Abstract
Renal arteriovenous fistula is classified into idiopathic, acquired and congenital. Endovascular therapy has become the gold standard compared to surgery. We present the embolization procedure of a renal fistula postbiopsy in a 57-year-old female patient through the use of St. Jude Medical Amplatzer vascular Plug IV. Although numerous embolizing agents are available, plug type IV has numerous advantages in terms of procedure times, speed of embolization and precision of the occlusion, but with a relative increase in costs.
Keywords: Transcatheter embolization; Coils Endovascular device; Renal embolization; Plug, interventional radiology
Introduction
Renal arteriovenous fistula is a rare pathological condition characterized by an abnormal connection between an arterial and venous vessel. Its forms are classified according to etiology into idiopathic, acquired and congenital. Idiopathic and congenital forms represent 30% of all renal fistulas [1], [2], [3]. Acquired renal arteriovenous fistula can be secondary to traumatic events, carcinoma, arteritis, biopsy [1], [2], [3]. The treatment has been surgical for years and involves partial or total nephrectomy and ligation of the arterial vessel [3,4]. Endovascular therapy allows to selectively occlude the afferent vessel and has progressively replaced surgery. Over the years, numerous embolizing materials have been proposed such as glues, coils and plugs. The plug represents a vascular occlusion system initially used in cardiac district (oval foramen occlusion or DIA) and subsequently extended in the peripheral area with the necessary structural changes. Currently, there are 4 models of St. Jude Medical Amplatzer vascular Plug that share 2 common components: a vascular plug and a delivery wire. In addition, all devices have a self-expanding system, a platinum radiopaque marker and a controlled and fast release mechanism. The structural characteristics of the different types of AVP are as follows: AVP I has a single lobe and single layer, AVP II has 3 lobes and multilayer, AVP III is bilobed and multilayer, AVP IV, is bilobed and single layer [5,6]. The indications are different: AVP I is indicated for vessels that have a reduced landing area, AVP II is very ductile due to the variability of available calibres and is indicated when rapid occlusion of the flow is required; contraindicated when there is a poor “landig zone,” the AVP III is used in medium-sized vessels, with a tortuous course and which have a high flow. AVP IV, available from 2009, with sizes from 4 to 8 mm represents the recent solution of the AVP family. The device can be released through a diagnostic 5 Fr catheter with compatible guide 0.038, the structural characteristics allow both the release in irregular course vessels and a rapid occlusion of the target [5,6] . We present the excellent result of embolization of a renal fistula postbiopsy in a 57-year-old female patient through the use of St. Jude Medical Amplatzer vascular Plug IV.
Case report
A 57-year-old female patient had been performing instrumental clinical follow-up for a nephrotic syndrome she was suffering from 10 years. About 5 years ago he had a left kidney biopsy resulting in the development of intrarenal fistula. Although nephrotic syndrome was well compensated, renal fistula had experienced clinical signs of heart failure in the past 2 years, worsened in the past 3 months. Interventional radiology consultancy was performed with indication of embolization of the renal fistula already known in the anamnesis. Blood count, liver function and coagulation indices were normal. Preliminary abdominal angio-CT examination was performed and it confirmed the presence of direct high flow fistula Fig. 1. After percutaneous common right femoral access, a left renal selective angiography confirmed a meso-renal high flow AVF involving second order branches. Using a 6Fr 45 cm introducer positioned in the main left renal artery, and AVP IV 7 × 12 mm was released at the site of the fistula. After 10 minutes residual fistula's flow with minimum cortical perfusion opacification defect was not observed Fig. 2. The patient developed a postembolization syndrome characterized by pain and fever that was resolved with medical therapy. There were no complications during and after the procedure. Eco color Doppler exams (immediately after the procedure, 30 days and 3 months later) confirmed the resolution of the AVF. Angio CT scan at 6 months did not show the fistula with renal focal infarction <20% of renal volume. The signs of heart failure decreased significantly. The renal function was normal. The patient currently performs clinical instrumental follow-up and is in good physical condition.
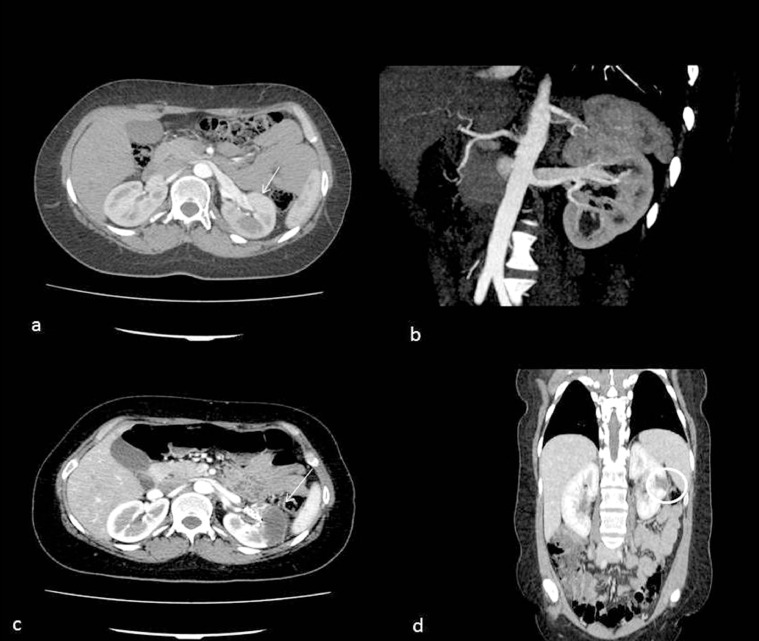
Fig. 1.
Angio-CT of the renal arteriovenous fistula (AVF). (a) Axial angio-CT of the high flow AVF (arrow) and reconstruction (b). Angio-CT of the postembolization AVF on the axial (c) and coronal (d) plane with parenchymal infarction <20% of the kidney (arrow and circle).
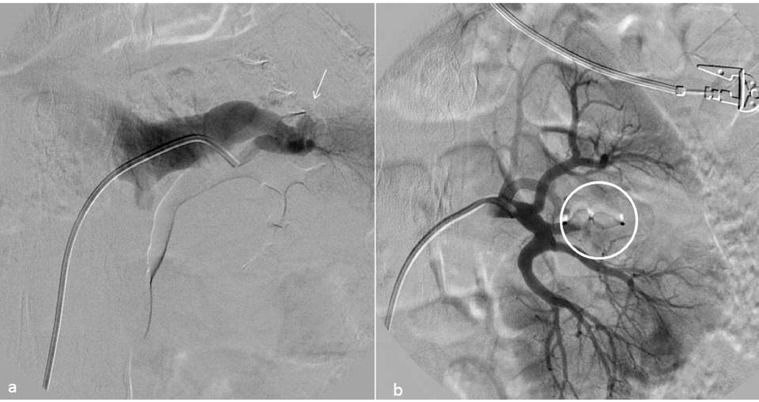
Fig. 2.
AVF treatment. (a) High flow fistula angiography (arrow) and subsequent treatment with release of AVP IV (circle) at the corresponding afferent arterial branch and flow stop (b).
Discussion
Renal arteriovenous fistula is a rare and very often incidental pathology. It can be classified according to the etiological characteristics in idiopathic, congenital and acquired. The forms acquired make up 70%-80% of the total [3,7,8]. The most frequent signs and symptoms are: heart failure, tachycardia, hypertension, hematuria, abdominal pain and are found mainly in the late stages of the pathology [2,3,7,9]. Diagnostic imaging has an important role [[10], [11], [12]]: Eco-color-Doppler examinations provide accurate information on the blood flow and the extent of the fistula; CT-angiography or MR-angiography allow a detailed study of the angio-architecture of the renal arteriovenous fistula with the possibility of 3-dimensional reconstructions; angiography in the past was gold standard for diagnosis, nowadays it is exclusively used for therapeutic treatment that, in the last decades, has become the first line treatment for it. The endovascular embolization has a high success rate, low risk of short and long-term complications as well as a reduction in hospitalization days and operating costs [3], [4], [5], [6], [7], [8], [9]. There are various agents for embolization and the choice of different materials depends on the characteristics of the renal fistula: size, site and degree of flow. Coils are the most frequently used for easy availability, low cost and ease of use. However, they present a risk of migration in the venous and pulmonary circulation and in general several spirals are needed for the complete occlusion of the vessel. The plugs have been introduced in clinical activity in recent decades initially for the treatment of heart diseases and subsequently for peripheral vessels. The structural characteristics of AVP IV allow the release both in irregular and high flow course vessels and a rapid occlusion of the target [13], [14], [15]. In our experience, the use of AVP IV has shown an excellent result without finding any complications during and after the interventional procedure. A single plug was used that perfectly adapted to the renal arteriovenous fistula's afferent artery. Plug IV can be released in tortuous and small-sized vessels and results in complete occlusion of the vessel in a short time. Furthermore, the use of the single device significantly reduces the procedural times with respect to the positioning of coils; in fact, very often more spirals are needed for the complete occlusion of the vessel. Although the cost of the single plug is often higher than the single coil, the use of multiple spirals and above all of the technologically more advanced ones (3D or 4D controlled release) involves an overall expenditure equal to or higher. The plug was released at the arteriovenous communication without any involvement of the nontarget arteries. The technical characteristics of the device allow to perform very accurate preliminary checks that guarantee the selective occlusion of the target vascular segment with a very low risk of migration. The release of the plug occurred in seconds and complete occlusion of the fistula occurred in 10 minutes. The release system is quick and safe. The structural characteristics of the device determine a complete embolization within 10 minutes from the release since it progressively adapts to the target vessel causing a slowing down of the blood flow until its complete stop. The postprocedural follow-up involves the execution of an ECD examination approximately 3-6 and 12 months after the interventional procedure. It is also advisable to perform an angio-CT examination about 6 months after the surgery to view more accurately the location of the device and the angioarchitecture of the organ. Monitoring of heart function and kidney function are further elements of assessment of the effectiveness of the treatment. Subsequently it is sufficient to carry out checks with ECD and annual cardiac and renal function tests.
In our case, ECD exams and CT performed 6 months later the treatment did not show arterial and fistula recanalization with regular opacification of the remaining vascular angioarchitecture. The area of infarction appeared on CT slightly reduced in volume with clearer margins for postembolization adaptation. In conclusion AVP IV, although still not widespread, has growing potential and numerous advantages: procedure times, speed of embolization and precision of occlusion, with minimal increase in costs.
Footnotes
Competing Interests: The authors declare that they have no conflicts of interest and the study was approved by the ethics committee of the institution.
References
- 1.Osawa T, Watarai Y, Morita K, Kakizaki H, Nonomura K. Surgery for giant high-flow renal arteriovenous fistula: experience in one institution. BJU Int. 2006;97(4):794–798. doi: 10.1111/j.1464-410X.2006.06108.x. [DOI] [PubMed] [Google Scholar]
- 2.Khawaja AT, McLean GK, Srinivasan V. Successful intervention for high-output cardiac failure caused by massive renal arteriovenous fistula-a case report. Angiology. 2004;55(2):205–208. doi: 10.1177/000331970405500213. [DOI] [PubMed] [Google Scholar]
- 3.Abdel-Aal AK, Elsabbagh A, Soliman H, Hamed M, Underwood E, Saddekni S. Percutaneous embolization of postnephrectomy arteriovenous fistula with intervening pseudoaneurym using the Amplatzer vascular plug 2. Vasc Endovascular Surg. 2014;48(7-8):516–521. doi: 10.1177/1538574414561230. [DOI] [PubMed] [Google Scholar]
- 4.Matos A, Moreira A, Mendonc¸a M. Renal arteriovenous fistula after nephrectomy. Ann Vasc Surg. 1992;6(4):378–380. doi: 10.1007/BF02008797. [DOI] [PubMed] [Google Scholar]
- 5.Wang W, Li H, Tam MD, Zhou D, Wang DX, Spain J. The amplatzer vascular plug: a review of the device and its clinical applications. Cardiovasc Intervent Radiol. 2012;35(4):725–740. doi: 10.1007/s00270-012-0387-z. [DOI] [PubMed] [Google Scholar]
- 6.Ramakrishnan S. Vascular plugs - A key companion to Interventionists - 'Just Plug it'. Indian Heart J. 2015;67(4):399–405. doi: 10.1016/j.ihj.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shih CH, Liang PC, Chiang FT, Tseng CD, Tseng YZ, Hsu KL. Transcatheter embolization of a huge renal arteriovenous fistula with Amplatzer Vascular Plug. Heart Vessels. 2010;25(4):356–358. doi: 10.1007/s00380-009-1210-x. [DOI] [PubMed] [Google Scholar]
- 8.Kayser O, Scha¨fer P. Transcatheter Amplatzer vascular plugembolization of a giant postnephrectomy arteriovenous fistula combined with an aneurysm of the renal pedicle by throughand- through, arteriovenous access. Ger Med Sci. 2013;11 doi: 10.3205/000169. Doc01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bozgeyik Z, Ozdemir H, Orhan I, Cihangiroglu M, Cetinkaya Z. Pseudoaneurysm and renal arteriovenous fistula after nephrectomy: two cases treated by transcatheter coil embolization. Emerg Radiol. 2008;15(2):119–122. doi: 10.1007/s10140-007-0646-5. [DOI] [PubMed] [Google Scholar]
- 10.Corvino A, Rosa D, Sbordone C, Nunziata A, Corvino F, Varelli C. Diastasis of rectus abdominis muscles: patterns of anatomical variation as demonstrated by ultrasound. Pol J Radiol. 2019;84:e542–e548. doi: 10.5114/pjr.2019.91303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corvino A, Pignata S, Campanino MR, Corvino F, Giurazza F, Tafuri D. Thyroglossal duct cysts and site-specific differential diagnoses: imaging findings with emphasis on ultrasound assessment. J Ultrasound. Feb 12 2020 doi: 10.1007/s40477-020-00433-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Catelli Antonio, Ponsiglione Andrea, Capaldo Iolanda, Corvino Antonio, Radice Leonardo, Venetucci Pietro. Giant endometriod ovarian cancer: the role of dagnostic imaging and figo staging. Euro Medit Biomed J. 2020;15(22):94–96. [Google Scholar]
- 13.Wang W, Li H, Tam MD, Zhou D, Wang DX, Spain J. The amplatzer vascular plug: a review of the device and its clinical applications. Cardiovasc Intervent Radiol. 2012;35(4):725–740. doi: 10.1007/s00270-012-0387-z. Epub 2012 Apr 21. [DOI] [PubMed] [Google Scholar]
- 14.Ramakrishnan S. Vascular plugs - a key companion to Interventionists - ‘Just Plug it’. Indian Heart J. Jul-Aug 2015;67(4):399–405. doi: 10.1016/j.ihj.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venetucci P, Quarantelli M, Iaccarino V. A rare case of recurrent hematuria from right kidney: radiologic diagnosis and treatment. ISRN Urol. 2011;2011 doi: 10.5402/2011/159104. [DOI] [PMC free article] [PubMed] [Google Scholar]