Graphical abstract
Keywords: Locally advanced pancreatic cancer, Magnetic resonance guided radiation therapy, Online adaptive radiotherapy
Highlights
-
•
Locally Advanced Pancreatic Cancer is one of most aggressive tumors.
-
•
Limitations of SABR are represented by the possible toxicity to the OARs surrounding the lesion.
-
•
MRgRT offers better soft tissue contrast and accurate management of organ motion during RT.
-
•
Online adaptive RT allows to adapt the treatment plan based on the daily anatomical changes.
-
•
Feasibility and the benefit of the online adaptive procedures has been demonstrated.
Abstract
Introduction
Magnetic Resonance-guided Radiation Therapy (MRgRT) allows online adaptations (OA) of the treatment plan to optimize daily dose distribution based on patient's anatomy, just before fraction delivery. The aim of this study is to evaluate feasibility and the dosimetric improvement of the OA workflow implemented in our institution for locally advanced pancreatic cancer (LAPC) patients, in terms of target coverage and organs at risk (OARs) sparing.
Methods
We retrospectively analysed 8 LAPC patients treated with MRgRT in combination with the OA approach, using video-assisted inspiratory breath-hold for a total of 38 fractions with a dose ranging from 30 Gy to 40 Gy in 5 fractions.
Dose distribution of the baseline plan was first calculated based on daily anatomy, obtaining a “predicted” plan to assess the dosimetric improvement. If the dose distribution did not meet the constraints set in the planning phase, PTV, GTV and OARs were re-contoured within a distance of 3 cm from the PTV external edge and a new online “adaptive” plan was generated. Other clinical and planning parameters were also evaluated to assess the feasibility and the dosimetic benefit of the online adaptive workflow.
Results
Out of 38 total fractions, 26 (68.4%) were adapted online and 12 (31.6%) were delivered using the baseline plan. The use of the adaptive workflow resulted to be feasible in our clinical practice and advantageous in all the patients: mean PTV V95% increased by 10.8% (5.7–20.8) while mean CTV V98% of 12.6% (7.3–17.7). Also OARs V33 and V25 showed a positive trend avoiding unnecessary irradiation.
Conclusion
OA workflow improves the dosimetric benefit of MRgRT, preventing the occurrence of high-doses to OARs and increasing the safety of stereotactic treatment for LAPC, without any drawback for our daily clinical practice routine.
Introduction
Pancreatic cancer is one of most aggressive tumours with a five-year overall survival (OS) rate of 9.3% [1]. More than half of the patients affected by unresectable locally advanced pancreatic cancer, have a poor prognosis and low response to standard-of-care chemotherapy or chemoradiotherapy treatment, with a median OS of only 12–15 months [2]. Pancreatic adenocarcinoma is very radioresistant and local recurrences are common in disease progression (30%–50%) [3], which can often lead to the onset of severe symptoms such as pain, obstruction and other complications that could compromise patients’ quality of life.
Therefore, innovative strategies to optimize local control can play an important role to maximise therapeutic effects [4], [5]. Several clinical trials have shown that the use of hypo-fractionated radiation therapy, combined with chemotherapy, can increase OS [6], [7], [8], [9]. Stereotactic ablative radiotherapy (SABR) offers the advantage of effective dose delivery [10], [11]; sharper dose gradients minimize dose to surrounding organs at risk (OARs) in one to five fractions over one to two weeks under increased biological image guidance [3], [12], [13]. Many SABR phase I and phase II trials reported improvement in abdominal pain control and one-year local control rates ranging from 75 to 100% [14], [15].
Nevertheless, one of the SABR limitations is represented by the possible toxicity to the OARs surrounding the lesion, which are particularly radio-sensitive. The duodenum and adjacent to the pancreas are definitely the most dose-limiting organs, and a dose dependent correlation with the frequency of grade ≥ 2 toxicity has been reported [16], [17].
The overall management of SABR is limited by the difficulty to accurately identify the therapy volumes. This is related to limited soft-tissue contrast provided by computed tomography (CT), cone beam computed tomography (CBCT) or mega voltage computed tomography (MVCT) images, generally used in abdominal radiotherapy (RT) [18], [19], especially for pre-treatment positioning. Motion management solutions in conventional radiotherapy often require larger planning target volume (PTV) margins or the use of the internal target volume (ITV), limiting the possibility to perform any kind of dose escalation protocols, since toxicity to surrounding organs remains a limiting factor.
Hybrid RT systems, which combine a radiotherapy delivery unit with an magnetic resonance image (MRI) scanner, have recently been introduced in clinical practice, enhancing even more personalized radiotherapy. The on-board MR scanner, allows more precise identification of the therapy volumes and reduced clinical tumor volume (CTV) to PTV margins with remarkable results in terms of OARs sparing because of superior soft tissue contrast [20], [21], [22]. Fiducial markers, visible on x-ray imaging as a surrogate for target volume, are often used to guide radiotherapy treatment. This is an invasive procedure bearing complication risks, which can be avoided when an on-board MR imaging is available.
Furthermore, these systems ensure a more accurate management of organ motion during RT, which can be carried out at three different levels of complexity: image alignment, online adaptive radiotherapy (OART) for inter-fraction modifications and intra-fraction motion management. Image alignment is performed by a 25 second MRI (0.35 T) able to offer a 3D MRI image to further improve patient positioning and therefore, single target/OARs positioning. Often, OAR variation regarding position and size cannot be compensated by patient positioning especially in the upper and lower GI as well as in the pelvis district. In this case, inter-fraction modification of target/OARs contours and optimization of the dose distribution on the new anatomy is possible thanks to the OART methods. Finally, an online Cine MRI (only sagittal view) allows the intra-fraction target motion management during treatment delivery. In particular, treatment delivery is allowed only if the target is within a boundary around the CTV, with a margin defined by a multidisciplinary team consisting of a radiation oncologist (RO), medical physicist (MP) and radiotherapy technologist (RTT), just before the delivery.
OART allows to adapt the treatment plan in response to a specific anatomical and/or biological change that may occur also during the course of treatment [22].
It appears evident that locally advanced pancreatic cancer (LAPC) patients could greatly benefit from the OART technique.
Aim of this study was to evaluate the dosimetric impact on the treatment of pancreatic cancer patients of a newly introduced OA procedure in our institution, in terms of target coverage and organs at risk (OARs) sparing using a 0.35 T MR-guided RT hybrid machine.
Materials and methods
Population
The first eight patients affected by biopsy-proven inoperable LAPC treated in our institution with MRIdian® Tri-Cobalt system (ViewRay Inc., Mountain View, CA, USA) were retrospectively included in this analysis.
All patients underwent radiological staging and disease resectability was assessed according to the degree of contact between the tumour and vessels (superior mesenteric artery, coeliac axis, hepatic artery, superior mesenteric vein and portal vein) and the presence or absence of metastases [23].
All patients underwent induction chemotherapy, and were confirmed unresectable at the post-chemotherapy re-evaluation performed with a contrast-enhanced CT or with a 18 fluorodeoxyglucose PET-CT scan.
Induction chemotherapy regimens included Gemcitabine plus Nabpaclitaxel, Folfiri, Folfirinox, Folfox and Cisplatin regimens. Patients did not undergo any prior RT treatment.
Each patient was evaluated by a RO to confirm the absence of previous abdominal RT treatments and clinical contraindications to RT and MR. Informed consent for radiotherapy and research participation was obtained from all participants included in the study.
Acute and late toxicity was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 scale. Acute toxicity was recorded during and up to six months after the final RT treatment visit. Late toxicity was recorded during planned follow-ups (approximately every 3–5 months during the first year). Survival outcome (OS), time to disease progression and rate of toxicities were considered from the start date of RT.
Online adaptive workflow
Treatment preparation
Each patient, underwent a 0.35 T MRI simulation (ViewRay Inc., Mountain View, CA, USA) subsequently (within 20 minutes) to a CT simulation (GE, Optima CT580 W, HiSpeed DX/I Spiral), both acquired with the same immobilization and positioning devices.
Patients were immobilized in supine position with both arms above their head, using a dedicated MR compatible modular immobilization device (Fluxboard™, Macromedics®, The Netherlands).
Additionally, during the MRI simulation, a cine MRI was acquired to evaluate patient’s compliance to properly maintain deep inspiration breath hold (DIBH) to decide if breath-hold treatment was suitable. Two parameters were set to quantitatively evaluate DIBH patient’s compliance: boundary and ROI%. Boundary is a defined margin from CTV, taking target intra-fraction maximum allowed motion into account. ROI% is the maximum allowed percentage of the target volume outside the defined boundary to keep the delivery going: if this threshold exceeds, the beam delivery is interrupted. Usually, ROI% for LAPC OA MRgRT was set to 5%, boundary to 3 mm. DIBH evaluation was performed only during the MRI simulation and consequentially the CT simulation was acquired accordingly, avoiding extra CT scan acquisition, in particular 4D CT.
Planning
CTV and OARs (i.e. duodenum, small bowel, liver, left kidney, right kidney, liver, stomach and spinal cord) were contoured on the acquired MRI simulation (25 second, TRUFI pulse sequence) in combination with post-chemotherapy imaging and according to internal department guidelines.
PTV was created adding an isotropic margin of 3 mm to the CTV.
All patients were treated with 5 fractions, with different prescription doses of 40 Gy (5 patients), 35 Gy (2 patients) or 30 Gy (1 patient), resulting in biologically effective doses (BED) of 72 Gy, 59,5 Gy and 48 Gy, respectively, if considering a tumoral alpha/beta ratio of 10.
Dose prescription was modulated in consideration of the OARs tolerance.
Planning quality was assessed quantifying the V95% of PTV and V98% of CTV for target coverage and using the OARs constraints recommended by AAPM Task group 101 [24].
A baseline treatment plan was calculated with MRIdian treatment planning system (TPS). The simulation CT images were used to provide the electron density map required for dose calculation. Dose calculation was carried out with the Monte Carlo algorithm, with a statistical uncertainty of 1% using a calculation grid of 0.3 cm × 0.3 cm × 0.3 cm [25].
Online adaptive treatment
For all enrolled patients, an OA workflow was foreseen: This procedure was applied only if the daily anatomy (OARs and target) was not matching the reference anatomy, after patient positioning. The reference image was automatically set as the last adapted fraction image to reduce the inter-fraction variability. Decision to perform an OART was discussed online by the multidisciplinary team. Once OART process was agreed, RO defined the target volumes (CTV and PTV) and the OAR within a 3 cm radius from PTV [26].
Then, dose distribution of the original plan (original) was calculated on the daily anatomy (predicted), without any further optimization. If the dose constraints and the target coverage parameters were not met, an online re-optimization was performed by MP, modifying the optimization cost function when necessary to obtain a daily adapted plan (adapted).
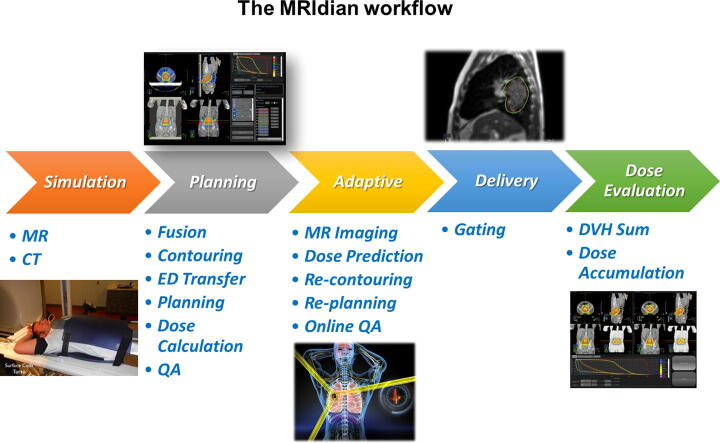
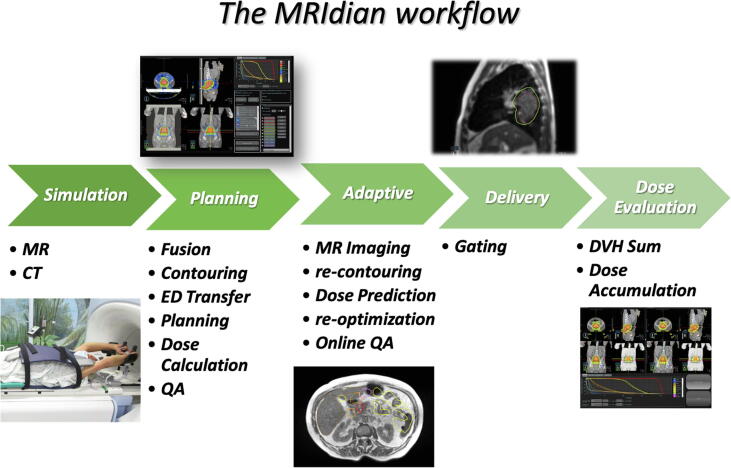
Fig. 1 summarizes the used workflow.
Fig. 1.
MRIdian workflow is divided in the following steps: simulation, planning, adaptive, delivery and dose evaluation.
Once the dose distribution of the day was defined and approved by RO, an online and independent quality assurance (QA) procedure was performed on the dose distribution in terms of gamma analysis (3%, 3 mm, gamma passing rate set to > 95%).
DIBH with visual feedback was used in all patients, as motion management technique. In particular, during the entire treatment process, patients were able to see, through a mirror, a screen showing the online cine MRI and therefore personally contribute to “place” the target within the boundary.
Calculation
Analysed parameters
Several clinical and planning parameters were considered in this study to overall evaluate the OA workflow. On a daily basis, log file generation of delivered plans was performed, to evaluate the following parameters:
-
•
couch shift (correction vector) after daily MRI registration
-
•
OARs and target volume (cc)
-
•
beam-on time (multi leaf collimator (MLC) movement + gating + beam-on) for each adaptive fraction
-
•
distance between CTV and duodenum/stomach centre of mass
The daily couch shift tracks along the entire treatment the accuracy of the patient positioning; the daily OARs and target volume variation highlight the need of re-contouring ROI, even if the patient positioning is suitable for treatment; daily beam-on time expresses the time variation of the treatment due to a new optimized dose distribution on the new anatomy or a worst patient compliance in terms of DIBH; the daily distance between the CTV and the two closest OARs (duodenum/stomach) centre of mass, expresses the daily organ motion, stressing even more the need of a daily re- contouring of the anatomy.
Dose Volume Histogram (DVH) metrics (target coverage as PTV V95% and CTV V98%, and OARs specific dose-volume constraint) were collected in order to compute the dosimetric comparison of the CTV, PTV and OARs between original plan (original) and adaptive plan (adaptive), and then between predicted plan (predicted) and adaptive plan to show the dosimetric benefit of the OART workflow using Whisker plot. A Whisker plot is a standardized way of displaying the distribution of data based on a five-number summary: minimum or lower whisker, lower quartile, median, upper quartile and maximum or upper whisker. We used Boxplot to display outliers, outlier values, dataset symmetry, how tightly the data is grouped, and if and how the data is skewed. Finally, the number of delivered OA fractions with predicted or adapted dose, were also recorded.
Results
Patient characteristics
Eight patients, 5 (62.5%) male and 3 (37.5%) female, affected by biopsy-proven, inoperable, pancreatic cancer were retrospectively analysed. Median age at diagnosis was 64 (range 53–76) years. Patient characteristics are shown in Table 1.
Table 1.
Patient’s characteristics.
| Parameters | Characteristics | N° (%) | |
|---|---|---|---|
| Median age at diagnosis | 64 (range 53–76) | ||
| Sex | M | 5 | (62.5) |
| F | 3 | (37.5) | |
| Location of the tumer | Head-isthmus | 7 | (87.5) |
| Tail | 1 | (12.5) | |
| Chemotherapy | Gemcitabine + Nab Paclitaxel → Folfox → Folfiri | 1 | (12,5) |
| Gemcitabine + Nab Paclitaxel → Folfirinox → Cisplatin | 1 | (12,5) | |
| Gemcitabine + Nab Paclitaxel | 4 | (50) | |
| Folfirinox | 1 | (12,5) | |
| None | 1 | (12,5) | |
| M + Diagnosis | Y | 2 | (25) |
| N | 6 | (75) | |
Of these 8 patients 6 were stage III and 2 were stage IV oligometastatic patients.
Patients were treated in our institution with MRIdian® Tri-Cobalt system (ViewRay Inc. Mountain View, CA, USA) from January to August 2018.
Five patients received a radiation dose with BED10 > 70 Gy (high dose SABR) and three patients received a radiation dose with BED10 < 70 Gy (conventional SABR) [27].
All patients completed the treatment and underwent all 5 scheduled fractions, except 1 who ended the treatment early, due to clinical deterioration.
One patient did not undergo induction chemotherapy due to the onset of splenic sequestration thrombocytopenia.
OA workflow evaluation
Based on the 38 analysed fractions, the daily lateral, longitudinal and vertical couch shifts resulted in (mean ± SD: min/max): lateral: −0.15 ± 0.69 cm: −2.15/1.91; longitudinal: −0.23 ± 0.76 cm: −2.81/1.07; vertical: 0 ± 0.35 cm: −0.75/0.68 cm, respectively.
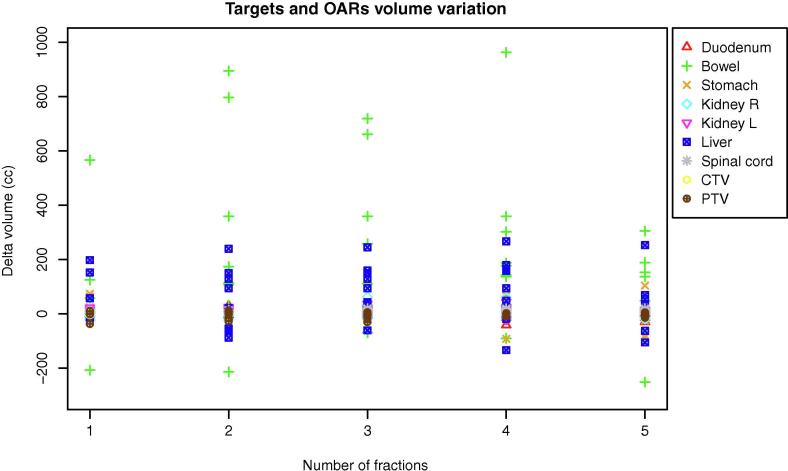
Average CTV and PTV sizes at baseline were, respectively, 17.81 cc (range 7.4–120.2 cc) and 35.47 cc (range 22.27–198.88 cc). Organs at risk and target volume variation in terms of difference of the planning and daily volumes are summarised in Fig. 1.
The volume changes compared to the pre-treatment situation were random and depicted in Fig. 2.
Fig. 2.
Targets and OARs single fraction volume variation (planning minus daily volume) for all eight patients.
The maximum/mean volume variations of the duodenum, small bowel and stomach were 31.7/3.06 cc, 894.04/186.31 cc and 142.62/12.33 cc, respectively.
Based on the online adjustment, the CTV average volume variation was −2.4 cc (range 2.5/ −19.09).
Out of 38 total fractions, 26 (68.4%) were adapted and 12 (31.6%) were delivered using the baseline plan, since dosimetric constraint (target coverage and OARs sparing) were met within the daily patient positioning and anatomy.
Daily fractions treatment time duration (beam-on), including the fraction with a re-optimized dose distribution, was (mean ± SD: min/max) 8,27 ± 2,87 min: 4,68/17,27 min. A reduction of the beam-on time equal to (mean ± SD: min/max) 2,51 ± 2,43 min: 0,09/6,67 min in comparison with the original plan had to be re-optimized in 38,46% of the cases (ten plans). In the remaining 16 plans (61,54%), an increased beam-on time equal to (mean ± SD: min/max) 1,14 ± 0,85 min: 0,12/2,89 min has been reported.
Excluding the patient positioning and the treatment delivery, the OA workflow (daily MR acquisition, re-contouring, re-optimization, plan quality evaluation and QA) average time was 23 minutes.
Dose volume histogram metrics results are shown in Table 2.
Table 2.
Targets and OARs DVH metrics in terms of minimum, maximum, median and standard deviation (SD) of the difference between the planning DVH parameters and the daily registered one: d = duodenum; b = small bowel; s = stomach; k = kidneys; l = liver.
| Δd V18 (cc) | Δd V12,5 (cc) | Δb V19,5 (cc) | Δs V18 (cc) | Δk V17,5 (cc) | Δl V21 (cc) | ΔPTV V95% | ΔCTV V98% | |
|---|---|---|---|---|---|---|---|---|
| Min | −2.2 | −5.2 | −8.0 | −31.1 | 0 | −6.6 | −16.9 | −6.9 |
| Max | 11.2 | 10.7 | 29.8 | 13.0 | 0.2 | 47.8 | 22.1 | 23.7 |
| Median | 3.6 | 1.8 | 7.0 | 0.8 | 0.1 | −0.2 | 0.0 | −0.2 |
| SD | 3.6 | 5.1 | 12.8 | 9.1 | 0.1 | 17.4 | 6.4 | 7.0 |
Maximum daily difference of CTV to duodenum/stomach centre of mass distance, compared to baseline resulted in 2,36 cm and 3,86 cm, respectively. SD of CTV to the duodenum/stomach centre of mass distance for all the evaluated 38 fractions were 1,03 cm and 1, 85 cm, respectively.
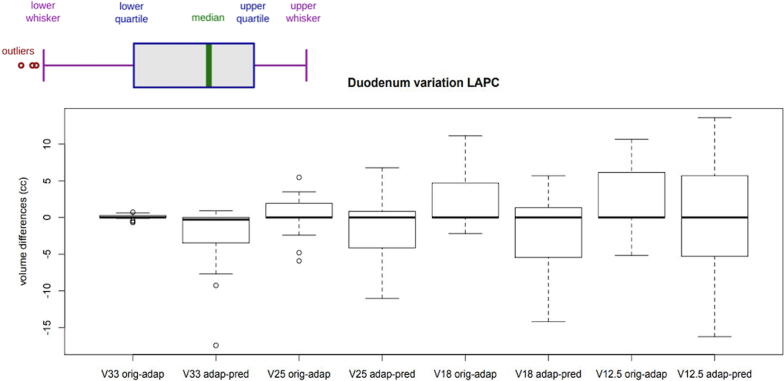
The plots of Fig. 3, Fig. 4, Fig. 5 compare the differences in terms of dose to the OARs and target coverage.
Fig. 3.
Duodenum V33, V25, V18 and V12,5 variation between original plan (orig) and adaptive plan (adap) and between adaptive plan and predicted plan (pred).
Fig. 4.
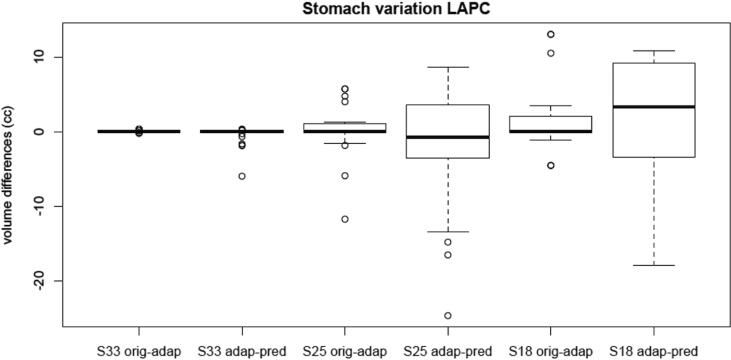
Stomach V33, V25 and V18 variation between original plan and adaptive plan and between adaptive plan and predicted plan.
Fig. 5.
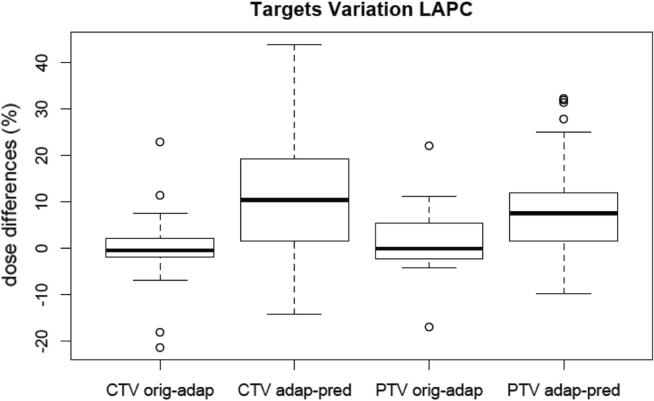
CTV and PTV percentage dose variation between original plan and adaptive plan and between adaptive plan and predicted plan.
Results are shown as Whisker boxplots (described in Fig. 3).
In particular, the stomach and duodenum were evaluated for their anatomical proximity to the target. Fig. 3 and Fig. 4 show the differences in volume (cc) of the V33, V25, V18 and V12.5 of the duodenum and of the stomach, respectively.
Toxicity
Radiation therapy treatment was well tolerated by all patients. One out of 8 patients developed acute grade 1 toxicity (diarrhoea G1 according to CTCAE 4.03). No late toxicity has been reported.
Survival
At last follow-up, 7 of 8 (87,5%) patients were alive, with a median follow-up time of 13 months (range 0 to 20). The median time to disease progression was 10,5 months (2–19) for 6 of 8 patients (75%). Among them, 2 patients experienced local recurrence, 2 experienced distant recurrence and 2 both types of recurrences.
All patients who received a BED10 > 70 showed overall survival after 1 year. Regarding the cohort of patients who received conventional SABR dose, one out of three patients was deceased 5 months after treatment end. This patient had interrupted the treatment after 3 fractions due to worsening of general conditions.
Discussion
With the introduction of MRgRT, the possibility to adapt the original treatment plan to daily anatomy has become a clinical reality.
In our experience, we evaluated the SABR MR guided treatment for 8 patients affected by LAPC, highlighting the importance of the OA workflow in terms of target coverage and respect of OARs constraints.
The results obtained by the daily lateral, longitudinal and vertical couch shifts highlight the low residual error of patient positioning in comparison with the reference planning image.
Inter-fraction OARs variation was observed in all analysed cases, both in terms of volume (Fig. 2) and position (the maximum daily difference of CTV to duodenum/stomach centre of mass distance, compared to the baseline resulted to be 2,36 cm and 3,86 cm, respectively), with remarkable consequences on the dosimetric point of view, as confirmed by the high number of adapted fractions (almost 70%).
The total beam-on treatment time (mean ± SD: 8,27 ± 2,87 min) was clinically affordable and well tolerated by all patients. Delivered treatment plans using the OA technique showed significant improvements in target coverage with adequate healthy tissue sparing. We did not observe major differences regarding target coverage comparing the original and the adapted plan. Instead, comparing the adaptive and the predicted plan, the median CTV and PTV had a positive value between 5% and 10% clearly indicating a systematic trend of higher target coverage (Fig. 4), which demonstrates what would have happened if the OA workflow have not been applied to the daily anatomy in terms of target coverage. The use of the adaptive workflow resulted to be advantageous in all patients: mean PTV V95% increased by 10.8% (5.7–20.8), while mean CTV V98% of 12.6% (7.3–17.7).
Fig. 3 and Fig. 4 shows differences in volume (cc) of the V33, V25, V18 and V12.5 of the duodenum and of the stomach, respectively. Especially for the duodenum, an advantage in dose reduction has been noted comparing the original plan and the adapted plan. This advantage was maintained comparing the predicted plan and the adapted plan with an even greater dosimetric difference. Therefore, we strongly recommend performing MRgRT with OA workflow for LAPC patients, which can be translated to the all clinical scenario where dose-escalation protocols can lead to increase local control and survival outcome.
Rudra et al. [27] recently published the results of a prospective phase II study evaluating outcomes of 44 patients who underwent adaptive MRgRT to treat inoperable pancreatic cancer. Patients were stratified into high-dose (BED10 > 70) and standard-dose (BED10 < 70) groups demonstrating an improved OS for patient with dose-escalated MRgRT treatment. Even though we investigated on a small sample size, results appear to be comparable with those published by Rudra et al. We observed a 1-year OS for 100% of patients who received a BED10 > 70 Gy (respect to 83.5% published in [27]); 66% for the patients who received a BED10 < 70 Gy (respect 50% reported in [27]).
Furthermore, the SABR for LAPC has shown an increasingly important role in pain control and, therefore, in quality of life improvement [28]. The authors showed that there were statistically significant differences regarding the physical and psychological health of patients in patients responding to therapy.
According to a recent review by Boldrini et al. [29], we identified future perspectives and current weaknesses of MRgRT in pancreatic cancer. Firstly, the future technology delivery improvements (higher MLC/gantry rotation speed and dose rate) and more robust dose accumulation algorithms may allow several improvements in the OA workflow, especially decreasing the time for OA procedure allowing more effective treatments. Moreover, imaging advancements, like faster MR protocols and functional imaging, will strongly support OART for LAPC patients opening new perspectives.
Another relevant weak point of OART is related to the time required to contour the daily anatomy. A large interest to use artificial intelligence improving daily OA processes could overcome this issue; more specifically, efficient auto-contouring solutions and quantitative analysis of the daily images (i.e. radiomics), allowing a daily therapy customization in the frame of the most innovative personalized medicine paradigm could reduce treatment time [30].
Nevertheless, this study presents some limitations. Firstly, the small sample size: for this reason, we are planning to repeat the study once we will at least triplicate the number of eligible patients to obtain more robust statistics. Secondary, we have observed a learning curve in the OA workflow, which we believe doesn’t affect dramatically the dosimetric results; nevertheless it could be a key parameter to be further compare in the next study.
Conclusions
As far as the authors are aware of, this study represents the first Italian experience of OA MRgRT in pancreatic cancer, using a 0.35 T MR-guided radiotherapy machine.
The OA workflow appeared to be feasible and affordable in clinical practice and the unique opportunities provided by the introduction of MRgRT system may be quickly translated in benefits for patients.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank Dr. Franziska Michaela Lohmeyer for critical and linguistic review of the manuscript.
References
- 1.Cancer of the Pancreas - Cancer Stat Facts. SEER n.d. https://seer.cancer.gov/statfacts/html/pancreas.html [accessed September 6, 2019].
- 2.Lidsky M.E., Sun Z., Nussbaum D.P., Adam M.A., Speicher P.J., Blazer D.G. Going the Extra Mile: Improved Survival for Pancreatic Cancer Patients Traveling to High-volume Centers. Ann Surg. 2017;266:333–338. doi: 10.1097/SLA.0000000000001924. [DOI] [PubMed] [Google Scholar]
- 3.Willett C.G., Czito B.G., Bendell J.C., Ryan D.P. Locally advanced pancreatic cancer. J Clin Oncol. 2005;23:4538–4544. doi: 10.1200/JCO.2005.23.911. [DOI] [PubMed] [Google Scholar]
- 4.Ceha H.M., van Tienhoven G., Gouma D.J., Veenhof C.H., Schneider C.J., Rauws E.A. Feasibility and efficacy of high dose conformal radiotherapy for patients with locally advanced pancreatic carcinoma. Cancer. 2000;89:2222–2229. doi: 10.1002/1097-0142(20001201)89:11<2222::aid-cncr10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 5.Chang S.T., Goodman K.A., Yang G.P., Koong A.C. Stereotactic body radiotherapy for unresectable pancreatic cancer. Front Radiat Ther Oncol. 2007;40:386–394. doi: 10.1159/000106048. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Josef E., Schipper M., Francis I.R., Hadley S., Ten-Haken R., Lawrence T. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2012;84:1166–1171. doi: 10.1016/j.ijrobp.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lominska C.E., Unger K., Nasr N.M., Haddad N., Gagnon G. Stereotactic body radiation therapy for reirradiation of localized adenocarcinoma of the pancreas. Radiat Oncol. 2012;7:74. doi: 10.1186/1748-717X-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammel P., Huguet F., van Laethem J.-L., Goldstein D., Glimelius B., Artru P. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA. 2016;315:1844–1853. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 9.Zhong J., Patel K., Switchenko J., Cassidy R.J., Hall W.A., Gillespie T. Outcomes for patients with locally advanced pancreatic adenocarcinoma treated with stereotactic body radiation therapy versus conventionally fractionated radiation. Cancer. 2017;123:3486–3493. doi: 10.1002/cncr.30706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang D.T., Schellenberg D., Shen J., Kim J., Goodman K.A., Fisher G.A. Stereotactic radiotherapy for unresectable adenocarcinoma of the pancreas. Cancer. 2009;115:665–672. doi: 10.1002/cncr.24059. [DOI] [PubMed] [Google Scholar]
- 11.Schellenberg D., Goodman K.A., Lee F., Chang S., Kuo T., Ford J.M. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678–686. doi: 10.1016/j.ijrobp.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 12.Mahadevan A., Jain S., Goldstein M., Miksad R., Pleskow D., Sawhney M. Stereotactic body radiotherapy and gemcitabine for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:735–742. doi: 10.1016/j.ijrobp.2009.08.046. [DOI] [PubMed] [Google Scholar]
- 13.Shaib W.L., Hawk N., Cassidy R.J., Chen Z., Zhang C., Brutcher E. A Phase 1 Study of Stereotactic Body Radiation Therapy Dose Escalation for Borderline Resectable Pancreatic Cancer After Modified FOLFIRINOX ( NCT01446458) Int J Radiat Oncol Biol Phys. 2016;96:296–303. doi: 10.1016/j.ijrobp.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Koong A.C., Le Q.T., Ho A., Fong B., Fisher G., Cho C. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Mahadevan A., Miksad R., Goldstein M., Sullivan R., Bullock A., Buchbinder E. Induction gemcitabine and stereotactic body radiotherapy for locally advanced nonmetastatic pancreas cancer. Int J Radiat Oncol Biol Phys. 2011;81:e615–e622. doi: 10.1016/j.ijrobp.2011.04.045. [DOI] [PubMed] [Google Scholar]
- 16.Goldsmith C., Price P., Cross T., Loughlin S., Cowley I., Plowman N. Dose-Volume Histogram Analysis of Stereotactic Body Radiotherapy Treatment of Pancreatic Cancer: A Focus on Duodenal Dose Constraints. Semin Radiat Oncol. 2016;26:149–156. doi: 10.1016/j.semradonc.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Murphy J.D., Christman-Skieller C., Kim J., Dieterich S., Chang D.T., Koong A.C. A dosimetric model of duodenal toxicity after stereotactic body radiotherapy for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2010;78:1420–1426. doi: 10.1016/j.ijrobp.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 18.Yan D., Georg D. Adaptive radiation therapy. Zeitschrift Für Medizinische Physik. 2018;28:173–174. doi: 10.1016/j.zemedi.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Disher B., Hajdok G., Wang A., Craig J., Gaede S., Battista J.J. Correction for “artificial” electron disequilibrium due to cone-beam CT density errors: implications for on-line adaptive stereotactic body radiation therapy of lung. Phys Med Biol. 2013;58:4157–4174. doi: 10.1088/0031-9155/58/12/4157. [DOI] [PubMed] [Google Scholar]
- 20.Park J.M., Park S.-Y., Kim J.-I., Kang H.-C., Choi C.H. A comparison of treatment plan quality between Tri-Co-60 intensity modulated radiation therapy and volumetric modulated arc therapy for cervical cancer. Phys Med. 2017;40:11–16. doi: 10.1016/j.ejmp.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Boldrini L., Placidi E., Dinapoli N., Azario L., Cellini F., Massaccesi M. Hybrid Tri-Co-60 MRI radiotherapy for locally advanced rectal cancer: An in silico evaluation. Tech Innovat Patient Support Radat Oncol. 2018;6:5–10. doi: 10.1016/j.tipsro.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Q.J., Li T., Wu Q., Yin F.-F. Adaptive radiation therapy: technical components and clinical applications. Cancer J. 2011;17:182–189. doi: 10.1097/PPO.0b013e31821da9d8. [DOI] [PubMed] [Google Scholar]
- 23.Callery M.P., Chang K.J., Fishman E.K., Talamonti M.S., William Traverso L., Linehan D.C. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol. 2009;16:1727–1733. doi: 10.1245/s10434-009-0408-6. [DOI] [PubMed] [Google Scholar]
- 24.Benedict S.H., Yenice K.M., Followill D., Galvin J.M., Hinson W., Kavanagh B. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys. 2010;37:4078–4101. doi: 10.1118/1.3438081. [DOI] [PubMed] [Google Scholar]
- 25.Mutic S., Dempsey J.F. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol. 2014;24:196–199. doi: 10.1016/j.semradonc.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Bohoudi O., Bruynzeel A.M.E., Senan S., Cuijpers J.P., Slotman B.J., Lagerwaard F.J. Fast and robust online adaptive planning in stereotactic MR-guided adaptive radiation therapy (SMART) for pancreatic cancer. Radiother Oncol. 2017;125:439–444. doi: 10.1016/j.radonc.2017.07.028. [DOI] [PubMed] [Google Scholar]
- 27.Rudra S., Jiang N., Rosenberg S.A., Olsen J.R., Roach M.C., Wan L. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med. 2019;8:2123–2132. doi: 10.1002/cam4.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosati L.M., Herman J.M. Role of Stereotactic Body Radiotherapy in the Treatment of Elderly and Poor Performance Status Patients With Pancreatic Cancer. J Oncol Pract. 2017;13:157–166. doi: 10.1200/JOP.2016.020628. [DOI] [PubMed] [Google Scholar]
- 29.Boldrini L., Cusumano D., Cellini F. Azario L, Mattiucci GC, Valentini V. Online adaptive magnetic resonance guided radiotherapy for pancreatic cancer: state of the art, pearls and pitfalls. Radiat Oncol. 2019;24 doi: 10.1186/s13014-019-1275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boldrini L., Cusumano D., Chiloiro G., Casà C., Masciocchi C., Lenkowicz J. Delta radiomics for rectal cancer response prediction with hybrid 0.35 T magnetic resonance-guided radiotherapy (MRgRT): a hypothesis-generating study for an innovative personalized medicine approach. Radiol Med. 2019;124:145–153. doi: 10.1007/s11547-018-0951-y. [DOI] [PMC free article] [PubMed] [Google Scholar]