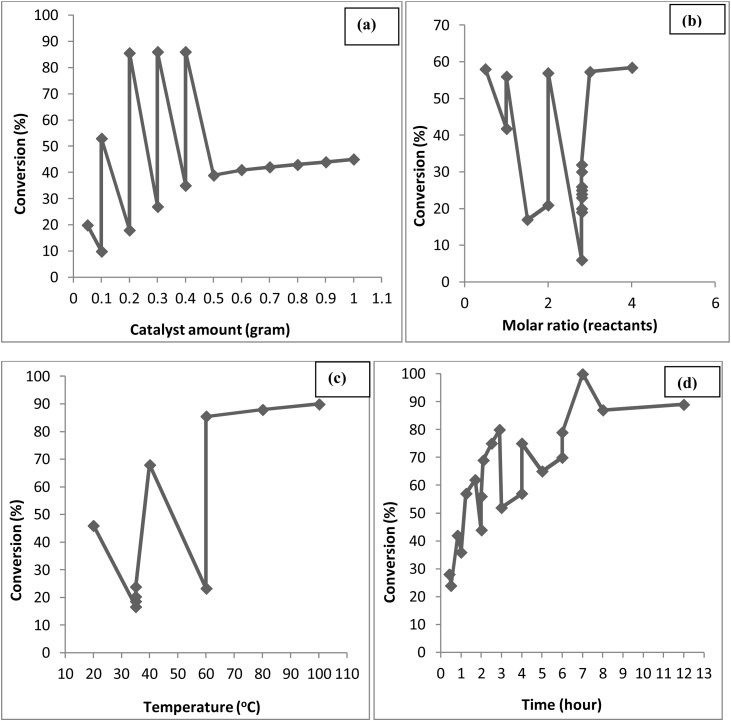
The effect of the molar ratio of the reactants used and the temperature of the reaction that affects the optimum yield of epichlorohydrin is presented in Fig. 6 based on literature survey [[57], [58], [59], [60]]. From the Fig. 6(a) the maximum yield 74.6 % of ECH is with 1.05 M ratio of reactants, as further increased in molar ratio lowered the yield of the product due to the internal rearrangement/ isomerization reaction of the reactants [59,61] and 6(b) the maximum yield of 73.8 % is at 50 °C as further increasing the temperature caused occurrence of side reactions leading to the lower yield of product epichlorohydrin [59].
Fig. 6.
Effect of (a) Molar ratio of Reactants and (b) Temperature on the production of epichlorohydrin.
2.2.2. Glycerol ethers
Glycerol ethers such as MTBE, GTBE are derivatives of crude glycerol, an effective oxygenated fuel and octane promoters. The reaction of crude glycerol and olefins like isobutylene or tert-butyl alcohol along with catalysts is called etherification [62]. GTBE is preferred to use in place of MTBE because MTBE is more toxic than GTBE. These fuel additives reduce the emission of particulates in the atmosphere. These are a group of solvents used in paints and cleaners. Different reaction conditions affecting the etherification reaction have been found out from the extensive literature survey and presented in Table 5.
Table 5.
Different reaction conditions that affect the etherification reaction.
| S. No. | Reactants(Molar Ratio) | Catalyst (amount) | Optimal Reaction condition(s) | Product(s) | Reference |
|---|---|---|---|---|---|
| 1. | Glycerol: 1-Butene (1:4) | Amberlyst-15, S100, S200 (10 wt. %) | 100 ◦C, 24 hours | MPGE, DPGE | [63] |
| 2. | Glycerol: Ethanol (1:8.62) | Acid catalysts – H-beta Zeolite and CER (5 w/v %) | 140 ◦C, 2 hours | Diethyl ether | [64] |
| 3. | Glycerol: tert-Butanol (1:15) | TPA (Tungsto-phosphoric acid) (20 wt. %) | 140 ◦C,120 minutes | ME, DE, TE | [48] |
| 4. | Glycerol:Short chain alkyl alcohols (1:4) | Lewis acid catalyst (6.5 mol %) | 150 ◦C, 24 hours | Monalkyl-glyceryl ethers | [65] |
| 5. | Glycerol:tert-Butanol (1:4) | Amberlyst-36, Amberlyst-15 (5.5 wt. %) | 82 ◦C, 8 hours | Glycerol alkyl ethers | [66] |
| 6. | Glycerol: Isobutene (1:2) | Ambelyst-15 (0.011 wt. %) | 92 ◦C, 480 minutes | GTBE | [67] |
| 7. | Glycerol: tert-Butanol (1:4) | Amberlyst-15, H-BEA (5 wt. %) | 90 ◦C, 180 minutes | Di-ethers | [68] |
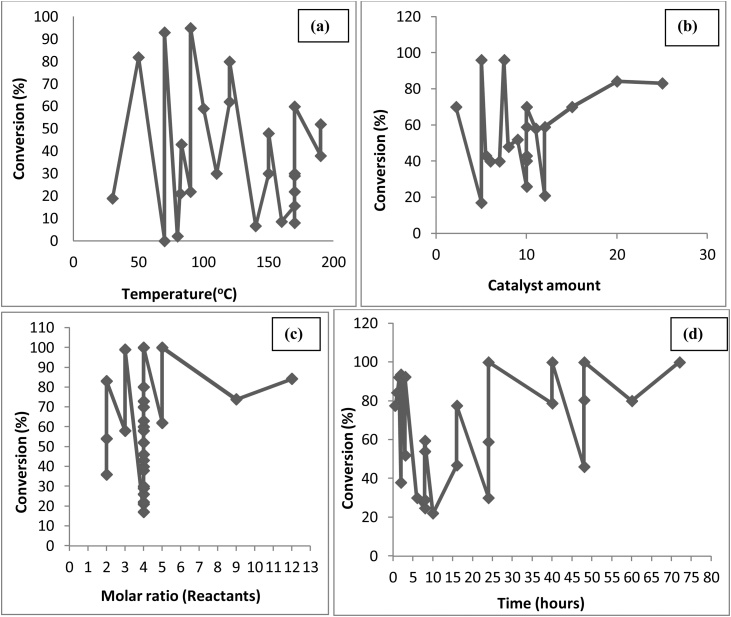
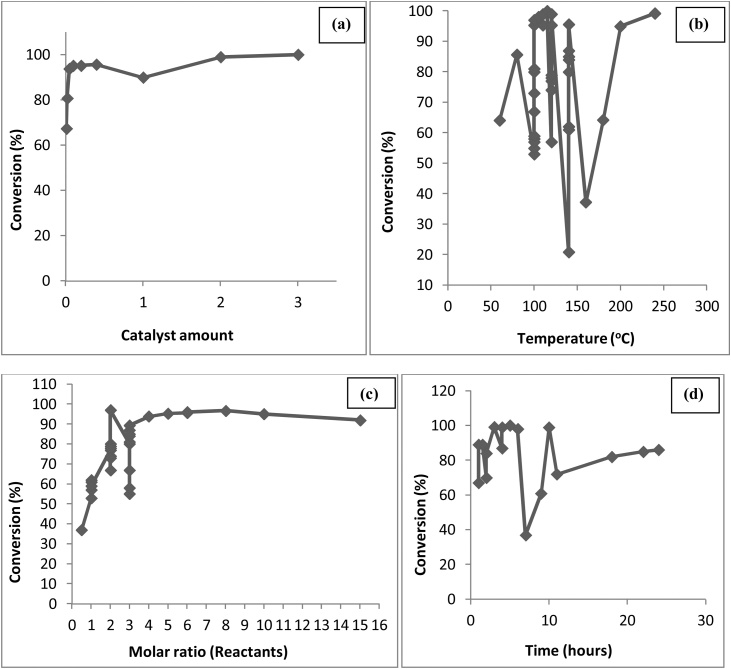
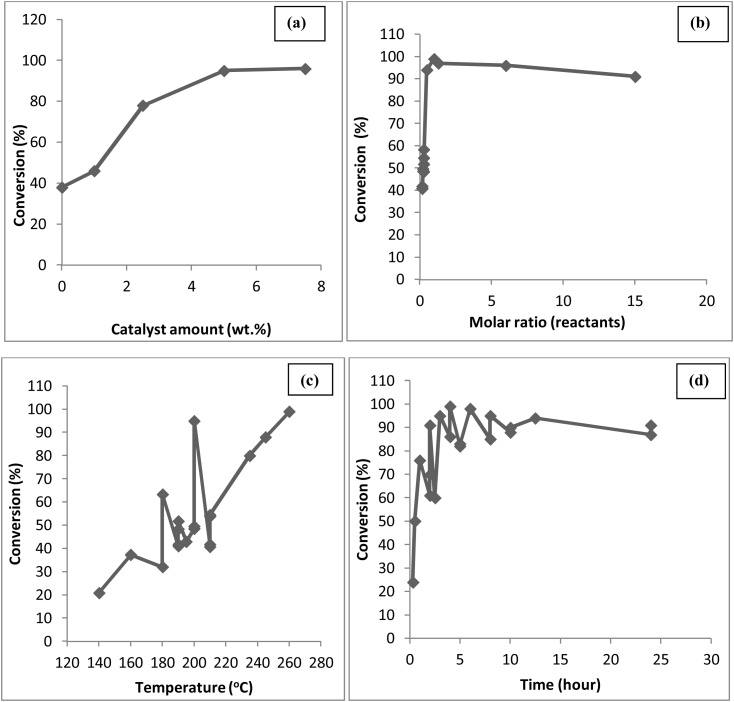
The technical assessment for the optimum yield of glycerol ethers with varying temperature, catalyst amount, the molar ratio of the reactants and time has been presented in Fig. 7(a), (b), (c) and (d), respectively [[63], [64], [65], [66], [67], [68]]. From Fig. 7(a), maximum 96 % conversion to glycerol ether is at 90 °C, because at lower temperature, catalyst favoured the by-product formation resulting lower yield of ethers [68,69]. In Fig. 7(b) maximum 96 % conversion is achieved using 5 wt. % and 7.5 wt. % catalyst as the amount of by-product appeared to level toward higher catalyst loadings [69]; (c) almost 100 % conversion is achieved using the molar ratio of 4–5 [63] and (d) One can nearly achieve a 100 % conversion as the reaction progresses with time to 72 h.
Fig. 7.
Effect of (a) Temperature, (b) Catalyst amount, (c) Molar ratio and (d) Time on etherification of glycerol.
2.2.3. Polyglycerols
Polyglycerols like diglycerol, triglycerol, tetraglycerol, hexaglycerol, decaglycerol are used in cosmetics, lubricants, additives in polymers and nutritionals. These can be produced via either by polymerization of glycerol or etherification of glycerol. Polyglycerols also have different biomedical applications. Different reaction conditions for the production of polyglycerol under different reaction conditions have been reviewed and presented in Table 6.
Table 6.
Different reaction conditions for the production of polyglycerols.
| S. No. |
Reactants | Catalyst (amount) | Optimal reaction condition(s) | Product(s) | Reference |
|---|---|---|---|---|---|
| 1. | Crude glycerol | No catalyst | 250 ◦C, 60 min | Polyglycerol(di-, tri-, tetra-, penta-) | [46] |
| 2. | Glycerol | MgAl mixed oxides | 220 ◦C, 24 hour, pH-7.7 | Diglycerol, Triglycerol | [70] |
| 3. | Glycerol | LiOH (2 wt.%) | 240 ◦C, 6 hour | Di-, tri-, Tetraglycerol | [71] |
| 4. | Glycerol | mesoporous catalyst- MCM-41 type (2 wt.%) | 260 ◦C | Diglycerol, Triglycerol | [72] |
| 5. | Glycerol | MCM-41 type mesoporous catalysts (2 wt.%) | 260 ◦C | Di-, tri-, Tetraglycerol | [73] |
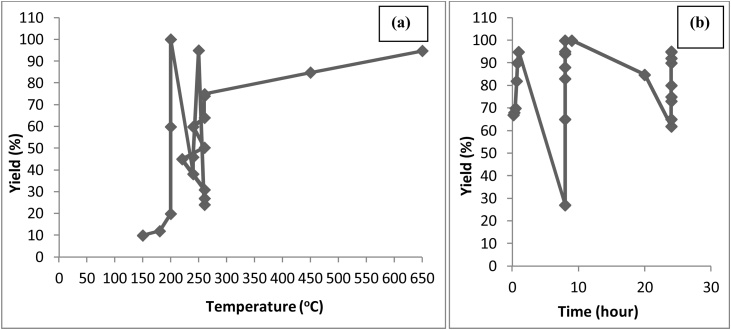
The technical assessment for optimum yield of polyglycerol using crude glycerol as feedstock have been worked out and presented in Fig. 8 to study the effect of temperature and time with respect to product yield [46,[70], [71], [72], [73]]. From Fig. 8, 100 % conversion is achieved at the 200 °C within 8−9 hours of the reaction [72]. At lower temperatures, the conversion is very less and it again starts decreasing after 200 °C and 9 h. The higher the temperature sustained for the reaction, the more is the reaction conversion resulting in a decrease of hydroxyl groups in reaction products [74].
Fig. 8.
Effect of (a) Temperature and (b) Time on production of polyglycerols.
2.2.4. Glycerol esters
Esterification reaction produces glycerol esters in which crude glycerol reacts with carboxylic acid or acetic acid to produce polyglycerol; esters or DAG, TAG; mono-, di- and tri-esters of glycerol acetates. Different catalysts employed in this reaction are homogeneous catalysts (viz. sulfuric acid (H2SO4), Hydrochloric acid (HCl), p-toluene sulfonic acid (PTSA), methane sulfonic acid (MSA) and titanate), ion exchange resins, metal oxides (zinc oxide, ferrous oxide and stannous oxide), zeolites, hetero polyacids, mesoporous silica etc. [75]. Triacetin (TAG) is used in plasticizer of cellulosic polymers as a solvent. TAG and DAG are valuable bio-additives for liquid fuels. The commercial supplier of the TAG is to M/s. React Chem. These esters can also be used as emulsifiers. Monoacetin (MA) and Diacetin (DA) are main elements in the preparation of polyesters and cryogenics. The use of Triacetin (TA) is as a fuel additive for improving fuel quality and as a moisturizer in the Cosmetic Industry [76]. Extensive literature has been reviewed in esterification process and factors affecting the reaction rate viz., reactant molar ratio, catalyst amount and optimal reaction conditions have been tabulated in Table 7.
Table 7.
Different reaction conditions affecting the esterification reaction.
| S.No. | Reactants(Molar ratio) | Catalyst (amount) | Optimal reaction condition(s) | Product(s) | Reference |
|---|---|---|---|---|---|
| 1. | Glycerol:Oleic acid (1:4) | SO4/ZrO2 (5 wt. %) | < 180 °C, 3–6 hours | GMO, GDO, GTO | [75] |
| 2. | Glycerol:Palm oil oleic acid (1:1) | MESA (Methyl Ester Sulfonate Acid) (0.5 %) | 240 °C, 180 minute | Glycerol ester | [77] |
| 3. | Glycerol:Acetic acid (1:6) | A70, A15, STA/S11, TPS/S11 (5 wt.%) | 105 °C, 4 hour | Monoacetin, Diacetin, Triacetin | [78] |
| 4. | Glycerol:FA [mystric acid(tetra-decanoic), stearic acid, Oleic acid, Lauric acid] (1:2) | DMC (Fe-Zn double-metal cyanide) (7 wt.%) | 180 °C, 8 hours | Glycerides (MG, DG, TG) | [79] |
| 5. | Glycerol:Acetic acid (1:8) | Double-SO3H functionalized ionic liquids (0.5 wt.%) | 100 °C, 30 minutes | Monoacetin, Diacetin, Triacetin | [80] |
| 6. | Glycerol:Olien (1:1) | Zinc oxide, ferrous oxide, stannous oxide (0.4 wt.%) | 170 °C, 6 hours | Component for fuel | [81] |
| 7. | Glycerol:Acetic acid (2g:20 ml) | PMo3_NaUSY (dodecamolybdophosphoric acid(PMo) engaged in NaUSY Zeolite) (1.9 wt.%) | 3 hour | Monoacetin, Diacetin, Triacetin | [82] |
| 8. | Glycerol:Acetic acid (1:1) | H-beta Zeolite (HBZ) | 100 °C, 1 bar pressure | Monoacetin | [76] |
| 9. | Glycerol:Acetic acid (3:1) | Bio-derived carbon catalyst (2 wt.%) | 110 ◦C, 3 hour | Monoacetin, Diacetin, Triacetin | [49] |
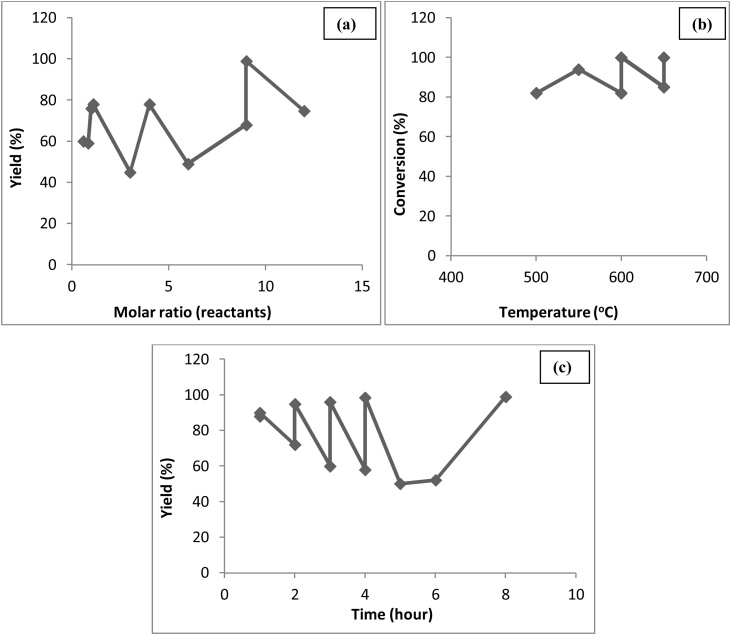
Technical assessment for optimal glycerol ester production with respect to catalyst amount, temperature, molar ratio and time has been worked out and presented in Fig. 9(a), (b), (c), and (d), respectively [49,[75], [76], [77], [78], [79], [80], [81], [82]]. From Fig. 9, (a) as the catalyst amount increases up to 3 wt.%, conversion increases and reaches to 100 % but using higher amounts of catalyst could lead to soap formation resulting lower concentrations of the product [83], (b) 100 % conversion is achieved at 115 °C and 120 °C as an increase in temperature favours the reaction by providing homogenization to the reaction mixture [75,84], (c) maximum 97 % conversion is achieved using 2 M ratios of the reactant to glycerol as it caused significant change in the reaction rate and excess molar ratio did not affect much on the conversion [85] and (d) 100 % conversion is achieved within 4−5 hours of reaction initiation.
Fig. 9.
Effect of (a) Catalyst amount, (b) Temperature, (c) Molar ratio and (d) Time.
2.3. Thermochemical conversion
Though thermal degradation or combustion of glycerol is a solution to consume a large amount of by-product of biodiesel industry named glycerol, but it leads to the emission of harmful toxic gases into the atmosphere which is harmful for the environment. Also, thermal degradation is not viable economically. Therefore, thermo chemical conversion of glycerol via pyrolysis, steam reforming, gasification, partial oxidation is more appropriate methods for converting glycerol into useful energy forms. Mainly three forms of products are produced by the process of pyrolysis or gasification of crude glycerol includes synthesis gas (CO + H2), liquid products and char. Carbon monoxide, hydrogen, carbon dioxide, methane, and syngas are major products produced by thermochemical conversion [23].
2.3.1. Hydrogen
Hydrogen can be produced by partial oxidation, auto-thermal reforming, steam reforming, supercritical water reforming, aqueous-phase reforming, pyrolysis and catalytic steam reforming of glycerol. The utmost common approach for producing hydrogen is reforming [86]. It is mainly used in fuel cells in the automotive industry for internal combustion engines. Different reaction conditions that affect the production of hydrogen via different catalytic processes along with various catalysts, optimal reaction conditions and molar ratio of reactants have been reviewed and tabulated in Table 8.
Table 8.
Different reaction conditions that affect the production of hydrogen.
| S. No. |
Reactants (Molar ratio) | Catalyst | Optimal reaction condition(s) | Product (s) | Reference |
|---|---|---|---|---|---|
| 1. | Glycerol:Water (1:9) | Ni/SiO2 | 600◦C | Hydrogen | [87] |
| 2. | 10 vol.% glycerol solution | Ni/ZrO2 | 500 ◦C | Hydrogen | [88] |
| 3. | Water:glycerol (12:1) | NiAl2O4 | 600◦C | Hydrogen | [89] |
| 4. | Concentrated glycerol solutions (50 wt. %) | Carbon-supported Pt-based bimetallic catalyst |
300◦C | Hydrogen | [90] |
| 5. | Water:glycerol (1:1) | – | 227 ◦C | Hydrogen and synthesis gas |
[91] |
| 6. | Water:glycerol (12:1) | Ni/CeO2 | 600◦C | Hydrogen | [92] |
| 7. | Steam:glycerol (9:1) | Co–Ni/HTls catalyst |
550 ◦C | Hydrogen | [93] |
Factors affecting the rates of hydrogen production from crude glycerol have been worked out with respect to molar ratio, time and temperature as presented in Fig. 10(a), (b) and (c), respectively [[87], [88], [89], [90], [91], [92], [93]]. From the Fig. 10, (a) maximum 99 % of yield is obtained with molar ratio of 9 of the reactants to glycerol as increased molar ratio favoured reverse water gas shift reaction leading to H2 formation [87]; (b) 100 % conversion is achieved at the temperature around 600 °C as hydrogen production via steam reforming occurs significantly only at higher temperatures. Also, catalytic activity could be enhanced at higher temperatures [87,94]. In Fig. 10(c), 99 % of yield is achieved after 8 h of reaction completion.
Fig. 10.
Effect of (a) Molar ratio, (b) Temperature and (c) Time on production of hydrogen.
Higher the temperatures, greater will be the yield of hydrogen. The best temperature was reported as 625 °C and water to a glycerol ratio of 9:1 because under these conditions, methane production was lessened and carbon production was apprehensive [20].
2.4. Catalytic conversion
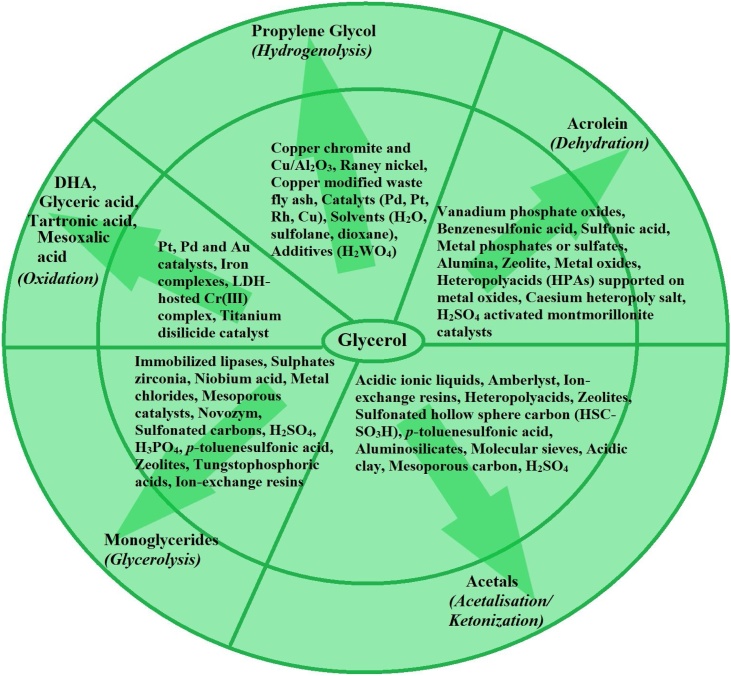
The transformation of glycerol mainly by using catalysts either basic or acidic, homogeneously or hetrogeneously is known as catalytic conversion [95]. Mostly, heterogeneous catalysts with high selectivity and conversion are preferred for the commercial conversion of glycerol due to their low cost operation and easy separation from the reaction mixture. Various catalytic conversion processes are: Selective oxidation of glycerol produces dihydroxyacetone (DHA), glyceric acid (GA), tartronic acid (TA), mesoxalic acid (MA) a potent hypoglycemic agent, (hydroxy pyruvic acid, glycolic acid, glyoxylic acid, oxalic acid, glyceraldehyde); dehydration produces acrolein, acrylonitrile; oxidative polymerization gives polyketomalonate (PKM); hydrogenolysis of glycerol produces propylene glycol, 1,2-propanediol which is used as a plasticizer and stabilizing agent; while glycerolysis produces monoglycerides [7]. However, the reaction conditions for different conversions are different that resulted in products with varying composition. Catalytic reaction pathways for conversion of glycerol to its derivatives are as shown in Fig. 11.
Fig. 11.
Catalytic reaction pathways to convert glycerol into its derivatives [[96], [97], [98], [99], [100], [101], [102], [103], [104], [105], [106]].
2.4.1. Propylene glycol
Propylene glycol is also known as 1,2-propanediol. Hydrogenolysis of crude glycerol gives 1,2-PDO. It can also be produced by hydrolysis of propylene oxide and de-hydroxylation of glycerol. Propylene glycol is the substitute of ethylene glycol as ethylene glycol is toxic in nature. Propylene glycol is a good antifreeze and de-icing agent. Polyester resins, functional fluids, pharmaceuticals, foods, detergents, enamels and animal feeds are some of the applications of PG [14]. Oleo-chemical company, Oleon, collaborated with BASF commercially produces 1,2-PDO. The parameters that affect the propylene glycol production viz., the molar ratio of reactants, catalyst type and amount, optimal reaction conditions and the selectivity of the yield have been reviewed and presented in Table 9.
Table 9.
Different reaction conditions affecting production of propylene glycol.
| S. No. |
Reactants (Molar ratio) | Catalyst (amount) | Optimal reaction condition(s) | Product (s) | Reference |
|---|---|---|---|---|---|
| 1. | Glycerol:Solvent (1:4) | Cu on acid treated fly ash (1 g) | 220 ◦C, 5 hour, 52 bar | 1,2-propanediol | [107] |
| 2. | 80 % glycerol solution | copper-chromite | 200 ◦C, 24 hour, 200 psi | propylene glycol | [108] |
| 3. | 40 wt.% of aqueous glycerol solution | Cu/SiO2 IE (1 g) | 200 ◦C, 15 bar, 7 hour | 1,2-propanediol | [109] |
| 4. | Glycerol in water | CuO–ZnO (5 mol%) | 180 ◦C, 80 bar, 48 hour | 1,3-propanediol, 1,2-propanediol | [110] |
| 5. | Aqueous glycerol | Cu/Zn/Al – 5 wt. % | 200 psig, 200 ◦C, 24 hour | Propylene Glycol | [111] |
| 6. | H2:Glycerol (2:1) | Mo and W catalysts (3 mL) | 60 bar, 24 hours, 325 ◦C | 1,2-propanediol | [112] |
| 7. | 1 wt.% glycerol solution | carbon-supported Ru and Pt catalysts | 200 ◦C, 40 bar H2, 5 hour |
Propylene Glycol | [113] |
| 8. | 10 vol% glycerol solution | Cu-Ni catalysts (1.25 g) | 250 ◦C, 40 bar, 6 hour | Propylene Glycol | [114] |
| 9. | 50 wt.% glycerol concentration | H-beta supported Ni-Zr catalyst (0.5 g) | 600 psi, 10 hour, 200 ◦C | Propylene Glycol | [115] |
| 10. | 10 wt% aqueous solution of glycerol | B2O3 Promoted Cu/Al2O3 Catalyst (2 g) |
250 ◦C, 6 MPa | 1,2-Propanediol | [116] |
| 11. | H2/Glycerol (10:1) | Copper over alumina and copper chromite (4 g) | 230◦C, 14 bar, 4 hour | 1,2-PDO | [117] |
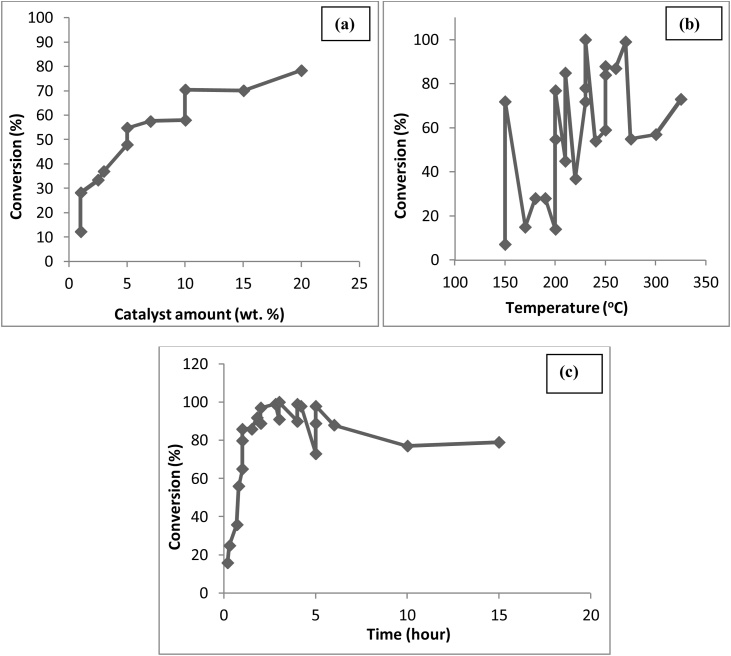
Technical assessment of the yield of the propylene glycol with varying catalyst, time and temperature has been presented in Fig. 12(a), (b) and (c) [[107], [108], [109], [110], [111], [112], [113], [114], [115], [116], [117]]. From the Fig. 12, conversion of glycerol increases with the increase in catalyst amount and it reaches maximum to 78.5 % with 20 wt. % of catalyst, higher concentration of which retarded the reaction [108] 100 % conversion was reportedly achieved at 230 °C, but after further increased in temperature decreased the conversion due to the conversion of the reactants into lower alcohols instead of PG within 3 h reaction time [108,117].
Fig. 12.
Effect of (a) Catalyst amount, (b) Temperature and (c) Time on the hydrogenolysis of glycerol for the production of propylene glycol.
2.4.2. Acrolein
Various catalysts, heterogeneous in nature, like zeolites and solid acid catalysts have been widely used for producing acrolein by glycerol dehydration. Dehydration is most important catalytic processes among all [118]. In heterogeneous acidic catalyzed reaction, the glycerol was evaporated, leaving behind the impurities in the liquid state and thus enhance the acrolein production. It is the most common intermediate required for the production of acrylic acid. Polymers used in pads and diapers with good absorbent properties are also produced from acrylic acid. The industrial scale production of acrolein has been yet not available. Different reaction conditions that affect the production of acrolein from glycerol is tabulated with respect to the molar concentration of reactants, catalyst types and amount, optimal reaction conditions and selectivity of the product yield as shown in Table 10.
Table 10.
Different reaction conditions affecting production of acrolein.
| S. No. |
Reactants (Molar ratio) | Catalyst (amount) | Optimal reaction conditions | Product (s) | Reference |
|---|---|---|---|---|---|
| 1. | 10 wt.% glycerol in water | Zirconium doped mesoporous silica catalysts (0.3 gm) | 325◦C, 5 hour | Acrolein, acetaldehyde and acetol | [119] |
| 2. | H2:H2O:glycerol (0.087:0.894:0.019) | Pd/LaY Zeolite (0.3 gm) | 573 K | Acrolein and acetol | [120] |
| 3. | Glycerol:Water (1:9) | Solid Acid-Base Catalyst | 315 ◦C | Acrolein | [121] |
| 4. | 1% glycerol in water | zinc sulfate | 360◦C, 60 second residence time | Acrolein | [122] |
| 5. | 20 % (w/w) glycerol solution | MUICaT-5 (1 g) | 225◦C, 4 hour | Acrolein | [123] |
Variation of product yield with respect to time and temperature has been presented in Fig. 13 [[119], [120], [121], [122], [123]]. From Fig. 13, (a) maximum 99 % of conversion is obtained at 325 °C [119] and (b) maximum of 98 % conversion is achieved at 1 h of reaction. However, after one hour with an increase in time, the conversion rate decreases to 9 %. 374 °C was reported as the critical temperature for the production of acrolein and at higher temperatures; there is a decrease in the product selectivity [124]. A complete conversion of glycerol with good selectivity (82 %) to acrolein was detected at 315 °C [125].
Fig. 13.
Effect of (a) Temperature and (b) Time on the dehydration of glycerol.
2.4.3. Dihydroxyacetone (DHA), glyceric acid (GA), tartronic acid (TA) and mesoxalic acid (MA)
These products can be derived from the oxidation of glycerol using catalyst. Glycerol oxidation is a complex reaction as compared to other catalytic conversion processes. Dihydroxyacetone (DHA) is used broadly in the cosmetic industry as in sunless tanning products and industries of alcohol, wine, beverages, and dietary supplement. In oxidation, secondary hydroxyl groups are involved in the reaction process. GA is a compound that has no commercial application but is an intermediate for the synthesis of TA and MA. It is produced by the oxidation of the primary hydroxyl group. TA is mainly used in the pharmaceutical area for the treatment of osteoporosis and obesity. It is produced from the oxidation of TA. The selectivity of the product formation with oxygenates has been tabulated in Table 11.
Table 11.
Different reaction conditions affecting the production of oxidation products.
| S. No. | Reactants (Molar ratio) | Catalyst (amount) | Optimal reaction condition(s) | Product(s) | Reference |
|---|---|---|---|---|---|
| 1. | H2O2:Glycerol (2.8:1) | Fe(BPA)2(OTf)2 | 25◦C, 90 minutes | DHA | [101] |
| 2. | Glycerol:Water | LDH-hosted Cr(III) complex (0.2 g) | 60◦C, 6 hour | DHA | [102] |
| 3. | NaOH:Glycerol (4:1) | Pt/Al2O3 catalyst (0.5 gm) | 60◦C, 3 hour | Products | [103] |
| 4. | NaOH/Glycerol (2:1) | Gold catalysts supported on carbon materials (Au/G-SGT) (0.5 g) | 60◦C, 1 hour | Glyceric acid, Oxalic acid | [126] |
| 5. | Glycerol/Air | Platinum-Bismuth (5 wt. %) | 50◦C, pH -2-4, 4 hour | DHA | [127] |
| 6. | Glycerol:NaOH (1:2) | Gold catalyst supported on carbon (Au/C) | 60◦C, 3 hour | Glyceric acid | [128] |
Effect of catalyst amount, the molar ratio of the reactants, temperature and time upon the oxidation of glycerol has been reviewed for optimal product yield and has been presented in Fig. 14(a), (b), (c) and (d), respectively [[101], [102], [103],[126], [127], [128]]. From the Fig. 14, (a) maximum 86 % of conversion is achieved using 0.4 g of catalyst as higher amount of catalyst favoured oxidation at primary alcohols rather than oxidation to GLA or Glyceraldehyde [129] (b) maximum of 58.4 % conversion is obtained with 4 M ratio of the reactant to glycerol, (c) Maximum 85.5 % conversion is there at 60 °C but as temperature was increased, the corresponding conversion was decreased, as further oxidation leads to the formation of GLA rather than DHA or Glyceraldehyde [102,129] and (d) 100 % conversion is achieved at 7 h of reaction.
Fig. 14.
Effect of (a) Catalyst amount, (b) Molar ratio, (c) Temperature and (d) Time on Oxidation of glycerol.
2.4.4. Monoglycerides
Glycerides are lipid esters of the glycerol molecule and fatty acids. Monoglycerides can be synthesized by chemical processes such as glycerolysis, enzymatic synthesis, alcoholysis and esterification [130]. The main function of the glycerides is as a defoamer. Two types of glycerides are normally formed viz. (1) Neutral glycerides, (2) Phosphoglycerates [104]. Different reaction conditions that affect the production of glycerides have been presented in Table 12.
Table 12.
Different reaction conditions affecting the production of glycerides.
| S.No. | Reactants (molar ratio) | Catalyst (amount) | Optimal reaction condition(s) | Product(s) | Reference |
|---|---|---|---|---|---|
| 1. | Glycerol:FA [mystric acid(tetra-decanoic), stearic acid, Oleic acid, Lauric acid] (1:2) | DMC (Fe-Zn double-metal cyanide) (7 wt. %) | 180 °C, 8 hours | Glycerides (MG, DG, TG) | [79] |
| 2. | Glycerol:Fatty acids (1:1) | ZnCl2 (0.3%) | 195 °C, 500 rpm | Glycerides (mono-, di- amd tri-) | [104] |
| 3. | Glycerol:Stearin (2.5:1) | – | 200 °C, 20 minutes, | Monoglycerides | [131] |
| 4. | Glycerol:Refined oil (1:1) | NaOH (0.13 g for 50 g) | 210 °C, 90 minutes | Monoglycerides | [132] |
| 5. | Glycerol:Lauric acid (1:1) | Sulphonted carbon catalysts (0.5 g) | 125 °C,7−24 hour | Monoglycerides | [105] |
Effect of catalyst amount, molar ratio, temperature and time upon the reaction rates glyceride formation have been presented in Fig. 15(a), (b), (c) and (d), respectively [79,104,105,131,132]. From Fig. 15, (a) conversion of glycerol increases as the amount of catalyst increases and reaches a maximum to 96 % with 7.5 wt. % catalyst [105], (b) 99 % of conversion is achieved with equimolar ratio, i.e. 1:1 of reactant to glycerol [105] (c) as the temperature increases the conversion of glycerol increases and reaches to 99 % with 260 °C as lower temperature gave lower yields of MG [105,118] and (d) 99 % of conversion is achieved at 4 h of reaction.
Fig. 15.
Effect of (a) Catalyst amount, (b) Molar ratio, (c) Temperature and (d) Time on production of glycerides from glycerol.
2.4.5. Acetals
The wide use of cyclic acetals is as flavours, disinfectants, surfactants, fuel additives for diesel fuels and reactants to produce enantiomerically pure compounds. These are also used for producing steroids, pharmaceuticals, and fragrances as reported [133]. Acetals are synthesized by acetalization/ketonization. Direct condensation reaction with aldehydes/ketones is called acetalization/ketonization while reaction with aldehyde by exchanging alcohol from another acetal is called trans-acetalization [106]. Different reaction conditions that affect the optimum production of acetals have been reviewed and Tabulated in Table 13.
Table 13.
Different reaction conditions affecting the production of acetals.
| S. No. | Molar ratio (reactants) | Catalyst (amount) | Optimal reaction condition(s) | Product(s) | Reference |
|---|---|---|---|---|---|
| 1. | Glycerol:Benzaldehyde – 1.5:1 | [BPy]HSO4 (0.88 g, 20 mol% based on PhCHO) | 70 °C, 2 hour | Cyclic acetals | [133] |
| 2. | Glycerol:DEE:Ethanol – 1:1:12 | Amberlyst 15 - 1.0 kg/m3 | < 45 °C, 24 hour | 6-membered acetal | [106] |
| 3. | Glycerol:Benzaldehyde – 1:2 | cationic acidic resin (270 mg) | 120 °C, 2 hour | Cyclic acetals | [107] |
| 4. | Glycerol:acetaldehyde | Amberlyst-15 | 30 °C, atmospheric pressure | Glycerol ethyl acetal (GEA) | [134] |
| 5. | Glycerol:acetaldehyde – 3:1 | Amberlyst 47 (2 wt. %) | 40 °C | 5-hydroxy-2-methyl-1,3 dioxane and 4-hydroxymethyl-2-methyl-1,3 dioxolane | [135] |
| 6. | Glycerol:Benzaldehyde | Amberlyst-36 (0.5 g) | Cyclic acetals | [136] | |
| 7. | Glycerol:Aldehyde – 1:1.05 | Amberlyst-15 (0.36 g) | 85 °C, 48 h. | Cyclic acetals | [137] |
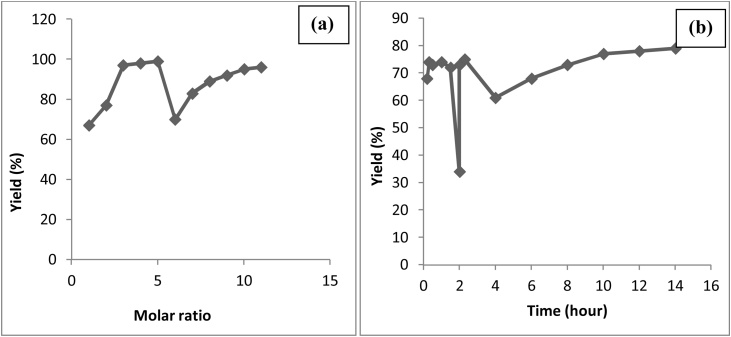
The effect of time and molar ratio for the production of acetals have been reviewed and shown in Fig. 16 [106,[133], [134], [135], [136], [137], [138]] respectively. From the Fig. 16, (a) the maximum yield of 97 % is obtained with a molar ratio of 1:2 i.e. using an excess of aldehyde and (b) the maximum yield of acetals is 79 % at 14 h. The acetalisation process can be best achieved at the possible mildest conditions even at low or room temperatures without solvent [139].
Fig. 16.
Effect of (a) Molar ratio and (b) Time on production of acetals.
2.5. Environmental and economic aspects of different routes
Pure glycerol has its own applications in different industries for example pharmaceuticals, food, cosmetics, etc. But, directly using the impure glycerol in industries is bounded due to the presence of impurities. Moreover, these can not be disposed directly in the environment due to their hazardous nature. Therefore, different routes are proposed by researchers for converting glycerol into value-added derivatives. But, each process has its own merits and demerits from both environmental as well as economics point of view.
Hydrogen production by microbial fermentation is a good alternative because production of hydrogen by combustion generates only water as a byproduct which is highly reducing CO2, NOx, particulate and other emissions, usually accompanied with the fossil fuel usage [26]. Various micro-organisms that occur naturally and metabolically developed, are capable of utilizing crude glycerol. Thus, economically, microbial conversion is more efficient and safer. Though, DHA production from microbes provides cost effective and environment friendly solution as compared to the chemical conversion, still it cannot be applied on a wide platform. Chemically, multiple reactions took place in a living cell when catalyzed by enzymes and have found to work ex-vivo even when isolated under both natural as well as non-natural environmental conditions. Glycerol oxidation by isolating enzymes can, however, overcome these limitations. The limitations of isolated enzymes like high production cost, expensive redox co-factors, stability, solubility, reusability, workability etc. limits the broad use of them. Thus, solutions to overcome these limitations is protein immobilization [20]. Immobilization of biocatalysts facilitates the easy separation of the product from the reaction mixture helps in the reduction of the process cost [140]. Less expensive, carried out at ambient temperature and pressure and simple reactors are some of the merits of the biological route [20]. Economically, the main drawback for hydrogen production is that at low temperature CH4 formation is favored. Most commonly, hydrogen is produced by steam reforming (SR) in which water vapor reacts with glycerol in the presence of catalyst giving hydrogen, CO2 and CO. Partial oxidation reforming (POR) is carried out exothermally under atmospheric pressure and its efficiency directly depends on the amount of oxygen entering the reaction mixture. Auto-thermal reforming (ATR) is similar to POR but in ATR hydrogen produced is more. The compactness and efficiency of this system help in the development of autonomous units for small scale decentralized production. Gasification, liquid phase reforming (LPR) and pyrolysis, provides a huge potential as circular economy, but have many limitations [20]. Long fermentation times, oxygen requirement, high energy consumption for sterilization, high concentration of broth product and substrate concentration, inhibitory effects on the cell growth, their pathogenicity etc. limt their broad application. Isolated enzymes can overcome these drawbacks which offers techno-economic and environmental advantages [20].
The potential environmental impacts of a process can be estimated by environmental factor assessments like Environmental Impact Assessment (EIA), Strategic Environmental Impact (SEA) and LCA (Life Cycle Assessment) [141]. The environmental issues caused by the low biodegradability of MTBE (pollution of aquifers and toxicity) led its replacement by ETBE [16]. Three major aspects in which modification is needed for enhancing the economic feasibility are the usage of raw materials in the process, the production method and the byproducts [12]. With reactors, drawbacks are generally associated while scaling-up. So, continuous flow reactors for production of solketal using heterogeneous catalyst is an effective solution. It provides higher heat and mass transfer efficiency, easy scaling-up giving with both environmental and economic advantages [12,142] (Table 14).
Table 14.
Unit Production Cost and GHG emission for various conversion processes of crude glycerol.
| S. No. | Process (Product name) | Unit production cost | GHG emission |
|---|---|---|---|
| 1. | Bioconversion - hydrogen | 3.49 $/kg | Reduce emission by 7.66 kg CO2 eq. |
| 2. | Chemical conversion - Epichlorohydrin | 1,282 €/t | |
| 3. | Chemical conversion - Esters | 1.7 €/kg | – |
| 4. | Catalytic conversion - Acrolein | 1.13 EUR/kg | – |
| 5. | Catalytic conversion - DHA | 17.02 RMB/kg | – |
| 6. | Catalytic conversion – Ketals/Acetals | 12.29US$/kg | – |
| 7. | Catalytic conversion – Propylene glycol | 1.326 $/kg | CO2 emissions - 1.21 million tons of CO2 eq. Reduce the CO2 emissions by 2.79% |
3. The commercialization of different products and economic viability
The current utility of the glycerol in the different chemical industry has been worked out and presented in Table 15.
Table 15.
Consumption of glycerol for different products and industries.
| Sector | Consumption (%) |
|---|---|
| Food | 8 |
| Polyether | 14 |
| Personal Care | 15 |
| Pharmaceuticals | 11 |
| Alkyd Resins | 8 |
| Detergents | 2 |
| Explosives | 2 |
| Paper | 1 |
| Tobacco | 4 |
| Triacetin | 10 |
| Resale | 15 |
| Others | 10-12 |
1,3-propanediol (PDO) can either be produced by bio-conversion or chemical conversion of crude glycerol. PDO produced via bioprocess consumes 40 % less energy than petroleum-based PDO [143]. Commercially, PDO is produced from acrolein or ethylene oxide. PDO is commercially used to produce polyester named polytrimethylene terephthalate (PTT) [143]. The major application of PTT is as carpet fibers, apparel fibers, in film and packaging industry, in the ETP area. The unique properties of PTT make it a better product than other polymers. The current market estimation of 1,3-propanediol is at ca. 400 kt y−1. In the near future, it is expected to raise by 10 % per year [144].
Lactic acid (LA) or 2-hydroxypropionic acid is the most commercially used hydroxycarboxylic acids [145]. It can be either produced biologically or chemically from crude glycerol. The prominent manufacturers of LA and PLA are Purac (Netherlands), Cargill, Henan Jindan Lactic acid Technology Co. Ltd. (China) uses dextrose derived from corn starch, Nature Works LLC (USA), Pyramid Bioplastics Guben GmBH (Germany) and Archer Daniels Midland Company (USA) [145,146]. The commercial use of lactic acid is in producing biodegradable thermostable polylactic acid (PLA), which is the major component of bioplastic polymers [146]. In 2012, this polymer was industrially produced in an amount of 180 Kton which will reach to 1Mton by 2020 [147]. In 2015, the estimated market size of lactic acid was 0.33 Mt and is predicted to expand by ca. 10 % annually until 2025 [144]. Worldwide, acrylic acid is produced from propylene at the 6 Mt y−1 scale and this figure is expected to increase to 8 Mt y−1 by 2020. In 2015, the market volume of Glycerol Carbonate was ca. 200 kt y−1 and is forecasted to reach ca. 3 Mt y−1 in 2020 [144]. Sensiva SC50 (3-[(2-ethylhexyl)oxy]-1,2-propanediol), a glycerol ether, is produced in Industry from epichlorohydrin (ECH) by M/s Schülke and Mayr [8] which is used in the preparation of other functioned ethers. The KAO Co. reportedly involved in the production of glycerol isostearyl ether which is also commercially known as Penetol GE-IS [8].
Propylene glycol (PPG) is produced by M/s Shell and Lyondell, using acid or base catalysed ring opening reaction of propylene oxide and approximately 80,000 tonnes/annum is being produced [148,149]. Shell and Degussa patented the conversion process of PPG produced from petrochemicals [149,150]. The DuPont along with companies like Genencor and Tate & Lyle commercially produces 1,3-propanediol via biological conversion of glycerol or glucose [149]. The production of Epichlorohydrin (ECH) via halogenation was commercialized by Solvay in France [150]. Epichlorohydrin obtained from glycerol-based dichloro-propanol is already being produced at the commercial level by M/s Solvay, Dow and Shandong Chemical Companies (Belgium), the United States and China [151]. ECH is a significant intermediate for the synthesis of epoxy resins. Biosuccinium has been producing from renewable carbon sources by Reverdia in Italy since 2012 [152], a joint venture between Corbion Purac and BASF built in 2013, a plant in Spain since 2013 and Myriant and Bioamber, operating in Canada and North America, respectively [152] with the capacity of 10 Kton/year. It turns out that an economic plant size is 26,000 t/yr [153].
Presently, the market of glycerol is likely to change unexpectedly and the price of glycerol market is directly dependent on the supply of biodiesel. An increase in biodiesel production can cause a fall in the price of glycerol. As expected, quantity of biodiesel is hiked by 5 billion liters, reaching a total of ca. 40 billion litres by 2020 [154]. It was projected in 2006 that by 2020, glycerol production would be 6 times more than the demand. The global production of biodiesel will rise by 70 %. Due to such a huge increment in biodiesel production, the commercial price of glycerol falls almost to half from 2004 to 2011 [139]. Glycerol supply is reliant on the performance of the biodiesel market. The surplus of glycerol present in the market raises the question on the sustainable use of the biodiesel [155]. Though one can use pure glycerol for the production of many chemicals with industrial applications, the purification of crude glycerol is costly and economically not viable. Hence, the discovery of new applications and the expansion of the glycerol market will help in growing up the biorefinery at large. The use of recent inventions in the traditional utilization of glycerol will help in the expansion of the glycerol market. Also, new geographical markets should appear as because the developing countries experience improvement in their standard of living with direct or indirect involvement in biorefinery set up, based on vegetable oil. The economic viability of the processes is assessed in terms of net profit, return on investment (ROI), net present value (NPV) and break-even price [155]. For the evaluation of a project, the economic model used is the discounted cash-flow rate of return (DCFROR) which considers the time value money. This model enables to calculate the net present value (NPV) and the minimum selling price by setting a target discount rate [152]. The production cost of a product includes the manufacturing costs and the management costs, which further comprises of raw materials cost, direct and indirect labor, utilities, supplies, maintenance, laboratory, equipment depreciation, insurance and taxes [154]. The total invested capital includes tied-up capital and working capital [154]. The tied-up capital constitutes the direct capital, building expenses, research, and start up and other expenses items. Direct capital is calculated as the sum between the fixed capital and the honoraries [154]. The economic analysis includes the estimation of capital expenditure (CAPEX), annual capital cost (CACC), annual operating cost (CAOC), and revenue (R) [141]. A profitable venture is indicated by positive NPV, a zero NPV indicates the venture breaks even, and loss of a venture is indicated by negative NPV [141] as depicted in (1), (2) and (3).
-
(1)
PV = FV/ {(1 + r)n} where, PV = Present value, FV = Future value, r = interest rate and n is the number of years of investment.
-
(2)
NPV where, NPV = Net Present Value, Cf = Cash Flow, n = year, r = discount rate, TPL = plant life
-
(3)
Cash Flow = Revenue – (Annual operating cost + Annual capital cost)
3.1. Factors affecting the cost and production of different products
The production cost of biodiesel increases by $0.021/L for every $0.22/kg cause reduction in glycerol selling price [26]. Mixed cultures have been applied under non sterile conditions to minimize the production cost of 1,3-propanediol [20]. The coproduction of succinic acid with the total production cost (TPC) is round about 4.37 M$/y [156]. TPC obtained in the production of succinic acid is approximately 3.86 M$/y, being within a range of variation of less than 12 % from the TPC reported in Vlysidis et al. [152]. The production cost of epichlorohydrin is estimated to be 36 M€/yr [153]. The cost of a unit of ECH production is 1,282 €/t according to the capacity of 26, 540 t/yr of the plant. The selling price of ECH is 1,976.22 €/t in 2014 [153]. The tied-up capital adds up to 55.6 M€. The lower price of glycerol as compared to sugars is a key to lower the material costs [144].
3.2. Barriers in the commercialization of different products
The high price of pure glycerol limits the economic viability of the glycerol transformation [20]. PHA’s represent naturally occurring bio-polymers produced via fermentation from several bacterial strains using the mineral medium named glycerol. Halomonas sp. SA8, a soil bacteria from Finnish soils and sediments have been reported for the intracellular accumulation of PHA (56 %). The produced PHA, identified as a PHB homopolymer, shows a great hindrance in the commercialised production of PHA’s [26]. The possible use of these organisms commercially could be limited due to many reasons such as pathogenicity, strict anaerobic conditions and impurities present in the substrate that may affect conversion rates [20]. The production of 1,3-propanediol by chemical synthesis involves the use of expensive catalysts, high pressure conditions and production of toxic intermediates that are not environmentally friendly which prevents the commercialisation of the process [20]. Similarly, the slow consumption rates of glycerol and unfavorable productivities cause the process unsuitable for industrialization, though fermentation of glycerol provides better yield of ethanol.
In most of the studies, the use of batch reactor for the production of 1,2-propanediol through hydrogenolysis, is a barrier for the commercialisation of the production process [116]. Though, there is wide usage of this process at large scale, but has some undesirable features mainly low yield [153]. Also, in chlorination and hypochlorination steps, it is expensive to dispose of the chlorinated organic, produced by the conventional production along with the waste water [153]. The toxic oxidants used in the oxidation process makes difficult to control the reaction pathway. Thus, bio-conversion is superior approach, but lower conversion and tedious extraction process limits its commercialisation [157]. Therefore, electrochemical oxidation is a green approach that overcomes all the limitations associated with oxidation of glycerol [157]. The main environment drawback of the Lactic acid production is the co-production of gypsum, whose disposal is a harmful [147]. The existence of bulky compounds leads to the deposition of carbon and coke, which is a barrier for the reforming of crude glycerol. This unreasonable coking leads to catalyst inactivation and lowers the yield of hydrogen [11]. Some systems need high running cost production along with high pressure, temperature, long reaction time, hinder the possible commercialisation [158]. Thus, to make the processes environmental and competitive, researchers should focus on the evolution of the catalysts with enhanced ability [144].
4. Conclusion and recommendations
The worldwide vast production of glycerol due to the biodiesel production stimulates the researchers to develop new commercially viable technologies. It helps in the valorisation of glycerol so that the glycerol glut does not alter the sustainability of the biodiesel industry, i.e. contribute positively to the environmental economy of the biodiesel industry. Thus, make this industry a profitable one and can lead to the biorefinery. There can be a change in the market scenario of biofuels due to the development of new technologies involving glycerine-to-methanol and glycerine-to-ethanol production pathway. Use of multi-step pathways and hybrid catalysts along with continuous flow reactor should be investigated on laboratory scale that can further be adapted on an industrial level. For addressing the issue of glycerol, various researchers have reported different processes for the conversion of glycerol into valuable chemicals such as PDO, acetate, butyrate, n-butanol, ethanol, lactic acid, hydrogen, propionic acid, trihalose, glyceric acid, citric acid, succinic acid, DHA, EPA, PHA, biogas, polyglycerols, solketal, MTBE, TTBG, ETBE, DAG, TAG, DCP, ECH, glycerol carbonate, glycerol esters, hydrogen, TA, MA, propylene glycol, monoglycerides, acrolein etc. All these chemicals have definite commercial value that can contribute to the biodiesel economy.
The recent advancement in the technology and commercial utilization of crude glycerol for value-added product formulation clearly establish the fact that crude glycerol can play a vital role in the realization of bio-refining industry based on oilseeds.
Declaration of Competing Interest
None.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00487.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Anitha M., Kamarudin S.K., Kofli N.T. The potential of glycerol as a value-added commodity. Chem. Eng. J. 2016;295:119–130. doi: 10.1016/j.cej.2016.03.012. [DOI] [Google Scholar]
- 2.Rastrogi A., Jha M.K., Sarma A.K. A comparative study of kinetics for combustion versus pyrolysis of Mesua ferrea husk, soya husk, and Jatropha curcas husk using thermogravimmetry and different methods. Energy Sources Part A Recovery Util. Environ. Eff. 2016;38(10):1355–1363. doi: 10.1080/15567036.2014.913094. [DOI] [Google Scholar]
- 3.Sharma N., Sharma N. Second generation bioethanol production from lignocellulosic waste and its future perspectives: a review. Int. J. Curr. Microbiol. Appl. Sci. 2018;7(5):1285–1290. [Google Scholar]
- 4.Robak K., Balcerek M. Review of second-generation bioethanol production from residual biomass. Food Technol. Biotechnol. 2018;56(2):174–187. doi: 10.17113/ftb.56.02.18.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J., Yang L., Luo W., Yang G., Miao C., Fu J. Sustainable biodiesel production via transesterification by using waste cooking oil by using CaO catalysts prepared from chicken manure. Energy Convers. Manage. 2016;123:487–497. doi: 10.1016/j.fuel.2017.02.007. [DOI] [Google Scholar]
- 6.Tran T.T.V., Kaiprommarat S., Kongparakul S., Reubroycharoen P., Guan G., Nguyen M.H. Green biodiesel production from waste cooking oil using an environmentally benign acid catalyst. Waste Manag. 2016;52:367–374. doi: 10.1016/j.wasman.2016.03.053. [DOI] [PubMed] [Google Scholar]
- 7.Kong P.S., Kheireddine M., Mohd W., Wan A. Conversion of crude and pure glycerol into derivatives : a feasibility evaluation. Renew. Sustain. Energy Rev. 2016;63:533–555. doi: 10.1016/j.rser.2016.05.054. [DOI] [Google Scholar]
- 8.Sutter M., Da Silva E., Duguet N., Raoul Y., Me E., Lemaire M. Glycerol ether synthesis : a bench test for green chemistry concepts and technologies. Chem. Rev. 2014;115(16):8609–8651. doi: 10.1021/cr5004002. [DOI] [PubMed] [Google Scholar]
- 9.Chouhan A.P.S., Sarma A.K. Modern heterogeneous catalysts for biodiesel production : a comprehensive review. Renew. Sustain. Energy Rev. 2011;15(9):4378–4799. doi: 10.1016/j.rser.2011.07.112. [DOI] [Google Scholar]
- 10.Ramos M., Paula A., Dias S., Puna J.F. Gomes J. Biodiesel production processes and sustainable raw materials. Energies. 2019;12(23):1–30. doi: 10.3390/en12234408. [DOI] [Google Scholar]
- 11.Rodrigues A. Upgrading the glycerol from biodiesel production as a source of energy carriers and chemicals. Energies. 2017;10(11):1–36. doi: 10.3390/en10111817. [DOI] [Google Scholar]
- 12.Talebian-kiakalaieh A., Aishah N., Amin S., Najaafi N., Tarighi S. A review on the catalytic acetalization of bio-renewable glycerol to fuel additives. Front. Chem. 2018;6:1–25. doi: 10.3389/fchem.2018.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vivek N., Sindhu R., Madhavan A., Jose A., Castro E., Faraco V. Recent advances in the production of value added chemicals and lipids utilizing biodiesel industry generated crude glycerol as a substrate – metabolic aspects, challenges and possibilities : an overview. Bioresour. Technol. 2017;239:507–517. doi: 10.1016/j.biortech.2017.05.056. [DOI] [PubMed] [Google Scholar]
- 14.Monteiro M.R., Kugelmeier C.L., Pinheiro R.S., Otávio M., César S. Glycerol from biodiesel production : technological paths for sustainability. Renew. Sustain. Energy Rev. 2018;88:109–122. doi: 10.1016/j.rser.2018.02.019. [DOI] [Google Scholar]
- 15.Tan H.W., Abdul Aziz A.R., Aroua M.K. Glycerol production and its applications as a raw material: a review. Renew. Sustain. Energy Rev. 2013;27:118–127. doi: 10.1016/j.rser.2013.06.035. [DOI] [Google Scholar]
- 16.Cornejo A., Barrio I., Campoy M., Lázaro J., Navarrete B. Oxygenated fuel additives from glycerol valorization. Main production pathways and e ff ects on fuel properties and engine performance : a critical review. Renew. Sustain. Energy Rev. 2017;79:1400–1413. doi: 10.1016/j.rser.2017.04.005. [DOI] [Google Scholar]
- 17.Wolfson A., Dlugy C., Shotland Y. Glycerol as a green solvent for high product yields and selectivities. Environ. Chem. Lett. 2007;5:67–71. doi: 10.1007/s10311-006-0080-z. [DOI] [Google Scholar]
- 18.Gholami Z., Zuhairi A., Lee K. Dealing with the surplus of glycerol production from biodiesel industry through catalytic upgrading to polyglycerols and other value-added products. Renew. Sustain. Energy Rev. 2014;39:327–341. doi: 10.1016/j.rser.2014.07.092. [DOI] [Google Scholar]
- 19.Ardi M.S., Aroua M.K., Hashim N.A. Progress, prospect and challenges in glycerol puri fi cation process : a review. Renew. Sustain. Energy Rev. 2015;42:1164–1173. doi: 10.1016/j.rser.2014.10.091. [DOI] [Google Scholar]
- 20.Pradima J., Kulkarni M.R. Review on enzymatic synthesis of value added products of glycerol, a by-product derived from biodiesel production. Resource-Efficient Technol. 2017;(4):394–405. doi: 10.1016/j.reffit.2017.02.009. [DOI] [Google Scholar]
- 21.Samul D., Leja K., Grajek W. Impurities of crude glycerol and their effect on metabolite production. Ann. Microbiol. 2014;64:891–898. doi: 10.1007/s13213-013-0767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo X., Ge X., Cui S., Li Y. Value-added processing of crude glycerol into chemicals and polymers. Bioresour. Technol. 2016;215:144–154. doi: 10.1016/j.biortech.2016.03.042. [DOI] [PubMed] [Google Scholar]
- 23.Yang F., Hanna M.A., Sun R. Value-added uses for crude glycerol – a byproduct of biodiesel production. Biotechnol. Biofuels. 2012;5(13):1–13. doi: 10.1186/1754-6834-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J., Yan S., Zhang X., Dayal R., Surampalli R.Y., Valéro J.R. Chemical and biological conversion of crude glycerol derived from waste cooking oil to biodiesel. Waste Manag. 2018;71:164–175. doi: 10.1016/j.wasman.2017.10.044. [DOI] [PubMed] [Google Scholar]
- 25.Hong A., Cheng K., Peng F., Zhou S., Sun Y., Liu C. Strain isolation and optimization of process parameters for bioconversion of glycerol to lactic acid. J. Chem. Technol. Biotechnol. 2009;84(10):1576–1581. doi: 10.1002/jctb.2209. [DOI] [Google Scholar]
- 26.Garlapati V.K., Shankar U., Budhiraja A. Bioconversion technologies of crude glycerol to value added industrial products. Biotechnol. Rep. 2016;9:9–14. doi: 10.1016/j.btre.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dikshit P.K., Padhi S.K., Moholkar V.S. Process optimization and analysis of product inhibition kinetics of crude glycerol fermentation for 1, 3 – dihydroxyacetone production. Bioresour. Technol. 2017;244:362–370. doi: 10.1016/j.biortech.2017.07.136. [DOI] [PubMed] [Google Scholar]
- 28.Kaur G., Srivastava A.K., Chand S. Bioconversion of glycerol to 1, 3-propanediol : a mathematical model-based nutrient feeding approach for high production using Clostridium diolis. Bioresour. Technol. 2013;142:82–87. doi: 10.1016/j.biortech.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 29.Khanna S., Jaiswal S., Goyal A., Moholkar V.S. Ultrasound enhanced bioconversion of glycerol by Clostridium pasteurianum : a mechanistic investigation. Chem. Eng. J. 2012;200–202:416–425. doi: 10.1016/j.cej.2012.06.040. [DOI] [Google Scholar]
- 30.Black C.S., Nair G.R. University of Waikato; Hamilton, New Zealand: 2013. Bioconversion of Glycerol to Dihydroxyacetone by Immobilized Gluconacetobacter Xylinus Cells; pp. 10–14. 4. [DOI] [Google Scholar]
- 31.Kumar P., Sharma R., Ray S., Mehariya S., Sanjay K.S., Lee J. Dark fermentative bioconversion of glycerol to hydrogen by Bacillus thuringiensis. Bioresour. Technol. 2015;182:383–388. doi: 10.1016/j.biortech.2015.01.138. [DOI] [PubMed] [Google Scholar]
- 32.Jinyang X., Xuebing Z., Wei D., Dehua L. Bioconversion of glycerol into lipids by Rhodosporidium toruloides in a two‐stage process and characterization of lipid properties. Eng. Life Sci. 2017;17(3):303–313. doi: 10.1002/elsc.201600062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nwachukwu R.E.S., Shahbazi A., Wang L., Ibrahim S., Worku M., Schimmel K. Bioconversion of glycerol to ethanol by a mutant Enterobacter aerogenes. AMB Express. 2012;2:1–6. doi: 10.1186/2191-0855-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheril S.N., Lai-Yee P., Toshinari M., Abd-Aziz Suraini W., Minato S., Yoshihto H. Mohd Ali, Bioconversion of glycerol for bioethanol production using isolated Escherichia coli ss1. Braz. J. Microbiol. 2012;43(2):506–516. doi: 10.1590/S1517-83822012000200011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sara M., Tayssir G., Rouissi T., Blais F. Valorization of raw glycerol and crustacean waste into value added products by Yarrowia lipolytica. Bioresour. Technol. 2017;243:57–68. doi: 10.1016/j.biortech.2017.06.074. [DOI] [PubMed] [Google Scholar]
- 36.Szymanowska-powa D., Leja K. An increasing of the efficiency of microbiological synthesis of 1, 3-propanediol from crude glycerol by the concentration of biomass. Electron. J. Biotechnol. 2014;17:72–78. doi: 10.1016/j.ejbt.2013.12.010. [DOI] [Google Scholar]
- 37.Chi Z., Pyle D., Wen Z., Frear C., Chen S. A laboratory study of producing docosahexaenoic acid from biodiesel-waste glycerol by microalgal fermentation. Process Biochem. 2007;42:1537–1545. doi: 10.1016/j.procbio.2007.08.008. [DOI] [Google Scholar]
- 38.Nwachukwu R.E.S., Shahbazi A., Wang L., Worku M., Ibrahim S. Optimization of cultural conditions for conversion of glycerol to ethanol by Enterobacter aerogenes. AMB Express. 2013;3:1–10. doi: 10.1186/2191-0855-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao Z., Ma Y., Wang Q., Zhang M., Wang J., Liu Y. Effect of crude glycerol impurities on lipid preparation by Rhodosporidium toruloides yeast. Bioresour. Technol. 2016;218:373–379. doi: 10.1016/j.biortech.2016.06.088. [DOI] [PubMed] [Google Scholar]
- 40.Kumar L.R., Kumar S., Tyagi R.D., Zhang X. A review on variation in crude glycerol composition, bio-valorization of crude and purified glycerol as carbon source for lipid production. Bioresour. Technol. 2019;293:122155. doi: 10.1016/j.biortech.2019.122155. [DOI] [PubMed] [Google Scholar]
- 41.Xu J., Banerjee A., Pan S., Jian Z. Galactose can be an inducer for production of therapeutic proteins by auto-induction using E. Coli BL21 strains. Protein Expr. Purif. 2012;83:30–36. doi: 10.1016/j.pep.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 42.Chemicals B., Nda-umar U.I., Ramli I., Taufiq-yap Y.H. An overview of recent research in the conversion of glycerol into biofuels, fuel additives and other bio-based chemicals. Catalysts. 2018;9:1–47. doi: 10.3390/catal9010015. [DOI] [Google Scholar]
- 43.Sun Y., Shen J., Yan L., Zhou J., Jiang L., Chen Y. Advances in bioconversion of glycerol to 1,3-propanediol: prospects and challenges. Process. Biochem. 2018;71:134–146. doi: 10.1016/j.procbio.2018.05.009. [DOI] [Google Scholar]
- 44.Almeida J.R.M., Fávaro L.C.L., Quirino B.F. Biodiesel biorefinery : opportunities and challenges for microbial production of fuels and chemicals from glycerol waste. Biotechnol. Biofuels. 2012;5:1–16. doi: 10.1186/1754-6834-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Industry C., Danilovic B., Savic D. Valorization of crude glycerol from biodiesel production. Chem. Ind. Chem. Eng. Q. 2016;22:19. doi: 10.2298/CICEQ160303019K. [DOI] [Google Scholar]
- 46.Siti N.I.K., Nek M., Din MAT Idris Z., Kian Y.S. Preparation of polyglycerol from palm biodiesel crude glycerine. J. Oil Palm Res. 2013;25:289–297. [Google Scholar]
- 47.Eze V.C., Harvey A.P. Continuous reactive coupling of glycerol and acetone - a strategy for triglyceride transesterification and in-situ valorisation of glycerol by-product. Chem. Eng. J. 2018;347:41–51. doi: 10.1016/j.cej.2018.04.078. [DOI] [Google Scholar]
- 48.Srinivas M., Sree R., Raveendra G., Kumar C.R., Prasad P.S.S., Lingaiah N. Selective etherification of glycerol with tert-butanol over 12-tungstophosphoric acid catalysts supported on Y-zeolite. Indian J. Chem. 2014;53:524–529. [Google Scholar]
- 49.Okoye P.U., Abdullah A.Z., Hameed B.H. Synthesis of oxygenated fuel additives via glycerol esterification with acetic acid over bio-derived carbon catalyst. Fuel. 2017;209:538–544. doi: 10.1016/j.fuel.2017.08.024. [DOI] [Google Scholar]
- 50.Wang K., Hawley M.C., DeAthos S.J. Conversion of glycerol to 1,3-Propanediol via selective dehydroxylation. Ind. Eng. Chem. Res. 2003;42:2913–2923. doi: 10.1021/ie020754h. [DOI] [Google Scholar]
- 51.Yang Z., Liting Y., Shanyu T., Longjia L., Wanjie W., Bingxin Y., Guanyu Y. Highly efficient acetalization and ketalization catalyzed by cobaloxime under solvent-free condition. Catalysts. 2018;8:1–10. doi: 10.3390/catal8020048. [DOI] [Google Scholar]
- 52.Guerrero-pérez M.O., Rosas J.M., Bedia J., Rodríguez-mirasol J., Cordero T. Recent inventions in glycerol transformations and processing. Recent Patents on Engineering. Benthem Sci. 2009;2:1–11. doi: 10.2174/1874478810902010011. [DOI] [Google Scholar]
- 53.Ishak Z.I., Sairi N.A., Alias Y., Kheireddine M., Aroua T., Yusoff R. Production of glycerol carbonate from glycerol with aid of ionic liquid as catalyst. Chem. Eng. J. 2016;297 doi: 10.1016/j.cej.2016.03.104. [DOI] [Google Scholar]
- 54.Behr A., Eilting J., Irawadi K., Leschinski J., Lindner F. Improved utilisation of renewable resources : new important derivatives of glycerol. Green Chem. 2008;10:13–30. doi: 10.1039/b710561d. [DOI] [Google Scholar]
- 55.Prakruthi H.R., Prakash B.S.J., Bhat Y.S. Microwave assisted synthesis of glycerol carbonate over LDH catalyst : activity restoration through rehydration and reconstruction. J. Mol. Catal. A Chem. 2015;408:214–220. doi: 10.1016/j.molcata.2015.07.036. [DOI] [Google Scholar]
- 56.Parameswaram G., Rao P.S.N., Srivani A., Rao G.N., Lingaiah N. Magnesia-ceria mixed oxide catalysts for the selective transesterification of glycerol to glycerol carbonate. Mol. Catal. 2017;451:135–142. doi: 10.1016/j.mcat.2017.12.006. [DOI] [Google Scholar]
- 57.Lari Giacomo M., Giorgio P., Cecilia M., Javier Perez-R. Towards sustainable manufacture of epichlorohydrin from glycerol using hydrotalcite-derived basic oxides. Green Chem. 2018;20:148–159. doi: 10.1039/C7GC02610B. [DOI] [Google Scholar]
- 58.Santacesaria E., Tesser R., Di Serio M., Casale L., Verde D., Uni V. New process for producing epichlorohydrin via glycerol chlorination. Ind. Eng. Chem. Res. 2010;49:964–970. [Google Scholar]
- 59.Wu D., Zhou S. A new coupling process for synthesis of epichlorohydrin from dichloropropanols. Adv. Eng. Res. 2016:715–720. doi: 10.2991/mmeceb-15.2016.141. [DOI] [Google Scholar]
- 60.Briggs J.R., Chambers S.M., Gaarenstroom P.D., Kearns K. Glycerin as a Renewable Feedstock for Epichlorohydrin Production. The GTE Process. CLEAN - Soil Air Water. 2008;36:657–661. doi: 10.1002/clen.200800067. [DOI] [Google Scholar]
- 61.Rahman H., Jayabaya U., Yunus R., Widyawati Y., Jayabaya U. kinetics of epichlorohydrin syntesis using dichloropropanol and sodium kinetics of epichlorohydrin syntesis using dichloropropanol and sodium hydroxide. Conference: The 3rd International Seminar on Chemstry. 2018 [Google Scholar]
- 62.Gu Y., Azzouzi A., Pouilloux Y., Jérôme F., Barrault J. Heterogeneously catalyzed etherification of glycerol: new pathways for transformation of glycerol to more valuable chemicals. Green Chem. 2008;10:164–167. doi: 10.1039/B715802E. [DOI] [Google Scholar]
- 63.Saengarun C., Petsom A., Tungasmita D.N. Etherification of glycerol with propylene or 1-Butene for fuel additives. Sci. World J. 2017:11. doi: 10.1155/2017/4089036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yadav V.P., Maity S.K., Shee D. Etherification of glycerol with ethanol over solid acid catalysts : kinetic study using cation exchange resin. Indian Chem. Eng. 2016;59:117–135. doi: 10.1080/00194506.2016.1139472. [DOI] [Google Scholar]
- 65.Liu F., De Oliveira Vigier K., Pera-Titus M., Pouilloux Y., Clacens J.-M.-M., Decampo F. Catalytic etherification of glycerol with short chain alkyl alcohols in the presence of Lewis acids. Green Chem. 2013;15:901–909. doi: 10.1039/c3gc36944g. [DOI] [Google Scholar]
- 66.Roze M., Kampars V., Teivena K., Kampare R., Liepins E. Catalytic etherification of glycerol with alcohols. Mater. Sci. Appl. Chem. 2013;28:67–72. doi: 10.7250/msac.2013.011. [DOI] [Google Scholar]
- 67.Di Serio M., Casale L., Tesser R., Santacesaria E. New process for the production of glycerol tert-butyl ethers. Energy Fuels. 2010;24:4668–4672. doi: 10.1021/ef901230r. [DOI] [Google Scholar]
- 68.Klepáčová K., Mravec D., Hajekova E., Bajus M. Etherification of glycerol. Pet. Coal J. 2003;45:1–2. [Google Scholar]
- 69.Dailey W. Production of ethers of glycerol from crude glycerol - the by-product of biodiesel production. Biomaterials. Commons. 2017:1–14. [Google Scholar]
- 70.García-sancho C., Moreno-tost R., Mérida-robles J.M., Santamaría-gonzález J., Jiménez-lópez A., Torres P.M. Etherification of glycerol to polyglycerols over MgAl mixed oxides. Catal. Today. 2011;167:84–90. doi: 10.1016/j.cattod.2010.11.062. [DOI] [Google Scholar]
- 71.Ayoub M., Khayoon M.S., Abdullah A.Z. Synthesis of oxygenated fuel additives via the solventless etherification of glycerol. Bioresour. Technol. 2012;112:308–312. doi: 10.1016/j.biortech.2012.02.103. [DOI] [PubMed] [Google Scholar]
- 72.Barrault J., Clacens J., Pouilloux Y. Selective oligomerization of glycerol over mesoporous catalysts. Top. Catal. 2004;27:137–142. [Google Scholar]
- 73.Clacens J., Pouilloux Y., Barrault J. Selective etherification of glycerol to polyglycerols over impregnated basic MCM-41 type mesoporous catalysts. Appl. Catal. A Gen. 2002;227:181–190. [Google Scholar]
- 74.Ardila-suárez C., Rojas-avellaneda D., Ramirez-caballero G.E. Effect of temperature and catalyst concentration on polyglycerol during synthesis. Int. J. Polym. Sci. 2015;5:1–8. [Google Scholar]
- 75.Kong P.S., Aroua M.K., Mohd W., Wan A. Catalytic esterification of bioglycerol to value-added products. Rev. Chem. Eng. 2015:437–451. doi: 10.1515/revce-2015-0004. [DOI] [Google Scholar]
- 76.Rastegari H., Ghaziaskar H.S. From glycerol as the by-product of biodiesel production to value-added monoacetin by continuous and selective esterification in acetic acid. J. Ind. Eng. Chem. 2015;21:856–861. doi: 10.1016/j.jiec.2014.04.023. [DOI] [Google Scholar]
- 77.Indrayanah S., Leung K.K., Yau Y.H. Esterification reaction of glycerol and Palm oil oleic acid using methyl ester sulfonate acid catalyst as drilling fluid formulation. IOP Conference Series: Materials Science and Engineering. 2017 doi: 10.1088/1742-6596/755/1/011001. [DOI] [Google Scholar]
- 78.Kale S., Armbruster U., Umbarkar S., Dongare M., Martin A. Esterification of glycerol with acetic acid for improved production of triacetin using toluene as an entrainer. 10th Green Chemistry Conference. 2013:70–71. [Google Scholar]
- 79.Kotwal M., Deshpande S.S., Srinivas D. Esterification of fatty acids with glycerol over Fe-Zn double-metal cyanide catalyst. Catal. Commun. 2011;12:1302–1306. doi: 10.1016/j.catcom.2011.05.008. [DOI] [Google Scholar]
- 80.Liu X., Ma H., Wu Y., Wang C., Yang M., Yan P. Esterification of glycerol with acetic acid using double SO3H-functionalized ionic liquids as recoverable catalysts. Green Chem. 2011;13:697–701. doi: 10.1039/c0gc00732c. [DOI] [Google Scholar]
- 81.Bombos D., Bombos M., Bolocan I., Vasilievici G., Zaharia E. Esterification of glycerol with technical olein in heterogeneous catalysis. Revista de Chimie. 2010;61:784–787. [Google Scholar]
- 82.Ferreira P., Fonseca I.M., Ramos A.M., Vital J., Castanheiro J.E. Esterification of glycerol with acetic acid over dodecamolybdophosphoric acid encaged in USY zeolite. Catal. Commun. 2009;10:481–484. doi: 10.1016/j.catcom.2008.10.015. [DOI] [Google Scholar]
- 83.HD H. Effects of molar ratio, catalyst concentration and temperature on transesterification of Triolein with ethanol under ultrasonic irradiation. J. Jpn. Pet. Inst. 2007;50:195–199. [Google Scholar]
- 84.Felizardo P.M., Gomes J.F. Study on the glycerolysis reaction of high free fatty acid oils for use as biodiesel feedstock. Fuel Process. Technol. 2011;92(6):1225–1229. doi: 10.1016/j.fuproc.2011.01.020. [DOI] [Google Scholar]
- 85.Kong P.S., Pérès Y., Mohd W., Wan A., Cognet P., Granger P. Esterification of glycerol with oleic acid over hydrophobic zirconia-silica acid catalyst and commercial acid catalyst : optimization and influence of catalyst acidity. Front. Chem. 2019;7:1–11. doi: 10.3389/fchem.2019.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reddy R., Patel S., Nair S., Suvikram Y. Preparation of hydrogen from glycerol via steam reforming process. Nuicone Org. 2011:8–10. [Google Scholar]
- 87.Sadanandam G., Sreelatha N., Sharma M.V.P., Reddy S.K., Srinivas B., Venkateswarlu K. Steam reforming of glycerol for hydrogen production over Ni / SiO 2 catalyst. Int. Sch. Res. Notices. 2012:10. doi: 10.5402/2012/591587. [DOI] [Google Scholar]
- 88.Manfro R.L., Ribeiro N.F.P., MVM Souza M. Production of hydrogen from steam reforming of glycerol using nickel catalysts supported on Al2O3, CeO2 and ZrO2. Catal. Sustain. Energy. 2013:60–70. doi: 10.2478/cse-2013-0001. [DOI] [Google Scholar]
- 89.Suffredini D.F.P., Thyssen V.V., De Almeida P.M.M., Gomes R.S., Borges M.C., Duarte A.M. Renewable hydrogen from glycerol reforming over nickel aluminate-based catalysts. Catal. Today. 2017;289:96–104. doi: 10.1016/j.cattod.2016.07.027. [DOI] [Google Scholar]
- 90.Kunkes E.L., Soares R.R., Simonetti D.A., Dumesic J.A. An integrated catalytic approach for the production of hydrogen by glycerol reforming coupled with water-gas shift. Appl. Catal. B Environ. 2009;90:693–698. doi: 10.1016/j.apcatb.2009.04.032. [DOI] [Google Scholar]
- 91.Valatkevi P. Glycerol steam reforming for hydrogen and synthesis gas production. Int. J. Hydrogen Energy. 2017:1–9. doi: 10.1016/j.ijhydene.2016.12.071. [DOI] [Google Scholar]
- 92.Adhikari S., Fernando S.D., To S.D.F., Bricka R.M., Steele P.H., Haryanto A. Conversion of glycerol to hydrogen via a steam reforming process over nickel catalysts. Energy Fuels. 2008;50:2367–2373. [Google Scholar]
- 93.Bineesh K.V., Kim D.K., Kim D.W., Cho H.J., Dae-Won P. 2010. Fuels of the Future Towards Efficient Hydrogen Production From Glycerol by Sorption Enhanced Steam Reforming. This paper is published as part of an Energy & Environmental Science Themed Issue on. [DOI] [Google Scholar]
- 94.Wang C., Dou B., Chen H., Song Y., Xu Y., Du X. Renewable hydrogen production from steam reforming of glycerol by Ni-Cu-Al, Ni-Cu-Mg, Ni-Mg catalysts. Int. J. Hydrogen Energy. 2013;8:1–9. doi: 10.1016/j.ijhydene.2013.01.042. [DOI] [Google Scholar]
- 95.Sudarsanam P., Zhong R., Van Den Bosch S. Functionalised heterogeneous catalysts for sustainable biomass valorisation. Chem. Soc. Rev. 2020;47:8349–8402. doi: 10.1039/c8cs00410b. [DOI] [PubMed] [Google Scholar]
- 96.Wang Y., Xiao Y., Xiao G. Sustainable value-added C3 chemicals from glycerol transformations: a mini review for heterogeneous catalytic processes. Chin. J. Chem. Eng. 2019;27:1536–1542. doi: 10.1016/j.cjche.2019.03.001. [DOI] [Google Scholar]
- 97.Tae Y., Jung K., Duck E. Microporous and Mesoporous Materials Gas-phase dehydration of glycerol over ZSM-5 catalysts. Microporous Mesoporous Mater. 2010;131:28–36. doi: 10.1016/j.micromeso.2009.11.037. [DOI] [Google Scholar]
- 98.Alhanash A., Kozhevnikova E.F., Kozhevnikov I.V. Gas-phase dehydration of glycerol to acrolein catalysed by caesium heteropoly salt. Appl. Catal. A Gen. 2010;378:11–18. doi: 10.1016/j.apcata.2010.01.043. [DOI] [Google Scholar]
- 99.Zhao H., Hui C., Mei L., Yi J., Li N., Min H. Dehydration of glycerol to acrolein over sulfuric acid-activated montmorillonite catalysts. Appl. Clay Sci. Catalytic. 2012:154–162. doi: 10.1016/j.clay.2012.09.011. [DOI] [Google Scholar]
- 100.Carrettin S., Mcmorn P., Johnston P., Griffin K., Kiely J., Hutchings G.J. Oxidation of glycerol using supported Pt, Pd and Au catalysts. Phys. Chem. Chem. Phys. 2003:1329–1336. doi: 10.1039/b212047j. [DOI] [Google Scholar]
- 101.Crotti C., Farnetti E. Selective oxidation of glycerol catalyzed by iron complexes. J. Mol. Catal. A Chem. 2015;396:353–359. doi: 10.1016/j.molcata.2014.10.021. [DOI] [Google Scholar]
- 102.Wu G., Wang X., Jiang T., Lin Q. Selective oxidation of glycerol with 3% H2O2 catalyzed by LDH-Hosted Cr(III) complex. Catalysts. 2015;5:2039–2351. doi: 10.3390/catal5042039. [DOI] [Google Scholar]
- 103.Skrzyńska E., Wondołowska-Grabowska A., Capron M., Dumeignil F. Crude glycerol as a raw material for the liquid phase oxidation reaction. Appl. Catal. A Gen. 2014;482:245–257. doi: 10.1016/j.apcata.2014.06.005. [DOI] [Google Scholar]
- 104.Mostafa N.A., Maher A., Abdelmoez W. Production of mono-, di-, and triglycerides from waste fatty acids through esterification with glycerol. Adv. Biosci. Biotechnol. 2013:900–907. [Google Scholar]
- 105.Konwar L.J., Mikkola N.K.J., Sarma A.K., Deka D. Selective esterification of fatty acids with glycerol to monoglycerides over – SO 3 H functionalized carbon catalysts. React. Kinet. Mech. Catal. 2016:121–138. doi: 10.1007/s11144-016-1040-7. [DOI] [Google Scholar]
- 106.Hong X., Mcgiveron O., Kolah A.K., Orjuela A., Peereboom L., Lira C.T. Reaction kinetics of glycerol acetal formation via transacetalization with 1, 1-diethoxyethane. Chem. Eng. J. 2013:374–381. doi: 10.1016/j.cej.2013.02.023. [DOI] [Google Scholar]
- 107.Rode C.V., Mane R.B., Potdar A.S., Patil P.B., Niphadkar P.S., Joshi P.N. Copper modified waste fly ash as a promising catalyst for glycerol hydrogenolysis. Catal. Today. 2012;190:31–37. doi: 10.1016/j.cattod.2011.11.038. [DOI] [Google Scholar]
- 108.Dasari M.A., Kiatsimkul P.P., Sutterlin W.R., Suppes G.J. Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl. Catal. A Gen. 2005;281:225–231. doi: 10.1016/j.apcata.2004.11.033. [DOI] [Google Scholar]
- 109.Bienholz A., Hofmann H., Claus P. Selective hydrogenolysis of glycerol over copper catalysts both in liquid and vapour phase: correlation between the copper surface area and the catalyst’s activity. Appl. Catal. A Gen. 2011;391:153–157. doi: 10.1016/j.apcata.2010.08.047. [DOI] [Google Scholar]
- 110.Chaminand J., Lauren Djakovitch, Gallezot P., Marion P., Pinel C., Rosier C. Glycerol hydrogenolysis on heterogeneous catalysts. Green Chem. 2004;6:359–361. doi: 10.1039/b407378a. [DOI] [Google Scholar]
- 111.Meher L.C., Gopinath R., Naik S.N., Dalai A.K. Catalytic hydrogenolysis of glycerol to propylene glycol over mixed oxides derived from a hydrotalcite-type precursor. Ind. Eng. Chem. Res. 2009;4:1840–1846. doi: 10.1021/ie8011424. [DOI] [Google Scholar]
- 112.Shozi M.L., Dasireddy VDBC Singh S., Mohlala P., Morgan D.J., Friedrich H.B. Hydrogenolysis of glycerol to Monoalcohols over supported Mo and W catalysts. ACS Sustain. Chem. Eng. 2016;4:5752–5760. doi: 10.1021/acssuschemeng.6b01675. [DOI] [Google Scholar]
- 113.Maris E.P., Davis R.J. Hydrogenolysis of glycerol over carbon-supported Ru and Pt catalysts. J. Catal. 2007;249:328–337. doi: 10.1016/j.jcat.2007.05.008. [DOI] [Google Scholar]
- 114.Freitas I.C., Manfro R.L., Souza M.M.V.M. Hydrogenolysis of glycerol to propylene glycol in continuous system without hydrogen addition over Cu-Ni catalysts. Appl. Catal. B Environ. 2018;220:31–41. doi: 10.1016/j.apcatb.2017.08.030. [DOI] [Google Scholar]
- 115.Kant A., He Y., Jawad A., Li X., Rezaei F., Smith J.D. Hydrogenolysis of glycerol over Ni, Cu, Zn, and Zr supported on H-beta. Chem. Eng. J. 2017;317:1–8. doi: 10.1016/j.cej.2017.02.064. [DOI] [Google Scholar]
- 116.Industry B., O B, Cu P, Catalysts AO. Selective Hydrogenolysis of Glycerol and Crude Glycerol (a By-Product or Waste Stream from the Biodiesel Industry) to 1,2-Propanediol over B2O3 Promoted Cu/Al2O3 Catalysts. Catalysts. 2017:1–16. doi: 10.3390/catal7070196. [DOI] [Google Scholar]
- 117.Manuale Debora L. Hydrogenolysis of glycerol to 1,2-propanediol in a continuous flow trickle bed reactor. J. Chem. Technol. Boiotechnol. 2017:1050–1064. doi:10.1002/j. [Google Scholar]
- 118.Zhao H., Hui C., Mei L., Yi J., Li N., Min H. Dehydration of glycerol to acrolein over hierarchical ZSM-5 zeolites: effects of mesoporosity and acidity. Renew. Sustain. Energy Rev. 2015;5:2548–2558. doi: 10.1021/cs5019953. [DOI] [Google Scholar]
- 119.García-Sancho C., Moreno-Tost R., Mérida-Robles J., Santamaría-González J., Jiménez-López A., Maireles-Torres P. Zirconium doped mesoporous silica catalysts for dehydration of glycerol to high added-value products. Appl. Catal. A Gen. 2012;433–434:179–187. doi: 10.1016/j.apcata.2012.05.015. [DOI] [Google Scholar]
- 120.Rosas I.P., Contreras L., Salmones J., Tapia C., Zeifert B., Navarrete J. Catalytic dehydration of glycerol to acrolein over a catalyst of Pd / LaY zeolite and comparison with the chemical equilibrium. Catalysts. 2017:1–29. doi: 10.3390/catal7030073. [DOI] [Google Scholar]
- 121.Chai S.H., Wang H.P., Liang Y., Xu B.Q. Sustainable production of acrolein: gas-phase dehydration of glycerol over Nb2O5 catalyst. J. Catal. 2007;250:342–349. doi: 10.1016/j.jcat.2007.06.016. [DOI] [Google Scholar]
- 122.Ott L., Bicker M., Vogel H. Catalytic dehydration of glycerol in sub- and supercritical water: a new chemical process for acrolein production. Green Chem. 2006;8:214–220. doi: 10.1039/b506285c. [DOI] [Google Scholar]
- 123.Yadav G.D., Sharma R.V., Katole S.O. Selective dehydration of glycerol to acrolein: development of efficient and robust solid acid catalyst MUICaT-5. Ind. Eng. Chem. Res. 2013;52:10133–10144. doi: 10.1021/ie401098n. [DOI] [Google Scholar]
- 124.Len C., Luque R. Continuous flow transformations of glycerol to valuable products: an overview. Sustain. Chem. Process. 2014;2:1–10. doi: 10.1186/2043-7129-2-1. [DOI] [Google Scholar]
- 125.Varma R.S., Len C. Glycerol valorization under continuous flow conditions-recent advances. Curr. Opin. Green Sustain. Chem. 2018;15:83–90. doi: 10.1016/j.cogsc.2018.11.003. [DOI] [Google Scholar]
- 126.Gil S., Marchena M., Sánchez-Silva L., Romero A., Sánchez P., Valverde J.L. Effect of the operation conditions on the selective oxidation of glycerol with catalysts based on Au supported on carbonaceous materials. Chem. Eng. J. 2011;178:423–435. doi: 10.1016/j.cej.2011.10.048. [DOI] [Google Scholar]
- 127.Kimura H. Selective oxidation of glycerol on a platinum-bismuth catalyst by using a fixed bed reactor. Appl. Catal. 1993;105:147–158. doi: 10.1016/0926-860X(93)80245-L. [DOI] [Google Scholar]
- 128.Carrettin S., McMorn P., Johnston P., Griffin K., Hutchings G.J., Besson M. Selective oxidation of glycerol to glyceric acid using a gold catalyst in aqueous sodium hydroxide. Chem. Commun. 2002;57:696–697. doi: 10.1039/b201112n. [DOI] [PubMed] [Google Scholar]
- 129.Li Y., Zaera F. Factors affecting activity and selectivity in the oxidation of glycerol promoted by platinum catalysts. Catal. Sci. Technol. 2015;5:3773–3781. doi: 10.1039/c5cy00586h. [DOI] [Google Scholar]
- 130.Rarokar N.R., Menghani S., Kerzare D., Khedekar P.B. Synthesis of monoglycerides for use in food and pharmaceuticals. J. Exp. Food Prog. 2017;3:1–6. doi: 10.4172/2472-0542.1000128. [DOI] [Google Scholar]
- 131.Chetpattananondh P., Tongurai C. Synthesis of high purity monoglycerides from crude glycerol and palm stearin. Songklanakarin J. Sci. Technol. 2008;30:515–521. [Google Scholar]
- 132.Atistella B., Ilho R.U.M.A.F., Process S., Ldps D. Optimization of distilled monoglycerides production. Appl. Biochem. Biotechnol. 2006;129:680–693. [PubMed] [Google Scholar]
- 133.Bo W., Yue S., Jiankui S., Runcang S. Conversion of platform chemical glycerol to cyclic acetals promoted by acidic ionic liquids. RSC Adv. 2014;4:18917–18923. doi: 10.1039/c4ra01443j. [DOI] [Google Scholar]
- 134.Faria R.P.V., Pereira C.S.M., Silva V.M.T.M., Loureiro J.M., Rodrigues A.E. Sorption enhanced reactive process for the synthesis of glycerol ethyl acetal. Chem. Eng. J. 2014;258:229–239. doi: 10.1016/j.cej.2014.07.073. [DOI] [Google Scholar]
- 135.Agirre I., Güemez M.B., Ugarte A., Requies J., Barrio V.L., Cambra J.F. Glycerol acetals as diesel additives : kinetic study of the reaction between glycerol and acetaldehyde. Fuel Process. Technol. 2013;116:182–188. doi: 10.1016/j.fuproc.2013.05.014. [DOI] [Google Scholar]
- 136.Deutsch J., Martin A., Lieske H. Investigations on heterogeneously catalysed condensations of glycerol to cyclic acetals. J. Catal. 2007;245:428–435. doi: 10.1016/j.jcat.2006.11.006. [DOI] [Google Scholar]
- 137.Silva P.H.R., Gonçalves V.L.C., Mota C.J.A. Glycerol acetals as anti-freezing additives for biodiesel. Bioresour. Technol. 2010;101:6225–6229. doi: 10.1016/j.biortech.2010.02.101. [DOI] [PubMed] [Google Scholar]
- 138.Yamamoto K., Kiyan A.M., Bagio J.C., Araújo K., Rossi B. Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin. Green Process. Synth. 2018:1–8. [Google Scholar]
- 139.Paul Ş, Pap T. Glycerol acetals and ketals as possible diesel additives. A review of their synthesis protocols. Renew. Sustain. Energy Rev. 2016;62:804–814. doi: 10.1016/j.rser.2016.05.013. [DOI] [Google Scholar]
- 140.Berthold-pluta A. Valorization of waste glycerol to dihydroxyacetone with biocatalysts obtained from Gluconobacter oxydans. Appl. Sci. 2018:1–16. doi: 10.3390/app8122517. [DOI] [Google Scholar]
- 141.White R., Navarro-pineda F.S., Cockerill T., Dupont V., Julio C. Techno-economic and life cycle impacts analysis of direct methanation of glycerol to bio-synthetic natural gas at a biodiesel refinery. Energies. 2019:1–20. doi: 10.3390/en12040678. [DOI] [Google Scholar]
- 142.Bagnato G., Iulianelli A., Sanna A., Basile A. Glycerol production and transformation : a critical review with particular emphasis on glycerol reforming reaction for producing hydrogen in conventional and membrane reactors. Membranes. 2017:1–31. doi: 10.3390/membranes7020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu H., Xu Y., Zheng Z., Liu D. 1,3‐Propanediol and its copolymers: Research, development and industrialization. Biotechnol. J. 2010;5(11):1137–1148. doi: 10.1002/biot.201000140. [DOI] [PubMed] [Google Scholar]
- 144.Sebastiano C.D., Agostino D., Cecilia M. Techno-economic analysis of a glycerol biorefinery. ACS Sustain. Chem. Eng. 2018;6(12):16563–16572. doi: 10.1021/acssuschemeng.8b03770. [DOI] [Google Scholar]
- 145.Komesu A., Allan J., De Oliveira R., Martins S. Lactic acid production to purification: a review. Bioresources. 2020;12(2):4364–4383. [Google Scholar]
- 146.Juturu V., Wu J.C. Microbial production of lactic acid : the latest development. Crit. Rev. Biotechnol. 2015:1–11. doi: 10.3109/07388551.2015.1066305. [DOI] [PubMed] [Google Scholar]
- 147.Witte J., Scherrer P., Eth I., Mondelli C., Papadokonstantakis S., Hungerb K. Environmental and economic assessment of lactic acid production from glycerol using cascade bio- and chemocatalysis. Energy Environ. Sci. 2014;8:558–567. doi: 10.1039/C4EE03352C. [DOI] [Google Scholar]
- 148.Magdouli S., Rouissi T., Brar K. 2016. Propylene Glycol : An Industrially Important C3 Platform Chemical. Author’ s personal copy. [DOI] [Google Scholar]
- 149.Van Haveren J., Scott E.L., Sanders J. Bulk chemicals from biomass. Biofuels Bioprod. Biorefining. 2008;2(1):41–57. doi: 10.1002/bbb. [DOI] [Google Scholar]
- 150.Talebian-kiakalaieh A., Aishah N., Amin S., Hezaveh H. Glycerol for renewable acrolein production by catalytic dehydration. Renew. Sustain. Energy Rev. 2014;40:28–59. doi: 10.1016/j.rser.2014.07.168. [DOI] [Google Scholar]
- 151.Sudhakar T., Tao L., Michael A.G. An overview of biorefinery derived platform chemicals from a cellulose and hemicellulose biorefinery. Clean Technol. Environ. Policy. 2018;20(7):1615–1630. doi: 10.1007/s10098-018-1568-5. doi: 10.100/s10098-018-1568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gargalo C.L., Carvalho A., Gernaey K.V., Sin G. A framework for techno-economic & environmental sustainability analysis by risk assessment for conceptual process evaluation. Biochem. Eng. J. 2016;116:146–156. doi: 10.1016/j.bej.2016.06.007. [DOI] [Google Scholar]
- 153.Almena A., Martín M. Techno-economic analysis of the production of epichlorohydrin from glycerol. Ind. Eng. Chem. Res. 2015 doi: 10.1021/acs.iecr.5b02555. [DOI] [Google Scholar]
- 154.Angelo S.C.D., Ara A.D., Mondelli C., Pe J., Papadokonstantakis S. Techno-economic analysis of a glycerol biorefinery. ACS Sustain. Chem. Eng. 2018;6(12):16563–16572. doi: 10.1021/acssuschemeng.8b03770. [DOI] [Google Scholar]
- 155.Simasatitkul L., Gani R., Arpornwichanop A. Optimal design of biodiesel production process from waste cooking palm oil. Proceedia Eng. 2012;42:1292–1301. doi: 10.1016/j.proeng.2012.07.521. [DOI] [Google Scholar]
- 156.Vlysidis A., Binns M., Webb C., Theodoropoulos C. A techno-economic analysis of biodiesel biore fi neries : assessment of integrated designs for the co-production of fuels and chemicals. Energy. 2011;36:4671–4683. doi: 10.1016/j.energy.2011.04.046. [DOI] [Google Scholar]
- 157.Zhou Y., Shen Y., Piao J. Sustainable conversion of glycerol into value-added chemicals by selective electro-oxidation on Pt-Based catalysts. ChemElectroChem. 2018;5(13):1636–1643. doi: 10.1002/celc.201800309. [DOI] [Google Scholar]
- 158.Houache M.S.E., Hughes K., Baranova E.A. Study on catalyst selection for electrochemical valorization of glycerol. Sustain. Energy Fuels. 2019;3:1892–1915. doi: 10.1039/c9se00108e. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.