Plastic pollution is one of the most visible and complex environmental issues today. Interested and concerned parties include researchers, governmental agencies, nongovernmental organizations, industry, media, and the general public. One key assumption behind the issue and the public outcry is that plastics last indefinitely in the environment, resulting in chronic exposure that harms animals and humans. But the data supporting this assumption are scant.
We don’t have a complete understanding of how long plastic consumer goods last in the environment. Here, artist Mark Dion displays pieces of plastic retrieved from the ocean. Image credit: Tanya Bonakdar Gallery and Mark Dion (artist).
An accurate understanding of the persistence of plastic goods in the environment is critical for many stakeholders. Consumers need reliable information about that persistence to make informed choices. Researchers need this information because persistence is a key factor in models that predict how much plastic waste is in the environment and where it resides (1, 2), as well as the risks associated with this pollution (3). Legislators need this information to develop evidence-based policy that bans the use of plastics at the local, national, and international level.
The ubiquity of these bans is rivaled only by the range of information that drives public perception of how long it takes for different types of plastic goods to degrade in the environment. Our fundamental belief that scientific evidence should inform the public and drive environmental policy led us to one seemingly simple question: What evidence underpins how long plastic goods last in the environment?
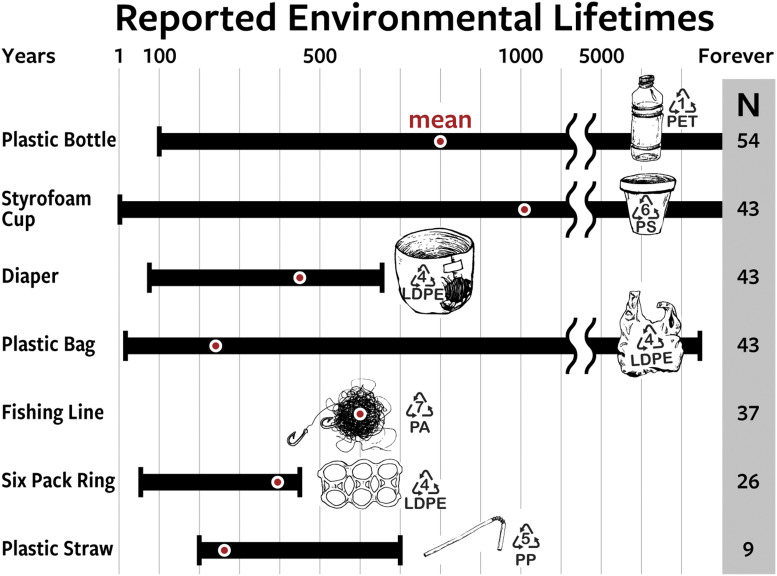
To answer this question, we reviewed information graphics and documents reporting the lifetime of different plastic goods in the environment (Figure 1, Table S1). These 57 graphics and documents were published by governmental agencies, nongovernmental organizations, academic institutions, peer-reviewed journals (4), college textbooks (5), reference books, lesson plans, nonprofit organizations, for-profit companies, and the print and online media. Many of the information graphics are displayed in public places such as parks, beaches, or aquaria. This review is global in nature, spanning 13 countries, three languages, and four continents. We report on four major findings.
Fig. 1.
Review of 57 information graphics and documents that report environmental lifetimes of common plastic consumer goods. The bars represent the range of estimates, the red circles represent the mean of estimates, and the number of estimates for each plastic good (N) is provided on the right (N = 255 in total). The recycling number corresponds to the base polymer of each good. PET = polyethylene terephthalate, PS = polystyrene, LDPE = low-density polyethylene, PA = polyamide, and PP = polypropylene. Individual lifetime estimates and additional details about the analysis are provided in Table S1 of the Supporting Information. Image credit: Natalie Reiner.
To be clear, none of these findings excuses the large and growing amount of plastic waste humans are producing. Cumulative plastic waste is estimated to rise from six to greater than 25 billion metric tons from 2015 to 2050 (6). Moreover, these findings do not excuse the ubiquitous plastic litter in the environment. Our sole intent here is to provide transparency on the quality of information currently being disseminated to stakeholders about the environmental persistence of plastic goods.
Wide Variation
First, estimates of the environmental lifetime of individual plastic goods vary substantially, in some cases from one year to “forever” (Fig. 1). For example, the lifetime of Styrofoam (expanded polystyrene) reported by the National Oceanic and Atmospheric Administration (NOAA) is 50 years (Table S1, Sources 1 and 2), whereas the United Nations Environmental Programme (UNEP) reports “thousands of years” (Table S1, Source 7). Closer to our home, the Woods Hole NOAA Sea Grant office located on Cape Cod, MA, reports a lifetime for plastic grocery bags of one to 20 years (Table S1, Source 2), whereas the local government of Nantucket, MA, reports a lifetime of 500 years (Table S1, Source 6). Sixty-five kilometers apart geographically, yet a 25- to 500-times difference in the lifetime of the same plastic good.
Second, not one of the 255 lifetime estimates from the 57 information graphics examined was based on peer-reviewed literature. In fact, 21 of 57 graphics (∼40%) didn’t provide any sources for the information they contain. For those that did provide such, we searched for the primary sources of the data they presented and found that it mainly originated from three sources: NOAA (Sea Grant and Marine Debris programs; Table S1, Sources 1 and 2), Mote Marine Laboratory and Aquarium (Sarasota, FL; Table S1, Sources 9 and 13–16), and the South Carolina Department of Health and Environmental Control (in coordination with NOAA Sea Grant and the Consortium for Ocean Science Exploration and Engagement; Table S1, Source 1). To be prudent, we contacted program directors at NOAA who confirmed that these estimates are not based on peer-reviewed science. Our literature search also revealed no record of environmental lifetime estimates for plastic goods conducted by researchers at Mote Marine Laboratory and Aquarium, a fact that was corroborated by conversations with a retired librarian from the institution.
Third, unlike recycling of plastic goods [only 9% of total plastic waste in 2015 was recycled (6)], recycling of this non–peer-reviewed information is extremely efficient. A prime example is the lifetime estimate of fishing line. We found 37 different information graphics with estimates for the environmental lifetime of fishing line, all providing the exact same number: 600 years. It is unlikely that 37 independent scientific studies arrived at the same result. Instead, it appears that all of these estimates were recycled from infographics originally published by NOAA, Mote Marine Laboratory and Aquarium, and the South Carolina Department of Health and Environmental Control (Table S1, Sources 1, 2, and 13–16). Moreover, the pervasiveness of these lifetime estimates is not limited to fishing line, but rather it is true for all plastic goods reviewed. For example, 40 of 54 estimates (∼75%) for the environmental lifetime of a plastic water bottle were 450 years (Table S1), an estimate that also appears to have originated from NOAA, Mote Marine Laboratory and Aquarium, and the South Carolina Department of Health and Environmental Control.
Finally, the wording in these infographics lacks precision, which could very well play a role in the wide range of lifetime estimates we saw. Without a definition of degradation, it is unclear what the plastics are degrading into. Are they degrading completely to carbon dioxide, degrading partially to different chemical products, or merely physically degrading into smaller pieces? These varied breakdown products have vastly different—not to mention poorly understood—risks to environmental and human health. Moreover, by not stating how or where the degradation occurs, the public is led to believe that lifetimes of plastic goods are the same in all environments.
Environmental Context
Recent peer-reviewed literature suggests that plastic in the environment may be more susceptible to degradation than previously recognized (7–12). In these studies, the common theme is that sunlight, rather than microbes, is sparking the degradation.
We recently reported environmental lifetime estimates of polystyrene, a type of plastic used in food containers and to make Styrofoam (7). We defined lifetime as complete degradation to carbon dioxide and partial degradation to new compounds. We found that, in the presence of sunlight, the lifetime of polystyrene is tens to hundreds of times shorter than in the absence of sunlight. This result led us to conclude that sunlight exposure, rather than microbial degradation, is one of the primary controls of the lifetime of polystyrene. Consequently, it is unlikely that polystyrene persists in the environment for thousands of years, as reported by UNEP (Table S1; Source 7).
This finding does not warrant disposal of polystyrene-based consumer goods in the environment. Nevertheless, it may be somewhat positive news that offers a path toward a more robust and comprehensive understanding of the persistence of other types of plastic goods in the environment.
At a Crossroads
The reality is that what the public and legislators know about the environmental persistence of plastic goods is often not based on solid science, despite the need for reliable information to form the foundation for a great many decisions, large and small.
Our intent is not to point fingers over the state of information regarding the environmental lifetime of plastic goods, especially because these early efforts
Rather, it is our hope that by bringing transparency to this environmental issue we will help improve the quality of information available to all stakeholders (e.g., consumers, researchers, and legislators) to make informed, sustainable decisions.
were presumably well intentioned. Again, these findings should not be interpreted as an endorsement of the mounting and unsustainable consumer demand of plastics (6), nor the ubiquitous, unpleasant, and possibly unhealthy plastic litter in the environment. Rather, it is our hope that by bringing transparency to this environmental issue we will help improve the quality of information available to all stakeholders (e.g., consumers, researchers, and legislators) to make informed, sustainable decisions.
We also recognize that this pressing question of environmental persistence is not going to be easy to answer. Plastic pieces in the environment are like snowflakes—no two are alike. They are a complex mixture of polymers and additives that come in all shapes and sizes (13, 14). In addition, the numerous natural processes that degrade plastics in the environment vary in their effectiveness across space and time, which should give members of the public and legislators pause when making broad statements about the lifetime of specific products. All of this underscores the fact that it will take substantial time and resources for the scientific community to answer this question of environmental persistence.
Nevertheless, we and many others are committed to quantifying the environmental persistence of plastic goods and are optimistic that we can offer stakeholders accurate, evidence-based information about the environmental lifetime of common plastic goods. Given the potential risks to environmental and human health, there’s plenty at stake.
Supplementary Material
Acknowledgments
We thank Briana Prado, Cassia Armstrong, and Anna Walsh for their help with the review, Kenneth Kostel, Katie Linehan, Daniel Ward, and Rose Cory for feedback on an earlier version of this piece, John Furfey for assistance with tracking down the original sources of the environmental lifetime estimates, and Natalie Reiner for help with Fig. 1. We acknowledge financial support from Woods Hole Oceanographic Institution (Woods Hole, MA) and the Seaver Institute (Los Angeles, CA).
Footnotes
The authors declare no competing interest.
Any opinions, findings, conclusions, or recommendations expressed in this work are those of the authors and have not been endorsed by the National Academy of Sciences.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2008009117/-/DCSupplemental.
References
- 1.Jambeck J. R., et al. , Marine pollution: Plastic waste inputs from land into the ocean. Science 347, 768–771 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Sherman P., Van Sebille E., Modeling marine surface microplastic transport to assess optimal removal locations. Environ. Res. Lett. 11, 1–6 (2016). [Google Scholar]
- 3.Gouin T., et al. , Toward the development and application of an environmental risk assessment framework for microplastic. Environ. Toxicol. Chem. 38, 2087–2100 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richards Z. T., Beger M., A quantification of the standing stock of macro-debris in Majuro lagoon and its effect on hard coral communities. Mar. Pollut. Bull. 62, 1693–1701 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Pipkin B. W., Trent D. D., Hazlett R. W., Bierman P., Geology and the Environment (Cengage Learning, Boston, MA, ed. 7, 2014). [Google Scholar]
- 6.Geyer R., Jambeck J. R., Law K. L., Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward C. P., Armstrong C. J., Walsh A. N., Jackson J. H., Reddy C. M., Sunlight converts polystyrene to carbon dioxide and dissolved organic carbon. Environ. Sci. Technol. Lett. 6, 669–674 (2019). [Google Scholar]
- 8.Gewert B., Plassmann M., Sandblom O., MacLeod M., Identification of chain scission products released to water by plastic exposed to ultraviolet light. Environ. Sci. Technol. Lett. 5, 272–276 (2018). [Google Scholar]
- 9.Rummel C. D., et al. , Effects of leachates from UV-weathered microplastic in cell-based bioassays. Environ. Sci. Technol. 53, 9214–9223 (2019). [DOI] [PubMed] [Google Scholar]
- 10.Khaled A., Rivaton A., Richard C., Jaber F., Sleiman M., Phototransformation of plastic containing brominated flame retardants: Enhanced fragmentation and release of photoproducts to water and air. Environ. Sci. Technol. 52, 11123–11131 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Zhu L., Zhao S., Bittar T. B., Stubbins A., Li D., Photochemical dissolution of buoyant microplastics to dissolved organic carbon: Rates and microbial impacts. J. Hazard. Mater. 383, 121065 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Royer S. J., Ferrón S., Wilson S. T., Karl D. M., Production of methane and ethylene from plastic in the environment. PLoS One 13, e0200574 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rochman C. M., et al. , Rethinking microplastics as a diverse contaminant suite. Environ. Toxicol. Chem. 38, 703–711 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Hanke U. M., Ward C. P., Reddy C. M., Leveraging lessons learned from black carbon research to study plastics in the environment. Environ. Sci. Technol. 53, 6599–6600 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.