Abstract
Cocaine is a known vasoactive drug associated with poor clinical outcomes and high in-hospital mortality related to aneurysmal subarachnoid hemorrhage; however, the association of prior cocaine use with the incidence of vasospasm and delayed cerebral ischemia remains controversial. We report a case of a 42-year-old male with a history of active cocaine use who presented with a severe headache. Imaging demonstrated diffuse cisternal subarachnoid hemorrhage due to a ruptured basilar apex aneurysm, which was successfully treated with endovascular coil embolization. Despite expedited endovascular treatment and an initially benign clinical course, he suffered from delayed cerebral ischemia resulting in cortical blindness due to bilateral posterior cerebral artery vasospasm secondary to repeat cocaine use weeks after his initial ictus. To our knowledge, the present case is the first to describe delayed cerebral ischemia resulting in a severe neurologic deficit due to repeat cocaine use weeks subsequent to aneurysm rupture. We review the current literature on the association of cocaine use with the incidence of vasospasm and delayed cerebral ischemia as well as the effects of cocaine on the cerebrovasculature.
Keywords: Aneurysm, cocaine, delayed cerebral ischemia, subarachnoid hemorrhage, vasospasm
Abbreviations: CT, computed tomography; DCI, delayed cerebral ischemia; CTA, computed tomography angiography; TCD, transcranial Doppler
Introduction
Cocaine is a highly vasoactive substance known to block the presynaptic reuptake of norepinephrine, serotonin, and dopamine [1]. Numerous reports have described the various neurologic sequelae associated with cocaine use including cerebral vasoconstriction, cerebral ischemia, intracranial hemorrhage, seizures, aneurysm formation, and aneurysm rupture as well as rerupture [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15]. Other less-recognized mechanisms leading to neurologic damage related to cocaine use involve cardiac dysfunction, a direct toxic effect on neurons, and increased platelet aggregation leading to thrombosis [1,[4], [5], [6],8]. Cocaine users who suffer from aneurysmal subarachnoid hemorrhage present at a younger age, with an increased amount of cisternal blood on computed tomography (CT) and tend to have worse clinical outcomes [[1], [2], [3], [4], [5],7,11]. However, whether a history of cocaine use prior to aneurysm rupture leads to worsened vasospasm and subsequent delayed cerebral ischemia (DCI) remains controversial [[2], [3], [4], [5],11,12,[14], [15]].
Here, we describe a case of a 42-year-old male with a history of active cocaine use found to have subarachnoid hemorrhage secondary to a ruptured basilar apex aneurysm. Despite successful aneurysm treatment and an initially benign clinical course, he suffered from delayed cerebral ischemia resulting in cortical blindness due to bilateral posterior cerebral artery vasospasm secondary to repeat cocaine use weeks after his initial ictus. This is the first report, to our knowledge, of DCI resulting in neurologic sequelae weeks subsequent to aneurysm rupture and directly related to a relapse in cocaine use. Moreover, we discuss the association of cocaine use with the incidence of vasospasm and delayed cerebral ischemia as well as the effects cocaine has on the cerebrovasculature.
Case report
A 42-year-old male with a history of active cocaine use presented with the sudden onset of a severe headache and nuchal rigidity consistent with Hunt-Hess grade 2 subarachnoid hemorrhage. Physical examination was unremarkable. He admitted to cocaine use 1-2 times per week for the past 3 years. Urine toxicology was negative for additional illicit substances. Noncontrast CT head revealed Fisher grade 4 diffuse cisternal subarachnoid hemorrhage (Fig. 1A). Computed tomography angiography (CTA) demonstrated an irregular saccular aneurysm arising from the basilar artery eccentric to the right (Figs. 1B and C). The patient was taken emergently to the endovascular suite for diagnosis and potential treatment. Digital subtraction angiography demonstrated a 4.0 × 4.4 mm saccular aneurysm projecting eccentrically to the right from the basilar artery apex with a small daughter sac projecting anterior-superior, which was successfully treated with endovascular coil embolization (Figs. 2A-D). Importantly, angiography demonstrated diffuse small caliber extracranial and intracranial vasculature, likely related to drug-induced vasospasm (Figs. 2E and F). He was monitored in the neurointensive care unit and underwent the basic aneurysmal subarachnoid hemorrhage protocol. Transcranial Doppler velocities began to elevate on post-procedure day three. The following day he developed acute onset altered mental status and blurry vision, prompting urgent cerebral angiogram. Angiography demonstrated moderate to severe vasospasm involving the distal basilar artery, bilateral supraclinoid segments of the internal carotid arteries, and bilateral M1 and A1 segments: all of which were treated with transarterial balloon angioplasty and intra-arterial verapamil infusion. He did not require return to the endovascular suite for the remainder of his initial hospitalization. On postprocedure day 11, magnetic resonance imaging of the brain was ordered to evaluate his persistent visual complaint; however, it was unremarkable for acute infarction. The next day, he opted to leave the hospital against medical advice. Prior to leaving, he was provided a prescription for nimodipine and educated numerous times by both neurosurgical staff and social workers regarding the potential catastrophic sequelae associated with cocaine relapse.
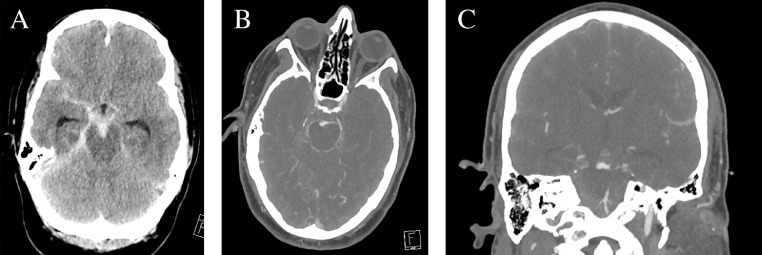
Fig. 1.
Imaging on presentation. (A) Axial CT demonstrated thick diffuse cisternal subarachnoid hemorrhage with clot within the interpeduncular cistern. (B) Axial and (C) coronal CT angiography revealed a large irregular saccular aneurysm arising from the basilar artery apex eccentric to the right.
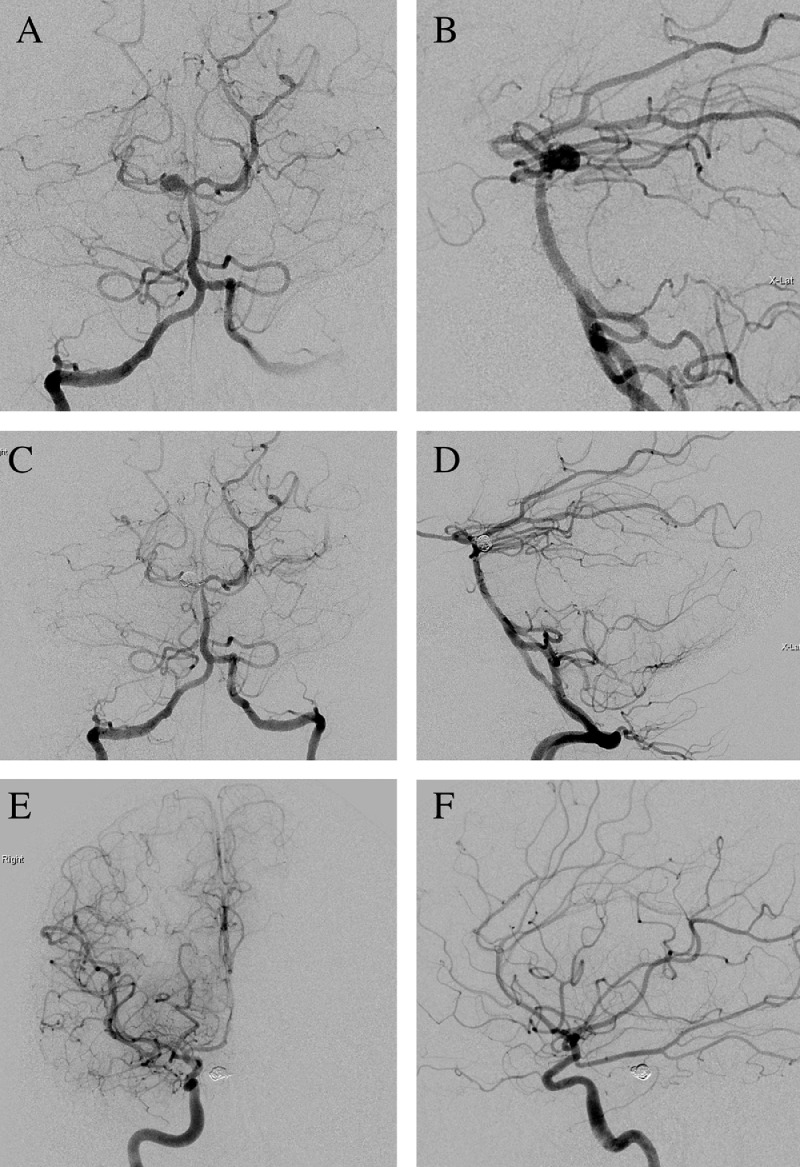
Fig. 2.
Digital subtraction angiography of a right vertebral artery injection pre- and postembolization revealed a 4.0 × 4.4 mm saccular aneurysm projecting eccentrically to the right from the basilar artery apex and small daughter sac projecting anterior-superior. (A) Anterior-posterior and (B) lateral views pre-embolization. (C) Anterior-posterior and (D) lateral views postembolization. (E) and (F) Digital subtraction angiography of a right internal carotid artery injection, which demonstrated diffuse small caliber cerebrovasculature and a fetal posterior-communicating artery.
Five days later, on postprocedure day 17, he presented to the emergency department with sudden onset total blindness. He admitted to cocaine relapse a few hours prior to the onset of blindness. CT head demonstrated extensive bilateral temporo-occipital lobe ischemic infarctions in posterior cerebral artery distributions (Fig. 3A). Emergent cerebral angiogram revealed diminutive flow through the bilateral posterior cerebral artery distributions, severe P2/3 junction vasospasm, sluggish flow through multiple P4 segments and mild diffuse vasospasm throughout the entire cerebrovasculature (Figs. 3B and C). Vasospasm of the posterior cerebral arteries was successfully treated with intra-arterial verapamil infusion. Over the next two days transcranial Doppler velocities were without significant elevation. Unfortunately, he remained cortically blind upon discharge to inpatient rehabilitation on post-procedure day 32.
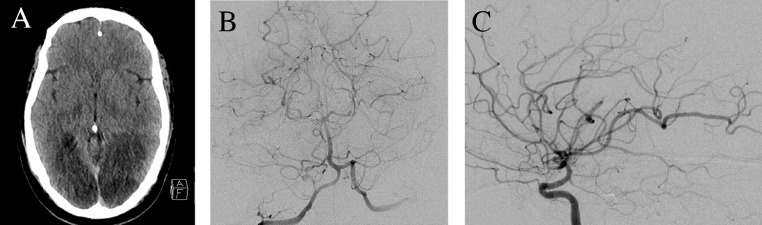
Fig. 3.
Imaging on representation on post-procedure day 17. (A) Axial CT revealed extensive ischemic infarction of the bilateral posterior cerebral artery distributions. (B) Digital subtraction angiography of a right vertebral artery injection anterior-posterior view with severe P2/3 junction vasospasm and sluggish flow through multiple P4 segments. (C) Right internal carotid artery injection lateral view with severe vasospasm involving the near fetal-type posterior communicating artery and diminutive flow through the posterior cerebral artery distributions.
Discussion
The vasoactive properties of cocaine derive from the facilitation of the monoamine neurotransmitters dopamine, serotonin, and norepinephrine [4,6,7,13]. Cocaine blocks presynaptic monoamine reuptake resulting in increased neurotransmitter concentration at the synapse and, among other potential sequelae, can lead to vasoconstriction downstream [1,2,4,6,7]. Independently, dopamine can activate alpha-1 receptors and lead to potent vasoconstriction [4,6]. Moreover, cocaine in the short term can upregulate synaptic dopamine receptor population and increase receptor sensitivity [4,6]. Other potential mechanisms of vasoconstriction include endothelial cell activation via endothelin-1 and increased calcium influx into vascular smooth muscle cells [2,8]. The metabolites of cocaine (benzoylecgonine and ecgonine) also exhibit vasoactive properties with longer half-lives than the parent drug, which may potentiate vasoconstrictive effects [1,2,6]. Though the potential systemic complications of cocaine are vast, its disastrous effects on the central nervous system include increased risk of cerebral aneurysm development, aneurysm rupture and rerupture, younger age at time of rupture, ischemic and hemorrhagic stroke, vasculitis, vasoconstriction, and platelet aggregation and thrombosis [[1], [2], [3], [4], [5], [6], [7],[10], [11], [12], [13]]. Moreover, Du et al identified a direct toxic effect of cocaine and its metabolites on neurons, resulting in increased intracellular calcium and cell death [8].
Whether the use of cocaine leads to an increase in radiographic or symptomatic vasospasm and subsequent DCI remains controversial. However, a history of recent cocaine use is reported in up to 33% of patients presenting with aneurysmal subarachnoid hemorrhage [2,4]. Several studies have demonstrated that, even in healthy subjects, the administration of cocaine will lead to potent vasospasm of the cerebral circulation [4,9,14]. This effect is compounded exponentially in those who have been previously exposed to cocaine [1,9]. These chronic cocaine users also demonstrate focal cerebrovascular dysfunction in the form of perfusion deficits, which are seen even after long-term abstinence [8,9,14]. Early studies evaluating vasospasm in aneurysmal subarachnoid hemorrhage patients with recent cocaine use (24-72 hours prior to ictus) demonstrated that those who used the drug exhibited a significant increase in vasospasm [[1], [2], [3], [4], [5],7,11]. Indeed, Howington et al retrospectively found a 2.8-fold greater risk of developing vasospasm and a 3.3-fold greater risk of poor clinical outcome (based on the Glasgow Outcome Scale) in the cocaine cohort, which had a total sample size of 150 patients [4]. Based on these data, the authors suggested a history of cocaine use to be considered equivalent to a major medical co-morbidity and be a factor when determining Hunt and Hess grade and overall prognosis [4]. A retrospective study by Chang et al found a 2.9-fold greater risk of in-hospital mortality and aneurysm rerupture in the cocaine cohort, which had a total sample size of 1,134 patients [2]. However, while the cocaine users exhibited an increase in vasospasm and DCI compared to the non-cocaine cohort, this association was not statistically significant when corrected for confounding variables [2]. Similarly, Alaraj et al reviewed 573 aneurysmal subarachnoid hemorrhage patients, 31 (5%) of which recently used cocaine, and found no statistically significant difference in vasospasm, functional outcome, Hunt-Hess or Fisher grade, incidence of symptomatic or radiologic vasospasm, stroke, or mortality in aneurysmal subarachnoid hemorrhage patients who used cocaine compared to nonusers [3]. Murthy et al reported a cohort of 102,174 aneurysmal subarachnoid hemorrhage patients of which 1702 (1.7%) reported cocaine use [15]. Cocaine users in this cohort were more commonly younger, males, African-American, and with more medical comorbidities compared to the non-cocaine group [15]. Cocaine users had a statistically significant higher incidence of hydrocephalus, seizures, longer length of stay, higher in-hospital mortality, and lower home discharge rates comparatively [15]. However, after adjusting for confounding variables, cocaine exhibited no effect on the incidence of symptomatic vasospasm [15].
As depicted prior, there is conflicting data in the literature regarding a correlation between a higher incidence of symptomatic vasospasm and active cocaine users with aneurysmal subarachnoid hemorrhage. Importantly, there are currently no studies that have assessed the potential effect of repeat cocaine use during the first 21 days following aneurysmal subarachnoid hemorrhage.
Conclusions
Cocaine is a highly vasoactive substance that is associated with catastrophic sequelae on the central nervous system. The available literature would argue that cocaine users with aneurysmal subarachnoid hemorrhage tend to have worse clinical outcomes. However, whether a history of cocaine use prior to aneurysm rupture leads to worsened vasospasm and subsequent delayed cerebral ischemia remains ambiguous. Here, we described a case of devastating delayed cerebral ischemia occurring weeks following aneurysm rupture and as a result of cocaine relapse. Indeed, further studies are necessary to understand the implications of active cocaine use following aneurysmal subarachnoid hemorrhage and its effects on vasospasm and delayed cerebral ischemia.
Footnotes
Conflicts of Interest: The authors declare that there are no conflicts of interest to report.
Disclosure Statement: The authors have no financial support, financial disclosures, or conflicts of interest of interest to report. The authors declare that there are no disclosures to report.
Ethics Statement: The patient described in this report provided written informed consent for its publication.
Funding sources: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions: All authors have made substantial contributions to all of the following: (1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, (2) drafting the article or revising it critically for important intellectual content, (3) final approval of the version to the submitted.
Contributor Information
Brendan J. Klein, Email: bklein1@carilionclinic.org.
Joshua A. Cuoco, Email: jacuoco@carilionclinic.org.
John J. Entwistle, Email: jjentwistle1@carilionclinic.org.
Eric A. Marvin, Email: eamarvin@carilionclinic.org.
Biraj M. Patel, Email: bmpatel@carilionclinic.org.
References
- 1.Si X., Luo J.J. Acute cocaine exposure and cerebrovascular diseases: a retrospective clinical study and literature Review. J Neurol Exp Neurosci. 2018;4(1):1–6. [Google Scholar]
- 2.Chang T.R., Kowalski R.G., Caserta F., Carhuapoma J.R., Tamargo R.J., Naval N.S. Impact of acute cocaine use on aneurysmal subarachnoid hemorrhage. Stroke. 2013;44(7):1825–1829. doi: 10.1161/STROKEAHA.111.000749. [DOI] [PubMed] [Google Scholar]
- 3.Alaraj A., Wallace A., Mander N., Aletich V., Charbel F.T., Amin-Hanjani S. Effect of acute cocaine use on vasospasm and outcome in aneurysmal subarachnoid hemorrhage. World Neurosurg. 2010;73(4):357–360. doi: 10.1016/j.wneu.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Howington J.U., Kutz S.C., Wilding G.E., Awasthi D. Cocaine use as a predictor of outcome in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2003;99(2):271–275. doi: 10.3171/jns.2003.99.2.0271. [DOI] [PubMed] [Google Scholar]
- 5.Vannemreddy P., Caldito G., Willis B., Nanda A. Influence of cocaine on ruptured intracranial aneurysms: a case control study of poor prognostic indicators. J Neurosurg. 2008;108(3):470–476. doi: 10.3171/JNS/2008/108/3/0470. [DOI] [PubMed] [Google Scholar]
- 6.Ciccarone D. Stimulant abuse: pharmacology, cocaine, methamphetamine, treatment, attempts at pharmacotherapy. Prim Care. 2011;38(1):41–58. doi: 10.1016/j.pop.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fessler R.D., Esshaki C.M., Stankewitz R.C., Johnson R.R., Diaz F.G. The neurovascular complications of cocaine. Surg Neurol. 1997;47(4):339–345. doi: 10.1016/s0090-3019(96)00431-4. [DOI] [PubMed] [Google Scholar]
- 8.Du C., Yu M., Volkow N.D., Koretsky A.P., Fowler J.S., Benveniste H. Cocaine increases the intracellular calcium concentration in brain independently of its cerebrovascular effects. J Neurosci. 2006;26(45):11522–11531. doi: 10.1523/JNEUROSCI.3612-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman M.J., Levin J.M., Ross M.H., Lange N., Rose S.L., Kukes T.J. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA. 1998;279(5):376–380. [PubMed] [Google Scholar]
- 10.Martin-Schild S., Albright K.C., Hallevi H., Barreto A.D., Philip M., Vivek M. Intracerebral hemorrhage in cocaine users. Stroke. 2010;41(4):680–684. doi: 10.1161/STROKEAHA.109.573147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conway J.E., Tamargo R.J. Cocaine use is an independent risk factor for cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2001;32(10):2338–2343. doi: 10.1161/hs1001.097041. [DOI] [PubMed] [Google Scholar]
- 12.Chang T.R., Kowalski R.G., Carhuapoma J.R., Tamargo R.J., Naval N.S. Cocaine use as an independent predictor of seizures after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2016;124(3):730–735. doi: 10.3171/2015.2.JNS142856. [DOI] [PubMed] [Google Scholar]
- 13.Buttner A., Mall G., Penning R., Sachs H., Weis S. The neuropathology of cocaine abuse. Leg Med (Tokyo) 2003;5(Suppl 1):S240–S242. doi: 10.1016/s1344-6223(02)00122-0. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Bermejo P., Rodriguez-Arias C., Crespo E., Perez-Fernandez S., Arenillas J.F., Martinez-Galdamez M. Severe cerebral vasospasm in chronic cocaine users during neurointerventional procedures: a report of two cases. Interv Neuroradiol. 2015;21(1):19–22. doi: 10.15274/INR-2014-10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murthy S., Shah S., Moradiya Y., Shastri A., Smirnakis S. Clinical outcomes of aneurysmal subarachnoid hemorrhage with cocaine use in the United States. Neurology. 2014;82(10 Suppl) S25.001. [Google Scholar]