Highlights
-
•
HVC was found in about half of CCTA patients.
-
•
Most of the HVC was AVC or MVC, and most MVC coexisted with AVC.
-
•
The association with CACS was found only in the presence of AVC.
-
•
Neither quantitative AVC nor MVC was associated with coronary atherosclerosis.
Keywords: Coronary CT angiography, Heart valve calcification, Coronary artery calcium score, Coronary artery disease
Abstract
Background
The concept of active atherosclerotic disease has been accepted for heart valve calcification (HVC). We investigated prevalence, distribution and related factors of HVC in patients who had undergone coronary CT angiography (CCTA).
Methods
Subjects were consecutive 200 patients who underwent CCTA. The prevalence and the distribution of HVC using ECG gated non-contrast CT were investigated. Logistic regression analysis and simple regression analysis for factors associated with presence of the calcification and quantitative calcification in the aortic and mitral valve were conducted.
Results
HVC was detected in 48.0%. Aortic valve calcification (AVC) was found in 92 cases, the most, followed by mitral valve calcification (MVC) in 25 cases, pulmonary valve in 3 cases, and tricuspid valve in 1 case. Although the left coronary cusp showed the most in 65.2%, no statistic significant difference for Agatston score was detected among each cusp in AVC. Multiple logistic regression analysis showed that age (OR:1.211, 95%C.I.:1.0716–1.1728, p < 0.0001) and coronary artery calcium score (CACS) grade (grade2 OR:7.3393, 95%C.I.:1.7699–30.4349, p = 0.0060, grade3 OR:7.2214, 95%C.I.:1.4376–36.2762, p = 0.0164) were significant factors associated with presence of AVC. The significant factors associated with quantitative AVC were age (p = 0.0043), dyslipidemia (p = 0.0117), and statin use (p = 0.0221). Only age (OR:1.1589, 95%C.I.:1.0726–1.2520, p = 0.0002) was significant factor related to presence of MVC. No significant related factor was found in quantitative MVC.
Conclusions
There was an association between presence of AVC and CACS, but not a significant association with presence of MVC. Neither quantitative AVC nor MVC had a significant association with CACS or coronary artery disease.
1. Introduction
It has been indicated that the heart valve calcification becomes not only a cause of valve disease[1], [2], but also association with occurrence of the aortic valve calcification and cardiovascular events [3], mitral annular calcium (MAC) and cerebral infarction [4], cardiac death [5], and atrial fibrillation [6]. However, the details of the mechanism and factors associated with the heart valve calcification haven’t been elucidated completely.
As for the calcification in the aortic or mitral valve, although there have been reports stating that significant differences were present in distribution of different races or gender, or valve leaflet [7], they were almost limited to Black people or Caucasian and there has been no report on the prevalence, distribution, and factors associated with the heart valve calcification in Asian people. Recently, evaluation of the coronary arterial calcification or stenosis using ECG gated CT has become possible and has been used widely at clinical situation. In these CT images, at the same time, evaluation of the heart valve calcification can be done and the relation of the coronary arterial calcification or stenosis with the heart valve calcification has been reported [8], [9], [10]. However, there has been almost none about Japanese case.
The purpose of this study was to investigate the prevalence of the heart valve calcification, the evaluation of the distribution in each valve leaflet and the factors associated with the heart valve calcification from patient’s background factors, the coronary arterial calcification and stenosis in patients who underwent scoring of the coronary arterial calcification score (CACS) and coronary CT angiography (CCTA).
2. Methods
2.1. Study population
This retrospective Health Insurance Portability and Accountability Act-compliant study was approved by the institutional human research ethics committee. Among patients aged 45 or older who were suspected coronary arterial diseases and underwent CCTA using 320-row CT during a period from December 18, 2018 to March 29, 2019, excluding patients with surgery of the heart valve, that of known coronary artery disease, and that of congenital bicuspid valve and hemodialysis, consecutive 200 patients were made the subjects to investigate the prevalence of the calcification cases and the distribution in each valve using ECG gated non-contrast axial CT scan taken at CACS.
2.2. CCTA acquisition
Patients with a pre-scan heart rate of ≥ 60 beats per minute were given 20 to 40 mg of metoprolol orally. If the heart rate remained ≥ 61 beats per minute after 1 h, they were given an intravenous injection of landiolol (0.125 mg/kg). Patients in whom beta-blockers were contraindicated (due to severe aortic stenosis, systolic blood pressure < 90 mmHg, bronchial asthma, symptomatic heart failure, or advanced atrioventricular block) did not receive these treatments.
The following devices were used: Aquilion ONE ViSION EditionTM or Aquilion ONE GENESIS EditionTM (320-ADCT, Canon Medical Systems Corporation, Otawara, Japan), a Dual Shot GX 7 (contrast injector, Nemoto Kyorindo Co., Ltd., Tokyo, Japan), a Model 7800 ECG monitor (Chronos Medical Devices Inc., Tokyo, Japan), and a Ziostation image analyzer (Zio M900, Ziosoft Inc., Tokyo, Japan).
Scanning was performed at a tube voltage of 100 kVp in patients with body mass indexes of < 30 kg/m2 and a tube voltage of 120 kVp in patients with body mass indexes of > 30 kg/m2. The mean tube current was calculated with automatic exposure control for a standard deviation (SD) of 20. Starting with a slice width of 0.5 mm and reconstruction interval of 0.25 mm, the minimum number of rows necessary to include all coronary arteries was selected from 200 rows (100 mm), 240 rows (120 mm), 256 rows (128 mm), 280 rows (140 mm), and 320 rows (160 mm), with reference to unenhanced CT images obtained to determine the coronary artery calcium score (CACS). All CACS data were evaluated on a workstation software (Zio M900 or ZioStation, Ziosoft). A calcified lesion was defined as ≥ 3 contiguous pixels with a peak attenuation of at least 130 Hounsfield units (HU). The CACS was determined using the following parameters: 120 kVp, 150 mA, and 3-mm thickness to calculate the Agatston score [11]. Moreover, CACS was classified into four categories: Grade 0 (0–10), Grade 1 (10–100), Grade 2 (100–400) and Grade 3 (400 < ).
Prospective CTA mode was used for all patients. The contrast agent iohexol (Omnipaque 350 mg/ml I; Daiichi Sankyo Company, Tokyo, Japan) or iopamidol (Iopamiron 300 mg/ml I; Bayer AG, Germany) was injected for 12 sec at 18.0 mg I/kg/s, followed by injection of 30 mL of saline at the same rate as contrast agent injection.
Real prep scanning with bolus tracking at the ascending aorta level was performed every 0.5 s beginning 10 s after the start of contrast agent injection. Scanning was started 6 s after when the contrast agent reached to 300HU at the ascending aorta. The reconstruction slice thickness was 0.5 mm and the increment was 0.25. A convolution kernel of FC04 was used with iterative reconstruction technology (FIRST).
2.3. CCTA interpretation
Both cross-sectional and longitudinal curved multiplanar reformation images were analyzed for plaque detection. Coronary artery segments with diameters of ≥ 2 mm were evaluated for the degree of stenosis. The percent degree of stenosis was determined by obtaining the percent ratio of the stenotic lumen to the normal vessel diameter proximal or distal to the stenosis. The stenosis was measured at the angle showing the narrowest degree of stenosis in still images taken from multiple projections. The degree of stenosis was evaluated by consensus of three experienced cardiologists who were unaware of the clinical data. Lesions with stenosis of > 50% were defined as significant. A stenotic lesion was defined as significant if calcification prevented access to the stenosis. As for coronary arterial disease staging, referring to the stage classification reported in the past[12], it was categorized into five stages as follows: In case of absence of arteriosclerosis, 0 (normal); in case of < 30% of stenosis in 1–2 vessels, 1 (mild); in case of 30–49% stenosis in 1–2 vessels or < 30% of stenosis in 3 vessels , 2 (moderate); ≧50% of stenosis in 1–2 vessels or 30–49% of stenosis in 3 vessels, 3 (severe); in case of ≧50% of stenosis in 2 vessels and stenosis in the proximal left anterior descending artery or 3 vessels, ≧50% of stenosis in 3 vessels or ≧50% of stenosis in the left main trunk, 4 (very severe).
2.4. Heart valve calcification
After the data was input in the workstation software (Zio M900 or ZioStation, Ziosoft), presence or absence of the calcification was investigated in individual valves of the aortic valve, mitral valve, tricuspid valve and pulmonary valve. Aortic valve calcification was defined as a calcification lesion just inferior to the origin of the coronary arteries and located at the aortic cusps, including the valvular point of attachment. Mitral valve calcification was defined as any calcification located at the junction between the left atrium and ventricle and includes the mitral leaflets.
In addition, Agatston Score was measured in the different valve cusps and commissure of the aortic valve, in the different anterior and posterior leaflets of the mitral valve and in the tricuspid valve and pulmonary valve without relation to the valve leaflet. In case of that the valve calcification fused between the cusps or the leaflets, the calcified lesion was cut at the researcher’s discretion and the Agatston Score was then measured in each valve.
2.5. Definition of risk factors
Hypertension was defined as either a systolic or diastolic blood pressure of ≥ 140/90 mmHg or the use of antihypertensive medications. Diabetes mellitus was defined when any of the following conditions were met fasting blood sugar of ≥ 126 mg/dl, postprandial blood sugar of ≥ 200 mg/dl, hemoglobin A1c of ≥ 6.5% (NGSP), or the use of medications. Dyslipidemia was defined when any of the following conditions were met total cholesterol of ≥ 220 mg/dl, low-density lipoprotein cholesterol of ≥ 140 mg/dl, fasting triglycerides of ≥ 150 mg/dl, high density cholesterol of < 40 mg/dl, or the use of lipid-lowering medications. Patients who had smoked during the past 1 year from the time of CCTA acquisition were defined as smokers.
2.6. Statistical analysis
Continuous data were expressed as the mean ± standard deviation (SD). If the variables were non-normally distributed, the median and quartile was used. When the median and quartile data were 0, the maximum and minimum results were added as the form of median [quartile; range]. Categorical data were expressed as frequencies (percentages).
Kruskal-Wallis test and Steel-Dwass test were used to investigate significant differences between the calcium scores of each aortic cusps and commissures and the t-test and Wilcoxon signed-rank test were used to investigate significant differences between mitral leaflets.
As for factors associated with presence or absence of the calcification in the aortic valve and mitral valve, the univariate logistic regression analysis was conducted, in which patient characteristics, CACS grade, and coronary arterial disease staging were made independent variables. Only for significantly related factors obtained the above, the multivariate logistic regression analysis was conducted. As for Agatston scores of the aortic valve and mitral valve, the simple regression analysis was conducted with patient characteristics, CACS grade, and coronary arterial disease staging as independent variables. p-Values of < 0.05 were considered significant.
The statistical analysis were performed using JMP Software for Windows (SAS Institute Inc., USA).
3. Results
3.1. Baseline characteristics
Table 1 shows the Baseline Characteristics in 200 cases (114 males, 57.0%).
Table 1.
Baseline clinical characteristics of all study patients.
 |
CACS: coronary artery calcium score.
The mean age was 66.93 ± 10.07. As for the coronary arteries, the significant stenosis of 50% or more was detected in 27.5% (55 cases), including the grade 3 (severe) in 24.5% (49 cases) and the grade 4 (very severe) in 3.0% (6 cases). As for the coronary arterial calcification, the mean CACS was 25.1[0–225.2] and the grade 0 was the most in 46.0% (92 cases).
3.2. Heart valve calcification
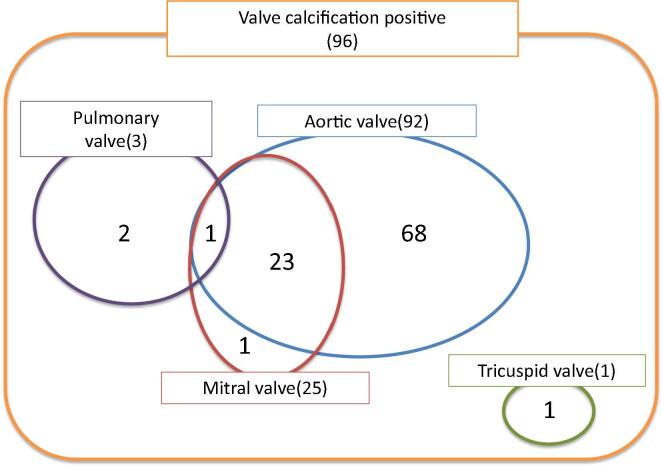
The calcification in the heart valve was detected in 48.0% (96/200 cases). Among those cases, the calcification in the aortic valve was found in 92 cases, the most, followed by the mitral valve in 25 cases, the pulmonary valve in 3 cases, and tricuspid valve in 1 case. The 24 cases among the 25 cases of the calcified mitral valve also showed the calcification in the aortic valve, and moreover, the one case had the calcification in the pulmonary valve. The case of calcified tricuspid valve had no calcification in the other heart valves (Fig. 1).
Fig. 1.
Distribution of heart valve calcification. Calcification in the aortic valve was found in 92 cases. The 24 cases among the 25 cases of the calcified mitral valve also showed the calcification in the aortic valve, and moreover, the one case had the calcification in the pulmonary valve. The cases of calcified tricuspid valve had no calcification in the other heart valves.
3.3. Aortic valve calcification
3.3.1. Distribution and quantitative assessment of aortic valve calcification
As for the distribution of the aortic valve calcification, the left coronary cusp showed the most in 65.2% (60 cases), followed by non-coronary cusp in 60.9% (56), the right coronary cusp in 46.7% (43), the LCC/NCC commissure in 33.7% (31), the NCC/RCC commissure in 22.8% (21), and the RCC/LCC commissure in 18.5% (17).
The Agatston Scores in each valve leaflet and commissure of the aortic valve were 0[0–18.24] in the left coronary cusp, 0[0–5.36] in the non-coronary cusp, 0[0–0;0–1774.09] in the right coronary cusp, 0[0–0;0–1911.64] in the RCC/LCC commissure, 0[0–0;0–928.66] in the LCC/NCC commissure, and 0[0–0;0–402.13] in the NCC/RCC commissure. Statistic significant difference was detected in each valve and commissure (p = 0.0008). However, there was no significant difference among each cusp. Figure regarding distribution of aortic valve calcification and statistical analysis for differences for the Agatston Score of aortic valve calcification among each valve and commissure were provided in supplementary material. (Fig. 1s, 2s.)
3.3.2. Analysis for prediction of the presence of aortic valve calcification
To investigate the factors associated with the presence of aortic valve calcification, the logistic regression analysis was used. Significantly related factors in the univariate analysis included age (Odds ratio (OR) 1.1311, 95% confidential interval (C.I.) 1.0878–1.1762, p < 0.0001), hypertension (OR 1.9254, 95%C.I. 1.0897–3.4019, p = 0.0230), dyslipidemia (OR 1.8286, 95%C.I. 1.0195–3.2796, p = 0.0411), statin use (OR 2.4793, 95%C.I. 1.3899–4.4227, p = 0.0019), CACS grade(grade1:OR 3.5354, 95%C.I. 1.5935–7.8434, p = 0.0019, grade2:OR 7.2727, 95%C.I. 3.3011–16.0229, p < 0.0001, grade 3:OR 9.5455, 95%C.I. 3.3714–27.0263, p < 0.0001), and coronary arterial disease staging (grade1:OR 3.4286, 95%C.I. 1.4673–8.0111,p = 0.0044, grade2:OR 2.6832, 95%C.I. 1.1386–6.3234, p = 0.0240, grade3:OR 8.5714, 95%C.I. 3.6293–20.2435, p < 0.0001, grade4:OR 6.8571, 95%C.I. 1.1349–41.4316, p = 0.0359) (Table 2(A)). In addition, the multivariate analysis was conducted in the significant factors detected by the univariate analysis and only the age(OR 1.1211, 95%C.I. 1.0716–1.1728, p < 0.0001), and CACS grade 2(OR 7.3393, 95%C.I. 1.7699–30.4349, p = 0.0060) and 3(OR 7.2214, 95%C.I. 1.4376–36.2762, p = 0.0164) were found to be significant factors (Table 2(B)).
Table 2.
(A) Univariate logistic regression analysis of the predictors associated with aortic valve calcification (B) Multivariate logistic regression analysis of the predictors associated with aortic valve calcification.
 |
CACS: coronary artery calcium score.
3.3.3. Analysis for related factors of the quantitative aortic valve calcification
The simple regression analysis was conducted to investigate the risk factors in the calcification score of the aortic valve. In the results, significant factors were age (p = 0.0043, R2 = 0.0400, Regression coefficient = 0.00318), dyslipidemia (p = 0.0117, R2 = 0.0323, Regression coefficient = 0.00102), and statin use (p = 0.0221, R2 = 0.0195, Regression coefficient = 0.00055), whereas CACS grade (p = 0.3642, R2 = 0.0161, Regression coefficient = −0.00044) or coronary arterial disease staging (p = 0.9944, R2 = 0.0011, Regression coefficient = −0.00011) was not significant factor. Table regarding simple regression analysis of the factors associated with quantitative aortic valve calcification was provided in supplementary material. (Table1s.)
3.4. Mitral valve calcification
3.4.1. Distribution and quantitative assessment of mitral valve calcification
In the mitral valve, the calcification was detected only at the posterior leaflet in 60.0% (15 cases), only at the anterior leaflet in 16.0% (4) and at both the anterior and posterior leaflets in 24.0% (6).
The calcification scores in the mitral valve were 0[0–0;0–4088.52] in the anterior leaflet and 0[0–0;0–5153.2] in the posterior leaflet and no significant difference was found in each leaflet (p = 0.3411).
3.4.2. Analysis for prediction of the presence of mitral valve calcification
The logistic regression analysis was used to investigate the factors associated with the presence of mitral valve calcification. In the univariate analysis, significantly related factors were age (OR 1.1778, 95%C.I. 1.0996–1.2616, p < 0.0001), eGFR (OR 0.9481, 95%C.I. 0.9149–0.9825, p = 0.0014) and CACS grade (grade2: OR 5.3514, 95%C.I. 1.5504–18.4704, p = 0.0080, grade3: OR 9.0588, 95%C.I. 2.3870–34.3789, p = 0.0012) (Table 3(A)). The multivariate analysis was conducted for the significant factors detected in the univariate analysis, and significant factor was found to be only age (OR 1.1589, 95%C.I. 1.0726–1.2520, p = 0.0002) (Table 3(B)).
Table 3.
(A) Univariate logistic regression analysis of the predictors associated with mitral valve calcification (B) Multivariate logistic regression analysis of the predictors associated with mitral valve calcification.
 |
CACS: coronary artery calcium score.
3.4.3. Analysis for related factors of the quantitative mitral valve calcification
The simple regression analysis was conducted to investigate risk factors in the calcification score of the mitral valve and in the result, no significant factor was found. Table regarding simple regression analysis of the factors associated with quantitative mitral valve calcification was provided in supplementary material. (Table2s).
4. Discussion
To our knowledge, this is the first study to summarize the prevalence, distribution and related factors in Japanese cases of heart valve calcification using the ECG gated CT. Because this study was conducted in the subjects of the patients who underwent CCTA, the relation to CACS grade or coronary arterial disease staging was also investigated. In this study, 48.0% of all the subjects had the calcification in any one of the valves. The incidence was 46.0% in the aortic valve, 12.5% in the mitral valve, 1.5% in the pulmonary valve, and 0.5% in the tricuspid valve. Because in this study, the subjects were the patients with CCTA, the population who had a high risk of atherosclerosis, it was inferred that the prevalence for the aortic and mitral valves might be higher than those reported in the past [3], [13], [14]. In addition, as for the distribution among the valve leaflets in the calcification of each valve, the prevalence tended to be higher at the left coronary cusp in the aortic valve and the posterior leaflet in the mitral valve. The findings were similar to a previous report [7]. In the quantitative evaluation using Agatston scores, no significant difference in the distribution among the cusps and leaflets was found in any valve. As for the difference in the distribution among the leaflets, there has been a report stating differently that the calcification was seen more at the non-coronary cusp in the aortic valve [14]. In the mitral valve, although the influence of left ventricular outflow has been investigated [7], no significant difference was found in the calcification resulting from the quantitative evaluation. Thus, furthermore studies will be required.
Recently, the concept of active atherosclerotic disease has been accepted for the heart valve calcification [15]. Thus, in this study, to evaluate the relationship between the coronary atherosclerosis and the valve calcification in Japanese population, the subjects were the patients who were measured CACS by ECG gated non-contrast CT and taken CCTA. Significant factors associated with the presence of aortic valve calcification were CACS grade and coronary arterial disease staging in addition to age, hypertension, dyslipidemia, statin use. Previously, coronary risk factors have been reported to be significant related factors when adjusted for age [16]. When the multivariate analysis was conducted for these factors, only age and CACS grade were significantly related factors. Although the relationship between the coronary arterial calcification and aortic valve calcifications has been already reported [8], [9], [10], this finding was observed similarly in Japanese population. A reason why a coronary risk factor or coronary arterial disease staging doesn’t become a significantly related factor is presumed because that may become a confounding factor of CACS grade.
On the other hand, as for the relation to the quantitative evaluation by the Agatston score of the aortic valve, significant factors were age, dyslipidemia, and statin use, whereas non-significant factors were indices of coronary arteriosclerosis such as CACS grade and coronary arterial disease staging.
Although the indices of coronary arteriosclerosis were significant factors for the presence of aortic valve calcification, the finding wasn’t related to the severity. The reason is assumed as follows: Although the initial stage of the aortic valve calcification conforms well with the concept of active atherosclerotic process of the aortic calcification accompanying the coronary arterial calcification [17], the relation to coronary risk factors is absent at the late stage and it conforms with the biological concept of afferent extension of calcified small nodes surrounded by osteoblast-like cells and grows faster by load of the calcification. Consequently, the presence of aortic valve calcification at the initial stage becomes a marker for presence of coronary arterial diseases and the range. However, as the calcification progresses, it may not become the marker [15]. In addition, as for statin use, it has been reported that statin induces regression of the coronary arterial plaque and also induces the coronary arterial calcification [18], [19]. However, statin use may not be a significant factor in multivariate analysis for the presence of calcification and may be a confounding factor in dyslipidemia. As for the relation to the quantitative evaluation by Agatston score of the aortic valve, statin use becomes a significantly related factor, being different from the indices of coronary arteriosclerosis such as CACS grade and coronary arterial disease staging. Thus, the possibility isn’t deniable that statin itself may influence the promotion of the calcification.
As for the presence of mitral valve calcification, although age, eGFR and CACS grade were significantly related factors, eGFR and CACS grade didn’t become significant factors after adjusting by age. Only age was a significantly related factor, being in consistency with past reports stating that the mitral valve calcification didn’t associate with the coronary arterial calcification or the severity of coronary arterial lesions [13], [20]. On the other hand, it has been reported that a significant relationship was shown between the mitral valve calcification and coronary arterial calcification [21]. In this study, as for the presence of mitral valve calcification, the univariate analysis revealed the relation to severe calcification of the coronary artery. However, any factor associated with the severity quantified by Agatston score was absent. Although previous reports on the mitral valve calcification have been limited almost to MAC [13], [16], [22], in this study on the mitral valve calcification, we observed not only MAC, but also the calcification of the leaflets, which may influence the difference from the previous reports. Although the mitral valve calcification was found in 25 cases, the 24 cases also had the aortic valve calcification. It is inferred that probably, while the active process of arteriosclerosis occurs, the aortic valve calcification progresses earlier than the mitral valve calcification. Gomel et al. reported that in the aortic valve, the compression and oscillatory shear on the fibrosa valve endothelial cells (VEC) and tensile strain on the valve interstitial cells (VIC) at the diastole phase and straight shear on the ventricularis VECs and bending forces on the VICs during systole at the systolic phase promote the start and progression of the calcification of the aortic valve [23]. Especially, it is assumed that compared to the mitral valve, the aortic valve has greater pressure load and consequently, starts the calcification earlier than that of the mitral valve. Thus it is assumed one of the reasons why mild calcification in the coronary artery doesn’t become a related factor and any factor isn’t related to the severity by Agatston score for the mitral valve calcification.
5. Limitations
There are some limitations in this study.
First, this is a single-center retrospective study with limited number of the cases. Secondly, because of the evaluation about the relationship between the heart valve calcification and coronary arterial calcification or coronary arterial diseases, the subjects were limited to the patients who underwent the CCTA, whose results may not be always applied to Japanese general population. Third, this is a cross sectional study, and the relation to the progress of valve calcification, appearance of severe valve disease accompanying it, and the outcome are thus unknown. Fourth, because of the cusps’ fusing caused by severe calcification and some consequent difficult evaluation cases for the borders of the cusps, the incidences of the calcification in individual cusps of the aortic valve may not be always accurate. Fifth, although it would be very interesting to evaluate a relationship between the distribution of the aortic calcification and aortic stenosis or change of the morphology of the aortic root, we could not evaluate a relationship between the distribution of the aortic calcification and aortic stenosis because there is no data for the degree of aortic stenosis such as echocardiography in all cases. In addition, we could not evaluate the relationship with the change of the morphology of the aortic root during heartbeat because we imaged a specific phase during one heartbeat using prospective ECG gated scan in order to minimize the radiation exposure with CCTA.
6. Conclusion
Heart valve calcification was found in about half of the patients with CCTA. The prevalence of the aortic valve calcification was the most, whereas the calcification of the pulmonary and tricuspid valves were almost none. There was an association between the presence of aortic valve calcification and coronary calcification, but not a significant association with the presence of mitral valve calcification. In addition, neither quantitative calcification of the aortic valve nor the mitral valve had a significant association with CACS grade or coronary artery disease staging.
Funding
This work was supported by Edwards Lifesciences Ltd.
CRediT authorship contribution statement
Yuki Kamo: Conceptualization, Methodology, Formal analysis, Data curation, Writing - original draft, Visualization. Shinichiro Fujimoto: Conceptualization, Methodology, Writing - review & editing, Supervision. Chihiro Aoshima: Investigation, Resources. Yuko O. Kawaguchi: Data curation. Yui Nozaki: Data curation. Ayako Kudo: Data curation. Daigo Takahashi: Data curation. Kazuhisa Takamura: Data curation. Makoto Hiki: Data curation. Nobuo Tomizawa: Data curation. Kanako K. Kumamaru: Funding acquisition. Shigeki Aoki: Writing - review & editing. Hiroyuki Daida: Writing - review & editing.
Declaration of Competing Interest
Dr. Fujimoto has a research agreement with Edwards Lifesciences Ltd. that is related to this study. He also has a research agreement with HeartFlow Japan G.K. and Canon Medical Systems Corporation that is not related to this study.
Dr. Aoki has a research agreement with Daiichi-Sankyo Company, Medi-Physics Co., FUJIFILM Toyama Chemical Co. and Bayer Holding Ltd. that is not related to this study.
Dr. Daida has received remuneration for lectures from Amgen Astellas Biopharma K.K., Sanofi K.K., Daiichi-Sankyo Pharmaceutical Company Ltd., Terumo Corporation, Boehringer Ingelheim GmbH., Bayer AG., MSD K.K., Mitsubishi Tanabe Parma, Fujifilm, Fuji Chemical Co, Pfizer Pharmaceutical Company Ltd. and Canon Medical Systems Corporation, and research grants from Nihon Medi-Physics Company Ltd., Kowa Pharmaceutical Company Ltd., Daiichi-Sankyo Company Ltd., Canon Medical Systems Corporation, Philips Respironics Ltd., Astellas Pharma Inc., Otsuka Pharmaceutical Company Ltd., Sanofi K.K., Boehringer Ingelheim GmbH., Bayer AG., Teijin Ltd., MSD K.K., Pfizer Pharmaceutical Company Ltd., HeartFlow, Novartis Pharma, Nihon Shinyaku, Fujifilm RI Pharm and Actelion Pharmaceuticals Japan Ltd. that is not related to this study. He has been in affiliation with some endowed departments including, Philips Respironics Ltd., Fukuda Denshi Company Ltd. and ResMed Ltd. that is not related to this study.
Footnotes
"This author takes responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation".
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100571.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Muddassir S.M., Pressman G.S. Mitral annular calcification as a cause of mitral valve gradients. Int. J. Cardiol. 2007;123(1):58–62. doi: 10.1016/j.ijcard.2006.11.142. [DOI] [PubMed] [Google Scholar]
- 2.Fulkerson P.K., Beaver B.M., Auseon J.C., Graber H.L. Calcification of the mitral annulus: etiology, clinical associateons, complications and therapy. Am. J. Med. 1979;66(6):967–977. doi: 10.1016/0002-9343(79)90452-2. [DOI] [PubMed] [Google Scholar]
- 3.Owens D.S., Budoff M.J., Katz R. Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. JACC Cardiovasc. Imag. 2012;6:619–625. doi: 10.1016/j.jcmg.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamin E.J., Plehn J.F., D’Agostino R.B. Mitral annular calcification and the risk of stroke in an elderly cohort. N. Engl. J. Med. 1992;327:374–379. doi: 10.1056/NEJM199208063270602. [DOI] [PubMed] [Google Scholar]
- 5.Kohsaka S., Jin Z., Rundek T. Impact of Mitral annular calcification on cardiovascular events in a multiethnic community: the Northern Manhattan Study. JACC Cardiovasc. Imag. 2008;1(5):617–623. doi: 10.1016/j.jcmg.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox C.S., Parise H., Vasan R.S. Mitral annular calcification is a predictor for incident atrial fibrillation. Atherosclerosis. 2004;173(2):291–294. doi: 10.1016/j.atherosclerosis.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 7.Koshkelashvili N., Codolsa J.N., Goykhman I., Romero-Corral A., Pressman G.S. Distribution of mitral annular and aortic valve calcium as assessed by unenhanced multidetector computed tomography. Am. J. Cardiol. 2015;116:1923–1927. doi: 10.1016/j.amjcard.2015.09.037. [DOI] [PubMed] [Google Scholar]
- 8.Nasir K., Katz R., Al-Mallah M. Relationship of aortic valve calcification with coronary artery calcium severity: The Multi-Ethnic Study of Atherosclerosis (MESA) J. Cardiovasc. Comput. Tomogr. 2010;4:41–46. doi: 10.1016/j.jcct.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Utsunomiya H., Yamamoto H., Kunita E. Combined presence of aortic valve calcification and mitral annular calcification as a marker of the extent and vulnerable characteristics of coronary artery plaque assessed by 64-multidetector computed tomography. Atherosclerosis. 2010;213:166–172. doi: 10.1016/j.atherosclerosis.2010.08.070. [DOI] [PubMed] [Google Scholar]
- 10.Mahabadi A.A., Bamberg F., Toepker M. Association of aortic valve calcification to the presence, extent, and composition of coronary artery plaque burden: ROMICAT study. Am. Heart J. 2009;158(4):562–568. doi: 10.1016/j.ahj.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 12.Arbab-Zadeh A., Fuster V. From detecting the vulnerable plaque to managing the vulnerable patient. JACC. 2019;74(12):1582–1593. doi: 10.1016/j.jacc.2019.07.062. [DOI] [PubMed] [Google Scholar]
- 13.Allison M.A., Cheung P., Criqui M.H., Langer R.D., Wright M. Mitral and aortic annular calcification are highly associated with systemic calcified Atherosclerosis. Circulation. 2006;113:861–866. doi: 10.1161/CIRCULATIONAHA.105.552844. [DOI] [PubMed] [Google Scholar]
- 14.Cujec B., Polick C. Isolated thickening of one aortic cusp: preferential thickening of the noncorenary cusp. J. Am. Soc. Echocardiogr. 1988;1:430–432. doi: 10.1016/s0894-7317(88)80025-7. [DOI] [PubMed] [Google Scholar]
- 15.Messika-Zeitoun D., Bielak L.F., Peyser P.A., Enriquez-Sarano M. Aortic valve calcification. Arterioscler. Thromb. Vasc. Biol. 2007;27:642–648. doi: 10.1161/01.ATV.0000255952.47980.c2. [DOI] [PubMed] [Google Scholar]
- 16.Boon A., Cheriex E., Lodder J., Kessels F. Cardiac valve calcification: characteristics of patients with calcification of the mitral annulus or aortic valve. Heart. 1997;78:472–474. doi: 10.1136/hrt.78.5.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien K.D. Pathogenesis of calcific aortic valve disease: a disease process comes of age (and a good deal more) Arterioscler. Thromb. Vasc. Biol. 2006;26:1721–1728. doi: 10.1161/01.ATV.0000227513.13697.ac. [DOI] [PubMed] [Google Scholar]
- 18.Okuyama H., Langsjoen P.H., Hamazki T. Statin stimulate atherosclerosis and heart failure: pharmacological mechanisms. Exp. Rev. Clin. Pharmacol. 2015;8(2):189–199. doi: 10.1586/17512433.2015.1011125. [DOI] [PubMed] [Google Scholar]
- 19.Kamada A., Ikeo T., Tamura I. Statin promotes mineralization potential in MC3T3-E1 nonmineralizing subclone. J. Oral Tissue Eng. 2006;3(3):169–174. [Google Scholar]
- 20.Yamamoto H., Shavelle D., Takasu J. Valvular and thoracic aortic calcium as a marker of the extent and severity of angiographic coronary artery disease. Am. Heart J. 2003;146:153–159. doi: 10.1016/S0002-8703(03)00105-4. [DOI] [PubMed] [Google Scholar]
- 21.Hamirani Y.S., Nasir K., Blumenthal R.S. Relation of mitral annular calcium and coronary calcium (from the multi-ethnic study of atherosclerosis [MESA]) Am. J. Cardiol. 2011;107:1291–1294. doi: 10.1016/j.amjcard.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Elmariah S., Budoff M.J., Delaney J.A.C. Risk factors associated with the incidence and progression of mitral annulus calcification: the multi-ethnic study of atherosclerosis. Am. Heart J. 2013;166:904–912. doi: 10.1016/j.ahj.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.M.A. Gomel, R. Lee, G.J. Allen, Comparing the role of mechanical forces in vascular and valvular calcification progression, Front. Cardiovasc. Med., 2019, 5, 197. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.