Abstract
Background
To achieve optimal bone formation one of the most influential parameters has been mentioned to be adequate blood supply. Vascular endothelial growth factor (VEGF) is hereby of particular interest in bone regeneration, because of its primary ability to induce neovascularization and chemokine affection for endothelial cells (EC), and is considered to be the main regulator of vascular formation. However, the growth factor has yet to be implemented in a clinical setting in orthopaedic intervention surgery. We hypothesised that the development of VEGF in vivo for bone formation in the last decade had progressed towards clinical application since the latest systematic review from 2008.
Objective
This systematic review recapped the last 13 years of in vivo bone regeneration using vascular endothelial growth factor (VEGF).
Method
A total of 1374 articles were identified using the PubMed search string (vegf or “vascular endothelial growth factor”) and (osteogen∗ or “bone formation” or “bone regeneration”). By 3 selection phases 24 published articles were included by the criteria of being in vivo, using only VEGF for bone formation, published after 2007 and written in English. Articles in vitro, written in different languages than English and older than 2007 was excluded. The most recent systematic review on this subject was published in 2008, with the latest included study from 01 to 11-2007. All included studies were classified based on animal, type of defect, scaffold, control group, type of VEGF, release rate, dosage of VEGF, time of evaluation and results. Each study was evaluated for risk of bias by modified CAMARADES quality assessment for the use in experimental animal studies. The score was calculated by peer review journal publication, use of control group, randomisation of groups, justified VEGF dosage, blinding of results, details on animal model, sample size calculation, comply with ethics and no conflict of interest.
Results
No clinical trials or human application studies were obtained from our search. Experimentally, 11 articles using solely VEGF for bone formation had a group or a timepoint significantly better than the corresponding control group. 18 articles revealed no significant difference of VEGF compared to the control group and 1 article reported a significant decreased bone growth using VEGF compared to control.
Conclusion
Based on these results no clinical studies have yet been performed. However, indications in the best use of VEGF from experimental studies could be made towards that the optimal release is within the first three weeks, in defect models, with the best effect before eight weeks. Future designs should incorporate this with standardised and reproducible models for verification towards clinical practice.
The translational potential of this article
This systematic review aims to assess the existing literature to focus on methodologies and outcomes that can provide future knowledge regarding the solitary use of VEGF for bone regeneration in a clinical setting.
Keywords: Angiogenesis, Biomaterials, Osteogenesis, Growth factors, Tissue engineering, Vascular endothelial growth factor
Introduction
Bone loss and defect due to trauma or infection is a very specialised area in the field of orthopaedics, and knowledge of the physiological parameters in bone formation is subject to an ongoing investigation. Research on bone formation has attracted increasing interest due to an increasing elderly population and fracture rate globally, where a significant percentage of fractures has inadequate defect healing due to infections, surgical procedures and more fragile bone structure [1,2]. One well-known factor in achieving sufficient bone healing is to secure sufficient blood vessel contribution to the defect, with some research on growth factors focusing on the use of the angiogenic protein vascular endothelial growth factor (VEGF).
VEGF in bone research
VEGF is of particular interest in bone regeneration due to its primary ability to induce neovascularisation [10,15,22]. The VEGF protein also carries an indirect ability to differentiate MSCs into the osteogenic lineage [3,7]. Furthermore, it has a chemokine effect on surrounding endothelial cells (ECs) that can increase the number of vessels in a localised area [8]. These effects contribute to achieving a more controlled use of both the stimulating effect of VEGF and regenerative effects of the MSCs. Notably, the inhibition of VEGF has led to non-union models [9], making the growth factor essential in the bone healing process.
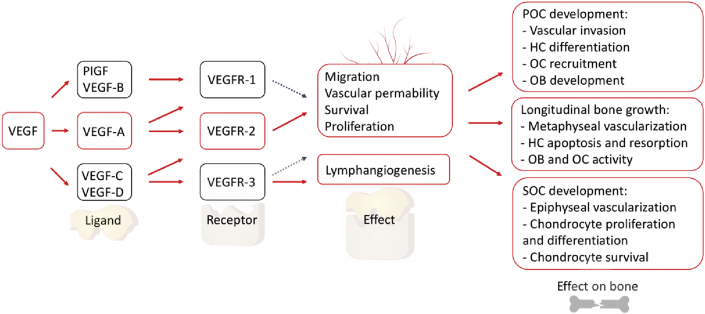
The VEGF family consists of different members, including VEGFA, -B, –C and -D, and placenta growth factor-1/2 (PIGF1/2) [8,10] (Fig. 1). The most commonly used isomer of VEGF in the field of bone research is the human VEGF165 (rVEGF165)—a member of VEGFA—due to its elevated potency and effect [24].
Fig. 1.
VEGF family and receptors for the signal pathway to vessel distribution. Primary ossification center (POC), hypertrophic chondrocyte (HC) [12].
Bone formation can be divided into intramembranous and endochondral ossification. The primary stimulator of VEGF expression in the osteoblast-like cell line is hypoxia-induced factor 1α (HIF-1α), while the release of HIF-1α is related to the initiation of fracture repair in the endochondral ossification [13,14]. The initial stage of endochondral bone formation is the cartilage base structure created by the osteochondral progenitor cells within regions of low blood perfusion. Then, the chondrocytes proliferate and enlarge. This initiates the attraction and incorporation of endothelial cells, osteoblastic precursor cells, haematopoietic cells and osteoclasts that result in cartilage becoming degraded and replaced by trabecular bone and bone marrow [15,16].
The challenges for using VEGF for bone regeneration
Generally, abundance of blood vessels around the healing site will enable more nutrients and regenerative cells to be transported from the bloodstream to the area of bone formation, with more waste products from the healing site being moved away from the desired location [17]. However, the VEGF protein appears to have a narrow therapeutic window [4], which implies that if the dosage of VEGF is too high, this can cause malformed and non-functional vessels as well as a potentially toxic effect [4,18].
Furthermore, VEGF has a half-life between 4 and 24 h [19,20]. This short period makes it difficult to use in the surgical setting if a long-term effect on the defect is desired without reaching the toxicity threshold. This issue has caused multiple delivery methods in experimental designs to prolong the release of VEGF [21].
Theoretically, the VEGF protein should have the capability to enhance the regeneration of bone defects, especially endochondral ossification. The growth factor is already applied in human clinical trials for other purposes [22] and would have the natural capability to transition to the field of bone research. However, to our knowledge, clinical trials using VEGF in this field have not yet been published.
Objective of this study
This systematic review aspires to collect all existing in vivo results on the solitary use of vascular endothelial growth factor for bone growth compared to control, as evaluated by new bone formation. Additionally, we evaluate whether these results indicate any promising progress towards release methods and dosages that could be applied in a focused experimental design and translated into human clinical use. Our hypothesis is that solitary VEGF stimulation in the current in vivo literature will demonstrate a pattern and method for human application in bone formation for a potential solution in the use in orthopaedic surgery.
Materials and methods
Design
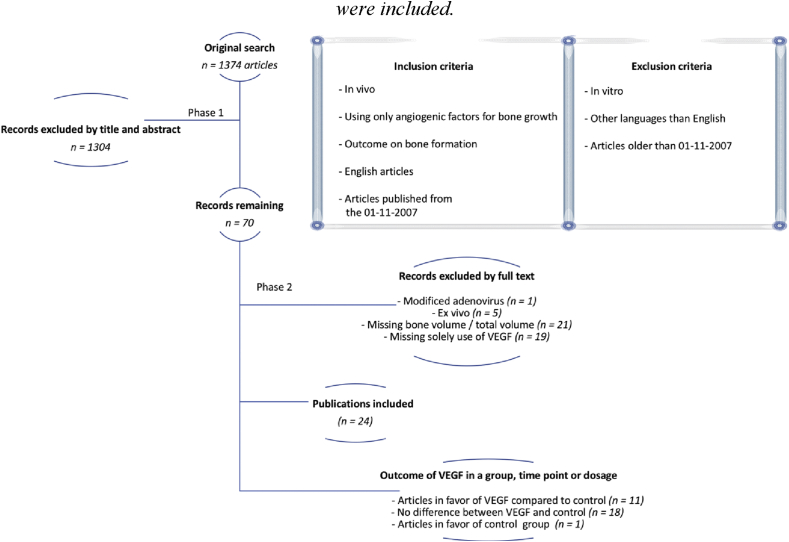
Inclusion criteria include in vivo studies in both animals and humans using VEGF compared to empty defect or empty control, where the outcome measure is bone growth within a region assessed either by micro-CT or histomorphometry. All human studies were included. If a study used VEGF in combination with other hormones or growth factors, only the solely VEGF groups were included. Articles were restricted to English articles with full text available that were published during the last thirteen years. Exclusion criteria were all ex vivo studies, reviews, explanatory articles, conference abstracts, lectures and newspaper articles (Fig. 2).
Fig. 2.
Illustration of the search strategy for the systematic review. A total of twenty-four articles were included.
Based on previous literature
A thirteen-year duration was selected due to an existing systematic literature review produced in 2008 on the effect of VEGF on bone formation [23]. The latest included article from this study was from 01 to 11/2007.
Studies were identified through PubMed using the search string (vegf or “vascular endothelial growth factor”) and (osteogen∗ or “bone formation” or “bone regeneration”). Studies were then filtered for full text only, human or animal studies, and publication date from 1 January 2007 to 01 July 2019. Studies were then screened by title and abstract, and the full texts of eligible studies were analysed for final inclusion (Fig. 2).
Phases for inclusion
Study selection was divided into different phases. In phase one, articles were evaluated by their title and abstract. In phase two, articles were assessed based on their full text by primary inclusion and exclusion criteria without any evaluation of results. In the final phase, relevant studies were subject to quality assessment using a scoring system.
The extracted data from each included article
The following data were extracted from the included studies: journal type (peer-review or not), animal model details, tissue type (e.g. cranial or ectopic bone formation), type of scaffold, type of control group, type of randomisation, type of VEGF and its release system, justification for VEGF dose and/or release rate, experiment duration, type and magnitude of outcome, type of blinding, sample size considerations, and ethics and disclosure statements. The corresponding author of the study was contacted if any of the aforementioned information was missing from the articles. Data extraction was performed by CHD and verified by KK. At any disagreements, the third or fourth authors were included, and a discussion was initiated until agreement by all authors.
Risk of bias assessment
Assessing the risk of bias among articles was inspired by Macleod et al., while the pooling of animal experimental data was provided by CAMARADES for experimental animals [24] (Table 1). This assessment consisted of 10 items, with each providing 1 point. Three items were removed, and two items were added to highlight the objective of this systematic review. This meant that questions regarding the control of temperature, the blinded induction of ischaemia, and the use of anaesthetic without significant intrinsic neuroprotective activity were removed. Instead, the justification of a control group and VEGF dosage were added.
Table 1.
Modified score of quality from CAMARADES for systemic reviews in experimental animal studies [24]: [1] peer-reviewed journal; [2] control group; [3] randomisation; [4]VEGF dose justified; [5] blinding; [6] details on animal model; [7] sample size calculation; [8] compliant with ethics; and [9] no conflicts of interest.
| References | 1) Peer review journal | 2) Control group | 3) Randomi -zation | 4) VEGF dose justified | 5) Blinding | 6) Details on animal model | 7) Sample size calculation | 8) Comply with ethics | 9) No conflict of interest | Quality Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Amirian 2015 [23] | X | X | — | — | — | X | — | X | — | 4 |
| Kenney 2009 [32] | X | X | — | — | — | — | — | X | — | 3 |
| Lohse 2015 [24] | X | X | — | X | — | — | — | — | — | 3 |
| Çakir-Özkan 2017 [34] | X | X | — | — | — | X | — | X | — | 4 |
| L Zhang 2014 [42] | X | X | — | — | X | X | — | X | X | 6 |
| Lv 2015 [35] | X | X | X | — | — | — | — | X | — | 4 |
| Khojasteh 2017 [43] | X | X | — | — | X | — | — | X | — | 4 |
| Moser 2017 [25] | X | X | X | X | X | — | — | X | X | 7 |
| W Zhang 2014 [36] | X | X | — | — | — | — | — | X | — | 3 |
| W Zhang 2011 [37] | X | X | X | — | — | — | — | X | — | 4 |
| Quinlan 2015 [26] | X | X | — | X | X | X | — | X | X | 7 |
| Schliephake 2015 [27] | X | X | — | — | X | X | X | X | X | 7 |
| Behr 2012 [33] | X | X | — | X | — | X | — | X | X | 6 |
| Geuze 2012 [44] | X | X | X | X | — | X | X | X | X | 8 |
| Hernández 2012 [38] | X | X | — | — | — | X | — | X | — | 4 |
| Kempen 2009 [28] | X | X | — | X | — | X | — | X | — | 4 |
| Casap 2008 [39] | X | X | X | — | X | X | — | X | — | 6 |
| Patel 2008 [29] | X | X | — | X | X | X | — | X | — | 6 |
| Yang 2010 [40] | X | X | — | — | X | X | — | X | — | 5 |
| Yonamine 2010 [30] | X | X | — | — | — | — | — | X | — | 3 |
| Wu 2012 [41] | X | X | X | — | X | X | — | X | X | 7 |
| Schmitt 2013 [46] | X | X | — | X | — | X | — | X | — | 5 |
| Du 2015 [45] | X | X | X | — | X | X | — | X | X | 7 |
| Das 2016 [31] | X | X | — | X | — | X | X | X | — | 5 |
The total assessment of the risk of bias was based on scientific quality according to peer-reviewed articles, control groups, random allocations to intervention groups, blinded evaluations and sample size calculation. Furthermore, the reproducibility of dosage and animal models, any conflict of interest and animal regulations by approval mentioned in the article.
Statistics
Statistics were calculated by one-way ANOVA for the comparison of different parameters, when applicable. A p-value of less than 0.05 was considered significant.
Results
A total of 1374 articles were identified using the PubMed search. During the title/abstract screening, 1304 articles were excluded, leaving 70 articles for the full-text analysis. The full-text analysis excluded 46 articles: 1 used a modified adenovirus, 5 were ex vivo studies, 21 were missing bone volume/total volume evaluation by either histomorphometry or microCT scan and 19 articles did not have a group with the solitary use of VEGF for bone formation (Fig. 2).
Data extracted from each article
The content of each article is illustrated in Table 2. No studies used VEGF in human studies.
Table 2.
The characteristics of included studies.
| Ref | Animal | Type | Scaffold | Control | Type of VEGF | Release | Dosage | TOE | Results | BV/TV | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amirian 2015 [25] | Rat | Cranial defect model | Pectin-biphasic calcium phosphate | Empty defect | rhVEGF | Gelatin hydrogel scaffolds | Total concentration VEGF: Unknown 75% released in 15 days |
2 weeks/4 weeks | MicroCT: VEGF significantly better than control No mentioned P-values. |
MicroCT: Control: 2w/4w 2.3%/3.3% VEGF: 2w/4w 6.4%/6.5% Histomorphometry: Control: 2w/4w 2.13%/3.75% VEGF: 2w/4w 6.74%/8.95% |
4 |
| Keeney 2010 [34] | Mice | Intra-femoral defect | Collagen/calcium phosphate | Only scaffold | Therapeutic plasmid VEGF165 | Plasmid DNA | 0.35ug/mm3 | 30 days | Significant more bone in scaffold + VEGF165 | Histomorphometry: Control: 9.8% VEGF (non-complexed): 24.2% VEGF (complexed): 17.1% |
3 |
| Lohse 2015 [26] | Rat | Mandible | calcium carbonate granules |
Scaffold and empty defect | rhVEGF165 | poly-dl-lactic acid (PDLLA) | 0.24ug/1.5ug /6ug. ≈50% release first 3 days, low constant release till 5 weeka. |
4 weeks/13 weeks | Only significantly better with 1.5ug after 4 weeks. NS other timepoint and dosages |
Histomorphometry: 4 weeks: 4.4%–7.3% 13 weeks: 2.7%–4.6% Blank scaffold and empty defect, 4 or 13 weeks: 0.6–1.8% |
3 |
| Çakır-Özkan 2017 [36] | Rabbit | Mandible | PLLA-PEG | Only scaffold | rhVEGF165 | Gelatin | 750ng/scaffold Release 60% first 2 days, constant release for more than 14 days. |
4 weeks/8 weeks | Significantly better than control. | Histomorphometry: Newly formed bone Control: 4w/8w 34%/17% VEGF: 4w/8w 53%/31% |
4 |
| L Zhang 2014 [44] | Beagle | Femoral neck fracture model | Cannulated (titanium) screws | Cannulated screw fibrin glue | VEGF | PLGA/Fibrin glue | Unknown total VEGF in fibrin glue. 21.5% released after 3 days. Steady with 1.71% till 42 days with 88% cumulative release. |
4 weeks/8 weeks/12 weeks | VEGF had significant better results in week 8 and week 12 (p < 0.01) | 4 weeks: Control 5.7% VEGF 8.0% 8 weeks: Control: 19.5% VEGF: 29.0% 12 weeks: Control: 32.3% VEGF 41.3% |
6 |
| Lv 2015 [37] | Rabbit | Femoral condyle defect model | Titanium scaffold or empty defect | Empty titanium scaffold | rhVEGF165 | Fibrin glue | 0.5ug VEGF. Steady 100% release in 96 h 0.6%/hour steadily from 12 to 96 h |
4 weeks | Significantly better than control | New bone: Control: 7.8–8.3% VEGF: 17.4% |
4 |
| Khojasteh 2017 [45] | Dog (mongrel) | Mandible defect | B-TCP | Scaffold only | VEGF | PLGA microspheres | Release: Burst 60 ng/ml 8h. Steady 14 days release total 200 ng/ml. |
8 weeks | No significant difference to control | Histomorphometry_ Control: 7.2% VEGF: 20% |
4 |
| Moser 2018 [27] | Rat | Ectopic | PDLLA/CaCO3 composite granules | Scaffold + granules | rhVEGF165 | PDLLA/CaCO3 | 25ug VEGF/g polymer Total dose: 1.5ug VEGF Burst release: 3 days 5.5%/6% total release in 29 days 100ug/g polymer /6ug VEGF Burst release: 3 days 6% total release in 29 days 10.5% |
4 seeks/13 weeks | No significant difference to control | Histomorphometry: 4 weeks: Control: 0–1% VEGF: 0–1% 13 weeks: Control: 0–1% VEGF: 0–1% |
7 |
| W Zhang 2014 [38] | Rabbit | Skull defect | Silk scaffold | Silk scaffold with water | VEGF | Silk scaffold + water absorption | 6ug/scaffold | 12 weeks | No significant difference to control | uCT BV/TV control: 4.12 VEGF: 10.14 Histomorphometry: Control: ~28% VEGF ~44% |
3 |
| W Zhang 2011 [39] | Rabbit | Sinus floor elevation surgery | Silk hydrogel | Silk gel alone | rhVEGF165 | Silk hydrogel | 1000ug/ml ∗ 0.200 ml = 4 ug per scaffold No burst release. At least 24 days release |
4 weeks/12 weeks | No signficant difference between VEGF and control | Histomorphometry 4 weeks: Control: 1.8% VEGF: 5.6% 12 weeks: Control 8.7% VEGF: 18.5% |
4 |
| Quinlan 2017 [28] | Rat | Calvarial defect model | Collagen-Hydroxyapatite scaffold | Empty defect and only scaffold | rhVEGF165 | Alginate microparticles | 1ug/mg (1.6 ug/scaffold) Burst release till day 7. Steady release till 8 weeks. |
8 weeks | Significantly better than empty defect. NS against scaffold alone. Significant more new bone in VEGF group |
uCT: Empty defect: 0.4% Empty scaffold: 1.8% VEGF: 3.2% Histomorphometry: (um2, 10ˆ5) Empty defect: 0.9 um2 x 10ˆ5 Empty scaffold: 1.9 um2 x 10ˆ5 VEGF: 5.4 um2 x 10ˆ5 |
7 |
| Schliephake 2015 [29] | Rat | Tibia head placement | Titanium implant | Empty implants Empty implants with empty DNA nucleotide surface |
rhVEGF165 | DNA oligonucleotide | 750ng/screw 53% released within week 1. |
1 week/4 weeks/13 weeks | Significant lower bone formation in week 4. NS in week 1 and 13. | Histomorphometry: 1 week: Empty control: 4.0% Surface control: 4.3% VEGF: 5.9% 4 weeks Empty control: 17.6% Surface control: 20.3% VEGF: 5.9% 13 weeks No values mentioned |
7 |
| Behr 2011 [35] | Mouse | Calvarial model | Collagen sponge | PBS soaked collagen sponges | VEGFA | Collagen sponge | 200ng/mouse | 2 weeks/4 weeks/8 weeks/12 weeks | VEGF significantly better than control. | uCT 2 weeks: Control:1.2% VEGF:81.0% 4 weeks: Control:0–2% VEGF: ~95% 8 weeks: Control:~12% VEGF: ~90% 12 weeks: Control: 18.3% VEGF: 95.1% Histomorphometry: 3 weeks: Control: ~2% VEGF: ~55% 12 weeks: Control: ~11% VEGF: ~65% |
6 |
| Geuze 2012 [46] | Beagle | Ectopic | BCP scaffold | Calcium phosphate BCP scaffold mixed with microparticles or hydrogel without growth factors | rhVEGF165 | Sustained release: PLGA microparticles. Fast release: Hydrogel (gelatin) |
0.4ug per ectopic implant | 9 weeks | No significant difference to control. | Histomorphometry: PLGA release: Control:0–1% VEGF:0–1% Hydrogel release: Control: 0-% VEGF: 3–4% |
8 |
| Hernandez 2012 [40] | Rabbit | Bone defect condyle femur | PLGA pororus scaffold | Empty defect and empty scaffold | rhVEGF165 | PLGA microspheres | 4 mg (0.35ug)/20 mg (1.75ug) 50% release after 4 days. Around 90% in 2 weeks (fig says 2 weeks, text 3 weeks fig. 3a) |
2 weeks/4 weeks/8 weeks/12 weeks | 2 weeks: VEGF (1.75) significantly better than all groups 4 weeks: significantly better in VEGF group. 8 weeks: no difference from week 4. Data not shown. 12 weeks: no difference in control and VEGF. |
4 weeks Control: 10% VEGF: 20% 12 weeks Control: 10–15% VEGF: 18–20% |
4 |
| Kempen 2009 [30] | Rat | Critical sized femur shaft model, subcutaneous model | Empty defects (only orthotopic) and empty scaffold | VEGF | Gelatine Hydrogel | 2.0ug/scaffold 58% release during first 3.5 days. Total release after 2 weeks |
8 weeks | Subcutaneous: No significant difference between control and VEGF. Orthotopic: No significant difference between VEGF and empty scaffold or empty defect. |
Subcutaneous: Empty scaffold: 0% VEGF: 0% Orthotopic¢: uCT new bone; Empty defect: 28mm3 Empty scaffold: 28mm3 VEGF: 30mm3 |
4 | |
| Casap 2008 [41] | Rabbit | Mandible distraction | Injections | No injection | rVEGF165 | — | After 14 days 5ug/uL for 4 days. | 60 days | No significant difference to control (p = 0.057) | MicroCT BV/TV: Control: 2.5% VEGF: 13% |
6 |
| Patel 2008 [55] | rat | Cranial defect | Gelatin microspheres in porous PPF scaffold | Blank Gelatin microspheres or empty defect | VEGF | Gelatin | 0.24ug/mm3 | 4 weeks/12 weeks | No significant difference to control | Histomorphometry scoring: Control 4 weeks: 0.5 12 weeks: 1 VEGF 4 weeks: 0.5 12 weeks: 1 MicroCT Empty defect: 4w: 7% 12w: 16% Control 4 weeks: 4% 12 weeks: 8% VEGF 4 weeks: 2% 12 weeks: 6% |
6 |
| Yang 2010 [42] | Rabbit | Radial diaphysis | BTCP coated with fibrin sealant | Scaffold and untreated | rhVEGF165 | Absorption fibrinogen | 2.6ug VEGF/scaffold 90% release after 7 days. |
4 weeks, 8 weeks, 12 weeks | Significant difference to control | microCT new bone/TV 4 weeks: Control: 18% VEGF:33% 8 weeks: Control:34% VEGF: 63% 12 weeks: Control: 55% VEGF: 18% |
5 |
| Yonamine 2010 [32] | Rat | calvaria | PLGA microspheres | Empty defect/sham surgery | VEGF165 | PLGA microspheres | 1ug per 500ul No release from day 0–7. Full release till day 21. |
12 weeks | No significant difference to control | X-ray: Control: 20% VEGF:27–28% |
3 |
| Wu 2012 [43] | rabbit | Mandibular distraction | Plasmid pIRES injection | pIRES and normal saline | hVEGF165 | Plasmid | 2ug | 2 weeks/4 weeks/8 weeks | Significant difference to control in both bone types. | Histomorphometry: Cortical 2 weeks: Saline:33% pIRES: 35% VEGF: 39% 4 weeks: Saline:81% pIRES: 84% VEGF: 93% 8 weeks: Control:91% pIRES: 93% VEGF: 96% Trabecular 2 weeks: Saline: 23% pIRES: 23% VEGF: 25% 4 weeks: Saline: 41% pIRES: 43% VEGF: 47% 8 weeks: Control:43% pIRES:46% VEGF: 53% |
7 |
| Schmitt 2013 [48] | pigs | Calvaria defect or vertical augmentation | Bio-oss | Bio-oss collagen carriers (empty scaffold) | rhVEGF165 | Fibrin glue | 8ug/ml, 3 ml total | 30 days/60 days | No significant difference to control | X-ray Critical size defect: 30 days: Control: 17% VEGF: 19% Vertical augmentation: 30 days: Control: 6% VEGF 4% 60 days Control: 10% VEGF: 11% |
5 |
| Du 2015 [47] | Beagle | Mandible defect | Nano-hydroxyapatite coral blocks | Only scaffold | rhVEGF165 | Absorption to scaffold | 3ug per scaffold block | 3 weeks/8 weeks | No significant difference to control | Histomorphometry 3 weeks: Control: 22% VEGF: 27% 8 weeks: Control: 33% VEGF: 39% |
7 |
| Das 2016 [33] | Rats | Mandible | PLGA microsphere | Empty defect | rhVEGF | PLGA microsphere | 200 ng steady release 3 weeks. | 12 weeks | No significant difference to control | Histomorphometry 12 weeks: Control: 1% VEGF 5% |
5 |
Experimental models
The experimental models were as follows: rats [[25], [26], [27], [28], [29], [30], [31], [32], [33]], mice [34,35], rabbits [[36], [37], [38], [39], [40], [41], [42], [43]], beagles [[44], [45], [46], [47]] and pigs [48]. The types of defect were as follows: cranial/calvaria/skull defects [25,28,31,32,35,44,48], intra-femoral implantation [34], mandible [26,33,36,41,43,45,47], femoral neck fracture model [44], femoral condyle defect [37,40], femoral shaft defect [30], ectopic [27,46], sinus floor elevation surgery [39], tibia head implant [29] and radial diaphysis [42].
Release methods for VEGF
VEGF was released by different delivery systems as follows: gelatine [30,31,34,36,46], plasmid DNA [34,43], poly-d,l-lactic acid (PDLLA) [26], fibrin glue [37,42,44], PLGA microspheres [32,33,40,45], PDLLA/CO3 [27], silk scaffold [38], hydrogel [39], alginate microparticles [28], PLGA microparticles [46], DNA oligonucleotide [29], Bio-Oss [48], hydroxyapatite [47], collagen sponge [35] and injection [41].
VEGF dosages
The dosage of VEGF was mentioned as either a total dosage, a dosage per area, or an amount in fluid. Doses were as follows: 0.24ug [26], 0.35ug [40], 0.4ug [46], 0.5ug [37], 1.5ug [26,27], 1.75ug [40], 2ug [30,43], 2.6ug [42], 3ug [47], 6ug [26,27,38], 20ug [39], 24ug [48], 200 ng [33,35], 750 ng [36], 200ng/100ul [45], 1ug/500ul [32], 5ug/ul [41], 75 ng/mm2 [29], 2.1ug/mm3 [34], 0.24ug/mm3 [31], 1ug/mg [28] and two studies did not mention dosage [25,44].
Studies in favour of using VEGF
The results were based on new bone regenerated in the bone defect region and compared to the control were as follows. Eleven articles had a group, time point or dosage with significantly better results in the VEGF group compared to the control (Fig. 2). Of which seven articles showed better bone growth at all time points and dosages for the VEGF group [25,[34], [35], [36], [37],42,43]. One article only showed significantly better results when compared to empty control and not an empty carrier [28]. One article favoured VEGF at 8 and 12 weeks [44]. One article favoured VEGF at week two, four, and eight [40], and another article favoured VEGF at a dosage of 1.5ug VEGF at week four [26]. Of these 11 articles, 6 had empty scaffold as the control group [25,26,35,36,42,43], while four articles had an empty defect as the control group [28,34,40,43] and four compared the combined scaffold and release method without VEGF [37,40,42,44]. Evaluations were conducted after an average of 5.8 weeks.
Studies not in favour of using VEGF
In total, 18 articles had a group, time point or dosage with no significant (NS) difference in bone formation in the VEGF groups compared the control group (Fig. 2). Twelve articles showed NS compared to control for all groups included in the study [27,28,[30], [31], [32], [33],38,39,41,[45], [46], [47], [48]]. One article showed NS compared to the scaffold [28]. One article with the dosage of 0.24ug and 6ug in weeks 4 and 13, and 1.5ug at week 4 [26], while 1 article was NS in the 4-week group [44]. One article in weeks 1 and 13 [29] and one in week 12 [40]. Of these results, eight had a control of only scaffold [26,27,32,33,38,[45], [46], [47]], two had an empty defect [30,41], two had the scaffold and release method combined [44,46], three had both the empty defect and scaffold group [28,30,40,48] and one had an empty defect and release material [31]. The average evaluation was conducted after eight weeks.
One article had a group in week four with significantly lower bone formation in the VEGF group compared to both empty defect and scaffold [29] (Fig. 2).
The time of evaluation was significantly lower in the 11 articles that showed better results with the use of VEGF compared to the 18 articles showing NS compared to the control (p < 0.05).
Risk of bias
The characteristics of included studies are illustrated in Table 2. The quality scores of the 24 articles range from 3 to 8 with an average of 5.08 points out of 10. The average quality score of the 11 studies that had a group, time point or dosage with a significantly better bone formation than the control was 4.81, whereas the average of the 18 articles with no significant difference was 5.3. No statistical differences were observed between the groups (p > 0.05). The only article with a lower quantity of bone formation with the use of VEGF had a score of 7.
Discussion
This article conducted a systematic review that collated and assessed recent progress in the solitary use of VEGF for bone formation over the past 13 years alongside any progress made towards clinical application.
A total of 1374 articles were found by the search criteria. Phase one included 70 articles by title and abstract, while phase two excluded 46 articles based on the full-text analysis. Ultimately, a total of 24 articles met the criteria for inclusion. These were quality scored using eight validated questions and one modified for the purpose of this review. Notably, the various models and methodologies used in these studies made statistical comparisons difficult. However, some very exciting indications could be extracted from the articles, such as the most efficient use of VEGF appearing to occur in defect models with a release of VEGF within the first three weeks, and evaluation studies with an early focus of eight weeks or less exhibiting the improved use of VEGF. For future study designs, this review serves as an inspiration for the modification and improvement of VEGF use for bone formation.
In 2008, a systematic review of the use of VEGF for bone formation was produced for future applications in bone research [23].
The study concluded that the existing evidence on the use of VEGF for bone formation is positive, but difficult to direct due the use of a lot of different models. The study recommended the potential use of VEGF in fracture healing and suggested a focus on future implementation.
The most recent study included in this review was from January 2007. To our knowledge, no systematic review has been performed on this matter since. This point highlights the relevance of a follow-up study to evaluate further progress regarding the clinical use of this growth factor in bone surgery to promote more efficient healing.
However, when comparing the conclusion from these two reviews, similar indications and conclusions of the beneficial use of VEGF for bone formation are present. Both studies have not been able to make statistical heterogeneity analysis due to both lack of descriptive methodology and different fracture and animal models. The conclusion from both studies is that VEGF has been seen in several studies to have a beneficial effect which could indicate potential use.
This review presents an overview of all articles using VEGF and approaches could hereby inspire and be compared for future designs. It must be implied that strategies for in vivo studies are based on previous literature and not local methods, experience, and regular descriptions. Based on these findings the lack of clinical trials can be correlated to a narrow window of the VEGF dosage and release, the insufficient focus of the research on specific type of bone healing processes i.e. POC, long bone, SOC etc, and specific location for the fracture in the case of mechanical strength and the cortical-trabecular ratio.
The consideration in using VEGF in vivo should in general be addressed to monitored effect on affected tissue to not induce irregulated tissue growth, as all stimulants in tissue engineering. Furthermore, isolated VEGF has been identified with malignant cell growth [49]. This is why general side effects and systemic evaluation must be addressed when using VEGF in designs with local approach [50].
When using VEGF locally, some dosages have shown to cause edema around the healing site and in later stages, non-union [4,18,29]. Before any clinical trials can be initiated reproducible results must be presented for a well-defined clinical treatment and side effect profile. In case of inspiration for this development, this review can be relevant in both developing and inventing future strategies.
A statistical comparison of all included studies proved difficult due to the inconsistencies in variables such as administration, model, defect, animal, release and dosage across studies. Moreover, an internal difference noted by Schliephake et al. had one group showing NS results in weeks 1 and 13, but a lower amount of bone in week 4. This illustrates that the use of the same design can yield different results depending on the time of observation. The time of observation was between 1 and 13 weeks across all included studies. Moreover, the majority of the included studies had an evaluation between 4 and 8 weeks (58%). Comparison according to outcome showed a significantly shorter evaluation period in the studies achieving a better effect of VEGF compared to the control. This could be an indication that the greatest effects of VEGF occur within the early stage of the bone regeneration process.
Although dosages were reported in different units, a general observation was that the lowest reported single dosage used in all included studies was 0.2ug [33,35], while the highest was 24ug [48]. The two dosages with 0.2ug were reported by Behr et al. (significantly better results with VEGF) and by Das et al. (no significant difference). Furthermore, Schmitt et al. used 24ug and noted no significant difference compared to control. In studies where VEGF showed significantly better results in all groups, 2.1ug/mm3 was used in a mouse intra-femoral implementation [34], 0.5ug in a rabbit femoral condyle defect [37], 200 ng in a mouse skull defect [35], 2ug in a rabbit mandible distraction model [43], 750 ng in a rabbit mandible defect [36] and 2.6ug in a rabbit radial diaphysis [42], while a rat cranial defect model used an unknown dosage [25]. The only group with a worse effect of VEGF than control used 750 ng/cm2 in a rat tibial head implant [29]. Based on these markers, it is not possible to provide a dose–response curve in any of the models. However, the highest single dosage with consistent positive response of VEGF was 2.6ug. This interval serves as a marker for future studies working with the same animal and defect model with the use of VEGF—even in combination studies.
In translational medicine, the dosages and release rates are essential to provide sufficient evidence for the clinical applications of products [40]. Release rates are defined as burst or continuous release. The release pattern of the growth factors has already been previously studied with a focus on prolonging the effect of VEGF [51]. In the use of VEGF on bone formation, the general consensus is that VEGF has the greatest effect when released in the natural systemic peak of 2–3 weeks [50] which, in smaller rodent and humans, is calculated to be at approximately week 1–3 [52,53]. Theoretically, VEGF will be concentrated in the inflammatory phase during the first week after a fracture within a defect. However, in the cartilage phase, inhibition has been shown to stimulate the osteogenic lineage [54]. These statements would suggest that VEGF had an optimal effect in endochondral ossification and that the release should be stopped before the cartilage phase of the bone formation process. This would imply that the optimal release occurs within the first weeks of a defect. By this assumption, we grouped the included studies into burst release groups, with full release occurring within the first three weeks [25,30,32,33,37,41,42,45] and continuous release occurring for more than three weeks [[26], [27], [28],39,44]. The remaining studies have no information regarding release after three weeks [29,36,40] or have an unknown release profile [31,34,35,38,43,[46], [47], [48]].
In the seven studies that had better bone formation than the control, three had a full release within the first three weeks [25,37,42] while the rest were unknown [[34], [35], [36],43]. The only group worse than control had no information on the release [29]. The assumption made by these results supports the existing literature, which suggests that VEGF should be released within the first 3 weeks.
The amount of new bone varied from 0 to 1% in a mouse ectopic model [27] up to 92% in a mouse calvarial model [35], and up to 96% in a rat mandible distraction model [43]. Since the physiological effects of VEGF seem to be best in the endochondral state of healing, it follows that the ectopic designs exhibit low amounts of new bone. The other ectopic design in this review had only 4% of total bone formation [46]. These results suggest that VEGF is not very osteogenic outside of defects models and the effectiveness is location-dependent. By this assumption, VEGF has the potential to optimise existing chemokine effects, but not to establish new pathways for enhancement. This could theoretically indicate that a focus towards fracture models would be preferable if the optimal use of VEGF should be established.
In the calvarial mouse model by Behr et al., VEGF had the same effect on bone formation as BMP-2. As such, the authors focused on comparing the dosages of BMP-2 to the clinical dosages that are already used for BMP-2 and could be used for VEGF. This study design focused on the translational purpose and the release method of the collagen sponge that has already been used for clinical studies. This study could serve as a marker for future designs in larger animal models with a dosage of 200ng/mouse. This also seems to correlate to the dosage range for growth factors in a small animal model. Furthermore, BMP-2 was adjusted from a clinical dosage into an experimental dosage. Notably, being able to translate between different models and areas to use the design for multiple purposes is an important aspect of optimal VEGF use.
Overall, 15 of the studies included in this review have the primary purpose of combining the angiogenic stimulation of VEGF with bone morphogenic protein (BMP) or mesenchymal stem cells [[25], [26], [27],30,33,[36], [37], [38], [39], [40],43,45,46,48,55], whereas the group with only angiogenic stimulation serves as a secondary objective. The general outcome is that 11 of these studies with a combination treatment using BMP [[25], [26], [27],30,33,[36], [37], [38],46,48,55] showed superior results to treatment using VEGF alone, as measured by new bone formation. Khojasteh et al. showed superior results with a combination of mesenchymal stem cells. Moreover, Wu et al. noted no difference between combination treatment and VEGF at week two, though the combination treatment was significantly better than VEGF alone at weeks four and eight. This illustrates that the time of treatment observation serves as an important factor in the measures of the outcome of bone formation with the use of these growth factors.
The majority of control groups in the included articles are empty defects, scaffolds only, or scaffold in combination with release material (Table 1). The clinical relevance for this approach must be considered limited since our intervention has the purpose of providing the same (or even better) results using existing procedures. However, while this can prove difficult in smaller animal models, it should be considered when translating into larger models to provide comparable results. An example of this is a study by Hernández et al., where the authors used the INFUSE model for the delivery of BMP-2—a method that is already established clinically and thus serves as a relevant control group.
In general, the use of VEGF in bone formation studies has shown strong potential over the last decade; however, no human trials have been performed to date. In order to evolve in the field, detailed description of methodologies would be particularly useful so that each study could be compared with existing results and developed to influence future designs. This would provide an opportunity to compare existing results using statistics, thereby creating the transparency necessary to build on existing literature. Notably, this field is currently delayed by the lack of reproducibility.
Conclusion
This review revealed that the development of VEGF is still progressing for the purpose of clinical application. The studies included in this systematic review provide valuable information on the use of VEGF for bone formation, such as:
The releases of VEGF appearing to be optimal within the first three weeks following fracture.
VEGF seems to have the best effect in fracture models, and in general seems to be better in defect models when compared to ectopic design. The highest single VEGF dosage for significant results was 2.6 ug per animal, regarding a variety of models and animals. Higher dosages did not have any improved effect compared to control.
The studies with an observation shorter than eight weeks seem to have the best outcome.
Studies that combined VEGF with other osteogenic factors generally had a higher percentage of new bone formation compared to VEGF and/or control; however, this is also correlated to possible higher costs, more preparation efforts, and higher side effect profiles such as malignant growth.
Future research using solely VEGF should be inspired by the existing literature and focus on the development of published methodologies and results. This will ensure progression in the area while making it possible to translate the results of different models and animals for clinical trials.
Funding statement
Funding support for compiling this article was jointly provided by The University of Southern Denmark, Odense University Hospital and the Chinese University of Hong Kong. The study was alongside produced at Region Zealand and with contribution from The Danish Orthopaedic Society, Knud Højgaards Fund, and The Danish Doctors Pension Fund – SEB pension.
Conflict of Interest
The authors have no conflicts of interest to disclose in relation to this article.
References
- 1.Svedbom A., Hernlund E., Ivergård M., Compston J., Cooper C., Stenmark J. Osteoporosis in the European Union: a compendium of country-specific reports. Arch Osteoporos. 2013;8(1–2) doi: 10.1007/s11657-013-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mills L., Tsang J., Hopper G., Keenan G., Simpson A.H.R.W. The multifactorial aetiology of fracture nonunion and the importance of searching for latent infection. Bone Jt Res. 2016;5(10):512–519. doi: 10.1302/2046-3758.510.BJR-2016-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Street J., Bao M., DeGuzman L., Bunting S., Peale F.V., Ferrara N. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002 Jul 23;99(15):9656–9661. doi: 10.1073/pnas.152324099. http://www.pnas.org/cgi/doi/10.1073/pnas.152324099 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wernike E., Montjovent M.O., Liu Y., Wismeijer D., Hunziker E.B., Siebenrock K.a. Vegf incorporated into calcium phosphate ceramics promotes vascularisation and bone formation in vivo. Eur Cell Mater. 2010;19:30–40. doi: 10.22203/ecm.v019a04. [DOI] [PubMed] [Google Scholar]
- 5.Gao C., Harvey E.J., Chua M., Chen B.P., Jiang F., Liu Y. MSC-seeded dense collagen scaffolds with a bolus dose of VEGF promote healing of large bone defects. Eur Cell Mater. 2013 Oct 13;26:195–207. doi: 10.22203/ecm.v026a14. http://www.ncbi.nlm.nih.gov/pubmed/24122654 [Internet] discussion 207. Available from: [DOI] [PubMed] [Google Scholar]
- 6.Portal-Núñez S., Lozano D., Esbrit P. Role of angiogenesis on bone formation. Histol Histopathol. 2012;27(5):559–566. doi: 10.14670/HH-27.559. [DOI] [PubMed] [Google Scholar]
- 7.Behr B., Tang C., Germann G., Longaker M.T., Quarto N. Locally applied vascular endothelial growth factor A increases the osteogenic healing capacity of human adipose-derived stem cells by promoting osteogenic and endothelial differentiation. Stem Cell. 2011;29(2):286–296. doi: 10.1002/stem.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer H., Bertram H., Lindenmaier W., Korff T., Weber H., Weich H. Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: autocrine and paracrine role on osteoblastic and endothelial differentiation. J Cell Biochem. 2005 Jul 1;95(4):827–839. doi: 10.1002/jcb.20462. http://doi.wiley.com/10.1002/jcb.20462 [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 9.Dimitriou R., Tsiridis E., Carr I., Simpson H., Giannoudis P.V. 2006. The role of inhibitory molecules in fracture healing; pp. 20–29. [DOI] [PubMed] [Google Scholar]
- 10.Hoeben A.N.N., Landuyt B., Highley M.S., Wildiers H., Oosterom A.T.V.A.N., Bruijn E.A.D.E. Vascular endothelial growth factor and angiogenesis. 2004;56(4):549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 12.Maes Christa, Carmeliet Geert. Springer; New York, NY: 2008. Vascular and nonvascular roles of VEGF in bone development; pp. 79–90. [Google Scholar]
- 13.Steinbrech D.S., Mehrara B.J., Saadeh P.B., Greenwald J.A., Spector J.A., Gittes G.K. VEGF expression in an osteoblast-like cell line is regulated by a hypoxia response mechanism. Am J Physiol Cell Physiol. 2000 Apr;278(4):C853–C860. doi: 10.1152/ajpcell.2000.278.4.C853. [DOI] [PubMed] [Google Scholar]
- 14.Amarilio R., Viukov S.V., Sharir A., Eshkar-oren I., Johnson R.S., Zelzer E. HIF1 regulation of Sox9 is necessary to maintain differentiation of hypoxic prechondrogenic cells during early skeletogenesis. 2007;3928:3917–3928. doi: 10.1242/dev.008441. [DOI] [PubMed] [Google Scholar]
- 15.Kronenberg H.M. Developmental regulation of the growth plate. Nature. 2003;423(6937):332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 16.Hu K., Olsen B.R. Vascular endothelial growth factor control mechanisms in skeletal growth and repair. Dev Dynam. 2016;10:1–8. doi: 10.1002/dvdy.24463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaigler D., Wang Z., Horger K., Mooney D.J., Krebsbach P.H. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res. 2006 Feb 6;21(5):735–744. doi: 10.1359/jbmr.060120. http://doi.wiley.com/10.1359/jbmr.060120 [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 18.Storkebaum E., Lambrechts D., Dewerchin M., Moreno-Murciano M.-P., Appelmans S., Oh H. Treatment of motoneuron degeneration by intracerebroventricular delivery of VEGF in a rat model of ALS. Nat Neurosci. 2005;8(1):85–92. doi: 10.1038/nn1360. [DOI] [PubMed] [Google Scholar]
- 19.Eckardt H., Ding M., Lind M., Hansen E.S., Christensen K.S., Hvid I. Recombinant human vascular endothelial growth factor enhances bone healing in an experimental nonunion model. J Bone Jt Surg Br. 2005;87(10):1434–1438. doi: 10.1302/0301-620X.87B10.16226. [DOI] [PubMed] [Google Scholar]
- 20.Levy N.S., Chung S., Furneaux H., Levy a P. Hypoxic stabilization of vascular endothelial growth factor mRNA by the RNA-binding protein HuR. J Biol Chem. 1998 Mar 13;273(11):6417–6423. doi: 10.1074/jbc.273.11.6417. http://www.jbc.org/cgi/doi/10.1074/jbc.273.11.6417 [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 21.Lee K., Silva E.A., Mooney D.J. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8(55):153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta R., Tongers J., Losordo D.W. Human studies of angiogenic gene therapy. Circ Res. 2009;105(8):724–736. doi: 10.1161/CIRCRESAHA.109.200386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keramaris N.C., Calori G.M., Nikolaou V.S., Schemitsch E.H., Giannoudis P.V. Fracture vascularity and bone healing: a systematic review of the role of VEGF. Injury. 2008;39(SUPPL.2) doi: 10.1016/S0020-1383(08)70015-9. [DOI] [PubMed] [Google Scholar]
- 24.Macleod M.R., O'Collins T., Howells D.W., Donnan G.A. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35(5):1203–1208. doi: 10.1161/01.STR.0000125719.25853.20. [DOI] [PubMed] [Google Scholar]
- 25.Amirian J., Linh N.T.B., Min Y.K., Lee B. Bone formation of a porous Gelatin-Pectin-biphasic calcium phosphate composite in presence of BMP-2 and VEGF. Int J Biol Macromol. 2015 May;76:10–24. doi: 10.1016/j.ijbiomac.2015.02.021. https://linkinghub.elsevier.com/retrieve/pii/S0141813015000926 [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 26.Lohse N., Moser N., Backhaus S., Annen T., Epple M., Schliephake H. Continuous delivery of rhBMP2 and rhVEGF165 at a certain ratio enhances bone formation in mandibular defects over the delivery of rhBMP2 alone - an experimental study in rats. J Contr Release. 2015;220:201–209. doi: 10.1016/j.jconrel.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 27.Moser N., Goldstein J., Kauffmann P., Epple M., Schliephake H. Experimental variation of the level and the ratio of angiogenic and osteogenic signaling affects the spatiotemporal expression of bone-specific markers and organization of bone formation in ectopic sites. Clin Oral Invest. 2018;22(3):1223–1234. doi: 10.1007/s00784-017-2202-3. [DOI] [PubMed] [Google Scholar]
- 28.Quinlan E., López-Noriega A., Thompson E.M., Hibbitts A., Cryan S.A., O'Brien F.J. Controlled release of vascular endothelial growth factor from spray-dried alginate microparticles in collagen-hydroxyapatite scaffolds for promoting vascularization and bone repair. J Tissue Eng Regen Med. 2017 Apr;11(4):1097–1109. doi: 10.1002/term.2013. http://doi.wiley.com/10.1002/term.2013 [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 29.Schliephake H., Rublack J., Förster A., Schwenzer B., Reichert J., Scharnweber D. Functionalization of titanium implants using a modular system for binding and release of VEGF enhances bone-implant contact in a rodent model. J Clin Periodontol. 2015 Mar;42(3):302–310. doi: 10.1111/jcpe.12370. http://doi.wiley.com/10.1111/jcpe.12370 [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 30.Kempen D.H.R., Lu L., Heijink A., Hefferan T.E., Creemers L.B., Maran A. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30(14):2816–2825. doi: 10.1016/j.biomaterials.2009.01.031. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 31.Patel Z.S., Young S., Tabata Y., Jansen J.A., Wong M.E.K., Mikos A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43(5):931–940. doi: 10.1016/j.bone.2008.06.019. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yonamine Y., Matsuyama T., Sonomura T., Takeuchi H., Furuichi Y., Uemura M. Effectable application of vascular endothelial growth factor to critical sized rat calvaria defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109(2):225–231. doi: 10.1016/j.tripleo.2009.09.010. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 33.Das A., Fishero B.A., Christophel J.J., Li C.J., Kohli N., Lin Y. Poly(lactic-co-glycolide) polymer constructs cross-linked with human BMP-6 and VEGF protein significantly enhance rat mandible defect repair. Cell Tissue Res. 2016;364(1):125–135. doi: 10.1007/s00441-015-2301-x. [DOI] [PubMed] [Google Scholar]
- 34.Keeney M., van den Beucken J.J.J.P., van der Kraan P.M., Jansen J.A., Pandit A. The ability of a collagen/calcium phosphate scaffold to act as its own vector for gene delivery and to promote bone formation via transfection with VEGF165. Biomaterials. 2010 Apr;31(10):2893–2902. doi: 10.1016/j.biomaterials.2009.12.041. https://linkinghub.elsevier.com/retrieve/pii/S0142961209014288 [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 35.Behr B., Sorkin M., Lehnhardt M., Renda A., Longaker M.T., Quarto N. A comparative analysis of the osteogenic effects of BMP-2, FGF-2, and VEGFA in a calvarial defect model. Tissue Eng Part A. 2011;18(9–10):1079–1086. doi: 10.1089/ten.tea.2011.0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Çakır-Özkan N., Eğri S., Bekar E., Altunkaynak B.Z., Kabak Y.B., Kıvrak E.G. The use of sequential VEGF- and BMP2-releasing biodegradable scaffolds in rabbit mandibular defects. J Oral Maxillofac Surg. 2017 Jan;75(1) doi: 10.1016/j.joms.2016.08.020. https://linkinghub.elsevier.com/retrieve/pii/S0278239116307546 [Internet] 221.e1-221221.e14. Available from: [DOI] [PubMed] [Google Scholar]
- 37.Lv J., Xiu P., Tan J., Jia Z., Cai H., Liu Z. Enhanced angiogenesis and osteogenesis in critical bone defects by the controlled release of BMP-2 and VEGF: implantation of electron beam melting-fabricated porous Ti6Al4V scaffolds incorporating growth factor-doped fibrin glue. Biomed Mater. 2015;10(3) doi: 10.1088/1748-6041/10/3/035013. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W., Zhu C., Wu Y., Ye D., Wang S., Zou D. VEGF and BMP-2 promote bone regeneration by facilitating bone marrow stem cell homing and differentiation. Eur Cell Mater. 2014 Jan 15;27:1–12. doi: 10.22203/ecm.v027a01. http://ecmjournal.org/journal/papers/vol027/pdf/v027a01.pdf [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 39.Zhang W., Wang X., Wang S., Zhao J., Xu L., Zhu C. The use of injectable sonication-induced silk hydrogel for VEGF165 and BMP-2 delivery for elevation of the maxillary sinus floor. Biomaterials. 2011 Dec;32(35):9415–9424. doi: 10.1016/j.biomaterials.2011.08.047. https://linkinghub.elsevier.com/retrieve/pii/S0142961211009744 [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hernández A., Reyes R., Sánchez E., Rodríguez-Évora M., Delgado A., Évora C. In vivo osteogenic response to different ratios of BMP-2 and VEGF released from a biodegradable porous system. J Biomed Mater Res Part A. 2012;100 A(9) doi: 10.1002/jbm.a.34183. http://doi.wiley.com/10.1002/jbm.a.34183 [Internet] n/a-n/a. Available from: [DOI] [PubMed] [Google Scholar]
- 41.Casap N., Venezia N.B., Wilensky A., Samuni Y. VEGF facilitates periosteal distraction-induced osteogenesis in rabbits: a micro-computerized tomography study. Tissue Eng - Part A. 2008;14(2):247–253. doi: 10.1089/tea.2007.0069. [DOI] [PubMed] [Google Scholar]
- 42.Yang P., Wang C., Shi Z., Huang X., Dang X., Li X. RhVEGF165 delivered in a porous β-tricalcium phosphate scaffold accelerates bridging of critical-sized defects in rabbit radii. J Biomed Mater Res - Part A. 2010;92(2):626–640. doi: 10.1002/jbm.a.32403. [DOI] [PubMed] [Google Scholar]
- 43.Wu G.P., He X.C., Hu C.B., Li D.P., Yang Z.H., Guo L. Effect of electroporation-mediated transfecting recombinant plasmid pIRES-hBMP2-hVEGF165 on mandibular distraction osteogenesis. Ann Plast Surg. 2012;69(3):316–325. doi: 10.1097/SAP.0b013e3182119275. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L., Zhang L., Lan X., Xu M., Mao Z., Lv H. Improvement in angiogenesis and osteogenesis with modified cannulated screws combined with VEGF/PLGA/fibrin glue in femoral neck fractures. J Mater Sci Mater Med. 2014;25(4):1165–1172. doi: 10.1007/s10856-013-5138-4. [DOI] [PubMed] [Google Scholar]
- 45.Khojasteh A., Fahimipour F., Jafarian M., Sharifi D., Jahangir S., Khayyatan F. Bone engineering in dog mandible: coculturing mesenchymal stem cells with endothelial progenitor cells in a composite scaffold containing vascular endothelial growth factor. J Biomed Mater Res B Appl Biomater. 2017;105(7):1767–1777. doi: 10.1002/jbm.b.33707. [DOI] [PubMed] [Google Scholar]
- 46.Geuze R.E., Theyse L.F.H., Kempen D.H.R., Hazewinkel H.A.W., Kraak H.Y.A., Oner F.C. A differential effect of bone morphogenetic protein-2 and vascular endothelial growth factor release timing on osteogenesis at ectopic and orthotopic sites in a large-animal model. Tissue Eng Part A. 2012;18(19–20):2052–2062. doi: 10.1089/ten.tea.2011.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Du B., Liu W., Deng Y., Li S., Liu X., Gao Y. Angiogenesis and bone regeneration of porous nano-hydroxyapatite/coralline blocks coated with rhVEGF165 in critical-size alveolar bone defects in vivo. Int J Nanomed. 2015;10:2555–2565. doi: 10.2147/IJN.S78331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitt C., Lutz R., Doering H., Lell M., Ratky J., Schlegel K.A. Bio-Oss® blocks combined with BMP-2 and VEGF for the regeneration of bony defects and vertical augmentation. Clin Oral Implants Res. 2013;24(4):450–460. doi: 10.1111/j.1600-0501.2011.02351.x. [DOI] [PubMed] [Google Scholar]
- 49.Das S., Ladell D.S., Podgrabinska S., Ponomarev V., Nagi C., Fallon J.T. Vascular endothelial growth factor-C induces lymphangitic carcinomatosis, an extremely aggressive form of lung metastases. Canc Res. 2010 Mar;70(5):1814–1824. doi: 10.1158/0008-5472.CAN-09-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dreyer C.H., Kjaergaard K., Ditzel N., Jørgensen N.R., Overgaard S., Ding M. Optimizing combination of vascular endothelial growth factor and mesenchymal stem cells on ectopic bone formation in SCID mice. J Biomed Mater Res Part A. 2017;105(12):3326–3332. doi: 10.1002/jbm.a.36195. http://doi.wiley.com/10.1002/jbm.a.36195 [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 51.Dimitriou R., Tsiridis E., Giannoudis P.V. 2005. Current concepts of molecular aspects of bone healing; pp. 1392–1404. [DOI] [PubMed] [Google Scholar]
- 52.Weiss S., Zimmermann G., Pufe T., Varoga D., Henle P. The systemic angiogenic response during bone healing. Arch Orthop Trauma Surg. 2009 Jul 27;129(7):989–997. doi: 10.1007/s00402-008-0777-5. http://link.springer.com/10.1007/s00402-008-0777-5 [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 53.Uchida S., Sakai A., Kudo H., Otomo H., Watanuki M., Tanaka M. Vascular endothelial growth factor is expressed along with its receptors during the healing process of bone and bone marrow after drill-hole injury in rats. 2003;32:491–501. doi: 10.1016/s8756-3282(03)00053-x. [DOI] [PubMed] [Google Scholar]
- 54.Hu K., Olsen B.R. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone. 2016;91:30–38. doi: 10.1016/j.bone.2016.06.013. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel Z.S., Young S., Tabata Y., Jansen J.A., Wong M.E.K., Mikos A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone. 2008;43(5):931–940. doi: 10.1016/j.bone.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]