Highlights
-
•
The possible impact of lifetime physical activity on the risk of ALS is debated.
-
•
Brain18F-FDG-PET is a marker of neuronal integrity in vivo.
-
•
We compared cases who did not practice sport (N), cases who did (Y) and controls.
-
•
N had more extensive changes in areas involved in ALS at the same disability level.
-
•
N might cope better with the neurodegenerative process compared to Y.
Keywords: Amyotrophic Lateral Sclerosis, Sport, 18F-FDG-PET
Abstract
Objective
To evaluate the metabolic correlates of lifetime sport practice in ALS through brain 18F-FDG-PET.
Methods
131 patients completed a questionnaire about lifetime exposures, including physical activity related to sports, hobbies and occupations, and underwent brain 18F-FDG-PET. Exposure to sports was expressed as MET (Metabolic Equivalent of Task). We considered only regular practice (at least 2 h/week, for at least three months). We compared brain metabolism between two groups: subjects who did not report regular sport practice during life (N-group) and patients who did (Y-group). The resulting significant clusters were used in each group as seed regions in an interregional correlation analysis (IRCA) to evaluate the impact of lifetime sport practice on brain networks typically involved by the neurodegenerative process of ALS. Each group was compared to healthy controls (HC, n = 40).
Results
We found a significant, relative cerebellar hypermetabolism in the N-group compared to the Y-group. The metabolism of such cerebellar cluster resulted correlated to more significant and widespread metabolic changes in areas known to be affected by ALS (i.e. frontotemporal regions and corticospinal tracts) in the N-group as compared to the Y-group, despite the same level of disability as expressed by the ALS FRS-R. Such findings resulted independent of age, sex, site of onset (bulbar/spinal), presence/absence of C9ORF72 expansion, cognitive status and physical activity related to hobbies and occupations. When compared to HC, the N-group showed more widespread metabolic changes than the Y-group in cortical regions known to be relatively hypometabolic in ALS patients as compared to HC.
Conclusions
We hypothesize that patients of the N-group might cope better with the neurodegenerative process, since they show more widespread metabolic changes as compared to the Y-group, despite the same level of disability. Nevertheless, further studies are necessary to corroborate this hypothesis.
1. Introduction
Amyotrophic Lateral Sclerosis (ALS) is a neurodegenerative disease affecting motor neurons, leading to death within approximately three years, usually due to respiratory failure (van Es et al., 2017). Heritability studies suggest that genetic factors account for approximately 60% of the risk of developing ALS, while the remaining 40% is due to environmental exposures (Al-Chalabi and Hardiman, 2013). The possible impact of lifetime physical activity (PA) on the risk of developing ALS has been investigated, since vigorous physical exercise might cause oxidative stress and glutamate excitotoxicity (Bastos et al., 2011, Harwood et al., 2009). Nevertheless, published studies about cumulative, leisure and occupational PA as a risk factor are inconclusive (Lacorte et al., 2016). The reasons of such heterogeneity of results include differences in PA quantification and study design, the difficulty of evaluating a single environmental factor among the others, the variability of exposures in space and time, and possible gene-environment interactions. A detrimental role of sport practice on the risk of ALS has been hypothesized by studies on former athletes, including Italian professional soccer players (Chiò et al., 2005) and American football players (Lehman et al., 2012). A recent multicentre, cross-cultural, population-based, case-control study performed in the context of the Euro-MOTOR Project showed a linear association between lifetime PA and the risk of ALS, that was independent of age, sex, education, smoking and alcohol. A detrimental effect was found for leisure activities (sports and hobbies), occupational activities and for all activities combined (Visser et al., 2018). We focused on the metabolic correlates, assessed through brain 18F-2-fluoro-2-deoxy-D-glucose-PET (18F-FDG-PET), of lifetime sport practice in a subset of the population-based ALS series recruited in Piedmont and Valle d’Aosta, Italy, in the context of the Euro-MOTOR Project.
2. Methods
2.1. Partecipants recruitment
The Euro-MOTOR Project was a multicentre, case-control, European study involving the Netherlands, Italy (Piedmont and Valle d’Aosta, Lombardy and Apulia regions) and Ireland, including incident cases diagnosed with definite, (laboratory-supported) probable or possible ALS according to the El Escorial revised criteria (Brooks et al., 2000) from February 2011 to February 2014 (D’Ovidio et al., 2017). A structured questionnaire was administered to 1557 patients and 2922 controls in order to collect exposome data about PA (sports, hobbies, occupations), and many other factors, including traumas and pharmacological therapies. At the ALS Centre of Turin, Piedmont, Italy, 262 patients were enrolled in the Euro-MOTOR Project. Of these, 131 (50%) underwent brain 18F-FDG-PET at diagnosis, in the context of the research activity of our Centre about the metabolic correlates of neurodegeneration in ALS (Pagani et al., 2016, Cistaro et al., 2014), and were included in the present study. The other cases of the Euro-MOTOR sample did not undergo 18F-FDG-PET for the following reasons: denial, orthopnoea or difficulties reaching the PET Centre.
Forty subjects who were referred to the PET Centre for a suspected diagnosis of lung cancer in whom no oncologic disease was disclosed with 18F-FDG-PET/CT and who had a normal neurological assessment served as controls. Exclusion criteria were presence of major systemic illness, major vision disturbances, psychiatric illnesses, and diseases affecting brain functioning and metabolism.
2.2. Assessment of lifetime sport practice
Patients were interviewed through a structured questionnaire including many exposome aspects, as mentioned above, so limiting recall bias as much as possible. To quantify the lifetime exposure to PA, the Euro-MOTOR Project employed the Compendium of Physical Activities (Ainsworth et al., 2000). Such tool allows to estimate the metabolic consumption for each PA, calculated as the ratio to a standard resting condition (sitting quietly). The energy expenditure is expressed as MET (Metabolic Equivalent of Task): 1 MET corresponds to 1 Kcal/Kg body weight/h. The following formula was used to calculate the lifetime scores per participant, where k represents a specific PA:
In the Euro-MOTOR Project such methodology allowed to quantify PA related to sport activities, hobbies and occupations with three distinct scores. All exposures were calculated up to 3 years before the enrolment in the survey, since over 95% of all participants of the Euro-MOTOR Study had a delay from disease onset to survey of<3 years (D’Ovidio et al., 2017). We considered only regular sport practice, i.e. physical exercise for at least 2 h per week, for at least three months. Patients were then classified into two groups: subjects who had never practiced sport regularly during their life (NO sport practice, i.e. N-group, for whom MET score related to sport was 0, n = 78), and those who had practiced sport regularly, regardless of the entity of the exposure (YES sport practice, i.e. Y-group, for whom MET score related to sports was > 0, n = 53).
2.3. 18F-FDG-PET acquisition
18F-FDG-PET was performed according to published guidelines (Varrone et al., 2009). Briefly, subjects fasted at least six hours before the exam. Blood glucose was < 7.2 mmol/l in all cases before the procedure. After a 20-minute rest, about 185 MBq of 18F-FDG were injected. The acquisition started 60 min after the injection. PET/CT scans were performed by a Discovery ST-E System (General Electric). Brain CT (thickness of 3.75 mm, 140 kVolt, 60–80 mAs) and PET scan (1 FOV of 30 transaxial centimetres) were sequentially acquired, being the former used for attenuation correction of PET data. Data were collected in 128 × 128 matrices with a reconstructed voxel of 2.34 × 2.34 × 2.00 mm.
2.4. Statistical analysis
Demographic and clinical characteristics and exposome data of the N-group and the Y-group were compared through Mann-Whitney and χ2 tests where applicable. Demographic characteristics of patient groups and healthy controls were compared through Mann-Whitney and χ2 tests where applicable. SPM8 implemented in Matlab 7.10.0 (MathWorks) was used for image normalization. A customized brain 18F-FDG-PET template, obtained from scans performed at the same centre, was utilized for spatial normalization. Intensity normalization was performed using the 0.8 default SPM value of grey matter threshold and images were subsequently smoothed with an 8-mm filter and submitted to statistical analysis. The 18F-FDG-PET data of the two groups were compared applying the two-sample t-test of SPM12, including age at PET, sex, site of onset (spinal/bulbar), presence/absence of C9ORF72 expansion, ALS FRS-R (ALS Functional Rating Scale-Revised) total score at the time of PET, MET score related to hobbies and MET score related to occupational activities as covariates. The height threshold was set at p < 0.01uncorrected, p < 0.05FWE-corrected at cluster level. Only clusters containing at least 100 contiguous voxels have been considered significant. Metabolic clusters showing a significant difference between the two groups were then used as seed regions in a multiple regression analysis for each group to identify cerebral regions whose metabolism was positively or negatively correlated with that of the seed clusters (i.e interregional correlation analysis, IRCA) (Morbelli et al., 2013). In the IRCA the height threshold was set at p < 0.005uncorrected; p < 0.05FWE-corrected at cluster level.
Then, each patient group (i.e. N-group and Y-group) was compared to healthy controls, setting the height threshold at p < 0.05FWE-corrected; p < 0.05FWE-corrected at cluster level.
Brodmann areas (BAs) were identified at a 0–2 mm range from the Talairach coordinates of the SPM output isocentres corrected by Talairach Client (http://www.talairach.org/index.html).
Since the neuropsychological assessment was not available for 14 subjects out of 131, we performed a sensitivity analysis on the remaining 117 patients: we repeated the comparison between the N-group and the Y-group adding the cognitive status to the covariates mentioned above. The neuropsychological battery and the testing procedure have been previously described (Montuschi et al., 2015). Based on the performance at the neuropsychological assessment, the cognitive status was classified as normal, frontotemporal dementia (FTD) and intermediate impairment (i.e. patients with cognitive and/or behavioural impairment not fulfilling criteria for full-blown FTD) according to published criteria (Strong et al., 2009).
2.5. Ethical issues
This work has been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and with Uniform Requirements for manuscripts submitted to Biomedical Journals. The study has been approved by the ethical committee “Comitato Etico Interaziendale Azienda Ospedaliero-Universitaria Città della Salute e della Scienza di Torino”. Patients signed a written informed consent.
2.6. Data availability statement
Data will be available upon request by interested researchers.
3. Results
3.1. Demographic and clinical characteristics and exposome data
A descriptive summary statistics of covariates stratified by sport practice (N-group versus Y-group) is reported in Table 1. We found a prevalence of males in the group who practiced sport (p = 0.001). The age at PET was lower in the Y-group as compared to the N-group (p < 0.05). No significant difference was found for site of onset (bulbar/spinal), presence/absence of C9ORF72 expansion, ALS FRS-R total score at the time of PET, MET score related to lifetime hobbies and MET score related to occupational activities.
Table 1.
Descriptive summary statistics of covariates, stratified by sport activity (N-group versus Y-group).
| Sport activity |
Total | p* | ||
|---|---|---|---|---|
| N-group | Y-group | |||
| Sex | ||||
| Female (%) | 43 (55.1) | 14 (26.4) | 57 (43.5) | p = 0.001 |
| Male (%) | 35 (44.9) | 39 (73.6) | 74 (56.5) | |
| Age, median (IQR) | 67.5 (60.7–73.6) | 62.2 (51.8–70.6) | 67 (58.3–72.8) | p < 0.05 |
| Site of onset | ||||
| Bulbar (%) | 35 (44.9) | 16 (30.2) | 51 (38.9) | p = 0.091 |
| Spinal (%) | 43 (55.1) | 37 (69.8) | 80 (61.1) | |
| C9ORF72 expansion (%) | 11 (14.1) | 5 (9.4) | 16 (12.2) | p = 0.423 |
| Total Occupation MET scores, median (IQR) | 3975 (2524–5775) | 3036 (2229–4860) | 3800 (2394–5670) | p = 0.159 |
| Total Hobby MET scores, median (IQR) | 425 (148–1210) | 365 (60–1237) | 396 (96–1237) | p = 0.449 |
| Total ALS FRS-R score, median (IQR) | 39 (36–43) | 41 (37–45) | 40 (36–44) | p = 0.346 |
| Total | 78 (1 0 0) | 53 (1 0 0) | 131 (1 0 0) | |
Significant differences are reported in bold. IQR, Interquartile Range; ALS FRS-R, ALS Functional Rating Scale-Revised.
* Mann-Whitney test and Chi-square test were performed for continuous and discrete variables respectively.
When considering only patients for whom the neuropsychological assessment was available (n = 117), the difference of cognitive status was not significant between N- and Y-group (p = 0.07; Table 2). Table 2 reports no difference between the two groups in terms of previous general and head traumas and exposure to drugs related to sport practice (the list of drugs is reported in Inline Supplementary Table 1).
Table 2.
Descriptive summary statistics of cognitive status, history of general and head traumas, and intake of sport-related drugs, stratified by sport activity (N-group versus Y-group).
| Sport activity |
Total | p* | ||
|---|---|---|---|---|
| N-group | Y-group | |||
| Cognitive status | ||||
| Normal (%) | 32 (47.1) | 32 (65.3) | 64 (54.7) | p = 0.070 |
| Intermediate (%) | 21 (30.8) | 13 (26.5) | 34 (29.1) | |
| FTD (%) | 15 (22.1) | 4 (8.2) | 19 (16.2) | |
| missing | 10 | 4 | 14 | |
| General traumas | ||||
| No (%) | 21 (26.9) | 8 (15.1) | 29 (22.1) | p = 0.109 |
| Yes (%) | 57 (73.1) | 45 (84.9) | 102 (77.9) | |
| Head traumas | ||||
| No (%) | 54 (69.2) | 36 (67.9) | 90 (68.7) | p = 0.874 |
| Yes (%) | 24 (30.8) | 17 (32.1) | 41 (31.3) | |
| Sport-related drugs | ||||
| No (%) | 68 (87.2) | 47 (88.7) | 115 (87.8) | p = 0.797 |
| Yes (%) | 10 (12.8) | 6 (11.3) | 16 (12.2) | |
| Total | 78 (1 0 0) | 53 (1 0 0) | 131 (1 0 0) | |
* Mann-Whitney test and Chi-square test were performed for continuous and discrete variables respectively.
Sex and age at PET were included among covariates in the comparison between N-group and Y-group, while cognitive status was considered as covariate in the sensitivity analysis.
The control group (n = 40) showed median age at PET of 66.5 years (interquartile range 55–72), and male/female ratio of 2.64 (29/11). As compared to the control group, the N-group did not show any significant difference of age at PET (p = 0.172), while the male/female ratio was significantly lower in the N-group (p = 0.004). As compared to the control group, the Y-group did not show any significant difference of age at PET (p = 0.592) and male/female ratio (p = 0.907). Anyway, both age at PET and sex were included among covariates in the comparisons between each patient group and healthy controls.
3.2. 18F-FDG-PET
We did not find any region of relative hypometabolism in the N-group compared to the Y-group.
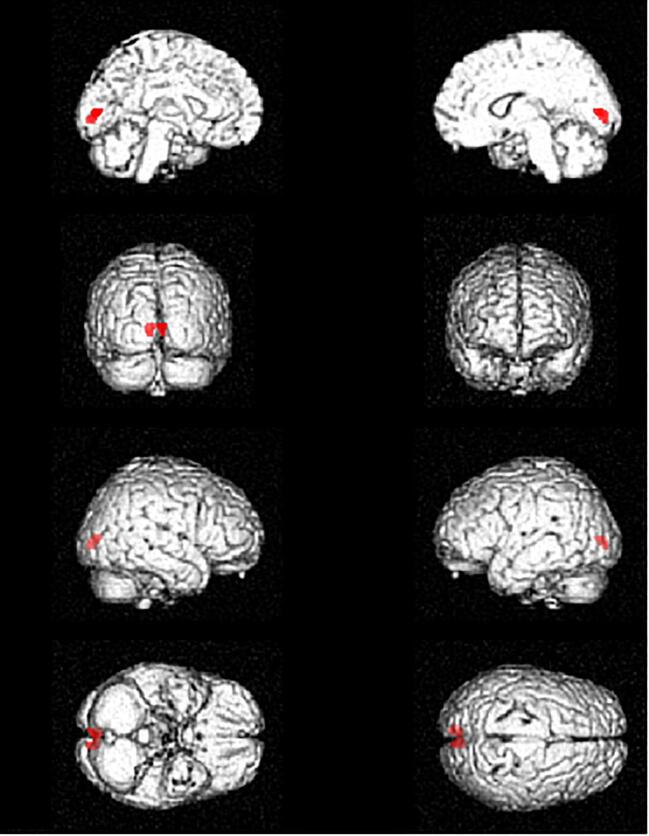
The N-group showed relative hypermetabolism compared to the Y-group in the cerebellum bilaterally (Fig. 1, Table 3).
Fig. 1.
Glass brain rendering of the comparison between the N-group and the Y-group. The clusters showing a statistically significant, relative hypermetabolism in the N-group as compared to the Y-group are projected on brain surface. N-group: NO sport practice. Y-group: YES sport practice.
Table 3.
Regions of relative hypermetabolism in the N-group as compared to the Y-group. N-group: NO sport practice. Y-group: YES sport practice.
| P (FWEcorrected) | Cluster extent | Z-score | Talairach coordinates | Cluster site | |||
|---|---|---|---|---|---|---|---|
| 0.001 |
1779 |
3.76 | −34 | −74 | −40 | Left Cerebellum | Posterior Lobe - Inferior Semi-Lunar Lobule |
| 3.67 | −10 | −50 | −26 | Left Cerebellum | Anterior Lobe - Dentate | ||
| 3.58 | −12 | −45 | −40 | Left Cerebellum | Posterior Lobe - Cerebellar Tonsil | ||
| 0.025 | 947 | 3.59 |
26 |
−72 |
−40 |
Right Cerebellum |
Posterior Lobe - Inferior Semi-Lunar Lobule |
| 3.35 | 8 | −56 | −26 | Right Cerebellum | Anterior Lobe - Dentate | ||
The sensitivity analysis showed substantially unchanged findings (data not shown).
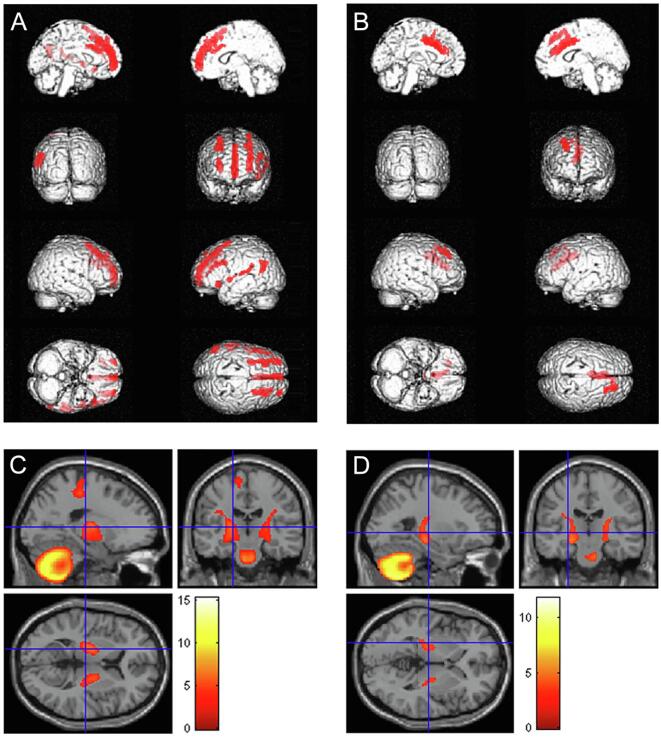
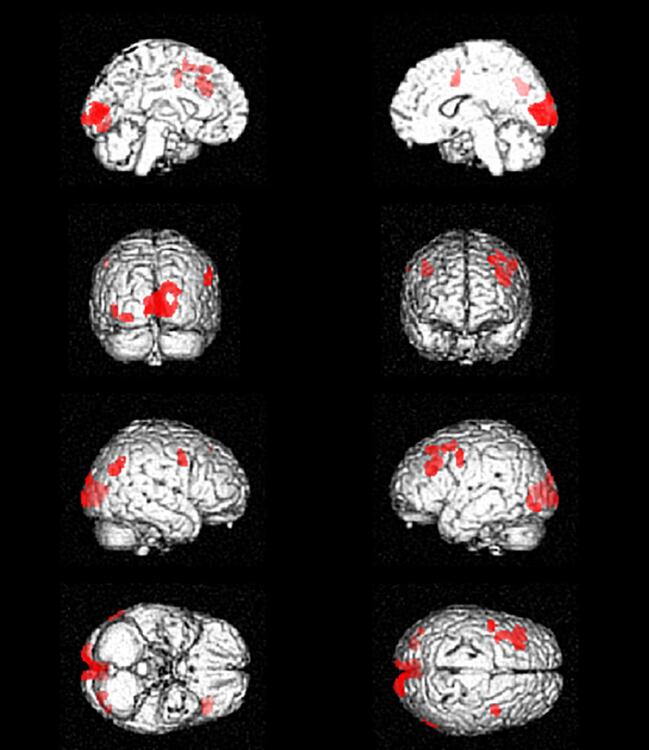
The IRCA showed that in the N-group metabolism in such cerebellar cluster negatively correlated with metabolism in left medial and inferior frontal gyrus, insula, superior, transverse and middle temporal gyrus and in bilateral superior and middle frontal gyrus (Fig. 2A, Table 4). In the Y-group the metabolism in this cerebellar cluster was negatively correlated with metabolism in smaller clusters including bilateral cingulate gyrus and right superior and middle frontal gyrus (Fig. 2B, Table 5). The metabolism of the cerebellar cluster showed a positive correlation in both groups with the metabolism in corticospinal tracts, that was larger in the N-group (Fig. 2C) as compared to the Y-group (Fig. 2D).
Fig. 2.
Results of the IRCA in the N-group, showing clusters of negative (A) and positive (C) correlation with metabolism of the cerebellar cluster of interest. Results of the IRCA in the Y-group, showing clusters of negative (B) and positive (D) correlation with metabolism of the cerebellar cluster of interest. N-group: NO sport practice. Y-group: YES sport practice.
Table 4.
Results of the IRCA in the N-group: regions whose metabolism resulted negatively correlated with that of the cerebellar cluster of interest. IRCA: interregional correlation analysis. N-group: NO sport practice. BA: Brodmann Area.
| P (FWEcorrected) | Cluster extent | Z-score | Talairach Coordinates | Cortical region | BA | ||
|---|---|---|---|---|---|---|---|
| 0.000 |
911 |
6.76 | −26 | 33 | 41 | Left Middle Frontal Gyrus | 8 |
| 6.44 | −30 | 50 | 21 | Left Superior Frontal Gyrus | 10 | ||
| 6.00 | −30 | 22 | 49 | Left Superior Frontal Gyrus | 8 | ||
| 0.000 | 240 | 6.65 | 30 | 57 | 12 | Right Superior Frontal Gyrus | 10 |
| 0.000 |
1568 |
6.53 | 0 | 56 | −6 | Left Medial Frontal Gyrus | 10 |
| 5.98 | −2 | 48 | 23 | Left Medial Frontal Gyrus | 9 | ||
| 5.86 | 0 | 31 | 37 | Left Medial Frontal Gyrus | 8 | ||
| 0.000 |
521 |
5.91 | 28 | 26 | 47 | Right Middle Frontal Gyrus | 8 |
| 5.62 | 26 | 13 | 56 | Right Middle Frontal Gyrus | 6 | ||
| 0.000 |
254 |
5.51 | −51 | −34 | 18 | Left Insula | 13 |
| 5.00 | −59 | −19 | 10 | Left Transverse Temporal Gyrus | 42 | ||
| 0.000 |
278 |
5.51 |
−53 | −59 | 21 | Left Superior Temporal Gyrus | 39 |
| 5.45 | −48 | −61 | 29 | Left Middle Temporal Gyrus | 39 | ||
| 0.000 |
269 |
5.47 | −46 | 26 | 24 | Left Middle Frontal Gyrus | 46 |
| 5.27 | −50 | 13 | 29 | Left Inferior Frontal Gyrus | 9 | ||
| 0.000 | 113 | 5.41 | −44 | 21 | −9 | Left Inferior Frontal Gyrus | 47 |
| 0.000 | 140 | 5.35 | −48 | 0 | 2 | Left Superior Temporal Gyrus | 22 |
Table 5.
Results of the IRCA in the Y-group: regions whose metabolism is negatively correlated with that of the cerebellar cluster of interest. IRCA: interregional correlation analysis. Y-group: YES sport practice. BA: Brodmann Area.
| P (FWEcorrected) | Cluster extent | Z-score | Talairach coordinates | Cortical region | BA | ||
|---|---|---|---|---|---|---|---|
| 0.014 |
393 |
4.83 | 26 | 41 | 38 | Right Superior Frontal Gyrus | 8 |
| 3.73 | 28 | 26 | 47 | Right Middle Frontal Gyrus | 8 | ||
| 0.000 | 877 | 4.19 |
−6 |
2 |
40 |
Left Cingulate Gyrus |
24 |
| 4.19 | 2 | 11 | 31 | Right Cingulate Gyrus | 24 | ||
The comparison between the N-group and healthy controls revealed a relative hypometabolism in the N-group in clusters including bilateral precentral gyrus, left middle frontal gyrus, left lingual gyrus, left inferior occipital gyrus, right angular gyrus, and bilateral cuneus (see Inline Supplementary Figure 1 and Inline Supplementary Table 2). No areas of relative hypermetabolism in the N-group were found.
The comparison between the Y-group and healthy controls revealed a relative hypometabolism in the Y-group in the cuneus bilaterally (see Inline Supplementary Figure 2 and Inline Supplementary Table 3). No areas of relative hypermetabolism in the Y-group were found.
4. Discussion
In the present study we compared the brain metabolic pattern, as assessed through 18F-FDG-PET, of two groups of ALS patients, i.e. subjects who had never practiced sports regularly during their life (N-group), and subjects who practiced sport activities regularly, with different entity of exposure (Y-group). We found a significant, relative cerebellar hypermetabolism in the N-group as compared to the Y-group. The metabolism of the cerebellar cluster showed a negative correlation with metabolism of frontotemporal regions that resulted more significant and more widespread in the N-group as compared to the Y-group. Furthermore, the metabolism of the same cerebellar cluster showed a positive correlation with the metabolism of the corticospinal tracts in both groups, with a larger size of the cluster in the N-group. Such findings resulted independent of age at 18F-FDG-PET, sex, site of onset (bulbar/spinal), presence/absence of C9ORF72 expansion, level of disability as assessed by ALS FRS-R total score, cognitive status and PA related to hobbies and occupations. Furthermore, when compared to healthy controls, the N-group showed more widespread cortical metabolic changes than the Y-group.
The finding of a cerebellar relative hypermetabolism has been already reported in ALS patients as compared to healthy controls (D’hulst et al., 2018). Furthermore, a study evaluating the brain metabolic correlates at 18F-FDG-PET of the different degrees of cognitive impairment in ALS reported a decreasing gradient of frontal lobe metabolism from cognitively spared patients to subjects with frontotemporal dementia (FTD), while patients with mild cognitive deficits showed an intermediate metabolic behaviour. Strikingly, frontal lobe relative hypometabolism resulted associated with relative hypermetabolism in cerebellum, corticospinal tracts and midbrain, with increasing statistical significance and cluster size as the cognitive impairment increased (Canosa et al., 2016).
To interpret our finding of a cerebellar, relative hypermetabolism in the N-group, we employed the identified cerebellar cluster as seed region to evaluate which brain regions showed a positive or negative correlation of metabolism (i.e. interregional correlation analysis, IRCA). In the N-group cerebellar metabolism resulted negatively correlated with metabolism in bilateral frontal and left temporal regions. In the Y-group cerebellar metabolism was negatively correlated with bilateral cingulate cortex and right superior and middle frontal gyrus. Furthermore, we observed a positive correlation between the metabolism of the cerebellar cluster of interest and that of corticospinal tracts in both N- and Y-group, that resulted more extensive in the N-group. Indeed, relative hypermetabolism at 18F-FDG-PET of corticospinal tracts has been already reported in ALS and has been usually ascribed to gliosis due to motor axons degeneration (Chiò et al., 2014). Based on the larger size and the higher statistical significance of the frontotemporal clusters of negative correlation and the more extensive positive correlation with the corticospinal tracts detected in the N-group in the IRCA, we hypothesize that in the N-group the relative, cerebellar hypermetabolism is related to lower metabolic levels in frontotemporal regions and higher metabolic levels in corticospinal tracts, both possibly due to a more severe neurodegenerative process in areas already known to be involved in ALS (Pagani et al., 2014, Van Laere et al., 2014). The cerebellum is known to be involved in both motor and extra-motor function (Reeber et al., 2013). Its role in ALS has been investigated in different studies, with conflicting results. A study including MRI longitudinal assessment of ALS and PLS patients has shown significant white matter damage over time in different regions, including the cerebellum (Menke et al., 2018). Otherwise, a recent multimodal MRI study has reported significantly decreased grey matter volume (GMV) in the left precentral gyrus, increased GMV in bilateral cerebellum, and decreased functional connectivity in the cerebellar anterior lobe in patients with ALS compared with healthy controls. The authors interpreted their findings as evidence of precentral degeneration and cerebellar compensation in ALS, suggesting that the white matter alterations mediate the relationship between the pathologies of the primary motor cortex and the cerebellum (Qiu et al., 2019). A possible explanation of such discrepancy has been hypothesized in a recent functional MRI study showing the coexistence of cerebellar circuits with decreased and increased connectivity, suggesting the concomitance of neurodegenerative and adaptive changes (Abidi et al., 2020).
Being the ALS FRS-R a score quantifying disability and functional impairment in ALS (Cedarbaum and Stambler, 1997), it can be considered as a measure of motor performance, albeit not accurate. So, we included it as a covariate in the analyses. Based on the results of the IRCA, we could hypothesize that N-patients can cope better with the neurodegenerative process due to ALS as compared to Y-patients, at the same level of disability as expressed by the ALS FRS-R score. Accordingly, when compared to healthy controls, the N-group showed more widespread metabolic changes than the Y-group in cortical regions known to be relatively hypometabolic in ALS patients as compared to healthy controls (Pagani et al., 2014), suggesting the presence of larger ALS-related brain metabolic changes in the N-group as compared to the Y-group, although the same level of motor disability.
Based on our results, we speculate that subjects who have never practiced sports are able to cope better with the neurodegenerative process. Since our patients underwent brain 18F-FDG-PET at diagnosis, the relative cerebellar hypermetabolism found in our study might represent an early adaptive mechanism to degeneration of frontotemporal regions and corticospinal tracts, probably destined to be overcome by neurodegeneration over time. If this resilience may be due to the preservation of compensatory mechanisms and/or brain reserve that can be exhausted by sport practice, can be subject to discussion. Indeed, this hypothesis could agree with the report of a lower age at ALS onset in professional athletes (Chiò et al., 2005). In this context, further aspects should be considered. Some studies suggest that subjects with relatively higher levels of cardiovascular (Turner et al., 2012) and physical (Mattsson et al., 2012) fitness show a higher risk of ALS. It is possible that PA is not a risk factor for ALS per se, but rather that a genetic profile promoting fitness also leads to increased ALS susceptibility (Huisman et al., 2013, Chiò and Mora, 2012), or that PA anticipates the occurrence of the disease in genetically predisposed individuals (Hamidou et al., 2014).
Nevertheless, this field needs further studies, overcoming the limitations of the present one. First, although the ALS FRS-R is a validated measure of disability caused by ALS, its total score does not provide an accurate estimation of motor performance. For such purpose the Walking Test or prolonged actigraphic registration may be possible tools. Second, the information resulting from 18F-FDG-PET, a valuable marker of neurodegeneration in vivo (Jack et al., 2012), might be complemented by the different brain MRI techniques (voxel-based morphometry, diffusion tensor imaging, resting state imaging) and spinal cord MRI. Third, the use of MET to quantify PA does not take into account differences among persons in body mass, body fat percentage, age, cardiorespiratory fitness levels, and can be inaccurate in measuring activities performed in different environmental conditions (Ainsworth et al., 2000). Anyway, albeit not free from limitations, the use of MET is suggested as a valuable tool to quantify lifetime exposure to PA (Lacorte et al., 2016). Fourth, the classification of patients into two groups, i.e. N- and Y-group, does not consider the different entity of the exposure to sport practice. We tried to subdivide the Y-group in tertiles or into two groups according to the median value of MET scores related to sport practice (above median value and below median value). The comparisons between each subgroup of the Y-group and the N-group provided a trend towards results overlapping those obtained comparing the whole Y-group and the N- group, but not reaching statistical significance probably due to the reduced size of the subgroups. Furthermore, we performed a multiple regression analysis in the Y-group to evaluate the relationship between brain metabolism and MET scores related to sport practice, but we did not find any significant cluster (data not shown). The issue of the different levels of exposure to sport practice should be considered in future studies. In the study by Visser et al. (Visser et al., 2018) a linear association between PA and ALS was reported: the more active a person has been, the higher the risk of developing ALS. Otherwise, other studies suggested that PA could have a different impact on ALS risk according to the level of exposure: a small amount could be protective and a higher level of activity detrimental Hamidou et al., 2014, Fang et al., 2016, Bandres-Ciga et al., 2019. Finally, the age of the exposure to PA should be considered. Indeed, PA can modulate brain plasticity (Fernandes et al., 2017), and it is well recognized that brain plasticity in response to environmental factors varies along the life course Li et al., 2014, Todorova and Blokland, 2017. As a consequence, PA might exert different effects according to the age of exposure.
On the other hand, the present study shows some strengths. To our knowledge, it is the first one employing neuroimaging to integrate epidemiological data in order to assess the impact of lifetime sport practice on the neurodegenerative process due to ALS. Second, the exposome data have been collected without stressing specific hypotheses about the risk of developing the disease, limiting recall bias as much as possible. Third, we were able to consider sport practice per se, eliminating the influence of PA due to hobbies and occupations. Fourth, we considered concomitant exposures (general traumas, head traumas, sport-related drugs) that can act as possible confounders, excluding their impact on our results. Actually, a potential impact of sport-related drugs cannot be completely ruled out, as the use of illicit drugs may have been under-reported by patients.
In conclusion, this is the first study about the brain metabolic correlates of lifetime sport practice in ALS and suggests that patients who never practiced sports regularly might cope better with the neurodegenerative process. Nevertheless, further studies are necessary to corroborate this hypothesis. They should stratify patients according to the age and the level of exposure to sport activity and employ more accurate tools to evaluate patients’ motor performance and further imaging techniques to estimate the damage due to neurodegeneration at different levels.
Disclosures
Antonio Canosa, Umberto Manera, Maria Claudia Torrieri, Rosario Vasta, Angelina Cistaro, Silvia Gallo, Barbara Iazzolino, Flavio Mariano Nobili, Federico Casale, Fabrizio D’Ovidio and Marco Pagani: no disclosures. Cristina Moglia has received research support from the Italian Ministry of Health (Ricerca Finalizzata). Andrea Calvo has received research support from the Italian Ministry of Health (Ricerca Finalizzata), and has received a research grant from Cytokinetics. Adriano Chiò serves on the editorial advisory board of Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, and Neurological Sciences; he has received research support from the Italian Ministry of Health (Ricerca Finalizzata), Regione Piemonte (Ricerca Finalizzata), University of Turin and the European Commission (Health Seventh Framework Programme); he serves on scientific advisory boards for Mitsubishi Tanabe, Roche, Biogen, Cytokinetics, and AveXis, and has received a research grant from Italfarmaco.
Funding
This work was in part supported by the Italian Ministry of Health (Ministero della Salute, Ricerca Sanitaria Finalizzata, grants RF-2010–2309849, RF-2011–02351193, GR-2010–2320550, and RF-2016–02362405), the European Commission’s Health Seventh Framework Programme (FP7/2007–2013 under grant agreement 259867), the Joint Programme - Neurodegenerative Disease Research (Brain-Mend project), granted by the Italian Ministry of Education, University and Research, Fondazione Mario ed Anna Magnetto and Associazione Piemontese per l’Assistenza alla SLA (APASLA). This study was performed under the Department of Excellence grant of the Italian Ministry of Education, University and Research to the “Rita Levi Montalcini” Department of Neuroscience, University of Turin, Turin, Italy.
Funding sources had no role in design and conduct of the study, collection, management, analysis, and interpretation of the data, preparation, review, or approval of the manuscript, and decision to submit the manuscript for publication.
CRediT authorship contribution statement
Antonio Canosa: Conceptualization, Data curation, Formal analysis, Methodology, Writing - original draft, Writing - review & editing, Supervision. Fabrizio D'Ovidio: Conceptualization, Data curation, Formal analysis, Methodology, Writing - original draft, Writing - review & editing, Supervision. Andrea Calvo: Data curation, Formal analysis, Funding acquisition, Writing - review & editing, Supervision. Cristina Moglia: Data curation, Writing - review & editing. Umberto Manera: Data curation, Writing - review & editing. Maria Claudia Torrieri: Data curation, Writing - review & editing. Rosario Vasta: Data curation, Writing - review & editing. Angelina Cistaro: Data curation, Writing - review & editing. Silvia Gallo: Data curation, Writing - review & editing. Barbara Iazzolino: Data curation, Writing - review & editing. Flavio Mariano Nobili: Formal analysis, Writing - review & editing. Federico Casale: Data curation, Writing - review & editing. Adriano Chiò: Conceptualization, Formal analysis, Funding acquisition, Methodology, Writing - original draft, Writing - review & editing, Supervision. Marco Pagani: Conceptualization, Data curation, Formal analysis, Methodology, Writing - original draft, Writing - review & editing, Supervision.
Acknowledgments
Acknowledgements
Contributors.
Euro-MOTOR Consortium: Giancarlo Logroscino, MD, PhD, Rosanna Tortelli, MD, PhD (Unit of Neurodegenerative Diseases, Department of Clinical Research in Neurology, “Aldo Moro” University of Bari, “Pia Fondazione Cardinale G. Panico”, Tricase, Lecce, Italy; Department of Basic Medical Sciences, Neurosciences and Sense Organs, “Aldo Moro” University of Bari, Bari, Italy); Chiara Zecca, MSc (Department of Clinical Research in Neurology, “Aldo Moro” University of Bari, “Pia Fondazione Cardinale G. Panico”, Tricase, Lecce, Italy); Ettore Beghi, MD, Elisabetta Pupillo, PharmD (Department of Neurosciences, IRCSS, Mario Negri Institute of Pharmacological Research, Milan, Italy); Giancarlo Comi, MD, Nilo Riva MD (IRCCS San Raffaele Hospital, Milan, Italy); Christian Lunetta, MD, Francesca Gerardi, DrBT (NEMO Clinical Center, Serena Onlus Foundation, Niguarda Ca’ Granda Hospital, Milan, Italy); Massimiliano Filosto, MD, Maria Sofia Cotelli, MD, Fabrizio Rinaldi, MD (Civil Hospital of Brescia, Brescia, Italy); Luca Chiveri, MD (Ospedale Valduce, Como, Italy); Maria Cristina Guaita, MD, Patrizia Perrone, MD (A.O. Civil Hospital, Legnano, Italy); Mauro Ceroni, MD, Luca Diamanti, MD (IRCCS National Neurological Institute “C. Mondino”, Pavia, Italy); Carlo Ferrarese, MD, PhD, Lucio Tremolizzo, MD (University of Milano-Bicocca, “San Gerardo” Hospital, Monza, Italy); Maria Luisa Delodovici, MD, Giorgio Bono, MD (“Ospedale di Circolo, Fondazione Macchi”, Varese, Italy); Orla Hardiman, MD, PhD, James Rooney, MSc, Mark Heverin, MSc, Alice Vajda, PhD (Academic Unit of Neurology, Trinity Biomedical Sciences Institute, Trinity College Dublin, Dublin, Ireland); Leonard H van den Berg, MD, PhD, Jan H Veldink, MD, PhD, Anne Visser, MD (Department of Neurology, University Medical Centre Utrecht, Utrecht, The Netherlands); Roel Vermeulen, PhD (Institute for Risk Assessment Sciences, Utrecht University, Utrecht, The Netherlands; University Medical Centre Utrecht, Julius Centre for Public Health Sciences and Primary Care, Utrecht, The Netherlands); Susan Peters, PhD (Institute for Risk Assessment Sciences, Utrecht University, Utrecht, The Netherlands; Department of Neurology, University Medical Centre Utrecht, Utrecht, The Netherlands); Jelle Vlaanderen, PhD, Lützen Portengen, PhD (Institute for Risk Assessment Sciences, Utrecht University, Utrecht, The Netherlands); Anneke J van der Kooi, MD, PhD (Department of Neurology, Amsterdam Medical Center, University of Amsterdam, Amsterdam, The Netherlands); Joost Raaphorst, MD, PhD (Department of Neurology, Donders Institute for Brain, Cognition and Behavior, Center for Neuroscience, Radboud University Nijmegen Medical Center, Nijmegen, The Netherlands); Giuseppe Fuda, MSc, Maurizio Grassano, MD, Paolina Salamone, MSc, PhD, Giuseppe Marrali, MSc, PhD (ALS Centre, “Rita Levi Montalcini” Department of Neuroscience, University of Turin, Turin, Italy); Letizia Mazzini, MD (ALS Centre, Department of Neurology, “Maggiore della Carità” University Hospital, Novara, Italy).
The authors thank the AFFIDEA-IRMET PET Centre of Turin for the execution of the 18F-FDG-PET brain scans.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2020.102312.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
Supplementary figure 2.
References
- van Es M.A., Hardiman O., Chio A. Amyotrophic lateral sclerosis. Lancet Lond Engl. 2017;390(10107):2084–2098. doi: 10.1016/S0140-6736(17)31287-4. [DOI] [PubMed] [Google Scholar]
- Al-Chalabi A., Hardiman O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat Rev Neurol. 2013;9(11):617–628. doi: 10.1038/nrneurol.2013.203. [DOI] [PubMed] [Google Scholar]
- Bastos A.F., Orsini M., Machado D. Amyotrophic lateral sclerosis: one or multiple causes? Neurol Int. 2011;3(1) doi: 10.4081/ni.2011.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C.A., McDermott C.J., Shaw P.J. Physical activity as an exogenous risk factor in motor neuron disease (MND): a review of the evidence. Amyotroph Lateral Scler Off Publ World Fed Neurol Res Group Mot Neuron Dis. 2009;10(4):191–204. doi: 10.1080/17482960802549739. [DOI] [PubMed] [Google Scholar]
- Lacorte E., Ferrigno L., Leoncini E., Corbo M., Boccia S., Vanacore N. Physical activity, and physical activity related to sports, leisure and occupational activity as risk factors for ALS: A systematic review. Neurosci Biobehav Rev. 2016;66:61–79. doi: 10.1016/j.neubiorev.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Chiò A., Benzi G., Dossena M., Mutani R., Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain J Neurol. 2005;128(Pt 3):472–476. doi: 10.1093/brain/awh373. [DOI] [PubMed] [Google Scholar]
- Lehman E.J., Hein M.J., Baron S.L., Gersic C.M. Neurodegenerative causes of death among retired National Football League players. Neurology. 2012;79(19):1970–1974. doi: 10.1212/WNL.0b013e31826daf50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser A.E., Rooney J.P.K., D’Ovidio F. Multicentre, cross-cultural, population-based, case-control study of physical activity as risk factor for amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2018;89(8):797–803. doi: 10.1136/jnnp-2017-317724. [DOI] [PubMed] [Google Scholar]
- Brooks B.R., Miller R.G., Swash M., Munsat T.L. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Mot Neuron Disord Off Publ. World Fed Neurol Res Group Mot Neuron Dis. 2000;1(5):293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- D’Ovidio F., Rooney J.P.K., Visser A.E. Critical issues in ALS case-control studies: the case of the Euro-MOTOR study. Amyotroph Lateral Scler Front Degener. 2017;18(5–6):411–418. doi: 10.1080/21678421.2017.1285939. [DOI] [PubMed] [Google Scholar]
- Pagani M., Öberg J., De Carli F. Metabolic spatial connectivity in amyotrophic lateral sclerosis as revealed by independent component analysis. Hum Brain Mapp. 2016;37(3):942–953. doi: 10.1002/hbm.23078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cistaro A., Pagani M., Montuschi A. The metabolic signature of C9ORF72-related ALS: FDG PET comparison with nonmutated patients. Eur J Nucl Med Mol Imaging. 2014;41(5):844–852. doi: 10.1007/s00259-013-2667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth B.E., Haskell W.L., Whitt M.C. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(9 Suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Varrone A., Asenbaum S., Vander Borght T. EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging. 2009;36(12):2103–2110. doi: 10.1007/s00259-009-1264-0. [DOI] [PubMed] [Google Scholar]
- Morbelli S., Perneczky R., Drzezga A. Metabolic networks underlying cognitive reserve in prodromal Alzheimer disease: a European Alzheimer disease consortium project. J Nucl Med Off Publ Soc Nucl Med. 2013;54(6):894–902. doi: 10.2967/jnumed.112.113928. [DOI] [PubMed] [Google Scholar]
- Montuschi A., Iazzolino B., Calvo A. Cognitive correlates in amyotrophic lateral sclerosis: a population-based study in Italy. J Neurol Neurosurg Psychiatry. 2015;86(2):168–173. doi: 10.1136/jnnp-2013-307223. [DOI] [PubMed] [Google Scholar]
- Strong M.J., Grace G.M., Freedman M. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Off Publ World Fed Neurol Res Group Mot Neuron Dis. 2009;10(3):131–146. doi: 10.1080/17482960802654364. [DOI] [PubMed] [Google Scholar]
- D’hulst L, Van Weehaeghe D, Chiò A, et al. Multicenter validation of [18F]-FDG PET and support-vector machine discriminant analysis in automatically classifying patients with amyotrophic lateral sclerosis versus controls. Amyotroph Lateral Scler Front Degener. 2018;19(7-8):570-577. doi:10.1080/21678421.2018.1476548. [DOI] [PubMed]
- Canosa A., Pagani M., Cistaro A. 18F-FDG-PET correlates of cognitive impairment in ALS. Neurology. 2016;86(1):44–49. doi: 10.1212/WNL.0000000000002242. [DOI] [PubMed] [Google Scholar]
- Chiò A., Pagani M., Agosta F., Calvo A., Cistaro A., Filippi M. Neuroimaging in amyotrophic lateral sclerosis: insights into structural and functional changes. Lancet Neurol. 2014;13(12):1228–1240. doi: 10.1016/S1474-4422(14)70167-X. [DOI] [PubMed] [Google Scholar]
- Pagani M., Chiò A., Valentini M.C. Functional pattern of brain FDG-PET in amyotrophic lateral sclerosis. Neurology. 2014;83(12):1067–1074. doi: 10.1212/WNL.0000000000000792. [DOI] [PubMed] [Google Scholar]
- Van Laere K., Vanhee A., Verschueren J. Value of 18fluorodeoxyglucose-positron- emission tomography in amyotrophic lateral sclerosis: a prospective study. JAMA Neurol. 2014;71(5):553–561. doi: 10.1001/jamaneurol.2014.62. [DOI] [PubMed] [Google Scholar]
- Reeber S.L., Otis T.S., Sillitoe R.V. New roles for the cerebellum in health and disease. Front Syst Neurosci. 2013;7:83. doi: 10.3389/fnsys.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke R a. L, Proudfoot M, Talbot K, Turner MR. The two-year progression of structural and functional cerebral MRI in amyotrophic lateral sclerosis. NeuroImage Clin. 2018;17:953-961. doi:10.1016/j.nicl.2017.12.025. [DOI] [PMC free article] [PubMed]
- Qiu T., Zhang Y., Tang X. Precentral degeneration and cerebellar compensation in amyotrophic lateral sclerosis: A multimodal MRI analysis. Hum Brain Mapp. 2019;40(12):3464–3474. doi: 10.1002/hbm.24609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abidi M., de Marco G., Couillandre A. Adaptive functional reorganization in amyotrophic lateral sclerosis: coexisting degenerative and compensatory changes. Eur J Neurol. 2020;27(1):121–128. doi: 10.1111/ene.14042. [DOI] [PubMed] [Google Scholar]
- Cedarbaum J.M., Stambler N. Performance of the Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS) in multicenter clinical trials. J Neurol Sci. 1997;152(Suppl 1):S1–S9. doi: 10.1016/s0022-510x(97)00237-2. [DOI] [PubMed] [Google Scholar]
- Turner M.R., Wotton C., Talbot K., Goldacre M.J. Cardiovascular fitness as a risk factor for amyotrophic lateral sclerosis: indirect evidence from record linkage study. J Neurol Neurosurg Psychiatry. 2012;83(4):395–398. doi: 10.1136/jnnp-2011-301161. [DOI] [PubMed] [Google Scholar]
- Mattsson P., Lönnstedt I., Nygren I., Askmark H. Physical fitness, but not muscle strength, is a risk factor for death in amyotrophic lateral sclerosis at an early age. J Neurol Neurosurg Psychiatry. 2012;83(4):390–394. doi: 10.1136/jnnp.2010.218982. [DOI] [PubMed] [Google Scholar]
- Huisman M.H.B., Seelen M., de Jong S.W. Lifetime physical activity and the risk of amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2013;84(9):976–981. doi: 10.1136/jnnp-2012-304724. [DOI] [PubMed] [Google Scholar]
- Chiò A., Mora G. Physical fitness and amyotrophic lateral sclerosis: dangerous liaisons or common genetic pathways? J Neurol Neurosurg Psychiatry. 2012;83(4):389. doi: 10.1136/jnnp-2012-302351. [DOI] [PubMed] [Google Scholar]
- Hamidou B., Couratier P., Besançon C., Nicol M., Preux P.M., Marin B. Epidemiological evidence that physical activity is not a risk factor for ALS. Eur J Epidemiol. 2014;29(7):459–475. doi: 10.1007/s10654-014-9923-2. [DOI] [PubMed] [Google Scholar]
- Jack C.R., Knopman D.S., Weigand S.D. An operational approach to National Institute on Aging-Alzheimer’s Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71(6):765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang F., Hållmarker U., James S. Amyotrophic lateral sclerosis among cross-country skiers in Sweden. Eur J Epidemiol. 2016;31(3):247–253. doi: 10.1007/s10654-015-0077-7. [DOI] [PubMed] [Google Scholar]
- Bandres-Ciga S, Noyce AJ, Hemani G, et al. Shared polygenic risk and causal inferences in amyotrophic lateral sclerosis. Ann Neurol. Published online February 5, 2019. doi:10.1002/ana.25431 [DOI] [PMC free article] [PubMed]
- Fernandes J., Arida R.M., Gomez-Pinilla F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci Biobehav Rev. 2017;80:443–456. doi: 10.1016/j.neubiorev.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Legault J., Litcofsky K.A. Neuroplasticity as a function of second language learning: anatomical changes in the human brain. Cortex J Devoted Study Nerv Syst Behav. 2014;58:301–324. doi: 10.1016/j.cortex.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Todorova V., Blokland A. Mitochondria and Synaptic Plasticity in the Mature and Aging Nervous System. Curr Neuropharmacol. 2017;15(1):166–173. doi: 10.2174/1570159x14666160414111821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be available upon request by interested researchers.